WO2010148256A1 - Fuel compositions comprising isoprene derivatives - Google Patents
Fuel compositions comprising isoprene derivatives Download PDFInfo
- Publication number
- WO2010148256A1 WO2010148256A1 PCT/US2010/039088 US2010039088W WO2010148256A1 WO 2010148256 A1 WO2010148256 A1 WO 2010148256A1 US 2010039088 W US2010039088 W US 2010039088W WO 2010148256 A1 WO2010148256 A1 WO 2010148256A1
- Authority
- WO
- WIPO (PCT)
- Prior art keywords
- isoprene
- cells
- composition
- polypeptides
- polypeptide
- Prior art date
Links
Classifications
-
- C—CHEMISTRY; METALLURGY
- C10—PETROLEUM, GAS OR COKE INDUSTRIES; TECHNICAL GASES CONTAINING CARBON MONOXIDE; FUELS; LUBRICANTS; PEAT
- C10L—FUELS NOT OTHERWISE PROVIDED FOR; NATURAL GAS; SYNTHETIC NATURAL GAS OBTAINED BY PROCESSES NOT COVERED BY SUBCLASSES C10G, C10K; LIQUEFIED PETROLEUM GAS; ADDING MATERIALS TO FUELS OR FIRES TO REDUCE SMOKE OR UNDESIRABLE DEPOSITS OR TO FACILITATE SOOT REMOVAL; FIRELIGHTERS
- C10L1/00—Liquid carbonaceous fuels
- C10L1/04—Liquid carbonaceous fuels essentially based on blends of hydrocarbons
-
- C—CHEMISTRY; METALLURGY
- C10—PETROLEUM, GAS OR COKE INDUSTRIES; TECHNICAL GASES CONTAINING CARBON MONOXIDE; FUELS; LUBRICANTS; PEAT
- C10L—FUELS NOT OTHERWISE PROVIDED FOR; NATURAL GAS; SYNTHETIC NATURAL GAS OBTAINED BY PROCESSES NOT COVERED BY SUBCLASSES C10G, C10K; LIQUEFIED PETROLEUM GAS; ADDING MATERIALS TO FUELS OR FIRES TO REDUCE SMOKE OR UNDESIRABLE DEPOSITS OR TO FACILITATE SOOT REMOVAL; FIRELIGHTERS
- C10L1/00—Liquid carbonaceous fuels
- C10L1/10—Liquid carbonaceous fuels containing additives
- C10L1/14—Organic compounds
- C10L1/16—Hydrocarbons
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B01—PHYSICAL OR CHEMICAL PROCESSES OR APPARATUS IN GENERAL
- B01J—CHEMICAL OR PHYSICAL PROCESSES, e.g. CATALYSIS OR COLLOID CHEMISTRY; THEIR RELEVANT APPARATUS
- B01J23/00—Catalysts comprising metals or metal oxides or hydroxides, not provided for in group B01J21/00
- B01J23/38—Catalysts comprising metals or metal oxides or hydroxides, not provided for in group B01J21/00 of noble metals
- B01J23/40—Catalysts comprising metals or metal oxides or hydroxides, not provided for in group B01J21/00 of noble metals of the platinum group metals
- B01J23/44—Palladium
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07C—ACYCLIC OR CARBOCYCLIC COMPOUNDS
- C07C11/00—Aliphatic unsaturated hydrocarbons
- C07C11/12—Alkadienes
- C07C11/173—Alkadienes with five carbon atoms
- C07C11/18—Isoprene
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07C—ACYCLIC OR CARBOCYCLIC COMPOUNDS
- C07C9/00—Aliphatic saturated hydrocarbons
- C07C9/14—Aliphatic saturated hydrocarbons with five to fifteen carbon atoms
Definitions
- isoprene is derived from petrochemical sources either directly by cracking of naphtha and other light petroleum fractions, or indirectly through chemical synthesis (See, for examples, H. Pommer and A. Nurrenbach, Industrial Synthesis of Terpene Compounds, Pure Appl Chem., 1975, 43, 527-551; H. M. Weitz and E. Loser, Isoprene, in Ullmann 's Encyclopedia of Industrial Chemistry, Seventh Edition, Electronic Release, Wiley- VCH Verlag GMBH, Weinheim, 2005; and H.M.
- isoprene derived from biological sources contains very few hydrocarbon impurities and instead contains a number of oxygenated compounds such as ethanol, acetaldehyde and acetone. Many of these compounds can be easily removed by contact with water or passage through alumina or other adsorbents.
- the invention provides a method for producing a fuel constituent from a bioisoprene composition comprising chemically transforming a substantial portion of the isoprene in the bioisoprene composition to non- isoprene compounds.
- the bioisoprene composition is chemically transformed by subjecting the bioisoprene composition to heat or catalytic conditions suitable for isoprene dimerization to produce an isoprene dimer and then catalytically hydrogenating the isoprene dimer to form a saturated ClO fuel constituent.
- the bioisoprene composition is chemically transformed by (i) partially hydrogenating the bioisoprene composition to produce an isoamylene, (ii) dimerizing the isoamylene with a mono-olefin selected from the group consisting of isoamylene, propylene and isobutene to form a dimate and (iii) completely hydrogenating the dimate to produce a fuel constituent.
- at least about 95% of isoprene in the bioisoprene composition is converted to non-isoprene compounds during the chemical transformation.
- the bioisoprene composition is heated to about 150 0 C to about 250 0 C to produce an unsaturated cyclic isoprene dimer and the unsaturated cyclic isoprene dimer is hydrogenated catalytically to produce a saturated cyclic isoprene dimer fuel constituent.
- the method comprises: (i) contacting the bioisoprene composition with a catalyst for catalyzing cyclo-dimerization of isoprene to produce an unsaturated cyclic isoprene dimer and the unsaturated cyclic isoprene dimer is hydrogenated catalytically to produce a saturated cyclic isoprene dimer fuel constituent.
- the catalyst for catalyzing cyclo- dimerization of isoprene comprising a catalyst selected from the group consisting of a nickel catalyst, iron catalysts and chromium catalysts.
- the step of partially hydrogenating the bioisoprene composition comprises contacting the bioisoprene composition with hydrogen gas and a catalyst for catalyzing partial hydrogenation of isoprene.
- the catalyst for catalyzing partial hydrogenation of isoprene comprises a palladium catalyst.
- the step of dimerizing the isoamylene with a mono- olefin comprises contacting the isoamylene with the mono-olefin in the presence of a catalyst for catalyzing dimerization of mono-olefin.
- the catalyst for catalyzing dimerization of mono-olefin comprises an acid catalyst.
- the method further comprises purifying the isoprene from the bioisoprene composition prior to chemically transforming the bioisoprene composition to a fuel constituent.
- the invention provides a system for producing a fuel constituent from a bioisoprene composition, wherein a substantial portion of the isoprene in the bioisoprene composition is chemically converted to non-isoprene compounds, the system comprising a bioisoprene composition and (a) (i) one or more chemicals capable of dimerizing isoprene in the bioisoprene composition or a source of heat capable of dimerizing isoprene in the bioisoprene composition; and (ii) a catalyst capable of hydrogenating the isoprene dimer to form a saturated ClO fuel constituent; or (b) (i) a chemical capable of partially hydrogenating isoprene in the bioisoprene composition to produce an isoamylene, (ii) a chemical capable of dimerizing the isoamylene with mono-olefins selected from the group consisting of isoamylene, propylene and isobutene to form
- the bioisoprene composition comprising greater than about 2 mg of isoprene and comprising greater than or about 99.94% isoprene by weight compared to the total weight of all C5 hydrocarbons in the composition.
- the one or more chemicals capable of dimerizing isoprene comprises catalyst for catalyzing cyclo-dimerization of isoprene comprising a catalyst selected from the group consisting of ruthenium catalysts, nickel catalysts, iron catalysts and chromium catalysts.
- the catalyst for hydrogenating the unsaturated isoprene dimers comprises a catalyst selected from the group consisting of palladium catalysts, nickel catalysts, ruthenium catalysts and rhodium catalysts.
- the chemical capable of partially hydrogenating isoprene comprises a palladium catalyst.
- the chemical capable of dimerizing the isoamylene with mono- olefins comprises an acid catalyst.
- the invention provides a fuel composition comprising a fuel constituent produced by the methods described herein.
- the fuel composition is substantially free of isoprene.
- the fuel composition has 5 13 C value which is greater than -22%o or within the range of -32%o to -24%o.
- the invention provides a system for producing a fuel constituent from isoprene comprising: (a) a commercially beneficial amount of highly pure isoprene; and (b) a fuel constituent produced from at least a portion of the highly pure isoprene; wherein at least a portion of the commercially beneficial amount of highly pure isoprene undergoes a chemical transformation.
- the commercially beneficial amount of highly pure isoprene comprises greater than about 2 mg of isoprene and comprising greater than or about 99.94% isoprene by weight compared to the total weight of all C5 hydrocarbons in the composition. In some embodiments, the commercially beneficial amount of highly pure isoprene comprises greater than about 2 mg of isoprene and comprising one or more compounds selected from the group consisting of ethanol, acetone, C5 prenyl alcohols, and isoprenoid compounds with 10 or more carbon atoms.
- the commercially beneficial amount of highly pure isoprene comprises greater than about 2 mg of isoprene and comprising one or more second compounds selected from the group consisting of ethanol, acetone, methanol, acetaldehyde, methacrolein, methyl vinyl ketone, 2-methyl-2-vinyloxirane, cis- and trans-3 -methyl- 1, 3 -pentadiene, a C5 prenyl alcohol, 2-heptanone, 6-methyl-5-hepten-2-one, 2,4,5-trimethylpyridine, 2,3,5-trimethylpyrazine, citronellal, methanethiol, methyl acetate, 1- propanol, diacetyl, 2-butanone, 2-methyl-3-buten-2-ol, ethyl acetate, 2-methyl-l-propanol, 3- methyl-1-butanal, 3-methyl-2-butanone, 1-butanol, 2-pentanone, 3 -methyl
- the commercially beneficial amount of highly pure isoprene comprises greater than about 2 mg of isoprene and comprising less than or about 0.5 ⁇ g/L per compound for any compound in the composition that inhibits the polymerization of isoprene. In some preferred embodiments, the commercially beneficial amount of highly pure isoprene is produced by a biological process.
- the fuel constituent comprises one or more compounds selected from the group consisting of cyclic isoprene dimers and trimers, linear isoprene oligomers, aromatic and alicyclic isoprene derivatives, and oxygenated isoprene derivatives.
- the oxygenated isoprene derivatives are compounds selected from the group consisting of alcohols, ketones, esters and ethers derived from isoprene.
- the fuel constituent comprises cyclic isoprene dimers and the chemical transformation comprises a dimerization reaction of isoprene. In some embodiments, the dimerization reaction is carried out by heating the commercially beneficial amount of highly pure isoprene.
- the dimerization reaction of isoprene produces a product comprising unsaturated isoprene dimers and the chemical transformation further comprises a hydrogenation reaction of the unsaturated isoprene dimers.
- the system further comprises a catalyst for catalyzing the hydrogenation reaction of the unsaturated isoprene dimers.
- the catalyst for catalyzing the hydrogenation reaction of the unsaturated isoprene dimers comprising a catalyst selected from the group consisting of palladium catalysts, nickel catalysts, ruthenium catalysts and rhodium catalysts.
- the dimerization reaction is carried out by contacting the commercially beneficial amount of highly pure isoprene with a catalyst for catalyzing cyclo- dimerization of isoprene.
- the catalyst for catalyzing cyclo-dimerization of isoprene comprising a catalyst selected from the group consisting of ruthenium catalysts, nickel catalysts, iron catalysts and chromium catalysts.
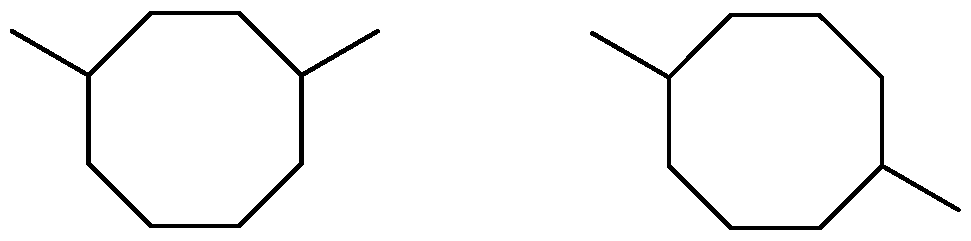
- the catalyst for catalyzing cyclo-dimerization of isoprene is a nickel catalyst and the fuel constituent comprising one or more eight-membered ring dimers of isoprene.
- the fuel constituent comprises linear and/or cyclic trimers of isoprene and the chemical transformation comprises catalytic trimerization of isoprene.
- the invention provides a method for producing a fuel constituent from isoprene comprising: (a) obtaining a commercially beneficial amount of highly pure isoprene; and (b) chemically transforming at least a portion of the commercially beneficial amount of highly pure isoprene to a fuel constituent.
- the commercially beneficial amount of highly pure isoprene comprises bioisoprene.
- the commercially beneficial amount of highly pure isoprene is obtained by the steps comprising: (i) culturing cells comprising a heterologous nucleic acid encoding an isoprene synthase polypeptide under suitable culture conditions for the production of isoprene, wherein the cells (1) produce greater than about 400 nmole/g wcm /hr of isoprene, (2) convert more than about 0.002 molar percent of the carbon that the cells consume from a cell culture medium into isoprene, or (3) have an average volumetric productivity of isoprene greater than about 0.1 mg/L broth /hr of isoprene, and (ii) producing isoprene.
- the cells further comprise a heterologous nucleic acid encoding an isoprene synthase polypeptide or an MVA pathway polypeptide.
- chemically transforming at least a portion of the commercially beneficial amount of highly pure isoprene to a fuel constituent comprises: (i) heating the commercially beneficial amount of highly pure isoprene to about 150 0 C to about 250 0 C; (ii) converting at least a portion of the commercially beneficial amount of highly pure isoprene to unsaturated cyclic isoprene dimers; (iii) hydrogenating the unsaturated cyclic isoprene dimers to produce saturated cyclic isoprene dimers; and (iv) producing the fuel constituent.
- at least about 20% to about 100% of isoprene in the commercially beneficial amount of highly pure isoprene is converted to unsaturated cyclic isoprene dimers.
- chemically transforming at least a portion of the commercially beneficial amount of highly pure isoprene to a fuel constituent comprises: (i) contacting the commercially beneficial amount of highly pure isoprene with a catalyst for catalyzing cyclo-dimerization of isoprene, (ii) converting at least a portion of the commercially beneficial amount of highly pure isoprene to cyclic isoprene dimers; and (iii) producing the fuel constituent.
- chemically transforming at least a portion of the commercially beneficial amount of highly pure isoprene to a fuel constituent comprises: (i) contacting the commercially beneficial amount of highly pure isoprene with a catalyst for catalyzing cyclo-dimerization of isoprene, (ii) converting at least a portion of the commercially beneficial amount of highly pure isoprene to unsaturated cyclic isoprene dimers; (iii) hydrogenating the unsaturated cyclic isoprene dimers to produce saturated cyclic isoprene dimers; and (iv) producing the fuel constituent.
- the catalyst for catalyzing cyclo-dimerization of isoprene comprising a catalyst selected from the group consisting of a nickel catalyst, iron catalysts and chromium catalysts.
- chemically transforming at least a portion of the commercially beneficial amount of highly pure isoprene to a fuel constituent comprises: (i) contacting the highly pure isoprene composition with a catalyst system; (ii) converting at least a portion of the starting isoprene composition to unsaturated isoprene dimers and/or trimers; and (iii) hydrogenating the unsaturated dimers and/or trimers to produce saturated ClO and/or C15 hydrocarbons.
- the method for producing a fuel constituent from isoprene comprises: (a) obtaining a commercially beneficial amount of any of the highly pure isoprene starting composition described herein; (b) converting at least a portion of the starting isoprene composition to oxygenated isoprene derivatives; and optionally (c) hydrogenating any unsaturated oxygenated isoprene derivatives to produce saturated oxygenates.
- the oxygenated isoprene derivatives are compounds selected from the group consisting of alcohols, ketones, esters and ethers derived from isoprene.
- any of the methods described herein further comprises purifying the commercially beneficial amount of highly pure isoprene prior to chemically transforming at least a portion of the commercially beneficial amount of highly pure isoprene to a fuel constituent.
- a continuous process for producing a fuel constituent from isoprene comprising: (a) continuously producing a commercially beneficial amount of highly pure isoprene; and (b) continuously transforming chemically at least a portion of the commercially beneficial amount of highly pure isoprene to a fuel constituent.
- the commercially beneficial amount of highly pure isoprene comprising a gas phase comprising isoprene.
- the method further comprises passing the gas phase comprising isoprene to a reactor for chemically transforming at least a portion of the commercially beneficial amount of highly pure isoprene to a fuel constituent.
- the commercially beneficial amount of highly pure isoprene comprises bioisoprene.
- a fuel composition comprising a fuel constituent produced by any of the methods described herein.
- the fuel constituent comprises less than or about 0.5 ⁇ g/L a product from a C5 hydrocarbon other than isoprene after undergoing the steps according to the methods described herein.
- the fuel constituent comprises one or more product from one or more compound selected from the group consisting of ethanol, acetone, methanol, acetaldehyde, methacrolein, methyl vinyl ketone, 2-methyl-2- vinyloxirane, cis- and tr ⁇ r ⁇ -3-methyl-l,3-pentadiene, a C5 prenyl alcohol, 2-heptanone, 6- methyl-5-hepten-2-one, 2,4,5-trimethylpyridine, 2,3,5-trimethylpyrazine, citronellal, methanethiol, methyl acetate, 1-propanol, diacetyl, 2-butanone, 2-methyl-3-buten-2-ol, ethyl acetate, 2-methyl- 1-propanol, 3 -methyl- 1-butanal, 3-methyl-2-butanone, 1-butanol, 2-pentanone, 3 -methyl- 1-butanol, ethyl isobutyrate
- the fuel composition comprises a fuel composition having 5 13 C value which is greater than -22%o. In some embodiments, the fuel composition has 5 13 C value which is within the range of -22%o to -10%o or -34%o to -24 %o. In some embodiments, the fuel composition has f M value which is greater than 0.9. Also provided is a blend of any of the fuel compositions described herein with a petroleum based fuel in the amount of from about 1% to about 95% by weight or volume, based on the total weight or volume of the total fuel composition.
- Figure 1 is the nucleotide sequence of a kudzu isoprene synthase gene codon-optimized for expression in E. coli (SEQ ID NO:1).
- the atg start codon is in italics, the stop codon is in bold and the added Pstl site is underlined.
- Figure 2 is a map of pTrcKudzu.
- Figures 3A-C are the nucleotide sequence of pTrcKudzu (SEQ ID NO:2).
- the RBS is underlined, the kudzu isoprene synthase start codon is in bold capitol letters and the stop codon is in bold, capital letters.
- the vector backbone is pTrcHis2B.
- Figure 4 is a map of pETNHisKudzu.
- Figures 5A-C are the nucleotide sequence of pETNHisKudzu (SEQ ID NO:3).
- Figure 6 is a map of pCL-lac-Kudzu.
- Figures 7A-C are the nucleotide sequence of pCL-lac-Kudzu (SEQ ID NO:4).
- Figure 8 A is a graph showing the production of isoprene in E. coli BL21 cells with no vector.
- Figure 8B is a graph showing the production of isoprene in E. coli BL21 cells with pCL-lac-Kudzu
- Figure 8C is a graph showing the production of isoprene in E. coli BL21 cells with pTrcKudzu.
- Figure 8D is a graph showing the production of isoprene in E. coli BL21 cells with pETN-HisKudzu.
- Figure 9A is a graph showing OD over time of fermentation of E. coli
- Figure 9B is a graph showing isoprene production over time of fermentation of E. coli BL21/pTrcKudzu in a 14 liter fed batch fermentation.
- Figure 1OA is a graph showing the production of isoprene in Panteoa citrea. Control cells without recombinant kudzu isoprene synthase. Grey diamonds represent isoprene synthesis, black squares represent ODOOO-
- Figure 1OB is a graph showing the production of isoprene in Panteoa citrea expressing pCL-lac Kudzu. Grey diamonds represent isoprene synthesis, black squares represent OD OOO - [0044]
- Figure 1OC is a graph showing the production of isoprene in Panteoa citrea expressing pTrcKudzu. Grey diamonds represent isoprene synthesis, black squares represent OD OOO - [0045]
- Figure 11 is a graph showing the production of isoprene in Bacillus subtilis expressing recombinant isoprene synthase.
- BG3594comK is a B. subtilis strain without plasmid (native isoprene production).
- CF443 is B. subtilis strain BG3594comK with pBSKudzu (recombinant isoprene production). IS on the y-axis indicates isoprene.
- Figures 12A-C are the nucleotide sequence of pBS Kudzu #2 (SEQ ID NO:5).
- Figure 13 is the nucleotide sequence of kudzu isoprene synthase codon-optimized for expression in Yarrowia (SEQ ID NO: 6).
- Figure 14 is a map of pTrex3g comprising a kudzu isoprene synthase gene codon- optimized for expression in Yarrowia.
- Figures 15A-C are the nucleotide sequence of vector pSPZl(MAP29Spb) (SEQ ID NO:7).
- Figure 16 is the nucleotide sequence of the synthetic kudzu (Pueraria montan ⁇ ) isoprene gene codon-optimized for expression in Yarrowia (SEQ ID NO: 8).
- Figure 17 is the nucleotide sequence of the synthetic hybrid poplar (Populus albax Populus tremul ⁇ ) isoprene synthase gene (SEQ ID NO:9). The ATG start codon is in bold and the stop codon is underlined.
- Figure 18A shows a schematic outlining construction of vectors pYLA 1, pYLl and pYL2 (SEQ ID NO:75, 73, 72, 71, 70, 69).
- Figure 18B shows a schematic outlining construction of the vector pYLA(POPl) (SEQ ID NO:68, 69).
- Figure 18C shows a schematic outlining construction of the vector pYLA(KZl)
- Figure 18D shows a schematic outlining construction of the vector pYLI(KZl) (SEQ ID NO:66, 67)
- Figure 18E shows a schematic outlining construction of the vector pYLI(MAP29)
- Figure 18F shows a schematic outlining construction of the vector pYLA(MAP29)
- Figure 19A shows the MVA and DXP metabolic pathways for isoprene (based on F. Bouvier et ah, Progress in Lipid Res. 44: 357-429, 2005). The following description includes alternative names for each polypeptide in the pathways and a reference that discloses an assay for measuring the activity of the indicated polypeptide (each of these references are each hereby incorporated by reference in their entireties, particularly with respect to assays for polypeptide activity for polypeptides in the MVA and DXP pathways).
- Mevalonate Pathway AACT; Acetyl-CoA acetyltransferase, MvaE, EC 2.3.1.9.
- DXP Pathway DXS; l-Deoxyxylulose-5 -phosphate synthase, dxs, EC 2.2.1.7.
- P and PP in the structural formula are phosphate and pyrophosphate, respectively. This figure was taken from Koga and Morii, Microbiology and MoI. Biology Reviews, 71:97-120, 2007, which is incorporated by reference in its entirety, particular with respect to nucleic acids and polypeptides of the modified MVA pathway.
- the modified MVA pathway is present, for example, in some archaeal organisms, such as Methanosarcina mazei.
- Figure 20 shows graphs representing results of the GC-MS analysis of isoprene production by recombinant Y. lipofytica strains without (left) or with (right) a kudzu isoprene synthase gene.
- the arrows indicate the elution time of the authentic isoprene standard.
- Figure 21 is a map of pTrcKudzu yIDI DXS Kan.
- Figures 22 A-D are the nucleotide sequence of pTrcKudzu yIDI DXS Kan (SEQ ID NO: 1]
- Figure 23 A is a graph showing production of isoprene from glucose in
- Time 0 is the time of induction with IPTG (400 ⁇ mol).
- the x-axis is time after induction; the y-axis is OD 6 Oo and the y2-axis is total productivity of isoprene ( ⁇ g/L headspace or specific productivity ( ⁇ g/L headspace/OD).
- Diamonds represent OD 6 Oo, circles represent total isoprene productivity ( ⁇ g/L) and squares represent specific productivity of isoprene ( ⁇ g/L/OD).
- Figure 23B is a graph showing production of isoprene from glucose in
- Time 0 is the time of induction with IPTG (400 ⁇ mol).
- the x-axis is time after induction; the y-axis is OD 6 oo and the y2-axis is total productivity of isoprene ( ⁇ g/L headspace or specific productivity ( ⁇ g/L headspace/OD).
- Diamonds represent OD 6 oo, circles represent total isoprene productivity ( ⁇ g/L) and squares represent specific productivity of isoprene ( ⁇ g/L/OD).
- Figure 23C is a graph showing production of isoprene from glucose in
- Time 0 is the time of induction with IPTG (400 ⁇ mol).
- the x-axis is time after induction; the y-axis is OD 6 oo and the y2-axis is total productivity of isoprene ( ⁇ g/L headspace or specific productivity ( ⁇ g/L headspace/OD).
- Diamonds represent OD 6 oo, circles represent total isoprene productivity ( ⁇ g/L) and squares represent specific productivity of isoprene ( ⁇ g/L/OD).
- Figure 23D is a graph showing production of isoprene from glucose in
- Time 0 is the time of induction with IPTG (400 ⁇ mol).
- the x- axis is time after induction; the y-axis is OD 6 oo and the y2-axis is total productivity of isoprene
- Figure 23E is a graph showing production of isoprene from glucose in BL21/pCL PtrcKudzu.
- Time 0 is the time of induction with IPTG (400 ⁇ mol).
- the x-axis is time after induction; the y-axis is OD 6 oo and the y2-axis is total productivity of isoprene ( ⁇ g/L headspace or specific productivity ( ⁇ g/L headspace/OD).
- Diamonds represent OD 6 Oo
- circles represent total isoprene productivity ( ⁇ g/L) and squares represent specific productivity of isoprene ( ⁇ g/L/OD).
- Figure 23F is a graph showing production of isoprene from glucose in BL21/pCL PtrcKudzu yIDI.
- Time 0 is the time of induction with IPTG (400 ⁇ mol).
- the x-axis is time after induction; the y-axis is OD 6 oo and the y2-axis is total productivity of isoprene ( ⁇ g/L headspace or specific productivity ( ⁇ g/L headspace/OD).
- Diamonds represent OD 6 oo
- circles represent total isoprene productivity ( ⁇ g/L) and squares represent specific productivity of isoprene ( ⁇ g/L/OD).
- Figure 23G is a graph showing production of isoprene from glucose in BL21/pCL PtrcKudzu DXS.
- Time 0 is the time of induction with IPTG (400 ⁇ mol).
- the x-axis is time after induction; the y-axis is OD 6 Oo and the y2-axis is total productivity of isoprene ( ⁇ g/L headspace or specific productivity ( ⁇ g/L headspace/OD).
- Diamonds represent OD 6 oo
- circles represent total isoprene productivity ( ⁇ g/L) and squares represent specific productivity of isoprene ( ⁇ g/L/OD).
- Figure 23H is a graph showing production of isoprene from glucose in BL21/pTrcKudzuIDIDXSkan.
- the arrow indicates the time of induction with IPTG (400 ⁇ mol).
- the x-axis is time after induction; the y-axis is OD 6O o and the y2-axis is total productivity of isoprene ( ⁇ g/L headspace or specific productivity ( ⁇ g/L headspace/OD).
- Black diamonds represent OD 60 O, black triangles represent isoprene productivity ( ⁇ g/L) and white squares represent specific productivity of isoprene ( ⁇ g/L/OD).
- Figure 24 is a map of pTrcKKDylklS kan.
- Figures 25 A-D are a nucleotide sequence of pTrcKKDylklS kan (SEQ ID NO: 11).
- Figure 26 is a map of pCL PtrcUpperPathway.
- Figures 27A-27D is a nucleotide sequence of pCL PtrcUpperPathway (SEQ ID NO: 12).
- Figure 28 shows a map of the cassette containing the lower MVA pathway and yeast idi for integration into the B. subtilis chromosome at the nprE locus.
- nprE upstream/downstream indicates 1 kb each of sequence from the nprE locus for integration.
- aprE promoter alkaline serine protease promoter indicates the promoter (-35, -10, +1 transcription start site, RBS) of the aprE gene.
- MVKl indicates the yeast mevalonate kinase gene.
- RBS-PMK indicates the yeast phosphomevalonate kinase gene with a Bacillus RBS upstream of the start site.
- RBS-MPD indicates the yeast diphosphomevalonate decarboxylase gene with a Bacillus RBS upstream of the start site.
- RBS-IDI indicates the yeast idi gene with a Bacillus RBS upstream of the start site.
- Terminator indicates the terminator alkaline serine protease transcription terminator from B. amyliquefaciens .
- SpecR indicates the spectinomycin resistance marker.
- "nprE upstream repeat for amp.” indicates a direct repeat of the upstream region used for amplification.
- Figures 29A-D are a nucleotide sequence of cassette containing the lower MVA pathway and yeast idi for integration into the B. subtilis chromosome at the nprE locus (SEQ ID NO:13).
- Figure 30 is a map of p9796-poplar.
- Figures 3 IA-B are a nucleotide sequence of p9796-poplar (SEQ ID NO: 14).
- Figure 32 is a map of pTrcPoplar.
- Figures 33A-C are a nucleotide sequence of pTrcPoplar (SEQ ID NO: 15).
- Figure 34 is a map of pTrcKudzu yIDI Kan.
- Figures 35A-C are a nucleotide sequence of pTrcKudzu yIDI Kan (SEQ ID NO: 16).
- Figure 36 is a map of pTrcKudzuDXS Kan.
- Figures 37A-C are a nucleotide sequence of pTrcKudzuDXS Kan (SEQ ID NO:17).
- Figure 38 is a map of pCL PtrcKudzu.
- Figures 39A-C are a nucleotide sequence of pCL PtrcKudzu (SEQ ID NO: 18).
- Figure 40 is a map of pCL PtrcKudzu A3.
- Figures 41 A-C are a nucleotide sequence of pCL PtrcKudzu A3 (SEQ ID NO: 19).
- Figure 42 is a map of pCL PtrcKudzu yIDI.
- Figures 43A-C are a nucleotide sequence of pCL PtrcKudzu yIDI (SEQ ID NO:20).
- Figure 44 is a map of pCL PtrcKudzu DXS.
- Figures 45 A-D are a nucleotide sequence of pCL PtrcKudzu DXS (SEQ ID NO:21).
- Figures 46A-E show graphs representing isoprene production from biomass feedstocks. Panel A shows isoprene production from corn stover, Panel B shows isoprene production from bagasse, Panel C shows isoprene production from softwood pulp, Panel D shows isoprene production from glucose, and Panel E shows isoprene production from cells with no additional feedstock. Grey squares represent OD 6 Oo measurements of the cultures at the indicated times post-inoculation and black triangles represent isoprene production at the indicated times post- inoculation.
- Figure 47A shows a graph representing isoprene production by BL21 ( ⁇ DE3) pTrcKudzu yIDI DXS (kan) in a culture with no glucose added. Squares represent OD 6 oo, and triangles represent isoprene produced ( ⁇ g/ml).
- Figure 47B shows a graph representing isoprene production from 1% glucose feedstock invert sugar by BL21 ( ⁇ DE3) pTrcKudzu yIDI DXS (kan). Squares represent OD 6 Oo, and triangles represent isoprene produced ( ⁇ g/ml).
- Figure 47C shows a graph representing isoprene production from 1% invert sugar feedstock by BL21 ( ⁇ DE3) pTrcKudzu yIDI DXS (kan). Squares represent OD 6 oo, and triangles represent isoprene produced ( ⁇ g/ml).
- Figure 47D shows a graph representing isoprene production from 1% AFEX corn stover feedstock by BL21 ( ⁇ DE3) pTrcKudzu yIDI DXS (kan). Squares represent OD 6 Oo, and triangles represent isoprene produced ( ⁇ g/ml).
- Figures 48 A-C show graphs demonstrating the effect of yeast extract of isoprene production.
- Panel A shows the time course of optical density within fermentors fed with varying amounts of yeast extract.
- Panel B shows the time course of isoprene titer within fermentors fed with varying amounts of yeast extract. The titer is defined as the amount of isoprene produced per liter of fermentation broth.
- Panel C shows the effect of yeast extract on isoprene production in E. coli grown in fed-batch culture.
- Figures 49 A-C show graphs demonstrating isoprene production from a 500 L bioreactor with E. coli cells containing the pTrcKudzu + yIDI + DXS plasmid.
- Panel A shows the time course of optical density within the 500-L bioreactor fed with glucose and yeast extract.
- Panel B shows the time course of isoprene titer within the 500-L bioreactor fed with glucose and yeast extract.
- the titer is defined as the amount of isoprene produced per liter of fermentation broth.
- Panel C shows the time course of total isoprene produced from the 500-L bioreactor fed with glucose and yeast extract.
- Figure 50 is a map of pJMupperpathway2.
- Figures 5 IA-C are the nucleotide sequence of pJMupperpathway2 (SEQ ID NO:22).
- Figure 52 is a map of pBS Kudzu #2.
- Figure 53A is a graph showing growth during fermentation time of Bacillus expressing recombinant kudzu isoprene synthase in 14 liter fed batch fermentation. Black diamonds represent a control strain (BG3594comK) without recombinant isoprene synthase (native isoprene production) and grey triangles represent CF443, Bacillus strain BG3594comK with pBSKudzu (recombinant isoprene production).
- Figure 53B is a graph showing isoprene production during fermentation time of
- Black diamonds represent a control strain (BG3594comK) without recombinant isoprene synthase (native isoprene production) and grey triangles represent CF443, Bacillus strain
- Figure 54 is a time course of optical density within the 15-L bioreactor fed with glucose.
- Figure 55 is a time course of isoprene titer within the 15-L bioreactor fed with glucose.
- the titer is defined as the amount of isoprene produced per liter of fermentation broth.
- Figure 56 is a time course of total isoprene produced from the 15-L bioreactor fed with glucose.
- Figure 57 is a time course of optical density within the 15-L bioreactor fed with glycerol.
- Figure 58 is a time course of isoprene titer within the 15-L bioreactor fed with glycerol.
- the titer is defined as the amount of isoprene produced per liter of fermentation broth.
- Figure 59 is a time course of total isoprene produced from the 15-L bioreactor fed with glycerol.
- Figures 60A-60C are the time courses of optical density, mevalonic acid titer, and specific productivity within the 150-L bioreactor fed with glucose.
- Figures 61A-61C are the time courses of optical density, mevalonic acid titer, and specific productivity within the 15-L bioreactor fed with glucose.
- Figures 62A-62C are the time courses of optical density, mevalonic acid titer, and specific productivity within the 15-L bioreactor fed with glucose.
- Figure 63A-63C are the time courses of optical density, isoprene titer, and specific productivity within the 15-L bioreactor fed with glucose.
- Figures 64A-64C are the time courses of optical density, isoprene titer, and specific productivity within the 15-L bioreactor fed with glucose.
- Figures 65A-65C are the time courses of optical density, isoprene titer, and specific productivity within the 15-L bioreactor fed with glucose.
- Figures 66A-66C are the time courses of optical density, isoprene titer, and specific productivity within the 15-L bioreactor fed with glucose.
- Figure 67A-67C are the time courses of optical density, isoprene titer, and specific productivity within the 15-L bioreactor fed with glucose.
- Figure 68 is a graph of the calculated adiabatic flame temperatures for Series A as a function of fuel concentration for various oxygen levels. The figure legend lists the curves in the order in which they appear in the graph. For example, the first entry in the figure legend
- Figure 69 is a graph of the calculated adiabatic flame temperatures for Series B as a function of fuel concentration for various oxygen levels with 4% water.
- the figure legend lists the curves in the order in which they appear in the graph.
- Figure 70 is a graph of the calculated adiabatic flame temperatures for Series C as a function of fuel concentration for various oxygen levels with 5% CCh.
- the figure legend lists the curves in the order in which they appear in the graph.
- Figure 71 is a graph of the calculated adiabatic flame temperatures for Series D as a function of fuel concentration for various oxygen levels with 10% CCh.
- the figure legend lists the curves in the order in which they appear in the graph.
- Figure 72 is a graph of the calculated adiabatic flame temperatures for Series E as a function of fuel concentration for various oxygen levels with 15% CCh. The figure legend lists the curves in the order in which they appear in the graph.
- Figure 73 is a graph of the calculated adiabatic flame temperatures for Series F as a function of fuel concentration for various oxygen levels with 20% CCh.
- the figure legend lists the curves in the order in which they appear in the graph.
- Figure 74 is a graph of the calculated adiabatic flame temperatures for Series G as a function of fuel concentration for various oxygen levels with 30% CCh. The figure legend lists the curves in the order in which they appear in the graph.
- Figure 75A is a table of the conversion of the CAFT Model results from weight percent to volume percent for series A.
- Figure 75B is a graph of the flammability results from the CAFT model for Series A in
- Figure 76A is a table of the conversion of the CAFT Model results from weight percent to volume percent for series B .
- Figure 76B is a graph of the flammability results from the CAFT model for Series B in
- Figure 77 is a figure depicting the flammability test vessel.
- Figure 78A is a graph of the flammability Curve for Test Series 1: 0% Steam, 0 psig, and 40 0 C.
- Figure 78B is a table summarizing the explosion and non-explosion data points for Test
- Figure 78C is a graph of the flammability curve for Test Series 1 compared with the
- Figure 79A is a graph of the flammability curve for Test Series 2: 4% Steam, 0 psig, and 40 0 C.
- Figure 79B is a table summarizing the explosion and non-explosion data points for Test
- Figure 79C is a graph of the flammability curve for Test Series 2 compared with the
- Figures 80A-B are a table of the detailed experimental conditions and results for Test
- Figure 81 is a table of the detailed experimental conditions and results for Test Series
- Figure 82 is a graph of the calculated adiabatic flame temperature plotted as a function of fuel concentration for various nitrogen/oxygen ratios at 3 atmospheres of pressure.
- Figure 83 is a graph of the calculated adiabatic flame temperature plotted as a function of fuel concentration for various nitrogen/oxygen ratios at 1 atmosphere of pressure.
- Figure 84 is a graph of the flammability envelope constructed using data from Figure
- Figure 85 is a graph of the flammability envelope constructed using data from Figure
- Figure 86A is a GC/MS chromatogram of fermentation off-gas.
- Figure 86B is an expansion of Fig 86A to show minor volatiles present in fermentation off-gas.
- Figure 87A is a GC/MS chromatogram of trace volatiles present in off-gas following cryo-trapping at -78°C.
- Figure 87B is a GC/MS chromatogram of trace volatiles present in off-gas following cryo-trapping at -196°C.
- Figure 87C is an expansion of Figure 87B.
- Figure 87D is an expansion of Figure 87C.
- Figures 88A-B are GC/MS chromatogram comparing C5 hydrocarbons from petroleum-derived isoprene ( Figure 88A) and biologically produced isoprene ( Figure 88B).
- the standard contains three C5 hydrocarbon impurities eluting around the main isoprene peak
- Figure 89 is a graph of the analysis of fermentation off-gas of an E. coli BL21 (DE3) pTrcIS strain expressing a Kudzu isoprene synthase and fed glucose with 3 g/L yeast extract.
- Figure 90 shows the structures of several impurities that are structurally similar to isoprene and may also act as polymerization catalyst poisons.
- Figure 91 is a map of pTrcHis2AUpperPathway (also called pTrcUpperMVA).
- Figures 92A-92C are the nucleotide sequence of pTrcHis2AUpperPathway (also called pTrcUpperMVA) (SEQ ID NO:23).
- Figure 93 is a time course of optical density within the 15-L bioreactor fed with glucose.
- Figure 94 is a time course of isoprene titer within the 15-L bioreactor fed with glucose.
- the titer is defined as the amount of isoprene produced per liter of fermentation broth.
- Figure 95 is a time course of total isoprene produced from the 15-L bioreactor fed with glucose.
- Figure 96 is a time course of optical density within the 15-L bioreactor fed with invert sugar.
- Figure 97 is a time course of isoprene titer within the 15-L bioreactor fed with invert sugar.
- the titer is defined as the amount of isoprene produced per liter of fermentation broth.
- Figure 98 is a time course of total isoprene produced from the 15-L bioreactor fed with invert sugar.
- Figure 99 is a time course of optical density within the 15-L bioreactor fed with glucose.
- Figure 100 is a time course of isoprene titer within the 15-L bioreactor fed with glucose.
- the titer is defined as the amount of isoprene produced per liter of fermentation broth.
- Figure 101 is a time course of isoprene specific activity from the 15-L bioreactor fed with glucose.
- Figure 102 is a map of pCLPtrcUpperPathwayHGS2.
- Figures 103A- 103 C are the nucleotide sequence of pCLPtrcUpperPathwayHGS2 (SEQ ID NO:24).
- Figure 104 is a time course of optical density within the 15-L bioreactor fed with glucose.
- Figure 105 is a time course of isoprene titer within the 15-L bioreactor fed with glucose. The titer is defined as the amount of isoprene produced per liter of fermentation broth.
- Figure 106 is a time course of total isoprene produced from the 15-L bioreactor fed with glucose.
- Figure 107 is a map of plasmid MCM330 (FRT-cm-FRT-gil .2-KKDy at attTn7).
- Figures 108A-108C are the nucleotide sequence of plasmid MCM330 (SEQ ID NO:25).
- Figure 109 is a map of pET24D-Kudzu.
- Figures 110A-B are the nucleotide sequence of pET24D-Kudzu (SEQ ID NO:26).
- Figure 11 IA is a time course of optical density within the 15-L bioreactor fed with glucose.
- Figure 11 IB is a time course of isoprene titer within the 15-L bioreactor fed with glucose.
- the titer is defined as the amount of isoprene produced per liter of fermentation broth.
- Figure 111C is a time course of specific productivity of isoprene in the 15-L bioreactor fed with glucose.
- Figure 112A is a map of the M. mazei archaeal Lower Pathway operon.
- Figures 112B-C are the nucleotide sequence of the M. mazei archaeal lower Pathway operon (SEQ ID NO:27).
- Figure 113A is a map of MCM382 - pTrcKudzuMVK(mazei).
- Figures 113B-C are the nucleotide sequence of MCM382 - pTrcKudzuMVK(mazei) (SEQ ID NO:28).
- Figure 114A is a map of MCM376 - MVK from M. mazei archaeal Lower in pET200D.
- Figures 114B-C are the nucleotide sequence of MCM376 - MVK from M. mazei archaeal Lower in pET200D (SEQ ID NO:29).
- Figures 115A-115D demonstrate that over-expression of MVK and isoprene synthase results in increased isoprene production.
- Accumulated isoprene and CO 2 from MCM401 and MCM343 during growth on glucose in 100 mL bioreactors with 100 and 200 ⁇ M IPTG induction of isoprene production was measured over a 22 hour time course.
- Figure 115A is a graph of the accumulated isoprene (%) from MCM343.
- Figure 115B is a graph of the accumulated isoprene (%) from MCM401.
- Figure 115C is a graph of the accumulated CO 2 (%) from MCM343.
- Figure 115D is a graph of the accumulated CO 2 (%) from MCM401.
- Figure 116 is a time course of optical density within the 15-L bioreactor fed with glucose.
- Figure 117 is a time course of isoprene titer within the 15-L bioreactor fed with glucose.
- the titer is defined as the amount of isoprene produced per liter of fermentation broth.
- Figure 118 is a time course of total isoprene produced from the 15-L bioreactor fed with glucose.
- Figure 119 is a graph of the total carbon dioxide evolution rate (TCER), or metabolic activity profile, within the 15-L bioreactor fed with glucose.
- TCER total carbon dioxide evolution rate
- Figure 120 is a graph of the cell viability during isoprene production within the 15-L bioreactor fed with glucose.
- TVC/OD is the total viable counts (colony forming units) in 1 mL of broth per optical density unit (OD 550 ).
- Figure 121 is a time course of optical density within the 15-L bioreactor fed with glucose.
- Figure 122 is a time course of isoprene titer within the 15-L bioreactor fed with glucose.
- the titer is defined as the amount of isoprene produced per liter of fermentation broth.
- Figure 123 is a time course of total isoprene produced from the 15-L bioreactor fed with glucose.
- Figure 124 is a time course of volumetric productivity within the 15-L bioreactor fed with glucose.
- the volumetric productivity is defined as the amount of isoprene produced per liter of broth per hour.
- Figure 125 is a time course of instantaneous yield within the 15-L bioreactor fed with glucose.
- the instantaneous yield is defined as the amount of isoprene (gram) produced per amount of glucose (gram) fed to the bioreactor (w/w) during the time interval between the data points.
- Figure 126 is a graph of the total carbon dioxide evolution rate (TCER), or metabolic activity profile, within the 15-L bioreactor fed with glucose.
- Figure 127 is cell viability during isoprene production within the 15-L bioreactor fed with glucose.
- TVC/OD is the total viable counts (colony forming units) in 1 mL of broth per optical density unit (OD 550 ).
- Figure 128 is a time course of optical density within the 15-L bioreactor fed with glucose.
- Figure 129 is a time course of isoprene titer within the 15-L bioreactor fed with glucose.
- the titer is defined as the amount of isoprene produced per liter of fermentation broth.
- Figure 130 is a time course of total isoprene produced from the 15-L bioreactor fed with glucose.
- Figure 131 is a graph of total carbon dioxide evolution rate (TCER), or metabolic activity profile, within the 15-L bioreactor fed with glucose.
- TCER total carbon dioxide evolution rate
- Figure 132 is a graph showing that a transient decrease in the airflow to the bioreactor caused a spike in the concentration of isoprene in the off-gas that did not cause a dramatic decrease in metabolic activity (TCER).
- TCER or metabolic activity, is the total carbon dioxide evolution rate.
- Figure 133 is a graph of the cell viability during isoprene production within the 15-L bioreactor fed with glucose.
- TVC/OD is the total viable counts (colony forming units) in 1 mL of broth per optical density unit (OD 550 ).
- Figure 134 is a time course of optical density within the 15-L bioreactor fed with glucose. Dotted vertical lines denote the time interval when isoprene was introduced into the bioreactor at a rate of 1 g/L/hr.
- Figure 135 is total carbon dioxide evolution rate (TCER), or metabolic activity profile, within the 15-L bioreactor fed with glucose. Dotted vertical lines denote the time interval when isoprene was introduced into the bioreactor at a rate of 1 g/L/hr.
- Figure 136 is cell viability during isoprene production within the 15-L bioreactor fed with glucose.
- TVC/OD is the total viable counts (colony forming units) in 1 mL of broth per optical density unit (OD 550 ).
- Dotted vertical lines denote the time interval when isoprene was introduced into the bioreactor at a rate of 1 g/L/hr.
- Figures 137A-B are the sequence o ⁇ Populus alba pET24a: isoprene synthase gene highlighted in bold letters (SEQ ID NO:30).
- Figures 137C-D are the sequence o ⁇ Populus nigra pET24a: isoprene synthase gene highlighted in bold letters (SEQ ID NO:31).
- Figures 137E-F are the sequence o ⁇ Populus tremuloides pET24a (SEQ ID NO:32).
- Figure 137G is the amino acid sequence o ⁇ Populus tremuloides isoprene synthase gene (SEQ ID NO:33).
- Figures 137H-I are the sequence of Populus trichocarpa pET24a: isoprene synthase gene highlighted in bold letters (SEQ ID NO:34).
- Figures 137J-K are the sequence of Populus tremula x Populus alba pET24a: isoprene synthase gene highlighted in bold letters (SEQ ID NO:35).
- Figure 137L is a map of MCM93 which contains the kudzu IspS coding sequence in a pCR2.1 backbone.
- Figures 137M-N are the sequence of MCM93 (SEQ ID NO:36).
- Figure 1370 is a map of pET24D-Kudzu.
- Figures 137P-Q are the sequence of pET24D-Kudzu (SEQ ID NO:37).
- Figure 138 is isoprene synthase expression data for various poplar species as measured in the whole cell head space assay.
- Y-axis is ⁇ g/L/OD of isoprene produced by 0.2 mL of a culture induced with IPTG.
- Figure 139 is relative activity of Poplar isoprene synthase enzymes as measured by
- Poplar enzymes have significantly higher activity than the isoprene synthase from Kudzu.
- Poplar [albax tremula] only had traces ( ⁇ 1%) of activity and is not shown in the plot.
- Figure 140 is a map of pDONR221: 19430 - hybrid HGS.
- Figure 141 is the nucleotide sequence of pDONR221 : 19430 - hybrid HGS, the sequence of Kudzu isoprene synthase codon-optimized for yeast (SEQ ID NO: 38).
- Figure 142 A is a map of pDW14.
- Figures 142B-C are the complete nucleotide sequence of pDW14 (SEQ ID NO:39).
- Figure 143 shows induced INVSc-I strains harboring pDW14 or pYES-DEST52.
- Figure 143 A A 4-12% bis tris gel (Novex, Invitrogen) of lysates generated from INVSc-I strains induced with galactose and stained with SimplyBlue SafeStain (Invitrogen).
- Figure 143B A 4-12% bis tris gel (Novex, Invitrogen) of lysates generated from INVSc-I strains induced with galactose and stained with SimplyBlue SafeStain (Invitrogen).
- Figure 143B A 4-12% bis tris gel (Novex, Invitrogen) of lysates generated from INVSc-I strains induced with galactose and stained with SimplyBlue SafeStain (Invitrogen).
- Figure 144 shows induced INVSc-I strains harboring pDW14 or pYES-DEST52.
- Figure 144A OD 6 Oo of galactose-induced strains prior to lysis. The y-axis is OD 6 Oo- Figure 144B.
- Figure 145A is a map of codon optimized isoprene synthase fluo-opt2v2.
- Figure 145B is the nucleotide sequence of codon optimized isoprene synthase fluo- opt2v2 (SEQ ID NO:40).
- Figure 146A is a map of pBBRlMCS5.
- Figures 146B-C are the nucleotide sequence of pBBRlMCS5 (SEQ ID NO:41).
- Figure 147A is a map of pBBR5HGSOpt2_2.
- Figures 147B-C are the nucleotide sequence of pBBR5HGSOpt2_2 (SEQ ID NO:42).
- Figure 148 is a graph of CER versus fermentation time for strain MCM401, uninduced, induced with IPTG (4 x 50 ⁇ mol) or IPTG (2 x 100 ⁇ mol).
- Figure 150 shows growth curves (OD 600 as a function of time) of Pseudomonas putida
- Figure 151 shows growth curves (OD 600 as a function of time) of E. coli BL21(DE3)
- Figure 152 is a map of plasmid pET24 P. alba HGS.
- Figure 153 A-B are the nucleotide sequence of plasmid pET24 P. alba HGS (SEQ ID NO: 1).
- Figure 154 is a schematic diagram showing restriction sites used for endonuclease digestion to construct plasmid EWL230 and compatible cohesive ends between BspHI and Ncol sites.
- Figure 155 is a map of plasmid EWL230.
- Figures 156A-B are the nucleotide sequence of plasmid EWL230 (SEQ ID NO:44).
- Figure 157 is a schematic diagram showing restriction sites used for endonuclease digestion to construct plasmid EWL244 and compatible cohesive ends between Nsil and Pstl sites.
- Figure 158 is a map of plasmid EWL244.
- Figures 159A-B are the nucleotide sequence of plasmid EWL244 (SEQ ID NO:45).
- Figure 160A is a map of the M. mazei archaeal Lower Pathway operon.
- Figures 160B-C are the nucleotide sequence of the M. mazei archaeal Lower Pathway operon (SEQ ID NO:46).
- Figure 161 A is a map of MCM376-MVK from M. mazei archaeal Lower in pET200D.
- Figures 161B-C are the nucleotide sequence of MCM376-MVK from M. mazei archaeal Lower in pET200D (SEQ ID NO:47).
- Figure 162 is a map of plasmid pBBRCMPGIl .5-pgl.
