WO2011104667A1 - Basic aminoacid salts of polyphenols - Google Patents
Basic aminoacid salts of polyphenols Download PDFInfo
- Publication number
- WO2011104667A1 WO2011104667A1 PCT/IB2011/050737 IB2011050737W WO2011104667A1 WO 2011104667 A1 WO2011104667 A1 WO 2011104667A1 IB 2011050737 W IB2011050737 W IB 2011050737W WO 2011104667 A1 WO2011104667 A1 WO 2011104667A1
- Authority
- WO
- WIPO (PCT)
- Prior art keywords
- polyphenol
- compound
- formula
- group
- solution
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Ceased
Links
- BWMDMTSNSXYYSP-UHFFFAOYSA-N CCCNC(N)=N Chemical compound CCCNC(N)=N BWMDMTSNSXYYSP-UHFFFAOYSA-N 0.000 description 2
- YUKZXAYRTOPCEJ-UHFFFAOYSA-N CC(C)(C)C1=CNCC=N1 Chemical compound CC(C)(C)C1=CNCC=N1 YUKZXAYRTOPCEJ-UHFFFAOYSA-N 0.000 description 1
- LUKBXSAWLPMMSZ-OWOJBTEDSA-N Oc1ccc(/C=C/c2cc(O)cc(O)c2)cc1 Chemical compound Oc1ccc(/C=C/c2cc(O)cc(O)c2)cc1 LUKBXSAWLPMMSZ-OWOJBTEDSA-N 0.000 description 1
Classifications
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07C—ACYCLIC OR CARBOCYCLIC COMPOUNDS
- C07C229/00—Compounds containing amino and carboxyl groups bound to the same carbon skeleton
- C07C229/02—Compounds containing amino and carboxyl groups bound to the same carbon skeleton having amino and carboxyl groups bound to acyclic carbon atoms of the same carbon skeleton
- C07C229/04—Compounds containing amino and carboxyl groups bound to the same carbon skeleton having amino and carboxyl groups bound to acyclic carbon atoms of the same carbon skeleton the carbon skeleton being acyclic and saturated
- C07C229/26—Compounds containing amino and carboxyl groups bound to the same carbon skeleton having amino and carboxyl groups bound to acyclic carbon atoms of the same carbon skeleton the carbon skeleton being acyclic and saturated having more than one amino group bound to the carbon skeleton, e.g. lysine
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07C—ACYCLIC OR CARBOCYCLIC COMPOUNDS
- C07C279/00—Derivatives of guanidine, i.e. compounds containing the group, the singly-bound nitrogen atoms not being part of nitro or nitroso groups
- C07C279/04—Derivatives of guanidine, i.e. compounds containing the group, the singly-bound nitrogen atoms not being part of nitro or nitroso groups having nitrogen atoms of guanidine groups bound to acyclic carbon atoms of a carbon skeleton
- C07C279/14—Derivatives of guanidine, i.e. compounds containing the group, the singly-bound nitrogen atoms not being part of nitro or nitroso groups having nitrogen atoms of guanidine groups bound to acyclic carbon atoms of a carbon skeleton being further substituted by carboxyl groups
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D233/00—Heterocyclic compounds containing 1,3-diazole or hydrogenated 1,3-diazole rings, not condensed with other rings
- C07D233/54—Heterocyclic compounds containing 1,3-diazole or hydrogenated 1,3-diazole rings, not condensed with other rings having two double bonds between ring members or between ring members and non-ring members
- C07D233/64—Heterocyclic compounds containing 1,3-diazole or hydrogenated 1,3-diazole rings, not condensed with other rings having two double bonds between ring members or between ring members and non-ring members with substituted hydrocarbon radicals attached to ring carbon atoms, e.g. histidine
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D311/00—Heterocyclic compounds containing six-membered rings having one oxygen atom as the only hetero atom, condensed with other rings
- C07D311/02—Heterocyclic compounds containing six-membered rings having one oxygen atom as the only hetero atom, condensed with other rings ortho- or peri-condensed with carbocyclic rings or ring systems
- C07D311/04—Benzo[b]pyrans, not hydrogenated in the carbocyclic ring
- C07D311/22—Benzo[b]pyrans, not hydrogenated in the carbocyclic ring with oxygen or sulfur atoms directly attached in position 4
- C07D311/26—Benzo[b]pyrans, not hydrogenated in the carbocyclic ring with oxygen or sulfur atoms directly attached in position 4 with aromatic rings attached in position 2 or 3
- C07D311/28—Benzo[b]pyrans, not hydrogenated in the carbocyclic ring with oxygen or sulfur atoms directly attached in position 4 with aromatic rings attached in position 2 or 3 with aromatic rings attached in position 2 only
- C07D311/30—Benzo[b]pyrans, not hydrogenated in the carbocyclic ring with oxygen or sulfur atoms directly attached in position 4 with aromatic rings attached in position 2 or 3 with aromatic rings attached in position 2 only not hydrogenated in the hetero ring, e.g. flavones
Definitions
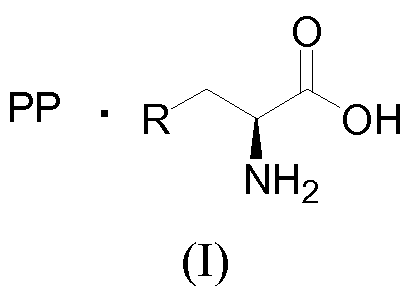
- the present disclosure relates to the basic amino acid salts of polyphenols that are having the general formula (I) with improved stability, solubility and pharmacological properties over parent polyphenols. More particularly, the present disclosure relates to L-Arginine salts of resveratrol and the methods of preparations thereof. The present disclosure also describes improved oral bioavailability of polyphenol derivatives.
- the present disclosure also provides a process for the preparation of the above said compounds of the general formula (I).
- Polyphenols such as resveratrol exhibits a wide variety of biological activities and are widely used as anti aging agent and also it is widely exploited as an antioxidant.
- compositions containing unprotected polyphenols are not likely to deliver their complete biological potential and the provision of protective packaging or special handling necessary to preserve their activity is too costly to be commercially feasible on a large scale.
- WO 2005/000780 Al describes about to compounds of general formula (I) having a trans configuration: wherein: R is selected from COOH and a group of formula (II):
- Ri is H, OH or R 2 , and R 2 is independently selected from OH, linear or branched 0- (Cl- C6) alkyl optionally substituted with a group selected from OH or O- (C1-C6) alkyl; R 3 is independently selected from H and linear or branched (C1-C6) alkyl optionally substituted with a group selected from OH or O- (C1-C6) alkyl.
