WO2011071860A2 - Compositions for nucleic acid delivery - Google Patents
Compositions for nucleic acid delivery Download PDFInfo
- Publication number
- WO2011071860A2 WO2011071860A2 PCT/US2010/059206 US2010059206W WO2011071860A2 WO 2011071860 A2 WO2011071860 A2 WO 2011071860A2 US 2010059206 W US2010059206 W US 2010059206W WO 2011071860 A2 WO2011071860 A2 WO 2011071860A2
- Authority
- WO
- WIPO (PCT)
- Prior art keywords
- lipid
- independently
- atom
- optionally substituted
- alkyl
- Prior art date
Links
- 0 CC*(*(C)C)*(*)C(*1)C1*(C)**(C*=N*C)N Chemical compound CC*(*(C)C)*(*)C(*1)C1*(C)**(C*=N*C)N 0.000 description 6
- JFHYMLALDQBBFE-UHFFFAOYSA-N CCC(C)(C=C)c1c[n](C)nn1 Chemical compound CCC(C)(C=C)c1c[n](C)nn1 JFHYMLALDQBBFE-UHFFFAOYSA-N 0.000 description 1
- VCVOSERVUCJNPR-SYDPRGILSA-N O[C@H](CCC1)[C@H]1O Chemical compound O[C@H](CCC1)[C@H]1O VCVOSERVUCJNPR-SYDPRGILSA-N 0.000 description 1
Classifications
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K47/00—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient
- A61K47/06—Organic compounds, e.g. natural or synthetic hydrocarbons, polyolefins, mineral oil, petrolatum or ozokerite
- A61K47/16—Organic compounds, e.g. natural or synthetic hydrocarbons, polyolefins, mineral oil, petrolatum or ozokerite containing nitrogen, e.g. nitro-, nitroso-, azo-compounds, nitriles, cyanates
- A61K47/18—Amines; Amides; Ureas; Quaternary ammonium compounds; Amino acids; Oligopeptides having up to five amino acids
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/70—Carbohydrates; Sugars; Derivatives thereof
- A61K31/7088—Compounds having three or more nucleosides or nucleotides
- A61K31/7105—Natural ribonucleic acids, i.e. containing only riboses attached to adenine, guanine, cytosine or uracil and having 3'-5' phosphodiester links
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/70—Carbohydrates; Sugars; Derivatives thereof
- A61K31/7088—Compounds having three or more nucleosides or nucleotides
- A61K31/713—Double-stranded nucleic acids or oligonucleotides
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K47/00—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient
- A61K47/06—Organic compounds, e.g. natural or synthetic hydrocarbons, polyolefins, mineral oil, petrolatum or ozokerite
- A61K47/22—Heterocyclic compounds, e.g. ascorbic acid, tocopherol or pyrrolidones
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K9/00—Medicinal preparations characterised by special physical form
- A61K9/10—Dispersions; Emulsions
- A61K9/127—Liposomes
- A61K9/1271—Non-conventional liposomes, e.g. PEGylated liposomes, liposomes coated with polymers
- A61K9/1272—Non-conventional liposomes, e.g. PEGylated liposomes, liposomes coated with polymers with substantial amounts of non-phosphatidyl, i.e. non-acylglycerophosphate, surfactants as bilayer-forming substances, e.g. cationic lipids
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K9/00—Medicinal preparations characterised by special physical form
- A61K9/14—Particulate form, e.g. powders, Processes for size reducing of pure drugs or the resulting products, Pure drug nanoparticles
- A61K9/19—Particulate form, e.g. powders, Processes for size reducing of pure drugs or the resulting products, Pure drug nanoparticles lyophilised, i.e. freeze-dried, solutions or dispersions
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07C—ACYCLIC OR CARBOCYCLIC COMPOUNDS
- C07C217/00—Compounds containing amino and etherified hydroxy groups bound to the same carbon skeleton
- C07C217/02—Compounds containing amino and etherified hydroxy groups bound to the same carbon skeleton having etherified hydroxy groups and amino groups bound to acyclic carbon atoms of the same carbon skeleton
- C07C217/04—Compounds containing amino and etherified hydroxy groups bound to the same carbon skeleton having etherified hydroxy groups and amino groups bound to acyclic carbon atoms of the same carbon skeleton the carbon skeleton being acyclic and saturated
- C07C217/06—Compounds containing amino and etherified hydroxy groups bound to the same carbon skeleton having etherified hydroxy groups and amino groups bound to acyclic carbon atoms of the same carbon skeleton the carbon skeleton being acyclic and saturated having only one etherified hydroxy group and one amino group bound to the carbon skeleton, which is not further substituted
- C07C217/08—Compounds containing amino and etherified hydroxy groups bound to the same carbon skeleton having etherified hydroxy groups and amino groups bound to acyclic carbon atoms of the same carbon skeleton the carbon skeleton being acyclic and saturated having only one etherified hydroxy group and one amino group bound to the carbon skeleton, which is not further substituted the oxygen atom of the etherified hydroxy group being further bound to an acyclic carbon atom
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07C—ACYCLIC OR CARBOCYCLIC COMPOUNDS
- C07C219/00—Compounds containing amino and esterified hydroxy groups bound to the same carbon skeleton
- C07C219/02—Compounds containing amino and esterified hydroxy groups bound to the same carbon skeleton having esterified hydroxy groups and amino groups bound to acyclic carbon atoms of the same carbon skeleton
- C07C219/04—Compounds containing amino and esterified hydroxy groups bound to the same carbon skeleton having esterified hydroxy groups and amino groups bound to acyclic carbon atoms of the same carbon skeleton the carbon skeleton being acyclic and saturated
- C07C219/08—Compounds containing amino and esterified hydroxy groups bound to the same carbon skeleton having esterified hydroxy groups and amino groups bound to acyclic carbon atoms of the same carbon skeleton the carbon skeleton being acyclic and saturated having at least one of the hydroxy groups esterified by a carboxylic acid having the esterifying carboxyl group bound to an acyclic carbon atom of an acyclic unsaturated carbon skeleton
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07C—ACYCLIC OR CARBOCYCLIC COMPOUNDS
- C07C229/00—Compounds containing amino and carboxyl groups bound to the same carbon skeleton
- C07C229/02—Compounds containing amino and carboxyl groups bound to the same carbon skeleton having amino and carboxyl groups bound to acyclic carbon atoms of the same carbon skeleton
- C07C229/04—Compounds containing amino and carboxyl groups bound to the same carbon skeleton having amino and carboxyl groups bound to acyclic carbon atoms of the same carbon skeleton the carbon skeleton being acyclic and saturated
- C07C229/06—Compounds containing amino and carboxyl groups bound to the same carbon skeleton having amino and carboxyl groups bound to acyclic carbon atoms of the same carbon skeleton the carbon skeleton being acyclic and saturated having only one amino and one carboxyl group bound to the carbon skeleton
- C07C229/10—Compounds containing amino and carboxyl groups bound to the same carbon skeleton having amino and carboxyl groups bound to acyclic carbon atoms of the same carbon skeleton the carbon skeleton being acyclic and saturated having only one amino and one carboxyl group bound to the carbon skeleton the nitrogen atom of the amino group being further bound to acyclic carbon atoms or to carbon atoms of rings other than six-membered aromatic rings
- C07C229/12—Compounds containing amino and carboxyl groups bound to the same carbon skeleton having amino and carboxyl groups bound to acyclic carbon atoms of the same carbon skeleton the carbon skeleton being acyclic and saturated having only one amino and one carboxyl group bound to the carbon skeleton the nitrogen atom of the amino group being further bound to acyclic carbon atoms or to carbon atoms of rings other than six-membered aromatic rings to carbon atoms of acyclic carbon skeletons
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07C—ACYCLIC OR CARBOCYCLIC COMPOUNDS
- C07C251/00—Compounds containing nitrogen atoms doubly-bound to a carbon skeleton
- C07C251/32—Oximes
- C07C251/34—Oximes with oxygen atoms of oxyimino groups bound to hydrogen atoms or to carbon atoms of unsubstituted hydrocarbon radicals
- C07C251/36—Oximes with oxygen atoms of oxyimino groups bound to hydrogen atoms or to carbon atoms of unsubstituted hydrocarbon radicals with the carbon atoms of the oxyimino groups bound to hydrogen atoms or to acyclic carbon atoms
- C07C251/40—Oximes with oxygen atoms of oxyimino groups bound to hydrogen atoms or to carbon atoms of unsubstituted hydrocarbon radicals with the carbon atoms of the oxyimino groups bound to hydrogen atoms or to acyclic carbon atoms to carbon atoms of an unsaturated carbon skeleton
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07C—ACYCLIC OR CARBOCYCLIC COMPOUNDS
- C07C271/00—Derivatives of carbamic acids, i.e. compounds containing any of the groups, the nitrogen atom not being part of nitro or nitroso groups
- C07C271/06—Esters of carbamic acids
- C07C271/08—Esters of carbamic acids having oxygen atoms of carbamate groups bound to acyclic carbon atoms
- C07C271/10—Esters of carbamic acids having oxygen atoms of carbamate groups bound to acyclic carbon atoms with the nitrogen atoms of the carbamate groups bound to hydrogen atoms or to acyclic carbon atoms
- C07C271/12—Esters of carbamic acids having oxygen atoms of carbamate groups bound to acyclic carbon atoms with the nitrogen atoms of the carbamate groups bound to hydrogen atoms or to acyclic carbon atoms to hydrogen atoms or to carbon atoms of unsubstituted hydrocarbon radicals
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07C—ACYCLIC OR CARBOCYCLIC COMPOUNDS
- C07C271/00—Derivatives of carbamic acids, i.e. compounds containing any of the groups, the nitrogen atom not being part of nitro or nitroso groups
- C07C271/06—Esters of carbamic acids
- C07C271/08—Esters of carbamic acids having oxygen atoms of carbamate groups bound to acyclic carbon atoms
- C07C271/10—Esters of carbamic acids having oxygen atoms of carbamate groups bound to acyclic carbon atoms with the nitrogen atoms of the carbamate groups bound to hydrogen atoms or to acyclic carbon atoms
- C07C271/20—Esters of carbamic acids having oxygen atoms of carbamate groups bound to acyclic carbon atoms with the nitrogen atoms of the carbamate groups bound to hydrogen atoms or to acyclic carbon atoms to carbon atoms of hydrocarbon radicals substituted by nitrogen atoms not being part of nitro or nitroso groups
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07C—ACYCLIC OR CARBOCYCLIC COMPOUNDS
- C07C323/00—Thiols, sulfides, hydropolysulfides or polysulfides substituted by halogen, oxygen or nitrogen atoms, or by sulfur atoms not being part of thio groups
- C07C323/23—Thiols, sulfides, hydropolysulfides or polysulfides substituted by halogen, oxygen or nitrogen atoms, or by sulfur atoms not being part of thio groups containing thio groups and nitrogen atoms, not being part of nitro or nitroso groups, bound to the same carbon skeleton
- C07C323/24—Thiols, sulfides, hydropolysulfides or polysulfides substituted by halogen, oxygen or nitrogen atoms, or by sulfur atoms not being part of thio groups containing thio groups and nitrogen atoms, not being part of nitro or nitroso groups, bound to the same carbon skeleton having the sulfur atoms of the thio groups bound to acyclic carbon atoms of the carbon skeleton
- C07C323/25—Thiols, sulfides, hydropolysulfides or polysulfides substituted by halogen, oxygen or nitrogen atoms, or by sulfur atoms not being part of thio groups containing thio groups and nitrogen atoms, not being part of nitro or nitroso groups, bound to the same carbon skeleton having the sulfur atoms of the thio groups bound to acyclic carbon atoms of the carbon skeleton the carbon skeleton being acyclic and saturated
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D317/00—Heterocyclic compounds containing five-membered rings having two oxygen atoms as the only ring hetero atoms
- C07D317/08—Heterocyclic compounds containing five-membered rings having two oxygen atoms as the only ring hetero atoms having the hetero atoms in positions 1 and 3
- C07D317/10—Heterocyclic compounds containing five-membered rings having two oxygen atoms as the only ring hetero atoms having the hetero atoms in positions 1 and 3 not condensed with other rings
- C07D317/14—Heterocyclic compounds containing five-membered rings having two oxygen atoms as the only ring hetero atoms having the hetero atoms in positions 1 and 3 not condensed with other rings with substituted hydrocarbon radicals attached to ring carbon atoms
- C07D317/28—Radicals substituted by nitrogen atoms
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D317/00—Heterocyclic compounds containing five-membered rings having two oxygen atoms as the only ring hetero atoms
- C07D317/08—Heterocyclic compounds containing five-membered rings having two oxygen atoms as the only ring hetero atoms having the hetero atoms in positions 1 and 3
- C07D317/44—Heterocyclic compounds containing five-membered rings having two oxygen atoms as the only ring hetero atoms having the hetero atoms in positions 1 and 3 ortho- or peri-condensed with carbocyclic rings or ring systems
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D317/00—Heterocyclic compounds containing five-membered rings having two oxygen atoms as the only ring hetero atoms
- C07D317/08—Heterocyclic compounds containing five-membered rings having two oxygen atoms as the only ring hetero atoms having the hetero atoms in positions 1 and 3
- C07D317/72—Heterocyclic compounds containing five-membered rings having two oxygen atoms as the only ring hetero atoms having the hetero atoms in positions 1 and 3 spiro-condensed with carbocyclic rings
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D491/00—Heterocyclic compounds containing in the condensed ring system both one or more rings having oxygen atoms as the only ring hetero atoms and one or more rings having nitrogen atoms as the only ring hetero atoms, not provided for by groups C07D451/00 - C07D459/00, C07D463/00, C07D477/00 or C07D489/00
- C07D491/02—Heterocyclic compounds containing in the condensed ring system both one or more rings having oxygen atoms as the only ring hetero atoms and one or more rings having nitrogen atoms as the only ring hetero atoms, not provided for by groups C07D451/00 - C07D459/00, C07D463/00, C07D477/00 or C07D489/00 in which the condensed system contains two hetero rings
- C07D491/10—Spiro-condensed systems
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N15/00—Mutation or genetic engineering; DNA or RNA concerning genetic engineering, vectors, e.g. plasmids, or their isolation, preparation or purification; Use of hosts therefor
- C12N15/09—Recombinant DNA-technology
- C12N15/87—Introduction of foreign genetic material using processes not otherwise provided for, e.g. co-transformation
- C12N15/88—Introduction of foreign genetic material using processes not otherwise provided for, e.g. co-transformation using microencapsulation, e.g. using amphiphile liposome vesicle
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K47/00—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient
- A61K47/06—Organic compounds, e.g. natural or synthetic hydrocarbons, polyolefins, mineral oil, petrolatum or ozokerite
- A61K47/26—Carbohydrates, e.g. sugar alcohols, amino sugars, nucleic acids, mono-, di- or oligo-saccharides; Derivatives thereof, e.g. polysorbates, sorbitan fatty acid esters or glycyrrhizin
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07C—ACYCLIC OR CARBOCYCLIC COMPOUNDS
- C07C2601/00—Systems containing only non-condensed rings
- C07C2601/02—Systems containing only non-condensed rings with a three-membered ring
Definitions
- the present invention relates to lipids, lipid particles, compositions including lipid particles, and methods for making and using these.
- Therapeutic nucleic acids include, e.g. , small interfering RNA (siRNA), microRNA (siRNA), microRNA (mRNA), and RNAse RNA (mRNA), microRNA (mRNA), mRNA (mRNA), mRNA (mRNA), microRNA (mRNA), mRNA (mRNA), mRNA (mRNA), mRNA (mRNA), mRNA (mRNA), mRNA (mRNA), mRNA), microporous RNA (mRNA), microporous RNA (mRNA), microporous RNA, RNA microRNA (mRNA), microRNA (mRNA), mRNA (mRNA), microRNA (mRNA), mRNA (mRNA), microRNA (mRNA), mRNA (mRNA), mRNA (mRNA), mRNA (mRNA), mRNA (mRNA), mRNA (mRNA), mRNA (mRNA), mRNA (mRNA), mRNA (mRNA), mRNA (mRNA), mRNA (mRNA), m
- RNA antisense oligonucleotides, ribozymes, plasmids, immune stimulating nucleic acids, antisense, antagomir, antimir, microRNA mimic, supermir, Ul adaptor, and aptamer.
- RNAi RNA interference
- the sense strand of the siRNA or miRNA is displaced from the RISC complex providing a template within RISC that can recognize and bind mRNA with a complementary sequence to that of the bound siRNA or miRNA. Having bound the complementary mRNA the RISC complex cleaves the mRNA and releases the cleaved strands.
- RNAi can provide down-regulation of specific proteins by targeting specific destruction of the corresponding mRNA that encodes for protein synthesis.
- RNAi The therapeutic applications of RNAi are extremely broad, since siRNA and miRNA constructs can be synthesized with any nucleotide sequence directed against a target protein.
- siRNA constructs have shown the ability to specifically down- regulate target proteins in both in vitro and in vivo models.
- siRNA constructs are currently being evaluated in clinical studies.
- two problems currently faced by siRNA or miRNA constructs are, first, their susceptibility to nuclease digestion in plasma and, second, their limited ability to gain access to the intracellular compartment where they can bind RISC when
- siRNA or miRNA administered systemically as the free siRNA or miRNA.
- These double- stranded constructs can be stabilized by incorporation of chemically modified nucleotide linkers within the molecule, for example, phosphothioate groups.
- these chemical modifications provide only limited protection from nuclease digestion and may decrease the activity of the construct.
- Intracellular delivery of siRNA or miRNA can be facilitated by use of carrier systems such as polymers, cationic liposomes or by chemical modification of the construct, for example by the covalent attachment of cholesterol molecules.
- carrier systems such as polymers, cationic liposomes or by chemical modification of the construct, for example by the covalent attachment of cholesterol molecules.
- improved delivery systems are required to increase the potency of siRNA and miRNA molecules and reduce or eliminate the requirement for chemical modification.
- Antisense oligonucleotides and ribozymes can also inhibit mRNA translation into protein.
- these single stranded deoxynucleic acids have a complementary sequence to that of the target protein mRNA and can bind to the mRNA by Watson-Crick base pairing. This binding either prevents translation of the target mRNA and/or triggers RNase H degradation of the mRNA transcripts, Consequently, antisense oligonucleotides have tremendous potential for specificity of action (i.e., down-regulation of a specific disease-related protein).
- Antisense can also affect cellular activity by hybridizing specifically with chromosomal DNA. Advanced human clinical assessments of several antisense drugs are currently underway. Targets for these drugs include the bcl2 and apolipoprotein B genes and mRNA products.
- Immune-stimulating nucleic acids include deoxyribonucleic acids and ribonucleic acids.
- deoxyribonucleic acids certain sequences or motifs have been shown to illicit immune stimulation in mammals. These sequences or motifs include the CpG motif, pyrimidine-rich sequences and palindromic sequences. It is believed that the CpG motif in deoxyribonucleic acids is specifically recognized by an endosomal receptor, tolllike receptor 9 (TLR-9), which then triggers both the innate and acquired immune stimulation pathway.
- TLR-9 endosomal receptor
- Certain immune stimulating ribonucleic acid sequences have also been reported. It is believed that these RNA sequences trigger immune activation by binding to toll-like receptors 6 and 7 (TLR-6 and TLR-7). In addition, double- stranded RNA is also reported to be immune stimulating and is believe to activate via binding to TLR-3.
- nucleotide base e.g., 5-propynyl-pyrimidines
- sugar e.g., 2' -modified sugars
- lipid-based carrier systems to deliver chemically modified or unmodified therapeutic nucleic acids.
- the authors refer to the use of anionic (conventional) liposomes, pH sensitive liposomes, immunoliposomes, fusogenic liposomes, and charged lipid/antisense aggregates.
- siRNA has been administered systemically in cationic liposomes, and these nucleic acid-lipid particles have been reported to provide improved down-regulation of target proteins in mammals including non-human primates (Zimmermann et al., Nature 441: 111-114 (2006)).
- these compositions would encapsulate nucleic acids with high-efficiency, have high drug: lipid ratios, protect the encapsulated nucleic acid from degradation and clearance in serum, be suitable for systemic delivery, and provide intracellular delivery of the encapsulated nucleic acid.
- these lipid-nucleic acid particles should be well-tolerated and provide an adequate therapeutic index, such that patient treatment at an effective dose of the nucleic acid is not associated with significant toxicity and/or risk to the patient.
- the present invention provides such compositions, methods of making the compositions, and methods of using the compositions to introduce nucleic acids into cells, including for the treatment of diseases.
- a method for delivering a nucleic acid to a cell can include contacting cells with a composition comprising a neutral lipid and a cationic lipid having the formula:
- Ri and R 2 are each independently for each occurrence a C 10 to C30 group having the formula -L la -(CR la R lb ) a -[L lb -(CR la R lb ) p ] y -L lc -R lc , wherein: L la is a
- Each R la and each R lb is H; halo; hydroxy; cyano; C1-C6 alkyl optionally substituted by halo, hydroxy, or alkoxy; C3-C8 cycloalkyl optionally substituted by halo, hydroxy, or alkoxy; -OR lc ; -NR lc R ld ; aryl; heteroaryl; or
- Each L lb independently, is a bond, -(CR la R lb )i- 2 -, -0-, -CO-, -NR ld -, -S-, , or a combination thereof; or has the formula
- j, k, and 1 are each independently 0, 1, 2, or 3, provided that the sum of j, k and 1 is at least 1 and no greater than 8; and R lf and R lg are each independently R lb , or adjacent R lf and R lg , taken together, are optionally a bond; or has the formula
- j and k are each independently 0, 1, 2, 3, or 4 provided that the sum of j and k is at least 1; and R and R g are each independently R , or adjacent R and R g , taken together, are optionally a bond;
- heterocyclylene or heteroarylene group optionally substituted by zero to six R la groups.
- L lc is -(CR la R lb ) ! _ 2 -, -0-, -CO-, -NR ld -, -S-,
- R lc is H; halo; hydroxy; cyano; Q-Cg alkyl optionally substituted by halo, hydroxy, or alkoxy; C3-C8 cycloalkyl optionally substituted by halo, hydroxy, or alkoxy; aryl; heteroaryl; or heterocyclyl; or R lc has the formula:
- R is H; halo; hydroxy; cyano; C1-C6 alkyl optionally substituted by halo, hydroxy, or alkoxy; C3-C8 cycloalkyl optionally substituted by halo, hydroxy, or alkoxy; aryl; heteroaryl; or heterocyclyl.
- a is 0-6; each ⁇ , independently, is 0-6; and ⁇ is 0-6. represents a connection between L 2 and Li which is:
- alkylene -N(Q)-, -C(O)-, -O(CO)-, -OC(0)N(Q)-, -N(Q)C(0)0-, -C(0)0,
- R a is H, alkyl, alkoxy, -OH, -N(Q)Q, or - SQ.
- L 2 has the formula
- X is the first atom of L 2
- Y is the second atom of L 2
- X and Y are each, independently, selected from the group consisting of -0-, -S-,
- alkylene -N(Q , -C(0 , -O(CO)-, -OC(0)N(Q)-, -N(Q)C(0)0-, -C(0)0,
- Zi and Z 4 are each, independently, -0-, -S-, -CH 2 -, -CHR 5 -, or -CR 5 R 5 -;
- Z 2 is CH or N;
- Z 3 is CH or N; or
- Z 2 and Z 3 taken together, are a single C atom.
- a ! and A 2 are each, independently, -0-, -S-, -CH 2 -, -CHR 5 -, or -CR 5 R 5 -.
- Each Z is N, C(R 5 ), or C(R 3 ).
- k is 0, 1, or 2; each m, independently, is 0 to 5; and each n, independently, is 0 to 5; where m and n taken together result in a 3, 4, 5, 6, 7 or 8 member ring.
- X is the first atom of Li
- Y is the second atom of Li
- X and Y are each, independently, selected from the group consisting of -0-, -S-,
- alkylene -N(Q)-, -C(0)-, -O(CO)-, -OC(0)N(Q)-, -N(Q)C(0)0-, -C(0)0,
- X is the first atom of Li
- Y is the second atom of Li
- X and Y are each, independently, selected from the group consisting of -0-, -S-, alkylene, -N(Q)-, -C(O)-, -O(CO)-, -OC(0)N(Q)-, -N(Q)C(0)0-, -C(0)0-,
- Ti is -CR 5 R 6 -, -N(Q)-, -0-, or -S-;
- T 2 is -CR 5 R 6 -, -N(Q)-, -0-, or -S-;
- L 2 is CR 5 or
- Each of x and y independently, is 0, 1, 2, 3, 4, or 5.
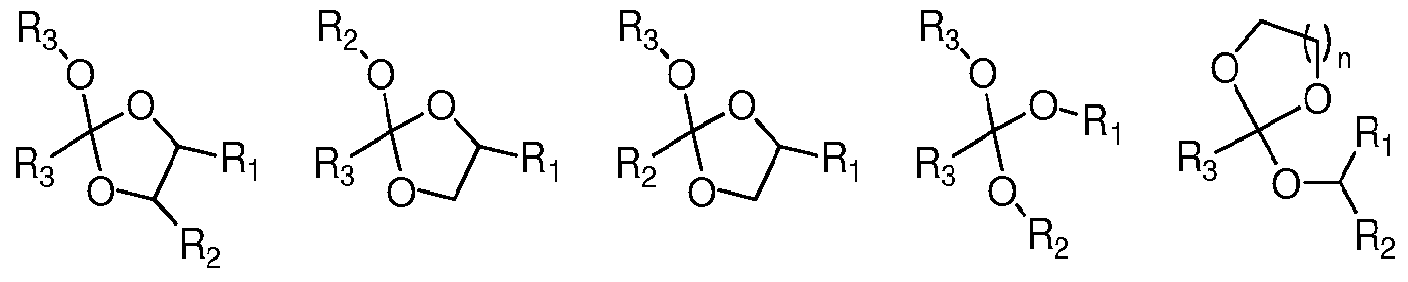
- R 3 has the formula:
- Yi is alkyl, cycloalkyl, aryl, aralkyl, or alkynyl, wherein Yi is optionally substituted by 0 to 6 R n ;
- Y 2 is alkyl, cycloalkyl, aryl, aralkyl, or alkynyl, wherein Y 2 is optionally substituted by 0 to 6 R n ;
- Y 3 is alkyl, cycloalkyl, aryl, aralkyl, or alkynyl, wherein Y 3 is optionally substituted by 0 to 6 R n ;
- Y 4 is alkyl, cycloalkyl, aryl, aralkyl, or alkynyl, wherein Y 4 is optionally substituted by 0 to 6 R n ; or any two of Yi, Y 2 , and Y 3 are taken together with the N atom to which they are attached to form a 3- to 8- member heterocycle optionally substituted by
- Each R n is H, halo, cyano, hydroxy, amino, alkyl, alkoxy, cycloalkyl, aryl, heteroaryl, or heterocyclyl.
- L 3 is a bond, -N(Q)-, -0-, -S-, -(CRyRs -, -C(O)-, or a combination of any two of these.
- L 4 is a bond, -N(Q)-, -0-, -S-, -(CR 7 R 8 ) a -, -C(O)-, or a combination of any two of these.
- L5 is a bond, -N(Q)-, -0-, -S-, -C(O)-, or a combination of any two of these.
- Each occurrence of R7 and Rg is, independently, H, halo, cyano, hydroxy, amino, alkyl, alkoxy, cycloalkyl, aryl, heteroaryl, or heterocyclyl; or two R 7 groups on adjacent carbon atoms are taken together to form a double bond between their respective carbon atoms; or two R 7 groups on adjacent carbon atoms and two R 8 groups on the same adjacent carbon atoms are taken together to form a triple bond between their respective carbon atoms.
- Each a is 0, 1, 2, or 3; wherein an R 7 or R 8 substituent from any of L 3 , L 4 , or L5 is optionally taken with an R 7 or R 8 substituent from any of L 3 , L4, or L5 to form a 3- to 8- member cycloalkyl, heterocyclyl, aryl, or heteroaryl group; and any one of Yi, Y 2 , or Y 3 , is optionally taken together with an R 7 or R 8 group from any of L 3 , L 4 , and L5, and atoms to which they are attached, to form a 3- to 8- member heterocyclyl group.
- Each occurrence of R5 and R 6 is, independently, H, halo, cyano, hydroxy, amino, alkyl, alkoxy, cycloalkyl, aryl, heteroaryl, or heterocyclyl.
- Each Q independently, is H, alkyl, acyl, cycloalkyl, alkenyl, alkynyl, aryl, heteroaryl or heterocyclyl; and each Q 2 , independently, is O, S, N(Q)Q, alkyl or alkoxy,
- the composition can further include a lipid capable of reducing aggregation.
- the composition can further include a nucleic acid.
- the nucleic acid can include a chemically modified nucleic acid.
- the nucleic acid can be 10 to 50 nucleotides long.
- the nucleic acid can be an oligonucleotide.
- the oligonucleotide can be 10 to 50 nucleotides long.
- the oligonucleotide can be double stranded or single stranded. More particularly, in some embodiments, the nucleic acid can be siRNA or mRNA.
- the nucleic acid can be an antisense nucleic acid, a microRNA, an antimicro RNA, an antagomir, a microRNA inhibitor or an immune stimulatory nucleic acid.
- the sample cells can be in suspension.
- the volume of the sample cells in suspension can be at least 0.050 L, at least 3 L, at least 25 L or at least 40 L.
- a method for delivering a nucleic acid to sample cells can further include culturing untreated control cells that have not been exposed to the composition.
- the cell density of the sample cells can increase after the sample cells have been exposed to the composition. In some circumstances, the cell density of the sample cells can increase exponentially for a period of time after the sample cells have been exposed to the composition. In some embodiments, the cell density of the sample cells can be greater than or equal to the cell density of the untreated control cells as measured three days after the sample cells have been exposed to the composition.
- the sample cell viability can be greater than 90% as measured three days after the sample cells have been exposed to the composition.
- a method for delivering a nucleic acid to sample cells can further include measuring a level of a protein in the sample cells and untreated control cells, the protein can be produced from an mRNA that an siRNA delivered into the sample cells was directed against.
- the protein level in the sample cells can be less than the protein level in the untreated control cells as measured at one day after the sample cells have been exposed to the composition. In some circumstances, the protein level in the sample cells can be less than 60% of the protein level in the untreated control cells as measured at one day after the sample cells have been exposed to the composition or as measured at one sample cell doubling time after the sample cells have been exposed to the composition. In some circumstances, the protein level in the sample cells can be less than 70% of the protein level in the untreated control cells as measured at three days after the sample cells have been exposed to the composition or as measured at three times the sample cell doubling time after the sample cells have been exposed to the composition.
- the protein level in the sample cells can be less than 75% of the protein level in the untreated control cells as measured at five days after the sample cells have been exposed to the composition or as measured at five times the sample cell doubling time after the sample cells have been exposed to the composition.
- a storage-stable composition can include a cryoprotectant selected from sucrose, trehalose, glucose, 2-hydroxypropyl-a-cyclodextrin, and sorbitol, and a cationic lipid having the formula described above.
- the composition can further include a neutral lipid; a sterol; and/or a lipid capable of reducing aggregation.
- the composition can include a nucleic acid.
- the cryoprotectant can be present at from 5 wt% to 25 wt%, or at from 7 wt% to 15 wt%.
- the cryoprotectant can include sucrose.
- the composition can be lyophilized, i.e., in a lyophilized state.
- a method for reconstituting a storage- stable composition can include resuspending the composition in a liquid. It can further include adding a lipid and/or a nucleic acid to the resuspended composition.
- FIG. 1 is a graph depicting relative gene expression in a knockdown experiment with varying concentrations of siRNA.
- FIG. 2 is a graph depicting relative gene expression in a knockdown experiment as a function of N/P ratio.
- FIG. 3 is a graph depicting relative gene expression in a knockdown experiment with varying concentrations of siRNA.
- FIG. 4 is a graph depicting expression knockdown measured using different transfection compositions.
- FIG. 5 is a graph depicting particle sizes of liposomes.
- FIGS. 6A-6D are graphs depicting expression knockdown measured using different transfection compositions.
- FIG. 7 is a graph depicting relative gene expression in a knockdown experiment with varying concentrations of siRNA and varying liposome compositions.
- FIG. 8 is a graph depicting expression knockdown measured using different transfection compositions.
- FIG. 9 is a graph depicting relative gene expression in a knockdown experiment with varying concentrations of siRNA and varying liposome compositions.
- FIG. 10 is a graph depicting expression knockdown measured using different transfection compositions.
- FIG. 11 is a graph depicting cell viability as a function of lipid concentration for various lipids.
- FIG. 12 is a graph depicting cell viability as a function of lipid concentration for various lipids.
- FIG. 13 is a graph depicting expression knockdown measured using different transfection compositions.
- FIG. 14 is a graph depicting percent GFP signal remaining after a GFP knockdown experiment using different transfection compositions to deliver siRNA directed against GFP mRNA.
- FIG. 15 is a graph depicting relative gene expression in a knockdown experiment using varying concentrations of a transfection reagent, K8.
- FIG. 16 is a graph depicting relative gene expression in a knockdown experiment using varying concentrations of two transfection reagents, K8 and P8.
- FIG. 17 is a graph depicting cell viability and cell density following exposure to lipid formulation P8.
- FIG. 18 is a graph depicting relative LDH activity as a function of time.
- FIG. 19 is a graph depicting cell viability and cell density following exposure to lipid formulation P8.
- FIG. 20 is a graph depicting relative LDH activity as a function of time.
- the present invention is based, in part, upon the discovery of charged lipids that provide advantages when used in lipid particles for the in vivo delivery of a therapeutic agent.
- the present invention provides nucleic acid-lipid particle compositions comprising a charged lipid according to the present invention.
- a composition described herein provides increased activity of the nucleic acid and/or improved tolerability of the compositions in vivo, which can result in a significant increase in therapeutic index as compared to lipid- nucleic acid particle compositions previously described. Additionally compositions and methods of use are disclosed that can provide for amelioration of the toxicity observed with certain therapeutic nucleic acid-lipid particles.
- the present invention specifically provides for improved compositions for the delivery of siRNA molecules. It is shown herein that these compositions are effective in down-regulating the protein levels and/or mRNA levels of target proteins. Furthermore, it is shown that the activity of these improved compositions is dependent on the presence of a certain charged lipids and that the molar ratio of charged lipid in the formulation can influence activity.
- the lipid particles and compositions of the present invention may be used for a variety of purposes, including the delivery of associated or encapsulated therapeutic agents to cells, both in vitro and in vivo. Accordingly, the present invention provides methods of treating diseases or disorders in a subject in need thereof, by contacting the subject with a lipid particle of the present invention associated with a suitable therapeutic agent.
- the lipid particles of the present invention are particularly useful for the delivery of nucleic acids, including, e.g. , siRNA molecules and plasmids. Therefore, the lipid particles and compositions of the present invention may be used to modulate the expression of target genes and proteins both in vitro and in vivo by contacting cells with a lipid particle of the present invention associated with a nucleic acid that reduces target gene expression (e.g., an siRNA) or a nucleic acid that may be used to increase expression of a desired protein (e.g. , a plasmid encoding the desired protein).
- a nucleic acid that reduces target gene expression e.g., an siRNA
- a nucleic acid that may be used to increase expression of a desired protein e.g. , a plasmid encoding the desired protein.
- the present invention provides novel lipids having certain design features, As shown in Figure 5, the lipid design features include at least one of the following: a head group with a quaternary amine, and optionally, a varying pKa, a cationic, , 2° and 3°, monoamine, di and triamine, oligoamine/polyamine, a low pKa head groups - imidazoles and pyridine, guanidinium, anionic, zwitterionic and hydrophobic tails can include symmetric and/or unsymmetric chains, long and shorter, saturated and unsaturated chain the back bone includes Backbone glyceride and other acyclic analogs, cyclic, spiro, bicyclic and polycyclic linkages with ethers, esters, phosphate and analogs, sulfonate and analogs, disulfides, pH sensitive linkages like acetals and ketals, imines and hydrazones, and oximes.
- Lipids can be advantageously used in lipid particles for the in vivo delivery of therapeutic agents to cells.
- li ids are those having the formula:
- Each of R 1 or R 2 is independently a C 10 to C30 group having the formula -L l - (CR la R lb )a-[L lb -(CR la R lb )p] y -L lc -R lc , where: L la is a bond, - CR la R lb -, -0-, -CO-, -NR ld -, -S-, or a combination thereof.
- Each R la and each R lb is H; halo; hydroxy; cyano; Q-C6 alkyl optionally substituted by halo, hydroxy, or alkoxy; C 3 -Cs cycloalkyl optionally ssuubbssttiittuutteedd bbyy halo, hydroxy, or alkoxy; -OR lc ; -NR lc R ld ; aryl; heteroaryl; or heterocyclyl;
- Each L lb is a bond, -(CR la R lb )i- , -0-, -CO-, -NR ld -, -S-, . , ! s , or a combination thereof, or has the formula
- j, k, and 1 are each independently 0, 1, 2, or 3, provided that the sum of j, k and 1 is at least 1 and no greater than 8; and R lf and R lg are each independently R lb , or adjacent R lf and R lg , taken together, are optionally a bond; or has the formula
- L 1C is --((TCPR lia RR l1bD )i_ 2 -, --0 ⁇ --, --C ⁇ O ⁇ --, --NNRR l10d --, --SS--, --,
- R lc is H; halo; hydroxy; cyano; Ci-C 6 alkyl optionally substituted by halo, hydroxy, or alkoxy; C3-C8 cycloalkyl optionally substituted by halo, hydroxy, or alkoxy; aryl; he e formula:
- R is H; halo; hydroxy; cyano; -C 6 alkyl optionally substituted by halo, hydroxy, or alkoxy; C3-C8 cycloalkyl optionally substituted by halo, hydroxy, or alkoxy; aryl; heteroaryl; or heterocyclyl.
- a is 0-6; each ⁇ , independently, is 0-6; and ⁇ is 0-6. represents a connection between L 2 and Li which is:
- Li is C(R a ), O, S or N(Q);
- alkylene -N(Q , -C(O)-, -O(CO)-, -OC(0)N(Q)-, -N(Q)C(0)0-, -C(0)0, -OC(0)0-, -0 S(0)(Q 2 )0-, and -OP(0)(Q 2 )0-.
- R a is H, alkyl, alkoxy, -OH, -N(Q)Q, or -SQ.
- L 2 has the formula
- X is the first atom of L 2
- Y is the second atom of L 2
- X and Y are each, independently, selected from the group consisting of -0-, -S-,
- alkylene -N(Q)-, -C(O)-, -O(CO)-, -OC(0)N(Q)-, -N(Q)C(0)0-, -C(0)0, -OC(0)0-, -0 S(0)(Q 2 )0-, and -OP(0)(Q 2 )0-; 3 ⁇ 4 and Z 4 are each, independently, -0-, -S-, -CH 2 -, - CHR 5 -, or -CR 5 R 5 -; Z 2 is CH or N; Z 3 is CH or N; or Z 2 and Z 3 , taken together, are a single C atom; Ai and A 2 are each, independently, -0-, -S-, -CH 2 -, -CHR 5 -, or -CR 5 R 5 -.
- Each Z is N, C(R 5 ), or C(R 3 ).
- k is 0, 1, or 2; each m, independently, is 0 to 5; each n, independently, is 0 to 5; where m and n taken together result in a 3, 4, 5, 6, 7 or 8 member ring.
- X is the first atom of Li
- Y is the second atom of Li
- X and Y are each, independently, selected from the group consisting of -0-, -S-,
- alkylene -N(Q)-, -C(O)-, -O(CO)-, -OC(0)N(Q)-, -N(Q)C(0)0-, -C(0)0, -OC(0)0-, S(0)(Q 2 )0-, and -OP(0)(Q 2 )0-;
- Ti is CH or N;
- T 2 is CH or N; or
- L 2 is CR 5 .
- X is the first atom of I
- Y is the second atom of L l 5 represents a single bond to the first atom of L 2
- X and Y are each, independently, selected from the group consisting of -0-, -S-,
- alkylene alkylene, -N(Q)-, -C(O)-, -O(CO)-, -OC(0)N(Q)-, -N(Q)C(0)0-, -C(0)0, -OC(0)0-, -0 S(0)(Q 2 )0-, and -OP(0)(Q 2 )0-;
- Ti is -CR 5 R 6 -, -N(Q)-, -0-, or -S-;
- T 2 is -CR 5 R 6 -, -N(Q)- , -0-, or -S-.
- L 2 is CR 5 or N; each of s and y, independently, is 0, 1, 2, 3, 4, or 5.
- R 3 has the formula:
- Yi is alkyl, cycloalkyl, aryl, aralkyl, or alkynyl, wherein Yi is optionally substituted by 0 to 6 R n .
- Y 2 is alkyl, cycloalkyl, aryl, aralkyl, or alkynyl, wherein Y 2 is optionally substituted by 0 to 6 R n .
- Y 3 is alkyl, cycloalkyl, aryl, aralkyl, or alkynyl, wherein Y 3 is optionally substituted by 0 to 6 R n .
- Y 4 is alkyl, cycloalkyl, aryl, aralkyl, or alkynyl, wherein Y 4 is optionally substituted by 0 to 6 R n ; or any two of Yi, Y 2 , and Y 3 are taken together with the N atom to which they are attached to form a 3- to 8- member heterocycle optionally substituted by 0 to 6 R n ; or Yi, Y 2 , and Y 3 are all be taken together with the N atom to which they are attached to form a bicyclic 5- to 12- member heterocycle optionally substituted by 0 to 6 R n .
- Each R n is H, halo, cyano, hydroxy, amino, alkyl, alkoxy, cycloalkyl, aryl, heteroaryl, or heterocyclyl.
- L 3 is a bond, -N(Q)-, -0-, -S-, -(CR Rs , - C(O)-, or a combination of any two of these.
- L4 is a bond, -N(Q)-, -0-, -S-, -(CR 7 R 8 ) a -, - C(O)-, or a combination of any two of these.
- L5 is a bond, -N(Q)-, -0-, -S-, -(CRvRs , - C(O)-, or a combination of any two of these.
- Each occurrence of R7 and Rg is, independently, H, halo, cyano, hydroxy, amino, alkyl, alkoxy, cycloalkyl, aryl, heteroaryl, or heterocyclyl; or two R 7 groups on adjacent carbon atoms are taken together to form a double bond between their respective carbon atoms; or two R7 groups on adjacent carbon atoms and two R 8 groups on the same adjacent carbon atoms are taken together to form a triple bond between their respective carbon atoms.
- Each a is 0, 1, 2, or 3; wherein an R7 or Rg substituent from any of L 3 , L 4 , or L5 is optionally taken with an R7 or Rg substituent from any of L 3 , L4, or L5 to form a 3- to 8- member cycloalkyl, heterocyclyl, aryl, or heteroaryl group; and any one of Yi, Y 2 , or Y 3 , is optionally taken together with an R7 or Rg group from any of L 3 , L4, and L5, and atoms to which they are attached, to form a 3- to 8- member heterocyclyl group.
- R5 and R 6 are, independently, H, halo, cyano, hydroxy, amino, alkyl, alkoxy, cycloalkyl, aryl, heteroaryl, or heterocyclyl.
- Each Q independently, is H, alkyl, acyl, cycloalkyl, alkenyl, alkynyl, aryl, heteroaryl or heterocyclyl.
- Each Q 2 is O, S, N(Q)Q, alkyl or alkoxy.
- Y 3 and/or Y 4 is absent, such that the lipid does not include a quaternary nitrogen atom.
- a compound can have the formula:
- Ri and R 2 are each independently for each occurrence optionally substituted C10-C30 alkyl, optionally substituted Cio-C 30 alkoxy, optionally substituted Cio-C 3 o alkenyl, optionally substituted Cio-C 3 o alkenyloxy, optionally substituted C10-C30 alkynyl, optionally substituted Cio-C 3 o alkynyloxy, or optionally substituted Cio-C 3 o acyl; represents a connection between L 2 and Li which is: (1) a single bond between one atom of L 2 and one atom of Li, wherein
- Li is C(R X ) or N
- L 2 is -CR5R6-, -0-, -S-, -N(Q ,
- X is the first atom of L 2
- Y is the second atom of L 2
- X and Y are each, independently, selected from the group consisting of -0-, -S-,
- alkylene -N(Q)-, -C(O)-, -O(CO)-, -OC(0)N(Q)-, -N(Q)C(0)0-, -C(0)0, -OC(0)0-, -0 S(0)(Q 2 )0-, and -OP(0)(Q 2 )0-;
- Zi and Z 4 are each, independently, -0-, -S-, -CH 2 -, -CHR 5 -, or -CR 5 R 5 -;
- Z 2 is CH or N
- Z 3 is CH or N
- Ai and A 2 are each, independently, -0-, -S-, -CH 2 -, -CHR 5 -, or -CR 5 R 5 -; each Z is N, C(R 5 ), or C(R 3 );
- k 0, l, or 2;
- each m independently, is 0 to 5;
- each n independently, is 0 to 5;
- X is the first atom of L l 5
- Y is the second atom of L represents a single bond to the first atom of L 2
- X and Y are each, independently, selected from the group consisting of -0-, -S-,
- alkylene -N(Q)-, -C(O)-, -O(CO)-, -OC(0)N(Q)-, -N(Q)C(0)0-, -C(0)0, -OC(0)0-, -0 S(0)(Q 2 )0-, and -OP(0)(Q 2 )0-;
- Ti is CH or N
- T 2 is CH or N
- L 2 is CR 5 ;
- X is the first atom of Li
- Y is the second atom of Li
- X and Y are each, independently, selected from the group consisting of -0-, -S-,
- alkylene -N(Q)-, -C(O)-, -O(CO)-, -OC(0)N(Q)-, -N(Q)C(0)0-, -C(0)0, -OC(0 )0-, -OS(0)(Q 2 )0-, and -OP(0)(Q 2 )0-;
- i is -CR5R5-, -N(Q)-, -0-, or -S-;
- T 2 is -CR5R5-, -N(Q)-, -0-, or -S-;
- L 2 is CR 5 or N
- R 3 has the formula
- each of Yi, Y 2 , Y 3 , and Y 4 independently, is alkyl, cycloalkyl, aryl, aralkyl, or alkynyl; or
- any two of Yi, Y 2 , and Y 3 are taken together with the N atom to which they are attached to form a 3- to 8- member heterocycle; or
- Yi, Y 2 , and Y 3 are all be taken together with the N atom to which they are attached to form a bicyclic 5- to 12- member heterocycle;
- each R n independently, is H, halo, cyano, hydroxy, amino, alkyl, alkoxy, cycloalkyl, aryl, heteroaryl, or heterocyclyl;
- L 3 is a bond, -N(Q)-, -0-, -S-, -(CRsRe , -C(O)-, or a combination of any two of these;
- L 4 is a bond, -N(Q)-, -0-, -S-, -(CR 5 R 6 ) a -, -C(O)-, or a combination of any two of these;
- L 5 is a bond, -N(Q)-, -0-, -S-, -(CR 5 R 6 ) a -, -C(O)-, or a combination of any two of these;
- each occurrence of R5 and R 6 is, independently, H, halo, cyano, hydroxy, amino, alkyl, alkoxy, cycloalkyl, aryl, heteroaryl, or heterocyclyl; or two R5 groups on adjacent carbon atoms are taken together to form a double bond between their respective carbon atoms; or two R 5 groups on adjacent carbon atoms and two R 6 groups on the same adjacent carbon atoms are taken together to form a triple bond between their respective carbon atoms;
- each a independently, is 0, 1, 2, or 3;
- an R5 or R6 substituent from any of L 3 , L 4 , or L5 is optionally taken with an R5 or R 6 substituent from any of L 3 , L 4 , or L5 to form a 3- to 8- member cycloalkyl, heterocyclyl, aryl, or heteroaryl group; and any one of Yi, Y 2 , or Y 3 , is optionally taken together with an R5 or R 6 group from any of L 3 , L 4 , and L5, and atoms to which they are attached, to form a 3- to 8- member heterocyclyl group;
- each Q independently, is H, alkyl, acyl, cycloalkyl, alkenyl, alkynyl, aryl, heteroaryl or heterocyclyl;
- each Q 2 independently, is 0, S, N(Q)(Q), alkyl or alkoxy.
