WO2008148839A2 - Long-acting polymeric prodrugs of exendin - Google Patents
Long-acting polymeric prodrugs of exendin Download PDFInfo
- Publication number
- WO2008148839A2 WO2008148839A2 PCT/EP2008/056981 EP2008056981W WO2008148839A2 WO 2008148839 A2 WO2008148839 A2 WO 2008148839A2 EP 2008056981 W EP2008056981 W EP 2008056981W WO 2008148839 A2 WO2008148839 A2 WO 2008148839A2
- Authority
- WO
- WIPO (PCT)
- Prior art keywords
- substituted
- poly
- exendin
- aryls
- branched
- Prior art date
Links
- 0 CC(CCCC(C)(C)CC(*)OC(*)=O)C(**)(**)OC(N)=O Chemical compound CC(CCCC(C)(C)CC(*)OC(*)=O)C(**)(**)OC(N)=O 0.000 description 4
Classifications
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K14/00—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof
- C07K14/435—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof from animals; from humans
- C07K14/575—Hormones
- C07K14/57563—Vasoactive intestinal peptide [VIP]; Related peptides
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K47/00—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient
- A61K47/50—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient the non-active ingredient being chemically bound to the active ingredient, e.g. polymer-drug conjugates
- A61K47/51—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient the non-active ingredient being chemically bound to the active ingredient, e.g. polymer-drug conjugates the non-active ingredient being a modifying agent
- A61K47/56—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient the non-active ingredient being chemically bound to the active ingredient, e.g. polymer-drug conjugates the non-active ingredient being a modifying agent the modifying agent being an organic macromolecular compound, e.g. an oligomeric, polymeric or dendrimeric molecule
- A61K47/59—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient the non-active ingredient being chemically bound to the active ingredient, e.g. polymer-drug conjugates the non-active ingredient being a modifying agent the modifying agent being an organic macromolecular compound, e.g. an oligomeric, polymeric or dendrimeric molecule obtained otherwise than by reactions only involving carbon-to-carbon unsaturated bonds, e.g. polyureas or polyurethanes
- A61K47/60—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient the non-active ingredient being chemically bound to the active ingredient, e.g. polymer-drug conjugates the non-active ingredient being a modifying agent the modifying agent being an organic macromolecular compound, e.g. an oligomeric, polymeric or dendrimeric molecule obtained otherwise than by reactions only involving carbon-to-carbon unsaturated bonds, e.g. polyureas or polyurethanes the organic macromolecular compound being a polyoxyalkylene oligomer, polymer or dendrimer, e.g. PEG, PPG, PEO or polyglycerol
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K47/00—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient
- A61K47/50—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient the non-active ingredient being chemically bound to the active ingredient, e.g. polymer-drug conjugates
- A61K47/51—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient the non-active ingredient being chemically bound to the active ingredient, e.g. polymer-drug conjugates the non-active ingredient being a modifying agent
- A61K47/56—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient the non-active ingredient being chemically bound to the active ingredient, e.g. polymer-drug conjugates the non-active ingredient being a modifying agent the modifying agent being an organic macromolecular compound, e.g. an oligomeric, polymeric or dendrimeric molecule
- A61K47/61—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient the non-active ingredient being chemically bound to the active ingredient, e.g. polymer-drug conjugates the non-active ingredient being a modifying agent the modifying agent being an organic macromolecular compound, e.g. an oligomeric, polymeric or dendrimeric molecule the organic macromolecular compound being a polysaccharide or a derivative thereof
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K47/00—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient
- A61K47/50—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient the non-active ingredient being chemically bound to the active ingredient, e.g. polymer-drug conjugates
- A61K47/51—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient the non-active ingredient being chemically bound to the active ingredient, e.g. polymer-drug conjugates the non-active ingredient being a modifying agent
- A61K47/62—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient the non-active ingredient being chemically bound to the active ingredient, e.g. polymer-drug conjugates the non-active ingredient being a modifying agent the modifying agent being a protein, peptide or polyamino acid
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K47/00—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient
- A61K47/50—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient the non-active ingredient being chemically bound to the active ingredient, e.g. polymer-drug conjugates
- A61K47/51—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient the non-active ingredient being chemically bound to the active ingredient, e.g. polymer-drug conjugates the non-active ingredient being a modifying agent
- A61K47/62—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient the non-active ingredient being chemically bound to the active ingredient, e.g. polymer-drug conjugates the non-active ingredient being a modifying agent the modifying agent being a protein, peptide or polyamino acid
- A61K47/64—Drug-peptide, drug-protein or drug-polyamino acid conjugates, i.e. the modifying agent being a peptide, protein or polyamino acid which is covalently bonded or complexed to a therapeutically active agent
- A61K47/643—Albumins, e.g. HSA, BSA, ovalbumin or a Keyhole Limpet Hemocyanin [KHL]
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K47/00—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient
- A61K47/50—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient the non-active ingredient being chemically bound to the active ingredient, e.g. polymer-drug conjugates
- A61K47/51—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient the non-active ingredient being chemically bound to the active ingredient, e.g. polymer-drug conjugates the non-active ingredient being a modifying agent
- A61K47/62—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient the non-active ingredient being chemically bound to the active ingredient, e.g. polymer-drug conjugates the non-active ingredient being a modifying agent the modifying agent being a protein, peptide or polyamino acid
- A61K47/65—Peptidic linkers, binders or spacers, e.g. peptidic enzyme-labile linkers
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P25/00—Drugs for disorders of the nervous system
- A61P25/28—Drugs for disorders of the nervous system for treating neurodegenerative disorders of the central nervous system, e.g. nootropic agents, cognition enhancers, drugs for treating Alzheimer's disease or other forms of dementia
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P3/00—Drugs for disorders of the metabolism
- A61P3/08—Drugs for disorders of the metabolism for glucose homeostasis
- A61P3/10—Drugs for disorders of the metabolism for glucose homeostasis for hyperglycaemia, e.g. antidiabetics
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P5/00—Drugs for disorders of the endocrine system
- A61P5/48—Drugs for disorders of the endocrine system of the pancreatic hormones
- A61P5/50—Drugs for disorders of the endocrine system of the pancreatic hormones for increasing or potentiating the activity of insulin
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K38/00—Medicinal preparations containing peptides
Definitions
- the present invention relates to a class of novel long-acting transient polymer conjugates of exendins and exendin agonists. These conjugates of exendins and exendin agonists following administration are capable of undergoing spontaneous chemical transformation in the living organism from an essentially inactive into a bioactive form.
- the invention also relates to polymer conjugates of exendins and exendin agonists bearing functional groups sensitive to neutral aqueous conditions, and to pharmaceutical compositions comprising them.
- the conjugates are particularly useful for prevention of hyperglycemia, treatment of diabetes mellitus, treatment of disorders which would be benefited with agents useful in delaying and/or slowing gastric emptying and treatment of obesity.
- Exendin-4 is a 39-amino acid peptide, isolated from the salivary secretions of the venomous GiIa monster (Heloderma suspectum). It has some sequence similarity to several members of the glucagon- like peptide family, with the highest homology of 53%, being to glucagon-like peptide-1 [7-36]-amide (GLP-I). Exendin-4 acts as a GLP-I agonist on the GLP-I receptor and bears GLP-I -like insulin sectretagogue action in isolated rat islets.
- Exendin-4 is a high potency agonist and truncated exendin-(9-39)-amide an antagonist at the glucagon-like peptide l-(7-36)-amide receptor of insulin-secreting beta-cells, (see e.g. J. Biol. Chem. 268(26): 19650-19655). Exendin-4 (“exenatide”) was approved recently in the US and EU for improving glycemic control in patients with type 2 diabetes taking metformin and/or a sulfonylurea but have not achieved adequate glycemic control.
- GLP-I is one of the intestinal peptide hormones that are released into the circulatory system after food intake. It augments the postprandial release of insulin, when nutritions (especially carbohydrates) are absorbed and their level postprandially elevated.
- GLP-I associates with GLP-I receptor sites located on pancreatic ⁇ -cells and elevates endogenous cAMP levels in a dose dependent manner. In isolated rat islets in the presence of above normoglycemic glucose levels, GLP-I stimulates the release of insulin.
- GLP-I A therapeutic potential for GLP-I in type 2 diabetes patients was suggested before, owing to the profound efficacy of this insulinotropic peptide to stimulate secretion of insulin when glucose levels are elevated and to cease doing so upon return to normoglycemia.
- the antidiabetogenic effect of glucagon-like peptide-1 (7-36) amide in normal subjects and patients with diabetes mellitus is described e. g. in N. Engl. J. Med. 326(20): 1316-1322.
- GLP-I improves insulin sensitivity and has an anabolic effect on pancreatic ⁇ -cells.
- GLP-I was also reported to suppress glucagon secretion, decelerate gastric emptying, and induce satiety, leading to weight loss if administered for weeks and months.
- Exendin-4 is reported to associate with GLP-I receptors located on pancreatic beta-cells with 2.5 times higher affinity than GLP-I. In isolated rat islets and beta-cells in presence of glucose, exendin enhances secretion of insulin in a dose-dependent fashion. Exendin-4 is a high potency agonist and truncated exendin-(9-39)-amide an antagonist at the glucagon- like peptide l-(7-36)-amide receptor of insulin-secreting beta-cells (see J. Biol. Chem. 268(26): 19650-19655). Studies in type 2 diabetic rodents revealed that exendin-4 is 5530- fold more potent than GLP-I in lowering blood glucose levels.
- exendin-4 reduces fasting and postprandial glucose and decreases energy intake in healthy volunteers (see e.g. Am. J. Physiol. Endocrinol. Metab. 281(1):E155-61).
- exenatide-4 therapy in diabetic patients recieving exendin- 4 and metformin include improved glycemic control as assessed by durable reductions in haemoglobin AIc (HbAIc) value, weight reduction and additional significant improvements of cardiovascular risk factors (see Ratner R. E., et al., (2006) , Diabetes Obes. Metab. 8(4):419-428).
- HbAIc haemoglobin AIc
- the usefulness of exendin-4 in type 1 diabetes therapy was shown (see e.g. Dupre J., et al., (2004), J. Clin. Endocrinol. Metab. 89(7):3469-3473).
- the peptide is formulated in a fashion that provides for a sustained plasma level in human for at least one week after application to a human body resulting in a once-weekly or longer injection frequency.
- FMS cleavage out of FMS3-exendin-4 leads to regeneration of the amino groups of exendins-4 with a half- life of 18 h after incubation in a human plasma model, with cleavage being complete at 40 h.
- FMS3-exendin-4 (10 ⁇ g/mouse subcutaneously) showed a sustained 50 % reduction in blood glucose, returning to baseline value after 45 h.
- FMS 3 -exendin-4 subject to rapid renal clearance due to its small size, may exert its prolonged effect due to precipitation or binding to plasma protein, e.g. albumin (see Shechter et al. (2003), Biochem. Biophys. Res. Commun. 305(2):386-391).