- A is a substituted or unsubstituted, saturated or unsaturated alkyl radical having from 1 to 20 carbon atoms, which is bonded to the polyphenol by: a carboxylic ester function on a hydroxyl function of the said polyphenol; or by means of an A' spacer, in which A is bonded to A' via a carboxylic ester function, and A' is bonded to the polyphenol via a carboxylic ester function on a hydroxyl function of the said polyphenol;
- n is an integer greater than or equal to 1 ;
- B is a precursor of a biologically active molecule which is bonded to the polyphenol by: a carboxylic ester function on a hydroxyl function of the said polyphenol; or by means of a B' spacer, in which B is bonded to B' via a carboxylic ester function, and B' is bonded to the polyphenol via a carboxylic ester function on a hydroxyl function of the said polyphenol;
- n is an integer greater than or equal to 1.
- the present disclosure provides a information about polyphenolic derivatives which are more water soluble and enhanced in their activity.
- PP polyphenol and R is selected from a group comprising;
- PP polyphenol and R is selected from a group comprising
- composition comprising acts of a) adding amino acid solution to a solution of polyphenol to obtain a mixture, b) heating the mixture to obtain the compound of formula (I) and c) optionally adding with pharmaceutically acceptable excipients; a composition comprising a compound of formula (I) along pharmaceutically acceptable excipients(s) to the compound of formula (I) to obtain a composition.
- PP polyphenol and R is selected from a group comprising
- Figure 1 shows comparison of pharmacokinetics parameters of Resveratrol and Resveratrol-L-Arginine salt.
- Figure 2 provides a graph of Resveratrol standard calibration curve at ⁇ ⁇ 306nm.
- Figure 3 provides a graph Resveratrol-L-arginine salt standard calibration curve at ⁇ max 306nm.
- PP is polyphenol
- R is selected from a group comprising
- the polyphenol is Resveratrol and the R is
- PP is polyphenol
- R is selected from a group comprising
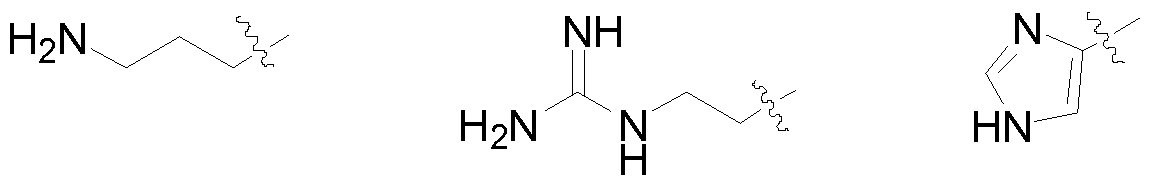
- the amino acid is a basic amino acid, selected from a group comprising Arginine, Lysine and Histidine.
- the amino acid solution is a solution in a solvent selected from a group comprising water, methanol, ethanol, propanol and butanol or combination thereof.
- the polyphenol solution is a solution in ethanol.
- the addition of amino acid solution is carried out at a temperature ranging from about 20°C to about 30°C, preferably at about 25°C .
- the heating is carried out at a temperature ranging from about 60°C to about 80°C preferably at about 70°C.
- the present disclosure is also in relation to a composition comprising a compound of formula (I) along with pharmaceutically acceptable excipients.
- the pharmaceutically acceptable excipients are selected but not limiting to a group comprising binders, disintegrants, diluents, lubricants, plasticizers, permeation enhancers and solubilizers.
- the composition is in a form selected but not limiting to a group comprising tablet, capsule, powder, syrup, solution, aerosol and suspension.
- the present disclosure is also in relation to a method for increasing the bioavailability of a polyphenol said method comprising an act of contacting an animal in need thereof with a compound of formula (I) or a pharmaceutical compositioncomprising compound of formula (I).
- the polyphenol is Resveratrol and the R are;
- PP represents polyphenols and polyphenols like Resveratrol, Quercetin, Luteolin, Curcumin as given below and each of them may be optionally substituted.
- R is selected from basic amino acid side chain such as:
- the poly phenols and amino acid may be optionally substituted with various substitutions possible for a person skilled in the art.
- the compound of formula (I) is particularly arginine salt of Resveratrol.
- the compounds are useful for methods for improving antioxidant properties by increasing the oral bioavailability of resveratrol andsolubility.
- the disclosure also provides a method of delayed release of resveratrol as derivatives which can improve half life and bio availability of resveratrol.
- Another embodiment of the present disclosure provides a method for rendering water- soluble an insoluble resveratrol which comprises salts of the insoluble polyphenol to an extent sufficient to render the polyphenol water-soluble.
- the present disclosure is also in relation to a pharmaceutical composition, comprising a compound of formula (I) along with pharmaceutically acceptable excipient selected but not limiting to a group comprising of binders, disintegrants, diluents, lubricants, plasticizers, permeation enhancers and solubilizers.
- the composition is in the form selected but not limiting to a group comprising of tablet, capsule, powder, syrup, solution, aerosol and suspension.
- Optionally substituted means that substitution is optional and therefore it is possible for the designated atom or molecule to be unsubstituted. In the event a substitution is desired, then such substitution means that any number of hydrogens on the designated atom is replaced with a selection from the indicated group, provided that the normal valency of the designated atom is not exceeded, and that the substitution results in a stable compound.
- Pharmaceutically acceptable salts include base addition salts such as alkali metal salts like Li, Na, and K salts; alkaline earth metal salts like Ca and Mg, salts of organic bases such as lysine, arginine, guanidine, diethanolamine, O-phenylethylamine, benzylamine, piperidine, morpholine, pyridine, hydroxyethylpyrrolidine, hydroxyethylpiperidine, choline, ammonium or substituted ammonium salts, aluminum salts. Salts also include amino acid salts such as glycine, alanine, cystine, cysteine, lysine, arginine, phenylalanine, guanidine.
- Salts may include acid addition salts where appropriate, which are sulphates, nitrates, phosphates, perchlorates, borates, hydrohalides, acetates, tartrates, maleates, citrates, succinates, palmoates, methanesulphonates, tosylates, benzoates, salicylates, hydroxynaphthoates, benzenesulfonates, ascorbates, glycerophosphates, ketoglutarates.
