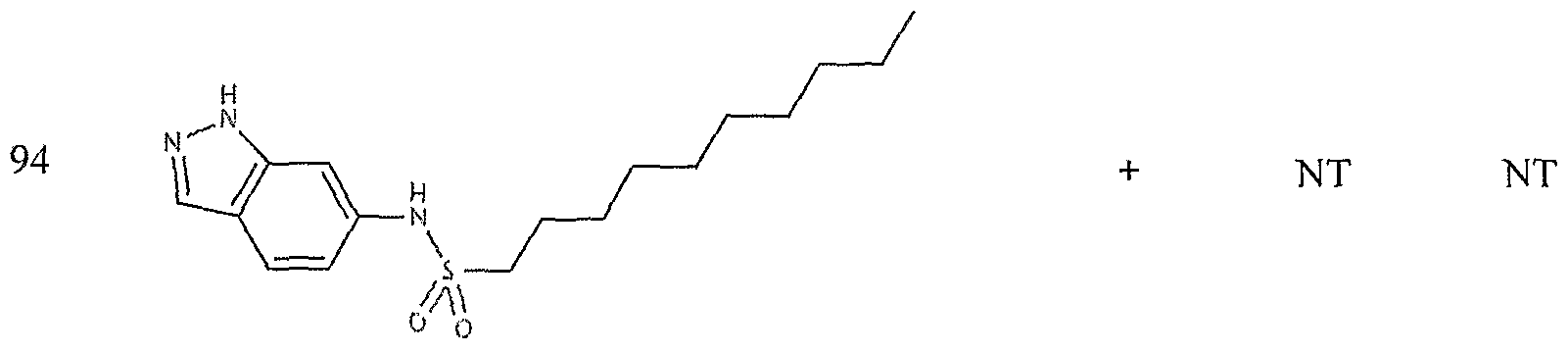WO2007008541A2 - Cellular cholesterol absorption modifiers - Google Patents
Cellular cholesterol absorption modifiers Download PDFInfo
- Publication number
- WO2007008541A2 WO2007008541A2 PCT/US2006/026242 US2006026242W WO2007008541A2 WO 2007008541 A2 WO2007008541 A2 WO 2007008541A2 US 2006026242 W US2006026242 W US 2006026242W WO 2007008541 A2 WO2007008541 A2 WO 2007008541A2
- Authority
- WO
- WIPO (PCT)
- Prior art keywords
- group
- optionally substituted
- c2nc3ccc
- cc3nc4
- heteroaryl
- Prior art date
Links
- 0 CCC(c1cc(*C)ccc1)=CCCC(C)=* Chemical compound CCC(c1cc(*C)ccc1)=CCCC(C)=* 0.000 description 35
- FNMFQFZQEDUXGY-UHFFFAOYSA-N C=CC(c1cc(NC(CSC(CCc2c3)=Cc2ccc3C(C(c2ccccc2C=C)=C)=C)=[ClH])ccc1)=O Chemical compound C=CC(c1cc(NC(CSC(CCc2c3)=Cc2ccc3C(C(c2ccccc2C=C)=C)=C)=[ClH])ccc1)=O FNMFQFZQEDUXGY-UHFFFAOYSA-N 0.000 description 1
- GSABXVGMZRWEGU-UHFFFAOYSA-N CC(C(c1ccc(C(C)(C)C)cc1)=C)c(cc1[s]2)ccc1nc2[I+]CC(NC1CC1)=N Chemical compound CC(C(c1ccc(C(C)(C)C)cc1)=C)c(cc1[s]2)ccc1nc2[I+]CC(NC1CC1)=N GSABXVGMZRWEGU-UHFFFAOYSA-N 0.000 description 1
- DLDCZXREYCYLJK-UHFFFAOYSA-N CC(C)(C)c(cc1)ccc1[S]([N+](c1cc(cccc2)c2cc1C([O+]=C)=N)=C)(=N)#[U] Chemical compound CC(C)(C)c(cc1)ccc1[S]([N+](c1cc(cccc2)c2cc1C([O+]=C)=N)=C)(=N)#[U] DLDCZXREYCYLJK-UHFFFAOYSA-N 0.000 description 1
- OFWIEIWXTCWGEX-UHFFFAOYSA-N CC(C)CNC(c1cc(NC(C(C)(C)C)=O)ccc1N1Cc2ccccc2CC1)=N Chemical compound CC(C)CNC(c1cc(NC(C(C)(C)C)=O)ccc1N1Cc2ccccc2CC1)=N OFWIEIWXTCWGEX-UHFFFAOYSA-N 0.000 description 1
- SNGJOAYHAOGIJI-UHFFFAOYSA-N CC(C)CNc1nc(Nc2cc(C)cc(C)c2)ccn1 Chemical compound CC(C)CNc1nc(Nc2cc(C)cc(C)c2)ccn1 SNGJOAYHAOGIJI-UHFFFAOYSA-N 0.000 description 1
- YLIZOTRBSUCRNT-UHFFFAOYSA-N CC(C)NC(c1c2nc(C)c(Cc(cc3)ccc3Cl)c(C)[n]2nc1)=O Chemical compound CC(C)NC(c1c2nc(C)c(Cc(cc3)ccc3Cl)c(C)[n]2nc1)=O YLIZOTRBSUCRNT-UHFFFAOYSA-N 0.000 description 1
- BCJOWPJUHPRVQB-UHFFFAOYSA-N CC(CO)(CS1)N/C1=N\C1(C)C=CC(S(N2CCCCC2)(=N)=O)=CC1 Chemical compound CC(CO)(CS1)N/C1=N\C1(C)C=CC(S(N2CCCCC2)(=N)=O)=CC1 BCJOWPJUHPRVQB-UHFFFAOYSA-N 0.000 description 1
- OSLMDZTZEPAYCP-UHFFFAOYSA-N CCC(NC1CCCCC1)=O Chemical compound CCC(NC1CCCCC1)=O OSLMDZTZEPAYCP-UHFFFAOYSA-N 0.000 description 1
- VDKXVEARTKOREE-UHFFFAOYSA-N CCCC1=C[Cl]=C(C)C=C1 Chemical compound CCCC1=C[Cl]=C(C)C=C1 VDKXVEARTKOREE-UHFFFAOYSA-N 0.000 description 1
- FIQCBOLSBNPGKJ-UHFFFAOYSA-N CCc1cc(-c2cncc(N(C)Cc3ccc(C)cc3)n2)cnc1 Chemical compound CCc1cc(-c2cncc(N(C)Cc3ccc(C)cc3)n2)cnc1 FIQCBOLSBNPGKJ-UHFFFAOYSA-N 0.000 description 1
- HEVAISJKFSCVHX-UHFFFAOYSA-N CN(C)S(Oc1cc(C(Nc(cc2)ccc2Cl)=O)ccc1)(=O)=O Chemical compound CN(C)S(Oc1cc(C(Nc(cc2)ccc2Cl)=O)ccc1)(=O)=O HEVAISJKFSCVHX-UHFFFAOYSA-N 0.000 description 1
- SWXULQRSEYINNH-UHFFFAOYSA-N CN(CCN1CCCC1)c(cc1)nnc1N1CC[N](C)(C)CC1 Chemical compound CN(CCN1CCCC1)c(cc1)nnc1N1CC[N](C)(C)CC1 SWXULQRSEYINNH-UHFFFAOYSA-N 0.000 description 1
- NQQGIWFILFOAQX-UHFFFAOYSA-N COc1cc(OC)c(-c2c[n](c3ccccc3[s]3)c3n2)c(Br)c1 Chemical compound COc1cc(OC)c(-c2c[n](c3ccccc3[s]3)c3n2)c(Br)c1 NQQGIWFILFOAQX-UHFFFAOYSA-N 0.000 description 1
- GODFLKGCXTYAKG-UHFFFAOYSA-N Cc(cc(cc1)OC)c1Nc1cc(C(NCc(cccc2)c2OC)=O)cc(OC)n1 Chemical compound Cc(cc(cc1)OC)c1Nc1cc(C(NCc(cccc2)c2OC)=O)cc(OC)n1 GODFLKGCXTYAKG-UHFFFAOYSA-N 0.000 description 1
- UZQJCQINVLELAG-UHFFFAOYSA-N Cc(cc1)ccc1[ClH]C Chemical compound Cc(cc1)ccc1[ClH]C UZQJCQINVLELAG-UHFFFAOYSA-N 0.000 description 1
- FHLYKVYZJHAXSS-UHFFFAOYSA-N Cc1c(N(C)c(cc2)ccc2OC)nc(N(C)Cc2ccccc2)nc1 Chemical compound Cc1c(N(C)c(cc2)ccc2OC)nc(N(C)Cc2ccccc2)nc1 FHLYKVYZJHAXSS-UHFFFAOYSA-N 0.000 description 1
- RYACPGWZSFNIGW-UHFFFAOYSA-O Cc1c(Nc2cc(F)ccc2)nc(NC2CCCC2)[nH+]c1 Chemical compound Cc1c(Nc2cc(F)ccc2)nc(NC2CCCC2)[nH+]c1 RYACPGWZSFNIGW-UHFFFAOYSA-O 0.000 description 1
- OORDYLBQLLDCJQ-UHFFFAOYSA-N Cc1ccc(CN(C)c2nc(N(C)c3cc(C)cc(C)c3)c(C)cn2)cc1 Chemical compound Cc1ccc(CN(C)c2nc(N(C)c3cc(C)cc(C)c3)c(C)cn2)cc1 OORDYLBQLLDCJQ-UHFFFAOYSA-N 0.000 description 1
- SJUIBIOOCRCYEA-UHFFFAOYSA-N Cc1ccc(CN(CCC(CC2)N(C)Cc3c[s]c(-c4ccc[s]4)n3)C2=O)cc1 Chemical compound Cc1ccc(CN(CCC(CC2)N(C)Cc3c[s]c(-c4ccc[s]4)n3)C2=O)cc1 SJUIBIOOCRCYEA-UHFFFAOYSA-N 0.000 description 1
Classifications
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/16—Amides, e.g. hydroxamic acids
- A61K31/18—Sulfonamides
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/63—Compounds containing para-N-benzenesulfonyl-N-groups, e.g. sulfanilamide, p-nitrobenzenesulfonyl hydrazide
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P3/00—Drugs for disorders of the metabolism
- A61P3/04—Anorexiants; Antiobesity agents
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P3/00—Drugs for disorders of the metabolism
- A61P3/06—Antihyperlipidemics
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P43/00—Drugs for specific purposes, not provided for in groups A61P1/00-A61P41/00
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P9/00—Drugs for disorders of the cardiovascular system
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P9/00—Drugs for disorders of the cardiovascular system
- A61P9/10—Drugs for disorders of the cardiovascular system for treating ischaemic or atherosclerotic diseases, e.g. antianginal drugs, coronary vasodilators, drugs for myocardial infarction, retinopathy, cerebrovascula insufficiency, renal arteriosclerosis
Definitions
- the present invention is directed to new compounds and compositions and their application as pharmaceuticals for the treatment of disease.
- Methods of modulation of cholesterol absorption activity in a human or animal subject are also provided for the treatment diseases such as vascular disease and atherosclerosis.
- a factor leading to development of vascular disease is elevated serum cholesterol. It is estimated that 19% of Americans between the ages of 20 and 74 years of age have high serum cholesterol.
- arteriosclerosis a condition associated with the thickening and hardening of the arterial wall.
- Arteriosclerosis of the large vessels is referred to as atherosclerosis.
- Atherosclerosis is the predominant underlying factor in vascular disorders such as coronary artery disease, aortic aneurysm, arterial disease of the lower extremities and cerebrovascular disease.
- Cholesteryl esters are a major component of atherosclerotic lesions and the major storage form of cholesterol in arterial wall cells. Formation of cholesteryl esters is also a step in the intestinal absorption of dietary cholesterol. Thus, inhibition of cholesteryl ester formation and reduction of serum cholesterol can inhibit the progression of atherosclerotic lesion formation, decrease the accumulation of cholesteryl esters in the arterial wall, and block the intestinal absorption of dietary cholesterol.
- the regulation of whole-body cholesterol homeostasis in mammals and animals involves the regulation of intestinal cholesterol absorption, cellular cholesterol trafficking, dietary cholesterol and modulation of cholesterol biosynthesis, bile acid biosynthesis, steroid biosynthesis and the catabolism of the cholesterol-containing plasma lipoproteins. Regulation of intestinal cholesterol absorption has proven to be an effective means by which to regulate serum cholesterol levels. For example, a cholesterol absorption inhibitor, ezetimibe has been shown to be effective in this regard. Ezetimibe is believed to prevent cholesterol absorption by inhibiting NPC I Ll . ( WO05015988 Al).
- NPCl Ll is an N-glycosylated protein comprising a a trans-golgi network to plasma membrane transport signal; see Bos, et al., (1993) EMBO J. 12:2219-2228; Humphrey, et al., (1993) J. Cell. Biol. 120: 1 123-1 135; Ponnambalam, et al., (1994) J. Cell. Biol. 125:253-268 and Rothman, et al., (1996) Science 272:227-234) which exhibits limited tissue distribution and gastrointestinal abundance.
- the human NPClLl promoter includes a Sterol Regulated Element Binding Protein 1 (SREBPl) binding consensus sequence (Athanikar, et al., (1998) Proc. Natl. Acad. Sci. USA 95:4935-4940; Ericsson, et al., (1996) Proc. Natl. Acad. Sci. USA 93:945-950; Metherall, et al., (1989) J. Biol. Chem. 264:15634- 15641 ; Smith, et al., (1990) J. Biol. Chem. 265:2306-2310; Bennett, et al., (1999) J. Biol. Chem.
- SREBPl Sterol Regulated Element Binding Protein 1
- MPClLl has 42% amino acid sequence homology to human NPCl (Genbank Accession No. AF002020), a receptor responsible for Niemann-
- NPCl and NPClLl each possess 13 transmembrane spanning segments as well as a sterol- sensing domain (SSD).
- SSD sterol- sensing domain
- HMG-R HMG-CoA Reductase
- PTC Patched
- SCAP Sterol Regulatory Element Binding Protein Cleavage-Activation Protein
- Novel compounds and pharmaceutical compositions that prevent cholesterol absorption by presumably inhibiting NPCl Ll , though the mechamism of action of these compounds are still to be confirmed, have been found together with methods of synthesizing and using the compounds including methods for inhibiting or modulating cholesterol absorption in a patient by administering the compounds.
- the present invention discloses a class of compounds, useful in treating NPC-I Ll -mediated disorders and conditions, defined by structural Formula I:
- R 1 and R 2 are independently selected from the group consisting of hydrogen, lower alkyl, lower alkoxy, lower alkylamino, lower alkenyl, lower alkynyl, lower alkoxyalkyl, lower alkylaminoalkyl, lower alkylcarbonyl, lower alkoxycarbonylalkyl, lower aminocarbonylalkyl, amido, lower aminoalkyl, aryl, arylalkyl, arylalkenyl, arylalkynyl, lower cycloalkyl, lower cycloalkylalkyl, lower haloalkyl, lower perhaloalkyl, lower alkylthioalkyl, lower alkylsulfonylalky], lower alkylsulfonyl, lower perhaloalkylsulfonyl, lower cycloalkylsulfonyl, heteroaryl, heteroarylalkyl, heteroarylalkenyl, and heterocycloalkyl, any
- R 3 is selected form the group consisting of lower alkyl, lower alkenyl, lower alkynyl, lower alkoxyalkyl, lower alkylaminoalkyl, lower aminoalkyl, aryl, arylalkyl, arylalkenyl, arylalkynyl, lower cycloalkyl, lower cycloalkylalkyl, lower haloalkyl, lower perhaloalkyl, heteroaryl, heteroarylalkyl, heteroarylalkenyl, and heterocycloalkyl, any of which may be optionally substituted; or R 1 and R 3 , together with the atoms to which they are attached, may be joined to form an optionally substituted heterocycloalkyl moiety.
- the present invention discloses a class of compounds, useful in treating NPC-I Ll -mediated disorders and conditions, defined by structural Formula II:
- X 1 is selected from the group consisting of C(R 4 ) and " N;
- X 2 is selected from the group consisting of C(R 5 ) and N;
- X 3 is selected from the group consisting of C(R 6 ) and N;
- X 4 is selected from the group consisting of C(R 7 ) and N; and
- R 4 , R 5 , R 6 , R 7 , R 8 , and R 9 are independently absent or selected from the group consisting of hydrogen, lower acyl, lower alkenyl, lower alkoxy, lower alkoxyalkyl, lower alkoxycarbonyl, lower alkyl, lower alkylamidoamino, lower alkylamino, lower alkylaminosulfonyl, lower alkylene, lower alkylsulfinyl, lower alkylsulfonyl, lower alkylsulfonylamino, lower alkylthio, lower alkylthioamido, amido, amino,
- the present invention further discloses a class of compounds, useful in treating NPC-I Ll- mediated disorders and conditions, defined by structural Formula III:
- R 10 is selected from the group consisting of aryl, cycloalkyl, heteroaryl, and heterocycloalkyl, any of which may be optionally substituted;
- R 1 1 and R 12 are independently selected from the group consisting of hydrogen, lower acyl, lower alkyl, aroyl, and heteroaroyl , any of which may be optionally substituted
- R b and R 14 are independently selected from the group consisting of hydrogen, hydroxy, halogen, amido, amino, aminocarbonyl, carboxy, cyano, nitro, sulfonate, thiol, lower acyl, lower alkenyl, lower alkoxy, lower alkoxyalkyl, lower alkoxycarbonyl, lower alkyl, lower alkylamidoamino, lower alkylamino, lower alkylaminosulfonyl, lower alkylenejower alkylsulfinyl, lower alkylsulfonyl, lower alkylsulfonylamino, lower alkylthio, lower alkylthioamido, lower cycloalkyl, lower cycloalkylalkyl, lower haloalk
- R 15 is selected from the group consisting of hydrogen, amido, aminocarbonyl, hydroxy, lower acyl, lower alkenyl, lower alkoxyalkyl, lower alkoxycarbonyl, lower alkyl, lower alkylamido, lower alkylamino, lower alkylaminosulfonyl, lower alkylene, lower alkylsulfinyl, lower alkylsulfonyl, lower aminoalkyl, aryl, arylalkoxy, arylalkyl, arylalkylamino, arylamido, arylamino, arylaminosulfonyl, arylsulfinyl, arylsulfonyl, lower cycloalkyl, lower cycloalkylalkyl, haloalkylcarbonyl, heteroaryl, heteroarylalkyl, heteroarylamido, heteroarylaminosulfonyl, heteroarylsulfonyl, heterocyclo
- the present invention further discloses a class of compounds, useful in treating NPC-I Ll- mediated disorders and conditions, defined by structural Formula IV:
- R 16 is selected from the group consisting of optionally substituted aryl, optionally substituted cycloalkyl, optionally substituted heteroaryl, and optionally substituted heterocycloalkyl;
- R 17 is selected from the group consisting of hydrogen, amido, aminocarbonyl, hydroxy, lower acyl, lower alkenyl, lower alkoxyalkyl, lower alkoxycarbonyl, lower alkyl, lower alkylamido, lower alkylamino, lower alkylaminosulfonyl, lower alkylene, lower alkylsulfinyl, lower alkylsulfonyl, lower aminoalkyl, aryl, arylalkoxy, arylalkyl, arylalkylamino, arylamido, arylamino, arylaminosulfonyl, arylsulf ⁇ nyl, arylsulfonyl, lower cycloalkyl, lower cycloalkylalkyl, lower haloalkylcarbonyl, heteroaryl, heteroarylalkyl, heteroarylamido, heteroarylaminosulfonyl, heteroarylsulfonyl, hetero
- R 18 and R 19 are independently selected from the group consisting of hydrogen, amido, amino, carboxy, cyano, halo, hydroxy, nitro, sulfonate, -SH, lower acyl, lower alkenyl, lower alkoxy, lower alkoxyalkyl, lower alkoxycarbonyl, lower alkyl, lower alkylamidoamino, lower alkylamino, lower alkylaminosulfonyl, lower alkylene, lower alkylsulfinyl, lower alkylsulfonyl, lower alkylsulfonylamino, lower alkylthio, lower alkylthioamidoaminoalkyl, aminocarbonyl, aryl, arylalkoxy, arylalkyl, arylalkylamino, arylalkylthio, arylamino, arylaminosulfonyl, aryloxy, arylsulfinyl, aryl
- R 20 and R 21 are independently selected from the group consisting of hydrogen, lower acyl, lower alkyl, lower alkylamido, aryl, arylalkyl, arylamido, lower cycloalkyl, lower cycloalkylalkyl, lower cycloalkylamido, heteroaryl, heteroarylalkyl, heteroarylamido, heterocycloalkyl, heterocycloalkylalkyl, and heterocycloalkylamido, any of which may be optionally substituted; and R 22 , R 23 , R 24 and R 25 are independently selected from the group consisting of hydrogen, amido, amino, carboxy, cyano, hydroxy, nitro, sulfonate, thio, halogen, aminocarbonyl, lower acyl, lower alkenyl, lower alkoxy, lower alkoxyalkyl, lower alkoxycarbonyl, lower alkyl, lower alkylamidoamino, lower alkylaniino, lower alky
- the present invention also provides pharmaceutical compositions comprising one or more compounds of the present invention together with a pharmaceutically acceptable carrier, as well as methods of making and using the compounds and compositions.
- the present invention provides methods for preventing cholesterol absorption by presumably inhibiting NPC 1 L 1.
- the present invention provides methods for treating a cholesterol absorption -mediated disorder in a patient in need of such treatment comprising administering to said patient a therapeutically effective amount of a compound or composition according to the present invention.
- the present invention also contemplates the use of compounds disclosed herein for use in the manufacture of a medicament for the treatment of a disease or condition ameliorated by the modulation of cholesterol absorption.
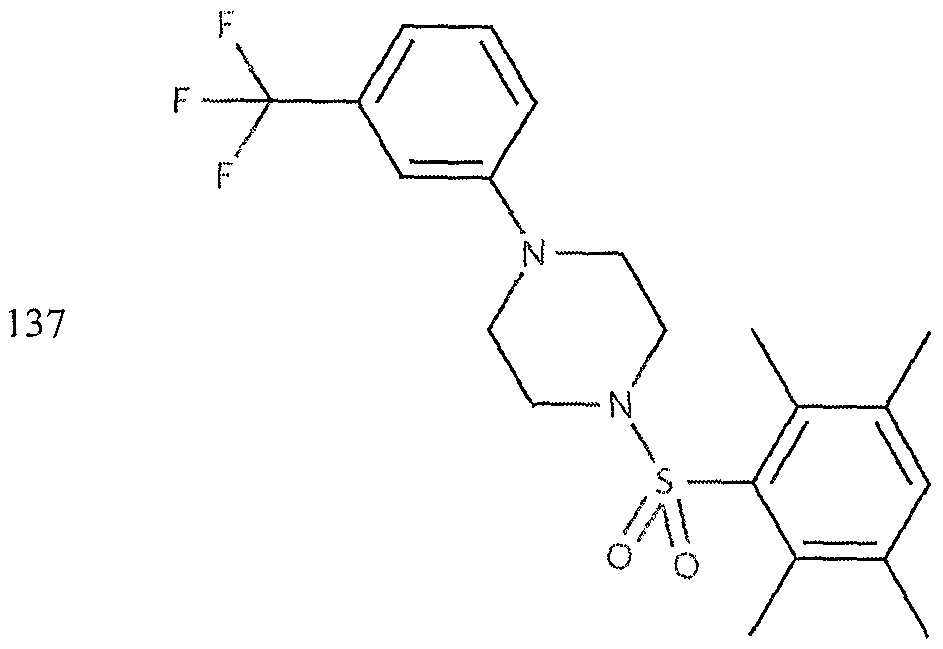
- the invention provides for compounds selected from the group consisting of Examples 1 to 740, as shown in Table 1.
- acyl refers to a carbonyl attached to an alkenyl, alkyl, aryl, cycloalkyl, heteroaryl, heterocycle, or any other moiety were the atom attached to the carbonyl is carbon.
- An "acetyl” group refers to a -C(O)CH 3 group.
- Examples of acyl groups include formyl, aikanoyl and aroyl radicals.
- acylamino embraces an amino radical substituted with an acyl group.
- An example of an “acylamino” radical is acetylamino (CH 3 C(O)NH-).
- alkenyl refers to a straight-chain or branched-chain hydrocarbon radical having one or more double bonds and containing from 2 to 20, preferably 2 to 6, carbon atoms.
- suitable alkenyl radicals include ethenyl, 1-propenyl, 2-propenyl (allyl), 2-methyl-l-propenyl, 2-methyl-2-propenyl (methylallyl), 3-methyl-2-butenyl (prenyl), 1 ,4-butadienyl and the like.
- alkoxy refers to an alkyl ether radical, wherein the term alkyl is as defined below.
- suitable alkyl ether radicals include methoxy, ethoxy, n-propoxy, isopropoxy, n-butoxy, iso-butoxy, sec-butoxy, tert-butoxy, and the like.
- alkoxyalkoxy refers to one or more alkoxy groups attached to the parent molecular moiety through another alkoxy group. Examples include ethoxyethoxy, methoxypropoxyethoxy, ethoxypentoxyethoxyethoxy and the like.
- alkoxy alkyl refers to an alkoxy group attached to the parent molecular moiety through an alkyl group.
- alkoxyalkyl also embraces alkoxyalkyl groups having one or more alkoxy groups attached to the alkyl group, that is, to form monoalkoxyalkyl and dialkoxyalkyl groups.
- alkoxycarbonyl refers to an alkoxy group attached to the parent molecular moiety through a carbonyl group.
- alkoxycarbonyl groups include methoxycarbonyl, ethoxycarbonyl, propoxycarbonyl, butoxy carbonyl and hexyloxycarbonyl.
- alkoxycarbonylalkyl embraces radicals having "alkoxycarbonyl", as defined above substituted to an alkyl radical. More preferred alkoxycarbonylalkyl radicals are "lower alkoxycarbonylalkyl” having lower alkoxycarbonyl radicals as defined above attached to one to six carbon atoms. Examples of such lower alkoxycarbonylalkyl radicals include methoxycarbonylmethyl.
- alkyl refers to a straight-chain or branched-chain alkyl radical containing from 1 to and including 20, preferably 1 to 10, and more preferably 1 to 6, carbon atoms. Alkyl groups may be optionally substituted as defined herein. Examples of alkyl radicals include methyl, ethyl, n-propyl, isopropyl, n-butyl, isobutyl, sec-butyl, tert-butyl, pentyl, iso-amyl, hexyl, octyl, noyl and the like.
- alkylene as used herein, alone or in combination, refers to a saturated aliphatic group derived from a straight or branched chain saturated hydrocarbon attached at two or more positions, such as methylene (-CH 2 -).
- alkylamino refers to an alkyl group attached to the parent molecular moiety through an amino group. Suitable alkylamino groups may be mono- or dialkylated, forming groups such as, for example, N-methylamino, N-ethylamino, N,N- dimethylamino, N,N-diethylamino and the like.
- alkylaminocarbonyl refers to an alkylamino group attached to the parent molecular moiety through a carbonyl group. Examples of such radicals include N-methylaminocarbonyl and N,M-dimethylcarbonyl.
- alkylcarbonyl and “alkanoyl,” as used herein, alone or in combination, refers to an alkyl group attached to the parent molecular moiety through a carbonyl group. Examples of such groups include methylcarbonyl and ethylcarbonyl.
- alkyl idene refers to an alkenyl group in which one carbon atom of the carbon-carbon double bond belongs to the moiety to which the alkenyl group is attached.
- alkylsulfinyl refers to an alkyl group attached to the parent molecular moiety through a sulfinyl group. Examples of alkylsulfinyl groups include methylsulfinyl, ethylsulfinyl, butylsulfinyl and hexylsulf ⁇ nyl.
- alkylsulfonyl refers to an alkyl group attached to the parent molecular moiety through a sulfonyl group.
- alkylsulfinyl groups include methanesulfonyl, ethanesulfonyl, tert-butanesulfonyl, and the like.
- alkylthio refers to an alkyl thioether (R-S- ) radical wherein the term alkyl is as defined above.
- suitable alkyl thioether radicals include methylthio, ethylthio, n-propylthio, isopropylthio, n-butylthio, iso-butylthio, sec-butylthio, tert-butylthio, ethoxyethylthio, methoxypropoxyethylthio, ethoxypentoxyethoxyethylthio and the like.
- alkylthioalkyl embraces alkylthio radicals attached to an alkyl radical.
- Alkylthioalkyl radicals include "lower alkylthioalkyl” radicals having alkyl radicals of one to six carbon atoms and an alkylthio radical as described above. Examples of such radicals include methylthiomethyl.
- alkynyl refers to a straight-chain or branched chain hydrocarbon radical having one or more triple bonds and containing from 2 to 20, preferably from 2 to 6, more preferably from 2 to 4, carbon atoms.
- Alkynylene refers to a carbon- carbon triple bond attached at two positions such as ethynylene (-C:::C-, -C ⁇ C-).
- alkynyl radicals examples include ethynyl, propynyl, hydroxypropynyl, butyn-1-yl, butyn-2-yl, pentyn-1 -yl, pentyn-2-yl, 4-methoxypentyn-2-yl, 3-methylbutyn-l-yl, hexyn-1 -yl, hexyn-2-yl, hexyn-3-yl, 3,3-dimethylbutyn-l -yl, and the like.
- the term "amido,” as used herein, alone or in combination, refers to an amino group as described below attached to the parent molecular moiety through a carbonyl group.
- amino refers to — NRR , wherein R and R are independently selected from the group consisting of hydrogen, alkenyl, alkoxy, alkoxyalkyl, alkoxycarbonyl, alkyl, alkylcarbonyl, aryl, arylalkenyl, arylalkyl, cycloalkyl, haloalkylcarbonyl, heteroaryl, heteroarylalkenyl, heteroarylalkyl, heterocycle, heterocycloalkeny], and heterocycloalkyl, wherein the aryl, the aryl part of the arylalkenyl, the arylalkyl, the heteroaryl, the heteroaryl part of the heteroarylalkenyl and the heteroarylalkyl, the heterocycle, and the heterocycle part of the heterocycloalkenyl and the heterocycloalkyl can be optionally substituted as defined herein with one, two, three, four, or five substitus, and the arylalkenyl and the heterocycl
- aminocarbonyl and “carbamoyl,” as used herein, alone or in combination, refer to an amino-substituted carbonyl group, wherein the amino group can be a primary or secondary amino group containing substituents selected from alkyl, aryl, aralkyl, cycloalkyl, cycloalkylalkyl radicals and the like.
- aminocarbonylalkyl refers to an aminocarbonyl radical attached to an alkyl radical, as described above.
- An example of such radicals is aminocarbonylmethyl.
- aminocarbonylalkyl denotes an -C(NH)NH 2 radical.
- cyanoamidino denotes an -C(N-CN)NH 2 radical.
- alkenyl or arylalkenyl, as used herein, alone or in combination, refers to an aryl group attached to the parent molecular moiety through an alkenyl group.
- aralkoxy or "arylalkoxy,” as used herein, alone or in combination, refers to an aryl group attached to the parent molecular moiety through an alkoxy group.
- aralkyl or “arylalkyl,” as used herein, alone or in combination, refers to an aryl group attached to the parent molecular moiety through an alkyl group.
- aralkylamino or “arylalkylamino,” as used herein, alone or in combination, refers to an arylalkyl group attached to the parent molecular moiety through a nitrogen atom, wherein the nitrogen atom is substituted with hydrogen.