- the disadvantage of this approach is the presence of several different FMS-exendin conjugates in vivo as the FMS linkers are sequentially cleaved from the FMS 3 -exendin-4 parent molecule resulting in heterogeneous pharmacology.
- a further approach is the permanent covalent attachment of a polymeric carrier molecule to the exendin peptide.
- Conjugation to polymeric carrier like poly(ethylene glycol) (PEG) or human serum albumin greatly reduces renal elimination and shields from proteases and the immune system.
- PEG poly(ethylene glycol)
- human serum albumin greatly reduces renal elimination and shields from proteases and the immune system.
- WO 2007/053946Al discloses a permanent human serum albumin conjugate of Exendin-4.
- conjugation of a polymer to a peptide results in significant loss of receptor affinity and bioactivity.
- N-terminal PEGylation of GLP-I with only PEG2kDa nearly completely abolished its activity on stimulating insulin release from rat pancreas islets (see Lee S. H., et al. (2005) Bioconjug. Chem. 16(2):377-382).
- N-terminal PEGylation of Exendin-4 results in only approximately 1% residual activity compared to the native peptide (see Tsubery, et al. (2004) J. Biol. Chem. 279(37):38118-38124).
- the distribution of the conjugate in the body differs significantly from the native peptide, potentially resulting in different pharmacological actions.
- exendin-4 was formulated in biodegradable poly-lactide-glycolide based microspheres.
- Clinical data after 15 weeks of once weekly injection revealed improved glycemic control and reduced side effects compared to bidaily injections (Kim D. et al. (2007) ; Diabetes Care. 30(6): 1487-1493).
- the advantage of this approach is the release of the native exendin molecule with its full and unchanged pharmacology.
- Transient polymer conjugation combines the advantages of prolonged circulation times due to polymer attachment and the recovery of the original pharmacology of the native peptide after release from the polymer conjugate.
- release kinetics would be independent from the presence of enzymes like proteases or esterases in body fluids to guarantee a consistent and homogenous release pattern.
- Shechter et al. presented a system of transiently PEGylated Exendin-4 based on a traceless FMS-linker. Upon subcutaneous injection, PEG40kDa-FMS-exendin-4 maintained a glucose lowering effect of 30 % for 24 h in normoglycaemic mice (see Shechter Y. et al. (2004) , J. Biol. Chem. 279(37):38118-38124). However, Exendin-4 was cleaved from PEG40kDa-FMS-exendin-4 with a half-life of 12 h in a human plasma model. This half- life is too short to achieve a sustained plasma level over one week and a once-weekly injection regime. Linker molecules with longer half-lives have to be used to achieve this goal.
- Linker molecules suitable for transient polymer conjugation have been described by Complex Biosystems for example in WO 2006/136586 (aliphatic prodrug linkers) and WO 2005/099768 (cyclic prodrug linkers). These linkers provide for slower cleavage kinetics as compared to the above mentioned FMS-linker and are useful for once-weekly injection regime.
- the present invention is directed to a polymeric compound of the general formula (I) PoI-L-E (I) wherein Pol is a polymer,
- L is a releasing linker undergoing autohydrolysis and E is exendin or an exendin agonist.
- the bond between L and E is hydro lysed under in vivo conditions at a pH-value between 7.0 and 7.5 and a temperature of 36° to 38° C and in human plasma with a half- life of 24 hours or more.
- Pol is a polyalkyloxy-based polymer
- L is a releasing linker consisting of neighbouring groups catalyzing hydrolysis of a transient linkage
- E is exendin or an exendin agonist.
- the bond between L and E is hydrolysed under in vivo conditions at a pH-value between 7.0 and 7.5 and a temperature of 36°C to 38°C and in human plasma with a half-life between 24 hours and 100 days.
- the half- life is between 2 days and 80 days, more preferably between 4 days and 60 days, even more preferably between 7 days and 40 days and most preferably the half-life is between 28 days and 31 days.
- Preferred embodiments of these polymers of formula (I) are the structures of the following five formulae Ia, Ib, Ic, Id, and Ie. These polymeric compounds are hydrolysed under in vivo conditions at a pH-value between 7.0 and 7.5 and a temperature of 36°C to 38°C and in human plasma with a half- life of 24 hours or more, and they all can thereby release the active principle E.
- the invention in particular relates to a polymeric compound having the following structure (Ia) :
- E is exendin or an exendin agonist
- X is a spacer moiety R13-Y2
- Y 2 is O, S, NR14, succinimide, maleimide, unsaturated carbon-carbon bonds or any heteratom containing a free electron pair or is absent
- Rl 3 is selected from substituted or non-substituted linear, branched or cyclical alkyl or heteroalkyl, aryls, substituted aryls, substituted or non-substituted heteroaryls,
- Rl 4 is selected from hydrogen, substituted or non-substituted linear, branched or cyclical alkyl or heteroalkyl, aryls, substituted aryls, substituted or non-substituted heteroaryls;
- R2 and R3 are selected independently from hydrogen or acyl groups
- R4 to Rl 2 are selected independently from hydrogen, X-PoI, substituted or non- substituted linear, branched or cyclical alkyl or heteroalkyl, aryls, substituted aryls, substituted or non-substituted heteroaryls, cyano, nitro, halogen, carboxy, carboxamide, and
- Pol is a polymer.
- Preferred polymeric compound have structure (Ia), wherein E is exendin or an exendin agonist; X is a spacer moiety R13-Y 2 ,
- Y 2 is O, S, NRl 4, succinimide, unsaturated carbon-carbon bonds or is absent,
- Rl 3 is selected from non-substituted linear, branched or cyclical Cl to C12 alkyl or heteroalkyl, aryls, aryls, or non-substituted heteroaryls,
- Rl 4 is selected from hydrogen, non- substituted linear, branched or cyclical Cl to C8 alkyl or heteroalkyl, aryls, or non-substituted heteroaryls;
- R2 and R3 are selected independently from hydrogen or Cl to C6 acyl groups.
- R4 to R12 are selected independently from hydrogen, X-PoI, non-substituted linear, branched or cyclical Cl to C8 alkyl or heteroalkyl, aryls, non- substituted heteroaryls, cyano, nitro, halogen, carboxy, carboxamide; and Pol is poly (propylene glycol), poly(ethylene glycol), dextran, chitosan, hyaluronic acid, alginate, xylan, mannan, carrageenan, agarose, cellulose, starch, hydroxyethyl starch (HES), poly(vinyl alcohols), poly (oxazo lines), poly(anhydrides), poly(ortho esters), poly(carbonates), poly(urethanes), poly(acrylic acids), poly(acrylamides), HMPA), poly(acrylates), poly(methacrylates), poly(organophosphazenes), poly(siloxanes), poly(vinylpyrrolidone),
- E is exendin or an exendin agonist
- X is a spacer moiety such as Rl 3-Y 2 ,
- Y 2 is O, S, NR14, succinimide, maleimide, unsaturated carbon-carbon bonds or any heteratom containing a free electron pair or is absent,
- Rl 3 is selected from substituted or non-substituted linear, branched or cyclical alkyl or heteroalkyl, aryls, substituted aryls, substituted or non-substituted heteroaryls,
- Rl 4 is selected from hydrogen, substituted or non-substituted linear, branched or cyclical alkyl or heteroalkyl, aryls, substituted aryls, substituted or non-substituted heteroaryls;
- R2 and R3 are selected independently from hydrogen or acyl groups
- R4 to Rl 2 are selected independently from hydrogen, X-PoI, substituted or non- substituted linear, branched or cyclical alkyl or heteroalkyl, aryls, substituted aryls, substituted or non-substituted heteroaryls, cyano, nitro, halogen, carboxy, carboxamide,
- Pol is a polymer.
- E is exendin or an exendin agonist
- X is a spacer moiety R13-Y 2
- Y 2 is O, S, NRl 4, succinimide, unsaturated carbon-carbon bonds or is absent
- Rl 3 is selected from non-substituted linear, branched or cyclical Cl to C 12 alkyl or heteroalkyl, aryls, or non-substituted heteroaryls,
- Rl 4 is selected from hydrogen, substituted or non-substituted linear, branched or cyclical alkyl or heteroalkyl, aryls, substituted aryls, substituted or non-substituted heteroaryls;
- R2 and R3 are selected independently from hydrogen or C 1 to C6 acyl groups
- R4 to Rl 2 are selected independently from hydrogen, X-PoI, non-substituted linear, branched or cyclical Cl to C8 alkyl or heteroalkyl, aryls, non-substituted heteroaryls, cyano, nitro, halogen, carboxy, carboxamide,
- Pol is poly (propylene glycol), poly(ethylene glycol), dextran, chitosan, hyaluronic acid, alginate, xylan, mannan, carrageenan, agarose, cellulose, starch, hydroxyethyl starch (HES), poly(vinyl alcohols), poly (oxazo lines), poly(anhydrides), poly(ortho esters), poly(carbonates), poly(urethanes), poly(acrylic acids), poly(acrylamides), HMPA), poly(acrylates), poly(methacrylates), poly(organophosphazenes), poly(siloxanes), poly(vinylpyrrolidone), poly(cyanoacrylates), poly(esters), poly(iminocarbonates), poly(amino acids), collagen, gelatin, or albumin.
- the invention in particular relates to a polymeric compound having the following structure structure (Ic) :
- E is exendin or an exendin agonist
- X is a spacer moiety such as Rl 3-Y 2 ,
- Y 2 is O, S, NR14, succinimide, maleimide, unsaturated carbon-carbon bonds or any heteratom containing a free electron pair or is absent,
- Rl 3 is selected from substituted or non-substituted linear, branched or cyclical alkyl or heteroalkyl, aryls, substituted aryls, substituted or non-substituted heteroaryls,
- Rl 4 is selected from hydrogen, non-substituted linear, branched or cyclical Cl to C8 alkyl or heteroalkyl, aryls, or non-substituted heteroaryls;
- R2 and R3 are selected independently from hydrogen or acyl groups
- R4 to R12 are selected independently from hydrogen, X-PoI, substituted or non- substituted linear, branched or cyclical alkyl or heteroalkyl, aryls, substituted aryls, substituted or non-substituted heteroaryls, cyano, nitro, halogen, carboxy, carboxamide, and
- Pol is a polymer.