- Pharmaceutically acceptable solvates may be hydrates or comprising of other solvents of crystallization such as alcohols.
- the term analog includes a compound, which differs from the parent structure by one or more C, N, O or S atoms.
- stereoisomer includes isomers that differ from one another in the way the atoms are arranged in space, but whose chemical formulas and structures are otherwise identical.
- Stereoisomers include enantiomers and diastereoisomers.
- tautomers include readily interconvertible isomeric forms of a compound in equilibrium.
- polymorphs include crystallographically distinct forms of compounds with chemically identical structures.
- pharmaceutically acceptable solvates includes combinations of solvent molecules with molecules or ions of the solute compound.
- derivative refers to a compound obtained from a compound according to formula (I), an analog, tautomeric form, stereoisomer, polymorph, hydrate, pharmaceutically acceptable salt or pharmaceutically acceptable solvate thereof, by a simple chemical process converting one or more functional groups, such as, by oxidation, hydrogenation, alkylation, esterification, halogenation.
- pharmacological properties includes but not limited to antioxidant properties, Type II diabetes or hyperglycemia, cancers including skin, breast, cervix, colon, lung, liver, lymphoma, prostate, heart diseases, optic neuritis and retinal degeneration, neurodegeneration, stroke and cardiac arrest, osteoporosis, kidney dysfunction and albuminuria, cataracts, inflammatory bowel diseases (e.g. colitis), COPD (emphysema).
- Type II diabetes or hyperglycemia cancers including skin, breast, cervix, colon, lung, liver, lymphoma, prostate, heart diseases, optic neuritis and retinal degeneration, neurodegeneration, stroke and cardiac arrest, osteoporosis, kidney dysfunction and albuminuria, cataracts, inflammatory bowel diseases (e.g. colitis), COPD (emphysema).
- the compounds of this disclosure may be prepared by the following process.
- Resveratrol-L-Arginine salt showed about 6 times higher Cmax and about 5 times higher AUC as compared to parent Resveratrol
- Resveratrol salt showed about 6 times increase in the maximum plasma concentrations (Cmax) and about 5 times increase in the AUC.
- the figure 1 provides the comparative analysis of Resveratrol and Resveratrol-L-arginine salt.
- Intrinsic solubility of Resveratrol and Resveratrol-L-arginine salt The test compound was allowed to saturate in an aqueous medium (Milli-Q water) and were equilibrated for about 8 hrs at 25° C. The equilibrated solution was centrifuged at 5,000 rpm for 15 min at 25° C and the supernatant was analyzed by UV spectrometer. A standard linearity curve was obtained at ⁇ max using UV spectrometer. The information is tabulated in table 2.
Landscapes
- Chemical & Material Sciences (AREA)
- Organic Chemistry (AREA)
- Organic Low-Molecular-Weight Compounds And Preparation Thereof (AREA)
- Acyclic And Carbocyclic Compounds In Medicinal Compositions (AREA)
Abstract
The present disclosure describes about basic amino acid salts of polyphenolic compounds of formula (I) and composition thereof having improved physicochemical and pharmacological prosperities. Also describes process for preparation of the same. Formula (I).
Description
BASIC AMINOACID SALTS OF POLYPHENOLS
TECHNICAL FIELD:
The present disclosure relates to the basic amino acid salts of polyphenols that are having the general formula (I) with improved stability, solubility and pharmacological properties over parent polyphenols. More particularly, the present disclosure relates to L-Arginine salts of resveratrol and the methods of preparations thereof. The present disclosure also describes improved oral bioavailability of polyphenol derivatives.
(I)
The present disclosure also provides a process for the preparation of the above said compounds of the general formula (I).
BACKGROUND OF DISCLOSURE AND PRIOR ART
Polyphenols such as resveratrol exhibits a wide variety of biological activities and are widely used as anti aging agent and also it is widely exploited as an antioxidant.
These classes of compounds are highly susceptible to degradation by exposure to heat or light. Oral bioavailability of polyphenols is low because it is rapidly metabolized in intestines and liver into conjugated forms - glucuronate and sulfonate.
Because of this inherent instability of this class of compounds, the true scope of its utility has not yet fully been realized. Compositions containing unprotected polyphenols are not likely to deliver their complete biological potential and the provision of protective packaging or special handling necessary to preserve their activity is too costly to be commercially feasible on a large scale. Thus, there continues to be a need for polyphenol- containing compositions with improved stability and enhanced biological activity.
WO 2005/000780 Al describes about to compounds of general formula (I) having a trans configuration: wherein: R is selected from COOH and a group of formula (II):
Ri is H, OH or R2, and R2 is independently selected from OH, linear or branched 0- (Cl- C6) alkyl optionally substituted with a group selected from OH or O- (C1-C6) alkyl; R3 is independently selected from H and linear or branched (C1-C6) alkyl optionally substituted with a group selected from OH or O- (C1-C6) alkyl.
The aforesaid compounds-hydroxy stilbenes with R = an aromatic radical and hydroxy cinnamic acids with R = COOH-have a delocalised phenolic or carboxylic type acidic group and may hence form a piperazinium salt.
As per this patent the piperazinium salts of hydroxycinnamic acids and hydroxy stilbenes are stable with respect to the trans/cis isomerisation of said compounds.
US 2009 /0215881 Al describes about A bioprecursor having the structural formula: [A]„— PP— [B]m in which: PP is a polyphenol radical in which each hydroxyl function is protected by a group A or a group B;
A is a substituted or unsubstituted, saturated or unsaturated alkyl radical having from 1 to 20 carbon atoms, which is bonded to the polyphenol by: a carboxylic ester function on a hydroxyl function of the said polyphenol; or by means of an A' spacer, in which A is
bonded to A' via a carboxylic ester function, and A' is bonded to the polyphenol via a carboxylic ester function on a hydroxyl function of the said polyphenol;
n is an integer greater than or equal to 1 ;
B is a precursor of a biologically active molecule which is bonded to the polyphenol by: a carboxylic ester function on a hydroxyl function of the said polyphenol; or by means of a B' spacer, in which B is bonded to B' via a carboxylic ester function, and B' is bonded to the polyphenol via a carboxylic ester function on a hydroxyl function of the said polyphenol;
and m is an integer greater than or equal to 1.
The present disclosure provides a information about polyphenolic derivatives which are more water soluble and enhanced in their activity.
