WO2009095395A2 - Pharmaceutical compositions - Google Patents
Pharmaceutical compositions Download PDFInfo
- Publication number
- WO2009095395A2 WO2009095395A2 PCT/EP2009/050924 EP2009050924W WO2009095395A2 WO 2009095395 A2 WO2009095395 A2 WO 2009095395A2 EP 2009050924 W EP2009050924 W EP 2009050924W WO 2009095395 A2 WO2009095395 A2 WO 2009095395A2
- Authority
- WO
- WIPO (PCT)
- Prior art keywords
- pharmaceutical composition
- bupropion
- combination
- pharmaceutically acceptable
- stabilizer
- Prior art date
Links
Classifications
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K9/00—Medicinal preparations characterised by special physical form
- A61K9/20—Pills, tablets, discs, rods
- A61K9/28—Dragees; Coated pills or tablets, e.g. with film or compression coating
- A61K9/2806—Coating materials
- A61K9/2833—Organic macromolecular compounds
- A61K9/286—Polysaccharides, e.g. gums; Cyclodextrin
- A61K9/2866—Cellulose; Cellulose derivatives, e.g. hydroxypropyl methylcellulose
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/13—Amines
- A61K31/135—Amines having aromatic rings, e.g. ketamine, nortriptyline
- A61K31/137—Arylalkylamines, e.g. amphetamine, epinephrine, salbutamol, ephedrine or methadone
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/33—Heterocyclic compounds
- A61K31/335—Heterocyclic compounds having oxygen as the only ring hetero atom, e.g. fungichromin
- A61K31/34—Heterocyclic compounds having oxygen as the only ring hetero atom, e.g. fungichromin having five-membered rings with one oxygen as the only ring hetero atom, e.g. isosorbide
- A61K31/343—Heterocyclic compounds having oxygen as the only ring hetero atom, e.g. fungichromin having five-membered rings with one oxygen as the only ring hetero atom, e.g. isosorbide condensed with a carbocyclic ring, e.g. coumaran, bufuralol, befunolol, clobenfurol, amiodarone
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/33—Heterocyclic compounds
- A61K31/395—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins
- A61K31/55—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins having seven-membered rings, e.g. azelastine, pentylenetetrazole
- A61K31/554—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins having seven-membered rings, e.g. azelastine, pentylenetetrazole having at least one nitrogen and one sulfur as ring hetero atoms, e.g. clothiapine, diltiazem
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K9/00—Medicinal preparations characterised by special physical form
- A61K9/20—Pills, tablets, discs, rods
- A61K9/28—Dragees; Coated pills or tablets, e.g. with film or compression coating
- A61K9/2806—Coating materials
- A61K9/2833—Organic macromolecular compounds
- A61K9/284—Organic macromolecular compounds obtained by reactions only involving carbon-to-carbon unsaturated bonds, e.g. polyvinyl pyrrolidone
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P25/00—Drugs for disorders of the nervous system
- A61P25/22—Anxiolytics
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P25/00—Drugs for disorders of the nervous system
- A61P25/24—Antidepressants
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K9/00—Medicinal preparations characterised by special physical form
- A61K9/20—Pills, tablets, discs, rods
- A61K9/2072—Pills, tablets, discs, rods characterised by shape, structure or size; Tablets with holes, special break lines or identification marks; Partially coated tablets; Disintegrating flat shaped forms
- A61K9/2077—Tablets comprising drug-containing microparticles in a substantial amount of supporting matrix; Multiparticulate tablets
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K9/00—Medicinal preparations characterised by special physical form
- A61K9/20—Pills, tablets, discs, rods
- A61K9/2072—Pills, tablets, discs, rods characterised by shape, structure or size; Tablets with holes, special break lines or identification marks; Partially coated tablets; Disintegrating flat shaped forms
- A61K9/2086—Layered tablets, e.g. bilayer tablets; Tablets of the type inert core-active coat
- A61K9/209—Layered tablets, e.g. bilayer tablets; Tablets of the type inert core-active coat containing drug in at least two layers or in the core and in at least one outer layer
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K9/00—Medicinal preparations characterised by special physical form
- A61K9/48—Preparations in capsules, e.g. of gelatin, of chocolate
- A61K9/50—Microcapsules having a gas, liquid or semi-solid filling; Solid microparticles or pellets surrounded by a distinct coating layer, e.g. coated microspheres, coated drug crystals
- A61K9/5084—Mixtures of one or more drugs in different galenical forms, at least one of which being granules, microcapsules or (coated) microparticles according to A61K9/16 or A61K9/50, e.g. for obtaining a specific release pattern or for combining different drugs
Definitions
- the present invention relates to novel once daily pharmaceutical compositions comprising combinations of escitalopram and bupropion or citalopram and bupropion and their use for the treatment of central nervous system disorders, such as for example mood disorders (e.g., major depressive disorder (MDD)-also known as major depression, unipolar depression, unipolar disorder, or clinical depression) and anxiety disorders (general anxiety disorder, social anxiety disorder, post traumatic stress disorder, or panic disorder).
- mood disorders e.g., major depressive disorder (MDD)- also known as major depression, unipolar depression, unipolar disorder, or clinical depression
- anxiety disorders general anxiety disorder, social anxiety disorder, post traumatic stress disorder, or panic disorder.
- the present invention also relates to novel once daily pharmaceutical compositions comprising a combination of bupropion and quetiapine fumarate.
- STAR*D Sequenced Treatment Alternatives to Relieve Depression
- Level 1 evaluated the effectiveness of the antidepressant citalopram alone.
- Citalopram and escitalopram (the s-enantiomer of citalopram) currently marketed in the United States as Celexa ® and Lexapro ® respectively, belong to the class of antidepressants known as selective serotonin reuptake inhibitors (SSRIs).
- SSRIs selective serotonin reuptake inhibitors
- Buspirone itself is not an antidepressant, but enhances the action of antidepressants.
- Bupropion on the other hand, is an antidepressant belonging to the chemical class of aminoketones. Bupropion, marketed in the U.S. as Wellbutrin ® , Wellbutrin ® SR, or Wellbutrin ® XL, is classified as an atypical antidepressant.
- Bupropion was chosen as the antidepressant of choice in Level 2 possibly for several reasons. For one, clinical studies have confirmed the efficacy of bupropion for MDD. (Fava M. et al. (2005). Prim Care Companion J Clin Psychiatry 7(3): 106-113). For another, bupropion, in contrast to nearly all other antidepressants, does not cause weight gain or sexual dysfunction (Zimmerman M. et al. (2005). J Clin Psychiatry 66(10): 1336-1339; Clayton AH. (2003). Primary Psychiatry 10(1): 55-61) and is more effective than SSRIs at improving symptoms of hypersomnia and fatigue in depressed patients (Baldwin et al. (2006).
- Citalopram primarily through its S-enantiomer, escitalopram, mediates its antidepressant effects by inhibiting re-uptake of serotonin (5-hydroxytryptamine [5-HT]) released into the synaptic cleft ⁇ Brcestrup C. and Sanchez C. (2004). Int J Psychiatry Clin Practice 8 (suppl 1): 11-13).
- a result of the inhibition of this uptake is that 5-HT persists in the synaptic cleft thereby stimulating receptors of postsynaptic neurons for an extended period in patients suffering from MDD.
- 5-HT 1A autoreceptors located at the cell body of neurons, exert a negative feedback response on the firing activity of serotonergic (5-HT) neurons by binding to excess 5-HT.
- 5-HT 1A autoreceptors after a period of time of treatment with the SSRI, become desensitized and allow 5-HT neurons to regain their normal firing rate in the presence of sustained reuptake inhibition (Blier, P. (2003) European Neuropsychopharmacology 13: 57-66).
- the time taken to desensitize 5-HT 1A autoreceptors about two to three weeks, is believed to represent the delay in onset of action of SSRIs.
- Bupropion has the ability to increase synaptic availability of norepinephrine (NE) and differentially effect dopamine (DA) release in various parts of the brain (Dong J. and Blier P. (2001). Psychopharmacology 155: 52-57; Mansari M. E. et al. (2008). Neuropharmacology 55: 1191- 1198). It is believed that this enhanced NE release results in an attenuation of firing of NE neurons due to an increased activation of inhibitory somatodendritic ( ⁇ -adrenoceptors located on NE neurons rather than due to the re-uptake inhibition of NE as previously thought.
- ⁇ -adrenoceptors inhibitory somatodendritic
- NE neurons gradually re-initiate firing to normal levels over a two-week period of bupropion administration as the ( ⁇ -adrenoceptors become desensitized.
- SSRIs and bupropion exert their action via different neuronal systems it appears that these systems work in concert in the antidepressant response.
- 5-HT and NE neurons have reciprocal connections.
- bupropion leads to a rapid and sustained increase in the firing rate of 5-HT neurons and conclude that this is a result of the desensitization of the 5-HT 1A autoreceptors after only two days of administration (Mansari et al. (2008). Neuropharmacology 55: 1191-1198).
- the enhanced NE releasing action by bupropion should counteract the decreased firing rate of NE neurons produced by long- term administration of SSRIs.
- treatment methods that affect both 5-HT and NE neuronal systems might be expected to benefit depressed patients regardless of whether their depression is a result of 5-HT and/or NE deficiency.
- Prica et al. evaluated the effects of co-administration of bupropion and SSRIs in mice using the forced swimming test, which is predictive of the antidepressant activity of drugs (Prica et al. Behav. Brain Res. (2008). 194: 92-99). The results suggest that bupropion might enhance the effectiveness of SSRIs and SNRIs but not NRIs. Their results also suggest that bupropion enhances only the serotonergic system, which is in agreement with the pre-clinical studies presented above.
- a pharmaceutical composition can be manufactured such that both citalopram or escitalopram and bupropion can be formulated into a single composition, which provides for the release of both drugs such that the drugs might be able to act on the 5 -HT and NE neuronal systems at or about the same time to maximize the expected synergistic antidepressant outcome.
- compositions for the delivery of combinations of drugs are not new in the art of drug delivery.
- US Pat. No. 4,449,983 (the '983 application) refers to 'an osmotic device for delivering two beneficial drugs to an environment of use'.
- the patent refers to a tri-layer tablet coated with a semi-permeable membrane with two separate orifices to allow for drug release.
- the semi-permeable membrane is substantially impermeable to the drugs.
- the first tablet layer contains the first active ingredient
- the second tablet layer forms a swellable (hydrogel) partition barrier
- the third tablet layer contains the second drug.
- the tablet is then coated with a semi-permeable membrane to form two drug-containing compartments in one tablet.
- the swellable (hydrogel) partition layer acts as a 'driving' layer. As the partition layer hydrates it expands and reduces the volume of each drug-containing compartment. The rate of drug release from this device is controlled by an osmotic pressure gradient within each drug- containing compartment.
- US Pat. No. 4,455,143 refers to a similar osmotic device to that described in the
- the partition layer is made of a material 'selected from the group consisting essentially of semi-permeable, microporous and impermeable materials'.
- the function of the partition layer is to 'maintain the integrity of the first and second compartments', (i.e. the drug containing compartments). The rate of drug release is controlled by the osmotic pressure within the drug compartment.
- US Pat. No. 4,601,894 refers to a matrix tablet composition for the controlled release of the triple drug combination of acetaminophen, pseudoephedrine sulfate and dexbrompheniramine maleate.
- the matrix composition contains the three actives but a choice of polymers (preferably hydroxypropyl methylcellulose (HPMC) ethers and ethylcellulose.
- HPMC hydroxypropyl methylcellulose
- the patent refers to a simple combination dosage form with unexpected release rates (based on very different drug solubilities) specific to three actives, 'acetaminophen, pseudoephedrine or a pharmaceutically acceptable salt thereof and dexbrompheniramine or a pharmaceutically acceptable salt thereof.
- the matrix tablet composition referred to is an uncoated matrix tablet, with drug release controlled by a combination of drug diffusion and polymer erosion.
- US Pat. No. 4,662,880 refers to an osmotic device for the controlled delivery of the two pharmaceutical actives pseudoephedrine and brompheniramine. Both actives are formulated in one tablet core; a semi-permeable membrane, which is substantially impermeable to the passage of drug, is applied followed by an immediate release active coat containing both pharmaceutical actives.
- US Pat. No. 4,844,907 refers to a 'multiphase (especially a bi-layered, optionally coated) tablet' composition for the delivery of a combination of a narcotic analgesic and a nonsteroidal anti-inflammatory.
- the patent refers to a bi -layer tablet consisting of two separate controlled release matrix layers, each layer containing one of the actives individually. There is no partition layer between the two active layers.
- US Pat. No. 5,866,164 refers to a similar method for the controlled delivery of an opioid and an opioid antagonist.
- US Pat. Nos. 4,814,181, and 4,915,954 refer to an osmotic pump dosage form for delivering actives at two different rates.
- the patents refer to a bi-layer tablet core coated with a semi-permeable membrane with a single passageway for osmotic drug release.
- the semipermeable membrane is substantially impermeable to the passage of the drug.
- the first drug layer (closest to the passageway) releases drug rapidly while the second drug layer releases active over a prolonged period of time.
- US Pat. Application No. 11/355,315 refers to an osmotic dual delivery technology containing a bi-layered core.
- the application purports to teach a dual controlled release of both drugs from a controlled release bi-layered core osmotic device.
- the arrangement of the layers of the bi-layer core can be stacked or the second layer can surround the first.
- the application refers to a first and second drug which can be released sequentially or in an overlapping manner when the osmotic device is exposed to an aqueous environment in a timed, targeted, pseudo-first order, first order, pseudo-zero order, zero-order, and/or delayed release profile.
- PCT International Application Number PCT/US2007/011186 (WO 2007/133583) refers to a solid dosage form for delivery of water-soluble pharmaceutical agents.
- the solid dosage form comprises a matrix core containing the pharmaceutical agent and a hydrophobic material, and a coating containing a hydrophilic pore-forming agent and a hydrophobic polymer.
- the dosage form exhibits a zero-order release profile upon dissolution.
- US Pat. Application Nos. 1 1/582,164 (the ' 164 application) and 11/549,714 both refer to stable once-a-day oral dosage forms containing escitalopram or pharmaceutically acceptable salt thereof and bupropion and pharmaceutically acceptable salt thereof.
- compositions comprising the drugs may be separated into separate discrete zones such as separate layers or the compositions may take the form of a plurality of escitalopram beads or tablets and a plurality of bupropion tablets or beads, where ate least one or both of the bead or tablet populations are coated.
- US Pat. No. 7,241,805 refers to combinations of bupropion hydrobromide with a second drug, which may be citalopram or escitalopram.
- the '805 patent refers to controlled release microparticulate compositions wherein combination products can be made by providing an overcoat comprising a second drug substantially surrounding a control-releasing coat of each microparticle core comprising bupropion hydrobromide.
- a pulsatile release of at least one other drug is achieved from the coated microparticles.
- the overcoat can be an immediate release overcoat that includes at least one other drug.
- this composition can provide an immediate release of at least one other drug from the overcoat in a first phase of drug release, and then a subsequent controlled release of the bupropion hydrobromide from the control-releasing coated microparticle in a second phase of drug release.
- the present invention relates to a once-daily pharmaceutical composition
- a tablet core comprising a combination of actives selected from the group consisting of bupropion hydrochloride and escitalopram oxalate, bupropion hydrobromide and citalopram hydrochloride, bupropion hydrobromide and escitalopram oxalate, and bupropion hydrobromide and quetiapine fumarate, optionally a stabilizer in an effective stabilizing amount, and at least one pharmaceutically acceptable excipient, and a control-releasing coat surrounding the tablet core, wherein said composition surprisingly provides for a synchronous release of the combination of active agents across the pH range i.e., 0.1N HCl, pH 4.5 acetate buffer, and pH 6.8 phosphate buffer in-vitro.
- the once daily pharmaceutical composition surprisingly also provides for enhanced absorption of bupropion hydrobromide when administered to a subject in need of such administration.
- the once-daily pharmaceutical composition provides an about 15-25% increase in the bioavailability of bupropion when compared to co-administration of single active agent pharmaceutical compositions of bupropion hydrobromide and citalopram hydrochloride or bupropion hydrobromide and escitalopram oxalate.
- the synchronous release of the combination of actives comprising the once-daily pharmaceutical compositions of the present invention is particularly surprising when one considers that the differing physicochemical characteristics of the active ingredients and the likely differences in the permeability coefficients for the combination of active drugs would result in a differing rate and extent of drug release for each of the drugs chosen to be part of the combination. Accordingly, it was expected that it would be difficult to optimize the release kinetics of the combination of drugs contemplated without one drug potentially negatively influencing the release kinetics of the other drug of the combination. However, it was surprisingly found that despite the differing physicochemical characteristics (shown below) for the actives used in the combinations described herein, the in-vitro rate and extent of drug release was substantially synchronous across the pH range.
- At least one embodiment of the present invention provides for a once-daily pharmaceutical composition
- a once-daily pharmaceutical composition comprising a homogenous core comprising a therapeutically effective combination of active agents selected from the group consisting of bupropion hydrochloride and escitalopram oxalate, bupropion hydrobromide and citalopram hydrochloride, and bupropion hydrobromide and escitalopram oxalate, a stabilizer in an effective stabilizing amount, and at least one pharmaceutically acceptable excipient, and a control- releasing coating surrounding said core, said coating comprising a water-insoluble water- permeable film-forming polymer, a water-soluble polymer and at least one plasticizer; wherein said composition provides for a synchronous release of the combination of active agents.
- the pharmaceutical compositions provide for a synchronous release of the combination of actives in 0. IN HCl, pH 4.5 acetate buffer, pH 6.8 phosphate buffer when measured in 900 ml of each aqueous solution at 37 0 C using USPl apparatus at 75 rpm.
- the stabilizer comprises at least one suitable pharmaceutically acceptable inorganic acid, at least one suitablem pharmaceutically acceptable organic acid, at least one suitable pharmaceutically acceptable salt of an organic base, at least one suitable pharmaceutically acceptable salt of an inorganic acid, at least one suitable pharmaceutically acceptable acid salt of an amino acid, potassium metabisulfite, sodium bisulfite, or at least one suitable pharmaceutically acceptable phenylated antioxidant, or any combination thereof.
- stabilizer comprises at least one suitable inorganic acid, which at a concentration of about 0.31% w/w/ forms an aqueous solution having a pH of from about 0.5 to about 0.4.
- the stabilizer comprises hydrochloric acid, phosphoric acid, nitric acid, or sulfuric acid, or any combination thereof.
- the stabilizer comprises at least one suitable organic acid that has a solubility in water at 2O 0 C of less than about 10g/100g water and that at a concentration of about 60% w/w forms an aqueous suspension having a pH of from about 0.9 to about 4.0.
- the stabilizer comprises at least one suitable dicarboxylic acid that has a solubility in water at 2O 0 C of less than about
- the stabilizer comprises hydrochloric acid, phosphoric acid, nitric acid, and sulfuric acid, or any combination thereof.
- the stabilizer comprises at least one suitable pharmaceutically acceptable salt of an organic base having an aqueous pH of from about 2.70 to about 3.10 at a concentration of about 10% w/w.
- the stabilizer comprises creatinine hydrochloride.
- the stabilizer comprises at least one suitable pharmaceutically acceptable salt of an organic base having an aqueous pH of from about 2.95 to about 3.05, at a concentration of about 20% w/w.
- the stabilizer comprises thiamine hydrochloride.
- the stabilizer comprises sat least one salt of an organic base having an aqueous pH of from about 2.70 to about 2.72, at a concentration of about 20% w/w.
- the stabilizer comprises thiamine hydrochloride.
- the stabilizer is citric acid.
- the stabilizer comprises at least one suitable pharmaceutically acceptable salt of an inorganic acid having an aqueous pH of from about 4.20 to about 4.30 at a concentration of about 10 w/w.
- the stabilizer comprises potassium phosphate monobasic.
- the stabilizer comprises at least one suitable pharmaceutically acceptable acid salt of an amino acid.
- the stabilizer comprises L- cysteine hydrochloride, L-cystine dihydrochloride, glycine hydrochloride or any combination thereof.
- the stabilizer comprises potassium metabisulfite, sodium bisulfite, or any combination thereof.
- the stabilizer comprises at least one suitable pharmaceutically acceptable phenylated antioxidant.
- the stabilizer comprises butlylated hydroxytoluene (BHT), butylated hydroxyanisole (BHA), or any combination thereof.
- BHT butlylated hydroxytoluene
- BHA butylated hydroxyanisole
- the stabilizer comprises butylated hydroxytoluene.
- the stabilizer comprises a combination of citric acid and butylated hydroxytoluene.
- the once-daily pharmaceutical composition comprises at least one pharmaceutically acceptable excipient selected from the group consisting of a binder, a lubricant, a filler, a glidant, or any combinations thereof.
- the water-insoluble water- permeable film-forming polymer comprises at least one cellulose ether, cellulose ester, methacrylic acid derivative, aqueous ethylcellulose dispersion, aqueous acrylic enteric system, or polyvinyl derivative, or any combination thereof.
- the water-soluble polymer comprising the control-releasing coat comprises at least one methylcellulose, hydroxypropylcellulose, hydroxypropylmethylcellulose, hydroxyethylcellulose, polyvinyl alcohol, or polyvinylpyrrolidone, or any combination thereof.
- the at least one plasticizer comprises a combination of two plasticizers.
- the at least one plasticizer comprises at least one ester, or a polyalkylene glycol, or any combination thereof.
- the plastizer is a combination of polyethylene glycol 3350 and dibutyl sebacate.
- the once-daily pharmaceutical composition is in the form of a tablet.
- the once-daily pharmaceutical composition when administered to a subject in need of such administration can provide an about 15-25% increase in the bioavailability of bupropion when compared to co -administration of single active agent pharmaceutical compositions of bupropion hydrobromide and citalopram hydrochloride or bupropion hydrobromide and escitalopram oxalate.