- aralkylidene or “arylalkylidene,” as used herein, alone or in combination, refers to an aryl group attached to the parent molecular moiety through an alkylidene group
- aralkylthio or "arylalkylthio,” as used herein, alone or in combination, refers to an arylalkyl group attached to the parent molecular moiety through a sulfur atom.
- aralkynyl or “arylalkynyl,” as used herein, alone or in combination, refers to an aryl group attached to the parent molecular moiety through an alkynyl group.
- aralkoxycarbonyl refers to a radical of the formula aralkyl-O-C(O)- in which the term "aralkyl,” has the significance given above.
- examples of an aralkoxycarbonyl radical are benzyloxycarbonyl (Z or Cbz) and 4-methoxyphenylmethoxycarbony! (MOS).
- aralkanoyl refers to an acyl radical derived from an aryl-substituted alka ⁇ ecarboxylic acid such as benzoyl, phenylacetyl, 3-phenylpropiony!
- hydrocinnamoyl 4-phenylbutyryl, (2-naphthyl)acelyl, 4-chlorohydrocinnamoyl, A- aminohydiOcinnamoyl, 4-inethoxyhydiOcinnamoyl, and the like.
- aroyl refers to an acyl radical derived from an arylcarboxylic acid, "aryl” having the meaning given below.
- aroyl radicals include substituted and unsubstituted benzoyl or napthoyl such as benzoyl, 4- chlorobenzoyl, 4-carboxybenzoyl, 4-(benzyloxycarbonyl)benzoyl, 1 -naphthoyl, 2-naphthoyl, 6-carboxy- 2-naphthoyl, 6-(benzyloxycarbonyl)-2 -naphthoyl, 3-benzyloxy-2-naphthoyl, 3-hydroxy-2-na ⁇ hthoyl, 3- (benzyloxyformamido)-2-naphthoyl, and the like.
- aryl as used herein, alone or in combination, means a carbocyclic aromatic system containing one, two or three rings wherein such rings may be attached together in a pendent manner or may be fused.
- aryl embraces aromatic radicals such as benzyl, phenyl, naphthyl, anthracenyl, phenanthryl, indanyl, indenyl, annulenyl, azulenyl, tetrahydronaphthyl, and biphenyl.
- arylamino as used herein, alone or in combination, refers to an aryl group attached to the parent moiety through an amino group, such as methylamino, N-phenylamino, and the like.
- arylcarbonyl and “aroyl,” as used herein, alone or in combination, refer to an aryl group attached to the parent molecular moiety through a carbonyl group.
- aryloxy refers to an aryl group attached to the parent molecular moiety through an oxygen atom.
- arylsulfonyl refers to an aryl group attached to the parent molecular moiety through a sulfonyl group.
- arylthio refers to an aryl group attached to the parent molecular moiety through a sulfur atom.
- carboxy or “carboxyl”, whether used alone or with other terms, such as
- Carboxyalkyl denotes -CO 2 H.
- O-carbamyl as used herein, alone or in combination, refers to a -OC(O)NR, group-with R as defined herein.
- N-carbamyl as used herein, alone or in combination, refers to a ROC(O)NH- group, with R as defined herein.
- carbonyl when alone includes formyl [-C(O)H] and in combination is a -C(O)- group.
- carboxy refers to -C(O)OH or the corresponding "carboxylate” anion, such as is in a carboxylic acid salt.
- An "O-carboxy” group refers to a RC(O)O- group, where R is as defined herein.
- a “C-carboxy” group refers to a — C(O)OR groups where R is as defined herein.
- cyano as used herein, alone or in combination, refers to -CN.
- cycloalkyl refers to a saturated or partially saturated monocyclic, bicyclic or tricyclic alkyl radical wherein each cyclic moiety contains from 3 to 12, preferably five to seven, carbon atom ring members and which may optionally be a benzo fused ring system which is optionally substituted as defined herein.
- cycloalkyl radicals include cyclopropyl, cyclobutyl, cyclopentyl, cyclohexyl, cycloheptyl, octahydronaphthyl, 2,3-dihydro-l H- indenyl, adamantyl and the like.
- Bicyclic and tricyclic as used herein are intended to include both fused ring systems, such as decahydonapthalene, octahydronapthalene as well as the multicyclic (multicentered) saturated or partially unsaturated type.
- the latter type of isomer is exemplified in general by bicyclo[2,2,2]octane, bicyclo[2,2,2]octane, bicyclo[l,l,l]pentane, camphor and bicyclo[3,2,l]octane.
- esteer as used herein, alone or in combination, refers to a carbonyl group bridging two moieties linked at carbon atoms.
- ether refers to an oxy group bridging two moieties linked at carbon atoms.
- halo or halogen, as used herein, alone or in combination, refers to fluorine, chlorine, bromine, or iodine.
- haloalkoxy refers to a haloalkyl group attached to the parent molecular moiety through an oxygen atom.
- haloalkyl refers to an alkyl radical having the meaning as defined above wherein one or more hydrogens are replaced with a halogen. Specifically embraced are monohaloalkyl, dihaloalkyl and polyhaloalkyl radicals.
- a monohaloalkyl radical for one example, may have either an iodo, bromo, chloro or fluoro atom within the radical.
- Dihalo and polyhaloalkyl radicals may have two or more of the same halo atoms or a combination of different halo radicals.
- haloalkyl radicals include fluoromethyl, difluoromethyl, trifluoromethyl, chloromethyl, dichloromethyl, trichloromethyl, trichloromethyl, pentafluoroethyl, heptafluoropropyl, difluorochloromethyl, dichlorofluoromethyl, difluoroethyl, difluoropropyl, dichloroethyl and dichloropropyl.
- Haloalkylene refers to a halohydrocarbyl group attached at two or more positions.
- Examples include fluoromethylene (— CFH- ), difluoromethylene (— CFj — ), chloromethylene (— CHCl-) and the like.
- haloalkyl radicals include chloromethyl, 1-bromoethyl, fluoromethyl, difluoromethyl, trifluoromethyl, 1 , 1 ,1-trifIuoroethyI, perfluorodecyl and the like.
- heteroalkyl refers to a stable straight or branched chain, or cyclic hydrocarbon radical, or combinations thereof, fully saturated or containing from 1 to 3 degrees of unsaturation, consisting of the stated number of carbon atoms and from one to three heteroatoms selected from the group consisting of O, N, and S, and wherein the nitrogen and sulfur atoms may optionally be oxidized and the nitrogen heteroatom may optionally be quaternized.
- the heteroatom(s) O, N and S may be placed at any interior position of the heteroalkyl group. Up to two heteroatoms may be consecutive, such as, for example, -CH2-NH-OCH3.
- heteroaryl refers to 3 to 7 membered, preferably 5 to 7 membered, unsaturated heterocyclic rings wherein at least one atom is selected from the group consisting of O, S, and N.
- Heteroaryl groups are exemplified by: unsaturated 3 to 7 membered heteromonocyclic groups containing 1 to 4 nitrogen atoms, for example, pyrrolyl, pyrrolinyl, imidazolyl, pyrazolyl, pyridyl, pyrimidinyl, pyrazinyl, pyridazinyl, triazolyl [e.g., 4H- 1 ,2,4-triazolyl, 1 H-1 ,2,3- triazolyl, 2H-l ,2,3-triazolyl, etc.]tetrazolyl [e.g.
- benzoxazolyl, benzoxadiazolyl, etc.] unsaturated 3 to 6-membered heteromonocyclic groups containing 1 to 2 sulfur atoms and I to 3 nitrogen atoms, for example, thiazolyl, thiadiazolyl [e.g., 1 ,2,4- thiadiazolyl, 1 ,3,4-thiadiazolyl, 1,2,5-thiadiazolyl, etc.]and isothiazolyl; unsaturated condensed heterocyclic groups containing ! to 2 sulfur atoms and 1 to 3 nitrogen atoms [e.g., benzothiazolyl, benzothiadiazolyl, etc.]and the like.
- thiazolyl, thiadiazolyl e.g., 1 ,2,4- thiadiazolyl, 1 ,3,4-thiadiazolyl, 1,2,5-thiadiazolyl, etc.
- isothiazolyl unsaturated condensed heterocyclic groups containing ! to
- heterocyclic radicals are fused with aryi radicals.
- fused bicyclic radicals include benzofuryl, benzothienyl, and the like.
- heteroarylke ⁇ yl or “heteroarylalkenyl,” as used herein, alone or in combination, refers to a heteroaryl group attached to the parent molecular moiety through an alkenyl group.
- heteroarylkoxy or “heteroarylalkoxy,” as used herein, alone or in combination, refers to a heteroaryl group attached to the parent molecular moiety through an alkoxy group.
- heteroarylalkyl refers to a heteroaryl group attached to the parent molecular moiety through an alkyl group.
- heteroarylkylidene or “heteroarylalkyiidene,” as used herein, alone or in combination, refers to a heteroaryl group attached to the parent molecular moiety through an alkylidene group.
- heteroaryloxy refers to a heteroaryl group attached to the parent molecular moiety through an oxygen atom.
- heteroarylsulfonyl refers to a heteroaryl group attached to the parent molecular moiety through a sulfonyl group.
- heterocycloalkyl and, interchangeably, “heterocycle,” as used herein, alone or in combination, each refer to a saturated, partially unsaturated, or fully unsaturated monocyclic, bicyclic, or tricyclic heterocyclic radical containing at least one, preferably 1 to 4, and more preferably 1 to 2 heteroatoms as ring members, wherein each said heteroatom may be independently selected from the group consisting of nitrogen, oxygen, and sulfur, and wherein there are preferably 3 to 8 ring members in each ring, more preferably 3 to 7 ring members in each ring, and most preferably 5 to 6 ring members in each ring.
- Heterocycloalkyl and “heterocycle” are intended to include sulfones, sulfoxides, N-oxides of tertiary nitrogen ring members, and carbocyclic fused and benzo fused ring systems; additionally, both terms also include systems where a heterocycle ring is fused to an aryl group, as defined herein, or an additional heterocycle group.
- Heterocycle groups of the invention are exemplified by aziridinyl, azetidinyl, 1 ,3-benzodioxolyl, dihydroisoindolyi, dihydroisoquinolinyl, dihydrocinnolinyl, dihydrobenzodioxinyl, dihydro[l ,3]oxazolo[4,5-b]pyridinyl, benzothiazolyl, dihydroindolyl, dihy- dropyridinyl, 1 ,3-dioxanyl, 1 ,4-dioxanyl, 1 ,3-dioxolanyl, isoindolinyl, morpholinyl, piperazinyl, pyrrolidinyl, tetrahydropyridinyl, piperidinyl, thiomorpholinyl, and the like.
- the heterocycle groups may be optionally substituted unless specifically prohibited.
- heterocycloalkoxy refers to a heterocycle group attached to the parent molecular group through an oxygen atom.
- heterocycloalkyl refers to an alkyl radical as defined above in which at least one hydrogen atom is replaced by a heterocyclo radical as defined above, such as pyrrolidinylmethyl, tetrahydrothienylmethyl, pyridylmethyl and the like.
- heterocycloalkylidene refers to a heterocycle group attached to the parent molecular moiety through an alkylidene group.
- hydrazinyl as used herein, alone or in combination, refers to two amino groups joined by a single bond, i.e., -N-M-.
- hydroxyalkyl refers to a linear or branched alkyl group having one to about ten carbon atoms any one of which may be substituted with one or more hydroxyl radicals.
- examples of such radicals include hydroxymethyl, hydroxyethyl, hydroxypropyl, hydroxybutyl and hydroxyhexyl.
- hydroxyalkyl refers to a hydroxy group attached to the parent molecular moiety through an alkyl group.
- the phrase "in the main chain” refers to the iongest contiguous or adjacent chain of carbon atoms starting at the point of attachment of a group to the compounds of this invention.
- isocyanato refers to a -NCO group.
- isothiocyanato refers to a -NCS group.
- linear chain of atoms refers to the longest straight chain of atoms independently selected from carbon, nitrogen, oxygen and sulfur.
- lower means containing from 1 to and including 6 carbon atoms.
- mercaptoalkyl as used herein, alone or in combination, refers to an R'SR- group, where R and R' are as defined herein.
- mercaptomercaptyl as used herein, alone or in combination, refers to a RSR'S- group, where R is as defined herein.
- mercaptyl as used herein, alone or in combination, refers to an RS- group, where R is as defined herein.
- null refers to a lone electron pair
- nitro refers to -NO 2 .
- oxy or “oxa,” as used herein, alone or in combination, refer to -O-.
- perhaloalkoxy refers to an alkoxy group where all of the hydrogen atoms are replaced by halogen atoms.
- perhaloalkyl refers to an alkyl group where all of the hydrogen atoms are replaced by halogen atoms.
- sulfonate refers the -SO 3 H group and its anion as the sulfonic acid is used in salt formation.
- sulfanyl as used herein, alone or in combination, refers to -S-.
- sulfinyl as used herein, alone or in combination, refers to -S(O)-.
- sulfonyl as used herein, alone or in combination, refers to -SO 2 -.
- thia and thio refer to a -S- group or an ether wherein the oxygen is replaced with sulfur. The oxidized derivatives of the thio group, namely sulfinyl and sulfonyl, are included in the definition of thia and thio.
- thioether refers to a thio group bridging two moieties linked at carbon atoms.
- thiol refers to an -SH group.
- thiocarbonyl when alone includes thioformyl -C(S)H and in combination is a -C(S)- group.
- N-thiocarbamyl refers to an ROC(S)NH- group, with R as defined herein.
- O-thiocarbamyl refers to a -OC(S)NR, group with R as defined herein.
- thiocyanato refers to a -CNS group.
- trihalomethanesulfonamido refers to a X 3 CS(O) 2 NR- group with X is a halogen and R as defined herein.
- trimethanesulfonyl refers to a X 3 CS(O) 2 - group where X is a halogen.
- trimethoxy refers to a X 3 CO- group where X is a halogen.
- trimethysilyl as used herein, alone or in combination, refers to a silicone group substituted at its three free valences with groups as listed herein under the definition of substituted amino. Examples include trimethysilyl, tert-butyldimethylsilyl, triphenylsilyl and the like.
- the term "optionally substituted” means the anteceding group may be substituted or unsubstituted.
- the substituents of an "optionally substituted” group may include, without limitation, one or more substituents independently selected from the following groups or a particular designated set of groups, alone or in combination: lower alkyl, lower alkenyl, lower alkynyl, lower heteroalkyl, lower heterocycloalkyl, lower haloalkyl, lower haloalkenyl, lower haloalkynyl, lower perhaloalkyl, lower perhaloalkoxy, lower cycloalkyl, phenyl, aryl, aryloxy, lower alkoxy, lower haloalkoxy, oxo, lower acyloxy, carbonyl, lower carboxyester, lower carboxamido, cyano, hydrogen, halogen, hydroxy, amino, lower alkylamino, arylamino, amido, thiol, lower alkyl
- Two substituents may be joined together to form a fused five-, six-, or seven-menbered carbocyclic or heterocyclic ring consisting of zero to three heteroatoms, for example forming methylenedioxy or ethylenedioxy.
- An optionally substituted group may be unsubstituted (e.g., - CH2CH 3 ), fully substituted (e.g., -CF 2 CF 3 ), monosubstituted (e.g., -CH 2 CH 2 F) or substituted at a level anywhere in-between fully substituted and monosubstituted (e.g., -CH 2 CF 3 ).
- substituent, or term e.g. aryl, heterocycle, R, etc.
- bonds refers to a covalent linkage between two atoms, or two moieties when the atoms joined by the bond are considered to be part of larger substructure.
- a bond may be single, double, or triple unless otherwise specified.
- combination therapy means the administration of two or more therapeutic agents to treat a therapeutic condition or disorder described in the present disclosure. Such administration encompasses co-administration of these therapeutic agents in a substantially simultaneous manner, such as in a single capsule having a fixed ratio of active ingredients or in multiple, separate capsules for each active ingredient. In addition, such administration also encompasses use of each type of therapeutic agent in a sequential manner. In either case, the treatment regimen will provide beneficial effects of the drug combination in treating the conditions or disorders described herein.
- “Cholesterol absorption inhibitor” is used herein to refer to a compound that exhibits an IC 50 with respect to downregulation of ABCAl transcription while upregulating the expression of HMG COA synthase activity of no more than about 100 ⁇ M and more typically not more than about 50 ⁇ M, as measured in the the luciferase reporter HEP ABCA l Luc assay described generally hereinbelow.
- IC 50 is that concentration of inhibitor which reduces the level of ABCAl expression to half-maximal level while increasing expression of HMG COA synthase.
- Representative compounds of the present invention have been discovered to exhibit inhibitory activity against cholesterol absorption, presumably by inhibiting NPCl Ll .
- Compounds of the present invention preferably exhibit an IC 50 with respect to downregulation of ABCAl transcription while upregulating the expression of HMG COA synthase activity of no more than about 10 ⁇ M, more preferably, no more than about 5 ⁇ M, even more preferably not more than about 1 ⁇ M, and most preferably, not more than about 200 nM, as measured in luciferase reporter HEP ABCAl Luc assay described herein.
- the phrase "therapeutically effective" is intended to qualify the amount of active ingredients used in the treatment of a disease or disorder. This amount will achieve the goal of reducing or eliminating the said disease or disorder.
- patient means all mammals including humans. Examples of patients include humans, cows, dogs, cats, goats, sheep, pigs, and rabbits. Preferably, the patient is a human.
- prodrug refers to a compound that is made more active in vivo.
- the present compounds can also exist as prodrugs, as described in Hydrolysis in Drug and Prodrug Metabolism : Chemisti ⁇ , Biochemistry, and Enzymology (Testa, Bernard and Mayer, Joachim M. Wiley-VHCA, Zurich, Switzerland 2003).
- Prodrugs of the compounds described herein are structurally modified forms of the compound that readily undergo chemical changes under physiological conditions to provide the compound.
- prodrugs can be converted to the compound by chemical or biochemical methods in an ex vivo environment. For example, prodrugs can be slowly converted to a compound when placed in a transdermal patch reservoir with a suitable enzyme or chemical reagent.
- Prodrugs are often useful because, in some situations, they may be easier to administer than the compound, or parent drug. They may, for instance, be bioavailable by oral administration whereas the parent drug is not. The prodrug may also have improved solubility in pharmaceutical compositions over the parent drug.
- a wide variety of prodrug derivatives are known in the art, such as those that rely on hydrolytic cleavage or oxidative activation of the prodrug.
- An example, without limitation, of a prodrug would be a compound which is administered as an ester (the "prodrug"), but then is metabolically hydrolyzed to the carboxylic acid, the active entity. Additional examples include peptidyl derivatives of a compound.
- therapeutically acceptable prodrug refers to those prodrugs or zwitterions which are suitable for use in contact with the tissues of patients without undue toxicity, irritation, and allergic response, are commensurate with a reasonable benefit/risk ratio, and are effective for their intended use.
- terapéuticaally acceptable salt represents salts or zwitterionic forms of the compounds of the present invention which are water or oil-soluble or dispersible; which are suitable for treatment of diseases without undue toxicity, irritation, and allergic-response; which are commensurate with a reasonable benefit/risk ratio; and which are effective for their intended use.
- the salts can be prepared during the final isolation and purification of the compounds or separately by reacting the appropriate compound in the form of the free base with a suitable acid.
- Representative acid addition salts include acetate, adipate, alginate, L-ascorbate, aspartate, benzoate, benzenesulfonate (besylate), bisulfate, butyrate, camphorate, camphorsulfonate, citrate, digluconate, formate, fumarate, gentisate, glutarate, glycerophosphate, glycolate, hemisulfate, heptanoate, hexanoate, hippurate, hydrochloride, hydrobromide, hydroiodide, 2-hydroxyethansulfonate (isethionate), lactate, maleate, malonate, DL-mandelate, mesitylenesulfonate, methanesulfonate, naphthylenesulfonate, nicotinate, 2- naphthalenesulfonate, oxalate, pamoate, pectinate, persulfate, 3-pheny
- basic groups in the compounds of the present invention can be quaternized with methyl, ethyl, propyl, and butyl chlorides, bromides, and iodides; dimethyl, diethyl, dibutyl, and diamyl sulfates; decyl, lauryl, myristyl, and steryl chlorides, bromides, and iodides; and benzyl and phenethyl bromides.
- acids which can be employed to form therapeutically acceptable addition salts include inorganic acids such as hydrochloric, hydrobromic, sulfuric, and phosphoric, and organic acids such as oxalic, maleic, succinic, and citric.
- Salts can also be formed by coordination of the compounds with an alkali metal or alkaline earth ion.
- the present invention contemplates sodium, potassium, magnesium, and calcium salts of the compounds of the compounds of the present invention and the like.
- Basic addition salts can be prepared during the final isolation and purification of the compounds by reacting a carboxy group with a suitable base such as the hydroxide, carbonate, or bicarbonate of a metal cation or with ammonia or an organic primary, secondary, or tertiary amine.
- the cations of therapeutically acceptable salts include lithium, sodium, potassium, calcium, magnesium, and aluminum, as well as nontoxic quaternary amine cations such as ammonium, tetramethylammonium, tetraethylammonium, methylamine, dimethylamine, trimethylamine, triethylamine, diethylamine, ethylamine, tributylamine, pyridine, /V./V-dimethylaniline, /V-methylpiperidine, /V-methylmorpholine, dicyclohexylamine, procaine, dibenzylamine, /V,/V-dibenzyIphenethylamine, 1 -ephenamine, and N,N'- dibenzylethylenediamine.
- Other representative organic amines useful for the formation of base addition salts include ethylenediamine, ethanolamine, diethanolamine, piperidine, and piperazine.
- the compounds of the present invention can exist as therapeutically acceptable salts.
- the present invention includes compounds listed above in the form of salts, in particular acid addition salts. Suitable salts include those formed with both organic and inorganic acids. Such acid addition salts will normally be pharmaceutically acceptable. However, salts of non-pharmaceutical Iy acceptable salts may be of utility in the preparation and purification of the compound in question. For a more complete discussion of the preparation and selection of salts, refer to Pharmaceutical Salts: Properties, Selection, ami Use (Slahl, P. Heinrich. Wiley-VCHA, Zurich, Switzerland, 2002).
- the subject invention provides a pharmaceutical formulation comprising a compound or a pharmaceutically acceptable salt, ester, prodrug or solvate thereof, together with one or more pharmaceutically acceptable carriers thereof and optionally one or more other therapeutic ingredients.
- the carrier(s) must be "acceptable” in the sense of being compatible with the other ingredients of the formulation and not deleterious to the recipient thereof. Proper formulation is dependent upon the route of administration chosen. Any of the well-known techniques, carriers, and excipients may be used as suitable and as understood in the art; e.g., in Remington's Pharmaceutical Sciences.
- compositions of the present invention may be manufactured in a manner that is itself known, e.g., by means of conventional mixing, dissolving, granulating, dragee-making, levigating, emulsifying, encapsulating, entrapping or compression processes.
- the formulations include those suitable for oral, parenteral (including subcutaneous, intradermal, intramuscular, intravenous, intraarticular, and intramedullary), intraperitoneal, transmucosal, transdermal, rectal and topical (including dermal, buccal, sublingual and intraocular) administration although the most suitable route may depend upon for example the condition and disorder of the recipient.
- the formulations may conveniently be presented in unit dosage form and may be prepared by any of the methods well known in the art of pharmacy. All methods include the step of bringing into association a compound of the subject invention or a pharmaceutically acceptable salt, ester, prodrug or solvate thereof ("active ingredient") with the carrier which constitutes one or more accessory ingredients. In general, the formulations are prepared by uniformly and intimately bringing into association the active ingredient with liquid carriers or finely divided solid carriers or both and then, if necessary, shaping the product into the desired formulation.
- Formulations of the present invention suitable for oral administration may be presented as discrete units such as capsules, cachets or tablets each containing a predetermined amount of the active ingredient; as a powder or granules; as a solution or a suspension in an aqueous liquid or a non-aqueous liquid; or as an oil-in-water liquid emulsion or a water-in-oil liquid emulsion.
- the active ingredient may also be presented as a bolus, electuary or paste.
- compositions which can be used orally include tablets, push-fit capsules made of gelatin, as well as soft, sealed capsules made of gelatin and a plasticizer, such as glycerol or sorbitol. Tablets may be made by compression or molding, optionally with one or more accessory ingredients. Compressed tablets may be prepared by compressing in a suitable machine the active ingredient in a free-flowing form such as a powder or granules, optionally mixed with binders, inert diluents, or lubricating, surface active or dispersing agents. Molded tablets may be made by molding in a suitable machine a mixture of the powdered compound moistened with an inert liquid diluent.
- the tablets may optionally be coated or scored and may be formulated so as to provide slow or controlled release of the active ingredient therein. All formulations for oral administration should be in dosages suitable for such administration.
- the push-fit capsules can contain the active ingredients in admixture with filler such as lactose, binders such as starches, and/or lubricants such as talc or magnesium stearate and, optionally, stabilizers.
- the active compounds may be dissolved or suspended in suitable liquids, such as fatty oils, liquid paraffin, or liquid polyethylene glycols.
- stabilizers may be added.
- Dragee cores are provided with suitable coatings.
- concentrated sugar solutions may be used, which may optionally contain gum arabic, talc, polyvinyl pyrrolidone, carbopol gel, polyethylene glycol, and/or titanium dioxide, lacquer solutions, and suitable organic solvents or solvent mixtures.
- Dyestuffs or pigments may be added to the tablets or dragee coatings for identification or to characterize different combinations of active compound doses.
- the compounds may be formulated for parenteral administration by injection, e.g., by bolus injection or continuous infusion.
- Formulations for injection may be presented in unit dosage form, e.g., in ampoules or in multi-dose containers, with an added preservative.
- the compositions may take such forms as suspensions, solutions or emulsions in oily or aqueous vehicles, and may contain formulatory agents such as suspending, stabilizing and/or dispersing agents.
- the formulations may be presented in unit-dose or multi-dose containers, for example sealed ampoules and vials, and may be stored in powder form or in a freeze-dried (lyophilized) condition requiring only the addition of the sterile liquid carrier, for example, saline or sterile pyrogen-free water, immediately prior to use.
- sterile liquid carrier for example, saline or sterile pyrogen-free water
- Extemporaneous injection solutions and suspensions may be prepared from sterile powders, granules and tablets of the kind previously described.
- Formulations for parenteral administration include aqueous and non-aqueous (oily) sterile injection solutions of the active compounds which may contain antioxidants, buffers, bacteriostats and solutes which render the formulation isotonic with the blood of the intended recipient; and aqueous and non-aqueous sterile suspensions which may include suspending agents and thickening agents.
- Suitable lipophilic solvents or vehicles include fatty oils such as sesame oil, or synthetic fatty acid esters, such as ethyl oleate or triglycerides, or liposomes.
- Aqueous injection suspensions may contain substances which increase the viscosity of the suspension, such as sodium carboxymethyl cellulose, sorbitol, or dextran.
- the suspension may also contain suitable stabilizers or agents which increase the solubility of the compounds to allow for the preparation of highly concentrated solutions.
- the compounds may also be formulated as a depot preparation. Such long acting formulations may be administered by implantation (for example subcutaneously or intramuscularly) or by intramuscular injection.
- the compounds may be formulated with suitable polymeric or hydrophobic materials (for example as an emulsion in an acceptable oil) or ion exchange resins, or as sparingly soluble derivatives, for example, as a sparingly soluble salt.
- compositions may take the form of tablets, lozenges, pastilles, or gels formulated in conventional manner.
- Such compositions may comprise the active ingredient in a flavored basis such as sucrose and acacia or tragacanth.
- the compounds may also be formulated in rectal compositions such as suppositories or retention enemas, e.g., containing conventional suppository bases such as cocoa butter, polyethylene glycol, or other glycerides.
- Compounds of the present invention may be administered topically, that is by non-systemic administration. This includes the application of a compound of the present invention externally to the epidermis or the buccal cavity and the instillation of such a compound into the ear, eye and nose, such that the compound does not significantly enter the blood stream.
- systemic administration refers to oral, intravenous, intraperitoneal and intramuscular administration.
- Formulations suitable for topical administration include liquid or semi-liquid preparations suitable for penetration through the skin to the site of inflammation such as gels, liniments, lotions, creams, ointments or pastes, and drops suitable for administration to the eye, ear or nose.
- the active ingredient may comprise, for topical administration, from 0.001 % to 10% w/w, for instance from 1 % to 2% by weight of the formulation. It may however comprise as much as 10% w/w but preferably will comprise less than 5% w/w, more preferably from 0.1 % to 1 % w/w of the formulation.
- the compounds according to the invention are conveniently delivered from an insufflator, nebulizer pressurized packs or other convenient means of delivering an aerosol spray.
- Pressurized packs may comprise a suitable propellant such as dichlorodifluoromethane, trichlorofluoromethane, dichlorotetrafluoroethane, carbon dioxide or other suitable gas.
- the dosage unit may be determined by providing a valve to deliver a metered amount.
- the compounds according to the invention may take the form of a dry powder composition, for example a powder mix of the compound and a suitable powder base such as lactose or starch.
- the powder composition may be presented in unit dosage form, in for example, capsules, cartridges, gelatin or blister packs from which the powder may be administered with the aid of an inhalator or insufflator.
- Preferred unit dosage formulations are those containing an effective dose, as herein below recited, or an appropriate fraction thereof, of the active ingredient.
- formulations of this invention may include other agents conventional in the art having regard to the type of formulation in question, for example those suitable for oral administration may include flavoring agents.
- the compounds of the invention may be administered orally or via injection at a dose of from 0.1 to 500 mg/kg per day.
- the dose range for adult humans is generally from 5 mg to 2 g/day.
- Tablets or other forms of presentation provided in discrete units may conveniently contain an amount of compound of the invention which is effective at such dosage or as a multiple of the same, for instance, units containing 5 mg to 500 mg, usually around 10 mg to 200 mg.
- the amount of active ingredient that may be combined with the carrier materials to produce a single dosage form will vary depending upon the host treated and the particular mode of administration.
- the compounds of the subject invention can be administered in various modes, e.g. orally, topically, or by injection.
- the precise amount of compound administered to a patient will be the responsibility of the attendant physician.
- the specific dose level for any particular patient will depend upon a variety of factors including the activity of the specific compound employed, the age, body weight, general health, sex, diets, time of administration, route of administration, rate of excretion, drug combination, the precise disorder being treated, and the severity of the indication or condition being treated.
- the route of administration may vary depending on the condition and its severity.
- the compounds described herein may be administered in combination with another therapeutic agent.
- another therapeutic agent such as a pharmaceutically acceptable salt, ester, or prodrug thereof.
- the therapeutic effectiveness of one of the compounds described herein may be enhanced by administration of an adjuvant (i.e., by itself the adjuvant may only have minimal therapeutic benefit, but in combination with another therapeutic agent, the overall therapeutic benefit to the patient is enhanced).
- the benefit of experienced by a patient may be increased by administering one of the compounds described herein with another therapeutic agent (which also includes a therapeutic regimen) that also has therapeutic benefit.
- another therapeutic agent which also includes a therapeutic regimen
- increased therapeutic benefit may result by also providing the patient with another therapeutic agent for diabetes.
- the overall benefit experienced by the patient may simply be additive of the two therapeutic agents or the patient may experience a synergistic benefit.