- E is exendin or an exendin agonist
- X is a spacer moiety R13-Y 2
- Y 2 is O, S, NRl 4, succinimide, unsaturated carbon-carbon or is absent
- Rl 3 is selected from non-substituted linear, branched or cyclical Cl to C 12 alkyl or heteroalkyl, aryls, or non-substituted heteroaryls,
- R14 is selected from hydrogen, non-substituted linear, branched or cyclical Cl to C8 alkyl or heteroalkyl, aryls, or non-substituted heteroaryls;
- R2 and R3 are selected independently from hydrogen or Cl to C6 acyl groups
- R4 to Rl 2 are selected independently from hydrogen, X-PoI, non-substituted linear, branched or cyclical Cl to C8 alkyl or heteroalkyl, aryls, non-substituted heteroaryls, cyano, nitro, halogen, carboxy, carboxamide;
- Pol is poly (propylene glycol), poly(ethylene glycol), dextran, chitosan, hyaluronic acid, alginate, xylan, mannan, carrageenan, agarose, cellulose, starch, hydroxyethyl starch (HES), poly(vinyl alcohols), poly (oxazo lines), poly(anhydrides), poly(ortho esters), poly(carbonates), poly(urethanes), poly(acrylic acids), poly(acrylamides), HMPA), poly(acrylates), poly(methacrylates), poly(organophosphazenes), poly(siloxanes), poly(vinylpyrrolidone), poly(cyanoacrylates), poly(esters), poly(iminocarbonates), poly(amino acids), collagen, gelatin, or albumin.
- the invention in particular relates to a polymeric compound having the following structure structure (Id):
- E is exendin or an exendin agonist
- X is a spacer moiety R13-Y2
- Yi is O, NRl 4, or is absent
- Y 2 is O, S, NRl 4, succinimide, unsaturated carbon-carbon bonds or is absent,
- Rl 5 is selected from hydrogen, substituted or non-substituted linear, branched or cyclical alkyl or heteroalkyl, aryls, substituted aryls, substituted or non-substituted heteroaryls, cyano, nitro, halogen, carboxy, carboxyalkyl, alkylcarbonyl, or carboxamidoalkyl;
- Rl is selected independently from hydrogen, substituted or non-substituted linear, branched or cyclical alkyl or heteroalkyl, aryl, substituted aryl, substituted or non- substituted heteroaryl, substituted or non-substituted linear, branched, or cyclical alkoxy, substituted or non-substituted linear, branched, or cyclical heteroalkyloxy, aryloxy, or heteroaryloxy, cyano, halogen;
- Rl 3 is selected from substituted or non-substituted linear, branched or cyclical alkyl or heteroalkyl, aryls, substituted aryls, substituted or non-substituted heteroaryls;
- Rl 4 is selected from hydrogen, substituted or non-substituted linear, branched or cyclical alkyl or heteroalkyl, aryls, substituted aryls, substituted or non-substituted heteroaryls;
- Pol is a polymer
- W is selected from substituted or non-substituted linear, branched or cyclical alkyl, aryls, substituted aryls, substituted or non-substituted linear, branched or cyclical heteroalkyl, substituted or nonsubstituted heteroaryls;
- Nu is a nucleophile
- n is zero or a positive integer (e.g. 1 to 10 or 1 to 5) and
- Ar is a multi-substituted aromatic hydrocarbon or a multi-substituted aromatic heterocycle.
- Polymeric compounds of structure (Id) are preferred wherein : E is exendin or an exendin agonist,
- X is a spacer moiety R13-Y 2 ,
- Yi is O, NRl 4, or is absent
- Y 2 is O, S, NRl 4, succinimide, unsaturated carbon-carbon bonds or is absent, Rl 5 is selected from hydrogen, non- substituted linear, branched or cyclical Cl to
- Rl is selected independently from hydrogen, non-substituted linear, branched or cyclical Cl to C6 alkyl or heteroalkyl, aryl, non-substituted heteroaryl, cyano, halogen;
- Rl 3 is selected from non- substituted linear, branched or cyclical alkyl or heteroalkyl, aryls, or non-substituted heteroaryls;
- Rl 4 is selected from hydrogen, non- substituted linear, branched or cyclical Cl to C8 alkyl or heteroalkyl, aryls, or non-substituted heteroaryls;
- Pol is poly (propylene glycol), poly(ethylene glycol), dextran, chitosan, hyaluronic acid, alginate, xylan, mannan, carrageenan, agarose, cellulose, starch, hydroxyethyl starch (HES), poly(vinyl alcohols), poly (oxazo lines), poly(anhydrides), poly(ortho esters), poly(carbonates), poly(urethanes), poly(acrylic acids), poly(acrylamides), HMPA), poly(acrylates), poly(methacrylates), poly(organophosphazenes), poly(siloxanes), polyvinylpyrrolidone), poly(cyanoacrylates), poly(esters), poly(iminocarbonates), poly(amino acids),
- W is selected from non-substituted linear, branched or cyclical Cl to C8 alkyl or heteroalkyl, aryls, or nonsubstituted heteroaryls;
- Nu is a primary, secondary or tertiary amino group; n is zero or a positive integer; and
- Ar is a multi-substituted C5 or C6 aromatic cycle or heterocycle.
- the invention in particular relates to a polymeric compound having the following structure structure (Ie) :
- E is exendin or an exendin agonist
- X is a spacer moiety R13-Y2
- Yi is O, NRl 4, or is absent
- Y 2 is O, S, NR14, succinimide unsaturated carbon-carbon bonds or any heteratom containing a free electron pair or is absent,
- Rl 5 and Rl 6 is selected independently from hydrogen, substituted or non- substituted linear, branched or cyclical alkyl or heteroalkyl, aryls, substituted aryls, substituted or non-substituted heteroaryls, cyano, nitro, halogen, carboxy, carboxyalkyl, alkylcarbonyl, or carboxamidoalkyl;
- Rl is selected independently from hydrogen, substituted or non-substituted linear, branched or cyclical alkyl or heteroalkyl, aryl, substituted aryl, substituted or non- substituted heteroaryl, substituted or non-substituted linear, branched, or cyclical alkoxy, substituted or non-substituted linear, branched, or cyclical heteroalkyloxy, aryloxy, or heteroaryloxy, cyano, halogen;
- Rl 3 is selected from substituted or non-substituted linear, branched or cyclical alkyl or heteroalkyl, aryls, substituted aryls, substituted or non-substituted heteroaryls;
- Rl 4 is selected from hydrogen, substituted or non-substituted linear, branched or cyclical alkyl or heteroalkyl, aryls, substituted aryls, substituted or non-substituted heteroaryls;
- Pol is a polymer
- W is selected from substituted or non-substituted linear, branched or cyclical alkyl, aryls, substituted aryls, substituted or non-substituted linear, branched or cyclical heteroalkyl, substituted or nonsubstituted heteroaryls;
- Nu is a nucleophile
- n is zero or a positive integer
- Ar is a multi-substituted aromatic hydrocarbon or a multi-substituted aromatic heterocycle.
- Preferred polymeric compounds of structure (Ie) are those, wherein:
- E is exendin or an exendin agonist
- X is a spacer moiety R13-Y 2 ,
- Yi is O, NRl 4, or is absent
- Y 2 is O, S, NRl 4, succinimide, unsaturated carbon-carbon bonds or is absent,
- Rl 5 is selected from hydrogen, non- substituted linear, branched or cyclical Cl to C8 alkyl or heteroalkyl, aryls,non-substituted heteroaryls, cyano, nitro, halogen, carboxy, carboxyalkyl, alkylcarbonyl, or carboxamidoalkyl;
- Rl is selected independently from hydrogen, non-substituted linear, branched or cyclical Cl to C6 alkyl or heteroalkyl, aryl, non-substituted heteroaryl, cyano, halogen;
- Rl 3 is selected from non- substituted linear, branched or cyclical alkyl or heteroalkyl, aryls, or non-substituted heteroaryls;
- Rl 4 is selected from hydrogen, non- substituted linear, branched or cyclical Cl to C8 alkyl or heteroalkyl, aryls, or non-substituted heteroaryls;
- Pol is poly (propylene glycol), poly(ethylene glycol), dextran, chitosan, hyaluronic acid, alginate, xylan, mannan, carrageenan, agarose, cellulose, starch, hydroxyethyl starch (HES), poly(vinyl alcohols), poly (oxazo lines), poly(anhydrides), poly(ortho esters), poly(carbonates), poly(urethanes), poly(acrylic acids), poly(acrylamides), HMPA), poly(acrylates), poly(methacrylates), poly(organophosphazenes), poly(siloxanes), polyvinylpyrrolidone), poly(cyanoacrylates), poly(esters), poly(iminocarbonates), poly(amino acids), collagen, gelatin, or albumin, W is selected from non-substituted linear, branched or cyclical Cl to C8 alkyl or heteroalkyl, aryls, or nonsubstituted
- Nu is a primary, secondary or tertiary amino group; n is zero or a positive integer; and Ar is a multi-substituted C5 or C6 aromatic cycle or heterocycle.
- substituted alkyl or heteroalkyl or substituted aryl or heteroaryl means substitution with one or more of any of the functional groups selected independently from hydroxyl, chloride, bromide, fluoride, carboxamide, carboxyl, amino, carbamate, urea, thiourea, thiocarbamate, oxime, cyano, carboxyl, or carbonyl.
- alkyl shall mean a monovalent straight chain or branched chain group of 1 or 2 or 3 or 4 or 5 or 6 or 7 or 8 or 9 or 10 or 11 or 12 carbon atoms including, but not limited to, methyl, ethyl, n-propyl, isopropyl, n-butyl, sec-butyl, tert-butyl and the like.
- cyclical alkyl shall mean a monovalent cyclic group of 3 or 4 or 5 or 6 or 7 carbon atoms including, but not limited to cyclopropyl, cyclopentyl, cyclohexyl and 4-methyl-cyclohexyl.
- aryl shall mean carbocyclic and heterocyclic aromatic groups including, but not limited to, phenyl, 1-naphthyl, 2-naphthyl, fluorenyl, (l,2)-dihydronaphthyl, indenyl, indanyl, thienyl, benzothienyl and thienopyridyl.
- heteroaryl shall mean heterocyclic aromatic groups including, but not limited to thienyl, furyl, benzothienyl and pyridyl.
- aralkyl (also called arylalkyl) shall mean an aryl group appended to an alkyl group including, but not limited to, benzyl, 1-naphthylmethyl, 2-naphthylmethyl, fluorobenzyl, chlorobenzyl, bromobenzyl, iodobenzyl, alkoxybenzyl (wherein “alkoxy” means methoxy, ethoxy, isopropoxy, n-butoxy, sec-butoxy, tert-butoxy an the like), hydroxybenzyl, aminobenzyl, nitrobenzyl, guanidinobenzyl, fluorenylmethyl, phenylmethyl(benzyl), 1-phenylethyl, 2-phenylethyl, 1-naphthylethyl.
- heteroalkyl in the context of the present invention denotes (linear, cyclical or branched) alkyl chains where the alkyl chains contain or are substituted with at any position one or more heteroatoms, selected independently from O, S, N, P, Si, Cl, F, Br, I, etc. or groups, selected independently from carboxamide, carboxylic ester, phosphonate ester, hydroxyl, phosphate ester, double or triple bonds, carbamate, urea, thiourea, thiocarbamate, oxime, cyano, carboxyl or carbonyl.