- At least one embodiment of the present invention provides for a method of treating a mood and/or anxiety disorder in a subject in need of such treatment comprising administering once daily to said subject any one of the pharmaceutical compositions of the invention.
- At least one embodiment of the present invention provides for a method of treating a mood and/or anxiety disorder in a subject in need of such treatment comprising administering a once daily pharmaceutical composition comprising a homogenous core comprising a therapeutically effective combination of active agents selected from the group consisting of bupropion hydrochloride and escitalopram oxalate, bupropion hydrobromide and citalopram hydrochloride, and bupropion hydrobromide and escitalopram oxalate, a stabilizer in an effective stabilizing amount, and at least one pharmaceutically acceptable excipient, and a control-releasing coating surrounding said core, said coating comprising a water-insoluble water- permeable film-forming polymer, a water-soluble polymer and at least one plasticizer.
- active agents selected from the group consisting of bupropion hydrochloride and escitalopram oxalate, bupropion hydrobromide and citalopram hydrochloride, and bupropion hydrobromide and es
- At least one embodiment of the present invention provides for a method of treating a mood and/or anxiety disorder in a subject in need of such treatment comprising administering a once daily pharmaceutical composition comprising a homogenous core comprising a therapeutically effective combination of active agents selected from the group consisting of bupropion hydrochloride and escitalopram oxalate, bupropion hydrobromide and citalopram hydrochloride, and bupropion hydrobromide and escitalopram oxalate, a stabilizer in an effective stabilizing amount, and at least one pharmaceutically acceptable excipient, and a control-releasing coating surrounding said core, said coating comprising a water-insoluble water- permeable film-forming polymer, a water-soluble polymer and at least one plasticizer, wherein said composition provides an about 15-25% increase in the bioavailability of bupropion when compared to co-administration of single active agent pharmaceutical compositions of bupropion hydrobromide and citalopram hydrochloride or bupropion hydro
- At least one embodiment of the present invention provides for a method of treating a mood and/or anxiety disorder in a subject in need of such treatment comprising administering a once daily pharmaceutical composition comprising a homogenous core comprising a therapeutically effective combination of active agents selected from the group consisting of bupropion hydrochloride and escitalopram oxalate, bupropion hydrobromide and citalopram hydrochloride, and bupropion hydrobromide and escitalopram oxalate, a stabilizer in an effective stabilizing amount, and at least one pharmaceutically acceptable excipient, and a control-releasing coating surrounding said core, said coating comprising a water-insoluble water- permeable film-forming polymer, a water-soluble polymer and at least one plasticizer; wherein said composition provides for a synchronous release of the combination of active agents.
- At least one embodiment of the present invention provides for a pharmaceutical composition
- a pharmaceutical composition comprising a controlled release matrix core, said controlled release matrix core comprising at least one hydrophilic control-releasing polymer present in a control-releasing amount, a therapeutically effective combination of active agents selected from the group consisting of bupropion hydrochloride and escitalopram oxalate, bupropion hydrobromide and citalopram hydrochloride, and bupropion hydrobromide and escitalopram oxalate, a stabilizer, and at least one pharmaceutically acceptable excipient; wherein said pharmaceutical composition provides for a synchronous release of the combination of active agents.
- the at least one hydrophilic control-releasing polymer comprising the controlled-release matrix core comprises at least one hydrophilic cellulose, ethylcellulose, polysaccharide, polyvinylpyrrolidone, polymethacrylate, or a mixture of polyvinyl acetate and polyvinylpyrrolidone, or any combination thereof.
- At least one embodiment of the present invention provides for a method of treating a mood and/or anxiety disorder in a subject in need of such treatment comprising administering once daily to said subject a pharmaceutical composition comprising a controlled release matrix core, said controlled release matrix core comprising at least one hydrophilic control-releasing polymer present in a control-releasing amount, a therapeutically effective combination of active agents selected from the group consisting of bupropion hydrochloride and escitalopram oxalate, bupropion hydrobromide and citalopram hydrochloride, and bupropion hydrobromide and escitalopram oxalate, a stabilizer, and at least one pharmaceutically acceptable excipient; wherein said composition provides for a synchronous release of the combination of actives.
- At least one embodiment of the present invention provides for a method of treating a mood and/or anxiety disorder in a subject in need of such treatment comprising administering once daily to said subject a pharmaceutical composition comprising a controlled release matrix core, said controlled release matrix core comprising at least one hydrophilic control-releasing polymer present in a control-releasing amount, a therapeutically effective combination of active agents selected from the group consisting of bupropion hydrochloride and escitalopram oxalate, bupropion hydrobromide and citalopram hydrochloride, and bupropion hydrobromide and escitalopram oxalate, a stabilizer, and at least one pharmaceutically acceptable excipient.
- At least one embodiment of the present invention provides for a method of treating a mood and/or anxiety disorder in a subject in need of such treatment comprising administering once daily to said subject a pharmaceutical composition comprising a controlled release matrix core, said controlled release matrix core comprising at least one hydrophilic control-releasing polymer present in a control-releasing amount, a therapeutically effective combination of active agents selected from the group consisting of bupropion hydrochloride and escitalopram oxalate, bupropion hydrobromide and citalopram hydrochloride, and bupropion hydrobromide and escitalopram oxalate, a stabilizer, and at least one pharmaceutically acceptable excipient, wherein said composition provides an about 15-25% increase in the bioavailability of bupropion when compared to co-administration of single active agent pharmaceutical compositions of bupropion hydrobromide and citalopram hydrochloride or bupropion hydrobromide and escitalopram oxalate.
- At least one embodiment provides for a pharmaceutical composition
- a pharmaceutical composition comprising a core comprising a first immediate release layer comprising a therapeutically effective amount of an active agent selected from the group consisting of bupropion hydrochloride and bupropion hydrobromide, optionally a stabilizer and at least one pharmaceutically acceptable excipient in direct contact with a second immediate release layer comprising an active agent selected from the group consisting of citalopram hydrochloride and escitalopram oxalate, optionally a stabilizer, and at least one pharmaceutically acceptable excipient, and a control-releasing coating surrounding said core, said coating comprising a water-insoluble water-permeable film-forming polymer, a water-soluble polymer and at least one plasticizer, wherein said composition provides for a synchronous release of the active agents.
- At least one embodiment of the present invention provides for a method of treating a mood and/or anxiety disorder in a subject in need of such treatment comprising administering once daily to said subject a pharmaceutical composition comprising a core comprising a first immediate release layer comprising a therapeutically effective amount of an active agent selected from the group consisting of bupropion hydrochloride and bupropion hydrobromide, optionally a stabilizer and at least one pharmaceutically acceptable excipient in direct contact with a second immediate release layer comprising an active agent selected from the group consisting of citalopram hydrochloride and escitalopram oxalate, optionally a stabilizer, and at least one pharmaceutically acceptable excipient, and a control-releasing coating surrounding said core, said coating comprising a water-insoluble water-permeable film-forming polymer, a water-soluble polymer and at least one plasticizer, wherein said composition provides for a synchronous release of the active agents.
- At least one embodiment of the present invention provides for a method of treating a mood and/or anxiety disorder in a subject in need of such treatment comprising administering once daily to said subject a pharmaceutical composition comprising administering once daily to said subject a pharmaceutical composition comprising a core comprising a first immediate release layer comprising a therapeutically effective amount of an active agent selected from the group consisting of bupropion hydrochloride and bupropion hydrobromide, optionally a stabilizer and at least one pharmaceutically acceptable excipient in direct contact with a second immediate release layer comprising an active agent selected from the group consisting of citalopram hydrochloride and escitalopram oxalate, optionally a stabilizer, and at least one pharmaceutically acceptable excipient, and a control-releasing coating surrounding said core, said coating comprising a water-insoluble water-permeable film-forming polymer, a water-soluble polymer and at least one plasticizer.
- At least one embodiment of the present invention provides for a method of treating a mood and/or anxiety disorder in a subject in need of such treatment comprising administering once daily to said subject a pharmaceutical composition comprising administering once daily to said subject a pharmaceutical composition comprising a core comprising a first immediate release layer comprising a therapeutically effective amount of an active agent selected from the group consisting of bupropion hydrochloride and bupropion hydrobromide, optionally a stabilizer and at least one pharmaceutically acceptable excipient in direct contact with a second immediate release layer comprising an active agent selected from the group consisting of citalopram hydrochloride and escitalopram oxalate, optionally a stabilizer, and at least one pharmaceutically acceptable excipient, and a control-releasing coating surrounding said core, said coating comprising a water-insoluble water-permeable film-forming polymer, a water-soluble polymer and at least one plasticizer, wherein said composition provides an about 15-25% increase in the bioavailability of bupropion when
- the once-daily pharmaceutical compositions of the invention avoid dose dumping of the combination of actives in the presence of food and/or alcohol.
- the once-daily pharmaceutical compositions of the invention are free of food-effect.
- At least one embodiment of the present invention provides for a method of treating a mood and/or anxiety disorder in a subject in need of such treatment comprising administering a once daily pharmaceutical composition comprising a homogenous core comprising a therapeutically effective combination of active agents selected from the group consisting of bupropion hydrochloride and escitalopram oxalate, bupropion hydrobromide and citalopram hydrochloride, and bupropion hydrobromide and escitalopram oxalate, a stabilizer in an effective stabilizing amount, and at least one pharmaceutically acceptable excipient, and a control-releasing coating surrounding said core, said coating comprising a water-insoluble water- permeable film-forming polymer, a water-soluble polymer and at least one plasticizer, wherein said composition provides an about 15-25% increase in the bioavailability of bupropion when compared
- At least one embodiment of the present invention provides for a method of treating a mood and/or anxiety disorder in a subject in need of such treatment comprising administering once daily to said subject a pharmaceutical composition comprising a controlled release matrix core, said controlled release matrix core comprising at least one hydrophilic control-releasing polymer present in a control-releasing amount, a therapeutically effective combination of active agents selected from the group consisting of bupropion hydrochloride and escitalopram oxalate, bupropion hydrobromide and citalopram hydrochloride, and bupropion hydrobromide and escitalopram oxalate, a stabilizer, and at least one pharmaceutically acceptable excipient, wherein said composition provides an about 15-25% increase in the bioavailability of bupropion when compared to co-administration of single active agent pharmaceutical compositions of bupropion hydrobromide and citalopram hydrochloride or bupropion hydrobromide and escitalopram oxalate and is free of food effect.
- At least one embodiment of the present invention provides for a method of treating a mood and/or anxiety disorder in a subject in need of such treatment comprising administering once daily to said subject a pharmaceutical composition comprising administering once daily to said subject a pharmaceutical composition comprising a core comprising a first immediate release layer comprising a therapeutically effective amount of an active agent selected from the group consisting of bupropion hydrochloride and bupropion hydrobromide, optionally a stabilizer and at least one pharmaceutically acceptable excipient in direct contact with a second immediate release layer comprising an active agent selected from the group consisting of citalopram hydrochloride and escitalopram oxalate, optionally a stabilizer, and at least one pharmaceutically acceptable excipient, and a control-releasing coating surrounding said core, said coating comprising a water-insoluble water-permeable film-forming polymer, a water-soluble polymer and at least one plasticizer, wherein said composition provides an about 15-25% increase in the bioavailability of bupropion when
- At least one embodiment of the present invention provides for a method of manufacturing a pharmaceutical composition, said method comprising the steps of: a) granulating an active agent selected from the group consisting of bupropion hydrobromide and bupropion hydrochloride by homogenously blending with a solution of at least one suitable binder and optionally a suitable stabilizer; b) drying said granules comprising either bupropion hydrobromide or bupropion hydrochloride and retaining said granules of a size between about 355 ⁇ m and about 800 ⁇ m; c) granulating an active agent selected from the group consisting of citalopram hydrochloride, escitalopram oxalate, and quetiapine fumarate by homogenously blending with a solution of at least one suitable binder and optionally at least one suitable stabilizer; d) drying said granules comprising either citalopram hydrochloride, escitalopram oxalate, and quet
- At least one embodiment of the present invention provides for a method of manufacturing a pharmaceutical composition
- a method of manufacturing a pharmaceutical composition comprising the steps of: a) granulating a first active selected from the group consisting of bupropion hydrochloride and bupropion hydrobromide with a second active selected from the group consisting of citalopram hydrochloride, escitalopram oxalate and quetiapine fumarate, in an amount equivalent to the desired dosage strength the first and second active by homogenously blending with a solution of at least one suitable binder and optionally at least suitable stabilizer; b) drying the granules obtained in (a) and retaining granules of ⁇ 1.00 ⁇ m) homogenously blending the granules obtained in (b) with at least one suitable lubricant; d) compressing the homogenously blended mixture obtained in (c) into a homogenous tablet core; and d) coating said homogenously blended tablet core with a control-releasing coat
- the amount of bupropion hydrobromide present is at least about 10% less than a single active agent pharmaceutical composition comprising bupropion hydrobromide.
- the amount of bupropion hydrobromide present is at least about 10% less than a single active agent pharmaceutical composition comprising 348mg bupropion hydrobromide.
- FIG. IA is a graph depicting the dissolution profile in 900 ml of 0. IN HCl using
- FIG. IB is a graph depicting the dissolution profile in 900 ml of pH 4.5 acetate buffer using USP Apparatus 1 at 75 rpm at 37 0 C of the composition described in Example 1.
- FIG. 1C is a graph depicting the dissolution profile in 900 ml of pH 6.8 phosphate buffer using USP Apparatus 1 at 75 rpm at 37 0 C of the composition described in Example 1.
- FIG. 2A is a graph depicting the dissolution profile in 900 ml of 0. IN HCl using
- FIG. 2B is a graph depicting the dissolution profile in 900 ml of pH 4.5 acetate buffer using USP Apparatus 1 at 75 rpm at 37 0 C of the composition described in Example 2.
- FIG. 2C is a graph depicting the dissolution profile in 900 ml of pH 6.8 phosphate buffer using USP Apparatus 1 at 75 rpm at 37 0 C of the composition described in
- FIG. 3A is a graph depicting the dissolution profile in 900 ml of 0. IN HCl using
- FIG. 3B is a graph depicting the dissolution profile in 900 ml of pH 4.5 acetate buffer using USP Apparatus 1 at 75 rpm at 37 0 C of the composition described in Example 3.
- FIG. 3C is a graph depicting the dissolution profile in 900 ml of pH 6.8 phosphate buffer using USP Apparatus 1 at 75 rpm at 37 0 C of the composition described in
- FIG. 4 is a graph depicting the dissolution profile in 900 ml of 0. IN HCl using
- FIG. 5 is a graph depicting the dissolution profile in 900 ml of 0. IN HCl using
- FIG. 6 is a graph depicting the dissolution profile in 900 ml of 0. IN HCl using
- FIG. 7 is a graph depicting the dissolution profile in 900 ml of 0. IN HCl using
- FIG. 8 is a graph depicting the dissolution profile in 900 ml of 0. IN HCl using
- FIG. 9 is a graph depicting the dissolution profile in 900 ml of 0. IN HCl using
- FIG. 10 is a graph depicting the dissolution profile in 900 ml of 0. IN HCl using
- FIG. 11 is a graph depicting the dissolution profile in 900 ml of 0. IN HCl using
- FIG. 12 is a graph depicting the dissolution profile in 900 ml of 0. IN HCl using
- FIG. 13 is a graph depicting the dissolution profile in 900 ml of 0. IN HCl using
- FIG. 14 is a graph depicting the dissolution profile in 900 ml of 0. IN HCl using
- FIG. 15 is a graph depicting the dissolution profile in 900 ml of 0. IN HCl using
- FIG. 16 is a graph depicting the dissolution profile in 900 ml of 0. IN HCl using
- FIG. 17A is a graph depicting the dissolution profile in 900 ml of 0. IN HCl using USP Apparatus 1 at 75 rpm at 37 0 C of the composition described in Example 17.
- FIG. 17B is a graph depicting the dissolution profile in 900 ml of pH 6.8 phosphate buffer using USP Apparatus 1 at 75 rpm at 37 0 C of the composition described in
- FIG. 18 is a graph depicting the dissolution profile in 900 ml of 0. IN HCl using
- FIG 19 is a graph depicting the dissolution profile in 900 ml of 0. IN HCl using
- FIG 2OA is a graph depicting the dissolution profile in 900 ml of 0. IN HCl using
- FIG. 2OB is a graph depicting the dissolution profile in 900 ml of pH 6.8 phosphate buffer using USP Apparatus 1 at 75 rpm at 37 0 C of the composition described in
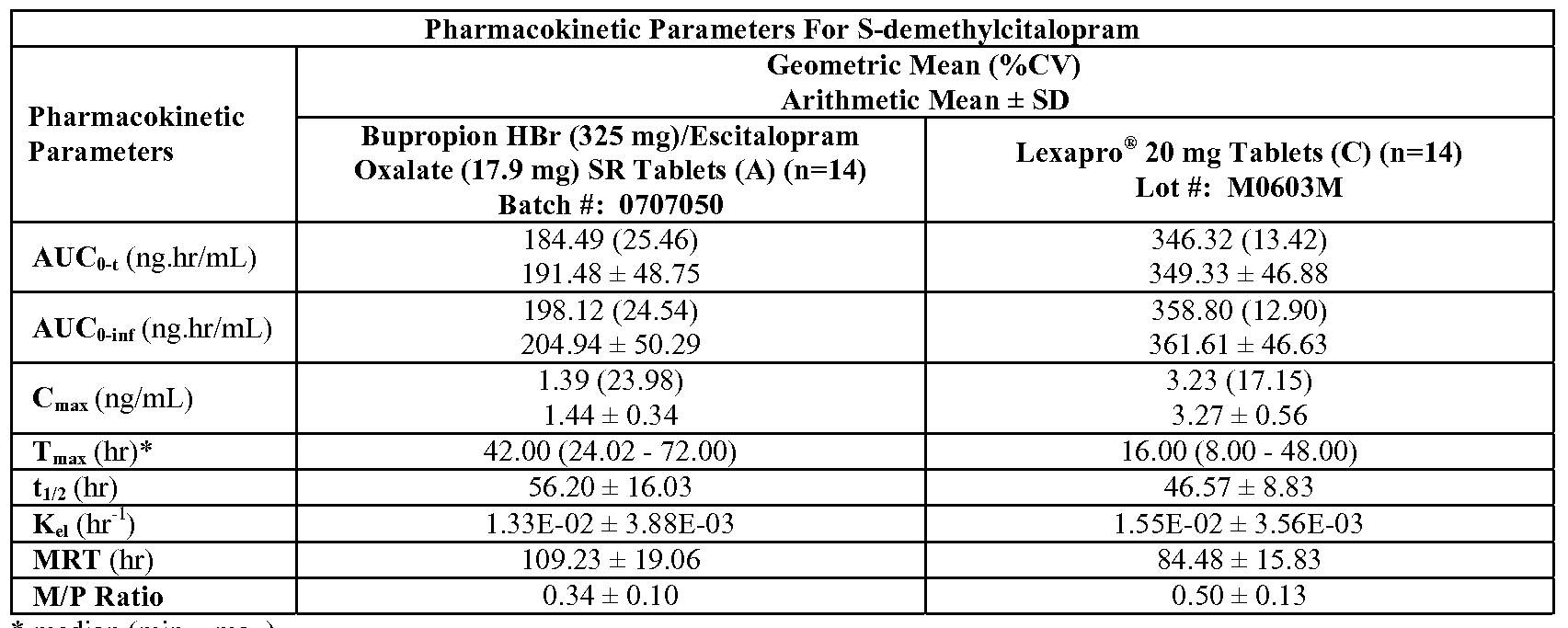
- FIG. 22B is a graph depicting the mean concentration-time profile of desmethylcitalopram during steady-state dosing of Celexa ® 20 mg alone and Celexa ® 20 mg plus
- FIG. 23C is a graph depicting the mean plasma bupropion threoamino alcohol concentration versus time profile for the study described in Example 23 (linear scale, Group 1
- FIG. 23D is a graph depicting the mean plasma bupropion erythroamino alcohol concentration versus time profile for the study described in Example 23 (linear scale, Group 1
- FIG. 23G is a graph depicting the mean plasma demethylcitalopram concentration versus time profile for the study described in Example 23 (linear scale, Group 1
- FIG. 23H is a graph depicting the mean plasma didemethylcitalopram concentration versus time profile for the study described in Example 23 (linear scale, Group 1
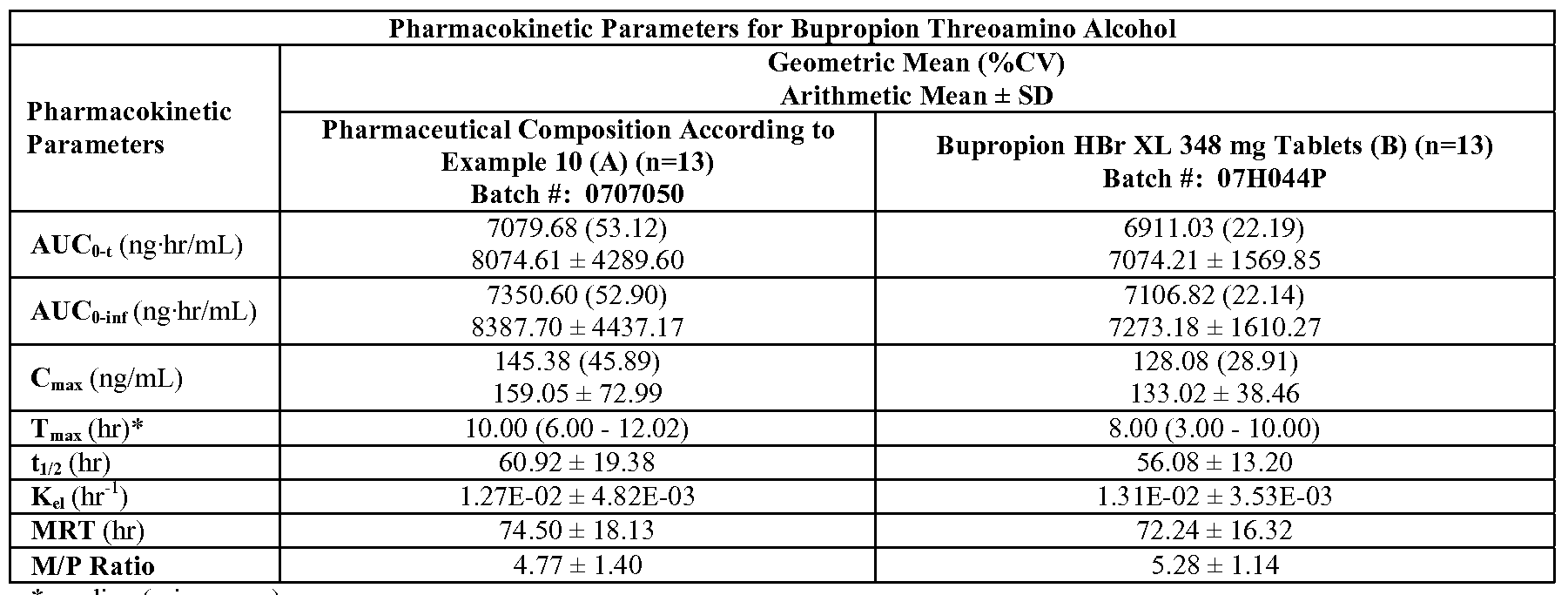
- FIG. 23K is a graph depicting the mean plasma bupropion threoamino alcohol concentration versus time profile for the study described in Example 23 (linear scale, Group 2
- FIG. 23L is a graph depicting the mean plasma bupropion erythroamino alcohol concentration versus time profile for the study described in Example 23 (linear scale, Group 2
- FIG. 23O is a graph depicting the mean plasma demethylcitalopram concentration versus time profile for the study described in Example 23 (linear scale, Group 2
- FIG. 23P is a graph depicting the mean plasma didemethylcitalopram concentration versus time profile for the study described in Example 23 (linear scale, Group 2
- FIG. 24C is a graph depicting the mean plasma bupropion threoamino alcohol concentration versus time profile for the study described in Example 24 (linear scale, Group 1
- FIG. 24D is a graph depicting the mean plasma bupropion erythroamino alcohol concentration versus time profile for the study described in Example 24 (linear scale, Group 1
- FIG. 24G is a graph depicting the mean plasma demethylcitalopram concentration versus time profile for the study described in Example 24 (linear scale, Group 1
- FIG. 24H is a graph depicting the mean plasma didemethylcitalopram concentration versus time profile for the study described in Example 24 (linear scale, Group 1
- FIG. 24K is a graph depicting the mean plasma bupropion threoamino alcohol concentration versus time profile for the study described in Example 24 (linear scale, Group 2
- FIG. 24L is a graph depicting the mean plasma bupropion erythroamino alcohol concentration versus time profile for the study described in Example 24 (linear scale, Group 2
- FIG. 24O is a graph depicting the mean plasma demethylcitalopram concentration versus time profile for the study described in Example 24 (linear scale, Group 2
- FIG. 24P is a graph depicting the mean plasma didemethylcitalopram concentration versus time profile for the study described in Example 24 (linear scale, Group 2
- FIG. 27 is a graph depicting the dissolution profile in 900 ml of pH 7.5 phosphate buffer using USP Apparatus 1 at 75 rpm at 37 0 C of the composition described in
- FIG. 28 is a graph depicting the dissolution profile in 900 ml of pH 7.5 phosphate buffer using USP Apparatus 1 at 75 rpm at 37 0 C of the composition described in
- FIG. 29A is a graph depicting the dissolution profile in 900 ml of 0. IN HCl using USP Apparatus 1 at 75 rpm at 37 0 C of the composition described in Example 29.