STATEMENT OF DISCLOSURE
(I)
(I)
wherein PP is polyphenol and R is selected from a group comprising
comprises acts of a) adding amino acid solution to a solution of polyphenol to obtain a mixture, b) heating the mixture to obtain the compound of formula (I) and c) optionally adding with pharmaceutically acceptable excipients; a composition comprising a compound of formula (I) along pharmaceutically acceptable excipients(s) to the compound of formula (I) to obtain a composition.,
(a) (b) (c)
and a method for delaying the release of polyphenol to improve half life and bioavailability of polyphenol said method comprising contacting an animal in need thereof with a compound of formula (I) or a pharmaceutical composition comprising compound of formula (I).
BRIEF DESCRIPTION OF ACCOMPANYING FIGURE:
The features of the present disclosure will become more fully apparent from the following description and appended claims, taken in conjunction with the accompanying figures. Understanding that these figures depict only several embodiments in accordance with the disclosure and are; therefore, not to be considered limiting of its scope, the
disclosure will be described with additional specificity and detail through use of the accompanying figures:
Figure 1: shows comparison of pharmacokinetics parameters of Resveratrol and Resveratrol-L-Arginine salt.
Figure 2: provides a graph of Resveratrol standard calibration curve at λ ^ 306nm.
Figure 3: provides a graph Resveratrol-L-arginine salt standard calibration curve at λ max 306nm.
DETAILED DESCRIPTION OF DISCLOSURE
(I)
wherein PP is polyphenol; and
(a) (b) (c) In an embodiment of the present disclosure, the polyphenol is Resveratrol and the R is
(R)
(I)
wherein PP is polyphenol; and
(a) (b) (c)
comprises acts of
a) adding amino acid solution to a solution of polyphenol to obtain a mixture, b) heating the mixture to obtain the compound of formula (I); and
c) optionally adding pharmaceutically acceptable excipients to the compound of formula (I) to obtain a composition.
In still another embodiment of the present disclosure, the amino acid is a basic amino acid, selected from a group comprising Arginine, Lysine and Histidine.
In yet another embodiment of the present disclosure, the amino acid solution is a solution in a solvent selected from a group comprising water, methanol, ethanol, propanol and butanol or combination thereof.
In yet another embodiment of the present disclosure, the polyphenol solution is a solution in ethanol.
In yet another embodiment of the present disclosure, the addition of amino acid solution is carried out at a temperature ranging from about 20°C to about 30°C, preferably at about 25°C .
In yet another embodiment of the present disclosure, the heating is carried out at a temperature ranging from about 60°C to about 80°C preferably at about 70°C.
The present disclosure is also in relation to a composition comprising a compound of formula (I) along with pharmaceutically acceptable excipients.
PP
In yet another embodiment of the present disclosure, the pharmaceutically acceptable excipients are selected but not limiting to a group comprising binders, disintegrants, diluents, lubricants, plasticizers, permeation enhancers and solubilizers.
In yet another embodiment of the present disclosure, the composition is in a form selected but not limiting to a group comprising tablet, capsule, powder, syrup, solution, aerosol and suspension.
The present disclosure is also in relation to a method for increasing the bioavailability of a polyphenol said method comprising an act of contacting an animal in need thereof with a compound of formula (I) or a pharmaceutical compositioncomprising compound of formula (I).
In yet another embodiment of the present disclosure, the polyphenol is Resveratrol and the R are;
(R)
The present disclosure provides a compound of formula (I);
Wherein PP represents polyphenols and polyphenols like Resveratrol, Quercetin, Luteolin, Curcumin as given below and each of them may be optionally substituted.
Quercetin Luteolin
(a) (b) (c)
In an embodiment of the present disclosure the relates various derivatives, analogs, tautomeric forms, stereo isomers, polymorphs, solvates, intermediates and metabolites of
the compound of present disclosure. The poly phenols and amino acid may be optionally substituted with various substitutions possible for a person skilled in the art.
In an embodiment of the present disclosure the compound of formula (I) is particularly arginine salt of Resveratrol.
In an embodiment of the present disclosure the compounds are useful for methods for improving antioxidant properties by increasing the oral bioavailability of resveratrol andsolubility. The disclosure also provides a method of delayed release of resveratrol as derivatives which can improve half life and bio availability of resveratrol.
Another embodiment of the present disclosure provides a method for rendering water- soluble an insoluble resveratrol which comprises salts of the insoluble polyphenol to an extent sufficient to render the polyphenol water-soluble.
The present disclosure is also in relation to a pharmaceutical composition, comprising a compound of formula (I) along with pharmaceutically acceptable excipient selected but not limiting to a group comprising of binders, disintegrants, diluents, lubricants, plasticizers, permeation enhancers and solubilizers.
In yet another embodiment of the present disclosure, the composition is in the form selected but not limiting to a group comprising of tablet, capsule, powder, syrup, solution, aerosol and suspension.
Reference now will be made in detail to the embodiments of the disclosure, one or more examples of which are set forth below. Each example is provided by way of explanation of the disclosure, not limitation of the disclosure. In fact, it will be apparent to those skilled in the art that various modifications and variations can be made in the present disclosure without departing from the scope or spirit of the disclosure.
For instance, features illustrated or described as part of one embodiment can be used on another embodiment to yield a still further embodiment. Thus, it is intended that the present disclosure cover such modifications and variations as come within the scope of the appended claims and their equivalents. Other objects, features and aspects of the present disclosure are disclosed in or are obvious from the following detailed description. It is to be understood by one of ordinary skill in the art that the present discussion is a description of exemplary embodiments only, and is not intended as limiting the broader aspects of the present disclosure. Abbreviations and Definitions
The term Optionally substituted' means that substitution is optional and therefore it is possible for the designated atom or molecule to be unsubstituted. In the event a substitution is desired, then such substitution means that any number of hydrogens on the designated atom is replaced with a selection from the indicated group, provided that the normal valency of the designated atom is not exceeded, and that the substitution results in a stable compound.
Pharmaceutically acceptable salts include base addition salts such as alkali metal salts like Li, Na, and K salts; alkaline earth metal salts like Ca and Mg, salts of organic bases such as lysine, arginine, guanidine, diethanolamine, O-phenylethylamine, benzylamine, piperidine, morpholine, pyridine, hydroxyethylpyrrolidine, hydroxyethylpiperidine, choline, ammonium or substituted ammonium salts, aluminum salts. Salts also include amino acid salts such as glycine, alanine, cystine, cysteine, lysine, arginine, phenylalanine, guanidine. Salts may include acid addition salts where appropriate, which are sulphates, nitrates, phosphates, perchlorates, borates, hydrohalides, acetates, tartrates, maleates, citrates, succinates, palmoates, methanesulphonates, tosylates, benzoates, salicylates, hydroxynaphthoates, benzenesulfonates, ascorbates, glycerophosphates, ketoglutarates. Pharmaceutically acceptable solvates may be hydrates or comprising of other solvents of crystallization such as alcohols.