- heteroalkyl groups are: -S-(CH2)-(CH2)-CO-NH-(CH2)-(CH2)-(CH2)-(CH2)-(CH2)- -S-(CH2)-(CH2)-CO-NH-(CH2)-(CH2)-(CH2)- -S-(CH2)-(CH2)-CO-NH-(CH2)-(CH2)- -S-(CH2)-(CH2)-NH-CO-(CH2)-O-(CH2)-
- PoI is a polymer.
- suitable polymers are polyalkyloxy-based polymers like poly(propylene glycol) or poly(ethylene glycol), dextran, chitosan, hyaluronic acid and derivatives, alginate, xylan, mannan, carrageenan, agarose, cellulose, starch, hydroxyethyl starch (HES) and other carbohydrate-based polymers, poly(vinyl alcohols), poly(oxazolines), poly(anhydrides), poly(ortho esters), poly(carbonates), poly(urethanes), poly(acrylic acids), poly(acrylamides) such as poly(hydroxypropyl- methacrylamide) (HMPA), poly(acrylates), poly(methacrylates) like poly(hydroxyethyl- methacrylate), poly(organophosphazenes), poly(siloxanes), polyvinylpyrrolidone), poly(cyanoacrylates), poly(esters) such as poly((prop
- the invention also relates to a polymeric compound wherein Pol is selected from poly(propylene glycol), poly(ethylene glycol), starch, hydroxyethyl starch (HES) poly(vinyl alcohols), poly(oxazoline, spoly(acrylic acids), poly(acrylamides), poly(acrylates), poly(methacrylates), poly(organophosphazenes), poly(siloxanes), poly(vinylpyrrolidone), poly(cyanoacrylates), poly(esters), poly(glutamic acid), collagen, or gelatin.
- Pol is often selected from poly(propylene glycol) and poly(ethylene glycol).
- the invention also relates to a polymeric compound wherein Pol is a hydrogel.
- the invention also relates to a polymeric compound wherein Pol is a branched or hyperbranched polymer.
- the invention also relates to a polymeric compound wherein Pol is a biopolymer.
- the invention also relates to a polymeric compound wherein Pol is a protein, preferably an albumin.
- the invention also relates to a polymeric compound wherein Pol is a linear or branched poly(ethylene glycol) with a molecular weight between 2,000 Da and 150,000 Dalton. Pol is preferably a linear or branched poly( ethylene glycol) with a molecular weight between 20,000 Da and 80,000 Da.
- the invention also relates to a polymeric compound according, wherein E is an exendin, an exendin agonist, an exendin analogue, an exendin derivative, an truncated exendin, a truncated exendin agonist, a truncated exendin derivative, a truncated exendin analogue, GLP-I, a GLP-I analogue, or a GLP-I derivative.
- E is exendin or an exendin agonist of sequence ID 1 to ID 20, and more preferred E is exendin-3 having sequence ID 2 or exendin-4 having sequence ID 1.
- a further aspect of the invention is the use of a polymeric compound as described for the preparation of a medicament, particularly for the treatment of diabetes mellitus or for the prevention of hyperglycemia. Also a medicament for the treatment of obesity or eating disorders can be provided.
- the invention also relates to the use of a polymeric compound for the preparation of a medicament for the treatment of central nervous system disorders, in particular for the treatment of Alzheimer's desease.
- the invention also relates to a pharmaceutical compositon comprising at least one polymeric compound as described together with a pharmaceutically acceptable carrier which is useful in a medicine.
- a pharmaceutical compositon comprising at least one polymeric compound as described together with a pharmaceutically acceptable carrier which is useful in a medicine.
- These compositions are prepared by mixing the polymeric compound with the pharmaceutically acceptable carrier.
- the invention also covers a method for the preparation of a polymeric compound of the general formula PoI-L-E, by first attaching the linker L to the exendin or exendin agonist E and then coupling of the polymer Pol to the conjugate L-E.
- An alternative method for the preparation of a polymeric compound of the general formula PoI-L-E consists of attaching a conjugate PoI-L of the polymer and the linker to the exendin or exendin agonist E.
- Hydrogels according to this invention may be defined as three-dimensional, hydrophilic or amphiphilic polymeric networks imbibing large quantities of water.
- the networks are composed of homopolymers or copolymers, are insoluble due to the presence of covalent chemical or physical (ionic, hydrophobic interactions, entanglements) crosslinks.
- the crosslinks provide the network structure and physical integrity.
- Hydrogels exhibit a thermodynamic compatibility with water which allows them to swell in aqueous media (see. N.A. Peppas, P. Bures, W. Leobandung, H. Ichikawa, Hydrogels in pharmaceutical formulations, Eur. J. Pharm. Biopharm. 2000,50, 27-46, WO 2006/003014).
- the chains of the network are connected in such a fashion that pores exist and that a substantial fraction of these pores are of dimensions of between 1 and 1000 nm.
- the hydrogel may be obtained in the form of an amorphous gel or as beaded resin. Such soft beads may have a diameter of between 1 and 1000 micrometer.
- Hydrogels may be synthesized from the polymers and copolymers listed above and physically cross-linked or chemically cross-linked by radical, anionic or cationic polymerization, by chemical reactions like condensation or addition reactions as described in Hennink W.E. and van Nostrum CF. (2002), Adv. Drug Del. Rev., 54, 13-36.
- branched and hyperbranched polymers examples include dendrimers and other dense star polymers.
- dendrimers and other dense star polymers examples include dendrimers and other dense star polymers.
- Pol can also be a biopolymer like a protein.
- Non-limiting examples of such polymers include albumin, antibodies, fibrin, casein, transferrin and other plasma proteins.
- Each Pol polymer can carry one or more biologically active substances linked to the polymer by conjugation with a second prodrug linker as described herein or any other linker known to the person skilled in the art.
- the polymers may have further substituents and may be functionalized for attachment to the spacer moiety X.
- Non-limiting examples of such functional groups comprise carboxylic acid and activated derivatives, amino, maleimide, thiol, sulfonic acid and derivatives, carbonate and derivatives, carbamate and derivatives, hydroxyl, aldehyde, ketone, hydrazine , isocyanate, isothiocyanate, phosphoric acid and derivatives, phosphonic acid and derivatives, haloacetyl, alkyl halides, acryloyl, arylating agents like aryl fluorides, hydroxylamine, disulfides like pyridyl disulfide, vinyl sulfone, vinyl ketone, diazoalkanes, diazoacetyl compounds, epoxide, oxirane, and aziridine.
- Preferred functional groups for the Pol polymer include but are not limited to thiol, maleimide, amino, carboxylic acid and derivatives, carbonate and derivatives, carbamate and derivatives, aldehyde, and haloacetyl.
- Especially preferred functional groups include thiol, maleimide, amino, carboxylic acid and derivatives, carbamate and derivatives, and carbonate and derivatives thereof.
- Non-limiting examples for suitable bonds or groups formed between X and Pol include disulfide, S-succinimido, amide, amino, carboxylic ester, sulfonamide, carbamate, carbonate, ether, oxime, hydrazone, urea, thiourea, phosphate, phosphonate, etc.
- Preferred bonds or groups formed between X and Pol comprise S-succinimido, amide, carbamate, and urea.
- the Pol polymers are well hydrated, degradable or excretable, nontoxic and non- immunogenic in mammals.
- Preferred Pol polymers include polyalkoxy-based polymers like poly( ethylene glycol) and poly(ethylene glycol) reagents as those described in Nektar Inc. 2003 catalog "Nektar Molecule Engineering - Polyethylene Glycol and Derivatives for Advanced PEGylation” and branched, hyperbranched, cross-linked polymers and hydrogels, and proteins like albumin.
- Preferred substituents of the compounds according to the invention are:
- R2 and R3 are preferably hydrogen or acetyl.
- R4 to Rl 2 are preferably selected independently from hydrogen, substituted or non- substituted linear, branched or cyclical Ci to Cs alkyl or heteroalkyl;
- R4 to Rl 2 are most preferably hydrogen.
- Rl 5 and Rl 6 are selected independently from hydrogen, substituted or non-substituted linear, branched or cyclical alkyl or heteroalkyl, aryls, substituted aryls, substituted or non- substituted heteroaryls, cyano, nitro, halogen, carboxy, carboxyalkyl, alkylcarbonyl, carboxamidoalkyl, etc.
- Rl 5 and Rl 6 are most preferably hydrogen.
- Each Rl substitution on Ar may be the same or different and is selected independently from hydrogen, substituted or non-substituted linear, branched or cyclical alkyl or heteroalkyl, aryl, substituted aryl, substituted or non-substituted heteroaryl, substituted or non-substituted linear, branched, or cyclical alkoxy, substituted or non-substituted linear, branched, or cyclical heteroalkyloxy, aryloxy, heteroaryloxy, cyano, halogen.
- Rl is selected preferably from small substituents such as hydrogen, methyl, ethyl, ethoxy, methoxy, and other Cl to C6 linear, cyclical or branched alkyls and heteroalkyls.
- Rl is selected most preferably from methyl, ethyl, propyl, isopropyl, methoxy, ethoxy and hydrogen.
- n is zero or a positive integer.
- n is preferably zero, one or two.
- Rl 3 is e.g. selected from substituted or non-substituted linear, branched or cyclical alkyl or heteroalkyl, aryls, substituted aryls, substituted or non-substituted heteroaryls,
- R 13 is preferably selected from linear or branched alkyl or heteroalkyl.
- Rl 4 is selected from hydrogen, substituted or non-substituted linear, branched or cyclical alkyl or heteroalkyl, aryls, substituted aryls, substituted or non-substituted heteroaryls, Nu- W.
- Rl 4 is selected preferably from hydrogen, methyl, ethyl or Nu-W.
- W is selected from substituted or non-substituted linear, branched or cyclical alkyl, aryls, substituted aryls, substituted or non-substituted linear, branched or cyclical heteroalkyl, substituted or nonsubstituted heteroaryls.
- W is selected preferably from linear or branched alkyls or heteroalkyls. At least one Nu is present in Nu-W.
- Nu is a nucleophile that can perform a nucleophilic attack at the carbonyl carbon of
- Preferred nucleophiles include primary, secondary and tertiary amino groups, thiol, carboxylic acid, hydroxylamine, hydrazine, and nitrogen containing heteroaryl.
- Especially preferred nucleophiles include primary, secondary and tertiary amino groups.
- the spacing between the nucleophile Nu and Yi is preferably between one and thirteen atoms.
- the spacing between Nu and Yi is between two and eight atoms.
- the at least one nucleophile Nu may be attached anywhere to W (e.g. at the terminus or in the middle of W) or may be part of W.
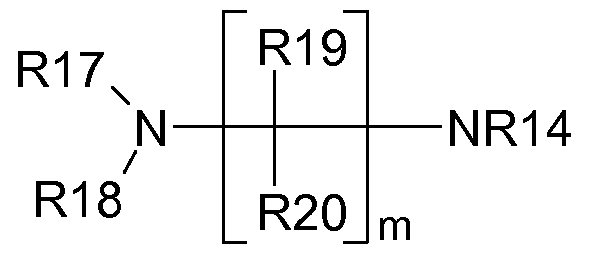
- Rl 7 to R20 are selected independently from hydrogen, non-substituted alkyl and Rl 7 and/or Rl 8 are not hydrogen.