- Ri and R 2 are each independently for each occurrence optionally substituted C10-C30 alkyl, optionally substituted C10-C30 alkoxy, optionally substituted C10-C30 alkenyl, optionally substituted C10-C30 alkenyloxy, optionally substituted C10-C30 alkynyl, optionally substituted C10-C30 alkynyloxy, or optionally substituted C10-C30 acyl;
- R 3 is independently for each occurrence H, optionally substituted C1-C10 alkyl, optionally substituted C2-C10 alkenyl, optionally substituted C2-C10 alkynyl, optionally substituted alkylheterocycle, optionally substituted heterocyclealkyl, optionally substituted alkylphosphate, optionally substituted phosphoalkyl, optionally substituted alkylphosphorothioate, optionally substituted phosphorothioalkyl, optionally substituted alkylphosphorodithioate, optionally substituted phosphorodithioalkyl, optionally substituted alkylphosphonate, optionally substituted phosphonoalkyl, optionally substituted amino, optionally substituted alkylamino, optionally substituted
- di(alkyl)amino optionally substituted aminoalkyl, optionally substituted alkylaminoalkyl, optionally substituted di(alkyl)aminoalkyl, optionally substituted hydroxyalkyl, optionally substituted polyethylene glycol (PEG, mw 100-40K), optionally substituted mPEG (mw 120-40K), optionally substituted heteroaryl, or optionally substituted heterocycle;
- At least one R 3 includes a quaternary amine
- X and Y are each independently -0-, -S-,
- alkylene -N(Q)-, -C(O)-, -O(CO)-, -OC(0)N(Q)-, -N(Q)C(0)0-, -C(0)0, -OC(0)0-, -0 S(0)(Q 2 )0-, or -OP(0)(Q 2 )0-;
- Q is H, alkyl, ⁇ -aminoalkyl, ro-(substituted)aminoalkyl, ⁇ -phosphoalkyl, or ⁇ -thiophosphoalkyl;
- Q 2 is independently for each occurrence O, S, N(Q)(Q), alkyl or alkoxy;
- Ai, A 2 , A 3 , A 4 , A5 and A 6 are each
- Ag is independently for each occurrence -CH 2 -, -CHR 5 -, -CR 5 R 5 -;
- N V N 1 1 ⁇ N y N , arylene, heteroarylene, cycloalkylene, or
- Z is N or C(R 3 );
- Z' is -0-, -S-, -N(Q)-, or alkylene
- each R', R", and R' independently, is H, alkyl, alkyl, heteroalkyl, aralkyl, cyclic alkyl, or heterocyclyl;
- R 5 is H, halo, cyano, hydroxy, amino, optionally substituted alkyl, optionally substituted alkoxy, or optionally substituted cycloalkyl; i and j are each independently 0-10; and
- a and b are each independently 0-2.
- a compound in another aspect, can be selected from the group consisting of:
- a composition can include a compound as described above, a neutral lipid, and a sterol.
- the composition can further include a nucleic acid.
- the nucleic acid can be RNA.
- E is O(CO), (CO)O, OC(0)N(R'), or N(R')C(0)0.
- Ri and R 2 are each independently for each occurrence optionally substituted C10-C30 alkyl, optionally substituted C10-C30 alkoxy, optionally substituted C10-C30 alkenyl, optionally substituted C10-C30 alkenyloxy, optionally substituted C10-C30 alkynyl, optionally substituted C10-C30 alkynyloxy, or optionally substituted C10-C30 acyl.
- R3 is independently for each occurrence H, optionally substituted C1-C10 alkyl, optionally substituted C2-C10 alkenyl, optionally substituted C2-C10 alkynyl, optionally substituted alkylheterocycle, optionally substituted heterocyclealkyl, optionally substituted alkylphosphate, optionally substituted phosphoalkyl, optionally substituted alkylphosphorothioate, optionally substituted phosphorothioalkyl, optionally substituted alkylphosphorodithioate, optionally substituted phosphorodithioalkyl, optionally substituted alkylphosphonate, optionally substituted phosphonoalkyl, optionally substituted amino, optionally substituted alkylamino, optionally substituted
- di(alkyl)amino optionally substituted aminoalkyl, optionally substituted alkylaminoalkyl, optionally substituted di(alkyl)aminoalkyl, optionally substituted hydroxyalkyl, optionally substituted polyethylene glycol (PEG, mw 100-40K), optionally substituted mPEG (mw 120-40K), optionally substituted heteroaryl, or optionally substituted heterocycle.
- PEG polyethylene glycol
- mw 100-40K optionally substituted mPEG (mw 120-40K)
- heteroaryl optionally substituted heterocycle.
- At least one R3 includes a quaternary amine.
- X and Y are each independently -0-, -S-,
- alkylene -N(Q)-, -C(0)-, -O(CO)-, -OC(0)N(Q)-, -N(Q)C(0)0-, -C(0)0, -OC(0)0-, -0 S(0)(Q 2 )0-, or -OP(0)(Q 2 )0-.
- Q is H, alkyl, ⁇ -aminoalkyl, ro-(substituted)aminoalkyl, ⁇ -phosphoalkyl, or ⁇ -thiophosphoalkyl.
- Q 2 is independently for each occurrence 0, S, N(Q)(Q), alkyl or alkoxy.
- a A 2 , A 3 , A 4 , A 5 and A 6 are each
- a 8 is independently for each occurrence -CH 2 -, -CHR 5 -, -CR 5 R 5 -.
- Z is N or C(R 3 ).
- Z' is -0-, -S-, -N(Q , or alkylene.
- Each R', R", and R' independently, is H, alkyl, alkyl, heteroalkyl, aralkyl, cyclic alkyl, or heterocyclyl.
- R 5 is H, halo, cyano, hydroxy, amino, optionally substituted alkyl, optionally substituted alkoxy, or optionally substituted cycloalkyl.
- i and j are each independently 0-10.
- a and b are each independently 0-2.
- R 3 is ro-(substituted)aminoalkyl.
- the ⁇ -amino group can be a quaternary amine.
- Examples quaternary ro-(substituted)aminoalkyl groups include 2-(trimethylamino)ethyl, 3-(triisopropylamino)propyl, or
- R 3 has the formula:
- each of Yi, Y 2 , and Y 3 is independently, alkyl, cycloalkyl, aryl, aralkyl, or alkynyl.
- each of Y l f Y 2 , and Y 3 is independently Ci-C 6 alkyl or C 3 -C6 cycloalkyl, C 1 -C4 alkyl, or Ci-C 3 alkyl.
- Yi, Y 2 , and Y 3 can be taken together with the N atom to which they are attached to form a 3- to 8- member heterocycle.
- Yi, and Y 2 can be taken together with the N atom to which they are attached to form a pyrrolidine, a pyrrole, an oxazole, an imidazole, a pyridine, a piperidine, or other N-containing heterocycles.
- Yi, Y 2 , and Y 3 can all be taken together with the N atom to which they are attached to form a bicyclic 5- to 12- member heterocycle.
- Yi, Y 2 , and Y 3 can all be taken together the N atom to which they are attached to form a quinuclidine, a tropane, a l,4-diazabicyclo[2.2.2]octane, or other bicyclic heterocycles.
- Each Yi, Y 2 , Y 3 can be optionally substituted alkyl, optionally substituted cycloalkyl, optionally substituted aryl, optionally substituted aralkyl, or optionally substituted alkynyl.
- the heterocyclic group can be optionally substituted.
- L 3 is a bond, -N(Q)-, -0-, -S-, -(CRsRe , -C(O)-, or a combination of any two of these;
- L 4 is a bond, -N(Q)-, -0-, -S-, -(CR5R 6 ) a -, -C(O)-, or a combination of any two of these;
- L 5 is a bond, -N(Q)-, -0-, -S-, -(CR5R 6 ) a -, -C(O)-, or a combination of any two of these;
- Each of R5 and R 6 is, independently, H, halo, cyano, hydroxy, amino, alkyl, alkoxy, cycloalkyl, aryl, heteroaryl, or heterocyclyl.
- Two R5 groups on adjacent carbon atoms can be taken together to form a double bond between their respective carbon atoms.
- Two R5 groups on adjacent carbon atoms and two R6 groups on the same adjacent carbon atoms can be taken together to form a triple bond between their respective carbon atoms.
- Each R5 and R 6, independently, can be optionally substituted alkyl, optionally substituted alkoxy, optionally substituted cycloalkyl, optionally substituted aryl, optionally substituted heteroaryl, or optionally substituted heterocyclyl.
- Each a independently, is 0, 1, 2, or 3.
- an R5 or R6 substituent from L 3 can be taken with an R5 or R 3 ⁇ 4 substituent from L 4 to form a 3- to 8- member cycloalkyl or heterocycle group.
- an R 5 or R 6 substituent from L 3 can be taken with an R 5 or R 6 substituent from L 5 to form a 3- to 8- member cycloalkyl or heterocycle group; or an R 5 or R 6 substituent from L 4 can be taken with an R5 or R 6 substituent from L5 to form a 3- to 8- member cycloalkyl or heterocycle group.
- a cycloalkyl group or heterocycle group formed by R5 or R 6 substituents from L 3 , L 4 , or L5 can be optionally substituted.
- one exemplary R 3 group having this structural feature includes
- Yi, Y 2 , or Y 3 can be taken together with an R 5 or R6 group from any of L 3 , L 4 , and L5, and atoms to which they are attached, to form a 3- to 8- member heterocycle.
- R 3 groups having this structural feature include (N,N-dimethylpyrrolidin-2-yl)methyl, and
- X and Y can be independently -0-, -S-, alkylene, or -N(Q)-. It has been found that charged lipids comprising unsaturated alkyl chains are particularly useful for forming lipid nucleic acid particles with increased membrane fluidity.
- at least one of Ri or R 2 comprises at least one, at least two or at least three sites of unsaturation, e.g. double bond or triple bond.
- only one of Ri or R 2 comprises at least one, at least two or at least three sites of unsaturation.
- Ri and R 2 both comprise at least one, at least two or at least three sites of unsaturation.
- Ri and R 2 comprise different numbers of unsaturation, e.g., one of Ri and R 2 has one site of unsaturation and the other has two or three sites of unsaturation.
- Ri and R 2 both comprise the same number of unsaturation sites. In one embodiment, Ri and R 2 comprise different types of unsaturation, e.g. unsaturation in one of Ri and R 2 is double bond and in the other unsaturation is triple bond.
- Ri and R 2 both comprise the same type of unsaturation, e.g. double bond or triple bond.
- At least one of Ri or R 2 comprises at least one double bond and at least one triple bond.
- only one of Ri or R 2 comprises at least one double bond and at least one triple bond.
- Ri and R 2 both comprise at least one double bond and at least one triple bond.
- Ri and R 2 are both same, e.g. Ri and R 2 are both linoleyl (CI 8) or Ri and R 2 are both heptadeca-9-enyl.
- Ri and R 2 are different from each other.
- At least one of Ri and R 2 is cholesterol.
- At least one of Ri or R 2 comprises at least one methylene group where one or both H atoms are replaced by F, e.g. fluoromethylene or
- both Ri and R 2 comprise at least one methylene group with one or two H replaced by F, e.g. . fluoromethylene or difluoromethylene.
- only one of Ri and R 2 comprises at least one methylene group with one or both H replaced by F.
- At least one of Ri or R 2 terminates in fluoromethyl, difluormethyl, or trifluoromethyl. In one embodiment, both Ri and R 2 terminate in fluoromethyl, difluormethyl, or trifluoromethyl.
- At least one of Ri or R 2 is -(CF 2 ) y -Z"-(CH 2 ) y -CH 3 , wherein each y is independently 1-10 and Z" is O, S or N(Q).
- both of Ri and R 2 are -(CF 2 ) y -Z"-(CH 2 ) y -CH 3 , wherein each y is independently 1-10 and Z' ' is O, S or N(Q).
- At least one of Ri or R 2 is -(CH 2 ) y -Z"-(CF 2 ) y -CF 3 , wherein each y is independently 1-10 and Z" is O, S or N(Q).
- both of Ri and R 2 are -(CH 2 ) y -Z"-(CF 2 ) y -CF 3 , wherein each y is independently 1-10 and Z" is O, S or N(Q).
- at least one of Ri or R 2 is -(CF 2 ) y -(CF 2 ) y -CF 3 , wherein each y is independently 1-10.
- both of Ri and R 2 are -(CF 2 ) y -(CF 2 ) y -CF 3 , wherein each y is independently 1-10.
- Ri and R 2 are, independently, selected from the group consisting of lineolyl, ⁇ -linoenyl, n-octadecanyl, n-decanyl, n-dodecanyl, and
- the lipid can have (R l 5 R 2 ) selected from the group consisting of (lineolyl, lineolyl), ( ⁇ -linoenyl, ⁇ -linoenyl), (lineolyl, n-octadecanyl), (lineolyl, n-decanyl), (lineolyl, n-dodecanyl), and (9-methyloctadecanyl,
- At least one R 3 is co-aminoalkyl or ⁇ -(substituted)aminoalkyl.
- At least one R 3 is co-aminoalkyl or ⁇ -(substituted)aminoalkyl.
- the lipid is a racemic mixture.
- the lipid is enriched in one diastereomer, e.g. the lipid has at least 95%, at least 90%, at least 80% or at least 70% diastereomeric excess.
- the lipid is enriched in one enantiomer, e.g. the lipid has at least 95%, at least 90%, at least 80% or at least 70% enantiomer excess.
- the lipid is chirally pure, e.g. is a single optical isomer.
- the lipid is enriched for one optical isomer.
- a double bond e.g., a carbon-carbon double bond or carbon-nitrogen double bond
- isomerism in the configuration about the double bond (i.e. cis/trans or E/Z isomerism).
- the configuration of a double bond is illustrated in a chemical structure, it is understood that the corresponding isomer can also be present.
- the amount of isomer present can vary, depending on the relative stabilities of the isomers and the energy required to convert between the isomers.
- the invention features a compound of formula XXXIVa, XXXIVb, XXXIVc, XXXIVd, or XXXIVe, salts or isomers thereof:
- Ri and R 2 are each independently for each occurrence optionally substituted C10-C30 alkyl, optionally substituted C10-C30 alkenyl, or optionally substituted C10-C30 alkynyl;
- R 3 is defined as above,
- n 1, 2, or 3.
- R 3 is optionally substituted heterocyclealkyl, optionally substituted amino, optionally substituted alkylamino, optionally substituted
- di(alkyl)amino optionally substituted aminoalkyl, optionally substituted alkylaminoalkyl, optionally substituted di(alkyl)aminoalkyl, or optionally substituted heterocycle.
- the lipid is a compound of formula Xllla:
- Ri and R 2 are each independently for each occurrence optionally substituted
- C10-C30 alkyl optionally substituted Cio-C 3 o alkenyl, or optionally substituted Cio-C 3 o alkynyl.
- At least one of R 3 and R 3 > includes a quaternary amine.
- R 3 and R are independently for each occurrence defined as R 3 above;
- R 3 and R can be taken together with the atoms to which they are attached to form an optionally substituted carbocyclyl, optionally substituted heterocyclyl, optionally substituted aryl or optionally substituted heteroaryl; each of which is substituted with 0-4 occurrences of R4;
- each R4 is independently selected from optionally substituted C1-C10 alkyl, optionally substituted C2-C10 alkenyl, optionally substituted C2-C10 alkynyl, optionally substituted amino, optionally substituted alkylamino, optionally substituted
- di(alkyl)amino optionally substituted aminoalkyl, optionally substituted alkylaminoalkyl, optionally substituted di(alkyl)aminoalkyl, optionally substituted hydroxyalkyl, optionally substituted aryl, optionally substituted heteroaryl, or optionally substituted heterocycle;
- X and Y are each independently -0-, -S-, alkylene, or -N(Q)-;
- Q is H, alkyl, ⁇ -aminoalkyl, ro-(substituted)aminoalkyl, co-phosphoalkyl, or co-thiophosphoalkyl;
- Ai and A 2 are each independently -0-, -S-, or -CR 5 R 3 -;
- R 5 is H, halo, cyano, hydroxy, amino, optionally substituted alkyl, optionally substituted alkoxy, or optionally substituted cycloalkyl;
- Z and Z' are each independently selected from -0-, -S-, -N(Q)-, alkylene or absent;
- a and b are each independently 0-2.
- X and Y are each independently O.
- the sum of a and b is 1, 2, or 3.
- Ai and A 2 are each independently -CR 5 R 5 -.
- Z and Z' are each a bond.
- R 3 and Ry can be taken together with the atoms to which they are attached to form an optionally substituted carbocyclyl, optionally substituted heterocyclyl, optionally substituted aryl or optionally substituted heteroaryl.
- R 3 and R 3 can be taken together with the atoms to which they are attached to form an optionally substituted carbocyclyl (e.g., optionally substituted with amino, alkylamino, or dialkylamino).
- R 3 and R 3 can be taken together with the atoms to which they are attached to form an optionally substituted heterocyclyl (e.g., a nitrogen containing heterocyclyl).
- an optionally substituted heterocyclyl e.g., a nitrogen containing heterocyclyl
- R 3 and Ry are taken together to form a carbocyclic ring
- R 3 and Ry are taken together to form a heterocyclic ring (e.g., piperidine) substituted with 0-3 occurrences of R 4 .
- a heterocyclic ring e.g., piperidine
- each R4 is independently selected from optionally optionally substituted amino, optionally substituted alkylamino, optionally substituted di(alkyl)amino, optionally substituted aminoalkyl, optionally substituted alkylaminoalkyl, optionally substituted di(alkyl)aminoalkyl, and optionally substituted hydroxyalkyl.
- the lipid is a compound of formula XXXIX, salts or isomers thereof:
- Ri and R 2 are each independently for each occurrence optionally substituted
- C 10 -C30 alkyl optionally substituted C 10 -C30 alkenyl, optionally substituted C 10 -C30 alkynyl, optionally substituted C 10 -C30 acyl.
- R 3 is defined as above.
- X and Y are each independently 0, C(0)0, S, alkyl or N(Q);
- Q is H, alkyl, ⁇ -aminoalkyl, ro-(substituted)aminoalkyl, ⁇ -phosphoalkyl or ⁇ -thiophosphoalkyl.
- the lipid is a compo formula XXXIII, salts or isomers thereof
- Ri and R 2 are each independently for each occurrence optionally substituted
- C 10 -C30 alkyl optionally substituted C 10 -C30 alkenyl, optionally substituted C 10 -C30 alkynyl, optionally substituted C 10 -C30 acyl;
- Q is H, alkyl, ⁇ -aminoalkyl, ro-(substituted)aminoalky, ⁇ -phosphoalkyl or ⁇ -thiophosphoalkyl.
- Ri and R2 are each independently for each occurrence optionally substituted C10-C30 alkyl, optionally substituted C10-C30 alkoxy, optionally substituted C10-C30 alkenyl, optionally substituted C10-C30 alkenyloxy, optionally substituted C10-C30 alkynyl, optionally substituted C10-C30 alkynyloxy, or optionally substituted Cio-C 30 acyl.
- the lipid is a compound of formula XXXIII, provided that
- the invention features a lipid of formula XXXVIII:
- N 1 arylene, heteroarylene, cycloalkylene, or
- R3 has the formula:
- Yi is alkyl, cycloalkyl, aryl, aralkyl, or alkynyl, wherein Y 1 is optionally substituted by 0 to 6 R n .
- Y 2 is alkyl, cycloalkyl, aryl, aralkyl, or alkynyl, wherein Y 2 is optionally substituted by 0 to 6 R n .
- Y3 is alkyl, cycloalkyl, aryl, aralkyl, or alkynyl, wherein Y 3 is optionally substituted by 0 to 6 R n .
- Y 4 is alkyl, cycloalkyl, aryl, aralkyl, or alkynyl, wherein Y 4 is optionally substituted by 0 to 6 R n ; or any two of Yi, Y 2 , and Y 3 are taken together with the N atom to which they are attached to form a 3- to 8- member heterocycle optionally substituted by 0 to 6 R n ; or Yi, Y 2 , and Y 3 are all be taken together with the N atom to which they are attached to form a bicyclic 5- to 12- member heterocycle optionally substituted by 0 to 6 R n .
- Each R n is H, halo, cyano, hydroxy, amino, alkyl, alkoxy, cycloalkyl, aryl, heteroaryl, or heterocyclyl.
- L 3 is a bond, -N(Q)-, -0-, -S-, -(CR 7 R8) a -, - C(O)-, or a combination of any two of these.
- L 4 is a bond, -N(Q)-, -0-, -S-, -(CR 7 Rs) a -, - C(O)-, or a combination of any two of these.
- L5 is a bond, -N(Q)-, -0-, -S-, -(CR 7 Rs) a -, - C(O)-, or a combination of any two of these.
- Each occurrence of R 7 and R 8 is, independently, H, halo, cyano, hydroxy, amino, alkyl, alkoxy, cycloalkyl, aryl, heteroaryl, or heterocyclyl; or two R 7 groups on adjacent carbon atoms are taken together to form a double bond between their respective carbon atoms; or two R 7 groups on adjacent carbon atoms and two Rg groups on the same adjacent carbon atoms are taken together to form a triple bond between their respective carbon atoms.
- Each a is 0, 1, 2, or 3; wherein an R 7 or Rg substituent from any of L 3 , L 4 , or L 5 is optionally taken with an R 7 or Rg substituent from any of L 3 , L4, or L 5 to form a 3- to 8- member cycloalkyl, heterocyclyl, aryl, or heteroaryl group; and any one of Yi, Y 2 , or Y 3 , is optionally taken together with an R 7 or R 8 group from any of L 3 , L , and L 5 , and atoms to which they are attached, to form a 3- to 8- member heterocyclyl group.
- Each occurrence of R5 and R 6 is, independently, H, halo, cyano, hydroxy, amino, alkyl, alkoxy, cycloalkyl, aryl, heteroaryl, or heterocyclyl.
- Each Q independently, is H, alkyl, acyl, cycloalkyl, alkenyl, alkynyl, aryl, heteroaryl or heterocyclyl.
- Each Q 2 is 0, S, N(Q)Q, alkyl or alkoxy.
- Q is H, alkyl, co-aminoalkyl, ro-(substituted)aminoalkyl, co-phosphoalkyl, or ⁇ -thiophosphoalkyl.
- Ri and R 2 and R x are each independently for each occurrence H, optionally substituted C1-C10 alkyl, optionally substituted C10-C30 alkyl, optionally substituted C10-C30 alkenyl, optionally substituted C10-C30 alkynyl, optionally substituted C10-C30 acyl. In some embodiments, at least one of Ri, R 2 and R x is not H.
- At least two of Ri, R 2 and R x is not H.
- R3 is defined as above.
- n 0, 1, 2, or 3.
- R 2 is C(0)0, R and one of R R 2 , or R x is H, then the remaining of R 2, or
- R x are not both linoleyl.
- each of Ri and R 2 is independently for each occurrence optionally substituted C10-C30 alkyl, optionally substituted C10-C30 alkenyl, optionally substituted C10-C30 alkynyl, optionally substituted C10-C30 acyl.
- R x is H or optionally substituted C1-C10 alkyl.
- R x is optionally substituted C10-C30 alkyl, optionally substituted C10-C30 alkenyl, optionally substituted C10-C30 alkynyl, optionally substituted C10-C30 acyl.
- the present invention comprises of synthesis of lipids described herein in racemic as well as in optically pure form.
- a lipid has formula LX, LXI, LXII, LXIII, LXIV, LXV, LXVI, LXVII, LXVIII, LXIX, LXX, LXXI, LXXII, or LXXIII:
- X and Y are each independently -0-, -S-, -CH 2 -, or -N(Q 3 )-; where Q is H, Me, Et, or -(CH 2 ) r -N(Q 3 )(Q 4 );
- Z is N, CH, C(Me), C(Et);
- Q 2 is O or S
- Each of Ai and A 2 are CH 2 , CHF, or CF 2 ;
- n, p and q are each independently 0 to 5.
- R 2 and R4 are each independently selected from the group consisting of alkyl groups having about 10 to 30 carbon atoms, wherein R ⁇ R 2 and R4 independently comprises of: fully saturated alkyl chain, at least one double bond, at least one triple bond, at least one hetero atom, at least one CF 2 , at least one CHF or at least one perfluoroalkylated chain. CF 2 /CHF could be on the lipid anchor or on the core.
- R 3 is defined as above.
- the lipid can be a compound having the formula:
- R a independently, is absent, H, alkyl, or cycloalkyl. In one embodiment, R a is alkyl or cycloalkyl for no more than two occurrences. In one embodiment, R a is alkyl or cycloalkyl for no more than one occurrence.
- R for at least 3 occurrences, is ⁇ R .
- Y is 0 or NR 4 . In one embodiment, Y is 0. In one embodiment, Y is 0 for each occurrence. In one embodiment, R 1 is H. In one embodiment, R 1 is H for each occurrence.
- R 1 is R 3 .wherein R 3 alkyl, alkenyl, alkynyl, heteroalkyl, heteroalkenyl, or heteroalkynyl, each of which is optionally substituted with one or more substituent (e.g., a hydrophilic substituent). In one embodiment, R 1 is ⁇ 3 , and R 3 alkyl optionally substituted with one or more substituent (e.g., a hydrophilic substituent). In one embodiment, R 3 is substituted with -OH.
- R 1 is , or .uß., ⁇ r.3
- R alkyl is optionally substituted with one or more substituent.
- R 3 is substituted with a hydrophilic substituent.
- R 4 for each occurrence is independently H alkyl, alkenyl, alkynyl, heteroalkyl, heteroalkenyl, or heteroalkynyl; each of which is optionally substituted with one or more substituent. In one embodiment, 3 2
- R is substituted with -OH.
- R is alkyl, alkenyl, or alkynyl.
- R is alkyl (e.g., C 6 -Ci 8 alkyl, e.g., C 8 -C 12 alkyl, e.g., C 10 alkyl).
- R for at least 3 (e.g., at least 4 or 5) occurrences is R .
- R is alkyl (e.g., C6-Ci 8 alkyl, e.g., C 8 -C 12 alkyl, e.g., C 10 alkyl). In one embodiment, R for at least 1 occurrence (e.g., 1 or 2 occurrences) is H.
- the lipid can be a compound having the formula:
- the lipid can be a quaternary lipid derived from the compounds disclosed in Akinc, A., et al., "Development of lipidoid-siRNA formulations for systemic delivery of RNAi therapeutics," Nat. Biotechnol.
- the lipid can have one of the following formulas:
- R a is alkyl or cycloalkyl for no more than two occurrences, or R a can be alkyl or cycloalkyl for no more than one occurrence. In some cases, R a is methyl.
- the lipid can have one of the following formulas:
- R a independently, is absent, H, alkyl, or cycloalkyl.
- R a is alkyl or cycloalkyl for no more than two occurrences, or R a can be alkyl or cycloalkyl for no more than one occurrence. In some cases, R a is methyl.
- the lipid can have the formula:
- Y is N, O or S; and when Y is O or S, then Q 4 and Q 5 are absent.
- Q 4 and (3 ⁇ 4 can independently be H, alkyl (e.g., a primary, secondary or tertiary alkyl, such as, for example, Me, Et, isopropyl, or ieri-butyl), alkenyl, alkynyl, aryl, aralkyl, cycloalkyl, or heterocyclyl.
- Q 4 or Q 5 includes a double or triple bond, the double or triple bond can be anywhere in the chain. When there are two or more double (or triple) bonds or combination of both then the double (or triple) bonds can be separated by at least 1 saturated carbon atom. In some occurrences, two or more multiple bonds can be conjugated. In some occurrences, two double bonds are tied to the same carbon atom. Double bonds can be cis, trans, or combination of cis and trans.
- Li, L 2 and L 3 are each independently alkyl (e.g., primary, secondary or tertiary alkyl, such as, for example, Me, Et, isopropyl, or tert- My ⁇ ), aryl, aralkyl, alkenyl or alkynyl.
- alkyl e.g., primary, secondary or tertiary alkyl, such as, for example, Me, Et, isopropyl, or tert- My ⁇
- aryl e.g., aryl, aralkyl, alkenyl or alkynyl.
- Ri is a C 6 to C 6 o group selected from alkyl, alkenyl, alkynyl, heteroalkyl, aralkyl, cycloalkyl, and heterocyclyl.
- the double or triple bond can be anywhere in the chain.
- the double (or triple) bonds can be separated by at least 1 saturated carbon atom.
- two or more multiple bonds can be conjugated.
- two double bonds are tied to the same carbon atom. Double bonds can be cis, trans, or combination of cis and trans.
- Ri and R 2 are independently branched alkyl. In one example branched alkyl include
- R', R", and R'" are independently H, or a Ci to C30 group selected from alkyl, alkenyl, alkynyl, heteroalkyl, aralkyl, cycloalkyl and heterocyclyl.
- R', R", or R'" includes a double or triple bond
- the double or triple bond can be anywhere in the chain.
- the double (or triple) bonds can be separated by at least 1 saturated carbon atom.
- two or more multiple bonds can be conjugated.
- two double bonds are tied to the same carbon atom. Double bonds can be cis, trans, or combination of cis and trans.
- Each of Z Z 2 , Z 3 , and Z 4 is independently H, F, XR N(Q 6 )(Q 7 ) or -[Y(Q 4 ,Q 5
- Ci a Ci to C 30 group selected from alkyl, substituted alkyl, heteroalkyl, aralkyl, cycloalkyl and heterocyclyl.
- p is 0 to 19.
- q is 0 to 20.
- r is 0 to 100.
- the lipid including a quaternary amine can be in the form of a salt, i.e. complexed with a counterion.
- the counterion can be any anion, such as an organic or inorganic anion. Suitable examples of such anions include tosylate, methanesulfonate, acetate, citrate, malonate, tartarate, succinate, benzoate, ascorbate, a-ketoglutarate, and a- glycerophosphate,
- Inorganic can include chloride, sulfate, nitrate, bicarbonate, and carbonate salts.
- a lipid including a quaternary amine can be prepared from a corresponding lipid that includes a tertiary amine.
- the tertiary amine is converted to a quaternary amine by, e.g., alkylation with an appropriate alkyl halide.
- a lipid including a dimethylamino group i.e., a tertiary amine
- a lipid including a dimethylamino group can be converted to the corresponding trimethylamino group by reaction with methyl chloride.
- lipids described in these applications are suitable for converting to the corresponding quaternary amine. See, for example, Table 1 of application no. PCT/US09/63933, filed November 10, 2009, at pages 33-50.
- the lipids are charged lipids.
- charged lipid is meant to include those lipids having one or two fatty acyl or fatty alkyl chains and a quaternary amino head group.
- the quaternary amine carries a permanent positive charge.
- the head group can optionally include a ionizable group, such as a primary, secondary, or tertiary amine that may be protonated at physiological pH.
- a charged lipid is referred to as an "amino lipid.”
- lipids would include those having alternative fatty acid groups and other quaternary groups, including those in which the alkyl substituents are different (e.g., N-ethyl-N-methylamino-, N-propyl-N-ethylamino- and the like),
- the alkyl substituents are different (e.g., N-ethyl-N-methylamino-, N-propyl-N-ethylamino- and the like)
- Ri and R 2 are both long chain alkyl or acyl groups
- they can be the same or different.
- lipids e.g., a charged lipid having less saturated acyl chains are more easily sized, particularly when the complexes are sized below about 0.3 microns, for purposes of filter sterilization.
- Charged lipids containing unsaturated fatty acids with carbon chain lengths in the range of C 10 to C 2 o are typical.
- Other scaffolds can also be used to separate the amino group (e.g., the amino group of the charged lipid) and the fatty acid or fatty alkyl portion of the charged lipid. Suitable scaffolds are known to those of skill in the art.
- charged lipids of the present invention have at least one protonatable or deprotonatable group, such that the lipid is positively charged at a pH at or below physiological pH (e.g. pH 7,4), and neutral at a second pH, preferably at or above physiological pH.
- lipids are also referred to as charged lipids.
- the addition or removal of protons as a function of pH is an equilibrium process, and that the reference to a charged or a neutral lipid refers to the nature of the predominant species and does not require that all of the lipid be present in the charged or neutral form.
- Lipids that have more than one protonatable or deprotonatable group, or which are zwiterrionic, are not excluded from use in the invention.
- protonatable lipids i.e., charged lipids
- lipids will have a pKa of about 4 to about 7, e.g., between about 5 and 7, such as between about 5.5 and 6.8, when incorporated into lipid particles.
- Such lipids will be cationic at a lower pH formulation stage, while particles will be largely (though not completely) surface neutralized at physiological pH around pH 7.4.
- pKa measurements of lipids within lipid particles can be performed, for example, by using the fluorescent probe 2-(p-toluidino)-6-napthalene sulfonic acid (TNS), using methods described in Cullis et al., (1986) Chem Phys Lipids 40, 127-144.
- TMS 2-(p-toluidino)-6-napthalene sulfonic acid
- compositions described herein can include mixtures of charged lipids.
- the compositions e.g., lipoplexes and/or lipid nanoparticles
- Suitable lipids for the compositions include those described in WO 2010/054406, WO 2010/054405, WO 2010/054401, WO 2010/054384, U.S. Application No. 61/309,697, filed March 2, 2010; U.S. Application No. 61/321,829, filed April 7, 2010; U.S. Application No.
- the formulations can include a cryoprotectant.
- a formulation can be suspended in a buffer containing a cryprotectant at a volume measured to obtain a final desired lipid concentration.
- the suspension can be agitated to thoroughly mix the cryoprotectant with the lipid nanoparticles.
- the suspension can be extruded or filtered to select nanoparticles of a given size. This can result in a final formulation, which can be stored under appropriate conditions until use.
- the lipid nanoparticles can be used for delivery of nucleic acids to cells without increased cell death or decreased delivery efficiency.
- a cryoprotectant can be a compound used to protect the formulation from damage due to cold, for example, freezing.
- a cryoprotectant can include a polyol, e.g., a carbohydrate, for example, sucrose, trehalose, glucose or a 2-hydroxypropyl-a- cyclodextrin.
- a sugar alcohol, such as sorbitol can also be included in a cryoprotectant.
- a cryprotectant can include a protein, a peptide or an amino acid.
- a cryoprotectant can include proline or hydroxyl proline.
- An organic compound, such as glycerol, ethylene glycol, or propylene glycol, can be included in a cryoprotectant.
- a cryoprotectant can include a polymer, for example,
- polyvinylpyrrolidone polyethylene glycol or gelatin or hydroxyethylcellulose.
- a formulation can be mixed and solvent can be removed, which can result in a residue.
- the residue can be resuspended in a buffer including a cryoprotectant, or the residue can be resuspended in a buffer and then a cryoprotectant can be added.
- the result can be a suspension of formulation in buffer.
- the formulation can include lipid nanoparticles.
- the lipid nanoparticles can also include a nucleic acid.
- the buffer can be a buffer solution or a buffered media.
- the pH of the buffer can be greater than 5.0, greater than 6.0, greater than 6.5, greater than 7.0, greater than 7.1, greater than 7.2, greater than 7.3, greater than 7.4, greater than 7.5, greater than 7.6, greater than 7.7, greater than 7.8, greater than 7.9, greater than 8.0 or greater than 9.0.
- the pH of the buffer can be less than 9.0, less than 8.0, less than 7.9, less than 7.8, less than 7.7, less than 7.6, less than 7.5, less than 7.4, less than 7.3, less than 7.2, less than 7.1, less than 7.0, less than 6.5, less than 6.0 or less than 5.0.
- the buffer can be pH 7.4.
- the suspension can include less than 20%, less than 15%, less than 12%, less than 10%, less than 9%, less than 8%, less than 7%, less than 6%, less than 5% or less than 3% cryoprotectant by volume.
- the suspension can include more than 3%, more than 5%, more than 6%, more than 7%, more than 8%, more than 9%, more than 10% more than 12%, more than 15%, or more than 20% cryoprotectant by volume.
- the suspension can include 5% or 10% cryoprotectant by volume.
- the lipid concentration within the suspension can be greater than 0.25 mg/mL, greater than 0.5 mg/mL, greater than 1.0 mg/niL or greater than 1.5 mg/mL.
- the lipid concentration within the suspension can be less than 2.0 mg/mL, less than 1.5 mg/niL, less than 1.0 mg/mL, less than 0.5 mg/mL, or less than 0.25 mg/mL. More specifically, the concentration can be 1.0 mg/mL.
- the suspension can be mixed or agitated to distribute the cryoprotectant throughout the suspension.
- the mixing or agitation can occur at 4 °C, 25 °C or 37 °C.
- the mixing or agitation can occur for greater than 5 minutes, greater than 10 minutes, greater than 15 minutes, greater than 30 minutes or greater than an hour.
- Mixing or agitation can occur by shaking, pipetting or stirring.
- the lipid nanoparticles contained in the suspension can be selected for size.
- the nanoparticles can be filtered or extruded.
- the resuspension can be extruded through a filter, for example a polycarbonate filter.
- the resuspension can also be syringe filtered.
- the filter can allow particles less than 0.5 ⁇ , less than 0.45 ⁇ , less than 0.4 ⁇ , less than 0.35 ⁇ , less than 0.3 ⁇ , less than 0.25 ⁇ , less than 0.2 ⁇ or less than 0.15 ⁇ to pass through.
- a filter can have a pore size of 0.45 ⁇ , 0.4 ⁇ , 0.22 ⁇ or 0.2 ⁇ .
- a final suspension of the formulation can contain only lipid nanoparticles smaller than the pore size of the filter.
- Lipid nanoparticle sizes can be less than 300 nm, less than 275 nm, less than 250 nm, less than 225 nm, less than 200 nm, less than 175 nm, less than 150 nm, less than 125 nm or less than 100 nm.
- the final suspension of the formulation can be stored at cold temperatures, for example, less than or equal to 25 °C, less than or equal to 4 °C, less than or equal to 0 °C, than or equal to -20 °C or less than or equal to -80 °C, until the formulation is used.
- the cold formulation can be prepared for use by warming the formulation (e.g., at room temperature) until the formulation is at room temperature or adequately thawed.
- the final suspension of the formulation can also be stored at low moisture.
- the formulation can be dried, lyophilized or freeze-dried. Freeze-drying can be accomplished by storing the formulation at -80 °C and then lyophilizing the formulation.
- the low moisture formulation can be prepared for use by rehydrating the formulation, for instance, by resuspending the formulation in a liquid,
- the liquid can be water, a buffer solution or cell culture media.
- the formulation can be stored at cold temperatures or low moisture for greater than lhour, greater than 2 hours, greater than 6 hours, greater than 12 hours, greater than 24 hours, greater than 2 days, greater than 3 days, greater than 4, greater than 5 days, greater than 6 days or greater than 1 week and still remain an effective transfection reagent.
- the formulation can be used for transfections after being stored at cold temperature or at low moisture.
- a lyophilized formulation can be reconstituted by warming following by resuspension; or simply by resuspension in a cold or warm liquid (e.g., water, buffer or media).
- a formulation stored as a cold or frozen solution can be reconstituted by warming to a desired temperature, e.g., 4 °C, room temperature, or 37 °C.
- Reconstitution can also include altering the
- Formulations may be stored with or without nucleic acids included; when stored without a nucleic acid present, reconsistitution can include adding a nucleic acid to the formulation. Altering the formulation can also include adding additional or different lipids to the formulation. The order of various steps of reconstitution may be varied.
- the formulations of the invention further comprise an apolipoprotein.
- apolipoprotein or “lipoprotein” refers to apolipoproteins known to those of skill in the art and variants and fragments thereof and to apolipoprotein agonists, analogues or fragments thereof described below.
- Suitable apolipoproteins include, but are not limited to, ApoA-I, ApoA-II, ApoA- IV, ApoA-V and ApoE, and active polymorphic forms, isoforms, variants and mutants as well as fragments or truncated forms thereof.
- the apolipoprotein is a thiol containing apolipoprotein.
- Thiol containing apolipoprotein refers to an apolipoprotein, variant, fragment or isoform that contains at least one cysteine residue.
- thiol containing apolipoproteins are ApoA-I Milano (ApoA-iM) and ApoA-I Paris (ApoA-I P ) which contain one cysteine residue (Jia et al., 2002, Biochem. Biophys. Res. Comm. 297: 206-13; Bielicki and Oda, 2002, Biochemistry 41 : 2089-96).
- ApoA-II, ApoE2 and ApoE3 are also thiol containing apolipoproteins. Isolated ApoE and/or active fragments and polypeptide analogues thereof, including recombinantly produced forms thereof, are described in U.S. Pat. Nos.
- the apolipoprotein can be in its mature form, in its preproapolipoprotein form or in its proapolipoprotein form.
- Homo- and heterodimers (where feasible) of pro- and mature ApoA-I Duverger et al., 1996, Arterioscler. Thromb. Vase. Biol. 16(12): 1424-29
- ApoA-I Milano Klon et al obsession 2000, Biophys. J. 79:(3)1679- 87; Franceschini et al., 1985, J. Biol. Chem. 260: 1632-35
- ApoA-I Paris Daum et al., 1999, J. Mol. Med. 77:614-22
- ApoA-II Shelness et al., 1985, J. Biol. Chem.
- the apolipoprotein can be a fragment, variant or isoform of the apolipoprotein.
- fragment refers to any apolipoprotein having an amino acid sequence shorter than that of a native apolipoprotein and which fragment retains the activity of native apolipoprotein, including lipid binding properties.
- variant is meant substitutions or alterations in the amino acid sequences of the apolipoprotein, which substitutions or alterations, e.g., additions and deletions of amino acid residues, do not abolish the activity of native apolipoprotein, including lipid binding properties.
- a variant can comprise a protein or peptide having a substantially identical amino acid sequence to a native apolipoprotein provided herein in which one or more amino acid residues have been conservatively substituted with chemically similar amino acids.
- conservative substitutions include the substitution of at least one
- hydrophobic residue such as isoleucine, valine, leucine or methionine for another.
- the present invention contemplates, for example, the substitution of at least one hydrophilic residue such as, for example, between arginine and lysine, between glutamine and asparagine, and between glycine and serine (see U.S. Pat. Nos. 6,004,925, 6,037,323 and 6,046,166).
- the term "isoform” refers to a protein having the same, greater or partial function and similar, identical or partial sequence, and may or may not be the product of the same gene and usually tissue specific (see Weisgraber 1990, J. Lipid Res. 31(8):1503- 11; Hixson and Powers 1991, J. Lipid Res. 32(9): 1529-35; Lackner et al., 1985, J. Biol.
- the methods and compositions of the present invention include the use of a chimeric construction of an apolipoprotein.
- a chimeric construction of an apolipoprotein can be comprised of an apolipoprotein domain with high lipid binding capacity associated with an apolipoprotein domain containing ischemia reperfusion protective properties.
- a chimeric construction of an apolipoprotein can be a construction that includes separate regions within an apolipoprotein (i.e., homologous construction) or a chimeric construction can be a construction that includes separate regions between different apolipoproteins (i.e., heterologous constructions).
- compositions comprising a chimeric construction can also include segments that are apolipoprotein variants or segments designed to have a specific character (e.g., lipid binding, receptor binding, enzymatic, enzyme activating, antioxidant or reduction- oxidation property) (see Weisgraber 1990, J. Lipid Res. 31(8):1503-11 ; Hixson and Powers 1991, J. Lipid Res. 32(9):1529-35; Lackner et al tension 1985, J. Biol. Chem.
- a specific character e.g., lipid binding, receptor binding, enzymatic, enzyme activating, antioxidant or reduction- oxidation property
- Apolipoproteins utilized in the invention also include recombinant, synthetic, semi-synthetic or purified apolipoproteins. Methods for obtaining apolipoproteins or equivalents thereof, utilized by the invention are well-known in the art.