- Figures 163 A-B are the nucleotide sequence of plasmid pBBRCMPGIl .5-pgl (SEQ ID NO:48).
- Figures 164A-F are graphs of isoprene production by E. coli strain expressing M. mazei mevalonate kinase, P. alba isoprene synthase, and pgl (RHMl 11608-2), and grown in fed-batch culture at the 15-L scale.
- Figure 164A shows the time course of optical density within the 15-L bioreactor fed with glucose.
- Figure 164B shows the time course of isoprene titer within the 15-L bioreactor fed with glucose. The titer is defined as the amount of isoprene produced per liter of fermentation broth.
- Figure 164C also shows the time course of isoprene titer within the 15-L bioreactor fed with glucose.
- Figure 164D shows the time course of total isoprene produced from the 15-L bioreactor fed with glucose.
- Figure 164E shows volumetric productivity within the 15-L bioreactor fed with glucose.
- Figure 164F shows carbon dioxide evolution rate (CER), or metabolic activity profile, within the 15-L bioreactor fed with glucose.
- CER carbon dioxide evolution rate
- Figures 165 A-B are graphs showing analysis of off-gas from fermentation in 15L bioreactors.
- Sample A is strain RMl 11608-2 sampled at 64.8 hours.
- Sample B is strain EWL256 was E. coli BL21 (DE3), pCL upper, cmR-gil.2-yKKDyI, pTrcAlba-mMVK sampled at 34.5 hours. Hydrogen is detected above the baseline (0.95 x 10 "8 torr) for both samples.
- Figure 166A shows an exemplary BioisopreneTM recovery unit.
- Figure 166B shows an exemplary BioisopreneTM desorption/condensation setup.
- Figure 167 shows a GC/FID chromatogram of a BioisopreneTM product. The material was determined to be 99.7% pure.
- Figure 168 A-C show the GC/FID chromatograms of a BioisopreneTM sample before (A) and after treatment with alumina (B) or silica (C). The isoprene peak is not shown in these chromatograms.
- Figure 169 shows a diagram of a process and associated apparatus for purifying isoprene from a fermentation off-gas.
- Figure 170 shows GC/FID chromatogram of partially hydrogenated BioisopreneTM monomer.
- compound 4 (RT
- Figure 171 shows the GC/MS Total Ion Chromatogram for products derved from the
- Figure 172 shows the GC/MS Total Ion Chramatogram for products derived from
- Figure 173 shows a process flow diagram for the conversion of a C5 stream into a
- the C5 stream comprises BiolsopreneTM monomer and/or C5 derivatives of BiolsopreneTM monomer.
- the invention provides, inter alia, compositions and methods for producing a fuel constituent from isoprene.
- fuel constituents or additives for example, cyclic isoprene dimers and trimers, linear isoprene oligomers, aromatic and alicyclic isoprene derivatives, and oxygenated isoprene derivatives.
- the fuel constituent can be produced by chemical transformations of a starting material comprising a commercially beneficial amount of highly pure isoprene.
- the commercially beneficial amount of highly pure isoprene comprises bioisoprene.
- a commercially beneficial amount of highly pure isoprene can be bioisoprene.
- a commercially beneficial amount of highly pure isoprene can be highly pure isoprene compositions produced by culturing cells expressing a heterologous isoprene synthase enzyme.
- highly pure isoprene undergoes oligomerization to form unsaturated isoprene oligomers such as cyclic dimers or trimers and linear oligomers.
- the unsaturated oligomers may be hydrogenated to produce saturated hydrocarbon fuel constituent.
- reaction of highly pure isoprene with alcohols in the presence of an acid catalyst produces fuel oxygenates.
- the highly pure isoprene is partially hydrogenated to produce isoamylenes.
- an isoamylene product derived from the highly pure isoprene undergoes dimerization to form isodecenes.
- isoamylene products derived from the highly pure isoprene react with alcohols in the presence of an acid catalyst to produce fuel oxygenates.
- Bioisoprene derived from renewable carbon can be converted to a variety of hydrocarbon fuels by chemical catalysis.
- Provided herein are methods for recovering isoprene from fermentation and subsequent conversion to hydrocarbon fuels by chemical catalysis to compounds of higher molecular weight. These methods include, but are not limited to, recovering and purifying isoprene from fermentation off-gas and subsequent gas or liquid phase catalysis to provide compounds with fuel value. Both continuous and batch mode processes are contemplated within the scope of the invention.
- a bioisoprene composition is distinguished from a petro- isoprene composition in that a bioisoprene composition is substantially free of any contaminating unsaturated C5 hydrocarbons that are usually present in petro-isoprene compositions, such as, but not limited to, 1,3-cyclopentadiene, tr ⁇ r ⁇ -l,3-pentadiene, cis-1,3- pentadiene, 1 ,4-pentadiene, 1-pentyne, 2-pentyne, 3-methyl-l-butyne, pent-4-ene-l-yne, trans- pent-3-ene-l-yne, and cz ' s-pent-3-ene-l-yne.
- any contaminating unsaturated C5 hydrocarbons are present in the bioisoprene starting material described herein, they are present in lower levels than that in petro-isoprene compositions. Accordingly, any fuel products derived from bioisoprene compositions described herein is essentially free of, or contains at lower levels than that in fuel products derived from petro-isoprene, any contaminating unsaturated C5 hydrocarbons or products derived from such contaminating unsaturated C5 hydrocarbons. In addition, the sulfur levels in a bioisoprene composition are lower than the sulfur levels in petro- isoprene compositions. Fuels products derived from bioisoprene compositions contain lower levels of sulfur than that in fuel products derived from petro-isoprene.
- Bioisoprene is distinguished from petro-isoprene in that bioisoprene is produced with other bio-byproducts (compounds derived from the biological sources and/or associated the biological processes that are obtained together with bioisoprene) that are not present or present in much lower levels in petro-isoprene compositions, such as alcohols, aldehydes, ketone and the like.
- the bio-byproducts may include, but are not limited to, ethanol, acetone, methanol, acetaldehyde, methacrolein, methyl vinyl ketone, 2-methyl-2-vinyloxirane, cis- and trans-3- methyl-l,3-pentadiene, a C5 prenyl alcohol (such as 3-methyl-3-buten-l-ol or 3-methyl-2-buten- l-ol), 2-heptanone, 6-methyl-5-hepten-2-one, 2,4,5-trimethylpyridine, 2,3,5-trimethylpyrazine, citronellal, methanethiol, methyl acetate, 1-propanol, diacetyl, 2-butanone, 2-methyl-3-buten-2- ol, ethyl acetate, 2-methyl- 1-propanol, 3-methyl-l-butanal, 3-methyl-2-butanone, 1-butanol, 2- pentanone, 3 -methyl- 1-
- Fuel products derived from bioisoprene contain one or more of the bio-byproducts or compounds derived from any of the bio-byproducts.
- fuel products derived from bioisoprene may contain compounds formed from these bio-byproducts during subsequent chemical conversion. Examples of such compounds include those derived from Diels- Alder cycloaddition of dienophiles to isoprene or fuel derivatives thereof, the oxidation of isoprene or fuel derivatives.
- bioisoprene is distinguished from petro-isoprene by carbon finger-printing.
- bioisoprene has a higher radioactive carbon- 14 ( 14 C) content or higher 14 C/ 12 C ratio than petro-isoprene.
- Bioisoprene is produced from renewable carbon sources, thus the 14 C content or the 14 C/ 12 C ratio in bioisoprene is the same as that in the present atmosphere.
- Petro- isoprene on the other hand, is derived from fossil fuels deposited thousands to millions of years ago, thus the 14 C content or the 14 C/ 12 C ratio is diminished due to radioactive decay.
- the fuel products derived from bioisoprene has higher 14 C content or 14 C/ 12 C ratio than fuel products derived from petro-isoprene.
- a fuel product derived from bioisoprene described herein has a 14 C content or 14 C/ 12 C ratio similar to that in the atmosphere.
- bioisoprene can be analytically distinguished from petro-isoprene by the stable carbon isotope ration ( 13 C/ 12 C), which can be reported as "delta values" represented by the symbol d 13 C.
- 13 C/ 12 C stable carbon isotope ration
- 5 13 C is about -22%o to about -24%o. This range is typical for light, unsaturated hydrocarbons derived from petroleum, and products derived from petroleum-based isoprene typically contain isoprenic units with the same 6 13 C.
- other carbon-containing nutrients e.g., yeast extract
- Compounds made by these methods include cyclic isoprene dimers and trimers, linear oligomers, aromatic and alicyclic derivatives. Diisoamylenes are made by methods comprising partial hydrogenation of bioisoprene compositons. These chemical derivatives of isoprene are useful as liquid transportation fuels (IsoFuelsTM) and as fuel additives.
- IsoFuelsTM liquid transportation fuels
- Also provided herein are methods for the production of oxygenated derivatives of isoprene including alcohols, ketones, esters and ethers. Methods for the synthesis of oxygenated derivatives of isoprene can also be performed in liquid or gas phase, using homogeneous and heterogeneous catalysts. Compounds of this chemical class are also useful as liquid transportation fuels, and can be used in fuel blends as fuel oxygenates for emissions reduction and as fuel modifiers, for example as cetane boosters for diesel.
- isoprene can be obtained by fractionating petroleum, the purification of this material is expensive and time-consuming. Petroleum cracking of the C5 stream of hydrocarbons produces only about 15% isoprene. Isoprene is also naturally produced by a variety of microbial, plant, and animal species. In particular, two pathways have been identified for the biosynthesis of isoprene: the mevalonate (MVA) pathway and the non-mevalonate (DXP) pathway. Genetically engineered cell cultures in bioreactors have produced isoprene more efficiently, in larger quantities, in higher purities and/or with unique impurity profiles, e.g. as described in U.S. provisional patent application Nos.
- isoprene refers to 2-methyl- 1 ,3-butadiene (CAS# 78-79-5 ), which is the direct and final volatile C5 hydrocarbon product from the elimination of pyrophosphate from 3,3-dimethylallyl pyrophosphate (DMAPP), and does not involve the linking or polymerization of [an] IPP molecule(s) to [a] DMAPP molecule(s).
- DMAPP 3,3-dimethylallyl pyrophosphate
- the term “isoprene” is not generally intended to be limited to its method of production unless indicated otherwise herein.
- biologically produced isoprene or “bioisoprene” is isoprene produced by any biological means, such as produced by genetically engineered cell cultures, natural microbials, plants or animals.
- a "bioisoprene composition” refers to a composition that can be produced by any biological means, such as systems (e.g., cells) that are engineered to produce isoprene. It contains isoprene and other compounds that are co-produced (including impurities) and/or isolated together with isoprene.
- a bioisoprene composition usually contains fewer hydrocarbon impurities than isoprene produced from petrochemical sources and often requires minimal treatment in order to be of polymerization grade. As detailed herein, bioisoprene composition also has a different impurity profile from a petrochemically produced isoprene composition.
- the isoprene starting composition can refer to at least about 1%, 5%, 10%, 15%, 20%, 25%, 30%, 35%, 40%, 45%, 50%, 55%, 60%, 65%, 70%, 75%, 80%, 85%, 90%, 91%, 92%, 93%, 94%, 95%, 96%, 97%, 98%, 99%, 99.5%, 99.6%, 99.7%, 99.8%, 99.9%, or 100% of the isoprene starting composition undergoing chemical transformation.
- IsoFuelsTM refers to fuels including liquid transportation fuels that are derived from isoprene.
- BioIsoFuelsTM refers to fuels including liquid transportation fuels that are derived from bioisoprene.
- oligomerization refers to a chemical process for combining two or more monomer units.
- Oligomerization produces a derivative of isoprene derived from two or more molecules of isoprene, such as linear dimers of isoprene, cyclic dimers of isoprene, linear trimers of isoprene, cyclic trimers of isoprene and the like.
- “Complete hydrogenation”, “Completely hydrogenate” or “Fully hydrogenate” is defined as the addition of hydrogen (H 2 ), typically in the presence of a hydrogenation catalyst, to all unsaturated functional groups, such as carbon-carbon double bonds, within a precursor compound to give fully saturated product compounds.
- H 2 hydrogen
- “Partial hydrogenation” or “Partially hydrogenate” is defined as the addition of hydrogen (H 2 ), typically in the presence of a hydrogenation catalyst, to at least one, but not all unsaturated functional groups, such as carbon-carbon double bonds, within a precursor compound.
- the product(s) of partial hydrogenation can be further completely hydrogenated to give fully saturated product compounds.
- Partial hydrogenation of a diene forms one or more mono-olefins.
- partial hydrogenation of isoprene can give 3 isomeric isopentenes (2-methylbut-l-ene, 2-methylbut-2-ene and 3-methylbut-l-ene) whereby 1 mole of H 2 is comsumed per mole of isoprene.
- Selective hydrogenation or “Selectively hydrogenate” is defined as the addition of hydrogen (H 2 ), typically in the presence of a hydrogenation catalyst, to at least one, but not all unsaturated functional groups, such as carbon-carbon double bonds, within a precursor compound whereby certain unsaturated functional groups are preferentially hydrogenated over other unsaturated groups under the chosen conditions.
- selective hydrogenation of isoprene may form preferentially 2-methyl-2-butene, 2-methyl-l-butene, 3 -methyl- 1-butene or a mixture thereof.
- polypeptides includes polypeptides, proteins, peptides, fragments of polypeptides, and fusion polypeptides.
- an "isolated polypeptide” is not part of a library of polypeptides, such as a library of 2, 5, 10, 20, 50 or more different polypeptides and is separated from at least one component with which it occurs in nature.
- An isolated polypeptide can be obtained, for example, by expression of a recombinant nucleic acid encoding the polypeptide.
- heterologous polypeptide is meant a polypeptide whose amino acid sequence is not identical to that of another polypeptide naturally expressed in the same host cell.
- a heterologous polypeptide is not identical to a wild-type polypeptide that is found in the same host cell in nature.
- Codon degeneracy refers to divergence in the genetic code permitting variation of the nucleotide sequence without affecting the amino acid sequence of an encoded polypeptide. The skilled artisan is well aware of the "codon-bias" exhibited by a specific host cell in usage of nucleotide codons to specify a given amino acid. Therefore, when synthesizing a nucleic acid for improved expression in a host cell, it is desirable in some embodiments to design the nucleic acid such that its frequency of codon usage approaches the frequency of preferred codon usage of the host cell.
- nucleic acid refers to two or more deoxyribonucleotides and/or ribonucleotides covalently joined together in either single or double-stranded form.
- recombinant nucleic acid is meant a nucleic acid of interest that is free of one or more nucleic acids (e.g., genes) which, in the genome occurring in nature of the organism from which the nucleic acid of interest is derived, flank the nucleic acid of interest.
- the term therefore includes, for example, a recombinant DNA which is incorporated into a vector, into an autonomously replicating plasmid or virus, or into the genomic DNA of a prokaryote or eukaryote, or which exists as a separate molecule (e.g., a cDNA, a genomic DNA fragment, or a cDNA fragment produced by PCR or restriction endonuclease digestion) independent of other sequences.
- a recombinant DNA which is incorporated into a vector, into an autonomously replicating plasmid or virus, or into the genomic DNA of a prokaryote or eukaryote, or which exists as a separate molecule (e.g., a cDNA, a genomic DNA fragment, or a cDNA fragment produced by PCR or restriction endonuclease digestion) independent of other sequences.
- heterologous nucleic acid is meant a nucleic acid whose nucleic acid sequence is not identical to that of another nucleic acid naturally found in the same host cell.
- a heterologous nucleic acid is not identical to a wild-type nucleic acid that is found in the same host cell in nature.
- a "vector” means a construct that is capable of delivering, and desirably expressing one or more nucleic acids of interest in a host cell.
- vectors include, but are not limited to, plasmids, viral vectors, DNA or RNA expression vectors, cosmids, and phage vectors.
- an "expression control sequence” means a nucleic acid sequence that directs transcription of a nucleic acid of interest.
- An expression control sequence can be a promoter, such as a constitutive or an inducible promoter, or an enhancer.
- An "inducible promoter” is a promoter that is active under environmental or developmental regulation.
- the expression control sequence is operably linked to the nucleic acid segment to be transcribed.
- selective marker or “selectable marker” refers to a nucleic acid capable of expression in a host cell that allows for ease of selection of those host cells containing an introduced nucleic acid or vector.
- selectable markers include, but are not limited to, antibiotic resistance nucleic acids (e.g., kanamycin, ampicillin, carbenicillin, gentamicin, hygromycin, phleomycin, bleomycin, neomycin, or chloramphenicol) and/or nucleic acids that confer a metabolic advantage, such as a nutritional advantage on the host cell.
- antibiotic resistance nucleic acids e.g., kanamycin, ampicillin, carbenicillin, gentamicin, hygromycin, phleomycin, bleomycin, neomycin, or chloramphenicol
- nucleic acids that confer a metabolic advantage such as a nutritional advantage on the host cell.
- Exemplary nutritional selective markers include those markers known in the art as amdS, argB, and pyr4.
- Isoprene derived from petrochemical sources usually is an impure C5 hydrocarbon fraction which requires extensive purification before the material is suitable for polymerization or other chemical transformations.
- impurities are particularly problematic given their structural similarity to isoprene and the fact that they can act as polymerization catalyst poisons.
- Such compounds include, but are not limited to, 1,3-cyclopentadiene, cis- and trans- 1,3- pentadiene, 1 ,4-pentadiene, 1-pentyne, 2-pentyne, 3-methyl-l-butyne, pent-4-ene-l-yne, trans- pent-3-ene-l-yne, and cz ' s-pent-3-ene-l-yne.
- biologically produced isoprene can be substantially free of any contaminating unsaturated C5 hydrocarbons without undergoing extensive purification.
- Some biologically produced isoprene compositions contain ethanol, acetone, and C5 prenyl alcohols.
- these components are more readily removed from the isoprene stream than the isomeric C5 hydrocarbon fractions that are present in isoprene compositions derived from petrochemical sources. Further, these impurities can be managed in the bioprocess, for example by genetic modification of the producing strain, carbon feedstock, alternative fermentation conditions, recovery process modifications and additional or alternative purification methods.
- the invention features compositions and systems for producing a fuel constituent from isoprene comprising: (a) a commercially beneficial amount of highly pure isoprene starting composition; and (b) a fuel constituent produced from at least a portion of the highly pure isoprene starting material; where at least a portion of the commercially beneficial amount of highly pure isoprene starting composition undergoes a chemical transformation.
- a highly pure isoprene starting material is subjected to chemical reactions to produce a commercially beneficial amount of product that is useful for making fuels.
- a commercially beneficial amount of highly pure isoprene comprises bioisoprene.
- a commercially beneficial amount of highly pure isoprene can be bioisoprene.
- the commercially beneficial amount of highly pure isoprene starting composition comprises greater than or about 2, 5, 10, 20, 30, 40, 50, 60, 70, 80, 90, 100, 200, 300, 400, 500, 600, 700, 800, 900, or 1000 mg of isoprene.
- the starting isoprene composition comprises greater than or about 2, 5, 10, 20, 30, 40, 50, 60, 70, 80, 90, 100 g of isoprene.
- the starting isoprene composition comprises greater than or about 0.2, 0.5, 1, 2, 5, 10, 20, 50, 100, 200, 500, 1000 kg of isoprene.
- the amount of isoprene in the starting composition is between about 2 to about 5,000 mg, such as between about 2 to about 100 mg, about 100 to about 500 mg, about 500 to about 1,000 mg, about 1,000 to about 2,000 mg, or about 2,000 to about 5,000 mg. In some embodiments, the amount of isoprene in the starting composition is between about 20 to about 5,000 mg, about 100 to about 5,000 mg, about 200 to about 2,000 mg, about 200 to about 1,000 mg, about 300 to about 1,000 mg, or about 400 to about 1,000 mg.
- the amount of isoprene in the starting composition is between about 2 to about 5,000 g, such as between about 2 to about 100 g, about 100 to about 500 g, about 500 to about 1,000 g, about 1,000 to about 2,000 g, or about 2,000 to about 5,000 g. In some embodiments, the amount of isoprene in the starting composition is between about 2 to about 5,000 kg, about 10 to about 2,000 kg, about 20 to about 1,000 kg, about 20 to about 500 kg, about 30 to about 200 kg, or about 40 to about 100 kg. In some embodiments, greater than or about 20, 25, 30, 40, 50, 60, 70, 80, 90, or 95% (w/w) of the volatile organic fraction of the starting composition is isoprene.
- the highly pure isoprene starting composition comprises greater than or about 98.0, 98.5, 99.0, 99.5, or 100% isoprene by weight compared to the total weight of all C5 hydrocarbons in the starting composition. In some embodiments, the highly pure isoprene starting composition comprises greater than or about 99.90, 99.92, 99.94, 99.96, 99.98, or 100% isoprene by weight compared to the total weight of all C5 hydrocarbons in the starting composition. In some embodiments, the starting composition has a relative detector response of greater than or about 98.0, 98.5, 99.0, 99.5, or 100% for isoprene compared to the detector response for all C5 hydrocarbons in the starting composition.
- the starting composition has a relative detector response of greater than or about 99.90, 99.91, 99.92, 99.93, 99.94, 99.95, 99.96, 99.97, 99.98, 99.99, or 100% for isoprene compared to the detector response for all C5 hydrocarbons in the starting composition.
- the starting isoprene composition comprises between about 98.0 to about 98.5, about 98.5 to about 99.0, about 99.0 to about 99.5, about 99.5 to about 99.8, about 99.8 to 100% isoprene by weight compared to the total weight of all C5 hydrocarbons in the starting composition.
- the starting isoprene composition comprises between about 99.90 to about 99.92, about 99.92 to about 99.94, about 99.94 to about 99.96, about 99.96 to about 99.98, about 99.98 to 100% isoprene by weight compared to the total weight of all C5 hydrocarbons in the starting composition.
- the highly pure isoprene starting composition comprises less than or about 2.0, 1.5, 1.0, 0.5, 0.2, 0.12, 0.10, 0.08, 0.06, 0.04, 0.02, 0.01, 0.005, 0.001, 0.0005, 0.0001, 0.00005, or 0.00001% C5 hydrocarbons other than isoprene (such 1,3-cyclopentadiene, czs-l,3-pentadiene, z ⁇ ms-l,3-pentadiene, 1 ,4-pentadiene, 1-pentyne, 2-pentyne, 1-pentene, 2- methyl-1-butene, 3-methyl-l-butyne, pent-4-ene-l-yne, jr ⁇ «s-pent-3-ene-l-yne, or cz ' s-pent-3- ene-1-yne) by weight compared to the total weight of all C5 hydrocarbons in the
- the starting composition has a relative detector response of less than or about 2.0, 1.5, 1.0, 0.5, 0.2, 0.12, 0.10, 0.08, 0.06, 0.04, 0.02, 0.01, 0.005, 0.001, 0.0005, 0.0001, 0.00005, or 0.00001% for C5 hydrocarbons other than isoprene compared to the detector response for all C5 hydrocarbons in the starting composition.
- the starting composition has a relative detector response of less than or about 2.0, 1.5, 1.0, 0.5, 0.2, 0.12, 0.10, 0.08, 0.06, 0.04, 0.02, 0.01, 0.005, 0.001, 0.0005, 0.0001, 0.00005, or 0.00001% for 1,3-cyclopentadiene, czs-l,3-pentadiene, z ⁇ ms-l,3-pentadiene, 1,4-pentadiene, 1-pentyne, 2- pentyne, 1-pentene, 2-methyl-l-butene, 3-methyl-l-butyne, pent-4-ene-l-yne, tr ⁇ r ⁇ -pent-3-ene- 1-yne, or cz ' s-pent-3-ene-l-yne compared to the detector response for all C5 hydrocarbons in the starting composition.
- the highly pure isoprene starting composition comprises between about 0.02 to about 0.04%, about 0.04 to about 0.06%, about 0.06 to 0.08%, about 0.08 to 0.10%, or about 0.10 to about 0.12% C5 hydrocarbons other than isoprene (such 1,3-cyclopentadiene, czs-l,3-pentadiene, trans- 1,3-pentadiene, 1,4-pentadiene, 1-pentyne, 2- pentyne, 1-pentene, 2-methyl-l-butene, 3-methyl-l-butyne, pent-4-ene-l-yne, tr ⁇ r ⁇ -pent-3-ene- 1-yne, or cz ' s-pent-3-ene-l-yne) by weight compared to the total weight of all C5 hydrocarbons in the starting composition.
- C5 hydrocarbons other than isoprene such 1,3-cyclopentadiene, cz
- the highly pure isoprene starting composition comprises less than or about 50, 40, 30, 20, 10, 5, 1, 0.5, 0.1, 0.05, 0.01, or 0.005 ⁇ g/L of a compound that inhibits the polymerization of isoprene for any compound in the starting composition that inhibits the polymerization of isoprene.
- the starting isoprene composition comprises between about 0.005 to about 50, such as about 0.01 to about 10, about 0.01 to about 5, about 0.01 to about 1, about 0.01 to about 0.5, or about 0.01 to about 0.005 ⁇ g/L of a compound that inhibits the polymerization of isoprene for any compound in the starting composition that inhibits the polymerization of isoprene.
- the starting isoprene composition comprises less than or about 50, 40, 30, 20, 10, 5, 1, 0.5, 0.1, 0.05, 0.01, or 0.005 ⁇ g/L of a hydrocarbon other than isoprene (such 1,3-cyclopentadiene, czs-l,3-pentadiene, drafts- 1, 3 -pentadiene, 1 ,4-pentadiene, 1-pentyne, 2-pentyne, 1-pentene, 2-methyl-l-butene, 3- methyl-1-butyne, pent-4-ene-l-yne, tr ⁇ r ⁇ -pent-3-ene-l-yne, or czs-pent-3-ene-l-yne).
- a hydrocarbon other than isoprene such 1,3-cyclopentadiene, czs-l,3-pentadiene, drafts- 1, 3 -pentadiene, 1 ,4-p
- the starting isoprene composition comprises between about 0.005 to about 50, such as about 0.01 to about 10, about 0.01 to about 5, about 0.01 to about 1, about 0.01 to about 0.5, or about 0.01 to about 0.005 ⁇ g/L of a hydrocarbon other than isoprene. In some embodiments, the starting isoprene composition comprises less than or about 50, 40, 30, 20, 10, 5, 1, 0.5, 0.1, 0.05, 0.01, or 0.005 ⁇ g/L of a protein or fatty acid (such as a protein or fatty acid that is naturally associated with natural rubber).
- a protein or fatty acid such as a protein or fatty acid that is naturally associated with natural rubber.
- the highly pure isoprene starting composition comprises less than or about 10, 5, 1, 0.8, 0.5, 0.1, 0.05, 0.01, or 0.005 ppm of alpha acetylenes, piperylenes, acetonitrile, or 1,3-cyclopentadiene. In some embodiments, the starting isoprene composition comprises less than or about 5, 1, 0.5, 0.1, 0.05, 0.01, or 0.005 ppm of sulfur or allenes.
- the starting isoprene composition comprises less than or about 30, 20, 15, 10, 5, 1, 0.5, 0.1, 0.05, 0.01, or 0.005 ppm of all acetylenes (such as 1-pentyne, 2-pentyne, 3-methyl-l- butyne, pent-4-ene-l-yne, trans -pent-3-ene-l-yne, and czs-pent-3-ene-l-yne).
- all acetylenes such as 1-pentyne, 2-pentyne, 3-methyl-l- butyne, pent-4-ene-l-yne, trans -pent-3-ene-l-yne, and czs-pent-3-ene-l-yne.
- the starting isoprene composition comprises less than or about 2000, 1000, 500, 200, 100, 50, 40, 30, 20, 10, 5, 1, 0.5, 0.1, 0.05, 0.01, or 0.005 ppm of isoprene dimers, such as cyclic isoprene dimers ⁇ e.g., cyclic ClO compounds derived from the dimerization of two isoprene units).
- the highly pure isoprene starting composition includes ethanol, acetone, methanol, acetaldehyde, methacrolein, methyl vinyl ketone, 2-methyl-2-vinyloxirane, cis- and zr ⁇ ms-3-methyl-l,3-pentadiene, a C5 prenyl alcohol (such as 3-methyl-3-buten-l-ol or 3- methyl-2-buten-l-ol), or any two or more of the foregoing.
- a C5 prenyl alcohol such as 3-methyl-3-buten-l-ol or 3- methyl-2-buten-l-ol
- the starting isoprene composition comprises greater than or about 0.005, 0.01, 0.05, 0.1, 0.5, 1, 5, 10, 20, 30, 40, 60, 80, 100, or 120 ⁇ g/L of ethanol, acetone, methanol, acetaldehyde, methacrolein, methyl vinyl ketone, 2-methyl-2-vinyloxirane, cis- and trans -3 -mothyl-l, 3- pentadiene, a C5 prenyl alcohol (such as 3-methyl-3-buten-l-ol or 3-methyl-2-buten-l-ol), or any two or more of the foregoing.
- ethanol acetone, methanol, acetaldehyde, methacrolein, methyl vinyl ketone, 2-methyl-2-vinyloxirane, cis- and trans -3 -mothyl-l, 3- pentadiene, a C5 prenyl alcohol (such as 3-methyl-3-buten-l-
- the isoprene composition comprises between about 0.005 to about 120, such as about 0.01 to about 80, about 0.01 to about 60, about 0.01 to about 40, about 0.01 to about 30, about 0.01 to about 20, about 0.01 to about 10, about 0.1 to about 80, about 0.1 to about 60, about 0.1 to about 40, about 5 to about 80, about 5 to about 60, or about 5 to about 40 ⁇ g/L of ethanol, acetone, methanol, acetaldehyde, methacrolein, methyl vinyl ketone, 2-methyl-2-vinyloxirane, cis- and tr ⁇ «s-3-methyl-l,3-pentadiene, a C5 prenyl alcohol, or any two or more of the foregoing.
- the highly pure isoprene starting composition includes one or more of the following components: 2-heptanone, 6-methyl-5-hepten-2-one, 2,4,5- trimethylpyridine, 2,3,5-trimethylpyrazine, citronellal, acetaldehyde, methanethiol, methyl acetate, 1-propanol, diacetyl, 2-butanone, 2-methyl-3-buten-2-ol, ethyl acetate, 2-methyl-l- propanol, 3-methyl-l-butanal, 3-methyl-2-butanone, 1-butanol, 2-pentanone, 3 -methyl- 1- butanol, ethyl isobutyrate, 3-methyl-2-butenal, butyl acetate, 3-methylbutyl acetate, 3-methyl-3- buten-1-yl acetate, 3-methyl-2-buten-l-yl acetate, 3-hexen-l-ol, 3-hex
- the amount of one of these components relative to amount of isoprene in units of percentage by weight is greater than or about 0.01, 0.02, 0.05, 0.1, 0.5, 1, 5, 10, 20, 30, 40, 50, 60, 70, 80, 90, 100, or 110% (w/w).
- the relative detector response for the second compound compared to the detector response for isoprene is greater than or about 0.01, 0.02, 0.05, 0.1, 0.5, 1, 5, 10, 20, 30, 40, 50, 60, 70, 80, 90, 100, or 110%.
- the amount of one of these components relative to amount of isoprene in units of percentage by weight is between about 0.01 to about 105 % (w/w), such as about 0.01 to about 90, about 0.01 to about 80, about 0.01 to about 50, about 0.01 to about 20, about 0.01 to about 10, about 0.02 to about 50, about 0.05 to about 50, about 0.1 to about 50, or 0.1 to about 20% (w/w).
- At least a portion of the highly pure isoprene starting composition is in a gas phase. In some embodiments, at least a portion of the highly pure isoprene starting composition is in a liquid phase (such as a condensate). In some embodiments, at least a portion of the highly pure isoprene starting composition is in a solid phase. In some embodiments, at least a portion of the highly pure isoprene starting composition is absorbed to a solid support, such as a support that includes silica and/or activated carbon. In some embodiments, the starting isoprene composition is mixed with one or more solvents. In some embodiments, the starting isoprene composition is mixed with one or more gases.
- the commercially beneficial amount of highly pure isoprene starting composition is produced by a biological process.
- the highly pure isoprene starting composition is a bioisoprene composition produced by culturing cells that produce greater than about 400 nmole of isoprene/gram of cells for the wet weight of the cells/hour (nmole/g wcm /hr) of isoprene.
- the bioisoprene composition is produced by culturing cells that convert more than about 0.002% of the carbon in a cell culture medium into isoprene.
- the cells have a heterologous nucleic acid that (i) encodes an isoprene synthase polypeptide, e.g. a naturally-occurring polypeptide from a plant such as Pueraria, and (ii) is operably linked to a promoter, e.g. a T7 promoter.
- a promoter e.g. a T7 promoter.
- Other isoprene synthase polypeptides for example, from poplar and variants of naturally-occurring as well as parent isoprene synthase, can be used to produce bioisoprene. Examples of isoprene synthase and its variants that can be used are described in U.S. Appl. No. 12/429,143, which is incorporated herein in its entirety.
- the cells are cultured in a culture medium that includes a carbon source, such as, but not limited to, a carbohydrate, glycerol, glycerine, dihydroxyacetone, one- carbon source, oil, animal fat, animal oil, fatty acid, lipid, phospholipid, glycerolipid, monoglyceride, diglyceride, triglyceride, renewable carbon source, polypeptide ⁇ e.g., a microbial or plant protein or peptide), yeast extract, component from a yeast extract, or any combination of two or more of the foregoing.
- the cells are cultured under limited glucose conditions.
- the cells further comprise a heterologous nucleic acid encoding an IDI polypeptide. In some embodiments, the cells further comprise a heterologous nucleic acid encoding an MDV pathway polypeptide.
- the starting isoprene composition is an isoprene composition as descried or is produced by culturing any of the cells described in U.S. provisional patent application Nos. 61/134,094, filed on July 2, 2008, WO 2010/003007, and U.S. patent application No. 12/335,071, filed December 15, 2008 (US 2009/0203102 Al), which are incorporated by reference in their entireties.
- the highly pure isoprene starting composition comprises a gas phase (off-gas) produced by cells in culture that produces isoprene.
- the gas phase has a nonflammable concentration of isoprene.
- the gas phase comprises less than about 9.5 % (volume) oxygen.
- the gas phase comprises greater than or about 9.5 % (volume) oxygen, and the concentration of isoprene in the gas phase is less than the lower flammability limit or greater than the upper fiammability limit.
- the portion of the gas phase other than isoprene comprises between about 0% to about 100% (volume) oxygen, such as between about 10% to about 100% (volume) oxygen.
- the portion of the gas phase other than isoprene comprises between about 0% to about 99% (volume) nitrogen. In some embodiments, the portion of the gas phase other than isoprene comprises between about 1% to about 50% (volume) CO 2 .
- the highly pure isoprene starting composition includes one or more of the following: an alcohol, an aldehyde, a ketone, or an ester (such as any of the alcohols, aldehydes, ketones or esters described herein).
- the isoprene composition includes (i) an alcohol and an aldehyde, (ii) an alcohol and a ketone, (iii) an aldehyde and a ketone, or (iv) an alcohol, an aldehyde, and a ketone. In some embodiments, any of the isoprene compositions further includes an ester.
- the highly pure isoprene starting composition derived from a biological source contains one or more of the following: methanol, acetaldehyde, ethanol, methanethiol, 1-butanol, 3-methyl-l-propanol, acetone, acetic acid, 2- butanone, 2 -methyl- 1-butanol, or indole.
- the starting isoprene composition contains 1 ppm or more of one or more of the following: methanol, acetaldehyde, ethanol, methanethiol, 1-butanol, 3-methyl-l-propanol, acetone, acetic acid, 2-butanone, 2- methyl- 1-butanol, or indole.
- the concentration of more of one or more of the following: methanol, acetaldehyde, ethanol, methanethiol, 1-butanol, 3-methyl-l-propanol, acetone, acetic acid, 2-butanone, 2-methyl- 1-butanol, or indole is between about 1 to about 10,000 ppm in a starting isoprene composition (such as off-gas before it is purified).
- the starting isoprene composition (such as off-gas after it has undergone one or more purification steps) includes one or more of the following: methanol, acetaldehyde, ethanol, methanethiol, 1-butanol, 3-methyl-l-propanol, acetone, acetic acid, 2-butanone, 2-methyl- 1- butanol, or indole, at a concentration between about 1 to about 100 ppm, such as about 1 to about 10 ppm, about 10 to about 20 ppm, about 20 to about 30 ppm, about 30 to about 40 ppm, about 40 to about 50 ppm, about 50 to about 60 ppm, about 60 to about 70 ppm, about 70 to about 80 ppm, about 80 to about 90 ppm, or about 90 to about 100 ppm.
- the starting isoprene composition contains less than 1 ppm of methanethiol (a potent catalyst poison and a source of sulfur in the final fuel product)
- Volatile organic compounds from cell cultures can be analyzed using standard methods such as those described herein or other standard methods such as proton transfer reaction-mass spectrometry (see, for example, Bunge et ah, Applied and Environmental Microbiology, 74(7):2179-2186, 2008 which is hereby incorporated by reference in its entirety, particular with respect to the analysis of volatile organic compounds).
- the invention also contemplates the use of highly pure isoprene starting composition that is derived from a biological source (such as a cell culture) the co-produces isoprene and hydrogen.
- the starting bioisoprene compositions comprise isoprene and hydrogen in ratios ranging from at least one molar percent of isoprene for every three molar percent of hydrogen to at least one molar percent of isoprene for every four molar percent of hydrogen.
- the starting bioisoprene compositions comprise isoprene and hydrogen in molar ratios of about 1 to 9, 2 to 8, 3 to 7, 4 to 6, 5 to 5, 6 to 4, 7 to 3, 8 to 2, or 9 to 1.
- the composition further comprises from 1 to 11 molar percent isoprene and from 4 to 44 molar percent hydrogen. In some embodiments, the composition further comprises oxygen, carbon dioxide, or nitrogen. In some embodiments, the composition further comprises from 0 to 21 molar percent oxygen, from 18 to 44 molar percent carbon dioxide, and from 0 to 78 molar percent nitrogen. In some embodiments, the composition further comprises 1.0 x 10 "4 molar percent or less of non-methane volatile impurities.
- the non-methane volatile impurities comprise one or more of the following: 2- heptanone, 6-methyl-5-hepten-2-one, 2,4,5-trimethylpyridine, 2,3,5-trimethylpyrazine, citronellal, acetaldehyde, methanethiol, methyl acetate, 1-propanol, diacetyl, 2-butanone, 2- methyl-3-buten-2-ol, ethyl acetate, 2-methyl- 1-propanol, 3 -methyl- 1-butanal, 3-methyl-2- butanone, 1-butanol, 2-pentanone, 3-methyl-l-butanol, ethyl isobutyrate, 3-methyl-2-butenal, butyl acetate, 3-methylbutyl acetate, 3-methyl-3-buten-l-yl acetate, 3-methyl-2-buten-l-yl acetate, 3-hexen-l-ol, 3-hexen-l-l, 3-
- the non-methane volatile impurities comprise one or more of the following: the isoprene composition includes one or more of the following: an alcohol, an aldehyde, or a ketone (such as any of the alcohols, aldehydes, or ketones described herein).
- the isoprene composition includes (i) an alcohol and an aldehyde, (ii) an alcohol and a ketone, (iii) an aldehyde and a ketone, or (iv) an alcohol, an aldehyde, and a ketone.
- the non-methane volatile impurities comprise one or more of the following: methanol, acetaldehyde, ethanol, methanethiol, 1-butanol, 3 -methyl- 1-propanol, acetone, acetic acid, 2-butanone, 2-methyl- 1-butanol, or indole.
- the efficiency of these systems is demonstrated by the conversion of about 2.2% of the carbon that the cells consume from a cell culture medium into isoprene. As shown in the Examples and Table 2, approximately 3 g of isoprene per liter of broth was generated. If desired, even greater amounts of isoprene can be obtained using other conditions, such as those described herein.
- a renewable carbon source is used for the production of isoprene.
- the production of isoprene is decoupled from the growth of the cells.
- the concentrations of isoprene and any oxidants are within the nonflammable ranges to reduce or eliminate the risk that a fire may occur during production or recovery of isoprene.
- compositions and methods are desirable because they allow high isoprene yield per cell, high carbon yield, high isoprene purity, high productivity, low energy usage, low production cost and investment, and minimal side reactions.
- This efficient, large scale, biosynthetic process for isoprene production provides an isoprene source for synthetic isoprene-based products such as rubber and provides a desirable, low-cost alternative to using natural rubber.
- the amount of isoprene produced by cells can be greatly increased by introducing a heterologous nucleic acid encoding an isoprene synthase polypeptide (e.g., a plant isoprene synthase polypeptide) into the cells.
- Isoprene synthase polypeptides convert dimethyl allyl diphosphate (DMAPP) into isoprene.
- DMAPP dimethyl allyl diphosphate
- a heterologous Pueraria Montana (kudzu) isoprene synthase polypeptide was expressed in a variety of host cells, such as Escherichia coli, Panteoa citrea, Bacillus subtilis, Yarrowia lipolytica, and Trichoderma reesei. All of these cells produced more isoprene than the corresponding cells without the heterologous isoprene synthase polypeptide. As illustrated in Tables 1 and 2, large amounts of isoprene are produced using the methods described herein. For example, B.
- subtilis cells with a heterologous isoprene synthase nucleic acid produced approximately 10-fold more isoprene in a 14 liter fermentor than the corresponding control B. subtilis cells without the heterologous nucleic acid (Table 2).
- the production of 300 mg of isoprene per liter of broth (mg/L, wherein the volume of broth includes both the volume of the cell medium and the volume of the cells) by E. coli and 30 mg/L by B. subtilis in fermentors indicates that significant amounts of isoprene can be generated (Table 2). If desired, isoprene can be produced on an even larger scale or other conditions described herein can be used to further increase the amount of isoprene.
- Table 1 Exemplary yields of isoprene from a shake flask using the cell cultures and methods described herein.
- the assay for measuring isoprene production is described in Example I, part II.
- a sample was removed at one or more time points from the shake flask and cultured for 30 minutes. The amount of isoprene produced in this sample was then measured.
- the headspace concentration and specific rate of isoprene production are listed in Table 1 and described further herein.
- Table 2 Exemplary yields of isoprene in a fermentor using the cell cultures and methods described herein.
- the assay for measuring isoprene production is described in Example I, part II.
- a sample of the off-gas of the fermentor was taken and analyzed for the amount of isoprene.
- the peak headspace concentration (which is the highest headspace concentration during the fermentation), titer (which is the cumulative, total amount of isoprene produced per liter of broth), and peak specific rate of isoprene production (which is the highest specific rate during the fermentation) are listed in Table 2 and described further herein.
- isoprene production by cells that contain a heterologous isoprene synthase nucleic acid can be enhanced by increasing the amount of a l-deoxy-D-xylulose-5- phosphate synthase (DXS) polypeptide and/or an isopentenyl diphosphate isomerase (IDI) polypeptide expressed by the cells.
- DXS l-deoxy-D-xylulose-5- phosphate synthase
- IDI isopentenyl diphosphate isomerase
- a DXS nucleic acid and/or an IDI nucleic acid can be introduced into the cells.
- the DXS nucleic acid may be a heterologous nucleic acid or a duplicate copy of an endogenous nucleic acid.
- the IDI nucleic acid may be a heterologous nucleic acid or a duplicate copy of an endogenous nucleic acid.
- the amount of DXS and/or IDI polypeptide is increased by replacing the endogenous DXS and/or IDI promoters or regulatory regions with other promoters and/or regulatory regions that result in greater transcription of the DXS and/or IDI nucleic acids.
- the cells contain both a heterologous nucleic acid encoding an isoprene synthase polypeptide (e.g., a plant isoprene synthase nucleic acid) and a duplicate copy of an endogenous nucleic acid encoding an isoprene synthase polypeptide.
- the encoded DXS and IDI polypeptides are part of the DXP pathway for the biosynthesis of isoprene ( Figure 19A). DXS polypeptides convert pyruvate and D- glyceraldehyde-3 -phosphate into l-deoxy-D-xylulose-5-phosphate.
- IDI polypeptides catalyze the interconversion of isopentenyl diphosphate (IPP) and dimethyl allyl diphosphate (DMAPP). While not intending to be bound by any particular theory, it is believed that increasing the amount of IDI polypeptide in cells increases the amount (and conversion rate) of IPP that is converted into DMAPP, which in turn is converted into isoprene.
- IPP isopentenyl diphosphate
- DMAPP dimethyl allyl diphosphate
- coli DXS nucleic acids was used to produce isoprene.
- the levels of isoprene varied from 50 to 300 ⁇ g/L over a time period of 15 hours (Example 7, part VII).
- the presence of heterologous or extra endogenous isoprene synthase, IDI, and DXS nucleic acids causes cells to grow more reproducibly or remain viable for longer compared to the corresponding cell with only one or two of these heterologous or extra endogenous nucleic acids.
- heterologous isoprene synthase, IDI, and DXS nucleic acids grew better than cells with only heterologous isoprene synthase and DXS nucleic acids or with only a heterologous isoprene synthase nucleic acid.
- heterologous isoprene synthase, IDI, and DXS nucleic acids were successfully operably linked to a strong promoter on a high copy plasmid that was maintained by E. coli cells, suggesting that large amounts of these polypeptides could be expressed in the cells without causing an excessive amount of toxicity to the cells.
- heterologous or extra endogenous isoprene synthase and IDI nucleic acids may reduce the amount of one or more potentially toxic intermediates that would otherwise accumulate if only a heterologous or extra endogenous DXS nucleic acid was present in the cells.
- the production of isoprene by cells that contain a heterologous isoprene synthase nucleic acid is augmented by increasing the amount of a MVA polypeptide expressed by the cells ( Figures 19A and 19B).
- MVA pathways polypeptides include any of the following polypeptides: acetyl-CoA acetyltransferase (AA-CoA thiolase) polypeptides, 3-hydroxy-3-methylglutaryl-CoA synthase (HMG-CoA synthase) polypeptides, 3- hydroxy-3-methylglutaryl-CoA reductase (HMG-CoA reductase) polypeptides, mevalonate kinase (MVK) polypeptides, phosphomevalonate kinase (PMK) polypeptides, diphosphomevalonate decarboxylase (MVD) polypeptides, phosphomevalonate decarboxylase (PMDC) polypeptides, isopentenyl phosphate kinase (IPK) polypeptides, IDI polypeptides, and polypeptides (e.g., fusion polypeptides) having an activity of two or more MVA pathway polypeptide
- one or more MVA pathway nucleic acids can be introduced into the cells.
- the cells contain the upper MVA pathway, which includes AA-CoA thiolase, HMG-CoA synthase, and HMG-CoA reductase nucleic acids.
- the cells contain the lower MVA pathway, which includes MVK, PMK, MVD, and IDI nucleic acids.
- the cells contain an entire MVA pathway that includes AA-CoA thiolase, HMG-CoA synthase, HMG-CoA reductase, MVK, PMK, MVD, and IDI nucleic acids.
- the cells contain an entire MVA pathway that includes AA-CoA thiolase, HMG-CoA synthase, HMG-CoA reductase, MVK, PMDC, IPK, and IDI nucleic acids.
- the MVA pathway nucleic acids may be heterologous nucleic acids or duplicate copies of endogenous nucleic acids.
- the amount of one or more MVA pathway polypeptides is increased by replacing the endogenous promoters or regulatory regions for the MVA pathway nucleic acids with other promoters and/or regulatory regions that result in greater transcription of the MVA pathway nucleic acids.
- the cells contain both a heterologous nucleic acid encoding an isoprene synthase polypeptide (e.g. , a plant isoprene synthase nucleic acid) and a duplicate copy of an endogenous nucleic acid encoding an isoprene synthase polypeptide.
- a heterologous nucleic acid encoding an isoprene synthase polypeptide (e.g. , a plant isoprene synthase nucleic acid) and a duplicate copy of an endogenous nucleic acid encoding an isoprene synthase polypeptide.
- E. coli cells with nucleic acids encoding Enterococcus faecalis AA-CoA thiolase, HMG-CoA synthase, and HMG-CoA reductase polypeptides produced 22 grams of mevalonic acid (an intermediate of the MVA pathway). A shake flask of these cells produced 2-4 grams of mevalonic acid per liter.
- heterologous MVA pathways nucleic acids are active in E. coli.
- E. coli cells that contain nucleic acids for both the upper MVA pathway and the lower MVA pathway as well as a kudzu isoprene synthase (strain MCM 127) produced significantly more isoprene (874 ⁇ g/L) compared to E. coli cells with nucleic acids for only the lower MVA pathway and the kudzu isoprene synthase (strain MCM 131) ⁇ see Table 3 and Example 8, part VIII).
- At least a portion of the cells maintain the heterologous isoprene synthase, DXS, IDI, and/or MVA pathway nucleic acid for at least about 5, 10, 20, 50, 75, 100, 200, 300, or more cell divisions in a continuous culture (such as a continuous culture without dilution).
- the nucleic acid comprising the heterologous or duplicate copy of an endogenous isoprene synthase, DXS, IDI, and/or MVA pathway nucleic acid also comprises a selective marker, such as a kanamycin, ampicillin, carbenicillin, gentamicin, hygromycin, phleomycin, bleomycin, neomycin, or chloramphenicol antibiotic resistance nucleic acid.
- a selective marker such as a kanamycin, ampicillin, carbenicillin, gentamicin, hygromycin, phleomycin, bleomycin, neomycin, or chloramphenicol antibiotic resistance nucleic acid.
- the amount of isoprene produced can be further increased by adding yeast extract to the cell culture medium.
- the amount of isoprene produced was linearly proportional to the amount of yeast extract in the cell medium for the concentrations tested ( Figure 48C). Additionally, approximately 0.11 grams of isoprene per liter of broth was produced from a cell medium with yeast extract and glucose (Example 7, part VIII). Both of these experiments used E. coli cells with kudzu isoprene synthase, S. cerevisia IDI, and E. coli DXS nucleic acids to produce isoprene.
- invert sugar was shown to function as a carbon source for the generation of isoprene ( Figures 47C and 96-98).
- 2.4 g/L of isoprene was produced from cells expressing MVA pathway polypeptides and a Kudzu isoprene synthase (Example 8, part XV).
- Glycerol was as also used as a carbon source for the generation of 2.2 mg/L of isoprene from cells expressing a Kudzu isoprene synthase (Example 8, part XIV).
- Expressing a DXS nucleic acid, an IDI nucleic acid, and/or one or more MVA pathway nucleic acids (such as nucleic acids encoding the entire MVA pathway) in addition to an isoprene synthase nucleic acid may increase the production of isoprene from glycerol.
- an oil is included in the cell medium.
- B. subtilis cells containing a kudzu isoprene synthase nucleic acid produced isoprene when cultured in a cell medium containing an oil and a source of glucose (Example 4, part III).
- more than one oil is included in the cell medium.
- the oil may increase the amount of carbon in the cells that is available for conversion to isoprene, (ii) the oil may increase the amount of acetyl-CoA in the cells, thereby increasing the carbon flow through the MVA pathway, and/or (ii) the oil may provide extra nutrients to the cells, which is desirable since much of the carbon in the cells is converted to isoprene rather than other products.
- cells that are cultured in a cell medium containing oil naturally use the MVA pathway to produce isoprene or are genetically modified to contain nucleic acids for the entire MVA pathway.
- the oil is partially or completely hydrolyzed before being added to the cell culture medium to facilitate the use of the oil by the host cells.
- the cells convert greater than or about 0.0015, 0.002, 0.005, 0.01, 0.02, 0.05, 0.1, 0.12, 0.14, 0.16, 0.2, 0.3, 0.4, 0.5, 0.6, 0.7, 0.8, 0.9, 1.0, 1.2, 1.4, 1.6, 1.8, 2.0, 2.5, 3.0, 3.5, 4.0, 5.0, 6.0, 7.0, or 8.0% of the carbon in the cell culture medium into isoprene.