- FIG. 29B is a graph depicting the dissolution profile in 900 ml of pH 6.8 phosphate buffer using USP Apparatus 1 at 75 rpm at 37 0 C of the composition described in
- the term “about” or “approximately” as used herein means within an acceptable range for the particular value as determined by one of ordinary skill in the art. An accetable range may depend on how the value is measured or determined, i.e., the limitations of the measurement system or on the desired properties sought to be obtained by the present invention.
- the term “active”, “active agent”, “active pharmaceutical agent”, “active drug” or “drug” as used herein means the active pharmaceutical ingredient (“API”), which can be either bupropion hydrobromide, bupropion hydrochloride, citalopram hydrochloride, escitalopram oxalate, or quetiapine fumarate alone or in combination.
- tablette core refers to the part of the once-daily pharmaceutical composition comprising the active agents, at least one pharmaceutically acceptable excipient, and optionally at least one stabilizer minus the control-releasing coat. More specifically, a tablet core can be a homogenous core, a controlled-release matrix core, or a bi- layered core.
- homogenous core refers to a composition in which the combination of active agents selected from the group consisting of bupropion hydrochloride (bupropion HCl) and escitalopram oxalate (escitalopram Ox), bupropion hydrobromide (bupropion HBr) and citalopram hydrochloride (citalopram HCl), bupropion HBr and escitalopram Oxalate, or bupropion hydrobromide and quetiapine fumarate are blended together with at least one other pharmaceutically acceptable excipient to form a homogenous solid core which has a uniform structure or composition throughout and is free of discreet zones or layers of the active agent combinations.
- active agents selected from the group consisting of bupropion hydrochloride (bupropion HCl) and escitalopram oxalate (escitalopram Ox), bupropion hydrobromide (bupropion HBr) and citalopram hydrochloride (citalopram HCl), bupropion
- controlled release matrix core refers to a composition comprising at least one hydrophilic control-releasing polymer present in a control-releasing amount, a combination of active agents selected from the group consisting of bupropion HCl and escitalopram Oxalate, bupropion HBr and citalopram HCl, bupropion HBr and escitalopram Oxalate, or bupropion hydrobromide and quetiapine fumarate, and at least one pharmaceutically acceptable excipient.
- control-releasing polymers can include, for example, hydrophilic celluloses, ethylcellulose, polysaccharides, polyvinylpyrrolidone, zein, ethylcellulose, polymethacrylates, and mixtures of polyvinyl acetate and polyvinylpyrrolidone, commercially available as Kollidon ® SR.
- the controlled release matrix core may comprise at least one other pharmaceutically acceptable excipient present in amounts that do not contribute to the control- release of the combination of actives, but are present for the ease of manufacture of the controlled release matrix core.
- the ingredients are blended together to form a homogenous solid core, which has a uniform structure or composition throughout and is free of discreet zones or layers of the active agent combinations.
- terapéuticaally effective refers to the amount or quantity of the combination of active agents enough for the required or desired therapeutic response or the amount which is sufficient to elicit an appreciable biological response, when administered to a patient in need of administration of the combination of drugs.
- the exact amount of the combination of active agents required will vary from subject to subject, depending on age, general condition of the subject, the severity of the condition being treated, and the particular combination of drugs administered. Thus, it is not possible to specify and exact “therapeutically effective” amount.
- the specific dosage for a given patient under specific conditions and for a specific disease will routinely vary, but determination of the optimum amount in each case can readily be accomplished by simple routine procedures.
- dose dumping refers to the unintended rapid release of the entire amount or a significant fraction of the active agents in a short period of time from a controlled release or modified-release dosage form in a fixed time relative to the release of the active agents that occurs when the same controlled release or modified-release dosage form is not subject to conditions which induces dose dumping.
- Conditions that may induce dose dumping include for concomitant ingestion of alcohol or food.
- control-releasing coating or "sustained release coating” as used herein refers to a functional coating which when applied onto a core comprising an active or combination of actives does not result in the immediate release of the active or combination of actives.
- the coating is permeable to the active or combination of actives in the absence of any monomeric pore forming agents and is free of any pre-formed pores.
- the coating when applied onto a core comprising an active or combination of actives modifies or controls the release of the active agents when compared to an uncoated core comprising the same active or combination of actives.
- controlled release includes any nonimmediate release pharmaceutical composition.
- a "controlled release” or “sustained release” pharmaceutical composition when administered orally or when placed in dissolution media, does not result in the immediate release of the active or combination of actives from the once-daily pharmaceutical composition.
- synchronous release refers to the substantially similar rate of release of the combination of active agents from the once-daily pharmaceutical composition in dissolution media in-vitro regardless of pH.
- plasticizer as used herein includes any compound or combination of compounds capable of plasticizing or softening a polymer or binder used in the present invention, The use of plasticizers is optional, and can be included in the dosage form to modify the properties of and characteristics of the polymers used in the control-releasing coating for convenient processing of the coat during manufacture of the coated pharmaceutical composition. Once the coated, plasticized pharmaceutical composition has been manufactured, the plasticizer can function to increase the hydrophilicity of the coat in the environment of use. During manufacture of the coated, plasticized pharmaceutical composition, the plasticizer(s) can lower the melting temperature or glass transition temperature (softening point temperature) of the polymer or combination of polymers used in the manufacture of the control-releasing coat.
- the plasticizer(s) can also broaden the average molecular weight of a polymer or combination of polymers used in the manufacture of the control-releasing coat, thereby also lowering the glass transition temperature of the control-releasing coat. Plasticizers can also reduce the viscosity of a polymer or combinations of polymers for convenient processing of the coat solution when manufacturing the control-releasing coat.
- tablette refers to a single dosage form comprising the combination of active agents to be administered to a patient in need of such administration.
- the term “tablet” also includes a tablet that may be a combination of one or more minitablets.
- single active agent pharmaceutical compositions refers to pharmaceutical compositions comprising only one active agent.
- a single active agent pharmaceutical composition of bupropion HBr contains only bupropion HBr and no other active agent.
- a single active agent pharmaceutical composition of bupropion HCl contains only bupropion HCl and no other active agent.
- the single active agent pharmaceutical composition of bupropion HCl described herein is commercially available as Wellbutrin ® XL in 150 mg and 300 mg dosage strengths in the US.
- the single active agent pharmaceutical composition of citalopram HCl contains only citalopram HCl and no other active agent.
- the single active agent pharmaceutical composition of citalopram HCl described herein is commercially available as Celexa ® in the US and is available in dosage strengths of 10 mg, 20 mg, and 40 mg of the base.
- the single active agent pharmaceutical composition of escitalopram Oxalate described herein contains escitalopram Oxalate as the sole active agent and is commercially available as Lexapro ® in the US in dosage strengths of 5 mg, 10 mg, and 20 mg of the base.
- co-administration refers to administering to a patient in need of such administration a first single active agent pharmaceutical composition together with a second single active agent pharmaceutical composition which may containing the same single active agent as the first single active agent pharmaceutical composition or a different single active agent pharmaceutical composition simultaneously.
- co -administration of 300 mg Wellbutrin ® XL and 20 mg Lexapro ® means that one 300 mg Wellbutrin ® XL tablet and one 20 mg Lexapro ® tablet are administered to a patient in need of such administration at the same time.
- immediate-release coat as used herein is defined to mean a coat, which has substantially no influence on the rate of release of an active or combination of actives from the once-daily pharmaceutical composition in-vitro or in-vivo when compared to a pharmaceutical composition comprising the same active or combination of actives.
- the excipients comprising the immediate release coat have no substantial controlled release, swelling, erosion, or erosion and swelling properties, which could lead to the non-immediate release of the active or combination of actives from the once-daily pharmaceutical composition.
- the immediate release coat can enhance the chemical, biological, physical stability, or the physical appearance of the once-daily pharmaceutical composition.
- immediate release core or “immediate release layer” as used herein refers to a core or immediate release layer within a core, which has substantially no influence on the rate of release of an active or combination of actives from the once-daily pharmaceutical composition in-vitro or in-vivo when compared to a controlled release matrix core comprising the same active or combination of actives.
- the excipients comprising the immediate release core or immediate release layer within a core have no substantial controlled release, swelling, erosion, or erosion and swelling properties, which could lead to the non-immediate release of the active or combination of actives from the immediate release core or immediate release layer within a core.
- Stabilizer means a compound when present in an effective stabilizing amount inhibits or prevents the degradation of the active agents, so that the stabilizer can be used in the once-daily pharmaceutical composition while retaining much of the active agents' potency over time.
- Stabilizers useful in accordance with the present invention retain at least about 80% of the potency of the active agents and preferably over 90% of potency after one year of storage at room temperature (59 - 77 0 C) at 35-60% humidity.
- the term “potency” means the weight of the active agent remaining in a pharmaceutical composition after a period of time has elapsed, for example about a year under ambient conditions or about 12 weeks at about 4O 0 C and about 75% relative humidity, expressed as a percentage of the initial weight of the active agents in the composition.
- the weight is measured by suitable quantitative analytical techniques known to one of ordinary skill in the art, such as for example an HPLC.
- Free of food effect means that the bioavailability of the desired combination of drug actives when administered using the once-daily pharmaceutical compositions of the present invention is not statistically significantly different between a fed and fasted study as described in the Guidance for Industry:Food-Effect Bioavailability and Fed Bioequivalence Studies, U.S. Department of Health and Human Services, Food and Drug Administration, Center for Drug Evaluation and Research (CDER), December 2002.
- the present invention relates to a pharmaceutical composition
- a pharmaceutical composition comprising a tablet core comprising a combination of actives selected from the group consisting of bupropion hydrochloride and escitalopram oxalate, bupropion hydrobromide and citalopram hydrochloride, bupropion hydrobromide and escitalopram oxalate, and bupropion hydrobromide and quetiapine fumarate, and at least one pharmaceutically acceptable excipient, and a control-releasing coat surrounding the tablet core, wherein said composition surprisingly provides for a synchronous release of the combination of active agents across the pH range i.e., 0.1N HCl, pH 4.5 acetate buffer, and pH 6.8 phosphate buffer in-vitro.
- the once-daily pharmaceutical composition surprisingly also provides for enhanced absorption of bupropion hydrobromide when administered to a subject in need of such administration.
- the once-daily pharmaceutical composition provides an about 15-25% increase in the bioavailability of bupropion when compared to co-administration of single active agent pharmaceutical compositions of bupropion hydrobromide and citalopram hydrochloride or bupropion hydrobromide and escitalopram oxalate.
- the tablet core comprises a combination of actives selected from the group consisting of a therapeutically effective combination of active agents selected from the group consisting of bupropion hydrochloride and escitalopram oxalate, bupropion hydrobromide and citalopram hydrochloride, bupropion hydrobromide and escitalopram oxalate, bupropion hydrobromide and quetiapine fumarate, and optionally a stabilizer, and at least one pharmaceutically acceptable excipient.
- actives selected from the group consisting of a therapeutically effective combination of active agents selected from the group consisting of bupropion hydrochloride and escitalopram oxalate, bupropion hydrobromide and citalopram hydrochloride, bupropion hydrobromide and escitalopram oxalate, bupropion hydrobromide and quetiapine fumarate, and optionally a stabilizer, and at least one pharmaceutically acceptable excipient.
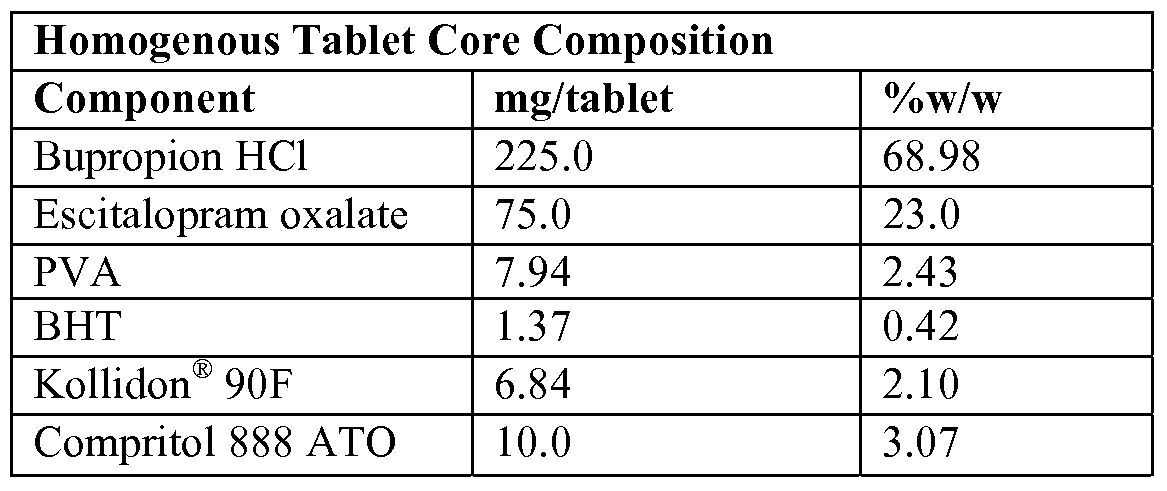
- the amount of bupropion hydrochloride present in the homogenous tablet core can be about 10%, about 20%, about 30%, about 40%, about 50%, about 60%, about 70%, about 80%, about 90%, or about 99% w/w of the dry tablet core weight, and the amount of escitalopram oxalate can be present at about 0.1%, about 0.2%, about 0.4%, about 0.6%, about 0.8%, about 1%, about 2%, about 4%, about 6%, ablout 8%, about 10%, about 20%, about 30%, about 40%, or about 50% w/w of the dry tablet core weight.
- the amount of bupropion hydrochloride is about 300 mg and the amount of escitalopram oxalate is about 20 mg (16mg escitalopram free base).
- the amount of bupropion hydrobromide present in the homogenous tablet core can be about 10%, about 20%, about 30%, about 40%, about 50%, about 60%, about 70%, about 80%, about 90%, or about 99% w/w of the dry tablet core weight, and the amount of escitalopram oxalate can be present at about 0.1%, about 0.2%, about 0.4%, about 0.6%, about 0.8%, about 1%, about 2%, about 4%, about 6%, ablout 8%, about 10%, about 20%, about 30%, about 40%, or about 50% w/w of the dry tablet core weight.
- the amount of bupropion hydrobromide is about 325 mg and the amount of escitalopram oxalate is about 16 mg. In at least one other embodiment of the present invention, the amount of bupropion hydrobromide is about 156 mg and the amount of escitalopram oxalate is about 8 mg.
- the amount of bupropion hydrobromide present in the homogenous tablet core can be about 10%, about 20%, about 30%, about 40%, about 50%, about 60%, about 70%, about 80%, about 90%, or about 99% w/w of the dry tablet core weight, and the amount of escitalopram oxalate can be present at about 0.1%, about 0.2%, about 0.4%, about 0.6%, about 0.8%, about 1%, about 2%, about 4%, about 6%, ablout 8%, about 10%, about 20%, about 30%, about 40%, or about 50% w/w of the dry tablet core weight.
- the amount of bupropion hydrobromide is about 348 mg and the amount of citalopram hydrochloride is about 22.2 mg.
- the amount of bupropion hydrobromide present in the homogenous tablet core can be about 10%, about 20%, about 30%, about 40%, about 50%, about 60%, about 70%, about 80%, about 90%, or about 99% w/w of the dry tablet core weight, and the amount of quetiapine fumarate can be present at about 1%, about 2%, about 4%, about 6%, about 8%, about 10%, about 20%, about 30%, about 40%, about 50%, about 60%, about 70%, about 80%, or about 90% w/w of the dry tablet core weight.
- the amount of bupropion hydrobromide is about 348 mg and the amount of quetiapine fumarate is about 23 mg.
- the tablet core can comprise a pharmaceutically acceptable suitable stabilizer. Stabilizers are used to inhibit degradation of the combination of active agents, thereby maintaining their potency over time (at least 12-months) and increasing shelf life of the finished pharmaceutical compositions of the invention. Stabilizers for bupropion hydrochloride or bupropion hydrobromide are optional. Stabilizers suitable for inhibiting degradation of bupropion hydrochloride or bupropion hydrobromide may be chosen based on the stabilizer's ability to provide an acidic environment in the once-daily pharmaceutical composition.
- Stabilizers suitable for inhibiting degradation of bupropion hydrochloride or bupropion hydrobromide include, for example, pharmaceutically acceptable inorganic acids, which at a concentration of about 0.31% w/w/ form an aqueous solution having a pH of from about 0.5 to about 0.4.
- inorganic acids include, but are not limited to, hydrochloric acid, phosphoric acid, nitric acid, and sulfuric acid, or combinations thereof.
- suitable organic acids that have a solubility in water at 2O 0 C of less than about 10g/100g water and that at a concentration of about 60% w/w form an aqueous suspension having a pH of from about 0.9 to about 4.0 can also function as suitable stabilizers.
- organic acids include, but are not limited to, dicarboxylic acids, such as for example, lactic, formic, acetic, oxalic, succinic, adipic, fumaric, and phthalic acid, or combinations thereof.
- Citric acid is another example of a suitable organic acid hat can be used as an effective stabilizer.
- Suitable stabilizers include salts of organic bases such as, creatinine hydrochloride, preferably having an aqueous pH of from about 2.70 to about 3.10 at a concentration of about 10% w/w, thiamine hydrochloride, preferably having an aqueous pH of from about 2.95 to about 3.05, at a concentration of about 20% w/w, pyridoxine hydrochloride, preferably having an aqueous pH of from about 2.70 to about 2.72, at a concentration of about 20% w/w, or combinations thereof.
- Suitable salts of inorganic acids can also function as stabilizers.
- Such a salt includes, but is not limited to potassium phosphate monobasic, preferably having an aqueous pH of from about 4.20 to about 4.30 at a concentration of about 10 w/w.
- Other stabilizers suitable for use include acid salts of amino acids such as L-cysteine hydrochloride, L-cystine dihydrochloride and glycine hydrochloride, or combinations thereof and sulfites such as potassium metabisulfite and sodium bisulfite, or combinations thereof.