- a) anti-diabetic agents such as insulin, insulin derivatives and mimetics; insulin secretagogues such as the sulfonylureas, e.g., Glipizide, glyburide and Amaryl; insulinotropic sulfonylurea receptor ligands such as meglitinides, e.g., nateglinide and repaglinide; insulin sensitizer such as protein tyrosine phosphatase-lB (PTP-I B) inhibitors such as PTP-1 12; GSK3 (glycogen synthase kinase-3) inhibitors such as SB-517955, SB-4195052, SB-216763, NN-57-05441 and NN-57-05445; RXR ligands such as GW-0791
- anti-diabetic agents such as insulin, insulin derivatives and mimetics
- insulin secretagogues such as the sulfonylurea
- hypolipidemic agents such as 3-hydroxy-3-methyl-glutaryl coenzyme A (HMG-CoA) reductase inhibitors, e.g., lovastatin, pravastatin, simvastatin, pravastatin, cerivastatin, mevastatin, velostatin, fluvastatin, dalvastatin, atorvastatin, rosuvastatin and rivastatin; squalene synthase inhibitors; FXR (famesoid X receptor) and LXR (liver X receptor) ligands; cholestyramine; fibrates; nicotinic acid and aspirin; c) an anti-obesity agent or appetite regulating agent such as phentermine, leptin, bromocriptine, dexamphetamine, amphetamine, fenfluramine, dexfenfluramine, sibutramine, orlist
- HMG-CoA 3-
- ECE inhibitors e.g. SLV306
- ACE/NEP inhibitors such as omapatrilat, sampatrilat and fasidotril
- angiotensin n antagonists such as candesartan, eprosartan, irbesaitan, losartan, tehnisartan and valsartan, in particular valsartan
- renin inhibitors such as aliskiren, terlakiren, ditekiren, RO 66- 1 132, RO-66-1 168
- ⁇ -adrenergic receptor blockers such as acebutolol, atenolol, betaxolol, bisoprolol, metoprolol, nadolol, propranolol, sotalol and timolol
- inotropic agents such as digoxin, dobutamine and milrin
- a chemotherapeutic agent such as aromatase inhibitors e.g. femara, anti -estrogens, topoisomerase I inhibitors, topoisomerase II inhibitors, microtubule active agents, alkylating agents, antineoplastic antimetabolites, platin compounds, compounds decreasing the protein kinase activity such as a PDGF receptor tyrosine kinase inhibitor preferably miatinib ( ⁇ N- ⁇ 5-[4-(4-methyl-piperazino- methyl)-benzoylamido]-2-methylphenyl ⁇ -4-(3-pyridyl)-2-pyrimidine-amine ⁇ ) described in the European patent application EPA-0564409 as example 21 or 4-Methyl- " N-[3-(4-methyl-imidazol-l-yl)-5- trifluoiOmethyl-phenyl]-3-(4-pyridin-3-yl-pyrimidin-2-yIamino)
- Most preferred combination partners are cholesterol absorption modulator such as Zetia ⁇ and KT6-971or hypolipidemic agents such as 3-hydroxy-3-methyl ⁇ glutaryl coenzyme A (HMG-CoA) reductase inhibitors, e.g., lovastatin, pravastatin, simvastatin, pravastatin, cerivastatin, mevastatin, velostatin, fluvastatin, dalvastatin, atorvastatin, rosuvastatin and rivastatin; squalene synthase inhibitors; FXR (famesoid X receptor) and LXR (liver X receptor) ligands; cholestyramine; fibrates; nicotinic acid and aspirin.
- HMG-CoA 3-hydroxy-3-methyl ⁇ glutaryl coenzyme A reductase inhibitors
- lovastatin e.g., lovastatin, pravastatin
- the multiple therapeutic agents may be administered in any order or even simultaneously. If simultaneously, the multiple therapeutic agents may be provided in a single, unified form, or in multiple forms (by way of example only, either as a single pill or as two separate pills). One of the therapeutic agents may be given in multiple doses, or both may be given as multiple doses. If not simultaneous, the timing between the multiple doses may be any duration of time ranging from a few minutes to four weeks.
- the present invention provides methods for treating NPC-I Ll -mediated disorders in a human or animal subject in need of such treatment comprising administering to said subject an amount of a compound of the present invention effective to reduce or prevent said disorder in the subject in combination with at least one additional agent for the treatment of said disorder that is known in the art.
- the present invention provides therapeutic compositions comprising at least one compound of the present invention in combination with one or more additional agents for the treatment of NPC-I Ll -mediated disorders.
- the present invention includes compounds listed above in the form of salts, in particular acid addition salts. Suitable salts include those formed with both organic and inorganic acids. Such acid addition salts will normally be pharmaceutically acceptable. However, salts of non-pharmaceutically acceptable salts may be of utility in the preparation and purification of the compound in question.
- preferred salts include hydrochloride, hydrobromide, sulfonate, citrate, tartrate, phosphonate, lactate, pyruvate, acetate, succinate, oxalate, fumarate, malate, oxaloacetate, methanesulfonate, ethanesuifonate, p-toluenesulfonate, benzenesulfonate and isethionate salts of compounds of the present invention.
- a salt of a compound can be made by reacting the appropriate compound in the form of the free base with the appropriate acid.
- Individual stereoisomers of compounds can be prepared synthetically from commer- daily available starting materials which contain chiral centers or by preparation of mixtures of enantiomeric products followed by separation such as conversion to a mixture of diastereomers followed by separation or recrystallization, chromatographic techniques, direct separation of enantiomers on chiral chromatographic columns, or any other appropriate method known in the art.
- Starting compounds of particular stereochemistry are either commercially available or can be made and resolved by techniques known in the art.
- the compounds of the present invention may exist as geometric isomers.
- the present invention includes all cis, trans, syn, anti,
- compounds may exist as tautomers; all tautomeric isomers are provided by this invention.
- the compounds of the present invention can exist in unsolvated as well as solvated forms with pharmaceutically acceptable solvents such as water, ethanol, and the like. In general, the solvated forms are considered equivalent to the unsolvated forms for the purposes of the present invention.
- the compounds and formulations of the present invention are also useful for veterinary treatment of companion animals, exotic animals and farm animals, including mammals, rodents, and the like. More preferred animals include horses, dogs, and cats.
- Solvent water, acetic acid, etlianol, tetrahydrofuran, 1,2-dichloroethane, toluen, or the like;
- Catalyst HCI, p-toluenesuHbnic acid, boron trifluoride, or the like.
- the compounds of the invention have an effect on ABCA-I gene expression. It is reported that compounds that effect cholesterol production and absorption such as statins and ezetimibe cause a decrease expression of ABCAl transcript. For example, in " NPC l Ll knockout animals (the target of Ezetimibe) and also in wild type animals treated with ezetimibe, a decrease in ABCAl transcript is seen. Therefore, a stable cell line with 2KB of the ABCA-I 5' promoter region fused to a luciferase reporter was used in a reporter assay to interrogate compounds for the ability to decrease ABCA- I expression.
- the promoter region was made by using PCR to obtain the fragment from human genomic DNA using the following primers; sense strand primer: ATAAGTTGGAGGTCTGGGAGTGGCTA and antisense strand primer : GCTCTGTTGGTG-CGCGGAGCT.
- the genomic fragment included approx 2KB of the genomic ABCAl (accession number Gl:21536375) including promoter elements important to the transcriptional regulation of this gene.
- a human hepatocyte cell line (HEPG2C3A ATCC#CRL- 1074; ATCC, Manassas, VA ) was stably transfected with the above described fragment cloned into a luciferase containing pGL3 vector.
- Transient transfections were done initially to insure activity in cells when stimulated with an RXR agonist, 9-cis retinoic acid.
- Stable cells were made using lenti viral clone construction of the above described ABCAl clone in a Lenti vival luciferase tagged vector with puromycin selection. Following viral production in 293T cells, HEPG2 C3A cells were infected and put under puromycin selective pressure until cells began to divide and behave normally in the presence of puromycin.
- NPCl Ll transcript is a protein shown previously to be impotant in cholesterol absorption: see patent WO05015988A1).
- RTPCR was used to determine the presence of NPCl Ll transcript (NM 13389) in this cell line before and after stable transfection using the following primers: Forward primer ATAGGCGCGCCATGGCGGAGGCCGGCCTGAG and Reverse primer
- RTPCR was performed using Trizol Reagent according to manufacturer's instructions (Invitrogen Corp, Carlsbad Ca) to extract total RNA from the HEPG2C3A cells.
- the mRNA from these total RNA preparations was amplified using Superscript II reverse transcriptase according to manufacturer's instructions (Invitrogen Corp, Carlsbad Ca) with the oligo dT primer and the random hexamer primer for first strand synthesis provided in the kit.
- Various primer sets, including those described above were used to confirm the presence of NPCl Ll transcript.
- the resulting cDNA of NPCl Ll was cloned into an appropriate prokaryotic vector and sequenced to confirm identity.
- HepG2C3A cells were found to have ample amounts of the NPCl Ll transcript before and after stable transfection. Following confirmation of the presence of " NPCl Ll , stably transfected cells were expanded for luciferase reporter assays under the selection of puromycin and assayed for luciferase activity following 9-cis retinoic acid stimulation. Luciferase reporter assays were run in high throughput 1536 well format using 5 ul of cells at a concentration of 500000 cells/ml of media.
- Branched DNA is a method of accurate RNA quantification that offers RNA quantification directly from cell lysate.
- the branched DNA technology introduces multiple labels onto a target nucleic acid.
- the sensitivity stems from the use of a set of branched reporter probes. Each probe has 15 branches, and each branch can react with up to three alkaline phosphatase-labeled detection probes. This leads to a high degree of labeling of the target and kits to perform branched DNA assays can be obtained from Genospectra, Inc (Fremont, CA).
- Branched DNA assays were performed with select compounds using specific probes for HMG COA synthase and ABCA 1 RNA.
- the HEPG2C3A cells described previously along with the human intestinal cell lines: CaCo2 (ATCC # HTB-237; colorectal adenocarcinoma; ATCC: Manassas, VA) and the human intestinal cell line FHs (ATCC # CCL-241 ; normal fetal small intestine, ATCC: Manassas, VA ) were plated in clear bottom 96 well format in 1% fetal bovine serum overnight and treated with compounds the following morning. Cells were plated at a concentration of 200000 cells/ ml media.
- DMSO vehicle used for compound dilution
- concentration of compounds was kept at 0.8%.
- Compound remained on cells for 20 hr and cells were lysed following compound incubation according to kit instructions. Lysed cells release mRNA in the presence of target probes. Target mRNA from lysed cells is captured by hybridization and transferred to the Genospectra Capture Plate. Signal amplification is performed by hybridization of the bDNA Amplifier and Label Probe. Addition of chemiluminescence substrate yields a signal that is proportional to the amount of mRNA present in the sample.
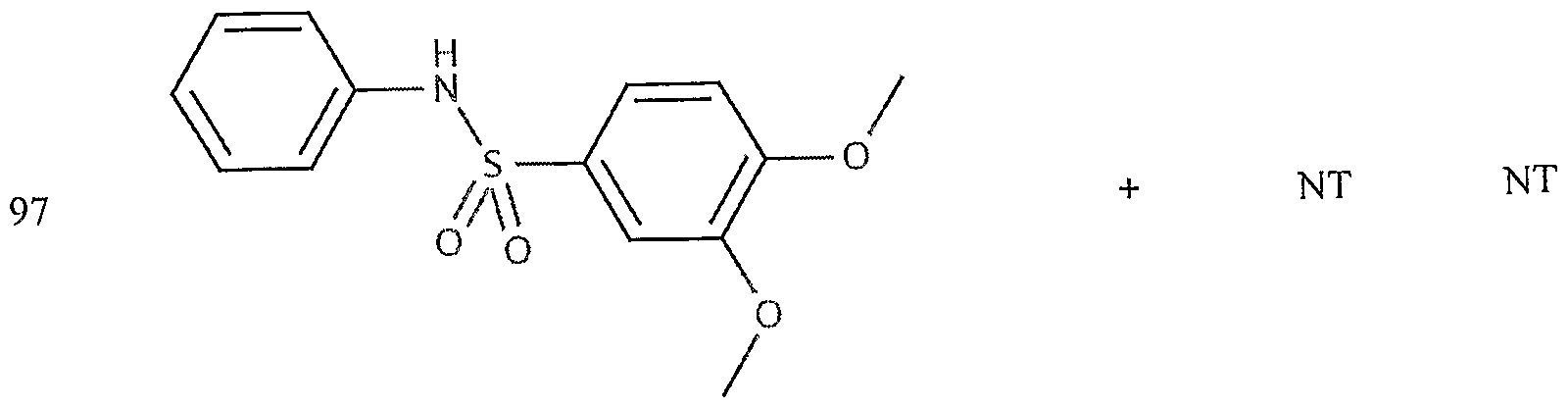
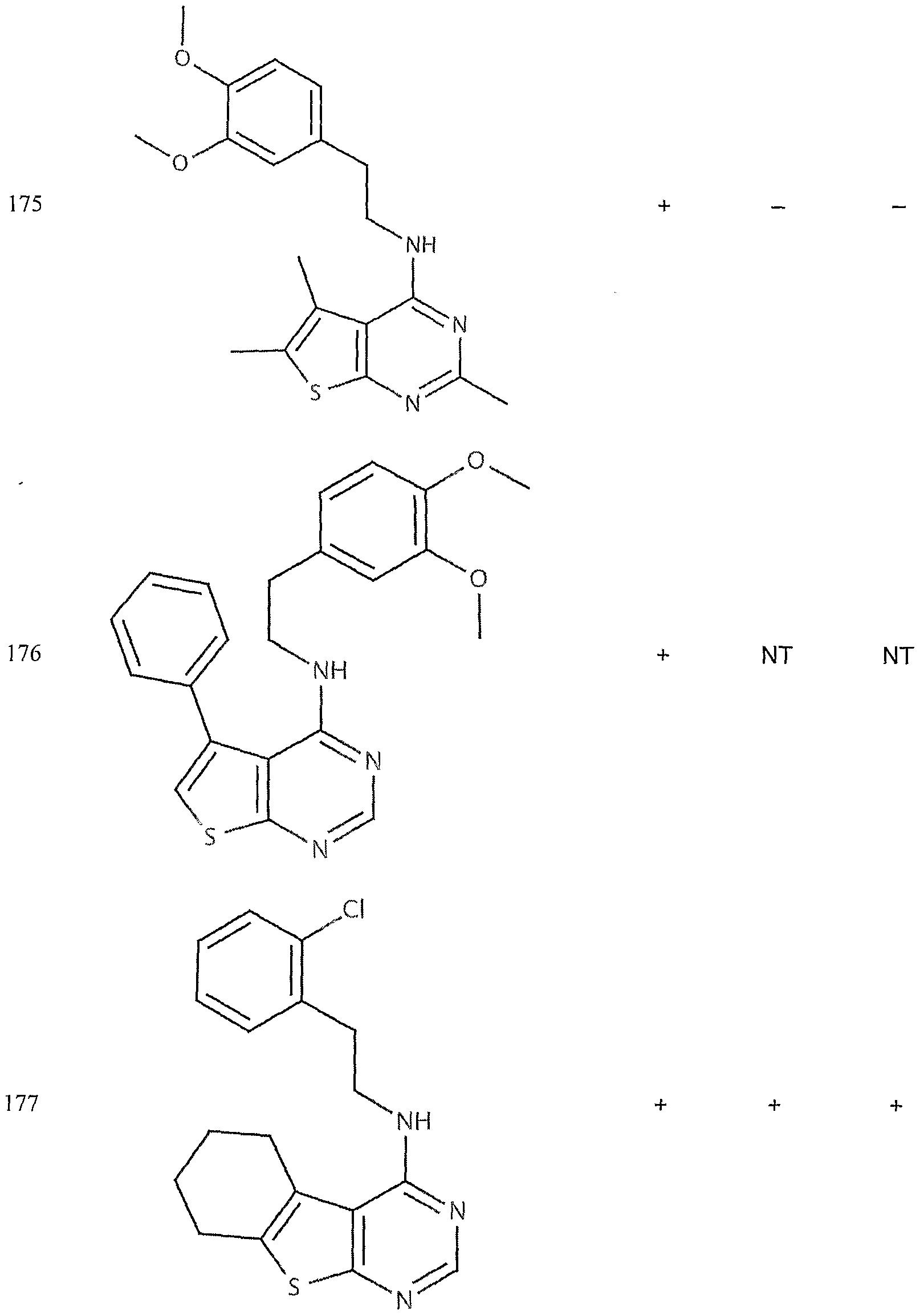
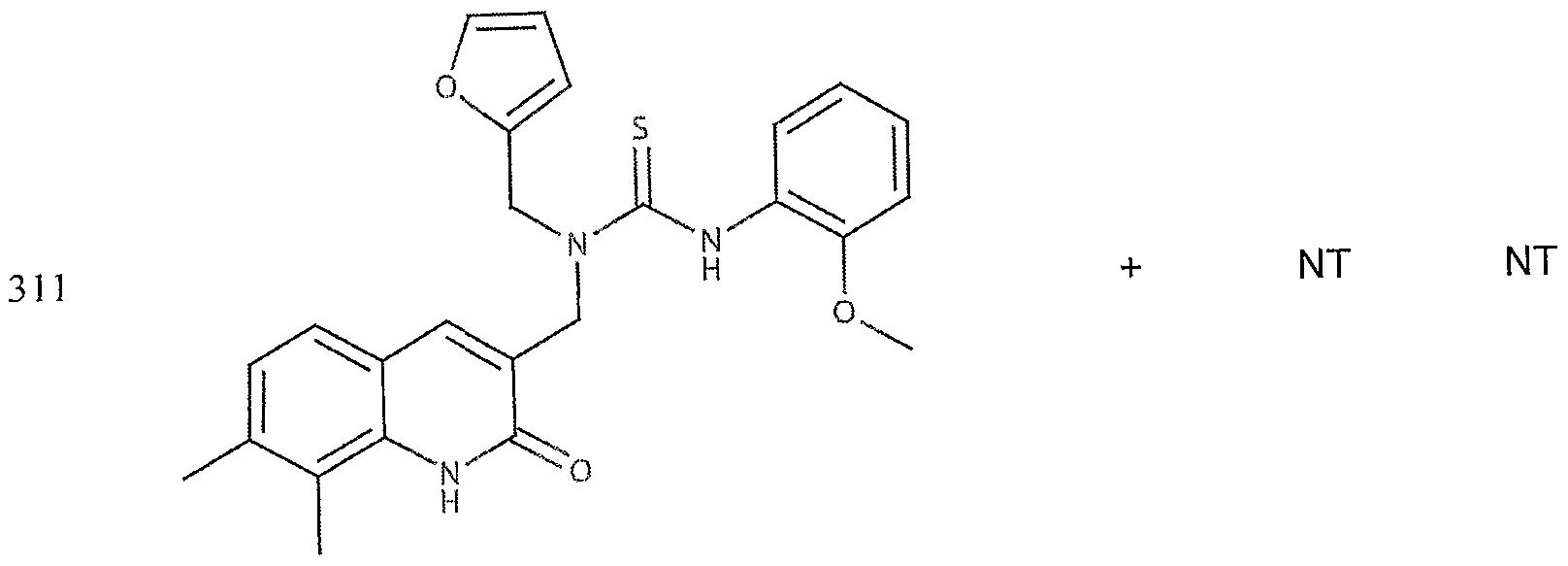
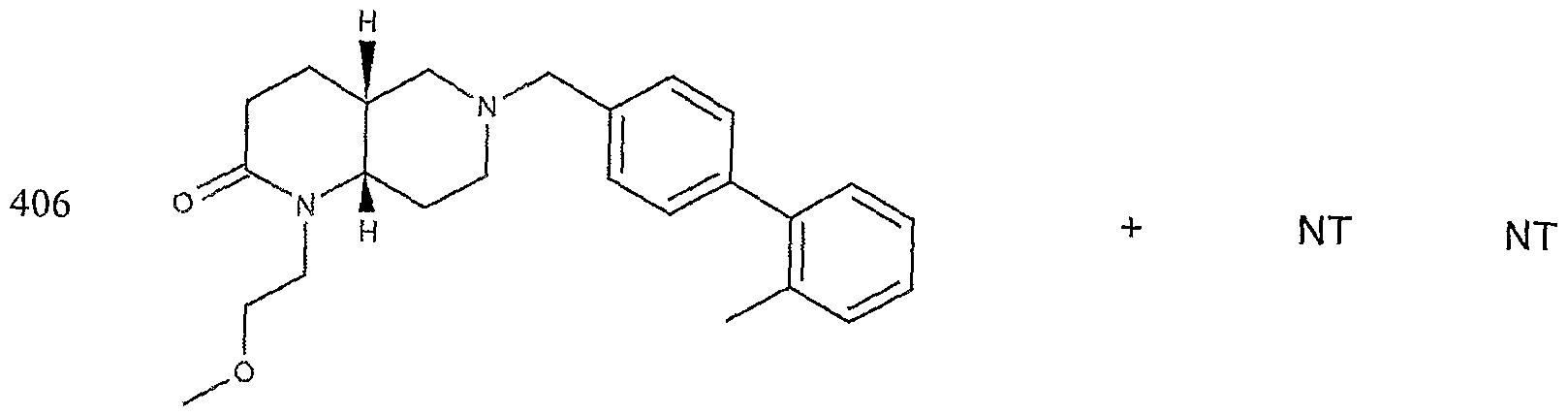
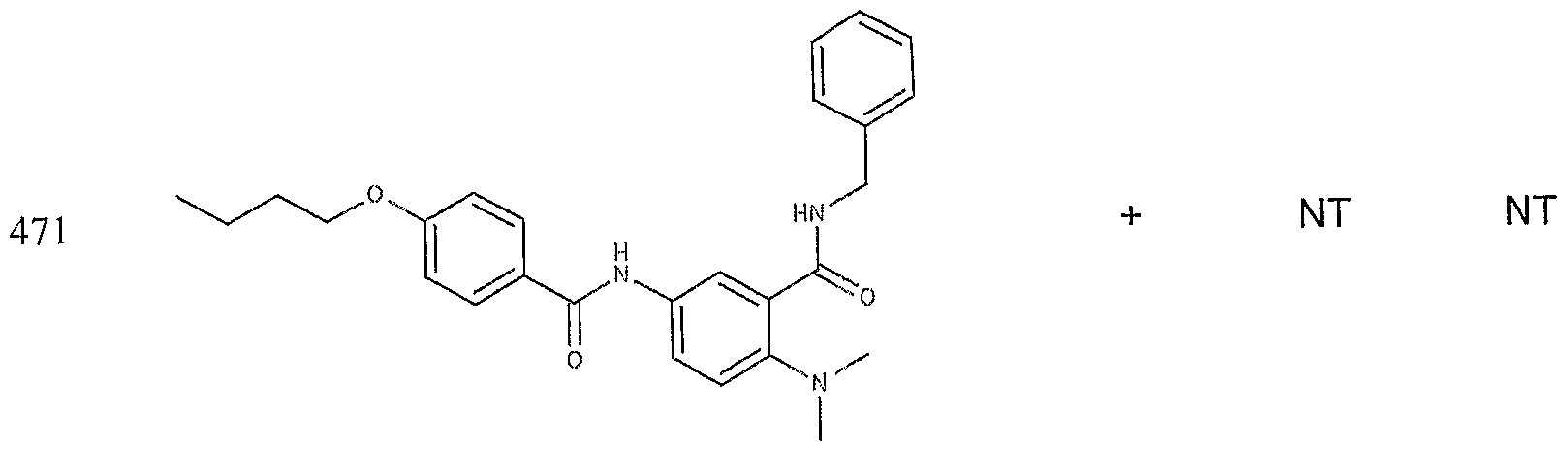
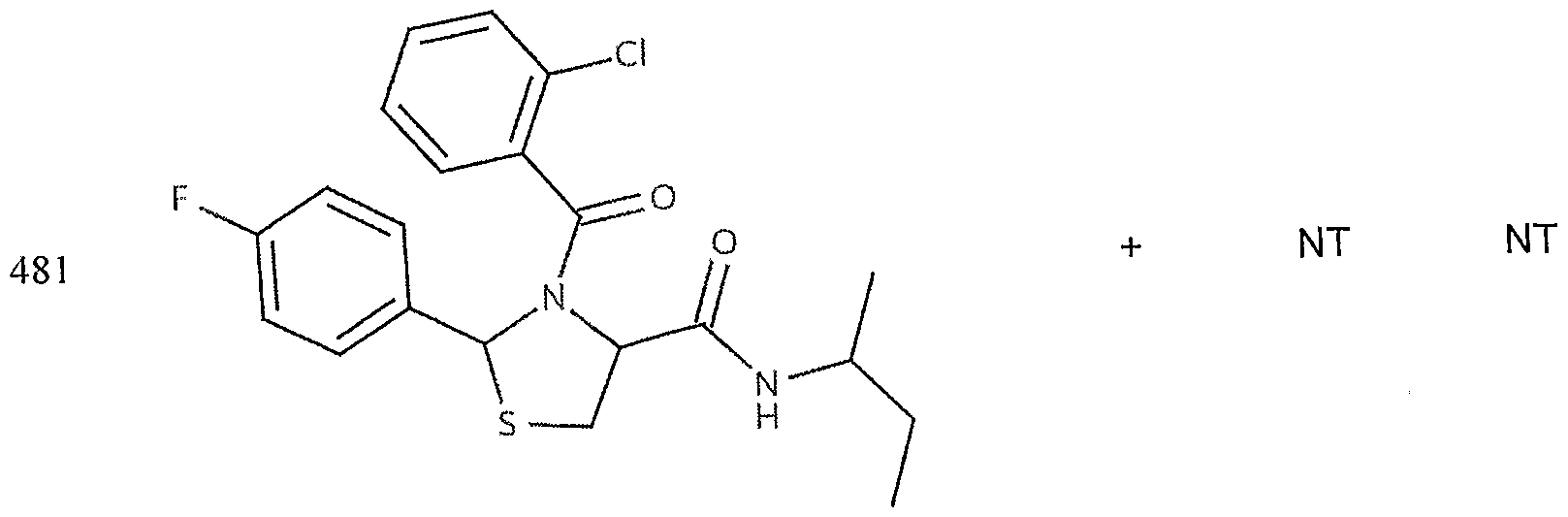
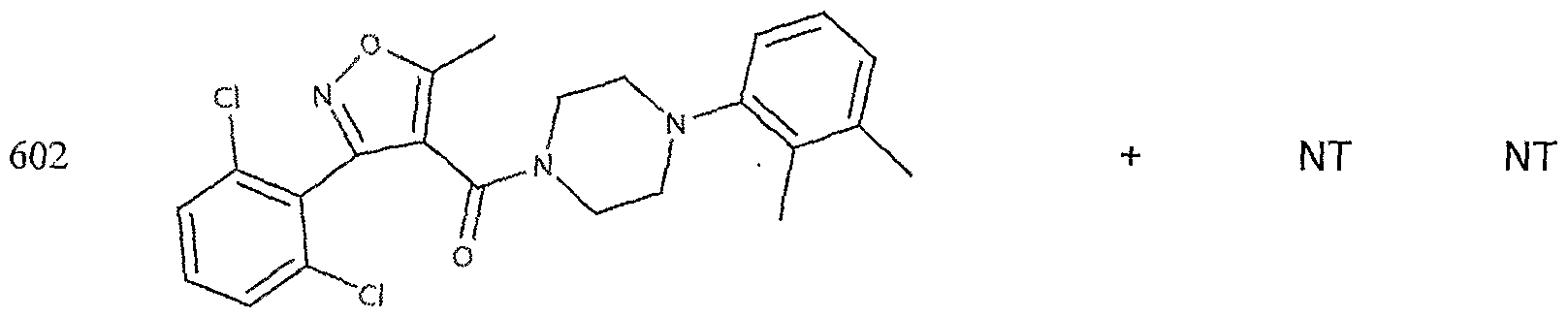
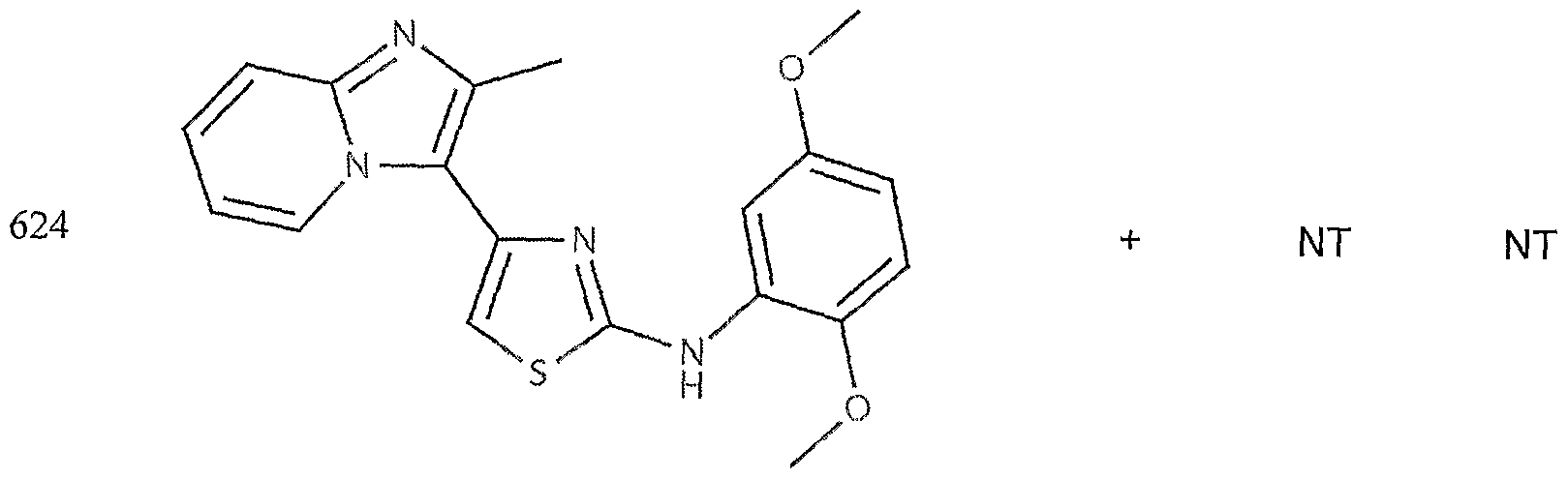
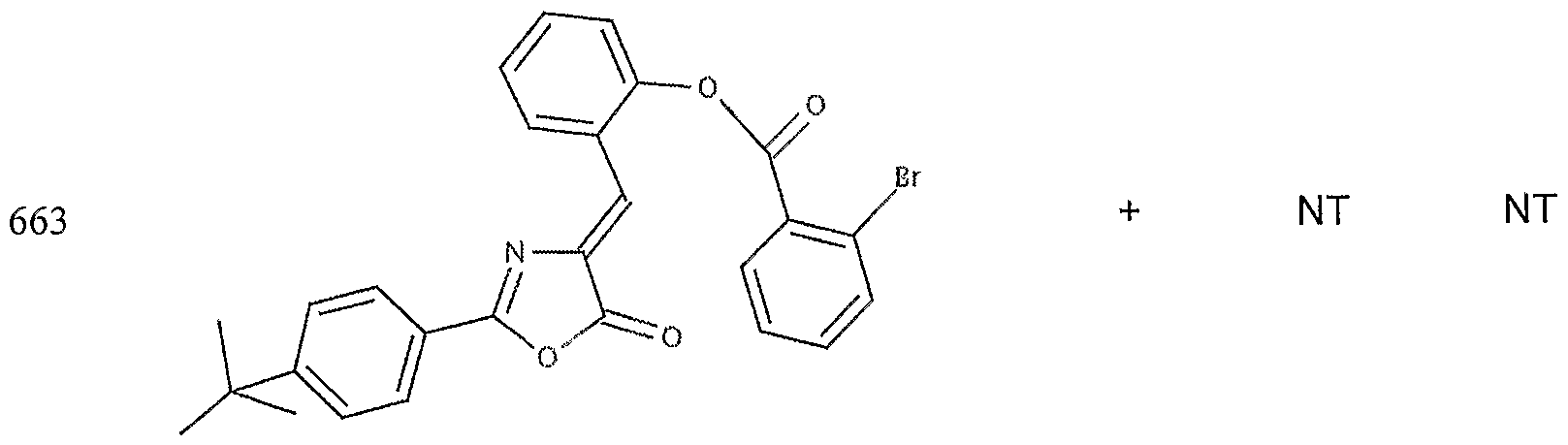
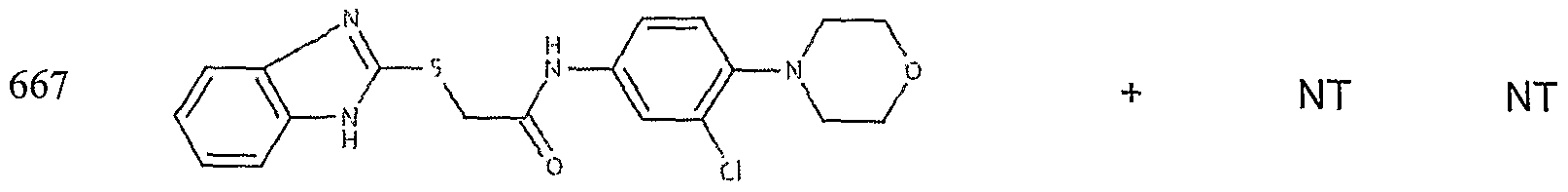
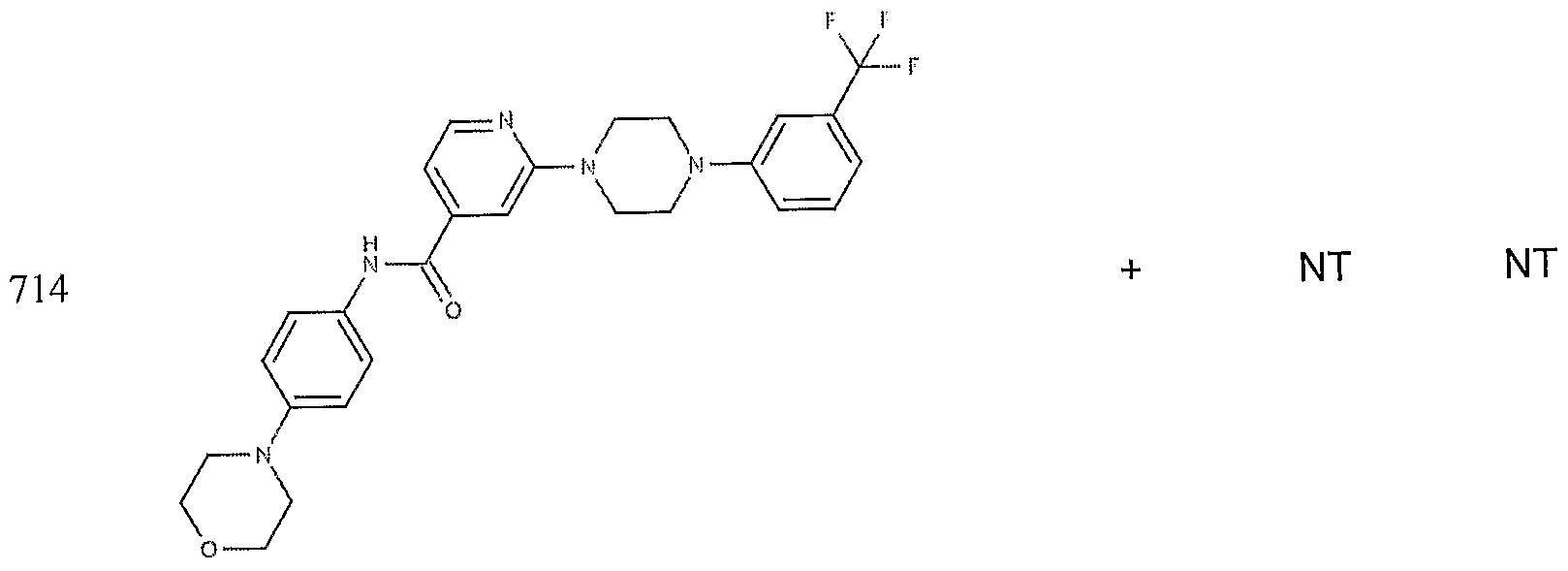
- the target probes used in this experiment were specific to HMG CoA synthase and ABCAl (NMJJ05502, cat #PA-10181) purchased from Genospectra and used according to kit instructions. Differential expression as seen with bDNA was qualitatively confirmed with immunofluorescence. Data is represented as (+ or -) for both ABCAl and HGMG CoA synthase, although to be positive in ABCAl assay required a minimal 2X decrease in overall transcriptional activity over vehicle control (DMSO) and to be positive in HMG coA synthase required a minimal 2X increase in transcriptional activity of the target gene over DMSO control. Compounds not tested in these assays are designated by "NT".