- Rl 9 and R20 are hydrogen.
- Rl 7 and Rl 8 are methyl or ethyl.
- n a positive integer
- m is preferably 2 or 3 or 4
- Ar of formula Id or Ie is a multi-substituted aromatic hydrocarbon or a multi-substituted aromatic heterocycle.
- the number of pi electrons must satisfy the H ⁇ ckel rule (4n+2) and the cycle has to be planar.
- the aromatic moieties include:
- Z in the structures of Ar is O, N, or S, independent from each other.
- Preferred moieties for Ar are mono- and dicyclic aromatic hydrocarbons or aromatic heterocycles. Especially preferred moieties are monocyclic five- or six-membered aromatic hydrocarbons or aromatic heterocycles.
- Ar is a phenyl group.
- E is an exendin or exendin agonist.
- exendin agonists as used herein are exendin-3 or exendin-4 agonists including but not limited to: (i) exendin-4 analogues and amidated exendin-4 analogues, in which sequences one or more amino acid residues have been replaced by different amino acid residues including N-terminal modifications,
- a prodrug or a pharmaceutically acceptable salt thereof comprising a drug linker conjugate D-L, wherein
- -D is a nitrogen containing biologically active moiety
- -L is a non-bio logically active linker moiety -L 1 represented by formula (I),
- X is C(R 4 R 4a ); N(R 4 ); O; C(R 4 R 4a )-C(R 5 R 5a ); C(R 5 R 5a )-C(R 4 R 4a ); C(R 4 R 4a )- N(R 6 ); N(R 6 )-C(R 4 R 4a ); C(R 4 R 4a )-O; or O-C(R 4 R 4a );
- X I is C; or S(O);
- X 2 is C(R 7 , R 7a ); or C(R 7 , R 7a )-C(R 8 , R 8a );
- R 1 , R la , R 2 , R 2a , R 3 , R 3a , R 4 , R 4a , R 5 , R 5a , R 6 , R 7 , R 7a , R 8 , R 8a are independently selected from the group consisting of H; and Ci_ 4 alkyl; or
- R 7a /R 8a form & chemical bond
- one or more of the pairs RVR la , R 2 /R 2a , R 4 /R 4a , R 5 /R 5a , R 7 /R 7a , R 8 /R 8a are joined together with the atom to which they are attached to form a C3-7 cycloalkyl; or 4 to 7 membered heterocyclyl;
- one or more of the pairs RVR 4 , RVR 5 , RVR 6 , R 4 /R 5 , R 7 /R 8 , R 2 /R 3 are joined together with the atoms to which they are attached to form a ring A;
- R 3 /R 3a are joined together with the nitrogen atom to which they are attached to form a 4 to 7 membered heterocycle;
- A is selected from the group consisting of phenyl; naphthyl; indenyl; indanyl; tetralinyl; C3_io cycloalkyl; 4 to 7 membered heterocyclyl; and 9 to 11 membered heterobicyclyl; and
- L 1 is substituted with one to four groups L 2 -Z and optionally further substituted, provided that the hydrogen marked with the asterisk in formula (I) is not replaced by a substituent;
- L 2 is a single chemical bond or a spacer
- Z is a carrier group.
- Hydrogel - A suitable example of hydrogel linker is:
- a polymeric prodrug comprising a hydrogel, a biologically active moiety and a reversible prodrug linker, wherein
- the prodrug linker covalently links the hydrogel and the biologically active moiety at a position; and - the hydrogel has a plurality of pores with openings on the surface of the hydrogel, wherein the diameter of the pores is larger than the biologically active moiety at least at all points of the pore between at least one of the openings and the position of the biologically active moiety.
- Exendin agonists mimics the activities of exendin-3 or exendin-4 by binding the receptor(s) at which exendin-3 or exendin-4 exerts its actions which are beneficial as insulinotropic and in the treatment of diabetes mellitus or by mimicking the effects of exendin on urine flow, slowing gastric emptying, inducing satiety, increasing urinary sodium excetion and/or decreasing urinary potassium concentration, by binding to the receptor(s) where exendin cause these effects.
- exendin or exendin agonists with the Sequence ID NOs: 1-20 can be used to prepare the long acting polymeric conjugates of the invention:
- HXaaEGTFTSDV SSYLEGQAAK EFIAWLVKGR-NH 2 Xaa T, ⁇ -aminobutyric acid, D-AIa, V, GIy
- R acetyl, pyroglutamyl, N-2-hydroxybenzoyl, N-trans-3-hexenoyl
- exendin is exendin-4 having sequence ID 1.
- exendin and exendin agonists derivatives of the invention will exert any and all activities exhibited by the parent non-modified molecule, but with a prolonged action.
- the derivative is administered as a prodrug being essentially non-active biologically but being capable of spontaneous and slow conversion to the original active drug molecule in its bioactive form under physiological conditions in the body, following administration.
- the present invention relates to a pharmaceutical composition
- a pharmaceutical composition comprising an exendin or exendin agonist conjugate of the invention, and a pharmaceutically acceptable carrier.
- These compositions are in use for any of the uses known for exendin and exendin agonists, for example, for prevention of hyperglycemia and for treatment of diabetes mellitus of any type, e.g. insulin-dependent diabetes mellitus, non insulin dependent diabetes mellitus or gestational diabetes mellitus, for prevention of metabolic syndrome and/or obesity and/or eating disorders, insulin resistance syndrome, lowering plasma lipid level, reducing the cardiac risk, reducing the appetite, reducing the body weight, etc.
- compositions useful in the invention may be presented in any suitable route of administration to humans such as formulations for parenteral, including intravenous, intramuscular and subcutaneous, or for intranasal or oral administration.
- suitable pharmaceutically acceptable carriers and excipients can be added by conventional methods known to those skilled in the art, for example as described in Remington: The Science and Practice of Pharmacy, A.R. Gennaro, ed., 20th edition, 2000.
- the present invention relates to a method for prevention or treatment of a condition, disease or disorder that can be prevented or treated with an exendin or exendin agonist, which comprises administering to an individual in need an effective amount of an exendin or exendin agonist derivative of the invention.
- the present invention relates to a method for prevention of hyperglycemia which comprises administering to an individual in need an effective insulinotropic amount of exendin or exendin agonist derivative of the invention.
- the present invention provides a method for treatment of diabetes mellitus which comprises administering to an individual in need an effective amount of an exendin or exendin agonist derivative of the invention.
- the diabetes mellitus may be non- insulin dependent diabetes mellitus, insulin dependent diabetes mellitus, or gestational diabetes mellitus.
- the present invention provides a method for treatment or prevention of metabolic syndrome and/or obesity and/or eating disorders, insulin resistance syndrome, lowering plasma lipid level, reducing the cardiac risk, reducing the appetite, reducing the weight which comprises administering to an individual in need an effective amount of an exendin or exendin agonist conjugate of the invention.
- the exendin and exendin conjugates may be obtained as described for GLP-I conjugates in WO 2006/136586 and WO 2005/099768.
- the PoI-L-E preferably wherein the Pol is PEG
- the PoI-L-E has an exendin activity which is less than 5% of the native exendin (E) without the Pol, more preferably less than 3%, even more preferably less than 1% and most preferably virtually inactive.
- the activity of the transiently conjugated exendin compounds can be expressed by measuring the glucose lowering effect in db/db mice of their permanently conjugated compound and comparing the permanently conjugated compound's activity to that of native exendin measured as the glucose lowering effect in db/db mice as described in example 17, 18 and 19.
- Figure 1 shows the glucose lowering effect of exendin-4 and PEG40k-BCBl-exendin-4 in db/db mice.
- the plasma glucose level (mg/dl) is shown as a function of time (hours).
- Figure 2 shows the absence of glucose lowering effects of saline and PEG40k-exendin-4 in db/db mice.
- the plasma glucose level (mg/dl) is shown as a function of time (hours).
- Figure 3 shows the pharmacokinetics of transient PEG40k-BCBl -exendin-4 in rat.
- the total exendin-4 concentration ( ⁇ M) is shown as a function of time (hours).
- Figure 4 shows the pharmacokinetics of transient PEG40k-CB3 -exendin-4 in rat.
- the total plasma exendin-4 concentration ( ⁇ M) is shown as a function of time (hours).
- Figure 5 shows the pharmacokinetics of transient PEG40k-exendin-4 in rat.
- the PEG40k- exendin-4 concentration ( ⁇ M) is shown as a function of time (hours).
- Figure 6 shows the absence of protease digestion of permanent PEG40k-exendin-4 in rat (ratio fluorescence 538 nm/620 nm as a function of time (hours)).
- 4OkDa methoxy poly(ethylene glycol) maleimido-propionamide(PEG40K-maleimide) was obtained from Chirotech Technology Ltd, Cambridge, UK.
- 2-Chlorotrityl chloride resin and amino acids were from Merck Biosciences GmbH, Schwalbach/Ts, Germany, if not stated otherwise.
- Fmoc-D-homocysteine(Trt)-OH and S- Trityl-3-mercaptopropionic acid (Trt-MPA) were obtained from Bachem AG, Bubendorf, Switzerland.
- Bodipy-TR-X SE was purchased from Invitrogen GmbH, Düsseldorf, Germany. All other chemicals were from Sigma-ALDRICH Chemie GmbH, Taufkirchen, Germany.
- Electrospray ionization mass spectrometry was performed on a Waters ZQ 4000 ESI instrument and spectra were, if necessary, interpreted by Waters software MaxEnt.
- RP-HPLC was done on 100x20 or 100x40 C18 ReproSil-Pur 300 ODS-3 5 ⁇ colum (Dr. Maisch, Ammerbuch, Germany) connected to a Waters 600 HPLC System and Water2487 Absorbance detector. Linear gradients were used between solution A (0,1 % TFA in H 2 O) and solution B (0,1 % TFA in acetonitrile)
- Size exclusion chromatography was performed using an Amersham Bioscience AEKTAbasic system equipped with a Superdex200 10/300 column (Amersham Bioscience/GE Healthcare), if not stated otherwise.
- SEC Size exclusion chromatography
- an Amersham Bioscience AEKTAbasic system was equipped with an Source 15S filled HR 16/ 10 column (Amersham Bioscience/GE Healthcare)
- mice Genetically diabetic mice (db/db mice, strain B6.Cg-m +/+ Lepr db /J, weight 37 - 42 g) were obtained from Jackson Laboratories (Bar Harbour, Me., USA). Mice were kept 3 weeks to habituate to vivarium conditions (21 - 23 0 C, 45-55 % relative humidity, 12: 12 hours lightdark cycle with lights on at 7:00 a.m.). Plasma glucose levels were measured using a OneTouch Ultra glucometer (LifeScan Inc., Miliptas CA, USA).