The term analog includes a compound, which differs from the parent structure by one or more C, N, O or S atoms. Hence, a compound in which one of the N atoms in the parent structure is replaced by an S atom is an analog of the former. The term stereoisomer includes isomers that differ from one another in the way the atoms are arranged in space, but whose chemical formulas and structures are otherwise identical. Stereoisomers include enantiomers and diastereoisomers. The term tautomers include readily interconvertible isomeric forms of a compound in equilibrium. The term polymorphs include crystallographically distinct forms of compounds with chemically identical structures.
The term pharmaceutically acceptable solvates includes combinations of solvent molecules with molecules or ions of the solute compound. The term derivative refers to a compound obtained from a compound according to formula (I), an analog, tautomeric form, stereoisomer, polymorph, hydrate, pharmaceutically acceptable salt or pharmaceutically acceptable solvate thereof, by a simple chemical process converting one or more functional groups, such as, by oxidation, hydrogenation, alkylation, esterification, halogenation. The term pharmacological properties includes but not limited to antioxidant properties, Type II diabetes or hyperglycemia, cancers including skin, breast, cervix, colon, lung, liver, lymphoma, prostate, heart diseases, optic neuritis and retinal degeneration, neurodegeneration, stroke and cardiac arrest, osteoporosis, kidney dysfunction and albuminuria, cataracts, inflammatory bowel diseases (e.g. colitis), COPD (emphysema).
The compounds of this disclosure may be prepared by the following process.
The present disclosure is provided by the examples given below, which are provided by the way of illustration only, and should not be considered to limit the scope of the disclosure.
Variation and changes, which are obvious to one skilled in the art, are intended to be within the scope and nature of the disclosure, which are defined in the appended claims.
Example 1
Preparation of Resveratrol-L-Arginine salt.
To a solution of resveratrol (10.0 g, 43.8 mmol) in ethanol (150 mL) is added drop wise solution of L-arginine (7.63 g, 43.8 mmol) in water (50 mL) at room temperature over a period of 10 min. The mixture is allowed to stir at 70 °C over a period of lh. Then volatiles were evaporated under reduced pressure to obtain crude product. The crude product was suspended in ethyl acetate and filtered to obtain product as brownish solid (15.2 g, 86%).
XH NMR (300 MHz, CD3OD) δ (ppm): 1.63-1.66 (m, 4H), 3.15-3.18 (m, 2H), 3.31-3.32 (m, 1H), 6.16-6.17 (m, 1H), 6.45 (d, J= 2.1 Hz, 2H), 6.76-6.99 (m, 4H), 7.36 (d, J= 8.4 Hz, 2H).
Example 2:
Oral pharmacokinetics studies in Sprague dawley male rats.
The oral pharmacokinetics studies of Resveratrol-L-arginine salt carried out in male Sprague dawley rats after obtaining the institution animal ethics committee (IAEC) permission. The objective of the study was to evaluate the oral absorption characteristics of Resveratrol derivatives as compared to parent Resveratrol.
Animals: Rats aged 7 to 8 weeks and weighing around 200 to 225 g were used. Animals were fasted overnight with free access to water. Animals were administered test substance by p.o (oral gavage) route with a dose of 0.25 mM/kg body weight (in a suitable formulation and dose volume). Feed was given 3 hrs post dosing to all animals. Blood samples (150-200 μΐ) were collected in a suitable anticoagulant at 0.25, 0.5, 1, 2, 4, 6 and 24 hr post dosing. The plasma separated from the blood samples were used to quantify the Resveratrol using LC-MS-MS (API 3200 Q Trap). The pharmacokinetics parameters were calculated using WinNonlin 5.2 software. Table 1 : Example of pharmacokinetics parameters of Resveratrol and Resveratrol-L- arginine salt
AUC % Extrap (%) .
Resveratrol-L-Arginine salt showed about 6 times higher Cmax and about 5 times higher AUC as compared to parent Resveratrol
As per above information, Resveratrol salt showed about 6 times increase in the maximum plasma concentrations (Cmax) and about 5 times increase in the AUC.
The figure 1 provides the comparative analysis of Resveratrol and Resveratrol-L-arginine salt.
Example 3:
Intrinsic solubility of Resveratrol and Resveratrol-L-arginine salt. The test compound was allowed to saturate in an aqueous medium (Milli-Q water) and were equilibrated for about 8 hrs at 25° C. The equilibrated solution was centrifuged at 5,000 rpm for 15 min at 25° C and the supernatant was analyzed by UV spectrometer. A standard linearity curve was obtained at λ max using UV spectrometer. The information is tabulated in table 2.
Table -2
The figures 2 and 3 further explains the information provided above.
Claims
(a) (b) (c).
2. The compound as claimed in claim 1 , wherein the polyphenol is Resveratrol and the R is
(R).
3. A process of preparation of compound of formula (I)
wherein PP is polyphenol; and
(a) (b) (c)
comprises acts of
a) adding amino acid solution to a solution of polyphenol to obtain a mixture; b) heating the mixture to obtain the compound of formula (I); and
c) optionally adding pharmaceutically acceptable excipients(s) to the compound of formula (I) to obtain a composition.
4. The process of preparation as claimed in claim 3, wherein the amino acid is a basic amino acid, selected from a group comprising Arginine, Lysine and Histidine.
5. The process as claimed in claim 3, wherein the amino acid solution is a solution in a solvent selected from a group comprising water, methanol, ethanol, propanol and butanol or combination thereof.
6. The process as claimed in claim 3, wherein the polyphenol solution is a solution in ethanol.
7. The process as claimed in claim 3, wherein the addition of amino acid solution is carried out at a temperature ranging from about 20°C to about 30°C, preferably about 25°C.
8. The process as claimed in claim 3, wherein the heating is carried out at a temperature ranging from about 60°C to about 80°C, preferably about 70°C.
9. A composition comprising a compound of formula (I) along with pharmaceutically acceptable excipients
(a) (b) (c)
10. The composition as claimed in claim 9, wherein the pharmaceutically acceptable excipients are selected but not limiting to a group comprising binders, disintegrants, diluents, lubricants, plasticizers, permeation enhancers and solubilizers.