- a significant portion of the carbon from the feedstock that is converted to downstream products is converted to isoprene.
- coli cells expressing MVA pathway and kudzu isoprene synthase nucleic acids exhibited decoupling of the production of isoprene or the intermediate mevalonic acid from growth, resulting in high carbon efficiency.
- mevalonic acid was formed from cells expressing the upper MVA pathway from Enterococcus faecalis .
- Isoprene was formed from cells expressing the upper MVA pathway from Enterococcus faecalis, the lower MVA pathway from Saccharomyces cerevisiae, and the isoprene synthase from Pueraria montana (Kudzu). This decoupling of isoprene or mevalonic acid production from growth was demonstrated in four different strains of E.
- E. coli BL21(LDE3), BL21(LDE3) Tuner, FM5, and MG 1655.
- the first two E. coli strains are B strains, and the latter two are Kl 2 strains. Decoupling of production from growth was also demonstrated in a variant of MG 1655 with ack and pta genes deleted. This variant also demonstrated less production of acetate.
- the fusion polypeptide includes part or all of a first polypeptide ⁇ e.g., an isoprene synthase, DXS, IDI, MVA pathway, hydrogenase, hydrogenase maturation or transcription factor polypeptide or catalytically active fragment thereof) and may optionally include part or all of a second polypeptide ⁇ e.g., a peptide that facilitates purification or detection of the fusion polypeptide, such as a His-tag).
- the fusion polypeptide has an activity of two or more MVA pathway polypeptides (such as AA-CoA thiolase and HMG- CoA reductase polypeptides).
- the polypeptide is a naturally-occurring polypeptide (such as the polypeptide encoded by an Enterococcus faecalis mvaE nucleic acid) that has an activity of two or more MVA pathway polypeptides.
- a polypeptide has at least or about 50, 100, 150, 175, 200, 250, 300, 350, 400, or more amino acids.
- the polypeptide fragment contains at least or about 25, 50, 75, 100, 150, 200, 300, or more contiguous amino acids from a full-length polypeptide and has at least or about 5%, 10%, 20%, 30%, 40%, 50%, 60%, 70%, 80%, 90%, 95%, or 100% of an activity of a corresponding full-length polypeptide.
- the polypeptide includes a segment of or the entire amino acid sequence of any naturally-occurring isoprene synthase, DXS, IDI, MVA pathway, hydrogenase, hydrogenase maturation or transcription factor polypeptide.
- the polypeptide has one or more mutations compared to the sequence of a wild-type ⁇ i.e., a sequence occurring in nature) isoprene synthase, DXS, IDI, MVA pathway, hydrogenase, hydrogenase maturation or transcription factor polypeptide.
- the polypeptide is an isolated polypeptide.
- the polypeptide is a heterologous polypeptide.
- the nucleic acid is a recombinant nucleic acid.
- an isoprene synthase, DXS, IDI, MVA pathway, hydrogenase, hydrogenase maturation or transcription factor nucleic acid is operably linked to another nucleic acid encoding all or a portion of another polypeptide such that the recombinant nucleic acid encodes a fusion polypeptide that includes an isoprene synthase, DXS, IDI, MVA pathway, hydrogenase, hydrogenase maturation or transcription factor polypeptide and all or part of another polypeptide (e.g., a peptide that facilitates purification or detection of the fusion polypeptide, such as a His- tag).
- part or all of a recombinant nucleic acid is chemically synthesized. It is to be understood that mutations, including single nucleotide mutations, can occur within a nucleic acid
- the nucleic acid is a heterologous nucleic acid.
- the nucleic acid includes a segment of or the entire nucleic acid sequence of any naturally-occurring isoprene synthase, DXS, IDI, MVA pathway, hydrogenase, hydrogenase maturation or transcription factor nucleic acid.
- the nucleic acid includes at least or about 50, 100, 150, 200, 300, 400, 500, 600, 700, 800, or more contiguous nucleotides from a naturally-occurring isoprene synthase nucleic acid DXS, IDI, MVA pathway, hydrogenase, hydrogenase maturation or transcription factor nucleic acid.
- the nucleic acid has one or more mutations compared to the sequence of a wild-type (i.e., a sequence occurring in nature) isoprene synthase, DXS, IDI, MVA pathway, hydrogenase, hydrogenase maturation or transcription factor nucleic acid.
- the nucleic acid has one or more mutations (e.g. , a silent mutation) that increase the transcription or translation of isoprene synthase, DXS, IDI, MVA pathway, hydrogenase, or transcription factor nucleic acid.
- the nucleic acid is a degenerate variant of any nucleic acid encoding an isoprene synthase, DXS, IDI, MVA pathway, hydrogenase, hydrogenase maturation or transcription factor polypeptide.
- accession numbers of exemplary isoprene synthase, DXS, IDI, and/or MVA pathway polypeptides and nucleic acids are listed in Appendix 1 (the accession numbers of Appendix 1 and their corresponding sequences are herein incorporated by reference in their entireties, particularly with respect to the amino acid and nucleic acid sequences of isoprene synthase, DXS, IDI, and/or MVA pathway polypeptides and nucleic acids).
- the Kegg database also contains the amino acid and nucleic acid sequences of numerous exemplary isoprene synthase, DXS, IDI, and/or MVA pathway polypeptides and nucleic acids (see, for example, the world- wide web at "genome.jp/kegg/pathway/map/map00100.html" and the sequences therein, which are each hereby incorporated by reference in their entireties, particularly with respect to the amino acid and nucleic acid sequences of isoprene synthase, DXS, IDI, and/or MVA pathway polypeptides and nucleic acids).
- one or more of the isoprene synthase, DXS, IDI, and/or MVA pathway polypeptides and/or nucleic acids have a sequence identical to a sequence publicly available on December 12, 2007 or September 14, 2008 such as any of the sequences that correspond to any of the accession numbers in Appendix 1 or any of the sequences present in the Kegg database. Additional exemplary isoprene synthase, DXS, IDI, and/or MVA pathway polypeptides and nucleic acids are described further below.
- isoprene synthase polypeptides convert dimethyl allyl diphosphate (DMAPP) into isoprene.
- exemplary isoprene synthase polypeptides include polypeptides, fragments of polypeptides, peptides, and fusions polypeptides that have at least one activity of an isoprene synthase polypeptide. Standard methods can be used to determine whether a polypeptide has isoprene synthase polypeptide activity by measuring the ability of the polypeptide to convert DMAPP into isoprene in vitro, in a cell extract, or in vivo.
- cell extracts are prepared by growing a strain (e.g., the E. co/z ' /pTrcKudzu strain described herein) in the shake flask method as described in Example 1. After induction is complete, approximately 10 mL of cells are pelleted by centrifugation at 7000 x g for 10 minutes and re-suspended in 5 ml of PEB without glycerol. The cells are lysed using a French Pressure cell using standard procedures. Alternatively the cells are treated with lysozyme (Ready -Lyse lysozyme solution; EpiCentre) after a freeze/thaw at -80 0 C.
- lysozyme Ready -Lyse lysozyme solution; EpiCentre
- Isoprene synthase polypeptide activity in the cell extract can be measured, for example, as described in Silver et ah, J. Biol. Chem. 270:13010-13016, 1995 and references therein, which are each hereby incorporated by reference in their entireties, particularly with respect to assays for isoprene synthase polypeptide activity.
- DMAPP Sigma is evaporated to dryness under a stream of nitrogen and re-hydrated to a concentration of 100 mM in 100 mM potassium phosphate buffer pH 8.2 and stored at -20 0 C.
- a solution of 5 ⁇ L of IM MgCl 2 , 1 mM (250 ⁇ g/ml) DMAPP, 65 ⁇ L of Plant Extract Buffer (PEB) (50 mM Tris-HCl, pH 8.0, 20 mM MgCl 2 , 5% glycerol, and 2 mM DTT) is added to 25 ⁇ L of cell extract in a 20 ml Headspace vial with a metal screw cap and teflon coated silicon septum (Agilent Technologies) and cultured at 37 0 C for 15 minutes with shaking.
- the reaction is quenched by adding 200 ⁇ L of 250 mM EDTA and quantified by GC/MS as described in Example 1, part II.
- Exemplary isoprene synthase nucleic acids include nucleic acids that encode a polypeptide, fragment of a polypeptide, peptide, or fusion polypeptide that has at least one activity of an isoprene synthase polypeptide.
- Exemplary isoprene synthase polypeptides and nucleic acids include naturally-occurring polypeptides and nucleic acids from any of the source organisms described herein as well as mutant polypeptides and nucleic acids derived from any of the source organisms described herein.
- the isoprene synthase polypeptide or nucleic acid is from the family Fabaceae, such as the Faboideae subfamily.
- the isoprene synthase polypeptide or nucleic acid is a polypeptide or nucleic acid from Pueraria montana (kudzu) (Sharkey et al., Plant Physiology 137: 700-712, 2005), Pueraria lobata, poplar (such as Populus alba, Populus nigra, Populus trichocarpa, or Populus alba x tremula (CAC35696) Miller et ah, Planta 213: 483-487, 2001) aspen (such as Populus tremuloides) Silver et al, JBC 270(22): 13010-1316, 1995), or English Oak (Quercus robur) (Zimmer et al, WO 98/02550),
- Pueraria montana kud
- Suitable isoprene synthases include, but are not limited to, those identified by Genbank Accession Nos. AY341431, AY316691, AY279379, AJ457070, and AY182241, which are each hereby incorporated by reference in their entireties, particularly with respect to sequences of isoprene synthase nucleic acids and polypeptides.
- the isoprene synthase polypeptide or nucleic acid is not a naturally-occurring polypeptide or nucleic acid from Quercus robur ⁇ i.e., the isoprene synthase polypeptide or nucleic acid is an isoprene synthase polypeptide or nucleic acid other than a naturally-occurring polypeptide or nucleic acid from Quercus robur).
- the isoprene synthase nucleic acid or polypeptide is a naturally-occurring polypeptide or nucleic acid from poplar.
- the isoprene synthase nucleic acid or polypeptide is not a naturally-occurring polypeptide or nucleic acid from poplar.
- DXS polypeptides convert pyruvate and D-glyceraldehyde-3 -phosphate into l-deoxy-D-xylulose-5-phosphate.
- exemplary DXS polypeptides include polypeptides, fragments of polypeptides, peptides, and fusions polypeptides that have at least one activity of a DXS polypeptide.
- Standard methods can be used to determine whether a polypeptide has DXS polypeptide activity by measuring the ability of the polypeptide to convert pyruvate and D- glyceraldehyde-3 -phosphate into 1 -deoxy-D-xylulose-5 -phosphate in vitro, in a cell extract, or in vivo.
- Exemplary DXS nucleic acids include nucleic acids that encode a polypeptide, fragment of a polypeptide, peptide, or fusion polypeptide that has at least one activity of a DXS polypeptide.
- Exemplary DXS polypeptides and nucleic acids include naturally-occurring polypeptides and nucleic acids from any of the source organisms described herein as well as mutant polypeptides and nucleic acids derived from any of the source organisms described herein.
- Isopentenyl diphosphate isomerase polypeptides catalyses the interconversion of isopentenyl diphosphate (IPP) and dimethyl allyl diphosphate (DMAPP) ⁇ e.g., converting IPP into DMAPP and/or converting DMAPP into IPP).
- IDI polypeptides include polypeptides, fragments of polypeptides, peptides, and fusions polypeptides that have at least one activity of an IDI polypeptide.
- Standard methods can be used to determine whether a polypeptide has IDI polypeptide activity by measuring the ability of the polypeptide to interconvert IPP and DMAPP in vitro, in a cell extract, or in vivo.
- IDI nucleic acids include nucleic acids that encode a polypeptide, fragment of a polypeptide, peptide, or fusion polypeptide that has at least one activity of an IDI polypeptide.
- Exemplary IDI polypeptides and nucleic acids include naturally-occurring polypeptides and nucleic acids from any of the source organisms described herein as well as mutant polypeptides and nucleic acids derived from any of the source organisms described herein.
- Exemplary MVA pathway polypeptides include acetyl-CoA acetyltransferase (AA- CoA thiolase) polypeptides, 3-hydroxy-3-methylglutaryl-CoA synthase (HMG-CoA synthase) polypeptides, 3-hydroxy-3-methylglutaryl-CoA reductase (HMG-CoA reductase) polypeptides, mevalonate kinase (MVK) polypeptides, phosphomevalonate kinase (PMK) polypeptides, diphosphomevalonate decarboxylase (MVD) polypeptides, phosphomevalonate decarboxylase (PMDC) polypeptides, isopentenyl phosphate kinase (IPK) polypeptides, IDI polypeptides, and polypeptides ⁇ e.g., fusion polypeptides) having an activity of two or more MVA pathway polypeptides.
- MVK
- MVA pathway polypeptides include polypeptides, fragments of polypeptides, peptides, and fusions polypeptides that have at least one activity of an MVA pathway polypeptide.
- Exemplary MVA pathway nucleic acids include nucleic acids that encode a polypeptide, fragment of a polypeptide, peptide, or fusion polypeptide that has at least one activity of an MVA pathway polypeptide.
- Exemplary MVA pathway polypeptides and nucleic acids include naturally-occurring polypeptides and nucleic acids from any of the source organisms described herein as well as mutant polypeptides and nucleic acids derived from any of the source organisms described herein.
- acetyl-CoA acetyltransferase polypeptides convert two molecules of acetyl-CoA into acetoacetyl-CoA.
- Standard methods (such as those described herein) can be used to determine whether a polypeptide has AA-CoA thiolase polypeptide activity by measuring the ability of the polypeptide to convert two molecules of acetyl-CoA into acetoacetyl-CoA in vitro, in a cell extract, or in vivo.
- HMG-CoA synthase or HMGS 3-hydroxy-3-methylglutaryl-CoA synthase
- HMGS 3-hydroxy-3-methylglutaryl-CoA synthase
- Standard methods can be used to determine whether a polypeptide has HMG-CoA synthase polypeptide activity by measuring the ability of the polypeptide to convert acetoacetyl- CoA into 3-hydroxy-3-methylglutaryl-CoA in vitro, in a cell extract, or in vivo.
- HMG-CoA reductase or HMGR 3-hydroxy-3-methylglutaryl-CoA reductase
- HMGR 3-hydroxy-3-methylglutaryl-CoA reductase
- Standard methods can be used to determine whether a polypeptide has HMG-CoA reductase polypeptide activity by measuring the ability of the polypeptide to convert 3-hydroxy- 3-methylglutaryl-CoA into mevalonate in vitro, in a cell extract, or in vivo.
- MVK Mevalonate kinase
- Standard methods can be used to determine whether a polypeptide has MVK polypeptide activity by measuring the ability of the polypeptide to convert mevalonate into mevalonate-5-phosphate in vitro, in a cell extract, or in vivo.
- Phosphomevalonate kinase (PMK) polypeptides phosphorylates mevalonate-5- phosphate to form mevalonate-5-diphosphate.
- Standard methods can be used to determine whether a polypeptide has PMK polypeptide activity by measuring the ability of the polypeptide to convert mevalonate-5-phosphate into mevalonate-5-diphosphate in vitro, in a cell extract, or in vivo.
- Diphosphomevalonate decarboxylase (MVD or DPMDC) polypeptides convert mevalonate-5 -diphosphate into isopentenyl diphosphate (IPP).
- Standard methods can be used to determine whether a polypeptide has MVD polypeptide activity by measuring the ability of the polypeptide to convert mevalonate-5-diphosphate into IPP in vitro, in a cell extract, or in vivo.
- PMDC Phosphomevalonate decarboxylase
- Standard methods can be used to determine whether a polypeptide has IPK polypeptide activity by measuring the ability of the polypeptide to convert IP into IPP in vitro, in a cell extract, or in vivo.
- Exemplary IDI polypeptides and nucleic acids are described above.
- Hydrogenase polypeptides catalyze the reaction: 2H + + 2e ⁇ ⁇ -» H 2 . In vitro that reaction is reversible, but certain hydrogenases may work in only one direction in vivo, either oxidizing H 2 or reducing H + . Hydrogenase polypeptides can be oxygen-sensitive, contain complex metal cofactors as part of their catalytic center and sometimes consist of multiple subunits, with hydrogenase gene expression sometimes involving additional accessory polypeptides, such as 'maturation' factors or transcription regulatory factors (i.e., activators or repressors).
- additional accessory polypeptides such as 'maturation' factors or transcription regulatory factors (i.e., activators or repressors).
- Hydrogenases are classified into at least three broad groups based upon the type of metal cofactor in their catalytic center: (1) nickel-iron (“NiFe”) hydrogenases have a nickel/iron cofactor; (2) iron-iron hydrogenases (“FeFe”) have an iron/iron cofactor; and (3) iron/sulfur-free (“Fe”) hydrogenases, which lack the 4Fe4S clusters found in groups (1) and (2), have an iron cofactor and a methenyl-tetrahydromethanopterin electron carrier. See, e.g., Chung- Jung Chou et al., "Hydrogenesis in hyperthermophilic microorganisms: implications for biofuels," Metabol. Eng.
- the catalytic center of NiFe hydrogenases consists of a nickel atom and an iron atom, each with two carbon monoxide (CO) and two cyanide (CN " ) ligands.
- the NiFe hydrogenases all comprise at least a second subunit containing multiple iron-sulfur (Fe-S) centers for the transfer of electrons to and from the catalytic center.
- NiFe hydrogenases can be subdivided into four main classes: (1) respiratory enzymes, which are part of multi-enzyme systems that couple the oxidation of H 2 to reduction of terminal electron acceptors such as SO 4 2" or NO 3 " under anaerobic conditions, or to O 2 in aerobic microorganisms; (2) H 2 sensors, which activate expression of the metabolically active NiFe hydrogenases; (3) cytoplasmic hydrogenases, containing multiple subunits able to utilize NADP + , which are readily reversible in vitro, but in vivo may only oxidize H 2 ; and (4) membrane-bound, energy-conserving multi-enzyme complexes also found in bacteria and Archaea. Chung- Jung Chou et al., "Hydrogenesis in hyperthermophilic microorganisms: implications for biofuels," Metabol Eng. 10:394-404 (2008).
- the catalytic center of FeFe hydrogenases contains a catalytic "H cluster" which coordinates a binuclear (FeFe) site bridged to a [4Fe-4S] center by a single protein (cysteine) ligand.
- the two iron atoms of the binuclear center each have two carbon monoxide (CO) and two cyanide (CN " ) ligands, and are also bridged by two sulfur atoms which are part of a small organic molecule.
- Most FeFe hydrogenases are monomeric enzymes of about 50 kilodaltons (kDa), and appear to function in vivo primarily to dispose of excess reducing equivalents by reducing protons to hydrogen gas.
- Exemplary hydrogenase polypeptides include, but are not limited to, the E. coli hydrogenase- 1 (Hyd-1) polypeptides, E. coli hydrogenase-2 (Hyd-2) polypeptides, E. coli hydrogenase-3 (Hyd-3) polypeptides, E. coli hydrogenase-4 (Hyd-4) polypeptides, E.
- FHL formate hydrogen lyase
- FHL formate hydrogen lyase
- Akihito Yoshida et al. "Efficient induction of formate hydrogen lyase of aerobically grown Escherichia coli in a three-step biohydrogen production process," Appl. Microbiol. Biotechnol. 74:754-760 (2007), which is incorporated herein by reference in its entirety, particularly with respect to the induction of expression of formate hydrogen lyase in E. coli), Ralstonia eutropha Hl 6 hydrogenase (R.
- hydrogenase polypeptides include polypeptides, fragments of polypeptides, peptides, and fusion polypeptides that have at least one activity of a hydrogenase polypeptide.
- Exemplary hydrogenase nucleic acids include nucleic acids that encode a polypeptide, fragment of a polypeptide, peptide, or fusion polypeptide that has at least one activity of a hydrogenase polypeptide, or at least one activity necessary for expression, processing, or maturation of a hydrogenase polypeptide.
- Exemplary hydrogenase polypeptides and nucleic acids include naturally-occurring polypeptides and nucleic acids from any of the source organisms described herein as well as mutant polypeptides and nucleic acids derived from any of the source organisms described herein.
- coli Hyd-3 which is part of the anaerobic formate hydrogen lyase (FHL) complex, is encoded by the hyc operon (comprising the hycA, hycB, hycC, hycD, hycE, hycF, hycG, hycH, and hycl genes).
- FHL anaerobic formate hydrogen lyase
- coli Hyd-4 is encoded by the hyf operon (comprising the hyfA, hyfB, hyfC, hyfD, hyfE, hyfF, hyfG, hyfli, hyfl, hyfJ, and hyfR genes).
- E. coli FHL is encoded by six genes from the hyc operon (hycB, hycC, hycD, hycE, hycF and hycG) and the fdhF gene (encoding formate dehydrogenase H (Fdh-H)).
- Expression of the FHL complex can further involve expression of pyruvate formate lyase (pfl), FhIA, a transcription factor that activates transcription of fdhF and the hyc operon, or deletion/inactivation of HycA, a transcription factor encoded by the hyc A gene that negatively regulates transcription of FHL.
- pfl pyruvate formate lyase
- FhIA a transcription factor that activates transcription of fdhF and the hyc operon
- HycA a transcription factor encoded by the hyc A gene that negatively regulates transcription of FHL.
- Co-production of isoprene and hydrogen can be improved by expression or inactivation/deletion of additional proteins involved in the regulation of gene expression for hydrogenases and other enzymes, such as, for example, iron-sulfur complex transcriptional regulator (iscR) (Kalim-Akhtar et al., "Deletion of iscR stimulates recombinant Clostridial Fe/Fe hydrogenase activity and H 2 - accumulation in Escherichia coli EL21(DE3) " Appl. Microbiol. Biotechnol. 78:853-862 (2008), which is incorporated herein by reference in its entirety, particularly with reference to stimulation of Clostridial Fe/Fe hydrogenase activity and hydrogen accumulation in E. coli by deleting the iscR gene).
- iscR iron-sulfur complex transcriptional regulator
- Exemplary ferredoxin-dependent hydrogenase polypeptides include, but are not limited to, Clostridium acetobutulicum hydrogenase A (HydA) (see, e.g., P. W. King et al., "Functional studies of [FeFe] hydrogenase maturation in an Escherichia coli biosynthetic system," J Bacteriol.
- HydA Clostridium acetobutulicum hydrogenase A
- HydE HydA and three HydA-associated maturation enzymes
- HydE HydA and three HydA-associated maturation enzymes
- HydE HydA and three HydA-associated maturation enzymes
- HydE HydA and three HydA-associated maturation enzymes
- HydE HydA and three HydA-associated maturation enzymes
- HydE HydA and three HydA-associated maturation enzymes
- NFOR Bacillus subtilis NADPH ferredoxin oxidoreductase
- PCT Publication No. WO/2007/089901 which is incorporated herein by reference in its entirety, particularly with respect to optimization ofii.
- Clostridium kluyveri NADH ferredoxin oxidoreductase (Henning Seedorf et al., "The genome of Clostridium kluyveri, a strict anaerobe with unique metabolic features," Proc. Nat'l Acad. Sci. U.S.A.
- ferredoxin-dependent hydrogenase polypeptides include polypeptides, fragments of polypeptides, peptides, and fusion polypeptides that have at least one activity of a ferredoxin-dependent hydrogenase polypeptide.
- NADPH-dependent hydrogenase polypeptides include, but are not limited to thermophilic hydrogenase polypeptides such as Pyrococcus furiosus hydrogenase (see, e.g., J. Woodward et al., "Enzymatic production of biohydrogen," Nature 405(6790):1014-1015 (2000)), and polypeptides (e.g., fusion polypeptides) having an activity of two or more NADPH- dependent hydrogenase polypeptides.
- thermophilic hydrogenase polypeptides such as Pyrococcus furiosus hydrogenase (see, e.g., J. Woodward et al., "Enzymatic production of biohydrogen," Nature 405(6790):1014-1015 (2000)
- polypeptides e.g., fusion polypeptides having an activity of two or more NADPH- dependent hydrogenase polypeptides.
- NADPH-dependent hydrogenase polypeptides include polypeptides, fragments of polypeptides, peptides, and fusion polypeptides that have at least one activity of a NADPH-dependent hydrogenase polypeptide.
- oxygen-tolerant or oxygen-insensitive hydrogenases include, but are not limited to, Rubrivivax gelatinosus hydrogenase (see, e.g., P. C. Maness et al., "Characterization of the oxygen tolerance of a hydrogenase linked to a carbon monoxide oxidation pathway in Rubrivivax gelatinosus," Appl. Environ. Microbiol.
- heterologous nucleic acids encoding hydrogenase polypeptides can be mutagenized and screened for 02-tolerance or Ch-insensitivity using standard methods and assays (see, e.g., L. E. Nagy et al., "Application of gene-shuffling for the rapid generation of novel [FeFe] -hydrogenase libraries," Biotechnol. Letts. 29(3)421-430 (2007), which is incorporated herein by reference, particularly with respect to mutagenesis and screening for oxygen tolerant hydrogenase polypeptides).
- Standard methods can be used to determine whether a polypeptide has hydrogenase activity by measuring the ability of the polypeptide to produce hydrogen gas in vitro, in a cell extract, or in vivo.
- co-production of isoprene and hydrogen can be improved by inactivation of anaerobic biosynthetic pathways, thereby blocking the carbon flow to a variety of metabolites (i.e., fermentation side products) produced under oxygen-limited or anaerobic conditions, including, but not limited to, lactate, acetate, pyruvate, ethanol, succinate, and glycerol.
- Exemplary polypeptides involved in the production of fermentation side products include formate dehydrogenase N, alpha subunit (fdnG), formate dehydrogenase O, large subunit (fdoG), nitrate reductase (narG), formate transporter A (focA), formate transporter B (focB), pyruvate oxidase (poxE), pyruvate dehydrogenase El component ackA/pta (aceE), alcohol dehydrogenase (adhE), fumarate reductase membrane protein (frdC), and lactate dehydrogenase (idhA).
- Exemplary polypeptides involved in the regulation or expression of genes involved in the production of fermentation side products that may also be inactivated to improve co-production of isoprene and hydrogen include, but are not limited to, repressor of formate hydrogen lyase (hycA), fumarate reductase regulator (fnr), acetyl-coenzyme A synthetase (acs), and formate dehydrogenase regulatory protein (hycA), which regulates expression of the transcriptional regulator fhlA (formate hydrogen lyase transcriptional activator).
- hycA formate hydrogen lyase
- fnr fumarate reductase regulator
- acs acetyl-coenzyme A synthetase
- hycA formate dehydrogenase regulatory protein
- Exemplary polypeptides involved in hydrogen re-uptake that may also be inactivated to improve co-production of isoprene and hydrogen include, but are not limited to, E. coli hydrogenase-1 (Hyd-1) (hya operon) and i ⁇ 1 . coli hydrogenase-2 (Hyd-2) (hyb operon).
- E. coli Hyd-1 is encoded by the hya operon (comprising the hyaA, hyaB, hyaC, hyaD, hyaE, and hyaF genes).
- E. coli Hyd-1 is encoded by the hya operon (comprising the hyaA, hyaB, hyaC, hyaD, hyaE, and hyaF genes).
- coli Hyd-2 is encoded by the hyb operon (comprising the hybA, hybB, hybC, hybD, hybE, hybF, hybG, and hybO genes).
- Isoprene synthase, DXS, IDI, MVA pathway, hydrogenase, hydrogenase maturation and/or transcription factor nucleic acids can be isolated using standard methods. Methods of obtaining desired nucleic acids from a source organism of interest (such as a bacterial genome) are common and well known in the art of molecular biology (see, for example, WO 2004/033646 and references cited therein, which are each hereby incorporated by reference in their entireties, particularly with respect to the isolation of nucleic acids of interest).
- telomere sequence For example, if the sequence of the nucleic acid is known (such as any of the known nucleic acids described herein), suitable genomic libraries may be created by restriction endonuclease digestion and may be screened with probes complementary to the desired nucleic acid sequence. Once the sequence is isolated, the DNA may be amplified using standard primer directed amplification methods such as polymerase chain reaction (PCR) (U.S. Patent No. 4,683,202, which is incorporated by reference in its entirety, particularly with respect to PCR methods) to obtain amounts of DNA suitable for transformation using appropriate vectors.
- PCR polymerase chain reaction
- isoprene synthase, DXS, IDI, MVA pathway, hydrogenase, hydrogenase maturation and/or transcription factor nucleic acids can be chemically synthesized using standard methods.
- isoprene synthase, DXS, IDI, MVA pathway, hydrogenase, hydrogenase maturation and/or transcription factor polypeptides and nucleic acids which may be suitable for use in the compositions and methods described herein can be identified using standard methods.
- cosmid libraries of the chromosomal DNA of organisms known to produce isoprene naturally can be constructed in organisms such as E. coli, and then screened for isoprene production.
- cosmid libraries may be created where large segments of genomic DNA (35-45 kb) are packaged into vectors and used to transform appropriate hosts.
- Cosmid vectors are unique in being able to accommodate large quantities of DNA.
- cosmid vectors have at least one copy of the cos DNA sequence which is needed for packaging and subsequent circularization of the heterologous DNA.
- these vectors also contain an origin of replication such as CoIEI and drug resistance markers such as a nucleic acid resistant to ampicillin or neomycin.
- cosmid vectors for the transformation of suitable bacterial hosts are well described in Sambrook et ah, Molecular Cloning: A Laboratory Manual, 2 nd ed., Cold Spring Harbor, 1989, which is hereby incorporated by reference in its entirety, particularly with respect to transformation methods.
- heterologous DNA is isolated using the appropriate restriction endonucleases and ligated adjacent to the cos region of the cosmid vector using the appropriate ligases.
- Cosmid vectors containing the linearized heterologous DNA are then reacted with a DNA packaging vehicle such as bacteriophage.
- the cos sites are cleaved and the heterologous DNA is packaged into the head portion of the bacterial viral particle. These particles are then used to transfect suitable host cells such as E. coli. Once injected into the cell, the heterologous DNA circularizes under the influence of the cos sticky ends. In this manner, large segments of heterologous DNA can be introduced and expressed in host cells.
- Additional methods for obtaining isoprene synthase, DXS, IDI, MVA pathway, hydrogenase, hydrogenase maturation and/or transcription factor nucleic acids include screening a metagenomic library by assay (such as the headspace assay described herein) or by PCR using primers directed against nucleotides encoding for a length of conserved amino acids (for example, at least 3 conserved amino acids). conserved amino acids can be identified by aligning amino acid sequences of known isoprene synthase, DXS, IDI, MVA pathway, hydrogenase, hydrogenase maturation and/or transcription factor polypeptides.
- conserveed amino acids for isoprene synthase polypeptides can be identified based on aligned sequences of known isoprene synthase polypeptides.
- An organism found to produce isoprene naturally can be subjected to standard protein purification methods (which are well known in the art) and the resulting purified polypeptide can be sequenced using standard methods.
- Other methods are found in the literature (see, for example, Julsing et ah, Applied. Microbiol. Biotechnol. 75: 1377-84, 2007; Withers et al., Appl Environ Microbiol. 73(19):6277-83, 2007, which are each hereby incorporated by reference in their entireties, particularly with respect to identification of nucleic acids involved in the synthesis of isoprene).
- standard sequence alignment and/or structure prediction programs can be used to identify additional DXS, IDI, MVA pathway, hydrogenase, hydrogenase maturation and/or transcription factor polypeptides and nucleic acids based on the similarity of their primary and/or predicted polypeptide secondary structure with that of known DXS, IDI, MVA pathway, hydrogenase, hydrogenase maturation and/or transcription factor polypeptides and nucleic acids.
- Standard databases such as the swissprot-trembl database (world-wide web at "expasy.org", Swiss Institute of Bioinformatics Swiss-Prot group CMU - 1 rue Michel Servet CH- 1211 Geneva 4, Switzerland) can also be used to identify isoprene synthase, DXS, IDI, MVA pathway, hydrogenase, hydrogenase maturation and/or transcription regulatory polypeptides and nucleic acids.
- the secondary and/or tertiary structure of an isoprene synthase, DXS, IDI, MVA pathway, hydrogenase, hydrogenase maturation and/or transcription factor polypeptide can be predicted using the default settings of standard structure prediction programs, such as PredictProtein (630 West, 168 Street, BB217, New York, N. Y. 10032, USA).
- standard structure prediction programs such as PredictProtein (630 West, 168 Street, BB217, New York, N. Y. 10032, USA).
- the actual secondary and/or tertiary structure of an isoprene synthase, DXS, IDI, MVA pathway, hydrogenase, hydrogenase maturation and/or transcription factor polypeptide can be determined using standard methods.
- Additional isoprene synthase, DXS, IDI, MVA pathway, hydrogenase, hydrogenase maturation and/or transcription factor nucleic acids can also be identified by hybridization to probes generated from known isoprene synthase, DXS, IDI, MVA pathway, hydrogenase, hydrogenase maturation and/or transcription factor nucleic acids.
- any of the isoprene synthase, DXS, IDI, MVA pathway, hydrogenase, hydrogenase maturation and/or transcription factor nucleic acids described herein can be included in one or more vectors. Accordingly, also described herein are vectors with one more nucleic acids encoding any of the isoprene synthase, DXS, IDI, MVA pathway, hydrogenase, hydrogenase maturation and/or transcription factor polypeptides that are described herein. In some embodiments, the vector contains a nucleic acid under the control of an expression control sequence.
- the vector contains a selective marker or selectable marker.
- Markers useful in vector systems for transformation of Trichoderma are known in the art ⁇ see, e.g., Finkelstein, Chapter 6 in Biotechnology of Filamentous Fungi, Finkelstein et ah, Eds. Butterworth-Heinemann, Boston, MA, Chap. 6., 1992; and Kinghorn et ah, Applied Molecular Genetics of Filamentous Fungi, Blackie Academic and Professional, Chapman and Hall, London, 1992, which are each hereby incorporated by reference in their entireties, particularly with respect to selective markers).
- the selective marker is the amdS nucleic acid, which encodes the enzyme acetamidase, allowing transformed cells to grow on acetamide as a nitrogen source.
- amdS nucleic acid which encodes the enzyme acetamidase, allowing transformed cells to grow on acetamide as a nitrogen source.
- A. nidulans amdS nucleic acid as a selective marker is described in Kelley et al., EMBO J. 4:475 - 479, 1985 and Penttila et al, Gene 61:155-164, 1987 (which are each hereby incorporated by reference in their entireties, particularly with respect to selective markers).
- an isoprene synthase, DXS, IDI, MVA pathway, hydrogenase, hydrogenase maturation, or transcription regulatory nucleic acid integrates into a chromosome of the cells without a selective marker.
- Suitable vectors are those which are compatible with the host cell employed. Suitable vectors can be derived, for example, from a bacterium, a virus (such as bacteriophage T7 or a M- 13 derived phage), a cosmid, a yeast, or a plant.
- Promoters are well known in the art. Any promoter that functions in the host cell can be used for expression of an isoprene synthase, DXS, IDI, MVA pathway, hydrogenase, hydrogenase maturation and/or transcription factor nucleic acid in the host cell.
- Initiation control regions or promoters which are useful to drive expression of isoprene synthase, DXS, IDI, MVA pathway, hydrogenase, hydrogenase maturation and/or transcription factor nucleic acids in various host cells are numerous and familiar to those skilled in the art ⁇ see, for example, WO 2004/033646 and references cited therein, which are each hereby incorporated by reference in their entireties, particularly with respect to vectors for the expression of nucleic acids of interest).
- Virtually any promoter capable of driving these nucleic acids can be used including, but not limited to, CYCl, HIS3, GALl, GALlO, ADHl, PGK, PHO5, GAPDH, ADCI, TRPl, URA3, LEU2, ENO, and TPI (useful for expression in Saccharomyces); AOXl (useful for expression in Pichia); and lac, trp, ⁇ P L , ⁇ P R , T7, tac, and trc (useful for expression in E. coll).
- a glucose isomerase promoter is used (see, for example, U.S. Patent No.
- Reported glucose isomerase promoter mutants can be used to vary the level of expression of the polypeptide encoded by a nucleic acid operably linked to the glucose isomerase promoter (U.S. Patent No. 7,132,527).
- the glucose isomerase promoter is contained in a low, medium, or high copy plasmid (U.S. Patent No. 7,132,527).
- an isoprene synthase, DXS, IDI, MVA pathway, hydrogenase, hydrogenase maturation and/or transcription factor nucleic acid is contained in a low copy plasmid (e.g., a plasmid that is maintained at about 1 to about 4 copies per cell), medium copy plasmid (e.g., a plasmid that is maintained at about 10 to about 15 copies per cell), or high copy plasmid (e.g., a plasmid that is maintained at about 50 or more copies per cell).
- a low copy plasmid e.g., a plasmid that is maintained at about 1 to about 4 copies per cell
- medium copy plasmid e.g., a plasmid that is maintained at about 10 to about 15 copies per cell
- high copy plasmid e.g., a plasmid that is maintained at about 50 or more copies per cell.
- the heterologous or extra endogenous isoprene synthase, DXS, IDI, MVA pathway, hydrogenase, hydrogenase maturation and/or transcription factor nucleic acid is operably linked to a T7 promoter.
- the heterologous or extra endogenous isoprene synthase, DXS, IDI, MVA pathway, hydrogenase, hydrogenase maturation and/or transcription factor nucleic acid operably linked to a T7 promoter is contained in a medium or high copy plasmid.
- the heterologous or extra endogenous isoprene synthase, DXS, IDI, MVA pathway, hydrogenase, hydrogenase maturation and/or transcription factor nucleic acid is operably linked to a Trc promoter.
- the heterologous or extra endogenous isoprene synthase, DXS, IDI, MVA pathway, hydrogenase, hydrogenase maturation and/or transcription factor nucleic acid operably linked to a Trc promoter is contained in a medium or high copy plasmid.
- the heterologous or extra endogenous isoprene synthase, DXS, IDI, MVA pathway, hydrogenase, hydrogenase maturation and/or transcription factor nucleic acid is operably linked to a Lac promoter.
- the heterologous or extra endogenous isoprene synthase, DXS, IDI, MVA pathway, hydrogenase, hydrogenase maturation and/or transcription factor nucleic acid operably linked to a Lac promoter is contained in a low copy plasmid.
- the heterologous or extra endogenous isoprene synthase, DXS, IDI, MVA pathway, hydrogenase, hydrogenase maturation and/or transcription factor nucleic acid is operably linked to an endogenous promoter, such as an endogenous Escherichia, Panteoa, Bacillus, Yarrowia, Streptomyces, or Trichoderma promoter or an endogenous alkaline serine protease, isoprene synthase, DXS, IDI, MVA pathway, hydrogenase, hydrogenase maturation and/or transcription factor promoter.
- an endogenous promoter such as an endogenous Escherichia, Panteoa, Bacillus, Yarrowia, Streptomyces, or Trichoderma promoter or an endogenous alkaline serine protease
- isoprene synthase DXS, IDI, MVA pathway, hydrogenase, hydrogenase maturation and/or transcription
- the heterologous or extra endogenous isoprene synthase, DXS, IDI, MVA pathway, hydrogenase, hydrogenase maturation and/or transcription factor nucleic acid operably linked to an endogenous promoter is contained in a high copy plasmid.
- the vector is a replicating plasmid that does not integrate into a chromosome in the cells. In some embodiments, part or all of the vector integrates into a chromosome in the cells.
- the vector is any vector which when introduced into a fungal host cell is integrated into the host cell genome and is replicated.
- FGSC Fungal Genetics Stock Center Catalogue of Strains
- fgsc.net the world-wide web at "fgsc.net” and the references cited therein, which are each hereby incorporated by reference in their entireties, particularly with respect to vectors. Additional examples of suitable expression and/or integration vectors are provided in Sambrook et ah, Molecular Cloning: A Laboratory Manual, 2 nd ed., Cold Spring Harbor, 1989, Current Protocols in Molecular Biology (F. M. Ausubel et al.
- vectors include pFB6, pBR322, PUC 18, pUClOO, and pENTR/D.
- an isoprene synthase, DXS, IDI, MVA pathway, hydrogenase, hydrogenase maturation and/or transcription factor nucleic acid is operably linked to a suitable promoter that shows transcriptional activity in a fungal host cell.
- the promoter may be derived from one or more nucleic acids encoding a polypeptide that is either endogenous or heterologous to the host cell.
- the promoter is useful in a Trichoderma host. Suitable non-limiting examples of promoters include cbhl, cbhl, egll, egl2,pepA, hfb ⁇ , hfbl, xyn ⁇ , and amy.
- the promoter is one that is native to the host cell.
- the promoter when T. reesei is the host, the promoter is a native T. reesei promoter.
- the promoter is T. reesei cbhl, which is an inducible promoter and has been deposited in GenBank under Accession No. D86235, which is incorporated by reference in its entirety, particularly with respect to promoters.
- the promoter is one that is heterologous to the fungal host cell.
- Other examples of useful promoters include promoters from the genes of A.
- awamori and A niger glucoamylase (glaA) (Nunberg et ah, MoI. Cell Biol. 4:2306-2315, 1984 and Boel et al., EMBO J. 3:1581-1585, 1984, which are each hereby incorporated by reference in their entireties, particularly with respect to promoters); Aspergillus niger alpha amylases, Aspergillus oryzae TAKA amylase, T. reesei xlnl, and the T. reesei cellobiohydrolase 1 (EP 137280, which is incorporated by reference in its entirety, particularly with respect to promoters).
- the expression vector also includes a termination sequence. Termination control regions may also be derived from various genes native to the host cell. In some embodiments, the termination sequence and the promoter sequence are derived from the same source. In another embodiment, the termination sequence is endogenous to the host cell.
- a particularly suitable terminator sequence is cbhl derived from a Trichoderma strain (such as T. reesei). Other useful fungal terminators include the terminator from an A. niger or A. awamori glucoamylase nucleic acid (Nunberg et ah, MoI. Cell Biol. 4:2306-2315, 1984 and Boel et al., EMBOJ.
- DNA encoding the polypeptide are linked operably through initiation codons to selected expression control regions such that expression results in the formation of the appropriate messenger RNA.
- the promoter, coding, region, and terminator all originate from the isoprene synthase, DXS, IDI, MVA pathway, hydrogenase, hydrogenase maturation and/or transcription factor nucleic acid to be expressed.
- the coding region for an isoprene synthase, DXS, IDI, MVA pathway, hydrogenase, hydrogenase maturation and/or transcription factor nucleic acid is inserted into a general-purpose expression vector such that it is under the transcriptional control of the expression construct promoter and terminator sequences.
- genes or part thereof are inserted downstream of the strong cbhl promoter.
- An isoprene synthase, DXS, IDI, MVA pathway, hydrogenase, hydrogenase maturation and/or transcription factor nucleic acid can be incorporated into a vector, such as an expression vector, using standard techniques (Sambrook et al, Molecular Cloning: A Laboratory Manual, Cold Spring Harbor, 1982, which is hereby incorporated by reference in its entirety, particularly with respect to the screening of appropriate DNA sequences and the construction of vectors).
- Methods used to ligate the DNA construct comprising a nucleic acid of interest such as an isoprene synthase, DXS, IDI, MVA pathway, hydrogenase, hydrogenase maturation and/or transcription factor nucleic acid), a promoter, a terminator, and other sequences and to insert them into a suitable vector are well known in the art.
- restriction enzymes can be used to cleave the isoprene synthase, DXS, IDI, MVA pathway, hydrogenase, hydrogenase maturation and/or transcription factor nucleic acid and the vector.
- the compatible ends of the cleaved isoprene synthase, DXS, IDI, MVA pathway, hydrogenase, hydrogenase maturation and/or transcription factor nucleic acid and the cleaved vector can be ligated.
- Linking is generally accomplished by ligation at convenient restriction sites.
- oligonucleotide linkers are used in accordance with conventional practice (see, Sambrook et ah, Molecular Cloning: A Laboratory Manual, 2 nd ed., Cold Spring Harbor, 1989, and Bennett and Lasure, More Gene Manipulations in Fungi, Academic Press, San Diego, pp 70-76, 1991, which are each hereby incorporated by reference in their entireties, particularly with respect to oligonucleotide linkers). Additionally, vectors can be constructed using known recombination techniques (e.g., Invitrogen Life Technologies, Gateway Technology).
- under-express e.g., mutate, inactivate, or delete
- isoprene synthase DXS, IDI, MVA pathway
- hydrogenase hydrogenase maturation
- transcription factor polypeptide-encoding nucleic acids at levels far below that those currently found in naturally-occurring cells.
- This result may be accomplished by the mutation or inactivation of transcriptional regulatory proteins required for expression of isoprene synthase, DXS, IDI, MVA pathway, hydrogenase, hydrogenase maturation and/or transcription factor nucleic acids, by deletion of the isoprene synthase, DXS, IDI, MVA pathway, hydrogenase, hydrogenase maturation and/or transcription factor nucleic acids, or by placing those nucleic acids under the control of a strong repressible promoter.
- Isoprene synthase, DXS, IDI, MVA pathway, hydrogenase, hydrogenase maturation and/or transcription factor nucleic acids can be obtained from any organism that naturally contains isoprene synthase, DXS, IDI, MVA pathway, hydrogenase, hydrogenase maturation and/or transcription factor nucleic acids.
- isoprene is formed naturally by a variety of organisms, such as bacteria, yeast, plants, and animals. Organisms contain the MVA pathway, DXP pathway, or both the MVA and DXP pathways for producing isoprene ( Figures 19A and 19B).
- DXS nucleic acids can be obtained, e.g., from any organism that contains the DXP pathway or contains both the MVA and DXP pathways.
- IDI and isoprene synthase nucleic acids can be obtained, e.g., from any organism that contains the MVA pathway, DXP pathway, or both the MVA and DXP pathways.
- MVA pathway nucleic acids can be obtained, e.g., from any organism that contains the MVA pathway or contains both the MVA and DXP pathways.
- Hydrogenase nucleic acids can be obtained, e.g., from any organism that oxidizes hydrogen or reduces hydrogen ions.
- Fermentation side product genes can be obtained or identified, e.g., from any organism that undergoes oxygen-limited or anaerobic respiration, such as glycolysis.
- the nucleic acid sequence of the isoprene synthase, DXS, IDI, MVA pathway, hydrogenase, hydrogenase maturation and/or transcription factor nucleic is identical to the sequence of a nucleic acid that is produced by any of the following organisms in nature.
- the amino acid sequence of the isoprene synthase, DXS, IDI, MVA pathway, hydrogenase, hydrogenase maturation and/or transcription factor polypeptide is identical to the sequence of a polypeptide that is produced by any of the following organisms in nature.
- the isoprene synthase, DXS, IDI, MVA pathway, hydrogenase, hydrogenase maturation and/or transcription factor nucleic acid or polypeptide is a mutant nucleic acid or polypeptide derived from any of the organisms described herein.
- "derived from” refers to the source of the nucleic acid or polypeptide into which one or more mutations is introduced.
- a polypeptide that is "derived from a plant polypeptide” refers to polypeptide of interest that results from introducing one or more mutations into the sequence of a wild-type (i.e., a sequence occurring in nature) plant polypeptide.
- the source organism is a fungus, examples of which are species of Aspergillus such as A. oryzae and A. niger, species of Saccharomyces such as S. cerevisiae, species of Schizosaccharomyces such as S. pombe, and species of Trichoderma such as T. reesei.
- the source organism is a filamentous fungal cell.
- filamentous fungi refers to all filamentous forms of the subdivision Eumycotina (see, Alexopoulos, C. J. (1962), Introductory Mycology, Wiley, New York).
- filamentous fungal parent cell may be a cell of a species of, but not limited to, Trichoderma, (e.g., Trichoderma reesei, the asexual morph of Hypocrea jecorina, previously classified as T.
- Fusarium sp. e.g., F. roseum, F. graminum F. cerealis, F. oxysporuim, ox F. venenatum
- Neurospora sp. e.g., N. crassa
- Hypocrea sp. Mucor sp., (e.g., M.
- Trichoderma ox "Trichoderma sp.” ox "Trichoderma spp.” refer to any fungal genus previously or currently classified as Trichoderma.
- the fungus is A. nidulans, A. awamori, A. oryzae, A. aculeatus, A. niger, A.japonicus, T. reesei, T. viride, F. oxysporum, ox F. solani.
- Aspergillus strains are disclosed in Ward et al., Appl. Microbiol. Biotechnol. 39:738-743, 1993 and Goedegebuur et al., Curr Gene 41:89-98, 2002, which are each hereby incorporated by reference in their entireties, particularly with respect to fungi.
- the fungus is a strain of Trichoderma, such as a strain of T. reesei.
- Strains of T. reesei are known and non-limiting examples include ATCC No. 13631, ATCC No. 26921, ATCC No. 56764, ATCC No. 56765, ATCC No. 56767 ', and NRRL 15709, which are each hereby incorporated by reference in their entireties, particularly with respect to strains of T. reesei.
- the host strain is a derivative of RL-P37.
- RL-P37 is disclosed in Sheir-Neiss et ah, Appl. Microbiol. Biotechnology 20:46-53, 1984, which is hereby incorporated by reference in its entirety, particularly with respect to strains of T. reesei.
- the source organism is a yeast, such as Saccharomyces sp., Schizosaccharomyces sp., Pichia sp., or Candida sp.
- Saccharomyces sp. is Saccharomyces cerevisiae.
- the source organism is a bacterium, such as strains of Bacillus such as B. lichenformis or B. subtilis, strains of Pantoea such as P. citrea, strains of Pseudomonas such as P. alcaligenes, P. putida, or P. fluorescens, strains of Streptomyces such as S. lividans or S. rubiginosus, strains of Corynebacterium sp. such as Corynebacterium glutamicum, strains of Rhodopseudomonas sp. such as Rhodopseudomonas palustris, or strains of Escherichia such as E. coli.
- bacterium such as strains of Bacillus such as B. lichenformis or B. subtilis, strains of Pantoea such as P. citrea, strains of Pseudomonas such as P. alcaligenes, P. putida, or P
- the genus Bacillus ' includes all species within the genus ""Bacillus,” as known to those of skill in the art, including but not limited to B. subtilis, B. licheniformis, B. lentus, B. brevis, B. stearothermophilus, B. alkalophilus, B. amyloliquefaciens, B. clausii, B. halodurans, B. megaterium, B. coagulans, B. circulans, B. lautus, and B. thuringiensis. It is recognized that the genus Bacillus continues to undergo taxonomical reorganization.
- the genus include species that have been reclassified, including but not limited to such organisms as B. stearothermophilus, which is now named "Geobacillus stearothermophilus.”
- B. stearothermophilus which is now named "Geobacillus stearothermophilus.”
- the production of resistant endospores in the presence of oxygen is considered the defining feature of the genus Bacillus, although this characteristic also applies to the recently named Alicyclobacillus, Amphibacillus, Aneurinibacillus, Anoxybacillus, Brevibacillus, Filobacillus, Gracilibacillus, Halobacillus, Paenibacillus, Salibacillus, Thermobacillus, Ureibacillus, and Virgibacillus.
- the source organism is a gram-positive bacterium.
- Non-limiting examples include strains of Streptomyces ⁇ e.g., S. lividans, S. coelicolor, ox S. griseus) and Bacillus.
- the source organism is a gram-negative bacterium, such as E. coli., Rhodopseudomonas sp. such as Rhodopseudomonas palustris, or Pseudomonas sp., such as
- P. alcaligenes P. putida, or P. fluorescens.
- the source organism is a plant, such as a plant from the family
- the source organism is kudzu, poplar (such as Populus albax tremula CAC35696), aspen (such as Populus tremuloides), or Quercus robur.
- the source organism is an algae, such as a green algae, red algae, glaucophytes, chlorarachniophytes, euglenids, chromista, or dino flagellates.
- an algae such as a green algae, red algae, glaucophytes, chlorarachniophytes, euglenids, chromista, or dino flagellates.
- the source organism is a cyanobacteria, such as cyanobacteria classified into any of the following groups based on morphology: Chroococcales,
- Pleurocapsales, Oscillatoriales, Nostocales, or Stigonematales Pleurocapsales, Oscillatoriales, Nostocales, or Stigonematales .