- apolipoproteins can be separated from plasma or natural products by, for example, density gradient centrifugation or immunoaffinity chromatography, or produced synthetically, semi-synthetically or using recombinant DNA techniques known to those of the art (see, e.g., Mulugeta et al., 1998, J. Chromatogr. 798(1-2): 83-90; Chung et al., 1980, J. Lipid Res.
- Apolipoproteins utilized in the invention further include apolipoprotein agonists such as peptides and peptide analogues that mimic the activity of ApoA-I, ApoA-I Milano (ApoA-I M ), ApoA-I Paris (ApoA-I P ), ApoA-II, ApoA-IV, and ApoE.
- apolipoprotein can be any of those described in U.S. Pat. Nos. 6,004,925, 6,037,323, 6,046,166, and 5,840,688, the contents of which are incorporated herein by reference in their entireties.
- Apolipoprotein agonist peptides or peptide analogues can be synthesized or manufactured using any technique for peptide synthesis known in the art including, e.g., the techniques described in U.S. Pat. Nos. 6,004,925, 6,037,323 and 6,046,166.
- the peptides may be prepared using the solid-phase synthetic technique initially described by Merrifield (1963, J. Am. Chem. Soc. 85:2149-2154).
- Other peptide synthesis techniques may be found in Bodanszky et al., Peptide Synthesis, John Wiley & Sons, 2d Ed., (1976) and other references readily available to those skilled in the art.
- Peptides may also be synthesized by solution methods as described in The Proteins, Vol. II, 3d Ed., Neurath et. al., Eds., p. 105-237, Academic Press, New York, N.Y. (1976). Appropriate protective groups for use in different peptide syntheses are described in the above- mentioned texts as well as in McOmie, Protective Groups in Organic Chemistry, Plenum Press, New York, N.Y. (1973).
- the peptides of the present invention might also be prepared by chemical or enzymatic cleavage from larger portions of, for example, apolipoprotein A-I.
- the apolipoprotein can be a mixture of apolipoproteins. In one embodiment, the apolipoprotein can be a homogeneous mixture, that is, a single type of apolipoprotein. In another embodiment, the apolipoprotein can be a heterogeneous mixture of apolipoproteins, that is, a mixture of two or more different apolipoproteins. Embodiments of heterogenous mixtures of apolipoproteins can comprise, for example, a mixture of an apolipoprotein from an animal source and an apolipoprotein from a semisynthetic source. In certain embodiments, a heterogenous mixture can comprise, for example, a mixture of ApoA-I and ApoA-I Milano. In certain embodiments, a
- heterogeneous mixture can comprise, for example, a mixture of ApoA-I Milano and
- the apolipoprotein is obtained from natural sources, it can be obtained from a plant or animal source. If the apolipoprotein is obtained from an animal source, the apolipoprotein can be from any species. In certain embodiments, the apolipoprotien can be obtained from an animal source. In certain embodiments, the apolipoprotein can be obtained from a human source. In preferred embodiments of the invention, the
- apolipoprotein is derived from the same species as the individual to which the
- apolipoprotein is administered.
- a method for delivering a nucleic acid to a cell can include exposing sample cells to a composition containing a charged lipid.
- the charged lipid can include the charged lipids described herein.
- a sample cell can include a eukaryotic cell.
- the eukaryotic cell can be a stem cell, primary cell or a cell in a cell line.
- the cell line can be a primary cell line, a secondary cell line or an immortalized cell line.
- Exemplary cell lines can include Chinese hamster ovary (CHO) cells, HeLa cells, U20S cells, Caco-2 cells, HT29 cells, NIH3T3 cells, PC12 cells, HepG2 cells, U937 cells, Vero cells, BH cells, ME-180 cells, A549 cells, HEK-293 cells, MCF-7 cells, Jurkat cells, Mdck cells, 3T3 cells, COS-7 cells or GH3 cells.
- CHO Chinese hamster ovary
- the cell line can include GFP-CHO cells or DG44-CHO cells.
- the cells can be non-adherent suspension cells, including, but not limited to, suspension CHO cells, suspension BHK cells, suspension NSO cells, suspension HeLa cells and suspension HEK293 cells.
- Exposing sample cells to a composition can include contacting the cells with the composition, adding the composition to the media the cells are cultured in or incubating the cells in a solution containing the composition.
- a method for delivering a nucleic acid to sample cells can include forming the composition and exposing the sample cells to the composition.
- the composition can be purchased, provided or formed.
- Charged lipids can be prepared for use in transfection by forming into liposomes and mixing with the macromolecules to be introduced into the cell.
- Macromolecules that can be delivered to cells with the transfection reagents can be macromolecules having at least one negative charge in the molecule.
- Such macromolecules can include, but are not limited to, proteins, polypeptides and nucleic acids, such as RNA and DNA.
- Methods of forming liposomes can include, but are not limited to, sonication, extrusion, extended vortexing, reverse evaporation, and homogenization, which can include microfulidization. Additional methods of forming liposomes are well known in the art.
- Sonication can produce small, unilamellar vesicles (SUV) with diameters in the range of 15-50 nm.
- Bath sonicators can be instrumentation used for preparation of SUV (Avanti Polar Lipids, Inc., 700 Industrial Park Drive, Alabaster, Ala. 35007). Sonication can be accomplished by placing a test tube containing the suspension in a bath sonicator (or placing the tip of a sonicator in the test tube) and sonicating for 5-10 minutes above the gel-liquid crystal transition temperature of the lipid.
- Mean size and uniformity can be influenced by lipid composition and concentration, temperature, sonication time, power, volume, and sonicator tuning.
- Reverse evaporation can be used to form larger liposome vesicles (>1000 nm) known as giant unilamellar vesicles (GUV's).
- Lipid extrusion can be a technique in which a lipid suspension is forced through a
- polycarbonate filter with a defined pore size to yield particles having a diameter near the pore size of the filter used.
- Extrusion through filters with pores having an approximately 100 nm diameter typically can yield large, unilamellar vesicles (LUV) with a mean diameter of 120 nm-140 nm.
- Mean particle size can also depend on lipid composition and can be reproducible from batch to batch.
- the formed liposomes can be approximately 120 nm to 800 nm in diameter.
- the composition can further include a nucleic acid.
- the nucleic acid can be deoxyribonucleic acid (DNA) or ribonucleic acid (RNA).
- the nucleic acid can include a chemically modified nucleic acid. Chemical modifications can include methylation, acetylation, oxidation, intercalation, thymine dimerization, PEGylation or phosphorylation. The chemical modifications can include using, for example, a phosphorothioate, methyl phosphonate or phosphoramidate linkage at the internucleotide phosphodiester bridge. Additionally, the chemical modifications can include a modification of the nucleotide base, for example, 5-propynyl-pyrimidine, or of the sugar, for example, 2'modified sugars.
- the nucleic acid can be 10 to 50 nucleotides long.
- the nucleic acid can be an oligonucleotide.
- the oligonucleotide can be 10 to 50 nucleotides long.
- the oligonucleotide can be double stranded or single stranded. More particularly, in some embodiments, the nucleic acid can be siRNA or mRNA. The siRNA can be single stranded or double stranded. In other embodiments, the nucleic acid can be a shRNA, an antisense nucleic acid, a microRNA, an antimicro RNA, an antagomir, a microRNA inhibitor or an immune stimulatory nucleic acid.
- the sample cells can be in suspension.
- the volume of the sample cells in suspension can be at least 0.050 L, at least 0.1 L, at least 0.5 L, at least 1L, at least 3 L, at least 5 L, at least 10 L, at least 25 L, at least 40 L or more than 40 L.
- the suspension can be cultured in a bioreactor, a flask, a tube or a tank.
- the suspension can be cultured with or without serum.
- a method for delivering a nucleic acid to sample cells can further include culturing untreated control cells that have not been exposed to the composition.
- a culture of cells is divided into at least two groups of cells including the sample cells and the untreated control cells.
- the sample cells are exposed to the composition.
- the untreated control cells are not exposed to the composition and provide a negative control to compare sample cell results against.
- the untreated control cells can indicate results that are independent of treatment with the composition.
- a cell density of the sample cells can increase after the sample cells have been exposed to the composition.
- the cell density can be greater than O. lxlO 6 cells/mL, greater than 0.5xl0 "6 cells/mL, greater than l.OxlO "6 cells/mL, greater than 1 ,5xl0 "6 cells/mL, greater than 2.0xl0 "6 cells/mL, greater than 2.5xl0 "6 cells/mL, greater than 3.0xl0 "6 cells/mL or greater than 3.5xl0 "6 cells/mL.
- the cell density can increase by greater than O.
- the cell density of the sample cells can increase exponentially for a period of time after the sample cells have been exposed to the composition.
- the cell density of the sample cells can be greater than or equal to the cell density of the untreated control cells.
- the cell density measurement can be taken one day, two days, three days, four days, five days, six days, 1 week or greater than 1 week after the sample cells have been exposed to the composition.
- the sample cell viability can be greater than 75%, greater than 80%, greater than 85%, greater than 90% or greater than 95%.
- the sample cell viability can be measured one day, two days, three days, four days, five days or greater than five days after the sample cells have been exposed to the composition.
- a method for delivering a nucleic acid to sample cells can further include measuring a level of a protein in the sample cells and untreated control cells, the protein can be produced from an mRNA that an siRNA delivered into the sample cells is directed against.
- the mRNA that an siRNA molecule is directed against can be determined by the sequence of the siRNA.
- the siRNA sequence can be complementary to the sequence of its target mRNA. Therefore, when the siRNA is incorporated into the RISC complex, the RISC complex can bind to the mRNA with the sequence complementary to the siRNA and the RISC complex can cleave the mRNA. This decreases the level of that mRNA in the cell, and consequently, it can decrease the level of protein translated from that mRNA.
- An siRNA can target RNA other than an mRNA.
- An siRNA can have a sequence that is directed against more than one mRNA, thereby affecting the levels of more than one mRNA and more than one protein.
- Measuring a level of a protein can include measuring the quantity of the protein, measuring an activity of the protein or measuring a downstream effect of the protein.
- the downstream effect can include activation of another molecule, modification of another molecule or the presence or absence of another molecule.
- Measuring a level of the protein can be accomplished using techniques well known in the art.
- Techniques for measuring the quantity of a protein can include an ultraviolet absorption assay, for example 260nm and 280nm absorbance reading, a Bradford assays, a Lowry, a Biuret assay, a bicinchoninic assay, or a quantitative Western blot.
- Techniques for measuring the activity of a protein can include a SDS-Page, a Western blot, a BIAcore assay or an enzyme-linked immunosorbent assay (ELISA).
- the protein level in the sample cells can be less than the protein level in the untreated control cells.
- the protein level in the sample cells can be less than 40%, less than 50%, less than 60%, less than 70%, less than 75% or less than 80% of the protein level in the untreated control cells.
- the protein level in the sample cells and the protein level in the untreated control cells can be measured one day, two days, three days, four days, five days, six days, 1 week or greater than 1 week after the sample cells have been exposed to the composition.
- the protein level in the sample cells and the protein level in the untreated control cells can be measured one doubling time, two doubling times, three doubling times, four doubling times, five doubling times or greater than five doubling times after the sample cells have been exposed to the composition.
- a doubling time can be the period of time required for the quantity of cells to double. For example, if it takes one cell 24 hours to grow and divide to two cells, the doubling time is 24 hours.
- the cell density of the sample cells can increase after the sample cells have been exposed to the composition.
- the cell density can be greater than O. lxlO 6 cells/mL, greater than 0.5xl0 "6 cells/mL, greater than l.OxlO "6 cells/mL, greater than 1.5xl0 ⁇ 6 cells/mL, greater than 2.0xl0 ⁇ 6 cells/mL, greater than 2.5xl0 ⁇ 6 cells/mL, greater than 3.0xl0 ⁇ 6 cells/mL or greater than 3.5xl0 "6 cells/mL.
- the cell density can increase by greater than O.
- the cell density of the sample cells can increase exponentially for a period of time after the sample cells have been exposed to the composition.
- the cell density of the sample cells can increase exponentially for a period of time after the sample cells have been exposed to the composition.
- the cell density of the sample cells can be greater than or equal to the cell density of the control cells.
- the cell density measurement can be taken one day, two days, three days, four days, five days, six days, 1 week or greater than 1 week after the sample cells have been exposed to the composition.
- the sample cell viability can be greater than 75%, greater than 80%, greater than 85%, greater than 90% or greater than 95%.
- the sample cell viability can be measured one day, two days, three days, four days, five days or greater than five days after the sample cells have been exposed to the composition.
- a method for delivering a nucleic acid to sample cells can further include measuring a level of a protein in the sample cells and the untreated control cells, where the nucleic acid can be an siRNA and the protein can be produced from an mRNA that the siRNA is directed against.
- the mRNA that an siRNA molecule is directed against can be determined by the sequence of the siRNA.
- the siRNA sequence can be complementary to the sequence of its target mRNA. Therefore, when the siRNA is incorporated into the RISC complex, the RISC complex can bind to the mRNA with the sequence complementary to the siRNA and the RISC complex can cleave the mRNA. This decreases the level of that mRNA in the cell, and consequently, it can decrease the level of protein translated from that mRNA.
- An siRNA can target RNA other than an mRNA.
- An siRNA can have a sequence that is directed against more than one mRNA, thereby affecting the levels of more than one mRNA and more than one protein.
- Measuring a level of a protein can include measuring the quantity of the protein, measuring an activity of the protein or measuring a downstream effect of the protein.
- the downstream effect can include activation of another molecule, modification of another molecule or the presence or absence of another molecule.
- Measuring a level of the protein can be accomplished using techniques well known in the art.
- Techniques for measuring the quantity of a protein can include an ultraviolet absorption assay, for example 260nm and 280nm absorbance reading, a Bradford assays, a Lowry, a Biuret assay, a bicinchoninic assay, or a quantitative Western blot.
- Techniques for measuring the activity of a protein can include a SDS-Page, a Western blot, a BIAcore assay or an enzyme-linked immunosorbent assay (ELISA).
- the protein level in the sample cells can be less than the protein level in the control cells.
- the protein level in the sample cells can be less than 40%, less than 50%, less than 60%, less than 70%, less than 75% or less than 80% of the protein level in the control cells.
- the protein level in the sample cells and the protein level in the control cells can be measured one day, two days, three days, four days, five days, six days, 1 week or greater than 1 week after the sample cells have been exposed to the composition.
- the protein level in the sample cells and the protein level in the control cells can be measured one doubling time, two doubling times, three doubling times, four doubling times, five doubling times or greater than five doubling times after the sample cells have been exposed to the composition.
- a doubling time can be the period of time required for the quantity of cells to double. For example, if it takes one cell 24 hours to grow and divide to two cells, the doubling time is 24 hours.
- the transfection methods can be applied to in vitro and in vivo transfection of cells, particularly to transfection of eukaryotic cells including animal cells.
- the methods can be used to generate transfected cells which express useful gene products.
- the methods can also be employed as a step in the production of transgenic animals.
- the methods are useful as a step in any therapeutic method requiring introducing of nucleic acids into cells. In particular, these methods are useful in cancer treatment, in in vivo and ex vivo gene therapy, and in diagnostic methods.
- the transfection compositions can be employed as research reagents in any transfection of cells done for research purposes.
- Nucleic acids that can be transfected by the methods of include DNA and RNA from any source comprising natural bases or non-natural bases, and include those encoding and capable of expressing therapeutic or otherwise useful proteins in cells, those which inhibit undesired expression of nucleic acids in cells, those which inhibit undesired enzymatic activity or activate desired enzymes, those which catalyze reactions (Ribozymes), and those which function in diagnostic assays.
- the reagents and methods provided herein can are also readily adapted to introduce biologically active anionic macromolecules other than nucleic acids including, among others, polyamines, polyamine acids, polypeptides, proteins, biotin, and polysaccharides into cells.
- biologically active anionic macromolecules other than nucleic acids including, among others, polyamines, polyamine acids, polypeptides, proteins, biotin, and polysaccharides into cells.
- Other materials useful, for example as therapeutic agents, diagnostic materials and research reagents can be complexed by the polycharged lipid aggregates and delivered into cells by the methods of this invention.
- one or more cells are contacted with a test compound after the macromolecule, particularly an expression vector, is introduced into the one or more cells.
- the one or more cells are contacted with the test compound for a selected time, for example within 5 days, after the macromolecule is introduced into the one or more cells.
- the present invention also provides lipid particles comprising one or more of the charged lipids described above.
- a complex of nucleic acid and lipid particles can be referred to as an association complex.
- An association complex of nucleic acid and lipid particle may be a liposome, a nanoparticle, an ion pair, a lipoplex, or a combination thereof.
- Lipoplexes are composed of charged lipid bilayers sandwiched between DNA layers, as described, e.g. , in Feigner, Scientific American.
- Lipid particles include, but are not limited to, liposomes.
- a liposome is a structure having lipid- containing membranes enclosing an aqueous interior. Liposomes may have one or more lipid membranes.
- the invention contemplates both single-layered liposomes, which are referred to as unilamellar, and multi-layered liposomes, which are referred to as multilamellar.
- the lipid particles of the present invention may further comprise one or more additional lipids and/or other components such as cholesterol.
- Other lipids may be included in the liposome compositions of the present invention for a variety of purposes, such as to prevent lipid oxidation or to attach ligands onto the liposome surface. Any of a number of lipids may be present in liposomes of the present invention, including amphipathic, neutral, cationic, and anionic lipids, Such lipids can be used alone or in combination. Specific examples of additional lipid components that may be present are described below.
- Additional components that may be present in a lipid particle of the present invention include bilayer stabilizing components such as polyamide oligomers (see, e.g. , U.S. Patent No. 6,320,017), peptides, proteins, detergents, lipid-derivatives, such as PEG coupled to phosphatidylethanolamine and PEG conjugated to ceramides (see, U.S. Patent No. 5,885,613).
- bilayer stabilizing components such as polyamide oligomers (see, e.g. , U.S. Patent No. 6,320,017), peptides, proteins, detergents, lipid-derivatives, such as PEG coupled to phosphatidylethanolamine and PEG conjugated to ceramides (see, U.S. Patent No. 5,885,613).
- the lipid particles include one or more of a second amino lipid or charged lipid, a neutral lipid, and a sterol.
- Neutral lipids when present in the lipid particle, can be any of a number of lipid species which exist either in an uncharged or neutral zwitterionic form at physiological pH.
- lipids include, for example phosphocholines (PC), phosphatidylethanolamines (PE), phosphatidylserines (PS), cardiolipins, diacylphosphatidylcholine,
- the neutral lipid component is a lipid having two acyl groups, (i.e., diacylphosphatidylcholine and
- lipids having a variety of acyl chain groups of varying chain length and degree of saturation are available or may be isolated or synthesized by well-known techniques. In one group of embodiments, lipids containing saturated fatty acids with carbon chain lengths in the range of C 10 to C20 are preferred. In another group of embodiments, lipids with mono or diunsaturated fatty acids with carbon chain lengths in the range of C 10 to C20 are used. Additionally, lipids having mixtures of saturated and unsaturated fatty acid chains can be used.
- the neutral lipids used in the present invention are DOPE, DSPC, POPC, DPPC or any related phosphatidylcholine.
- the neutral lipids useful in the present invention may also be composed of
- sphingomyelin dihydrosphingomyeline
- phospholipids with other head groups such as serine and inositol.
- the sterol component of the lipid mixture when present, can be any of those sterols conventionally used in the field of liposome, lipid vesicle or lipid particle preparation.
- a preferred sterol is cholesterol,
- protonatable lipids which carry a net positive charge at about physiological pH, in addition to those specifically described above, may also be included in lipid particles of the present invention.
- Such protonatable lipids include, but are not limited to, N,N-dioleyl-N,N-dimethylammonium chloride ("DODAC”); N-(2,3- dioleyloxy)propyl-N,N-N-triethylammonium chloride (“DOTMA”); N,N-distearyl-N,N- dimethylammonium bromide (“DDAB”); N-(2,3-dioleoyloxy)propyl)-N,N,N- trimethylammonium chloride (“DOTAP”); l,2-Dioleyloxy-3-trimethylaminopropane chloride salt (“DOTAP.C1"); 3P-(N-(N',N'-dimethylaminoethane)-carbamoyl)cholesterol (“DC
- DOGS carboxyspermine
- DOPE l,2-dileoyl-sn-3-phosphoethanolamine
- DOPE 1,2- dioleoyl-3-dimethylammonium propane
- DODAP 1,2- dioleoyl-3-dimethylammonium propane
- DODMA N, N-dimethyl-2,3- dioleyloxy)propylamine
- DMRIE N-(l,2-dimyristyloxyprop-3-yl)-N,N- dimethyl-N-hydroxyethyl ammonium bromide
- LIPOFECTIN including DOTMA and DOPE, available from GIBCO/BRL
- LIPOFECTAMINE comprising DOSPA and DOPE, available from GIBCO/BRL.
- Anionic lipids suitable for use in lipid particles include, but are not limited to, phosphatidylglycerol, cardiolipin, diacylphosphatidylserine, diacylphosphatidic acid, N- dodecanoyl phosphatidylethanoloamine, N-succinyl phosphatidylethanolamine, N- glutaryl phosphatidylethanolamine, lysylphosphatidylglycerol, and other anionic modifying groups joined to neutral lipids,
- amphipathic lipids are included in lipid particles of the present invention.
- “Amphipathic lipids” refer to any suitable material, wherein the hydrophobic portion of the lipid material orients into a hydrophobic phase, while the hydrophilic portion orients toward the aqueous phase.
- Such compounds include, but are not limited to, phospholipids, aminolipids, and sphingolipids.
- Representative phospholipids include sphingomyelin, phosphatidylcholine, phosphatidylethanolamine, phosphatidylserine, phosphatidylinositol, phosphatidic acid, palmitoyloleoyl
- phosphatdylcholine lysophosphatidylcholine, lysophosphatidylethanolamine, dipalmitoylphosphatidylcholine, dioleoylphosphatidylcholine,
- distearoylphosphatidylcholine or dilinoleoylphosphatidylcholine.
- Other phosphorus- lacking compounds such as sphingolipids, glycosphingolipid families, diacylglycerols, and ⁇ -acyloxyacids, can also be used. Additionally, such amphipathic lipids can be readily mixed with other lipids, such as triglycerides and sterols.
- the lipid particle can include a lipid selected to reduce aggregation of lipid particles during formation, which may result from steric stabilization of particles which prevents charge-induced aggregation during formation.
- PEG polyethylene glycol
- PAO polyamide oligomers
- ATTA-lipids are described, e.g. , in U.S. Patent No, 6,320,017
- PEG-lipid conjugates are described, e.g., in U.S. Patent Nos.
- the concentration of the lipid component selected to reduce aggregation is about 1 to 15% (by mole percent of lipids).
- Specific examples of PEG-modified lipids (or lipid-polyoxyethylene conjugates) that are useful in the present invention can have a variety of "anchoring" lipid portions to secure the PEG portion to the surface of the lipid vesicle.
- PEG-modified lipids examples include PEG-modified phosphatidylethanolamine and phosphatidic acid, PEG-ceramide conjugates (e.g., PEG-CerC14 or PEG-CerC20) which are described in copending USSN 08/486,214, incorporated herein by reference, PEG-modified
- dialkylamines and PEG-modified l,2-diacyloxypropan-3-amines Particularly preferred are PEG-modified diacylglycerols and dialkylglycerols.
- a sterically-large moiety such as PEG or ATTA are conjugated to a lipid anchor
- the selection of the lipid anchor depends on what type of association the conjugate is to have with the lipid particle. It is well known that mPEG (mw2000)-diastearoylphosphatidylethanolamine (PEG-DSPE) will remain associated with a liposome until the particle is cleared from the circulation, possibly a matter of days. Other conjugates, such as PEG-CerC20 have similar staying capacity. PEG- CerC14, however, rapidly exchanges out of the formulation upon exposure to serum, with a Ti/2 less than 60 mins. in some assays. As illustrated in U.S. Pat.
- Compounds having suitable variations of these features may be useful for the invention.
- aggregation preventing compounds do not necessarily require lipid conjugation to function properly. Free PEG or free ATTA in solution may be sufficient to prevent aggregation. If the particles are stable after formulation, the PEG or ATTA can be dialyzed away before administration to a subject.
- lipid particles of the present invention are programmable fusion lipids.
- Such lipid particles have little tendency to fuse with cell membranes and deliver their pay load until a given signal event occurs. This allows the lipid particle to distribute more evenly after injection into an organism or disease site before it starts fusing with cells.
- the signal event can be, for example, a change in H, temperature, ionic environment, or time. In the latter case, a fusion delaying or
- cloaking component such as an ATTA-lipid conjugate or a PEG-lipid conjugate
- ATTA-lipid conjugate or a PEG-lipid conjugate can simply exchange out of the lipid particle membrane over time. By the time the lipid particle is suitably distributed in the body, it has lost sufficient cloaking agent so as to be fusogenic. With other signal events, it is desirable to choose a signal that is associated with the disease site or target cell, such as increased temperature at a site of inflammation.
- lipid particles of this invention it is desirable to target the lipid particles of this invention using targeting moieties that are specific to a cell type or tissue.
- targeting moieties such as ligands, cell surface receptors, glycoproteins, vitamins (e.g., riboflavin) and monoclonal antibodies, has been previously described (see, e.g. , U.S. Patent Nos. 4,957,773 and 4,603,044).
- the targeting moieties can comprise the entire protein or fragments thereof.
- Targeting mechanisms generally require that the targeting agents be positioned on the surface of the lipid particle in such a manner that the target moiety is available for interaction with the target, for example, a cell surface receptor.
- lipid particles i.e. , liposomes
- hydrophilic polymer chains such as polyethylene glycol (PEG) chains
- a ligand such as an antibody, for targeting the lipid particle is linked to the polar head group of lipids forming the lipid particle.
- the targeting ligand is attached to the distal ends of the PEG chains forming the hydrophilic polymer coating (Klibanov, et al. , Journal of Liposome Research 2: 321-334 (1992); Kirpotin et al., FEBS Letters 388 115-118 (1996)).
- Standard methods for coupling the target agents can be used.
- phosphatidylethanolamine which can be activated for attachment of target agents
- derivatized lipophilic compounds such as lipid-derivatized bleomycin
- Antibody-targeted liposomes can be constructed using, for instance, liposomes that incorporate protein A (see, Renneisen, et al. , J. Bio. Chem., 265: 16337-16342 (1990) and Leonetti, et al, Proc. Natl. Acad. Sci. (USA), 87:2448-2451 (1990).
- Other examples of antibody conjugation are disclosed in U.S. Patent No.
- targeting moieties can also include other proteins, specific to cellular components, including antigens associated with neoplasms or tumors. Proteins used as targeting moieties can be attached to the liposomes via covalent bonds (see, Heath, Covalent Attachment of Proteins to
- Liposomes 149 Methods in Enzymology 111-119 (Academic Press, Inc. 1987)).
- Other targeting methods include the biotin-avidin system.
- the lipid particle comprises a mixture of a charged lipid of the present invention, one or more different neutral lipids, and a sterol (e.g., cholesterol).
- the lipid mixture consists of or consists essentially of a charged lipid as described herein, a neutral lipid, and cholesterol.
- the lipid particle consists of or consists essentially of the above lipid mixture in molar ratios of about 50-90% charged lipid, 0-50% neutral lipid, and 0-10% cholesterol.
- the lipid particle can further include a PEG-modified lipid (e.g., a PEG-DMG or PEG-DMA).
- the lipid particle consists of a charged lipid (e.g., a quaternary nitrogen containing lipid) and a protonatable lipid, a neutral lipid or a steroid, or a combination thereof.
- the particles can be formulated with a nucleic acid therapeutic agent so as to attain a desired N/P ratio.
- the N/P ratio is the ratio of number of molar equivalent of cationic nitrogen (N) atoms present in the lipid particle to the number of molar equivalent of anionic phosphate (P) of the nucleic acid backbone.
- the N/P ratio can be in the range of about 1 to about 50. In one example, the range is about 1 to about 20, about 1 to about 10, about 1 to about 5.
- the lipid particle consists of or consists essentially of a charged lipid, DOPE, and cholesterol.
- the particle includes lipids in the following mole percentages: charged lipid, 45-63 mol %; DOPE, 35-55 mol %; and cholesterol, 0-10 mol %.
- the particles can be formulated with a nucleic acid therapeutic agent so as to attain a desired N/P ratio.
- the N/P ratio is the ratio of number of moles cationic nitrogen (N) atoms (i.e., charged lipids) to the number of molar equivalents of anionic phosphate (P) backbone groups of the nucleic acid.
- the N to P ratio can be in the range of about 5: 1 to about 1 :1.
- the charged lipid is chosen from compound 205, 201, or 204 (see Scheme 1 below).
- the neutral lipid, DOPE, in these compositions is replaced with POPC, DPPC, DPSC or SM.
- the present invention includes compositions comprising a lipid particle of the present invention and an active agent, wherein the active agent is associated with the lipid particle.
- the active agent is a therapeutic agent.
- the active agent is encapsulated within an aqueous interior of the lipid particle.
- the active agent is present within one or more lipid layers of the lipid particle.
- the active agent is bound to the exterior or interior lipid surface of a lipid particle.
- “Fully encapsulated” as used herein indicates that the nucleic acid in the particles is not significantly degraded after exposure to serum or a nuclease assay that would significantly degrade free nucleic acids. In a fully encapsulated system, preferably less than 25% of particle nucleic acid is degraded in a treatment that would normally degrade 100% of free nucleic acid, more preferably less than 10% and most preferably less than 5% of the particle nucleic acid is degraded. Alternatively, full encapsulation may be determined by an Oligreen ® assay. Oligreen ® is an ultra-sensitive fluorescent nucleic acid stain for quantitating oligonucleotides and single-stranded DNA in solution
- Active agents include any molecule or compound capable of exerting a desired effect on a cell, tissue, organ, or subject. Such effects may be biological, physiological, or cosmetic, for example. Active agents may be any type of molecule or compound, including e.g. , nucleic acids, peptides and polypeptides, including, e.g., antibodies, such as, e.g., polyclonal antibodies, monoclonal antibodies, antibody fragments; humanized antibodies, recombinant antibodies, recombinant human antibodies, and PrimatizedTM antibodies, cytokines, growth factors, apoptotic factors, differentiation- inducing factors, cell surface receptors and their ligands; hormones; and small molecules, including small organic molecules or compounds.
- nucleic acids e.g., nucleic acids, peptides and polypeptides
- antibodies such as, e.g., polyclonal antibodies, monoclonal antibodies, antibody fragments
- the active agent is a therapeutic agent, or a salt or derivative thereof
- therapeutic agent derivatives may be therapeutically active themselves or they may be prodrugs, which become active upon further modification.
- a therapeutic agent derivative retains some or all of the therapeutic activity as compared to the unmodified agent, while in another embodiment, a therapeutic agent derivative lacks therapeutic activity.
- therapeutic agents include any therapeutically effective agent or drug, such as anti-inflammatory compounds, anti-depressants, stimulants, analgesics, antibiotics, birth control medication, antipyretics, vasodilators, anti- angiogenics, cytovascular agents, signal transduction inhibitors, cardiovascular drugs, e.g. , anti- arrhythmic agents, vasoconstrictors, hormones, and steroids.
- therapeutically effective agent or drug such as anti-inflammatory compounds, anti-depressants, stimulants, analgesics, antibiotics, birth control medication, antipyretics, vasodilators, anti- angiogenics, cytovascular agents, signal transduction inhibitors, cardiovascular drugs, e.g. , anti- arrhythmic agents, vasoconstrictors, hormones, and steroids.
- the therapeutic agent is an oncology drug, which may also be referred to as an anti-tumor drug, an anti-cancer drug, a tumor drug, an antineoplastic agent, or the like.
- oncology drugs that may be used according to the invention include, but are not limited to, adriamycin, alkeran, allopurinol, altretamine, amifostine, anastrozole, araC, arsenic trioxide, azathioprine, bexarotene, biCNU, bleomycin, busulfan intravenous, busulfan oral, capecitabine (Xeloda), carboplatin, carmustine, CCNU, celecoxib, chlorambucil, cisplatin, cladribine, cyclosporin A, cytarabine, cytosine arabinoside, daunorubicin, Cytoxan, daunorubicin, dexamethasone, de
- oncology drugs that may be used according to the invention are ellipticin and ellipticin analogs or derivatives, epothilones, intracellular kinase inhibitors and camptothecins.
- Nucleic Acid-Lipid Particles Nucleic Acid-Lipid Particles
- lipid particles of the present invention are associated with a nucleic acid, resulting in a nucleic acid-lipid particle.
- the nucleic acid is fully encapsulated in the lipid particle.
- nucleic acid is meant to include any oligonucleotide or polynucleotide. Fragments containing up to 50 nucleotides are generally termed oligonucleotides, and longer fragments are called polynucleotides. In particular embodiments, oligonucletoides of the present invention are 15-50 nucleotides in length.
- polynucleotide and “oligonucleotide” refer to a polymer or oligomer of nucleotide or nucleoside monomers consisting of naturally occurring bases, sugars and intersugar (backbone) linkages.
- polynucleotide and oligonucleotide also includes polymers or oligomers comprising non-naturally occurring monomers, or portions thereof, which function similarly. Such modified or substituted oligonucleotides are often preferred over native forms because of properties such as, for example, enhanced cellular uptake and increased stability in the presence of nucleases.
- the nucleic acid that is present in a lipid-nucleic acid particle according to this invention includes any form of nucleic acid that is known.
- the nucleic acids used herein can be single-stranded DNA or RNA, or double-stranded DNA or RNA, or DNA-RNA hybrids.
- double- stranded DNA include structural genes, genes including control and termination regions, and self -replicating systems such as viral or plasmid DNA.
- double-stranded RNA include siRNA and other RNA interference reagents.
- Single-stranded nucleic acids include, e.g., antisense oligonucleotides, ribozymes, microRNA, siRNA, antimicroRNA, antagomirs, microRNA inhibitor, supermirs, and triplex-forming oligonucleotides.
- the nucleic acid that is present in a lipid-nucleic acid particle of this invention may include one or more of the
- Nucleic acids of the present invention may be of various lengths, generally dependent upon the particular form of nucleic acid.
- plasmids or genes may be from about 1,000 to 100,000 nucleotide residues in length.
- oligonucleotides may range from about 10 to 100 nucleotides in length.
- oligonucleotides, single-stranded, double-stranded, and triple-stranded may range in length from about 10 to about 50 nucleotides, from about 20 o about 50 nucleotides, from about 15 to about 30 nucleotides, from about 20 to about 30 nucleotides in length.
- the oligonucleotide (or a strand thereof) of the present invention specifically hybridizes to or is complementary to a target polynucleotide.
- oligonucleotide and “complementary” are terms which are used to indicate a sufficient degree of complementarity such that stable and specific binding occurs between the DNA or RNA target and the oligonucleotide. It is understood that an oligonucleotide need not be 100% complementary to its target nucleic acid sequence to be specifically hybridizable. An oligonucleotide is specifically hybridizable when binding of the oligonucleotide to the target interferes with the normal function of the target molecule to cause a loss of utility or expression therefrom, and there is a sufficient degree of complementarity to avoid non-specific binding of the oligonucleotide to non-target sequences under conditions in which specific binding is desired, i.e.
- this oligonucleotide includes 1, 2, or 3 base substitutions, e.g. mismatches, as compared to the region of a gene or mRNA sequence that it is targeting or to which it specifically hybridizes.
- nucleic acid-lipid particles of the present invention are associated with RNA interference (RNAi) molecules.
- RNA interference methods using RNAi molecules may be used to disrupt the expression of a gene or polynucleotide of interest.
- Small interfering RNA siRNA has essentially replaced antisense ODN and ribozymes as the next generation of targeted oligonucleotide drugs under development.
- SiRNAs are RNA duplexes normally 16-30 nucleotides long that can associate with a cytoplasmic multi-protein complex known as RNAi-induced silencing complex (RISC).
- RISC RNAi-induced silencing complex
- siRNA function through a natural mechanism evolved to control gene expression through non-coding RNA. This is generally considered to be the reason why their activity is more potent in vitro and in vivo than either antisense ODN or ribozymes.
- RNAi reagents including siRNAs targeting clinically relevant targets, are currently under pharmaceutical development, as described, e.g., in de Fougerolles, A. et al , Nature Reviews 6:443-453 (2007).
- RNAi molecules While the first described RNAi molecules were RNA:RNA hybrids comprising both an RNA sense and an RNA antisense strand, it has now been demonstrated that DNA sense:RNA antisense hybrids, RNA sense:DNA antisense hybrids, and DNA:DNA hybrids are capable of mediating RNAi (Lamberton, J.S. and Christian, A.T., (2003) Molecular Biotechnology 24:111-119). Thus, the invention includes the use of RNAi molecules comprising any of these different types of double-stranded molecules. In addition, it is understood that RNAi molecules may be used and introduced to cells in a variety of forms.
- RNAi molecules encompasses any and all molecules capable of inducing an RNAi response in cells, including, but not limited to, double- stranded oligonucleotides comprising two separate strands, i.e. a sense strand and an antisense strand, e.g., small interfering RNA (siRNA); double-stranded
- oligonucleotide comprising two separate strands that are linked together by non- nucleotidyl linker; oligonucleotides comprising a hairpin loop of complementary sequences, which forms a double- stranded region, e.g. , shRNAi molecules, and expression vectors that express one or more polynucleotides capable of forming a double- stranded polynucleotide alone or in combination with another polynucleotide.
- a "single strand siRNA compound” as used herein, is an siRNA compound which is made up of a single molecule. It may include a duplexed region, formed by intra-strand pairing, e.g., it may be, or include, a hairpin or pan-handle structure. Single strand siRNA compounds may be antisense with regard to the target molecule
- a single strand siRNA compound may be sufficiently long that it can enter the RISC and participate in RISC mediated cleavage of a target mRNA.
- a single strand siRNA compound is at least 14, and in other embodiments at least 15, 20, 25, 29, 35, 40, or 50 nucleotides in length. In certain embodiments, it is less than 200, 100, or 60 nucleotides in length.
- Hairpin siRNA compounds will have a duplex region equal to or at least 17, 18, 19, 29, 21, 22, 23, 24, or 25 nucleotide pairs.
- the duplex region will may be equal to or less than 200, 100, or 50, in length. In certain embodiments, ranges for the duplex region are 15-30, 17 to 23, 19 to 23, and 19 to 21 nucleotides pairs in length.
- the hairpin may have a single strand overhang or terminal unpaired region. In certain embodiments, the overhangs are 2-3 nucleotides in length. In some embodiments, the overhang is at the sense side of the hairpin and in some embodiments on the antisense side of the hairpin.
- a "double stranded siRNA compound” as used herein, is an siRNA compound which includes more than one, and in some cases two, strands in which interchain hybridization can form a region of duplex structure.
- the antisense strand of a double stranded siRNA compound may be equal to or at least, 14, 15, 16 17, 18, 19, 25, 29, 40, or 60 nucleotides in length. It may be equal to or less than 200, 100, or 50, nucleotides in length. Ranges may be 17 to 25, 19 to 23, and 19 to21 nucleotides in length.
- antisense strand means the strand of an siRNA compound that is sufficiently complementary to a target molecule, e.g. a target RNA.
- the sense strand of a double stranded siRNA compound may be equal to or at least 14, 15, 16 17, 18, 19, 25, 29, 40, or 60 nucleotides in length. It may be equal to or less than 200, 100, or 50, nucleotides in length. Ranges may be 17 to 25, 19 to 23, and 19 to 21 nucleotides in length.
- the double strand portion of a double stranded siRNA compound may be equal to or at least, 14, 15, 16 17, 18, 19, 20, 21, 22, 23, 24, 25, 29, 40, or 60 nucleotide pairs in length, It may be equal to or less than 200, 100, or 50, nucleotides pairs in length, Ranges may be 15-30, 17 to 23, 19 to 23, and 19 to 21 nucleotides pairs in length.
- the siRNA compound is sufficiently large that it can be cleaved by an endogenous molecule, e.g., by Dicer, to produce smaller siRNA compounds, e.g., siRNAs agents
- the sense and antisense strands may be chosen such that the double-stranded siRNA compound includes a single strand or unpaired region at one or both ends of the molecule.
- a double- stranded siRNA compound may contain sense and antisense strands, paired to contain an overhang, e.g., one or two 5' or 3' overhangs, or a 3' overhang of 1 - 3 nucleotides.
- the overhangs can be the result of one strand being longer than the other, or the result of two strands of the same length being staggered. Some embodiments will have at least one 3' overhang. In one embodiment, both ends of an siRNA molecule will have a 3' overhang. In some embodiments, the overhang is 2 nucleotides.
- the length for the duplexed region is between 15 and 30, or 18, 19, 20, 21, 22, and 23 nucleotides in length, e.g., in the ssiRNA compound range discussed above.
- ssiRNA compounds can resemble in length and structure the natural Dicer processed products from long dsiRNAs.
- Embodiments in which the two strands of the ssiRNA compound are linked, e.g., covalently linked are also included. Hairpin, or other single strand structures which provide the required double stranded region, and a 3' overhang are also within the invention.
- the siRNA compounds described herein, including double-stranded siRNA compounds and single- stranded siRNA compounds can mediate silencing of a target RNA, e.g., mRNA, e.g., a transcript of a gene that encodes a protein.
- mRNA e.g., a transcript of a gene that encodes a protein.
- mRNA to be silenced e.g., a transcript of a gene that encodes a protein.
- mRNA to be silenced e.g., a transcript of a gene that encodes a protein.
- mRNA to be silenced e.g., a transcript of a gene that encodes a protein.
- mRNA to be silenced e.g., a transcript of a gene that encodes a protein.
- a gene e.g., a gene that encodes a protein.
- the RNA to be silenced is an endogenous gene or a pathogen gene
- RNAi refers to the ability to silence, in a sequence specific manner, a target RNA. While not wishing to be bound by theory, it is believed that silencing uses the RNAi machinery or process and a guide RNA, e.g., an ssiRNA compound of 21 to 23 nucleotides.
- an siRNA compound is "sufficiently complementary" to a target RNA, e.g., a target mRNA, such that the siRNA compound silences production of protein encoded by the target mRNA.
- the siRNA compound is "exactly complementary" to a target RNA, e.g., the target RNA and the siRNA compound anneal, for example to form a hybrid made exclusively of Watson-Crick base pairs in the region of exact complementarity.
- a "sufficiently complementary" target RNA can include an internal region (e.g., of at least 10 nucleotides) that is exactly complementary to a target RNA.
- the siRNA compound specifically discriminates a single-nucleotide difference. In this case, the siRNA compound only mediates RNAi if exact complementary is found in the region (e.g., within 7 nucleotides of) the single-nucleotide difference.
- miRNAs are a highly conserved class of small RNA molecules that are transcribed from DNA in the genomes of plants and animals, but are not translated into protein.
- Processed miRNAs are single stranded -17-25 nucleotide (nt) RNA molecules that become incorporated into the RNA-induced silencing complex (RISC) and have been identified as key regulators of development, cell proliferation, apoptosis and differentiation. They are believed to play a role in regulation of gene expression by binding to the 3 '-untranslated region of specific mRNAs.
- RISC mediates down- regulation of gene expression through translational inhibition, transcript cleavage, or both. RISC is also implicated in transcriptional silencing in the nucleus of a wide range of eukaryotes.
- miRNA sequences identified to date is large and growing, illustrative examples of which can be found, for example, in: "miRBase: microRNA sequences, targets and gene nomenclature' ' Griffiths- Jones S, Grocock RJ, van Dongen S, Bateman A, Enright AJ. NAR, 2006, 34, Database Issue, D140-D144; "The microRNA Registry” Griffiths -Jones S. NAR, 2004, 32, Database Issue, D109-D111; and also at http ://microrna. sanger.ac.uk/sequences/.
- a nucleic acid is an antisense oligonucleotide directed to a target polynucleotide.
- antisense oligonucleotide or simply “antisense” is meant to include oligonucleotides that are complementary to a targeted polynucleotide sequence.
- Antisense oligonucleotides are single strands of DNA or RNA that are complementary to a chosen sequence, e.g. a target gene mRNA. Antisense
- oligonucleotides are thought to inhibit gene expression by binding to a complementary mRNA. Binding to the target mRNA can lead to inhibition of gene expression by through making the either by preventing translation of complementary mRNA strands by binding to it or by leading to degradation of the target mRNA
- Antisense DNA can be used to target a specific, complementary (coding or non-coding) RNA. If binding takes places this DNA/RNA hybrid can be degraded by the enzyme RNase H.
- antisense oligonucleotides contain from about 10 to about 50 nucleotides, more preferably about 15 to about 30 nucleotides. The term also encompasses antisense oligonucleotides that may not be exactly complementary to the desired target gene.
- the invention can be utilized in instances where non-target specific-activities are found with antisense, or where an antisense sequence containing one or more mismatches with the target sequence is the most preferred for a particular use.
- Antisense oligonucleotides have been demonstrated to be effective and targeted inhibitors of protein synthesis, and, consequently, can be used to specifically inhibit protein synthesis by a targeted gene.
- the efficacy of antisense oligonucleotides for inhibiting protein synthesis is well established. For example, the synthesis of polygalactauronase and the muscarine type 2 acetylcholine receptor are inhibited by antisense oligonucleotides directed to their respective mRNA sequences (U. S. Patent 5,739,119 and U. S. Patent 5,759,829).
- antisense constructs have also been described that inhibit and can be used to treat a variety of abnormal cellular proliferations, e.g. cancer (U. S. Patent 5,747,470; U. S. Patent 5,591,317 and U. S. Patent 5,783,683).
- antisense oligonucleotides are known in the art and can be readily adapted to produce an antisense oligonucleotide that targets any polynucleotide sequence. Selection of antisense oligonucleotide sequences specific for a given target sequence is based upon analysis of the chosen target sequence and determination of secondary structure, T m , binding energy, and relative stability.
- Antisense oligonucleotide sequences specific for a given target sequence is based upon analysis of the chosen target sequence and determination of secondary structure, T m , binding energy, and relative stability.