11.The composition as claimed in claim 9, wherein the composition is in a form selected but not limiting to a group comprising tablet, capsule, powder, syrup, solution, aerosol and suspension.
12. A method for increasing bioavailability of a polyphenol said method comprising an act of contacting an animal in need thereof with a compound of formula (I) of claim 1 or a pharmaceutical composition of claim 9.
13. The method for delaying the release of polyphenol as claimed in claim 12, wherein the polyphenol is Resveratrol and R is
(R)
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| IN498CH2010 | 2010-02-25 | ||
| IN498/CHE/2010 | 2010-02-25 |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| WO2011104667A1 true WO2011104667A1 (en) | 2011-09-01 |
Family
ID=44506179
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| PCT/IB2011/050737 Ceased WO2011104667A1 (en) | 2010-02-25 | 2011-02-23 | Basic aminoacid salts of polyphenols |
Country Status (1)
| Country | Link |
|---|---|
| WO (1) | WO2011104667A1 (en) |
Cited By (12)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| ITPG20120030A1 (en) * | 2012-06-27 | 2013-12-29 | Bernard Fioretti | HYBRID INORGANIC RESVERATROL |
| CN105078944A (en) * | 2014-05-11 | 2015-11-25 | 复旦大学 | Application of Isopaucifloral F in preparation of anti-osteoporosis drug |
| US9782375B2 (en) * | 2015-10-28 | 2017-10-10 | Shil Kothari | Methods and compositions for improving microvascular function, suppressing cyclooxygenase activity, reducing platelet aggregation and increasing levels of resveratrol in plasma |
| US9844526B2 (en) * | 2015-10-28 | 2017-12-19 | Gateway Health Alliances, Inc. | Methods and compositions for improving microvascular function, suppressing cyclooxygenase activity, reducing platelet aggregation and increasing levels of resveratrol in plasma |
| CN112218641A (en) * | 2018-04-23 | 2021-01-12 | 阿尔卑斯药品工业株式会社 | Composition of O-glycosidyl flavonoids |
| US10918654B1 (en) | 2019-09-23 | 2021-02-16 | Alps Pharmaceutical Ind. Co., Ltd. | Rutin compositions |
| WO2021042001A1 (en) * | 2019-08-30 | 2021-03-04 | Natural Extraction Systems, LLC | Compositions and methods related to dissolved oxides |
| WO2021158574A1 (en) * | 2020-02-03 | 2021-08-12 | Natural Extraction Systems, LLC | Compositions and methods related to pharmaceutical excipients |
| US11110109B2 (en) | 2019-10-22 | 2021-09-07 | Alps Pharmaceutical Ind. Co., Ltd. | Water soluble O-glycosyl flavonoid compositions and methods for preparing same |
| US11241396B2 (en) * | 2015-10-28 | 2022-02-08 | Gateway Health Alliances, Inc. | Methods and compositions for improving microvascular function, suppressing cyclooxygenase activity, reducing platelet aggregation and increasing levels of resveratrol in plasma |
| IT202100014966A1 (en) | 2021-06-08 | 2022-12-08 | S&R Farm S P A | Use of a stoichiometric combination blend of resveratrol and bile acids for the topical treatment of neurogenic inflammation and pruritus |
| US12349706B2 (en) | 2020-07-31 | 2025-07-08 | Natural Extraction Systems, LLC | Compositions and methods related to excipients and cannabinoid formulations |
Citations (18)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| FR3600M (en) * | 1964-01-03 | 1965-10-11 | Rhone Poulenc Sa | New Quinic Acid Salts and Compositions Containing Them. |
| FR5695M (en) * | 1966-08-29 | 1968-01-08 | ||
| FR2104992A1 (en) * | 1970-09-09 | 1972-04-28 | Melle Bezons | Recovering organic arginine salts - by discharging a spray of soln into hot air current |
| US4285964A (en) * | 1979-08-30 | 1981-08-25 | Continental Pharma | Salts of (+)-catechine, their preparation and use, and compositions containing these salts |
| WO1985000517A1 (en) * | 1983-07-20 | 1985-02-14 | "Continental Pharma" | Drug for treating affections related to an undesirable histamine level, of the gastroduodenal mucosa and allergic affections |
| EP0256566B1 (en) * | 1986-06-25 | 1991-11-06 | ISCOFAR Sas di Paolo E. Ghirardi | Use of usnic acid or derivatives thereof in the treatment of dental caries |
| US5118705A (en) * | 1990-02-28 | 1992-06-02 | Gruppo Lepetit S.P.A. | Water soluble salts of purpuromycin and pharmaceutical formulations thereof |
| WO1993008797A1 (en) * | 1991-11-01 | 1993-05-13 | New York University | Hypericin compositions for treating t-cell mediated diseases |
| WO1993015607A1 (en) * | 1992-02-14 | 1993-08-19 | Yeda Research And Development Co. Ltd. | Ion pairs of hypericin compounds having antiviral activity |
| US5514714A (en) * | 1990-08-23 | 1996-05-07 | New York University | Methods and polycyclic aromatic compound containing compositions for treating T-cell-mediated diseases |
| RU2094045C1 (en) * | 1994-01-10 | 1997-10-27 | Научно-исследовательский институт фармакологии Томского научного центра РАМН | Heme stimulating agent |
| CN1666739A (en) * | 2004-03-08 | 2005-09-14 | 山东绿叶天然药物研究开发有限公司 | Application of Silibinin or its salts in preparation of medicine for treating coronary heart disease or strengthening the effect of coronary heart disease treatment |
| CN1679542A (en) * | 2004-04-06 | 2005-10-12 | 山东绿叶天然药物研究开发有限公司 | Frozen powder injection of silybin and its preparing method |
| CN1778295A (en) * | 2004-11-22 | 2006-05-31 | 山东绿叶天然药物研究开发有限公司 | New medical application of silybin or its salt |
| CN1966481A (en) * | 2005-11-18 | 2007-05-23 | 山东绿叶制药有限公司 | Red sage root water-soluble extract salt and its preparing process |
| CN101108869A (en) * | 2006-07-21 | 2008-01-23 | 徐广爱 | Mangiferin salt and method of preparing the same and use thereof |