- the source organism is an anaerobic organism.
- Anaerobic organisms can include, but are not limited to, obligate anaerobes, facultatitive anaerobes, and aerotolerant anaerobes. Such organisms can be any of the organisms listed above, bacteria, yeast, etc.
- the obligate anaerobes can be any one or combination selected from the group consisting of Clostridium ljungdahlii, Clostridium autoethanogenum, Eurobacterium limosum, Clostridium carboxydivorans, Peptostreptococcus productus, and Butyribacterium methylotrophicum. It is to be understood that any combination of any of the source organisms described herein can be used for other embodiments of the invention.
- a variety of host cells can be used to express isoprene synthase, DXS, IDI, MVA pathway, hydrogenase, hydrogenase maturation and/or transcription factor polypeptides and to co-produce isoprene and hydrogen in the methods described herein.
- Exemplary host cells include cells from any of the organisms listed in the prior section under the heading "Exemplary Source Organisms. ' " The host cell may be a cell that naturally produces isoprene or a cell that does not naturally produce isoprene.
- the host cell naturally produces isoprene using the DXP pathway, and an isoprene synthase, DXS, and/or IDI nucleic acid is added to enhance production of isoprene using this pathway.
- the host cell naturally produces isoprene using the MVA pathway, and an isoprene synthase and/or one or more MVA pathway nucleic acids are added to enhance production of isoprene using this pathway.
- the host cell naturally produces isoprene using the DXP pathway and one or more MVA pathway nucleic acids are added to produce isoprene using part or all of the MVA pathway as well as the DXP pathway.
- the host cell naturally produces isoprene using both the DXP and MVA pathways and one or more isoprene synthase, DXS, IDI, or MVA pathway nucleic acids are added to enhance production of isoprene by one or both of these pathways.
- the host cell naturally produces isoprene using both the DXP and MVA pathways, and one or more isoprene synthase, DXS, IDI, or MVA pathway nucleic acids are added to enhance production of isoprene by one or both of these pathways, one or more hydrogenase nucleic acids are added to enhance hydrogen production and one or more fermentation side product-producing genes are inactivated or deleted to limit production of fermentation side products.
- the host cell naturally co-produces isoprene and hydrogen using both the DXP and MVA pathways and one or more isoprene synthase, DXS, IDI, or MVA pathway nucleic acids are added to enhance production of isoprene by one or both of these pathways, one or more hydrogenase nucleic acids are added to enhance hydrogen production, one or more fermentation side product-producing genes are inactivated or deleted to limit production of fermentation side products, and one or more hydrogen reuptake genes are inactivated or deleted to increase hydrogen production.
- the host cell naturally co-produces isoprene and hydrogen using both the DXP and MVA pathways and a hydrogenase, and one or more isoprene synthase, DXS, IDI, or MVA pathway nucleic acids are added to enhance production of isoprene by one or both of these pathways, one or more hydrogenase nucleic acids are added to enhance hydrogen production, one or more hydrogenase maturation nucleic acids are added to enhance hydrogen production, one or more fermentation side product-producing genes are inactivated or deleted to limit production of fermentation side products, and one or more hydrogen reuptake genes are inactivated or deleted to increase hydrogen production.
- the host cell naturally co-produces isoprene and hydrogen using both the DXP and MVA pathways and one or more isoprene synthase, DXS, IDI, or MVA pathway nucleic acids are added to enhance production of isoprene by one or both of these pathways, one or more hydrogenase nucleic acids are added to enhance hydrogen production, one or more hydrogenase maturation nucleic acids are added to enhance hydrogen production, one or more transcription factor nucleic acids are added or inactivated or deleted to enhance hydrogenase production, one or more fermentation side product-producing genes are inactivated or deleted to limit production of fermentation side products, and one or more hydrogen reuptake genes are inactivated or deleted to increase hydrogen production.
- Isoprene synthase, DXS, IDI, MVA pathway, hydrogenase, hydrogenase maturation and/or transcription factor nucleic acids or vectors containing them can be inserted into a host cell ⁇ e.g., a plant cell, a fungal cell, a yeast cell, or a bacterial cell described herein) using standard techniques for expression of the encoded isoprene synthase, DXS, IDI, MVA pathway, hydrogenase, hydrogenase maturation and/or transcription factor polypeptide.
- a host cell e.g., a plant cell, a fungal cell, a yeast cell, or a bacterial cell described herein
- Introduction of a DNA construct or vector into a host cell can be performed using techniques such as transformation, electroporation, nuclear microinjection, transduction, transfection ⁇ e.g., lipofection mediated or DEAE-Dextrin mediated transfection or transfection using a recombinant phage virus), incubation with calcium phosphate DNA precipitate, high velocity bombardment with DNA-coated microprojectiles, and protoplast fusion.
- General transformation techniques are known in the art ⁇ see, e.g., Current Protocols in Molecular Biology (F. M. Ausubel et al.
- the introduced nucleic acids may be integrated into chromosomal DNA or maintained as extrachromosomal replicating sequences.
- any method known in the art may be used to select transformants.
- stable transformants including an amdS marker are distinguished from unstable transformants by their faster growth rate and the formation of circular colonies with a smooth, rather than ragged outline on solid culture medium containing acetamide.
- a further test of stability is conducted by growing the transformants on a solid non- selective medium (e.g., a medium that lacks acetamide), harvesting spores from this culture medium, and determining the percentage of these spores which subsequently germinate and grow on selective medium containing acetamide.
- fungal cells are transformed by a process involving protoplast formation and transformation of the protoplasts followed by regeneration of the cell wall in a known manner.
- the preparation of Trichoderma sp. for transformation involves the preparation of protoplasts from fungal mycelia (see, Campbell et al, Curr. Genet. 16:53-56, 1989, which is incorporated by reference in its entirety, particularly with respect to transformation methods).
- the mycelia are obtained from germinated vegetative spores. The mycelia are treated with an enzyme that digests the cell wall resulting in protoplasts. The protoplasts are then protected by the presence of an osmotic stabilizer in the suspending medium.
- These stabilizers include sorbitol, mannitol, potassium chloride, magnesium sulfate, and the like. Usually the concentration of these stabilizers varies between 0.8 M and 1.2 M. It is desirable to use about a 1.2 M solution of sorbitol in the suspension medium.
- Uptake of DNA into the host Trichoderma sp. strain is dependent upon the calcium ion concentration. Generally, between about 10 mM CaCl 2 and 50 mM CaCl 2 is used in an uptake solution. In addition to the calcium ion in the uptake solution, other compounds generally included are a buffering system such as TE buffer (10 Mm Tris, pH 7.4; 1 mM EDTA) or 10 mM MOPS, pH 6.0 buffer (morpholinepropanesulfonic acid) and polyethylene glycol (PEG). While not intending to be bound to any particular theory, it is believed that the polyethylene glycol acts to fuse the cell membranes, thus permitting the contents of the medium to be delivered into the cytoplasm of the Trichoderma sp. strain and the plasmid DNA to be transferred to the nucleus. This fusion frequently leaves multiple copies of the plasmid DNA integrated into the host chromosome.
- TE buffer 10 Mm Tris, pH 7.4; 1 mM EDTA
- MOPS
- a suspension containing the Trichoderma sp. protoplasts or cells that have been subjected to a permeability treatment at a density of 10 5 to 10 7 /mL (such as 2 x 10 6 /mL) are used in the transformation.
- a volume of 100 ⁇ L of these protoplasts or cells in an appropriate solution e.g., 1.2 M sorbitol and 50 mM CaCl 2
- an appropriate solution e.g., 1.2 M sorbitol and 50 mM CaCl 2
- PEG is added to the uptake solution.
- From 0.1 to 1 volume of 25% PEG 4000 can be added to the protoplast suspension. In some embodiments, about 0.25 volumes are added to the protoplast suspension.
- Additives such as dimethyl sulfoxide, heparin, spermidine, potassium chloride, and the like may also be added to the uptake solution and aid in transformation. Similar procedures are available for other fungal host cells (see, e.g., U.S. Patent Nos. 6,022,725 and 6,268,328, which are each hereby incorporated by reference in their entireties, particularly with respect to transformation methods).
- the mixture is then cultured at approximately 0 0 C for a period of between 10 to 30 minutes. Additional PEG is then added to the mixture to further enhance the uptake of the desired nucleic acid sequence.
- the 25% PEG 4000 is generally added in volumes of 5 to 15 times the volume of the transformation mixture; however, greater and lesser volumes may be suitable.
- the 25% PEG 4000 is desirably about 10 times the volume of the transformation mixture.
- the transformation mixture is then cultured either at room temperature or on ice before the addition of a sorbitol and CaCl 2 solution.
- the protoplast suspension is then further added to molten aliquots of a growth medium.
- the growth medium includes a growth selection (e.g., acetamide or an antibiotic) it permits the growth of transformants only.
- transformation of bacterial cells may be performed according to conventional methods, e.g., as described in Sambrook et ah, Molecular Cloning: A Laboratory Manual, Cold Spring Harbor, 1982, which is hereby incorporated by reference in its entirety, particularly with respect to transformation methods.
- cells in culture is meant two or more cells in a solution (e.g., a cell growth medium) that allows the cells to undergo one or more cell divisions.
- Cells in culture do not include plant cells that are part of a living, multicellular plant containing cells that have differentiated into plant tissues. In various embodiments, the cell culture includes at least or about 10, 20, 50, 100, 200, 500, 1,000, 5,000, 10,000 or more cells.
- cells in oxygen- limited culture is meant two or more cells in a solution (e.g., a cell growth medium) that allows the cell to under go one or more cell divisions, wherein the solution contains a limiting amount of oxygen.
- a solution e.g., a cell growth medium
- oxygen-limited culture means that the culture is either anoxic or contains less than the required amount of oxygen to support respiration via the biological transfer of reducing equivalents to oxygen, and also encompasses anaerobic cultures.
- oxygen-limited culture conditions some electrons derived from carbon metabolism cannot be accepted because oxygen concentrations are too low, causing cells to switch to hydrogen production if they comprise the appropriate metabolic pathways for doing so.
- Oxygen-limited culture conditions occur when the oxygen transfer rate (“OTR") is less than the oxygen uptake rate ("OUR”) indicated by dissolved oxygen concentrations of close to zero in culture medium.
- OTR oxygen transfer rate
- OUR oxygen uptake rate
- Any carbon source can be used to cultivate the host cells.
- the term "carbon source” refers to one or more carbon-containing compounds capable of being metabolized by a host cell or organism.
- the cell medium used to cultivate the host cells may include any carbon source suitable for maintaining the viability or growing the host cells.
- the carbon source is a carbohydrate (such as monosaccharide, disaccharide, oligosaccharide, or polysaccharides), invert sugar (e.g., enzymatically treated sucrose syrup), glycerol, glycerine (e.g., a glycerine byproduct of a biodiesel or soap-making process), dihydroxyacetone, one-carbon source, oil (e.g., a plant or vegetable oil such as corn, palm, or soybean oil), animal fat, animal oil, fatty acid (e.g., a saturated fatty acid, unsaturated fatty acid, or polyunsaturated fatty acid), lipid, phospholipid, glycerolipid, monoglyceride, diglyceride, triglyceride, polypeptide (e.g., a microbial or plant protein or peptide), renewable carbon source (e.g., a biomass carbon source such as a hydrolyzed biomass carbon
- Exemplary monosaccharides include glucose and fructose; exemplary oligosaccharides include lactose and sucrose, and exemplary polysaccharides include starch and cellulose.
- Exemplary carbohydrates include C6 sugars (e.g., fructose, mannose, galactose, or glucose) and C5 sugars (e.g., xylose or arabinose).
- the cell medium includes a carbohydrate as well as a carbon source other than a carbohydrate (e.g., glycerol, glycerine, dihydroxyacetone, one-carbon source, oil, animal fat, animal oil, fatty acid, lipid, phospholipid, glycerolipid, monoglyceride, diglyceride, triglyceride, renewable carbon source, or a component from a yeast extract).
- the cell medium includes a carbohydrate as well as a polypeptide (e.g., a microbial or plant protein or peptide).
- the microbial polypeptide is a polypeptide from yeast or bacteria.
- the plant polypeptide is a polypeptide from soy, corn, canola, jatropha, palm, peanut, sunflower, coconut, mustard, rapeseed, cottonseed, palm kernel, olive, safflower, sesame, or linseed.
- the concentration of the carbohydrate is at least or about 5 grams per liter of broth (g/L, wherein the volume of broth includes both the volume of the cell medium and the volume of the cells), such as at least or about 10, 15, 20, 30, 40, 50, 60, 80, 100, 150, 200, 300, 400, or more g/L.
- the concentration of the carbohydrate is between about 50 and about 400 g/L, such as between about 100 and about 360 g/L, between about 120 and about 360 g/L, or between about 200 and about 300 g/L. In some embodiments, this concentration of carbohydrate includes the total amount of carbohydrate that is added before and/or during the culturing of the host cells.
- the cells are cultured under limited glucose conditions.
- limited glucose conditions is meant that the amount of glucose that is added is less than or about 105% (such as about 100%) of the amount of glucose that is consumed by the cells.
- the amount of glucose that is added to the culture medium is approximately the same as the amount of glucose that is consumed by the cells during a specific period of time.
- the rate of cell growth is controlled by limiting the amount of added glucose such that the cells grow at the rate that can be supported by the amount of glucose in the cell medium.
- glucose does not accumulate during the time the cells are cultured.
- the cells are cultured under limited glucose conditions for greater than or about 1, 2, 3, 5, 10, 15, 20, 25, 30, 35, 40, 50, 60, or 70 hours. In various embodiments, the cells are cultured under limited glucose conditions for greater than or about 5, 10, 15, 20, 25, 30, 35, 40, 50, 60, 70, 80, 90, 95, or 100% of the total length of time the cells are cultured. While not intending to be bound by any particular theory, it is believed that limited glucose conditions may allow more favorable regulation of the cells. [0399] In some embodiments, the cells are cultured in the presence of an excess of glucose.
- the amount of glucose that is added is greater than about 105% (such as about or greater than 110, 120, 150, 175, 200, 250, 300, 400, or 500%) or more of the amount of glucose that is consumed by the cells during a specific period of time. In some embodiments, glucose accumulates during the time the cells are cultured.
- Exemplary lipids are any substance containing one or more fatty acids that are C4 and above fatty acids that are saturated, unsaturated, or branched.
- Exemplary oils are lipids that are liquid at room temperature.
- the lipid contains one or more C4 or above fatty acids (e.g., contains one or more saturated, unsaturated, or branched fatty acid with four or more carbons).
- the oil is obtained from soy, corn, canola, jatropha, palm, peanut, sunflower, coconut, mustard, rapeseed, cottonseed, palm kernel, olive, safflower, sesame, linseed, oleagineous microbial cells, Chinese tallow, or any combination of two or more of the foregoing.
- Exemplary fatty acids include compounds of the formula RCOOH, where "R" is a hydrocarbon.
- Exemplary unsaturated fatty acids include compounds where "R” includes at least one carbon-carbon double bond.
- Exemplary unsaturated fatty acids include, but are not limited to, oleic acid, vaccenic acid, linoleic acid, palmitoleic acid, and arachidonic acid.
- Exemplary polyunsaturated fatty acids include compounds where "R” includes a plurality of carbon-carbon double bonds.
- Exemplary saturated fatty acids include compounds where "R" is a saturated aliphatic group.
- the carbon source includes one or more C12-C22 fatty acids, such as a C 12 saturated fatty acid, a C H saturated fatty acid, a Ci 6 saturated fatty acid, a C 18 saturated fatty acid, a C20 saturated fatty acid, or a C22 saturated fatty acid.
- the fatty acid is palmitic acid.
- the carbon source is a salt of a fatty acid (e.g., an unsaturated fatty acid), a derivative of a fatty acid (e.g., an unsaturated fatty acid), or a salt of a derivative of fatty acid (e.g., an unsaturated fatty acid).
- Suitable salts include, but are not limited to, lithium salts, potassium salts, sodium salts, and the like.
- Di- and triglycerols are fatty acid esters of glycerol.
- the concentration of the lipid, oil, fat, fatty acid, monoglyceride, diglyceride, or triglyceride is at least or about 1 gram per liter of broth (g/L, wherein the volume of broth includes both the volume of the cell medium and the volume of the cells), such as at least or about 5, 10, 15, 20, 30, 40, 50, 60, 80, 100, 150, 200, 300, 400, or more g/L.
- the concentration of the lipid, oil, fat, fatty acid, monoglyceride, diglyceride, or triglyceride is between about 10 and about 400 g/L, such as between about 25 and about 300 g/L, between about 60 and about 180 g/L, or between about 75 and about 150 g/L. In some embodiments, the concentration includes the total amount of the lipid, oil, fat, fatty acid, monoglyceride, diglyceride, or triglyceride that is added before and/or during the culturing of the host cells.
- the carbon source includes both (i) a lipid, oil, fat, fatty acid, monoglyceride, diglyceride, or triglyceride and (ii) a carbohydrate, such as glucose.
- a lipid, oil, fat, fatty acid, monoglyceride, diglyceride, or triglyceride and (ii) a carbohydrate, such as glucose.
- the ratio of the lipid, oil, fat, fatty acid, monoglyceride, diglyceride, or triglyceride to the carbohydrate is about 1:1 on a carbon basis (i.e., one carbon in the lipid, oil, fat, fatty acid, monoglyceride, diglyceride, or triglyceride per carbohydrate carbon).
- the amount of the lipid, oil, fat, fatty acid, monoglyceride, diglyceride, or triglyceride is between about 60 and 180 g/L, and the amount of the carbohydrate is between about 120 and 360 g/L.
- Exemplary microbial polypeptide carbon sources include one or more polypeptides from yeast or bacteria.
- Exemplary plant polypeptide carbon sources include one or more polypeptides from soy, corn, canola, jatropha, palm, peanut, sunflower, coconut, mustard, rapeseed, cottonseed, palm kernel, olive, safflower, sesame, or linseed.
- Exemplary renewable carbon sources include cheese whey permeate, cornsteep liquor, sugar beet molasses, barley malt, and components from any of the foregoing.
- Exemplary renewable carbon sources also include glucose, hexose, pentose and xylose present in biomass, such as corn, switchgrass, sugar cane, cell waste of fermentation processes, and protein byproduct from the milling of soy, corn, or wheat.
- the biomass carbon source is a lignocellulosic, hemicellulosic, or cellulosic material such as, but are not limited to, a grass, wheat, wheat straw, bagasse, sugar cane bagasse, soft wood pulp, corn, corn cob or husk, corn kernel, fiber from corn kernels, corn stover, switch grass, rice hull product, or a by-product from wet or dry milling of grains (e.g., corn, sorghum, rye, triticate, barley, wheat, and/or distillers grains).
- Exemplary cellulosic materials include wood, paper and pulp waste, herbaceous plants, and fruit pulp.
- the carbon source includes any plant part, such as stems, grains, roots, or tubers. In some embodiments, all or part of any of the following plants are used as a carbon source: corn, wheat, rye, sorghum, triticate, rice, millet, barley, cassava, legumes, such as beans and peas, potatoes, sweet potatoes, bananas, sugarcane, and/or tapioca. In some embodiments, the carbon source is a biomass hydrolysate, such as a biomass hydrolysate that includes both xylose and glucose or that includes both sucrose and glucose.
- the renewable carbon source (such as biomass) is pretreated before it is added to the cell culture medium.
- the pretreatment includes enzymatic pretreatment, chemical pretreatment, or a combination of both enzymatic and chemical pretreatment (see, for example, Farzaneh et ah, Bioresource Technology 96 (18): 2014-2018, 2005; U.S. Patent No. 6,176,176; U.S. Patent No. 6,106,888; which are each hereby incorporated by reference in their entireties, particularly with respect to the pretreatment of renewable carbon sources).
- the renewable carbon source is partially or completely hydrolyzed before it is added to the cell culture medium.
- the renewable carbon source (such as corn stover) undergoes ammonia fiber expansion (AFEX) pretreatment before it is added to the cell culture medium (see, for example, Farzaneh et al, Bioresource Technology 96 (18): 2014-2018, 2005).
- AFEX ammonia fiber expansion
- a renewable carbon source is treated with liquid anhydrous ammonia at moderate temperatures (such as about 60 to about 100 0 C) and high pressure (such as about 250 to about 300 psi) for about 5 minutes. Then, the pressure is rapidly released.
- AFEX pretreatment has the advantage that nearly all of the ammonia can be recovered and reused, while the remaining serves as nitrogen source for microbes in downstream processes. Also, a wash stream is not required for AFEX pretreatment. Thus, dry matter recovery following the AFEX treatment is essentially 100%.
- AFEX is basically a dry to dry process. The treated renewable carbon source is stable for long periods and can be fed at very high solid loadings in enzymatic hydrolysis or fermentation processes.
- the concentration of the carbon source is equivalent to at least or about 0.1, 0.5, 1, 1.5 2, 3, 4, 5, 10, 15, 20, 30, 40, or 50% glucose (w/v).
- the equivalent amount of glucose can be determined by using standard HPLC methods with glucose as a reference to measure the amount of glucose generated from the carbon source.
- the concentration of the carbon source is equivalent to between about 0.1 and about 20% glucose, such as between about 0.1 and about 10% glucose, between about 0.5 and about 10% glucose, between about 1 and about 10% glucose, between about 1 and about 5% glucose, or between about 1 and about 2% glucose.
- the carbon source includes yeast extract or one or more components of yeast extract.
- the concentration of yeast extract is at least 1 gram of yeast extract per liter of broth (g/L, wherein the volume of broth includes both the volume of the cell medium and the volume of the cells), such at least or about 5, 10, 15, 20, 30, 40, 50, 60, 80, 100, 150, 200, 300, or more g/L.
- the concentration of yeast extract is between about 1 and about 300 g/L, such as between about 1 and about 200 g/L, between about 5 and about 200 g/L, between about 5 and about 100 g/L, or between about 5 and about 60 g/L.
- the concentration includes the total amount of yeast extract that is added before and/or during the culturing of the host cells.
- the carbon source includes both yeast extract (or one or more components thereof) and another carbon source, such as glucose.
- the ratio of yeast extract to the other carbon source is about 1:5, about 1:10, or about 1:20 (w/w).
- the carbon source may also be one-carbon substrates such as carbon dioxide, or methanol. Glycerol production from single carbon sources (e.g., methanol, formaldehyde, or formate) has been reported in methylotrophic yeasts (Yamada et al., Agric. Biol.
- the pathway of carbon assimilation can be through ribulose monophosphate, through serine, or through xylulose- momophosphate (Gottschalk, Bacterial Metabolism, Second Edition, Springer- Verlag: New York, 1986, which is hereby incorporated by reference in its entirety, particularly with respect to carbon sources).
- the ribulose monophosphate pathway involves the condensation of formate with ribulose-5 -phosphate to form a six carbon sugar that becomes fructose and eventually the three carbon product glyceraldehyde-3 -phosphate.
- the serine pathway assimilates the one-carbon compound into the glycolytic pathway via methylenetetrahydrofolate.
- methylotrophic organisms are also known to utilize a number of other carbon containing compounds such as methylamine, glucosamine and a variety of amino acids for metabolic activity.
- methylotrophic yeast are known to utilize the carbon from methylamine to form trehalose or glycerol (Bellion et al., Microb. Growth Cl Compd., [Int. Symp.], 7 th ed., 415-32. Editors: Murrell et al, Publisher: Intercept, Andover, UK, 1993, which is hereby incorporated by reference in its entirety, particularly with respect to carbon sources).
- cells are cultured in a standard medium containing physiological salts and nutrients (see, e.g., Pourquie, J. et al., Biochemistry and Genetics of Cellulose Degradation, eds. Aubert et al., Academic Press, pp. 71-86, 1988 and Ilmen et al., Appl. Environ. Microbiol.
- Exemplary growth media are common commercially prepared media such as Luria Bertani (LB) broth, Sabouraud Dextrose (SD) broth, or Yeast medium (YM) broth.
- LB Luria Bertani
- SD Sabouraud Dextrose
- YM Yeast medium
- Other defined or synthetic growth media may also be used, and the appropriate medium for growth of particular host cells are known by someone skilled in the art of microbiology or fermentation science.
- the cell medium desirably contains suitable minerals, salts, cofactors, buffers, and other components known to those skilled in the art suitable for the growth of the cultures or the enhancement of isoprene production (see, for example, WO 2004/033646 and references cited therein and WO 96/35796 and references cited therein, which are each hereby incorporated by reference in their entireties, particularly with respect cell medias and cell culture conditions).
- an isoprene synthase, DXS, IDI, and/or MVA pathway nucleic acid is under the control of an inducible promoter
- the inducing agent e.g., a sugar, metal salt or antimicrobial
- cell medium has an antibiotic (such as kanamycin) that corresponds to the antibiotic resistance nucleic acid (such as a kanamycin resistance nucleic acid) on a vector that has one or more DXS, IDI, or MVA pathway nucleic acids.
- the cells are cultured in a culture medium under conditions permitting the expression of one or more isoprene synthase, DXS, IDI, or MVA pathway polypeptides encoded by a nucleic acid inserted into the host cells.
- Standard cell culture conditions can be used to culture the cells (see, for example, WO 2004/033646 and references cited therein, which are each hereby incorporated by reference in their entireties, particularly with respect to cell culture and fermentation conditions).
- Cells are grown and maintained at an appropriate temperature, gas mixture, and pH (such as at about 20 0 C to about 37°C, at about 6% to about 84% CO 2 , and at a pH between about 5 to about 9).
- cells are grown at 35°C in an appropriate cell medium.
- cultures are cultured at approximately 28 0 C in appropriate medium in shake cultures or fermentors until the desired amount of isoprene and hydrogen co-production is achieved.
- the pH ranges for fermentation are between about pH 5.0 to about pH 9.0 (such as about pH 6.0 to about pH 8.0 or about 6.5 to about 7.0). Reactions may be performed under aerobic, anoxic, or anaerobic conditions based on the requirements of the host cells.
- the cells are cultured under oxygen-limited conditions. In some embodiments, the cells are cultured in the presence of oxygen under conditions where 0.5 moles of oxygen are taken up per mole of isoprene produced. In some embodiments, the cells are cultured under anaerobic conditions.
- Exemplary culture conditions for a given filamentous fungus are known in the art and may be found in the scientific literature and/or from the source of the fungi such as the American Type Culture Collection and Fungal Genetics Stock Center.
- the cells are grown using any known mode of fermentation, such as batch, fed-batch, or continuous processes.
- a batch method of fermentation is used.
- Classical batch fermentation is a closed system where the composition of the media is set at the beginning of the fermentation and is not subject to artificial alterations during the fermentation. Thus, at the beginning of the fermentation the cell medium is inoculated with the desired host cells and fermentation is permitted to occur adding nothing to the system.
- batch fermentation is batch with respect to the addition of carbon source and attempts are often made at controlling factors such as pH and oxygen concentration.
- the metabolite and biomass compositions of the system change constantly until the time the fermentation is stopped.
- cells moderate through a static lag phase to a high growth log phase and finally to a stationary phase where growth rate is diminished or halted.
- cells in log phase are responsible for the bulk of the isoprene production.
- cells in stationary phase produce isoprene.
- a variation on the standard batch system is used, such as the Fed- Batch system.
- Fed-Batch fermentation processes comprise a typical batch system with the exception that the carbon source is added in increments as the fermentation progresses.
- Fed- Batch systems are useful when catabolite repression is apt to inhibit the metabolism of the cells and where it is desirable to have limited amounts of carbon source in the cell medium.
- Fed-batch fermentations may be performed with the carbon source (e.g. , glucose) in a limited or excess amount. Measurement of the actual carbon source concentration in Fed-Batch systems is difficult and is therefore estimated on the basis of the changes of measurable factors such as pH, dissolved oxygen, and the partial pressure of waste gases such as CO 2 .
- Continuous fermentation is an open system where a defined fermentation medium is added continuously to a bioreactor and an equal amount of conditioned medium is removed simultaneously for processing. Continuous fermentation generally maintains the cultures at a constant high density where cells are primarily in log phase growth.
- Continuous fermentation allows for the modulation of one factor or any number of factors that affect cell growth or isoprene production.
- one method maintains a limiting nutrient such as the carbon source or nitrogen level at a fixed rate and allows all other parameters to moderate.
- a number of factors affecting growth can be altered continuously while the cell concentration (e.g., the concentration measured by media turbidity) is kept constant.
- Continuous systems strive to maintain steady state growth conditions. Thus, the cell loss due to media being drawn off is balanced against the cell growth rate in the fermentation.
- cells are immobilized on a substrate as whole cell catalysts and subjected to fermentation conditions for isoprene production.
- bottles of liquid culture are placed in shakers in order to introduce oxygen to the liquid and maintain the uniformity of the culture.
- an incubator is used to control the temperature, humidity, shake speed, and/or other conditions in which a culture is grown.
- the simplest incubators are insulated boxes with an adjustable heater, typically going up to ⁇ 65 0 C. More elaborate incubators can also include the ability to lower the temperature (via refrigeration), or the ability to control humidity or CO 2 levels.
- Most incubators include a timer; some can also be programmed to cycle through different temperatures, humidity levels, etc. Incubators can vary in size from tabletop to units the size of small rooms.
- the cell medium can be changed to replenish nutrients and/or avoid the build up of potentially harmful metabolic byproducts and dead cells.
- cells can be separated from the media by centrifuging or filtering the suspension culture and then resuspending the cells in fresh media.
- adherent cultures the media can be removed directly by aspiration and replaced.
- the cell medium allows at least a portion of the cells to divide for at least or about 5, 10, 20, 40, 50, 60, 65, or more cell divisions in a continuous culture (such as a continuous culture without dilution).
- a constitutive or leaky promoter such as a Trc promoter
- a compound such as IPTG
- a compound such as IPTG
- a compound such as IPTG
- a compound such as IPTG
- a compound is added to induce expression of the isoprene synthase, DXS, IDI, or MVA pathway nucleic acid(s) operably linked to the promoter.
- Exemplary Methods for Decoupling Isoprene Production from Cell Growth carbon from the feedstock is converted to isoprene rather than to the growth and maintenance of the cells.
- the cells are grown to a low to medium OD ⁇ oo, then production of isoprene is started or increased. This strategy permits a large portion of the carbon to be converted to isoprene.
- cells reach an optical density such that they no longer divide or divide extremely slowly, but continue to make isoprene for several hours (such as about 2, 4, 6, 8, 10, 15, 20, 25, 30, or more hours).
- Figs. 60A-67C illustrate that cells may continue to produce a substantial amount of mevalonic acid or isoprene after the cells reach an optical density such that they no longer divide or divide extremely slowly.
- the optical density at 550 nm decreases over time (such as a decrease in the optical density after the cells are no longer in an exponential growth phase due to cell lysis), and the cells continue to produce a substantial amount of mevalonic acid or isoprene.
- the optical density at 550 nm of the cells increases by less than or about 50% (such as by less than or about 40, 30, 20, 10, 5, or 0%) over a certain time period (such as greater than or about 5, 10, 15, 20, 25, 30, 40, 50 or 60 hours), and the cells produce isoprene at greater than or about 1, 10, 25, 50, 100, 150, 200, 250, 300, 400, 500, 600, 700, 800, 900, 1,000, 1,250, 1,500, 1,750, 2,000, 2,500, 3,000, 4,000, 5,000, or more nmole of isoprene/gram of cells for the wet weight of the cells/hour (nmole/g wcm /hr) during this time period.
- the amount of isoprene is between about 2 to about 5,000 nmole/g wcm /hr, such as between about 2 to about 100 nmole/g wcm /hr, about 100 to about 500 nmole/g wcm /hr, about 150 to about 500 nmole/g wcm /hr, about 500 to about 1,000 nmole/g wcm /hr, about 1,000 to about 2,000 nmole/g wcm /hr, or about 2,000 to about 5,000 nmole/g wcm /hr.
- the amount of isoprene is between about 20 to about 5,000 nmole/g wcm /hr, about 100 to about 5,000 nmole/g wcm /hr, about 200 to about 2,000 nmole/g wcm /hr, about 200 to about 1,000 nmole/g wcm /hr, about 300 to about 1,000 nmole/g wcm /hr, or about 400 to about 1,000 nmole/g wcm /hr.
- the optical density at 550 nm of the cells increases by less than or about 50% (such as by less than or about 40, 30, 20, 10, 5, or 0%) over a certain time period (such as greater than or about 5, 10, 15, 20, 25, 30, 40, 50 or 60 hours), and the cells produce a cumulative titer (total amount) of isoprene at greater than or about 1, 10, 25, 50, 100, 150, 200, 250, 300, 400, 500, 600, 700, 800, 900, 1,000, 1,250, 1,500, 1,750, 2,000, 2,500, 3,000, 4,000, 5,000, 10,000, 50,000, 100,000, or more mg of isoprene/L of broth (mg/L brot h, wherein the volume of broth includes the volume of the cells and the cell medium) during this time period.
- mg/L brot h wherein the volume of broth includes the volume of the cells and the cell medium
- the amount of isoprene is between about 2 to about 5,000 mg/L broth , such as between about 2 to about 100 mg/Lb ro th, about 100 to about 500 mg/Lbroth, about 500 to about 1,000 mg/Lbroth, about 1,000 to about 2,000 mg/Lb ro th, or about 2,000 to about 5,000 mg/Lbroth- In some embodiments, the amount of isoprene is between about 20 to about 5,000 mg/Lb ro th, about 100 to about 5,000 mg/L br oth, about 200 to about 2,000 mg/L br oth, about 200 to about 1,000 mg/Lbroth, about 300 to about 1,000 mg/Lbroth, or about 400 to about 1,000 mg/Lbroth- [0427] In some embodiments, the optical density at 550 nm of the cells increases by less than or about 50% (such as by less than or about 40, 30, 20, 10, 5, or 0%) over a certain time period (such as greater than or about 5, 10, 15, 20, 25, 30,
- the percent conversion of carbon into isoprene is between such as about 0.002 to about 4.0%, about 0.002 to about 3.0%, about 0.002 to about 2.0%, about 0.002 to about 1.6%, about 0.002 to about 0.005%, about 0.005 to about 0.01%, about 0.01 to about 0.05%, about 0.05 to about 0.15%, 0.15 to about 0.2%, about 0.2 to about 0.3%, about 0.3 to about 0.5%, about 0.5 to about 0.8%, about 0.8 to about 1.0%, or about 1.0 to about 1.6%.
- the percent conversion of carbon into isoprene is between about 0.002 to about 0.4%, 0.002 to about 0.16%, 0.04 to about 0.16%, about 0.005 to about 0.3%, about 0.01 to about 0.3%, or about 0.05 to about 0.3%.
- isoprene is only produced in stationary phase. In some embodiments, isoprene is produced in both the growth phase and stationary phase. In various embodiments, the amount of isoprene produced (such as the total amount of isoprene produced or the amount of isoprene produced per liter of broth per hour per OD OOO ) during stationary phase is greater than or about 2, 3, 4, 5, 10, 20, 30, 40, 50, or more times the amount of isoprene produced during the growth phase for the same length of time.
- greater than or about 5, 10, 20, 30, 40, 50, 60, 70, 80, 90, 95, 99% or more of the total amount of isoprene that is produced (such as the production of isoprene during a fermentation for a certain amount of time, such as 20 hours) is produced while the cells are in stationary phase.
- greater than or about 5, 10, 20, 30, 40, 50, 60, 70, 80, 90, 95, 99% or more of the total amount of isoprene that is produced (such as the production of isoprene during a fermentation for a certain amount of time, such as 20 hours) is produced while the cells divide slowly or not at all such that the optical density at 550 nm of the cells increases by less than or about 50% (such as by less than or about 40, 30, 20, 10, 5, or 0%).
- isoprene is only produced in the growth phase.
- one or more MVA pathway, IDI, DXP, or isoprene synthase nucleic acids are placed under the control of a promoter or factor that is more active in stationary phase than in the growth phase.
- a promoter or factor that is more active in stationary phase than in the growth phase.
- one or more MVA pathway, IDI, DXP, or isoprene synthase nucleic acids may be placed under control of a stationary phase sigma factor, such as RpoS.
- one or more MVA pathway, IDI, DXP, or isoprene synthase nucleic acids are placed under control of a promoter inducible in stationary phase, such as a promoter inducible by a response regulator active in stationary phase.
- isoprene within safe operating levels simplifies the design and construction of commercial facilities, vastly improves the ability to operate safely, and limits the potential for fires to occur.
- the optimal ranges for the production of isoprene are within the safe zone, i.e., the nonflammable range of isoprene concentrations.
- described herein is a method for the production of isoprene within the nonflammable range of isoprene concentrations (outside the fiammability envelope of isoprene).
- the fiammability envelope is characterized by the lower fiammability limit (LFL), the upper flammability limit (UFL), the limiting oxygen concentration (LOC), and the limiting temperature.
- LFL lower fiammability limit
- UNL upper flammability limit
- LOC limiting oxygen concentration
- a minimum amount of fuel such as isoprene
- oxidant typically oxygen.
- the LFL is the minimum amount of isoprene that must be present to sustain burning, while the UFL is the maximum amount of isoprene that can be present. Above this limit, the mixture is fuel rich and the fraction of oxygen is too low to have a flammable mixture. The LOC indicates the minimum fraction of oxygen that must also be present to have a flammable mixture.
- the limiting temperature is based on the flash point of isoprene and is that lowest temperature at which combustion of isoprene can propagate. These limits are specific to the concentration of isoprene, type and concentration of oxidant, inerts present in the system, temperature, and pressure of the system.
- Test Suite 1 isoprene: 0 wt% - 14 wt% O 2 : 6 wt% - 21 wt% N 2 : 79 wt% - 94 wt%
- Test Suite 2 isoprene: 0 wt% - 14 wt% O 2 : 6 wt% - 21 wt% N 2 : 79 wt% - 94 wt% Saturated with H 2 O
- Test Suite 3 isoprene: O wt% - 14 wt% O 2 : 6 wt% - 21 wt% N 2 : 79 wt% - 94 wt% CO 2 : 5 wt% - 30 wt%
- Test Suite 1 isoprene: 0 wt% - 14 wt% O 2 : 6 wt% - 21 wt% N 2 : 79 wt% - 94 wt%
- Test Suite 2 isoprene: 0 wt% - 14 wt% O 2 : 6 wt% - 21 wt% N 2 : 79 wt% - 94 wt% Saturated with H 2 O
- the LOC was determined to be 9.5 vol% for an isoprene, O 2 , N 2 , and CO 2 mixture at 40 0 C and 1 atmosphere.
- the addition of up to 30% CO 2 did not significantly affect the flammability characteristics of an isoprene, O 2 , and N 2 mixture. Only slight variations in flammability characteristics were shown between a dry and water saturated isoprene, O 2 , and N 2 system.
- the limiting temperature is about -54 0 C. Temperatures below about -54 0 C are too low to propagate combustion of isoprene.
- the LFL of isoprene ranges from about 1.5 vol.% to about 2.0 vol%, and the UFL of isoprene ranges from about 2.0 vol.% to about 12.0 vol.%, depending on the amount of oxygen in the system.
- the LOC is about 9.5 vol% oxygen.
- the LFL of isoprene is between about 1.5 vol.% to about 2.0 vol%
- the UFL of isoprene is between about 2.0 vol.% to about 12.0 vol.%
- the LOC is about 9.5 vol% oxygen when the temperature is between about 25 0 C to about 55 0 C (such as about 40 0 C) and the pressure is between about 1 atmosphere and 3 atmospheres.
- isoprene is produced in the presence of less than about 9.5 vol% oxygen (that is, below the LOC required to have a flammable mixture of isoprene).
- the isoprene concentration is below the LFL (such as below about 1.5 vol.%).
- the amount of isoprene can be kept below the LFL by diluting the isoprene composition with an inert gas (e.g., by continuously or periodically adding an inert gas such as nitrogen to keep the isoprene composition below the LFL).
- the isoprene concentration is above the UFL (such as above about 12 vol.%).
- the amount of isoprene can be kept above the UFL by using a system (such as any of the cell culture systems described herein) that produces isoprene at a concentration above the UFL.
- a relatively low level of oxygen can be used so that the UFL is also relatively low. In this case, a lower isoprene concentration is needed to remain above the UFL.
- the isoprene concentration is within the flammability envelope (such as between the LFL and the UFL).
- one or more steps are performed to reduce the probability of a fire or explosion.
- one or more sources of ignition such as any materials that may generate a spark
- one or more steps are performed to reduce the amount of time that the concentration of isoprene remains within the flammability envelope.
- a sensor is used to detect when the concentration of isoprene is close to or within the flammability envelope.
- the concentration of isoprene can be measured at one or more time points during the culturing of cells, and the cell culture conditions and/or the amount of inert gas can be adjusted using standard methods if the concentration of isoprene is close to or within the flammability envelope.
- the cell culture conditions such as fermentation conditions
- the amount of isoprene is kept below the LFL by diluting the isoprene composition with an inert gas (such as by continuously or periodically adding an inert gas to keep the isoprene composition below the LFL).
- the amount of flammable volatiles other than isoprene is at least about 2, 5, 10, 50, 75, or 100-fold less than the amount of isoprene produced.
- the portion of the gas phase other than isoprene gas comprises between about 0% to about 100% (volume) oxygen, such as between about 0% to about 10%, about 10% to about 20%, about 20% to about 30%, about 30% to about 40%, about 40% to about 50%, about 50% to about 60%, about 60% to about 70%, about 70% to about 80%, about 90% to about 90%, or about 90% to about 100% (volume) oxygen.
- the portion of the gas phase other than isoprene gas comprises between about 0% to about 99% (volume) nitrogen, such as between about 0% to about 10%, about 10% to about 20%, about 20% to about 30%, about 30% to about 40%, about 40% to about 50%, about 50% to about 60%, about 60% to about 70%, about 70% to about 80%, about 90% to about 90%, or about 90% to about 99% (volume) nitrogen.
- the portion of the gas phase other than isoprene gas comprises between about 1% to about 50% (volume) CO 2 , such as between about 1% to about 10%, about 10% to about 20%, about 20% to about 30%, about 30% to about 40%, or about 40% to about 50% (volume) CO 2 .
- an isoprene composition also contains ethanol.
- ethanol may be used for extractive distillation of isoprene, resulting in compositions (such as intermediate product streams) that include both ethanol and isoprene.
- the amount of ethanol is outside the flammability envelope for ethanol.
- the LOC of ethanol is about 8.7 vol%, and the LFL for ethanol is about 3.3 vol% at standard conditions, such as about 1 atmosphere and about 60 F (NFPA 69 Standard on Explosion Prevention Systems, 2008 edition, which is hereby incorporated by reference in its entirety, particularly with respect to LOC, LFL, and UFL values).
- compositions that include isoprene and ethanol are produced in the presence of less than the LOC required to have a flammable mixture of ethanol (such as less than about 8.7% vol%). In some embodiments in which compositions that include isoprene and ethanol are produced in the presence of greater than or about the LOC required to have a flammable mixture of ethanol, the ethanol concentration is below the LFL (such as less than about 3.3 vol.%).
- the amount of oxidant is below the LOC of any fuel in the system (such as isoprene or ethanol). In various embodiments, the amount of oxidant (such as oxygen) is less than about 60, 40, 30, 20, 10, or 5% of the LOC of isoprene or ethanol. In various embodiments, the amount of oxidant (such as oxygen) is less than the LOC of isoprene or ethanol by at least 2, 4, 5, or more absolute percentage points (vol %).
- the amount of oxygen is at least 2 absolute percentage points (vol %) less than the LOC of isoprene or ethanol (such as an oxygen concentration of less than 7.5 vol% when the LOC of isoprene is 9.5 vol%).
- the amount of fuel (such as isoprene or ethanol) is less than or about 25, 20, 15, 10, or 5% of the LFL for that fuel.
- Methods are provided herein of producing isoprene comprising a) culturing cells under suitable conditions for production of isoprene; and b) producing isoprene, wherein the liquid phase concentration of isoprene is less than about 200 mg/L.
- the liquid phase concentration of isoprene in the culture is less than about any of 175 mg/L, 150 mg/L, 125 mg/L, 100 mg/L, 75 mg/L, 50 mg/L, 25 mg/L, 20 mg/L, 15 mg/L, 10 mg/L, 5 mg/L, or 2.5 mg/L.
- the liquid phase concentration of isoprene in culture is between about any of 0.1 mg/L to 200 mg/L, 1 mg/L to 200 mg/L, 1 mg/L to 150 mg/L, 1 mg/L to 100 mg/L, 1 mg/L to 50 mg/L, 1 mg/L to 25 mg/L, 1 mg/L to 20 mg/L, or 10 mg/L to 20 mg/L.
- the isoprene produced is any concentration or amount disclosed in the section entitled "Exemplary Production of Isoprene.”
- the liquid phase concentration is below the solubility limit of isoprene.
- the cells produce greater than about 400 nmole/gwcm/hour of isoprene.
- the amount of isoprene is between about any of 400 nmole/g wcm /hour to 1 mole/g wcm /hour, 400 nmole/g wcm /hour to 1 mmole/g wcm /hour, 400 nmole/g wcm /hour to 40 mmole/g wcm /hour, 400 nmole/g wcm /hour to 4 mmole/g wcm /hour, 1 mmole/g wcm /hour to 1.5 mmole/g wcm /hour, 1.5 mmole/g wcm /hour to 3 mmole/g wcm /hour, 3 mmole/g wcm /hour to 5 mmole/g
- the amount of isoprene is about any of 1 mmole/g wcm /hour, 1.5 mmole/g wcm /hour, 2 mmole/g wcm /hour, 3 mmole/g wcm /hour, 4 mmole/g wcm /hour, or 5 mmole/g wcm /hour.
- the low value for Henry's coefficient means that isoprene can be recovered from fermentation broth by gas stripping at low sparging rates, for example 0.01 vvm to 2 vvm.
- the gas sparging rate is between about any of 0.1 vvm to 1 vvm, 0.01 vvm to 0.5 vvm, 0.2 wm to 1 vvm, or 0.5 vvm to 1 vvm.
- the gas sparging rate is about any of 0.1 vvm, 0.25 vvm, 0.5 vvm, 0.75 vvm, 1 vvm, 1.25 vvm, 1.5 vvm, 1.75 vvm, or 2 vvm.
- the low sparging rates are maintained for the entire course of the fermentation run, during growth phase, or during stationary phase. In some embodiments, the low sparging rates are maintained for between about any of 1 hour to 5 hours, 5 hours to 10 hours, 10 hours to 20 hours, 20 hours to 30 hours, 30 hours to 40 hours, 40 hours to 50 hours, or 50 hours to 60 hours.
- the lower desirable gas sparge limit is defined by the point at which the aqueous phase becomes saturated with isoprene and a liquid organic phase forms. This can only occur below the boiling point of isoprene (34.1 0 C at 1 atm), above which a liquid isoprene phase will never form. At temperatures below the boiling point of isoprene, the formation of a liquid phase is determined by the aqueous solubility of isoprene, which is approximately 650 mg/L at 25 0 C. While it is highly desirable to avoid the formation of a liquid isoprene phase, it is not absolutely required provided that the cells can tolerate the presence of liquid isoprene without toxic effects.
- the oxygen, CO 2 , and isoprene are any of the amounts or concentrations discussed in the section entitled "Production of Isoprene with Safe Operating Ranges.”
- all the oxygen is consumed by the cells while maintaining fully aerobic metabolism.
- an excess of oxygen is used in order to satisfy the oxygen demands of the cells. Desirable ranges of oxygen in the off-gas are less than 20%, or less than 15% or less than 10% (v/v). Levels of oxygen below the limiting oxygen concentration required for combustion of isoprene (9.5% v/v at 1 atm) are particularly desirable.
- oxygen-enriched air is utilized with the purpose of allowing minimal gas sweep rates while satisfying the cellular oxygen demand.
- the portion of the gas phase of the gas sweep comprises between about 0.1% to about 10%, about 10% to about 20%, or about 20% to about 30% (volume) oxygen.
- isoprene fermentations are performed under high pressure in order minimize the amount of excess oxygen required to maintain the required dissolved oxygen levels in the liquid phase.
- the reduction of the gas sweep rate through the fermentor is advantageous for an integrated isoprene production process in that such conditions enrich the off-gas isoprene levels up to about 30,000 ⁇ g/L (about 1% v/v) without adversely affecting the physiology of the cells.
- reduced gas-sparge rates do not significantly adversely affect the physiology of the cells.
- the carbon dioxide evolution rate of cells in culture with reduced gas-sparge rates is between about any of 1 x 10 "18 mmol/L/hour to about 1 mol/L/hour, 1 mmol/L/hour to 1 mol/L/hour, 25 mmol/L/hour to 750 mmol/L/hour, 25 mmol/L/hour to 75 mmol/L/hour, 250 mmol/L/hour to 750 mmol/L/hour, or 450 mmol/L/hour to 550 mmol/L/hour.
- the carbon dioxide evolution rate is about any of 50 mmol/L/hour, 100 mmol/L/hour, 150 mmol/L/hour, 200 mmol/L/hour, 250 mmol/L/hour, 300 mmol/L/hour, 350 mmol/L/hour, 400 mmol/L/hour, 450 mmol/L/hour, or 500 mmol/L/hour.
- cell viability with reduced gas-sparge rates is reduced by less than about any of 1.75-fold, 1.5-fold, 1.25-fold, 1-fold, 0.75-fold, 0.5-fold, or 0.25-fold. In some embodiments, cell viability with reduced gas-sparge rates is reduced by about 2-fold.
- cell viability with reduced gas-sparge rates of a cell expressing a MVA pathway and/or DXP pathway RNA and/or protein from one or more of a heterologous and/or duplicate copy of a MVA pathway and/or DXP pathway nucleic acid is compared to a control cell lacking one or more of a heterologous and/or duplicate copy of a MVA pathway and/or DXP pathway nucleic acid with reduced gas-sparge rates.
- cell viability with reduced gas-sparge rates of a cell expressing a MVA pathway and/or DXP pathway RNA and/or protein from one or more of a heterologous and/or duplicate copy of a MVA pathway and/or DXP pathway nucleic acid under the control of an inducible promoter, wherein the promotor is induced is compared to a control cell containing one or more of a heterologous and/or duplicate copy of a MVA pathway and/or DXP pathway nucleic acid under the control of an inducible promoter, wherein the promotor is not induced (uninduced) with reduced gas-sparge rates.
- the inducible promoter is a beta-galactosidase promotor.
- the fermentation of a genetically modified host organism that converts at least 5% of the total carbon consumed by the organism into a volatile, unsaturated hydrocarbon.
- the production of an unsaturated hydrocarbon at such a rate as to be present in the fermentation off-gas at a level of at least about any of 100 ⁇ g/L, 500 ⁇ g/L, 1000 ⁇ g/L, 2, 500 ⁇ g/L, 5,000 ⁇ g/L, 7,500 ⁇ g/L, or 10,000 ⁇ g/L.
- the unsaturated hydrocarbon is recovered from the off-gas stream in a manner that is suited to high-rates of production, which correspond to concentrations in the off-gas of at least about any of 100 ⁇ g/L, 500 ⁇ g/L, 1000 ⁇ g/L, 2,500 ⁇ g/L, 5,000 ⁇ g/L, 7,500 ⁇ g/L, or 10,000 ⁇ g/L.
- the continuous extraction and recovery of an unsaturated hydrocarbon from the fermentation off-gas in particular at low gas sweep rates such that the resulting off-gas is enriched in the volatile component of interest.
- recovery of the volatile hydrocarbon by methods that depend on elevated concentrations of the volatile.
- the method comprises: a) culturing cells under suitable conditions for production of the compound, wherein gas is added (such as the addition of gas to a system such as a fermentation system) at a gas sparging rate between about 0.01 vvm to about 2 vvm; and b) producing the compound.