- Ezetimibe was used as positive control in bDNA assays as well as antibody experiments.
- the EC50 of ezetimibe in these assays was calculated to be less than 10OnM with an R 2 value of 0.89.
- Several of compounds tested here show similar ECSOs. Briefly, cells were plated and treated with compound as described above and fixed with formaldehyde after compound incubation. Qualitative visualization of protein changes were made (data not shown) using antibodies to HMG CoA synthase protein (chicken polyclonal #AB 14302; Abeam Inc,
- Ezetimibe (I uM final concentration) was used as positive control in antibody experiments and showed a diminished signal with ABCAl antibody in Hep G2C3 A and CaCo2 cells and was completely absent by antibody staining in FhS cells when compared to DMSO treated cells.
- the ezetimibe treated cells showed a moderate increase in staining with the HMG CoA synthase antibody when compared to DMSO treated controls.
- SMILES Simplified Molecular Input Line Entry System
- SMILES is a modern chemical notation system, developed by David Weininger and Daylight Chemical Information Systems, Inc., that is built into all major commercial chemical structure drawing software packages. Software is not needed to interpret SMILES text strings, and an explanation of how to translate SMILES into structures can be found in Weininger, D., J. Chem. Inf. Comput. ScL 1988, 28, 31 -36.
Landscapes
- Health & Medical Sciences (AREA)
- General Health & Medical Sciences (AREA)
- Animal Behavior & Ethology (AREA)
- Chemical & Material Sciences (AREA)
- Veterinary Medicine (AREA)
- Public Health (AREA)
- Medicinal Chemistry (AREA)
- Pharmacology & Pharmacy (AREA)
- Life Sciences & Earth Sciences (AREA)
- Engineering & Computer Science (AREA)
- Bioinformatics & Cheminformatics (AREA)
- General Chemical & Material Sciences (AREA)
- Organic Chemistry (AREA)
- Nuclear Medicine, Radiotherapy & Molecular Imaging (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Epidemiology (AREA)
- Hematology (AREA)
- Obesity (AREA)
- Cardiology (AREA)
- Heart & Thoracic Surgery (AREA)
- Diabetes (AREA)
- Urology & Nephrology (AREA)
- Vascular Medicine (AREA)
- Child & Adolescent Psychology (AREA)
- Pharmaceuticals Containing Other Organic And Inorganic Compounds (AREA)
Abstract
The present invention relates to compounds and methods useful as inhibitors of cholesterol absorption for the treatment or prevention of vascular disease and atherosclerosis.
Description
CELULLAR CHOLESTEROL ABSORPTION MODIFIERS
FIELD OF THE INVENTION
The present invention is directed to new compounds and compositions and their application as pharmaceuticals for the treatment of disease. Methods of modulation of cholesterol absorption activity in a human or animal subject are also provided for the treatment diseases such as vascular disease and atherosclerosis.
BACKGROUND OF THE INVENTION
A factor leading to development of vascular disease, a leading cause of death in industrialized nations, is elevated serum cholesterol. It is estimated that 19% of Americans between the ages of 20 and 74 years of age have high serum cholesterol. The most prevalent form of vascular disease is arteriosclerosis, a condition associated with the thickening and hardening of the arterial wall. Arteriosclerosis of the large vessels is referred to as atherosclerosis. Atherosclerosis is the predominant underlying factor in vascular disorders such as coronary artery disease, aortic aneurysm, arterial disease of the lower extremities and cerebrovascular disease.
Cholesteryl esters are a major component of atherosclerotic lesions and the major storage form of cholesterol in arterial wall cells. Formation of cholesteryl esters is also a step in the intestinal absorption of dietary cholesterol. Thus, inhibition of cholesteryl ester formation and reduction of serum cholesterol can inhibit the progression of atherosclerotic lesion formation, decrease the accumulation of cholesteryl esters in the arterial wall, and block the intestinal absorption of dietary cholesterol.
The regulation of whole-body cholesterol homeostasis in mammals and animals involves the regulation of intestinal cholesterol absorption, cellular cholesterol trafficking, dietary cholesterol and modulation of cholesterol biosynthesis, bile acid biosynthesis, steroid biosynthesis and the catabolism of the cholesterol-containing plasma lipoproteins. Regulation of intestinal cholesterol absorption has proven to be an effective means by which to regulate serum cholesterol levels. For example, a cholesterol absorption inhibitor, ezetimibe has been shown to be effective in this regard. Ezetimibe is believed to prevent cholesterol absorption by inhibiting NPC I Ll . ( WO05015988 Al). Furthermore, data has been presented that transcription of two cholesterol modifying proteins, HMG COA Synthase and ABCAl (ATPase binding cassette protein Family Al) are regulated by NPCl Ll, see Davis, et al, (2004) JBC 32:, .33586-33592. In this paper, both NPCl Ll knockout animals and normal animals treated with Ezetimibe show decreased expression of HMG COA synthase and increased expression of ABCAl . It should be noted that other cholesterol modifying agents, such as statins, show a similar transcriptional profile in regards to ABCAl ; see Wong, et al., (2004) Arteriosclerosis, Thrombosis, and Vascular Biol. 24:2365, suggesting that this is a common compensatory theme in effective cholesterol modifying agents.
NPCl Ll is an N-glycosylated protein comprising a a trans-golgi network to plasma membrane transport signal; see Bos, et al., (1993) EMBO J. 12:2219-2228; Humphrey, et al., (1993) J. Cell. Biol. 120: 1 123-1 135; Ponnambalam, et al., (1994) J. Cell. Biol. 125:253-268 and Rothman, et al., (1996) Science 272:227-234) which exhibits limited tissue distribution and gastrointestinal abundance. Also, the human NPClLl promoter includes a Sterol Regulated Element Binding Protein 1 (SREBPl) binding consensus sequence (Athanikar, et al., (1998) Proc. Natl. Acad. Sci. USA 95:4935-4940; Ericsson, et al., (1996) Proc. Natl. Acad. Sci. USA 93:945-950; Metherall, et al., (1989) J. Biol. Chem. 264:15634- 15641 ; Smith, et al., (1990) J. Biol. Chem. 265:2306-2310; Bennett, et al., (1999) J. Biol. Chem. 274:13025-13032 and Brown, et al., (1997) Cell 89:331-340). MPClLl has 42% amino acid sequence homology to human NPCl (Genbank Accession No. AF002020), a receptor responsible for Niemann-
Pick Cl disease (Carstea, et al., (1997) Science 277:228-231). Niemann-Pick Cl disease is a rare genetic disorder in humans which results in accumulation of low density lipoprotein (LDL)-derived unesterified cholesterol in lysosomes (Pentchev, et al., (1994) Biochim. Biophys. Acta. 1225: 235-243 and Vanier, et al., (1991) Biochim. Biophys. Acta. 1096:328-337). In addition, cholesterol accumulates in the trans- golgi network of npcl .sup- cells, and relocation of cholesterol, to and from the plasma membrane, is delayed. NPCl and NPClLl each possess 13 transmembrane spanning segments as well as a sterol- sensing domain (SSD). Several other proteins, including HMG-CoA Reductase (HMG-R), Patched (PTC) and Sterol Regulatory Element Binding Protein Cleavage-Activation Protein (SCAP), include an SSD which is involved in sensing cholesterol levels possibly by a mechanism which involves direct cholesterol binding (Gil, et al., (1985) Cell 41 :249-258; Kumagai, et al., (1995) J. Biol. Chem. 270: 19107-191 13 and Hua, et al., (1996) Cell 87:415-426).
SUMMARY OF THE INVENTION Novel compounds and pharmaceutical compositions that prevent cholesterol absorption by presumably inhibiting NPCl Ll , though the mechamism of action of these compounds are still to be confirmed, have been found together with methods of synthesizing and using the compounds including methods for inhibiting or modulating cholesterol absorption in a patient by administering the compounds. The present invention discloses a class of compounds, useful in treating NPC-I Ll -mediated disorders and conditions, defined by structural Formula I:
(0 or a salt, ester, or prodrug thereof, wherein:
R1 and R2 are independently selected from the group consisting of hydrogen, lower alkyl, lower alkoxy, lower alkylamino, lower alkenyl, lower alkynyl, lower alkoxyalkyl, lower
alkylaminoalkyl, lower alkylcarbonyl, lower alkoxycarbonylalkyl, lower aminocarbonylalkyl, amido, lower aminoalkyl, aryl, arylalkyl, arylalkenyl, arylalkynyl, lower cycloalkyl, lower cycloalkylalkyl, lower haloalkyl, lower perhaloalkyl, lower alkylthioalkyl, lower alkylsulfonylalky], lower alkylsulfonyl, lower perhaloalkylsulfonyl, lower cycloalkylsulfonyl, heteroaryl, heteroarylalkyl, heteroarylalkenyl, and heterocycloalkyl, any of which may be optionally substituted; or R.' and R2, together with the atoms to which they are attached, may be joined to form an optionally substituted heterocycloalkyl moiety; and
R3 is selected form the group consisting of lower alkyl, lower alkenyl, lower alkynyl, lower alkoxyalkyl, lower alkylaminoalkyl, lower aminoalkyl, aryl, arylalkyl, arylalkenyl, arylalkynyl, lower cycloalkyl, lower cycloalkylalkyl, lower haloalkyl, lower perhaloalkyl, heteroaryl, heteroarylalkyl, heteroarylalkenyl, and heterocycloalkyl, any of which may be optionally substituted; or R1 and R3, together with the atoms to which they are attached, may be joined to form an optionally substituted heterocycloalkyl moiety.
The present invention discloses a class of compounds, useful in treating NPC-I Ll -mediated disorders and conditions, defined by structural Formula II:
(II)
or a salt, ester, or prodrug thereof, wherein:
X1 is selected from the group consisting of C(R4) and "N; X2 is selected from the group consisting of C(R5) and N; X3 is selected from the group consisting of C(R6) and N; X4 is selected from the group consisting of C(R7) and N; and R4, R5, R6, R7, R8, and R9 are independently absent or selected from the group consisting of hydrogen, lower acyl, lower alkenyl, lower alkoxy, lower alkoxyalkyl, lower alkoxycarbonyl, lower alkyl, lower alkylamidoamino, lower alkylamino, lower alkylaminosulfonyl, lower alkylene, lower alkylsulfinyl, lower alkylsulfonyl, lower alkylsulfonylamino, lower alkylthio, lower alkylthioamido, amido, amino, lower aminoalkyl, aminocarbonyl, aryl, arylalkoxy, arylalkyl, arylalkylamino, arylalkylthio, arylamino, arylaminosulfonyl, aryloxy, arylsulfinyl, arylsulfonyl, arylsulfonylamino, arylthio, carboxy, cyano, lower cycloalkyl, lower cycloalkylalkyl, halo, lower haloalkoxy, lower haloalkyl, lower haloalkylcarbonyl, heteroaryl, heteroarylalkoxy, heteroarylalkyl, heteroarylamino, heteroarylaminosulfonyl, heteroaryloxy, heteroarylsulfinyl, heteroarylsulfonyl, heleroarylsulfonylamino, heterocyclo, heterocycloalkoxy, heterocycloalkyl, hydroxy, hydroxyalkyl, nitro, sulfonate, thio, and tiϊsubstituled silyl; alternatively, R4, R5, R6, R7, R8, and R9 may be linked with any of the other R4, R5,
R6, R7, R8, and R9 sites to form an optionally-substituted polycyclic cycloalkyl, aryl, heteroaryl, or heterocyclic ring independent of any other non-adjacent site; and provided that the number of X1"4 that are nitrogen is 2 or 3.
The present invention further discloses a class of compounds, useful in treating NPC-I Ll- mediated disorders and conditions, defined by structural Formula III:
(III) or a salt, ester, or prodrug thereof, wherein:
R10 is selected from the group consisting of aryl, cycloalkyl, heteroaryl, and heterocycloalkyl, any of which may be optionally substituted;
R1 1 and R12 are independently selected from the group consisting of hydrogen, lower acyl, lower alkyl, aroyl, and heteroaroyl , any of which may be optionally substituted Rb and R14 are independently selected from the group consisting of hydrogen, hydroxy, halogen, amido, amino, aminocarbonyl, carboxy, cyano, nitro, sulfonate, thiol, lower acyl, lower alkenyl, lower alkoxy, lower alkoxyalkyl, lower alkoxycarbonyl, lower alkyl, lower alkylamidoamino, lower alkylamino, lower alkylaminosulfonyl, lower alkylenejower alkylsulfinyl, lower alkylsulfonyl, lower alkylsulfonylamino, lower alkylthio, lower alkylthioamido, lower cycloalkyl, lower cycloalkylalkyl, lower haloalkoxy, lower haloalkyl, lower haloalkylcarbonyl, lower hydroxyalkyl, and trisubstituted silyl, any of which may be optionally substituted; and
R15 is selected from the group consisting of hydrogen, amido, aminocarbonyl, hydroxy, lower acyl, lower alkenyl, lower alkoxyalkyl, lower alkoxycarbonyl, lower alkyl, lower alkylamido, lower alkylamino, lower alkylaminosulfonyl, lower alkylene, lower alkylsulfinyl, lower alkylsulfonyl, lower aminoalkyl, aryl, arylalkoxy, arylalkyl, arylalkylamino, arylamido, arylamino, arylaminosulfonyl, arylsulfinyl, arylsulfonyl, lower cycloalkyl, lower cycloalkylalkyl, haloalkylcarbonyl, heteroaryl, heteroarylalkyl, heteroarylamido, heteroarylaminosulfonyl, heteroarylsulfonyl, heterocyclo, heterocycloalkoxy, heterocycloalkyl, heterocycloamido, and lower hydroxyalkyl, any of which may be optionally substituted.
The present invention further discloses a class of compounds, useful in treating NPC-I Ll- mediated disorders and conditions, defined by structural Formula IV:
(IV)
or a salt, ester, or prodrug thereof, wherein:
R16 is selected from the group consisting of optionally substituted aryl, optionally substituted cycloalkyl, optionally substituted heteroaryl, and optionally substituted heterocycloalkyl;
R17 is selected from the group consisting of hydrogen, amido, aminocarbonyl, hydroxy, lower acyl, lower alkenyl, lower alkoxyalkyl, lower alkoxycarbonyl, lower alkyl, lower alkylamido, lower alkylamino, lower alkylaminosulfonyl, lower alkylene, lower alkylsulfinyl, lower alkylsulfonyl, lower aminoalkyl, aryl, arylalkoxy, arylalkyl, arylalkylamino, arylamido, arylamino, arylaminosulfonyl, arylsulfϊnyl, arylsulfonyl, lower cycloalkyl, lower cycloalkylalkyl, lower haloalkylcarbonyl, heteroaryl, heteroarylalkyl, heteroarylamido, heteroarylaminosulfonyl, heteroarylsulfonyl, heterocycloalkyl, heterocycloalkoxy, heterocycloamido, and lower hydroxyalkyl, any of which may be optionally substituted;
R18 and R19 are independently selected from the group consisting of hydrogen, amido, amino, carboxy, cyano, halo, hydroxy, nitro, sulfonate, -SH, lower acyl, lower alkenyl, lower alkoxy, lower alkoxyalkyl, lower alkoxycarbonyl, lower alkyl, lower alkylamidoamino, lower alkylamino, lower alkylaminosulfonyl, lower alkylene, lower alkylsulfinyl, lower alkylsulfonyl, lower alkylsulfonylamino, lower alkylthio, lower alkylthioamidoaminoalkyl, aminocarbonyl, aryl, arylalkoxy, arylalkyl, arylalkylamino, arylalkylthio, arylamino, arylaminosulfonyl, aryloxy, arylsulfinyl, arylsulfonyl, arylsulfonylainino, arylthio, lower cycloalkyl, lower cycloalkylalkyl, lower haloalkoxy, lower haloalkyl, lower perhaloalkyl, lower haloalkylcarbonyl, heteroaryl, heteroarylalkoxy, heteroarylalkyl, heteroarylamino, heteroarylaminosulfonyl, heteroaryloxy, heteroarylsulfinyl, heteroarylsulfonyl, heteroarylsulfonylamino, heterocycloalkyl, heterocycloalkoxy, lower hydroxyalkyl, and trisubstituted silyl, any of which may be optionally substituted; or both R16 groups may be joined in a optionally substituted lower cycloalkyl, optionally substituted heteroaryl, or optionally substituted heterocycloalkyl ring;
R20 and R21 are independently selected from the group consisting of hydrogen, lower acyl, lower alkyl, lower alkylamido, aryl, arylalkyl, arylamido, lower cycloalkyl, lower cycloalkylalkyl, lower cycloalkylamido, heteroaryl, heteroarylalkyl, heteroarylamido, heterocycloalkyl, heterocycloalkylalkyl, and heterocycloalkylamido, any of which may be optionally substituted; and
R22, R23, R24 and R25 are independently selected from the group consisting of hydrogen, amido, amino, carboxy, cyano, hydroxy, nitro, sulfonate, thio, halogen, aminocarbonyl, lower acyl, lower alkenyl, lower alkoxy, lower alkoxyalkyl, lower alkoxycarbonyl, lower alkyl, lower alkylamidoamino, lower alkylaniino, lower alkylaminosulfonyl, lower alkylene, lower alkylsulfinyl, lower alkylsulfonyl, lower alkylsulfonylamino, lower alkylthio, lower alkylthioamido, lower aminoalkyl, aryl, arylalkoxy, arylalkyl, arylalkylamino, arylalkylthio, arylamino, arylaminosulfonyl, aryloxy, arylsulfinyl, arylsulfonyl, arylsulfonylamino, arylthio, lower cycloalkyl, lower cycloalkylalkyl, lower haloalkoxy, lower haloalkyl, lower perhaloalkyl, lower haloalkylcarbonyl, heteroaryl, heteroarylalkoxy, heteroarylalkyl, heteroarylamino, heteroarylaminosulfonyl, heteroaryloxy, heteroarylsulfinyl, heteroarylsulfonyl, heteroarylsulfonylamino, heterocycloalkyl, heterocycloalkoxy, lower hydroxyalkyl, and trisubstituted silyl, any of which may be optionally substituted; or R18 and R19, together with the atoms to which they are attached, may be joined to form an optionally substituted aryl, optionally substituted heteroaryl, optionally substituted heterocycloalkyl or optionally substituted cycloalkyl moiety; wherein at least one of R20 and R2' is hydrogen.
Compounds according to the present invention possess useful NPC-I Ll inhibiting or cholesterol absorption-inhibiting activity, and may be used in the treatment or prophylaxis of a disease or condition in which cholesterol absorption plays an active role. Thus, in broad aspect, the present invention also provides pharmaceutical compositions comprising one or more compounds of the present invention together with a pharmaceutically acceptable carrier, as well as methods of making and using the compounds and compositions. In certain embodiments, the present invention provides methods for preventing cholesterol absorption by presumably inhibiting NPC 1 L 1. In other embodiments, the present invention provides methods for treating a cholesterol absorption -mediated disorder in a patient in need of such treatment comprising administering to said patient a therapeutically effective amount of a compound or composition according to the present invention. The present invention also contemplates the use of compounds disclosed herein for use in the manufacture of a medicament for the treatment of a disease or condition ameliorated by the modulation of cholesterol absorption.
DETAILED DESCRIPTION OF THE INVENTION
In another embodiment, the invention provides for compounds selected from the group consisting of Examples 1 to 740, as shown in Table 1.
As used herein, the terms below have the meanings indicated. The term "acyl," as used herein, alone or in combination, refers to a carbonyl attached to an alkenyl, alkyl, aryl, cycloalkyl, heteroaryl, heterocycle, or any other moiety were the atom attached to the carbonyl is carbon. An "acetyl" group refers to a -C(O)CH3 group. Examples of acyl groups include formyl, aikanoyl and aroyl radicals.
The term "acylamino" embraces an amino radical substituted with an acyl group. An example of an "acylamino" radical is acetylamino (CH3C(O)NH-).
The term "alkenyl," as used herein, alone or in combination, refers to a straight-chain or branched-chain hydrocarbon radical having one or more double bonds and containing from 2 to 20, preferably 2 to 6, carbon atoms. Alkenylene refers to a carbon-carbon double bond system attached at two or more positions such as ethenylene [(-CH=CH-),(-C::C-)]. Examples of suitable alkenyl radicals include ethenyl, 1-propenyl, 2-propenyl (allyl), 2-methyl-l-propenyl, 2-methyl-2-propenyl (methylallyl), 3-methyl-2-butenyl (prenyl), 1 ,4-butadienyl and the like.
The term "alkoxy," as used herein, alone or in combination, refers to an alkyl ether radical, wherein the term alkyl is as defined below. Examples of suitable alkyl ether radicals include methoxy, ethoxy, n-propoxy, isopropoxy, n-butoxy, iso-butoxy, sec-butoxy, tert-butoxy, and the like.
The term "alkoxyalkoxy," as used herein, alone or in combination, refers to one or more alkoxy groups attached to the parent molecular moiety through another alkoxy group. Examples include ethoxyethoxy, methoxypropoxyethoxy, ethoxypentoxyethoxyethoxy and the like. The term "alkoxy alkyl," as used herein, alone or in combination, refers to an alkoxy group attached to the parent molecular moiety through an alkyl group. The term "alkoxyalkyl" also embraces alkoxyalkyl groups having one or more alkoxy groups attached to the alkyl group, that is, to form monoalkoxyalkyl and dialkoxyalkyl groups.
The term "alkoxycarbonyl," as used herein, alone or in combination, refers to an alkoxy group attached to the parent molecular moiety through a carbonyl group. Examples of such "alkoxycarbonyl" groups include methoxycarbonyl, ethoxycarbonyl, propoxycarbonyl, butoxy carbonyl and hexyloxycarbonyl.
The term "alkoxycarbonylalkyl" embraces radicals having "alkoxycarbonyl", as defined above substituted to an alkyl radical. More preferred alkoxycarbonylalkyl radicals are "lower alkoxycarbonylalkyl" having lower alkoxycarbonyl radicals as defined above attached to one to six carbon atoms. Examples of such lower alkoxycarbonylalkyl radicals include methoxycarbonylmethyl.
The term "alkyl," as used herein, alone or in combination, refers to a straight-chain or branched-chain alkyl radical containing from 1 to and including 20, preferably 1 to 10, and more preferably 1 to 6, carbon atoms. Alkyl groups may be optionally substituted as defined herein. Examples of alkyl radicals include methyl, ethyl, n-propyl, isopropyl, n-butyl, isobutyl, sec-butyl, tert-butyl, pentyl, iso-amyl, hexyl, octyl, noyl and the like. The term "alkylene," as used herein, alone or in combination, refers to a saturated aliphatic group derived from a straight or branched chain saturated hydrocarbon attached at two or more positions, such as methylene (-CH2-).
The term "alkylamino," as used herein, alone or in combination, refers to an alkyl group attached to the parent molecular moiety through an amino group. Suitable alkylamino groups may be mono- or dialkylated, forming groups such as, for example, N-methylamino, N-ethylamino, N,N- dimethylamino, N,N-diethylamino and the like.
The term "alkylaminocarbonyl" as used herein, alone or in combination, refers to an alkylamino group attached to the parent molecular moiety through a carbonyl group. Examples of such radicals include N-methylaminocarbonyl and N,M-dimethylcarbonyl.
The term "alkylcarbonyl" and "alkanoyl," as used herein, alone or in combination, refers to an alkyl group attached to the parent molecular moiety through a carbonyl group. Examples of such groups include methylcarbonyl and ethylcarbonyl.
The term "alkyl idene," as used herein, alone or in combination, refers to an alkenyl group in which one carbon atom of the carbon-carbon double bond belongs to the moiety to which the alkenyl group is attached. The term "alkylsulfinyl," as used herein, alone or in combination, refers to an alkyl group attached to the parent molecular moiety through a sulfinyl group. Examples of alkylsulfinyl groups include methylsulfinyl, ethylsulfinyl, butylsulfinyl and hexylsulfϊnyl.
The term "alkylsulfonyl," as used herein, alone or in combination, refers to an alkyl group attached to the parent molecular moiety through a sulfonyl group. Examples of alkylsulfinyl groups include methanesulfonyl, ethanesulfonyl, tert-butanesulfonyl, and the like.
The term "alkylthio," as used herein, alone or in combination, refers to an alkyl thioether (R-S- ) radical wherein the term alkyl is as defined above. Examples of suitable alkyl thioether radicals include methylthio, ethylthio, n-propylthio, isopropylthio, n-butylthio, iso-butylthio, sec-butylthio, tert-butylthio, ethoxyethylthio, methoxypropoxyethylthio, ethoxypentoxyethoxyethylthio and the like. The term "alkylthioalkyl" embraces alkylthio radicals attached to an alkyl radical.
Alkylthioalkyl radicals include "lower alkylthioalkyl" radicals having alkyl radicals of one to six carbon atoms and an alkylthio radical as described above. Examples of such radicals include methylthiomethyl.
The term "alkynyl," as used herein, alone or in combination, refers to a straight-chain or branched chain hydrocarbon radical having one or more triple bonds and containing from 2 to 20, preferably from 2 to 6, more preferably from 2 to 4, carbon atoms. "Alkynylene" refers to a carbon- carbon triple bond attached at two positions such as ethynylene (-C:::C-, -C≡C-). Examples of alkynyl radicals include ethynyl, propynyl, hydroxypropynyl, butyn-1-yl, butyn-2-yl, pentyn-1 -yl, pentyn-2-yl, 4-methoxypentyn-2-yl, 3-methylbutyn-l-yl, hexyn-1 -yl, hexyn-2-yl, hexyn-3-yl, 3,3-dimethylbutyn-l -yl, and the like. The term "amido," as used herein, alone or in combination, refers to an amino group as described below attached to the parent molecular moiety through a carbonyl group. The term "C-amido" as used herein, alone or in combination, refers to a -C(=O)-NR2 group with R as defined herein. The term "N-amido" as used herein, alone or in combination, refers to a RC(=O)NH- group, with R as defined herein. The term "amino," as used herein, alone or in combination, refers to — NRR , wherein R and R are independently selected from the group consisting of hydrogen, alkenyl, alkoxy, alkoxyalkyl, alkoxycarbonyl, alkyl, alkylcarbonyl, aryl, arylalkenyl, arylalkyl, cycloalkyl, haloalkylcarbonyl, heteroaryl, heteroarylalkenyl, heteroarylalkyl, heterocycle, heterocycloalkeny], and heterocycloalkyl,
wherein the aryl, the aryl part of the arylalkenyl, the arylalkyl, the heteroaryl, the heteroaryl part of the heteroarylalkenyl and the heteroarylalkyl, the heterocycle, and the heterocycle part of the heterocycloalkenyl and the heterocycloalkyl can be optionally substituted as defined herein with one, two, three, four, or five substituents. The term "aminoalkyl," as used herein, alone or in combination, refers to an amino group attached to the parent molecular moiety through an alkyl group. Examples include aminomethyl, aminoethyl and aminobutyl.