- the amount of stabilizer appropriate for inhibiting degradation of bupropion hydrochloride or bupropion hydrobromide can be about 0.1%, about 0.2%, about 0.4%, about 0.6%, about 0.8%, about 1%, about 2%, about 4%, about 6%, about 8%, about 10%, about 15%, about 20%, about 25%, or about 30% w/w of the dry tablet core weight. In at least one embodiment of the present invention, the amount of stabilizer appropriate for inhibiting degradation of bupropion hydrochloride or bupropion hydrobromide can be about 5% w/w of the dry tablet core. Stabilizers for citalopram hydrochloride and escitalopram oxalate are optional.
- suitable stabilizers can be added to stabilize the citalopram hydrochloride or escitalopram oxalate when the citalopram hydrochloride or escitalopram oxalate is in intimate contact with either bupropion hydrochloride or bupropion hydrobromide.
- the suitable stabilizers can be selected from the class of phenylated antioxidants. Non-limiting examples of such phenylated antioxidants include butlylated hydroxytoluene (BHT), butylated hydroxyanisole (BHA), or combinations thereof.
- BHT is the preferred stabilizer and can be present at about 0.01%, about 0.02%, about 0.04%, about 0.06%, about 0.08%, about 1%, about 1.5%, about 2%, about 2.5%, about 3%, about 3.5%, about 4%, about 4.5%, or about 5% w/w of the dry tablet core weight. In at least one embodiment of the present invention, BHT comprises about 0.1% w/w of the dry tablet core weight.
- the tablet core can comprise at least one pharmaceutically acceptable excipient conventional in the pharmaceutical arts.
- pharmaceutically acceptable excipients include spheronization aids, solubility enhancers, disintegrating agents, diluents, lubricants, binders, fillers, glidants, etc.
- excipients to be used in formulating compositions are subcategorized into different groups. However, one excipient can affect the properties of a composition in a series of ways, and many excipients used in compositions can thus be described as being multifunctional.
- the tablet cores can comprise at least one diluent.
- any suitable diluent conventional in the pharmaceutical art can be used.
- suitable diluents suitable for use in the present invention include, lactose, microcrystalline cellulose, mannitol, and combinations thereof.
- the lactose can be lactose anhydrous (direct tabletting).
- the microcrystalline cellulose can be, for example, AVICEL ® , such as AVICEL ® PHlOl or AVICEL ® PH 102.
- the tablet cores can comprise at least one binder.
- Any suitable binder conventional in the pharmaceutical art can be used.
- a binder also sometimes called adhesive
- Binders can be added to a drug-filler mixture to increase the mechanical strength of the tablet cores.
- Binders can be added to the formulation in different ways: (1) as a dry powder, which is mixed with other ingredients before wet agglomeration, (2) as a solution, which is used as agglomeration liquid during wet agglomeration, and is referred to as a solution binder, and (3) as a dry powder, which is mixed with the other ingredients before compaction. In this form the binder is referred to as a dry binder.
- Solution binders are a common way of incorporating a binder into granules.
- the binder used in the tablet cores is in the form of a solution binder.
- binders useful for the tablet cores include hydrogenated vegetable oil, castor oil, paraffin, higher aliphatic alcohols, higher alphatic acids, long chain fatty acids, fatty acid esters, wax-like materials such as fatty alcohols, fatty acid esters, fatty acid glycerides, hydrogenated fats, hydrocarbons, normal waxes, stearic acid, stearyl alcohol, hydrophobic and hydrophilic polymers having hydrocarbon backbones, and mixtures thereof.
- water-soluble polymer binders include modified starch, gelatin, polyvinylpyrrolidone, cellulose derivatives (such as for example hydroxypropyl methylcellulose (HPMC) and hydroxypropyl cellulose (HPC)), polyvinyl alcohol and mixtures thereof.
- the binder is polyvinylpyrrolidone (KOLLIDON ® 9OF, KOLLIDON ® K29/32, or combinations thereof).
- the amount of binder present can be present at about 0.1%, about 0.2%, about 0.4%, about 0.6%, about 0.8%, about 1%, about 2%, about 4%, about 6%, about 8%, about 10%, about 12%, about 14%, about 16%, about 18%, or about 20% w/w of the dry tablet core weight. In at least one embodiment, the binder is present at about 3% w/w of the tablet dry weight.
- Certain embodiments of the present invention can comprise at least one lubricant.
- lubricants useful for the tablet cores include glyceryl behenate, stearic acid, hydrogenated vegetable oils (such as hydrogenated cottonseed oil (STEROTEX ® ), hydrogenated soybean oil (STEROTEX ® HM) and hydrogenated soybean oil & castor wax (STEROTEX ® K), stearyl alcohol, leucine, polyethylene glycol (MW 1450, suitably 4000, and higher), magnesium stearate, glyceryl monostearate, stearic acid, polyethylene glycol, ethylene oxide polymers (CARBOWAX ® ), sodium lauryl sulfate, magnesium lauryl sulfate, sodium oleate, sodium stearyl fumarate, DL-leucine, colloidal silica, mixtures thereof and others as known in the art.
- glyceryl behenate such as hydrogenated cottonseed oil (STEROTEX ® ), hydrogenated soybean oil (STEROTEX ® HM) and hydrogenated soybean
- the lubricant can be glyceryl behenate (for example, COMPRITOL ® 888 ATO).
- the amount of lubricant present can be about 0.1%, about 0.2%, about 0.4%, about 0.6%, about 0.8%, about 1%, about 2%, about 4%, about 6%, about 8%, or about 10% w/w of the dry tablet core weight. In at least one embodiment, the lubricant is present at about 3% w/w of the tablet dry weight.
- one or both active agents may be granulated for use in this invention to manufacture the tablet core.
- Well known granulation methods can be used to manufacture the tablet core, including wet mass granulation (such as high shear and top-spray granulation), dry granulation (such as roller compaction and slugging) and hot-melt granulation.
- the active agents can be granulated individually and then combined, in order to be compressed into a tablet core or they can be co-granulated (both actives granulated together into the one granule) for incorporation into a tablet core.
- both pharmaceutical actives may be incorporated directly into the tablet blend.
- one active may need to be granulated (as described above) while the second active is added directly to the tablet blend.
- both actives are granulated, the active granules (either dispensed separately or as a co-granule) are incorporated into the tablet blend.
- the tablet blend is made using conventional tablet blend technologies (e.g. low shear blending using v-blenders or bowl blenders or high-shear blending).
- the actives are combined with a tablet lubricant.
- the tablet blend is compressed to the required shape, weight and hardness using a standard tablet press.
- the bupropion hydrochloride or bupropion hydrochloride is uniformly granulated by spraying the active agents with an aqueous mixture comprising a binder, such as for example polyvinyl alcohol, and optionally a stabilizer, such as for example citric acid in a fluid bed processor or other suitable apparatus known in the art.
- a binder such as for example polyvinyl alcohol
- a stabilizer such as for example citric acid
- the bupropion hydrochloride or bupropion hydrobromide granules thus formed are then dried and screened for granules between about 355 ⁇ m and about 800 ⁇ m. These appropriately seized bupropion hydrochloride or bupropion hydrobromide granules are retained for manufacture of the tablet core.
- the citalopram hydrochloride or escitalopram oxalate is uniformly granulated by spraying the active agents with solvent based mixture comprising a binder, such as for example polyvinylpyrrolidone, and optionally a stabilizer, such as for example BHT in a fluid bed processor or other suitable apparatus known in the art.
- a binder such as for example polyvinylpyrrolidone
- a stabilizer such as for example BHT
- the citalopram hydrochloride or citalopram hydrobromide granules thus formed are then dried and screened for granules between about 355 ⁇ m and about 800 ⁇ m.
- the quetiapine fumarate can be granulated by spraying with an aqueous solution of polyvinyl alcohol in a suitable granulating apparatus and subsequently dried. The resulting granules are screened and granules between about 355 ⁇ m about 800 ⁇ m are retained for use in the tablet core.
- a homogenous tablet core an appropriate amount of each of the sized granulated active agents, equivalent to the dosage strength desired, for the combination is mixed uniformly with a lubricant, such as for example glyceryl behenate, to obtain a homogenous mixture of granules of the two actives and lubricant.
- a lubricant such as for example glyceryl behenate
- the homogenous mixture is then compressed into a homogenous tablet core using a tablet press to a hardness of about 130N using 9mm round normal concave shaped tablet tooling.
- the resulting immediate release homogenous tablet core is ready to be coated with a control-releasing coat.
- the granulation solution is first prepared by combining an aqueous solution of a binder, such as for example, polyvinyl alcohol and optionally a stabilizer, such as for example, citric acid together with a solvent based solution comprising a binder, such as for example, polyvinylpyrrolidone and optionally a stabilizer, such as for example BHT.
- a binder such as for example, polyvinyl alcohol and optionally a stabilizer, such as for example, citric acid
- a solvent based solution comprising a binder, such as for example, polyvinylpyrrolidone and optionally a stabilizer, such as for example BHT.
- the BHT becomes finely dispersed in the PV A/citric acid solution.
- the combination of active agents is charged to the granulation chamber of a suitable apparatus in the required ratio to give the desired final dosage strengths of each active.
- the combination of active agents are then sprayed and simultaneously uniformly mixed for a period of time with the granulation solution to obtain a homogenously mixed co-granulate of the combination of active agents.
- the granules thus obtained are screened through a 1.00mm screen, and the material ⁇ 1.00mm retained for use in the tablet core.
- the dried co-granules obtained by the above described co- granulation method are then uniformly combined with a lubricant (e.g., glyceryl behenate) to obtain a homogenous tablet core which is then compressed to a target tablet hardness of 130N using 9mm round normal concave shaped tablet tooling.
- a lubricant e.g., glyceryl behenate
- control-releasing coated homogenous tablet cores of the present invention can avoid the dose dumping of the combination of active agents in the presence of food and/or alcohol regardless of whether the homogenous tablet core is manufactured by the separate granulation or co -granulation methods described herein.
- the tablet core comprises a controlled-release matrix core.
- a controlled release matrix core is provided from which the kinetics of drug release from the matrix core are dependent at least in part upon the diffusion and/or erosion properties of excipients within the tablet core.
- the controlled release matrix core comprises a therapeutically effective amount of a combination of bupropion hydrochloride or bupropion hydrobromide and citalopram hydrochloride or escitalopram oxalate, bupropion hydrochloride, or bupropion hydrobromide and quetiapine fumarate, optionally a stabilizer, and at least one pharmaceutically acceptable excipient.
- the amount of the bupropion salt present in the controlled release matrix can be about 10%, about 20%, about 30%, about 40%, about 50%, about 60%, about 70%, or about 80% w/w of the dry controlled-release matrix core.
- the amount of the citalopram hydrochloride or escitalopram oxalate present in the controlled release matrix can be about 0.1%, about 0.2%, about 0.4%, about 0.6%, about 0.8%, about 1%, about 2%, about 4%, about 6%, about 8%, about 10%, about 20%, about 30%, about 40%, or about 50% w/w of the dry controlled-release matrix core.
- the amount of quetiapine fumarate present in the controlled release matrix can be about 0.1%, about 0.2%, about 0.4%, about 0.6%, about 0.8%, about 1%, about 2%, about 4%, about 6%, about 8%, about 10%, about 20%, about 30%, about 40%, or about 50% w/w of the dry controlled-release matrix core.
- the controlled release matrix is preferably uniparticulate, and can be uncoated or further coated with at least one control-releasing or non- functional coating.
- Functional coatings can include, by way of example, controlled release polymeric coatings, enteric polymeric coatings, and the like.
- Non-functional coatings are coatings that do not affect drug release but which affect other properties (e.g., they may enhance the chemical, biological, or the physical appearance of the controlled release formulation).
- Those skilled in the pharmaceutical art and the design of medicaments are well aware of controlled release matrices conventionally used in oral pharmaceutical compositions adopted for controlled release and means for their preparation. Examples of controlled release matrices are described in U.S. Pat. Nos.
- Suitable excipient materials for use in such controlled release matrices include, by way of example, release-resistant or controlled release materials such as hydrophobic polymers, hydrophilic polymers, lipophilic materials and mixtures thereof.
- hydrophobic, or lipophilic components include glyceryl monostearate, mixtures of glyceryl monostearate and glyceryl monopalmitate (Myvaplex, Eastman Fine Chemical Company), glycerylmonooleate, a mixture of mono, di and tri-glycerides (ATMUL 84S), glycerylmonolaurate, paraffin, white wax, long chain carboxylic acids, long chain carboxylic acid esters, long chain carboxylic acid alcohols, and mixtures thereof.
- the long chain carboxylic acids can contain from 6 to 30 carbon atoms; in certain embodiments at least 12 carbon atoms, and in other embodiments from 12 to 22 carbon atoms. In some embodiments this carbon chain is fully saturated and unbranched, while others contain one or more double bonds. In at least one embodiment the long chain carboxylic acids contain 3 -carbon rings or hydroxyl groups.
- Non- limiting examples of saturated straight chain acids include n-dodecanoic acid, n-tetradecanoic acid, n-hexadecanoic acid, caproic acid, caprylic acid, capric acid, lauric acid, myristic acid, palmitic acid, stearic acid, arachidic acid, behenic acid, montanic acid and melissic acid.
- unsaturated monoolefinic straight chain monocarboxylic acids Non-limiting examples of these include oleic acid, gadoleic acid and erucic acid.
- unsaturated (polyolefinic) straight chain monocaboxyic acids include n-dodecanoic acid, n-tetradecanoic acid, n-hexadecanoic acid, caproic acid, caprylic acid, capric acid, lauric acid, myristic acid, palmitic acid, stearic acid, arachidic acid, behenic acid, montanic acid and melis
- Non-limiting examples of these include linoleic acid, linolenic acid, arachidonic acid and behenolic acid.
- Useful branched acids include, for example, diacetyl tartaric acid.
- Non-limiting examples of long chain carboxylic acid esters include glyceryl monostearates; glyceryl monopalmitates; mixtures of glyceryl monostearate and glyceryl monopalmitate (Myvaplex 600, Eastman Fine Chemical Company); glyceryl monolinoleate; glyceryl monooleate; mixtures of glyceryl monopalmitate, glyceryl monostearate glyceryl monooleate and glyceryl monolinoleate (Myverol 18-92, Eastman Fine Chemical Company); glyceryl monolinolenate; glyceryl monogadoleate; mixtures of glyceryl monopalmitate, glyceryl monostearate,
- waxes can be useful alone or in combination with the materials listed above, as excipient materials for the controlled release matrix embodiments of the present invention.
- Non-limiting examples of these include white wax, paraffin, microcrystalline wax, carnauba wax, and mixtures thereof.
- the lipophilic agent can be present in an amount of from 5% to 90% by weight of the controlled release matrix dosage form.
- the lipophilic agent is present in an amount of from 10% to 85%, and in other embodiments from 30% to 60% by weight of the controlled release matrix dosage form.
- hydrophilic polymers that can be used in certain embodiments of the controlled release matrix dosage form include hydro xypropylmethylcel lulose (HPMC), hydroxypropylcellulose (HPC), hydroxyethylceflulose (HEC), carboxymethylcellulose (CMC) or other cellulose ethers, polyoxyethylene, alginic acid, acrylic acid derivatives such as polyacmylic acid, Carbopol (B. F. Goodrich, Cleveland, Ohio), polymethacrylate polymer such as EUDRAGIT ® RL, RS, R.
- HPMC hydro xypropylmethylcel lulose
- HPC hydroxypropylcellulose
- HEC hydroxyethylceflulose
- CMC carboxymethylcellulose
- polyoxyethylene alginic acid
- acrylic acid derivatives such as polyacmylic acid
- Carbopol B. F. Goodrich, Cleveland, Ohio
- polymethacrylate polymer such as EUDRAGIT ® RL, RS, R.
- control-release matrix core is uncoated and comprises hydroxypropyl cellulose as the hydrophilic polymer.
- the hydrophilic polymer can be present in an amount of from 10% to 90% by weight of the controlled release matrix tablet core.
- the hydrophilic polymer can be present in an amount of from 20% to 75%, and in other embodiments from 30% to 60% by weight of the controlled release matrix tablet core.
- the controlled release matrix tablet core can comprise hydroxypropylmethylcellulose (HPMC).
- HPMC hydroxypropylmethylcellulose
- hydroxypropyl methylcelluloses that are commercially available include METHOCEL ® E (USP type 2910), METHOCEL ® F (USP type 2906), METHOCEL ® J (USP type 1828), METHOCEL ® K (USP type 2201), and METHOCEL ® 310 Series, products of The Dow Chemical Company, Midland, Mich., USA.
- the average degree of methoxyl substitution in these products can range from 1.3 to 1.9 (of the three positions on each unit of the cellulose polymer that are available for substitution) while the average degree of hydroxypropyl substitution per unit expressed in molar terms can range from 0.13 to 0.82.
- the controlled release matrix tablet core can comprise different HPMC grades having different viscosities.
- the size of a HPMC polymer is expressed not as molecular weight but instead in terms of its viscosity as a 2% solution by weight in water. Different HPMC grades can be combined to achieve the desired viscosity characteristics.
- the at least one pharmaceutically acceptable polymer can comprise two HPMC polymers such as for example METHOCEL ® K3 LV (which has a viscosity of 3 cps) and METHOCEL ® K100M CR (which has a viscosity of 100,000 cps).
- the polymer can comprise two hydroxypropylcellulose forms such as KLUCEL ® LF and KLUCEL ® EF.
- the at least one polymer can comprise a mixture of a KLUCEL ® and a METHOCEL ® .
- the controlled release matrix tablet core can comprise a polyethylene oxide (PEO).
- PEO is a linear polymer of unsubstituted ethylene oxide.
- poly(ethylene oxide) polymers having viscosity -average molecular weights of 100,000 daltons and higher can be used.
- Non-limiting examples of poly(ethylene oxide)s that are commercially available include: POLYOX ® NF, grade WSR Coagulant, molecular weight 5 million; POLYOX ® grade WSR 301, molecular weight 4 million; POLYOX ® grade WSR 303, molecular weight 7 million; POLYOX ® grade WSR N-60 K, molecular weight 2 million; and mixtures thereof.
- polyethylene oxides are products of Dow Chemical Company, Midland, Mich., USA.
- polyethylene oxides exist and can likewise be used.
- the required molecular weight for the PEO can be obtained by mixing PEO of differing molecular weights that are available commercially.
- HPMC can be combined within the same controlled release matrix.
- the polyethylene oxides can have molecular weights ranging from 2,000,000 to 10,000,000 Da.
- the polyethylene oxides can have molecular weights ranging from 4,000,000 to 7,000,000 Da.
- the HPMC polymers have a viscosity within the range of 4,000 centipoise to 200,000 centipoise.
- the HPMC polymers can have a viscosity of from 50,000 centipoise to 200,000 centipoise, and in other embodiments from 80,000 centipoise to 120,000 centipoise.
- the relative amounts of PEO and HPMC within the controlled release matrix tablet core can vary within the scope of the invention.
- the PEO:HPMC weight ratio can be from about 1:3 to about 3:1.
- the PEO:HPMC weight ratio is from about 1:2 to about 2: 1.
- the total amount of polymer relative to the entire controlled release matrix tablet core this can vary as well and can depend on the desired drug loading.
- the total amount of polymer in the controlled release matrix tablet core can constitute from 15% to 90% by weight of the controlled release matrix tablet core.
- the total amount of polymer in the controlled release matrix tablet core can be from 20% to 75%, in other embodiments from 30% to 60%, and in still other embodiments from 10% to 20% by weight of the controlled release matrix tablet core.
- the controlled release matrix tablet core can comprise a hydrophobic polymer such as ethylcellulose.
- the viscosity of ethylcellulose can be selected in order to influence of rate the drug release.
- the ethylcellulose has a viscosity from 7 to 100 cP (when measured as a 5% solution at 25. degree. C. in an Ubbelohde viscometer, using a 80:20 toluene :ethanol solvent.)
- the hydrophobic polymer can constitute from 10% to 90% by weight of the controlled release matrix core.
- the hydrophobic polymer can constitutes from 20% to 75%, and in other embodiments from 30% to 60% by weight of the controlled release matrix dosage tablet core.
- the controlled release matrix tablet core can comprise at least one lubricant.
- lubricants include stearic acid, hydrogenated vegetable oils (such as hydrogenated cottonseed oil (STEROTEX ® ), hydrogenated soybean oil (STEROTEX ® HM) and hydrogenated soybean oil & castor wax (STEROTEX ® K)) stearyl alcohol, leucine, polyethylene glycol (MW 1450, suitably 4000, and higher), magnesium stearate, glyceryl monostearate, stearic acid, glycerylbehenate, polyethylene glycol, ethylene oxide polymers (for example, available under the registered trademark CARBOWAX ® from Union Carbide, Inc., Danbury, Conn.), sodium lauryl sulfate, magnesium lauryl sulfate, sodium oleate, sodium stearyl fumarate, DL-leucine, colloidal silica, and mixtures thereof.
- the controlled release matrix tablet core comprises a plasticizer.
- plasticizers include dibutyl sebacate, diethyl phthalate, triethyl citrate, tributyl citrate, triacetin, citric acid esters such as triethyl citrate NF XVI, tributyl citrate, dibutyl phthalate, 1,2-propylene glycol, polyethylene glycols, propylene glycol, diethyl phthalate, castor oil, acetylated monoglycerides, phthalate esters, and mixtures thereof.
- the plasticizer can be present in an amount of from 1% to 70% by weight of the controlled release polymer in the controlled release matrix tablet core.
- the plasticizer can be present in an amount of from 5% to 50%, and in other embodiments from 10% to 40% by weight of the controlled release polymer in the controlled release matrix tablet core.
- the controlled release matrix tablet core can comprise at least one diluent, non-limiting examples of which include di calcium phosphate, calcium sulfate, lactose or sucrose or other disaccharides, cellulose, cellulose derivatives, kaolin, mannitol, dry starch, glucose or other monosaccharides, dextrin or other polysaccharides, sorbitol, inositol, sucralfate, calcium hydroxyl-apatite, calcium phosphates and fatty acid salts such as magnesium stearate.