| CN101497570A (en) * | 2008-01-31 | 2009-08-05 | 烟台靶点药物研究有限公司 | Salvianolic acid A amino-acid salt, preparation, powder injection composition and use |
| WO2010068861A1 (en) * | 2008-12-11 | 2010-06-17 | Axcentua Pharmaceutucals Ab | Crystalline forms of genistein |
-
2011
- 2011-02-23 WO PCT/IB2011/050737 patent/WO2011104667A1/en not_active Ceased
Patent Citations (18)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| FR3600M (en) * | 1964-01-03 | 1965-10-11 | Rhone Poulenc Sa | New Quinic Acid Salts and Compositions Containing Them. |
| FR5695M (en) * | 1966-08-29 | 1968-01-08 | ||
| FR2104992A1 (en) * | 1970-09-09 | 1972-04-28 | Melle Bezons | Recovering organic arginine salts - by discharging a spray of soln into hot air current |
| US4285964A (en) * | 1979-08-30 | 1981-08-25 | Continental Pharma | Salts of (+)-catechine, their preparation and use, and compositions containing these salts |
| WO1985000517A1 (en) * | 1983-07-20 | 1985-02-14 | "Continental Pharma" | Drug for treating affections related to an undesirable histamine level, of the gastroduodenal mucosa and allergic affections |
| EP0256566B1 (en) * | 1986-06-25 | 1991-11-06 | ISCOFAR Sas di Paolo E. Ghirardi | Use of usnic acid or derivatives thereof in the treatment of dental caries |
| US5118705A (en) * | 1990-02-28 | 1992-06-02 | Gruppo Lepetit S.P.A. | Water soluble salts of purpuromycin and pharmaceutical formulations thereof |
| US5514714A (en) * | 1990-08-23 | 1996-05-07 | New York University | Methods and polycyclic aromatic compound containing compositions for treating T-cell-mediated diseases |
| WO1993008797A1 (en) * | 1991-11-01 | 1993-05-13 | New York University | Hypericin compositions for treating t-cell mediated diseases |
| WO1993015607A1 (en) * | 1992-02-14 | 1993-08-19 | Yeda Research And Development Co. Ltd. | Ion pairs of hypericin compounds having antiviral activity |
| RU2094045C1 (en) * | 1994-01-10 | 1997-10-27 | Научно-исследовательский институт фармакологии Томского научного центра РАМН | Heme stimulating agent |
| CN1666739A (en) * | 2004-03-08 | 2005-09-14 | 山东绿叶天然药物研究开发有限公司 | Application of Silibinin or its salts in preparation of medicine for treating coronary heart disease or strengthening the effect of coronary heart disease treatment |
| CN1679542A (en) * | 2004-04-06 | 2005-10-12 | 山东绿叶天然药物研究开发有限公司 | Frozen powder injection of silybin and its preparing method |
| CN1778295A (en) * | 2004-11-22 | 2006-05-31 | 山东绿叶天然药物研究开发有限公司 | New medical application of silybin or its salt |
| CN1966481A (en) * | 2005-11-18 | 2007-05-23 | 山东绿叶制药有限公司 | Red sage root water-soluble extract salt and its preparing process |
| CN101108869A (en) * | 2006-07-21 | 2008-01-23 | 徐广爱 | Mangiferin salt and method of preparing the same and use thereof |
| CN101497570A (en) * | 2008-01-31 | 2009-08-05 | 烟台靶点药物研究有限公司 | Salvianolic acid A amino-acid salt, preparation, powder injection composition and use |
| WO2010068861A1 (en) * | 2008-12-11 | 2010-06-17 | Axcentua Pharmaceutucals Ab | Crystalline forms of genistein |
Non-Patent Citations (11)
| Title |
|---|
| DATABASE CA PLUS "Maniferin salts combined with L-Arginine or L-Lysine", Database accession no. 2008: 221931 * |
| DATABASE CA PLUS "Salvianolic acid A combined with L-Arginine or L-Lysine or L-Histidine", Database accession no. 2009:962742 * |
| DATABASE CA PLUS "Scutellarin (or Brieviscapine) salts combined with L-Arginine or L-Lysine", Database accession no. 2008: 398286 * |
| FU W. ET AL: "Preparation of quercetin-arginine complex", ZHONGCAOYAO, vol. 33, no. 8, 2002, pages 695 - 697 * |
| GERSON F. ET AL: "Electron-acceptor properties of hypericin and its salts: an ESR/ENDOR and electrochemical study", J. AMERICAN CHEMICAL SOCIETY, vol. 117, no. 48, 1995, pages 11861 - 11866 * |
| KAR, R. ET AL.: "A study on synthetic humic acids", J. IND. CHEM. SOC., vol. 65, no. 12, 1988, pages 834 - 837 * |
| KINSEL G. ET AL: "Arginine/2,5-dihydroxybenzoic acid clusters: an experimental and computational study of gas-phase and solid-state systems", J. PHYS. CHEM. A., vol. 108, no. 15, 2004, pages 3153 - 3161 * |
| RAZINA, T ET AL.: "Semisynthetic flavonoid of the baikal scutellaria as a means for increasing the effectiveness of the experimental tumor chemotherapy", EKSPERIMENTAL'NAYA I KLINICHESKAYA FARMAKOLOGIYA, vol. 61, no. 2, 1998, pages 54 - 56 * |
| SAWAMURA ET AL.: "Inhibitory effects of Ellagic acid on glucosyltransferases from mutans streptococci", BIOSCI. BIOTECH. BIOCHEM., vol. 56, no. 5, 1992, pages 766 - 768 * |
| WAUTERS, P. ET AL.: "Oxidation products are responsible for the resistance to the action of collagenase conferred on collagen by (+)-catechin", BIOCHEM. PHARM., vol. 35, no. 17, 1986, pages 2971 - 2973 * |
| WEINER, L. ET AL.: "EPR studies of Hypericin. Photogeneration of free radicals and superoxide", J. CHEM. SOC., PERKINS TRANS., vol. 2, no. 9, 1992, pages 1439 - 1442 * |
Cited By (17)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| EP2679243A1 (en) | 2012-06-27 | 2014-01-01 | Bernard Fioretti | Co-precipitate of one or more stilbene polyphenols and their derivatives in lamellar anionic solids, it's applications and related preparation method |
| ITPG20120030A1 (en) * | 2012-06-27 | 2013-12-29 | Bernard Fioretti | HYBRID INORGANIC RESVERATROL |
| CN105078944A (en) * | 2014-05-11 | 2015-11-25 | 复旦大学 | Application of Isopaucifloral F in preparation of anti-osteoporosis drug |
| US11241396B2 (en) * | 2015-10-28 | 2022-02-08 | Gateway Health Alliances, Inc. | Methods and compositions for improving microvascular function, suppressing cyclooxygenase activity, reducing platelet aggregation and increasing levels of resveratrol in plasma |