- oligonucleotides may be selected based upon their relative inability to form dimers, hairpins, or other secondary structures that would reduce or prohibit specific binding to the target mRNA in a host cell.
- Highly preferred target regions of the mRNA include those regions at or near the AUG translation initiation codon and those sequences that are substantially complementary to 5' regions of the mRNA.
- Antagomirs are RNA-like oligonucleotides that harbor various modifications for RNAse protection and pharmacologic properties, such as enhanced tissue and cellular uptake. They differ from normal RNA by, for example, complete 2'-0-methylation of sugar, phosphorothioate backbone and, for example, a cholesterol-moiety at 3'-end. Antagomirs may be used to efficiently silence endogenous miRNAs by forming duplexes comprising the antagomir and endogenous miRNA, thereby preventing miRNA-induced gene silencing.
- antagomir-mediated miRNA silencing is the silencing of miR-122, described in Krutzfeldt et al, Nature, 2005, 438: 685-689, which is expressly incorporated by reference herein in its entirety.
- Antagomir RNAs may be synthesized using standard solid phase oligonucleotide synthesis protocols. See U.S. Patent
- An antagomir can include ligand-conjugated monomer subunits and monomers for oligonucleotide synthesis. Exemplary monomers are described in U.S. Application No. 10/916,185, filed on August 10, 2004.
- An antagomir can have a ZXY structure, such as is described in PCT Application No. PCT/US2004/07070 filed on March 8, 2004.
- An antagomir can be complexed with an amphipathic moiety. Exemplary amphipathic moieties for use with oligonucleotide agents are described in PCT Application
- Aptamers are nucleic acid or peptide molecules that bind to a particular molecule of interest with high affinity and specificity (Tuerk and Gold, Science 249:505 (1990); Ellington and Szostak, Nature 346:818 (1990)).
- DNA or RNA aptamers have been successfully produced which bind many different entities from large proteins to small organic molecules. See Eaton, Curr. Opin. Chem. Biol. 1: 10-16 (1997), Famulok, Curr. Opin. Struct. Biol. 9:324-9(1999), and Hermann and Patel, Science 287:820-5 (2000).
- Aptamers may be RNA or DNA based, and may include a riboswitch.
- a riboswitch is a part of an mRNA molecule that can directly bind a small target molecule, and whose binding of the target affects the gene's activity.
- an mRNA that contains a riboswitch is directly involved in regulating its own activity, depending on the presence or absence of its target molecule.
- aptamers are engineered through repeated rounds of in vitro selection or equivalently, SELEX (systematic evolution of ligands by exponential enrichment) to bind to various molecular targets such as small molecules, proteins, nucleic acids, and even cells, tissues and organisms.
- the aptamer may be prepared by any known method, including synthetic, recombinant, and purification methods, and may be used alone or in combination with other aptamers specific for the same target. Further, as described more fully herein, the term “aptamer” specifically includes "secondary aptamers” containing a consensus sequence derived from comparing two or more known aptamers to a given target.
- nucleic acid- lipid particles are associated with ribozymes.
- Ribozymes are RNA molecules complexes having specific catalytic domains that possess endonuclease activity (Kim and Cech, Proc Natl Acad Sci U S A. 1987 Dec;84(24):8788-92; Forster and Symons, Cell. 1987 Apr 24;49(2):211-20).
- a large number of ribozymes accelerate phosphoester transfer reactions with a high degree of specificity, often cleaving only one of several phosphoesters in an oligonucleotide substrate (Cech et al, Cell.
- enzymatic nucleic acids act by first binding to a target RNA. Such binding occurs through the target binding portion of a enzymatic nucleic acid which is held in close proximity to an enzymatic portion of the molecule that acts to cleave the target RNA. Thus, the enzymatic nucleic acid first recognizes and then binds a target RNA through complementary base-pairing, and once bound to the correct site, acts enzymatically to cut the target RNA.
- RNA Strategic cleavage of such a target RNA will destroy its ability to direct synthesis of an encoded protein. After an enzymatic nucleic acid has bound and cleaved its RNA target, it is released from that RNA to search for another target and can repeatedly bind and cleave new targets.
- the enzymatic nucleic acid molecule may be formed in a hammerhead, hairpin, a hepatitis ⁇ virus, group I intron or RNaseP RNA (in association with an RNA guide sequence) or Neurospora VS RNA motif, for example.
- a hammerhead hairpin
- a hepatitis ⁇ virus group I intron or RNaseP RNA (in association with an RNA guide sequence)
- Neurospora VS RNA motif for example.
- hairpin motifs are described by Hampel et al. (Eur. Pat. Appl. Publ. No. EP 0360257), Hampel and Tritz, Biochemistry 1989 Jun 13;28(12):4929- 33; Hampel et al, Nucleic Acids Res. 1990 Jan 25;18(2):299-304 and U. S. Patent 5,631,359.
- An example of the hepatitis ⁇ virus motif is described by Perrotta and Been, Biochemistry. 1992 Dec 1 ;31(47): 11843-52; an example of the RNaseP motif is described by Guerrier-Takada et al , Cell. 1983 Dec;35(3 Pt 2):849-57; Neurospora VS RNA ribozyme motif is described by Collins (Saville and Collins, Cell. 1990 May
- enzymatic nucleic acid molecules used according to the invention have a specific substrate binding site which is complementary to one or more of the target gene DNA or RNA regions, and that they have nucleotide sequences within or surrounding that substrate binding site which impart an RNA cleaving activity to the molecule.
- the ribozyme constructs need not be limited to specific motifs mentioned herein.
- Ribozymes may be designed as described in Int. Pat. Appl. Publ. No. WO 93/23569 and Int. Pat. Appl. Publ. No. WO 94/02595, each specifically incorporated herein by reference, and synthesized to be tested in vitro and in vivo, as described therein.
- Ribozyme activity can be optimized by altering the length of the ribozyme binding arms or chemically synthesizing ribozymes with modifications that prevent their degradation by serum ribonucleases (see e.g. , Int. Pat. Appl. Publ. No. WO 92/07065; Int. Pat. Appl. Publ. No. WO 93/15187; Int. Pat. Appl. Publ. No. WO 91/03162; Eur. Pat. Appl. Publ. No. 92110298.4; U. S. Patent 5,334,711 ; and Int. Pat. Appl. Publ. No. WO 94/13688, which describe various chemical modifications that can be made to the sugar moieties of enzymatic RNA molecules), modifications which enhance their efficacy in cells, and removal of stem ⁇ bases to shorten RNA synthesis times and reduce chemical requirements.
- Nucleic acids associated with lipid particles of the present invention may be immunostimulatory, including immunostimulatory oligonucleotides (ISS; single-or double- stranded) capable of inducing an immune response when administered to a subject, which may be a mammal or other patient.
- ISS immunostimulatory oligonucleotides
- ISS include, e.g., certain palindromes leading to hairpin secondary structures (see Yamamoto S., et al. (1992) J. Immunol. 148: 4072-4076), or CpG motifs, as well as other known ISS features (such as multi-G domains, see WO 96/11266).
- the immune response may be an innate or an adaptive immune response.
- the immune system is divided into a more innate immune system, and acquired adaptive immune system of vertebrates, the latter of which is further divided into humoral cellular components.
- the immune response may be mucosal,
- an immunostimulatory nucleic acid is only immunostimulatory when administered in combination with a lipid particle, and is not immunostimulatory when administered in its "free form.” According to the present invention, such an oligonucleotide is considered to be immunostimulatory.
- Immunostimulatory nucleic acids are considered to be non-sequence specific when it is not required that they specifically bind to and reduce the expression of a target polynucleotide in order to provoke an immune response.
- immunostimulatory nucleic acids may comprise a seuqence correspondign to a region of a naturally occurring gene or mRNA, but they may still be considered non-sequence specific immunostimulatory nucleic acids.
- the immunostimulatory nucleic acid or oligonucleotide comprises at least one CpG dinucleotide.
- the oligonucleotide or CpG dinucleotide may be unmethylated or methylated.
- the immunostimulatory nucleic acid comprises at least one CpG dinucleotide having a methylated cytosine.
- the nucleic acid comprises a single CpG dinucleotide, wherein the cytosine in said CpG dinucleotide is methylated.
- the nucleic acid comprises the sequence 5' TAACGTTGAGGGGCAT 3'.
- the nucleic acid comprises at least two CpG dinucleotides, wherein at least one cytosine in the CpG dinucleotides is methylated. In a further embodiment, each cytosine in the CpG dinucleotides present in the sequence is methylated. In another embodiment, the nucleic acid comprises a plurality of CpG dinucleotides, wherein at least one of said CpG dinucleotides comprises a methylated cytosine.
- the nucleic acid comprises the sequence 5'
- the nucleic acid sequence comprises the sequence 5' TCCATGACGTTCCTGACGT 3', wherein the two cytosines indicated in bold are methylated.
- the ODN is selected from a group of ODNs consisting of ODN #1, ODN #2, ODN #3, ODN #4, ODN #5, ODN #6, ODN #7, ODN #8, and ODN #9, as shown below.
- ODN 14 is a 15-mer
- oligonucleotide and ODN 1 is the same oligonucleotide having a
- ODNs oligonucleotides
- oligonucleotides bearing the consensus binding sequence of a specific transcription factor can be used as tools for manipulating gene expression in living cells.
- This strategy involves the intracellular delivery of such "decoy oligonucleotides", which are then recognized and bound by the target factor. Occupation of the transcription factor's DNA-binding site by the decoy renders the transcription factor incapable of subsequently binding to the promoter regions of target genes. Decoys can be used as therapeutic agents, either to inhibit the expression of genes that are activated by a transcription factor, or to upregulate genes that are suppressed by the binding of a transcription factor. Examples of the utilization of decoy oligonucleotides may be found in Mann et al., J. Clin. Invest, 2000, 106: 1071-1075, which is expressly incorporated by reference herein, in its entirety.
- a supermir refers to a single stranded, double stranded or partially double stranded oligomer or polymer of ribonucleic acid (RNA) or deoxyribonucleic acid (DNA) or both or modifications thereof, which has a nucleotide sequence that is substantially identical to an miRNA and that is antisense with respect to its target, This term includes oligonucleotides composed of naturally-occurring nucleobases, sugars and covalent internucleoside (backbone) linkages and which contain at least one non-naturally- occurring portion which functions similarly. Such modified or substituted
- oligonucleotides are preferred over native forms because of desirable properties such as, for example, enhanced cellular uptake, enhanced affinity for nucleic acid target and increased stability in the presence of nucleases.
- the supermir does not include a sense strand, and in another preferred embodiment, the supermir does not self -hybridize to a significant extent.
- An supermir featured in the invention can have secondary structure, but it is substantially single-stranded under physiological conditions.
- An supermir that is substantially single-stranded is single-stranded to the extent that less than about 50% ⁇ e.g., less than about 40%, 30%, 20%, 10%, or 5%) of the supermir is duplexed with itself.
- the supermir can include a hairpin segment, e.g., sequence, preferably at the 3' end can self hybridize and form a duplex region, e.g., a duplex region of at least 1, 2, 3, or 4 and preferably less than 8, 7, 6, or n nucleotides, e.g., 5 nuclotides.
- the duplexed region can be connected by a linker, e.g., a nucleotide linker, e.g., 3, 4, 5, or 6 dTs, e.g., modified dTs.
- the supermir is duplexed with a shorter oligo, e.g., of 5, 6, 7, 8, 9, or 10 nucleotides in length, e.g., at one or both of the 3' and 5' end or at one end and in the non-terminal or middle of the supermir. miRNA mimics
- miRNA mimics represent a class of molecules that can be used to imitate the gene silencing ability of one or more miRNAs.
- miRNA mimic refers to synthetic non-coding RNAs (i.e. the miRNA is not obtained by purification from a source of the endogenous miRNA) that are capable of entering the RNAi pathway and regulating gene expression.
- miRNA mimics can be designed as mature molecules (e.g. single stranded) or mimic precursors (e.g., pri- or pre-miRNAs).
- miRNA mimics can be comprised of nucleic acid (modified or modified nucleic acids) including oligonucleotides comprising, without limitation, RNA, modified RNA, DNA, modified DNA, locked nucleic acids, or 2'-0,4'-C-ethylene-bridged nucleic acids (ENA), or any combination of the above (including DNA-RNA hybrids).
- miRNA mimics can comprise conjugates that can affect delivery, intracellular compartmentalization, stability, specificity, functionality, strand usage, and/or potency.
- miRNA mimics are double stranded molecules (e.g., with a duplex region of between about 16 and about 31 nucleotides in length) and contain one or more sequences that have identity with the mature strand of a given miRNA.
- Modifications can comprise 2' modifications (including 2'-0 methyl modifications and 2' F modifications) on one or both strands of the molecule and internucleotide modifications (e.g. phorphorthioate modifications) that enhance nucleic acid stability and/or specificity.
- miRNA mimics can include overhangs. The overhangs can consist of 1-6 nucleotides on either the 3' or 5' end of either strand and can be modified to enhance stability or functionality. In one
- a miRNA mimic comprises a duplex region of between 16 and 31 nucleotides and one or more of the following chemical modification patterns: the sense strand contains 2'-0-methyl modifications of nucleotides 1 and 2 (counting from the 5' end of the sense oligonucleotide), and all of the Cs and Us; the antisense strand modifications can comprise 2' F modification of all of the Cs and Us, phosphorylation of the 5' end of the oligonucleotide, and stabilized internucleotide linkages associated with a 2 nucleotide 3 ' overhang.
- antimir microRNA inhibitor
- miR inhibitor miR inhibitor
- inhibitor refers to oligonucleotides or modified oligonucleotides that interfere with the ability of specific miRNAs.
- the inhibitors are nucleic acid or modified nucleic acids in nature including oligonucleotides comprising RNA, modified RNA, DNA, modified DNA, locked nucleic acids (LNAs), or any combination of the above.
- Modifications include 2' modifications (including 2'-0 alkyl modifications and 2' F modifications) and internucleotide modifications (e.g. phosphorothioate modifications) that can affect delivery, stability, specificity, intracellular compartmentalization, or potency.
- miRNA inhibitors can comprise conjugates that can affect delivery, intracellular compartmentalization, stability, and/or potency.
- microRNA inhibitors comprise contain one or more sequences or portions of sequences that are complementary or partially complementary with the mature strand (or strands) of the miRNA to be targeted, in addition, the miRNA inhibitor may also comprise additional sequences located 5' and 3' to the sequence that is the reverse complement of the mature miRNA.
- the additional sequences may be the reverse complements of the sequences that are adjacent to the mature miRNA in the pri-miRNA from which the mature miRNA is derived, or the additional sequences may be arbitrary sequences (having a mixture of A, G, C, or U).
- one or both of the additional sequences are arbitrary sequences capable of forming hairpins.
- the sequence that is the reverse complement of the miRNA is flanked on the 5' side and on the 3' side by hairpin structures.
- Micro-RNA inhibitors when double stranded, may include mismatches between nucleotides on opposite strands.
- micro-RNA inhibitors may be linked to conjugate moieties in order to facilitate uptake of the inhibitor into a cell.
- a micro-RNA inhibitor may be linked to cholesteryl 5-(bis(4- methoxyphenyl)(phenyl)methoxy)-3 hydroxypentylcarbamate) which allows passive uptake of a micro-RNA inhibitor into a cell.
- Micro-RNA inhibitors including hairpin miRNA inhibitors, are described in detail in Vermeulen et al., "Double-Stranded Regions Are Essential Design Components Of Potent Inhibitors of RISC Function," RNA 13: 723- 730 (2007) and in WO2007/095387 and WO 2008/036825 each of which is incorporated herein by reference in its entirety.
- a person of ordinary skill in the art can select a sequence from the database for a desired miRNA and design an inhibitor useful for the methods disclosed herein.
- Ul adaptor inhibit polyA sites and are bifunctional oligonucleotides with a target domain complementary to a site in the target gene's terminal exon and a 'Ul domain' that binds to the Ul smaller nuclear RNA component of the Ul snRNP (Goraczniak, et al., 2008, Nature Biotechnology, 27(3), 257-263, which is expressly incorporated by reference herein, in its entirety).
- Ul snRNP is a ribonucleoprotein complex that functions primarily to direct early steps in spliceosome formation by binding to the pre-mRNA exon- intron boundary (Brown and Simpson, 1998, Annu Rev Plant Physiol Plant Mol Biol 49:77-95). Nucleotides 2-11 of the 5'end of Ul snRNA base pair bind with the 5'ss of the pre mRNA.
- oligonucleotides of the invention are Ul adaptors. In one embodiment,
- the Ul adaptor can be administered in combination with at least one other iRNA agent.
- Unmodified oligonucleotides may be less than optimal in some applications, e.g., unmodified oligonucleotides can be prone to degradation by e.g., cellular nucleases, Nucleases can hydrolyze nucleic acid phosphodiester bonds. However, chemical modifications of oligonucleotides can confer improved properties, and, e.g., can render oligonucleotides more stable to nucleases.
- oligonucleotides are polymers of subunits or monomers, many of the modifications described below occur at a position which is repeated within an
- oligonucleotide e.g., a modification of a base, a sugar, a phosphate moiety, or the non- bridging oxygen of a phosphate moiety. It is not necessary for all positions in a given oligonucleotide to be uniformly modified, and in fact more than one of the
- the modification will occur at all of the subject positions in the oligonucleotide but in many, and in fact in most cases it will not.
- a modification may only occur at a 3' or 5' terminal position, may only occur in the internal region, may only occur in a terminal regions, e.g. at a position on a terminal nucleotide or in the last 2, 3, 4, 5, or 10 nucleotides of an oligonucleotide.
- a modification may occur in a double strand region, a single strand region, or in both.
- oligonucleotide or may only occur in a single strand region of a double- stranded oligonucleotide.
- a phosphorothioate modification at a non-bridging oxygen position may only occur at one or both termini, may only occur in a terminal regions, e.g., at a position on a terminal nucleotide or in the last 2, 3, 4, 5, or 10 nucleotides of a strand, or may occur in double strand and single strand regions, particularly at termini.
- the 5' end or ends can be phosphorylated.
- a modification described herein may be the sole modification, or the sole type of modification included on multiple nucleotides, or a modification can be combined with one or more other modifications described herein.
- the modifications described herein can also be combined onto an oligonucleotide, e.g. different nucleotides of an oligonucleotide have different modifications described herein.
- Modifications can include, e.g., the use of modifications at the 2' OH group of the ribose sugar, e.g., the use of deoxyribonucleotides, e.g., deoxythymidine, instead of ribonucleotides, and modifications in the phosphate group, e.g., phosphothioate modifications.
- Overhangs need not be homologous with the target sequence.
- the phosphate group is a negatively charged species. The charge is distributed equally over the two non-bridging oxygen atoms. However, the phosphate group can be modified by replacing one of the oxygens with a different substituent. One result of this modification to RNA phosphate backbones can be increased resistance of the
- oligoribonucleotide to nucleolytic breakdown.
- modified phosphate groups include phosphorothioate,
- one of the non-bridging phosphate oxygen atoms in the phosphate backbone moiety can be replaced by any of the following: S, Se, BR 3 (R is hydrogen, alkyl, aryl), C (i.e. an alkyl group, an aryl group, etc...), H, NR 2 (R is hydrogen, alkyl, aryl), or OR (R is alkyl or aryl).
- the phosphorous atom in an unmodified phosphate group is achiral.
- the stereogenic phosphorous atom can possess either the "R" configuration (herein Rp) or the "S” configuration (herein Sp).
- Phosphorodithioates have both non-bridging oxygens replaced by sulfur.
- the phosphorus center in the phosphorodithioates is achiral which precludes the formation of oligoribonucleotides diastereomers.
- the non-bridging oxygens can be independently any one of S, Se, B, C, H, N, or OR (R is alkyl or aryl).
- the phosphate linker can also be modified by replacement of bridging oxygen, (i.e. oxgen that links the phosphate to the nucleoside), with nitrogen (bridged
- the replacement can occur at the either linking oxygen or at both the linking oxygens.
- the bridging oxygen is the 3 '-oxygen of a nucleoside, replcament with carbobn is preferred.
- replcament with nitrogen is preferred.
- the phosphate group can be replaced by non-phosphorus containing connectors. While not wishing to be bound by theory, it is believed that since the charged
- phosphodiester group is the reaction center in nucleolytic degradation, its replacement with neutral structural mimics should impart enhanced nuclease stability.
- moieties which can replace the phosphate group include methyl phosphonate, hydroxylamino, siloxane, carbonate, carboxymethyl, carbamate, amide, thioether, ethylene oxide linker, sulfonate, sulfonamide, thioformacetal, formacetal, oxime, methyleneimino, methylenemethylimino, methylenehydrazo,
- Preferred replacements include the methylenecarbonylamino and methylenemethylimino groups.
- Modified phosphate linkages where at least one of the oxygens linked to the phosphate has been replaced or the phosphate group has been replaced by a non- phosphorous group are also referred to as "non phosphodiester backbone linkage.” Replacement of Ribophosphate Backbone
- Oligonucleotide- mimicking scaffolds can also be constructed wherein the phosphate linker and ribose sugar are replaced by nuclease resistant nucleoside or nucleotide surrogates. While not wishing to be bound by theory, it is believed that the absence of a repetitively charged backbone diminishes binding to proteins that recognize polyanions (e.g. nucleases). Again, while not wishing to be bound by theory, it can be desirable in some embodiment, to introduce alterations in which the bases are tethered by a neutral surrogate backbone. Examples include the mophilino, cyclobutyl, pyrrolidine and peptide nucleic acid (PNA) nucleoside surrogates. A preferred surrogate is a PNA surrogate.
- a modified RNA can include modification of all or some of the sugar groups of the ribonucleic acid, E.g., the 2' hydroxyl group (OH) can be modified or replaced with a number of different "oxy" or "deoxy” substituents. While not being bound by theory, enhanced stability is expected since the hydroxyl can no longer be deprotonated to form a 2'-alkoxide ion.
- the 2'-alkoxide can catalyze degradation by intramolecular nucleophilic attack on the linker phosphorus atom.
- oligonucleotides containing only the methoxy ethyl group (MOE), (OCH 2 CH 2 OCH 3 , a PEG derivative), exhibit nuclease stabilities comparable to those modified with the robust phosphorothioate modification.
- “Deoxy” modifications include hydrogen (i.e. deoxyribose sugars, which are of particular relevance to the overhang portions of partially ds RNA); halo (e.g., fluoro); amino (e.g.
- Preferred substitutents are 2'-methoxyethyl, 2'-OCH3, 2'-0-
- an oligonucleotide can include nucleotides containing e.g., arabinose, as the sugar.
- the monomer can have an alpha linkage at the position on the sugar, e.g., alpha- nucleosides.
- Oligonucleotides can also include "abasic" sugars, which lack a nucleobase at C- . These abasic sugars can also be further containing modifications at one or more of the constituent sugar atoms.
- Oligonucleotides can also contain one or more sugars that are in the L form, e.g. L-nucleosides. Terminal Modifications
- the 3' and 5' ends of an oligonucleotide can be modified. Such modifications can be at the 3' end, 5' end or both ends of the molecule. They can include modification or replacement of an entire terminal phosphate or of one or more of the atoms of the phosphate group.
- the 3' and 5' ends of an oligonucleotide can be conjugated to other functional molecular entities such as labeling moieties, e.g., fluorophores (e.g., pyrene, TAMRA, fluorescein, Cy3 or Cy5 dyes) or protecting groups (based e.g., on sulfur, silicon, boron or ester).
- labeling moieties e.g., fluorophores (e.g., pyrene, TAMRA, fluorescein, Cy3 or Cy5 dyes) or protecting groups (based e.g., on sulfur, silicon, boron or ester).
- the functional molecular entities can be attached to the sugar through a phosphate group and/or a linker.
- the terminal atom of the linker can connect to or replace the linking atom of the phosphate group or the C-3' or C-5' O, N, S or C group of the sugar.
- the linker can connect to or replace the terminal atom of a nucleotide surrogate (e.g., PNAs).
- PNAs nucleotide surrogate
- Terminal modifications useful for modulating activity include modification of the 5' end with phosphate or phosphate analogs.
- antisense strands of dsRNAs are 5' phosphorylated or include a phosphoryl analog at the 5' prime terminus.
- 5 '-phosphate modifications include those which are compatible with RISC mediated gene silencing. Suitable modifications include: 5 '-monophosphate
- Terminal modifications can also be useful for monitoring distribution, and in such cases the preferred groups to be added include fluorophores, e.g., fluorscein or an Alexa dye, e.g., Alexa 488. Terminal modifications can also be useful for enhancing uptake, useful modifications for this include cholesterol. Terminal modifications can also be useful for cross-linking an RNA agent to another moiety; modifications useful for this include mitomycin C.
- Adenine, guanine, cytosine and uracil are the most common bases found in RNA. These bases can be modified or replaced to provide RNA's having improved properties.
- nuclease resistant oligoribonucleotides can be prepared with these bases or with synthetic and natural nucleobases (e.g., inosine, thymine, xanthine, hypoxanthine, nubularine, isoguanisine, or tubercidine) and any one of the above modifications.
- substituted or modified analogs of any of the above bases e.g., "unusual bases", “modified bases”, “non-natual bases” and “universal bases” described herein, can be employed.
- Examples include without limitation 2-aminoadenine, 6-methyl and other alkyl derivatives of adenine and guanine, 2-propyl and other alkyl derivatives of adenine and guanine, 5-halouracil and cytosine, 5-propynyl uracil and cytosine, 6-azo uracil, cytosine and thymine, 5-uracil (pseudouracil), 4-thiouracil, 5-halouracil, 5-(2- aminopropyl)uracil, 5-amino allyl uracil, 8-halo, amino, thiol, thioalkyl, hydroxyl and other 8-substituted adenines and guanines, 5-trifluoromethyl and other 5-substituted uracils and cytosines, 7-methylguanine, 5-substituted pyrimidines, 6-azapyrimidines and N-2, N-6 and 0-6 substituted purines, including
- Modifications to oligonucleotides can also include attachment of one or more cationic groups to the sugar, base, and/or the phosphorus atom of a phosphate or modified phosphate backbone moiety.
- a cationic group can be attached to any atom capable of substitution on a natural, unusual or universal base.
- a preferred position is one that does not interfere with hybridization, i.e., does not interfere with the hydrogen bonding interactions needed for base pairing.
- a cationic group can be attached e.g., through the C2' position of a sugar or analogous position in a cyclic or acyclic sugar surrogate.
- AMINE NH 2 ; alkylamino, dialkylamino, heterocyclyl, arylamino, diaryl amino, heteroaryl amino,or diheteroaryl amino).
- modifications may preferably be included on an oligonucleotide at a particular location, e.g., at an internal position of a strand, or on the 5' or 3' end of an oligonucleotide.
- a preferred location of a modification on an oligonucleotide may confer preferred properties on the agent.
- preferred locations of particular modifications may confer optimum gene silencing properties, or increased resistance to endonuclease or exonuclease activity.
- One or more nucleotides of an oligonucleotide may have a 2' -5' linkage.
- One or more nucleotides of an oligonucleotide may have inverted linkages, e.g. 3'-3', 5'-5', 2'-2' or 2'-3' linkages.
- a double-stranded oligonucleotide may include at least one 5'-uridine-adenine-3' (5'-UA-3') dinucleotide wherein the uridine is a 2'-modified nucleotide, or a terminal 5'- uridine-guanine-3' (5'-UG-3') dinucleotide, wherein the 5 '-uridine is a 2 '-modified nucleotide, or a terminal 5'-cytidine-adenine-3' (5'-CA-3') dinucleotide, wherein the 5'- cytidine is a 2'-modified nucleotide, or a terminal 5' -uridine -uridine-3' (5'-UU-3') dinucleotide, wherein the 5'-uridine is a 2'-modified nucleotide, or a terminal 5'-cytidine- cytidine-3' (5'-CC-3') dinu
- oligoribonucleotides and oligoribonucleosides used in accordance with this invention may be synthesized with solid phase synthesis, see for example "Oligonucleotide synthesis, a practical approach", Ed. M. J. Gait, IRL Press, 1984;
- Methyloligoribonucleotide- s synthesis and applications, Chapter 4, Phosphorothioate oligonucleotides, Chapter 5, Synthesis of oligonucleotide phosphorodithioates, Chapter 6, Synthesis of oligo-2'-deoxyribonucleoside methylphosphonates, and. Chapter 7,
- Oligodeoxynucleotides containing modified bases are described in Martin, P., Helv. Chim. Acta, 1995, 78, 486-504; Beaucage, S. L. and Iyer, R. P., Tetrahedron, 1992, 48, 2223-2311 and Beaucage, S. L. and Iyer, R. P., Tetrahedron, 1993, 49, 6123-6194, or references referred to therein. Modification described in WO 00/44895, WOOl/75164, or WO02/44321 can be used herein. The disclosure of all publications, patents, and published patent applications listed herein are hereby incorporated by reference.
- MMI linked oligoribonucleosides also identified herein as MMI linked oligoribonucleosides, methylenedimethylhydrazo linked oligoribonucleosides, also identified herein as MDH linked oligoribonucleosides, and methylenecarbonylamino linked oligonucleosides, also identified herein as amide-3 linked oligoribonucleosides, and methyleneaminocarbonyl linked oligonucleosides, also identified herein as amide-4 linked oligoribonucleosides as well as mixed backbone compounds having, as for instance, alternating MMI and PO or PS linkages can be prepared as is described in U.S. Pat. Nos. 5,378,825, 5,386,023, 5,489,677 and in published PCT applications
- PCT/US92/04294 and PCT/US92/04305 (published as WO 92/20822 WO and 92/20823, respectively).
- Formacetal and thioformacetal linked oligoribonucleosides can be prepared as is described in U.S. Pat. Nos. 5,264,562 and 5,264,564.
- Ethylene oxide linked oligoribonucleosides can be prepared as is described in U.S. Pat. No. 5,223,618.
- Siloxane replacements are described in CormierJ.F. et al. Nucleic Acids Res. 1988, 16, 4583. Carbonate replacements are described in Tittensor, J.R. /. Chem. Soc. C 1971, 1933.
- Carboxymethyl replacements are described in Edge, M.D. et al. J. Chem. Soc. Perkin Trans. 1 1972, 1991. Carbamate replacements are described in Stirchak, E.P. Nucleic Acids Res. 1989, 17, 6129.
- Cyclobutyl sugar surrogate compounds can be prepared as is described in U.S. Pat. No. 5,359,044. Pyrrolidine sugar surrogate can be prepared as is described in U.S. Pat. No. 5,519,134. Morpholino sugar surrogates can be prepared as is described in U.S. Pat. Nos. 5,142,047 and 5,235,033, and other related patent disclosures.
- Peptide Nucleic Acids (PNAs) are known per se and can be prepared in accordance with any of the various procedures referred to in Peptide Nucleic Acids (PNA): Synthesis, Properties and Potential Applications, Bioorganic & Medicinal Chemistry, 1996, 4, 5-23. They may also be prepared in accordance with U.S. Pat. No. 5,539,083. Terminal Modification References
- N-2 substitued purine nucleoside amidites can be prepared as is described in U.S. Pat. No. 5,459,255.
- 3-Deaza purine nucleoside amidites can be prepared as is described in U.S. Pat. No. 5,457,191.
- 5,6-Substituted pyrimidine nucleoside amidites can be prepared as is described in U.S. Pat. No. 5,614,617.
- 5-Propynyl pyrimidine nucleoside amidites can be prepared as is described in U.S. Pat. No. 5,484,908.
- linker means an organic moiety that connects two parts of a compound.
- Linkers typically comprise a direct bond or an atom such as oxygen or sulfur, a unit such as NR 1 , C(O), C(0)NH, SO, S0 2 , S0 2 NH or a chain of atoms, such as substituted or unsubstituted alkyl, substituted or unsubstituted alkenyl, substituted or unsubstituted alkynyl, arylalkyl, arylalkenyl, arylalkynyl, heteroarylalkyl, heteroarylalkenyl, heteroarylalkynyl, heterocyclylalkyl, heterocyclylalkenyl, heterocyclylalkynyl, aryl, heteroaryl, heterocyclyl, cycloalkyl, cycloalkenyl, alkylarylalkyl, alkylarylalkenyl, alkylarylalkynyl, alkenyl
- alkynylarylalkyl alkynylarylalkenyl, alkynylarylalkynyl, alkylheteroarylalkyl, alkylheteroary lalkenyl , alkylheteroarylalkynyl, alkeny lheteroarylalkyl ,
- alkenylheteroarylalkenyl alkenylheteroarylalkynyl, alkynylheteroarylalkyl,
- alkynylheteroarylalkenyl alkynylheteroarylalkynyl, alkylheterocyclylalkyl,
- alkylheterocyclylalkenyl alkylhererocyclylalkynyl, alkenylheterocyclylalkyl,
- alkenylheterocyclylalkenyl alkenylheterocyclylalkynyl, alkynylheterocyclylalkyl, alkynylheterocyclylalkenyl, alkynylheterocyclylalkynyl, alkylaryl, alkenylaryl, alkynylaryl, alkylheteroaryl, alkenylheteroaryl, alkynylhereroaryl, where one or more methylenes can be interrupted or terminated by O, S, S(O), SO 2 , NCR 1 ⁇ , C(O), cleavable linking group, substituted or unsubstituted aryl, substituted or unsubstituted heteroaryl, substituted or unsubstituted heterocyclic; where R 1 is hydrogen, acyl, aliphatic or substituted aliphatic.
- the linker is -[(P-Q-R) q -X-(P'-Q'-R') q '] q "-T-, wherein: P, R, T, P', R' and T are each independently for each occurrence absent, CO, NH, 0, S, OC( a )C(0), -C(0)-CH(R a )-NH-, or heterocyclyl;
- Q and Q' are each independently for each occurrence absent, -(CH 2 ) n -, - C ⁇ XR'XCH ⁇ -, -(CH ⁇ C ⁇ XR 2 )-, -(CH 2 CH 2 0) m CH 2 CH 2 -, or -
- X is absent or a cleavable linking group
- R a is H or an amino acid side chain
- R 1 and R 2 are each independently for each occurrence H, CH 3 , OH, SH or N(R N ) 2 ;
- R is independently for each occurrence H, methyl, ethyl, propyl, isopropyl, butyl or benzyl;
- q, q' and q" are each independently for each occurrence 0-20 and wherein the repeating unit can be the same or different;
- n is independently for each occurrence 1-20;
- n is independently for each occurrence 0-50.
- the linker comprises at least one cleavable linking group.
- the linker is a branched linker.
- the branchpoint of the branched linker may be at least trivalent, but may be a tetravalent, pentavalent or hexavalent atom, or a group presenting such multiple valencies.
- the branchpoint is , -N, -N(Q)-C, -O-C, -S-C, -SS-C, -C(0)N(Q)-C, -OC(0)N(Q)-C, - N(Q)C(0)-C, or -N(Q)C(0)0-C; wherein Q is independently for each occurrence H or optionally substituted alkyl.
- the branchpoint is glycerol or glycerol derivative.
- a cleavable linking group is one which is sufficiently stable outside the cell, but which upon entry into a target cell is cleaved to release the two parts the linker is holding together.
- the cleavable linking group is cleaved at least 10 times or more, preferably at least 100 times faster in the target cell or under a first reference condition (which can, e.g., be selected to mimic or represent intracellular conditions) than in the blood of a subject, or under a second reference condition (which can, e.g., be selected to mimic or represent conditions found in the blood or serum).
- Cleavable linking groups are susceptible to cleavage agents, e.g., pH, redox potential or the presence of degradative molecules.
- cleavage agents are more prevalent or found at higher levels or activities inside cells than in serum or blood.
- degradative agents include: redox agents which are selected for particular substrates or which have no substrate specificity, including, e.g., oxidative or reductive enzymes or reductive agents such as mercaptans, present in cells, that can degrade a redox cleavable linking group by reduction; esterases; endosomes or agents that can create an acidic environment, e.g., those that result in a pH of five or lower; enzymes that can hydrolyze or degrade an acid cleavable linking group by acting as a general acid, peptidases (which can be substrate specific), and phosphatases.
- redox agents which are selected for particular substrates or which have no substrate specificity, including, e.g., oxidative or reductive enzymes or reductive agents such as mercaptans, present in cells, that can degrade a redox cleavable linking group
- a cleavable linkage group such as a disulfide bond can be susceptible to pH.
- the pH of human serum is 7.4, while the average intracellular pH is slightly lower, ranging from about 7.1-7.3. Endosomes have a more acidic pH, in the range of 5.5-6.0, and lysosomes have an even more acidic pH at around 5.0.
- Some linkers will have a cleavable linking group that is cleaved at a preferred pH, thereby releasing the charged lipid from the ligand inside the cell, or into the desired compartment of the cell.
- a linker can include a cleavable linking group that is cleavable by a particular enzyme.
- the type of cleavable linking group incorporated into a linker can depend on the cell to be targeted. For example, liver targeting ligands can be linked to the charged lipids through a linker that includes an ester group. Liver cells are rich in esterases, and therefore the linker will be cleaved more efficiently in liver cells than in cell types that are not esterase-rich. Other cell-types rich in esterases include cells of the lung, renal cortex, and testis.
- Linkers that contain peptide bonds can be used when targeting cell types rich in peptidases, such as liver cells and synoviocytes.
- the suitability of a candidate cleavable linking group can be evaluated by testing the ability of a degradative agent (or condition) to cleave the candidate linking group. It will also be desirable to also test the candidate cleavable linking group for the ability to resist cleavage in the blood or when in contact with other non-target tissue.
- a degradative agent or condition
- the candidate cleavable linking group for the ability to resist cleavage in the blood or when in contact with other non-target tissue.
- the evaluations can be carried out in cell free systems, in cells, in cell culture, in organ or tissue culture, or in whole animals.
- useful candidate compounds are cleaved at least 2, 4, 10 or 100 times faster in the cell (or under in vitro conditions selected to mimic intracellular conditions) as compared to blood or serum (or under in vitro conditions selected to mimic extracellular conditions).
- cleavable linking groups are redox cleavable linking groups that are cleaved upon reduction or oxidation.
- An example of reductively cleavable linking group is a disulphide linking group (-S-S-).
- a candidate cleavable linking group is a suitable "reductively cleavable linking group," or for example is suitable for use with a particular iRNA moiety and particular targeting agent one can look to methods described herein.
- a candidate can be evaluated by incubation with dithiothreitol (DTT), or other reducing agent using reagents know in the art, which mimic the rate of cleavage which would be observed in a cell, e.g., a target cell.
- the candidates can also be evaluated under conditions which are selected to mimic blood or serum conditions.
- candidate compounds are cleaved by at most 10% in the blood,
- useful candidate compounds are degraded at least 2, 4, 10 or 100 times faster in the cell (or under in vitro conditions selected to mimic intracellular conditions) as compared to blood (or under in vitro conditions selected to mimic extracellular conditions).
- the rate of cleavage of candidate compounds can be determined using standard enzyme kinetics assays under conditions chosen to mimic intracellular media and compared to conditions chosen to mimic extracellular media.
- Phosphate-based cleavable linking groups are cleaved by agents that degrade or hydrolyze the phosphate group.
- An example of an agent that cleaves phosphate groups in cells are enzymes such as phosphatases in cells.
- Examples of phosphate-based linking groups are -0-P(0)(ORk)-0-, -0-P(S)(ORk)-0-, -0-P(S)(SRk)-0-, -S-P(0)(ORk)-0-, -
- Preferred embodiments are -0-P(0)(OH)-0-, -0-P(S)(OH)-0-, -0-P(S)(SH)-0-, -S- P(0)(OH)-0-, -0-P(0)(OH)-S-, -S-P(0)(OH)-S-, -0-P(S)(OH)-S-, -S-P(S)(OH)-0-, -0- ⁇ (0)( ⁇ )-0-, -0-P(S)(H)-0-, -S-P(0)(H)-0-, -S-P(S)(H)-0-, -S-P(0)(H)-S-, -0-P(S)(H)- S-.
- a preferred embodiment is -0-P(0)(OH)-0-.
- Acid cleavable linking groups are linking groups that are cleaved under acidic conditions.
- acid cleavable linking groups are cleaved in an acidic environment with a pH of about 6.5 or lower (e.g., about 6.0, 5.5, 5,0, or lower), or by agents such as enzymes that can act as a general acid.
- specific low pH organelles such as endosomes and lysosomes can provide a cleaving environment for acid cleavable linking groups.
- acid cleavable linking groups include but are not limited to hydrazones, esters, and esters of amino acids.
- a preferred embodiment is when the carbon attached to the oxygen of the ester (the alkoxy group) is an aryl group, substituted alkyl group, or tertiary alkyl group such as dimethyl pentyl or t-butyl.
- Ester-based cleavable linking groups are cleaved by enzymes such as esterases and amidases in cells.
- Examples of ester-based cleavable linking groups include but are not limited to esters of alkylene, alkenylene and alkynylene groups.
- Ester cleavable linking groups have the general formula -C(0)0-, or -OC(O)-. These candidates can be evaluated using methods analogous to those described above.
- Peptide-based cleavable linking groups are cleaved by enzymes such as peptidases and proteases in cells.
- Peptide-based cleavable linking groups are peptide bonds formed between amino acids to yield oligopeptides (e.g., dipeptides, tripeptides etc.) and polypeptides.
- Peptide-based cleavable groups do not include the amide group (-C(O)NH- ).
- the amide group can be formed between any alkylene, alkenylene or alkynelene.
- a peptide bond is a special type of amide bond formed between amino acids to yield peptides and proteins.
- the peptide based cleavage group is generally limited to the peptide bond (i.e., the amide bond) formed between amino acids yielding peptides and proteins and does not include the entire amide functional group.
- Peptide -based cleavable linking groups have the general formula - NHCHR A C(0)NHCHR B C(0)-, where R A and R are the R groups of the two adjacent amino acids. These candidates can be evaluated using methods analogous to those described above.
- moieties are ligands, which are coupled, preferably covalently, either directly or indirectly via an intervening tether.
- a ligand alters the distribution, targeting or lifetime of the molecule into which it is incorporated.
- a ligand provides an enhanced affinity for a selected target, e.g. , molecule, cell or cell type, compartment, e.g. , a cellular or organ compartment, tissue, organ or region of the body, as, e.g., compared to a species absent such a ligand,
- Ligands providing enhanced affinity for a selected target are also termed targeting ligands.
- Preferred ligands for conjugation to the lipids of the present invention are targeting ligands.
- Some ligands can have endosomolytic properties.
- the endosomolytic ligands promote the lysis of the endosome and/or transport of the composition of the invention, or its components, from the endosome to the cytoplasm of the cell.
- the endosomolytic ligand may be a polyanionic peptide or peptidomimetic which shows pH-dependent membrane activity and fusogenicity.
- the endosomolytic ligand assumes its active conformation at endosomal pH.
- the "active" conformation is that conformation in which the endosomolytic ligand promotes lysis of the endosome and/or transport of the composition of the invention, or its components, from the endosome to the cytoplasm of the cell.
- Exemplary endosomolytic ligands include the GALA peptide (Subbarao et al., Biochemistry, 1987, 26: 2964-2972), the EALA peptide (Vogel et al dislike J. Am. Chem. Soc, 1996, 118: 1581-1586), and their derivatives (Turk et al., Biochem. Biophys. Acta, 2002, 1559: 56-68).
- the endosomolytic component may contain a chemical group (e.g., an amino acid) which will undergo a change in charge or protonation in response to a change in pH.
- the endosomolytic component may be linear or branched. Exemplary primary sequences of peptide based endosomolytic ligands are shown in Table 4. Table 4: List of peptides with endosomolytic activity.
- Preferred ligands can improve transport, hybridization, and specificity properties and may also improve nuclease resistance of the resultant natural or modified
- oligoribonucleotide or a polymeric molecule comprising any combination of monomers described herein and/or natural or modified ribonucleotides.
- Ligands in general can include therapeutic modifiers, e.g., for enhancing uptake; diagnostic compounds or reporter groups e.g., for monitoring distribution; cross-linking agents; and nuclease-resistance conferring moieties.
- therapeutic modifiers e.g., for enhancing uptake
- diagnostic compounds or reporter groups e.g., for monitoring distribution
- cross-linking agents e.g., for monitoring distribution
- nuclease-resistance conferring moieties lipids, steroids, vitamins, sugars, proteins, peptides, polyamines, and peptide mimics.
- Ligands can include a naturally occurring substance, such as a protein (e.g., human serum albumin (HSA), low-density lipoprotein (LDL), high-density lipoprotein (HDL), or globulin); an carbohydrate (e.g., a dextran, pullulan, chitin, chitosan, inulin, cyclodextrin or hyaluronic acid); or a lipid.
- the ligand may also be a recombinant or synthetic molecule, such as a synthetic polymer, e.g., a synthetic polyamino acid, an oligonucleotide (e.g. an aptamer).
- polyamino acids examples include polyamino acid is a poly lysine (PLL), poly L-aspartic acid, poly L- glutamic acid, styrene-maleic acid anhydride copolymer, poly(L-lactide-co-glycolied) copolymer, divinyl ether-maleic anhydride copolymer, N-(2-hydroxypropyl)methacrylamide copolymer (HMPA), polyethylene glycol (PEG), polyvinyl alcohol (PVA), polyurethane, poly(2-ethylacryllic acid), N-isopropylacrylamide polymers, or polyphosphazine.
- PLL poly lysine
- poly L-aspartic acid poly L- glutamic acid
- styrene-maleic acid anhydride copolymer poly(L-lactide-co-glycolied) copolymer
- divinyl ether-maleic anhydride copolymer divinyl
- polyamines include: polyethylenimine, polylysine (PLL), spermine, spermidine, polyamine, pseudopeptide-polyamine, peptidomimetic polyamine, dendrimer polyamine, arginine, amidine, protamine, charged lipid, cationic porphyrin, quaternary salt of a polyamine, or an alpha helical peptide.
- Ligands can also include targeting groups, e.g., a cell or tissue targeting agent, e.g., a lectin, glycoprotein, lipid or protein, e.g., an antibody, that binds to a specified cell type such as a kidney cell.