- the diluent can be added in an amount so that the combination of the diluent and the combination of active agent comprises up to 60%, and in other embodiments up to 50%, by weight of the composition.
- the controlled release matrix tablet core can comprise a solubilizer.
- the solubilizer can act to increase the instantaneous solubility of the bupropion salt.
- the solubilizer can be selected from hydrophilic surfactants or lipophilic surfactants or mixtures thereof.
- the surfactants can be anionic, nonionic, cationic, and zwitterionic surfactants.
- the hydrophilic non-ionic surfactants can be selected from the group comprised of, but not limited to: polyethylene glycol sorbitan fatty acid esters and hydrophilic trans esterification products of a polyol with at least one member of the group from triglycerides, vegetable oils, and hydrogenated vegetable oils such as glycerol, ethylene glycol, polyethylene glycol, sorbitol, propylene glycol, pentaerythritol, or a saccharide, d- ⁇ -iocopheryl polyethylene glycol 1000 succinate.
- polyethylene glycol sorbitan fatty acid esters and hydrophilic trans esterification products of a polyol with at least one member of the group from triglycerides, vegetable oils, and hydrogenated vegetable oils such as glycerol, ethylene glycol, polyethylene glycol, sorbitol, propylene glycol, pentaerythritol, or a saccharide, d- ⁇ -iocopheryl
- the ionic surfactants can be selected from the group comprised of, but not limited to: alkylammonium salts; fusidic acid salts; fatty acid derivatives of amino acids, oligopeptides, and polypeptides; glyceride derivatives of amino acids, oligopeptides, and polypeptides; lecithins and hydrogenated lecithins; lysolecithins and hydrogenated lysolecithins; phospholipids and derivatives thereof; lysophospholipids and derivatives thereof; carnitine fatty acid ester salts; salts of alkyisulfates ; fatty acid salts; sodium docusate; acyl lactylates; mono- and di-acetylated tartaric acid esters of mono- and di- glycericles; succinylated mono-and di- glycerides; citric acid esters of mono-and di- glycerides; and mixtures thereof.
- the lipophilic surfactants can be selected from the group comprised of, but not limited to: fatty alcohols; glycerol fatty acid esters; acetylated glycerol fatty acid esters; lower alcohol fatty acids esters; propylene glycol fatty acid esters; sorbitan fatty acid esters; polyethylene glycol sorbitan fatty acid esters; sterols and sterol derivatives: polyoxyethylated sterols and sterol derivatives; polyethylene glycol alkyl ethers; sugar esters; sugar ethers; lactic acid derivatives of mono-and di-glycerides; hydrophobic transesterification products of a polyol with at least one member of the group from glycerides, vegetable oils, hydrogenated vegetable oils, fatty acids and sterols; oil- soluble vitamins/vitamin derivatives; PEG sorbitan fatty acid esters, PEG glycerol fatty acid esters, polyglycerized fatty acid,
- the solubilizer can be selected from: PEG-20-glyceryl stearate, PEG-40 hydrogenated castor oil, PEG 6 corn oil, lauryl macrogol-32 glyceride, stearoyl macrogol glyceride, poly glyceryl- 10 mono dioleate, propylene glycol olcate, Propylene glycol dioctanoate, Propylene glycol caprylate/caprate, Glyceryl monooleate, Glycerol monolinoleate, Glycerol monostearate, PEG-20 sorbitan monolaurate, PEG-4 lauryl ether, Sucrose distearate, Sucrose monopalmitate, polyoxyethylene- polyoxypropylene block copolymer, polyethylene glycol 660 hydroxystearate, Sodium lauryl sulfate, Sodium dodecyl sulphate, Dioctyl suphosuccinate, L-hydroxypropyl cellulose,
- the solubilizer can be selected from PEG-40 hydrogenated castor oil, lauryl macrogol-32 glyceride, stearoyl macrogol glyceride, PEG-20 sorbitan monolaurate, PEG-4 lauryl ether, polyoxyethylene- polyoxypropylene block copolymer, Sodium lauryl sulphate, Sodium dodecyl sulphate, polyethylene glycol, and mixtures thereof.
- the controlled release matrix tablet core comprises a swelling enhancer.
- Swelling enhancers are members of a special category of excipients that swell rapidly to a large extent resulting in an increase in the size of the tablet. At lower concentrations, these excipients can be used as superdisintegrants; however at concentrations above 5% w/w these agents can function as swelling enhancers and help increase the size of the controlled release matrix tablet core.
- examples of swelling enhancers include but are not limited to: low-substituted hydroxypropyl cellulose, microcrystalline cellulose, cross- linked sodium or calcium carboxymethyl cellulose, cellulose fiber, cross-linked polyvinyl pyrrolidone, cross-linked polyacrylic acid, cross-linked amberlite resin, alginates, colloidal magnesium-aluminum silicate, corn starch granules, rice starch granules, potato starch granules, pregelatinised starch, sodium carboxymethyl starch and mixtures thereof.
- the swelling enhancer is cross-linked polyvinylpyrrolidone.
- the amount of the swelling enhancer can be from 5% to 90% by weight of the controlled release matrix tablet core.
- the swelling enhancer is present in an amount of from 10% to 70%, and in other embodiments from 15% to 50% by weight of the controlled release matrix tablet core.
- the controlled release matrix tablet core comprises additives for allowing water to penetrate into the core of the preparation (hereinafter referred to as "hydrophilic base").
- the amount of water required to dissolve 1 g of the hydrophilic base is not more than 5 ml, and in other embodiments is not more than 4 ml at the temperature of 20 0 C ⁇ 5°C.
- the hydrophilic base includes, inter alia, hydrophilic polymers such as polyethylene glycol (PEG); (e.g.
- PEG400 PEG 1500, PEG4000, PEG6000 and PEG20000, produced by Nippon Oils and Fats Co.
- PVP polyvinylpyrrolidone
- sugar alcohols such as D- sorbitol, xylitol, or the like
- sugars such as sucrose, anhydrous maltose, D-fructose, dextran (e.g. dextran 40), glucose or the like
- surfactants such as polyoxyethylene-hydrogenated castor oil (HCO; e.g.
- Cremophor RH40 produced by BASF, HCO-40 and HCO-60 produced by Nikko Chemicals Co.
- polyoxyethylene-polyoxypropylene glycol e.g. Pluronic F68 produced by Asahi Denka Kogyo K.K.
- polyoxyethylene-sorbitan high molecular fatty acid ester Teween; e.g. Tween 80 produced by Kanto Kagaku K.K.
- salts such as sodium chloride, magnesium chloride, or the like
- organic acids such as citric acid, tartaric acid, or the like
- amino acids such as glycine, ⁇ -alanine, lysine hydrochloride, or the like
- amino sugars such as meglumine.
- the hydrophilic base is PEG6000, PVP, D-sorbitol, or mixtures thereof.
- the controlled release matrix tablet core of the present invention can further contain one or more pharmaceutically acceptable excipients such as, granulating aids or agents, colorants, flavorants, pH adjusters, anti-adherents, glidants and like excipients conventionally used in pharmaceutical compositions.
- pharmaceutically acceptable excipients such as, granulating aids or agents, colorants, flavorants, pH adjusters, anti-adherents, glidants and like excipients conventionally used in pharmaceutical compositions.
- a controlled release matrix tablet core comprising the combination of actives incorporated within the homogeneous controlled release matrix tablet core, which include effective amounts of at least two polymers having opposing wettability characteristics, wherein at least one polymer is selected which demonstrates a stronger tendency towards hydrophobicity and the other polymer(s) is selected such that it demonstrates a stronger tendency towards hydrophilicity.
- the polymer demonstrating a stronger tendency towards hydrophobicity can be ethylcellulose (EC) whereas the polymer demonstrating a stronger tendency towards hydrophilicity can be hydroxyethylcellulose (HEC) and/or hydroxypropyl methylcellulose (HPMC).
- the pharmaceutical composition of the present invention can be provided as a controlled release matrix tablet core, which can be optionally encased in the controlled-release coating described herein.
- a controlled release matrix tablet core which can be optionally encased in the controlled-release coating described herein.
- coated controlled-release matrix core tablets avoid the dose dumping of the combination of active agents in the presence of food and/or alcohol.
- Certain embodiments of the present invention provide for a method for preparing the controlled release of the combination of actives, the method comprising blending the combination of actives with 5% to 25% by weight of hydrophillic polymer, and 1% to 25% by weight of hydrophobic polymer, adding suitable pharmaceutical excipients, surface active agents and lubricants, granulating the mixture with solvents such as isopropyl alcohol, drying the granular mixture, milling the dried mixture, adding from 5% to 70% by weight of ethylcellulose, adding a lubricant and optionally a glidant and compressing the granules into matrices.
- the controlled- release matrices can optionally be encased in a gastrointestinal resistant coat or a pharmaceutically acceptable film coat.
- a swellable controlled release matrix tablet core in which the combination of actives is dispersed in a polymeric matrix that is water-swellable rather than merely hydrophilic, that has an erosion rate that is substantially slower than its swelling rate, and that releases the combination of actives primarily by diffusion.
- the rate of diffusion of the combination of actives out of the swellable matrix can be slowed by increasing the drug particle size, by the choice of polymer used in the matrix, and/or by the choice of molecular weight of the polymer.
- the swellable matrix can comprise a relatively high molecular weight polymer that swells upon ingestion.
- the swellable matrix swells upon ingestion to a size that is at least twice its unswelled volume, and that promotes gastric retention during the fed mode.
- the swellable matrix can also convert over a prolonged period of time from a glassy polymer to a polymer that is rubbery in consistency, or from a crystalline polymer to a rubbery one.
- the penetrating fluid then causes release of the combination of actives in a gradual and prolonged manner by the process of solution diffusion, i.e., dissolution of the combination of actives in the penetrating fluid and diffusion of the dissolved combination of actives back out of the swellable matrix.
- the swellable matrix itself is solid prior to administration and, once administered, remains undissolved in (i.e., is not eroded by) the environment of use for a period of time sufficient to permit the majority of the combination of actives to be released by the solution diffusion process during the fed mode.
- the rate-limiting factor in the release of the combination of actives from the swellable matrix is therefore controlled diffusion of the combination of actives from the swellable matrix rather than erosion, dissolving or chemical decomposition of the swellable matrix.
- the combination of actives in the swellable matrix can be present in a therapeutically effective amount of from 0.1% to 99% by weight of the dried controlled release matrix tablet core in a ratio according to the desired dosage strengths.
- the combination of actives is present in the swellable matrix in an amount of from 5% to 90%, in still other embodiments from 10% to 80%, and in even still other embodiments from 25% to 80% by weight of the swellable matrix in a ratio according to the desired dosage strengths.
- the water-swellable polymer forming the swellable matrix tablet core in accordance with the embodiments of the present invention can be any polymer that is non-toxic, that swells in a dimensionally unrestricted manner upon imbibition of water, and that provides for a synchronous release of the combination of actives.
- Non-limiting examples of polymers suitable for use in the swellable matrix include cellulose polymers and their derivatives (such as for example, hydroxyethylcellulose, hydroxypropylcellulose, carboxymethylcellulose, and microcrystalline cellulose, polysaccharides and their derivatives, polyalkylene oxides, polyethylene glycols, chitosan, poly(vinyl alcohol), xanthan gum, maleic anhydride copolymers, poly(vinyl pyrrolidone), starch and starch-based polymers, poly (2-ethyl-2-oxazoline), poly(ethyleneimine), polyurethane hydrogels, and crosslinked polyacrylic acids and their derivatives, and mixtures thereof.
- cellulose polymers and their derivatives such as for example, hydroxyethylcellulose, hydroxypropylcellulose, carboxymethylcellulose, and microcrystalline cellulose, polysaccharides and their derivatives, polyalkylene oxides, polyethylene glycols, chitosan, poly(vinyl alcohol),
- copolymers of the polymers listed in the preceding sentence include block copolymers and grafted polymers.
- specific examples of copolymers include PLURONIC ® and TECTONIC ® which are polyethylene oxide-polypropylene oxide block copolymers available from BASF Corporation, Chemicals Div., Wyandotte, Mich., USA.
- controlled release matrices of the present invention can be manufactured by methods known in the art such as those described in the patents listed above (e.g. U.S. Pat. No. 5,965,161).
- Other examples of methods of manufacturing controlled release matrices include wet granulation, dry granulation (e.g. slugging, roller compaction), direct compression, melt granulation, and rotary granulation.
- controlled release matrix tablet cores can optionally be coated with the control-releasing coat or a non- functional aesthetic coat using well-known coating methods.
- the tablet core can comprise a bi-layer tablet core, wherein the first layer comprises an immediate release composition comprising one drug of the desired combination, optionally a stabilizer and at least one pharmaceutically acceptable excipient and the second layer comprises an immediate release composition comprising the second drug of the desired combination, optionally a stabilizer, and at least one pharmaceutically acceptable excipient.
- the two layers are in direct contact.
- This bi- layer tablet core can subsequently be coated with a control-releasing coat such as the one described herein.
- the layers can be manufactured according to the separately granulated method described herein and subsequently compressed into a bi-layer tablet core using methods and equipment well known in the art (e.g., using a bi-layer press).
- each layer can be directly compressed using methods well known in the art and subsequently compressed to form the bi-layer tablet core using methods and equipment well known in the art.
- the immediate release bi-layer tablet core is subsequently coated with a control-releasing coat.
- the pharmaceutical compositions comprising a bi-layer tablet core avoid dose dumping of the combination of active agents in the presence of food and/or alcohol regardless of whether the homogenous tablet core is manufactured by the separate granulation or co -granulation methods described herein.
- control-releasing coat is a semipermeable coat, which comprises a water-insoluble water-permeable film forming polymer, a water-soluble polymer and at least one plasticizer.
- the coat is permeable to both the passage of the actives and water and is free of any preformed pores.
- the water-insoluble water-permeable film-forming polymer can include, a cellulose ether, such as for example, ethylcellulose; a cellulose ester, such as for example, cellulose acetate; methacrylic acid derivatives, such as for example EUDRAGIT ® NE30D or NE40D; aqueous ethylcellulose dispersions, such as for example, Surelease ® ; aqueous acrylic enteric systems, such as for example, Acryl-EZE ® , Kollicoat ® MAE30DP, and Kollicoat ® MAElOOP; and polyvinyl derivatives, such as for example, Kollidon ® SR, Kollicoat ® SR30D, and Kollicoat ® EMM30D.
- a cellulose ether such as for example, ethylcellulose
- a cellulose ester such as for example, cellulose acetate
- methacrylic acid derivatives such as for example EUDRAGIT ® NE30D
- ethylcellulose is used as the water- insoluble, water-permeable film-forming polymer.
- Ethylcelluloses of a variety of viscosities can be utilized.
- Non-limiting examples of the ethylcellulose that can be used include, for example, ETHOCELTM Standard Premium 4, 7, 10, 20, 45 and 100 or ETHOCELTM Standard FP Premium 7, 10, and 100. Any combination of these ethylcelluloses can be used.
- ETHOCELTM Standard FP Premium 100 is the water-insoluble water-permeable film- forming polymer.
- the amount of the water-insoluble, water-permeable film- forming polymer can be present at about 10%, about 20%, about 30%, about 40%, about 50%, about 60%, about 70%, about 80%, or about 90% w/w of the dry coat weight.
- the amount of water-insoluble water-permeable film- forming polymer, such as for example ETHOCELTM Standard FP Premium 100 used can be about 50%, about 50.5%, about 51%, about 51.5%, or about 52% w/w of the dry coat weight.
- the amount of ETHOCELTM Standard FP Premium 100 used is about 51.2% w/w of the dry coat weight.
- the water-soluble polymer can be a partially or substantially water-soluble hydrophilic substance intended to modulate film permeability to both the medium and the actives in the environment of use.
- Non-limiting examples of the water- soluble polymers can be water-soluble cellulose ethers, vinylic polymers and combinations thereof.
- Non-limiting examples of the water-soluble cellulose ethers that can be used in the manufacture of the control-releasing coat can include, for example, cellulose ethers such as methylcellulose, hydroxypropylmethylcellulose, non-ionic water-soluble cellulose ethers, and any combinations thereof.
- Non-limiting examples of the vinylic polymers that can be used in the manufacture of the control-releasing coat include, for example, polyvinyl alcohol, polyvinylpyrrolidone, and any combinations thereof.
- the amount of the water-soluble polymer present can be about 1%, about 2%, about 4%, about 6%, about 8%, about 10%, about 20%, about 30%, about 40%, about 50% or about 60% w/w of the dry coat weight.
- the preferred water-soluble polymer is polyvinylpyrrolidone, such as for example, KOLLIDON ® as supplied by BASF and is present at about 32% w/w of the dry coat weight. Similar polyvinylpyrrolidones are also available from other suppliers.
- Plasticizers are generally added to film coatings to modify the physical properties of a polymer or polymer combinations used during manufacture of a particular coating system.
- the amount and choice of the plasticizer contributes to the hardness of the tablet and can even affect its dissolution or disintegration characteristics, as well as the chemical and physical stability of the coated tablet.
- Certain plasticizers can increase the elasticity and/or pliability of a coat, thereby decreasing the coat's brittleness.
- certain plasticizers can function to increase the hydrophilicity of the coat in the environment of use. Therefore, plasticizers can function to enhance processing of coating formulations during manufacture as well as affect release characteristics of a coating system.
- Non-limiting examples of plasticizers that can be used in the control-releasing coat described herein include acetylated monoglycerides; acetyltributyl citrate, butyl phthalyl butyl glycolate; dibutyl tartrate; diethyl phthalate; dimethyl phthalate; ethyl phthalyl ethyl glycolate; glycerin; propylene glycol; triacetin; tripropioin; diacetin; dibutyl phthalate; acetyl monoglyceride; acetyltriethyl citrate, polyethylene glycols; castor oil; rape seed oil, olive oil, sesame oil, triethyl citrate; polyhydric alcohols, glycerol, glycerin sorbitol, acetate esters, gylcerol triacetate, acetyl triethyl citrate, dibenzyl phthalate, dihexyl
- polyethylene glycol of various molecular weights, and mixtures thereof. It is contemplated and within the scope of the invention, that a combination of plasticizers can be used in the present composition.
- the plastizer is a combination of polyethylene glycol 3350 and dibutyl sebacate. In certain other embodiments, the plasticizer is dibutyl sebacate.
- the amount of plasticizer can be present at about 0.1%, about 0.2%, about 0.4%, about 0.6%, about 0.8%, about 1%, about 2%, about 4%, about 6%, about 8%, about 10%, about 15%, about 20%, about 25%, about 30%, about 35%, or about 40% w/w of the dry coat weight.
- the amount of plasticizer used can be present at about 3%, about 5%, about 7%, about 9%, about 11%, about 12%, or about 15% of the dry coat weight in a ratio of about 2:1 (polyethylene glycol 3350 : dibutyl sebacate). In embodiments where the plasticizer is dibutyl sebabcate, the amount of dibutyl sebacate is about 5% w/w of the dry coat weight.
- control-releasing coating will affect the release profile of the actives from the immediate release tablet cores described herein. This is true even if a controlled-releasing coat is applied onto the controlled-release matrix core described herein. Not only can the relative proportions of the preferred polymer coat ingredients, notably the ratio of the water-insoluble water-permeable film- forming polymer : plasticizer : water-soluble polymer, be varied to alter the permeability of the control-releasing coat, but so can the type and viscosity of the polymers used as well as the thickness of coating applied.
- the ratio of water-insoluble water-permeable film- forming polymer: water-soluble polymer and/or the amount of coating applied would be increased.
- the polymers being used are of a lower viscosity, the amount of coating to be applied can be increased to obtain the desired release profile when compared to a control-release coating formulation with a higher viscosity polymer. Addition of other excipients to the tablet core can also alter the permeability of the control- releasing coat.
- the amount of plasticizer in the control-releasing coat should be increased to make the coat more pliable as the pressure exerted on a less pliable control-releasing coat by the expanding agent would rupture the coat.
- Other excipients such as taste masking agents and pigments can also be added to the control-releasing coat.
- the weight gained after coating the tablet cores with the control-releasing coat can be about 4%, about 6%, about 8%, about 10%, about 12%, about 14%, about 16%, about 18%, about 20%, about 22%, about 24%, or about 25% w/w of the dry tablet core. In at least one embodiment of the present invention, the weight gained is about 5% of the dry coat weight.
- Preparation and application of the control-releasing coat are well known in the art. Nevertheless, an exemplary process for preparing the coating solution can be as follows.
- the water-insoluble water-permeable film-forming polymer e.g. ethylcellulose
- the plasticizer or plasticizers e.g. PEG 3350 or PEG 3350 and dibutyl sebacate
- an organic solvent e.g. ethyl alcohol
- a mixture of organic solvents and water e.g. ethyl alcohol, propanol, and water.
- the water-soluble polymer e.g. polyvinylpyrrolidone
- the resulting solution can be, if necessary, homogenized by passing it through a high-pressure homogenizer.
- the coating solution is then spray coated onto the tablet cores using a tablet coater, fluidized bed apparatus, or any other suitable coating apparatus known in the art until the desired weight gain is achieved.
- the tablet cores coated with the control-releasing mixture are subsequently dried.
- the controlled-release pharmaceutical formulations prepared by the process described above surprisingly exhibit a synchronous release of the combination of actives at least in-vitro.
- the synchronous release is observed in 900 ml of 0.1N HCl, pH4.5 acetate buffer, or pH 6.8 phosphate buffer using a USP Apparatus 1 at 75 rpm at 37 0 C.
- the control-release pharmaceutical compositions of the invention surprisingly also provide an about 15% to about 26% enhanced absorption of bupropion hydrobromide in the plasma when compared to co-administration of single active agent pharmaceutical compositions of bupropion hydrobromide and citalopram hydrochloride or bupropion hydrobromide and escitalopram oxalate.
- the pharmaceutical compositions of the invention can avoid the dose-dumping phenomenon when the compositions are administered with food and/or alcohol.