| US9782375B2 (en) * | 2015-10-28 | 2017-10-10 | Shil Kothari | Methods and compositions for improving microvascular function, suppressing cyclooxygenase activity, reducing platelet aggregation and increasing levels of resveratrol in plasma |
| US9844526B2 (en) * | 2015-10-28 | 2017-12-19 | Gateway Health Alliances, Inc. | Methods and compositions for improving microvascular function, suppressing cyclooxygenase activity, reducing platelet aggregation and increasing levels of resveratrol in plasma |
| US20180243252A1 (en) * | 2015-10-28 | 2018-08-30 | Shil Kothari | Methods and compositions for improving microvascular function, suppressing cyclooxygenase activity, reducing platelet aggregation and increasing levels of resveratrol in plasma |
| US10406129B2 (en) * | 2015-10-28 | 2019-09-10 | Shil Kothari | Methods and compositions for improving microvascular function, suppressing cyclooxygenase activity, reducing platelet aggregation and increasing levels of resveratrol in plasma |
| US10632090B2 (en) * | 2015-10-28 | 2020-04-28 | Shil Kothari | Methods and compositions for improving microvascular function, suppressing cyclooxygenase activity, reducing platelet aggregation and increasing levels of resveratrol in plasma |
| CN112218641A (en) * | 2018-04-23 | 2021-01-12 | 阿尔卑斯药品工业株式会社 | Composition of O-glycosidyl flavonoids |
| US11266671B2 (en) | 2018-04-23 | 2022-03-08 | Alps Pharmaceutical Ind. Co., Ltd. | Compositions of O-glycosyl flavonoids |
| WO2021042001A1 (en) * | 2019-08-30 | 2021-03-04 | Natural Extraction Systems, LLC | Compositions and methods related to dissolved oxides |
| US10918654B1 (en) | 2019-09-23 | 2021-02-16 | Alps Pharmaceutical Ind. Co., Ltd. | Rutin compositions |
| US11110109B2 (en) | 2019-10-22 | 2021-09-07 | Alps Pharmaceutical Ind. Co., Ltd. | Water soluble O-glycosyl flavonoid compositions and methods for preparing same |
| WO2021158574A1 (en) * | 2020-02-03 | 2021-08-12 | Natural Extraction Systems, LLC | Compositions and methods related to pharmaceutical excipients |
| US12349706B2 (en) | 2020-07-31 | 2025-07-08 | Natural Extraction Systems, LLC | Compositions and methods related to excipients and cannabinoid formulations |
| IT202100014966A1 (en) | 2021-06-08 | 2022-12-08 | S&R Farm S P A | Use of a stoichiometric combination blend of resveratrol and bile acids for the topical treatment of neurogenic inflammation and pruritus |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| WO2011104667A1 (en) | Basic aminoacid salts of polyphenols | |
| KR102405650B1 (en) | Salts of treprostinil | |
| CN101255119A (en) | New Tetrahydrocurcumin Derivatives and Salts | |
| US20160347713A1 (en) | 2-Aminopyridine-based Selective Neuronal Nitric Oxide Synthase Inhibitors | |
| US20100152493A1 (en) | BIS(ARYLMETHYLIDENE)ACETONE COMPOUND, ANTI-CANCER AGENT, CARCINOGENESIS-PREVENTIVE AGENT, INHIBITOR OF EXPRESSION OF Ki-Ras, ErbB2, c-Myc AND CYCLINE D1, BETA-CATENIN-DEGRADING AGENT, AND p53 EXPRESSION ENHANCER | |
| KR20170061616A (en) | New salt of fimasartan | |
| KR102502749B1 (en) | Liver Delivery Entecavir Prodrug Nucleotide Cyclophosphate Compounds and Applications | |
| JP5515078B2 (en) | Ischemic injury inhibitor | |
| US9328084B2 (en) | Nitrogen-containing biphenyl compounds, pharmaceutical compositions of same, preparation methods and anti-HIV-1 uses thereof | |
| US20220041538A1 (en) | Cannabigerol quinone acid and salts thereof | |
| CN107129517A (en) | A kind of pregnenolone derivative with alpha, beta unsaturated ketone structure fragment and application thereof | |
| WO2013114040A1 (en) | Novel compounds and compositions used as anticancer agents | |
| US11377418B2 (en) | Compounds and methods for treating influenza | |
| EP3179998B1 (en) | Compounds for the treatment of hpv-induced carcinoma | |
| JP2012097003A (en) | Bixin derivative and cytoprotective agent | |
| IL280870B1 (en) | Phenoxy(hetero)aryl ethers of antiproliferative activity | |
| WO2017050969A1 (en) | Heterocyclic ho-1 inducers, their use in the treatment of inflammatory or cardiovascular diseases and their process of preparation | |
| RU2435767C1 (en) | -5-4-chlorophenyl-3-[2-(4-chlorophenyl)ethenyl]-2-oxaspiro[5,6]dodec-3-en-1-one, having analgesic activity | |
| EP4512797A1 (en) | Chiral aryl propionic acid derivative and pharmaceutical composition thereof, and use | |
| JP2008543941A (en) | Ansamycin formulations and methods of use | |
| US20150284353A1 (en) | Sesquiterpene lactone-based pharmaceutical composition for treating gastrointestinal diseases | |
| US20250002436A1 (en) | Naphthoquinone-based chalcone derivatives and uses thereof | |
| EP3353186B1 (en) | Highly efficient nrf2 activators-co-releasing molecule hybrids, their use in the treatment of inflammatory or cardiovascular diseases and their process of preparation | |
| KR102302055B1 (en) | Amorphous form of a thiocolchicine derivative | |
| US10450328B2 (en) | Crystals of thiadiazole derivative DPP-IV inhibitors and uses thereof |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| 121 | Ep: the epo has been informed by wipo that ep was designated in this application |
Ref document number: 11746944 Country of ref document: EP Kind code of ref document: A1 |
|
| NENP | Non-entry into the national phase |
Ref country code: DE |
|
| 122 | Ep: pct application non-entry in european phase |
Ref document number: 11746944 Country of ref document: EP Kind code of ref document: A1 |