- gas such as the addition of gas to a system such as a fermentation system
- the amount of the compound that partitions into the cell mass is not included in the liquid phase solubility values. In some embodiments, the liquid phase concentration is below the solubility limit of compound.
- the compounds can be continuously recovered from fermentation broth by gas stripping at moderate to low gas sparging rates, in particular those compounds with Henry's law coefficients of about any of less than 250 M/atm, 200 M/atm, 150 M/atm, 100 M/atm, 75 M/atm, 50 M/atm, 25 M/atm, 10 M/atm, 5 M/atm, or 1 M/atm.
- Examples include aldehydes such as acetaldehyde (15 M/atm), ketones such as acetone (30 M/atm) or 2- butanone (20 M/atm), or alcohols including methanol (220 M/atm), ethanol (200 M/atm), 1- butanol (120 M/atm) or C5 alcohols including 3-methyl-3-buten-l-ol, and 3-methyl-2-buten-l-ol (50- 100 M/atm).
- Esters of alcohols generally have lower Henry's constants than the respective alcohols, for example ethyl acetate (6-9 M/atm) or the acetyl esters of C5 alcohols ( ⁇ 5 M/atm).
- Compounds with Henry's law coefficients of less than 1 M/atm are particularly desirable.
- Examples include hemiterpenes, monoterpenes, or sesquiterpenes, in addition to other hydrocarbons such as Cl to C5 hydrocarbons (e.g., methane, ethane, ethylene, or propylene).
- the hydrocarbons such as Cl to C5 hydrocarbons are saturated, unsaturated, or branched.
- the gas sparging rate is between about any of 0.1 vvm to 1 vvm, 0.2 vvm to 1 vvm, or 0.5 vvm to 1 vvm. In some embodiments, the gas sparging rate is about any of 0.1 vvm, 0.25 vvm, 0.5 vvm, 0.75 vvm, 1 vvm, 1.25 vvm, 1.5 vvm, 1.75 vvm, or 2 vvm. In some embodiments, the low sparging rates are maintained for the entire course of the fermentation run, during growth phase, or during stationary phase.
- the low sparging rates are maintained for between about any of 1 hour to 5 hours, 5 hours to 10 hours, 10 hours to 20 hours, 20 hours to 30 hours, 30 hours to 40 hours, 40 hours to 50 hours, or 50 hours to 60 hours.
- Any of the systems described herein can be used in the methods of producing a compound described above. Standard methods would be used to purify such as those described in the section entitled “Exemplary Purification Methods.” Separation can be performed post- recovery for example, by distillation or selective adsorption techniques.
- the cells are cultured in a culture medium under conditions permitting the production of isoprene by the cells.
- peak absolute productivity is meant the maximum absolute amount of isoprene in the off-gas during the culturing of cells for a particular period of time ⁇ e.g., the culturing of cells during a particular fermentation run).
- peak absolute productivity time point is meant the time point during a fermentation run when the absolute amount of isoprene in the off-gas is at a maximum during the culturing of cells for a particular period of time ⁇ e.g., the culturing of cells during a particular fermentation run).
- the isoprene amount is measured at the peak absolute productivity time point.
- the peak absolute productivity for the cells is about any of the isoprene amounts disclosed herein.
- peak specific productivity is meant the maximum amount of isoprene produced per cell during the culturing of cells for a particular period of time ⁇ e.g., the culturing of cells during a particular fermentation run).
- peak specific productivity time point is meant the time point during the culturing of cells for a particular period of time ⁇ e.g., the culturing of cells during a particular fermentation run) when the amount of isoprene produced per cell is at a maximum.
- the peak specific productivity is determined by dividing the total productivity by the amount of cells, as determined by optical density at 600nm (OD OOO )- In some embodiments, the isoprene amount is measured at the peak specific productivity time point. In some embodiments, the peak specific productivity for the cells is about any of the isoprene amounts per cell disclosed herein.
- peak volumetric productivity is meant the maximum amount of isoprene produced per volume of broth (including the volume of the cells and the cell medium) during the culturing of cells for a particular period of time (e.g., the culturing of cells during a particular fermentation run).
- peak specific volumetric productivity time point is meant the time point during the culturing of cells for a particular period of time (e.g., the culturing of cells during a particular fermentation run) when the amount of isoprene produced per volume of broth is at a maximum.
- the peak specific volumetric productivity is determined by dividing the total productivity by the volume of broth and amount of time. In some embodiments, the isoprene amount is measured at the peak specific volumetric productivity time point. In some embodiments, the peak specific volumetric productivity for the cells is about any of the isoprene amounts per volume per time disclosed herein.
- peak concentration is meant the maximum amount of isoprene produced during the culturing of cells for a particular period of time (e.g., the culturing of cells during a particular fermentation run).
- peak concentration time point is meant the time point during the culturing of cells for a particular period of time (e.g., the culturing of cells during a particular fermentation run) when the amount of isoprene produced per cell is at a maximum.
- the isoprene amount is measured at the peak concentration time point.
- the peak concentration for the cells is about any of the isoprene amounts disclosed herein.
- average volumetric productivity is meant the average amount of isoprene produced per volume of broth (including the volume of the cells and the cell medium) during the culturing of cells for a particular period of time (e.g., the culturing of cells during a particular fermentation run).
- the average volumetric productivity is determined by dividing the total productivity by the volume of broth and amount of time.
- the average specific volumetric productivity for the cells is about any of the isoprene amounts per volume per time disclosed herein.
- cumulative total productivity is meant the cumulative, total amount of isoprene produced during the culturing of cells for a particular period of time (e.g., the culturing of cells during a particular fermentation run). In some embodiments, the cumulative, total amount of isoprene is measured. In some embodiments, the cumulative total productivity for the cells is about any of the isoprene amounts disclosed herein.
- relative detector response refers to the ratio between the detector response (such as the GC/MS area) for one compound (such as isoprene) to the detector response (such as the GC/MS area) of one or more compounds (such as all C5 hydrocarbons).
- the detector response may be measured as described herein, such as the GC/MS analysis performed with an Agilent 6890 GC/MS system fitted with an Agilent HP-5MS GC/MS column (30 m x 250 ⁇ m; 0.25 ⁇ m film thickness). If desired, the relative detector response can be converted to a weight percentage using the response factors for each of the compounds.
- This response factor is a measure of how much signal is generated for a given amount of a particular compound (that is, how sensitive the detector is to a particular compound).
- This response factor can be used as a correction factor to convert the relative detector response to a weight percentage when the detector has different sensitivities to the compounds being compared.
- the weight percentage can be approximated by assuming that the response factors are the same for the compounds being compared. Thus, the weight percentage can be assumed to be approximately the same as the relative detector response.
- the cells in culture produce isoprene at greater than or about 1, 10, 25, 50, 100, 150, 200, 250, 300, 400, 500, 600, 700, 800, 900, 1,000, 1,250, 1,500, 1,750, 2,000, 2,500, 3,000, 4,000, 5,000, 10,000, 12,500, 20,000, 30,000, 40,000, 50,000, 75,000, 100,000, 125,000, 150,000, 188,000, or more nmole of isoprene/gram of cells for the wet weight of the cells/hour (nmole/g wcm /hr).
- the amount of isoprene is between about 2 to about 200,000 nmole/g wcm /hr, such as between about 2 to about 100 nmole/g wcm /hr, about 100 to about 500 nmole/g wcm /hr, about 150 to about 500 nmole/g wcm /hr, about 500 to about 1,000 nmole/g wcm /hr, about 1,000 to about 2,000 nmole/g wcm /hr, or about 2,000 to about 5,000 nmole/g wcm /hr, about 5,000 to about 10,000 nmole/g wcm /hr, about 10,000 to about 50,000 nmole/g wcm /hr, about 50,000 to about 100,000 nmole/g wcm /hr, about 100,000 to about 150,000 nmole/g wcm /hr, or about 150,000 to about 200,000
- the amount of isoprene is between about 20 to about 5,000 nmole/g wcm /hr, about 100 to about 5,000 nmole/g wcm /hr, about 200 to about 2,000 nmole/g wcm /hr, about 200 to about 1,000 nmole/g wcm /hr, about 300 to about 1,000 nmole/g wcm /hr, or about 400 to about 1,000 nmole/g wcm /hr, about 1,000 to about 5,000 nmole/g wcm /hr, about 2,000 to about 20,000 nmole/g wcm /hr, about 5,000 to about 50,000 nmole/g wcm /hr, about 10,000 to about 100,000 nmole/g wcm /hr, about 20,000 to about 150,000 nmole/g wcm /hr, or about 20,000 to about 200,000
- the amount of isoprene in units of nmole/g wcm /hr can be measured as disclosed in U.S. Patent No. 5,849,970, which is hereby incorporated by reference in its entirety, particularly with respect to the measurement of isoprene production.
- two mL of headspace are analyzed for isoprene using a standard gas chromatography system, such as a system operated isothermally (85 0 C) with an n-octane/porasil C column (Alltech Associates, Inc., Deerfield, 111.) and coupled to a RGD2 mercuric oxide reduction gas detector (Trace Analytical, Menlo Park, CA) (see, for example, Greenberg et al, Atmos. Environ.
- a standard gas chromatography system such as a system operated isothermally (85 0 C) with an n-octane/porasil C column (Alltech Associates, Inc., Deerfield, 111.) and coupled to a RGD2 mercuric oxide reduction gas detector (Trace Analytical, Menlo Park, CA) (see, for example, Greenberg et al, Atmos. Environ.
- the gas chromatography area units are converted to nmol isoprene via a standard isoprene concentration calibration curve.
- the value for the grams of cells for the wet weight of the cells is calculated by obtaining the A 6 Oo value for a sample of the cell culture, and then converting the A 6 Oo value to grams of cells based on a calibration curve of wet weights for cell cultures with a known A 6 Oo value.
- the grams of the cells is estimated by assuming that one liter of broth (including cell medium and cells) with an A 6 Oo value of 1 has a wet cell weight of 1 gram. The value is also divided by the number of hours the culture has been incubating for, such as three hours.
- the cells in culture produce isoprene at greater than or about 1, 10, 25, 50, 100, 150, 200, 250, 300, 400, 500, 600, 700, 800, 900, 1,000, 1,250, 1,500, 1,750, 2,000, 2,500, 3,000, 4,000, 5,000, 10,000, 100,000, or more ng of isoprene/gram of cells for the wet weight of the cells/hr (ng/g wcm /h).
- the amount of isoprene is between about 2 to about 5,000 ng/g wcm /h, such as between about 2 to about 100 ng/g wcm /h, about 100 to about 500 ng/g wcm /h, about 500 to about 1,000 ng/g wcm /h, about 1,000 to about 2,000 ng/g wcm /h, or about 2,000 to about 5,000 ng/g wcm /h.
- the amount of isoprene is between about 20 to about 5,000 ng/g wcm /h, about 100 to about 5,000 ng/g wcm /h, about 200 to about 2,000 ng/g wcm /h, about 200 to about 1,000 ng/g wcm /h, about 300 to about 1,000 ng/g wcm /h, or about 400 to about 1,000 ng/g wcm /h.
- the amount of isoprene in ng/g wcm /h can be calculated by multiplying the value for isoprene production in the units of nmole/g wcm /hr discussed above by 68.1 (as described in Equation 5 below).
- the cells in culture produce a cumulative titer (total amount) of isoprene at greater than or about 1, 10, 25, 50, 100, 150, 200, 250, 300, 400, 500, 600, 700, 800, 900, 1,000, 1,250, 1,500, 1,750, 2,000, 2,500, 3,000, 4,000, 5,000, 10,000, 50,000, 100,000, or more mg of isoprene/L of broth (mg/Lbroth, wherein the volume of broth includes the volume of the cells and the cell medium).
- the amount of isoprene is between about 2 to about 5,000 mg/Lb ro th, such as between about 2 to about 100 mg/Lbroth, about 100 to about 500 mg/Lbroth, about 500 to about 1,000 mg/Lb ro th, about 1,000 to about 2,000 mg/Lbroth, or about 2,000 to about 5,000 mg/Lb ro th- In some embodiments, the amount of isoprene is between about 20 to about 5,000 mg/L br oth, about 100 to about 5,000 mg/L br oth, about 200 to about 2,000 mg/Lbroth, about 200 to about 1,000 mg/Lbroth, about 300 to about 1,000 mg/Lb ro th, or about 400 to about 1 ,000 mg/Lbroth-
- the specific productivity of isoprene in mg of isoprene/L of headspace from shake flask or similar cultures can be measured by taking a 1 ml sample from the cell culture at an OD ⁇ oo value of approximately 1.0, putting it in a 20 mL vial, incubating for 30 minutes, and then measuring the amount of isoprene in the headspace (as described, for example, in Example I, part II). If the OD OOO value is not 1.0, then the measurement can be normalized to an OD ⁇ oo value of 1.0 by dividing by the OD ⁇ oo value.
- the value of mg isoprene/L headspace can be converted to mg/Lbroth/hr/OD6oo of culture broth by multiplying by a factor of 38.
- the value in units of mg/Lbroth/hr/OD 6 oo can be multiplied by the number of hours and the OD 6 oo value to obtain the cumulative titer in units of mg of isoprene/L of broth.
- the cells in culture have an average volumetric productivity of isoprene at greater than or about 0.1, 1.0, 10, 25, 50, 100, 150, 200, 250, 300, 400, 500, 600, 700, 800, 900, 1,000, 1100, 1200, 1300, 1,400, 1,500, 1,600, 1,700, 1,800, 1,900, 2,000, 2,100, 2,200, 2,300, 2,400, 2,500, 2,600, 2,700, 2,800, 2,900, 3,000, 3,100, 3,200, 3,300, 3,400, 3,500, or more mg of isoprene/L of broth/hr (mg/L broth /hr, wherein the volume of broth includes the volume of the cells and the cell medium).
- the average volumetric productivity of isoprene is between about 0.1 to about 3,500 mg/L broth /hr, such as between about 0.1 to about 100 mg/L broth /hr, about 100 to about 500 mg/L broth /hr, about 500 to about 1,000 mg/L broth /hr, about 1,000 to about 1,500 mg/L broth /hr, about 1,500 to about 2,000 mg/L broth /hr, about 2,000 to about 2,500 mg/L broth /hr, about 2,500 to about 3,000 mg/L broth /hr, or about 3,000 to about 3,500 mg/L broth /hr.
- the average volumetric productivity of isoprene is between about 10 to about 3,500 mg/L broth /hr, about 100 to about 3,500 mg/L broth /hr, about 200 to about 1,000 mg/L broth /hr, about 200 to about 1,500 mg/L broth /hr, about 1,000 to about 3,000 mg/L broth /hr, or about 1,500 to about 3,000 mg/L broth /hr.
- the cells in culture have a peak volumetric productivity of isoprene at greater than or about 0.5, 1.0, 10, 25, 50, 100, 150, 200, 250, 300, 400, 500, 600, 700, 800, 900, 1,000, 1100, 1200, 1300, 1,400, 1,500, 1,600, 1,700, 1,800, 1,900, 2,000, 2,100, 2,200, 2,300, 2,400, 2,500, 2,600, 2,700, 2,800, 2,900, 3,000, 3,100, 3,200, 3,300, 3,400, 3,500, 3,750, 4,000, 4,250, 4,500, 4,750, 5,000, 5,250, 5,500, 5,750, 6,000, 6,250, 6,500, 6,750, 7,000, 7,250, 7,500, 7,750, 8,000, 8,250, 8,500, 8,750, 9,000, 9,250, 9,500, 9,750, 10,000, 12,500, 15,000, or more mg of isoprene/L of broth/hr (mg/L broth /hr, wherein the volume
- the peak volumetric productivity of isoprene is between about 0.5 to about 15,000 mg/L broth /hr, such as between about 0.5 to about 10 mg/L broth /hr, about 1.0 to about 100 mg/L broth /hr, about 100 to about 500 mg/L broth /hr, about 500 to about 1,000 mg/L broth /hr, about 1,000 to about 1,500 mg/L broth /hr, about 1,500 to about 2,000 mg/L broth /hr, about 2,000 to about 2,500 mg/L broth /hr, about 2,500 to about 3,000 mg/L broth /hr, about 3,000 to about 3,500 mg/L broth /hr, about 3,500 to about 5,000 mg/L broth /hr, about 5,000 to about 7,500 mg/L broth /hr, about 7,500 to about 10,000 mg/L broth /hr, about 10,000 to about 12,500 mg/Lbroth/h, or about 12,500 to about 15,000 mg/Lbroth/hr.
- the peak volumetric productivity of isoprene is between about 10 to about 15,000 mg/L broth /hr, about 100 to about 2,500 mg/L broth /hr, about 1,000 to about 5,000 mg/L broth /hr, about 2,500 to about 7,500 mg/L broth /hr, about 5,000 to about 10,000 mg/L broth /hr, about 7,500 to about 12,500 mg/L broth /hr, or about 10,000 to about 15,000 mg/L broth /hr.
- the instantaneous isoprene production rate in mg/L broth /hr in a fermentor can be measured by taking a sample of the fermentor off-gas, analyzing it for the amount of isoprene (in units such as mg of isoprene per L gas ) as described, for example, in Example I, part II and multiplying this value by the rate at which off-gas is passed though each liter of broth (e.g., at 1 vvm (volume of air/volume of broth/minute) this is 60 L gas per hour).
- an off-gas level of 1 mg/Lgas corresponds to an instantaneous production rate of 60 mg/Lbroth/hr at air flow of 1 vvm.
- the value in the units mg/L broth /hr can be divided by the OD OOO value to obtain the specific rate in units of mg/Lb ro th/hr/OD.
- the average value of mg isoprene/L gas can be converted to the total product productivity (grams of isoprene per liter of fermentation broth, mg/Lbroth) by multiplying this average off-gas isoprene concentration by the total amount of off-gas sparged per liter of fermentation broth during the fermentation.
- an average off-gas isoprene concentration of 0.5 mg/L broth /hr over 10 hours at 1 vvm corresponds to a total product concentration of 300 mg isoprene/Lbroth-
- the cells in culture convert greater than or about 0.0015, 0.002, 0.005, 0.01, 0.02, 0.05, 0.1, 0.12, 0.14, 0.16, 0.2, 0.3, 0.4, 0.5, 0.6, 0.7, 0.8, 0.9, 1.0, 1.2, 1.4, 1.6, 1.8, 2.0, 2.2, 2.4, 2.6, 3.0, 4.0, 5.0, 6.0, 7.0, 8.0, 9.0, 10.0, 11.0, 12.0, 13.0, 14.0, 15.0, 16.0, 17.0, 18.0, 19.0, 20.0, 21.0, 22.0, 23.0, 23.2, 23.4, 23.6, 23.8, 24.0, 25.0, 30.0, 31.0, 32.0, 33.0, 35.0, 37.5, 40.0, 45.0, 47.5, 50.0, 55.0, 60.0, 65.0, 70.0, 75.0, 80.0, 85.0, or 90.0 molar % of the carbon in the cell culture medium into isoprene
- the percent conversion of carbon into isoprene is between about 0.002 to about 90.0 molar %, such as about 0.002 to about 0.005%, about 0.005 to about 0.01%, about 0.01 to about 0.05%, about 0.05 to about 0.15%, 0.15 to about 0.2%, about 0.2 to about 0.3%, about 0.3 to about 0.5%, about 0.5 to about 0.8%, about 0.8 to about 1.0%, about 1.0 to about 1.6%, about 1.6 to about 3.0%, about 3.0 to about 5.0%, about 5.0 to about 8.0%, about 8.0 to about 10.0%, about 10.0 to about 15.0%, about 15.0 to about 20.0%, about 20.0 to about 25.0%, about 25.0% to 30.0%, about 30.0% to 35.0%, about 35.0% to 40.0%, about 45.0% to 50.0%, about 50.0% to 55.0%, about 55.0% to 60.0%, about 60.0% to 65.0%, about 65.0% to 70.0%, about 75.0% to 80
- the percent conversion of carbon into isoprene is between about 0.002 to about 0.4 molar %, 0.002 to about 0.16 molar %, 0.04 to about 0.16 molar %, about 0.005 to about 0.3 molar %, about 0.01 to about 0.3 molar %, about 0.05 to about 0.3 molar %, about 0.1 to 0.3 molar %, about 0.3 to about 1.0 molar %, about 1.0 to about 5.0 molar %, about 2 to about 5.0 molar %, about 5.0 to about 10.0 molar %, about 7 to about 10.0 molar %, about 10.0 to about 20.0 molar %, about 12 to about 20.0 molar %, about 16 to about 20.0 molar %, about 18 to about 20.0 molar %, about 18 to 23.2 molar %, about 18 to 23.6 molar %, about 18 to about 23.8 molar %,
- the percent conversion of carbon into isoprene (also referred to as "% carbon yield") can be measured by dividing the moles carbon in the isoprene produced by the moles carbon in the carbon source (such as the moles of carbon in batched and fed glucose and yeast extract). This number is multiplied by 100% to give a percentage value (as indicated in Equation 1).
- yeast extract can be assumed to contain 50% w/w carbon.
- the percent conversion of carbon into isoprene can be calculated as shown in Equation 2. Equation 2
- Example 7 For the two 500 liter fermentations described herein (Example 7, parts VII and VIII), the percent conversion of carbon into isoprene was between 0.04-0.06%. A 0.11-0.16% carbon yield has been achieved using 14 liter systems as described herein. Example 11, part V describes the 1.53% conversion of carbon to isoprene using the methods described herein. [0476] One skilled in the art can readily convert the rates of isoprene production or amount of isoprene produced into any other units. Exemplary equations are listed below for interconverting between units.
- Equation 10 can be used to convert any of the units that include the wet weight of the cells into the corresponding units that include the dry weight of the cells.
- Dry weight of cells (wet weight of cells)/3.3
- Equation 11 can be used to convert between units of ppm and ⁇ g/L.
- ppm means parts per million defined in terms of ⁇ g/g (w/w).
- Concentrations of gases can also be expressed on a volumetric basis using "ppmv" (parts per million by volume), defined in terms of uL/L (vol/vol).
- Conversion of ⁇ g/L to ppm can be performed by determining the mass per L of off-gas (i.e., the density of the gas). For example, a liter of air at standard temperature and pressure (STP; 101.3 kPa (1 bar) and 273.15K).
- ppm ( ⁇ g/g) has a density of approximately 1.29 g/L.
- a concentration of 1 ppm ( ⁇ g/g) equals 1.29 ⁇ g/L at STP (equation 11).
- the conversion of ppm ( ⁇ g/g) to ⁇ g/L is a function of both pressure, temperature, and overall composition of the off-gas.
- 1 ppm ( ⁇ g/g) equals 1.29 ⁇ g/L at standard temperature and pressure (STP; 101.3 kPa (1 bar) and 273.15K).
- PV nRT, where "P” is pressure, “V” is volume, “n” is moles of gas, “R” is the Universal gas constant, and “T” is temperature in Kelvin.
- the amount of impurities in isoprene compositions are typically measured herein on a weight per volume (w/v) basis in units such as ⁇ g/L. If desired, measurements in units of ⁇ g/L can be converted to units of mg/m 3 using equation 13.
- a cell comprising a heterologous nucleic acid encoding an isoprene synthase polypeptide produces an amount of isoprene that is at least or about 2-fold, 3-fold, 5-fold, 10-fold, 25-fold, 50-fold, 100-fold, 150-fold, 200-fold, 400-fold, or greater than the amount of isoprene produced from a corresponding cell grown under essentially the same conditions without the heterologous nucleic acid encoding the isoprene synthase polypeptide.
- a cell comprising a heterologous nucleic acid encoding an isoprene synthase polypeptide and one or more heterologous nucleic acids encoding a DXS, IDI, and/or MVA pathway polypeptide produces an amount of isoprene that is at least or about 2-fold, 3-fold, 5-fold, 10-fold, 25-fold, 50-fold, 100-fold, 150-fold, 200-fold, 400-fold, or greater than the amount of isoprene produced from a corresponding cell grown under essentially the same conditions without the heterologous nucleic acids.
- the isoprene composition comprises greater than or about 99.90, 99.92, 99.94, 99.96, 99.98, or 100% isoprene by weight compared to the total weight of all C5 hydrocarbons in the composition.
- the composition has a relative detector response of greater than or about 99.90, 99.91, 99.92, 99.93, 99.94, 99.95, 99.96, 99.97, 99.98, 99.99, or 100% for isoprene compared to the detector response for all C5 hydrocarbons in the composition.
- the isoprene composition comprises between about 99.90 to about 99.92, about 99.92 to about 99.94, about 99.94 to about 99.96, about 99.96 to about 99.98, about 99.98 to 100% isoprene by weight compared to the total weight of all C5 hydrocarbons in the composition.
- the isoprene composition comprises less than or about 0.12, 0.10, 0.08, 0.06, 0.04, 0.02, 0.01, 0.005, 0.001, 0.0005, 0.0001, 0.00005, or 0.00001% C5 hydrocarbons other than isoprene (such as 1,3-cyclopentadiene, cis-l,3-pentadiene, trans-1,3- pentadiene, 1 ,4-pentadiene, 1-pentyne, 2-pentyne, 1-pentene, 2-methyl-l-butene, 3-methyl-l- butyne, pent-4-ene-l-yne, trans-pent-3-ene-l-yne, or cis-pent-3-ene-l-yne) by weight compared to the total weight of all C5 hydrocarbons in the composition.
- C5 hydrocarbons other than isoprene such as 1,3-cyclopentadiene, cis-l,3-pent
- the composition has a relative detector response of less than or about 0.12, 0.10, 0.08, 0.06, 0.04, 0.02, 0.01, 0.005, 0.001, 0.0005, 0.0001, 0.00005, or 0.00001% for C5 hydrocarbons other than isoprene compared to the detector response for all C5 hydrocarbons in the composition.
- the composition has a relative detector response of less than or about 0.12, 0.10, 0.08, 0.06, 0.04, 0.02, 0.01, 0.005, 0.001, 0.0005, 0.0001, 0.00005, or 0.00001% for 1,3- cyclopentadiene, cis-l,3-pentadiene, trans- 1,3-pentadiene, 1 ,4-pentadiene, 1-pentyne, 2-pentyne, 1-pentene, 2-methyl-l-butene, 3-methyl-l-butyne, pent-4-ene-l-yne, trans-pent-3-ene-l-yne, or cis-pent-3-ene-l-yne compared to the detector response for all C5 hydrocarbons in the composition.
- the isoprene composition comprises between about 0.02 to about 0.04%, about 0.04 to about 0.06%, about 0.06 to 0.08%, about 0.08 to 0.10%, or about 0.10 to about 0.12% C5 hydrocarbons other than isoprene (such as 1,3-cyclopentadiene, cis-1,3- pentadiene, trans- 1,3-pentadiene, 1 ,4-pentadiene, 1-pentyne, 2-pentyne, 1-pentene, 2-methyl-l- butene, 3-methyl-l-butyne, pent-4-ene-l-yne, trans-pent-3-ene-l-yne, or cis-pent-3-ene-l-yne) by weight compared to the total weight of all C5 hydrocarbons in the composition.
- C5 hydrocarbons other than isoprene such as 1,3-cyclopentadiene, cis-1,3- pentadiene, trans
- the isoprene composition comprises less than or about 50, 40, 30, 20, 10, 5, 1, 0.5, 0.1, 0.05, 0.01, or 0.005 ⁇ g/L of a compound that inhibits the polymerization of isoprene for any compound in the composition that inhibits the polymerization of isoprene.
- the isoprene composition comprises between about 0.005 to about 50, such as about 0.01 to about 10, about 0.01 to about 5, about 0.01 to about 1, about 0.01 to about 0.5, or about 0.01 to about 0.005 ⁇ g/L of a compound that inhibits the polymerization of isoprene for any compound in the composition that inhibits the polymerization of isoprene.
- the isoprene composition comprises less than or about 50, 40, 30, 20, 10, 5, 1, 0.5, 0.1, 0.05, 0.01, or 0.005 ⁇ g/L of a hydrocarbon other than isoprene (such as 1,3- cyclopentadiene, cis-l,3-pentadiene, trans- 1,3-pentadiene, 1 ,4-pentadiene, 1-pentyne, 2-pentyne, 1-pentene, 2-methyl-l-butene, 3-methyl-l-butyne, pent-4-ene-l-yne, trans-pent-3-ene-l-yne, or cis-pent-3-ene-l-yne).
- a hydrocarbon other than isoprene such as 1,3- cyclopentadiene, cis-l,3-pentadiene, trans- 1,3-pentadiene, 1 ,4-pentadiene, 1-penty
- the isoprene composition comprises between about 0.005 to about 50, such as about 0.01 to about 10, about 0.01 to about 5, about 0.01 to about 1, about 0.01 to about 0.5, or about 0.01 to about 0.005 ⁇ g/L of a hydrocarbon other than isoprene. In some embodiments, the isoprene composition comprises less than or about 50, 40, 30, 20, 10, 5, 1, 0.5, 0.1, 0.05, 0.01, or 0.005 ⁇ g/L of a protein or fatty acid (such as a protein or fatty acid that is naturally associated with natural rubber).
- a protein or fatty acid such as a protein or fatty acid that is naturally associated with natural rubber.
- the isoprene composition comprises less than or about 10, 5, 1, 0.8, 0.5, 0.1, 0.05, 0.01, or 0.005 ppm of alpha acetylenes, piperylenes, acetonitrile, or 1,3- cyclopentadiene. In some embodiments, the isoprene composition comprises less than or about 5, 1, 0.5, 0.1, 0.05, 0.01, or 0.005 ppm of sulfur or allenes.
- the isoprene composition comprises less than or about 30, 20, 15, 10, 5, 1, 0.5, 0.1, 0.05, 0.01, or 0.005 ppm of all acetylenes (such as 1-pentyne, 2-pentyne, 3-methyl-l-butyne, pent-4-ene-l-yne, trans-pmt- 3-ene-l-yne, and czs-pent-3-ene-l-yne).
- all acetylenes such as 1-pentyne, 2-pentyne, 3-methyl-l-butyne, pent-4-ene-l-yne, trans-pmt- 3-ene-l-yne, and czs-pent-3-ene-l-yne.
- the isoprene composition comprises less than or about 2000, 1000, 500, 200, 100, 50, 40, 30, 20, 10, 5, 1, 0.5, 0.1, 0.05, 0.01, or 0.005 ppm of isoprene dimers, such as cyclic isoprene dimmers (e.g., cyclic ClO compounds derived from the dimerization of two isoprene units).
- isoprene dimers such as cyclic isoprene dimmers (e.g., cyclic ClO compounds derived from the dimerization of two isoprene units).
- the isoprene composition includes ethanol, acetone, a C5 prenyl alcohol (such as 3-methyl-3-buten-l-ol or 3-methyl-2-buten-l-ol), or any two or more of the foregoing.
- the isoprene composition comprises greater than or about 0.005, 0.01, 0.05, 0.1, 0.5, 1, 5, 10, 20, 30, 40, 60, 80, 100, or 120 ⁇ g/L of ethanol, acetone, a C5 prenyl alcohol (such as 3-methyl-3-buten-l-ol or 3-methyl-2-buten-l-ol), or any two or more of the foregoing.
- the isoprene composition comprises between about 0.005 to about 120, such as about 0.01 to about 80, about 0.01 to about 60, about 0.01 to about 40, about 0.01 to about 30, about 0.01 to about 20, about 0.01 to about 10, about 0.1 to about 80, about 0.1 to about 60, about 0.1 to about 40, about 5 to about 80, about 5 to about 60, or about 5 to about 40 ⁇ g/L of ethanol, acetone, a C5 prenyl alcohol, or any two or more of the foregoing.
- the isoprene composition includes one or more of the following components: 2-heptanone, 6-methyl-5-hepten-2-one, 2,4,5-trimethylpyridine, 2,3,5- trimethylpyrazine, citronellal, acetaldehyde, methanethiol, methyl acetate, 1-propanol, diacetyl, 2-butanone, 2-methyl-3-buten-2-ol, ethyl acetate, 2-methyl- 1-propanol, 3-methyl-l-butanal, 3- methyl-2-butanone, 1-butanol, 2-pentanone, 3-methyl-l-butanol, ethyl isobutyrate, 3-methyl-2- butenal, butyl acetate, 3-methylbutyl acetate, 3-methyl-3-buten-l-yl acetate, 3-methyl-2-buten-l-yl acetate, 3-hexen-l-ol, 3-hexen-l-ol, 3-hexen
- the amount of one of these components relative to amount of isoprene in units of percentage by weight is greater than or about 0.01, 0.02, 0.05, 0.1, 0.5, 1, 5, 10, 20, 30, 40, 50, 60, 70, 80, 90, 100, or 110% (w/w).
- the relative detector response for the second compound compared to the detector response for isoprene is greater than or about 0.01, 0.02, 0.05, 0.1, 0.5, 1, 5, 10, 20, 30, 40, 50, 60, 70, 80, 90, 100, or 110%.
- the amount of one of these components relative to amount of isoprene in units of percentage by weight is between about 0.01 to about 105 % (w/w), such as about 0.01 to about 90, about 0.01 to about 80, about 0.01 to about 50, about 0.01 to about 20, about 0.01 to about 10, about 0.02 to about 50, about 0.05 to about 50, about 0.1 to about 50, or 0.1 to about 20% (w/w).
- the isoprene composition includes one or more of the following: an alcohol, an aldehyde, or a ketone (such as any of the alcohols, aldehydes, or ketones described herein).
- the isoprene composition includes (i) an alcohol and an aldehyde, (ii) an alcohol and a ketone, (iii) an aldehyde and a ketone, or (iv) an alcohol, an aldehyde, and a ketone.
- the isoprene composition contains one or more of the following: methanol, acetaldehyde, ethanol, methanethiol, 1-butanol, 3 -methyl- 1-propanol, acetone, acetic acid, 2-butanone, 2-methyl- 1-butanol, or indole. In some embodiments, the isoprene composition contains 1 ppm or more of one or more of the following: methanol, acetaldehyde, ethanol, methanethiol, 1-butanol, 3 -methyl- 1-propanol, acetone, acetic acid, 2-butanone, 2- methyl- 1-butanol, or indole.
- the concentration of more of one or more of the following: methanol, acetaldehyde, ethanol, methanethiol, 1-butanol, 3 -methyl- 1-propanol, acetone, acetic acid, 2-butanone, 2-methyl- 1-butanol, or indole is between about 1 to about 10,000 ppm in an isoprene composition (such as off-gas before it is purified).
- the isoprene composition (such as off-gas after it has undergone one or more purification steps) includes one or more of the following: methanol, acetaldehyde, ethanol, methanethiol, 1-butanol, 3 -methyl- 1-propanol, acetone, acetic acid, 2-butanone, 2-methyl- 1- butanol, or indole, at a concentration between about 1 to about 100 ppm, such as about 1 to about 10 ppm, about 10 to about 20 ppm, about 20 to about 30 ppm, about 30 to about 40 ppm, about 40 to about 50 ppm, about 50 to about 60 ppm, about 60 to about 70 ppm, about 70 to about 80 ppm, about 80 to about 90 ppm, or about 90 to about 100 ppm.
- Volatile organic compounds from cell cultures can be analyzed using standard methods such as those described herein or other standard methods such as proton transfer reaction-mass spectrometry (see, for example, Bunge et ah, Applied and Environmental Microbiology, 74(7):2179-2186, 2008 which is hereby incorporated by reference in its entirety, particular with respect to the analysis of volatile organic compounds).
- the composition comprises greater than about 2 mg of isoprene, such as greater than or about 5, 10, 20, 30, 40, 50, 60, 70, 80, 90, 100, 200, 300, 400, 500, 600, 700, 800, 900, or 1000 mg of isoprene. In some embodiments, the composition comprises greater than or about 2, 5, 10, 20, 30, 40, 50, 60, 70, 80, 90, 100 g of isoprene. In some embodiments, the amount of isoprene in the composition is between about 2 to about 5,000 mg, such as between about 2 to about 100 mg, about 100 to about 500 mg, about 500 to about 1,000 mg, about 1,000 to about 2,000 mg, or about 2,000 to about 5,000 mg.
- the amount of isoprene in the composition is between about 20 to about 5,000 mg, about 100 to about 5,000 mg, about 200 to about 2,000 mg, about 200 to about 1,000 mg, about 300 to about 1,000 mg, or about 400 to about 1,000 mg. In some embodiments, greater than or about 20, 25, 30, 40, 50, 60, 70, 80, 90, or 95% by weight of the volatile organic fraction of the composition is isoprene.
- the composition includes ethanol.
- the composition includes between about 75 to about 90% by weight of ethanol, such as between about 75 to about 80%, about 80 to about 85%, or about 85 to about 90% by weight of ethanol.
- the composition also includes between about 4 to about 15% by weight of isoprene, such as between about 4 to about 8%, about 8 to about 12%, or about 12 to about 15% by weight of isoprene.
- a cell comprising one or more heterologous nucleic acids encoding an isoprene synthase polypeptide, DXS polypeptide, IDI polypeptide, and/or MVA pathway polypeptide produces an amount of an isoprenoid compound (such as a compound with 10 or more carbon atoms that is formed from the reaction of one or more IPP molecules with one or more DMAPP molecules) that is greater than or about 2-fold, 3 -fold, 5- fold, 10-fold, 25-fold, 50-fold, 100-fold, 150-fold, 200-fold, 400-fold, or greater than the amount of the isoprenoid compound produced from a corresponding cell grown under essentially the same conditions without the one or more heterologous nucleic acids.
- an isoprenoid compound such as a compound with 10 or more carbon atoms that is formed from the reaction of one or more IPP molecules with one or more DMAPP molecules
- a cell comprising one or more heterologous nucleic acids encoding an isoprene synthase polypeptide, DXS polypeptide, IDI polypeptide, and/or MVA pathway polypeptide produces an amount of a C5 prenyl alcohol (such as 3-methyl-3-buten-l-ol or 3-methyl-2-buten- l-ol) that is greater than or about 2-fold, 3-fold, 5-fold, 10-fold, 25-fold, 50-fold, 100-fold, 150- fold, 200-fold, 400-fold, or greater than the amount of the C5 prenyl alcohol produced from a corresponding cell grown under essentially the same conditions without the one or more heterologous nucleic acids.
- a C5 prenyl alcohol such as 3-methyl-3-buten-l-ol or 3-methyl-2-buten- l-ol
- any of the isoprene-producing cells described herein that comprise one or more heterologous nucleic acids encoding an isoprene synthase polypeptide, a DXS polypeptide, an IDI polypeptide, and/or an MVA pathway polypeptide operably linked to a promoter further comprise a heterologous nucleic acid also operably linked to a promoter encoding one or more hydrogenase polypeptides or one or more polypeptides involved in the regulation or expression of hydrogenase polypeptides ⁇ e.g., hydrogenase maturation proteins or transcription factors).
- any of the isoprene-producing cells described herein that comprise one or more heterologous nucleic acids encoding an isoprene synthase polypeptide, a DXS polypeptide, an IDI polypeptide, an MVA pathway polypeptide, one or more hydrogenase polypeptides or one or more polypeptides involved in the regulation or expression of hydrogenase polypeptides operably linked to a promoter further comprise a mutation or deletion inactivating one or more polypeptides involved in the production of fermentation side products, one or more polypeptides involved in the regulation or expression of genes for the production of fermentation side products, or one or more polypeptides involved in hydrogen reuptake.
- the cells can co-produce isoprene and hydrogen.
- the cells are bacterial cells, such as gram-positive bacterial cells ⁇ e.g., Bacillus cells such as Bacillus subtilis cells or Streptomyces cells such as Streptomyces lividans, Streptomyces coelicolor, or Streptomyces griseus cells).
- the cells are gram- negative bacterial cells ⁇ e.g., Escherichia cells such as Escherichia coli cells, Rhodopseudomonas sp.
- Rhodopseudomonas palustris cells such as Rhodopseudomonas palustris cells, Pseudomonas sp. such as Pseudomonas fluorescens cells or Pseudomonas putida cells, or Pantoea cells such as Pantoea citrea cells).
- the cells are fungal, cells such as filamentous fungal cells ⁇ e.g., Trichoderma cells such as Trichoderma reesei cells or Aspergillus cells such as Aspergillus oryzae and Aspergillus niger) or yeast cells ⁇ e.g., Yarrowia cells such as Yarrowia lipolytica cells or Sacchraomyces cells such as Saccaromyces cerevisiae).
- filamentous fungal cells ⁇ e.g., Trichoderma cells such as Trichoderma reesei cells or Aspergillus cells such as Aspergillus oryzae and Aspergillus niger
- yeast cells ⁇ e.g., Yarrowia cells such as Yarrowia lipolytica cells or Sacchraomyces cells such as Saccaromyces cerevisiae).
- the isoprene synthase polypeptide is a polypeptide from a plant such as Pueraria ⁇ e.g., Pueraria montana or Pueraria lobata) or Populus ⁇ e.g., Populus tremuloides, Populus alba, Populus nigra, Populus trichocarpa, or the hybrid, Populus alba x Populus tremula).
- the cells further comprise a heterologous nucleic acid encoding an IDI polypeptide. In some embodiments of any of the aspects described herein, the cells further comprise an insertion of a copy of an endogenous nucleic acid encoding an IDI polypeptide. In some embodiments of any of the aspects described herein, the cells further comprise a heterologous nucleic acid encoding a DXS polypeptide. In some embodiments of any of the aspects described herein, the cells further comprise an insertion of a copy of an endogenous nucleic acid encoding a DXS polypeptide.
- the cells further comprise one or more nucleic acids encoding an IDI polypeptide and a DXS polypeptide.
- one nucleic acid encodes the isoprene synthase polypeptide, IDI polypeptide, and DXS polypeptide.
- one vector encodes the isoprene synthase polypeptide, IDI polypeptide, and DXS polypeptide.
- the vector comprises a selective marker or a selectable marker, such as an antibiotic resistance nucleic acid.
- the cells further comprise a heterologous nucleic acid encoding an MVA pathway polypeptide (such as an MVA pathway polypeptide from Saccharomyces cerevisia or Enterococcus faecalis). In some embodiments of any of the aspects described herein, the cells further comprise an insertion of a copy of an endogenous nucleic acid encoding an MVA pathway polypeptide (such as an MVA pathway polypeptide from Saccharomyces cerevisia ox Enterococcus faecalis). In some embodiments of any of the aspects described herein, the cells comprise an isoprene synthase, DXS, and MVA pathway nucleic acid. In some embodiments of any of the aspects described herein, the cells comprise an isoprene synthase nucleic acid, a DXS nucleic acid, an IDI nucleic acid, and a MVA pathway nucleic acid.
- an MVA pathway polypeptide such as an MVA pathway polypeptide from Sac
- the isoprene-producing cells described herein further comprise a heterologous nucleic acid encoding a hydrogenase polypeptide operably linked to a promoter.
- the hydrogenase polypeptide comprises E. coli hydrogenase- 1 (Hyd-1), E. coli hydrogenase-2 (Hyd-2), E. coli hydrogenase-3 (Hyd-3), E. coli hydrogenase-4 (Hyd-4), E. coli formate hydrogen lyase (FHL) complex, which produces hydrogen gas from formate and CO 2 under anaerobic conditions at acidic pH, Rhodococcus opacus MRIl hydrogenase (R.
- the isoprene-producing cells furthercomprising a heterologous nucleic acid encoding a hydrogenase polypeptide operably linked to a promoter further comprise E. coli hydrogenase-3 (Hyd-3), E. coli pyruvate formate lyase (pfl), and E. coli formate hydrogen lyase (FHL) complex.
- the hydrogenase polypeptide encodes a ferredoxin-dependent hydrogenase polypeptide.
- the ferredoxin-dependent hydrogenase polypeptide comprises Clostridium acetobutulicum hydrogenase A (HydA), which can be expressed in conjunction with one or more of: (1) Bacillus subtilis NADPH ferredoxin oxidoreductase (NFOR) or Clostridium kluyveri NADH ferredoxin oxidoreductase (RnfCDGEAB), Clostridium pasteuranium ferredoxin oxidoreductase (F dx); (2) glyceraldehyde-6-phosphate ferredoxin oxidoreductase (GAPOR); or (3) pyruvate ferredoxin oxidoreductase (POR).
- HydA Clostridium acetobutulicum hydrogenase A
- the ferredoxin-dependent hydrogenase polypeptide Clostridium acetobutulicum hydrogenase A is expressed with three HydA- associated maturation enzymes (HydE, HydG, and HydF), and further in conjunction with one or more of: (1) Bacillus subtilis NADPH ferredoxin oxidoreductase (NFOR) or Clostridium kluyveri NADH ferredoxin oxidoreductase (RnfCDGEAB), Clostridium pasteuranium ferredoxin oxidoreductase (Fdx); (2) glyceraldehyde-6-phosphate ferredoxin oxidoreductase (GAPOR); or (3) pyruvate ferredoxin oxidoreductase (POR).
- NFOR Bacillus subtilis NADPH ferredoxin oxidoreductase
- RnfCDGEAB Clostridium kluyveri NADH ferredoxin oxid
- the hydrogenase polypeptide encodes an NADPH-dependent hydrogenase polypeptide.
- the NADPH-dependent hydrogenase polypeptide comprises Pyrococcus furiosus hydrogenase.
- the hydrogenase polypeptide encodes an oxygen-tolerant hydrogenase.
- the oxygen-tolerant hydrogenase comprises Rubrivivax gelatinosus hydrogenase, and Ralstonia eutropha hydrogenase.
- the isoprene-producing cells described herein further comprise a mutation or deletion inactivating a gene involved in regulation of hydrogenase activity, such as iron-sulfur complex transcriptional regulator (iscR) (Kalim-Akhtar et al, "Deletion of iscR stimulates recombinant Clostridial Fe/Fe hydrogenase activity and ⁇ -accumulation in Escherichia coli BL21(DE3)," Appl. Microbiol. Biotechnol. 78:853-862 (2008), which is incorporated herein by reference in its entirety, particularly with reference to stimulation of Clostridial Fe/Fe hydrogenase activity and hydrogen accumulation in E. coli by deleting the iscR gene).
- iscR iron-sulfur complex transcriptional regulator
- the isoprene-producing cells described herein further comprise a mutation or deletion inactivating a gene encoding one or more cellular polypeptides involved in production of fermentation side products, such as lactate, acetate, pyruvate, ethanol, succinate, and glycerol.
- the inactivated polypeptides involved in production of fermentation side products comprise one or more polypeptides encoding formate dehydrogenase N, alpha subunit (fdnG), formate dehydrogenase O, large subunit (fdoG), nitrate reductase (narG), formate transporter A (focA), formate transporter B (focB), pyruvate oxidase (poxB), pyruvate dehydrogenase El component ackA/pta (aceE), alcohol dehydrogenase (adhE), fumarate reductase membrane protein (frdC), or lactate dehydrogenase (UhA).
- the isoprene-producing cells described herein further comprise a mutation or deletion inactivating a gene encoding one or more cellular polypeptides involved in the regulation or expression of genes involved in production of fermentation side products.
- the inactivated polypeptides involved in the regulation or expression of genes involved in production of fermentation side products comprise repressor of formate hydrogen lyase (hycA), fumarate reductase regulator (fnr), acetyl-coenzyme A synthetase (acs), and formate dehydrogenase regulatory protein (hycA).
- the isoprene-producing cells described herein further comprise a mutation or deletion inactivating a gene encoding one or more cellular polypeptides involved in hydrogen re-uptake.
- the inactivated polypeptides involved in hydrogen re-uptake comprise E. coli hydrogenase- 1 (Hyd-1) (hya operon) and E. coli hydrogenase-2 (Hy d- 2) (hyb operon).
- the heterologous isoprene synthase, DXS polypeptide, IDI polypeptide, MVA pathway, hydrogenase, hydrogenase maturation or transcription factor polypeptide or nucleic acid is operably linked to a T7 promoter, such as a T7 promoter contained in a medium or high copy plasmid.
- the heterologous isoprene synthase, DXS polypeptide, IDI polypeptide, MVA pathway, hydrogenase, hydrogenase maturation or transcription factor nucleic acid is operably linked to a Trc promoter, such as a Trc promoter contained in a medium or high copy plasmid.
- the heterologous isoprene synthase, DXS polypeptide, IDI polypeptide, MVA pathway, hydrogenase, hydrogenase maturation or transcription factor nucleic acid is operably linked to a Lac promoter, such as a Lac promoter contained in a low copy plasmid.
- the heterologous isoprene synthase, DXS polypeptide, IDI polypeptide, MVA pathway, hydrogenase, hydrogenase maturation or transcription factor nucleic acid is operably linked to an endogenous promoter, such as an endogenous alkaline serine protease promoter.
- an endogenous promoter such as an endogenous alkaline serine protease promoter.
- the heterologous isoprene synthase, DXS polypeptide, IDI polypeptide, MVA pathway, hydrogenase, hydrogenase maturation or transcription factor nucleic acid integrates into a chromosome of the cells without a selective marker or without a selectable marker.
- one or more MVA pathway, IDI, DXS, isoprene synthase, hydrogenase, hydrogenase maturation or transcription factor nucleic acids are placed under the control of a promoter or factor that is more active in stationary phase than in the growth phase.
- one or more MVA pathway, IDI, DXS, isoprene synthase, hydrogenase, hydrogenase maturation or transcription factor nucleic acids may be placed under control of a stationary phase sigma factor, such as RpoS.
- one or more MVA pathway, IDI, DXS, isoprene synthase, hydrogenase, hydrogenase maturation or transcription factor nucleic acids are placed under control of a promoter inducible in stationary phase, such as a promoter inducible by a response regulator active in stationary phase.
- At least a portion of the cells maintain the heterologous isoprene synthase, DXS polypeptide, IDI polypeptide, MVA pathway, hydrogenase, hydrogenase maturation or transcription factor nucleic acid for at least or about 5, 10, 20, 40, 50, 60, 65, or more cell divisions in a continuous culture (such as a continuous culture without dilution).
- the nucleic acid comprising the isoprene synthase, DXS polypeptide, IDI polypeptide, MVA pathway, hydrogenase, hydrogenase maturation or transcription factor nucleic acid also comprises a selective marker or a selectable marker, such as an antibiotic resistance nucleic acid.
- a selective marker or a selectable marker such as an antibiotic resistance nucleic acid.
- any of the cells described herein are grown in oxygen-limited culture and co-produce isoprene and hydrogen.
- the cells in oxygen- limited culture produce isoprene at a rate greater than about 400 nmole/g wcm /hr, and produce hydrogen at a rate greater than about 125 nmole/g wcm /hr.
- the cells in oxygen-limited culture produce isoprene at a rate between about 400 nmole/g wcm /hr to about 2.0 x lO 5 nmole/g wcm /hr and hydrogen at a rate between about 125 nmole/g wcm /hr to about 1.25 x 10 4 nmole/g wcm /hr.
- the cells in oxygen-limited culture produce isoprene at a rate between about 400 nmole/g wcm /hr and about 2.0 ⁇ 10 5 nmole/g wcm /hr, between about 500 nmole/g wcm /hr and about 1.5 x 10 5 nmole/g wcm /hr, between about 750 nmole/g wcm /hr and about 1 x 10 5 nmole/g wcm /hr, between about 1000 nmole/g wcm /hr and about 1 x 10 5 nmole/g wcm /hr, between about 2500 nmole/g wcm /hr and about 1 x 10 5 nmole/g wcm /hr, between about 5000 nmole/g wcm /hr and about 1 x 10 5 nmole/g wcm /hr,
- the cells in oxygen-limited culture produce greater than about 400, 500, 600, 700, 800, 900, 1,000, 1,250, 1,500, 1,750, 2,000, 2,500, 3,000, 4,000, 5,000, or more nmole/g wcm /hr isoprene.