- Glatt GPCG 1 (6 inch chamber). The theoretical batch size was 3270.6g.
- the powder bed temperature was maintained between 38-45 0 C, and the liquid spray rate maintained between 5-7 g/min throughout the granulation process.
- the granules were fluid bed dried to an LOD (loss on drying) level of ⁇ 1%.
- the granules were screened and the granules with a particle size of between about 355 ⁇ m and about 800 ⁇ m were retained for manufacture of the homogenous tablet core.
- Glatt GPCG 1 (6 inch chamber). The theoretical batch size was 3216g.
- the powder bed temperature was maintained between 20-35 0 C, and the liquid spray rate maintained between 13 - 17 g/min throughout the granulation process.
- the homogenous tablet core comprising about 348mg of bupropion HBr (equivalent to about 260mg of bupropion base) and about 22.2mg of citalopram HCl (equivalent to about 20mg citalopram base)
- a blend with the following composition was prepared: about 91.3% bupropion HBr granules (manufactured as described above), about 5.8% citalopram HCl granules (manufactured as described above) and about 2.9% Compritol 888 ATO (screened through a 500 ⁇ m screen).
- a homogenous blend of about 150Og was manufactured by dispensing about 1369.5g of bupropion HBr granules, about 87.Og of citalopram HCl granules, and about 43.5g of the screened Compritol 888 ATO.
- the material was added to a v-blender (15 litre shell of a Pharmatech AB-050 v-blender) in the following order:
- the tablet core components were homogenous Iy blended for about 10 minutes, with the v-shell speed set to 25 rpm and the intensifier bar turned off.
- the homogenous blend was discharged from the v-shell and charged to a tablet press (Riva Picolla 10 station rotary tablet press) and compressed to a target tablet weight of about 416mg and a target tablet hardness of about 130N using 9mm round normal concave shaped tablet tooling.
- the resulting product comprises the homogenous tablet core, which at this point is an immediate release core having the following composition:
- the homogenous IR tablet cores were coated with an ethylcellulose based film by preparing an organic solvent solution consisting of about 4.61% ethocel standard IOOFP premium, about 2.86% Kollidon ® 9OF, about 1.03% carbowax sentry polyethylene glycol 3350 granular NF FCC grade, about 0.5% dibutyl sebacate NF, about 8.68% 2-propanol, about 81.86% absolute ethanol, and about 0.46% purified water.
- the plasticized polymer solution was applied to about 1 kg of the final tablet cores using an O'Hara Labcoat I tablet coating machine (12" pan) until about a 10% weight gain was obtained.
- the product temperature was maintained between about 38-44 0 C, and the liquid spray rate was maintained between about 7 - 9 g/min throughout the coating process.
- the controlled release coated tablets were then cured for about 10 minutes (inlet air is set at about 35 0 C, pan speed set at 5 rpm).
- Dissolution Conditions 900 ml 0.1N HCl, USP Apparatus 1,75 rpm, 37 0 C
- Glatt GPCG 1 (6 inch chamber). The theoretical batch size was 2725g.
- the powder bed temperature was maintained between 15-3O 0 C, and the liquid spray rate maintained between 15 - 19 g/min throughout the granulation process.
- the granules When spraying of the granulation solution was stopped, the granules were fluid bed dried to an LOD (loss on drying) level of ⁇ 1%. The granules were screened and the granules with a particle size of between about 355 ⁇ m and about 800 ⁇ m were retained for use to manufacture the homogenous tablet core.
- a homogenous tablet blend with the following composition was prepared; 90.46% bupropion HBr granules (manufactured as outlined in Example 1), 6.63% escitalopram Oxalate granules (manufactured as outlined above) and 2.91% Compritol 888 ATO (screened through a 500 ⁇ m screen).
- a homogenous tablet blend of 150Og was manufactured by dispensing 1356.9Og of bupropion HBr granules, 99.45g of escitalopram Oxalate granules, and 43.65g of the screened Compritol 888 ATO.
- the material was added to a v-blender (15 litre shell of a Pharmatech AB-050 v-blender) in the following order:
- the tablet components were blended for 10 minutes, with the v-shell speed set to 25 rpm and the intensifier bar turned off.
- the homogenous tablet blend was discharged from the v-shell and charged to a tablet press (Riva Bilayer 11 station rotary tablet press) and compressed to a target tablet weight of 419.4mg and a target tablet hardness of IOON using 9mm round normal concave shaped tablet tooling.
- the resulting product comprises the homogenous tablet core, which at this point is an immediate release core having the following composition:
- the dissolution profile of the above pharmaceutical composition was determined under the dissolution conditions described below in Tables 2A, 2B, and 2C.
- the results of the dissolution testing are presented as a % of the total bupropion HBr and escitalopram Oxalate in the controlled release tablet (batch no. 0705037) and are also depicted in FIGs. 2A, 2B, and 2C.
- PV A/citric acid solution and mixed for a minimum of 20 minutes.
- the BHT became finely dispersed in the PVA / citric acid solution.
- the final composition of the granulation suspension was 4.82% PVA, 7.50% citric acid, 1.67% 2- propanol, 0.14% BHT, 85.87% purified water.
- the buproion HBR and escitalopram Oxalate were charged to the granulation chamber in the required ratio to give the desired final dosage strengths of each active.
- the actives were dispensed such that the material in the granulation chamber consisted of 93.2% bupropion HBr and 6.8% escitalopram Ox.
- 3kg of material was granulated, 2796g of bupropion HBr mixed with 204g of escitalopram Ox.
- the granules When spraying of the granulation solution was stopped, the granules were fluid bed dried to an LOD (loss on drying) level of ⁇ 1%. The granules were screened through a 1.00mm screen, and the material ⁇ 1.00mm retained for manufacture of the homogenous tablet core.
- a homogenous tablet blend with the following composition was prepared; 97.1% bupropion HBr / escitalopram Oxalate co-granules (manufactured as outlined above), and 2.9% Compritol 888 ATO (screened through a 500 ⁇ m screen).
- a homogenous tablet blend of 150Og was manufactured by dispensing 1456.5g of bupropion HBr / escitalopram Oxalate co-granules, and 43.5g of the screened Compritol 888 ATO. The material was added to a v-blender (15 litre shell of a Pharmatech AB-050 v-blender) in the following order:
- the tablet components were blended for 10 minutes, with the v-shell speed set to 25 rpm and the intensifier bar turned off.
- the homogenous tablet blend was discharged from the v-shell and charged to a tablet press (Riva Bilayer 11 station rotary tablet press) and compressed to a target tablet weight of 420mg and a target tablet hardness of 130N using 9mm round normal concave shaped tablet tooling.
- the resulting product comprises the homogenous tablet core, which at this point is an immediate release core has the following composition:
- the dissolution profile of the above pharmaceutical composition was determined under the dissolution conditions described below in Tables 3A, 3B, and 3C.
- the results of the dissolution testing are presented as a % of the total bupropion HBr and escitalopram Oxalate in the controlled release tablet (batch no. 0704028) and are also depicted in FIGs. 3A, 3B, and 3C.
- the homogenous tablet core composition comprises a mixture of bupriopion HBr
- the homogenous tablet core was made according to the co-granulation method described in Example 3 with citalopram HCl instead of escitalopram Ox.
- the resulting product comprises the homogenous tablet core, which at this point is an immediate release tablet core has the following composition:
- control-releasing coat was manufactured according to the method described in Example 1 in the absence of the plasticizer PEG 3350.
- the resulting control-releasing coat has the following composition:
- E2240 was determined under the dissolution conditions described below in Table 4. The result of the dissolution testing is presented as a % of the total bupropion HBr and citalopram HCl in the controlled release tablet and is also depicted in FIG. 4.
- the homogenous tablet core was manufactured according to the method described in Example 3 with the following bupropion HBr/es citalopram Oxalate co-granule and homogenous tablet core composition:
- control-releasing coat was manufactured according to the method described in Example 1 accept that the polyvinylpyrrolidone (Kollidon ® 90F) was replaced with the lower viscosity Kollidon ® K29/32 at the same %w/w.
- the resulting control-releasing coat has the following composition:
- the dissolution profile of the above pharmaceutical composition was determined under the dissolution conditions described below in Table 5. The result of the dissolution testing is presented as a % of the total bupropion HBr and escitalopram Oxalate in the controlled release tablet and is also depicted in FIG. 5.
- the homogenous tablet core was manufactured according to the method described in Example 3 with the following co-granule and homogenous tablet core composition:
- control-releasing coat was manufactured according to the method described in Example 1 accept that the grade of ethylcellulose polymer was changed from Ethocel Std 100 PREM (Dow Chemical Company) to the lower viscosity grade, Ethocel Std 10 PREM.
- the resulting control-releasing coat has the following composition:
- the dissolution profile of the above pharmaceutical composition was determined under the dissolution conditions described below in Table 6. The result of the dissolution testing is presented as a % of the total bupropion HBr and escitalopram Oxalate in the controlled release tablet (batch no. E2828) and is also depicted in FIG. 6.
- the homogenous tablet core was manufactured according to the method and composition described in Example 2.
- the homogenous IR tablet cores were coated with a polymethacrylate based film by preparing an aqueous suspension consisting of about 26.97% aqueous disperion of Eudragit NE30D, 5.3% Talc, 2.47% Hydroxypropyl Methylcellulose, 2.25% PEG4000, 0.31% Somethicone C, 0.23% Tween 80 and 62.47% purified water. Approximately 60% of the required water was heated to approximately 65 0 C using a paddle mixer, to which the hydroxypropyl methylcellulose, Tween ® 80 and Simethicone ® C were added.
- the control-releasing coating formulation has the following composition:
- the homogenous tablet core was manufactured according to the method and composition described in Example 5.
- control-releasing coat was manufactured according to the method described in Example 1 accept that the polyvinylpyrrolidone (Kollidon ® 90F) was reduced by 50%.
- the resulting control-releasing coat has the following composition:
- the dissolution profile of the above pharmaceutical composition was determined under the dissolution conditions described below in Table 8. The result of the dissolution testing is presented as a % of the total bupropion HBr and escitalopram oxalate in the controlled release tablet and is also depicted in FIG. 8.
- PV A/citric acid solution and mixed for a minimum of 20 minutes.
- the BHT became finely dispersed in the PVA / citric acid solution.
- the final composition of the granulation suspension was 4.82% PVA, 7.50% citric acid, 1.67% 2- propanol, 0.1% BHT, 85.91% purified water.
- the bupropion HBR and escitalopram oxalate were charged to the granulation chamber in the required ratio to give the desired final dosage strengths of each active.
- the actives were dispensed such that the material in the granulation chamber consisted of 93.6% bupropion HBr and 6.4% escitalopram oxalate.
- 3kg of material was granulated, 2815.5g of bupropion HBr mixed with 193.73g of escitalopram oxalate.
- the granulation suspension was sprayed onto the 3 kg mix of bupropion HBr to a weight gain of 9.1% to produce a granule comprising of 86% bupropion HBr, 5.6% escitalopam oxalate, 3.3% PVA, 5.0% citric acid, and 0.1% BHT.
- the powder bed temperature was maintained between 40 - 45 0 C, and the liquid spray rate maintained between 5 - 7 g/min throughout the granulation process.
- a homogenous tablet blend with the following composition was prepared; 97% bupropion HBr / escitalopram oxalate co-granules (manufactured as outlined above), and 3% Compritol 888 ATO (screened through a 500 ⁇ m screen).
- a homogenous tablet blend of 150Og was manufactured by dispensing 1455g of bupropion HBr / escitalopram oxalate co- granules, and 45g of the screened Compritol 888 ATO. The material was added to a v-blender (15 litre shell of a Pharmatech AB-050 v-blender) in the following order:
- the tablet components were blended for 10 minutes, with the v-shell speed set to 25 rpm and the intensifier bar turned off.
- the homogenous tablet blend was discharged from the v-shell and charged to a tablet press (Riva Bilayer 11 station rotary tablet press) and compressed to a target tablet weight of 373.5mg and a target tablet hardness of 130N using 9mm round normal concave shaped tablet tooling.
- the resulting product comprises the homogenous tablet core, which at this point is an immediate release core having the following composition:
- the homogenous IR tablet cores were coated with Kollicoat SR30D (a polyvinyl acetate dispersion with 27% polyvinyl acetate, 2.7% povidone and 0.3% sodium lauryl sulfate) by preparing an aqueous suspension consisting of about 50% aqueous disperion of Kollidon SR30D, 3.5% Talc, 1.5% Triethyl citrate and 45% purified water. Talc was added to approximately 80% of the water and the dispersion mixed with high shear for approximately 15 minutes.
- Kollicoat SR30D a polyvinyl acetate dispersion with 27% polyvinyl acetate, 2.7% povidone and 0.3% sodium lauryl sulfate
- the dissolution profile of the above pharmaceutical composition was determined under the dissolution conditions described below in Table 9. The result of the dissolution testing is presented as a % of the total bupropion HBr and escitalopram oxalate in the controlled release tablet and is also depicted in FIG. 9.
- the homogenous tablet core was manufactured according to the method described in Example 3 with the following co-granule and homogenous tablet core composition:
- control-releasing coat was manufactured according to the method and composition described in Example 1.
- the dissolution profile of the above pharmaceutical composition was determined under the dissolution conditions described below in Table 10. The result of the dissolution testing is presented as a % of the total bupropion HBr and escitalopram Oxalate in the controlled release tablet and is also depicted in FIG. 10.
- the homogenous tablet core was manufactured according to the method described in Example 3 with the following co-granule and homogenous tablet core composition:
- Tablet Coat Composition and Method of Manufacture [00322] The control-releasing coat was manufactured according to the method and composition described in Example 1.
- the dissolution profile of the above pharmaceutical composition was determined under the dissolution conditions described below in Table 11. The result of the dissolution testing is presented as a % of the total bupropion HBr and escitalopram Oxalate in the controlled release tablet and is also depicted in FIG. 11.
- the homogenous tablet core was manufactured according to the method described in Example 3 with the following co-granule and homogenous tablet core composition:
- control-releasing coat was manufactured according to the method and composition described in Example 1.
- the dissolution profile of the above pharmaceutical composition was determined under the dissolution conditions described below in Table 12. The result of the dissolution testing is presented as a % of the total bupropion HBr and escitalopram Oxalate in the controlled release tablet and is also depicted in FIG. 12.
- the homogenous tablet core was manufactured according to the method described in Example 3 with the following co-granule and homogenous tablet core composition:
- control-releasing coat was manufactured according to the method and composition described in Example 1 , except that the plasticized polymer solution was applied until about a 18% weight gain was obtained.
- the dissolution profile of the above pharmaceutical composition was determined under the dissolution conditions described below in Table 13. The result of the dissolution testing is presented as a % of the total bupropion HBr and escitalopram Oxalate in the controlled release tablet and is also depicted in FIG. 13.
- the homogenous tablet core was manufactured according to the method described in Example 3 with the following co-granule and homogenous tablet core composition:
- control-releasing coat was manufactured according to the method and composition described in Example 1 , except that the plasticized polymer solution was applied until about a 12% weight gain was obtained.
- the dissolution profile of the above pharmaceutical composition was determined under the dissolution conditions described below in Table 14. The result of the dissolution testing is presented as a % of the total bupropion HBr and escitalopram Oxalate in the controlled release tablet and is also depicted in FIG. 14.
- compositions comprising a homogenous controlled-release matrix comprising about 300mg of bupropion HCl (equivalent to about 260mg of bupropion base) and about 25.5mg of escitalopram oxalate (equivalent to about 20mg escitalopram base) were prepared by direct blending of Bupropion HCl granule fines (initially manufactured as granules described in Example 18 and screened through a 45 mesh screen. Particles ⁇ 355 ⁇ m were retained for the manufacturing the controlled-release matrix pharmaceutical composition) with escitalopram oxalate powder and other excipients with the following compositon.
- a homogenous blend of about 15Og was manufactured by dispensing about 61.44 g of bupropion HCl granule fines, about 5.04g of escitalopram oxalate, about 39.56g of hydroxypropyl cellulose, about 24.73g of lactose, about 14.28g of microcrystalline cellulose, about 0.49g of silicon dioxide, and about 4.45g of magenisium stearate. All the excipients were pre-screened through the 30mesh screen prior to dispensing. Manual bag mixing was applied according to the following order.
- the homogenous blend was further compressed using Natoli Single Station Press equipped with 0.706" x 0.329" capsule shaped tablet tooling.
- the target tablet weight was 758.3 mg.
- the hardness of the table was about 210N. 2.25 tons of compression force was applied.
- the dissolution results of the homogenous controlled-release matrix pharmaceutical copmposition (batch no. BUPHCL/ESC-300/25.5-03-07) was determined under the dissolution conditions described below in Table 15.
- the result of the dissolution testing is presented as a % of the total bupropion HCl and escitalopram oxalate in the controlled release composition.
- the dissolution profile determined under the dissolution conditions is also depicted in Figure 15.
- HCl equivalent to about 130mg of bupropion base
- escitalopram oxalate equivalent to about 118mg escitalopram base
- two separate blends with the following compositions were prepared separately: 1) about 47.23% bupropion HCl granules (manufactured according to the separate granulation method) with about 1.52% Compritol 888 ATO (screened through a 500 ⁇ m screen); 2) about 49.73% escitalopram oxalate granules (manufactured according to the separate granulation method, potency of the escitalopram oxalate granules is 90.1%) with about 1.52% Compritol 888 ATO (screened through a 500 ⁇ m screen).
- the theoretical batch size was about 10Og.
- About 47.2g of bupropion HCl granules were lubricated with about 1.52g of the screened Compritol 888 ATO by bag mixing for 2 minutes, and about 49.7g of escitalopram oxalate granules were lubricated with about 1.52g of the screened Compritol 888 ATO by bag mixing for 2 minutes.
- the bi-layer tablet cores were prepared using Natoli Single Station Press equipped with 9mm round concave shaped tablet tooling by pre-compressing the lubricated escitalopram oxalate granules as the first layer and followed by compressing the lubricated bupropion HCl granules as second layer.
- the target tablet weight was 332 mg and the target tablet hardness was about 175N. 0.5 tons of pre-compression force and 3 tons of compression force was applied.
- the resulting product comprises the bi-layer tablet core (batch no. BUPHCL/ESC- 150- 150-01-07), which at this point is an immediate release core having the following composition:
- the bi-layer tablet cores were coated separately with an ethylcellulose based film by preparing an organic solvent solution consisting of about 4.61% ethocel standard IOOFP premium, about 2.86% Kollidon ® 9OF, about 1.03% carbowax sentry polyethylene glycol 3350 granular NF FCC grade, about 0.5% dibutyl sebacate NF, about 8.68% 2-propanol, about 81.86% absolute ethanol, and about 0.46% purified water.
- an organic solvent solution consisting of about 4.61% ethocel standard IOOFP premium, about 2.86% Kollidon ® 9OF, about 1.03% carbowax sentry polyethylene glycol 3350 granular NF FCC grade, about 0.5% dibutyl sebacate NF, about 8.68% 2-propanol, about 81.86% absolute ethanol, and about 0.46% purified water.
- the plasticized polymer solution is applied to about 1.53 kg of the tablet cores, including about 35g of active tablet cores and about 1.5kg placebo tablets (comprising 69% lactose monohydrate, 30% microcrystalline cellulose and 1% magnesium stearate), using an O'Hara Labcoat II-X tablet coater (15" pan) until about a 10% weight gain is obtained.
- the product temperature is maintained between about 30-33 0 C, and the liquid spray rate is maintained between about 20-22 g/min throughout the coating process.
- the controlled release coated tablets are then cured for about 25 minutes (inlet air is set at about 5O 0 C, pan speed set at 3 rpm).
- the dissolution profile of the controlled release coated bi-layer tablets (batch no.
- BUPHCL/ESC-150-150-02-07 was determined under the dissolution conditions described below in Table 16. The result of the dissolution testing is presented as a % of the total bupropion HCl and escitalopram oxalate in the controlled release tablet and is also depicted in FIG. 16.
- Bupropion HBr granules were manufactured as described in example 1.
- the pharmaceutical active, Quetiapine fumarate was top-spray granulated using a Glatt GPCG 1 (6 inch chamber). The theoretical batch size was 207Og.
- An aqueous (purified water) solution of polyvinyl alcohol (PVA) (4.8% of solution) was sprayed onto 2 kg of Quetiapine fumarate to a weight gain of 3.5% to produce a granule comprising of 96.62% Quetiapine fumarate, and 3.38% PVA.
- the powder bed temperature was maintained between 38 - 45 0 C, and the liquid spray rate maintained between 5 - 10 g/min throughout the granulation process.
- a homogenous tablet blend of 40Og was manufactured by dispensing 363.16g of Bupropion HBr granules, 22.84g of Quetiapine fumarate granules, 13.6Og of Compritol 888 ATO, and 0.4Og of lake green blend.
- the material was added to a v-blender (4 quart shell of a PK labmaster v-blender) in the following order:
- the tablet components were blended for 10 minutes, with the intensifier bar turned off.
- the homogenous tablet blend was discharged from the v-shell and charged to a tablet press (Riva Bilayer 11 station rotary tablet press) and compressed to a target tablet weight of 418mg and a target tablet hardness of 120N using 9mm round deep concave shaped tablet tooling.
- the resulting product comprises the homogenous tablet core, which at this point is an immediate release core having the following composition:
- the homogenous IR tablet cores were next coated with the control-releasing coat as described in Example 1.
- the dissolution profile of the above pharmaceutical composition was determined under the dissolution conditions described below in Tables 17A and 17B. The results of the dissolution testing are presented as a % of the total bupropion HBr and quetiapine fumarate in the controlled release tablet and are also depicted in FIGs. 17A and 17B.
- Aeromatic MP8 Fluid Bed The theoretical batch size was 310.6kg.
- the powder bed temperature was maintained between 48-52 0 C, and the liquid spray rate maintained between 1500 g/min throughout the granulation process.