- a cell or tissue targeting agent e.g., a lectin, glycoprotein, lipid or protein, e.g., an antibody, that binds to a specified cell type such as a kidney cell.
- a targeting group can be a thyrotropin, melanotropin, lectin, glycoprotein, surfactant protein A, Mucin carbohydrate, multivalent lactose, multivalent galactose, N-acetyl-galactosamine, N-acetyl-gulucosamine multivalent mannose, multivalent fucose, glycosylated polyaminoacids, multivalent galactose, transferrin, bisphosphonate, polyglutamate, polyaspartate, a lipid, cholesterol, a steroid, bile acid, folate, vitamin B 12, biotin, an RGD peptide, an RGD peptide mimetic or an aptamer.
- Table 5 shows some examples of targeting ligands and their associated receptors.
- ligands include dyes, intercalating agents (e.g. acridines), cross-linkers (e.g. psoralene, mitomycin C), porphyrins (TPPC4, texaphyrin, Sapphyrin), polycyclic aromatic hydrocarbons (e.g., phenazine, dihydrophenazine), artificial endonucleases (e.g.
- intercalating agents e.g. acridines
- cross-linkers e.g. psoralene, mitomycin C
- porphyrins TPPC4, texaphyrin, Sapphyrin
- polycyclic aromatic hydrocarbons e.g., phenazine, dihydrophenazine
- artificial endonucleases e.g.
- EDTA lipophilic molecules, e.g, cholesterol, cholic acid, adamantane acetic acid, 1-pyrene butyric acid, dihydrotestosterone, 1,3-Bis- 0(hexadecyl)glycerol, geranyloxyhexyl group, hexadecylglycerol, borneol, menthol, 1,3- propanediol, heptadecyl group, palmitic acid, myristic acid,03-(oleoyl)lithocholic acid, 03-(oleoyl)cholenic acid, dimethoxytrityl, or phenoxazine)and peptide conjugates (e.g., antennapedia peptide, Tat peptide), alkylating agents, phosphate, amino, mercapto, PEG (e.g., PEG-40K), MPEG, [MPEG] 2 , polyamino, alkyl, substituted alky
- biotin e.g., aspirin, vitamin E, folic acid
- transport/absorption facilitators e.g., aspirin, vitamin E, folic acid
- synthetic ribonucleases e.g., imidazole, bisimidazole, histamine, imidazole clusters, acridine-imidazole conjugates, Eu3+ complexes of
- Ligands can be proteins, e.g., glycoproteins, or peptides, e.g., molecules having a specific affinity for a co-ligand, or antibodies e.g., an antibody, that binds to a specified cell type such as a cancer cell, endothelial cell, or bone cell. Ligands may also include hormones and hormone receptors.
- the ligand can be, for example, a lipopolysaccharide, an activator of p38 MAP kinase, or an activator of NF-KB.
- the ligand can be a substance, e.g, a drug, which can increase the uptake of the iRNA agent into the cell, for example, by disrupting the cell's cytoskeleton, e.g., by disrupting the cell's microtubules, microfilaments, and/or intermediate filaments.
- the drug can be, for example, taxon, vincristine, vinblastine, cytochalasin, nocodazole, japlakinolide, latrunculin A, phalloidin, swinholide A, indanocine, or myoservin.
- the ligand can increase the uptake of the iRNA agent into the cell by activating an inflammatory response, for example.
- exemplary ligands that would have such an effect include tumor necrosis factor alpha (TNFalpha), interleukin-1 beta, or gamma interferon.
- the ligand is a lipid or lipid-based molecule.
- a lipid or lipid-based molecule preferably binds a serum protein, e.g., human serum albumin (HSA).
- HSA binding ligand allows for distribution of the conjugate to a target tissue, e.g., a non- kidney target tissue of the body,
- the target tissue can be the liver, including parenchymal cells of the liver.
- Other molecules that can bind HSA can also be used as ligands. For example, neproxin or aspirin can be used.
- a lipid or lipid-based ligand can (a) increase resistance to degradation of the conjugate, (b) increase targeting or transport into a target cell or cell membrane, and/or (c) can be used to adjust binding to a serum protein, e.g., HSA.
- a serum protein e.g., HSA.
- a lipid based ligand can be used to modulate, e.g., control the binding of the conjugate to a target tissue.
- a lipid or lipid-based ligand that binds to HSA more strongly will be less likely to be targeted to the kidney and therefore less likely to be cleared from the body.
- a lipid or lipid-based ligand that binds to HSA less strongly can be used to target the conjugate to the kidney.
- the lipid based ligand binds HSA.
- it binds HSA with a sufficient affinity such that the conjugate will be preferably distributed to a non-kidney tissue.
- the affinity it is preferred that the affinity not be so strong that the HSA-ligand binding cannot be reversed.
- the lipid based ligand binds HSA weakly or not at all, such that the conjugate will be preferably distributed to the kidney.
- Other moieties that target to kidney cells can also be used in place of or in addition to the lipid based ligand.
- the ligand is a moiety, e.g., a vitamin, which is taken up by a target cell, e.g., a proliferating cell.
- a target cell e.g., a proliferating cell.
- vitamins include vitamin A, E, and K.
- B vitamin e.g., folic acid, B12, riboflavin, biotin, pyridoxal or other vitamins or nutrients taken up by cancer cells.
- HAS low density lipoprotein
- HDL high-density lipoprotein
- the ligand is a cell-permeation agent, preferably a helical cell- permeation agent.
- the agent is amphipathic.
- An exemplary agent is a peptide such as tat or antennopedia. If the agent is a peptide, it can be modified, including a pep tidy lmimetic, invertomers, non-peptide or pseudo-peptide linkages, and use of D- amino acids.
- the helical agent is preferably an alpha-helical agent, which preferably has a lipophilic and a lipophobic phase.
- the ligand can be a peptide or peptidomimetic.
- a peptidomimetic also referred to herein as an oligopeptidomimetic is a molecule capable of folding into a defined three- dimensional structure similar to a natural peptide.
- the peptide or peptidomimetic moiety can be about 5-50 amino acids long, e.g., about 5, 10, 15, 20, 25, 30, 35, 40, 45, or 50 amino acids long (see Table 6, for example).
- a peptide or peptidomimetic can be, for example, a cell permeation peptide, cationic peptide, amphipathic peptide, or hydrophobic peptide ⁇ e.g., consisting primarily of Tyr, Trp or Phe).
- the peptide moiety can be a dendrimer peptide, constrained peptide or crosslinked peptide.
- the peptide moiety can include a hydrophobic membrane translocation sequence (MTS).
- An exemplary hydrophobic MTS-containing peptide is RFGF having the amino acid sequence
- RFGF analogue ⁇ e.g., amino acid sequence
- AALLPVLLAAP containing a hydrophobic MTS can also be a targeting moiety.
- the peptide moiety can be a "delivery" peptide, which can carry large polar molecules including peptides, oligonucleotides, and protein across cell membranes.
- sequences from the HIV Tat protein (GRKKRRQRRRPPQ) and the Drosophila Antennapedia protein (RQIKIWFQNRRMKWKK) have been found to be capable of functioning as delivery peptides.
- a peptide or peptidomimetic can be encoded by a random sequence of DNA, such as a peptide identified from a phage-display library, or one -bead-one-compound (OBOC) combinatorial library (Lam et al, Nature, 354:82-84, 1991).
- OBOC -bead-one-compound
- the peptide or peptidomimetic tethered to an iRNA agent via an incorporated monomer unit is a cell targeting peptide such as an arginine-glycine-aspartic acid (RGD) -peptide, or RGD mimic.
- RGD arginine-glycine-aspartic acid
- a peptide moiety can range in length from about 5 amino acids to about 40 amino acids.
- the peptide moieties can have a structural modification, such as to increase stability or direct conformational properties. Any of the structural modifications described below can be utilized.
- An RGD peptide moiety can be used to target a tumor cell, such as an endothelial tumor cell or a breast cancer tumor cell (Zitzmann et al., Cancer Res., 62:5139-43, 2002).
- An RGD peptide can facilitate targeting of an iRNA agent to tumors of a variety of other tissues, including the lung, kidney, spleen, or liver (Aoki et al., Cancer Gene Therapy 8:783-787, 2001).
- the RGD peptide will facilitate targeting of an iRNA agent to the kidney.
- the RGD peptide can be linear or cyclic, and can be modified, e.g., glycosylated or methylated to facilitate targeting to specific tissues.
- a glycosylated RGD peptide can deliver an iRNA agent to a tumor cell expressing ⁇ 3 (Haubner et al, Jour. Nucl. Med., 42:326-336, 2001).
- RGD containing peptides and pep tidomime tics can target cancer cells, in particular cells that exhibit an ⁇ 3 integrin.
- RGD one can use other moieties that target the ⁇ 3 integrin ligand.
- such ligands can be used to control proliferating cells and angiogeneis.
- a "cell permeation peptide” is capable of permeating a cell, e.g., a microbial cell, such as a bacterial or fungal cell, or a mammalian cell, such as a human cell.
- a microbial cell-permeating peptide can be, for example, an a-helical linear peptide (e.g., LL-37 or Ceropin PI), a disulfide bond-containing peptide ⁇ e.g., a -defensin, ⁇ -defensin or bactenecin), or a peptide containing only one or two dominating amino acids (e.g., PR-39 or indolicidin).
- a cell permeation peptide can also include a nuclear localization signal (NLS).
- NLS nuclear localization signal
- a cell permeation peptide can be a bipartite amphipathic peptide, such as MPG, which is derived from the fusion peptide domain of HIV-1 gp41 and the NLS of SV40 large T antigen (Simeoni et al., Nucl. Acids Res. 31:2717-2724, 2003).
- a targeting peptide tethered to an iRNA agent and/or the carrier oligomer can be an amphipathic a-helical peptide.
- exemplary amphipathic a- helical peptides include, but are not limited to, cecropins, lycotoxins, paradaxins, buforin, CPF, bombinin-like peptide (BLP), cathelicidins, ceratotoxins, S.
- clava peptides hagfish intestinal antimicrobial peptides (HFIAPs), magainines, brevinins-2, dermaseptins, melittins, pleurocidin, 3 ⁇ 4A peptides, Xenopus peptides, esculentinis-1, and caerins.
- HFIAPs hagfish intestinal antimicrobial peptides
- magainines brevinins-2
- dermaseptins melittins
- pleurocidin 3 ⁇ 4A peptides
- Xenopus peptides esculentinis-1, and caerins.
- a number of factors will preferably be considered to maintain the integrity of helix stability. For example, a maximum number of helix stabilization residues will be utilized (e.g., leu, ala, or lys), and a minimum number helix destabilization residues will be utilized (e.g., proline, or cyclic monomeric units.
- the capping residue will be considered (for example Gly is an exemplary N-capping residue and/or C-terminal amidation can be used to provide an extra H-bond to stabilize the helix.
- Formation of salt bridges between residues with opposite charges, separated by i + 3, or i ⁇ 4 positions can provide stability.
- cationic residues such as lysine, arginine, homo-arginine, ornithine or histidine can form salt bridges with the anionic residues glutamate or aspartate.
- Peptide and peptidomimetic ligands include those having naturally occurring or modified peptides, e.g., D or L peptides; ⁇ , ⁇ , or ⁇ peptides; N-methyl peptides;
- azapeptides peptides having one or more amide, i.e., peptide, linkages replaced with one or more urea, thiourea, carbamate, or sulfonyl urea linkages; or cyclic peptides.
- the targeting ligand can be any ligand that is capable of targeting a specific receptor. Examples are: folate, GalNAc, galactose, mannose, mannose-6P, clusters of sugars such as GalNAc cluster, mannose cluster, galactose cluster, or an apatamer. A cluster is a combination of two or more sugar units.
- the targeting ligands also include integrin receptor ligands, Chemokine receptor ligands, transferrin, biotin, serotonin receptor ligands, PSMA, endothelin, GCPII, somatostatin, LDL and HDL ligands.
- the ligands can also be based on nucleic acid, e.g., an aptamer.
- the aptamer can be unmodified or have any combination of modifications disclosed herein.
- Endosomal release agents include imidazoles, poly or oligoimidazoles, PEIs, peptides, fusogenic peptides, polycaboxylates, polyacations, masked oligo or poly cations or anions, acetals, polyacetals, ketals/polyketyals, orthoesters, polymers with masked or unmasked cationic or anionic charges, dendrimers with masked or unmasked cationic or anionic charges.
- PK modulator stands for pharmacokinetic modulator.
- PK modulator include lipophiles, bile acids, steroids, phospholipid analogues, peptides, protein binding agents, PEG, vitamins etc.
- Examplary PK modulator include, but are not limited to, cholesterol, fatty acids, cholic acid, lithocholic acid, dialkylglycerides, diacylglyceride, phospholipids, sphingolipids, naproxen, ibuprofen, vitamin E, biotin etc.
- Oligonucleotides that comprise a number of phosphorothioate linkages are also known to bind to serum protein, thus short oligonucleotides, e.g.
- oligonucleotides of about 5 bases, 10 bases, 15 bases or 20 bases, comprising multiple of phosphorothioate linkages in the backbaone are also amenable to the present invention as ligands (e.g. as PK modulating ligands).
- aptamers that bind serum components are also amenable to the present invention as PK modulating ligands.
- the ligands can all have same properties, all have different properties or some ligands have the same properties while others have different properties.
- a ligand can have targeting properties, have endosomolytic activity or have PK modulating properties.
- all the ligands have different properties.
- Ligands can be coupled to the oligonucleotides various places, for example, 3'- end, 5'-end, and/or at an internal position.
- the ligand is attached to the oligonucleotides via an intervening tether.
- the ligand or tethered ligand may be present on a monomer when said monomer is incorporated into the growing strand.
- the ligand may be incorporated via coupling to a
- a monomer having, e.g., an amino-terminated tether (i.e., having no associated ligand), e.g., ⁇ -(03 ⁇ 4) ⁇ ⁇ 2 may be incorporated into a growing sense or antisense strand.
- a ligand having an electrophilic group e.g., a pentafluorophenyl ester or aldehyde group
- a ligand having an electrophilic group can subsequently be attached to the precursor monomer by coupling the electrophilic group of the ligand with the terminal nucleophilic group of the precursor monomer's tether.
- ligands can be attached to one or both strands.
- a double-stranded iRNA agent contains a ligand conjugated to the sense strand.
- a double-stranded iRNA agent contains a ligand conjugated to the antisense strand.
- lignad can be conjugated to nucleobases, sugar moieties, or internucleosidic linkages of nucleic acid molecules. Conjugation to purine nucleobases or derivatives thereof can occur at any position including, endocyclic and exocyclic atoms.
- the 2-, 6-, 7-, or 8-positions of a purine nucleobase are attached to a conjugate moiety. Conjugation to pyrimidine nucleobases or derivatives thereof can also occur at any position. In some embodiments, the 2-, 5-, and 6-positions of a pyrimidine nucleobase can be substituted with a conjugate moiety. Conjugation to sugar moieties of nucleosides can occur at any carbon atom. Example carbon atoms of a sugar moiety that can be attached to a conjugate moiety include the 2', 3', and 5' carbon atoms. The ⁇ position can also be attached to a conjugate moiety, such as in an abasic residue.
- Internucleosidic linkages can also bear conjugate moieties.
- the conjugate moiety can be attached directly to the phosphorus atom or to an 0, N, or S atom bound to the phosphorus atom.
- the conjugate moiety can be attached to the nitrogen atom of the amine or amide or to an adjacent carbon atom.
- an oligomeric compound is attached to a conjugate moiety by contacting a reactive group (e.g., OH, SH, amine, carboxyl, aldehyde, and the like) on the oligomeric compound with a reactive group on the conjugate moiety.
- a reactive group e.g., OH, SH, amine, carboxyl, aldehyde, and the like
- one reactive group is electrophilic and the other is nucleophilic.
- an electrophilic group can be a carbonyl-containing functionality and a nucleophilic group can be an amine or thiol.
- Methods for conjugation of nucleic acids and related oligomeric compounds with and without linking groups are well described in the literature such as, for example, in Manoharan in Antisense Research and Applications, Crooke and LeBleu, eds., CRC Press, Boca Raton, Fla., 1993, Chapter 17, which is incorporated herein by reference in its entirety.
- oligonucleotide conjugates include, but are not limited to, U.S. Pat. Nos. 4,828,979; 4,948,882; 5,218, 105; 5,525,465; 5,541,313; 5,545,730; 5,552,538; 5,578, 717, 5,580,731; 5,580,731 ; 5,591,584; 5,109,124; 5,118, 802; 5,138,045; 5,414,077; 5,486,603; 5,512,439; 5,578, 718; 5,608,046; 4,587,044; 4,605,735; 4,667,025; 4,762, 779; 4,789,737; 4,824,941; 4,835,263; 4,876,335; 4,904, 582; 4,958,013; 5,082,830; 5,112,963; 5,214,136; 5,082, 830; 5,112,963; 5,149,782; 5,214,136;
- the present invention relates to methods and
- compositions for producing lipid-encapsulated nucleic acid particles in which nucleic acids are encapsulated within a lipid layer are compositions for producing lipid-encapsulated nucleic acid particles in which nucleic acids are encapsulated within a lipid layer.
- nucleic acid-lipid particles Such nucleic acid-lipid particles
- incorporating siRNA oligonucleotides are characterized using a variety of biophysical parameters including: (l)drug to lipid ratio; (2) encapsulation efficiency; and (3) particle size.
- High drug to lipid rations, high encapsulation efficiency, good nuclease resistance and serum stability and controllable particle size, generally less than 200 nm in diameter are desirable.
- the nature of the nucleic acid polymer is of significance, since the modification of nucleic acids in an effort to impart nuclease resistance adds to the cost of therapeutics while in many cases providing only limited resistance. Unless stated otherwise, these criteria are calculated in this specification as follows:
- Nucleic acid to lipid ratio is the amount of nucleic acid in a defined volume of preparation divided by the amount of lipid in the same volume. This may be on a mole per mole basis or on a weight per weight basis, or on a weight per mole basis.
- the nucleic acid:lipid ratio is calculated after dialysis, chromatography and/or enzyme (e.g., nuclease) digestion has been employed to remove as much of the external nucleic acid as possible;
- Encapsulation efficiency refers to the drug to lipid ratio of the starting mixture divided by the drug to lipid ratio of the final, administration competent formulation. This is a measure of relative efficiency. For a measure of absolute efficiency, the total amount of nucleic acid added to the starting mixture that ends up in the administration competent formulation, can also be calculated. The amount of lipid lost during the formulation process may also be calculated. Efficiency is a measure of the wastage and expense of the formulation; and Size indicates the size (diameter) of the particles formed. Size distribution may be determined using quasi-elastic light scattering (QELS) on a Nicomp Model 370 sub- micron particle sizer. Particles under 200 nm are preferred for distribution to neo- vascularized (leaky) tissues, such as neoplasms and sites of inflammation.
- QELS quasi-elastic light scattering
- the lipid particles of present invention may beformulated as a pharmaceutical composition, e.g., which further comprises a pharmaceutically acceptable diluent, excipient, or carrier, such as physiological saline or phosphate buffer, selected in accordance with the route of administration and standard pharmaceutical practice.
- a pharmaceutically acceptable diluent, excipient, or carrier such as physiological saline or phosphate buffer, selected in accordance with the route of administration and standard pharmaceutical practice.
- compositions comprising the lipid- nucleic acid particles of the invention are prepared according to standard techniques and further comprise a pharmaceutically acceptable carrier.
- a pharmaceutically acceptable carrier e.g., normal saline will be employed as the pharmaceutically acceptable carrier.
- suitable carriers include, e.g., water, buffered water, 0.9% saline, 0.3% glycine, and the like, including glycoproteins for enhanced stability, such as albumin, lipoprotein, globulin, etc.
- the carrier is preferably added following lipid particle formation.
- the compositions can be diluted into pharmaceutically acceptable carriers such as normal saline.
- the resulting pharmaceutical preparations may be sterilized by conventional, well known sterilization techniques.
- the aqueous solutions can then be packaged for use or filtered under aseptic conditions and lyophilized, the lyophilized preparation being combined with a sterile aqueous solution prior to administration.
- the compositions may contain pharmaceutically acceptable auxiliary substances as required to approximate physiological conditions, such as pH adjusting and buffering agents, tonicity adjusting agents and the like, for example, sodium acetate, sodium lactate, sodium chloride, potassium chloride, calcium chloride, etc.
- the lipidic suspension may include lipid-protective agents which protect lipids against free-radical and lipid- peroxidative damages on storage. Lipophilic free-radical quenchers, such as a-tocopherol and water-soluble iron-specific chelators, such as ferrioxamine, are suitable.
- compositions can vary widely, i.e. , from less than about 0.01%, usually at or at least about 0.05-5% to as much as 10 to 30% by weight and will be selected primarily by fluid volumes, viscosities, etc., in accordance with the particular mode of administration selected.
- concentration may be increased to lower the fluid load associated with treatment. This may be particularly desirable in patients having atherosclerosis-associated congestive heart failure or severe hypertension.
- complexes composed of irritating lipids may be diluted to low concentrations to lessen inflammation at the site of administration.
- the nucleic acid will have an attached label and will be used for diagnosis (by indicating the presence of complementary nucleic acid).
- the amount of complexes administered will depend upon the particular label used, the disease state being diagnosed and the judgement of the clinician but will generally be between about 0.01 and about 50 mg per kilogram of body weight, preferably between about 0.1 and about 5 mg/kg of body weight.
- the lipid-therapeutic agent e.g., nucleic acid
- the lipid-therapeutic agent may include polyethylene glycol (PEG)-modified phospholipids, PEG- ceramide, or ganglioside GMI -modified lipids or other lipids effective to prevent or limit aggregation. Addition of such components does not merely prevent complex aggregation. Rather, it may also provide a means for increasing circulation lifetime and increasing the delivery of the lipid-nucleic acid composition to the target tissues.
- PEG polyethylene glycol
- PEG-ceramide polyethylene glycol
- ganglioside GMI -modified lipids or other lipids effective to prevent or limit aggregation additive of such components does not merely prevent complex aggregation. Rather, it may also provide a means for increasing circulation lifetime and increasing the delivery of the lipid-nucleic acid composition to the target tissues.
- the present invention also provides lipid-therapeutic agent compositions in kit form.
- the kit will typically be comprised of a container that is compartmentalized for holding the various elements of the kit.
- the kit will contain the particles or
- compositions of the present invention preferably in dehydrated or concentrated form, with instructions for their rehydration or dilution and administration.
- the particles comprise the active agent, while in other embodiments, they do not.
- the methods and compositions of the invention make use of certain charged lipids, the synthesis, preparation and characterization of which is described below and the accompanying Examples.
- the present invention provides methods of preparing lipid particles, including those associated with a therapeutic agent, e.g. , a nucleic acid.
- a mixture of lipids is combined with a buffered aqueous solution of nucleic acid to produce an intermediate mixture containing nucleic acid encapsulated in lipid particles wherein the encapsulated nucleic acids are present in a nucleic acid/lipid ratio of about 3 wt% to about 25 wt , preferably 5 to 15 wt%.
- the intermediate mixture may optionally be sized to obtain lipid-encapsulated nucleic acid particles wherein the lipid portions are unilamellar vesicles, preferably having a diameter of 30 to 150 nm, more preferably about 40 to 90 nm.
- the pH is then raised to neutralize at least a portion of the surface charges on the lipid-nucleic acid particles, thus providing an at least partially surface-neutralized lipid-encapsulated nucleic acid composition.
- protonatable lipids are amino lipids that are charged at a pH below the pK a of the amino group and substantially neutral at a pH above the pK a .
- These protonatable lipids are termed titratable cationic lipids and can be used in the formulations of the invention using a two-step process.
- lipid vesicles can be formed at the lower pH with titratable cationic lipids and other vesicle components in the presence of nucleic acids. In this manner, the vesicles will encapsulate and entrap the nucleic acids.
- the surface charge of the newly formed vesicles can be neutralized by increasing the pH of the medium to a level above the pK a of the titratable cationic lipids present, i.e. , to physiological pH or higher.
- Particularly advantageous aspects of this process include both the facile removal of any surface adsorbed nucleic acid and a resultant nucleic acid delivery vehicle which has a neutral surface. Liposomes or lipid particles having a neutral surface are expected to avoid rapid clearance from circulation and to avoid certain toxicities which are associated with cationic liposome preparations. Additional details concerning these uses of such titratable cationic lipids in the formulation of nucleic acid-lipid particles are provided in U.S. Patent 6,287,591 and U.S. Patent 6,858,225, incorporated herein by reference.
- the vesicles formed in this manner provide formulations of uniform vesicle size with high content of nucleic acids. Additionally, the vesicles have a size range of from about 30 to about 150 nm, more preferably about 30 to about 90 nm.
- nucleic acid encapsulation is a result of electrostatic interaction at low pH.
- acidic pH e.g. pH 4.0
- the vesicle surface is charged and binds a portion of the nucleic acids through electrostatic interactions.
- a more neutral buffer e.g. pH 7.5
- the surface of the lipid particle or liposome is neutralized, allowing any external nucleic acid to be removed.
- the present invention provides methods of preparing lipid/nucleic acid formulations.
- a mixture of lipids is combined with a buffered aqueous solution of nucleic acid to produce an intermediate mixture containing nucleic acid encapsulated in lipid particles, e.g., wherein the encapsulated nucleic acids are present in a nucleic acid/lipid ratio of about 10 wt% to about 20 wt%.
- the intermediate mixture may optionally be sized to obtain lipid- encapsulated nucleic acid particles wherein the lipid portions are unilamellar vesicles, preferably having a diameter of 30 to 150 nm, more preferably about 40 to 90 nm.
- the pH is then raised to neutralize at least a portion of the surface charges on the lipid-nucleic acid particles, thus providing an at least partially surface-neutralized lipid-encapsulated nucleic acid composition.
- the mixture of lipids includes at least two lipid components: a first lipid component of the present invention that is selected from among lipids which have a pKa such that the lipid is cationic at pH below the pKa and neutral at pH above the pKa, and a second lipid component that is selected from among lipids that prevent particle aggregation during lipid-nucleic acid particle formation.
- the amino lipid is a novel charged lipid of the present invention.
- the mixture of lipids is typically a solution of lipids in an organic solvent.
- This mixture of lipids can then be dried to form a thin film or lyophilized to form a powder before being hydrated with an aqueous buffer to form liposomes.
- the lipid mixture can be solubilized in a water miscible alcohol, such as ethanol, and this ethanolic solution added to an aqueous buffer resulting in spontaneous liposome formation.
- the alcohol is used in the form in which it is commercially available.
- ethanol can be used as absolute ethanol (100%), or as 95% ethanol, the remainder being water. This method is described in more detail in U.S. Patent 5,976,567).
- the mixture of lipids is a mixture of charged lipids, neutral lipids (other than a charged lipid), a sterol (e.g., cholesterol) and a PEG-modified lipid (e.g., a PEG-DMG or PEG-DMA) in an alcohol solvent.
- the lipid mixture consists essentially of a charged lipid, a neutral lipid, cholesterol and a PEG-modified lipid in alcohol, more preferably ethanol.
- the first solution consists of the above lipid mixture in molar ratios of about 20-70% charged lipid: 5-45% neutral lipid:20-55% cholesterol:0.5-15%
- the first solution consists essentially of a lipid chosen from Table 1 or Table 2, DSPC, Choi and PEG-DMG or PEG-DMA, more preferably in a molar ratio of about 20-60% charged lipid: 5-25% DSPC:25-55% Chol:0.5-15% PEG-DMG or PEG-DMA.
- the molar lipid ratio is approximately 40/10/40/10 (mol% charged lipid/DSPC/Chol/PEG- DMG or PEG-DMA), 35/15/40/10 (mol% charged lipid/DSPC/Chol/PEG-DMG or PEG- DMA) or 52/13/30/5 (mol% charged lipid/DSPC/Chol/PEG-DMG or PEG-DMA).
- the neutral lipid in these compositions is replaced with POPC, DPPC, DOPE or SM.
- the lipid mixture is combined with a buffered aqueous solution that may contain the nucleic acids.
- the buffered aqueous solution of is typically a solution in which the buffer has a pH of less than the pK a of the protonatable lipid in the lipid mixture.
- suitable buffers include citrate, phosphate, acetate, and MES.
- a particularly preferred buffer is citrate buffer.
- Preferred buffers will be in the range of 1-1000 mM of the anion, depending on the chemistry of the nucleic acid being encapsulated, and optimization of buffer concentration may be significant to achieving high loading levels (see, e.g., U.S. Patent 6,287,591 and U.S. Patent 6,858,225).
- pure water acidified to pH 5-6 with chloride, sulfate or the like may be useful.
- it may be suitable to add 5% glucose, or another non- ionic solute which will balance the osmotic potential across the particle membrane when the particles are dialyzed to remove ethanol, increase the pH, or mixed with a pharmaceutically acceptable carrier such as normal saline.
- the amount of nucleic acid in buffer can vary, but will typically be from about 0.01 mg/mL to about 200 mg/mL, more preferably from about 0.5 mg/mL to about 50 mg/mL,
- the mixture of lipids and the buffered aqueous solution of therapeutic nucleic acids is combined to provide an intermediate mixture.
- the intermediate mixture is typically a mixture of lipid particles having encapsulated nucleic acids. Additionally, the intermediate mixture may also contain some portion of nucleic acids which are attached to the surface of the lipid particles (liposomes or lipid vesicles) due to the ionic attraction of the negatively-charged nucleic acids and positively-charged lipids on the lipid particle surface (the amino lipids or other lipid making up the protonatable first lipid component are positively charged in a buffer having a pH of less than the pK a of the protonatable group on the lipid).
- the mixture of lipids is an alcohol solution of lipids and the volumes of each of the solutions is adjusted so that upon combination, the resulting alcohol content is from about 20% by volume to about 45% by volume.
- the method of combining the mixtures can include any of a variety of processes, often depending upon the scale of formulation produced. For example, when the total volume is about 10-20 mL or less, the solutions can be combined in a test tube and stirred together using a vortex mixer. Large-scale processes can be carried out in suitable production scale glassware.
- the lipid-encapsulated therapeutic agent e.g., nucleic acid
- the compositions provided herein will be sized to a mean diameter of from about 70 to about 200 nm, more preferably about 90 to about 130 nm.
- Several techniques are available for sizing liposomes to a desired size. One sizing method is described in U.S. Pat. No. 4,737,323, incorporated herein by reference.
- Sonicating a liposome suspension either by bath or probe sonication produces a progressive size reduction down to small unilamellar vesicles (SUVs) less than about 0.05 microns in size.
- Homogenization is another method which relies on shearing energy to fragment large liposomes into smaller ones.
- multilamellar vesicles are recirculated through a standard emulsion homogenizer until selected liposome sizes, typically between about 0.1 and 0.5 microns, are observed.
- the particle size distribution can be monitored by conventional laser-beam particle size determination.
- extrusion is used to obtain a uniform vesicle size
- Extrusion of liposome compositions through a small-pore polycarbonate membrane or an asymmetric ceramic membrane results in a relatively well-defined size distribution.
- the suspension is cycled through the membrane one or more times until the desired liposome complex size distribution is achieved.
- the liposomes may be extruded through successively smaller-pore membranes, to achieve a gradual reduction in liposome size.
- the lipid-nucleic acid compositions which are formed can be used without any sizing.
- methods of the present invention further comprise a step of neutralizing at least some of the surface charges on the lipid portions of the lipid- nucleic acid compositions.
- unencapsulated nucleic acid is freed from the lipid particle surface and can be removed from the composition using conventional techniques.
- unencapsulated and surface adsorbed nucleic acids are removed from the resulting compositions through exchange of buffer solutions.
- buffer solutions For example, replacement of a citrate buffer (pH about 4.0, used for forming the compositions) with a HEPES -buffered saline (HBS pH about 7.5) solution, results in the neutralization of liposome surface and nucleic acid release from the surface.
- the released nucleic acid can then be removed via chromatography using standard methods, and then switched into a buffer with a pH above the pKa of the lipid used.
- the lipid vesicles can be formed by hydration in an aqueous buffer and sized using any of the methods described above prior to addition of the nucleic acid.
- the aqueous buffer should be of a pH below the pKa of the amino lipid.
- a solution of the nucleic acids can then be added to these sized, preformed vesicles.
- the mixture should contain an alcohol, such as ethanol. In the case of ethanol, it should be present at a concentration of about 20% (w/w) to about 45% (w/w).
- nucleic acid encapsulation process it may be necessary to warm the mixture of pre-formed vesicles and nucleic acid in the aqueous buffer-ethanol mixture to a temperature of about 25° C to about 50° C depending on the composition of the lipid vesicles and the nature of the nucleic acid. It will be apparent to one of ordinary skill in the art that optimization of the encapsulation process to achieve a desired level of nucleic acid in the lipid vesicles will require manipulation of variable such as ethanol concentration and temperature. Examples of suitable conditions for nucleic acid encapsulation are provided in the Examples. Once the nucleic acids are encapsulated within the prefromed vesicles, the external pH can be increased to at least partially neutralize the surface charge. Unencapsulated and surface adsorbed nucleic acids can then be removed as described above. Method of Use
- the lipid particles of the present invention may be used to deliver a therapeutic agent to a cell, in vitro or in vivo.
- the therapeutic agent is a nucleic acid, which is delivered to a cell using a nucleic acid- lipid particles of the present invention. While the following description o various methodsof using the lipid particles and related pharmaceutical compositions of the present invention are exemplified by description related to nucleic acid- lipid particles, it is understood that these methods and compositions may be readily adapted for the delivery of any therapeutic agent for the treatment of any disease or disorder that would benefit from such treatment.
- the present invention provides methods for introducing a nucleic acid into a cell.
- Preferred nucleic acids for introduction into cells are siRNA, immune-stimulating oligonucleotides, plasmids, antisense and ribozymes. These methods may be carried out by contacting the particles or compositions of the present invention with the cells for a period of time sufficient for intracellular delivery to occur.
- compositions of the present invention can be adsorbed to almost any cell type.
- the nucleic acid- lipid particles can either be endocytosed by a portion of the cells, exchange lipids with cell membranes, or fuse with the cells. Transfer or incorporation of the nucleic acid portion of the complex can take place via any one of these pathways. Without intending to be limited with respect to the scope of the invention, it is believed that in the case of particles taken up into the cell by endocytosis the particles then interact with the endosomal membrane, resulting in destabilization of the endosomal membrane, possibly by the formation of non-bilayer phases, resulting in introduction of the encapsulated nucleic acid into the cell cytoplasm.
- the liposome membrane is integrated into the cell membrane and the contents of the liposome combine with the intracellular fluid.
- Contact between the cells and the lipid-nucleic acid compositions when carried out in vitro, will take place in a biologically compatible medium.
- concentration of compositions can vary widely depending on the particular application, but is generally between about 1 ⁇ and about 10 mmol.
- treatment of the cells with the lipid-nucleic acid compositions will generally be carried out at physiological temperatures (about 37°C) for periods of time from about 1 to 24 hours, preferably from about 2 to 8 hours.
- the delivery of nucleic acids can be to any cell grown in culture, whether of plant or animal origin, vertebrate or invertebrate, and of any tissue or type.
- the cells will be animal cells, more preferably mammalian cells, and most preferably human cells.
- lipid- nucleic acid particle suspension is added to
- the lipid particles of the invention can be may be used to deliver a nucleic acid to a cell or cell line (for example, a tumor cell line).
- a cell or cell line for example, a tumor cell line.
- cell lines include: HELA (ATCC Cat N: CCL-2), KB (ATCC Cat N: CCL-17), HEP3B (ATCC Cat N: HB-8064), SKOV-3 (ATCC Cat N: HTB-77), HCT-116 (ATCC Cat N: CCL-247), HT-29 (ATCC Cat N: HTB-38), PC-3 (ATCC Cat N: CRL- 1435), A549 (ATCC Cat N: CCL-185), MDA-MB-231 (ATCC Cat N: HTB-26).
- Typical applications include using well known procedures to provide intracellular delivery of siRNA to knock down or silence specific cellular targets.
- Alternatively applications include delivery of DNA or mRNA sequences that code for therapeutically useful polypeptides.
- therapy is provided for genetic diseases by supplying deficient or absent gene products (i.e., for Duchenne's dystrophy, see Kunkel, et al, Brit. Med. Bull. 45(3):630-643 (1989), and for cystic fibrosis, see Goodfellow, Nature
- compositions of the present invention include introduction of antisense oligonucleotides in cells (see, Bennett, et al, Mol. Pharm. 41: 1023-1033 (1992)).
- compositions of the present invention can also be used for deliver of nucleic acids to cells in vivo, using methods which are known to those of skill in the art.
- delivery of DNA or mRNA sequences Zhu, et al, Science 261 :209-211 (1993), incorporated herein by reference, describes the intravenous delivery of cytomegalovirus (CMV)-chloramphenicol acetyltransferase (CAT) expression plasmid using DOTMA-DOPE complexes.
- CMV cytomegalovirus
- CAT chloramphenicol acetyltransferase
- CTR cystic fibrosis transmembrane conductance regulator
- Brigham, et al, Am. J. Med. Sci. 298:278-281 (1989), incorporated herein by reference describes the in vivo transfection of lungs of mice with a functioning prokaryotic gene encoding the intracellular enzyme, chloramphenicol acetyltransferase (CAT).
- CAT chloramphenicol acetyltransferase
- the pharmaceutical compositions are preferably administered parenterally, i.e., intraarticularly, intravenously, intraperitoneally, subcutaneously, or intramuscularly.
- the pharmaceutical compositions are administered intravenously or intraperitoneally by a bolus injection.
- a bolus injection for one example, see Stadler, et al , U.S. Patent No. 5,286,634, which is incorporated herein by reference. Intracellular nucleic acid delivery has also been discussed in Straubringer, et al, METHODS IN ENZYMOLOGY, Academic Press, New York. 101 :512-527 (1983);
- the pharmaceutical preparations may be contacted with the target tissue by direct application of the preparation to the tissue,
- the application may be made by topical, "open” or “closed” procedures.
- topical it is meant the direct application of the pharmaceutical preparation to a tissue exposed to the environment, such as the skin, oropharynx, external auditory canal, and the like.
- Open procedures are those procedures which include incising the skin of a patient and directly visualizing the underlying tissue to which the pharmaceutical preparations are applied. This is generally accomplished by a surgical procedure, such as a thoracotomy to access the lungs, abdominal laparotomy to access abdominal viscera, or other direct surgical approach to the target tissue.
- “Closed” procedures are invasive procedures in which the internal target tissues are not directly visualized, but accessed via inserting instruments through small wounds in the skin.
- the preparations may be administered to the peritoneum by needle lavage.
- the pharmaceutical preparations may be administered to the meninges or spinal cord by infusion during a lumbar puncture followed by appropriate positioning of the patient as commonly practiced for spinal anesthesia or metrazamide imaging of the spinal cord.
- the preparations may be administered through endoscopic devices.
- the lipid-nucleic acid compositions can also be administered in an aerosol inhaled into the lungs (see, Brigham, et al, Am. J. Sci. 298(4):278-281 (1989)) or by direct injection at the site of disease (Culver, Human Gene Therapy, Mary Ann Liebert, Inc., Publishers, New York, pp.70-71 (1994)).
- the methods of the present invention may be practiced in a variety of hosts.
- Preferred hosts include mammalian species, such as humans, non-human primates, dogs, cats, cattle, horses, sheep, and the like.
- Dosages for the lipid-therapeutic agent particles of the present invention will depend on the ratio of therapeutic agent to lipid and the administrating physician's opinion based on age, weight, and condition of the patient.
- the present invention provides a method of modulating the expression of a target polynucleotide or polypeptide. These methods generally comprise contacting a cell with a lipid particle of the present invention that is associated with a nucleic acid capable of modulating the expression of a target polynucleotide or polypeptide.
- modulating refers to altering the expression of a target polynucleotide or polypeptide. In different embodiments, modulating can mean increasing or enhancing, or it can mean decreasing or reducing.
- Methods of measuring the level of expression of a target polynucleotide or polypeptide include, e.g., methods employing reverse transcription-polymerase chain reaction (RT-PCR) and immunohistochemical techniques.
- RT-PCR reverse transcription-polymerase chain reaction
- the level of expression of a target polynucleotide or polypeptide is increased or reduced by at least 10%, 20%, 30%, 40%, 50%, or greater than 50% as compared to an appropriate control value.
- the nucleic acid may be an expression vector that includes a polynucleotide that encodes the desired polypeptide.
- the nucleic acid may be, e.g., an antisense oligonucleotide, siRNA, or microRNA that comprises a polynucleotide sequence that specifically hybridizes to a polnucleotide that encodes the target polypeptide, thereby disrupting expression of the target polynucleotide or polypeptide.
- the nucleic acid may be a plasmid that expresses such an antisense oligonucletoide, siRNA, or microRNA.
- the present invention provides a method of modulating the expression of a polypeptide by a cell, comprising providing to a cell a lipid particle that consists of or consists essentially of a lipid chosen from Table 1 or Table 2, DSPC, Choi and PEG-DMG or PEG-DMA, e.g., in a molar ratio of about 20- 60% charged lipid: 5-25% DSPC:25-55% Chol:0.5-15% PEG-DMG or PEG-DMA, wherein the lipid particle is assocated with a nucleic acid capable of modulating the expression of the polypeptide.
- the molar lipid ratio is approximately 40/10/40/10 (mol% charged lipid/DSPC/Chol/PEG-DMG or PEG-DMA), 35/15/40/10 (mol% charged lipid/DSPC/Chol/PEG-DMG or PEG-DMA) or 52/13/30/5 (mol% charged lipid/DSPC/Chol/PEG-DMG or PEG-DMA).
- the neutral lipid in these compositions is replaced with POPC, DPPC, DOPE or SM.
- the therapeutic agent is selected from an siRNA, a microRNA, an antisense oligonucleotide, and a plasmid capable of expressing an siRNA, a microRNA, or an antisense oligonucleotide, and wherein the siRNA, microRNA, or antisense RNA comprises a polynucleotide that specifically binds to a polynucleotide that encodes the polypeptide, or a complement thereof, such that the expression of the polypeptide is reduced.
- the nucleic acid is a plasmid that encodes the polypeptide or a functional variant or fragment thereof, such that expression of the polypeptide or the functional variant or fragment thereof is increased.
- the present invention provides a method of treating a disease or disorder characterized by overexpression of a polypeptide in a subject, comprising providing to the subject a pharmaceutical composition of the present invention, wherein the therapeutic agent is selected from an siRNA, a microRNA, an antisense oligonucleotide, and a plasmid capable of expressing an siRNA, a microRNA, or an antisense oligonucleotide, and wherein the siRNA, microRNA, or antisense RNA comprises a polynucleotide that specifically binds to a polynucleotide that encodes the polypeptide, or a complement thereof.
- the therapeutic agent is selected from an siRNA, a microRNA, an antisense oligonucleotide, and a plasmid capable of expressing an siRNA, a microRNA, or an antisense oligonucleotide
- the siRNA, microRNA, or antisense RNA comprises a polynucleotide that specifically
- the pharmaceutical composition comprises a lipid particle that consists of or consists essentially of a lipid chosen from Table 1 or Table 2, DSPC, Choi and PEG-DMG or PEG-DMA, e.g. , in a molar ratio of about 20-60% charged lipid: 5-25% DSPC:25-55% Chol:0.5-15% PEG-DMG or PEG-DMA, wherein the lipid particle is assocated with the therapeutic nucleic acid.
- a lipid particle that consists of or consists essentially of a lipid chosen from Table 1 or Table 2, DSPC, Choi and PEG-DMG or PEG-DMA, e.g. , in a molar ratio of about 20-60% charged lipid: 5-25% DSPC:25-55% Chol:0.5-15% PEG-DMG or PEG-DMA, wherein the lipid particle is assocated with the therapeutic nucleic acid.
- the molar lipid ratio is approximately 40/10/40/10 (mol% charged lipid/DSPC/Chol/PEG-DMG or PEG- DMA), 35/15/40/10 (mol% charged lipid/DSPC/Chol/PEG-DMG or PEG-DMA) or 52/13/30/5 (mol charged lipid/DSPC/Chol/PEG-DMG or PEG-DMA).
- the neutral lipid in these compositions is replaced with POPC, DPPC, DOPE or SM.
- the present invention includes a method of treating a disease or disorder characterized by underexpression of a polypeptide in a subject, comprising providing to the subject a pharmaceutical composition of the present invention, wherein the therapeutic agent is a plasmid that encodes the polypeptide or a functional variant or fragment thereof.
- the pharmaceutical composition comprises a lipid particle that consists of or consists essentially of a lipid chosen from Table 1 or Table 2, DSPC, Choi and PEG-DMG or PEG-DMA, e.g. , in a molar ratio of about 20-60% charged lipid: 5-25% DSPC:25-55% Chol:0.5-15% PEG-DMG or PEG-DMA, wherein the lipid particle is assocated with the therapeutic nucleic acid.
- a lipid particle that consists of or consists essentially of a lipid chosen from Table 1 or Table 2, DSPC, Choi and PEG-DMG or PEG-DMA, e.g. , in a molar ratio of about 20-60% charged lipid: 5-25% DSPC:25-55% Chol:0.5-15% PEG-DMG or PEG-DMA, wherein the lipid particle is assocated with the therapeutic nucleic acid.
- the molar lipid ratio is approximately 40/10/40/10 (mol% charged lipid/DSPC/Chol/PEG-DMG or PEG- DMA), 35/15/40/10 (mol% charged lipid/DSPC/Chol/PEG-DMG or PEG-DMA) or 52/13/30/5 (mol% charged lipid/DSPC/Chol/PEG-DMG or PEG-DMA).
- the neutral lipid in these compositions is replaced with POPC, DPPC, DOPE or SM.
- the present invention further provides a method of inducing an immune response in a subject, comprising providing to the subject the pharmaceutical composition of the present invention, wherein the therapeutic agent is an immunostimulatory
- the immune response is a humoral or mucosal immune response.