- the cells in oxygen-limited culture produce hydrogen at a rate between about 125 nmole/g wcm /hr to about 1.25 x 10 4 nmole/g wcm /hr, between about 250 nmole/g wcm /hr to about 1.25 x 10 4 nmole/g wcm /hr, between about 500 nmole/g wcm /hr to about 1.25 x 10 4 nmole/g wcm /hr, between about 750 nmole/g wcm /hr to about 1.25 x 10 4 nmole/g wcm /hr, between about 1000 nmole/g wcm /hr to about 1.25 x 10 4 nmole/g wcm /hr, between about 1250 nmole/g wcm /hr to about 1.25 ⁇ 10 4 nmole/g wc
- the cells in oxygen-limited culture produce greater than about 125, 250, 500, 750, 1000, 1,250, 1,500, 1,750, 2,000, 2,500, 3,000, 4,000, 5,000, 7,500, 10,000, or more nmole/g wcm /hr hydrogen.
- any of the cells described herein are grown in oxygen-limited culture and co-produce isoprene and hydrogen.
- the cells in oxygen- limited culture have an average volumetric productivity of isoprene greater than about 0.1 mg/L broth /hr and an average volumetric productivity of hydrogen greater than about 0.005 mg/Lbroth/hr.
- the cells in oxygen-limited culture have a peak volumetric productivity of isoprene greater than about 1000 mg/L broth /hr and a peak volumetric productivity of hydrogen greater than about 5 mg/L broth /hr.
- the cells in oxygen-limited culture have a peak volumetric productivity of isoprene greater than about 3000 mg/L broth /hr and a peak volumetric productivity of hydrogen greater than about 5 mg/L broth /hr. In some embodiments, the cells in oxygen-limited culture have a peak volumetric productivity of isoprene greater than about 5000 mg/L broth /hr and a peak volumetric productivity of hydrogen greater than about 5 mg/L broth /hr.
- the cells in oxygen-limited culture have an average volumetric productivity of isoprene between about 0.1 mg/L broth /hr and about 5000 mg/L broth /hr, and an average volumetric productivity of hydrogen between about 0.005 mg/Lbroth/hr and about 5 mg/Lbroth/hr.
- the cells in oxygen-limited culture have an average volumetric productivity of isoprene between about 1 mg/L broth /hr and about 5000 mg/L broth /hr, between about 5 mg/L broth /hr and about 5000 mg/L broth /hr, between about 10 mg/L broth /hr and about 5000 mg/L broth /hr, between about 25 mg/L broth /hr and about 5000 mg/L broth /hr, between about 50 mg/L broth /hr and about 5000 mg/L broth /hr, between about 100 mg/L broth /hr and about 5000 mg/L broth /hr, between about 250 mg/L broth /hr and about 5000 mg/L broth /hr, between about 500 mg/L broth /hr and about 5000 mg/L broth /hr, between about 1000 mg/L broth /hr and about 5000 mg/L broth /hr, and between about 2500 mg/L broth /hr and about
- any of the cells described herein are grown in oxygen-limited culture and co-produce isoprene and hydrogen.
- the cells in oxygen- limited culture convert more than about 0.002 molar percent of the carbon that the cells consume from a cell culture medium into isoprene, and produce hydrogen equivalent to more than about 0.024 molar percent of the carbon that the cells consume from a cell culture medium.
- the cells in oxygen-limited culture convert more than about 0.002 molar percent of the carbon that the cells consume from a cell culture medium into isoprene, and produce hydrogen equivalent to more than about 400 molar percent of the carbon that the cells consumer from a cell culture medium.
- any of the cells described herein that co-produce isoprene and hydrogen are grown in oxygen-limited culture.
- the cells in oxygen- limited culture co-produce isoprene and hydrogen in a ratio ranging from at least one molar percent of isoprene for every three molar percent of hydrogen to at least one molar percent of isoprene for every four molar percent of hydrogen.
- the cells in oxygen- limited culture produce an off-gas containing from 1 to 11 molar percent isoprene and from 3 to 33 molar percent hydrogen.
- the cells produce from 1 to 11 molar percent isoprene and from 4 to 44 molar percent hydrogen.
- the cells in oxygen- limited culture also produce an off-gas containing oxygen, carbon dioxide, or nitrogen. In some embodiments, the cells in oxygen limited culture produce an off-gas containing from 0 to 21 molar percent oxygen, from 18 to 44 molar percent carbon dioxide, and from 0 to 78 molar percent nitrogen.
- cells in oxygen-limited culture that co-produce isoprene and hydrogen comprising a heterologous nucleic acid encoding an isoprene synthase polypeptide, wherein the cells: (i) produce isoprene at a rate greater than about 400 nmole/g wcm /hr and produce hydrogen at a rate greater than about 125 nmole/g wcm /hr; (ii) have an average volumetric productivity of isoprene greater than about 0.1 mg/L broth /hr and an average volumetric productivity of hydrogen greater than about 0.005 mg/L broth /hr; or (iii) convert more than about 0.002 molar percent of the carbon that the cells consume from a cell culture medium into isoprene, and produce hydrogen equivalent to more than about 0.024 molar percent of the carbon that the cells consume from a cell culture medium.
- the cells in oxygen-limited culture comprise a heterologous nucleic acid encoding an isoprene synthase polypeptide, wherein the heterologous nucleic acid is operably linked to a promoter, and wherein the cells produce greater than about 400 nmole/g wcm /hr of isoprene and greater than about 125 nmole/g wcm /hr of hydrogen.
- the cells in oxygen-limited culture comprise a heterologous nucleic acid encoding an isoprene synthase polypeptide, wherein the heterologous nucleic acid is operably linked to a promoter, and wherein the cells have an average volumetric productivity of isoprene greater than about 0.1 mg/L broth /hr and an average volumetric productivity of hydrogen greater than about 0.005 mg/Lbroth/hr.
- the cells in oxygen-limited culture comprise a heterologous nucleic acid encoding an isoprene synthase polypeptide, wherein the heterologous nucleic acid is operably linked to a promoter, and wherein the cells convert more than about 0.002 molar percent of the carbon that the cells consume from a cell culture medium into isoprene, and more than about 0.024 molar percent of the carbon that the cells consume from a cell culture medium into hydrogen.
- the isoprene synthase polypeptide is a plant isoprene synthase polypeptide.
- the cells in oxygen-limited culture comprising a heterologous nucleic acid encoding an isoprene synthase polypeptide produce isoprene at a rate between about 400 nmole/g wcm /hr and about 2.0 x 10 5 nmole/g wcm /hr, between about 500 nmole/g wcm /hr and about 1.5 x 10 5 nmole/g wcm /hr, between about 750 nmole/g wcm /hr and about 1 x 10 5 nmole/g wcm /hr, between about 1000 nmole/g wcm /hr and about 1 x 10 5 nmole/g wcm /hr, between about 2500 nmole/g wcm /hr and about 1 x 10 5 nmole/g wcm /hr, between about 5000
- methods of co-producing isoprene and hydrogen comprising: (a) culturing cells under conditions suitable for the co- production of isoprene and hydrogen; and (b) co-producing isoprene and hydrogen, wherein the cells produce greater than about 400 nmole/g wcm /hour of isoprene, and wherein the cells produce greater than about 125 nmole/g wcm /hr of hydrogen.
- the cells in oxygen-limited culture comprising a heterologous nucleic acid encoding an isoprene synthase polypeptide produce isoprene at a rate between about 400 nmole/g wcm /hr and about 2.O x 10 5 nmole/g wcm /hr, between about 500 nmole/g wcm /hr and about 1.5 x 10 5 nmole/g wcm /hr, between about 750 nmole/g wcm /hr and about 1 x 10 5 nmole/g wcm /hr, between about 1000 nmole/g wcm /hr and about 1 x 10 5 nmole/g wcm /hr, between about 2500 nmole/g wcm /hr and about 1 x 10 5 nmole/g wcm /hr, between about 5000
- methods of co-producing isoprene and hydrogen comprising: (a) culturing cells under conditions suitable for the co- production of isoprene and hydrogen; and (b) co-producing isoprene and hydrogen, wherein the cells have an average volumetric productivity of isoprene greater than about 0.1 mg/L broth /hr and an average volumetric productivity of hydrogen greater than about 0.005 mg/L broth /hr.
- methods of co-producing isoprene and hydrogen comprising: (a) culturing cells under conditions suitable for the co- production of isoprene and hydrogen; and (b) co-producing isoprene and hydrogen, wherein the cells convert more than about 0.002 molar percent of the carbon that the cells consume from a cell culture medium into isoprene, and produce hydrogen equivalent to more than about 0.024 molar percent of the carbon that the cells consume from a cell culture medium.
- compositions comprising isoprene and hydrogen in a ratio ranging from at least one molar percent of isoprene for every three molar percent of hydrogen to at least one molar percent of isoprene for every four molar percent of hydrogen, and 0.1 molar percent or less of volatile impurities.
- the compositions further comprise from 1 to 11 molar percent isoprene and from 4 to 44 molar percent hydrogen.
- the compositions further comprise oxygen, carbon dioxide, or nitrogen.
- the compositions further comprise from 0 to 21 molar percent oxygen, from 18 to 44 molar percent carbon dioxide, and from 0 to 78 molar percent nitrogen.
- the composition further comprises 1.0 ⁇ 10 "4 molar percent or less of non-methane volatile impurities.
- the non-methane volatile impurities comprise one or more of the following: 2-heptanone, 6-methyl-5-hepten-2- one, 2,4,5-trimethylpyridine, 2,3,5-trimethylpyrazine, citronellal, acetaldehyde, methanethiol, methyl acetate, 1-propanol, diacetyl, 2-butanone, 2-methyl-3-buten-2-ol, ethyl acetate, 2-methyl- 1-propanol, 3 -methyl- 1-butanal, 3-methyl-2-butanone, 1-butanol, 2-pentanone, 3-methyl-l- butanol, ethyl isobutyrate, 3-methyl-2-butenal, butyl acetate, 3-methylbutyl acetate, 3-methyl-3- buten-1-yl acetate, 3-
- the non-methane volatile impurities comprise one or more of the following: the isoprene composition includes one or more of the following: an alcohol, an aldehyde, or a ketone (such as any of the alcohols, aldehydes, or ketones described herein).
- the isoprene composition includes (i) an alcohol and an aldehyde, (ii) an alcohol and a ketone, (iii) an aldehyde and a ketone, or (iv) an alcohol, an aldehyde, and a ketone.
- the non-methane volatile impurities comprise one or more of the following: methanol, acetaldehyde, ethanol, methanethiol, 1-butanol, 3-methyl-l-propanol, acetone, acetic acid, 2-butanone, 2-methyl-l- butanol, or indole.
- Also provided herein are methods of co-producing isoprene and hydrogen comprising: a) culturing cells under conditions suitable for the co-production of isoprene and hydrogen; and b) co-producing isoprene and hydrogen, wherein the peak concentration of the isoprene produced by the cells in oxygen-limited culture is greater than about 10 ng/Lb ro th and the hydrogen evolution rate of the cells is greater than about 0.0025 mmol/L broth /hour.
- the hydrogen evolution rate is between about any of 0.0025 mmol/L broth /hr and about 10 mmol/L broth /hr, between about 0.0025 mmol/L broth /hr and about 5 mmol/L broth /hr, between about 0.0025 mmol/L broth /hr and about 2.5 mmol/L broth /hr, between about 0.0025 mmol/L broth /hr and about 1 mmol/L broth /hr, between about 0.0025 mmol/L broth /hr and about 0.5 mmol/L broth /hr, between about 0.0025 mmol/L broth /hr and about 0.25 mmol/L broth /hr, between about 0.0025 mmol/L broth /hr and about 0.025 mmol/L broth /hr, between about 0.025 mmol/L broth /hr and about 0.5 mmol/L broth /hr, between about 0.0025 mmol/L
- co-producing isoprene and hydrogen comprising a) culturing cells under conditions suitable for the co-production of isoprene and hydrogen; and b) co-producing isoprene and hydrogen, wherein the liquid phase concentration of isoprene is less than about 200 mg/L, the cells produce greater than about 400 nmole/g wcm /hour of isoprene, and the hydrogen evolution rate of the cells is greater than about 0.0025 mmol/L/hour.
- the liquid phase concentration of isoprene in the culture is less than about any of 175 mg/L, 150 mg/L, 125 mg/L, 100 mg/L, 75 mg/L, 50 mg/L, 25 mg/L, 20 mg/L, 15 mg/L, 10 mg/L, 5 mg/L, or 2.5 mg/L. In some embodiments, the liquid phase concentration of isoprene in culture is between about any of 0.1 mg/L to 200 mg/L, 1 mg/L to 200 mg/L, 1 mg/L to 150 mg/L, 1 mg/L to 100 mg/L, 1 mg/L to 50 mg/L, 1 mg/L to 25 mg/L, 1 mg/L to 20 mg/L, or 10 mg/L to 20 mg/L.
- the hydrogen evolution rate is between about any of 0.0025 mmol/L broth /hr and about 10 mmol/L broth /hr, between about 0.0025 mmol/L broth /hr and about 5 mmol/L broth /hr, between about 0.0025 mmol/L broth /hr and about 2.5 mmol/L broth /hr, between about 0.0025 mmol/L broth /hr and about 1 mmol/L broth /hr, between about 0.0025 mmol/L broth /hr and about 0.5 mmol/L broth /hr, between about 0.0025 mmol/L broth /hr and about 0.25 mmol/L broth /hr, between about 0.0025 mmol/L broth /hr and about 0.025 mmol/L broth /hr, between about 0.025 mmol/L broth /hr and about 0.5 mmol/L broth /hr, between about 0.0025 mmol/L
- the oxygen-limited culture is anaerobic.
- the cells in oxygen-limited culture produce greater than about 400 nmole/g wcm /hr of isoprene and greater than about 125 nmole/g wcm /hr of hydrogen.
- the cells have a heterologous nucleic acid that (i) encodes an isoprene synthase polypeptide and (ii) is operably linked to a promoter.
- the cells are cultured in a culture medium that includes a carbon source, such as, but not limited to, a carbohydrate, glycerol, glycerine, dihydroxyacetone, one-carbon source, oil, animal fat, animal oil, fatty acid, lipid, phospholipid, glycerolipid, monoglyceride, diglyceride, triglyceride, renewable carbon source, polypeptide (e.g., a microbial or plant protein or peptide), yeast extract, component from a yeast extract, or any combination of two or more of the foregoing.
- the cells are cultured under limited glucose conditions.
- cells in oxygen-limited culture that convert more than about 0.002% of the carbon in a cell culture medium into isoprene and produce hydrogen equivalent to more than about 0.024 molar percent of the carbon in a cell culture medium.
- the oxygen-limited culture is anaerobic.
- the cells have a heterologous nucleic acid that (i) encodes an isoprene synthase polypeptide and (ii) is operably linked to a promoter.
- the cells are cultured in a culture medium that includes a carbon source, such as, but not limited to, a carbohydrate, glycerol, glycerine, dihydroxyacetone, one-carbon source, oil, animal fat, animal oil, fatty acid, lipid, phospholipid, glycerolipid, monoglyceride, diglyceride, triglyceride, renewable carbon source, polypeptide (e.g., a microbial or plant protein or peptide), yeast extract, component from a yeast extract, or any combination of two or more of the foregoing.
- the cells are cultured under limited glucose conditions.
- cells in oxygen-limited culture that comprise a heterologous nucleic acid encoding an isoprene synthase polypeptide.
- the oxygen-limited culture is anaerobic.
- the cells have a heterologous nucleic acid that (i) encodes an isoprene synthase polypeptide and (ii) is operably linked to a promoter.
- the cells are cultured in a culture medium that includes a carbon source, such as, but not limited to, a carbohydrate, glycerol, glycerine, dihydroxyacetone, one-carbon source, oil, animal fat, animal oil, fatty acid, lipid, phospholipid, glycerolipid, monoglyceride, diglyceride, triglyceride, renewable carbon source, polypeptide (e.g., a microbial or plant protein or peptide), yeast extract, component from a yeast extract, or any combination of two or more of the foregoing.
- the cells are cultured under limited glucose conditions.
- the method involves culturing cells under oxygen-limited conditions sufficient to produce greater than about 400 nmole/g wcm /hr of isoprene and greater than about 125 nmole/g wcm /hr of hydrogen.
- the oxygen-limited culture is anaerobic.
- the method also includes recovering the isoprene and hydrogen produced by the cells.
- the method further includes purifying the isoprene and the hydrogen produced by the cells.
- the method includes polymerizing the isoprene.
- the cells have a heterologous nucleic acid that (i) encodes an isoprene synthase polypeptide and (ii) is operably linked to a promoter.
- the cells are cultured in a culture medium that includes a carbon source, such as, but not limited to, a carbohydrate, glycerol, glycerine, dihydroxyacetone, one-carbon source, oil, animal fat, animal oil, fatty acid, lipid, phospholipid, glycerolipid, monoglyceride, diglyceride, triglyceride, renewable carbon source, polypeptide (e.g., a microbial or plant protein or peptide), yeast extract, component from a yeast extract, or any combination of two or more of the foregoing.
- the cells are cultured under limited glucose conditions.
- the amount of isoprene produced (such as the total amount of isoprene produced or the amount of isoprene produced per liter of broth per hour per OD OOO ) during stationary phase is greater than or about 2 or more times the amount of isoprene produced during the growth phase for the same length of time.
- the method includes culturing cells under oxygen-limited conditions sufficient to convert more than about 0.002% of the carbon (mol/mol) in a cell culture medium into isoprene and to produce hydrogen equivalent to more than about 0.024 molar percent of the carbon in a cell culture medium.
- the oxygen-limited culture is anaerobic.
- the method also includes recovering isoprene and hydrogen produced by the cells.
- the method further includes purifying isoprene and hydrogen produced by the cells.
- the method includes polymerizing the isoprene.
- the cells have a heterologous nucleic acid that (i) encodes an isoprene synthase polypeptide and (ii) is operably linked to a promoter.
- the cells are cultured in a culture medium that includes a carbon source, such as, but not limited to, a carbohydrate, glycerol, glycerine, dihydroxyacetone, one-carbon source, oil, animal fat, animal oil, fatty acid, lipid, phospholipid, glycerolipid, monoglyceride, diglyceride, triglyceride, renewable carbon source, polypeptide (e.g., a microbial or plant protein or peptide), yeast extract, component from a yeast extract, or any combination of two or more of the foregoing.
- a carbon source such as, but not limited to, a carbohydrate, glycerol, glycerine, dihydroxyacetone, one-carbon source, oil, animal fat, animal oil, fatty acid, lipid, phospholipid
- the microbial polypeptide carbon source includes one or more polypeptides from yeast or bacteria.
- the plant polypeptide carbon source includes one or more polypeptides from soy, corn, canola, jatropha, palm, peanut, sunflower, coconut, mustard, rapeseed, cottonseed, palm kernel, olive, safflower, sesame, or linseed.
- isoprene and hydrogen are only co-produced in stationary phase. In some embodiments, isoprene and hydrogen are co-produced in both the growth phase and stationary phase.
- the amount of isoprene produced (such as the total amount of isoprene produced or the amount of isoprene produced per liter of broth per hour per OD OOO ) during stationary phase is greater than or about 2, 3, 4, 5, 10, 20, 30, 40, 50, or more times the amount of isoprene produced during the growth phase for the same length of time.
- the amount of hydrogen produced (such as the total amount of hydrogen produced or the amount of hydrogen produced per liter of broth per hour per OD OOO ) during stationary phase is greater than or about 2, 3, 4, 5, 10, 20, 30, 40, 50, or more times the amount of hydrogen produced during the growth phase for the same length of time.
- compositions provided herein comprise hydrogen and greater than or about 99.90, 99.92, 99.94, 99.96, 99.98, or 100% isoprene by weight compared to the total weight of all C5 hydrocarbons in the composition.
- the composition comprises less than or about 0.12, 0.10, 0.08, 0.06, 0.04, 0.02, 0.01, 0.005, 0.001, 0.0005, 0.0001, 0.00005, or 0.00001% C5 hydrocarbons other than isoprene (such as 1,3- cyclopentadiene, cis-l,3-pentadiene, trans- 1,3-pentadiene, 1 ,4-pentadiene, 1-pentyne, 2-pentyne, 1-pentene, 2-methyl-l-butene, 3-methyl-l-butyne, pent-4-ene-l-yne, trans-pent-3-ene-l-yne, or cis-pent-3-ene-l-yne) by weight compared to the total weight of all C5 hydrocarbons in the composition.
- isoprene such as 1,3- cyclopentadiene, cis-l,3-pentadiene, trans- 1,3-p
- the composition has less than or about 0.12, 0.10, 0.08, 0.06, 0.04, 0.02, 0.01, 0.005, 0.001, 0.0005, 0.0001, 0.00005, or 0.00001% for 1,3- cyclopentadiene, cis-l,3-pentadiene, trans- 1,3-pentadiene, 1 ,4-pentadiene, 1-pentyne, 2-pentyne, 1-pentene, 2-methyl-l-butene, 3-methyl-l-butyne, pent-4-ene-l-yne, trans-pent-3-ene-l-yne, or cis-pent-3-ene-l-yne by weight compared to the total weight of all C5 hydrocarbons in the composition.
- the composition has greater than about 2 mg of isoprene and has greater than or about 99.90, 99.92, 99.94, 99.96, 99.98, or 100% isoprene by weight compared to the total weight of all C5 hydrocarbons in the composition.
- the composition has less than or about 50, 40, 30, 20, 10, 5, 1, 0.5, 0.1, 0.05, 0.01, or 0.005 ⁇ g/L of a compound that inhibits the polymerization of isoprene for any compound in the composition that inhibits the polymerization of isoprene.
- the composition also comprises greater than about 2 mg of isoprene and greater than about 0.48 mg of hydrogen.
- the volatile organic fraction of the gas phase has less than or about 50, 40, 30, 20, 10, 5, 1, 0.5, 0.1, 0.05, 0.01, or 0.005 ⁇ g/L of a compound that inhibits the polymerization of isoprene for any compound in the volatile organic fraction of the gas phase that inhibits the polymerization of isoprene.
- the volatile organic fraction of the gas phase also has greater than about 2 mg of isoprene and greater than about 0.48 mg of hydrogen.
- the systems include any of the cells and/or compositions described herein.
- the system includes a reactor that chamber comprises cells in oxygen-limited culture that produce greater than about 400, 500, 600, 700, 800, 900, 1,000, 1,250, 1,500, 1,750, 2,000, 2,500, 3,000, 4,000, 5,000, or more nmole/g wcm /hr isoprene and greater than about 125, 250, 500, 750, 1000, 1,250, 1,500, 1,750, 2,000, 2,500, 3,000, 4,000, 5,000, 7,500, 10,000, or more nmole/g wcm /hr hydrogen.
- the system is not a closed system.
- at least a portion of the isoprene is removed from the system.
- the system includes a gas phase comprising isoprene and hydrogen.
- the gas phase comprises any of the compositions described herein.
- featured herein is a product produced by any of the compositions or methods described herein.
- Isoprene is a hydrophobic molecule secreted by many plants, animals, and microbes. Bacteria, such as Bacillus, produce isoprene at fairly low levels. While there is some evidence that plants secrete isoprene to help with thermoprotection, it has been hypothesized that isoprene may act antagonistically to cyanobacteria or fungi, or as an antimicrobial agent. See, e.g., Ladygina et al., Process Biochemistry 41:1001-1014 (2006), which is incorporated by reference in its entirety, particularly with respect to isoprene acting antagonistically. Since the very low production levels happening in nature are sufficient to be anti-microbial, it was of great concern that the titers and productivity levels of isoprene necessary for commercialization of isoprene would kill the host microbe.
- the isoprene produced is any concentration or amount disclosed in the section entitled "Exemplary Production of Isoprene.” In some embodiments, the amount of isoprene is between about any of 400 nmole/g wcm /hour to 1 mole/g wcm /hour, 400 nmole/g wcm /hour to 1 mmole/g wcm /hour, 400 nmole/g wcm /hour to 40 mmole/g wcm /hour, 400 nmole/g wcm /hour to 4 mmole/g wcm /hour, 1 mmole/g wcm /hour to 1.5 mmole/g wcm /hour, 1.5 mmole/g wcm /hour to 3 mmole/g wcm /hour, 3 mmole/g wcm /hour to 5 mmole/g
- the amount of isoprene is about any of 1 mmole/g wcm /hour, 1.5 mmole/g wcm /hour, 2 mmole/g wcm /hour, 3 mmole/g wcm /hour, 4 mmole/g wcm /hour, or 5 mmole/g wcm /hour.
- the carbon dioxide evolution rate is between about any of 1 x 10 "18 mmol/L/hour to about 1 mol/L/hour, 1 mmol/L/hour to 1 mol/L/hour, 25 mmol/L/hour to 750 mmol/L/hour, 25 mmol/L/hour to 75 mmol/L/hour, 250 mmol/L/hour to 750 mmol/L/hour, or 450 mmol/L/hour to 550 mmol/L/hour.
- the carbon dioxide evolution rate is about any of 50 mmol/L/hour, 100 mmol/L/hour, 150 mmol/L/hour, 200 mmol/L/hour, 250 mmol/L/hour, 300 mmol/L/hour, 350 mmol/L/hour, 400 mmol/L/hour, 450 mmol/L/hour, or 500 mmol/L/hour.
- isoprene comprising: a) culturing cells under suitable conditions for production of isoprene; and b) producing isoprene, wherein cells produce greater than about 400 nmole/g wcm /hour of isoprene, and cell viability is reduced by less than about two-fold.
- the isoprene produced is any concentration or amount disclosed in the section entitled "Exemplary Production of Isoprene.” In some embodiments, the amount of isoprene is between about any of 400 nmole/g wcm /hour to 1 mole/g wcm /hour, 400 nmole/g wcm /hour to 1 mmole/g wcm /hour, 400 nmole/g wcm /hour to 40 mmole/g wcm /hour, 400 nmole/g wcm /hour to 4 mmole/g wcm /hour, 1 mmole/g wcm /hour to 1.5 mmole/g wcm /hour, 1.5 mmole/g wcm /hour to 3 mmole/g wcm /hour, 3 mmole/g wcm /hour to 5 mmole/g
- the amount of isoprene is about any of 1 mmole/g wcm /hour, 1.5 mmole/g wcm /hour, 2 mmole/g wcm /hour, 3 mmole/g wcm /hour, 4 mmole/g wcm /hour, or 5 mmole/g wcm /hour.
- cell viability is reduced by less than about any of 1.75-fold, 1.5-fold, 1.25-fold, 1-fold, 0.75-fold, 0.5-fold, or 0.25-fold. In some embodiments, cell viability is reduced by about 2-fold.
- isoprene comprising: a) culturing cells under suitable conditions for production of isoprene; and b) producing isoprene, wherein the cumulative total productivity of the isoprene produced by the cells in culture is greater than about 0.2 mg/L broth /hour and the carbon dioxide evolution rate of the cells is greater than about 1 x 10 "18 mmol/L/hour.
- the cumulative total productivity of isoprene is any concentration or amount disclosed in the section entitled "Exemplary Production of Isoprene.” In some embodiments, the cumulative total productivity of the isoprene is between about any of 0.2 mg/L broth /hour to 5 g/L broth /hour, 0.2 mg/L broth /hour to 1 g/L broth /hour, 1 g/L broth /hour to 2.5 g/L broth /hour, 2.5 g/L broth /hour to 5 g/L broth /hour.
- the carbon dioxide evolution rate is between about any of 1 x 10 "18 mmol/L/hour to about 1 mol/L/hour, 1 mmol/L/hour to 1 mol/L/hour, 25 mmol/L/hour to 750 mmol/L/hour, 25 mmol/L/hour to 75 mmol/L/hour, 250 mmol/L/hour to 750 mmol/L/hour, or 450 mmol/L/hour to 550 mmol/L/hour.
- the carbon dioxide evolution rate is about any of 50 mmol/L/hour, 100 mmol/L/hour, 150 mmol/L/hour, 200 mmol/L/hour, 250 mmol/L/hour, 300 mmol/L/hour, 350 mmol/L/hour, 400 mmol/L/hour, 450 mmol/L/hour, or 500 mmol/L/hour.
- isoprene comprising: a) culturing cells under suitable conditions for production of isoprene; and b) producing isoprene, wherein the cumulative total productivity of the isoprene produced by the cells in culture is greater than about 0.2 mg/L broth /hour and cell viability is reduced by less than about two-fold.
- the cumulative total productivity of isoprene is any concentration or amount disclosed in the section entitled "Exemplary Production of Isoprene.” In some embodiments, the cumulative total productivity of the isoprene is between about any of 0.2 mg/L broth /hour to 5 g/L broth /hour, 0.2 mg/L broth /hour to 1 g/L broth /hour, 1 g/L broth /hour to 2.5 g/L broth /hour, 2.5 g/L broth /hour to 5 g/L broth /hour. In some embodiments, cell viability is reduced by less than about any of 1.75-fold, 1.5-fold, 1.25-fold, 1-fold, 0.75-fold, 0.5-fold, or 0.25-fold.
- Methods of producing isoprene comprising: a) culturing cells under suitable conditions for production of isoprene; and b) producing isoprene, wherein the peak concentration of the isoprene produced by the cells in culture is greater than about 10 ng/L bro th and the carbon dioxide evolution rate of the cells is greater than about 1 x 10 "18 mmol/L/hour.
- the peak concentration of isoprene is any concentration or amount disclosed in the section entitled "Exemplary Production of Isoprene.” In some embodiments, the peak concentration of isoprene is between about any of 10 ng/L bro th to 500 ng/L brot h, 500 ng/L brot h to 1 ⁇ g/L broth , 1 ⁇ g/L broth to 5 ⁇ g/L broth , 5 ⁇ g/L broth to 50 ⁇ g/L broth , 5 ⁇ g/L broth to 100 ⁇ g/L broth , 5 ⁇ g/L broth to 250 ⁇ g/L broth , 250 ⁇ g/L broth to 500 ⁇ g/L broth , 500 ⁇ g/L broth to 1 mg/L brot h, 1 mg/L brot h to 50 mg/L brot h, 1 mg/L brot h to 100 mg/L brot h, 1 mg/L brot h to 200 mg/L brot h, 10 ng/L brot
- the carbon dioxide evolution rate is between about any of 1 x 10 "18 mmol/L/hour to about 1 mol/L/hour, 1 mmol/L/hour to 1 mol/L/hour, 25 mmol/L/hour to 750 mmol/L/hour, 25 mmol/L/hour to 75 mmol/L/hour, 250 mmol/L/hour to 750 mmol/L/hour, or 450 mmol/L/hour to 550 mmol/L/hour.
- the carbon dioxide evolution rate is about any of 50 mmol/L/hour, 100 mmol/L/hour, 150 mmol/L/hour, 200 mmol/L/hour, 250 mmol/L/hour, 300 mmol/L/hour, 350 mmol/L/hour, 400 mmol/L/hour, 450 mmol/L/hour, or 500 mmol/L/hour.
- methods of producing isoprene comprising: a) culturing cells under suitable conditions for production of isoprene; and b) producing isoprene, wherein the peak concentration of the isoprene produced by the cells in culture is greater than about 10 ng/Lbroth and cell viability is reduced by less than about two-fold.
- the peak concentration of isoprene is any concentration or amount disclosed in the section entitled “Exemplary Production of Isoprene.” In some embodiments, the peak concentration of isoprene is between about any of 10 ng/Lb ro th to 500 ng/Lb ro th, 500 ng/Lb ro th to 1 ⁇ g/Lbroth, 1 ⁇ g/Lb ro th to 5 ⁇ g/L bro th, 5 ⁇ g/L br oth to 50 ⁇ g/L bro th, 5 ⁇ g/L br oth to 100 ⁇ g/L bro th, 5 ⁇ g/Lb r oth to 250 ⁇ g/Lb r oth, 250 ⁇ g/L bro th to 500 ⁇ g/L bro th, 500 ⁇ g/L bro th to 1 mg/L bro th, 1 mg/L bro th to 50 mg/Lb r oth, 1 mg/L
- Cells in culture are also provided herein comprising a nucleic acid encoding an isoprene synthase polypeptide, wherein the cells produce greater than about 400 nmole/g wcm /hour of isoprene and carbon dioxide evolution rate of the cells is greater than about 1 x 10 "18 mmol/L/hour.
- the isoprene produced is any concentration or amount disclosed in the section entitled "Exemplary Production of Isoprene.” In some embodiments, the amount of isoprene is between about any of 400 nmole/g wcm /hour to 1 mole/g wcm /hour, 400 nmole/g wcm /hour to 1 mmole/g wcm /hour, 400 nmole/g wcm /hour to 40 mmole/g wcm /hour, 400 nmole/g wcm /hour to 4 mmole/g wcm /hour, 1 mmole/g wcm /hour to 1.5 mmole/g wcm /hour, 1.5 mmole/g wcm /hour to 3 mmole/g wcm /hour, 3 mmole/g wcm /hour to 5 mmole/g
- the amount of isoprene is about any of 1 mmole/g wcm /hour, 1.5 mmole/g wcm /hour, 2 mmole/g wcm /hour, 3 mmole/g wcm /hour, 4 mmole/g wcm /hour, or 5 mmole/g wcm /hour.
- the carbon dioxide evolution rate is between about any of 1 x 10 ⁇ 18 mmol/L/hour to about 1 mol/L/hour, 1 mmol/L/hour to 1 mol/L/hour, 25 mmol/L/hour to 750 mmol/L/hour, 25 mmol/L/hour to 75 mmol/L/hour, 250 mmol/L/hour to 750 mmol/L/hour, or 450 mmol/L/hour to 550 mmol/L/hour.
- the carbon dioxide evolution rate is about any of 50 mmol/L/hour, 100 mmol/L/hour, 150 mmol/L/hour, 200 mmol/L/hour, 250 mmol/L/hour, 300 mmol/L/hour, 350 mmol/L/hour, 400 mmol/L/hour, 450 mmol/L/hour, or 500 mmol/L/hour.
- cells in culture comprising a nucleic acid encoding an isoprene synthase polypeptide, wherein cumulative total productivity of the isoprene produced by the cells in culture is greater than about 0.2 mg/L broth /hour and carbon dioxide evolution rate of the cells is greater than about 1 x 10 "18 mmol/L/hour.
- the cumulative total productivity of isoprene is any concentration or amount disclosed in the section entitled "Exemplary Production of Isoprene.” In some embodiments, the cumulative total productivity of the isoprene is between about any of 0.2 mg/L broth /hour to 5 g/L broth /hour, 0.2 mg/L broth /hour to 1 g/L broth /hour, 1 g/L broth /hour to 2.5 g/L broth /hour, 2.5 g/L broth /hour to 5 g/L broth /hour.
- the carbon dioxide evolution rate is between about any of 1 x 10 "18 mmol/L/hour to about 1 mol/L/hour, 1 mmol/L/hour to 1 mol/L/hour, 25 mmol/L/hour to 750 mmol/L/hour, 25 mmol/L/hour to 75 mmol/L/hour, 250 mmol/L/hour to 750 mmol/L/hour, or 450 mmol/L/hour to 550 mmol/L/hour.
- the carbon dioxide evolution rate is about any of 50 mmol/L/hour, 100 mmol/L/hour, 150 mmol/L/hour, 200 mmol/L/hour, 250 mmol/L/hour, 300 mmol/L/hour, 350 mmol/L/hour, 400 mmol/L/hour, 450 mmol/L/hour, or 500 mmol/L/hour.
- cells in culture comprising a nucleic acid encoding an isoprene synthase polypeptide, wherein peak concentration of the isoprene produced by the cells in culture is greater than about 10 ng/Lb ro th and carbon dioxide evolution rate of the cells is greater than about 1 x 10 "18 mmol/L/hour.
- the peak concentration of isoprene is any concentration or amount disclosed in the section entitled “Exemplary Production of Isoprene.” In some embodiments, the peak concentration of isoprene is between about any of 10 ng/Lbroth to 500 ng/L bro th, 500 ng/L bro th to 1 ⁇ g/L bro th, 1 ⁇ g/L bro th to 5 ⁇ g/L bro th, 5 ⁇ g/L bro th to 50 ⁇ g/Lbroth, 5 ⁇ g/Lb ro th to 100 ⁇ g/L bro th, 5 ⁇ g/L bro th to 250 ⁇ g/L bro th, 250 ⁇ g/L bro th to 500 ⁇ g/L bro th, 500 ⁇ g/Lbroth to 1 mg/Lbroth, 1 mg/L br oth to 50 mg/L br oth, 1 mg/L br oth to 100 mg/L br oth, 1 mg/L br oth, 1 mg
- the carbon dioxide evolution rate is between about any of 1 x 10 "18 mmol/L/hour to about 1 mol/L/hour, 1 mmol/L/hour to 1 mol/L/hour, 25 mmol/L/hour to 750 mmol/L/hour, 25 mmol/L/hour to 75 mmol/L/hour, 250 mmol/L/hour to 750 mmol/L/hour, or 450 mmol/L/hour to 550 mmol/L/hour.
- the carbon dioxide evolution rate is about any of 50 mmol/L/hour, 100 mmol/L/hour, 150 mmol/L/hour, 200 mmol/L/hour, 250 mmol/L/hour, 300 mmol/L/hour, 350 mmol/L/hour, 400 mmol/L/hour, 450 mmol/L/hour, or 500 mmol/L/hour.
- carbon dioxide evolution rate and/or cell viability of a cell expressing a MVA pathway and/or DXP pathway RNA and/or protein from one or more of a heterologous and/or duplicate copy of a MVA pathway and/or DXP pathway nucleic acid is compared to a control cell lacking one or more of a heterologous and/or duplicate copy of a MVA pathway and/or DXP pathway nucleic acid.
- carbon dioxide evolution rate and/or cell viability of a cell expressing a MVA pathway and/or DXP pathway RNA and/or protein from one or more of a heterologous and/or duplicate copy of a MVA pathway and/or DXP pathway nucleic acid under the control of an inducible promoter, wherein the promotor is induced is compared to a control cell containing one or more of a heterologous and/or duplicate copy of a MVA pathway and/or DXP pathway nucleic acid under the control of an inducible promoter, wherein the promotor is not induced (uninduced).
- the inducible promoter is a beta-galactosidase promoter.
- the methods of producing isoprene comprise: a) culturing cells under suitable conditions for production of isoprene; and b) producing isoprene, wherein cells produce greater than about 400 nmole/g wcm /hour of isoprene, and the carbon dioxide evolution rate of the cells is greater than about 1 x 10 "18 mmol/L/hour.
- isoprene comprising: a) culturing cells under suitable conditions for production of isoprene; and b) producing isoprene, wherein the cumulative total productivity of the isoprene produced by the cells in culture is greater than about 0.2 mg/L broth /hour and the carbon dioxide evolution rate of the cells is greater than about 1 x 10 "18 mmol/L/hour.
- Methods of producing isoprene comprising: a) culturing cells under suitable conditions for production of isoprene; and b) producing isoprene, wherein the peak concentration of the isoprene produced by the cells in culture is greater than about 10 ng/Lb ro th and the carbon dioxide evolution rate of the cells is greater than about 1 x 10 "18 mmol/L/hour.
- the carbon dioxide evolution rate is between about any of 1 x 10 ⁇ 18 mmol/L/hour to about 1 mol/L/hour, 1 mmol/L/hour to 1 mol/L/hour, 25 mmol/L/hour to 750 mmol/L/hour, 25 mmol/L/hour to 75 mmol/L/hour, 250 mmol/L/hour to 750 mmol/L/hour, or 450 mmol/L/hour to 550 mmol/L/hour. In some embodiments, the carbon dioxide evolution rate is about 50 mmol/L/hour or about 500 mmol/L/hour.
- cells in culture comprising a nucleic acid encoding an isoprene synthase polypeptide, wherein the cells produce greater than about 400 nmole/g wcm /hour of isoprene and carbon dioxide evolution rate of the cells is greater than about 1 x 10 "18 mmol/L/hour.
- cells in culture comprising a nucleic acid encoding an isoprene synthase polypeptide, wherein cumulative total productivity of the isoprene produced by the cells in culture is greater than about 0.2 mg/L broth /hour and carbon dioxide evolution rate of the cells is greater than about 1 x 10 "18 mmol/L/hour.
- cells in culture comprising a nucleic acid encoding an isoprene synthase polypeptide, wherein peak concentration of the isoprene produced by the cells in culture is greater than about 10 ng/Lb ro th and carbon dioxide evolution rate of the cells is greater than about 1 x 10 "18 mmol/L/hour.
- the carbon dioxide evolution rate is between about any of 1 x 10 "18 mmol/L/hour to about 1 mol/L/hour, 1 mmol/L/hour to 1 mol/L/hour, 25 mmol/L/hour to 750 mmol/L/hour, 25 mmol/L/hour to 75 mmol/L/hour, 250 mmol/L/hour to 750 mmol/L/hour, or 450 mmol/L/hour to 550 mmol/L/hour. In some embodiments, the carbon dioxide evolution rate is about 50 mmol/L/hour or about 500 mmol/L/hour.
- isoprene comprising a) culturing cells under suitable conditions for production of isoprene; and b) producing isoprene, wherein the liquid phase concentration of isoprene is less than about 200 mg/L and the cells produce greater than about 400 nmole/g wcm /hour of isoprene.
- the liquid phase concentration of isoprene in the culture is less than about any of 175 mg/L, 150 mg/L, 125 mg/L, 100 mg/L, 75 mg/L, 50 mg/L, 25 mg/L, 20 mg/L, 15 mg/L, 10 mg/L, 5 mg/L, or 2.5 mg/L.
- the liquid phase concentration of isoprene in culture is between about any of 0.1 mg/L to 200 mg/L, 1 mg/L to 200 mg/L, 1 mg/L to 150 mg/L, 1 mg/L to 100 mg/L, 1 mg/L to 50 mg/L, 1 mg/L to 25 mg/L, 1 mg/L to 20 mg/L, or 10 mg/L to 20 mg/L.
- Also provided herein are methods of producing a compound, wherein the compound has one or more characteristics selected from the group consisting of (a) a Henry's law coefficient of less than about 250 M/atm and (b) a solubility in water of less than about 100 g/L.
- the method comprises: a) culturing cells under suitable conditions for production of the compound, wherein gas is added (such as the addition of gas to a system such as a fermentation system) at a gas sparging rate between about 0.01 vvm to about 2 vvm; and b) producing the compound.
- gas such as the addition of gas to a system such as a fermentation system
- the Henry's law coefficient of the compound is less than about any of 200 M/atm, 150 M/atm, 100 M/atm, 75 M/atm, 50 M/atm, 25 M/atm, 10 M/atm, 5 M/atm, or 1 M/atm.
- the solubility in water of the compound is less than about any of 75 g/L, 50 g/L, 25 g/L, 10 g/L, 5 g/L, or lg/L.
- the compound is selected from a group consisting of isoprene, an aldehyde (e.g., acetaldehyde), a ketone (e.g., acetone or 2-butanone), an alcohol (e.g., methanol, ethanol, 1-butanol, or C5 alcohols such as 3-methyl-3-buten-l-ol or 3-methyl-2-buten-l-ol), an ester of an alcohol (e.g., ethyl acetate or acetyl esters of C5 alcohols), a hemiterpene, a monoterpene, a sesquiterpene, and Cl to C5 hydrocarbons (e.g., methane, ethane, ethylene,
- C5 hydrocarbons
- the Cl to C5 hydrocarbons are saturated, unsaturated, or branched.
- the compound is isoprene.
- the gas sparging rate is between about any of 0.1 vvm to 1 vvm, 0.2 vvm to 1 vvm, or 0.5 vvm to 1 vvm.
- cells in culture are used to produce isoprene.
- the cells in culture produce greater than about 400 nmole of isoprene/gram of cells for the wet weight of the cells/hour (nmole/g wcm /hr) of isoprene.
- the cells have a heterologous nucleic acid that (i) encodes an isoprene synthase polypeptide and (ii) is operably linked to a promoter.
- the cells are cultured in a culture medium that includes a carbon source, such as, but not limited to, a carbohydrate, glycerol, glycerine, dihydroxyacetone, one-carbon source, oil, animal fat, animal oil, fatty acid, lipid, phospholipid, glycerolipid, monoglyceride, diglyceride, triglyceride, renewable carbon source, polypeptide (e.g., a microbial or plant protein or peptide), yeast extract, component from a yeast extract, or any combination of two or more of the foregoing.
- the cells are cultured under limited glucose conditions.
- the cells in culture convert more than about 0.002% of the carbon in a cell culture medium into isoprene.
- the cells have a heterologous nucleic acid that (i) encodes an isoprene synthase polypeptide and (ii) is operably linked to a promoter.
- the cells are cultured in a culture medium that includes a carbon source, such as, but not limited to, a carbohydrate, glycerol, glycerine, dihydroxyacetone, one-carbon source, oil, animal fat, animal oil, fatty acid, lipid, phospholipid, glycerolipid, monoglyceride, diglyceride, triglyceride, renewable carbon source, polypeptide (e.g., a microbial or plant protein or peptide), yeast extract, component from a yeast extract, or any combination of two or more of the foregoing.
- the cells are cultured under limited glucose conditions.
- the cells in culture comprise a heterologous nucleic acid encoding an isoprene synthase polypeptide.
- the cells have a heterologous nucleic acid that (i) encodes an isoprene synthase polypeptide and (ii) is operably linked to a promoter.
- the cells are cultured in a culture medium that includes a carbon source, such as, but not limited to, a carbohydrate, glycerol, glycerine, dihydroxyacetone, one-carbon source, oil, animal fat, animal oil, fatty acid, lipid, phospholipid, glycerolipid, monoglyceride, diglyceride, triglyceride, renewable carbon source, polypeptide (e.g., a microbial or plant protein or peptide), yeast extract, component from a yeast extract, or any combination of two or more of the foregoing.
- the cells are cultured under limited glucose conditions.
- described herein are methods of producing isoprene, such as methods of using any of the cells described herein to produce isoprene.
- the method involves culturing cells under conditions sufficient to produce greater than about 400 nmole/g wcm /hr of isoprene.
- the method also includes recovering isoprene produced by the cells.
- the method includes purifying isoprene produced by the cells.
- the method includes polymerizing the isoprene.
- the cells have a heterologous nucleic acid that (i) encodes an isoprene synthase polypeptide and (ii) is operably linked to a promoter.
- the cells are cultured in a culture medium that includes a carbon source, such as, but not limited to, a carbohydrate, glycerol, glycerine, dihydroxyacetone, one-carbon source, oil, animal fat, animal oil, fatty acid, lipid, phospholipid, glycerolipid, monoglyceride, diglyceride, triglyceride, renewable carbon source, polypeptide (e.g., a microbial or plant protein or peptide), yeast extract, component from a yeast extract, or any combination of two or more of the foregoing.
- a carbon source such as, but not limited to, a carbohydrate, glycerol, glycerine, dihydroxyacetone, one-carbon source, oil, animal fat, animal oil, fatty acid, lipid, phospholipid
- the cells are cultured under limited glucose conditions.
- the amount of isoprene produced (such as the total amount of isoprene produced or the amount of isoprene produced per liter of broth per hour per OD OOO ) during stationary phase is greater than or about 2 or more times the amount of isoprene produced during the growth phase for the same length of time.
- the gas phase comprises greater than or about 9.5 % (volume) oxygen, and the concentration of isoprene in the gas phase is less than the lower flammability limit or greater than the upper flammability limit.
- the concentration of isoprene in the gas phase is less than the lower flammability limit or greater than the upper flammability limit, and (ii) the cells produce greater than about 400 nmole/g wcm /hr of isoprene.
- the method includes culturing cells under conditions sufficient to convert more than about 0.002% of the carbon (mol/mol) in a cell culture medium into isoprene. In some embodiments, the method also includes recovering isoprene produced by the cells. In some embodiments, the method includes purifying isoprene produced by the cells. In some embodiments, the method includes polymerizing the isoprene. In some embodiments, the cells have a heterologous nucleic acid that (i) encodes an isoprene synthase polypeptide and (ii) is operably linked to a promoter.
- the cells are cultured in a culture medium that includes a carbon source, such as, but not limited to, a carbohydrate, glycerol, glycerine, dihydroxyacetone, one-carbon source, oil, animal fat, animal oil, fatty acid, lipid, phospholipid, glycerolipid, monoglyceride, diglyceride, triglyceride, renewable carbon source, polypeptide (e.g., a microbial or plant protein or peptide), yeast extract, component from a yeast extract, or any combination of two or more of the foregoing.
- the cells are cultured under limited glucose conditions.
- isoprene is only produced in stationary phase. In some embodiments, isoprene is produced in both the growth phase and stationary phase. In various embodiments, the amount of isoprene produced (such as the total amount of isoprene produced or the amount of isoprene produced per liter of broth per hour per OD OOO ) during stationary phase is greater than or about 2, 3, 4, 5, 10, 20, 30, 40, 50, or more times the amount of isoprene produced during the growth phase for the same length of time.
- compositions and systems that comprise isoprene.
- the composition comprises greater than or about 2, 5, 10, 20, 30, 40, 50, 60, 70, 80, 90, 100, 200, 300, 400, 500, 600, 700, 800, 900, or 1000 mg of isoprene.
- the composition comprises greater than or about 2, 5, 10, 20, 30, 40, 50, 60, 70, 80, 90, 100 g of isoprene(w/w) of the volatile organic fraction of the composition is isoprene.
- the composition comprises greater than or about 99.90, 99.92, 99.94, 99.96, 99.98, or 100% isoprene by weight compared to the total weight of all C5 hydrocarbons in the composition.
- the composition comprises less than or about 0.12, 0.10, 0.08, 0.06, 0.04, 0.02, 0.01, 0.005, 0.001, 0.0005, 0.0001, 0.00005, or 0.00001% C5 hydrocarbons other than isoprene (such as 1,3-cyclopentadiene, cis-1,3- pentadiene, trans- 1,3-pentadiene, 1 ,4-pentadiene, 1-pentyne, 2-pentyne, 1-pentene, 2-methyl-l- butene, 3-methyl-l-butyne, pent-4-ene-l-yne, trans-pent-3-ene-l-yne, or cis-pent-3-ene-l
- the composition has less than or about 0.12, 0.10, 0.08, 0.06, 0.04, 0.02, 0.01, 0.005, 0.001, 0.0005, 0.0001, 0.00005, or 0.00001% for 1,3-cyclopentadiene, cis- 1,3 -pentadiene, trans- 1,3 -pentadiene, 1 ,4-pentadiene, 1-pentyne, 2-pentyne, 1-pentene, 2-methyl-l-butene, 3- methyl-1-butyne, pent-4-ene-l-yne, trans-pent-3-ene-l-yne, or cis-pent-3-ene-l-yne by weight compared to the total weight of all C5 hydrocarbons in the composition.
- the composition has greater than about 2 mg of isoprene and has greater than or about 99.90, 99.92, 99.94, 99.96, 99.98, or 100% isoprene by weight compared to the total weight of all C5 hydrocarbons in the composition.
- the composition has less than or about 50, 40, 30, 20, 10, 5, 1, 0.5, 0.1, 0.05, 0.01, or 0.005 ⁇ g/L of a compound that inhibits the polymerization of isoprene for any compound in the composition that inhibits the polymerization of isoprene. In particular embodiments, the composition also has greater than about 2 mg of isoprene.
- the composition has one or more compounds selected from the group consisting of ethanol, acetone, C5 prenyl alcohols, and isoprenoid compounds with 10 or more carbon atoms.
- the composition has greater than or about 0.005, 0.01, 0.05, 0.1, 0.5, 1, 5, 10, 20, 30, 40, 60, 80, 100, or 120 ⁇ g/L of ethanol, acetone, a C5 prenyl alcohol (such as 3-methyl-3-buten-l-ol or 3-methyl-2-buten-l-ol), or any two or more of the foregoing.
- the composition has greater than about 2 mg of isoprene and has one or more compounds selected from the group consisting of ethanol, acetone, C5 prenyl alcohols, and isoprenoid compounds with 10 or more carbon atoms.