- Linker building block 1 was synthesized as described in WO 2006/136586.
- Trt-MPA (698 mg, 2.0 mmol) was dissolved in 5 ml DCM and mixed with N- hydroxysuccinimide (276 mg, 2.4 mmol), collidine (1,3 ml, 10.0 mmol) and DCC (495 mg, 2.4 mmol). Mixture was stirred for 2 h at RT and a solution of Fmoc-D-Lys-OH • TFA (482 mg, 1.0 mmol), DMAP (41 mg, 0.33 mmol) and DIEA (350 ⁇ l, 2.0 mmol) in 1 ml DMF was added and stirred for further 20 min. The mixture was filtered and volatiles were removed in vacuo.
- Linker building block 4 was synthesized as described for 3 except for protecting hydroxyl groups as acetate.
- resin with hydroxyethyl compound was incubated overnight with a mixture of acetic acid (3 ml), pyridine (3 ml) and DMF (6 ml).
- Linker building block 5 was synthesized as described for 3, starting from Fmoc-L-Lys-OH.
- Linker building block 8 was synthesized as described for 3, starting from Fmoc-D-Orn-OH.
- Conjugate lib was purified by cation exchange chromatography and analyzed by SEC
- Compounds 12a and 12b were synthesized according to Example 2 using building block 2.
- Conjugate 22b was purified by cation exchange chromatography and analyzed by SEC
- Bodipy-NHS ester 50 ⁇ l 3 mM Bodipy-NHS ester (Molecular Probes) in DMSO were mixed with 10 mg cystamine dihydrochloride in 150 ⁇ l DMSO and 10 ⁇ l DIEA. The solution was incubated for 30 min and than 30 mg DTT were added. 3 ml 0.5 M sodium phosphate buffer pH 7 were added and the solution was incubated for 10 min.
- Bodipy-SH intermediate was dissolved in 0.5 ml 1/1 (v/v) water/acetonitrile and 50 mg PEG40-maleimide in 1.5 ml 1/1 (v/v) water/acetonitrile and 0.5 ml sodium phosphate buffer pH 7 were were added. The solution was incubated for 20 min at room temperature and than 2 ⁇ l mercaptoethanol were added. The product was purified by SEC. Yield 28 mg (700 nmol).
- Exendin-4 Release of Exendin-4 from conjugate l ib, 12b, 13c, 15, cl6c, 17c, 18c, 19c, and 20b in vitro. Release of Exendin-4 from conjugates l ib, 12b, 13c, 15, cl ⁇ c, 17c, 18c, 19c, and 20b was effected by hydrolysis in buffer (15 mM HEPES, 150 mM NaCl, 3 mM EDTA, 0.005% Tween 20) at pH 7.4 and 37°C. Samples were taken at time intervals and analyzed by RP- HPLC. Peaks correlating with the retention time of Exendin-4 were integrated and plotted against incubation time, and curve-fitting software was applied to estimate the corresponding half- life of release.
- buffer 15 mM HEPES, 150 mM NaCl, 3 mM EDTA, 0.005% Tween 20
- EIA signals reflect mainly PEG40k-linker-exendin-4 conjugate pharmakokinetics . All conjugates showed Tmax values of about 24 h and terminal half- lives of about 24 h.
Abstract
Description
Claims
Priority Applications (4)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| AU2008258548A AU2008258548B2 (en) | 2007-06-08 | 2008-06-05 | Long-acting transient polymer conjugates of exendin |
| US12/663,628 US9353170B2 (en) | 2007-06-08 | 2008-06-05 | Long-acting transient polymer conjugates of exendin |
| CA2689909A CA2689909C (en) | 2007-06-08 | 2008-06-05 | Long-acting polymeric prodrugs of exendin |
| EP08760559A EP2164519A2 (en) | 2007-06-08 | 2008-06-05 | Long-acting polymeric prodrugs of exendin |
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| EP07109895 | 2007-06-08 | ||
| EP07109895.8 | 2007-06-08 |
Publications (2)
| Publication Number | Publication Date |
|---|---|
| WO2008148839A2 true WO2008148839A2 (en) | 2008-12-11 |
| WO2008148839A3 WO2008148839A3 (en) | 2009-08-13 |
Family
ID=38654779
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| PCT/EP2008/056981 WO2008148839A2 (en) | 2007-06-08 | 2008-06-05 | Long-acting polymeric prodrugs of exendin |
Country Status (5)
| Country | Link |
|---|---|
| US (1) | US9353170B2 (en) |
| EP (1) | EP2164519A2 (en) |
| AU (1) | AU2008258548B2 (en) |
| CA (1) | CA2689909C (en) |
| WO (1) | WO2008148839A2 (en) |
Cited By (36)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2011012718A1 (en) * | 2009-07-31 | 2011-02-03 | Ascendis Pharma As | Prodrugs comprising an insulin linker conjugate |
| WO2011012719A1 (en) * | 2009-07-31 | 2011-02-03 | Ascendis Pharma As | Long acting insulin composition |
| WO2011107494A1 (en) | 2010-03-03 | 2011-09-09 | Sanofi | Novel aromatic glycoside derivatives, medicaments containing said compounds, and the use thereof |
| DE102010015123A1 (en) | 2010-04-16 | 2011-10-20 | Sanofi-Aventis Deutschland Gmbh | New benzylamidic diphenylazetidinone compounds, useful for treating lipid disorders, hyperlipidemia, atherosclerotic manifestations or insulin resistance, and for reducing serum cholesterol levels |
| WO2011161030A1 (en) | 2010-06-21 | 2011-12-29 | Sanofi | Heterocyclic substituted methoxyphenyl derivatives having an oxo group, method for producing same, and use thereof as gpr40 receptor modulators |
| WO2012004270A1 (en) | 2010-07-05 | 2012-01-12 | Sanofi | Spirocyclically substituted 1,3-propane dioxide derivatives, methods for the production thereof and use of the same as medicament |
| WO2012004269A1 (en) | 2010-07-05 | 2012-01-12 | Sanofi | (2-aryloxy-acetylamino)-phenyl-propionic acid derivatives, method for producing same and use thereof as pharmaceuticals |
| WO2012010413A1 (en) | 2010-07-05 | 2012-01-26 | Sanofi | Aryloxy-alkylene substituted hydroxyphenyl hexynoic acids, methods for the production thereof and use of the same as medicament |
| WO2012035139A1 (en) * | 2010-09-17 | 2012-03-22 | Sanofi-Aventis Deutschland Gmbh | Prodrugs comprising an exendin linker conjugate |
| EP2567959A1 (en) | 2011-09-12 | 2013-03-13 | Sanofi | 6-(4-Hydroxy-phenyl)-3-styryl-1H-pyrazolo[3,4-b]pyridine-4-carboxylic acid amide derivatives as kinase inhibitors |
| WO2013037390A1 (en) | 2011-09-12 | 2013-03-21 | Sanofi | 6-(4-hydroxy-phenyl)-3-styryl-1h-pyrazolo[3,4-b]pyridine-4-carboxylic acid amide derivatives as kinase inhibitors |
| WO2013045413A1 (en) | 2011-09-27 | 2013-04-04 | Sanofi | 6-(4-hydroxy-phenyl)-3-alkyl-1h-pyrazolo[3,4-b]pyridine-4-carboxylic acid amide derivatives as kinase inhibitors |
| WO2014064215A1 (en) | 2012-10-24 | 2014-05-01 | INSERM (Institut National de la Santé et de la Recherche Médicale) | TPL2 KINASE INHIBITORS FOR PREVENTING OR TREATING DIABETES AND FOR PROMOTING β-CELL SURVIVAL |
| RU2574667C2 (en) * | 2009-07-31 | 2016-02-10 | Санофи-Авентис Дойчланд Гмбх | Prodrugs containing insulin linker conjugate |
| US9353170B2 (en) | 2007-06-08 | 2016-05-31 | Sanofi-Aventis Deutschland Gmbh | Long-acting transient polymer conjugates of exendin |
| WO2016151018A1 (en) | 2015-03-24 | 2016-09-29 | INSERM (Institut National de la Santé et de la Recherche Médicale) | Method and pharmaceutical composition for use in the treatment of diabetes |
| WO2016193371A1 (en) | 2015-06-05 | 2016-12-08 | Sanofi | Prodrugs comprising an glp-1/glucagon dual agonist linker hyaluronic acid conjugate |
| US9670261B2 (en) | 2012-12-21 | 2017-06-06 | Sanofi | Functionalized exendin-4 derivatives |
| US9694053B2 (en) | 2013-12-13 | 2017-07-04 | Sanofi | Dual GLP-1/glucagon receptor agonists |
| US9750788B2 (en) | 2013-12-13 | 2017-09-05 | Sanofi | Non-acylated exendin-4 peptide analogues |
| US9751926B2 (en) | 2013-12-13 | 2017-09-05 | Sanofi | Dual GLP-1/GIP receptor agonists |
| US9758561B2 (en) | 2014-04-07 | 2017-09-12 | Sanofi | Dual GLP-1/glucagon receptor agonists derived from exendin-4 |
| US9771406B2 (en) | 2014-04-07 | 2017-09-26 | Sanofi | Peptidic dual GLP-1/glucagon receptor agonists derived from exendin-4 |
| US9775904B2 (en) | 2014-04-07 | 2017-10-03 | Sanofi | Exendin-4 derivatives as peptidic dual GLP-1/glucagon receptor agonists |
| US9789165B2 (en) | 2013-12-13 | 2017-10-17 | Sanofi | Exendin-4 peptide analogues as dual GLP-1/GIP receptor agonists |
| US9932381B2 (en) | 2014-06-18 | 2018-04-03 | Sanofi | Exendin-4 derivatives as selective glucagon receptor agonists |
| US9982029B2 (en) | 2015-07-10 | 2018-05-29 | Sanofi | Exendin-4 derivatives as selective peptidic dual GLP-1/glucagon receptor agonists |
| WO2018100174A1 (en) | 2016-12-02 | 2018-06-07 | Sanofi | Conjugates comprising an glp-1/glucagon dual agonist, a linker and hyaluronic acid |
| KR20180096733A (en) * | 2015-12-23 | 2018-08-29 | 더 존스 홉킨스 유니버시티 | A long-acting GLF-1R agonist as a treatment for the nervous system and neurodegenerative conditions |
| WO2019229225A1 (en) | 2018-05-30 | 2019-12-05 | Sanofi | Conjugates comprising an glp-1/glucagon/gip triple receptor agonist, a linker and hyaluronic acid |
| US10751417B2 (en) | 2017-04-20 | 2020-08-25 | Novartis Ag | Sustained release delivery systems comprising traceless linkers |
| US10758592B2 (en) | 2012-10-09 | 2020-09-01 | Sanofi | Exendin-4 derivatives as dual GLP1/glucagon agonists |