The terms "aminocarbonyl" and "carbamoyl," as used herein, alone or in combination, refer to an amino-substituted carbonyl group, wherein the amino group can be a primary or secondary amino group containing substituents selected from alkyl, aryl, aralkyl, cycloalkyl, cycloalkylalkyl radicals and the like.
The term "aminocarbonylalkyl," as used herein, alone or in combination, refers to an aminocarbonyl radical attached to an alkyl radical, as described above. An example of such radicals is aminocarbonylmethyl. The term "amidino" denotes an -C(NH)NH2 radical. The term "cyanoamidino" denotes an -C(N-CN)NH2 radical.
The term "aralkenyl" or "arylalkenyl," as used herein, alone or in combination, refers to an aryl group attached to the parent molecular moiety through an alkenyl group.
The term "aralkoxy" or "arylalkoxy," as used herein, alone or in combination, refers to an aryl group attached to the parent molecular moiety through an alkoxy group. The term "aralkyl" or "arylalkyl," as used herein, alone or in combination, refers to an aryl group attached to the parent molecular moiety through an alkyl group.
The term "aralkylamino" or "arylalkylamino," as used herein, alone or in combination, refers to an arylalkyl group attached to the parent molecular moiety through a nitrogen atom, wherein the nitrogen atom is substituted with hydrogen. The term "aralkylidene" or "arylalkylidene," as used herein, alone or in combination, refers to an aryl group attached to the parent molecular moiety through an alkylidene group
The term "aralkylthio" or "arylalkylthio," as used herein, alone or in combination, refers to an arylalkyl group attached to the parent molecular moiety through a sulfur atom.
The term "aralkynyl" or "arylalkynyl," as used herein, alone or in combination, refers to an aryl group attached to the parent molecular moiety through an alkynyl group.
The term "aralkoxycarbonyl," as used herein, alone or in combination, refers to a radical of the formula aralkyl-O-C(O)- in which the term "aralkyl," has the significance given above. Examples of an aralkoxycarbonyl radical are benzyloxycarbonyl (Z or Cbz) and 4-methoxyphenylmethoxycarbony! (MOS). The term "aralkanoyl," as used herein, alone or in combination, refers to an acyl radical derived from an aryl-substituted alkaπecarboxylic acid such as benzoyl, phenylacetyl, 3-phenylpropiony! (hydrocinnamoyl), 4-phenylbutyryl, (2-naphthyl)acelyl, 4-chlorohydrocinnamoyl, A- aminohydiOcinnamoyl, 4-inethoxyhydiOcinnamoyl, and the like. The term "aroyl" refers to an acyl
radical derived from an arylcarboxylic acid, "aryl" having the meaning given below. Examples of such aroyl radicals include substituted and unsubstituted benzoyl or napthoyl such as benzoyl, 4- chlorobenzoyl, 4-carboxybenzoyl, 4-(benzyloxycarbonyl)benzoyl, 1 -naphthoyl, 2-naphthoyl, 6-carboxy- 2-naphthoyl, 6-(benzyloxycarbonyl)-2 -naphthoyl, 3-benzyloxy-2-naphthoyl, 3-hydroxy-2-naρhthoyl, 3- (benzyloxyformamido)-2-naphthoyl, and the like.
The term "aryl," as used herein, alone or in combination, means a carbocyclic aromatic system containing one, two or three rings wherein such rings may be attached together in a pendent manner or may be fused. The term "aryl" embraces aromatic radicals such as benzyl, phenyl, naphthyl, anthracenyl, phenanthryl, indanyl, indenyl, annulenyl, azulenyl, tetrahydronaphthyl, and biphenyl. The term "arylamino" as used herein, alone or in combination, refers to an aryl group attached to the parent moiety through an amino group, such as methylamino, N-phenylamino, and the like.
The terms "arylcarbonyl" and "aroyl," as used herein, alone or in combination, refer to an aryl group attached to the parent molecular moiety through a carbonyl group.
The term "aryloxy," as used herein, alone or in combination, refers to an aryl group attached to the parent molecular moiety through an oxygen atom.
The term "arylsulfonyl," as used herein, alone or in combination, refers to an aryl group attached to the parent molecular moiety through a sulfonyl group.
The term "arylthio," as used herein, alone or in combination, refers to an aryl group attached to the parent molecular moiety through a sulfur atom. The terms "carboxy" or "carboxyl", whether used alone or with other terms, such as
"carboxyalkyl", denotes -CO2H.
The terms "benzo" and "benz," as used herein, alone or in combination, refer to the divalent radical Q^= derived from benzene. Examples include benzothiophene and benzimidazole.
The term "O-carbamyl" as used herein, alone or in combination, refers to a -OC(O)NR, group-with R as defined herein.
The term "N-carbamyl" as used herein, alone or in combination, refers to a ROC(O)NH- group, with R as defined herein.
The term "carbonyl," as used herein, when alone includes formyl [-C(O)H] and in combination is a -C(O)- group. The term "carboxy," as used herein, refers to -C(O)OH or the corresponding "carboxylate" anion, such as is in a carboxylic acid salt. An "O-carboxy" group refers to a RC(O)O- group, where R is as defined herein. A "C-carboxy" group refers to a — C(O)OR groups where R is as defined herein.
The term "cyano," as used herein, alone or in combination, refers to -CN.
The term "cycloalkyl," as used herein, alone or in combination, refers to a saturated or partially saturated monocyclic, bicyclic or tricyclic alkyl radical wherein each cyclic moiety contains from 3 to 12, preferably five to seven, carbon atom ring members and which may optionally be a benzo fused ring system which is optionally substituted as defined herein. Examples of such cycloalkyl radicals include cyclopropyl, cyclobutyl, cyclopentyl, cyclohexyl, cycloheptyl, octahydronaphthyl, 2,3-dihydro-l H-
indenyl, adamantyl and the like. "Bicyclic" and "tricyclic" as used herein are intended to include both fused ring systems, such as decahydonapthalene, octahydronapthalene as well as the multicyclic (multicentered) saturated or partially unsaturated type. The latter type of isomer is exemplified in general by bicyclo[2,2,2]octane, bicyclo[2,2,2]octane, bicyclo[l,l,l]pentane, camphor and bicyclo[3,2,l]octane. The term "ester," as used herein, alone or in combination, refers to a carbonyl group bridging two moieties linked at carbon atoms.
The term "ether," as used herein, alone or in combination, refers to an oxy group bridging two moieties linked at carbon atoms.
The term "halo," or "halogen," as used herein, alone or in combination, refers to fluorine, chlorine, bromine, or iodine.
The term "haloalkoxy," as used herein, alone or in combination, refers to a haloalkyl group attached to the parent molecular moiety through an oxygen atom.
The term "haloalkyl," as used herein, alone or in combination, refers to an alkyl radical having the meaning as defined above wherein one or more hydrogens are replaced with a halogen. Specifically embraced are monohaloalkyl, dihaloalkyl and polyhaloalkyl radicals. A monohaloalkyl radical, for one example, may have either an iodo, bromo, chloro or fluoro atom within the radical. Dihalo and polyhaloalkyl radicals may have two or more of the same halo atoms or a combination of different halo radicals. Examples of haloalkyl radicals include fluoromethyl, difluoromethyl, trifluoromethyl, chloromethyl, dichloromethyl, trichloromethyl, trichloromethyl, pentafluoroethyl, heptafluoropropyl, difluorochloromethyl, dichlorofluoromethyl, difluoroethyl, difluoropropyl, dichloroethyl and dichloropropyl. "Haloalkylene" refers to a halohydrocarbyl group attached at two or more positions. Examples include fluoromethylene (— CFH- ), difluoromethylene (— CFj — ), chloromethylene (— CHCl-) and the like. Examples of such haloalkyl radicals include chloromethyl, 1-bromoethyl, fluoromethyl, difluoromethyl, trifluoromethyl, 1 , 1 ,1-trifIuoroethyI, perfluorodecyl and the like. The term "heteroalkyl," as used herein, alone or in combination, refers to a stable straight or branched chain, or cyclic hydrocarbon radical, or combinations thereof, fully saturated or containing from 1 to 3 degrees of unsaturation, consisting of the stated number of carbon atoms and from one to three heteroatoms selected from the group consisting of O, N, and S, and wherein the nitrogen and sulfur atoms may optionally be oxidized and the nitrogen heteroatom may optionally be quaternized. The heteroatom(s) O, N and S may be placed at any interior position of the heteroalkyl group. Up to two heteroatoms may be consecutive, such as, for example, -CH2-NH-OCH3.
The term "heteroaryl," as used herein, alone or in combination, refers to 3 to 7 membered, preferably 5 to 7 membered, unsaturated heterocyclic rings wherein at least one atom is selected from the group consisting of O, S, and N. Heteroaryl groups are exemplified by: unsaturated 3 to 7 membered heteromonocyclic groups containing 1 to 4 nitrogen atoms, for example, pyrrolyl, pyrrolinyl, imidazolyl, pyrazolyl, pyridyl, pyrimidinyl, pyrazinyl, pyridazinyl, triazolyl [e.g., 4H- 1 ,2,4-triazolyl, 1 H-1 ,2,3- triazolyl, 2H-l ,2,3-triazolyl, etc.]tetrazolyl [e.g. 1 H-tetrazolyl, 2H-tetrazoly!, etc.], etc.; unsaturated condensed heterocyclic group containing 1 to 5 nitrogen atoms, for example, indolyl, isoindolyl,
indolizinyl, benzimidazolyi, quinolyl, isoquinolyl, indazolyl, benzotriazolyl, tetrazolopyridazinyl [e.g., tetrazolo[l ,5-b]pyridaziny], etc.], etc.; unsaturated 3 to 6-membered heteromonocyclic groups containing an oxygen atom, for example, pyranyl, furyl, etc.; unsaturated 3 to 6-membered heteromonocyclic groups containing a sulfur atom, for example, thienyl, etc.; unsaturated 3- to 6-membered heteromonocyciic groups containing 1 to 2 oxygen atoms and I to 3 nitrogen atoms, for example, oxazolyl, isoxazoly], oxadiazolyl [e.g., 1,2,4-oxadiazolyl, 1 ,3,4-oxadiazolyl, 1 ,2,5-oxadiazolyl, etc.]etc; unsaturated condensed heterocyclic groups containing 1 to 2 oxygen atoms and 1 to 3 nitrogen atoms [e.g. benzoxazolyl, benzoxadiazolyl, etc.]; unsaturated 3 to 6-membered heteromonocyclic groups containing 1 to 2 sulfur atoms and I to 3 nitrogen atoms, for example, thiazolyl, thiadiazolyl [e.g., 1 ,2,4- thiadiazolyl, 1 ,3,4-thiadiazolyl, 1,2,5-thiadiazolyl, etc.]and isothiazolyl; unsaturated condensed heterocyclic groups containing ! to 2 sulfur atoms and 1 to 3 nitrogen atoms [e.g., benzothiazolyl, benzothiadiazolyl, etc.]and the like. The term also embraces radicals where heterocyclic radicals are fused with aryi radicals. Examples of such fused bicyclic radicals include benzofuryl, benzothienyl, and the like. The term "heteroaralkeπyl" or "heteroarylalkenyl," as used herein, alone or in combination, refers to a heteroaryl group attached to the parent molecular moiety through an alkenyl group.
The term "heteroaralkoxy" or "heteroarylalkoxy," as used herein, alone or in combination, refers to a heteroaryl group attached to the parent molecular moiety through an alkoxy group.
The term "heteroarylalkyl," as used herein, alone or in combination, refers to a heteroaryl group attached to the parent molecular moiety through an alkyl group.
The term "heteroaralkylidene" or "heteroarylalkyiidene," as used herein, alone or in combination, refers to a heteroaryl group attached to the parent molecular moiety through an alkylidene group.
The term ''heteroaryloxy," as used herein, alone or in combination, refers to a heteroaryl group attached to the parent molecular moiety through an oxygen atom.
The term "heteroarylsulfonyl," as used herein, alone or in combination, refers to a heteroaryl group attached to the parent molecular moiety through a sulfonyl group.
The terms "heterocycloalkyl" and, interchangeably, "heterocycle," as used herein, alone or in combination, each refer to a saturated, partially unsaturated, or fully unsaturated monocyclic, bicyclic, or tricyclic heterocyclic radical containing at least one, preferably 1 to 4, and more preferably 1 to 2 heteroatoms as ring members, wherein each said heteroatom may be independently selected from the group consisting of nitrogen, oxygen, and sulfur, and wherein there are preferably 3 to 8 ring members in each ring, more preferably 3 to 7 ring members in each ring, and most preferably 5 to 6 ring members in each ring. "Heterocycloalkyl" and "heterocycle" are intended to include sulfones, sulfoxides, N-oxides of tertiary nitrogen ring members, and carbocyclic fused and benzo fused ring systems; additionally, both terms also include systems where a heterocycle ring is fused to an aryl group, as defined herein, or an additional heterocycle group. Heterocycle groups of the invention are exemplified by aziridinyl, azetidinyl, 1 ,3-benzodioxolyl, dihydroisoindolyi, dihydroisoquinolinyl, dihydrocinnolinyl,
dihydrobenzodioxinyl, dihydro[l ,3]oxazolo[4,5-b]pyridinyl, benzothiazolyl, dihydroindolyl, dihy- dropyridinyl, 1 ,3-dioxanyl, 1 ,4-dioxanyl, 1 ,3-dioxolanyl, isoindolinyl, morpholinyl, piperazinyl, pyrrolidinyl, tetrahydropyridinyl, piperidinyl, thiomorpholinyl, and the like. The heterocycle groups may be optionally substituted unless specifically prohibited. The term "heterocycloalkenyl," as used herein, alone or in combination, refers to a heterocycle group attached to the parent molecular moiety through an alkenyl group.
The term "heterocycloalkoxy," as used herein, alone or in combination, refers to a heterocycle group attached to the parent molecular group through an oxygen atom.
The term "heterocycloalkyl," as used herein, alone or in combination, refers to an alkyl radical as defined above in which at least one hydrogen atom is replaced by a heterocyclo radical as defined above, such as pyrrolidinylmethyl, tetrahydrothienylmethyl, pyridylmethyl and the like.
The term "heterocycloalkylidene," as used herein, alone or in combination, refers to a heterocycle group attached to the parent molecular moiety through an alkylidene group.
The term "hydrazinyl" as used herein, alone or in combination, refers to two amino groups joined by a single bond, i.e., -N-M-.
The term "hydroxy," as used herein, alone or in combination, refers to -OH.
The term "hydroxyalkyl" as used herein, alone or in combination, refers to a linear or branched alkyl group having one to about ten carbon atoms any one of which may be substituted with one or more hydroxyl radicals. Examples of such radicals include hydroxymethyl, hydroxyethyl, hydroxypropyl, hydroxybutyl and hydroxyhexyl.
The term "hydroxyalkyl," as used herein, alone or in combination, refers to a hydroxy group attached to the parent molecular moiety through an alkyl group.
The term "imino," as used herein, alone or in combination, refers to =N-.
The term "iminohydroxy," as used herein, alone or in combination, refers to =N(OH) and =N- O-.
The phrase "in the main chain" refers to the iongest contiguous or adjacent chain of carbon atoms starting at the point of attachment of a group to the compounds of this invention.
The term "isocyanato" refers to a -NCO group.
The term "isothiocyanato" refers to a -NCS group. The phrase "linear chain of atoms" refers to the longest straight chain of atoms independently selected from carbon, nitrogen, oxygen and sulfur.
The term "lower," as used herein, alone or in combination, means containing from 1 to and including 6 carbon atoms.
The term "mercaptoalkyl" as used herein, alone or in combination, refers to an R'SR- group, where R and R' are as defined herein.
The term "mercaptomercaptyl" as used herein, alone or in combination, refers to a RSR'S- group, where R is as defined herein.
The term "mercaptyl" as used herein, alone or in combination, refers to an RS- group, where R is as defined herein.
The term "null" refers to a lone electron pair.
The term "nitro," as used herein, alone or in combination, refers to -NO2. The terms "oxy" or "oxa," as used herein, alone or in combination, refer to -O-.
The term "oxo," as used herein, alone or in combination, refers to =0. The term "perhaloalkoxy" refers to an alkoxy group where all of the hydrogen atoms are replaced by halogen atoms.
The term "perhaloalkyl" as used herein, alone or in combination, refers to an alkyl group where all of the hydrogen atoms are replaced by halogen atoms.
The terms "sulfonate," "sulfonic acid," and "sulfonic," as used herein, alone or in combination, refer the -SO3H group and its anion as the sulfonic acid is used in salt formation.
The term "sulfanyl," as used herein, alone or in combination, refers to -S-. The term "sulfinyl," as used herein, alone or in combination, refers to -S(O)-. The term "sulfonyl," as used herein, alone or in combination, refers to -SO2-.
The term "N-sulfonamido" refers to a RS(=O)2NH- group with R as defined herein. The term "S-sulfonamido" refers to a -SC=O)2NR2, group, with R as defined herein. The terms "thia" and "thio," as used herein, alone or in combination, refer to a -S- group or an ether wherein the oxygen is replaced with sulfur. The oxidized derivatives of the thio group, namely sulfinyl and sulfonyl, are included in the definition of thia and thio.
The term "thioether," as used herein, alone or in combination, refers to a thio group bridging two moieties linked at carbon atoms.
The term "thiol," as used herein, alone or in combination, refers to an -SH group. The term "thiocarbonyl," as used herein, when alone includes thioformyl -C(S)H and in combination is a -C(S)- group.
The term "N-thiocarbamyl" refers to an ROC(S)NH- group, with R as defined herein. The term "O-thiocarbamyl" refers to a -OC(S)NR, group with R as defined herein. The term "thiocyanato" refers to a -CNS group.
The term "trihalomethanesulfonamido" refers to a X3CS(O)2NR- group with X is a halogen and R as defined herein.
The term "trihalomethanesulfonyl" refers to a X3CS(O)2- group where X is a halogen. The term "trihalomethoxy" refers to a X3CO- group where X is a halogen. The term "trisubstituted silyl," as used herein, alone or in combination, refers to a silicone group substituted at its three free valences with groups as listed herein under the definition of substituted amino. Examples include trimethysilyl, tert-butyldimethylsilyl, triphenylsilyl and the like.
The term "optionally substituted" means the anteceding group may be substituted or unsubstituted. When substituted, the substituents of an "optionally substituted" group may include, without limitation, one or more substituents independently selected from the following groups or a
particular designated set of groups, alone or in combination: lower alkyl, lower alkenyl, lower alkynyl, lower heteroalkyl, lower heterocycloalkyl, lower haloalkyl, lower haloalkenyl, lower haloalkynyl, lower perhaloalkyl, lower perhaloalkoxy, lower cycloalkyl, phenyl, aryl, aryloxy, lower alkoxy, lower haloalkoxy, oxo, lower acyloxy, carbonyl, lower carboxyester, lower carboxamido, cyano, hydrogen, halogen, hydroxy, amino, lower alkylamino, arylamino, amido, thiol, lower alkylthio, arylthio, lower alkylsulfinyl, lower alkylsulfonyl, arylsulfϊnyl, arylsulfonyl, arylthio, sulfonate, sulfonic acid, trisubstituted silyl, N3, NHCH3, N(CH3)2, SH, SCH3, CO2CH3, C(O)NH2, pyridiπyl, thiophene, furanyl, lower carbamate, and lower urea. Two substituents may be joined together to form a fused five-, six-, or seven-menbered carbocyclic or heterocyclic ring consisting of zero to three heteroatoms, for example forming methylenedioxy or ethylenedioxy. An optionally substituted group may be unsubstituted (e.g., - CH2CH3), fully substituted (e.g., -CF2CF3), monosubstituted (e.g., -CH2CH2F) or substituted at a level anywhere in-between fully substituted and monosubstituted (e.g., -CH2CF3). Where substituents are recited without qualification as to substitution, both substituted and unsubstituted forms are encompassed. Where a substituent is qualified as "substituted," the substituted form is specifically intended. Additionally, different sets of optional substituents to a particuar moiety may be defined as needed; in these cases, the optional substitution will be as defined, often immediately following the phrase, "optionally substituted with."
The term R or the term R', appearing by itself and without a number designation, unless otherwise defined, refers to a moiety selected from the group consisting of alkyl, cycloalkyl, heteroalkyl, aryl, heteroaryl and heterocycloalkyl. Such R and R' groups should be understood to be optionally substituted as defined herein. Whether an R group has a number designation or not, every R group, including R, R' and R11 where n=(l , 2, 3, ...n), every substituent, and every term should be understood to be independent of every other in terms of selection from a group. Should any variable, substituent, or term (e.g. aryl, heterocycle, R, etc.) occur more than one time in a formula or generic structure, its definition at each occurrence is independent of the definition at every other occurrence.
The term "bond" refers to a covalent linkage between two atoms, or two moieties when the atoms joined by the bond are considered to be part of larger substructure. A bond may be single, double, or triple unless otherwise specified.
The term "combination therapy" means the administration of two or more therapeutic agents to treat a therapeutic condition or disorder described in the present disclosure. Such administration encompasses co-administration of these therapeutic agents in a substantially simultaneous manner, such as in a single capsule having a fixed ratio of active ingredients or in multiple, separate capsules for each active ingredient. In addition, such administration also encompasses use of each type of therapeutic agent in a sequential manner. In either case, the treatment regimen will provide beneficial effects of the drug combination in treating the conditions or disorders described herein.
"Cholesterol absorption inhibitor" is used herein to refer to a compound that exhibits an IC50 with respect to downregulation of ABCAl transcription while upregulating the expression of HMG COA synthase activity of no more than about 100 μM and more typically not more than about 50 μM, as
measured in the the luciferase reporter HEP ABCA l Luc assay described generally hereinbelow. "IC50" is that concentration of inhibitor which reduces the level of ABCAl expression to half-maximal level while increasing expression of HMG COA synthase. Representative compounds of the present invention have been discovered to exhibit inhibitory activity against cholesterol absorption, presumably by inhibiting NPCl Ll . Compounds of the present invention preferably exhibit an IC50 with respect to downregulation of ABCAl transcription while upregulating the expression of HMG COA synthase activity of no more than about 10 μM, more preferably, no more than about 5 μM, even more preferably not more than about 1 μM, and most preferably, not more than about 200 nM, as measured in luciferase reporter HEP ABCAl Luc assay described herein. The phrase "therapeutically effective" is intended to qualify the amount of active ingredients used in the treatment of a disease or disorder. This amount will achieve the goal of reducing or eliminating the said disease or disorder.
As used herein, reference to "treatment" of a patient is intended to include prophylaxis. The term "patient" means all mammals including humans. Examples of patients include humans, cows, dogs, cats, goats, sheep, pigs, and rabbits. Preferably, the patient is a human.
The term "prodrug" refers to a compound that is made more active in vivo. The present compounds can also exist as prodrugs, as described in Hydrolysis in Drug and Prodrug Metabolism : Chemistiγ, Biochemistry, and Enzymology (Testa, Bernard and Mayer, Joachim M. Wiley-VHCA, Zurich, Switzerland 2003). Prodrugs of the compounds described herein are structurally modified forms of the compound that readily undergo chemical changes under physiological conditions to provide the compound. Additionally, prodrugs can be converted to the compound by chemical or biochemical methods in an ex vivo environment. For example, prodrugs can be slowly converted to a compound when placed in a transdermal patch reservoir with a suitable enzyme or chemical reagent. Prodrugs are often useful because, in some situations, they may be easier to administer than the compound, or parent drug. They may, for instance, be bioavailable by oral administration whereas the parent drug is not. The prodrug may also have improved solubility in pharmaceutical compositions over the parent drug. A wide variety of prodrug derivatives are known in the art, such as those that rely on hydrolytic cleavage or oxidative activation of the prodrug. An example, without limitation, of a prodrug would be a compound which is administered as an ester (the "prodrug"), but then is metabolically hydrolyzed to the carboxylic acid, the active entity. Additional examples include peptidyl derivatives of a compound. The term "therapeutically acceptable prodrug," refers to those prodrugs or zwitterions which are suitable for use in contact with the tissues of patients without undue toxicity, irritation, and allergic response, are commensurate with a reasonable benefit/risk ratio, and are effective for their intended use.
The term "therapeutically acceptable salt," as used herein, represents salts or zwitterionic forms of the compounds of the present invention which are water or oil-soluble or dispersible; which are suitable for treatment of diseases without undue toxicity, irritation, and allergic-response; which are commensurate with a reasonable benefit/risk ratio; and which are effective for their intended use. The
salts can be prepared during the final isolation and purification of the compounds or separately by reacting the appropriate compound in the form of the free base with a suitable acid. Representative acid addition salts include acetate, adipate, alginate, L-ascorbate, aspartate, benzoate, benzenesulfonate (besylate), bisulfate, butyrate, camphorate, camphorsulfonate, citrate, digluconate, formate, fumarate, gentisate, glutarate, glycerophosphate, glycolate, hemisulfate, heptanoate, hexanoate, hippurate, hydrochloride, hydrobromide, hydroiodide, 2-hydroxyethansulfonate (isethionate), lactate, maleate, malonate, DL-mandelate, mesitylenesulfonate, methanesulfonate, naphthylenesulfonate, nicotinate, 2- naphthalenesulfonate, oxalate, pamoate, pectinate, persulfate, 3-phenylproρrionate, phosphonate, picrate, pivalate, propionate, pyroglutamate, succinate, sulfonate, tartrate, L-tartrate, trichloroacetate, trifluoroacetate, phosphate, glutamate, bicarbonate, para-toluenesulfonate (p-tosylate), and undecanoate. Also, basic groups in the compounds of the present invention can be quaternized with methyl, ethyl, propyl, and butyl chlorides, bromides, and iodides; dimethyl, diethyl, dibutyl, and diamyl sulfates; decyl, lauryl, myristyl, and steryl chlorides, bromides, and iodides; and benzyl and phenethyl bromides. Examples of acids which can be employed to form therapeutically acceptable addition salts include inorganic acids such as hydrochloric, hydrobromic, sulfuric, and phosphoric, and organic acids such as oxalic, maleic, succinic, and citric. Salts can also be formed by coordination of the compounds with an alkali metal or alkaline earth ion. Hence, the present invention contemplates sodium, potassium, magnesium, and calcium salts of the compounds of the compounds of the present invention and the like. Basic addition salts can be prepared during the final isolation and purification of the compounds by reacting a carboxy group with a suitable base such as the hydroxide, carbonate, or bicarbonate of a metal cation or with ammonia or an organic primary, secondary, or tertiary amine. The cations of therapeutically acceptable salts include lithium, sodium, potassium, calcium, magnesium, and aluminum, as well as nontoxic quaternary amine cations such as ammonium, tetramethylammonium, tetraethylammonium, methylamine, dimethylamine, trimethylamine, triethylamine, diethylamine, ethylamine, tributylamine, pyridine, /V./V-dimethylaniline, /V-methylpiperidine, /V-methylmorpholine, dicyclohexylamine, procaine, dibenzylamine, /V,/V-dibenzyIphenethylamine, 1 -ephenamine, and N,N'- dibenzylethylenediamine. Other representative organic amines useful for the formation of base addition salts include ethylenediamine, ethanolamine, diethanolamine, piperidine, and piperazine.
The compounds of the present invention can exist as therapeutically acceptable salts. The present invention includes compounds listed above in the form of salts, in particular acid addition salts. Suitable salts include those formed with both organic and inorganic acids. Such acid addition salts will normally be pharmaceutically acceptable. However, salts of non-pharmaceutical Iy acceptable salts may be of utility in the preparation and purification of the compound in question. For a more complete discussion of the preparation and selection of salts, refer to Pharmaceutical Salts: Properties, Selection, ami Use (Slahl, P. Heinrich. Wiley-VCHA, Zurich, Switzerland, 2002).
While it may be possible for the compounds of the subject invention to be administered as the raw chemical, it is also possible to present them as a pharmaceutical formulation. Accordingly, the subject invention provides a pharmaceutical formulation comprising a compound or a pharmaceutically
acceptable salt, ester, prodrug or solvate thereof, together with one or more pharmaceutically acceptable carriers thereof and optionally one or more other therapeutic ingredients. The carrier(s) must be "acceptable" in the sense of being compatible with the other ingredients of the formulation and not deleterious to the recipient thereof. Proper formulation is dependent upon the route of administration chosen. Any of the well-known techniques, carriers, and excipients may be used as suitable and as understood in the art; e.g., in Remington's Pharmaceutical Sciences. The pharmaceutical compositions of the present invention may be manufactured in a manner that is itself known, e.g., by means of conventional mixing, dissolving, granulating, dragee-making, levigating, emulsifying, encapsulating, entrapping or compression processes. The formulations include those suitable for oral, parenteral (including subcutaneous, intradermal, intramuscular, intravenous, intraarticular, and intramedullary), intraperitoneal, transmucosal, transdermal, rectal and topical (including dermal, buccal, sublingual and intraocular) administration although the most suitable route may depend upon for example the condition and disorder of the recipient. The formulations may conveniently be presented in unit dosage form and may be prepared by any of the methods well known in the art of pharmacy. All methods include the step of bringing into association a compound of the subject invention or a pharmaceutically acceptable salt, ester, prodrug or solvate thereof ("active ingredient") with the carrier which constitutes one or more accessory ingredients. In general, the formulations are prepared by uniformly and intimately bringing into association the active ingredient with liquid carriers or finely divided solid carriers or both and then, if necessary, shaping the product into the desired formulation.
Formulations of the present invention suitable for oral administration may be presented as discrete units such as capsules, cachets or tablets each containing a predetermined amount of the active ingredient; as a powder or granules; as a solution or a suspension in an aqueous liquid or a non-aqueous liquid; or as an oil-in-water liquid emulsion or a water-in-oil liquid emulsion. The active ingredient may also be presented as a bolus, electuary or paste.
Pharmaceutical preparations which can be used orally include tablets, push-fit capsules made of gelatin, as well as soft, sealed capsules made of gelatin and a plasticizer, such as glycerol or sorbitol. Tablets may be made by compression or molding, optionally with one or more accessory ingredients. Compressed tablets may be prepared by compressing in a suitable machine the active ingredient in a free-flowing form such as a powder or granules, optionally mixed with binders, inert diluents, or lubricating, surface active or dispersing agents. Molded tablets may be made by molding in a suitable machine a mixture of the powdered compound moistened with an inert liquid diluent. The tablets may optionally be coated or scored and may be formulated so as to provide slow or controlled release of the active ingredient therein. All formulations for oral administration should be in dosages suitable for such administration. The push-fit capsules can contain the active ingredients in admixture with filler such as lactose, binders such as starches, and/or lubricants such as talc or magnesium stearate and, optionally, stabilizers. In soft capsules, the active compounds may be dissolved or suspended in suitable liquids, such as fatty oils, liquid paraffin, or liquid polyethylene glycols. In addition, stabilizers may be added.