- the granules were fluid bed dried to a LOD (loss on drying) level of ⁇ 1%.
- the granules were screened and the granules with a particle size of between about 355 ⁇ m and about 850 ⁇ m were retained for manufacture of the homogenous tablet core.
- the pharmaceutical active, escitalopram oxalate was top-spray granulated using an Aeromatic fluid bed MPl.
- the theoretical batch size was 1943.5g.
- An organic solvent (2- propanol) solution of Kollidon 9OF (6.0% of solution) and butylated hydroxytoluene (BHT) (1.2% of solution) was sprayed onto about 1783.0 g of escitalopram oxalate to a weight gain of about 8.3% to produce a granule comprising of about 91.74% citalopram HCl, 6.88% Kollidon 9OF, and 1.38% BHT.
- the powder bed temperature was maintained between 35-45 0 C, and the liquid spray rate maintained between 13 - 17 g/min throughout the granulation process.
- the granules are fluid bed dried to a LOD (loss on drying) level of ⁇ 1%.
- the granules were screened and the granules with a particle size of between about 355 ⁇ m and about 850 ⁇ m were retained for manufacture of the homogenous tablet core.
- the homogenous tablet core comprising about 300mg of bupropion HCl (equivalent to about 260mg of bupropion base) and about 25.5mg of escitalopram oxalate (equivalent to about 20mg escitalopram base)
- a blend with the following composition was prepared: about 88.9% bupropion HCl granules (manufactured as described above), about 8.1% escitalopram oxalate granules (manufactured as described above, potency of the escitalopram oxalate granules is 90.1%) and about 3.0% Compritol 888 ATO (screened through a 500 ⁇ m screen).
- a homogenous blend of about lOOOg was manufactured by dispensing about 889.Og of bupropion HCl granules, about 81.Og of escitalopram oxalate granules, and about 30.3g of the screened Compritol ATO.
- the material was added to a 8 qt. v-blender in the following order:
- the tablet core components were homogenously blended for about 10 minutes, with the v-shell speed set to 25 rpm.
- the homogenous blend was discharged from the v-shell and charged to a tablet press (Manesty Betapress 16 station) and compressed to a target tablet weight of about 349.5mg and a target tablet hardness of about 13ON using 9mm round normal concave shaped tablet tooling.
- the resulting product comprises the homogenous tablet core, which at this point is an immediate release core having the following composition:
- the homogenous IR tablet cores were coated with an ethylcellulose based film by preparing an organic solvent solution consisting of about 4.61% ethocel standard IOOFP premium, about 2.86% Kollidon ® 9OF, about 1.03% carbowax sentry polyethylene glycol 3350 granular NF FCC grade, about 0.5% dibutyl sebacate NF, about 8.68% 2-propanol, about 81.86% absolute ethanol, and about 0.46% purified water.
- the plasticized polymer solution is applied to about 1.7 kg of the tablet cores, including about 0.2kg of active tablet cores and about 1.5kg placebo tablets (comprising 69% lactose monohydrate, 30% microcrystalline cellulose and 1% magnesium stearate), using an O'Hara Labcoat II-X tablet coater (15" pan) until about a 10% weight gain is obtained.
- the product temperature is maintained between about 30-33 0 C, and the liquid spray rate is maintained between about 20-22 g/min throughout the coating process.
- the controlled release coated tablets are then cured for about 25 minutes (inlet air is set at about 5O 0 C, pan speed set at 3 rpm).
- the homogenous tablet core comprising about 225mg of bupropion HCl
- escitalopram oxalate (equivalent to about 195mg of bupropion base) and about 75mg of escitalopram oxalate (equivalent to about 58.8mg escitalopram base) was prepared by blending and tabletting the following compositions: about 71.42% bupropion HCl granules (manufactured according to the separate granulation method)), and about 25.51% escitalopram oxalate granules (manufactured according to the separate granulation method), potency of the escitalopram oxalate granules is 90.1%), and about 3.07% Compritol 888 ATO (screened through a 500 ⁇ m screen).
- a homogenous blend of about lOOg was manufactured by dispensing about 71.4g of bupropion HCl granules, about 25.5g of escitalopram oxalate granules, and about 3.07g of the screened Compritol 888 ATO, and manually bag mixing for 2 minutes.
- the resulting homogenous tablet core (batch no. BUPHCL/ESC-225-75-01-07) has the following composition.
- the homogenous tablet cores were coated separately with an ethylcellulose based film by preparing an organic solvent solution consisting of about 4.61% ethocel standard IOOFP premium, about 2.86% Kollidon ® 9OF, about 1.03% carbowax sentry polyethylene glycol 3350 granular NF FCC grade, about 0.5% dibutyl sebacate NF, about 8.68% 2-propanol, about 81.86% absolute ethanol, and about 0.46% purified water.
- an organic solvent solution consisting of about 4.61% ethocel standard IOOFP premium, about 2.86% Kollidon ® 9OF, about 1.03% carbowax sentry polyethylene glycol 3350 granular NF FCC grade, about 0.5% dibutyl sebacate NF, about 8.68% 2-propanol, about 81.86% absolute ethanol, and about 0.46% purified water.
- the plasticized polymer solution was applied to about 1.53 kg of the tablet cores, including about 30g of active tablet cores and about 1.5kg placebo tablets (comprising 69% lactose monohydrate, 30% microcrystalline cellulose and 1% magnesium stearate), using an O'Hara Labcoat II-X tablet coater (15" pan) until about a 10% weight gain is obtained.
- the product temperature was maintained between about 30-33 0 C, and the liquid spray rate was maintained between about 20-22 g/min throughout the coating process.
- the controlled release coated tablets were then cured for about 25 minutes (inlet air is set at about 5O 0 C, pan speed set at 3 rpm).
- BUPHCL/ESC-225-75-02-07 are presented as a % of the total bupropion HCl and escitalopram oxalate released under the conditions described in Table 19.
- the dissolution profile is also depicted in Fig.19.
- HCl (equivalent to about 263.5mg of tramadol base) and about 25.5mg of escitalopram oxalate (equivalent to about 20mg escitalopram base)
- a blend with the following composition was prepared: about 88.9% tramadol HCl powder, about 7.6% escitalopram oxalate powder, about 3.1% Compritol 888 ATO (screened through a 500 ⁇ m screen) and 0.5% magnesium stearate (screened through a 500 ⁇ m screen).
- a homogenous blend of about 93.4g was manufactured by dispensing about 83.0g of tramadol HCl powder, about 7.1g of escitalopram oxalate, about 2.9g of the screened Compritol 888 ATO, and about 0.47g of magnesium stearate. The material was manually blended by bag mixing for 2 minutes.
- the homogenous blend was compressed using Natoli Single Station Press equipped with 9mm round concave shaped tablet tooling to a target tablet weight of about 337.5mg and a target tablet hardness of about 9ON. 1.75 tons of compression force is applied.
- the resulting homogenous tablet core (batch no. 08029T) has the following composition.
- the homogenous IR tablet cores were coated with an ethylcellulose based film by preparing an organic solvent solution consisting of about 4.61% ethocel standard IOOFP premium, about 2.86% Kollidon ® 9OF, about 1.03% carbowax sentry polyethylene glycol 3350 granular NF FCC grade, about 0.5% dibutyl sebacate NF, about 8.68% 2-propanol, about 81.86% absolute ethanol, and about 0.46% purified water.
- the plasticized polymer solution was applied to about 1.84 kg of the tablet cores, including about 40.8g of active tablet cores and about 1.8kg placebo tablets (comprising 69% lactose monohydrate, 30% microcrystalline cellulose and 1% magnesium stearate), using an O'Hara Labcoat II-X tablet coater (15" pan) until about a 10% weight gain is obtained.
- the product temperature was maintained between about 30-34 0 C, and the liquid spray rate was maintained between about 20-22 g/min throughout the coating process.
- the controlled release coated tablets were then cured for about 25 minutes (inlet air is set at about 48 0 C, pan speed set at 3 rpm).
- the dissolution profile of the above pharmaceutical composition was determined under the dissolution conditions described below in Table 2OA and 2OB.
- the results of the dissolution testing as presented as a % of the total tramadol HCl and escitalopram Oxalate in the controlled release tablet (batch no. 08029C) and is also depicted in Fig.2OA and 2OB.
- Day 13 0.00 (pre-dose), 1.00, 2.00, 3.00, 4.00, 5.00, 6.00, 8.00, 10.00, 12.00, 16.00, and 24.00 hours post-dose.
- Day 33 0.00 (pre-dose), 1.00, 2.00, 3.00, 4.00, 5.00, 6.00, 8.00, 10.00, 12.00, 16.00, and 24.00 hours post-dose.
- Bupropion and its metabolites - hydroxybupropion, bupropion erythroamino alcohol, and bupropion threoamino alcohol, and the internal standard, l-(3-chlorophenyl)- piperazine, were extracted by solid phase extraction into an organic media from 0.50 mL of human plasma. An aliquot of this extract was injected into a High Performance Liquid Chromatography system and detected using a tandem mass spectrometer. The analytes were separated by reverse phase chromatography.
- Bupropion, hydro xybupropion, erythro- hydrobupropion, threo-hydrobupropion, citalopram, desmethylcitalopram (demethycitalopram), and didesmethylcitalopram (didemethylcitaopram) were included in the analysis.
- PAWC potency corrected molar concentration summed for bupropion and its metabolites
- PAWC potency corrected molar concentration summed for bupropion and its metabolites
- T max values of citalopram, demethylcitalopram, and didemethyl citalopram were compared (Celexa ® + Wellbutrin XL ® vs. Celexa ® alone) using nonparametric methods; a significant difference was defined a priori as p ⁇ 0.05.
- the objectives of this study were: a) to determine and compare the rate and extent of absorption of bupropion and citalopram from a test fixed dose combination tablet formulation of Bupropion HBr XL (sustained release) 348 mg/Citalopram HCl Immediate Release (IR) 20 mg versus Bupropion HBr XL 348 mg tablets given concomitantly with CelexaTM 20 mg tablets under fasting conditions, (Group 1) and, b) to determine the effect of food on the rate and extent of absorption of a fixed dose combination tablet formulation of Bupropion HBr XL 348 mg/Citalopram HCl IR 20 mg (Group T).
- the bupropion HBr XL (sustained release) tablet used in this study was manufactured as described in US Patent No. 7,241,805.
- the bupropion HBr XL tablet is over coated with an immediate release coating comprising 20 mg citalopram HCl according to methods well known in the art. Normal, healthy, non-smoking male and female subjects between the ages of 18 and 55 years were included in the study.
- Bupropion, its metabolites - hydroxybupropion, bupropion erythroamino alcohol, and bupropion threoamino alcohol, and the internal standard, l-(3-chlorophenyl)-piperazine, were extracted by solid phase extraction into an organic media from 0.50 mL of human plasma. An aliquot of this extract was injected into a High Performance Liquid Chromatography system and detected using a tandem mass spectrometer. The analytes were separated by reverse phase chromatography.
- Citalopram, its metabolites - demethylcitalopram and didemethylcitalopram, and the internal standards, citalopram analog, demethylcitalopram analog, and didemethylcitalopram analog, were extracted by liquid-liquid extraction into an organic media from 1.00 mL of human plasma. An aliquot of this extract was injected into a High Performance Liquid Chromatography system and detected using a tandem mass spectrometer. The analytes were separated by reverse phase chromatography.
- Statistical analysis was carried out using General Linear Model (GLM) procedures in Statistical Analysis System (SAS), analysis of variance (ANOVA) was performed on In-transformed AUCo-t, AUC 0 - m f, and C max and on untransformed IQ, t./ 2 , MRT, and M/P ratio at the significance level of 0.05.
- the intra-subject coefficient of variation (CV) was calculated using the Mean Square Error (MSE) from the ANOVA table.
- MSE Mean Square Error
- the ratio of geometric means and the 90% geometric confidence interval (90% C.I.) were calculated based on the difference in the Least Squares Means of the In -transformed AUCo-t, AUCo- m f, and C max between the test and reference formulations. T max was analyzed using nonparametric methods.
- Example 1 Composition of Example 1, Lot #: 0612087, administered orally under fasting conditions, and Treatment C: One (1) Pharmaceutical Composition of Example 1, Lot #: 0612087, administered orally under fed conditions.
- SAS Analysis System
- ANOVA analysis of variance
- Treatment A One (1) Bupropion HBr (348 mg) / Escitalopram Oxalate (25.5 mg) SR Tablet [Formulation A], Lot #: 0704028, administered orally;
- Treatment B One (1) Bupropion HBr (348 mg) / Escitalopram Oxalate (25.5 mg) SR Tablet [Formulation B], Lot #: 0705037, administered orally;
- Treatment C One (1) Bupropion HBr XL 349 mg Tablet, Lot # 07D042P and one (1) Lexapro ® 20 mg Tablet, Lot#: M0603M, administered orally; AND Treatment D: One (1) Lexapro ® 20 mg Tablet, Lot M0603M, administered orally [00427] There were 24 subjects dosed in Period I, 13 of whom completed the study.
- Bupropion and its metabolites and citalopram and its metabolites were assayed as follows.
- Bupropion, hydroxybupropion, bupropion erythroamino alcohol, bupropion threoamino alcohol, and the internal standard, l-(3-chlorophenyl)-piperazine were extracted from human plasma (0.50 mL), by solid phase extraction (SPE) into an organic medium.
- SPE solid phase extraction
- the analytes were separated by High Performance Liquid Chromatography (HPLC) system using reverse phase chromatography conditions, detected using an API 3000 tandem mass spectrometer.
- HPLC High Performance Liquid Chromatography
- Method sensitivity and selectivity were achieved by detecting distinct precursor to production mass transitions for bupropion (240.3— > 184.0), hydroxybupropion (256.3— >238.0), bupropion erythroamino alcohol (242.4— > 168.1), bupropion threoamino alcohol (242.4— > 168.1) and the internal standard, l-(3-chlorophenyl)-piperazine (197.3— »153.8), at defined retention time.
- Citalopram, demethylcitalopram, didemethylcitalopram and the internal standards, citalopram analog, demethylcitalopram analog, and didemethylcitalopram analog, were extracted from human plasma (0.75 mL), using sodium heparin as an anticoagulant, by liquid-liquid extraction into an organic medium followed by back extraction into a dilute acid. An aliquot of this extract was injected into a High Performance Liquid Chromatography system and detected using a TSQ Quantum tandem mass spectrometer. The analytes were separated by reverse phase chromatography.
- citalopram 325.1— >109.0
- demethylcitalopram 31 l.l ⁇ 109.0
- didemethylcitalopram 297.1 ⁇ 109.0 and 297.1 ⁇ 260.0
- citalopram analog 341.1— »125.0
- demethylcitalopram analog 327.1— »125.0
- didemethylcitalopram analog 313.1— » 125.0
- Peak area ratios were used to determine the concentration of the standards, quality control samples, and the unknown study samples from the calibration curves.
- the pharmacokinetic and statistical analyses were performed on data for bupropion and its metabolites from 14 subjects, 13 of who completed the 4 study periods and 1 for whom there were sufficient data in at least 2 periods to potentially allow for a meaningful analysis.
- the pharmacokinetic and statistical analysis was performed data for citalopram and its metabolites on 13 subjects who completed the 4 study periods.
Landscapes
- Health & Medical Sciences (AREA)
- Veterinary Medicine (AREA)
- Chemical & Material Sciences (AREA)
- Medicinal Chemistry (AREA)
- Pharmacology & Pharmacy (AREA)
- Life Sciences & Earth Sciences (AREA)
- Animal Behavior & Ethology (AREA)
- General Health & Medical Sciences (AREA)
- Public Health (AREA)
- Epidemiology (AREA)
- Bioinformatics & Cheminformatics (AREA)
- Engineering & Computer Science (AREA)
- General Chemical & Material Sciences (AREA)
- Biomedical Technology (AREA)
- Neurology (AREA)
- Neurosurgery (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Emergency Medicine (AREA)
- Nuclear Medicine, Radiotherapy & Molecular Imaging (AREA)
- Organic Chemistry (AREA)
- Pain & Pain Management (AREA)
- Psychiatry (AREA)
- Medicinal Preparation (AREA)
- Pharmaceuticals Containing Other Organic And Inorganic Compounds (AREA)
- Acyclic And Carbocyclic Compounds In Medicinal Compositions (AREA)
Abstract
Description
Claims
Priority Applications (5)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| CA2710838A CA2710838A1 (en) | 2008-01-28 | 2009-01-28 | Pharmaceutical compositions |
| AU2009209629A AU2009209629A1 (en) | 2008-01-28 | 2009-01-28 | Pharmaceutical compositions |
| US12/864,453 US20110052684A1 (en) | 2008-01-28 | 2009-01-28 | Pharmaceutical composition |
| EP09704958A EP2234604A2 (en) | 2008-01-28 | 2009-01-28 | Pharmaceutical compositions |
| IL206487A IL206487A0 (en) | 2008-01-28 | 2010-06-20 | Pharmaceutical compositions |
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US2395108P | 2008-01-28 | 2008-01-28 | |
| US61/023,951 | 2008-01-28 |
Publications (2)
| Publication Number | Publication Date |
|---|---|
| WO2009095395A2 true WO2009095395A2 (en) | 2009-08-06 |
| WO2009095395A3 WO2009095395A3 (en) | 2010-03-18 |
Family
ID=40548683
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| PCT/EP2009/050924 WO2009095395A2 (en) | 2008-01-28 | 2009-01-28 | Pharmaceutical compositions |
Country Status (6)
| Country | Link |
|---|---|
| US (2) | US20090246276A1 (en) |
| EP (1) | EP2234604A2 (en) |
| AU (1) | AU2009209629A1 (en) |
| CA (1) | CA2710838A1 (en) |
| IL (1) | IL206487A0 (en) |
| WO (1) | WO2009095395A2 (en) |
Cited By (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2024165628A1 (en) * | 2023-02-07 | 2024-08-15 | Kinast Lasse | An immediate-release oral pharmaceutical form of escitalopram or racemate thereof with increased api content |
Families Citing this family (3)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| PT2271348T (en) * | 2008-03-28 | 2018-04-16 | Paratek Pharm Innc | Oral tablet formulation of tetracycline compound |
| EP2389923B1 (en) * | 2010-05-19 | 2013-01-30 | Uni-Pharma Kleon Tsetis Pharmaceutical Laboratories S.A. | Stable ready to use injectable paracetamol formulation |
| EP3697389A1 (en) * | 2017-10-16 | 2020-08-26 | Council of Scientific and Industrial Research | Sustained release formulations ofcrocus sativus |
Citations (55)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US4132753A (en) | 1965-02-12 | 1979-01-02 | American Cyanamid Company | Process for preparing oral sustained release granules |
| US4230687A (en) | 1978-05-30 | 1980-10-28 | Griffith Laboratories U.S.A., Inc. | Encapsulation of active agents as microdispersions in homogeneous natural polymeric matrices |
| US4343789A (en) | 1979-07-05 | 1982-08-10 | Yamanouchi Pharmaceutical Co., Ltd. | Sustained release pharmaceutical composition of solid medical material |
| US4344431A (en) | 1969-03-24 | 1982-08-17 | University Of Delaware | Polymeric article for dispensing drugs |
| US4346709A (en) | 1980-11-10 | 1982-08-31 | Alza Corporation | Drug delivery devices comprising erodible polymer and erosion rate modifier |
| US4421736A (en) | 1982-05-20 | 1983-12-20 | Merrel Dow Pharmaceuticals Inc. | Sustained release diethylpropion compositions |
| US4449983A (en) | 1982-03-22 | 1984-05-22 | Alza Corporation | Simultaneous delivery of two drugs from unit delivery device |
| US4455143A (en) | 1982-03-22 | 1984-06-19 | Alza Corporation | Osmotic device for dispensing two different medications |
| US4601894A (en) | 1985-03-29 | 1986-07-22 | Schering Corporation | Controlled release dosage form comprising acetaminophen, pseudoephedrine sulfate and dexbrompheniramine maleate |
| US4662880A (en) | 1986-03-14 | 1987-05-05 | Alza Corporation | Pseudoephedrine, brompheniramine therapy |
| US4764378A (en) | 1986-02-10 | 1988-08-16 | Zetachron, Inc. | Buccal drug dosage form |