| US11185570B2 (en) | 2017-02-08 | 2021-11-30 | Bristol-Myers Squibb Company | Method of treating cardiovascular disease and heart failure with modified relaxin polypeptides |
| US11311605B2 (en) | 2010-08-17 | 2022-04-26 | Ambrx, Inc. | Methods of treating heart failure and fibrotic disorders using modified relaxin polypeptides |
| US11389541B2 (en) | 2018-10-03 | 2022-07-19 | Novartis Ag | Sustained delivery of angiopoetin-like 3 polypeptides |
| US11439710B2 (en) | 2010-08-17 | 2022-09-13 | Ambrx, Inc. | Nucleic acids encoding modified relaxin polypeptides |
Families Citing this family (7)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US8758780B2 (en) | 2009-10-06 | 2014-06-24 | Ascendis Pharma As | Subcutaneous paliperidone composition |
| JP5893566B2 (en) | 2009-10-29 | 2016-03-23 | アセンディス ファーマ エー/エス | Sterilization of biodegradable hydrogels |
| SG181648A1 (en) | 2009-12-15 | 2012-07-30 | Ascendis Pharma As | Dry growth hormone composition transiently linked to a polymer carrier |
| EP2525831B1 (en) | 2010-01-22 | 2019-05-15 | Ascendis Pharma A/S | Carrier-linked carbamate prodrug linkers |
| DK2525830T3 (en) | 2010-01-22 | 2016-08-15 | Ascendis Pharma As | DIPEPTID-BASED PRODRUG LINKERS TO ALIFATIC AMINE-CONTAINING MEDICINES |
| WO2011089215A1 (en) | 2010-01-22 | 2011-07-28 | Ascendis Pharma As | Dipeptide-based prodrug linkers for aromatic amine-containing drugs |
| US20140256626A1 (en) * | 2011-10-18 | 2014-09-11 | Prolynx Llc | Peg conjugates of exenatide |
Citations (3)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2005099768A2 (en) * | 2004-03-23 | 2005-10-27 | Complex Biosystems Gmbh | Polymeric prodrug with a self-immolative linker |
| WO2006136586A2 (en) * | 2005-06-22 | 2006-12-28 | Complex Biosystems Gmbh | N, n-bis- (2-hydroxyethyl) glycine amide as linker in polymer conjugated prodrugs |
| WO2008116913A2 (en) * | 2007-03-28 | 2008-10-02 | Novo Nordisk A/S | Peptide compounds with transient biodegradable pegylation |
Family Cites Families (17)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US5424286A (en) | 1993-05-24 | 1995-06-13 | Eng; John | Exendin-3 and exendin-4 polypeptides, and pharmaceutical compositions comprising same |
| US7157555B1 (en) | 1997-08-08 | 2007-01-02 | Amylin Pharmaceuticals, Inc. | Exendin agonist compounds |
| ES2294822T3 (en) | 1997-11-14 | 2008-04-01 | Amylin Pharmaceuticals, Inc. | NEW COMPOUNDS OF EXENDINE AGONISTS. |
| BR9814189A (en) | 1997-11-14 | 2000-10-03 | Amylin Pharmaceuticals Inc | "exendin agonist compounds" |
| EP1054594B1 (en) | 1998-02-13 | 2007-07-04 | Amylin Pharmaceuticals, Inc. | Inotropic and diuretic effects of exendin and glp-1 |
| EP1175443A1 (en) | 1999-04-30 | 2002-01-30 | Amylin Pharmaceuticals, Inc. | Modified exendins and exendin agonists |
| US6506724B1 (en) | 1999-06-01 | 2003-01-14 | Amylin Pharmaceuticals, Inc. | Use of exendins and agonists thereof for the treatment of gestational diabetes mellitus |
| DK1651615T3 (en) * | 2003-07-29 | 2010-05-25 | High Point Pharmaceuticals Llc | Pyridazinyl-piperazines and their use as histamine H3 receptor ligands |
| EP1525890A1 (en) | 2003-10-02 | 2005-04-27 | Complex Biosystems GmbH | Protein-Proteophore complexes |
| EP1586334A1 (en) | 2004-04-15 | 2005-10-19 | TRASTEC scpa | G-CSF conjugates with peg |
| US7968085B2 (en) | 2004-07-05 | 2011-06-28 | Ascendis Pharma A/S | Hydrogel formulations |
| EP2505207B1 (en) * | 2005-01-14 | 2015-04-22 | Wuxi Grandchamp Pharmaceutical Technology Co., Ltd. | Modified exendins and uses thereof |
| AU2006215566A1 (en) * | 2005-02-16 | 2006-08-24 | Novo Nordisk A/S | Insulinotropic agents conjugated with structurally well defined branched polymers |
| DK1881850T3 (en) * | 2005-05-13 | 2011-01-03 | Lilly Co Eli | GLP-1-PEGylated Compounds |
| US8039432B2 (en) | 2005-11-09 | 2011-10-18 | Conjuchem, Llc | Method of treatment of diabetes and/or obesity with reduced nausea side effect |
| EP2364735A3 (en) | 2005-12-16 | 2012-04-11 | Nektar Therapeutics | Branched PEG conjugates of GLP-1 |
| AU2008258548B2 (en) | 2007-06-08 | 2014-07-10 | Sanofi-Aventis Deutschland Gmbh | Long-acting transient polymer conjugates of exendin |
-
2008
- 2008-06-05 AU AU2008258548A patent/AU2008258548B2/en not_active Ceased
- 2008-06-05 WO PCT/EP2008/056981 patent/WO2008148839A2/en active Application Filing
- 2008-06-05 CA CA2689909A patent/CA2689909C/en not_active Expired - Fee Related
- 2008-06-05 US US12/663,628 patent/US9353170B2/en active Active
- 2008-06-05 EP EP08760559A patent/EP2164519A2/en not_active Withdrawn
Patent Citations (3)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2005099768A2 (en) * | 2004-03-23 | 2005-10-27 | Complex Biosystems Gmbh | Polymeric prodrug with a self-immolative linker |
| WO2006136586A2 (en) * | 2005-06-22 | 2006-12-28 | Complex Biosystems Gmbh | N, n-bis- (2-hydroxyethyl) glycine amide as linker in polymer conjugated prodrugs |
| WO2008116913A2 (en) * | 2007-03-28 | 2008-10-02 | Novo Nordisk A/S | Peptide compounds with transient biodegradable pegylation |
Non-Patent Citations (5)
| Title |
|---|
| GREIG NIGEL H ET AL: "New therapeutic strategies and drug candidates for neurodegenerative diseases: p53 and TNF-alpha inhibitors, and GLP-1 receptor agonists." ANNALS OF THE NEW YORK ACADEMY OF SCIENCES DEC 2004, vol. 1035, December 2004 (2004-12), pages 290-315, XP002458396 ISSN: 0077-8923 * |
| LINNEBJERG H ET AL: "Exenatide: Effect of injection time on postprandial glucose in patients with Type 2 diabetes" DIABETIC MEDICINE 2006 UNITED KINGDOM, vol. 23, no. 3, 2006, pages 240-245, XP002458395 ISSN: 0742-3071 1464-5491 cited in the application * |
| RATNER R E ET AL: "Long-term effects of exenatide therapy over 82 weeks on glycaemic control and weight in over-weight metformin-treated patients with type 2 diabetes mellitus." DIABETES, OBESITY & METABOLISM JUL 2006, vol. 8, no. 4, July 2006 (2006-07), pages 419-428, XP002458394 ISSN: 1462-8902 cited in the application * |
| See also references of EP2164519A2 * |
| TSUBERY H ET AL: "PROLONGING THE ACTION OF PROTEIN AND PEPTIDE DRUGS BY A NOVEL APPROACH OF REVERSIBLE POLYETHYLENE GLYCOL MODIFICATION" JOURNAL OF BIOLOGICAL CHEMISTRY, AMERICAN SOCIETY OF BIOLOCHEMICAL BIOLOGISTS, BIRMINGHAM,, US, vol. 279, no. 37, 10 September 2004 (2004-09-10), pages 38118-38124, XP008040599 ISSN: 0021-9258 cited in the application * |
Cited By (59)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US9353170B2 (en) | 2007-06-08 | 2016-05-31 | Sanofi-Aventis Deutschland Gmbh | Long-acting transient polymer conjugates of exendin |
| US9457066B2 (en) | 2009-07-31 | 2016-10-04 | Sanofi-Aventis Deutschland Gmbh | Prodrugs comprising an insulin linker conjugate |
| AU2010277559B2 (en) * | 2009-07-31 | 2016-08-11 | Sanofi-Aventis Deutschland Gmbh | Prodrugs comprising an insulin linker conjugate |
| US9138462B2 (en) | 2009-07-31 | 2015-09-22 | Sanofi-Aventis Deutschland Gmbh | Prodrugs comprising an insulin linker conjugate |
| RU2574667C2 (en) * | 2009-07-31 | 2016-02-10 | Санофи-Авентис Дойчланд Гмбх | Prodrugs containing insulin linker conjugate |
| KR101759499B1 (en) | 2009-07-31 | 2017-07-19 | 사노피-아벤티스 도이칠란트 게엠베하 | Long acting insulin composition |
| CN102548583B (en) * | 2009-07-31 | 2015-04-22 | 赛诺菲-安万特德国有限公司 | Prodrugs comprising an insulin linker conjugate |
| WO2011012718A1 (en) * | 2009-07-31 | 2011-02-03 | Ascendis Pharma As | Prodrugs comprising an insulin linker conjugate |
| US9265723B2 (en) | 2009-07-31 | 2016-02-23 | Sanofi-Aventis Deutschland Gmbh | Long acting insulin composition |
| WO2011012719A1 (en) * | 2009-07-31 | 2011-02-03 | Ascendis Pharma As | Long acting insulin composition |
| CN102548583A (en) * | 2009-07-31 | 2012-07-04 | 赛诺菲-安万特德国有限公司 | Prodrugs comprising an insulin linker conjugate |
| JP2013500951A (en) * | 2009-07-31 | 2013-01-10 | サノフィ−アベンティス・ドイチュラント・ゲゼルシャフト・ミット・ベシュレンクテル・ハフツング | Prodrugs containing insulin linker conjugates |
| WO2011107494A1 (en) | 2010-03-03 | 2011-09-09 | Sanofi | Novel aromatic glycoside derivatives, medicaments containing said compounds, and the use thereof |
| DE102010015123A1 (en) | 2010-04-16 | 2011-10-20 | Sanofi-Aventis Deutschland Gmbh | New benzylamidic diphenylazetidinone compounds, useful for treating lipid disorders, hyperlipidemia, atherosclerotic manifestations or insulin resistance, and for reducing serum cholesterol levels |
| WO2011161030A1 (en) | 2010-06-21 | 2011-12-29 | Sanofi | Heterocyclic substituted methoxyphenyl derivatives having an oxo group, method for producing same, and use thereof as gpr40 receptor modulators |
| WO2012010413A1 (en) | 2010-07-05 | 2012-01-26 | Sanofi | Aryloxy-alkylene substituted hydroxyphenyl hexynoic acids, methods for the production thereof and use of the same as medicament |