Dragee cores are provided with suitable coatings. For this purpose, concentrated sugar solutions may be used, which may optionally contain gum arabic, talc, polyvinyl pyrrolidone, carbopol gel, polyethylene glycol, and/or titanium dioxide, lacquer solutions, and suitable organic solvents or solvent mixtures. Dyestuffs or pigments may be added to the tablets or dragee coatings for identification or to characterize different combinations of active compound doses.
The compounds may be formulated for parenteral administration by injection, e.g., by bolus injection or continuous infusion. Formulations for injection may be presented in unit dosage form, e.g., in ampoules or in multi-dose containers, with an added preservative. The compositions may take such forms as suspensions, solutions or emulsions in oily or aqueous vehicles, and may contain formulatory agents such as suspending, stabilizing and/or dispersing agents. The formulations may be presented in unit-dose or multi-dose containers, for example sealed ampoules and vials, and may be stored in powder form or in a freeze-dried (lyophilized) condition requiring only the addition of the sterile liquid carrier, for example, saline or sterile pyrogen-free water, immediately prior to use. Extemporaneous injection solutions and suspensions may be prepared from sterile powders, granules and tablets of the kind previously described.
Formulations for parenteral administration include aqueous and non-aqueous (oily) sterile injection solutions of the active compounds which may contain antioxidants, buffers, bacteriostats and solutes which render the formulation isotonic with the blood of the intended recipient; and aqueous and non-aqueous sterile suspensions which may include suspending agents and thickening agents. Suitable lipophilic solvents or vehicles include fatty oils such as sesame oil, or synthetic fatty acid esters, such as ethyl oleate or triglycerides, or liposomes. Aqueous injection suspensions may contain substances which increase the viscosity of the suspension, such as sodium carboxymethyl cellulose, sorbitol, or dextran. Optionally, the suspension may also contain suitable stabilizers or agents which increase the solubility of the compounds to allow for the preparation of highly concentrated solutions. In addition to the formulations described previously, the compounds may also be formulated as a depot preparation. Such long acting formulations may be administered by implantation (for example subcutaneously or intramuscularly) or by intramuscular injection. Thus, for example, the compounds may be formulated with suitable polymeric or hydrophobic materials (for example as an emulsion in an acceptable oil) or ion exchange resins, or as sparingly soluble derivatives, for example, as a sparingly soluble salt.
For buccal or sublingual administration, the compositions may take the form of tablets, lozenges, pastilles, or gels formulated in conventional manner. Such compositions may comprise the active ingredient in a flavored basis such as sucrose and acacia or tragacanth.
The compounds may also be formulated in rectal compositions such as suppositories or retention enemas, e.g., containing conventional suppository bases such as cocoa butter, polyethylene glycol, or other glycerides.
Compounds of the present invention may be administered topically, that is by non-systemic administration. This includes the application of a compound of the present invention externally to the
epidermis or the buccal cavity and the instillation of such a compound into the ear, eye and nose, such that the compound does not significantly enter the blood stream. In contrast, systemic administration refers to oral, intravenous, intraperitoneal and intramuscular administration.
Formulations suitable for topical administration include liquid or semi-liquid preparations suitable for penetration through the skin to the site of inflammation such as gels, liniments, lotions, creams, ointments or pastes, and drops suitable for administration to the eye, ear or nose. The active ingredient may comprise, for topical administration, from 0.001 % to 10% w/w, for instance from 1 % to 2% by weight of the formulation. It may however comprise as much as 10% w/w but preferably will comprise less than 5% w/w, more preferably from 0.1 % to 1 % w/w of the formulation. For administration by inhalation the compounds according to the invention are conveniently delivered from an insufflator, nebulizer pressurized packs or other convenient means of delivering an aerosol spray. Pressurized packs may comprise a suitable propellant such as dichlorodifluoromethane, trichlorofluoromethane, dichlorotetrafluoroethane, carbon dioxide or other suitable gas. In the case of a pressurized aerosol, the dosage unit may be determined by providing a valve to deliver a metered amount. Alternatively, for administration by inhalation or insufflation, the compounds according to the invention may take the form of a dry powder composition, for example a powder mix of the compound and a suitable powder base such as lactose or starch. The powder composition may be presented in unit dosage form, in for example, capsules, cartridges, gelatin or blister packs from which the powder may be administered with the aid of an inhalator or insufflator. Preferred unit dosage formulations are those containing an effective dose, as herein below recited, or an appropriate fraction thereof, of the active ingredient.
It should be understood that in addition to the ingredients particularly mentioned above, the formulations of this invention may include other agents conventional in the art having regard to the type of formulation in question, for example those suitable for oral administration may include flavoring agents.
The compounds of the invention may be administered orally or via injection at a dose of from 0.1 to 500 mg/kg per day. The dose range for adult humans is generally from 5 mg to 2 g/day. Tablets or other forms of presentation provided in discrete units may conveniently contain an amount of compound of the invention which is effective at such dosage or as a multiple of the same, for instance, units containing 5 mg to 500 mg, usually around 10 mg to 200 mg.
The amount of active ingredient that may be combined with the carrier materials to produce a single dosage form will vary depending upon the host treated and the particular mode of administration.
The compounds of the subject invention can be administered in various modes, e.g. orally, topically, or by injection. The precise amount of compound administered to a patient will be the responsibility of the attendant physician. The specific dose level for any particular patient will depend upon a variety of factors including the activity of the specific compound employed, the age, body weight, general health, sex, diets, time of administration, route of administration, rate of excretion, drug
combination, the precise disorder being treated, and the severity of the indication or condition being treated. Also, the route of administration may vary depending on the condition and its severity.
In certain instances, it may be appropriate to administer at least one of the compounds described herein (or a pharmaceutically acceptable salt, ester, or prodrug thereof) in combination with another therapeutic agent. By way of example only, if one of the side effects experienced by a patient upon receiving one of the compounds herein is hypertension, then it may be appropriate to administer an antihypertensive agent in combination with the initial therapeutic agent. Or, by way of example only, the therapeutic effectiveness of one of the compounds described herein may be enhanced by administration of an adjuvant (i.e., by itself the adjuvant may only have minimal therapeutic benefit, but in combination with another therapeutic agent, the overall therapeutic benefit to the patient is enhanced). Or, by way of example only, the benefit of experienced by a patient may be increased by administering one of the compounds described herein with another therapeutic agent (which also includes a therapeutic regimen) that also has therapeutic benefit. By way of example only, in a treatment for diabetes involving administration of one of the compounds described herein, increased therapeutic benefit may result by also providing the patient with another therapeutic agent for diabetes. In any case, regardless of the disease, disorder or condition being treated, the overall benefit experienced by the patient may simply be additive of the two therapeutic agents or the patient may experience a synergistic benefit.
Specific, non-limiting examples of possible combination therapies include use of a compound of the present invention as defined above or a pharmaceutically acceptable salt thereof; and at least one active ingredient selected from: a) anti-diabetic agents such as insulin, insulin derivatives and mimetics; insulin secretagogues such as the sulfonylureas, e.g., Glipizide, glyburide and Amaryl; insulinotropic sulfonylurea receptor ligands such as meglitinides, e.g., nateglinide and repaglinide; insulin sensitizer such as protein tyrosine phosphatase-lB (PTP-I B) inhibitors such as PTP-1 12; GSK3 (glycogen synthase kinase-3) inhibitors such as SB-517955, SB-4195052, SB-216763, NN-57-05441 and NN-57-05445; RXR ligands such as GW-0791 and AGN-194204; sodium-dependent glucose co-transporter inhibitors such as T-1095; glycogen phosphorylase A inhibitors such as BAY R3401 ; biguanides such as metformin; alpha- glucosidase inhibitors such as acarbose; GLP-I (glucagon like peptide-1), GLP-I analogs such as Exendin-4 and GLP-I mimetics; DPPIV (dipeptidyl peptidase IV) inhibitors such as DPP728, LAF237 (vildagliptin - Example 1 of WO 00/34241), MK-0431, saxagliptin, GSK23A ; an AGE breaker; a thiazolidone derivative (glitazone) such as pioglitazone, rosiglitazone, or (/?)-l-{4-[5-methyl-2-(4- trifluoromethyl-phenyl)-oxazol-4-ylmethoxy]-benzenesulfonyl}2,3-dihydro-l//-indole-2-carboxylic acid described in the patent application WO 03/043985, as compound 19 of Example 4, a non-glitazone type PPARδ agonist e.g. GI-262570; b) hypolipidemic agents such as 3-hydroxy-3-methyl-glutaryl coenzyme A (HMG-CoA) reductase inhibitors, e.g., lovastatin, pravastatin, simvastatin, pravastatin, cerivastatin, mevastatin, velostatin, fluvastatin, dalvastatin, atorvastatin, rosuvastatin and rivastatin; squalene synthase inhibitors;
FXR (famesoid X receptor) and LXR (liver X receptor) ligands; cholestyramine; fibrates; nicotinic acid and aspirin; c) an anti-obesity agent or appetite regulating agent such as phentermine, leptin, bromocriptine, dexamphetamine, amphetamine, fenfluramine, dexfenfluramine, sibutramine, orlistat, dexfenfluramine, mazindol, phentermine, phendimetrazine, diethylpropion, fluoxetine, bupropion, topiramate, diethylpropion, benzphetamine, phenylpropanolamine or ecopipam, ephedrine, pseudoephedrine or cannabinoid receptor antagonists; d) anti-hypertensive agents, e.g., loop diuretics such as ethacrynic acid, furosemide and torsemide; diuretics such as thiazide derivatives, chlorithiazide, hydrochlorothiazide, amiloride; angiotensin converting enzyme (ACE) inhibitors such as benazepril, captopril, enalapril, fosinopril, lisinopril, moexipril, perinodopril, quinapril, ramipril and trandolapril; inhibitors of the Na-K-ATPase membrane pump such as digoxin; neutral endopeptidase (NEP) inhibitors e.g. thiorphan, terteo- thiorphan, SQ29072; ECE inhibitors e.g. SLV306; ACE/NEP inhibitors such as omapatrilat, sampatrilat and fasidotril; angiotensin n antagonists such as candesartan, eprosartan, irbesaitan, losartan, tehnisartan and valsartan, in particular valsartan; renin inhibitors such as aliskiren, terlakiren, ditekiren, RO 66- 1 132, RO-66-1 168; β-adrenergic receptor blockers such as acebutolol, atenolol, betaxolol, bisoprolol, metoprolol, nadolol, propranolol, sotalol and timolol; inotropic agents such as digoxin, dobutamine and milrinone; calcium channel blockers such as amlodipine, bepridil, diltiazem, felodipine, nicardipine, nimodipine, nifedipine, nisoldipine and verapamil; aldosterone receptor antagonists; and aldosterone synthase inhibitors; e) a HDL increasing compound; f) Cholesterol absorption modulator such as Zetia® and KT6-971 ; g) Apo-Al analogues and mimetics; h) thrombin inhibitors such as Ximelagatran; i) aldosterone inhibitors such as anastrazole, fadrazole, epierenone; j) Inhibitors of platelet aggregation such as aspirin, clopidogrel bisulfate; k) estrogen, testosterone, a selective estrogen receptor modulator, a selective androgen receptor modulator;
1) a chemotherapeutic agent such as aromatase inhibitors e.g. femara, anti -estrogens, topoisomerase I inhibitors, topoisomerase II inhibitors, microtubule active agents, alkylating agents, antineoplastic antimetabolites, platin compounds, compounds decreasing the protein kinase activity such as a PDGF receptor tyrosine kinase inhibitor preferably miatinib ({ N-{5-[4-(4-methyl-piperazino- methyl)-benzoylamido]-2-methylphenyl}-4-(3-pyridyl)-2-pyrimidine-amine}) described in the European patent application EPA-0564409 as example 21 or 4-Methyl-"N-[3-(4-methyl-imidazol-l-yl)-5- trifluoiOmethyl-phenyl]-3-(4-pyridin-3-yl-pyrimidin-2-yIamino)-benzamide described in the patent application WO 04/005281 as example 92; and
m) an agent interacting with a 5-HT3 receptor and/or an agent interacting with 5-HT4 receptor such as tegaserod described in the US patent No. 5510353 as example 13, tegaserod hydrogen maleate, cisapride, cilansetron; or, in each case a pharmaceutically acceptable salt thereof; and optionally a pharmaceutically acceptable carrier.
Most preferred combination partners are cholesterol absorption modulator such as Zetia© and KT6-971or hypolipidemic agents such as 3-hydroxy-3-methyl~glutaryl coenzyme A (HMG-CoA) reductase inhibitors, e.g., lovastatin, pravastatin, simvastatin, pravastatin, cerivastatin, mevastatin, velostatin, fluvastatin, dalvastatin, atorvastatin, rosuvastatin and rivastatin; squalene synthase inhibitors; FXR (famesoid X receptor) and LXR (liver X receptor) ligands; cholestyramine; fibrates; nicotinic acid and aspirin.
In any case, the multiple therapeutic agents (at least one of which is a compound of the present invention) may be administered in any order or even simultaneously. If simultaneously, the multiple therapeutic agents may be provided in a single, unified form, or in multiple forms (by way of example only, either as a single pill or as two separate pills). One of the therapeutic agents may be given in multiple doses, or both may be given as multiple doses. If not simultaneous, the timing between the multiple doses may be any duration of time ranging from a few minutes to four weeks.
Thus, in another aspect, the present invention provides methods for treating NPC-I Ll -mediated disorders in a human or animal subject in need of such treatment comprising administering to said subject an amount of a compound of the present invention effective to reduce or prevent said disorder in the subject in combination with at least one additional agent for the treatment of said disorder that is known in the art. In a related aspect, the present invention provides therapeutic compositions comprising at least one compound of the present invention in combination with one or more additional agents for the treatment of NPC-I Ll -mediated disorders. The present invention includes compounds listed above in the form of salts, in particular acid addition salts. Suitable salts include those formed with both organic and inorganic acids. Such acid addition salts will normally be pharmaceutically acceptable. However, salts of non-pharmaceutically acceptable salts may be of utility in the preparation and purification of the compound in question.
Thus, preferred salts include hydrochloride, hydrobromide, sulfonate, citrate, tartrate, phosphonate, lactate, pyruvate, acetate, succinate, oxalate, fumarate, malate, oxaloacetate, methanesulfonate, ethanesuifonate, p-toluenesulfonate, benzenesulfonate and isethionate salts of compounds of the present invention. A salt of a compound can be made by reacting the appropriate compound in the form of the free base with the appropriate acid.
Asymmetric centers exist in the compounds of the present invention. These centers are designated by the symbols "R" or "S," depending on the configuration of substituents around the chiral carbon atom. It should be understood that the invention encompasses all stereochemical isomeric forms, including diaslereomeric, enantiomeric, and epimeric forms,as well as d-isomers and 1 -isomers, and mixtures thereof. Individual stereoisomers of compounds can be prepared synthetically from commer-
daily available starting materials which contain chiral centers or by preparation of mixtures of enantiomeric products followed by separation such as conversion to a mixture of diastereomers followed by separation or recrystallization, chromatographic techniques, direct separation of enantiomers on chiral chromatographic columns, or any other appropriate method known in the art. Starting compounds of particular stereochemistry are either commercially available or can be made and resolved by techniques known in the art. Additionally, the compounds of the present invention may exist as geometric isomers. The present invention includes all cis, trans, syn, anti, entgegen (E), and zusammen (Z) isomers as well as the appropriate mixtures thereof. Additionally, compounds may exist as tautomers; all tautomeric isomers are provided by this invention. Additionally, the compounds of the present invention can exist in unsolvated as well as solvated forms with pharmaceutically acceptable solvents such as water, ethanol, and the like. In general, the solvated forms are considered equivalent to the unsolvated forms for the purposes of the present invention.
Besides being useful for human treatment, the compounds and formulations of the present invention are also useful for veterinary treatment of companion animals, exotic animals and farm animals, including mammals, rodents, and the like. More preferred animals include horses, dogs, and cats.
All references, patents or applications, U.S. or foreign, cited in the application are hereby incorporated by reference as if written herein.
GENERAL SYNTHETIC METHODS FOR PREPARING COMPOUNDS
The following schemes can be used to practice the present invention.
Scheme I
Scheme 2
Scheme 3
"
(RlU7 = H, alkyl) (J = Bi-, I, Cl, OSO2CF3) (Solvent = water, cthanol,
DMH, or the like)
Scheme 5
1 1223;
Solvent = water, acetic acid, etlianol, tetrahydrofuran, 1,2-dichloroethane, toluen, or the like;
Catalyst = HCI, p-toluenesuHbnic acid, boron trifluoride, or the like.
The activity of the compounds in Examples 1-450 as cholesterol-absorption inhibitors and presumably, though the authors do not wish to be held to this theory, NPCl Ll inhibitors, is illustrated in the following assays. The other compounds listed above, which have not yet been made and/or tested, are predicted to have activity in these assays as well.
Biological Activity Assays
Hepatocyte (HEPG2C3A) Luciferase Reporter Assay
The compounds of the invention have an effect on ABCA-I gene expression. It is reported that compounds that effect cholesterol production and absorption such as statins and ezetimibe cause a decrease expression of ABCAl transcript. For example, in "NPC l Ll knockout animals (the target of Ezetimibe) and also in wild type animals treated with ezetimibe, a decrease in ABCAl transcript is seen. Therefore, a stable cell line with 2KB of the ABCA-I 5' promoter region fused to a luciferase reporter was used in a reporter assay to interrogate compounds for the ability to decrease ABCA- I expression. The promoter region was made by using PCR to obtain the fragment from human genomic DNA using the following primers; sense strand primer: ATAAGTTGGAGGTCTGGGAGTGGCTA and antisense strand primer : GCTCTGTTGGTG-CGCGGAGCT. The genomic fragment included approx 2KB of the genomic ABCAl (accession number Gl:21536375) including promoter elements important to the transcriptional regulation of this gene. To perform the luciferase assays, a human hepatocyte cell line (HEPG2C3A ATCC#CRL- 1074; ATCC, Manassas, VA ) was stably transfected with the above described fragment cloned into a luciferase containing pGL3 vector. Transient transfections were done initially to insure activity in cells when stimulated with an RXR agonist, 9-cis retinoic acid. Stable cells were made using lenti viral clone construction of the above described ABCAl clone in a Lenti vival luciferase tagged vector with puromycin selection. Following viral production in 293T cells, HEPG2 C3A cells were infected and put under puromycin selective pressure until cells began to divide and behave normally in the presence of puromycin. As these cells were to be used for an assay to find inhibitors of cholesterol absorption, the presence of "NPCl Ll transcript was deemed a necessity (NPCl Ll is a protein shown previously to be impotant in cholesterol absorption: see patent WO05015988A1). RTPCR was used to determine the presence of NPCl Ll transcript (NM 13389) in this cell line before and after stable transfection using the following primers: Forward primer ATAGGCGCGCCATGGCGGAGGCCGGCCTGAG and Reverse primer
TATGGCGCGCCTCAGAACTGCCGCCCATTGT). RTPCR was performed using Trizol Reagent according to manufacturer's instructions (Invitrogen Corp, Carlsbad Ca) to extract total RNA from the HEPG2C3A cells. The mRNA from these total RNA preparations was amplified using Superscript II reverse transcriptase according to manufacturer's instructions (Invitrogen Corp, Carlsbad Ca) with the oligo dT primer and the random hexamer primer for first strand synthesis provided in the kit. Various primer sets, including those described above were used to confirm the presence of NPCl Ll transcript.
The resulting cDNA of NPCl Ll was cloned into an appropriate prokaryotic vector and sequenced to confirm identity. HepG2C3A cells were found to have ample amounts of the NPCl Ll transcript before and after stable transfection. Following confirmation of the presence of "NPCl Ll , stably transfected cells were expanded for luciferase reporter assays under the selection of puromycin and assayed for luciferase activity following 9-cis retinoic acid stimulation. Luciferase reporter assays were run in high throughput 1536 well format using 5 ul of cells at a concentration of 500000 cells/ml of media. Briefly, cells were plated in MEM media (Invitrogen, Carlsbad, California) supplemented with 1% fetal bovine serum (Invitrogen) for 6 hr in white opaque tissue culture treated Greiner 1536 well plates (USA Scientific, Inc; Ocala, FLA) to promote attachment. Plated cells were then incubated with compound for 12 hr and stimulated with 60OnM 9-cis retinoic acid (RA) (Sigma, St Louis MO) following the 12 hr incubation with compound. Cells stimulated with 9-cis RA were incubated for 20 h and then Britelite (PerkinElmer Life And Analytical Sciences, Inc; Boston, MA) was added to determine the total amount of luciferase production as driven by the ABCAl promoter region described herein. Compounds were profiled in the same cell based assay in duplicate assays in five point dose response format to determine confirmation. To determine potential toxicity of compounds and to insure against "false positives" ATPlite was used in place of Britelite (both products of Perkin Elmer) under the same assay conditions described above. Compounds were chosen on the basis of non-toxic activity and reproducibility of results.
Quantitative Gene Expression Profiling from Cell Lysates by Branched DNA
The compounds of the invention modify HMG CoA synthase expression and ABCAl expression. Branched DNA (bDNA) is a method of accurate RNA quantification that offers RNA quantification directly from cell lysate. The branched DNA technology introduces multiple labels onto a target nucleic acid. The sensitivity stems from the use of a set of branched reporter probes. Each probe has 15 branches, and each branch can react with up to three alkaline phosphatase-labeled detection probes. This leads to a high degree of labeling of the target and kits to perform branched DNA assays can be obtained from Genospectra, Inc (Fremont, CA). Branched DNA assays were performed with select compounds using specific probes for HMG COA synthase and ABCA 1 RNA. The HEPG2C3A cells described previously along with the human intestinal cell lines: CaCo2 (ATCC # HTB-237; colorectal adenocarcinoma; ATCC: Manassas, VA) and the human intestinal cell line FHs (ATCC # CCL-241 ; normal fetal small intestine, ATCC: Manassas, VA ) were plated in clear bottom 96 well format in 1% fetal bovine serum overnight and treated with compounds the following morning. Cells were plated at a concentration of 200000 cells/ ml media. DMSO (vehicle used for compound dilution) concentration of compounds was kept at 0.8%. Compound remained on cells for 20 hr and cells were lysed following compound incubation according to kit instructions. Lysed cells release mRNA in the presence of target probes. Target mRNA from lysed cells is captured by hybridization and transferred to
the Genospectra Capture Plate. Signal amplification is performed by hybridization of the bDNA Amplifier and Label Probe. Addition of chemiluminescence substrate yields a signal that is proportional to the amount of mRNA present in the sample. The target probes used in this experiment were specific to HMG CoA synthase and ABCAl (NMJJ05502, cat #PA-10181) purchased from Genospectra and used according to kit instructions. Differential expression as seen with bDNA was qualitatively confirmed with immunofluorescence. Data is represented as (+ or -) for both ABCAl and HGMG CoA synthase, although to be positive in ABCAl assay required a minimal 2X decrease in overall transcriptional activity over vehicle control (DMSO) and to be positive in HMG coA synthase required a minimal 2X increase in transcriptional activity of the target gene over DMSO control. Compounds not tested in these assays are designated by "NT". Ezetimibe was used as positive control in bDNA assays as well as antibody experiments. The EC50 of ezetimibe in these assays was calculated to be less than 10OnM with an R2 value of 0.89. Several of compounds tested here show similar ECSOs. Briefly, cells were plated and treated with compound as described above and fixed with formaldehyde after compound incubation. Qualitative visualization of protein changes were made (data not shown) using antibodies to HMG CoA synthase protein (chicken polyclonal #AB 14302; Abeam Inc,
Cambridge, MA) and ABCAl protein (mouse monoclonal #AB 18180; Abeam Inc, Cambridge, MA) and observing cells microscopically after fluorescent labeling. Qualitative antibody labeling cooresponded well with quantitative bDNA results. Ezetimibe (I uM final concentration) was used as positive control in antibody experiments and showed a diminished signal with ABCAl antibody in Hep G2C3 A and CaCo2 cells and was completely absent by antibody staining in FhS cells when compared to DMSO treated cells. The ezetimibe treated cells showed a moderate increase in staining with the HMG CoA synthase antibody when compared to DMSO treated controls.
The following are examples of compounds and assay data disclosed by the present invention.
Table 1.
HEP
ABCAl HMG
Example LUC CoA
Reporter ABCAl Synthase
Structure Assay bDNA bDNA
NT
201 NT
NT NT
258
NT NT
263
NT NT
383 NT NT
All units of measurement are in counts/sec luminescent units
"+" means "IC50 between 100nM~5uM" for the luciferase reporter assay
"+" means "> or = 2X fold decrease" in ABCAl transcript for the ABCAl bDNA
"+" means "> or = 2X fold increase" in HMG CoA Syn transcript for the HMG bDNA
NT means not tested
All results are compared to cells treated with the vehicle control: DMSO
The compounds below have not yet been made, but can generally be made using both literature methods and those methods described above. It is expected that these compounds, when made, will have activity similar to those that have been described in the examples above. The compounds are represented herein using the Simplified Molecular Input Line Entry System, or SMILES. SMILES is a modern chemical notation system, developed by David Weininger and Daylight Chemical Information Systems, Inc., that is built into all major commercial chemical structure drawing software packages.
Software is not needed to interpret SMILES text strings, and an explanation of how to translate SMILES into structures can be found in Weininger, D., J. Chem. Inf. Comput. ScL 1988, 28, 31 -36.