- the pharmaceutical composition comprises a lipid particle that consists of or consists essentially of a lipid chosen from Table 1 or Table 2, DSPC, Choi and PEG-DMG or PEG-DMA, e.g. , in a molar ratio of about 20-60% charged lipid: 5-25% DSPC:25-55% Chol:0.5-15% PEG-DMG or PEG-DMA, wherein the lipid particle is assocated with the therapeutic nucleic acid.
- the molar lipid ratio is approximately 40/10/40/10 (mol% charged lipid/DSPC/Chol/PEG-DMG or PEG-DMA), 35/15/40/10 (mol% charged
- the pharmaceutical composition is provided to the subject in combination with a vaccine or antigen.
- the present invention itself provides vaccines comprising a lipid particle of the present invention, which comprises an immunostimulatory oligonucleotide, and is also associated with an antigen to which an immune response is desired.
- the antigen is a tumor antigen or is associated with an infective agent, such as, e.g. , a virus, bacteria, or parasiste.
- antigens suitable for use in the present invention include, but are not limited to, polypeptide antigens and DNA antigens.
- specific examples of antigens are Hepatitis A, Hepatitis B, small pox, polio, anthrax, influenza, typhus, tetanus, measles, rotavirus, diphtheria, pertussis, tuberculosis, and rubella antigens.
- the antigen is a Hepatitis B recombinant antigen.
- the antigen is a Hepatitis A recombinant antigen.
- the antigen is a tumor antigen. Examples of such tumor-associated antigens are MUC-1, EBV antigen and antigens associated with Burkitt's lymphoma.
- the antigen is a tyrosinase-related protein tumor antigen recombinant antigen. Those of skill in the art will know of other antigens suitable for use in the present invention.
- Tumor-associated antigens suitable for use in the subject invention include both mutated and non-mutated molecules that may be indicative of single tumor type, shared among several types of tumors, and/or exclusively expressed or overexpressed in tumor cells in comparison with normal cells.
- tumor-specific patterns of expression of carbohydrates, gangliosides, glycolipids and mucins have also been documented.
- Exemplary tumor-associated antigens for use in the subject cancer vaccines include protein products of oncogenes, tumor suppressor genes and other genes with mutations or rearrangements unique to tumor cells, reactivated embryonic gene products, oncofetal antigens, tissue-specific (but not tumor-specific) differentiation antigens, growth factor receptors, cell surface carbohydrate residues, foreign viral proteins and a number of other self proteins.
- tumor-associated antigens include, e.g., mutated antigens such as the protein products of the Ras p21 protooncogenes, tumor suppressor p53 and BCR-abl oncogenes, as well as CDK4, MUM1, Caspase 8, and Beta catenin;
- overexpressed antigens such as galectin 4, galectin 9, carbonic anhydrase, Aldolase A, PRAME, Her2/neu, ErbB-2 and KSA, oncofetal antigens such as alpha fetoprotein (AFP), human chorionic gonadotropin (hCG); self antigens such as carcinoembryonic antigen (CEA) and melanocyte differentiation antigens such as Mart 1/Melan A, gplOO, gp75, Tyrosinase, TRP1 and TRP2; prostate associated antigens such as PSA, PAP, PSMA, PSM-P1 and PSM-P2; reactivated embryonic gene products such as MAGE 1, MAGE 3, MAGE 4, GAGE 1, GAGE 2, BAGE, RAGE, and other cancer testis antigens such as NY-ESOl, SSX2 and SCP1 ; mucins such as Muc-1 and Muc-2; gangliosides such as GM2, GD2 and
- Pathogens include, but are not limited to, infectious agents, e.g., viruses, that infect mammals, and more particularly humans.
- infectious virus include, but are not limited to: Retroviridae (e.g., human immunodeficiency viruses, such as HIV-1 (also referred to as HTLV-III, LAV or HTLV-III/LAV, or HIV-III; and other isolates, such as HIV-LP; Picornaviridae (e.g., polio viruses, hepatitis A virus; enteroviruses, human Coxsackie viruses, rhinoviruses, echoviruses); Calciviridae (e.g., strains that cause gastroenteritis); Togaviridae (e.g., equine encephalitis viruses, rubella viruses); Flaviridae (e.g., dengue viruses, encephalitis viruses, yellow fever viruses); Coronoviridae (e.g., coronaviruses); Rhabdoviradae (
- Coronaviridae e.g., coronaviruses
- Rhabdoviridae e.g., vesicular stomatitis viruses, rabies viruses
- Filoviridae e.g., ebola viruses
- Paramyxoviridae e.g., parainfluenza viruses, mumps virus, measles virus, respiratory syncytial virus
- Orthomyxoviridae e.g., influenza viruses
- Bungaviridae e.g., Hantaan viruses, bunga viruses, phleboviruses and Nairo viruses
- Arena viridae hemorrhagic fever viruses
- Reoviridae e.g., reoviruses, orbiviurses and rotaviruses
- Birnaviridae Hepadnaviridae (Hepatitis B virus); Parvovirida (parvoviruses); Papovaviridae (pap
- Adenoviridae most adenoviruses
- Poxviridae variola viruses, vaccinia viruses, pox viruses
- Iridoviridae e.g., African swine fever virus
- gram negative and gram positive bacteria serve as antigens in vertebrate animals.
- Such gram positive bacteria include, but are not limited to Pasteurella species, Staphylococci species, and Streptococcus species.
- Gram negative bacteria include, but are not limited to, Escherichia coli, Pseudomonas species, and Salmonella species.
- infectious bacteria include but are not limited to: Helicobacterpyloris, Borelia burgdorferi, Legionella pneumophilia, Mycobacteria sps (e.g., M. tuberculosis, M, avium, M. intracellulare, M. kansaii, M.
- Streptococcus (viridans group), Streptococcusfaecalis, Streptococcus bovis,
- Streptococcus (anaerobic sps.), Streptococcus pneumoniae, pathogenic Campylobacter sp., Enterococcus sp., Haemophilus infuenzae, Bacillus antracis, corynebacterium diphtheriae, corynebacterium sp., Erysipelothrix rhusiopathiae, Clostridium perfringers, Clostridium tetani, Enterobacter aerogenes, Klebsiella pneumoniae, Pasturella multocida, Bacteroides sp., Fusobacterium nucleatum, Streptobacillus moniliformis, Treponema pallidium, Treponema permur, Leptospira, Rickettsia, and Actinomyces israelii.
- infectious fungi examples include, but are not limited to, infectious fungi that infect mammals, and more particularly humans.
- infectious fingi include, but are not limited to: Cryptococcus neoformans, Histoplasma capsulatum, Coccidioides immitis, Blastomyces dermatitidis, Chlamydia trachomatis, Candida albicans.
- infectious parasites include Plasmodium such as Plasmodium falciparum, Plasmodium malariae, Plasmodium ovale, and Plasmodium vivax.
- Other infectious organisms i.e. , protists
- Other infectious organisms include Toxoplasma gondii.
- the formulations of the invention can be used to silence or modulate a target gene such as but not limited to FVII, Eg5, PCSK9, TPX2, apoB, SAA, TTR, RSV, PDGF beta gene, Erb-B gene, Src gene, CRK gene, GRB2 gene, RAS gene, MEKK gene, JNK gene, RAF gene, Erkl/2 gene, PCNA(p21) gene, MYB gene, JUN gene, FOS gene, BCL-2 gene, Cyclin D gene, VEGF gene, EGFR gene, Cyclin A gene, Cyclin E gene, WNT-1 gene, beta-catenin gene, c-MET gene, PKC gene, NFKB gene, STAT3 gene, survivin gene, Her2/Neu gene, SORT1 gene, XBP1 gene, topoisomerase I gene, topoisomerase II alpha gene, p73 gene, p21(WAFl/CIPl) gene, p27(KIPl) gene
- AMLl/ETO fusion gene alpha v-integrin gene, Flt-1 receptor gene, tubulin gene, Human Papilloma Virus gene, a gene required for Human Papilloma Virus replication, Human Immunodeficiency Virus gene, a gene required for Human Immunodeficiency Virus replication, Hepatitis A Virus gene, a gene required for Hepatitis A Virus replication, Hepatitis B Virus gene, a gene required for Hepatitis B Virus replication, Hepatitis C Virus gene, a gene required for Hepatitis C Virus replication, Hepatitis D Virus gene, a gene required for Hepatitis D Virus replication, Hepatitis E Virus gene, a gene required for Hepatitis E Virus replication, Hepatitis F Virus gene, a gene required for Hepatitis F Virus replication, Hepatitis G Virus gene, a gene required for Hepatitis G Virus replication, Hepatitis H Virus gene,
- Cytomegalovirus replication herpes Epstein Barr Virus gene, a gene that is required for herpes Epstein Barr Virus replication, Kaposi's Sarcoma- associated Herpes Virus gene, a gene that is required for Kaposi's Sarcoma-associated Herpes Virus replication, JC Virus gene, human gene that is required for JC Virus replication, myxovirus gene, a gene that is required for myxovirus gene replication, rhinovirus gene, a gene that is required for rhinovirus replication, coronavirus gene, a gene that is required for coronavirus replication, West Nile Virus gene, a gene that is required for West Nile Virus replication, St. Louis Encephalitis gene, a gene that is required for St.
- Tick-borne encephalitis virus gene a gene that is required for Tick-borne encephalitis virus replication, Murray Valley encephalitis virus gene, a gene that is required for Murray Valley encephalitis virus replication, dengue virus gene, a gene that is required for dengue virus gene replication, Simian Virus 40 gene, a gene that is required for Simian Virus 40 replication, Human T Cell Lymphotropic Virus gene, a gene that is required for Human T Cell Lymphotropic Virus replication, Moloney-Murine Leukemia Virus gene, a gene that is required for Moloney-Murine Leukemia Virus replication, encephalomyocarditis virus gene, a gene that is required for encephalomyocarditis virus replication, measles virus gene, a gene that is required for measles virus replication, Vericella zoster virus gene, a gene that is required for Vericella zoster virus replication, adenovirus gene, a gene
- CMBKR5v AIF-1 gene, 1-309 gene, a gene to a component of an ion channel, a gene to a neurotransmitter receptor, a gene to a neurotransmitter ligand, amyloid-family gene, presenilin gene, HD gene, DRPLA gene, SCA1 gene, SCA2 gene, MJD1 gene,
- CACNL1A4 gene SCA7 gene, SCA8 gene, allele gene found in LOH cells, or one allele gene of a polymorphic gene.
- Alkyl means a straight chain or branched, noncyclic or cyclic, saturated aliphatic hydrocarbon containing from 1 to 24 carbon atoms.
- Representative saturated straight chain alkyls include methyl, ethyl, n-propyl, n-butyl, n-pentyl, n-hexyl, and the like; while saturated branched alkyls include isopropyl, sec-butyl, isobutyl, tert-butyl, isopentyl, and the like.
- saturated cyclic alkyls include cyclopropyl, cyclobutyl, cyclopentyl, cyclohexyl, and the like; while unsaturated cyclic alkyls include cyclopentenyl and cyclohexenyl, and the like.
- Alkenyl means an alkyl, as defined above, containing at least one double bond between adjacent carbon atoms. Alkenyls include both cis and trans isomers.
Landscapes
- Chemical & Material Sciences (AREA)
- Organic Chemistry (AREA)
- Health & Medical Sciences (AREA)
- Life Sciences & Earth Sciences (AREA)
- Engineering & Computer Science (AREA)
- General Health & Medical Sciences (AREA)
- Genetics & Genomics (AREA)
- Animal Behavior & Ethology (AREA)
- Pharmacology & Pharmacy (AREA)
- Public Health (AREA)
- Veterinary Medicine (AREA)
- Medicinal Chemistry (AREA)
- Epidemiology (AREA)
- Molecular Biology (AREA)
- Bioinformatics & Cheminformatics (AREA)
- Biochemistry (AREA)
- Wood Science & Technology (AREA)
- Biotechnology (AREA)
- General Engineering & Computer Science (AREA)
- Biomedical Technology (AREA)
- Zoology (AREA)
- Biophysics (AREA)
- General Chemical & Material Sciences (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Oil, Petroleum & Natural Gas (AREA)
- Microbiology (AREA)
- Physics & Mathematics (AREA)
- Plant Pathology (AREA)
- Proteomics, Peptides & Aminoacids (AREA)
- Dispersion Chemistry (AREA)
- Pharmaceuticals Containing Other Organic And Inorganic Compounds (AREA)
- Medicinal Preparation (AREA)
- Medicines That Contain Protein Lipid Enzymes And Other Medicines (AREA)
Abstract
Description
Claims
Priority Applications (7)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US13/514,352 US9687550B2 (en) | 2009-12-07 | 2010-12-07 | Compositions for nucleic acid delivery |
| AU2010328336A AU2010328336B2 (en) | 2009-12-07 | 2010-12-07 | Compositions for nucleic acid delivery |
| CA2783372A CA2783372C (en) | 2009-12-07 | 2010-12-07 | Compositions for nucleic acid delivery |
| EP10836504.0A EP2509636B1 (en) | 2009-12-07 | 2010-12-07 | Compositions for nucleic acid delivery |
| AU2017202153A AU2017202153A1 (en) | 2009-12-07 | 2017-03-31 | Compositions for nucleic acid delivery |
| US15/596,845 US20180369384A1 (en) | 2009-12-07 | 2017-05-16 | Compositions for nucleic acid delivery |
| AU2018275020A AU2018275020A1 (en) | 2009-12-07 | 2018-12-10 | Compositions for nucleic acid delivery |
Applications Claiming Priority (6)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US26741909P | 2009-12-07 | 2009-12-07 | |
| US61/267,419 | 2009-12-07 | ||
| US33439810P | 2010-05-13 | 2010-05-13 | |
| US61/334,398 | 2010-05-13 | ||
| US38430310P | 2010-09-19 | 2010-09-19 | |
| US61/384,303 | 2010-09-19 |
Related Child Applications (2)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| US13/514,352 A-371-Of-International US9687550B2 (en) | 2009-12-07 | 2010-12-07 | Compositions for nucleic acid delivery |
| US15/596,845 Continuation US20180369384A1 (en) | 2009-12-07 | 2017-05-16 | Compositions for nucleic acid delivery |
Publications (2)
| Publication Number | Publication Date |
|---|---|
| WO2011071860A2 true WO2011071860A2 (en) | 2011-06-16 |
| WO2011071860A3 WO2011071860A3 (en) | 2011-09-09 |
Family
ID=44146135
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| PCT/US2010/059206 WO2011071860A2 (en) | 2009-12-07 | 2010-12-07 | Compositions for nucleic acid delivery |
Country Status (6)
| Country | Link |
|---|---|
| US (2) | US9687550B2 (en) |
| EP (2) | EP2509636B1 (en) |
| AU (3) | AU2010328336B2 (en) |
| CA (2) | CA3044884A1 (en) |
| HK (1) | HK1252488A1 (en) |
| WO (1) | WO2011071860A2 (en) |
Cited By (43)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2013006825A1 (en) | 2011-07-06 | 2013-01-10 | Novartis Ag | Liposomes having useful n:p ratio for delivery of rna molecules |
| EP3110401A2 (en) | 2014-02-25 | 2017-01-04 | Merck Sharp & Dohme Corp. | Lipid nanoparticle vaccine adjuvants and antigen delivery systems |
| EP3336082A1 (en) | 2011-06-08 | 2018-06-20 | Translate Bio, Inc. | Cleavable lipids |
| WO2019207061A1 (en) | 2018-04-25 | 2019-10-31 | Ethris Gmbh | Cryoprotective agents for particulate formulations |
| US10493060B2 (en) | 2015-04-03 | 2019-12-03 | Recurium Ip Holdings, Llc | Spirocyclic compounds |
| US10934304B2 (en) | 2016-10-05 | 2021-03-02 | Recurium Ip Holdings, Llc | Spirocyclic compounds |
| US11046658B2 (en) | 2018-07-02 | 2021-06-29 | Incyte Corporation | Aminopyrazine derivatives as PI3K-γ inhibitors |
| WO2021195218A1 (en) | 2020-03-24 | 2021-09-30 | Generation Bio Co. | Non-viral dna vectors and uses thereof for expressing gaucher therapeutics |
| WO2021195214A1 (en) | 2020-03-24 | 2021-09-30 | Generation Bio Co. | Non-viral dna vectors and uses thereof for expressing factor ix therapeutics |
| EP3283125B1 (en) | 2015-04-17 | 2021-12-29 | CureVac Real Estate GmbH | Lyophilization of rna |
| WO2022023284A1 (en) | 2020-07-27 | 2022-02-03 | Anjarium Biosciences Ag | Compositions of dna molecules, methods of making therefor, and methods of use thereof |
| US20220125723A1 (en) | 2010-07-06 | 2022-04-28 | Glaxosmithkline Biologicals Sa | Lipid formulations with viral immunogens |
| US11433027B2 (en) | 2015-05-20 | 2022-09-06 | Curevac Ag | Dry powder composition comprising long-chain RNA |
| WO2022223556A1 (en) | 2021-04-20 | 2022-10-27 | Anjarium Biosciences Ag | Compositions of dna molecules encoding amylo-alpha-1, 6-glucosidase, 4-alpha-glucanotransferase, methods of making thereof, and methods of use thereof |
| WO2022232286A1 (en) | 2021-04-27 | 2022-11-03 | Generation Bio Co. | Non-viral dna vectors expressing anti-coronavirus antibodies and uses thereof |
| WO2022232289A1 (en) | 2021-04-27 | 2022-11-03 | Generation Bio Co. | Non-viral dna vectors expressing therapeutic antibodies and uses thereof |
| US11534405B2 (en) | 2015-05-20 | 2022-12-27 | Curevac Ag | Dry powder composition comprising long-chain RNA |
| WO2023001894A1 (en) | 2021-07-20 | 2023-01-26 | Ags Therapeutics Sas | Extracellular vesicles from microalgae, their preparation, and uses |
| US11590229B2 (en) | 2011-12-07 | 2023-02-28 | Alnylam Pharmaceuticals, Inc. | Biodegradable lipids for the delivery of active agents |
| US11596645B2 (en) | 2010-07-06 | 2023-03-07 | Glaxosmithkline Biologicals Sa | Delivery of RNA to trigger multiple immune pathways |
| US11639370B2 (en) | 2010-10-11 | 2023-05-02 | Glaxosmithkline Biologicals Sa | Antigen delivery platforms |
| WO2023081526A1 (en) | 2021-11-08 | 2023-05-11 | Orna Therapeutics, Inc. | Lipid nanoparticle compositions for delivering circular polynucleotides |
| US11655475B2 (en) | 2010-07-06 | 2023-05-23 | Glaxosmithkline Biologicals Sa | Immunisation of large mammals with low doses of RNA |
| WO2023135273A2 (en) | 2022-01-14 | 2023-07-20 | Anjarium Biosciences Ag | Compositions of dna molecules encoding factor viii, methods of making thereof, and methods of use thereof |
| WO2023144127A1 (en) | 2022-01-31 | 2023-08-03 | Ags Therapeutics Sas | Extracellular vesicles from microalgae, their biodistribution upon administration, and uses |
| US11759422B2 (en) | 2010-08-31 | 2023-09-19 | Glaxosmithkline Biologicals Sa | Pegylated liposomes for delivery of immunogen-encoding RNA |
| WO2023177655A1 (en) | 2022-03-14 | 2023-09-21 | Generation Bio Co. | Heterologous prime boost vaccine compositions and methods of use |
| WO2023183616A1 (en) * | 2022-03-25 | 2023-09-28 | Senda Biosciences, Inc. | Novel ionizable lipids and lipid nanoparticles and methods of using the same |
| WO2023230098A1 (en) | 2022-05-23 | 2023-11-30 | Logicbio Therapeutics, Inc. | Gene therapy compositions and methods of use thereof |
| WO2023232976A1 (en) | 2022-06-03 | 2023-12-07 | Ags Therapeutics Sas | Extracellular vesicles from genetically-modified microalgae containing endogenously-loaded cargo, their preparation, and uses |
| WO2023239756A1 (en) | 2022-06-07 | 2023-12-14 | Generation Bio Co. | Lipid nanoparticle compositions and uses thereof |
| US11896636B2 (en) | 2011-07-06 | 2024-02-13 | Glaxosmithkline Biologicals Sa | Immunogenic combination compositions and uses thereof |
| WO2024040222A1 (en) | 2022-08-19 | 2024-02-22 | Generation Bio Co. | Cleavable closed-ended dna (cedna) and methods of use thereof |
| US11926616B2 (en) | 2018-03-08 | 2024-03-12 | Incyte Corporation | Aminopyrazine diol compounds as PI3K-γ inhibitors |
| WO2024088808A1 (en) | 2022-10-24 | 2024-05-02 | Ags Therapeutics Sas | Extracellular vesicles from microalgae, their biodistribution upon intranasal administration, and uses thereof |
| WO2024102730A1 (en) | 2022-11-08 | 2024-05-16 | Orna Therapeutics, Inc. | Lipids and nanoparticle compositions for delivering polynucleotides |
| WO2024102677A1 (en) | 2022-11-08 | 2024-05-16 | Orna Therapeutics, Inc. | Circular rna compositions |
| WO2024102762A1 (en) | 2022-11-08 | 2024-05-16 | Orna Therapeutics, Inc. | Lipids and lipid nanoparticle compositions for delivering polynucleotides |
| WO2024119074A1 (en) | 2022-12-01 | 2024-06-06 | Generation Bio Co. | Stealth lipid nanoparticle compositions for cell targeting |
| WO2024119051A1 (en) | 2022-12-01 | 2024-06-06 | Generation Bio Co. | Novel polyglycerol-conjugated lipids and lipid nanoparticle compositions comprising the same |
| WO2024119103A1 (en) | 2022-12-01 | 2024-06-06 | Generation Bio Co. | Lipid nanoparticles comprising nucleic acids and lipid-anchored polymers |
| WO2024119039A2 (en) | 2022-12-01 | 2024-06-06 | Generation Bio Co. | Stealth lipid nanoparticles and uses thereof |
| WO2024205657A2 (en) | 2023-03-29 | 2024-10-03 | Orna Therapeutics, Inc. | Lipids and lipid nanoparticle compositions for delivering polynucleotides |
Families Citing this family (135)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| SG10201901089TA (en) | 2008-11-10 | 2019-03-28 | Arbutus Biopharma Corp | Novel lipids and compositions for the delivery of therapeutics |
| WO2012019168A2 (en) | 2010-08-06 | 2012-02-09 | Moderna Therapeutics, Inc. | Engineered nucleic acids and methods of use thereof |
| CA2821992A1 (en) | 2010-10-01 | 2012-04-05 | Moderna Therapeutics, Inc. | Engineered nucleic acids and methods of use thereof |
| US8979797B2 (en) * | 2010-12-16 | 2015-03-17 | Ams Research Corporation | High pressure delivery system and method for treating pelvic disorder using large molecule therapeutics |
| DE12722942T1 (en) | 2011-03-31 | 2021-09-30 | Modernatx, Inc. | RELEASE AND FORMULATION OF MANIPULATED NUCLEIC ACIDS |
| CA2833269C (en) * | 2011-04-15 | 2020-04-14 | Molecular Transfer, Inc. | Agents for improved delivery of nucleic acids to eukaryotic cells |
| US9464124B2 (en) | 2011-09-12 | 2016-10-11 | Moderna Therapeutics, Inc. | Engineered nucleic acids and methods of use thereof |
| EP3492109B1 (en) | 2011-10-03 | 2020-03-04 | ModernaTX, Inc. | Modified nucleosides, nucleotides, and nucleic acids, and uses thereof |
| JP2015501844A (en) | 2011-12-16 | 2015-01-19 | モデルナ セラピューティクス インコーポレイテッドModerna Therapeutics,Inc. | Modified nucleosides, nucleotides and nucleic acid compositions |
| US9283287B2 (en) | 2012-04-02 | 2016-03-15 | Moderna Therapeutics, Inc. | Modified polynucleotides for the production of nuclear proteins |
| US9878056B2 (en) | 2012-04-02 | 2018-01-30 | Modernatx, Inc. | Modified polynucleotides for the production of cosmetic proteins and peptides |
| EP2833923A4 (en) | 2012-04-02 | 2016-02-24 | Moderna Therapeutics Inc | Modified polynucleotides for the production of proteins |
| US9572897B2 (en) | 2012-04-02 | 2017-02-21 | Modernatx, Inc. | Modified polynucleotides for the production of cytoplasmic and cytoskeletal proteins |
| LT2922554T (en) | 2012-11-26 | 2022-06-27 | Modernatx, Inc. | Terminally modified rna |
| EP2971010B1 (en) | 2013-03-14 | 2020-06-10 | ModernaTX, Inc. | Formulation and delivery of modified nucleoside, nucleotide, and nucleic acid compositions |
| EP3757570B1 (en) | 2013-03-15 | 2023-10-11 | Translate Bio, Inc. | Synergistic enhancement of the delivery of nucleic acids via blended formulations |
| US8980864B2 (en) | 2013-03-15 | 2015-03-17 | Moderna Therapeutics, Inc. | Compositions and methods of altering cholesterol levels |
| WO2015048744A2 (en) | 2013-09-30 | 2015-04-02 | Moderna Therapeutics, Inc. | Polynucleotides encoding immune modulating polypeptides |
| EP3052521A1 (en) | 2013-10-03 | 2016-08-10 | Moderna Therapeutics, Inc. | Polynucleotides encoding low density lipoprotein receptor |
| CA3236835A1 (en) | 2013-11-22 | 2015-05-28 | Mina Therapeutics Limited | C/ebp alpha short activating rna compositions and methods of use |
| HRP20221536T1 (en) | 2014-06-25 | 2023-02-17 | Acuitas Therapeutics Inc. | Novel lipids and lipid nanoparticle formulations for delivery of nucleic acids |
| WO2016030863A1 (en) | 2014-08-29 | 2016-03-03 | Glaxosmithkline Intellectual Property Development Limited | Compounds and methods for treating viral infections |
| SI3313829T1 (en) | 2015-06-29 | 2024-09-30 | Acuitas Therapeutics Inc. | Lipids and lipid nanoparticle formulations for delivery of nucleic acids |
| HUE057613T2 (en) | 2015-09-17 | 2022-05-28 | Modernatx Inc | Compounds and compositions for intracellular delivery of therapeutic agents |
| WO2018081480A1 (en) | 2016-10-26 | 2018-05-03 | Acuitas Therapeutics, Inc. | Lipid nanoparticle formulations |
| CN113636947A (en) | 2015-10-28 | 2021-11-12 | 爱康泰生治疗公司 | Novel lipid and lipid nanoparticle formulations for delivery of nucleic acids |
| PL3386484T3 (en) | 2015-12-10 | 2022-07-25 | Modernatx, Inc. | Compositions and methods for delivery of therapeutic agents |
| DK3394030T3 (en) | 2015-12-22 | 2022-03-28 | Modernatx Inc | COMPOUNDS AND COMPOSITIONS FOR INTRACELLULAR RELEASE OF FUNDS |
| EP3825400A1 (en) | 2016-04-08 | 2021-05-26 | Translate Bio Ma, Inc. | Multimeric coding nucleic acid and uses thereof |
| JP2019522047A (en) | 2016-06-13 | 2019-08-08 | トランスレイト バイオ, インコーポレイテッド | Messenger RNA therapy for the treatment of ornithine transcarbamylase deficiency |
| EP3538067A1 (en) | 2016-11-08 | 2019-09-18 | Modernatx, Inc. | Stabilized formulations of lipid nanoparticles |
| FI3596041T3 (en) | 2017-03-15 | 2023-01-31 | Compound and compositions for intracellular delivery of therapeutic agents | |
| US11969506B2 (en) | 2017-03-15 | 2024-04-30 | Modernatx, Inc. | Lipid nanoparticle formulation |
| CA3056133A1 (en) | 2017-03-15 | 2018-09-20 | Modernatx, Inc. | Crystal forms of amino lipids |
| WO2018191719A1 (en) | 2017-04-13 | 2018-10-18 | Acuitas Therapeutics, Inc. | Lipid delivery of therapeutic agents to adipose tissue |
| WO2018191657A1 (en) | 2017-04-13 | 2018-10-18 | Acuitas Therapeutics, Inc. | Lipids for delivery of active agents |
| AU2018256877B2 (en) | 2017-04-28 | 2022-06-02 | Acuitas Therapeutics, Inc. | Novel carbonyl lipids and lipid nanoparticle formulations for delivery of nucleic acids |
| WO2018232120A1 (en) | 2017-06-14 | 2018-12-20 | Modernatx, Inc. | Compounds and compositions for intracellular delivery of agents |
| US11786607B2 (en) | 2017-06-15 | 2023-10-17 | Modernatx, Inc. | RNA formulations |
| WO2019036008A1 (en) | 2017-08-16 | 2019-02-21 | Acuitas Therapeutics, Inc. | Lipids for use in lipid nanoparticle formulations |
| US11524932B2 (en) | 2017-08-17 | 2022-12-13 | Acuitas Therapeutics, Inc. | Lipids for use in lipid nanoparticle formulations |
| WO2019036028A1 (en) | 2017-08-17 | 2019-02-21 | Acuitas Therapeutics, Inc. | Lipids for use in lipid nanoparticle formulations |
| WO2019036000A1 (en) | 2017-08-17 | 2019-02-21 | Acuitas Therapeutics, Inc. | Lipids for use in lipid nanoparticle formulations |
| WO2019046809A1 (en) | 2017-08-31 | 2019-03-07 | Modernatx, Inc. | Methods of making lipid nanoparticles |
| CA3075205A1 (en) | 2017-09-08 | 2019-03-14 | Mina Therapeutics Limited | Stabilized hnf4a sarna compositions and methods of use |
| US20200208152A1 (en) | 2017-09-08 | 2020-07-02 | Mina Therapeutics Limited | Stabilized sarna compositions and methods of use |
| CA3076751A1 (en) | 2017-09-26 | 2019-04-04 | Aquestive Therapeutics, Inc. | Delivery pharmaceutical compositions including permeation enhancers |
| WO2019089828A1 (en) | 2017-10-31 | 2019-05-09 | Acuitas Therapeutics, Inc. | Lamellar lipid nanoparticles |
| CA3084061A1 (en) | 2017-12-20 | 2019-06-27 | Translate Bio, Inc. | Improved composition and methods for treatment of ornithine transcarbamylase deficiency |
| CN110283223B (en) * | 2018-03-19 | 2022-01-04 | 广州市锐博生物科技有限公司 | Cationic lipid molecule and application thereof in nucleic acid delivery |
| EP4242307A3 (en) | 2018-04-12 | 2023-12-27 | MiNA Therapeutics Limited | Sirt1-sarna compositions and methods of use |
| CN108441483B (en) * | 2018-04-25 | 2021-09-10 | 江南大学 | Method for promoting bovine rhinotracheitis virus replication |
| EP3852728B1 (en) | 2018-09-20 | 2024-09-18 | ModernaTX, Inc. | Preparation of lipid nanoparticles and methods of administration thereof |
| EP3852911A2 (en) | 2018-09-21 | 2021-07-28 | Acuitas Therapeutics, Inc. | Systems and methods for manufacturing lipid nanoparticles and liposomes |
| MX2021008358A (en) | 2019-01-11 | 2021-09-30 | Acuitas Therapeutics Inc | Lipids for lipid nanoparticle delivery of active agents. |
| US20220211740A1 (en) | 2019-04-12 | 2022-07-07 | Mina Therapeutics Limited | Sirt1-sarna compositions and methods of use |
| CN110083592A (en) * | 2019-05-08 | 2019-08-02 | 中国建筑第八工程局有限公司 | Architectural engineering sample managing method based on BIM |
| WO2020254337A1 (en) * | 2019-06-19 | 2020-12-24 | Rhodia Operations | New quaternary ammonium compounds |
| CA3147875A1 (en) | 2019-07-19 | 2021-01-28 | Flagship Pioneering Innovations Vi, Llc | Recombinase compositions and methods of use |
| DK4013385T3 (en) | 2019-08-14 | 2024-09-02 | Acuitas Therapeutics Inc | Improved lipid nanoparticles for delivery of nucleic acids |
| US11066355B2 (en) | 2019-09-19 | 2021-07-20 | Modernatx, Inc. | Branched tail lipid compounds and compositions for intracellular delivery of therapeutic agents |
| AU2020352552A1 (en) | 2019-09-23 | 2022-03-17 | Omega Therapeutics, Inc. | Compositions and methods for modulating hepatocyte nuclear factor 4-alpha (HNF4α) gene expression |
| AU2020355000A1 (en) | 2019-09-23 | 2022-03-17 | Omega Therapeutics, Inc. | Compositions and methods for modulating apolipoprotein B (APOB) gene expression |
| JP2023517326A (en) | 2020-03-11 | 2023-04-25 | オメガ セラピューティクス, インコーポレイテッド | Compositions and methods for modulating forkhead box P3 (FOXP3) gene expression |
| KR20220161316A (en) | 2020-03-30 | 2022-12-06 | 비온테크 에스이 | RNA composition targeting CLAUDIN-18.2 |
| AU2021273502A1 (en) * | 2020-05-15 | 2023-02-02 | Translate Bio, Inc. | Lipid nanoparticle formulations for mRNA delivery |
| IL298362A (en) | 2020-05-20 | 2023-01-01 | Flagship Pioneering Innovations Vi Llc | Coronavirus antigen compositions and their uses |
| CA3180101A1 (en) | 2020-05-29 | 2021-12-02 | Flagship Pioneering Innovations Vi, Llc | Trem compositions and methods relating thereto |
| EP4158032A2 (en) | 2020-05-29 | 2023-04-05 | Flagship Pioneering Innovations VI, LLC | Trem compositions and methods relating thereto |
| EP4164668A1 (en) | 2020-06-15 | 2023-04-19 | Research Institute at Nationwide Children's Hospital | Adeno-associated virus vector delivery for muscular dystrophies |
| US11976019B2 (en) | 2020-07-16 | 2024-05-07 | Acuitas Therapeutics, Inc. | Cationic lipids for use in lipid nanoparticles |
| GB2603454A (en) | 2020-12-09 | 2022-08-10 | Ucl Business Ltd | Novel therapeutics for the treatment of neurodegenerative disorders |
| US20240175020A1 (en) | 2020-12-23 | 2024-05-30 | Flagship Pioneering Innovations Vi, Llc | Compositions of modified trems and uses thereof |
| US11524023B2 (en) | 2021-02-19 | 2022-12-13 | Modernatx, Inc. | Lipid nanoparticle compositions and methods of formulating the same |
| JP2024511092A (en) | 2021-03-26 | 2024-03-12 | ミナ セラピューティクス リミテッド | TMEM173saRNA composition and method of use |
| WO2022212784A1 (en) | 2021-03-31 | 2022-10-06 | Flagship Pioneering Innovations V, Inc. | Thanotransmission polypeptides and their use in treating cancer |
| EP4319803A1 (en) | 2021-04-08 | 2024-02-14 | Vaxthera SAS | Coronavirus vaccine comprising a mosaic protein |
| WO2023283359A2 (en) | 2021-07-07 | 2023-01-12 | Omega Therapeutics, Inc. | Compositions and methods for modulating secreted frizzled receptor protein 1 (sfrp1) gene expression |
| EP4377457A1 (en) | 2021-07-26 | 2024-06-05 | Flagship Pioneering Innovations VI, LLC | Trem compositions and uses thereof |
| WO2023023055A1 (en) | 2021-08-16 | 2023-02-23 | Renagade Therapeutics Management Inc. | Compositions and methods for optimizing tropism of delivery systems for rna |
| EP4402121A1 (en) | 2021-09-14 | 2024-07-24 | Renagade Therapeutics Management Inc. | Acyclic lipids and methods of use thereof |
| WO2023044333A1 (en) | 2021-09-14 | 2023-03-23 | Renagade Therapeutics Management Inc. | Cyclic lipids and methods of use thereof |
| CA3232635A1 (en) | 2021-09-17 | 2023-03-23 | Flagship Pioneering Innovations Vi, Llc | Compositions and methods for producing circular polyribonucleotides |
| AR127312A1 (en) | 2021-10-08 | 2024-01-10 | Suzhou Abogen Biosciences Co Ltd | LIPID COMPOUNDS AND LIPID NANOPARTICLE COMPOSITIONS |
| AU2022370530A1 (en) | 2021-10-18 | 2024-05-02 | Flagship Pioneering Innovations Vi, Llc | Compositions and methods for purifying polyribonucleotides |
| EP4426832A1 (en) | 2021-11-03 | 2024-09-11 | The J. David Gladstone Institutes, A Testamentary Trust Established under The Will of J. David Gladstone | Precise genome editing using retrons |
| KR20240122774A (en) | 2021-11-16 | 2024-08-13 | 세일 바이오메디슨스, 인크. | Novel ionizable lipids and lipid nanoparticles, and methods of using the same |
| AU2022391677A1 (en) | 2021-11-22 | 2024-06-06 | Sail Biomedicines, Inc. | Novel ionizable lipids and lipid nanoparticles and methods of using the same |
| AU2022397292A1 (en) | 2021-11-24 | 2024-05-30 | Flagship Pioneering Innovations Vi, Llc | Varicella-zoster virus immunogen compositions and their uses |
| CN118555966A (en) | 2021-11-24 | 2024-08-27 | 旗舰创业创新六公司 | Immunogenic compositions and uses thereof |
| EP4436984A1 (en) | 2021-11-24 | 2024-10-02 | Flagship Pioneering Innovations VI, LLC | Coronavirus immunogen compositions and their uses |
| WO2023099884A1 (en) | 2021-12-01 | 2023-06-08 | Mina Therapeutics Limited | Pax6 sarna compositions and methods of use |
| GB202117758D0 (en) | 2021-12-09 | 2022-01-26 | Ucl Business Ltd | Therapeutics for the treatment of neurodegenerative disorders |
| AR128002A1 (en) | 2021-12-17 | 2024-03-20 | Flagship Pioneering Innovations Vi Llc | CIRCULAR RNA ENRICHMENT METHODS UNDER DENATURALING CONDITIONS |
| TW202340461A (en) | 2021-12-22 | 2023-10-16 | 美商旗艦先鋒創新有限責任公司 | Compositions and methods for purifying polyribonucleotides |
| WO2023122789A1 (en) | 2021-12-23 | 2023-06-29 | Flagship Pioneering Innovations Vi, Llc | Circular polyribonucleotides encoding antifusogenic polypeptides |
| WO2023122752A1 (en) | 2021-12-23 | 2023-06-29 | Renagade Therapeutics Management Inc. | Constrained lipids and methods of use thereof |
| WO2023141602A2 (en) | 2022-01-21 | 2023-07-27 | Renagade Therapeutics Management Inc. | Engineered retrons and methods of use |
| AU2023212857A1 (en) | 2022-01-27 | 2024-07-04 | BioNTech SE | Pharmaceutical compositions for delivery of herpes simplex virus antigens and related methods |
| KR102465349B1 (en) | 2022-02-25 | 2022-11-11 | 주식회사 테르나테라퓨틱스 | Novel Ionizable Lipids and Lipid Nanoparticles Comprising Thereof |
| WO2023170435A1 (en) | 2022-03-07 | 2023-09-14 | Mina Therapeutics Limited | Il10 sarna compositions and methods of use |
| WO2023196931A1 (en) | 2022-04-07 | 2023-10-12 | Renagade Therapeutics Management Inc. | Cyclic lipids and lipid nanoparticles (lnp) for the delivery of nucleic acids or peptides for use in vaccinating against infectious agents |
| WO2023196634A2 (en) | 2022-04-08 | 2023-10-12 | Flagship Pioneering Innovations Vii, Llc | Vaccines and related methods |
| WO2023220083A1 (en) | 2022-05-09 | 2023-11-16 | Flagship Pioneering Innovations Vi, Llc | Trem compositions and methods of use for treating proliferative disorders |
| WO2023218431A1 (en) | 2022-05-13 | 2023-11-16 | BioNTech SE | Rna compositions targeting hiv |
| WO2023220729A2 (en) | 2022-05-13 | 2023-11-16 | Flagship Pioneering Innovations Vii, Llc | Double stranded dna compositions and related methods |
| WO2023230295A1 (en) | 2022-05-25 | 2023-11-30 | BioNTech SE | Rna compositions for delivery of monkeypox antigens and related methods |
| WO2023232747A1 (en) | 2022-05-30 | 2023-12-07 | BioNTech SE | Complexes for delivery of nucleic acids |
| WO2023250112A1 (en) | 2022-06-22 | 2023-12-28 | Flagship Pioneering Innovations Vi, Llc | Compositions of modified trems and uses thereof |
| WO2024030856A2 (en) | 2022-08-01 | 2024-02-08 | Flagship Pioneering Innovations Vii, Llc | Immunomodulatory proteins and related methods |
| WO2024035952A1 (en) | 2022-08-12 | 2024-02-15 | Remix Therapeutics Inc. | Methods and compositions for modulating splicing at alternative splice sites |
| WO2024049979A2 (en) | 2022-08-31 | 2024-03-07 | Senda Biosciences, Inc. | Novel ionizable lipids and lipid nanoparticles and methods of using the same |
| WO2024064934A1 (en) | 2022-09-23 | 2024-03-28 | BioNTech SE | Compositions for delivery of plasmodium csp antigens and related methods |
| WO2024064931A1 (en) | 2022-09-23 | 2024-03-28 | BioNTech SE | Compositions for delivery of liver stage antigens and related methods |
| WO2024063788A1 (en) | 2022-09-23 | 2024-03-28 | BioNTech SE | Compositions for delivery of malaria antigens and related methods |
| WO2024063789A1 (en) | 2022-09-23 | 2024-03-28 | BioNTech SE | Compositions for delivery of malaria antigens and related methods |
| WO2024077191A1 (en) | 2022-10-05 | 2024-04-11 | Flagship Pioneering Innovations V, Inc. | Nucleic acid molecules encoding trif and additionalpolypeptides and their use in treating cancer |
| WO2024074634A1 (en) | 2022-10-06 | 2024-04-11 | BioNTech SE | Rna compositions targeting claudin-18.2 |
| WO2024074211A1 (en) | 2022-10-06 | 2024-04-11 | BioNTech SE | Rna compositions targeting claudin-18.2 |
| WO2024097664A1 (en) | 2022-10-31 | 2024-05-10 | Flagship Pioneering Innovations Vi, Llc | Compositions and methods for purifying polyribonucleotides |
| WO2024102799A1 (en) | 2022-11-08 | 2024-05-16 | Flagship Pioneering Innovations Vi, Llc | Compositions and methods for producing circular polyribonucleotides |
| WO2024129988A1 (en) | 2022-12-14 | 2024-06-20 | Flagship Pioneering Innovations Vii, Llc | Compositions and methods for delivery of therapeutic agents to bone |
| WO2024134199A1 (en) | 2022-12-22 | 2024-06-27 | Mina Therapeutics Limited | Chemically modified sarna compositions and methods of use |
| WO2024141955A1 (en) | 2022-12-28 | 2024-07-04 | BioNTech SE | Rna compositions targeting hiv |
| US20240252520A1 (en) | 2023-01-09 | 2024-08-01 | Beth Israel Deaconess Medical Center, Inc. | Therapeutic agents and their use for treating chronic wounds |
| US20240238473A1 (en) | 2023-01-09 | 2024-07-18 | Beth Israel Deaconess Medical Center, Inc. | Recombinant nucleic acid molecules and their use in wound healing |
| WO2024151687A1 (en) | 2023-01-09 | 2024-07-18 | Flagship Pioneering Innovations V, Inc. | Genetic switches and their use in treating cancer |
| WO2024151583A2 (en) | 2023-01-09 | 2024-07-18 | Flagship Pioneering Innovations Vii, Llc | Vaccines and related methods |
| WO2024167885A1 (en) | 2023-02-06 | 2024-08-15 | Flagship Pioneering Innovations Vii, Llc | Immunomodulatory compositions and related methods |