| US4801460A (en) | 1986-04-11 | 1989-01-31 | Basf Aktiengesellschaft | Preparation of solid pharmaceutical forms |
| US4806337A (en) | 1984-07-23 | 1989-02-21 | Zetachron, Inc. | Erodible matrix for sustained release bioactive composition |
| US4814181A (en) | 1987-09-03 | 1989-03-21 | Alza Corporation | Dosage form comprising fast agent delivery followed by slow agent delivery |
| US4828836A (en) | 1986-06-05 | 1989-05-09 | Euroceltique S.A. | Controlled release pharmaceutical composition |
| US4834984A (en) | 1986-06-10 | 1989-05-30 | Euroceltique S.A. | Controlled release dihydrocodeine composition |
| US4844907A (en) | 1985-08-28 | 1989-07-04 | Euroceltique, S.A. | Pharmaceutical composition comprising analgesic and anti-inflammatory agent |
| US4844909A (en) | 1986-10-31 | 1989-07-04 | Euroceltique, S.A. | Controlled release hydromorphone composition |
| US4861598A (en) | 1986-07-18 | 1989-08-29 | Euroceltique, S.A. | Controlled release bases for pharmaceuticals |
| US4915954A (en) | 1987-09-03 | 1990-04-10 | Alza Corporation | Dosage form for delivering a drug at two different rates |
| US4959208A (en) | 1987-10-19 | 1990-09-25 | Ppg Industries, Inc. | Active agent delivery device |
| US4970075A (en) | 1986-07-18 | 1990-11-13 | Euroceltique, S.A. | Controlled release bases for pharmaceuticals |
| US5007790A (en) | 1989-04-11 | 1991-04-16 | Depomed Systems, Inc. | Sustained-release oral drug dosage form |
| US5023089A (en) | 1988-07-18 | 1991-06-11 | Shionogi & Co., Ltd. | Sustained-release preparations and the process thereof |
| US5073379A (en) | 1988-09-07 | 1991-12-17 | Basf Aktiengesellschaft | Continuous preparation of solid pharmaceutical forms |
| US5126145A (en) | 1989-04-13 | 1992-06-30 | Upsher Smith Laboratories Inc | Controlled release tablet containing water soluble medicament |
| US5178868A (en) | 1988-10-26 | 1993-01-12 | Kabi Pharmacia Aktiebolaq | Dosage form |
| US5183690A (en) | 1990-06-25 | 1993-02-02 | The United States Of America, As Represented By The Secretary Of Agriculture | Starch encapsulation of biologically active agents by a continuous process |
| US5202128A (en) | 1989-01-06 | 1993-04-13 | F. H. Faulding & Co. Limited | Sustained release pharmaceutical composition |
| US5266331A (en) | 1991-11-27 | 1993-11-30 | Euroceltique, S.A. | Controlled release oxycodone compositions |
| US5273758A (en) | 1991-03-18 | 1993-12-28 | Sandoz Ltd. | Directly compressible polyethylene oxide vehicle for preparing therapeutic dosage forms |
| US5283065A (en) | 1989-09-21 | 1994-02-01 | American Cyanamid Company | Controlled release pharmaceutical compositions from spherical granules in tabletted oral dosage unit form |
| US5350584A (en) | 1992-06-26 | 1994-09-27 | Merck & Co., Inc. | Spheronization process using charged resins |
| US5378462A (en) | 1992-08-19 | 1995-01-03 | Kali-Chemie Pharma Gmbh | Pancreatin micropellets prepared with polyethylene glycol 4000, paraffin and a lower alcohol by extrusion and rounding |
| US5395626A (en) | 1994-03-23 | 1995-03-07 | Ortho Pharmaceutical Corporation | Multilayered controlled release pharmaceutical dosage form |
| US5403593A (en) | 1991-03-04 | 1995-04-04 | Sandoz Ltd. | Melt granulated compositions for preparing sustained release dosage forms |
| US5443846A (en) | 1990-04-28 | 1995-08-22 | Takeda Chemical Industries, Ltd. | Granulated preparations and method of producing the same |
| US5453283A (en) | 1990-10-08 | 1995-09-26 | Schwarz Pharma Ag | Orally administered solvent-free pharmaceutical preparation with delayed active-substance release, and a method of preparing the preparation |
| US5476528A (en) | 1993-12-20 | 1995-12-19 | Tennessee Valley Authority | System for improving material release profiles |
| US5510114A (en) | 1993-05-18 | 1996-04-23 | Instituto Biochimico Italiano Giovanni Lorenzini S.P.A. | Slow release pharmaceutical composition containing a bile acid as an active ingredient |
| US5552159A (en) | 1991-11-23 | 1996-09-03 | Basf Aktiengesellschaft | Solid depot drug form |
| US5567439A (en) | 1994-06-14 | 1996-10-22 | Fuisz Technologies Ltd. | Delivery of controlled-release systems(s) |
| US5591452A (en) | 1993-05-10 | 1997-01-07 | Euro-Celtique, S.A. | Controlled release formulation |
| US5849240A (en) | 1993-11-23 | 1998-12-15 | Euro-Celtique, S.A. | Method of preparing sustained release pharmaceutical compositions |
| US5866164A (en) | 1996-03-12 | 1999-02-02 | Alza Corporation | Composition and dosage form comprising opioid antagonist |
| US5891471A (en) | 1993-11-23 | 1999-04-06 | Euro-Celtique, S.A. | Pharmaceutical multiparticulates |
| US5919826A (en) | 1996-10-24 | 1999-07-06 | Algos Pharmaceutical Corporation | Method of alleviating pain |
| US5958452A (en) | 1994-11-04 | 1999-09-28 | Euro-Celtique, S.A. | Extruded orally administrable opioid formulations |
| US6129933A (en) | 1991-12-24 | 2000-10-10 | Purdue Pharma Lp | Stabilized controlled release substrate having a coating derived from an aqueous dispersion of hydrophobic polymer |
| US6156342A (en) | 1998-05-26 | 2000-12-05 | Andex Pharmaceuticals, Inc. | Controlled release oral dosage form |
| US6306438B1 (en) | 1997-07-02 | 2001-10-23 | Euro-Celtique, S.A. | Stabilized sustained release tramadol formulations |
| US6340475B2 (en) | 1997-06-06 | 2002-01-22 | Depomed, Inc. | Extending the duration of drug release within the stomach during the fed mode |
| US6576260B2 (en) | 1999-08-31 | 2003-06-10 | Gruenenthal Gmbh | Sustained-release form of administration containing tramadol saccharinate |
| US7241805B2 (en) | 2005-06-27 | 2007-07-10 | Biovail Laboratories, Inc. | Modified release formulations of a bupropion salt |
| WO2007133583A2 (en) | 2006-05-09 | 2007-11-22 | Mallinckrodt Inc. | Zero-order modified release solid dosage forms |
Family Cites Families (4)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US20050112198A1 (en) * | 2003-10-27 | 2005-05-26 | Challapalli Prasad V. | Bupropion formulation for sustained delivery |
| US20050250838A1 (en) * | 2004-05-04 | 2005-11-10 | Challapalli Prasad V | Formulation for sustained delivery |
| CA2626025A1 (en) * | 2005-10-14 | 2007-04-26 | Forest Laboratories, Inc. | Stable pharmaceutical formulations containing escitalopram and bupropion |
| EP1954257A4 (en) * | 2005-10-14 | 2009-05-20 | Lundbeck & Co As H | Methods of treating central nervous system disorders with a low dose combination of escitalopram and bupropion |
-
2009
- 2009-01-27 US US12/360,673 patent/US20090246276A1/en not_active Abandoned
- 2009-01-28 AU AU2009209629A patent/AU2009209629A1/en not_active Abandoned
- 2009-01-28 CA CA2710838A patent/CA2710838A1/en not_active Abandoned
- 2009-01-28 EP EP09704958A patent/EP2234604A2/en not_active Withdrawn
- 2009-01-28 WO PCT/EP2009/050924 patent/WO2009095395A2/en active Application Filing
- 2009-01-28 US US12/864,453 patent/US20110052684A1/en not_active Abandoned
-
2010
- 2010-06-20 IL IL206487A patent/IL206487A0/en unknown
Patent Citations (63)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US4132753A (en) | 1965-02-12 | 1979-01-02 | American Cyanamid Company | Process for preparing oral sustained release granules |
| US4344431A (en) | 1969-03-24 | 1982-08-17 | University Of Delaware | Polymeric article for dispensing drugs |
| US4230687A (en) | 1978-05-30 | 1980-10-28 | Griffith Laboratories U.S.A., Inc. | Encapsulation of active agents as microdispersions in homogeneous natural polymeric matrices |
| US4343789A (en) | 1979-07-05 | 1982-08-10 | Yamanouchi Pharmaceutical Co., Ltd. | Sustained release pharmaceutical composition of solid medical material |
| US4346709A (en) | 1980-11-10 | 1982-08-31 | Alza Corporation | Drug delivery devices comprising erodible polymer and erosion rate modifier |
| US4449983A (en) | 1982-03-22 | 1984-05-22 | Alza Corporation | Simultaneous delivery of two drugs from unit delivery device |
| US4455143A (en) | 1982-03-22 | 1984-06-19 | Alza Corporation | Osmotic device for dispensing two different medications |
| US4421736A (en) | 1982-05-20 | 1983-12-20 | Merrel Dow Pharmaceuticals Inc. | Sustained release diethylpropion compositions |
| US4806337A (en) | 1984-07-23 | 1989-02-21 | Zetachron, Inc. | Erodible matrix for sustained release bioactive composition |
| US4601894A (en) | 1985-03-29 | 1986-07-22 | Schering Corporation | Controlled release dosage form comprising acetaminophen, pseudoephedrine sulfate and dexbrompheniramine maleate |
| US4844907A (en) | 1985-08-28 | 1989-07-04 | Euroceltique, S.A. | Pharmaceutical composition comprising analgesic and anti-inflammatory agent |
| US4764378A (en) | 1986-02-10 | 1988-08-16 | Zetachron, Inc. | Buccal drug dosage form |
| US4662880A (en) | 1986-03-14 | 1987-05-05 | Alza Corporation | Pseudoephedrine, brompheniramine therapy |
| US4801460A (en) | 1986-04-11 | 1989-01-31 | Basf Aktiengesellschaft | Preparation of solid pharmaceutical forms |
| US4828836A (en) | 1986-06-05 | 1989-05-09 | Euroceltique S.A. | Controlled release pharmaceutical composition |
| US4834984A (en) | 1986-06-10 | 1989-05-30 | Euroceltique S.A. | Controlled release dihydrocodeine composition |
| US4861598A (en) | 1986-07-18 | 1989-08-29 | Euroceltique, S.A. | Controlled release bases for pharmaceuticals |
| US4970075A (en) | 1986-07-18 | 1990-11-13 | Euroceltique, S.A. | Controlled release bases for pharmaceuticals |
| US4844909A (en) | 1986-10-31 | 1989-07-04 | Euroceltique, S.A. | Controlled release hydromorphone composition |
| US4915954A (en) | 1987-09-03 | 1990-04-10 | Alza Corporation | Dosage form for delivering a drug at two different rates |
| US4814181A (en) | 1987-09-03 | 1989-03-21 | Alza Corporation | Dosage form comprising fast agent delivery followed by slow agent delivery |
| US4959208A (en) | 1987-10-19 | 1990-09-25 | Ppg Industries, Inc. | Active agent delivery device |
| US5023089A (en) | 1988-07-18 | 1991-06-11 | Shionogi & Co., Ltd. | Sustained-release preparations and the process thereof |
| US5073379A (en) | 1988-09-07 | 1991-12-17 | Basf Aktiengesellschaft | Continuous preparation of solid pharmaceutical forms |
| US5178868A (en) | 1988-10-26 | 1993-01-12 | Kabi Pharmacia Aktiebolaq | Dosage form |
| US5202128A (en) | 1989-01-06 | 1993-04-13 | F. H. Faulding & Co. Limited | Sustained release pharmaceutical composition |
| US5007790A (en) | 1989-04-11 | 1991-04-16 | Depomed Systems, Inc. | Sustained-release oral drug dosage form |
| US5126145A (en) | 1989-04-13 | 1992-06-30 | Upsher Smith Laboratories Inc | Controlled release tablet containing water soluble medicament |
| US5283065A (en) | 1989-09-21 | 1994-02-01 | American Cyanamid Company | Controlled release pharmaceutical compositions from spherical granules in tabletted oral dosage unit form |
| US5443846A (en) | 1990-04-28 | 1995-08-22 | Takeda Chemical Industries, Ltd. | Granulated preparations and method of producing the same |
| US5183690A (en) | 1990-06-25 | 1993-02-02 | The United States Of America, As Represented By The Secretary Of Agriculture | Starch encapsulation of biologically active agents by a continuous process |
| US5453283A (en) | 1990-10-08 | 1995-09-26 | Schwarz Pharma Ag | Orally administered solvent-free pharmaceutical preparation with delayed active-substance release, and a method of preparing the preparation |
| US5403593A (en) | 1991-03-04 | 1995-04-04 | Sandoz Ltd. | Melt granulated compositions for preparing sustained release dosage forms |
| US5273758A (en) | 1991-03-18 | 1993-12-28 | Sandoz Ltd. | Directly compressible polyethylene oxide vehicle for preparing therapeutic dosage forms |
| US5552159A (en) | 1991-11-23 | 1996-09-03 | Basf Aktiengesellschaft | Solid depot drug form |
| US5266331A (en) | 1991-11-27 | 1993-11-30 | Euroceltique, S.A. | Controlled release oxycodone compositions |
| US6905709B2 (en) | 1991-12-24 | 2005-06-14 | Purdue Pharma, Lp | Stabilized controlled release substrate having a coating derived from an aqueous dispersion of hydrophobic polymer |
| US6129933A (en) | 1991-12-24 | 2000-10-10 | Purdue Pharma Lp | Stabilized controlled release substrate having a coating derived from an aqueous dispersion of hydrophobic polymer |
| US5350584A (en) | 1992-06-26 | 1994-09-27 | Merck & Co., Inc. | Spheronization process using charged resins |
| US5378462A (en) | 1992-08-19 | 1995-01-03 | Kali-Chemie Pharma Gmbh | Pancreatin micropellets prepared with polyethylene glycol 4000, paraffin and a lower alcohol by extrusion and rounding |
| US5591452A (en) | 1993-05-10 | 1997-01-07 | Euro-Celtique, S.A. | Controlled release formulation |
| US6326027B1 (en) | 1993-05-10 | 2001-12-04 | Euro-Celtique S.A. | Controlled release formulation |
| US6254887B1 (en) | 1993-05-10 | 2001-07-03 | Euro-Celtique S.A. | Controlled release tramadol |
| US5510114A (en) | 1993-05-18 | 1996-04-23 | Instituto Biochimico Italiano Giovanni Lorenzini S.P.A. | Slow release pharmaceutical composition containing a bile acid as an active ingredient |
| US5965163A (en) | 1993-11-23 | 1999-10-12 | Euro-Celtique, S.A. | Substained release compositions and a method of preparing pharmaceutical compositions |
| US5849240A (en) | 1993-11-23 | 1998-12-15 | Euro-Celtique, S.A. | Method of preparing sustained release pharmaceutical compositions |
| US6162467A (en) | 1993-11-23 | 2000-12-19 | Euro-Celtique, S.A. | Sustained release compositions and a method of preparing pharmaceutical compositions |
| US5891471A (en) | 1993-11-23 | 1999-04-06 | Euro-Celtique, S.A. | Pharmaceutical multiparticulates |
| US5476528A (en) | 1993-12-20 | 1995-12-19 | Tennessee Valley Authority | System for improving material release profiles |
| US5474786A (en) | 1994-03-23 | 1995-12-12 | Ortho Pharmaceutical Corporation | Multilayered controlled release pharmaceutical dosage form |
| US5395626A (en) | 1994-03-23 | 1995-03-07 | Ortho Pharmaceutical Corporation | Multilayered controlled release pharmaceutical dosage form |
| US5567439A (en) | 1994-06-14 | 1996-10-22 | Fuisz Technologies Ltd. | Delivery of controlled-release systems(s) |
| US5958452A (en) | 1994-11-04 | 1999-09-28 | Euro-Celtique, S.A. | Extruded orally administrable opioid formulations |
| US5965161A (en) | 1994-11-04 | 1999-10-12 | Euro-Celtique, S.A. | Extruded multi-particulates |
| US5866164A (en) | 1996-03-12 | 1999-02-02 | Alza Corporation | Composition and dosage form comprising opioid antagonist |
| US5919826A (en) | 1996-10-24 | 1999-07-06 | Algos Pharmaceutical Corporation | Method of alleviating pain |
| US6340475B2 (en) | 1997-06-06 | 2002-01-22 | Depomed, Inc. | Extending the duration of drug release within the stomach during the fed mode |
| US6306438B1 (en) | 1997-07-02 | 2001-10-23 | Euro-Celtique, S.A. | Stabilized sustained release tramadol formulations |
| US6645527B2 (en) | 1997-07-02 | 2003-11-11 | Euro-Celtique S.A. | Stabilized sustained release tramadol formulations |
| US6156342A (en) | 1998-05-26 | 2000-12-05 | Andex Pharmaceuticals, Inc. | Controlled release oral dosage form |
| US6576260B2 (en) | 1999-08-31 | 2003-06-10 | Gruenenthal Gmbh | Sustained-release form of administration containing tramadol saccharinate |
| US7241805B2 (en) | 2005-06-27 | 2007-07-10 | Biovail Laboratories, Inc. | Modified release formulations of a bupropion salt |
| WO2007133583A2 (en) | 2006-05-09 | 2007-11-22 | Mallinckrodt Inc. | Zero-order modified release solid dosage forms |
Non-Patent Citations (17)
| Title |
|---|
| BALDWIN ET AL., J CLIN PSYCHIATRY, vol. 67, no. 6, 2006, pages 9 - 15 |
| BLIER, P., EUROPEAN NEUROPSYCHOPHARMACOLOGY, vol. 13, 2003, pages 57 - 66 |
| CLAYTON AH., PRIMARY PSYCHIATRY, vol. 10, no. 1, 2003, pages 55 - 61 |
| DONG J.; BLIER P., PSYCHOPHARMACOLOGY, vol. 155, 2001, pages 52 - 57 |
| FAVA M. ET AL., PRIM CARE COMPANION J CLIN PSYCHIATRY, vol. 7, no. 3, 2005, pages 106 - 113 |
| LAM R.W., J CLIN PSYCHIATRY, vol. 65, no. 3, 2004, pages 337 - 340 |
| MANSARI ET AL., NEUROPHARMACOLOGY, vol. 55, 2008, pages 1191 - 1198 |
| MANSARI M.E. ET AL., NEUROPHARMACOLOGY, vol. 55, 2008, pages 1191 - 1198 |
| MICHAEL, T ET AL., PSYCHIATRY, vol. 6, no. 4, 2007, pages 136 - 142 |
| MURRAY C.J. ET AL., LANCET, vol. 349, 1997, pages 1436 - 1442 |
| PINEYRO G.; BLIER P., PHARMACOL REV, vol. 51, no. 3, 1999, pages 533 - 591 |
| PRICA ET AL., BEHAV. BRAIN RES., vol. 194, 2008, pages 92 - 99 |
| RUSH A.J. ET AL., AM J PSYCHIATRY, vol. 163, 2006, pages 1905 - 1917 |
| SZABO S.T. ET AL., INT J NEUROPSYCHOPHARMACOL, vol. 3, 2000, pages 1 - 11 |
| WEISSMAN, M.M. ET AL., AM MED ASS, vol. 276, 1996, pages 293 - 299 |
| ZIMMERMAN M. ET AL., J CLIN PSYCHIATRY, vol. 66, no. 10, 2005, pages 1336 - 1339 |
| ZISOOK S ET AL., BIOL PSYCHIATRY, vol. 59, no. 3, 2006, pages 203 - 210 |
Cited By (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2024165628A1 (en) * | 2023-02-07 | 2024-08-15 | Kinast Lasse | An immediate-release oral pharmaceutical form of escitalopram or racemate thereof with increased api content |
Also Published As
| Publication number | Publication date |
|---|---|
| IL206487A0 (en) | 2010-12-30 |
| CA2710838A1 (en) | 2009-08-06 |
| US20110052684A1 (en) | 2011-03-03 |
| EP2234604A2 (en) | 2010-10-06 |
| US20090246276A1 (en) | 2009-10-01 |
| WO2009095395A3 (en) | 2010-03-18 |
| AU2009209629A1 (en) | 2009-08-06 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| KR101087464B1 (en) | Modified-Release Tablet of Bupropion Hydrochloride | |
| AU2014265327B2 (en) | Cenicriviroc compositions and methods of making and using the same | |
| US7674479B2 (en) | Sustained-release bupropion and bupropion/mecamylamine tablets | |
| KR100958045B1 (en) | Pharmaceutical containing 3-3-dimethylamino-1-ethyl-2-methyl-propylphenol and providing delayed release of the active ingredient | |
| US20140030249A1 (en) | Pharmaceutical Compositions | |
| US20140242063A1 (en) | Pharmaceutical compositions | |
| US7741374B1 (en) | Methods of use of fenofibric acid | |
| US9717701B2 (en) | Compositions for overcoming resistance to tramadol | |
| KR101858797B1 (en) | Pharmaceutical compositions comprising hydromorphone and naloxone | |
| JP2013501810A (en) | Pharmaceutical composition | |
| EP3265126B1 (en) | Tesofensine and metoprolol combination formulation | |
| JPWO2006118265A1 (en) | Composition containing anti-dementia drug | |
| US20120208773A1 (en) | Pharmaceutical compositions with tetrabenazine | |
| WO2011098483A1 (en) | Pharmaceutical compositions comprising a combination of metformin and sitagliptin | |
| US20080138411A1 (en) | Modified Release Formulations Of Selective Serotonin Re-Uptake Inhibitors | |
| WO2011039686A1 (en) | Latrepirdine oral sustained release dosage forms | |
| WO2009095395A2 (en) | Pharmaceutical compositions | |
| US20150148388A1 (en) | Chemical composition | |
| KR20080059212A (en) | 3-(2-dimethylaminomethyl cyclohexyl) phenol retard formulation | |
| US20100008956A1 (en) | Composition and combinations of carboxylic acid losartan in dosage forms | |
| US20100203128A1 (en) | Bupropion hydrobromide and therapeutic applications | |
| KR20200104256A (en) | A single dosage form of a pharmaceutical composition for the treatment or prevention of hypertension and hyperlipidemia | |
| JP2011140510A (en) | Modified-release tablet of bupropion hydrochloride |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| 121 | Ep: the epo has been informed by wipo that ep was designated in this application |
Ref document number: 09704958 Country of ref document: EP Kind code of ref document: A2 |
|
| WWE | Wipo information: entry into national phase |
Ref document number: 2009704958 Country of ref document: EP |
|
| WWE | Wipo information: entry into national phase |
Ref document number: 4457/DELNP/2010 Country of ref document: IN |
|
| WWE | Wipo information: entry into national phase |
Ref document number: 2710838 Country of ref document: CA |
|
| WWE | Wipo information: entry into national phase |
Ref document number: 2009209629 Country of ref document: AU |
|
| WWE | Wipo information: entry into national phase |
Ref document number: PI 2010002982 Country of ref document: MY |
|
| ENP | Entry into the national phase |
Ref document number: 2009209629 Country of ref document: AU Date of ref document: 20090128 Kind code of ref document: A |
|
| NENP | Non-entry into the national phase |
Ref country code: DE |