CCOcI CC(CCCl Cl)C2Nc3ccc(cc3NC4=C2C(=O)CCC4)C(=O)c5ccccc5
Oc 1 ccc(cc 1 C#N)C2Nc3 ccc(Oc4ccccc4)cc3NC5=C2C(=O)CCC5 CIc 1 cccc(c 1 )C2"Nc3ccc(0Cc4ccccc4)cc3NC5=C2C(O)CCC5
CC(C)Ocl ccc(cclC(F)(F)F)C2Nc3ccc(COc4ccccc4)cc3NC5=C2C(=O)CCC5
COc 1 cc(cc(OC)c 10)C2Nc3ccc(Sc4ccccc4)cc3NC5=C2C(=O)CCC5
CCOclcc(OCC)cc(cl)C2Nc3ccc(cc3NC4=C2C(=O)CCC4)S(=O)(=O)c5ccccc5
CN(cl cccccl)c2ccc3NC(C4=C(CCCC4=O)Nc3c2)c5ccc(Cl)c(Cl)c5 O=C 1 CCCC2=C 1 C(Nc3ccc(cc3N2)S(=O)(=O)Nc4ccccc4)c5ccc6CCCc6c5
Brc 1 cncc(c 1 )C2Nc3ccc(cc3NC4=C2C(=O)CCC4)C(=O)Nc5ccccc5
CCOC(=O)c 1 cc(ccn 1 )C2Nc3 ccc(cc3 NC4=C2C(=O)CCC4)N(C)C(=O)c5ccccc5
O=C(Oc I ccccc 1 )c2ccc3NC(C4=C(CCCC4=0)Nc3c2)c5oc(nn5)C6CC6
CN(C(=O)Oc 1 ccccc 1 )c2ccc3NC(C4=C(CCCC4=O)Nc3c2 )c5cncc6ncccc56 CCOcI cc(cccl O)C2Nc3ccc(cc3MC4=C2C(=O)CCC4)C(=O)c5ccccc5
CCOc 1 cc(ccc 10)C2Nc3ccc(Oc4ccccc4)cc3NC5=C2C(=O)CCC5
CCOcl cc(ccclO)C2Nc3ccc(OCc4ccccc4)cc3NC5=C2C(=O)CCC5
CCOc 1 Cc(CCC 10)C2Nc3ccc(COc4ccccc4)cc3NC5=C2C(=O)CCC5
CCOcI cc(cccl O)C2Nc3ccc(Sc4ccccc4)cc3NC5=C2C(=O)CCC5 CCOc 1 CC(CCC 10)C2Nc3ccc(cc3NC4=C2C(=O)CCC4)S(=O)(=O)c5ccccc5
CCOc 1 cc(ccc 10)C2Nc3ccc(cc3NC4=C2C(=O)CCC4)N(C)c5ccccc5
CCOcI cc(cccl O)C2Nc3ccc(cc3NC4=C2C(=O)CCC4)S(=O)(=O)Nc5ccccc5
CCOcI cc(cccl O)C2Nc3ccc(cc3NC4=C2C(=O)CCC4)C(=O)"Nc5ccccc5
CCOcI cc(cccl O)C2Nc3ccc(cc3NC4=C2C(=O)CCC4)N(C)C(=O)c5ccccc5 CCOc 1 cc(ccc 10)C2Nc3ccc(cc3NC4=C2C(=O)CCC4)C(=O)Oc5ccccc5
CCOcl cc(ccclO)C2Nc3ccc(cc3NC4=C2C(=O)CCC4)N(C)C(=O)Oc5ccccc5
CCOcl cc(cccl O)C2Nc3ccc(cc3NC4=C2C(=O)CC(C)(C)C4)C(=O)c5ccccc5 CCOc 1 CC(CCc 1 O)C2Nc3cc(Oc4ccccc4)ccc3NC5=C2C(=O)CC(CC)C5 CCOc 1 CC(CCcI O)C2Nc3ccc(OCc4cncs4)cc3NC5=C2C(=O)CC(C5)c6ccccc6 CCOcI CC(CCcI O)C2Nc3cc(CCOc4ccncn4)ccc3NC5=C2C(=O)COC5 CCOc 1 Cc(CCC 10)C2Nc3ccc(Sc4ccccc4)cc3NC5=C2C(=O)CSC5
CC0cl cc(cccl O)C2'Nc3cc(CS(=0)(=0)c4ccccc4)ccc3'NC5=C2C(=O)CC(=O)C5 CCOcI cc(cccl O)C2Nc3cc(ccc3NC4=C2C(=O)CC(O)C4)N(C)c5ccccc5 CCOcI CC(CCc 1 O)C2NC3CCC(CC3NC4=C2C(=O)CC(C4)OC)S(=O)(=O)MC5CCCCC5 CCOc 1 Cc(CCCl O)C2Nc3ccc(cc3NC4=C2C(=O)CC5(CCCC5)C4)C(=O)Nc6ccccc6 CCOcI cc(ccclO)C2Nc3ccc(cc3NC4=C2C(=O)CNC4)N(C)C(=O)c5ccccc5
CCOclcc(ccclO)C2"Nc3ccc(cc3NC4=C2C(=O)CM(C4)C=O)C(=O)OC(C)c5ccccc5
CCOc 1 Cc(CCC 10)C2Nc3cc(ccc3NC4=C2C(=O)CN(C4)c5ccccc5)N(CC6CCC6)C(=O)Oc7ccccc7
CC(=0)C 1 =C(C)Nc2cc(Oc3ccccc3)ccc2NC 1 c4ccc(O)c(c4)C#N
CCOC(=O)Cl=C(C)Nc2cc(OCc3ccccc3)ccc2NCl c4ccc(C)c(Cl)c4 CC(C)Oc 1 CCc(CCl C(F)(F)F)C2Nc3ccc(COc4ccccc4)cc3NC(=C2C(=O)C)NC(C)(C)C
COclcc(cc(OC)clO)C2Nc3ccc(Sc4ccccc4)cc3NC(=C2C(=O)C(C)C)OC(C)(C)c5ccccc5 CCOc 1 cc(OCC)cc(c 1 )C2Nc3ccc(cc3NC(=C2C(=O)N4CCCC4)CC)S(=O)(=O)c5ccccc5 CC0C(=0)C 1 =C(Nc2cc(ccc2NC 1 c3 ccc(C I)c(Cl)c3 )N(C)c4ccccc4)OCC CC(=O)Cl=C(Nc2cc(ccc2NCl c3ccc4CCCc4c3)S(=O)(=O)Nc5ccccc5)C#Η CC(=O)Cl=CNc2cc(ccc2NClc3cncc(Br)c3)C(=O)Nc4ccccc4
CC0C(=O)clcc(ccnl)C2Nc3ccc(cc3NC(=C2C(=0)C)Sc4ccccc4)N(C)C(=O)c5ccccc5 CN(C(=O)Oc I ccccc 1 )c2ccc3NC(C(=C(Nc3 c2)C(=O)C)C(=O)C)c4cncc5ncccc45 CCCCS(=O)(=O)Cl=C(C(Nc2ccc(cc2N1)C(=O)Oc3ccccc3)c4oc(nn4)C5CC5)C(=O)C Clcl CCC(Cd Cl)C2Nc3ccc(cc3NC4=C2C(=O)CCC4)C(=O)c5ccccc5 Clclcccc(cl)C2Nc3ccc(cc3NC4=C2C(=O)CCC4)C(=O)c5ccccc5
COc 1 cc(cc(OC)c 10)C2Nc3ccc(cc3"NC4=C2C(=O)CCC4)C(=O)c5ccccc5
CCOcI cc(ccc 1 Cl)C2Nc3ccc(COc4ccccc4)cc3NC5=C2C(=O)CCC5 CCOcI cc(ccc1 Cl)C2Nc3ccc(cc31MC4=C2C(=O)CCC4)S(=O)(=O)Nc5ccccc5 CCOc 1 cc(cccl Cl)C2Nc3ccc(cc3'NC4=C2C(=O)CCC4)N(C)C(=O)c5ccccc5 CCOcI Cc(CCCl CI)C2Nc3ccc(cc3NC4=C2C(=O)CCC4)C(=O)OC(C)c5ccccc5 OcI ccc(cc 1 C#N)C2NC3CCC(CC3NC4=C2C(=O)CCC4)S(=O)(=O)NC5CCCCC5 CN(C(=O)clcccccl)c2ccc3NC(C4=C(CCCC4=O)Nc3c2)c5ccc(O)c(c5)C#N Oc 1 ccc(ccl C#TM)C2Nc3ccc(cc3NC4=C2C(=0)CCC4)C(=0)0c5ccccc5 OcI ccc(ccl C#M)C2Mc3ccc(OCc4cncs4)cc3NC5=C2C(=O)CCC5 Clcl cccc(cl)C2Nc3ccc(Oc4ccccc4)cc3NC5=C2C(==O)CCC5 CIc 1 CCCc(C 1 )C2Nc3ccc(cc3NC4=C2C(=O)CCC4)S(=O)(=O)c5ccccc5
CC(C)OcICCc(CClC(F)(F)F)C2"Nc3ccc(OCc4ccccc4)cc3-NC5=C2C(=O)CCC5 CC(C)Oclccc(cclC(F)(F)F)C2Nc3ccc(cc3NC4=C2C(=O)CCC4)S(=O)(=O)Nc5ccccc5 CC(C)Oc1CCC(Cc1C(F)(F)F)C2Nc3ccc(cc3NC4=C2C(=O)CCC4)N(C)C(=O)c5ccccc5 CC(C)OcIccc(cc1C(F)(F)F)C2NC3CCC(CC3NC4=C2C(=O)CCC4)C(=O)OC5CCCCC5 CC(C)Oc 1 ccc(cc 1 C(F)(F)F)C2Nc3ccc(OCc4cncs4)cc3NC5=C2C(=O)CCC5 COc 1 cc(cc(0C)c 10)C2Nc3ccc(COc4ccccc4)cc3NC5=C2C(=O)CCC5 COcI cc(cc(0C)cl O)C2"Nc3ccc(cc3NC4=C2C(=O)CCC4)N(C)c5ccccc5 COclcc(cc(0C)cI0)C2Nc3ccc(cc3NC4=C2C(=O)CCC4)S(=0)(=0)Nc5ccccc5 COcl cc(cc(OC)cl O)C2Nc3ccc(cc3NC4=C2C(=O)CCC4)N(C)C(=O)c5ccccc5 COc 1 cc(cc(0C)cl O)C2Nc3ccc(cc3NC4=C2C(=O)CCC4)C(=O)OC(C)c5ccccc5 CCOc 1 cc(0CC)cc(cl )C2Nc3ccc(cc3NC4=C2C(=O)CCC4)N(C)c5ccccc5 CCOc 1 CC(OCC)Cc(C 1 )C2Nc3 ccc(cc3NC4=C2C(=O)CCC4)C(=O)Nc5ccccc5 CCOcl cc(OCC)cc(cl)C2Nc3ccc(cc3NC4=C2C(=O)CCC4)C(=O)Oc5ccccc5 Clclccc(cclCl)C2Nc3ccc(cc3NC4=C2C(=O)CCC4)S(=O)(=O)Nc5ccccc5 CIc 1 ccc(cc 1 Cl)C2Nc3ccc(cc3NC4=C2C(=O)CCC4)C(=O)Nc5ccccc5 CIc 1 CCc(CCl Cl)C2Nc3ccc(cc3NC4=C2C(=O)CCC4)C(=O)Oc5ccccc5
O=C(Cl ccccci )c2ccc3NC(C4=C(CCCC4=O)Nc3c2)c5ccc6CCCc6c5
O=C 1 CCCC2=C 1 C(Nc3ccc(Oc4ccccc4)cc3"N2)c5ccc6CCCc6c5
O=C 1 CCCC2=C 1 C(Nc3 ccc(COc4ccccc4)cc3N2)c5ccc6CCCc6c5
O=C(Nc 1 ccccc 1 )c2ccc3NC(C4=C(CCCC4=O)Nc3 c2)c5ccc6CCCc6c5 CN(C(=O)c 1 cccccl )c2ccc3"NC(C4=C(CCCC4=O)Nc3c2)c5ccc6CCCc6c5
CN(C(=O)Oc 1 ccccc 1 )c2ccc3NC(C4=C(CCCC4=O)Nc3c2)c5ccc6CCCc6c5
O=Cl CCCC2=C 1 C(Nc3ccc(OCc4cncs4)cc3N2)c5ccc6CCCc6c5
CC(OC(=O)c 1 ccc2NC(C3=C(CCCC3=O)Nc2cl )c4ccc5CCCc5c4)c6ccccc6
Brc 1 cπcc(c 1 )C2Nc3ccc(COc4ccccc4)cc31MC5=C2C(=O)CCC5 CN(C(=O)cl ccccc 1 )c2ccc3NC(C4=C(CCCC4=O)Nc3c2)c5cncc(Br)c5
Brcl cncc(cl)C2TMc3ccc(OCc4cncs4)cc3NC5=C2C(=O)CCC5
CC(OC(=O)c 1 ccc2NC(C3=C(CCCC3=O)Nc2c 1 )c4cncc(Br)c4)c5ccccc5
CCOC(=O)c 1 cc(ccn 1 )C2Nc3 ccc(cc3NC4=C2C(=O)CCC4)C(=O)c5ccccc5
CCOC(=O)c 1 cc(ccn 1 )C2Nc3 ccc(cc3NC4=C2C(=O)CCC4)C(=O)Oc5ccccc5 CCOC(=O)c lcc(ccnl)C2Nc3ccc(cc3NC4=C2C(=0)CCC4)N(C)C(=0)0c5ccccc5
CCOC(=O)clcc(ccnl )C2Nc3ccc(OCc4cncs4)cc3MC5=C2C(=O)CCC5
O=C 1 CCCC2=C 1 C(Nc3ccc(Oc4ccccc4)cc3N2)c5oc(nn5)C6CC6
O=C 1 CCCC2=C 1 C(Nc3ccc(COc4ccccc4)cc3N2)c5oc(nn5)C6CC6
O=C(Nc I ccccc l)c2ccc3NC(C4=C(CCCC4=O)Nc3c2)c5oc(nn5)C6CC6 CN(C(=0)cl ccccc l)c2ccc3NC(C4=C(CCCC4=O)Nc3c2)c5oc(nn5)C6CC6
O=C 1 CCCC2=C 1 C(Nc3ccc(OCc4cncs4)cc3N2)c5oc(nn5)C6CC6
CC(OC(=0)clccc2NC(C3=C(CCCC3=0)Nc2cl)c4oc(nn4)C5CC5)c6ccccc6
O=ClCCCC2=Cl C(Nc3ccc(Oc4ccccc4)cc3N2)c5cncc6ncccc56
O=Cl CCCC2=Cl C(Nc3ccc(COc4ccccc4)cc3M2)c5cncc6ncccc56 O=C(Nc 1 ccccc 1 )c2ccc3NC(C4=C(CCCC4=O)Nc3c2)c5cncc6ncccc56
O=C!CCCC2=ClC(Nc3ccc(OCc4cncs4)cc3N2)c5cncc6ncccc56
CC(OC(=O)cl ccc2NC(C3=C(CCCC3=O)Nc2cl)c4cncc5ncccc45)c6ccccc6 CCOc 1 cc(ccc 10)C2Nc3ccc(OCc4cncs4)cc3"NC5=C2C(=O)CCC5 CcI CCC(Cc 1 C1)C2NC3CCC(CC3NC4=C2C(=O)CCC4)C(=O)C5CCCCC5 Cclccc(ccl Cl)C2"Nc3ccc(OCc4ccccc4)cc3NC5=C2C(=O)CCC5 CN(C(=O)cl cccccl )c2ccc3NC(C4=C(CCCC4=O)Nc3c2)c5ccc(C)c(Cl)c5 CC(OC(=O)c 1 ccc2NC(C3=C(CCCC3=O)Nc2c 1 )c4ccc(C)c(Cl)c4)c5ccccc5 CCOclcc(ccclCl)C2Nc3ccc(cc3NC4=C2C(=O)CC(C)(C)C4)C(=O)c5ccccc5 CCOc 1 cc(ccc 1 Cl)C2Ηc3ccc(cc3NC4=C2C(=O)CC(C)(C)C4)N(C)C(=O)Oc5ccccc5 CCOclcc(cccl Cl)C2Nc3ccc(OCc4cncs4)cc3NC5=C2C(=O)CC(C)(C)C5 CCl (C)CC2=C(C(Nc3ccc(OCc4ccccc4)cc3N2)c5ccc(O)c(c5)C#N)C(=O)Cl
CCI (C)CC2=C(C(Nc3ccc(cc3N2)C(=O)Nc4ccccc4)c5ccc(O)c(c5)C#N)C(=O)Cl CC(OC(=O)cl ccc2NC(C3=C(CC(C)(C)CC3=O)Ηc2cl)c4ccc(O)c(c4)C#N)c5ccccc5 CCl(C)CC2=C(C(Nc3ccc(cc3N2)C(=O)c4ccccc4)c5cccc(Cl)c5)C(=Oj)Cl CC 1 (C)CC2=C(C(Nc3ccc(COc4ccccc4)cc3N2)c5cccc(Cl)c5)C(=O)C 1 CCl(C)CC2=C(C(Ηc3ccc(cc3N2)C(=O)Nc4ccccc4)c5cccc(Cl)c5)C(=O)Cl CN(C(=O)c 1 ccccc 1 )c2ccc3NC(C4=C(CC(C)(C)CC4=O)Nc3c2)c5cccc(Cl)c5 CC(OC(=O)clccc2NC(C3=C(CC(C)(C)CC3=O)Nc2c1)c4cccc(Cl)c4)c5ccccc5 CC(C)OcI ccc(cc 1 C(F)(F)F)C2NC3CCC(CC3"NC4=C2C(=O)CC(C)(C)C4)C(=O)C5CCCCC5 CC(C)Oclccc(cclC(F)(F)F)C2Mc3ccc(cc3NC4=C2C(=O)CC(C)(C)C4)N(C)C(=O)Oc5ccccc5 COcI cc(cc(OC)c 10)C2Nc3ccc(cc3NC4=C2C(=O)CC(C)(C)C4)C(=O)c5ccccc5 COcI CC(CC(OC)C] O)C2Nc3ccc(OCc4ccccc4)cc3NC5=C2C(=O)CC(C)(C)C5 COcIcc(cc(OC)clO)C2Nc3ccc(cc3'NC4=C2C(=O)CC(C)(C)C4)N(C)C(=O)Oc5ccccc5 COcI cc(cc(OC)c 10)C2Nc3ccc(OCc4cncs4)cc3NC5=C2C(=O)CC(C)(C)C5 CCOcI cc(OCC)cc(c 1 )C2Nc3ccc(Oc4ccccc4)cc3NC5=C2C(=O)CC(C)(C)C5 CCOc 1 cc(OCC)cc(c 1 )C2Mc3ccc(COc4ccccc4)cc3NC5=C2C(=O)CC(C)(C)C5
CCOcl cc(OCC)cc(cl)C2Nc3ccc(cc3NC4=C2C(=O)CC(C)(C)C4)S(=O)(=O)Nc5ccccc5
CCOclcc(OCC)cc(cl)C2Nc3ccc(cc3NC4=C2C(=O)CC(C)(C)C4)C(=O)OC(C)c5ccccc5 CC 1 (C)CC(=O)C2=C(C 1 )Nc3cc(ccc3NC2c4ccc(Cl)c(Cl)c4)S(=O)(=O)c5ccccc5 CN(c 1 ccccc 1 )c2ccc3NC(C4=C(CC(C)(C)CC4=O)Nc3c2)c5ccc(Cl)c(Cl)c5 CC I (C)CC(=O)C2=C(C 1 )Nc3cc(ccc3NC2c4ccc5CCCc5c4)C(=O)c6ccccc6 CC 1 (C)CC(=O)C2=C(C 1 )Nc3cc(OCc4ccccc4)ccc3NC2c5ccc6CCCc6c5 CN(Cl cccccl)c2ccc3NC(C4=C(CC(C)(C)CC4=O)Nc3c2)c5ccc6CCCc6c5 CCl(C)CC(=O)C2=C(Cl)Nc3cc(ccc3"NC2c4ccc5CCCc5c4)S(=O)(=O)Nc6ccccc6 CCl (C)CC(=O)C2=C(Cl)Nc3cc(ccc3NC2c4ccc5CCCc5c4)C(=O)Nc6ccccc6 CC 1 (C)CC(=O)C2=C(C1 )Nc3cc(ccc3NC2c4ccc5CCCc5c4)C(=O)Oc6ccccc6 CC 1 (C)CC2=C(C(Nc3 ccc(Oc4ccccc4)cc3 N2)c5cncc(Br)c5)C(=O)C 1 CC 1 (C)CC2=C(C(Nc3ccc(OCc4ccccc4)cc3N2)c5cncc(Br)c5)C(=O)C 1 CCl (C)CC(=O)C2=C(Cl)Nc3cc(ccc3NC2c4cncc(Br)c4)S(=O)(=O)Nc5ccccc5 CC 1 (C)CC2=C(C(Nc3ccc(cc3N2)C(=O)Oc4ccccc4)c5cncc(Br)c5)C(=O)C 1 CN(C(=O)Ocl ccccc l)c2ccc3NC(C4=C(CC(C)(C)CC4=O)Nc3c2)c5cncc(Br)c5 CCOC(=O)clcc(ccnl)C2Nc3ccc(Oc4ccccc4)cc3NC5=C2C(=O)CC(C)(C)C5 CCOC(=O)c 1 Cc(CCiI 1 )C2Nc3ccc(COc4ccccc4)cc3NC5=C2C(=O)CC(C)(C)C5 CCOC(=O)c) cc(ccn l)C2Nc3ccc(cc3NC4=C2C(=O)CC(C)(C)C4)C(=O)Nc5ccccc5 CCOC(=O)cl cc(ccnl)C2Nc3ccc(cc3NC4=C2C(=O)CC(C)(C)C4)C(=O)OC(C)c5ccccc5 CCl(C)CC(=0)C2=C(Cl)>Jc3cc(ccc3NC2c4oc(nn4)C5CC5)C(=0)c6ccccc6 CN(c 1 cccccl )c2ccc3"NC(C4=C(CC(C)(C)CC4=O)Nc3c2)c5oc(nn5)C6CC6
CC 1 (C)CC(=O)C2=C(C 1 )Nc3 cc(ccc3NC2c4oc(nn4)C5CC5)C(=0)Oc6ccccc6 CN(C(=O)Oclcccccl)c2ccc3NC(C4=C(CC(C)(C)CC4=O)Nc3c2)c5oc(nn5)C6CC6 CC 1 (C)CC2=C(C(Nc3ccc(cc3N2)C(=O)c4ccccc4)c5cncc6ncccc56)C(=O)C 1 CN(cl cccccl)c2ccc3NC(C4=C(CC(C)(C)CC4=O)Nc3c2)c5cncc6ncccc56 CC 1 (C)CC2=C(C(Nc3ccc(cc3N2)C(=O)Oc4ccccc4)c5cncc6ncccc56)C(=O)C 1
CN(C(=O)Oclcccccl)c2ccc3NC(C4=C(CC(C)(C)CC4=O)Nc3c2)c5cncc6ncccc56
CCOcI CC(CCcI O)C2Nc3ccc(cc3'NC4=C2C(=O)CC(C)(C)C4)S(=O)(=O)c5ccccc5 CCOclcc(ccclO)C2Nc3ccc(cc3NC4=C2C(=O)CC(C)(C)C4)N(C)C(=O)c5ccccc5 Cc I ccc(cc 1 Cl)C2Nc3ccc(0c4ccccc4)cc3NC5=C2C(=O)CC(C)(C)C5 Cc I ccc(cc 1 Cl)C2Nc3ccc(cc3NC4=C2C(=O)CC(C)(C)C4)S(=O)(=O)Nc5ccccc5 CcI ccc(cc 1 Cl)C2Nc3ccc(cc3NC4=C2C(=O)CC(C)(C)C4)C(=O)Oc5ccccc5
CN(C(=O)Ocl cccccl)c2ccc3TMC(C4=C(CC(C)(C)CC4=O)Mc3c2)c5ccc(C)c(Cl)c5
Ccl ccc(cclCl)C2'Nc3ccc(OCc4cncs4)cc3'NC5=C2C(=O)CC(C)(C)C5
CCOc 1 Cc(CCCl Cl)C2"Nc3ccc(Oc4ccccc4)cc3NC5=C2C(=O)CC(CC)C5
CCOc 1 cc(ccc 1 Cl)C2Nc3ccc(cc3NC4=C2C(=O)CC(CC)C4)S(=O)(=O)c5ccccc5 CCC 1 CC(=O)C2=C(C 1 )Nc3cc(ccc3NC2c4ccc(O)c(c4)C#N)C(=O)c5ccccc5 CCCl CC2=C(C(Nc3ccc(Oc4ccccc4)cc3N2)c5ccc(O)c(c5)C#N)C(=O)C 1 CCC 1 CC(=O)C2=C(C 1 )Nc3cc(COc4ccccc4)ccc3NC2c5ccc(O)c(c5)C#N CCClCC2=C(C(Nc3ccc(cc3N2)N(C)C(=O)Oc4ccccc4)c5ccc(O)c(c5)C#N)C(=O)C l CCC 1 CC2=C(C(Nc3ccc(cc3N2)C(=0)OC(C)c4ccccc4)c5ccc(O)c(c5)C#N)C(=O)C 1 CCC 1 CC2=C(C(Nc3ccc(cc3N2)C(=O)c4ccccc4)c5cccc(Cl)c5)C(=O)C 1 CCC 1 CC2=C(C(Nc3ccc(cc3N2)N(C)c4ccccc4)c5cccc(Cl)c5)C(=O)Cl CCCl CC(=O)C2=C(Cl)Nc3cc(ccc3NC2c4cccc(Cl)c4)S(=O)(=O)Nc5ccccc5 CCClCC2=C(C(Nc3ccc(cc3N2)C(=O)Oc4ccccc4)c5cccc(Cl)c5)C(=O)Cl CCClCC2=C(C(Nc3ccc(OCc4cncs4)cc3N2)c5cccc(CI)c5)C(=O)Cl CCClCC2=C(C(Nc3ccc(Oc4ccccc4)cc3N2)c5ccc(OC(C)C)c(c5)C(F)(F)F)C(=O)Cl
CCC 1 CC2=C(C(Nc3ccc(cc3N2)C(=O)OC(C)c4ccccc4)c5ccc(OC(C)C)c(c5)C(F)(F)F)C(=O)C 1 CCC 1 CC2=C(C(Nc3ccc(Oc4ccccc4)cc3N2)c5cc(OC)c(O)c(OC)c5)C(=O)C 1 CCCl CC2=C(C(Nc3ccc(cc3N2)C(=O)Nc4ccccc4)c5cc(OC)c(O)c(OC)c5)C(=O)Cl CCC 1 CC2=C(C(Nc3ccc(cc3N2)C(=O)Oc4ccccc4)c5cc(OC)c(O)c(OC)c5)C(=O)C 1 CCOc 1 cc(OCC)cc(c 1 )C2Nc3ccc(cc3NC4=C2C(=O)CC(CC)C4)C(=O)c5ccccc5 CCOc 1 cc(OCC)cc(c 1 )C2Nc3ccc(Oc4ccccc4)cc3NC5=C2C(=O)CC(CC)C5
CCOc 1 cc(OCC)cc(cl )C2Nc3ccc(OCc4ccccc4)cc3NC5=C2C(=O)CC(CC)C5 CCOclcc(OCC)cc(c1)C2Nc3ccc(cc3NC4=C2C(=O)CC(CC)C4)Η(C)C(=O)c5ccccc5 CCOcl cc(OCC)cc(cl)C2Nc3ccc(cc3NC4=C2C(=0)CC(CC)C4)N(C)C(=0)Oc5ccccc5 CCOc 1 cc(OCC)cc(c 1 )C2Nc3 ccc(OCc4cncs4)cc3NC5=C2C(=O)CC(CC)C5 CCCl CC2=C(C(Nc3ccc(OCc4ccccc4)cc3N2)c5ccc(Cl)c(Cl)c5)C(=O)Cl CCC 1CC(=O)C2=C(C1)NC3CC(CCC31MC2C4CCC(C1)C(C1)C4)C(=O)NC5CCCCC5 CCC 1 CC2=C(C(NC3CCC(CC3N2)N(C)C(=O)OC4CCCCC4)C5CCC(C1)C(C1)C5)C(=O)C1 CCC 1 CC2=C(C(Nc3ccc(cc3N2)C(=O)OC(C)c4ccccc4)c5ccc(Cl)c(Cl)c5)C(=O)C 1 CCCiCC(=O)C2=C(Cl)Nc3cc(Oc4ccccc4)ccc3NC2c5ccc6CCCc6c5 CCC 1 CC(=O)C2=C(Cl)Nc3cc(COc4ccccc4)ccc3NC2c5ccc6CCCc6c5
CCClCC(=O)C2=C(Cl)Nc3cc(ccc3NC2c4ccc5CCCc5c4)S(=O)(=O)c6ccccc6 CCClCC(=O)C2=C(Cl)Nc3cc(ccc3NC2c4ccc5CCCc5c4)S(=O)(=O)Nc6ccccc6 CCCl CC(=O)C2=C(Cl)Nc3cc(ccc3NC2c4ccc5CCCc5c4)C(=O)Nc6ccccc6 CCClCC(=O)C2=C(Cl)Nc3cc(ccc3NC2c4ccc5CCCc5c4)N(C)C(=O)c6ccccc6 CCClCC(=O)C2=C(Cl)Nc3cc(ccc3NC2c4ccc5CCCc5c4)C(=O)Oc6ccccc6
CCC 1 CC2=C(C(Nc3ccc(cc3N2)C(=O)OC(C)c4ccccc4)c5ccc6CCCc6c5)C(=O)C 1 CCCl CC2=C(C(Nc3ccc(cc3N2)C(=O)c4ccccc4)c5cncc(Br)c5)C(=O)Cl CCCl CC2=C(C(Nc3ccc(cc3N2)N(C)c4ccccc4)c5cncc(Br)c5)C(=O)Cl CCOC(=O)clcc(ccnl)C2Nc3ccc(Oc4ccccc4)cc3NC5=C2C(=O)CC(CC)C5 CCOC(=O)c 1 cc(ccn 1 )C2Nc3ccc(cc3NC4=C2C(=O)CC(CC)C4)S(=O)(=O)Nc5ccccc5 CCOC(=O)c1cc(ccnl)C2Nc3ccc(cc3NC4=C2C(=0)CC(.CC)C4)N(C)C(=0)c5ccccc5 CCC 1 CC(=O)C2=C(C 1 )Nc3cc(OCc4ccccc4)ccc3NC2c5oc(nn5)C6CC6 CCC lCC(=0)C2=C(Cl)Nc3cc(ccc3NC2c4oc(nn4)C5CC5)S(=0)(=0)c6ccccc6 CCC 1 CC(=O)C2=C(C 1 )Nc3cc(ccc3NC2c4oc(nn4)C5CC5)~N(C)c6ccccc6 CCCl CC2=C(C(Nc3ccc(cc3N2)S(=O)(=O)c4ccccc4)c5cncc6ncccc56)C(=O)Cl CCClCC2=C(C(Nc3ccc(cc3N2)N(C)C(=O)c4ccccc4)c5cncc6ncccc56)C(=O)Cl
CCOc 1 cc(ccc 1 O)C2Nc3 ccc(cc3NC4=C2C(=O)CC(CC)C4)N(C)c5ccccc5 CCOc 1 cc(ccc 1 O)C2Nc3ccc(cc3NC4=C2C(=O)CC(CC)C4)S(=O)(=O)Nc5ccccc5 CCOcl cc(cccl O)C2Nc3ccc(cc3NC4=C2C(=O)CC(CC)C4)C(=O)OC(C)c5ccccc5 CCCl CC2=C(C(Nc3ccc(Oc4ccccc4)cc3N2)c5ccc(C)c(Cl)c5)C(=O)CI CCClCC(=O)C2=C(Cl)Nc3cc(ccc3NC2c4ccc(C)c(Cl)c4)S(:=O)(=O)c5ccccc5 CCClCC2=C(C(Nc3ccc(cc3N2)N(C)C(=O)c4ccccc4)c5ccc(C)c(CI)c5)C(=O)C] CCOclcc(ccclCl)C2Nc3ccc(cc3NC4=C2C(=O)CC(C4)c5ccccc5)S(=O)(=O)c6ccccc6 CCOclcc(ccclCl)C2Nc3ccc(cc3NC4=C2C(=O)CC(C4)c5ccccc5)N(C)c6ccccc6 OcI CCc(CCl C#N)C2Nc3ccc(Oc4ccccc4)cc3NC5=C2C(=O)CC(C5)c6ccccc6 Oclccc(cclC#N)C2Nc3ccc(COc4ccccc4)cc3NC5=C2C(=O)CC(C5)c6ccccc6
Ocl ccc(ccl C#N)C2Nc3ccc(cc3NC4=C2C(=O)CC(C4)c5ccccc5)C(=O)Nc6ccccc6 CM(C(=O)Oclcccccl)c2ccc3NC(C4=C(CC(CC4=O)c5ccccc5)Nc3c2)c6ccc(O)c(c6)C#N CC(OC(=O)cl ccc2NC(C3=C(CC(CC3=O)c4ccccc4)Nc2c 1 )c5ccc(O)c(c5)C#N)c6ccccc6 Clcl cccc(c 1 )C2Nc3ccc(cc3NC4=C2C(=O)CC(C4)c5ccccc5)C(=O)c6ccccc6 Clcl cccc(cl )C2Nc3ccc(OCc4ccccc4)cc3NC5=C2C(=O)CC(C5)c6ccccc6 CN(c 1 ccccc 1 )c2ccc3NC(C4=C(CC(CC4=O)c5ccccc5)Nc3 c2)c6cccc(Cl)c6 Clcl cccc(c 1)C2NC3CCC(CC3NC4=C2C(=O)CC(C4)C5CCCCC5)S(=O)(=O)NC6CCCCC6 CIc 1 cccc(c 1 )C2Nc3 ccc(cc3NC4=C2C(=O)CC(C4)c5ccccc5)C(=O)Oc6ccccc6 CN(C(=O)Oc 1 ccccc 1 )c2ccc3NC(C4=C(CC(CC4=O)c5ccccc5)Nc3c2)c6cccc(Cl)c6 CC(C)OcI ccc(cc 1 C(F)(F)F)C2NC3CCC(COC4CCCCC4)CC3NC5=C2C(=O)CC(C5)C6CCCCC6 CC(C)OcI ccc(cc 1 C(F)(F)F)C2NC3CCC(CC3NC4=C2C(=O)CC(C4)C5CCCCC5)"N(C)C6CCCCC6 CC(C)Oclccc(ccl C(F)(F)F)C2Nc3ccc(cc3NC4=C2C(=O)CC(C4)c5ccccc5)C(=O)Nc6ccccc6 COcI cc(cc(OC)c 10)C2Nc3ccc(cc3NC4=C2C(=O)CC(C4)c5ccccc5)C(=O)c6ccccc6 COclcc(cc(OC)cl O)C2Nc3ccc(OCc4ccccc4)cc3NC5=C2C(=0)CC(C5)c6ccccc6 COcI cc(cc(OC)cl O)C2Nc3ccc(cc3NC4=C2C(=O)CC(C4)c5ccccc5)C(=O)Oc6ccccc6