| WO2024167958A1 (en) * | 2023-02-06 | 2024-08-15 | Archimmune Therapeutics, Inc. | Antigen-capturing nanoparticles and formulations for immunotherapy |
| US20240293318A1 (en) | 2023-02-13 | 2024-09-05 | Flagship Pioneering Innovations Vii, Llc | Cleavable linker-containing ionizable lipids and lipid carriers for therapeutic compositions |
| WO2024173828A1 (en) | 2023-02-17 | 2024-08-22 | Flagship Pioneering Innovations Vii, Llc | Dna compositions comprising modified uracil |
| US20240293582A1 (en) | 2023-02-17 | 2024-09-05 | Flagship Pioneering Innovations Vii, Llc | Dna compositions comprising modified cytosine |
| WO2024192422A1 (en) | 2023-03-15 | 2024-09-19 | Flagship Pioneering Innovations Vi, Llc | Immunogenic compositions and uses thereof |
| WO2024192420A1 (en) | 2023-03-15 | 2024-09-19 | Flagship Pioneering Innovations Vi, Llc | Compositions comprising polyribonucleotides and uses thereof |
Citations (151)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US3687808A (en) | 1969-08-14 | 1972-08-29 | Univ Leland Stanford Junior | Synthetic polynucleotides |
| US3993754A (en) | 1974-10-09 | 1976-11-23 | The United States Of America As Represented By The United States Energy Research And Development Administration | Liposome-encapsulated actinomycin for cancer chemotherapy |
| US4145410A (en) | 1976-10-12 | 1979-03-20 | Sears Barry D | Method of preparing a controlled-release pharmaceutical preparation, and resulting composition |
| US4224179A (en) | 1977-08-05 | 1980-09-23 | Battelle Memorial Institute | Process for the preparation of liposomes in aqueous solution |
| US4235871A (en) | 1978-02-24 | 1980-11-25 | Papahadjopoulos Demetrios P | Method of encapsulating biologically active materials in lipid vesicles |
| US4469863A (en) | 1980-11-12 | 1984-09-04 | Ts O Paul O P | Nonionic nucleic acid alkyl and aryl phosphonates and processes for manufacture and use thereof |
| US4522803A (en) | 1983-02-04 | 1985-06-11 | The Liposome Company, Inc. | Stable plurilamellar vesicles, their preparation and use |
| US4587044A (en) | 1983-09-01 | 1986-05-06 | The Johns Hopkins University | Linkage of proteins to nucleic acids |
| US4588578A (en) | 1983-08-08 | 1986-05-13 | The Liposome Company, Inc. | Lipid vesicles prepared in a monophase |
| US4603044A (en) | 1983-01-06 | 1986-07-29 | Technology Unlimited, Inc. | Hepatocyte Directed Vesicle delivery system |
| US4605735A (en) | 1983-02-14 | 1986-08-12 | Wakunaga Seiyaku Kabushiki Kaisha | Oligonucleotide derivatives |
| WO1986004920A1 (en) | 1985-02-13 | 1986-08-28 | Biotechnology Research Partners, Limited | Human metallothionein-ii promoter in mammalian expression system |
| WO1987002062A1 (en) | 1985-10-04 | 1987-04-09 | Biotechnology Research Partners, Ltd. | Recombinant apolipoproteins and methods |
| US4667025A (en) | 1982-08-09 | 1987-05-19 | Wakunaga Seiyaku Kabushiki Kaisha | Oligonucleotide derivatives |
| US4737323A (en) | 1986-02-13 | 1988-04-12 | Liposome Technology, Inc. | Liposome extrusion method |
| US4762779A (en) | 1985-06-13 | 1988-08-09 | Amgen Inc. | Compositions and methods for functionalizing nucleic acids |
| US4824941A (en) | 1983-03-10 | 1989-04-25 | Julian Gordon | Specific antibody to the native form of 2'5'-oligonucleotides, the method of preparation and the use as reagents in immunoassays or for binding 2'5'-oligonucleotides in biological systems |
| US4828979A (en) | 1984-11-08 | 1989-05-09 | Life Technologies, Inc. | Nucleotide analogs for nucleic acid labeling and detection |
| US4835263A (en) | 1983-01-27 | 1989-05-30 | Centre National De La Recherche Scientifique | Novel compounds containing an oligonucleotide sequence bonded to an intercalating agent, a process for their synthesis and their use |
| US4876335A (en) | 1986-06-30 | 1989-10-24 | Wakunaga Seiyaku Kabushiki Kaisha | Poly-labelled oligonucleotide derivative |
| US4904582A (en) | 1987-06-11 | 1990-02-27 | Synthetic Genetics | Novel amphiphilic nucleic acid conjugates |
| EP0360257A2 (en) | 1988-09-20 | 1990-03-28 | The Board Of Regents For Northern Illinois University | RNA catalyst for cleaving specific RNA sequences |
| US4948882A (en) | 1983-02-22 | 1990-08-14 | Syngene, Inc. | Single-stranded labelled oligonucleotides, reactive monomers and methods of synthesis |
| US4957773A (en) | 1989-02-13 | 1990-09-18 | Syracuse University | Deposition of boron-containing films from decaborane |
| US4958013A (en) | 1989-06-06 | 1990-09-18 | Northwestern University | Cholesteryl modified oligonucleotides |
| US4987071A (en) | 1986-12-03 | 1991-01-22 | University Patents, Inc. | RNA ribozyme polymerases, dephosphorylases, restriction endoribonucleases and methods |
| WO1991003162A1 (en) | 1989-08-31 | 1991-03-21 | City Of Hope | Chimeric dna-rna catalytic sequences |
| US5013556A (en) | 1989-10-20 | 1991-05-07 | Liposome Technology, Inc. | Liposomes with enhanced circulation time |
| US5023243A (en) | 1981-10-23 | 1991-06-11 | Molecular Biosystems, Inc. | Oligonucleotide therapeutic agent and method of making same |
| US5059528A (en) | 1987-05-28 | 1991-10-22 | Ucb, S.A. | Expression of human proapolipoprotein a-i |
| US5082830A (en) | 1988-02-26 | 1992-01-21 | Enzo Biochem, Inc. | End labeled nucleotide probe |
| US5109124A (en) | 1988-06-01 | 1992-04-28 | Biogen, Inc. | Nucleic acid probe linked to a label having a terminal cysteine |
| WO1992007065A1 (en) | 1990-10-12 | 1992-04-30 | MAX-PLANCK-Gesellschaft zur Förderung der Wissenschaften e.V. | Modified ribozymes |
| US5112963A (en) | 1987-11-12 | 1992-05-12 | Max-Planck-Gesellschaft Zur Foerderung Der Wissenschaften E.V. | Modified oligonucleotides |
| US5116739A (en) | 1984-10-16 | 1992-05-26 | Mitsubishi Chemical Industries Limited | Process for the production of human apolipoprotein e, and transformed hosts and products thereof |
| US5118802A (en) | 1983-12-20 | 1992-06-02 | California Institute Of Technology | DNA-reporter conjugates linked via the 2' or 5'-primary amino group of the 5'-terminal nucleoside |
| US5130302A (en) | 1989-12-20 | 1992-07-14 | Boron Bilogicals, Inc. | Boronated nucleoside, nucleotide and oligonucleotide compounds, compositions and methods for using same |
| US5138045A (en) | 1990-07-27 | 1992-08-11 | Isis Pharmaceuticals | Polyamine conjugated oligonucleotides |
| US5142047A (en) | 1985-03-15 | 1992-08-25 | Anti-Gene Development Group | Uncharged polynucleotide-binding polymers |
| US5149782A (en) | 1988-08-19 | 1992-09-22 | Tanox Biosystems, Inc. | Molecular conjugates containing cell membrane-blending agents |
| WO1992020823A1 (en) | 1991-05-21 | 1992-11-26 | Isis Pharmaceuticals, Inc. | Backbone modified oligonucleotide analogs |
| US5168045A (en) | 1989-08-18 | 1992-12-01 | The Scripps Research Institute | Diagnostic systems and methods using polypeptide analogs of apolipoprotein e |
| EP0519463A1 (en) | 1991-06-20 | 1992-12-23 | Europäisches Laboratorium Für Molekularbiologie (Embl) | Synthetic, catalytic oligonucleotides |
| US5177189A (en) | 1989-08-18 | 1993-01-05 | The Scripps Research Institute | Polypeptide analogs of Apolipoprotein E |
| US5177198A (en) | 1989-11-30 | 1993-01-05 | University Of N.C. At Chapel Hill | Process for preparing oligoribonucleoside and oligodeoxyribonucleoside boranophosphates |
| US5182364A (en) | 1990-02-26 | 1993-01-26 | The Scripps Research Institute | Polypeptide analogs of apolipoprotein E |
| US5214136A (en) | 1990-02-20 | 1993-05-25 | Gilead Sciences, Inc. | Anthraquinone-derivatives oligonucleotides |
| US5218105A (en) | 1990-07-27 | 1993-06-08 | Isis Pharmaceuticals | Polyamine conjugated oligonucleotides |
| US5223618A (en) | 1990-08-13 | 1993-06-29 | Isis Pharmaceuticals, Inc. | 4'-desmethyl nucleoside analog compounds |
| WO1993015187A1 (en) | 1992-01-31 | 1993-08-05 | Massachusetts Institute Of Technology | Nucleozymes |
| US5235033A (en) | 1985-03-15 | 1993-08-10 | Anti-Gene Development Group | Alpha-morpholino ribonucleoside derivatives and polymers thereof |
| US5245022A (en) | 1990-08-03 | 1993-09-14 | Sterling Drug, Inc. | Exonuclease resistant terminally substituted oligonucleotides |
| US5254469A (en) | 1989-09-12 | 1993-10-19 | Eastman Kodak Company | Oligonucleotide-enzyme conjugate that can be used as a probe in hybridization assays and polymerase chain reaction procedures |
| US5256775A (en) | 1989-06-05 | 1993-10-26 | Gilead Sciences, Inc. | Exonuclease-resistant oligonucleotides |
| US5258506A (en) | 1984-10-16 | 1993-11-02 | Chiron Corporation | Photolabile reagents for incorporation into oligonucleotide chains |
| US5262536A (en) | 1988-09-15 | 1993-11-16 | E. I. Du Pont De Nemours And Company | Reagents for the preparation of 5'-tagged oligonucleotides |
| US5264564A (en) | 1989-10-24 | 1993-11-23 | Gilead Sciences | Oligonucleotide analogs with novel linkages |
| US5264562A (en) | 1989-10-24 | 1993-11-23 | Gilead Sciences, Inc. | Oligonucleotide analogs with novel linkages |
| WO1993023569A1 (en) | 1992-05-11 | 1993-11-25 | Ribozyme Pharmaceuticals, Inc. | Method and reagent for inhibiting viral replication |
| US5272250A (en) | 1992-07-10 | 1993-12-21 | Spielvogel Bernard F | Boronated phosphoramidate compounds |
| WO1994002595A1 (en) | 1992-07-17 | 1994-02-03 | Ribozyme Pharmaceuticals, Inc. | Method and reagent for treatment of animal diseases |
| US5286634A (en) | 1989-09-28 | 1994-02-15 | Stadler Joan K | Synergistic method for host cell transformation |
| US5292873A (en) | 1989-11-29 | 1994-03-08 | The Research Foundation Of State University Of New York | Nucleic acids labeled with naphthoquinone probe |
| US5317098A (en) | 1986-03-17 | 1994-05-31 | Hiroaki Shizuya | Non-radioisotope tagging of fragments |
| WO1994013688A1 (en) | 1992-12-08 | 1994-06-23 | Gene Shears Pty. Limited | Dna-armed ribozymes and minizymes |
| US5359044A (en) | 1991-12-13 | 1994-10-25 | Isis Pharmaceuticals | Cyclobutyl oligonucleotide surrogates |
| US5366878A (en) | 1990-02-15 | 1994-11-22 | The Worcester Foundation For Experimental Biology | Method of site-specific alteration of RNA and production of encoded polypeptides |
| US5371241A (en) | 1991-07-19 | 1994-12-06 | Pharmacia P-L Biochemicals Inc. | Fluorescein labelled phosphoramidites |
| US5386023A (en) | 1990-07-27 | 1995-01-31 | Isis Pharmaceuticals | Backbone modified oligonucleotide analogs and preparation thereof through reductive coupling |
| US5391723A (en) | 1989-05-31 | 1995-02-21 | Neorx Corporation | Oligonucleotide conjugates |
| US5414077A (en) | 1990-02-20 | 1995-05-09 | Gilead Sciences | Non-nucleoside linkers for convenient attachment of labels to oligonucleotides using standard synthetic methods |
| US5451463A (en) | 1989-08-28 | 1995-09-19 | Clontech Laboratories, Inc. | Non-nucleoside 1,3-diol reagents for labeling synthetic oligonucleotides |
| US5457191A (en) | 1990-01-11 | 1995-10-10 | Isis Pharmaceuticals, Inc. | 3-deazapurines |
| US5459255A (en) | 1990-01-11 | 1995-10-17 | Isis Pharmaceuticals, Inc. | N-2 substituted purines |
| US5473039A (en) | 1989-08-18 | 1995-12-05 | The Scripps Research Institute | Polypeptide analogs of apolipoprotein E, diagnostic systems and methods using the analogs |
| US5476925A (en) | 1993-02-01 | 1995-12-19 | Northwestern University | Oligodeoxyribonucleotides including 3'-aminonucleoside-phosphoramidate linkages and terminal 3'-amino groups |
| US5484908A (en) | 1991-11-26 | 1996-01-16 | Gilead Sciences, Inc. | Oligonucleotides containing 5-propynyl pyrimidines |
| US5486603A (en) | 1990-01-08 | 1996-01-23 | Gilead Sciences, Inc. | Oligonucleotide having enhanced binding affinity |
| US5489677A (en) | 1990-07-27 | 1996-02-06 | Isis Pharmaceuticals, Inc. | Oligonucleoside linkages containing adjacent oxygen and nitrogen atoms |
| US5508270A (en) | 1993-03-06 | 1996-04-16 | Ciba-Geigy Corporation | Nucleoside phosphinate compounds and compositions |
| WO1996011266A2 (en) | 1994-10-05 | 1996-04-18 | Amgen Inc. | Method for inhibiting smooth muscle cell proliferation and oligonucleotides for use therein |
| US5510475A (en) | 1990-11-08 | 1996-04-23 | Hybridon, Inc. | Oligonucleotide multiple reporter precursors |
| US5512439A (en) | 1988-11-21 | 1996-04-30 | Dynal As | Oligonucleotide-linked magnetic particles and uses thereof |
| US5512667A (en) | 1990-08-28 | 1996-04-30 | Reed; Michael W. | Trifunctional intermediates for preparing 3'-tailed oligonucleotides |
| US5514785A (en) | 1990-05-11 | 1996-05-07 | Becton Dickinson And Company | Solid supports for nucleic acid hybridization assays |
| US5519134A (en) | 1994-01-11 | 1996-05-21 | Isis Pharmaceuticals, Inc. | Pyrrolidine-containing monomers and oligomers |
| US5525472A (en) | 1991-06-26 | 1996-06-11 | Bio-Technology General Corp. | Method for production and purification or recombinant Apolipoprotein E from bacteria |
| US5525465A (en) | 1987-10-28 | 1996-06-11 | Howard Florey Institute Of Experimental Physiology And Medicine | Oligonucleotide-polyamide conjugates and methods of production and applications of the same |
| US5532130A (en) | 1993-07-20 | 1996-07-02 | Dyad Pharmaceutical Corporation | Methods and compositions for sequence-specific hybridization of RNA by 2'-5' oligonucleotides |
| US5534499A (en) | 1994-05-19 | 1996-07-09 | The University Of British Columbia | Lipophilic drug derivatives for use in liposomes |
| US5539083A (en) | 1994-02-23 | 1996-07-23 | Isis Pharmaceuticals, Inc. | Peptide nucleic acid combinatorial libraries and improved methods of synthesis |
| US5545730A (en) | 1984-10-16 | 1996-08-13 | Chiron Corporation | Multifunctional nucleic acid monomer |
| US5565552A (en) | 1992-01-21 | 1996-10-15 | Pharmacyclics, Inc. | Method of expanded porphyrin-oligonucleotide conjugate synthesis |
| US5574142A (en) | 1992-12-15 | 1996-11-12 | Microprobe Corporation | Peptide linkers for improved oligonucleotide delivery |
| US5578718A (en) | 1990-01-11 | 1996-11-26 | Isis Pharmaceuticals, Inc. | Thiol-derivatized nucleosides |
| US5580731A (en) | 1994-08-25 | 1996-12-03 | Chiron Corporation | N-4 modified pyrimidine deoxynucleotides and oligonucleotide probes synthesized therewith |
| US5585481A (en) | 1987-09-21 | 1996-12-17 | Gen-Probe Incorporated | Linking reagents for nucleotide probes |
| US5587371A (en) | 1992-01-21 | 1996-12-24 | Pharmacyclics, Inc. | Texaphyrin-oligonucleotide conjugates |
| US5591317A (en) | 1994-02-16 | 1997-01-07 | Pitts, Jr.; M. Michael | Electrostatic device for water treatment |
| US5595726A (en) | 1992-01-21 | 1997-01-21 | Pharmacyclics, Inc. | Chromophore probe for detection of nucleic acid |
| US5597696A (en) | 1994-07-18 | 1997-01-28 | Becton Dickinson And Company | Covalent cyanine dye oligonucleotide conjugates |
| US5599928A (en) | 1994-02-15 | 1997-02-04 | Pharmacyclics, Inc. | Texaphyrin compounds having improved functionalization |
| US5608046A (en) | 1990-07-27 | 1997-03-04 | Isis Pharmaceuticals, Inc. | Conjugated 4'-desmethyl nucleoside analog compounds |
| US5610288A (en) | 1993-01-27 | 1997-03-11 | Hekton Institute For Medical Research | Antisense polynucleotide inhibition of epidermal human growth factor receptor expression |
| US5614617A (en) | 1990-07-27 | 1997-03-25 | Isis Pharmaceuticals, Inc. | Nuclease resistant, pyrimidine modified oligonucleotides that detect and modulate gene expression |
| US5631359A (en) | 1994-10-11 | 1997-05-20 | Ribozyme Pharmaceuticals, Inc. | Hairpin ribozymes |
| US5672685A (en) | 1995-10-04 | 1997-09-30 | Duke University | Source of apolipoprotein E and method of isolating apolipoprotein E |
| US5672662A (en) | 1995-07-07 | 1997-09-30 | Shearwater Polymers, Inc. | Poly(ethylene glycol) and related polymers monosubstituted with propionic or butanoic acids and functional derivatives thereof for biotechnical applications |
| US5688941A (en) | 1990-07-27 | 1997-11-18 | Isis Pharmaceuticals, Inc. | Methods of making conjugated 4' desmethyl nucleoside analog compounds |
| US5714166A (en) | 1986-08-18 | 1998-02-03 | The Dow Chemical Company | Bioactive and/or targeted dendrimer conjugates |
| US5718709A (en) | 1988-09-24 | 1998-02-17 | Considine; John | Apparatus for removing tumours from hollow organs of the body |
| US5721114A (en) | 1992-12-11 | 1998-02-24 | Pharmacia & Upjohn Aktiebolag | Expression system for producing apolipoprotein AI-M |
| US5739119A (en) | 1996-11-15 | 1998-04-14 | Galli; Rachel L. | Antisense oligonucleotides specific for the muscarinic type 2 acetylcholine receptor MRNA |
| US5747470A (en) | 1995-06-07 | 1998-05-05 | Gen-Probe Incorporated | Method for inhibiting cellular proliferation using antisense oligonucleotides to gp130 mRNA |
| US5759829A (en) | 1986-03-28 | 1998-06-02 | Calgene, Inc. | Antisense regulation of gene expression in plant cells |
| US5783683A (en) | 1995-01-10 | 1998-07-21 | Genta Inc. | Antisense oligonucleotides which reduce expression of the FGFRI gene |
| US5789573A (en) | 1990-08-14 | 1998-08-04 | Isis Pharmaceuticals, Inc. | Antisense inhibition of ICAM-1, E-selectin, and CMV IE1/IE2 |
| US5801154A (en) | 1993-10-18 | 1998-09-01 | Isis Pharmaceuticals, Inc. | Antisense oligonucleotide modulation of multidrug resistance-associated protein |
| US5820873A (en) | 1994-09-30 | 1998-10-13 | The University Of British Columbia | Polyethylene glycol modified ceramide lipids and liposome uses thereof |
| US5834596A (en) | 1995-03-03 | 1998-11-10 | Pharmacia & Upjohn Ab | Process for purifying ApoA or ApoE |
| US5840688A (en) | 1994-03-22 | 1998-11-24 | Research Corporation Technologies, Inc. | Eating suppressant peptides |
| US5876968A (en) | 1991-12-13 | 1999-03-02 | Pharmacia & Upjohn Aktiebolag | Dimer of molecular variant of apolipoprotein and processes for the production thereof |
| US5885613A (en) | 1994-09-30 | 1999-03-23 | The University Of British Columbia | Bilayer stabilizing components and their use in forming programmable fusogenic liposomes |
| US5976567A (en) | 1995-06-07 | 1999-11-02 | Inex Pharmaceuticals Corp. | Lipid-nucleic acid particles prepared via a hydrophobic lipid-nucleic acid complex intermediate and use for gene transfer |
| US6004925A (en) | 1997-09-29 | 1999-12-21 | J. L. Dasseux | Apolipoprotein A-I agonists and their use to treat dyslipidemic disorders |
| US6027726A (en) | 1994-09-30 | 2000-02-22 | Inex Phamaceuticals Corp. | Glycosylated protein-liposome conjugates and methods for their preparation |
| US6037323A (en) | 1997-09-29 | 2000-03-14 | Jean-Louis Dasseux | Apolipoprotein A-I agonists and their use to treat dyslipidemic disorders |
| US6046166A (en) | 1997-09-29 | 2000-04-04 | Jean-Louis Dasseux | Apolipoprotein A-I agonists and their use to treat dyslipidemic disorders |
| WO2000044895A1 (en) | 1999-01-30 | 2000-08-03 | Roland Kreutzer | Method and medicament for inhibiting the expression of a defined gene |
| US6153737A (en) | 1990-01-11 | 2000-11-28 | Isis Pharmaceuticals, Inc. | Derivatized oligonucleotides having improved uptake and other properties |
| US6172208B1 (en) | 1992-07-06 | 2001-01-09 | Genzyme Corporation | Oligonucleotides modified with conjugate groups |
| US6287591B1 (en) | 1997-05-14 | 2001-09-11 | Inex Pharmaceuticals Corp. | Charged therapeutic agents encapsulated in lipid particles containing four lipid components |
| US6300319B1 (en) | 1998-06-16 | 2001-10-09 | Isis Pharmaceuticals, Inc. | Targeted oligonucleotide conjugates |
| WO2001075164A2 (en) | 2000-03-30 | 2001-10-11 | Whitehead Institute For Biomedical Research | Rna sequence-specific mediators of rna interference |
| US6320017B1 (en) | 1997-12-23 | 2001-11-20 | Inex Pharmaceuticals Corp. | Polyamide oligomers |
| US6335437B1 (en) | 1998-09-07 | 2002-01-01 | Isis Pharmaceuticals, Inc. | Methods for the preparation of conjugated oligomers |
| US6335434B1 (en) | 1998-06-16 | 2002-01-01 | Isis Pharmaceuticals, Inc., | Nucleosidic and non-nucleosidic folate conjugates |
| US6372886B1 (en) | 1992-06-23 | 2002-04-16 | Arch Development Corp. | Expression and purification of kringle domains of human apolipoprotein (a) in E. coli |
| US6395437B1 (en) | 1999-10-29 | 2002-05-28 | Advanced Micro Devices, Inc. | Junction profiling using a scanning voltage micrograph |
| WO2002044321A2 (en) | 2000-12-01 | 2002-06-06 | MAX-PLANCK-Gesellschaft zur Förderung der Wissenschaften e.V. | Rna interference mediating small rna molecules |
| US6444806B1 (en) | 1996-04-30 | 2002-09-03 | Hisamitsu Pharmaceutical Co., Inc. | Conjugates and methods of forming conjugates of oligonucleotides and carbohydrates |
| US6486308B2 (en) | 1995-04-03 | 2002-11-26 | Epoch Biosciences, Inc. | Covalently linked oligonucleotide minor groove binder conjugates |
| US6528631B1 (en) | 1993-09-03 | 2003-03-04 | Isis Pharmaceuticals, Inc. | Oligonucleotide-folate conjugates |
| US6559279B1 (en) | 2000-09-08 | 2003-05-06 | Isis Pharmaceuticals, Inc. | Process for preparing peptide derivatized oligomeric compounds |
| WO2007095387A2 (en) | 2006-02-17 | 2007-08-23 | Dharmacon, Inc. | Compositions and methods for inhibiting gene silencing by rna interference |
| WO2008036825A2 (en) | 2006-09-22 | 2008-03-27 | Dharmacon, Inc. | Duplex oligonucleotide complexes and methods for gene silencing by rna interference |
| WO2010054401A1 (en) | 2008-11-10 | 2010-05-14 | Alnylam Pharmaceuticals, Inc. | Novel lipids and compositions for the delivery of therapeutics |
| WO2010054384A1 (en) | 2008-11-10 | 2010-05-14 | Alnylam Pharmaceuticals, Inc. | Lipids and compositions for the delivery of therapeutics |
| US9204305B2 (en) | 2009-08-27 | 2015-12-01 | Siemens Aktiengesellschaft | Method for transmitting data in a sensor network, sensor node and central processor |
| US9204294B2 (en) | 2010-07-09 | 2015-12-01 | Telecommunication Systems, Inc. | Location privacy selector |
| US11598905B2 (en) | 2020-03-19 | 2023-03-07 | Korea Institute Of Science And Technology | Inverted nanocone structure for optical device and method of producing the same |
Family Cites Families (21)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US4007281A (en) * | 1971-04-16 | 1977-02-08 | Colgate-Palmolive Company | Pharmaceutical compositions containing quaternary ammonium compounds |
| US4054604A (en) | 1974-01-10 | 1977-10-18 | American Cyanamid Company | Amide derivatives of 9-oxo-13-trans-prostenoic acid |
| DE3402146A1 (en) * | 1984-01-23 | 1985-07-25 | Henkel KGaA, 4000 Düsseldorf | Novel quaternary ammonium compounds, their preparation and use as textile softeners |
| JP2563435B2 (en) * | 1988-02-10 | 1996-12-11 | 花王株式会社 | Acaricide |
| US5429755A (en) * | 1994-06-16 | 1995-07-04 | Lever Brothers Company | Fabric conditioning molecules derived from glycerol and betaine |
| JP3563473B2 (en) | 1995-02-24 | 2004-09-08 | 花王株式会社 | Novel quaternary ammonium salt and method for producing the same |
| US5902604A (en) | 1995-06-06 | 1999-05-11 | Board Of Regents, The University Of Texas System | Submicron liposome suspensions obtained from preliposome lyophilizates |
| JPH11507352A (en) * | 1995-06-07 | 1999-06-29 | ジンタ・インコーポレイテッド | New carbamate-based cationic lipids |
| DE19605279A1 (en) * | 1996-02-13 | 1997-08-14 | Hoechst Ag | Target cell-specific vectors for the introduction of genes into cells, drugs containing such vectors and their use |
| DE19605175A1 (en) * | 1996-02-13 | 1997-08-14 | Sourovoi Andrej Dr | Lipid compounds and their use |
| FR2756824B1 (en) * | 1996-12-11 | 1999-01-15 | Oreal | ESTER-FUNCTIONAL HYDROXYPROPYL QUATERNARY AMMONIUM DERIVATIVES, COSMETIC AND DERMATOLOGICAL COMPOSITIONS CONTAINING THEM |
| JP2002521423A (en) | 1998-07-31 | 2002-07-16 | コリア インスティチュート オブ サイエンス アンド テクノロージ | Lipid emulsions and solid lipid microparticles as gene or drug carriers |
| US6200599B1 (en) * | 1999-10-07 | 2001-03-13 | The Regents Of The University Of California | Ortho ester lipids |
| WO2002009678A2 (en) * | 2000-07-31 | 2002-02-07 | Ottawa Heart Institute Research Corporation | Charged phospholipid compositions and methods for their use |
| JP5042863B2 (en) | 2005-02-14 | 2012-10-03 | サーナ・セラピューティクス・インコーポレイテッド | Lipid nanoparticle-based compositions and methods for delivering biologically active molecules |
| EP1844772A1 (en) * | 2006-04-06 | 2007-10-17 | Polyplus-Transfection SA | Compositions for transfection of oligonucleotides active for gene silencing and their biological and therapeutical applications |
| WO2009086558A1 (en) | 2008-01-02 | 2009-07-09 | Tekmira Pharmaceuticals Corporation | Improved compositions and methods for the delivery of nucleic acids |
| WO2009132131A1 (en) * | 2008-04-22 | 2009-10-29 | Alnylam Pharmaceuticals, Inc. | Amino lipid based improved lipid formulation |
| JP2014501489A (en) | 2009-07-06 | 2014-01-23 | アルナイラム ファーマシューティカルズ, インコーポレイテッド | Cell-based bioprocessing |
| EP2451823A4 (en) * | 2009-07-06 | 2013-07-03 | Alnylam Pharmaceuticals Inc | Compositions and methods for enhancing production of a biological product |
| JP5806451B2 (en) | 2010-07-21 | 2015-11-10 | キヤノン株式会社 | Image forming apparatus |
-
2010
- 2010-12-07 WO PCT/US2010/059206 patent/WO2011071860A2/en active Application Filing
- 2010-12-07 EP EP10836504.0A patent/EP2509636B1/en active Active
- 2010-12-07 AU AU2010328336A patent/AU2010328336B2/en active Active
- 2010-12-07 CA CA3044884A patent/CA3044884A1/en not_active Abandoned
- 2010-12-07 EP EP17001185.2A patent/EP3296398A1/en not_active Withdrawn
- 2010-12-07 US US13/514,352 patent/US9687550B2/en active Active
- 2010-12-07 CA CA2783372A patent/CA2783372C/en active Active
-
2017
- 2017-03-31 AU AU2017202153A patent/AU2017202153A1/en not_active Abandoned
- 2017-05-16 US US15/596,845 patent/US20180369384A1/en not_active Abandoned
-
2018
- 2018-09-13 HK HK18111797.2A patent/HK1252488A1/en unknown
- 2018-12-10 AU AU2018275020A patent/AU2018275020A1/en not_active Abandoned
Patent Citations (166)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US3687808A (en) | 1969-08-14 | 1972-08-29 | Univ Leland Stanford Junior | Synthetic polynucleotides |
| US3993754A (en) | 1974-10-09 | 1976-11-23 | The United States Of America As Represented By The United States Energy Research And Development Administration | Liposome-encapsulated actinomycin for cancer chemotherapy |
| US4145410A (en) | 1976-10-12 | 1979-03-20 | Sears Barry D | Method of preparing a controlled-release pharmaceutical preparation, and resulting composition |
| US4224179A (en) | 1977-08-05 | 1980-09-23 | Battelle Memorial Institute | Process for the preparation of liposomes in aqueous solution |
| US4235871A (en) | 1978-02-24 | 1980-11-25 | Papahadjopoulos Demetrios P | Method of encapsulating biologically active materials in lipid vesicles |
| US4469863A (en) | 1980-11-12 | 1984-09-04 | Ts O Paul O P | Nonionic nucleic acid alkyl and aryl phosphonates and processes for manufacture and use thereof |
| US5023243A (en) | 1981-10-23 | 1991-06-11 | Molecular Biosystems, Inc. | Oligonucleotide therapeutic agent and method of making same |
| US4667025A (en) | 1982-08-09 | 1987-05-19 | Wakunaga Seiyaku Kabushiki Kaisha | Oligonucleotide derivatives |
| US4789737A (en) | 1982-08-09 | 1988-12-06 | Wakunaga Seiyaku Kabushiki Kaisha | Oligonucleotide derivatives and production thereof |
| US4603044A (en) | 1983-01-06 | 1986-07-29 | Technology Unlimited, Inc. | Hepatocyte Directed Vesicle delivery system |
| US4835263A (en) | 1983-01-27 | 1989-05-30 | Centre National De La Recherche Scientifique | Novel compounds containing an oligonucleotide sequence bonded to an intercalating agent, a process for their synthesis and their use |
| US4522803A (en) | 1983-02-04 | 1985-06-11 | The Liposome Company, Inc. | Stable plurilamellar vesicles, their preparation and use |
| US4605735A (en) | 1983-02-14 | 1986-08-12 | Wakunaga Seiyaku Kabushiki Kaisha | Oligonucleotide derivatives |
| US5541313A (en) | 1983-02-22 | 1996-07-30 | Molecular Biosystems, Inc. | Single-stranded labelled oligonucleotides of preselected sequence |
| US4948882A (en) | 1983-02-22 | 1990-08-14 | Syngene, Inc. | Single-stranded labelled oligonucleotides, reactive monomers and methods of synthesis |
| US4824941A (en) | 1983-03-10 | 1989-04-25 | Julian Gordon | Specific antibody to the native form of 2'5'-oligonucleotides, the method of preparation and the use as reagents in immunoassays or for binding 2'5'-oligonucleotides in biological systems |
| US4588578A (en) | 1983-08-08 | 1986-05-13 | The Liposome Company, Inc. | Lipid vesicles prepared in a monophase |
| US4587044A (en) | 1983-09-01 | 1986-05-06 | The Johns Hopkins University | Linkage of proteins to nucleic acids |
| US5118802A (en) | 1983-12-20 | 1992-06-02 | California Institute Of Technology | DNA-reporter conjugates linked via the 2' or 5'-primary amino group of the 5'-terminal nucleoside |
| US5258506A (en) | 1984-10-16 | 1993-11-02 | Chiron Corporation | Photolabile reagents for incorporation into oligonucleotide chains |
| US5578717A (en) | 1984-10-16 | 1996-11-26 | Chiron Corporation | Nucleotides for introducing selectably cleavable and/or abasic sites into oligonucleotides |
| US5116739A (en) | 1984-10-16 | 1992-05-26 | Mitsubishi Chemical Industries Limited | Process for the production of human apolipoprotein e, and transformed hosts and products thereof |
| US5545730A (en) | 1984-10-16 | 1996-08-13 | Chiron Corporation | Multifunctional nucleic acid monomer |
| US5552538A (en) | 1984-10-16 | 1996-09-03 | Chiron Corporation | Oligonucleotides with cleavable sites |
| US4828979A (en) | 1984-11-08 | 1989-05-09 | Life Technologies, Inc. | Nucleotide analogs for nucleic acid labeling and detection |
| WO1986004920A1 (en) | 1985-02-13 | 1986-08-28 | Biotechnology Research Partners, Limited | Human metallothionein-ii promoter in mammalian expression system |
| US5142047A (en) | 1985-03-15 | 1992-08-25 | Anti-Gene Development Group | Uncharged polynucleotide-binding polymers |
| US5235033A (en) | 1985-03-15 | 1993-08-10 | Anti-Gene Development Group | Alpha-morpholino ribonucleoside derivatives and polymers thereof |
| US4762779A (en) | 1985-06-13 | 1988-08-09 | Amgen Inc. | Compositions and methods for functionalizing nucleic acids |
| WO1987002062A1 (en) | 1985-10-04 | 1987-04-09 | Biotechnology Research Partners, Ltd. | Recombinant apolipoproteins and methods |
| US4737323A (en) | 1986-02-13 | 1988-04-12 | Liposome Technology, Inc. | Liposome extrusion method |
| US5317098A (en) | 1986-03-17 | 1994-05-31 | Hiroaki Shizuya | Non-radioisotope tagging of fragments |
| US5759829A (en) | 1986-03-28 | 1998-06-02 | Calgene, Inc. | Antisense regulation of gene expression in plant cells |
| US4876335A (en) | 1986-06-30 | 1989-10-24 | Wakunaga Seiyaku Kabushiki Kaisha | Poly-labelled oligonucleotide derivative |
| US5714166A (en) | 1986-08-18 | 1998-02-03 | The Dow Chemical Company | Bioactive and/or targeted dendrimer conjugates |
| US4987071A (en) | 1986-12-03 | 1991-01-22 | University Patents, Inc. | RNA ribozyme polymerases, dephosphorylases, restriction endoribonucleases and methods |
| US5059528A (en) | 1987-05-28 | 1991-10-22 | Ucb, S.A. | Expression of human proapolipoprotein a-i |
| US4904582A (en) | 1987-06-11 | 1990-02-27 | Synthetic Genetics | Novel amphiphilic nucleic acid conjugates |
| US5585481A (en) | 1987-09-21 | 1996-12-17 | Gen-Probe Incorporated | Linking reagents for nucleotide probes |
| US5525465A (en) | 1987-10-28 | 1996-06-11 | Howard Florey Institute Of Experimental Physiology And Medicine | Oligonucleotide-polyamide conjugates and methods of production and applications of the same |
| US5112963A (en) | 1987-11-12 | 1992-05-12 | Max-Planck-Gesellschaft Zur Foerderung Der Wissenschaften E.V. | Modified oligonucleotides |
| US5082830A (en) | 1988-02-26 | 1992-01-21 | Enzo Biochem, Inc. | End labeled nucleotide probe |
| US5109124A (en) | 1988-06-01 | 1992-04-28 | Biogen, Inc. | Nucleic acid probe linked to a label having a terminal cysteine |
| US5149782A (en) | 1988-08-19 | 1992-09-22 | Tanox Biosystems, Inc. | Molecular conjugates containing cell membrane-blending agents |
| US5262536A (en) | 1988-09-15 | 1993-11-16 | E. I. Du Pont De Nemours And Company | Reagents for the preparation of 5'-tagged oligonucleotides |
| EP0360257A2 (en) | 1988-09-20 | 1990-03-28 | The Board Of Regents For Northern Illinois University | RNA catalyst for cleaving specific RNA sequences |
| US5718709A (en) | 1988-09-24 | 1998-02-17 | Considine; John | Apparatus for removing tumours from hollow organs of the body |
| US5512439A (en) | 1988-11-21 | 1996-04-30 | Dynal As | Oligonucleotide-linked magnetic particles and uses thereof |
| US4957773A (en) | 1989-02-13 | 1990-09-18 | Syracuse University | Deposition of boron-containing films from decaborane |
| US5599923A (en) | 1989-03-06 | 1997-02-04 | Board Of Regents, University Of Tx | Texaphyrin metal complexes having improved functionalization |
| US5391723A (en) | 1989-05-31 | 1995-02-21 | Neorx Corporation | Oligonucleotide conjugates |
| US5256775A (en) | 1989-06-05 | 1993-10-26 | Gilead Sciences, Inc. | Exonuclease-resistant oligonucleotides |
| US4958013A (en) | 1989-06-06 | 1990-09-18 | Northwestern University | Cholesteryl modified oligonucleotides |
| US5416203A (en) | 1989-06-06 | 1995-05-16 | Northwestern University | Steroid modified oligonucleotides |
| US5177189A (en) | 1989-08-18 | 1993-01-05 | The Scripps Research Institute | Polypeptide analogs of Apolipoprotein E |
| US5168045A (en) | 1989-08-18 | 1992-12-01 | The Scripps Research Institute | Diagnostic systems and methods using polypeptide analogs of apolipoprotein e |
| US5473039A (en) | 1989-08-18 | 1995-12-05 | The Scripps Research Institute | Polypeptide analogs of apolipoprotein E, diagnostic systems and methods using the analogs |
| US5451463A (en) | 1989-08-28 | 1995-09-19 | Clontech Laboratories, Inc. | Non-nucleoside 1,3-diol reagents for labeling synthetic oligonucleotides |
| WO1991003162A1 (en) | 1989-08-31 | 1991-03-21 | City Of Hope | Chimeric dna-rna catalytic sequences |
| US5254469A (en) | 1989-09-12 | 1993-10-19 | Eastman Kodak Company | Oligonucleotide-enzyme conjugate that can be used as a probe in hybridization assays and polymerase chain reaction procedures |
| US5286634A (en) | 1989-09-28 | 1994-02-15 | Stadler Joan K | Synergistic method for host cell transformation |
| US5013556A (en) | 1989-10-20 | 1991-05-07 | Liposome Technology, Inc. | Liposomes with enhanced circulation time |
| US5264562A (en) | 1989-10-24 | 1993-11-23 | Gilead Sciences, Inc. | Oligonucleotide analogs with novel linkages |
| US5264564A (en) | 1989-10-24 | 1993-11-23 | Gilead Sciences | Oligonucleotide analogs with novel linkages |
| US5292873A (en) | 1989-11-29 | 1994-03-08 | The Research Foundation Of State University Of New York | Nucleic acids labeled with naphthoquinone probe |
| US5177198A (en) | 1989-11-30 | 1993-01-05 | University Of N.C. At Chapel Hill | Process for preparing oligoribonucleoside and oligodeoxyribonucleoside boranophosphates |
| US5130302A (en) | 1989-12-20 | 1992-07-14 | Boron Bilogicals, Inc. | Boronated nucleoside, nucleotide and oligonucleotide compounds, compositions and methods for using same |
| US5486603A (en) | 1990-01-08 | 1996-01-23 | Gilead Sciences, Inc. | Oligonucleotide having enhanced binding affinity |
| US5578718A (en) | 1990-01-11 | 1996-11-26 | Isis Pharmaceuticals, Inc. | Thiol-derivatized nucleosides |
| US5457191A (en) | 1990-01-11 | 1995-10-10 | Isis Pharmaceuticals, Inc. | 3-deazapurines |
| US6153737A (en) | 1990-01-11 | 2000-11-28 | Isis Pharmaceuticals, Inc. | Derivatized oligonucleotides having improved uptake and other properties |
| US5459255A (en) | 1990-01-11 | 1995-10-17 | Isis Pharmaceuticals, Inc. | N-2 substituted purines |
| US5366878A (en) | 1990-02-15 | 1994-11-22 | The Worcester Foundation For Experimental Biology | Method of site-specific alteration of RNA and production of encoded polypeptides |
| US5214136A (en) | 1990-02-20 | 1993-05-25 | Gilead Sciences, Inc. | Anthraquinone-derivatives oligonucleotides |
| US5414077A (en) | 1990-02-20 | 1995-05-09 | Gilead Sciences | Non-nucleoside linkers for convenient attachment of labels to oligonucleotides using standard synthetic methods |
| US5182364A (en) | 1990-02-26 | 1993-01-26 | The Scripps Research Institute | Polypeptide analogs of apolipoprotein E |
| US5514785A (en) | 1990-05-11 | 1996-05-07 | Becton Dickinson And Company | Solid supports for nucleic acid hybridization assays |
| US5688941A (en) | 1990-07-27 | 1997-11-18 | Isis Pharmaceuticals, Inc. | Methods of making conjugated 4' desmethyl nucleoside analog compounds |
| US5614617A (en) | 1990-07-27 | 1997-03-25 | Isis Pharmaceuticals, Inc. | Nuclease resistant, pyrimidine modified oligonucleotides that detect and modulate gene expression |
| US5608046A (en) | 1990-07-27 | 1997-03-04 | Isis Pharmaceuticals, Inc. | Conjugated 4'-desmethyl nucleoside analog compounds |
| US5218105A (en) | 1990-07-27 | 1993-06-08 | Isis Pharmaceuticals | Polyamine conjugated oligonucleotides |
| US5138045A (en) | 1990-07-27 | 1992-08-11 | Isis Pharmaceuticals | Polyamine conjugated oligonucleotides |
| US5386023A (en) | 1990-07-27 | 1995-01-31 | Isis Pharmaceuticals | Backbone modified oligonucleotide analogs and preparation thereof through reductive coupling |
| US5489677A (en) | 1990-07-27 | 1996-02-06 | Isis Pharmaceuticals, Inc. | Oligonucleoside linkages containing adjacent oxygen and nitrogen atoms |
| US5378825A (en) | 1990-07-27 | 1995-01-03 | Isis Pharmaceuticals, Inc. | Backbone modified oligonucleotide analogs |
| US5245022A (en) | 1990-08-03 | 1993-09-14 | Sterling Drug, Inc. | Exonuclease resistant terminally substituted oligonucleotides |
| US5567810A (en) | 1990-08-03 | 1996-10-22 | Sterling Drug, Inc. | Nuclease resistant compounds |