- the composition includes isoprene and one or more second compounds selected from the group consisting of 2-heptanone, 6-methyl-5-hepten-2-one, 2,4,5- trimethylpyridine, 2,3,5-trimethylpyrazine, citronellal, acetaldehyde, methanethiol, methyl acetate, 1-propanol, diacetyl, 2-butanone, 2-methyl-3-buten-2-ol, ethyl acetate, 2-methyl-l- propanol, 3-methyl-l-butanal, 3-methyl-2-butanone, 1-butanol, 2-pentanone, 3 -methyl- 1- butanol, ethyl isobutyrate, 3-methyl-2-butenal, butyl acetate, 3-methylbutyl acetate, 3-methyl-3- buten-1-yl acetate, 3-methyl-2-buten-l-yl acetate, 3-hexen-l-ol, 3-
- the amount of one of these second components relative to the amount of isoprene in units of percentage by weight is at greater than or about 0.01, 0.02, 0.05, 0.1, 0.5, 1, 5, 10, 20, 30, 40, 50, 60, 70, 80, 90, 100, or 110% (w/w).
- the composition comprises (i) a gas phase that comprises isoprene and (ii) cells in culture that produce greater than about 400 nmole/g wcm /hr of isoprene.
- the composition comprises a closed system, and the gas phase comprises greater than or about 5. 10, 20, 30, 40, 50, 60, 70, 80, 90, 100 ⁇ g/L of isoprene when normalized to 1 mL of 1 OD ⁇ oo cultured for 1 hour.
- the composition comprises an open system, and the gas phase comprises greater than or about 5, 10, 20, 30, 40, 50, 60, 70, 80, 90, 100 ⁇ g/L of isoprene when sparged at a rate of 1 vvm.
- the volatile organic fraction of the gas phase comprises greater than or about 99.90, 99.92, 99.94, 99.96, 99.98, or 100% isoprene by weight compared to the total weight of all C5 hydrocarbons in the volatile organic fraction.
- the volatile organic fraction of the gas phase comprises less than or about 0.12, 0.10, 0.08, 0.06, 0.04, 0.02, 0.01, 0.005, 0.001, 0.0005, 0.0001, 0.00005, or 0.00001% C5 hydrocarbons other than isoprene (such as 1,3- cyclopentadiene, cis-l,3-pentadiene, trans- 1,3-pentadiene, 1 ,4-pentadiene, 1-pentyne, 2-pentyne, 1-pentene, 2-methyl-l-butene, 3-methyl-l-butyne, pent-4-ene-l-yne, trans-pent-3-ene-l-yne, or cis-pent-3-ene-l-yne) by weight compared to the total weight of all C5 hydrocarbons in the volatile organic fraction.
- isoprene such as 1,3- cyclopentadiene, cis-l,3-pentad
- the volatile organic fraction of the gas phase has less than or about 0.12, 0.10, 0.08, 0.06, 0.04, 0.02, 0.01, 0.005, 0.001, 0.0005, 0.0001, 0.00005, or 0.00001% for 1,3-cyclopentadiene, cis-l,3-pentadiene, trans-l,3-pentadiene, 1 ,4-pentadiene, 1-pentyne, 2-pentyne, 1-pentene, 2-methyl-l-butene, 3-methyl-l-butyne, pent-4-ene-l-yne, trans- pent-3-ene-l-yne, or cis-pent-3-ene-l-yne by weight compared to the total weight of all C5 hydrocarbons in the volatile organic fraction.
- the volatile organic fraction of the gas phase has greater than about 2 mg of isoprene and has greater than or about 99.90, 99.92, 99.94, 99.96, 99.98, or 100% isoprene by weight compared to the total weight of all C5 hydrocarbons in the volatile organic fraction.
- the volatile organic fraction of the gas phase has less than or about 50, 40, 30, 20, 10, 5, 1, 0.5, 0.1, 0.05, 0.01, or 0.005 ⁇ g/L of a compound that inhibits the polymerization of isoprene for any compound in the volatile organic fraction of the gas phase that inhibits the polymerization of isoprene.
- the volatile organic fraction of the gas phase also has greater than about 2 mg of isoprene.
- the volatile organic fraction of the gas phase has one or more compounds selected from the group consisting of ethanol, acetone, C5 prenyl alcohols, and isoprenoid compounds with 10 or more carbon atoms.
- the volatile organic fraction of the gas phase has greater than or about 0.005, 0.01, 0.05, 0.1, 0.5, 1, 5, 10, 20, 30, 40, 60, 80, 100, or 120 ⁇ g/L of ethanol, acetone, a C5 prenyl alcohol (such as 3-methyl-3-buten-l-ol or 3-methyl-2-buten-l-ol), or any two or more of the foregoing.
- the volatile organic fraction of the gas phase has greater than about 2 mg of isoprene and has one or more compounds selected from the group consisting of ethanol, acetone, C5 prenyl alcohols, and isoprenoid compounds with 10 or more carbon atoms.
- the volatile organic fraction of the gas phase has includes isoprene and one or more second compounds selected from the group consisting of 2-heptanone, 6-methyl-5-hepten-2-one, 2,4,5-trimethylpyridine, 2,3,5-trimethylpyrazine, citronellal, acetaldehyde, methanethiol, methyl acetate, 1-propanol, diacetyl, 2-butanone, 2-methyl-3-buten- 2-ol, ethyl acetate, 2-methyl- 1-propanol, 3 -methyl- 1-butanal, 3-methyl-2-butanone, 1-butanol, 2- pentanone, 3 -methyl- 1-butanol, ethyl isobutyrate, 3-methyl-2-butenal, butyl acetate, 3- methylbutyl acetate, 3-methyl-3-buten-l-yl acetate, 3-methyl-2-buten-l-yl acetate, 3-he
- the amount of one of these second components relative to amount of isoprene in units of percentage by weight is at greater than or about 0.01, 0.02, 0.05, 0.1, 0.5, 1, 5, 10, 20, 30, 40, 50, 60, 70, 80, 90, 100, or 110% (w/w) in the volatile organic fraction of the gas phase.
- At least a portion of the isoprene is in a gas phase. In some embodiments, at least a portion of the isoprene is in a liquid phase (such as a condensate). In some embodiments, at least a portion of the isoprene is in a solid phase. In some embodiments, at least a portion of the isoprene is adsorbed to a solid support, such as a support that includes silica and/or activated carbon. In some embodiments, the composition includes ethanol.
- the composition includes between about 75 to about 90% by weight of ethanol, such as between about 75 to about 80%, about 80 to about 85%, or about 85 to about 90% by weight of ethanol. In some embodiments, the composition includes between about 4 to about 15% by weight of isoprene, such as between about 4 to about 8%, about 8 to about 12%, or about 12 to about 15% by weight of isoprene. [0568] In some embodiments, the systems include any of the cells and/or compositions described herein.
- the system includes a reactor that chamber comprises cells in culture that produce greater than about 400, 500, 600, 700, 800, 900, 1,000, 1,250, 1,500, 1,750, 2,000, 2,500, 3,000, 4,000, 5,000, or more nmole/g wcm /hr isoprene.
- the system is not a closed system.
- at least a portion of the isoprene is removed from the system.
- the system includes a gas phase comprising isoprene.
- the gas phase comprises any of the compositions described herein.
- a nonflammable concentration of isoprene in the gas phase is produced.
- the gas phase comprises less than about 9.5 % (volume) oxygen.
- the gas phase comprises greater than or about 9.5 % (volume) oxygen, and the concentration of isoprene in the gas phase is less than the lower fiammability limit or greater than the upper fiammability limit.
- the portion of the gas phase other than isoprene comprises between about 0% to about 100% (volume) oxygen, such as between about 10% to about 100% (volume) oxygen.
- the portion of the gas phase other than isoprene comprises between about 0% to about 99% (volume) nitrogen. In some embodiments, the portion of the gas phase other than isoprene comprises between about 1% to about 50% (volume) CO 2 .
- the cells in culture produce isoprene at greater than or about 400, 500, 600, 700, 800, 900, 1,000, 1,250, 1,500, 1,750, 2,000, 2,500, 3,000, 4,000, 5,000, or more nmole/g wcm /hr isoprene.
- the cells in culture convert greater than or about 0.002, 0.005, 0.01, 0.02, 0.05, 0.1, 0.12, 0.14, 0.16, 0.2, 0.3, 0.4, 0.5, 0.6, 0.7, 0.8, 0.9, 1.0, 1.2, 1.4, 1.6%, or more of the carbon in the cell culture medium into isoprene.
- the cells in culture produce isoprene at greater than or about 1, 10, 25, 50, 100, 150, 200, 250, 300, 400, 500, 600, 700, 800, 900, 1,000, 1,250, 1,500, 1,750, 2,000, 2,500, 3,000, 4,000, 5,000, 10,000, 100,000, or more ng of isoprene/gram of cells for the wet weight of the cells /hr (ng/g wcm /h).
- the cells in culture produce a cumulative titer (total amount) of isoprene at greater than or about 1, 10, 25, 50, 100, 150, 200, 250, 300, 400, 500, 600, 700, 800, 900, 1,000, 1,250, 1,500, 1,750, 2,000, 2,500, 3,000, 4,000, 5,000, 10,000, 50,000, 100,000, or more mg of isoprene/L of broth (mg/Lbroth, wherein the volume of broth includes the volume of the cells and the cell medium).
- mg/Lbroth wherein the volume of broth includes the volume of the cells and the cell medium.
- the cells further comprise a heterologous nucleic acid encoding an IDI polypeptide. In some embodiments of any of the aspects described herein, the cells further comprise an insertion of a copy of an endogenous nucleic acid encoding an IDI polypeptide. In some embodiments of any of the aspects described herein, the cells further comprise a heterologous nucleic acid encoding a DXS polypeptide. In some embodiments of any of the aspects described herein, the cells further comprise an insertion of a copy of an endogenous nucleic acid encoding a DXS polypeptide.
- the cells further comprise one or more nucleic acids encoding an IDI polypeptide and a DXS polypeptide.
- one nucleic acid encodes the isoprene synthase polypeptide, IDI polypeptide, and DXS polypeptide.
- one vector encodes the isoprene synthase polypeptide, IDI polypeptide, and DXS polypeptide.
- the vector comprises a selective marker, such as an antibiotic resistance nucleic acid.
- the heterologous isoprene synthase nucleic acid is operably linked to a T7 promoter, such as a T7 promoter contained in a medium or high copy plasmid. In some embodiments of any of the aspects described herein, the heterologous isoprene synthase nucleic acid is operably linked to a Trc promoter, such as a Trc promoter contained in a medium or high copy plasmid. In some embodiments of any of the aspects described herein, the heterologous isoprene synthase nucleic acid is operably linked to a Lac promoter, such as a Lac promoter contained in a low copy plasmid.
- the heterologous isoprene synthase nucleic acid is operably linked to an endogenous promoter, such as an endogenous alkaline serine protease promoter.
- an endogenous promoter such as an endogenous alkaline serine protease promoter.
- the heterologous isoprene synthase nucleic acid integrates into a chromosome of the cells without a selective marker.
- one or more MVA pathway, IDI, DXP, or isoprene synthase nucleic acids are placed under the control of a promoter or factor that is more active in stationary phase than in the growth phase.
- a promoter or factor that is more active in stationary phase than in the growth phase.
- one or more MVA pathway, IDI, DXP, or isoprene synthase nucleic acids may be placed under control of a stationary phase sigma factor, such as RpoS.
- one or more MVA pathway, IDI, DXP, or isoprene synthase nucleic acids are placed under control of a promoter inducible in stationary phase, such as a promoter inducible by a response regulator active in stationary phase.
- the cells maintain the heterologous isoprene synthase nucleic acid for at least or about 5, 10, 20, 40, 50, 60, 65, or more cell divisions in a continuous culture (such as a continuous culture without dilution).
- the nucleic acid comprising the isoprene synthase, IDI, or DXS nucleic acid also comprises a selective marker, such as an antibiotic resistance nucleic acid.
- the cells further comprise a heterologous nucleic acid encoding an MVA pathway polypeptide (such as an MVA pathway polypeptide from Saccharomyces cerevisia or Enterococcus faecalis). In some embodiments of any of the aspects described herein, the cells further comprise an insertion of a copy of an endogenous nucleic acid encoding an MVA pathway polypeptide (such as an MVA pathway polypeptide from Saccharomyces cerevisia ox Enterococcus faecalis). In some embodiments of any of the aspects described herein, the cells comprise an isoprene synthase, DXS, and MVA pathway nucleic acid.
- the cells comprise an isoprene synthase nucleic acid, a DXS nucleic acid, an IDI nucleic acid, and a MVA pathway nucleic (in addition to the IDI nucleic acid).
- the isoprene synthase polypeptide is a polypeptide from a plant such as Pueraria ⁇ e.g., Pueraria montana or Pueraria lobata) or Populus ⁇ e.g., Populus tremuloides, Populus alba, Populus nigra, Populus trichocarpa, or the hybrid, Populus alba x Populus tremula).
- the cells are bacterial cells, such as gram-positive bacterial cells ⁇ e.g., Bacillus cells such as Bacillus subtilis cells or Streptomyces cells such as Streptomyces lividans, Streptomyces coelicolor, or Streptomyces griseus cells).
- the cells are gram- negative bacterial cells ⁇ e.g., Escherichia cells such as Escherichia coli cells, Rhodopseudomonas sp. such as Rhodopseudomonas palustris cells, Pseudomonas sp.
- the cells are fungal, cells such as filamentous fungal cells ⁇ e.g., Trichoderma cells such as Trichoderma reesei cells or Aspergillus cells such as Aspergillus oryzae and Aspergillus niger) or yeast cells ⁇ e.g., Yarrowia cells such as Yarrowia lipolytica cells or Sacchraomyces cells such as Saccaromyces cerevisiae).
- filamentous fungal cells ⁇ e.g., Trichoderma cells such as Trichoderma reesei cells or Aspergillus cells such as Aspergillus oryzae and Aspergillus niger
- yeast cells ⁇ e.g., Yarrowia cells such as Yarrowia lipolytica cells or Sacchraomyces cells such as Saccaromyces cerevisiae).
- the microbial polypeptide carbon source includes one or more polypeptides from yeast or bacteria.
- the plant polypeptide carbon source includes one or more polypeptides from soy, corn, canola, jatropha, palm, peanut, sunflower, coconut, mustard, rapeseed, cottonseed, palm kernel, olive, safflower, sesame, or linseed.
- the bioisoprene composition is produced in anaerobic microorganisms using synthesis gas as an energy source as described in US Provisional Patent Application Nos. 61/289,347 and 61/289,355, filed on December 22, 2009, the disclosures of which are incorporated herein by reference in their entireties.
- Production of isoprene from syngas by anaerobic organisms may provide a number of advantages over production of isoprene from sugars by aerobic organisms.
- the anaerobic organisms do not have excess reducing power in the form of NAD(P)H that must be turned over via cell growth, formation of byproducts (such as glycerol, lactic acid, or ethanol) or oxidation using molecular oxygen. Without this NAD(P)H turnover requirement, anaerobic organisms can have higher energy yield, lower oxygen demand, lower heat of fermentation, and lower utility costs to run the process.
- anaerobic organisms can have greater isoprene concentration in the offgas, lower probability of creating a flammable isoprene-oxygen mixture, easier recovery, and higher isoprene quality.
- the anaerobic organisms can be more easily grown by using existing infrastructure, such as existing plants designed for production of bioethanol.
- Anaerobic organisms useful for isoprene production can include obligate anaerobes, facultatitive anaerobes, and aerotolerant anaerobes.
- the obligate anaerobes can be any one or combination selected from the group consisting of Clostridium ljungdahlii, Clostridium autoethanogenum, Eurobacterium limosum, Clostridium carboxydivorans, Peptostreptococcus productus, and Butyribacterium methylotrophicum.
- Syngas useful as energy source for production of isoprene can be derived from a feedstock by a variety of processes, including methane reforming, coal liquefaction, co-firing, fermentative reactions, enzymatic reactions, and biomass gasification.
- any of the methods described herein further include recovering the co-produced compounds. In some embodiments, any of the methods described herein further include recovering the isoprene. In some embodiments, any of the methods described herein further include recovering the hydrogen by cryogenic membrane, adsorption matrix-based separation methods.
- the isoprene and hydrogen produced using the compositions and methods described herein can be recovered using standard techniques, such as gas stripping, membrane enhanced separation, fractionation, adsorption/desorption, pervaporation, thermal or vacuum desorption of isoprene from a solid phase, or extraction of isoprene immobilized or absorbed to a solid phase with a solvent (see, for example, U.S. Patent Nos.
- the recovery of isoprene involves the isolation of isoprene in a liquid form (such as a neat solution of isoprene or a solution of isoprene in a solvent).
- Gas stripping involves the removal of isoprene vapor from the fermentation off-gas stream in a continuous manner. Such removal can be achieved in several different ways including, but not limited to, adsorption to a solid phase, partition into a liquid phase, or direct condensation (such as condensation due to exposure to a condensation coil or do to an increase in pressure).
- membrane enrichment of a dilute isoprene vapor stream above the dew point of the vapor resulting in the condensation of liquid isoprene isoprene.
- the isoprene is compressed and condensed.
- the recovery of isoprene may involve one step or multiple steps.
- the removal of isoprene vapor from the fermentation off-gas and the conversion of isoprene to a liquid phase are performed simultaneously.
- isoprene can be directly condensed from the off-gas stream to form a liquid.
- the removal of isoprene vapor from the fermentation off-gas and the conversion of isoprene to a liquid phase are performed sequentially.
- isoprene may be adsorbed to a solid phase and then extracted from the solid phase with a solvent.
- the recovery of hydrogen may involve one step or multiple steps.
- the removal of hydrogen gas from the fermentation off-gas and the conversion of hydrogen to a liquid phase are performed simultaneously.
- the removal of hydrogen gas from the fermentation off-gas and the conversion of hydrogen to a liquid phase are performed sequentially.
- hydrogen may be adsorbed to a solid phase and then desorbed from the solid phase by a pressure swing.
- recovered hydrogen gas is concentrated and compressed.
- any of the methods described herein further include purifying the isoprene.
- the isoprene produced using the compositions and methods described herein can be purified using standard techniques. Purification refers to a process through which isoprene is separated from one or more components that are present when the isoprene is produced. In some embodiments, the isoprene is obtained as a substantially pure liquid. Examples of purification methods include (i) distillation from a solution in a liquid extractant and (ii) chromatography. As used herein, "purified isoprene” means isoprene that has been separated from one or more components that are present when the isoprene is produced.
- the isoprene is at least about 20%, by weight, free from other components that are present when the isoprene is produced. In various embodiments, the isoprene is at least or about 25%, 30%, 40%, 50%, 60%, 70%, 75%, 80%, 90%, 95%, or 99%, by weight, pure. Purity can be assayed by any appropriate method, e.g., by column chromatography, HPLC analysis, or GC- MS analysis.
- any of the methods described herein further include purifying the hydrogen.
- the hydrogen produced using the compositions and methods described herein can be purified using standard techniques. Purification refers to a process through which hydrogen is separated from one or more components that are present when the hydrogen is produced. In some embodiments, the hydrogen is obtained as a substantially pure gas. In some embodiments, the hydrogen is obtained as a substantially pure liquid. Examples of purification methods include (i) cryogenic condensation and (ii) solid matrix adsorption. As used herein, "purified hydrogen” means hydrogen that has been separated from one or more components that are present when the hydrogen is produced. In some embodiments, the hydrogen is at least about 20%, by weight, free from other components that are present when the hydrogen is produced.
- the hydrogen is at least or about 25%, 30%, 40%, 50%, 60%, 70%, 75%, 80%, 90%, 95%, or 99%, by weight, pure. Purity can be assayed by any appropriate method, e.g., by column chromatography or GC-MS analysis.
- at least a portion of the gas phase remaining after one or more recovery steps for the removal of isoprene is recycled by introducing the gas phase into a cell culture system (such as a fermentor) for the production of isoprene.
- a bioisoprene composition from a fermentor off-gas may contain bioisoprene with volatile impurities and bio-byproduct impurities.
- a bioisoprene composition from a fermentor off-gas is purified using a method comprising: (a) contacting the fermentor off-gas with a solvent in a first column to form: an isoprene-rich solution comprising the solvent, a major portion of the isoprene and a major portion of the bio-byproduct impurity; and a vapor comprising a major portion of the volatile impurity; (b) transferring the isoprene- rich solution from the first column to a second column; and (c) stripping isoprene from the isoprene-rich solution in the second column to form: an isoprene-lean solution comprising a major portion of the bio-byproduct impurity; and a purified isopene composition.
- FIG. 169 illustrates an exemplary method of purifying isoprene and an exemplary apparatus.
- Fermentor off-gas comprising isoprene may be generated from renewable resources (e.g., carbon sources) by any method in the art for example, as described in U.S. provisional patent application Nos. 61/187,944, the content of which is hereby incorporated by reference, particularly with respect to the methods of generating fermentor off-gas comprising isoprene.
- the fermentor off-gas generated from one or more individual fermentors 12 e.g., 1, 2, 3, 4, 5, 6, 7, 8, or more fermentors connected in series and/or in parallel
- the fermentor off-gas may be directed through an isolation unit 16 and/or compressed by a compression means, such as compression system 18. Additionally, the temperature of the fermentor off-gas may optionally be reduced at any point, for example, to form a condensate or partial condensate prior to contact with the solvent (which may aid in solubiliztion of one or more off-gas components, such as isoprene).
- the fermentor off-gas may be contacted (e.g., absorbed) at column 14 with a solvent (e.g., any solvent described herein, such as a non-polar high boiling-point solvent).
- the volatile impurities having less propensity for absorption in the solvent are separated from the remaining solvent/fermentor off-gas mixture, resulting in a vapor comprising a major portion of the volatile impurity (e.g., exiting at port 20), and an isoprene-rich solution having a major portion of the isoprene and a major portion of the bio-byproduct impurity (e.g., at port 22).
- the solvent may optionally be heated by any suitable means (e.g., by steam) prior to, simultaneously, and/or after contact with the fermentor off-gas, which may aid in separation of the volatile impurity from the remaining solution.
- Steam may be directed through the column (at any suitable location, such as near entry of the off-gas and/or the opposite end of the volatile impurity exit as shown in Figure 169) to provide a sweeping vapor phase which may aid in the removal of the volatile impurity.
- the isoprene-rich solution having a major portion of the isoprene and a major portion of the bio-byproduct impurity may be directed to a second column 24.
- the second column may be isolated from the first column 14 (as shown in Figure 169) or may be part of a single column comprising both the first and second columns (e.g. , a tandem column wherein the solvent enters the first column at or near one end, and exits the second column at or near an opposite end).
- the isoprene may be stripped from the isoprene-rich solution in the second column to generate a purified isopene composition (e.g., at port 26) and an isoprene-lean solution comprising a major portion of the bio-byproduct impurity (e.g., at port 28).
- the isoprene-rich solution may be heated by any suitable means (e.g., by steam), which may aid in stripping of the isoprene from the remaining solution. Steam may be directed through the column (at any suitable location, such as the opposite end of the entry point of the isoprene-rich solution and/or the near the end of the isoprene-lean solution exit as shown in Figure 169).
- the columns may be conventional and of any suitable size.
- Exemplary types of columns are commercially available from manufacturers including Koch Modular Process Systems (Paramus, NJ), Fluor Corporation (Irving, TX), Kuhni USA (Mount Holly, NC).
- Suitable packing materials include both random and structured types based on metal, glass, polymer and ceramic materials.
- Exemplary random packing types include Raschig rings, Pall rings, A-PAK rings, Saddle rings, Pro-Pak, Heli-Pak, Ceramic saddles and FLEXIRINGS R .
- Structured packings include wire mesh and perforated metal plate type materials.
- Manfacturers specializing in column packings include ACS Separations & Mass-Transfer Products (Houston, TX), Johnson Bros. Metal Forming Co. (Berkeley, IL) and Koch Glitsch, Inc. Knight Div. (East Canton, OH).
- the efficiency of a gas stripping column is expressed in terms of the theoretical plate height and the total number of plates in the column. In general, the greater the number of theoretical plates present, the greater the efficiency of the column.
- Laboratory scale columns can be purchased from Ace Glass (Vineland, NJ), Sigma- Aldrich (St. Louis, MO) and Chemglass (Vineland, NJ).
- Suitable types of glass column include Vigreux, Snyder, Hemple and Perforated-plate type columns. Columns can include packing materals, or contain features designed to maximize vapor/liquid contact.
- a laboratory scale gas scrubber unit (part # CG-1830-10) is available from Chemglass and consists of a packed glass column, solvent reservoir and solvent recirculation pump.
- the purified isoprene composition from the second column 24 may be further purified by any suitable means (e.g. , by using a reflux condenser 34 and/or an adsorption system 36, such as a silica adsorption system). The reflux reduces the solvent composition in the isoprene product.
- the isoprene-lean solution may be recycled back to the first column for reuse (e.g., as shown in Figure 169 at port 30).
- the isoprene-lean solution may be purified by any suitable means (e.g., by liquid-liquid extraction and/or an adsorption system 32, such as a silica adsorption system) prior to recycling to the first column 14 to reduce to amount of bio-byproduct.
- the temperature of the isoprene-lean solution may be reduced by any suitable means prior to recycling to the first column 14 (e.g., prior to, simultaneously, and/or after optionally purifying the isoprene solution).
- Figure 169 shows an example of reducing the temperature of the isoprene-lean solution at port 40 prior to purification of the isoprene-lean solution (in this case, using coolant for temperature reduction).
- the vapor comprising a major portion of the volatile impurity may comprise a minor portion of isoprene (e.g., residual isoprene not remaining in the isoprene-rich solution).
- the residual isoprene may be recollected for use from the vapor comprising a major portion of the volatile impurity by any suitable means (e.g., an adsorption system 38, such as an activated carbon adsorption system) and in some cases, as shown in Figure 169, may be combined with the purified isoprene composition (e.g., prior to, during, or after additional purification, such as an adsorption system similar to system 36).
- Figure 169 also shows an optional capture device 42 (e.g., a thermal oxidizer and/or CO 2 capture system) capable of reducing the amount of undesirable components released into the atmosphere (e.g. , CO 2 ) from the vapor.
- an optional capture device 42 e.g., a thermal oxidizer and/or CO 2 capture system
- isoprene is a reactive conjugated diene and undergoes a varieties of chemical transformations to form oxygenates and higher molecular weight hydrocarbons.
- Palladium(O) complexes Pd(acac) 2 -Pli 3 P and Pd(OAc) 2 -Ph 3 P
- isoprene dimers e.g. 2,7-dimethyl- 1,3,7-octatriene
- methoxydimethyloctadienes Zakharkin, L. I. and Babich, S. A.
- JP59065026A (1984) reported preparation of l,6-dimethyl-l,5-cyclooctadiene by cyclic dimerization of isoprene in the presence of catalysts comprising Fe carboxylates or ⁇ -diketone compounds, organo-Al or Mg compounds, and 2,2'-dipyridyl derivatives having electron- donating groups.
- Dimethylcyclooctadiene was prepared by cyclodimerization of isoprene over 3-component catalysts containing Ni carboxylates or ⁇ -ketones, organoaluminum or organomagnesium compounds and substituted triphenylphosphite (JP58055434A, 1983.)
- 1,5- Dimethyl-l,5-cyclooctadiene was prepared by cyclodimerization of isoprene at 100-300° in an inert organic solvent in the presence of a homogeneous catalyst containing Fe(3) salt, organoaluminum compound and an activator (SU615056A1, 1978), in the presence of a homogeneous catalyst containing Ni acetylacetonate, a triarylphosphite and perhydroalumophenolene (SU493455A1, 1975), in the presence of a catalyst containing a mixture of a Ni carboxylate or carboxylate or chelate compounds of Ni and l-
- European Patent No. 2411(1981) disclosed cyclodimerization of isoprene over a Fe(NO) 2 Cl-bis(l,5-cyclooctadiene)nickel catalyst at from -5° to +20° to give 1-methyl- and 2- methyl-4-isopropenyl-l-cyclohexene and 1,4- and 2,4-dimethyl-4-vinyl-l-cyclohexene.
- U.S. Patent No. 4,189,403 disclosed preparation of l,5-dimethyl-l,5-cyclooctadiene and 1,4- dimethyl-4-vinyl-l-cyclohexene by contacting isoprene with a mixed catalyst of a tris(substituted hydrocarbyl) phosphite, arsenite, or antimonite and a Group VIII metal(O) compound (e.g. Ni acetylacetonate). Jackstell, R.; Grotevendt, A.; Michalik, D.; El Firdoussi, L.; Beller, M. J. Organometallic Chem.
- a commercially beneficial amount of highly pure isoprene starting material undergoes catalytic chemical transformation to give dimers and trimers.
- Catalyst systems are identified using methods known in the art.
- Preferred catalysts include those known to convert isoprene to dimers and trimers with high efficiency. Examples include those based upon Palladium, Nickel, Cobalt, Iron and Chromium.
- a commercially beneficial amount of highly pure isoprene starting material undergoes thermal or catalytic dimerization.
- the isoprene starting composition is converted to a mixture of dimers that includes unsaturated 6 and 8-membered rings by heating the starting composition under pressure.
- the starting isoprene composition is heated to over or about 100, 125, 150, 175, 200, 225 or 250 0 C under pressure.
- the starting isoprene composition is heated in the presence of an antioxidant (e.g. 2,6-di-tert-butyl-4-methylphenol) to prevent radical-mediated polymerization.
- an antioxidant e.g. 2,6-di-tert-butyl-4-methylphenol
- the reaction is accelerated by one or more catalysts selected from catalysts known in the art for catalyzing cyclic dimerization of isoprene, e.g., iron nitrosyl halide catalysts described in US patents 4,144,278, 4,181,707, 5,545,789 and European patent EP0397266A2.
- catalysts include but are not limited to Fe(NO) 2 Cl 2 and Cr 3+ -montmorillonite.
- an isoprene starting composition is converted to ClO cyclic dimers (e.g. a mixture of dimethyl-cyclooctadienes) using a ruthenium catalyst (Itoh, Kenji; Masuda, Katsuyuki; Fukahori, Takahiko; Nakano, Katsumasa; Aoki, Katsuyuki; Nagashima, Hideo, Organometallics (1994), 13(3), 1020-9.) [0602]
- a isoprene starting composition in a liquid state is heated to over 100 0 C under pressure and in the presence of an antioxidant to produce a mixture of dimers that includes 6 and 8-membered rings, for examples, limonene (l-methyl-4-(prop-l-en-2- yl)cyclohex- 1 -ene), 1 -methyl-5 -(prop- 1 -en-2-yl)cyclohex- 1 -ene, 1 ,
- a commercially beneficial amount of highly pure isoprene starting material undergoes photo-dimerization to give 4-membered ring dimers.
- the isoprene starting composition is converted by light irradiation to a mixture comprising one or more of 1 ,2-di(prop- 1 -en-2-yl)cyclobutane, 1 ,3-di(prop- 1 -en-2-yl)cyclobutane, 1 -methyl- 1 -vinyl-3 - (prop- 1 -en-2-yl)cyclobutane, 1 -methyl- 1 -vinyl-2-(prop- 1 -en-2-yl)cyclobutane, 1 ,3-dimethyl- 1,3- divinylcyclobutane, l,2-dimethyl-l,2-divinylcyclobutane and stereoisomers thereof .
- the highly pure isoprene starting material is dimerized in the presence of a catalyst (e.g. a nickel catalyst) or a photosensitizer (e.g. benzophenone) to yield 4-membered ring dimers, e.g. cis-2-isopropenyl-l-methylvinylcyclobutane, in addition to 6- and 8-membered rings
- a catalyst e.g. a nickel catalyst
- a photosensitizer e.g. benzophenone
- a commercially beneficial amount of highly pure isoprene starting material is converted to a cyclic trimer in the presence of a catalyst system.
- the cyclic trimer is trimethylcyclododecatriene.
- the catalyst system comprises a nickel catalyst.
- the catalyst system comprises a titanium compound.
- the catalyst system comprises a nickel catalyst and a titanium compound.
- the catalyst system comprises one or more organometallic compound and a Group VA compound.
- the catalytic transformation is conducted in a hydroxyl group-containing solvent.
- a starting isoprene composition is converted to trimethylcyclododecatriene in the presence of a catalyst system comprising Ni and/or Ti, one or more organometallic compound, and a Group VA compound, and that the reaction is conducted in a hydroxyl group -containing solvent.
- a commercially beneficial amount of highly pure isoprene starting material undergoes thermal or catalytic conversion to hydrocarbons in the C7 to C 14 range by treatment with a C2 to C5 unsaturated hydrocarbon.
- the C2 to C5 hydrocarbon can be an alkene, a diene or an alkyne.
- isoprene is contacted with ethylene and an appropriate catalyst to produce C7 compounds.
- isoprene is contacted with 1,3-butadiene to form cyclic C9 hydrocarbons. Hydrogenation of these C7 to C 14 hydrocarbons results in compositions suitable for use as jet fuels and other aviation fuels.
- unsaturated C7 to C14 hydrocarbons derived from isoprene and one or more unsaturated hydrocarbons undergo dehydrogenation to form aromatic derivatives suitable for use as jet fuels and other aviation fuels, or as blendstocks for such fuels.
- a commercially beneficial amount of highly pure isoprene starting material is converted to a mixture of linear dimers and trimers using a catalyst system in alcohol solvents. This reaction also produces alkoxy derivatives when performed in methanol or ethanol.
- the catalyst system is a palladium-based catalyst system, e.g., a palladium acetylacetonate - triphenylphosphine system or a palladium acetate - triphenylphosphine system.
- the alcohol solvent is methanol, ethanol or isopropyl alcohol. The nature of the products depends on the catalyst and the solvent used. For example, when a palladium-based catalyst system, e.g., Pd(acac) 2 -Pli 3 P or Pd(OAc) 2 -Ph 3 P, is used in isopropyl alcohol solvent, linear dimers of isoprene is formed, e.g.
- isoprene such as a-farnesene (3,7,11-trimethyl- 1,3, 6,10-dodecatetraene), ⁇ -farnesene (7,1 l-dimethyl-3 -methylene- 1, 6,10- dodecatriene) and other positional isomers (e.g. from tail to tail and head to head addition of isoprene units).
- isoprene is converted to linear and cyclic C 15 trimers using a chromium 7V,7V-bis(diarylphosphino)amine catalyst (Bowen, L.; Charernsuk, M.; Wass, D. F. Chem. Commun. (2007) 2835-2837.)
- a commercially beneficial amount of highly pure isoprene starting material is converted to fuel oxygenates by reaction with ethanol and other alcohols in the presence of an acid catalyst.
- the acid catalyst is sulfuric acid.
- the acid catalyst is a solid phase sulfuric acid (e.g., Dowex Marathon®).
- Other catalysts include both liquid and solid-phase fiuorosulfonic acids, for example, trifluoromethanesulfonic acid and Nafion-H (DuP ont).
- Zeolite catalysts can also been used, for example beta-zeolite, under conditions similar to those described by Hensel et al.
- a highly pure isoprene starting material is converted to alcohols and esters by a hydroxylation/esterification process or other known reaction of alkenes in the art, for example peroxidation to epoxides with peracids such as peracetic acid and 3-chloroperbenzoic acid; and hydration to give alcohols and diols with i) water and acid catalysts and ii) hydroboration methods.
- Such reactions are described in, for example, Michael B. Smith and Jerry March, Advanced Organic Chemistry: Reactions, Mechanisms, and Structure, Sixth Edition, John Wiley & Sons, 2007.
- Figures 3 and 4 show examples of alcohols and oxygenates that can be produced from the starting isoprene compositions.
- a commercially beneficial amount of highly pure isoprene starting material is partially hydrogenated to a mono-olefin (e.g. 2-methylbut-l-ene, 3-methyl- but-1-ene and 2-methylbut-2-ene).
- a mono-olefin e.g. 2-methylbut-l-ene, 3-methyl- but-1-ene and 2-methylbut-2-ene.
- the mono-olefin undergoes dimerization or reaction with other olefins using traditional hydrocarbon cationic catalysis, such as that used to covert isobutylene to isooctane. See, for example, H. M. Lybarger. Isoprene in Kirk-Othmer Encyclopedia of Chemical Technology, 4th ed., Wiley, New York (1995), 14, 934- 952.
- the highly pure isoprene starting material is a bioisoprene composition.
- a commercially beneficial amount of highly pure isoprene starting material undergoes partial hydrogenation to form mono-olefins (e.g. 2-methylbut-l-ene, 3-methyl-but-l-ene and 2-methylbut-2-ene).
- the highly pure isoprene starting material is a bioisoprene composition.
- partial hydrogenation of the isoprene starting material produces high yields of mono-olefins with minimal conversion to isopentane and low residual isoprene levels.
- a mixture of mono-olefin and isopentane is produced, with low levels of residual isoprene.
- the partial hydrogenation is selective hydrogenation where a particular mono-olefin such as 2-methylbut-2-ene, 2-methylbut-l-ene, or 3-methylbut-l-ene is preferentially produced.
- Preferred hydrogenation catalysts give high catalytic activity, maintain catalytic activity over time, and are highly selective for the conversion of isoprene to mono- olefins.
- Suitable catalysts for partial hydrogenation of the isoprene starting composition may contain a platinum-group metal such as platinum, palladium, rhodium, or ruthenium, or a transition metal such as nickel, cobalt, copper, iron, molybdenum, or a noble metal such as silver or gold in elemental form, or a salt or complex with organic and inorganic ligands. Alloys of these metals can also be used in some circumatances. These metals and their derivatives can be pure or mixed with other active and inert materials. Catalysts may be adsorbed to a support material (such as activated carbon, alumina, or silica) in order to maximize the effective surface area.
- a support material such as activated carbon, alumina, or silica
- Preferred hydrogenation catalysts include heterogeneous palladium catalysts such as palladium on carbon (Pd/C), palladium on alumina (PdZAl 2 O 3 ), or palladium on silica (Pd/SiO 2 ) in grades ranging from 0.1% Pd to 20% Pd (w/w) relative to the support material.
- Pd/C palladium on carbon
- PdZAl 2 O 3 palladium on alumina
- Pd/SiO 2 palladium on silica
- An example of a suitable selective hydrogenation catalyst is LD 2773, a sulfur tolerant promoted Pd on alumina (Axens, Rueil-Malmaison Cedex, France).
- Partial hydrogenation catalysts that allow the conversion of isoprene into isoamylenes with minimal conversion to isopentane include the Lindlar catalyst (Pd/BaSC> 4 treated with quinoline), Pd/C treated with the triphenyl derivative of a group 15 element (N, P, As), Pd/C treated with a sulfur- containing compound, molybdenum sulfide, and Pd/Fe alloys.
- One preferred catalyst comprises palladium adsorbed to egg-shell alumina (d-Al 2 O 3 ).
- Catalysts used in the refining industry for the removal of diolefins and alkynes from pyro lysis gasoline are particularly preferred, for example Ni/ Al 2 O 3 and Pd/ Al 2 O 3 based catalysts.
- Another suitable class of catalyst for the conversion of isoprene to isoamylenes are those used in industry for the hydrotreating of pyrolysis gasoline. See for example US Patent Nos. 7,014,750 and 6,9949,686, and references cited therein.
- the catalysts used in pyrolysis gas hydrotreament allow for selective conversion of acetlyenes and diolefins (e.g. isoprene and piperylenes) into monoolefins.
- the hydrogen source can be hydrogen gas or a hydrogen source including, but not limited to, hydrogen gas (H 2 ), formic acid, hydrazine, or isopropanol.
- the hydrogen source can be either chemically or biologically derived.
- the hydrogen used for hydrogenation is co-produced with isoprene during fermentation.
- the hydrogenation can be performed at hydrogen pressures ranging from 0.5 atm to 200 atm, or higher.
- the temperature can range from 0 0 C to 200 0 C.
- Products from partial hydrogenation of a bioisoprene composition are expected to contain certain impurities originally present in the starting isoprene composition and/or hydrogenated derivatives of the imputities such as acetone, ethanol, amyl alcohol, ethyl acetate, isoamyl acetate, methyl ethyl ketone and other saturated polar impurities.
- the isoprene starting composition undergoes partial hydrogenation or selective hydrogenation in the presence of a palladium catalyst.
- palladium catalysts such as Pd/CaCO 3 , Pd/BaSO 4 , Pd/C, Pd black, Pd/SiO3, Pd/Al 2 O 3 , or Pd/SiO 2 , were shown to convert isoprene to mono-olefins with a selectivity of greater than 95% over fully reduced C5 alkanes.
- Use of these palladium catalysts gave a mixture of mono-olefin products, with 5%Pd/CaCO3 having the greatest selectivity for 3-methylbut-l-ene and 5% Pd/SiO 2 having the greatest selectivity for 2-methylbut-2-ene. (See G. C. Bond and A.F. Rawle. J. MoI.
- Catalysis A Chemical 109 (1996) 261-271.) Bond and Rawle have also shown that palladium-gold and palladium-silver catalysts (e.g., Pd-Au/SiO 2 and Pd-Ag/SiO 2 ) have high selectivity for reducing isoprene to mono-olefins over fully reduced C5 alkanes.
- palladium-gold and palladium-silver catalysts e.g., Pd-Au/SiO 2 and Pd-Ag/SiO 2
- silica-supported polyamidoamine (PAMAM) dendrimer-palladium complexes have been used to selectively catalyze the reduction of cyclic and acyclic dienes to a mixture of mono-olefin isomers.
- reduction of isoprene to mono-olefins can be carried out using a Group VIB metal on an inorganic support (e.g., a metal zeolite) as a catalyst.
- a Mo/Al 2 O 3 catalyst was used to selectively reduce 1,3-butadiene to the respective mono-olefins.
- isoprene can be reduced to mono- olefins using a Group VIII metal catalyst promoted by a metal from Group IB, VIB, VIIB, or zinc to reduce poisoning of the catalyst. (See US 6,949,686 B2).
- isoprene can be reduced to mono-olefins using a monolithic catalyst bed, which may be in a honeycomb configuration.
- Catalyst support materials for the monolithic catalyst bed may include metals such as nickel, platinum, palladium, rhodium, ruthenium, silver, iron, copper, cobalt, chromium, iridium, tin, and alloys or mixtures thereof.
- isoprene can be reduced to mono-olefins using an eggshell PdZd-Al 2 O 3 catalyst, particularly if the reaction is free of water.
- the eggshell PdZd-Al 2 O 3 catalyst is selective for mono-olefins over fully reduced alkanes and 2-methylbut-2-ene is the thermodynamically favorable isomer.
- Light olefins can be readily dimerized using acid catalysts to give higher olefins (C6-C12) also known as dimate.
- acid catalysts including sulfuric, phosphoric and other mineral acids, sulfonic acids, fiuorosulfonic acids, zeolites and acidic clays.
- the isoprene starting composition undergoes partial hydrogenation or selective hydrogenation to form a mono-olefin, and the mono-olefin undergoes dimerization or reaction with other olefins using traditional hydrocarbon cationic catalysis, such as that used to covert isobutylene to isooctane, e.g. sulfuric, phosphoric and other mineral acids, sulfonic acids, fiuorosulfonic acids, zeolites and acidic clays. See, for example, H. M. Lybarger. Isoprene in Kirk-Othmer Encyclopedia of Chemical Technology, 4th ed., Wiley, New York (1995), 14, 934-952.
- the highly pure isoprene starting material is a bioisoprene composition.
- Acid catalyst in both liquid and solid forms can be used.
- Acid resins are preferred and include Amberlyst 15, 35, XE586, XNlOlO (Rohm and Haas) and similar acidic ion-exchange resins.
- Acidic molecular sieves are also preferred catalysts including a medium-pore acid molecular sieve such as ZSM-5, ferrierite, ZSM-22 and ZSM-23.
- the isoprene starting composition undergoes partial hydrogenation or selective hydrogenation to form isoamylenes.