| WO2012004270A1 (en) | 2010-07-05 | 2012-01-12 | Sanofi | Spirocyclically substituted 1,3-propane dioxide derivatives, methods for the production thereof and use of the same as medicament |
| WO2012004269A1 (en) | 2010-07-05 | 2012-01-12 | Sanofi | (2-aryloxy-acetylamino)-phenyl-propionic acid derivatives, method for producing same and use thereof as pharmaceuticals |
| US11786578B2 (en) | 2010-08-17 | 2023-10-17 | Ambrx, Inc. | Modified relaxin polypeptides and their uses |
| US11311605B2 (en) | 2010-08-17 | 2022-04-26 | Ambrx, Inc. | Methods of treating heart failure and fibrotic disorders using modified relaxin polypeptides |
| US11439710B2 (en) | 2010-08-17 | 2022-09-13 | Ambrx, Inc. | Nucleic acids encoding modified relaxin polypeptides |
| WO2012035139A1 (en) * | 2010-09-17 | 2012-03-22 | Sanofi-Aventis Deutschland Gmbh | Prodrugs comprising an exendin linker conjugate |
| EP2438930A1 (en) * | 2010-09-17 | 2012-04-11 | Sanofi-Aventis Deutschland GmbH | Prodrugs comprising an exendin linker conjugate |
| US9133276B2 (en) | 2010-09-17 | 2015-09-15 | Sanofi-Aventis Deutschland Gmbh | Prodrugs comprising an exendin linker conjugate |
| EP2567959A1 (en) | 2011-09-12 | 2013-03-13 | Sanofi | 6-(4-Hydroxy-phenyl)-3-styryl-1H-pyrazolo[3,4-b]pyridine-4-carboxylic acid amide derivatives as kinase inhibitors |
| WO2013037390A1 (en) | 2011-09-12 | 2013-03-21 | Sanofi | 6-(4-hydroxy-phenyl)-3-styryl-1h-pyrazolo[3,4-b]pyridine-4-carboxylic acid amide derivatives as kinase inhibitors |
| WO2013045413A1 (en) | 2011-09-27 | 2013-04-04 | Sanofi | 6-(4-hydroxy-phenyl)-3-alkyl-1h-pyrazolo[3,4-b]pyridine-4-carboxylic acid amide derivatives as kinase inhibitors |
| US10758592B2 (en) | 2012-10-09 | 2020-09-01 | Sanofi | Exendin-4 derivatives as dual GLP1/glucagon agonists |
| WO2014064215A1 (en) | 2012-10-24 | 2014-05-01 | INSERM (Institut National de la Santé et de la Recherche Médicale) | TPL2 KINASE INHIBITORS FOR PREVENTING OR TREATING DIABETES AND FOR PROMOTING β-CELL SURVIVAL |
| US9745360B2 (en) | 2012-12-21 | 2017-08-29 | Sanofi | Dual GLP1/GIP or trigonal GLP1/GIP/glucagon agonists |
| US9670261B2 (en) | 2012-12-21 | 2017-06-06 | Sanofi | Functionalized exendin-4 derivatives |
| US10253079B2 (en) | 2012-12-21 | 2019-04-09 | Sanofi | Functionalized Exendin-4 derivatives |
| US9751926B2 (en) | 2013-12-13 | 2017-09-05 | Sanofi | Dual GLP-1/GIP receptor agonists |
| US9789165B2 (en) | 2013-12-13 | 2017-10-17 | Sanofi | Exendin-4 peptide analogues as dual GLP-1/GIP receptor agonists |
| US9750788B2 (en) | 2013-12-13 | 2017-09-05 | Sanofi | Non-acylated exendin-4 peptide analogues |
| US9694053B2 (en) | 2013-12-13 | 2017-07-04 | Sanofi | Dual GLP-1/glucagon receptor agonists |
| US9758561B2 (en) | 2014-04-07 | 2017-09-12 | Sanofi | Dual GLP-1/glucagon receptor agonists derived from exendin-4 |
| US9771406B2 (en) | 2014-04-07 | 2017-09-26 | Sanofi | Peptidic dual GLP-1/glucagon receptor agonists derived from exendin-4 |
| US9775904B2 (en) | 2014-04-07 | 2017-10-03 | Sanofi | Exendin-4 derivatives as peptidic dual GLP-1/glucagon receptor agonists |
| US9932381B2 (en) | 2014-06-18 | 2018-04-03 | Sanofi | Exendin-4 derivatives as selective glucagon receptor agonists |
| WO2016151018A1 (en) | 2015-03-24 | 2016-09-29 | INSERM (Institut National de la Santé et de la Recherche Médicale) | Method and pharmaceutical composition for use in the treatment of diabetes |
| WO2016193371A1 (en) | 2015-06-05 | 2016-12-08 | Sanofi | Prodrugs comprising an glp-1/glucagon dual agonist linker hyaluronic acid conjugate |
| US10806797B2 (en) | 2015-06-05 | 2020-10-20 | Sanofi | Prodrugs comprising an GLP-1/glucagon dual agonist linker hyaluronic acid conjugate |
| US9982029B2 (en) | 2015-07-10 | 2018-05-29 | Sanofi | Exendin-4 derivatives as selective peptidic dual GLP-1/glucagon receptor agonists |
| AU2016379403B2 (en) * | 2015-12-23 | 2020-03-12 | The Johns Hopkins University | Long-acting GLP-1r agonist as a therapy of neurological and neurodegenerative conditions |
| CN108697768B (en) * | 2015-12-23 | 2022-07-22 | 约翰霍普金斯大学 | Long-acting GLP-1R agonists as methods of treatment for neurological and neurodegenerative conditions |
| EP3393496A4 (en) * | 2015-12-23 | 2019-07-31 | The Johns Hopkins University | Long-acting glp-1r agonist as a therapy of neurological and neurodegenerative conditions |
| US11123405B2 (en) | 2015-12-23 | 2021-09-21 | The Johns Hopkins University | Long-acting GLP-1R agonist as a therapy of neurological and neurodegenerative conditions |
| KR102508651B1 (en) * | 2015-12-23 | 2023-03-13 | 더 존스 홉킨스 유니버시티 | Long-acting GLF-1R agonists as therapy of the nervous system and neurodegenerative conditions |
| CN108697768A (en) * | 2015-12-23 | 2018-10-23 | 约翰霍普金斯大学 | Therapy of the long-acting GLP-1 R agonists as nervous system symptom and nervus retrogression symptom |
| KR20180096733A (en) * | 2015-12-23 | 2018-08-29 | 더 존스 홉킨스 유니버시티 | A long-acting GLF-1R agonist as a treatment for the nervous system and neurodegenerative conditions |
| WO2018100174A1 (en) | 2016-12-02 | 2018-06-07 | Sanofi | Conjugates comprising an glp-1/glucagon dual agonist, a linker and hyaluronic acid |
| US10792367B2 (en) | 2016-12-02 | 2020-10-06 | Sanofi | Conjugates comprising an GLP-1/glucagon dual agonist, a linker and hyaluronic acid |
| US11141489B2 (en) | 2016-12-02 | 2021-10-12 | Sanofi | Conjugates comprising an GLP-1/Glucagon dual agonist, a linker and hyaluronic acid |
| US11185570B2 (en) | 2017-02-08 | 2021-11-30 | Bristol-Myers Squibb Company | Method of treating cardiovascular disease and heart failure with modified relaxin polypeptides |
| US11364281B2 (en) | 2017-02-08 | 2022-06-21 | Bristol-Myers Squibb Company | Modified relaxin polypeptides comprising a pharmacokinetic enhancer and pharmaceutical compositions thereof |
| US10751417B2 (en) | 2017-04-20 | 2020-08-25 | Novartis Ag | Sustained release delivery systems comprising traceless linkers |
| WO2019229225A1 (en) | 2018-05-30 | 2019-12-05 | Sanofi | Conjugates comprising an glp-1/glucagon/gip triple receptor agonist, a linker and hyaluronic acid |
| US11389541B2 (en) | 2018-10-03 | 2022-07-19 | Novartis Ag | Sustained delivery of angiopoetin-like 3 polypeptides |
Also Published As
| Publication number | Publication date |
|---|---|
| AU2008258548B2 (en) | 2014-07-10 |
| CA2689909A1 (en) | 2008-12-11 |
| AU2008258548A1 (en) | 2008-12-11 |
| CA2689909C (en) | 2016-04-05 |
| WO2008148839A3 (en) | 2009-08-13 |
| US20110009315A1 (en) | 2011-01-13 |
| EP2164519A2 (en) | 2010-03-24 |
| US9353170B2 (en) | 2016-05-31 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| CA2689909C (en) | Long-acting polymeric prodrugs of exendin | |
| EP2616102B1 (en) | Prodrugs comprising an exendin linker conjugate | |
| EP2459171B1 (en) | Long acting insulin composition | |
| EP2459228B1 (en) | Prodrugs comprising an insulin linker conjugate | |
| EP3302567B1 (en) | Prodrugs comprising an glp-1/glucagon dual agonist linker hyaluronic acid conjugate | |
| JP2006520818A (en) | Polyethylene glycol-linked GLP-1 compound | |
| TW201204382A (en) | An insulin conjugate using an immunoglobulin fragment | |
| JP2006520818A5 (en) | ||
| TW201832783A (en) | Conjugates comprising an glp-1/glucagon dual agonist, a linker and hyaluronic acid | |
| KR20020001719A (en) | Method for Glucagon Suppression | |
| WO2017101883A1 (en) | Degradable hydrogel under physiological conditions | |
| KR102005294B1 (en) | Exenatide water plants and uses thereof | |
| TW201808346A (en) | Long-acting oxyntomodulin formulation and methods of producing and administering same | |
| RU2798085C9 (en) | Prodrug containing a self-cleavable linker | |
| RU2798085C2 (en) | Prodrug containing a self-cleavable linker |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| 121 | Ep: the epo has been informed by wipo that ep was designated in this application |
Ref document number: 08760559 Country of ref document: EP Kind code of ref document: A2 |
|
| WWE | Wipo information: entry into national phase |
Ref document number: 2008258548 Country of ref document: AU |
|
| WWE | Wipo information: entry into national phase |
Ref document number: 2689909 Country of ref document: CA |
|
| NENP | Non-entry into the national phase |
Ref country code: DE |
|
| ENP | Entry into the national phase |
Ref document number: 2008258548 Country of ref document: AU Date of ref document: 20080605 Kind code of ref document: A |
|
| WWE | Wipo information: entry into national phase |
Ref document number: 2008760559 Country of ref document: EP |
|
| WWE | Wipo information: entry into national phase |
Ref document number: 12663628 Country of ref document: US |