| US5223618A (en) | 1990-08-13 | 1993-06-29 | Isis Pharmaceuticals, Inc. | 4'-desmethyl nucleoside analog compounds |
| US5789573A (en) | 1990-08-14 | 1998-08-04 | Isis Pharmaceuticals, Inc. | Antisense inhibition of ICAM-1, E-selectin, and CMV IE1/IE2 |
| US5512667A (en) | 1990-08-28 | 1996-04-30 | Reed; Michael W. | Trifunctional intermediates for preparing 3'-tailed oligonucleotides |
| WO1992007065A1 (en) | 1990-10-12 | 1992-04-30 | MAX-PLANCK-Gesellschaft zur Förderung der Wissenschaften e.V. | Modified ribozymes |
| US5510475A (en) | 1990-11-08 | 1996-04-23 | Hybridon, Inc. | Oligonucleotide multiple reporter precursors |
| WO1992020823A1 (en) | 1991-05-21 | 1992-11-26 | Isis Pharmaceuticals, Inc. | Backbone modified oligonucleotide analogs |
| WO1992020822A1 (en) | 1991-05-21 | 1992-11-26 | Isis Pharmaceuticals, Inc. | Backbone modified oligonucleotide analogues |
| EP0519463A1 (en) | 1991-06-20 | 1992-12-23 | Europäisches Laboratorium Für Molekularbiologie (Embl) | Synthetic, catalytic oligonucleotides |
| US5334711A (en) | 1991-06-20 | 1994-08-02 | Europaisches Laboratorium Fur Molekularbiologie (Embl) | Synthetic catalytic oligonucleotide structures |
| US5525472A (en) | 1991-06-26 | 1996-06-11 | Bio-Technology General Corp. | Method for production and purification or recombinant Apolipoprotein E from bacteria |
| US5371241A (en) | 1991-07-19 | 1994-12-06 | Pharmacia P-L Biochemicals Inc. | Fluorescein labelled phosphoramidites |
| US5484908A (en) | 1991-11-26 | 1996-01-16 | Gilead Sciences, Inc. | Oligonucleotides containing 5-propynyl pyrimidines |
| US5876968A (en) | 1991-12-13 | 1999-03-02 | Pharmacia & Upjohn Aktiebolag | Dimer of molecular variant of apolipoprotein and processes for the production thereof |
| US5359044A (en) | 1991-12-13 | 1994-10-25 | Isis Pharmaceuticals | Cyclobutyl oligonucleotide surrogates |
| US5565552A (en) | 1992-01-21 | 1996-10-15 | Pharmacyclics, Inc. | Method of expanded porphyrin-oligonucleotide conjugate synthesis |
| US5595726A (en) | 1992-01-21 | 1997-01-21 | Pharmacyclics, Inc. | Chromophore probe for detection of nucleic acid |
| US5587371A (en) | 1992-01-21 | 1996-12-24 | Pharmacyclics, Inc. | Texaphyrin-oligonucleotide conjugates |
| WO1993015187A1 (en) | 1992-01-31 | 1993-08-05 | Massachusetts Institute Of Technology | Nucleozymes |
| WO1993023569A1 (en) | 1992-05-11 | 1993-11-25 | Ribozyme Pharmaceuticals, Inc. | Method and reagent for inhibiting viral replication |
| US6372886B1 (en) | 1992-06-23 | 2002-04-16 | Arch Development Corp. | Expression and purification of kringle domains of human apolipoprotein (a) in E. coli |
| US6172208B1 (en) | 1992-07-06 | 2001-01-09 | Genzyme Corporation | Oligonucleotides modified with conjugate groups |
| US5272250A (en) | 1992-07-10 | 1993-12-21 | Spielvogel Bernard F | Boronated phosphoramidate compounds |
| WO1994002595A1 (en) | 1992-07-17 | 1994-02-03 | Ribozyme Pharmaceuticals, Inc. | Method and reagent for treatment of animal diseases |
| WO1994013688A1 (en) | 1992-12-08 | 1994-06-23 | Gene Shears Pty. Limited | Dna-armed ribozymes and minizymes |
| US5721114A (en) | 1992-12-11 | 1998-02-24 | Pharmacia & Upjohn Aktiebolag | Expression system for producing apolipoprotein AI-M |
| US5574142A (en) | 1992-12-15 | 1996-11-12 | Microprobe Corporation | Peptide linkers for improved oligonucleotide delivery |
| US5610288A (en) | 1993-01-27 | 1997-03-11 | Hekton Institute For Medical Research | Antisense polynucleotide inhibition of epidermal human growth factor receptor expression |
| US5476925A (en) | 1993-02-01 | 1995-12-19 | Northwestern University | Oligodeoxyribonucleotides including 3'-aminonucleoside-phosphoramidate linkages and terminal 3'-amino groups |
| US5508270A (en) | 1993-03-06 | 1996-04-16 | Ciba-Geigy Corporation | Nucleoside phosphinate compounds and compositions |
| US5532130A (en) | 1993-07-20 | 1996-07-02 | Dyad Pharmaceutical Corporation | Methods and compositions for sequence-specific hybridization of RNA by 2'-5' oligonucleotides |
| US6528631B1 (en) | 1993-09-03 | 2003-03-04 | Isis Pharmaceuticals, Inc. | Oligonucleotide-folate conjugates |
| US5801154A (en) | 1993-10-18 | 1998-09-01 | Isis Pharmaceuticals, Inc. | Antisense oligonucleotide modulation of multidrug resistance-associated protein |
| US5519134A (en) | 1994-01-11 | 1996-05-21 | Isis Pharmaceuticals, Inc. | Pyrrolidine-containing monomers and oligomers |
| US5599928A (en) | 1994-02-15 | 1997-02-04 | Pharmacyclics, Inc. | Texaphyrin compounds having improved functionalization |
| US5591317A (en) | 1994-02-16 | 1997-01-07 | Pitts, Jr.; M. Michael | Electrostatic device for water treatment |
| US5539083A (en) | 1994-02-23 | 1996-07-23 | Isis Pharmaceuticals, Inc. | Peptide nucleic acid combinatorial libraries and improved methods of synthesis |
| US5840688A (en) | 1994-03-22 | 1998-11-24 | Research Corporation Technologies, Inc. | Eating suppressant peptides |
| US5534499A (en) | 1994-05-19 | 1996-07-09 | The University Of British Columbia | Lipophilic drug derivatives for use in liposomes |
| US5597696A (en) | 1994-07-18 | 1997-01-28 | Becton Dickinson And Company | Covalent cyanine dye oligonucleotide conjugates |
| US5580731A (en) | 1994-08-25 | 1996-12-03 | Chiron Corporation | N-4 modified pyrimidine deoxynucleotides and oligonucleotide probes synthesized therewith |
| US5591584A (en) | 1994-08-25 | 1997-01-07 | Chiron Corporation | N-4 modified pyrimidine deoxynucleotides and oligonucleotide probes synthesized therewith |
| US5820873A (en) | 1994-09-30 | 1998-10-13 | The University Of British Columbia | Polyethylene glycol modified ceramide lipids and liposome uses thereof |
| US5885613A (en) | 1994-09-30 | 1999-03-23 | The University Of British Columbia | Bilayer stabilizing components and their use in forming programmable fusogenic liposomes |
| US6027726A (en) | 1994-09-30 | 2000-02-22 | Inex Phamaceuticals Corp. | Glycosylated protein-liposome conjugates and methods for their preparation |
| WO1996011266A2 (en) | 1994-10-05 | 1996-04-18 | Amgen Inc. | Method for inhibiting smooth muscle cell proliferation and oligonucleotides for use therein |
| US5631359A (en) | 1994-10-11 | 1997-05-20 | Ribozyme Pharmaceuticals, Inc. | Hairpin ribozymes |
| US5783683A (en) | 1995-01-10 | 1998-07-21 | Genta Inc. | Antisense oligonucleotides which reduce expression of the FGFRI gene |
| US5834596A (en) | 1995-03-03 | 1998-11-10 | Pharmacia & Upjohn Ab | Process for purifying ApoA or ApoE |
| US6486308B2 (en) | 1995-04-03 | 2002-11-26 | Epoch Biosciences, Inc. | Covalently linked oligonucleotide minor groove binder conjugates |
| US5747470A (en) | 1995-06-07 | 1998-05-05 | Gen-Probe Incorporated | Method for inhibiting cellular proliferation using antisense oligonucleotides to gp130 mRNA |
| US5976567A (en) | 1995-06-07 | 1999-11-02 | Inex Pharmaceuticals Corp. | Lipid-nucleic acid particles prepared via a hydrophobic lipid-nucleic acid complex intermediate and use for gene transfer |
| US5672662A (en) | 1995-07-07 | 1997-09-30 | Shearwater Polymers, Inc. | Poly(ethylene glycol) and related polymers monosubstituted with propionic or butanoic acids and functional derivatives thereof for biotechnical applications |
| US5672685A (en) | 1995-10-04 | 1997-09-30 | Duke University | Source of apolipoprotein E and method of isolating apolipoprotein E |
| US6444806B1 (en) | 1996-04-30 | 2002-09-03 | Hisamitsu Pharmaceutical Co., Inc. | Conjugates and methods of forming conjugates of oligonucleotides and carbohydrates |
| US5739119A (en) | 1996-11-15 | 1998-04-14 | Galli; Rachel L. | Antisense oligonucleotides specific for the muscarinic type 2 acetylcholine receptor MRNA |
| US6287591B1 (en) | 1997-05-14 | 2001-09-11 | Inex Pharmaceuticals Corp. | Charged therapeutic agents encapsulated in lipid particles containing four lipid components |
| US6858225B2 (en) | 1997-05-14 | 2005-02-22 | Inex Pharmaceuticals Corporation | Lipid-encapsulated polyanionic nucleic acid |
| US6046166A (en) | 1997-09-29 | 2000-04-04 | Jean-Louis Dasseux | Apolipoprotein A-I agonists and their use to treat dyslipidemic disorders |
| US6004925A (en) | 1997-09-29 | 1999-12-21 | J. L. Dasseux | Apolipoprotein A-I agonists and their use to treat dyslipidemic disorders |
| US6037323A (en) | 1997-09-29 | 2000-03-14 | Jean-Louis Dasseux | Apolipoprotein A-I agonists and their use to treat dyslipidemic disorders |
| US6320017B1 (en) | 1997-12-23 | 2001-11-20 | Inex Pharmaceuticals Corp. | Polyamide oligomers |
| US6335434B1 (en) | 1998-06-16 | 2002-01-01 | Isis Pharmaceuticals, Inc., | Nucleosidic and non-nucleosidic folate conjugates |
| US6300319B1 (en) | 1998-06-16 | 2001-10-09 | Isis Pharmaceuticals, Inc. | Targeted oligonucleotide conjugates |
| US6525031B2 (en) | 1998-06-16 | 2003-02-25 | Isis Pharmaceuticals, Inc. | Targeted Oligonucleotide conjugates |
| US6335437B1 (en) | 1998-09-07 | 2002-01-01 | Isis Pharmaceuticals, Inc. | Methods for the preparation of conjugated oligomers |
| WO2000044895A1 (en) | 1999-01-30 | 2000-08-03 | Roland Kreutzer | Method and medicament for inhibiting the expression of a defined gene |
| US6395437B1 (en) | 1999-10-29 | 2002-05-28 | Advanced Micro Devices, Inc. | Junction profiling using a scanning voltage micrograph |
| WO2001075164A2 (en) | 2000-03-30 | 2001-10-11 | Whitehead Institute For Biomedical Research | Rna sequence-specific mediators of rna interference |
| US6559279B1 (en) | 2000-09-08 | 2003-05-06 | Isis Pharmaceuticals, Inc. | Process for preparing peptide derivatized oligomeric compounds |
| WO2002044321A2 (en) | 2000-12-01 | 2002-06-06 | MAX-PLANCK-Gesellschaft zur Förderung der Wissenschaften e.V. | Rna interference mediating small rna molecules |
| WO2007095387A2 (en) | 2006-02-17 | 2007-08-23 | Dharmacon, Inc. | Compositions and methods for inhibiting gene silencing by rna interference |
| WO2008036825A2 (en) | 2006-09-22 | 2008-03-27 | Dharmacon, Inc. | Duplex oligonucleotide complexes and methods for gene silencing by rna interference |
| WO2010054401A1 (en) | 2008-11-10 | 2010-05-14 | Alnylam Pharmaceuticals, Inc. | Novel lipids and compositions for the delivery of therapeutics |
| WO2010054405A1 (en) | 2008-11-10 | 2010-05-14 | Alnylam Pharmaceuticals, Inc. | Novel lipids and compositions for the delivery of therapeutics |
| WO2010054406A1 (en) | 2008-11-10 | 2010-05-14 | Alnylam Pharmaceuticals, Inc. | Novel lipids and compositions for the delivery of therapeutics |
| WO2010054384A1 (en) | 2008-11-10 | 2010-05-14 | Alnylam Pharmaceuticals, Inc. | Lipids and compositions for the delivery of therapeutics |
| US9204305B2 (en) | 2009-08-27 | 2015-12-01 | Siemens Aktiengesellschaft | Method for transmitting data in a sensor network, sensor node and central processor |
| US9204294B2 (en) | 2010-07-09 | 2015-12-01 | Telecommunication Systems, Inc. | Location privacy selector |
| US11598905B2 (en) | 2020-03-19 | 2023-03-07 | Korea Institute Of Science And Technology | Inverted nanocone structure for optical device and method of producing the same |
Non-Patent Citations (150)
| Title |
|---|
| "Oligonucleotide synthesis, a practical approach", 1984, IRL PRESS |
| "Peptide Nucleic Acids (PNA): Synthesis, Properties and Potential Applications", BIOORGANIC & MEDICINAL CHEMISTRY, vol. 4, 1996, pages 5 - 23 |
| "the Concise Encyclopedia Of Polymer Science And Engineering", 1990, JOHN WILEY & SONS, pages: 858 - 859 |
| "The Proteins", vol. II, 1976, ACADEMIC PRESS, pages: 105 - 237 |
| ABRA, RM ET AL., J. LIPOSOME RES, vol. 12, 2002, pages 1 - 3 |
| AGRAWAL, TRENDS IN BIOTECH., vol. 14, 1996, pages 376 - 387 |
| AKINC, A. ET AL.: "Development of lipidoid-siRNA formulations for systemic delivery of RNAi therapeutics", NAT. BIOTECHNOL., vol. 26, 2008, pages 561 - 569 |
| ALLEN ET AL., BIOCHIMICA ET BIOPHYSICA ACTA, vol. 1237, 1995, pages 99 - 108 |
| ALTSCHUL ET AL., NUCLEIC ACIDS RES., vol. 25, no. 17, 1997, pages 3389 - 3402 |
| AN, H ET AL., J. ORG. CHEM., vol. 66, 2001, pages 2789 - 2801 |
| AOKI ET AL., CANCER GENE THERAPY, vol. 8, 2001, pages 783 - 787 |
| AVIRAM ET AL., ARTERIOSCLER. THROMB. VASE. BIOL, vol. 18, no. 10, 1998, pages 1617 - 1624 |
| AVIRAM ET AL., J. CLIN. INVEST., vol. 101, no. 8, 1998, pages 1581 - 1590 |
| BEAUCAGE, S. L.; IYER, R. P., TETRAHEDRON, vol. 48, 1992, pages 2223 - 2311 |
| BEAUCAGE, S. L.; IYER, R. P., TETRAHEDRON, vol. 49, 1993, pages 6123 - 6194 |
| BEHR, ACC. CHEM. RES., vol. 26, 1993, pages 274 - 278 |
| BENNETT ET AL., MOL. PHARM., vol. 41, 1992, pages 1023 - 1033 |
| BIELICKI; ODA, BIOCHEMISTRY, vol. 41, 2002, pages 2089 - 2096 |
| BILLECKE ET AL., DRUG METAB. DISPOS, vol. 28, no. 11, 2000, pages 1335 - 1342 |
| BLUME ET AL., BIOCHIMICA ET BIOPHYSICA ACTA, vol. 1149, 1993, pages 180 - 184 |
| BODANSZKY ET AL.: "Peptide Synthesis", 1976, JOHN WILEY & SONS |
| BRIGHAM ET AL., AM. J. MED. SCI, vol. 298, 1989, pages 278 - 281 |
| BRIGHAM ET AL., AM. J. SCI, vol. 298, no. 4, 1989, pages 278 - 281 |
| BROWN; SIMPSON, ANNU REV PLANT PHYSIOL PLANT MOL BIOL, vol. 49, 1998, pages 77 - 95 |
| CECH ET AL., CELL, vol. 27, December 1981 (1981-12-01), pages 487 - 496 |
| CHEUNG ET AL., J. LIPID RES., vol. 28, no. 8, 1987, pages 913 - 929 |
| CHUNG ET AL., J. LIPID RES., vol. 21, no. 3, 1980, pages 284 - 291 |
| COLLINS; OLIVE, BIOCHEMISTRY, vol. 32, no. 11, 23 March 1993 (1993-03-23), pages 2795 - 2799 |
| CORMIER,J.F. ET AL., NUCLEIC ACIDS RES., vol. 16, 1988, pages 4583 |
| CROSSTICK ET AL., TETRAHEDRON LETT., vol. 30, 1989, pages 4693 |
| CULLIS ET AL., CHERN PHYS LIPIDS, vol. 40, 1986, pages 127 - 144 |
| CULVER: "Human Gene Therapy", 1994, MARYANN LIEBERT, INC., PUBLISHERS, pages: 70 - 71 |
| DAUM ET AL., J. MOL. MED., vol. 77, 1999, pages 614 - 622 |
| DE FOUGEROLLES, A. ET AL., NATURE REVIEWS, vol. 6, 2007, pages 443 - 453 |
| DEFREES ET AL., JOURNAL OF THE AMERICAN CHEMISTRY SOCIETY, vol. 118, 1996, pages 6101 - 6104 |
| DRAGANOV ET AL., J. BIOL. CHEM., vol. 275, no. 43, 2000, pages 33435 - 33442 |
| DUVERGER ET AL., ARTERIOSCLER. THROMB. VASE. BIOL, vol. 16, no. 12, 1996, pages 1424 - 1429 |
| DUVERGER ET AL., EURO. J. BIOCHEM., vol. 201, no. 2, 1991, pages 373 - 383 |
| DYER ET AL., J. LIPID RES, vol. 36, no. 1, 1995, pages 80 - 88 |
| DYER ET AL., J. LIPID RES., vol. 36, no. 1, 1995, pages 80 - 88 |
| DYER, J. BIOL. CHEM., vol. 266, no. 23, 1991, pages 150009 - 150015 |
| EATON, CURR. OPIN. CHEM. BIOL., vol. 1, 1997, pages 10 - 16 |
| EDGE, M.D. ET AL., J. CHEM. SOC. PERKIN TRANS. 1, 1972, pages 1991 |
| ELLINGTON; SZOSTAK, NATURE, vol. 346, 1990, pages 818 |
| ENGLISCH ET AL., ANGEWANDTE CHEMIE, INTERNATIONAL EDITION, vol. 30, 1991, pages 613 |
| F. ECKSTEIN: "Oligonucleotides and Analogues,A Practical Approach", 1991, IRL PRESS, article "Oligonucleotides and Analogues, A Practical Approach" |
| FAMULOK, CURR. OPIN. STRUCT. BIOL., vol. 9, 1999, pages 324 - 329 |
| FEIGNER, SCIENTIFIC AMERICAN |
| FORSTER; SYMONS, CELL, vol. 49, no. 2, 24 April 1987 (1987-04-24), pages 211 - 220 |
| FRANCESCHINI ET AL., J. BIOL. CHEM., vol. 260, 1985, pages 1632 - 1635 |
| GALBRAITH ET AL., ANTISENSE NUCL. ACID DRUG DES., vol. 4, 1994, pages 201 - 206 |
| GONG ET AL., J. BIOL. CHEM., vol. 277, no. 33, 2002, pages 29919 - 29926 |
| GOODFELLOW, NATURE, vol. 341, 1989, pages 102 - 103 |
| GORACZNIAK ET AL., NATURE BIOTECHNOLOGY, vol. 27, no. 3, 2008, pages 257 - 263 |
| GORDON ET AL., J. BIOL. CHEM., vol. 259, no. 1, 1984, pages 468 - 474 |
| GREEN, T.W.: "PROTECTIVE GROUPS IN ORGANIC SYNTHESIS", 1999, WILEY-INTERSCIENCE |
| GRIFFITHS-JONES S.: "The microRNA Registry", NAR, vol. 32, 2004, pages D 109 - D 111, Retrieved from the Internet <URL:http://microma.sanger.ac.uk/sequences> |
| GRIFFITHS-JONES S; GROCOCK RJ; VAN DONGEN S; BATEMAN A; ENRIGHT AJ.: "miRBase: microRNA sequences, targets and gene nomenclature", NAR, vol. 34, 2006, pages D140 - D144 |
| GUERRIER-TAKADA ET AL., CELL, vol. 35, December 1983 (1983-12-01), pages 849 - 857 |
| HAMPEL ET AL., NUCLEIC ACIDS RES., vol. 18, no. 2, 25 January 1990 (1990-01-25), pages 299 - 304 |
| HAMPEL; TRITZ, BIOCHEMISTRY, vol. 28, no. 12, 13 June 1989 (1989-06-13), pages 4929 - 4933 |
| HAUBNER ET AL., JOUR. NUCL. MED., vol. 42, 2001, pages 326 - 336 |
| HEATH: "Methods in Enzymology", vol. 149, 1987, ACADEMIC PRESS, INC, article "Covalent Attachment of Proteins to Liposomes", pages: 111 - 119 |
| HERMANN; PATEL, SCIENCE, vol. 287, 2000, pages 820 - 825 |
| HILL, J. BIOL. CHEM., vol. 273, no. 47, 1998, pages 30979 - 30984 |
| HIXSON A; POWERS, J. LIPID RE, vol. 32, no. 9, 1991, pages 1529 - 1535 |
| HIXSON; POWERS, J. LIPID RES, vol. 32, no. 9, 1991, pages 1529 - 1535 |
| HOEG ET AL., J. BIOL. CHEM., vol. 261, no. 9, 1986, pages 3911 - 3914 |
| HYDE ET AL., NATURE, vol. 362, 1993, pages 250 - 256 |
| JASKULSKI ET AL., SCIENCE, vol. 240, no. 4858, 10 June 1988 (1988-06-10), pages 1544 - 1546 |
| JIA ET AL., BIOCHEM. BIOPHYS. RES. COMM, vol. 297, 2002, pages 206 - 213 |
| KAWASAKI, J. MED. CHEM., vol. 36, 1993, pages 831 - 841 |
| KIM; CECH, PROC NATL ACAD SCI USA., vol. 84, no. 24, December 1987 (1987-12-01), pages 8788 - 8792 |
| KIRPOTIN ET AL., FEBSLETTERS, vol. 388, 1996, pages 115 - 118 |
| KLIBANOV ET AL., JOURNAL OF LIPOSOME RESEARCH, vol. 2, 1992, pages 321 - 334 |
| KLON ET AL., BIOPHYS. J., vol. 79, no. 3, 2000, pages 1679 - 1687 |
| KRUTZFELDT ET AL., NATURE, vol. 438, 2005, pages 685 - 689 |
| KUNKEL ET AL., BRIT. MED. BULL., vol. 45, no. 3, 1989, pages 630 - 643 |
| LACKNER ET AL., J. BIOL. CHEM., vol. 260, no. 2, 1985, pages 703 - 706 |
| LAM ET AL., NATURE, vol. 354, 1991, pages 82 - 84 |
| LAMBERTON, J.S.; CHRISTIAN, A.T., MOLECULAR BIOTECHNOLOGY, vol. 24, 2003, pages 111 - 119 |
| LEONETTI ET AL., PROC. NATL. ACAD. SCI. (USA), vol. 87, 1990, pages 2448 - 2451 |
| LOVE, K.T. ET AL.: "Lipid-like materials for low-dose, in vivo gene silencing", PNAS, vol. 107, no. 5, 2010, pages 1864 - 1869 |
| MAHON, K.P. ET AL.: "Combinatorial approach to determine functional group effects on lipidoid-mediated siRNA delivery", BIOCONJUG CHEM, vol. 21, no. 8, 18 August 2010 (2010-08-18), pages 1448 - 1454 |
| MANN ET AL., J. CLIN. INVEST, vol. 106, 2000, pages 1071 - 1075 |
| MANNINO ET AL., BIOTECHNIQUES, vol. 6, 1988, pages 682 - 690 |
| MANOHARAN, M. ET AL., ANTISENSE AND NUCLEIC ACID DRUG DEVELOPMENT, vol. 12, 2002, pages 103 - 128 |
| MANOHARAN: "Antisense Research and Applications", 1993, CRC PRESS |
| MARTIN, P., HELV. CHIM. ACTA, vol. 78, 1995, pages 486 - 504 |
| MARTIN, P., HELV. CHIM. ACTA, vol. 79, 1996, pages 1930 - 1938 |
| MCLEAN ET AL., J. BIOL. CHEM., vol. 258, no. 14, 1983, pages 8993 - 9000 |
| MCOMIE: "Protective Groups in Organic Chemistry", 1973, PLENUM PRESS |
| MERRIFIELD, J. AM. CHEM. SOC., vol. 85, 1963, pages 2149 - 2154 |
| MICHEL; WESTHOF, J MOL BIOL., vol. 216, no. 3, 5 December 1990 (1990-12-05), pages 585 - 610 |
| MULUGETA ET AL., J. CHROMATOGR., vol. 798, no. 1-2, 1998, pages 83 - 90 |
| NICOLAU ET AL., CRIT. REV. THER. DRUG CARRIER SYST, vol. 6, 1989, pages 239 - 271 |
| OBIKA, S. ET AL., BIOORGANIC & MEDICINAL CHEMISTRY, vol. 9, no. 2, 2001, pages 245 - 254 |
| OHTA ET AL., J. BIOL. CHEM., vol. 259, no. 23, 1984, pages 14888 - 14893 |
| PALGUNACHARI, ARTERIOSCLER. THROB. VASE. BIOL., vol. 16, no. 2, 1996, pages 328 - 338 |
| PERIS ET AL., BRAIN RES MOL BRAIN RES, vol. 57, no. 2, 15 June 1998 (1998-06-15), pages 310 - 320 |
| PERROTTA; BEEN, BIOCHEMISTRY, vol. 31, no. 47, 1 December 1992 (1992-12-01), pages 11843 - 11852 |
| PERSSON ET AL., J. CHROMATOGR, vol. 711, 1998, pages 97 - 109 |
| POWELL ET AL., CELL, vol. 50, no. 6, 1987, pages 831 - 840 |
| RAIL ET AL.: "Structural basis for receptor binding heterogeneity of apolipoprotein E from type III hyperlipoproteinemic subjects", PROC. NAT. ACAD. SCI., vol. 79, 1982, pages 4696 - 4700 |
| RANEY ET AL., JOURNAL OF PHARMACOLOGY AND EXPERIMENTAL THERAPEUTICS, vol. 298, 2001, pages 1185 - 1192 |
| REINHOLD-HUREK; SHUB, NATURE, vol. 357, no. 6374, 14 May 1992 (1992-05-14), pages 173 - 176 |
| RENNEISEN ET AL., J. BIO. CHEM., vol. 265, 1990, pages 16337 - 16342 |
| ROOSJEN, A. ET AL., EUR J ORG CHEM, vol. 7, pages 1271 - 1277 |
| ROSSI ET AL., NUCLEIC ACIDS RES., vol. 20, no. 17, 11 September 1992 (1992-09-11), pages 4559 - 4565 |
| SACRE ET AL., FEBS LETT., vol. 540, no. 1-3, 2003, pages 181 - 187 |
| SAPRA, P.; ALLEN, TM, PROG. LIPID RES., vol. 42, no. 5, 2003, pages 439 - 462 |
| SAVILLE; COLLINS, CELL, vol. 61, no. 4, 18 May 1990 (1990-05-18), pages 685 - 696 |
| SAVILLE; COLLINS, PROC NATL ACAD SCI U S A., vol. 88, no. 19, 1 October 1991 (1991-10-01), pages 8826 - 8830 |
| See also references of EP2509636A4 |
| SHELNESS ET AL., J. BIOL. CHEM., vol. 259, no. 15, 1984, pages 9929 - 9935 |
| SHELNESS ET AL., J. BIOL. CHEM., vol. 260, no. 14, 1985, pages 8637 - 8646 |
| SIMEONI ET AL., NUCL. ACIDS RES, vol. 31, 2003, pages 2717 - 2724 |
| SORENSON ET AL., ARTERIOSCLER. THROMB. VASE. BIOL., vol. 19, no. 9, 1999, pages 2214 - 2225 |
| SPROAT ET AL., NUCLEOSIDES NUCLEOTIDES, vol. 7, 1988, pages 651 |
| STEINMETZ; UTERMANN, J. BIOL. CHEM., vol. 260, no. 4, 1985, pages 2258 - 2264 |
| STIRCHAK, E.P., NUCLEIC ACIDS RES., vol. 17, 1989, pages 6129 |
| STRAUBRINGER ET AL.: "METHODS IN ENZYMOLOGY", vol. 101, 1983, ACADEMIC PRESS, pages: 512 - 527 |
| STUART; YOUNG: "Solid Phase Peptide. Synthesis", 1984, PIERCE CHEMICAL COMPANY |
| SUBBARAO ET AL., BIOCHEMISTRY, vol. 26, 1987, pages 2964 - 2972 |
| THIERRY, A.R. ET AL.: "Gene Regulation: Biology of Antisense RNA and DNA", 1992, RAVEN PRESS, pages: 147 - 161 |
| THURBERG ET AL., J. BIOL. CHEM., vol. 271, no. 11, pages 6062 - 6070 |
| TITTENSOR, J.R., J. CHEM. SOC. C, 1971, pages 1933 |
| TUERK; GOLD, SCIENCE, vol. 249, 1990, pages 505 |
| TURK ET AL., BIOCHEM. BIOPHYS. ACTA, vol. 1559, 2002, pages 56 - 68 |
| UHLMANN E. ET AL.: "Antisense: Chemical Modifications. Encyclopedia of Cancer", vol. X, 1997, ACADEMIC PRESS INC., pages: 64 - 81 |
| VASANTHAKUMAR; AHMED, CANCER COMMUN., vol. 1, no. 4, 1989, pages 225 - 232 |
| VERMA, S. ET AL., ANNU. REV. BIOCHEM, vol. 67, 1998, pages 99 - 134 |
| VERMEULEN ET AL.: "Double-Stranded Regions Are Essential Design Components Of Potent Inhibitors of RISC Function", RNA, vol. 13, 2007, pages 723 - 730 |
| VLASSOV ET AL., BIOCHIM. BIOPHYS. ACTA, vol. 1197, 1994, pages 95 - 1082 |
| VOGEL ET AL., J. AM. CHEM. SOC., vol. 118, 1996, pages 1581 - 1586 |
| WEERS ET AL., BIOPHYS. CHEM., vol. 100, no. 1-3, 2003, pages 481 - 492 |
| WEISGRABER ET AL.: "Human E apoprotein heterogeneity: cysteine-arginine interchanges in the amino acid sequence of the apo-E isoforms", J. BIOL. CHEM., vol. 256, 1981, pages 9077 - 9083 |
| WEISGRABER, J. LIPID RES, vol. 31, no. 8, 1990, pages 1503 - 1511 |
| WEISGRABER, J. LIPID RES., vol. 31, no. 8, 1990, pages 1503 - 1511 |
| WENGEL, J. ACC. CHEM. RES, vol. 32, 1999, pages 301 - 310 |
| WIDLER ET AL., J. BIOL. CHEM., vol. 255, no. 21, 1980, pages 10464 - 10471 |
| YAMAMOTO S. ET AL., J. IMMUNOL., vol. 148, 1992, pages 4072 - 4076 |
| ZALIPSKY, BIOCONJUGATE CHEMISTRY, vol. 4, 1993, pages 296 - 299 |
| ZALIPSKY, FEBS LETTERS, vol. 353, 1994, pages 71 - 74 |
| ZALIPSKY: "Stealth Liposomes", 1995, CRC PRESS |
| ZELPHATI, O. ET AL., ANTISENSE. RES. DEV., vol. 3, 1993, pages 323 - 338 |
| ZELPHATI, O; SZOKA, F.C., J. CONTR. REL., vol. 41, 1996, pages 99 - 119 |
| ZHU ET AL., SCIENCE, vol. 261, 1993, pages 209 - 211 |
| ZIMMERMANN ET AL., NATURE, vol. 441, 2006, pages 111 - 114 |
| ZITZMANN ET AL., CANCER RES., vol. 62, 2002, pages 5139 - 5143 |
Cited By (91)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US11845925B2 (en) | 2010-07-06 | 2023-12-19 | Glaxosmithkline Biologicals Sa | Immunisation of large mammals with low doses of RNA |
| US11786467B2 (en) | 2010-07-06 | 2023-10-17 | Glaxosmithkline Biologicals Sa | Lipid formulations with immunogens |
| US11666534B2 (en) | 2010-07-06 | 2023-06-06 | Glaxosmithkline Biologicals Sa | Methods of administering lipid formulations with viral immunogens |
| US11638693B2 (en) | 2010-07-06 | 2023-05-02 | Glaxosmithkline Biologicals Sa | Vaccine for eliciting immune response comprising RNA encoding an immunogen and lipid formulations comprising mole percentage of lipids |
| US11913001B2 (en) | 2010-07-06 | 2024-02-27 | Glaxosmithkline Biologicals Sa | Immunisation of large mammals with low doses of RNA |
| US11638694B2 (en) | 2010-07-06 | 2023-05-02 | Glaxosmithkline Biologicals Sa | Vaccine for eliciting immune response comprising lipid formulations and RNA encoding multiple immunogens |
| US11905514B2 (en) | 2010-07-06 | 2024-02-20 | Glaxosmithkline Biological Sa | Immunisation of large mammals with low doses of RNA |
| US11690862B1 (en) | 2010-07-06 | 2023-07-04 | Glaxosmithkline Biologicals Sa | Delivery of RNA to trigger multiple immune pathways |
| US11690861B2 (en) | 2010-07-06 | 2023-07-04 | Glaxosmithkline Biologicals Sa | Delivery of RNA to trigger multiple immune pathways |
| US11891608B2 (en) | 2010-07-06 | 2024-02-06 | Glaxosmithkline Biologicals Sa | Immunization of large mammals with low doses of RNA |
| US11883534B2 (en) | 2010-07-06 | 2024-01-30 | Glaxosmithkline Biologicals Sa | Immunisation with lipid formulations with RNA encoding immunogens |
| US11865080B2 (en) | 2010-07-06 | 2024-01-09 | Glaxosmithkline Biologicals Sa | Delivery of RNA to trigger multiple immune pathways |
| US11857681B2 (en) | 2010-07-06 | 2024-01-02 | Glaxosmithkline Biologicals Sa | Lipid formulations with RNA encoding immunogens |
| US11857562B2 (en) | 2010-07-06 | 2024-01-02 | Glaxosmithkline Biologicals Sa | Delivery of RNA to trigger multiple immune pathways |
| US11850305B2 (en) | 2010-07-06 | 2023-12-26 | Glaxosmithkline Biologicals Sa | Method of making lipid formulations with RNA encoding immunogens |
| US11851660B2 (en) | 2010-07-06 | 2023-12-26 | Glaxosmithkline Biologicals Sa | Immunisation of large mammals with low doses of RNA |
| US11690863B2 (en) | 2010-07-06 | 2023-07-04 | Glaxosmithkline Biologicals Sa | Delivery of RNA to trigger multiple immune pathways |
| US11839686B2 (en) | 2010-07-06 | 2023-12-12 | Glaxosmithkline Biologicals Sa | Lipid formulations with viral immunogens |
| US20220125723A1 (en) | 2010-07-06 | 2022-04-28 | Glaxosmithkline Biologicals Sa | Lipid formulations with viral immunogens |
| US11596645B2 (en) | 2010-07-06 | 2023-03-07 | Glaxosmithkline Biologicals Sa | Delivery of RNA to trigger multiple immune pathways |
| US11690865B2 (en) | 2010-07-06 | 2023-07-04 | Glaxosmithkline Biologicals Sa | Delivery of RNA to trigger multiple immune pathways |
| US11655475B2 (en) | 2010-07-06 | 2023-05-23 | Glaxosmithkline Biologicals Sa | Immunisation of large mammals with low doses of RNA |
| US11690864B2 (en) | 2010-07-06 | 2023-07-04 | Glaxosmithkline Biologicals Sa | Delivery of RNA to trigger multiple immune pathways |
| US11696923B2 (en) | 2010-07-06 | 2023-07-11 | Glaxosmithkline Biologicals, Sa | Delivery of RNA to trigger multiple immune pathways |
| US11773395B1 (en) | 2010-07-06 | 2023-10-03 | Glaxosmithkline Biologicals Sa | Immunization of large mammals with low doses of RNA |
| US11766401B2 (en) | 2010-07-06 | 2023-09-26 | Glaxosmithkline Biologicals Sa | Methods of administering lipid formulations with immunogens |
| US11707482B2 (en) | 2010-07-06 | 2023-07-25 | Glaxosmithkline Biologicals Sa | Delivery of RNA to trigger multiple immune pathways |
| US11759475B2 (en) | 2010-07-06 | 2023-09-19 | Glaxosmithkline Biologicals Sa | Delivery of RNA to trigger multiple immune pathways |
| US11739334B2 (en) | 2010-07-06 | 2023-08-29 | Glaxosmithkline Biologicals Sa | Immunisation of large mammals with low doses of RNA |
| US11730754B2 (en) | 2010-07-06 | 2023-08-22 | Glaxosmithkline Biologicals Sa | Delivery of RNA to trigger multiple immune pathways |
| US11717529B2 (en) | 2010-07-06 | 2023-08-08 | Glaxosmithkline Biologicals Sa | Delivery of RNA to trigger multiple immune pathways |
| US11759422B2 (en) | 2010-08-31 | 2023-09-19 | Glaxosmithkline Biologicals Sa | Pegylated liposomes for delivery of immunogen-encoding RNA |
| US11639370B2 (en) | 2010-10-11 | 2023-05-02 | Glaxosmithkline Biologicals Sa | Antigen delivery platforms |
| EP4074693A1 (en) * | 2011-06-08 | 2022-10-19 | Translate Bio, Inc. | Cleavable lipids |
| EP3998064A1 (en) * | 2011-06-08 | 2022-05-18 | Translate Bio, Inc. | Cleavable lipids |
| US11234936B2 (en) | 2011-06-08 | 2022-02-01 | Translate Bio, Inc. | Cleavable lipids |
| US10702478B2 (en) | 2011-06-08 | 2020-07-07 | Translate Bio, Inc. | Cleavable lipids |
| EP3336082B1 (en) | 2011-06-08 | 2020-04-15 | Translate Bio, Inc. | Cleavable lipids |
| US10507183B2 (en) | 2011-06-08 | 2019-12-17 | Translate Bio, Inc. | Cleavable lipids |
| US12102720B2 (en) | 2011-06-08 | 2024-10-01 | Translate Bio, Inc. | Cleavable lipids |
| EP3336082A1 (en) | 2011-06-08 | 2018-06-20 | Translate Bio, Inc. | Cleavable lipids |
| WO2013006825A1 (en) | 2011-07-06 | 2013-01-10 | Novartis Ag | Liposomes having useful n:p ratio for delivery of rna molecules |
| EP4014966A1 (en) * | 2011-07-06 | 2022-06-22 | GlaxoSmithKline Biologicals S.A. | Liposomes having useful n:p ratio for delivery of rna molecules |
| US11896636B2 (en) | 2011-07-06 | 2024-02-13 | Glaxosmithkline Biologicals Sa | Immunogenic combination compositions and uses thereof |
| EP4115876A1 (en) * | 2011-07-06 | 2023-01-11 | GlaxoSmithKline Biologicals S.A. | Liposomes having useful n:p ratio for delivery of rna molecules |
| EP3821879A1 (en) * | 2011-07-06 | 2021-05-19 | GlaxoSmithKline Biologicals S.A. | Liposomes having useful n:p ratio for delivery of rna molecules |
| EP4115875A1 (en) * | 2011-07-06 | 2023-01-11 | GlaxoSmithKline Biologicals S.A. | Liposomes having useful n:p ratio for delivery of rna molecules |
| EP2729126B1 (en) * | 2011-07-06 | 2020-12-23 | GlaxoSmithKline Biologicals SA | Liposomes having useful n:p ratio for delivery of rna molecules |
| US11633480B2 (en) | 2011-12-07 | 2023-04-25 | Alnylam Pharmaceuticals, Inc. | Biodegradable lipids for the delivery of active agents |
| US11590229B2 (en) | 2011-12-07 | 2023-02-28 | Alnylam Pharmaceuticals, Inc. | Biodegradable lipids for the delivery of active agents |
| US11612657B2 (en) | 2011-12-07 | 2023-03-28 | Alnylam Pharmaceuticals, Inc. | Biodegradable lipids for the delivery of active agents |
| US11633479B2 (en) | 2011-12-07 | 2023-04-25 | Alnylam Pharmaceuticals, Inc. | Biodegradable lipids for the delivery of active agents |
| US11679158B2 (en) | 2011-12-07 | 2023-06-20 | Alnylam Pharmaceuticals, Inc. | Biodegradable lipids for the delivery of active agents |
| EP3110401A2 (en) | 2014-02-25 | 2017-01-04 | Merck Sharp & Dohme Corp. | Lipid nanoparticle vaccine adjuvants and antigen delivery systems |
| US10525036B2 (en) | 2015-04-03 | 2020-01-07 | Recurium Ip Holdings, Llc | Spirocyclic compounds |
| US10493060B2 (en) | 2015-04-03 | 2019-12-03 | Recurium Ip Holdings, Llc | Spirocyclic compounds |
| US11491112B2 (en) | 2015-04-17 | 2022-11-08 | CureVac Manufacturing GmbH | Lyophilization of RNA |
| EP3283125B1 (en) | 2015-04-17 | 2021-12-29 | CureVac Real Estate GmbH | Lyophilization of rna |
| US11446250B2 (en) | 2015-04-17 | 2022-09-20 | Curevac Real Estate Gmbh | Lyophilization of RNA |
| US11433027B2 (en) | 2015-05-20 | 2022-09-06 | Curevac Ag | Dry powder composition comprising long-chain RNA |
| US11534405B2 (en) | 2015-05-20 | 2022-12-27 | Curevac Ag | Dry powder composition comprising long-chain RNA |
| US10934304B2 (en) | 2016-10-05 | 2021-03-02 | Recurium Ip Holdings, Llc | Spirocyclic compounds |
| US11926616B2 (en) | 2018-03-08 | 2024-03-12 | Incyte Corporation | Aminopyrazine diol compounds as PI3K-γ inhibitors |
| EP4311541A2 (en) | 2018-04-25 | 2024-01-31 | Ethris GmbH | Cryoprotective agents for particulate formulations |
| WO2019207061A1 (en) | 2018-04-25 | 2019-10-31 | Ethris Gmbh | Cryoprotective agents for particulate formulations |
| US11046658B2 (en) | 2018-07-02 | 2021-06-29 | Incyte Corporation | Aminopyrazine derivatives as PI3K-γ inhibitors |
| WO2021195214A1 (en) | 2020-03-24 | 2021-09-30 | Generation Bio Co. | Non-viral dna vectors and uses thereof for expressing factor ix therapeutics |
| WO2021195218A1 (en) | 2020-03-24 | 2021-09-30 | Generation Bio Co. | Non-viral dna vectors and uses thereof for expressing gaucher therapeutics |
| WO2022023284A1 (en) | 2020-07-27 | 2022-02-03 | Anjarium Biosciences Ag | Compositions of dna molecules, methods of making therefor, and methods of use thereof |
| WO2022223556A1 (en) | 2021-04-20 | 2022-10-27 | Anjarium Biosciences Ag | Compositions of dna molecules encoding amylo-alpha-1, 6-glucosidase, 4-alpha-glucanotransferase, methods of making thereof, and methods of use thereof |
| WO2022232289A1 (en) | 2021-04-27 | 2022-11-03 | Generation Bio Co. | Non-viral dna vectors expressing therapeutic antibodies and uses thereof |
| WO2022232286A1 (en) | 2021-04-27 | 2022-11-03 | Generation Bio Co. | Non-viral dna vectors expressing anti-coronavirus antibodies and uses thereof |
| WO2023001894A1 (en) | 2021-07-20 | 2023-01-26 | Ags Therapeutics Sas | Extracellular vesicles from microalgae, their preparation, and uses |
| WO2023081526A1 (en) | 2021-11-08 | 2023-05-11 | Orna Therapeutics, Inc. | Lipid nanoparticle compositions for delivering circular polynucleotides |
| WO2023135273A2 (en) | 2022-01-14 | 2023-07-20 | Anjarium Biosciences Ag | Compositions of dna molecules encoding factor viii, methods of making thereof, and methods of use thereof |
| WO2023144127A1 (en) | 2022-01-31 | 2023-08-03 | Ags Therapeutics Sas | Extracellular vesicles from microalgae, their biodistribution upon administration, and uses |
| WO2023177655A1 (en) | 2022-03-14 | 2023-09-21 | Generation Bio Co. | Heterologous prime boost vaccine compositions and methods of use |
| WO2023183616A1 (en) * | 2022-03-25 | 2023-09-28 | Senda Biosciences, Inc. | Novel ionizable lipids and lipid nanoparticles and methods of using the same |
| WO2023230098A1 (en) | 2022-05-23 | 2023-11-30 | Logicbio Therapeutics, Inc. | Gene therapy compositions and methods of use thereof |
| WO2023232976A1 (en) | 2022-06-03 | 2023-12-07 | Ags Therapeutics Sas | Extracellular vesicles from genetically-modified microalgae containing endogenously-loaded cargo, their preparation, and uses |
| WO2023239756A1 (en) | 2022-06-07 | 2023-12-14 | Generation Bio Co. | Lipid nanoparticle compositions and uses thereof |
| WO2024040222A1 (en) | 2022-08-19 | 2024-02-22 | Generation Bio Co. | Cleavable closed-ended dna (cedna) and methods of use thereof |
| WO2024088808A1 (en) | 2022-10-24 | 2024-05-02 | Ags Therapeutics Sas | Extracellular vesicles from microalgae, their biodistribution upon intranasal administration, and uses thereof |
| WO2024102730A1 (en) | 2022-11-08 | 2024-05-16 | Orna Therapeutics, Inc. | Lipids and nanoparticle compositions for delivering polynucleotides |
| WO2024102677A1 (en) | 2022-11-08 | 2024-05-16 | Orna Therapeutics, Inc. | Circular rna compositions |
| WO2024102762A1 (en) | 2022-11-08 | 2024-05-16 | Orna Therapeutics, Inc. | Lipids and lipid nanoparticle compositions for delivering polynucleotides |
| WO2024119074A1 (en) | 2022-12-01 | 2024-06-06 | Generation Bio Co. | Stealth lipid nanoparticle compositions for cell targeting |
| WO2024119051A1 (en) | 2022-12-01 | 2024-06-06 | Generation Bio Co. | Novel polyglycerol-conjugated lipids and lipid nanoparticle compositions comprising the same |
| WO2024119103A1 (en) | 2022-12-01 | 2024-06-06 | Generation Bio Co. | Lipid nanoparticles comprising nucleic acids and lipid-anchored polymers |
| WO2024119039A2 (en) | 2022-12-01 | 2024-06-06 | Generation Bio Co. | Stealth lipid nanoparticles and uses thereof |
| WO2024205657A2 (en) | 2023-03-29 | 2024-10-03 | Orna Therapeutics, Inc. | Lipids and lipid nanoparticle compositions for delivering polynucleotides |
Also Published As
| Publication number | Publication date |
|---|---|
| HK1252488A1 (en) | 2019-05-24 |
| CA3044884A1 (en) | 2011-06-16 |
| AU2010328336B2 (en) | 2017-03-02 |
| AU2018275020A1 (en) | 2019-01-03 |
| AU2010328336A1 (en) | 2012-07-05 |
| EP2509636A4 (en) | 2013-08-14 |
| CA2783372C (en) | 2019-07-16 |
| CA2783372A1 (en) | 2011-06-16 |
| EP2509636A2 (en) | 2012-10-17 |
| US20180369384A1 (en) | 2018-12-27 |
| EP2509636B1 (en) | 2017-07-19 |
| AU2017202153A1 (en) | 2017-04-20 |
| WO2011071860A3 (en) | 2011-09-09 |
| US9687550B2 (en) | 2017-06-27 |
| US20130338210A1 (en) | 2013-12-19 |
| EP3296398A1 (en) | 2018-03-21 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| US12042541B2 (en) | Lipids and compositions for the delivery of therapeutics | |
| AU2010328336B2 (en) | Compositions for nucleic acid delivery | |
| US20180221510A1 (en) | Methods and compositions for delivery of active agents | |
| WO2012016188A2 (en) | Methods and compositions for delivery of active agents |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| 121 | Ep: the epo has been informed by wipo that ep was designated in this application |
Ref document number: 10836504 Country of ref document: EP Kind code of ref document: A1 |
|
| ENP | Entry into the national phase |
Ref document number: 2783372 Country of ref document: CA |
|
| NENP | Non-entry into the national phase |
Ref country code: DE |
|
| WWE | Wipo information: entry into national phase |
Ref document number: 2010328336 Country of ref document: AU |
|
| REEP | Request for entry into the european phase |
Ref document number: 2010836504 Country of ref document: EP |
|
| WWE | Wipo information: entry into national phase |
Ref document number: 2010836504 Country of ref document: EP |
|
| ENP | Entry into the national phase |
Ref document number: 2010328336 Country of ref document: AU Date of ref document: 20101207 Kind code of ref document: A |
|
| WWE | Wipo information: entry into national phase |
Ref document number: 13514352 Country of ref document: US |