Landscapes
- Chemical & Material Sciences (AREA)
- Organic Chemistry (AREA)
- Oil, Petroleum & Natural Gas (AREA)
- Engineering & Computer Science (AREA)
- Chemical Kinetics & Catalysis (AREA)
- General Chemical & Material Sciences (AREA)
- Materials Engineering (AREA)
- Preparation Of Compounds By Using Micro-Organisms (AREA)
- Organic Low-Molecular-Weight Compounds And Preparation Thereof (AREA)
- Production Of Liquid Hydrocarbon Mixture For Refining Petroleum (AREA)
- Low-Molecular Organic Synthesis Reactions Using Catalysts (AREA)
- Catalysts (AREA)
Abstract
Description
Claims
Priority Applications (10)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| CN201080036083.XA CN102803185B (en) | 2009-06-17 | 2010-06-17 | Comprise the fuel composition of isoprene derivatives |
| JP2012516318A JP5448220B2 (en) | 2009-06-17 | 2010-06-17 | Fuel composition comprising isoprene derivative |
| MX2011013805A MX2011013805A (en) | 2009-06-17 | 2010-06-17 | Fuel compositions comprising isoprene derivatives. |
| CA2765916A CA2765916A1 (en) | 2009-06-17 | 2010-06-17 | Fuel compositions comprising isoprene derivatives |
| RU2012101453/04A RU2531623C2 (en) | 2009-06-17 | 2010-06-17 | Fuel compositions containing isoprene derivatives |
| SG2011093077A SG176875A1 (en) | 2009-06-17 | 2010-06-17 | Fuel compositions comprising isoprene derivatives |
| EP10790225.6A EP2448891A4 (en) | 2009-06-17 | 2010-06-17 | Fuel compositions comprising isoprene derivatives |
| AU2010262774A AU2010262774B2 (en) | 2009-06-17 | 2010-06-17 | Fuel compositions comprising isoprene derivatives |
| BRPI1011952A BRPI1011952A2 (en) | 2009-06-17 | 2010-06-17 | "fuel compositions comprising isoprene derivatives" |
| ZA2011/09375A ZA201109375B (en) | 2009-06-17 | 2011-12-20 | Fuel compositions comprising isoprene derivatives |
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US18795909P | 2009-06-17 | 2009-06-17 | |
| US61/187,959 | 2009-06-17 |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| WO2010148256A1 true WO2010148256A1 (en) | 2010-12-23 |
Family
ID=43356762
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| PCT/US2010/039088 WO2010148256A1 (en) | 2009-06-17 | 2010-06-17 | Fuel compositions comprising isoprene derivatives |
Country Status (16)
| Country | Link |
|---|---|
| US (2) | US8450549B2 (en) |
| EP (1) | EP2448891A4 (en) |
| JP (1) | JP5448220B2 (en) |
| KR (1) | KR20120047908A (en) |
| CN (1) | CN102803185B (en) |
| AR (1) | AR077114A1 (en) |
| AU (1) | AU2010262774B2 (en) |
| BR (1) | BRPI1011952A2 (en) |
| CA (1) | CA2765916A1 (en) |
| MX (1) | MX2011013805A (en) |
| MY (1) | MY155508A (en) |
| RU (1) | RU2531623C2 (en) |
| SG (2) | SG176875A1 (en) |
| TW (1) | TWI434921B (en) |
| WO (1) | WO2010148256A1 (en) |
| ZA (1) | ZA201109375B (en) |
Cited By (21)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2011160081A1 (en) | 2010-06-17 | 2011-12-22 | Danisco Us Inc. | Fuel compositions comprising isoprene derivatives |
| EP2443244A1 (en) * | 2009-06-17 | 2012-04-25 | Danisco US Inc. | Improved isoprene production using the dxp and mva pathway |
| WO2012088462A1 (en) | 2010-12-22 | 2012-06-28 | Danisco Us Inc. | Compositions and methods for improved isoprene production using two types of ispg enzymes |
| WO2012149491A2 (en) | 2011-04-29 | 2012-11-01 | Danisco Us Inc. | Recombinant microorganisms for enhanced production of mevalonate, isoprene, and isoprenoids |
| WO2012149469A1 (en) | 2011-04-29 | 2012-11-01 | Danisco Us Inc. | Production of mevalonate, isoprene, and isoprenoids using genes encoding polypeptides having thiolase, hmg-coa synthase and hmg-coa reductase enzymatic activities |
| WO2013020118A1 (en) | 2011-08-04 | 2013-02-07 | Danisco Us Inc. | Production of isoprene, isoprenoid precursors, and isoprenoids using acetoacetyl-coa synthase |
| WO2013066568A1 (en) | 2011-10-07 | 2013-05-10 | Danisco Us Inc. | Utilization of phosphoketolase in the production of mevalonate, isoprenoid precursors, and isoprene |
| WO2013149192A1 (en) | 2012-03-30 | 2013-10-03 | Danisco Us Inc. | Direct starch to fermentable sugar as feedstock for the production of isoprene, isoprenoid precursor molecules, and/or isoprenoids |
| WO2013166211A2 (en) | 2012-05-02 | 2013-11-07 | Danisco Us Inc. | Identification of isoprene synthase variants with improved properties for the production of isoprene |
| WO2013166320A1 (en) | 2012-05-02 | 2013-11-07 | Danisco Us Inc. | Legume isoprene synthase for production of isoprene |
| WO2013181647A2 (en) | 2012-06-01 | 2013-12-05 | Danisco Us Inc. | Compositions and methods of producing isoprene and/or industrrial bio-products using anaerobic microorganisms |
| US8691541B2 (en) | 2010-12-22 | 2014-04-08 | Danisco Us Inc. | Biological production of pentose sugars using recombinant cells |
| WO2014100726A2 (en) | 2012-12-21 | 2014-06-26 | Danisco Us Inc. | Production of isoprene, isoprenoid, and isoprenoid precursors using an alternative lower mevalonate pathway |
| US8778647B2 (en) | 2009-12-22 | 2014-07-15 | Danisco Us Inc. | Methods of producing isoprene comprising a membrane bioreactor |
| WO2014169144A2 (en) | 2013-04-10 | 2014-10-16 | Danisco Us Inc. | Phosphoketolases foe improved production of acetyl coenzyme a-derived metabolites, isoprene, isoprenoid precurosors, and isoprenoids |
| US8865442B2 (en) | 2011-12-23 | 2014-10-21 | Danisco Us Inc. | Production of isoprene under reduced oxygen inlet levels |
| WO2014205355A2 (en) | 2013-06-21 | 2014-12-24 | Danisco Us Inc. | Compositions and methods for clostridial transformation |
| US8975051B2 (en) | 2011-12-23 | 2015-03-10 | Danisco Us Inc. | Enhanced production of isoprene using host cells having decreased ispA activity |
| WO2015127305A2 (en) | 2014-02-20 | 2015-08-27 | Danisco Us Inc. | Recombinant microorganisms for the enhanced production of mevalonate, isoprene, isoprenoid precursors, isoprenoids, and acetyl-coa-derived products |
| WO2016015021A1 (en) | 2014-07-24 | 2016-01-28 | The Regents Of The University Of California | Oxidative starch degradation by a new family of pmos |
| US9273298B2 (en) | 2010-10-27 | 2016-03-01 | Danisco Us Inc. | Isoprene synthase variants for improved production of isoprene |
Families Citing this family (26)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| EP2382312A2 (en) * | 2008-12-30 | 2011-11-02 | Danisco US Inc. | Methods of producing isoprene and a co-product |
| US9522854B2 (en) * | 2009-07-29 | 2016-12-20 | The United States Of America As Represented By The Secretary Of The Navy | Process and apparatus for the selective dimerization of terpenes and poly-alpha-olefins with a single-stage reactor and a single-stage fractionation system |
| IN2012DN05187A (en) * | 2009-12-18 | 2015-10-23 | Danisco Us Inc | |
| EP2601300A1 (en) | 2010-08-06 | 2013-06-12 | Danisco US Inc. | Production of isoprene under neutral ph conditions |
| BR112014001939A2 (en) * | 2011-07-28 | 2017-02-21 | Glycos Biotechnologies Inc | compositions and methods for biofermentation of oil-containing feed stocks |
| US8951764B2 (en) | 2011-08-05 | 2015-02-10 | Danisco Us Inc. | Production of isoprenoids under neutral pH conditions |
| US9631210B2 (en) | 2011-09-14 | 2017-04-25 | The Regents Of The University Of California | Methods for increasing production of 3-methyl-2-butenol using fusion proteins |
| EP2898053B1 (en) | 2012-09-20 | 2021-08-25 | Danisco US Inc. | Microtiter plates for controlled release of culture components to cell cultures |
| BR112015018807B1 (en) * | 2013-02-07 | 2021-07-27 | Braskem S.A. | METHOD FOR SEPARATION AND PURIFICATION OF A CONJUGATED DIOLEPHINE PRODUCED BY FERMENTATION UNDER ANAEROBIC CONDITIONS |
| US9850512B2 (en) | 2013-03-15 | 2017-12-26 | The Research Foundation For The State University Of New York | Hydrolysis of cellulosic fines in primary clarified sludge of paper mills and the addition of a surfactant to increase the yield |
| WO2014156997A1 (en) * | 2013-03-29 | 2014-10-02 | 味の素株式会社 | Method for collecting isoprene contained in fermented gas, and method for producing purified isoprene |
| US9371258B1 (en) * | 2013-06-27 | 2016-06-21 | The United States Of America As Represented By The Secretary Of The Navy | High density fuels from isoprene |
| US9926245B2 (en) * | 2013-09-30 | 2018-03-27 | Pioneer Energy | Fuels and chemicals from lower alkanes |
| WO2015081406A1 (en) * | 2013-12-02 | 2015-06-11 | Braskem S.A. | Fermentation hydrocarbon gas products separation via membrane |
| US9951363B2 (en) | 2014-03-14 | 2018-04-24 | The Research Foundation for the State University of New York College of Environmental Science and Forestry | Enzymatic hydrolysis of old corrugated cardboard (OCC) fines from recycled linerboard mill waste rejects |
| BR112017010654A2 (en) * | 2014-11-26 | 2018-02-14 | Visolis Inc | processes for converting biologically derived mevalonic acid |
| DE102015000626A1 (en) * | 2015-01-22 | 2016-07-28 | Kibion Gmbh | Method for the detection of Helicobacter pylori |
| ES2901236T3 (en) * | 2016-02-11 | 2022-03-21 | Dow Technology Investments Llc | Processes for converting olefins into alcohols, ethers or combinations thereof |
| FR3062132B1 (en) | 2017-01-23 | 2020-12-04 | Eitl | FLAME-RETARDING PRODUCT, METHOD FOR MANUFACTURING SUCH A PRODUCT AND EXTINGUISHING DEVICE INCLUDING SUCH A PRODUCT |
| CN108998106B (en) * | 2018-08-27 | 2022-01-28 | 太原理工大学 | Desulfurization and deamination method for coking plant |
| CN113573798A (en) * | 2019-03-18 | 2021-10-29 | 诺沃梅尔公司 | Membrane separation system and use thereof |
| CN111909727B (en) * | 2020-09-22 | 2022-02-01 | 中国海洋大学 | Green synthesis method for preparing saturated hydrocarbon fuel from isoprene |
| CN112588279B (en) * | 2020-12-15 | 2022-08-02 | 华东理工大学 | Preparation method of catalyst for hydrogen production by methanol steam reforming, product and application thereof |
| US20230193148A1 (en) * | 2021-12-16 | 2023-06-22 | Battelle Memorial Institute | Method embodiments for partial hydrogenation of carbocyclic compounds to produce jet fuel blendstock |
| CN115557826B (en) * | 2022-10-09 | 2024-09-06 | 天津大学 | Preparation method of isoprene dimer, fuel and preparation method thereof |
| CN115845899B (en) * | 2022-12-02 | 2024-03-01 | 平顶山学院 | Preparation method of catalyst for catalyzing 1, 3-butadiene dicarbonyl to synthesize methyl adipate |
Citations (38)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US1338607A (en) | 1919-10-18 | 1920-04-27 | William E Bell | Electric switch |
| US3185739A (en) | 1960-05-19 | 1965-05-25 | Phillips Petroleum Co | Conversion of cyclotriene compounds |
| US3418386A (en) * | 1966-07-05 | 1968-12-24 | Columbian Carbon | Hydrogenation of cyclooctadienes to cyclooctenes |
| US3917730A (en) * | 1972-10-10 | 1975-11-04 | Elf Aquitaine Union Chimique | New catalyst for the dimerization of diolefins |
| US4166076A (en) * | 1977-01-19 | 1979-08-28 | Shell Oil Company | Cyclodimerization of isoprene |
| US4283305A (en) * | 1978-12-11 | 1981-08-11 | Institut Francais Du Petrole | Catalyst composition and its use for oligomerizing olefins |
| EP0137280A1 (en) | 1983-08-31 | 1985-04-17 | Cetus Oncology Corporation | Recombinant fungal cellobiohydrolases |
| US4570029A (en) | 1985-03-04 | 1986-02-11 | Uop Inc. | Process for separating isoprene |
| EP0215594A2 (en) | 1985-08-29 | 1987-03-25 | Genencor International, Inc. | Heterologous polypeptide expressed in filamentous fungi, processes for their preparation, and vectors for their preparation |
| US4683202A (en) | 1985-03-28 | 1987-07-28 | Cetus Corporation | Process for amplifying nucleic acid sequences |
| EP0238023A2 (en) | 1986-03-17 | 1987-09-23 | Novo Nordisk A/S | Process for the production of protein products in Aspergillus oryzae and a promoter for use in Aspergillus |
| US4703007A (en) | 1984-03-27 | 1987-10-27 | Ontario Research Foundation | Separation of volatiles from aqueous solutions by gas stripping |
| EP0244234A2 (en) | 1986-04-30 | 1987-11-04 | Alko Group Ltd. | Transformation of trichoderma |
| US4740222A (en) | 1982-05-03 | 1988-04-26 | Advanced Extraction Technologies, Inc. | Recovery and purification of hydrogen from refinery and petrochemical off-gas streams |
| US5329057A (en) * | 1991-04-19 | 1994-07-12 | The Dow Chemical Company | Process for the cyclodimerization of 1,3-butadienes to 4-vinylcyclohexenes |
| WO1996035796A1 (en) | 1995-05-12 | 1996-11-14 | E.I. Du Pont De Nemours And Company | Bioconversion of a fermentable carbon source to 1,3-propanediol by a single microorganism |
| WO1998002550A2 (en) | 1996-07-15 | 1998-01-22 | Fraunhofer-Gesellschaft zur Förderung der angewandten Forschung e.V. | Microbial isoprene generation |
| US5849970A (en) | 1995-06-23 | 1998-12-15 | The Regents Of The University Of Colorado | Materials and methods for the bacterial production of isoprene |
| US5874276A (en) | 1993-12-17 | 1999-02-23 | Genencor International, Inc. | Cellulase enzymes and systems for their expressions |
| US5906662A (en) * | 1996-07-16 | 1999-05-25 | Shell Oil Company | Liquid hydrocarbon fuel composition |
| US6022725A (en) | 1990-12-10 | 2000-02-08 | Genencor International, Inc. | Cloning and amplification of the β-glucosidase gene of Trichoderma reesei |
| US6106888A (en) | 1998-04-30 | 2000-08-22 | Board Of Trustees Operating Michigan State University | Process for treating cellulosic materials |
| US6188142B1 (en) | 1996-09-14 | 2001-02-13 | Braun Gmbh | Method and arrangement for disconnecting consumers |
| US6268328B1 (en) | 1998-12-18 | 2001-07-31 | Genencor International, Inc. | Variant EGIII-like cellulase compositions |
| WO2004033646A2 (en) | 2002-10-04 | 2004-04-22 | E.I. Du Pont De Nemours And Company | Process for the biological production of 1,3-propanediol with high yield |
| WO2005001036A2 (en) | 2003-05-29 | 2005-01-06 | Genencor International, Inc. | Novel trichoderma genes |
| US7132527B2 (en) | 2002-04-22 | 2006-11-07 | E. I. Du Pont De Nemours And Company | Promoter and plasmid system for genetic engineering |
| US7169588B2 (en) | 1995-05-12 | 2007-01-30 | E. I. Du Pont De Nemours And Company | Bioconversion of a fermentable carbon source to 1,3-propanediol by a single microorganism |
| WO2007089901A2 (en) | 2006-02-01 | 2007-08-09 | University Of Hawaii | Metabolically engineered organisms for the production of hydrogen and hydrogenase |
| US7262041B2 (en) | 2003-11-21 | 2007-08-28 | Genencor International, Inc. | Expression of granular starch hydrolyzing enzyme in Trichoderma |
| WO2009076676A2 (en) | 2007-12-13 | 2009-06-18 | Danisco Us Inc. | Compositions and methods for producing isoprene |
| US20100003716A1 (en) | 2008-04-23 | 2010-01-07 | Cervin Marguerite A | Isoprene synthase variants for improved microbial production of isoprene |
| WO2010003007A2 (en) | 2008-07-02 | 2010-01-07 | Danisco Us Inc. | Compositions and methods for producing isoprene free of c5 hydrocarbons under decoupling conditions and/or safe operating ranges |
| WO2010031076A2 (en) | 2008-09-15 | 2010-03-18 | Danisco Us Inc. | Conversion of prenyl derivatives to isoprene |
| WO2010031062A1 (en) | 2008-09-15 | 2010-03-18 | Danisco Us Inc. | Increased isoprene production using the archaeal lower mevalonate pathway |
| WO2010031079A1 (en) | 2008-09-15 | 2010-03-18 | Danisco Us Inc. | Systems using cell culture for production of isoprene |
| WO2010031077A1 (en) | 2008-09-15 | 2010-03-18 | Danisco Us Inc. | Increased isoprene production using mevalonate kinase and isoprene synthase |
| WO2010031068A1 (en) | 2008-09-15 | 2010-03-18 | Danisco Us Inc. | Reduction of carbon dioxide emission during isoprene production by fermentation |
Family Cites Families (61)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JPS507565B1 (en) * | 1969-12-24 | 1975-03-27 | ||
| JPS5233110B2 (en) | 1971-12-13 | 1977-08-26 | ||
| FR2225401B1 (en) | 1973-04-12 | 1976-05-21 | Aquitaine Petrole | |
| SU493455A1 (en) | 1973-07-03 | 1975-11-28 | Институт Химии Башкирского Филиала Ан Ссср | Method for preparing 1,5-dimethylcyclooctadiene 1.5 |
| JPS535642B2 (en) * | 1973-09-08 | 1978-03-01 | ||
| SU615056A1 (en) | 1976-08-01 | 1978-07-15 | Институт Химии Башкирского Филиала Ан Ссср | Method of obtaining 1,5-dimethylcyclooctadien-1,5 |
| US4189403A (en) | 1977-01-19 | 1980-02-19 | Shell Oil Company | Catalyst for cyclodimerization of isoprene |
| US4144278A (en) | 1977-07-20 | 1979-03-13 | Phillips Petroleum Company | Diolefin dimerization using nitrosyl halides of iron triad metals |
| US4181707A (en) | 1977-07-20 | 1980-01-01 | Phillips Petroleum Company | Diolefin dimerization catalyst and method for producing nitrosyl halides of iron triad metals |
| DD153820A1 (en) * | 1980-08-29 | 1982-02-03 | Ernst Alder | PROCESS FOR PREPARING ACYLIC DIHYDRODIMER OF 1.3-SERVES |
| JPS5855434A (en) | 1981-09-28 | 1983-04-01 | Mitsubishi Petrochem Co Ltd | Preparation of dimethylcyclooctadiene |
| JPS5965026A (en) | 1982-10-05 | 1984-04-13 | Mitsubishi Petrochem Co Ltd | Preparation of 1,6-dimethyl-1,5-cyclooctadiene |
| JPS59170192A (en) * | 1983-03-18 | 1984-09-26 | Nippon Petrochem Co Ltd | Fuel composition |
| DE3560049D1 (en) * | 1984-05-10 | 1987-02-19 | Charbonnages Ste Chimique | Process for dimerizung a conjugated diene |
| FR2564089B1 (en) * | 1984-05-10 | 1986-09-12 | Charbonnages Ste Chimique | BUTADIENE-1,3 DIMERIZATION PROCESS |
| US4652527A (en) | 1984-10-09 | 1987-03-24 | Celanese Corporation | Process for culturing methylophilus methylotrophus |
| US4818250A (en) | 1987-10-21 | 1989-04-04 | Lemco Energy, Inc. | Process for producing fuel from plant sources and fuel blends containing same |
| US4973568A (en) | 1989-05-08 | 1990-11-27 | The Dow Chemical Company | Preparation of a catalyst useful in the dimerization of butadiene |
| US4973787A (en) * | 1989-09-05 | 1990-11-27 | The Goodyear Tire & Rubber Company | Process for the thermal dimerization of isoprene |
| NL9201931A (en) * | 1992-11-05 | 1994-06-01 | Dsm Nv | Method for the dimerization of a conjugated diene. |
| JP3299585B2 (en) * | 1993-03-29 | 2002-07-08 | 株式会社東芝 | Paper feeder |
| FR2704222B1 (en) * | 1993-04-23 | 1995-06-30 | Inst Francais Du Petrole | Codimerization process for dienes and olefins. |
| WO1995004134A1 (en) | 1993-08-02 | 1995-02-09 | Genencor International, Inc. | Method of reducing complex carbohydrates in fermentation products |
| US5575822A (en) | 1994-05-04 | 1996-11-19 | Wilkins, Jr.; Joe S. | Engine fuels |
| US5446102A (en) | 1994-08-10 | 1995-08-29 | Bridgeston, Corporation | Olefin metathesis catalysts for degelling polymerization reactors |
| US6429349B1 (en) | 1996-08-12 | 2002-08-06 | Bp Corporation North America Inc. | Co-alkylation for gasoline RVP reduction |
| US6235954B1 (en) | 1999-09-29 | 2001-05-22 | Phillips Petroleum Company | Hydrocarbon hydrogenation catalyst and process |
| JP2003524697A (en) | 2000-02-14 | 2003-08-19 | ザ、プロクター、エンド、ギャンブル、カンパニー | Synthetic jet and diesel fuel compositions and methods |
| EP1245591B1 (en) * | 2000-06-30 | 2006-11-22 | Asahi Kasei Kabushiki Kaisha | Method for hydrogenation of diene polymers and copolymers |
| FR2814173B1 (en) | 2000-09-15 | 2005-09-16 | Inst Francais Du Petrole | COMPOSITIONS OF DIESEL FUELS CONTAINING OXYGEN COMPOUNDS DERIVED FROM TETRAHYDROFURFURYL |
| US6660898B1 (en) | 2000-11-03 | 2003-12-09 | Fortum Oil & Gas Oy | Process for dimerizing light olefins to produce a fuel component |
| JP4430869B2 (en) | 2001-04-04 | 2010-03-10 | ジェネンコー・インターナショナル・インク | Isolated production and catabolic pathways of host cells |
| RU2184720C1 (en) * | 2001-08-24 | 2002-07-10 | Попов Валерий Георгиевич | Olefin dimmer and oligomer production process |
| US7338541B2 (en) | 2001-11-20 | 2008-03-04 | The Procter & Gamble Company | Synthetic jet fuel and diesel fuel compositions and processes |
| US6949686B2 (en) | 2002-05-30 | 2005-09-27 | Equistar Chemicals, Lp | Pyrolysis gasoline stabilization |
| US20040004031A1 (en) | 2002-06-26 | 2004-01-08 | Boger Thorsten R. | System and process for pyrolysis gasoline hydrotreatment |
| US8329603B2 (en) | 2003-09-16 | 2012-12-11 | Uop Llc | Isoparaffin-olefin alkylation |
| US7521393B2 (en) | 2004-07-27 | 2009-04-21 | Süd-Chemie Inc | Selective hydrogenation catalyst designed for raw gas feed streams |
| DE102004054477A1 (en) | 2004-11-11 | 2006-05-24 | Degussa Ag | Process for the preparation of trimethylcyclododecatriene |
| JP5102943B2 (en) | 2005-05-25 | 2012-12-19 | Jx日鉱日石エネルギー株式会社 | Solid phosphoric acid catalyst and olefin dimerization reaction method using the same |
| US8299312B2 (en) * | 2005-10-28 | 2012-10-30 | Neste Oil Oyj | Process for dimerizing olefins |
| EP2038530A4 (en) | 2006-05-26 | 2011-04-27 | Amyris Biotechnologies Inc | Fuel components, fuel compositions and methods of making and using same |
| US20090099401A1 (en) | 2006-06-16 | 2009-04-16 | D Amore Michael B | Process for making isooctenes from aqueous isobutanol |
| US20080009656A1 (en) | 2006-06-16 | 2008-01-10 | D Amore Michael B | Process for making isooctenes from dry isobutanol |
| US7947478B2 (en) | 2006-06-29 | 2011-05-24 | The Regents Of The University Of California | Short chain volatile hydrocarbon production using genetically engineered microalgae, cyanobacteria or bacteria |
| DE102007023515A1 (en) * | 2006-07-05 | 2008-01-10 | Evonik Degussa Gmbh | Preparing 1,7-diolefin, useful to prepare alpha, omega-diamine, -diol and sebacic acid, comprises hydrodimerization of non-cyclic olefins with two conjugated double bonds in the presence of a reducing agent and a catalyst |
| US8049048B2 (en) | 2006-07-27 | 2011-11-01 | Swift Enterprises, Ltd. | Renewable engine fuel |
| US7399323B2 (en) | 2006-10-10 | 2008-07-15 | Amyris Biotechnologies, Inc. | Fuel compositions comprising farnesane and farnesane derivatives and method of making and using same |
| US20080236029A1 (en) | 2007-03-27 | 2008-10-02 | Wilkins Joe S | Engine fuel compositions |
| EP2152854A2 (en) | 2007-05-01 | 2010-02-17 | Acidophil LLC | Methods for the direct conversion of carbon dioxide into a hydrocarbon using a metabolically engineered photosynthetic microorganism |
| EP2173837A2 (en) | 2007-07-20 | 2010-04-14 | Amyris Biotechnologies, Inc. | Fuel compositions comprising tetramethylcyclohexane |
| US8523959B2 (en) | 2007-07-26 | 2013-09-03 | Chevron U.S.A. Inc. | Paraffinic biologically-derived distillate fuels with bio-oxygenates for improved lubricity and methods of making same |
| EP2203542A1 (en) | 2007-09-11 | 2010-07-07 | Sapphire Energy, Inc. | Methods of producing organic products with photosynthetic organisms and products and compositions thereof |
| WO2009064910A2 (en) | 2007-11-13 | 2009-05-22 | Synthetic Genomics, Inc. | Dimethyloctane as an advanced biofuel |
| US8211189B2 (en) | 2007-12-10 | 2012-07-03 | Wisys Technology Foundation, Inc. | Lignin-solvent fuel and method and apparatus for making same |
| CN102131935B (en) | 2008-06-30 | 2013-11-13 | 固特异轮胎和橡胶公司 | Polymers of isoprene from renewable resources |
| US20100099932A1 (en) | 2008-10-21 | 2010-04-22 | Ecoprene Llc | Isoprene Compositions and Methods of Use |
| EP2382312A2 (en) | 2008-12-30 | 2011-11-02 | Danisco US Inc. | Methods of producing isoprene and a co-product |
| TW201120213A (en) | 2009-06-17 | 2011-06-16 | Danisco Us Inc | Polymerization of isoprene from renewable resources |
| IN2012DN05187A (en) | 2009-12-18 | 2015-10-23 | Danisco Us Inc | |
| CN103025688A (en) * | 2010-06-17 | 2013-04-03 | 丹尼斯科美国公司 | Fuel compositions comprising isoprene derivatives |
-
2010
- 2010-06-15 TW TW099119620A patent/TWI434921B/en active
- 2010-06-16 AR ARP100102131A patent/AR077114A1/en unknown
- 2010-06-17 CN CN201080036083.XA patent/CN102803185B/en not_active Expired - Fee Related
- 2010-06-17 JP JP2012516318A patent/JP5448220B2/en not_active Expired - Fee Related
- 2010-06-17 EP EP10790225.6A patent/EP2448891A4/en not_active Withdrawn
- 2010-06-17 BR BRPI1011952A patent/BRPI1011952A2/en not_active IP Right Cessation
- 2010-06-17 AU AU2010262774A patent/AU2010262774B2/en not_active Ceased
- 2010-06-17 US US12/818,090 patent/US8450549B2/en not_active Expired - Fee Related
- 2010-06-17 KR KR1020127001228A patent/KR20120047908A/en not_active Application Discontinuation
- 2010-06-17 CA CA2765916A patent/CA2765916A1/en not_active Abandoned
- 2010-06-17 SG SG2011093077A patent/SG176875A1/en unknown
- 2010-06-17 RU RU2012101453/04A patent/RU2531623C2/en not_active IP Right Cessation
- 2010-06-17 SG SG10201507749UA patent/SG10201507749UA/en unknown
- 2010-06-17 WO PCT/US2010/039088 patent/WO2010148256A1/en active Application Filing
- 2010-06-17 MY MYPI2011006129A patent/MY155508A/en unknown
- 2010-06-17 MX MX2011013805A patent/MX2011013805A/en active IP Right Grant
-
2011
- 2011-12-20 ZA ZA2011/09375A patent/ZA201109375B/en unknown
-
2013
- 2013-04-26 US US13/872,006 patent/US20140148623A1/en not_active Abandoned
Patent Citations (41)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US1338607A (en) | 1919-10-18 | 1920-04-27 | William E Bell | Electric switch |
| US3185739A (en) | 1960-05-19 | 1965-05-25 | Phillips Petroleum Co | Conversion of cyclotriene compounds |
| US3418386A (en) * | 1966-07-05 | 1968-12-24 | Columbian Carbon | Hydrogenation of cyclooctadienes to cyclooctenes |
| US3917730A (en) * | 1972-10-10 | 1975-11-04 | Elf Aquitaine Union Chimique | New catalyst for the dimerization of diolefins |
| US4166076A (en) * | 1977-01-19 | 1979-08-28 | Shell Oil Company | Cyclodimerization of isoprene |
| US4283305A (en) * | 1978-12-11 | 1981-08-11 | Institut Francais Du Petrole | Catalyst composition and its use for oligomerizing olefins |
| US4740222A (en) | 1982-05-03 | 1988-04-26 | Advanced Extraction Technologies, Inc. | Recovery and purification of hydrogen from refinery and petrochemical off-gas streams |
| EP0137280A1 (en) | 1983-08-31 | 1985-04-17 | Cetus Oncology Corporation | Recombinant fungal cellobiohydrolases |
| US4703007A (en) | 1984-03-27 | 1987-10-27 | Ontario Research Foundation | Separation of volatiles from aqueous solutions by gas stripping |
| US4570029A (en) | 1985-03-04 | 1986-02-11 | Uop Inc. | Process for separating isoprene |
| US4683202A (en) | 1985-03-28 | 1987-07-28 | Cetus Corporation | Process for amplifying nucleic acid sequences |
| US4683202B1 (en) | 1985-03-28 | 1990-11-27 | Cetus Corp | |
| EP0215594A2 (en) | 1985-08-29 | 1987-03-25 | Genencor International, Inc. | Heterologous polypeptide expressed in filamentous fungi, processes for their preparation, and vectors for their preparation |
| EP0238023A2 (en) | 1986-03-17 | 1987-09-23 | Novo Nordisk A/S | Process for the production of protein products in Aspergillus oryzae and a promoter for use in Aspergillus |
| EP0244234A2 (en) | 1986-04-30 | 1987-11-04 | Alko Group Ltd. | Transformation of trichoderma |
| US6022725A (en) | 1990-12-10 | 2000-02-08 | Genencor International, Inc. | Cloning and amplification of the β-glucosidase gene of Trichoderma reesei |
| US5329057A (en) * | 1991-04-19 | 1994-07-12 | The Dow Chemical Company | Process for the cyclodimerization of 1,3-butadienes to 4-vinylcyclohexenes |
| US5874276A (en) | 1993-12-17 | 1999-02-23 | Genencor International, Inc. | Cellulase enzymes and systems for their expressions |
| WO1996035796A1 (en) | 1995-05-12 | 1996-11-14 | E.I. Du Pont De Nemours And Company | Bioconversion of a fermentable carbon source to 1,3-propanediol by a single microorganism |
| US7169588B2 (en) | 1995-05-12 | 2007-01-30 | E. I. Du Pont De Nemours And Company | Bioconversion of a fermentable carbon source to 1,3-propanediol by a single microorganism |
| US5849970A (en) | 1995-06-23 | 1998-12-15 | The Regents Of The University Of Colorado | Materials and methods for the bacterial production of isoprene |
| WO1998002550A2 (en) | 1996-07-15 | 1998-01-22 | Fraunhofer-Gesellschaft zur Förderung der angewandten Forschung e.V. | Microbial isoprene generation |
| US5906662A (en) * | 1996-07-16 | 1999-05-25 | Shell Oil Company | Liquid hydrocarbon fuel composition |
| US6188142B1 (en) | 1996-09-14 | 2001-02-13 | Braun Gmbh | Method and arrangement for disconnecting consumers |
| US6176176B1 (en) | 1998-04-30 | 2001-01-23 | Board Of Trustees Operating Michigan State University | Apparatus for treating cellulosic materials |
| US6106888A (en) | 1998-04-30 | 2000-08-22 | Board Of Trustees Operating Michigan State University | Process for treating cellulosic materials |
| US6268328B1 (en) | 1998-12-18 | 2001-07-31 | Genencor International, Inc. | Variant EGIII-like cellulase compositions |
| US7132527B2 (en) | 2002-04-22 | 2006-11-07 | E. I. Du Pont De Nemours And Company | Promoter and plasmid system for genetic engineering |
| WO2004033646A2 (en) | 2002-10-04 | 2004-04-22 | E.I. Du Pont De Nemours And Company | Process for the biological production of 1,3-propanediol with high yield |
| WO2005001036A2 (en) | 2003-05-29 | 2005-01-06 | Genencor International, Inc. | Novel trichoderma genes |
| US7262041B2 (en) | 2003-11-21 | 2007-08-28 | Genencor International, Inc. | Expression of granular starch hydrolyzing enzyme in Trichoderma |
| WO2007089901A2 (en) | 2006-02-01 | 2007-08-09 | University Of Hawaii | Metabolically engineered organisms for the production of hydrogen and hydrogenase |
| WO2009076676A2 (en) | 2007-12-13 | 2009-06-18 | Danisco Us Inc. | Compositions and methods for producing isoprene |
| US20090203102A1 (en) | 2007-12-13 | 2009-08-13 | Cervin Marguerite A | Compositions and methods for producing isoprene |
| US20100003716A1 (en) | 2008-04-23 | 2010-01-07 | Cervin Marguerite A | Isoprene synthase variants for improved microbial production of isoprene |
| WO2010003007A2 (en) | 2008-07-02 | 2010-01-07 | Danisco Us Inc. | Compositions and methods for producing isoprene free of c5 hydrocarbons under decoupling conditions and/or safe operating ranges |
| WO2010031076A2 (en) | 2008-09-15 | 2010-03-18 | Danisco Us Inc. | Conversion of prenyl derivatives to isoprene |
| WO2010031062A1 (en) | 2008-09-15 | 2010-03-18 | Danisco Us Inc. | Increased isoprene production using the archaeal lower mevalonate pathway |
| WO2010031079A1 (en) | 2008-09-15 | 2010-03-18 | Danisco Us Inc. | Systems using cell culture for production of isoprene |
| WO2010031077A1 (en) | 2008-09-15 | 2010-03-18 | Danisco Us Inc. | Increased isoprene production using mevalonate kinase and isoprene synthase |
| WO2010031068A1 (en) | 2008-09-15 | 2010-03-18 | Danisco Us Inc. | Reduction of carbon dioxide emission during isoprene production by fermentation |
Non-Patent Citations (81)
| Title |
|---|
| AKIHITO YOSHIDA ET AL.: "Efficient induction of formate hydrogen lyase of aerobically grown Escherichia coli in a three-step biohydrogen production process", APPL. MICROBIOL. BIOTECHNOL., vol. 74, 2007, pages 754 - 760, XP019489518 |
| AUSUBEL ET AL.,: "Current Protocols in Molecular Biology", 1994 |
| BELLION ET AL.: "Microb. Growth Cl Compd.[Tnt. Symp.], 7th ed.,", 1993, PUBLISHER: INTERCEPT, pages: 415 - 32 |
| BENNETT; LASURE: "More Gene Manipulations in Fungi", 1991, ACADEMIC PRESS, pages: 70 - 76 |
| BIOCHEMISTRY, vol. 33, 1994, pages 13355 - 13362 |
| BIOTECHNOLOGY, vol. 20, 1984, pages 46 - 53 |
| BOEL ET AL., EMBO J., vol. 3, 1984, pages 1581 - 1585 |
| BROCK: "Biotechnology: .4 Textbook of Industrial Microbiology, Second Edition", 1989, SINAUER ASSOCIATES, INC. |
| BROCK: "Biotechnology: A Textbook of Industrial Microbiology, Second Edition", 1989, SINAUER ASSOCIATES, INC. |
| BULIGE ET AL., APPLIED AND ENVIRONMENTAL MICROBIOLOGY, vol. 74, no. 7, 2008, pages 2179 - 2186 |
| BUNGE, APPLIED AND ENVIRONMENTAL MICROBIOLOGY, vol. 74, no. 7, 2008, pages 2179 - 2186 |
| CAMPBELL ET AL., CURR. GENET., vol. 16, 1989, pages 53 - 56 |
| CAO ET AL., SCI., vol. 9, 2000, pages 991 - 1001 |
| CHUNG-JUNG CHOU ET AL.: "Hydrogenesis in hyperthermophilic microorganisms: implications for biofuels", METABOL. ENG., vol. 10, 2008, pages 394 - 404 |
| CURR GENE, vol. 41, 2002, pages 89 - 98 |
| CURR GENET, vol. 19, 1991, pages 9 - 14 |
| CURRIE, L. A.: "Characterization of Environmental Particles", vol. I, 1992, LEWIS PUBLISHERS, INC, article "Source Apportionment of Atmospheric Particles", pages: 3 74 |
| EUR. J. BIOCHEM., vol. 269, 2002, pages 4446 - 4457 |
| F. BOUVIER ET AL., PROGRESS IN LIPID RES., vol. 44, 2005, pages 357 - 429 |
| F. M. AUSUBEL ET AL.: "Current Protocols in Molecular Biology", 1987, article "Chapter 9," |
| F. M. AUSUBEL ET AL.: "Current Protocols in Molecular Biology", vol. 30, 1987 |
| FARZANEH ET AL., BIORESOURCE TECHNOLOGY, vol. 96, no. 18, 2005, pages 2014 - 2018 |
| FARZANEH ET AL., BIORESOZIRCE TECHNOLOGY, vol. 96, no. 18, 2005, pages 2014 - 2018 |
| FARZANEH, BIORESOURCE TECHNOLOGY, vol. 96, no. 18, 2005, pages 2014 - 2018 |
| FINKELSTEIN ET AL.: "Biotechnology of Filamentous Fungi", 1992, BUTTERWORTH-HEINEMANN, article "Chap. 6.," |
| GERHARDT ET AL.,: "Manual of Methods for General Bacteriology", 1994, AMERICAN SOCIETY FOR MICROBIOLOGY |
| GÖNÜL VARDAR-SCHARA ET AL.: "Metabolically engineered bacteria for producing hydrogen via fermentation", MICROBIAL BIOTECHNOL., vol. 1, no. 2, 2008, pages 107 - 125 |
| GOTTSCHALK: "Bacterial Metabolism. Second Edition,", 1986, SPRINGER-VERLAG |
| GREENBERG ET AL., ATMOS. ENVIRON., vol. 27A, 1993, pages 2689 - 2692 |
| H. M. WEITZ; E. LOSER, ISOPRENE: "Ullmann's Encyclopedia of Industrial Chemistry", 2005, WILEY-VCH VERLAG GMBH |
| H. POMMER; A. NURRENBACH: "Industrial Synthesis of Terpene Compounds", PURE APPL. CHEM., vol. 43, 1975, pages 527 - 551 |
| H.M. LYBARGER: "Kirk-Othmer Encyclopedia of Chemical Technology, 4th ed.,", vol. 14, 1995, WILEY, article "Isoprene", pages: 934 - 952 |
| HARKKI ET AL., BIO TECHNOL., vol. 7, 1989, pages 596 - 603 |
| HARKKI ET AL., ENZYME MICROH. TECHNOL., vol. 13, 1991, pages 227 - 233 |
| HENNING SEEDORF ET AL.: "The genome of Clostridium kluyveri, a strict anaerobe with unique metabolic features", PROC. NAT'L ACAD. SCI. U.S.A., vol. 105, no. 6, 2008, pages 2128 - 2133, XP009119916, DOI: doi:10.1073/pnas.0711093105 |
| HSIEH, Y., SOIL SCI. SOC. AM J., vol. 56, 1992, pages 460 |
| HUNTER, BIOCHEMISTRY, vol. 24, 1985, pages 4148 - 4155 |
| ILMEN ET AL., APPL. ENVIRON. MICROBIAL., vol. 63, 1997, pages 1298 - 1306 |
| J. BACTERIOL., vol. 184, 2002, pages 2116 - 2122 |
| J. BACTERIOL., vol. 184, 2002, pages 4065 - 4070 |
| J. BIOL. CHEM., vol. 264, 1989, pages 19169 - 19175 |
| J. ORG. CHEM., vol. 70, 2005, pages 9168 - 9174 |
| J. WOODWARD ET AL.: "Enzymatic production ofbiohydrogen", NATURE, vol. 405, no. 6790, 2000, pages 1014 - 1015 |
| JACS, vol. 126, 2004, pages 12847 - 12855 |
| JULSING ET AL., APPLIED. MICROBIOL. BIOTECHNOL., vol. 75, 2007, pages 1377 - 84 |
| KALIM-AKHTAR ET AL.: "Deletion of iscR stimulates recombinant Clostridial Fe/Fe hydrogenase activity and H2- accumulation in Escherichia coli BL21(DE3", APPL. MICROBIOL. BIOTECHNOL., vol. 78, 2008, pages 853 - 862, XP019586354 |
| KALIM-AKHTAR ET AL.: "Deletion of iscR stimulates recombinant Clostridial Fe/Fe hydrogenase activity and H2-accumulation in Escherichia coli BL21(DE3", APPL. MICROBIOL. BIOTECHNOL., vol. 78, 2008, pages 853 - 862, XP019586354 |
| KELLEY ET AL., EALBO J., vol. 4, 1985, pages 475 - 479 |
| KINGHOM ET AL.: "Applied Molecular Genetics of Filamcntous Fungi", 1992, BLACKIE ACADEMIC AND PROFESSIONAL, CHAPMAN AND HALL |
| KOGA; MORII, MICROBIOLOGY AND MOL. BIOLOGY REVIEWS, vol. 71, 2007, pages 97 - 120 |
| KOVACH ET AL., GENE, vol. 166, 1995, pages 175 - 176 |
| KREIGLER: "Gene Transfer and Expression; A Laboratory Manual.", 1990 |
| L.E. NAGY ET AL.: "Application of gene-shuffling for the rapid generation of novel [FeFe]-hydrogenase libraries", BIOTECHNOL. LETTS, vol. 29, no. 3, 2007, pages 421 - 430, XP019477808 |
| LADYGINA ET AL., PROCESS BIOCHEMISTLY, vol. 41, 2006, pages 1001 - 1014 |
| MILLER ET AL., PLANTA, vol. 213, 2001, pages 483 - 487 |
| MOL CELL BIOL., vol. I1, 1991, pages 620 - 631 |
| NEVALAINEN ET AL.: "Molecular Industrial Mycology", 1992, MARCEL DEKKER INC., article "The Molecular Biology of Trichoderma and its Application to the Expression of Both Homologous and Heterologous Genes", pages: 129 - 148 |
| NUNBERG ET AL., MOL. CELL BIOL., vol. 4, 1984, pages 2306 - 2315 |
| P.C. MANESS ET AL.: "Characterization of the oxygen tolerance of a hydrogenase linked to a carbon monoxide oxidation pathway in Rubrivivax gelatinosus", APPL. ENVIRON. MICROBIOL., vol. 68, no. 6, 2002, pages 2633 - 2636, XP002506018, DOI: doi:10.1128/AEM.68.6.2633-2636 |
| P.W. KING ET AL.: "Functional studies of [FeFe] hydrogenase maturation in an Escherichia coli biosynthetic system", J. BACTERIOL., vol. 188, no. 6, 2006, pages 163 - 172, XP009096433, DOI: doi:10.1128/JB.188.6.2163-2172.2006 |
| PENTTILA ET AL., GENE, vol. 61, 1987, pages 155 - 164 |
| PNAS, vol. 94, 1997, pages 12857 - 62 |
| PNAS, vol. 96, 1999, pages 11758 - 11763 |
| PNAS, vol. 97, 2000, pages 1062 - 1067 |
| PNAS, vol. 97, 2000, pages 6451 - 6456 |
| POURQUIE, J. ET AL.: "Biochemistry and Genetics of Cellulose Degradation", 1988, ACADEMIC PRESS, pages: 71 - 86 |
| SAMBROOK ET AL.: "Molecular Cloning: A Laboratory Manual", 1982, COLD SPRING HARBOR |
| SAMBROOK ET AL.: "Molecular Cloning: A Laboratory Manual, 2ed ed.,", 1989, COLD SPRING HARBOR |
| SAMBROOK ET AL.: "Molecular Cloning: A Laboratory Manual, 2nd ed.,", 1989 |
| SAMBROOK: "Molecular Cloning: A Laboratory Manual, 2nd ed.,", 1989, COLD SPRING HARBOR |
| SHARKEY ET AL., PLANT PHYSIOLOGY, vol. 137, 2005, pages 700 - 712 |
| SILVER ET AL., J. BIOL. CHEM., vol. 270, 1995, pages 13010 - 13016 |
| SILVER ET AL., JBC, vol. 270, no. 22, 1995, pages 13010 - 1316 |
| SILVER ET AL., PLANT PHYSIOL., vol. 97, 1991, pages 1588 - 1591 |
| SULTER ET AL., ARCH. MICROBIOL., vol. 153, no. 5, 1990, pages 485 - 9 |
| T. BURGDORF ET AL.: "NiFe]-hydrogenases of Ralstonia eutropha H16: modular enzymes for oxygen-tolerant biological hydrogen oxidation", J. MOL. MICROBIOL. BIOTECHNOL., vol. 10, no. 2-4, 2005, pages 181 - 196, XP009109362, DOI: doi:10.1159/000091564 |
| TOSHINORI MAEDA ET AL.: "Enhanced hydrogen production from glucose by metabolically engineered Escherichia coli", APPL. MICROBIOL. BIOTECHNOL., vol. 77, no. 4, 2007, pages 879 - 890, XP019560746, DOI: doi:10.1007/s00253-007-1217-0 |
| VAN DEN HONDEL ET AL.: "More Gene Manipulations in Fungi", 1991, ACADEMIC PRESS, pages: 396 - 428 |
| WITHERS ET AL., APPL ENVIRON MICROBIOL., vol. 73, no. 19, 2007, pages 6277 - 83 |
| YAMADA ET AL., AGRIC. BIOL. CHEM., vol. 53, no. 2, 1989, pages 541 - 543 |
| YELTON ET AL., PROCEEDINGS. NATL. ACAD SCI. USA, vol. 81, 1984, pages 1470 - 1474 |
Cited By (47)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| EP2443244A1 (en) * | 2009-06-17 | 2012-04-25 | Danisco US Inc. | Improved isoprene production using the dxp and mva pathway |
| EP2443244A4 (en) * | 2009-06-17 | 2014-11-26 | Danisco Us Inc | Improved isoprene production using the dxp and mva pathway |
| US8507235B2 (en) | 2009-06-17 | 2013-08-13 | Danisco Us Inc. | Isoprene production using the DXP and MVA pathway |
| US8778647B2 (en) | 2009-12-22 | 2014-07-15 | Danisco Us Inc. | Methods of producing isoprene comprising a membrane bioreactor |
| WO2011160081A1 (en) | 2010-06-17 | 2011-12-22 | Danisco Us Inc. | Fuel compositions comprising isoprene derivatives |
| US9273298B2 (en) | 2010-10-27 | 2016-03-01 | Danisco Us Inc. | Isoprene synthase variants for improved production of isoprene |
| WO2012088462A1 (en) | 2010-12-22 | 2012-06-28 | Danisco Us Inc. | Compositions and methods for improved isoprene production using two types of ispg enzymes |
| US8691541B2 (en) | 2010-12-22 | 2014-04-08 | Danisco Us Inc. | Biological production of pentose sugars using recombinant cells |
| US8685702B2 (en) | 2010-12-22 | 2014-04-01 | Danisco Us Inc. | Compositions and methods for improved isoprene production using two types of ISPG enzymes |
| US8889383B2 (en) | 2011-04-29 | 2014-11-18 | Danisco Us Inc. | Production of mevalonate, isoprene, and isoprenoids using genes encoding polypeptides having thiolase, HMG-CoA synthase and HMG-CoA reductase enzymatic activities |
| US10975394B2 (en) | 2011-04-29 | 2021-04-13 | Danisco Us Inc. | Recombinant microorganisms for enhanced production of mevalonate, isoprene, and isoprenoids |
| US9121038B2 (en) | 2011-04-29 | 2015-09-01 | The Goodyear Tire & Rubber Company | Recombinant microorganisms for enhanced production of mevalonate, isoprene, and isoprenoids |
| US10364443B2 (en) | 2011-04-29 | 2019-07-30 | Danisco Us Inc. | Production of mevalonate, isoprene, and isoprenoids using genes encoding polypeptides having thiolase, HMG-CoA synthase and HMG-CoA reductase enzymatic activities |
| JP2017148063A (en) * | 2011-04-29 | 2017-08-31 | ダニスコ・ユーエス・インク | Recombinant microorganism for enhanced production of mevalonate, isoprene, and isoprenoid |
| WO2012149491A2 (en) | 2011-04-29 | 2012-11-01 | Danisco Us Inc. | Recombinant microorganisms for enhanced production of mevalonate, isoprene, and isoprenoids |
| US10138498B2 (en) | 2011-04-29 | 2018-11-27 | Danisco Us Inc. | Recombinant microorganisms for enhanced production of mevalonate, isoprene, and isoprenoids |
| WO2012149491A3 (en) * | 2011-04-29 | 2012-12-27 | Danisco Us Inc. | Recombinant microorganisms for enhanced production of mevalonate, isoprene, and isoprenoids |
| CN103930541A (en) * | 2011-04-29 | 2014-07-16 | 丹尼斯科美国公司 | Recombinant microorganisms for enhanced production of mevalonate, isoprene, and isoprenoids |
| US9752162B2 (en) | 2011-04-29 | 2017-09-05 | Danisco Us Inc. | Production of mevalonate, isoprene, and isoprenoids using genes encoding polypeptides having thiolase, HMG-CoA synthase and HMG-CoA reductase enzymatic activities |
| WO2012149469A1 (en) | 2011-04-29 | 2012-11-01 | Danisco Us Inc. | Production of mevalonate, isoprene, and isoprenoids using genes encoding polypeptides having thiolase, hmg-coa synthase and hmg-coa reductase enzymatic activities |
| WO2013020118A1 (en) | 2011-08-04 | 2013-02-07 | Danisco Us Inc. | Production of isoprene, isoprenoid precursors, and isoprenoids using acetoacetyl-coa synthase |
| EP3339426A1 (en) | 2011-10-07 | 2018-06-27 | Danisco US Inc. | Utilization of phosphoketolase in the production of mevalonate, isoprenoid precursors, and isoprene |
| US10113185B2 (en) | 2011-10-07 | 2018-10-30 | Danisco Us Inc. | Utilization of phosphoketolase in the production of mevalonate, isoprenoid precursors, and isoprene |
| US8993305B2 (en) | 2011-10-07 | 2015-03-31 | Danisco Us Inc. | Utilization of phosphoketolase in the production of mevalonate, isoprenoid precursors, and isoprene |
| WO2013066568A1 (en) | 2011-10-07 | 2013-05-10 | Danisco Us Inc. | Utilization of phosphoketolase in the production of mevalonate, isoprenoid precursors, and isoprene |
| US9493791B2 (en) | 2011-10-07 | 2016-11-15 | Danisco Us Inc. | Utilization of phosphoketolase in the production of mevalonate, isoprenoid precursors, and isoprene |
| US8865442B2 (en) | 2011-12-23 | 2014-10-21 | Danisco Us Inc. | Production of isoprene under reduced oxygen inlet levels |
| US8975051B2 (en) | 2011-12-23 | 2015-03-10 | Danisco Us Inc. | Enhanced production of isoprene using host cells having decreased ispA activity |
| US9388431B2 (en) | 2011-12-23 | 2016-07-12 | Danisco Us Inc. | Enhanced production of isoprene using host cells having decreased ispA activity |
| WO2013149192A1 (en) | 2012-03-30 | 2013-10-03 | Danisco Us Inc. | Direct starch to fermentable sugar as feedstock for the production of isoprene, isoprenoid precursor molecules, and/or isoprenoids |
| US9315831B2 (en) | 2012-03-30 | 2016-04-19 | Danisco Us Inc. | Direct starch to fermentable sugar as feedstock for the production of isoprene, isoprenoid precursor molecules, and/or isoprenoids |
| WO2013166320A1 (en) | 2012-05-02 | 2013-11-07 | Danisco Us Inc. | Legume isoprene synthase for production of isoprene |
| US9163263B2 (en) | 2012-05-02 | 2015-10-20 | The Goodyear Tire & Rubber Company | Identification of isoprene synthase variants with improved properties for the production of isoprene |
| WO2013166211A2 (en) | 2012-05-02 | 2013-11-07 | Danisco Us Inc. | Identification of isoprene synthase variants with improved properties for the production of isoprene |
| US8895277B2 (en) | 2012-05-02 | 2014-11-25 | Danisco Us Inc. | Legume isoprene synthase for production of isoprene |
| US20140234926A1 (en) * | 2012-06-01 | 2014-08-21 | E I Du Pont De Nemours And Company | Recombinant anaerobic acetogenic bacteria for production of isoprene and/or industrial bio-products using synthesis gas |
| WO2013181647A2 (en) | 2012-06-01 | 2013-12-05 | Danisco Us Inc. | Compositions and methods of producing isoprene and/or industrrial bio-products using anaerobic microorganisms |
| WO2014100726A2 (en) | 2012-12-21 | 2014-06-26 | Danisco Us Inc. | Production of isoprene, isoprenoid, and isoprenoid precursors using an alternative lower mevalonate pathway |
| EP3425049A1 (en) | 2013-04-10 | 2019-01-09 | Danisco US Inc. | Phosphoketolases for improved production of acetyl coenzyme a-derived metabolites, isoprene, isoprenoid precursors, and isoprenoids |
| WO2014169144A2 (en) | 2013-04-10 | 2014-10-16 | Danisco Us Inc. | Phosphoketolases foe improved production of acetyl coenzyme a-derived metabolites, isoprene, isoprenoid precurosors, and isoprenoids |
| US9926568B2 (en) | 2013-06-21 | 2018-03-27 | Danisco Us Inc. | Compositions and methods for clostridial transformation |
| US10364436B2 (en) | 2013-06-21 | 2019-07-30 | Danisco Us Inc. | Compositions and methods for clostridial transformation |
| WO2014205355A2 (en) | 2013-06-21 | 2014-12-24 | Danisco Us Inc. | Compositions and methods for clostridial transformation |
| US10648004B2 (en) | 2014-02-20 | 2020-05-12 | Danisco Us Inc. | Recombinant microorganisms for the enhanced production of mevalonate, isoprene, isoprenoid precursors, isoprenoids, and acetyl-CoA-derived products |
| WO2015127305A2 (en) | 2014-02-20 | 2015-08-27 | Danisco Us Inc. | Recombinant microorganisms for the enhanced production of mevalonate, isoprene, isoprenoid precursors, isoprenoids, and acetyl-coa-derived products |
| US11685937B2 (en) | 2014-02-20 | 2023-06-27 | Danisco Us Inc. | Recombinant microorganisms for the enhanced production of mevalonate, isoprene, isoprenoid precursors, isoprenoids, and acetyl-CoA-derived products |
| WO2016015021A1 (en) | 2014-07-24 | 2016-01-28 | The Regents Of The University Of California | Oxidative starch degradation by a new family of pmos |
Also Published As
| Publication number | Publication date |
|---|---|
| MX2011013805A (en) | 2012-07-20 |
| ZA201109375B (en) | 2014-03-26 |
| MY155508A (en) | 2015-10-30 |
| KR20120047908A (en) | 2012-05-14 |
| AU2010262774B2 (en) | 2015-12-03 |
| AU2010262774A1 (en) | 2012-01-19 |
| TW201120204A (en) | 2011-06-16 |
| EP2448891A4 (en) | 2013-07-24 |
| BRPI1011952A2 (en) | 2016-04-26 |
| US20140148623A1 (en) | 2014-05-29 |
| CN102803185A (en) | 2012-11-28 |
| SG10201507749UA (en) | 2015-10-29 |
| JP5448220B2 (en) | 2014-03-19 |
| US20110046422A1 (en) | 2011-02-24 |
| CA2765916A1 (en) | 2010-12-23 |
| RU2012101453A (en) | 2013-07-27 |
| CN102803185B (en) | 2016-01-06 |
| JP2012530716A (en) | 2012-12-06 |
| US8450549B2 (en) | 2013-05-28 |
| RU2531623C2 (en) | 2014-10-27 |
| SG176875A1 (en) | 2012-02-28 |
| AR077114A1 (en) | 2011-08-03 |
| TWI434921B (en) | 2014-04-21 |
| EP2448891A1 (en) | 2012-05-09 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| US8450549B2 (en) | Fuel compositions comprising isoprene derivatives | |
| US9752161B2 (en) | Methods of producing isoprene and a co-product | |
| US9121039B2 (en) | Systems using cell culture for production of isoprene | |
| US8945893B2 (en) | Conversion of prenyl derivatives to isoprene | |
| US20100048964A1 (en) | Compositions and methods for producing isoprene free of c5 hydrocarbons under decoupling conditions and/or safe operating ranges | |
| EP2337844A1 (en) | Reduction of carbon dioxide emission during isoprene production by fermentation | |
| AU2014200285A1 (en) | A biologically produced isoprene composition |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| WWE | Wipo information: entry into national phase |
Ref document number: 201080036083.X Country of ref document: CN |
|
| 121 | Ep: the epo has been informed by wipo that ep was designated in this application |
Ref document number: 10790225 Country of ref document: EP Kind code of ref document: A1 |
|
| WWE | Wipo information: entry into national phase |
Ref document number: 2010262774 Country of ref document: AU |
|
| WWE | Wipo information: entry into national phase |
Ref document number: MX/A/2011/013805 Country of ref document: MX |
|
| WWE | Wipo information: entry into national phase |
Ref document number: 2765916 Country of ref document: CA |
|
| NENP | Non-entry into the national phase |
Ref country code: DE |
|
| WWE | Wipo information: entry into national phase |
Ref document number: 2012516318 Country of ref document: JP |
|
| WWE | Wipo information: entry into national phase |
Ref document number: 78/DELNP/2012 Country of ref document: IN |
|
| ENP | Entry into the national phase |
Ref document number: 20127001228 Country of ref document: KR Kind code of ref document: A |
|
| REEP | Request for entry into the european phase |
Ref document number: 2010790225 Country of ref document: EP |
|
| WWE | Wipo information: entry into national phase |
Ref document number: 2010790225 Country of ref document: EP |
|
| WWE | Wipo information: entry into national phase |
Ref document number: 2012101453 Country of ref document: RU |
|
| ENP | Entry into the national phase |
Ref document number: 2010262774 Country of ref document: AU Date of ref document: 20100617 Kind code of ref document: A |
|
| REG | Reference to national code |
Ref country code: BR Ref legal event code: B01A Ref document number: PI1011952 Country of ref document: BR |
|
| ENP | Entry into the national phase |
Ref document number: PI1011952 Country of ref document: BR Kind code of ref document: A2 Effective date: 20111216 |