COc 1 cc(cc(OC)c 10)C2Nc3ccc(cc3NC4=C2C(=O)CC(C4)c5ccccc5)N(C)C(=O)Oc6ccccc6
COcl cc(cc(OC)cl O)C2Nc3ccc(OCc4cncs4)cc3ΗC5=C2C(=O)CC(C5)c6ccccc6 CCOcl cc(OCC)cc(cl)C2Nc3ccc(cc3NC4=C2C(=O)CC(C4)c5ccccc5)C(=O)c6ccccc6 CCOc 1 CC(OCC)Cc(C 1 )C2Nc3ccc(OCc4ccccc4)cc3NC5=C2C(=O)CC(C5)c6ccccc6 CCOc 1 CC(OCC)Cc(C 1 )C2Nc3 ccc(cc3NC4=C2C(=O)CC(C4)c5ccccc5)N(C)C(=O)c6ccccc6 CCOc 1 CC(OCC)Cc(C 1 )C2Nc3ccc(OCc4cncs4)cc3NC5=C2C(=O)CC(C5)c6ccccc6
CCOcl cc(OCC)cc(cl)C2Ηc3ccc(cc3NC4=C2C(=O)CC(C4)c5ccccc5)C(=O)OC(C)c6ccccc6 Clcl CCC(CcI Cl)C2Nc3ccc(cc3NC4=C2C(=O)CC(C4)c5ccccc5)C(=O)Nc6ccccc6 CN(C(=0)c 1 ccccc 1 )c2ccc3NC(C4=C(CC(CC4=O)c5ccccc5)Nc3c2)c6ccc(Cl)c(Cl)c6 CIc 1 CCc(CC 1 C!)C2Nc3ccc(OCc4cncs4)cc3NC5=C2C(=O)CC(C5)c6ccccc6 CC(0C(=0)c 1 ccc2NC(C3=C(CC(CC3=O)c4ccccc4)Nc2c 1 )c5ccc(Cl)c(Cl)c5)c6ccccc6 O=C(cl cccccl)c2ccc3NC(C4=C(CC(CC4=O)c5ccccc5)Nc3c2)c6ccc7CCCc7c6 O=C I CC(CC2=C1 C(Nc3ccc(OCc4ccccc4)cc3N2)c5ccc6CCCc6c5)c7ccccc7 CN(clcccccl)c2ccc3NC(C4=C(CC(CC4=O)c5ccccc5)Nc3c2)c6ccc7CCCc7c6 O=C 1 CC(CC2=C 1 C(Nc3ccc(cc3N2)S(=O)(=O)'Nc4ccccc4)c5ccc6CCCc6c5)c7ccccc7 O=C(Nc 1 ccccc 1 )c2ccc3NC(C4=C(CC(CC4=O)c5ccccc5)Nc3c2)c6ccc7CCCc7c6
CN(C(=O)clcccccl)c2ccc3NC(C4=C(CC(CC4=O)c5ccccc5)Nc3c2)c6ccc7CCCc7c6 O=C(Oc 1 ccccc 1)C2CCC3NC(C4=C(CC(CC4=O)C5CCCCC5)NC3C2)C6CCC7CCCC7C6 CC(OC(=O)cl ccc2NC(C3=C(CC(CC3=O)c4ccccc4)Nc2c 1 )c5ccc6CCCc6c5)c7ccccc7 Brclcncc(cl)C2Nc3ccc(cc3NC4=C2C(=O)CC(C4)c5ccccc5)C(=O)c6ccccc6 Brcl cncc(cl)C2Nc3ccc(OCc4ccccc4)cc3NC5=C2C(=O)CC(C5)c6ccccc6 CN(c 1 ccccc 1 )c2ccc3NC(C4=C(CC(CC4=O)c5ccccc5)Nc3c2)c6cncc(Br)c6 Brc 1 cncc(c 1 )C2Nc3ccc(cc3NC4=C2C(=O)CC(C4)c5ccccc5)C(=O)Oc6ccccc6 CN(C(=O)Oc 1 ccccc 1 )c2ccc3NC(C4=C(CC(CC4=O)c5ccccc5)Nc3c2)c6cncc(Br)c6 CC0C(=0)cl cc(ccn 1 )C2Nc3ccc(OCc4ccccc4)cc3NC5=C2C(=O)CC(C5)c6ccccc6 CCOC(=O)clcc(ccnl)C2Nc3ccc(cc3NC4=C2C(=O)CC(C4)c5ccccc5)S(=O)(=O)c6ccccc6 CCOC(=O)c lcc(ccnl)C2Nc3ccc(cc3NC4=C2C(=O)CC(C4)c5ccccc5)N(C)c6ccccc6
CN(clcccccl)c2ccc3NC(C4=C(CC(CC4=O)c5ccccc5)Nc3c2)c6oc(nn6)C7CC7 O=Cl CC(CC2=C 1 C(Nc3ccc(cc3N2)S(=O)(=O)Nc4ccccc4)c5oc(nn5)C6CC6)c7ccccc7 O=C(OcI ccccc l)c2ccc3"NC(C4=€(CC(CC4=O)c5ccccc5)"Nc3c2)c6oc(nn6)C7CC7 CN(C(=O)Oc 1 ccccc l )c2ccc3NC(C4=C(CC(CC4=O)c5ccccc5)Nc3c2)c6oc(τm6)C7CC7 CN(cl cccccl )c2ccc3NC(C4=C(CC(CC4=O)c5ccccc5)Nc3c2)c6cncc7ncccc67
O=C 1 CC(CC2=C 1 C(Nc3ccc(cc3N2)S(=O)(=O)Nc4ccccc4)c5cncc6ncccc56)c7ccccc7 O=C(Oc 1 ccccc 1 )c2ccc3N C(C4=C(CC(CC4=O)c5ccccc5)Nc3 c2)c6cn cc7n cccc67 CN(C(=O)Oclcccccl)c2ccc3NC(C4=C(CC(CC4=O)c5ccccc5)Nc3c2)c6cncc7ncccc67 CCOc 1 CC(CCc 1 O)C2Nc3ccc(COc4ccccc4)cc3NC5=C2C(=O)CC(C5)c6ccccc6 CCOc 1 cc(ccc 10)C2Nc3 ccc(cc3NC4=C2C(=O)CC(C4)c5ccccc5)N(C)c6ccccc6
CCOc 1 cc(ccc 10)C2Nc3ccc(cc3NC4=C2C(=O)CC(C4)c5ccccc5)C(=O)Nc6ccccc6 Cc 1 ccc(cc 1 Cl)C2Nc3 ccc(Oc4ccccc4)cc3NC5=C2C(=O)CC(C5)c6ccccc6 Cclccc(ccl Cl)C2Nc3ccc(cc3ΗC4=C2C(=O)CC(C4)c5ccccc5)S(=O)(=O)c6ccccc6 CCOcI Cc(CCC 1C1)C2NC3CCC(CC3NC4=C2C(=O)CC(=O)C4)C(=O)NC5CCCCC5 CCOcl cc(cccl Cl)C2Nc3ccc(cc3NC4=C2C(=O)CC(=O)C4)C(=O)Oc5ccccc5 Oc I CCC(Cc 1 C#N)C2NC3CCC(CC3NC4=C2C(=O)CC(=O)C4)S(=O)(=O)C5CCCCC5 CN(cl ccccc 1 )c2ccc3NC(C4=C(CC(=O)CC4=O)Nc3c2)c5ccc(O)c(c5)C#N Clcl cccc(cl)C2Nc3ccc(cc3NC4=C2C(=O)CC(=O)C4)C(=O)c5ccccc5 CIc 1 cccc(c 1 )C2Nc3ccc(cc3NC4=C2C(=O)CC(=O)C4)S(=O)(=O)Nc5ccccc5 CIc 1 cccc(c 1 )C2NC3CCC(CC3NC4=C2C(=O)CC(=O)C4)C(=O)NC5CCCCC5
CC(C)Oc 1 ccc(cc 1 C(F)(F)F)C2Nc3ccc(cc3NC4=C2C(=O)CC(=O)C4)S(=O)(=O)c5ccccc5
COcI cc(cc(OC)cl O)C2Nc3ccc(Oc4ccccc4)cc3NC5=C2C(=O)CC(=O)C5
COcI cc(cc(OC)c 10)C2Nc3ccc(cc3NC4=C2C(=O)CC(=O)C4)S(=O)(=O)c5ccccc5
CCOcl cc(OCC)cc(cl)C2Nc3ccc(cc3NC4=C2C(=O)CC(=O)C4)S(=O)(=O)c5ccccc5 CIc 1 ccc(cc 1 Cl)C2Nc3ccc(Oc4ccccc4)cc3NC5=C2C(=O)CC(=O)C5 CIcI ccc(ccl Cl)C2Nc3ccc(COc4ccccc4)cc3NC5=C2C(=O)CC(=O)C5
CIclccc(cclCl)C2Nc3ccc(cc3NC4=C2C(=O)CC(=O)C4)S(=O)(=O)c5ccccc5
O=CtCC(=O)C2=C(Cl)Nc3cc(OCc4ccccc4)ccc3MC2c5ccc6CCCc6c5
O=C 1 CC(=O)C2=C(C 1 )Nc3cc(ccc3NC2c4ccc5CCCc5c4)S(=O)(=O)c6ccccc6
CN(c 1 ccccc 1 )c2ccc3NC(C4=C(CC(=O)CC4=O)Nc3c2)c5ccc6CCCc6c5 O=C(Oc 1 ccccc 1 )c2ccc3NC(C4=C(CC(=O)CC4=O)Nc3 c2)c5ccc6CCCc6c5
Brclcncc(cl)C2Nc3ccc(cc3"NC4=C2C(=O)CC(=O)C4)S(=O)(=O)c5ccccc5
CN(c 1 ccccc 1 )c2ecc3NC(C4=C(CC(=O)CC4=O)Nc3c2)c5cncc(Br)c5
CCOC(=O)c 1 cc(ccn 1 )C2Nc3ccccc3NC4=C2C(=O)CC(=O)C4
O=C 1 CC(=O)C2=C(C 1 )Nc3cc(ccc3NC2c4oc(nn4)C5CC5)S(=0)(=0)c6ccccc6 O=C 1 CC2=C(C(Nc3ccccc3N2)c4cncc5ncccc45)C(=O)C 1
CCOc 1 cc(ccc 10)C2Nc3ccc(cc3NC4=C2C(=O)CC(=O)C4)C(=O)c5ccccc5
CCOcI cc(ccc 1 O)C2NC3CCC(OCC4CCCCC4)CC3NC5=C2C(=O)CC(=O)C5
CCOc 1 Cc(CCCl O)C2Nc3ccc(cc3NC4=C2C(=O)CC(=0)C4)C(=0)0c5ccccc5
CN(Cl ccccc 1 )C2CCC3NC(C4=C(CC(=O)CC4=O)ΗC3C2)C5CCC(C)C(C1)C5 CCOc 1 cc(ccc 1 Cl)C2Nc3 ccc(cc3NC4=C2C(=O)CC(O)C4)C(=O)c5ccccc5
CCOc 1 cc(ccc 1 Cl)C2Nc3ccc(OCc4ccccc4)cc3NC5=C2C(=O)CC(O)C5
OC 1 CC2=C(C(Nc3ccc(cc3N2)C(=O)c4ccccc4)c5ccc(O)c(c5)C#N)C(=O)C 1
OClCC2=C(C(Nc3ccc(Oc4ccccc4)cc3N2)c5ccc(O)c(c5)C#N)C(=O)Cl
OC 1 CC2=C(C(Nc3ccc(COc4ccccc4)cc3'N2)c5ccc(O)c(c5)C#N)C(=O)C 1 CC(OC(=O)clccc2NC(C3=C(CC(0)CC3=0)Nc2cl)c4ccc(O)c(c4)C#N)c5ccccc5
OCl CC2=C(C(Nc3ccc(COc4ccccc4)cc3N2)c5cccc(Cl)c5)C(=O)Cl
OCl CC2=C(CfNc3ccc(cc3N2)S(=O)(=O)c4ccccc4)c5cccc(Cl)c5)C(=O)Cl
CN (c 1 ccccc 1 )c2ccc3NC(C4=€(CC(O)CC4=O)Nc3 c2)c5cccc(Cl)c5
OC 1 CC2=C(C(Nc3ccc(cc3N2)C(=O)Nc4ccccc4)c5cccc(Cl)c5)C(=O)C 1 CN(C(=O)cl ccccc l)c2ccc3MC(C4=C(CC(O)CC4=O)Nc3c2)c5cccc(Cl)c5
CC(OC(=O)c 1 ccc2NC(C3=C(CC(O)CC3=O)Nc2c 1 )c4cccc(Cl)c4)c5ccccc5
CC(C)Oc 1 ccc(cc 1 C(F)(F)F)C2Nc3 ccc(COc4ccccc4)cc3NC5=C2C(=O)CC(O)C5 CC(C)Oc 1 CCC(Cd C(F)(F)F)C2Nc3ccc(cc3NC4=C2C(=O)CC(O)C4)N(C)c5ccccc5 CC(C)OcI CCc(CCl C(F)(F)F)C2Nc3ccc(cc3NC4=C2C(=O)CC(O)C4)C(=O)Nc5ccccc5 COcI cc(cc(OC)cl O)C2Nc3ccc(cc3NC4=C2C(=O)CC(O)C4)C(=O)c5ccccc5 COcl cc(cc(OC)clO)C2Nc3ccc(OCc4ccccc4)cc3NC5=C2C(=O)CC(O)C5 COcl cc(cc(OC)clO)C2Nc3ccc(Sc4ccccc4)cc3NC5=C2C(=O)CC(O)C5 COcI cc(cc(OC)c 10)C2Nc3ccc(cc3NC4=C2C(=O)CC(O)C4)C(=O)Oc5ccccc5 COc 1 cc(cc(OC)c 1 O)C2Nc3ccc(OCc4cncs4)cc3NC5=C2C(=O)CC(O)C5 CCOc 1 cc(0CC)cc(c 1 )C2Nc3ccc(Oc4ccccc4)cc3NC5=C2C(=O)CC(O)C5 CCOclcc(OCC)cc(cl)C2Nc3ccc(cc3NC4=C2C(=O)CC(O)C4)S(=O)(=O)Nc5ccccc5 CCOclcc(OCC)cc(cl)C2Nc3ccc(cc3NC4=C2C(=O)CC(O)C4)N(C)C(=O)c5ccccc5 CCOclcc(OCC)cc(cl)C2Nc3ccc(cc3NC4=C2C(=O)CC(O)C4)C(=O)OC(C)c5ccccc5 OCl CC2=C(C(Nc3ccc(cc3N2)C(=O)c4ccccc4)c5ccc(Cl)c(CI)c5)C(=O)Cl OC 1 CC2=C(C(Nc3ccc(Sc4ccccc4)cc3N2)c5ccc(Cl)c(Cl)c5)C(=O)C 1 OC 1 CC(=0)C2=C(C 1 )Nc3cc(ccc3NC2c4ccc(Cl)c(CI)c4)S(=O)(=O)Nc5ccccc5 CN(C(=O)Ocl cccccl)c2ccc3NC(C4=C(CC(O)CC4=O)Nc3c2)c5ccc(Cl)c(Cl)c5 OC lCC2=C(C(Nc3ccc(OCc4cncs4)cc3N2)c5ccc(Cl)c(Cl)c5)C(=O)C 1 OC 1 CC(=0)C2=C(C 1 )Nc3cc(ccc3NC2c4ccc5CCCc5c4)C(=O)c6ccccc6 OCl CC2=C(C(Nc3ccc(Oc4ccccc4)cc3N2)c5ccc6CCCc6c5)C(=O)Cl OC 1 CC(=0)C2=C(C 1 )Nc3cc(OCc4ccccc4)ccc3NC2c5ccc6CCCc6c5
OC 1 CC(=O)C2=C(C 1 )Nc3 cc(ccc3 NC2c4ccc5CCCc5c4)S(=O)(=O)Nc6ccccc6 OC 1 CC(=O)C2=C(C1)NC3CC(CCC3NC2C4CCC5CCCC5C4)C(=O)NC6CCCCC6 CN(C(=0)c 1 ccccc 1 )c2ccc3NC(C4=C(CC(O)CC4=O)Nc3c2)c5ccc6CCCc6c5 OC 1 CC(=0)C2=C(C 1 )Nc3cc(ccc3NC2c4ccc5CCCc5c4)C(=O)Oc6ccccc6 CC(OC(=O)cl ccc2NC(C3=C(CC(O)CC3=O)Nc2cl)c4ccc5CCCc5c4)c6ccccc6 OC 1 CC2=C(C(Nc3ccc(cc3N2)C(=O)c4ccccc4)c5cncc(Br)c5)C(=O)C 1
OC 1 CC2=C(C(Nc3ccc(Oc4ccccc4)cc3N2)c5cncc(Br)c5)C(=O)C 1 OCl CC2=C(C(Nc3ccc(OCc4ccccc4)cc3>J2)c5cncc(Br)c5)C(=O)Cl OCl CC2=C(CCNc3ccc(cc3N2)C(=O)Oc4ccccc4)c5cncc(Br)c5)C(=O)Cl CCOC(=O)clcc(ccnl)C2Nc3ccc(OCc4ccccc4)cc3NC5=C2C(=O)CC(O)C5 CCOC(=O)clcc(ccnl)C2Nc3ccc(cc3NC4=C2C(=O)CC(O)C4)S(=O)(=O)c5ccccc5 CCOC(=O)c 1 cc(ccn 1 )C2Nc3ccc(cc3NC4=C2C(=O)CC(O)C4)N(C)c5ccccc5 OCl CC2=C(C(Nc3ccc(cc3N2)C(=O)c4ccccc4)c5oc(nn5)C6CC6)C(=O)Cl OC 1 CC2=C(C(Nc3ccc(OCc4ccccc4)cc3N2)c5oc(nn5)C6CC6)C(=O)C 1 CN(cl cccccl)c2ccc3NC(C4=C(CC(O)CC4=O)Nc3c2)c5oc(nn5)C6CC6 OClCC2=C(C(Nc3ccc(cc3N2)C(=O)Oc4ccccc4)c5oc(nn5)C6CC6)C(=O)Cl OC 1 CC2=C(C(Nc3ccc(cc3N2)C(=O)c4ccccc4)c5cncc6ncccc56)C(=O)C 1 CM(clcccccl)c2ccc3NC(C4=C(CC(O)CC4=O)Nc3c2)c5cncc6ncccc56 CN(C(=O)c 1 ccccc 1 )c2ccc3NC(C4=C(CC(O)CC4=O)Nc3c2)c5cncc6ncccc56 OC 1 CC2=C(C(Nc3ccc(cc3N2)C(=O)Oc4ccccc4)c5cncc6ncccc56)C(=O)C 1 CCOc 1 CC(CCc 1 O)C2Nc3ccc(COc4ccccc4)cc3NC5=C2C(=O)CC(O)C5 CCOc 1 cc(ccc 10)C2Nc3ccc(cc3"NC4=C2C(=O)CC(O)C4)'N(C)c5ccccc5 CCOc 1 CC(CCC 10)C2Nc3ccc(cc3NC4=C2C(=O)CC(O)C4)C(=O)Nc5ccccc5 Cc 1 ccc(cc 1 Cl)C2Nc3 ccc(cc3NC4=C2C(=O)CC(O)C4)C(=O)Oc5ccccc5 Cc 1 CCc(CC 1 Cl)C2Nc3ccccc3NC4=C2C(=O)CC(O)C4 CCOcI cc(ccc 1 C1)C2NC3CCC(OCC4CCCCC4)CC3NC5=C2C(=O)CC(C5)OC
CCOc 1 cc(ccc 1 C!)C2Nc3ccc(cc3NC4=C2C(=O)CC(C4)OC)S(=O)(=O)c5ccccc5 COCl CC2=C(C(Nc3ccc(cc3N2)C(=O)c4ccccc4)c5ccc(O)c(c5)C#N)C(=O)Cl COCl CC2=C(CCNc3ccc(OCc4ccccc4)cc3M2)c5ccc(O)c(c5)C#N)C(=O)Cl ' COC 1 CC2=C(C(Nc3 ccc(cc3N2)N(C)C(=O)Oc4ccccc4)c5ccc(O)c(c5)C#N)C(=O)C 1 COCl CC2=C(C(Nc3ccc(cc3N2)C(=O)OC(C)c4ccccc4)c5ccc(O)c(c5)C#N)C(=O)Cl COC 1 CC2=C(C(Nc3ccc(cc3N2)C(=O)c4ccccc4)c5cccc(Cl)c5)C(=O)C 1
COC1CC2=C(C(Nc3ccc(COc4ccccc4)cc3N2)c5cccc(Cl)c5)C(=O)C1 COC1CC2=C(C(Nc3ccc(cc3N2)N(C)c4ccccc4)c5cccc(C1)c5)C(=O)C1 COC1CC2=C(C(Nc3ccc(cc3N2)C(=O)Nc4ccccc4)c5cccc(CI)c5)C(=O)Cl COC1CC2=C(C(Nc3ccc(cc3N2)N(C)C(=O)c4ccccc4)c5cccc(Cl)c5)C(=O)C1 COClCC2=C(CCNc3ccc(cc3N2)N(C)C(=O)Oc4ccccc4)c5cccc(CI)c5)C(=O)C1 COClCC2=C(C(Nc3ccc(OCc4cncs4)cc3N2)c5cccc(Cl)c5)C(=O)Cl COC1CC2=C(C(Nc3ccc(cc3Η2)C(=O)OC(C)c4ccccc4)c5cccc(Cl)c5)C(=O)C1 COClCC2=C(C(Nc3ccccc3N2)c4ccc(OC(C)C)c(c4)C(F)(F)F)C(=O)Cl COC1CC2=C(C(Nc3ccc(cc3N2)C(=O)c4ccccc4)c5cc(OC)c(O)c(OC)c5)C(=O)C1 COC1CC2=C(C(Nc3ccc(Oc4ccccc4)cc3N2)c5cc(OC)c(O)c(OC)c5)C(=O)C1 C0ClCC2=C(C(Nc3ccc(OCc4ccccc4)cc3N2)c5cc(0C)c(O)c(OC)c5)C(=0)Cl COClCC2=C(CG^c3ccc(cc3"N2)C(=O)Oc4ccccc4)c5cc(OC)c(O)c(OC)c5)C(=O)Cl COC1CC2=C(C(NC3CCC(CC3N2)N(C)C(=O)0C4CCCCC4)C5CC(0C)C(0)C(0C)C5)C(=0)C1 COClCC2=C(C(Nc3ccc(OCc4cncs4)cc3N2)c5cc(OC)c(O)c(OC)c5)C(=O)Cl CCOclcc(OCC)cc(cl )C2Nc3ccc(cc3NC4=C2C(=O)CC(C4)OC)C(=O)c5ccccc5 CCOc I Cc(OCC )cc(c 1 )C2Nc3ccc(Oc4ccccc4)cc3NC5=C2C(=O)CC(C5)OC CCOc 1 Cc(OCC)CC(C 1 )C2Nc3ccc(cc3NC4=C2C(=O)CC(C4)OC)N(C)C(=O)c5ccccc5 CCOcl cc(OCC)cc(cl)C2Nc3ccc(cc3NC4=C2C(=O)CC(C4)OC)N(C)C(=0)Oc5ccccc5 CCOcI cc(0CC)cc(cl )C2Nc3ccc(OCc4cncs4)cc3NC5=C2C(=O)CC(C5)OC CCOclcc(OCC)cc(cI )C2Nc3ccc(cc3NC4=C2C(=O)CC(C4)OC)C(=O)OC(C)c5ccccc5 COC1 CC2=C(C(NC3CCC(CC3N2)S(=O)(=O)NC4CCCCC4)C5CCC(C1)C(C1)C5)C(=O)C 1 COClCC2=C(C(Nc3ccc(cc3N2)C(=O)Oc4ccccc4)c5ccc(CI)c(CI)c5)C(=O)Cl COCl CC2=C(CCNc3ccc(Oc4ccccc4)cc3N2)c5ccc6CCCc6c5)C(=O)Cl COC 1 CC(=O)C2=C(Cl)Nc3cc(OCc4ccccc4)ccc3NC2c5ccc6CCCc6c5 COC 1 CC(=0)C2=C(C 1 )Nc3cc(ccc3NC2c4ccc5CCCc5c4)S(=O)(=O)c6ccccc6 COCl CC(=O)C2=C(Cl)Nc3cc(ccc3NC2c4ccc5CCCc5c4)S(=O)(=O)Ηc6ccccc6
COClCC(=O)C2=C(Cl)Nc3cc(ccc3NC2c4ccc5CCCc5c4)C(=O)Nc6ccccc6 COClCC(=O)C2=C(Cl)Nc3cc(ccc3NC2c4ccc5CCCc5c4)M(C)C(=O)c6ccccc6 COC 1 CC2=C(C(NC3CCC(CC3N2)C(=O)0C4GGCCC4)C5CCC6CCCC6C5)C(=O)C1 COCl CC2=C(C(Nc3ccc(cc3N2)C(=O)OC(C)c4ccccc4)c5ccc6CCCc6c5)C(=O)Cl COC 1 CC2=C(C(Nc3ccc(cc3N2)C(=O)c4ccccc4)c5cncc(Br)c5)C(=O)Cl COCl CC2=C(C(Nc3ccc(OCc4ccccc4)cc3N2)c5cncc(Br)c5)C(=O)Cl COC 1 CC2=C(C(Nc3ccc(Sc4ccccc4)cc3N2)c5cncc(Br)c5)C(=O)C 1 COC 1 CC2=C(C(Nc3ccc(cc3N2)C(=O)Oc4ccccc4)c5cricc(Br)c5)C(=O)C 1 CCOC(=O)c 1 cc(ccn 1 )C2Nc3ccc(Oc4ccccc4)cc3NC5=C2C(=O)CC(C5)OC CC0C(=0)c 1 cc(ccn I )C2Nc3 ccc(OCc4ccccc4)cc3NC5=C2C(=O)CC(C5)OC
CCOC(=O)c 1 cc(ccn 1 )C2Nc3 ccc(cc3NC4=C2C(=O)CC(C4)OC)S(=O)(=O)c5ccccc5 COC 1 CC2=C(C(Nc3ccc(cc3N2)C(=O)c4ccccc4)c5oc(nn5)C6CC6)C(=O)C 1 COClCC2=C(C(Nc3ccc(OCc4ccccc4)cc3N2)c5oφn5)C6CC6)C(=O)Cl COC 1 CC2=CtC(Nc3ccc(cc3M2)N(C)c4ccccc4)c5oc(nn5)C6CC6)C(=O)Cl COC 1 CC2=C(C(Nc3ccc(cc3N2)C(=O)c4ccccc4)c5cncc6ncccc56)C(=O)C 1 COC1 CC2=C(C(Nc3ccc(cc3N2)N(C)c4ccccc4)c5cncc6ncccc56)C(=O)Cl COCl CC2=C(C(Nc3ccc(cc3N2)N(C)C(=O)c4ccccc4)c5cncc6ncccc56)C(=O)Cl COC 1 CC2=C(C(Nc3ccc(cc3"N2)C(=O)Oc4ccccc4)c5cncc6ncccc56)C(=O)C 1 CCOcI cc(ccc 10)C2Mc3ccc(COc4ccccc4)cc3ΗC5=C2C(=O)CC(C5)OC CCOcI cc(cccl O)C2Nc3ccc(Sc4ccccc4)cc3NC5=C2C(=O)CC(C5)Oe COCl CC2=C(C(Nc3ccc(Oc4ccccc4)cc3N2)c5ccc(C)c(CI)c5)C(=O)Cl COClCC2=C(C(Nc3ccc(cc3N2)S(=O)(=O)c4ccccc4)c5ccc(C)c(Cljc5)C(=O)Cl CCOc] cc(cccl Cl)C2Nc3ccc(Oc4ccccc4)cc3NC5=C2C(=O)CC6(CCCC6)C5 CCOcI cc(ccc I Cl)C2Nc3ccc(cc3NC4=C2C(=O)CC5(CCCC5)C4)S(=O)(=O)c6ccccc6 Oc I ccc(cc 1 C#N)C2Nc3ccc(cc3NC4=C2C(=O)CC5(CCCC5)C4)C(=O)c6ccccc6 OcI ccc(cc ! C#N)C2Nc3ccc(Sc4ccccc4)cc3NC5=C2C(=O)CC6(CCCC6)C5
CN(C(=O)Oclcccccl)c2ccc3"NC(C4=C(CC5(CCCC5)CC4=O)Nc3c2)c6ccc(O)c(c6)C#N Clclcccc(cl)C2Nc3ccc(cc3NC4=C2C(=O)CC5(CCCC5)C4)S(=O)(=O)Nc6ccccc6 Clclcccc(cl )C2Nc3ccc(cc3NC4=C2C(=O)CC5(CCCC5)C4)C(=O)Oc6ccccc6 CN(C(=O)Oclcccccl)c2ccc3NC(C4=C(CC5(CCCC5)CC4=O)Nc3c2)c6cccc(Cl)c6 Clclcccc(cl)C2Nc3ccc(OCc4cncs4)cc3NC5=C2C(=O)CC6(CCCC6)C5
CC(C)Oc 1 ccc(cc 1 C(F)(F)F)C2"Nc3ccc(Oc4ccccc4)cc3NC5=C2C(=O)CC6(CCCC6)C5 CC(C)Oclccc(cclC(F)(F)F)C2Nc3ccc(cc3NC4=C2C(=O)CC5(CCCC5)C4)C(=O)Nc6ccccc6 CC(C)Oc 1 ccc(cc 1 C(F)(F)F)C2Nc3ccc(cc3NC4=C2C(=O)CC5(CCCC5)C4)C(=O)OC(C)c6ccccc6 COclcc(cc(OC)clO)C2Nc3ccc(Oc4ccccc4)cc3NC5=C2C(=O)CC6(CCCC6)C5 COclcc(cc(OC)clO)C2Nc3ccc(cc3NC4=C2C(=O)CC5(CCCC5)C4)S(=O)(=O)c6ccccc6 COclcc(cc(OC)cl O)C2Nc3ccc(cc3NC4=C2C(=O)CC5(CCCC5)C4)C(=O)Oc6ccccc6 CCOc 1 cc(OCC)cc(c 1 )C2Nc3ccc(cc3NC4=C2C(=O)CC5(CCCC5)C4)C(=O)c6ccccc6 CCOcI cc(OCC)cc(cl )C2Nc3ccc(cc3NC4=C2C(=O)CC5(CCCC5)C4)N(C)C(=O)c6ccccc6 CCOc 1 cc(OCC)cc(cl )C2Nc3ccc(cc3"NC4=C2C(=O)CC5(CCCC5)C4)N(C)C(=O)Oc6ccccc6 CIc 1 CCc(CC 1 Cl)C2Nc3ccc(Oc4ccccc4)cc3NC5=C2C(=O)CC6(CCCC6)C5 Clclccc(cclCl)C2Nc3ccc(OCc4ccccc4)cc3NC5=C2C(=O)CC6(CCCC6)C5 O=C 1 CC2(CCCC2)CC3=C1 C(Nc4ccc(cc4N3)S(=O)(=O)c5ccccc5)c6ccc7CCCc7c6 Brc 1 cncc(c 1 )C2Nc3 ccc(cc3NC4=C2C(=O)CC5(CCCC5)C4)C(=O)c6ccccc6 CCOC(=O)clcc(ccnl)C2Nc3ccc(cc3NC4=C2C(=O)CC5(CCCC5)C4)S(=O)(=O)c6ccccc6 CCOC(=O)c 1 cc(ccn 1 )C2Nc3ccc(cc3NC4=C2C(=O)CC5(CCCC5)C4)N(C)c6ccccc6 O=C 1 CC2(CCCC2)CC3=C 1 C(Nc4ccc(OCc5ccccc5)cc4M3)c6oc(nn6)C7CC7 O=Cl CC2(CCCC2)CC3=Cl C(Nc4ccc(cc4N3)S(=O)(=O)Nc5ccccc5)c6oc(nn6)C7CC7 O=C 1 CC2(CCCC2)CC3=C 1 C(Nc4ccccc4N3)c5oc(nn5)C6CC6 O=ClCC2(CCCC2)CC3=ClC(Nc4ccc(cc4N3)S(=O)(=O)Nc5ccccc5)c6cncc7ncccc67 CCOclcc(ccclO)C2Nc3ccc(COc4ccccc4)cc3NC5=C2C(=O)CC6(CCCC6)C5 CCOc 1 cc(ccc 10)C2Nc3ccc(cc3NC4=C2C(=O)CC5(CCCC5)C4)N(C)c6ccccc6
CCOc] CC(CCd O)C2TMc3ccc(cc3NC4=C2C(=O)CC5(CCCC5)C4)C(=O)OC(C)c6ccccc6 Cc 1 ccc(cc 1 Cl)C2Nc3 ccc(cc3 NC4=C2C(=O)CC5(CCCC5)C4)S(=O)(=O)c6ccccc6 CN(clcccccl)c2ccc3NC(C4=C(CC5(CCCC5)CC4=O)Nc3c2)c6ccc(C)c(Cl)c6 CcI CCc(CC lCl)C2Nc3ccc(cc3NC4=C2C(=0)CC5(CCCC5)C4)C(=O)Nc6ccccc6 CCOcIcc(ccclC!)C2Nc3cc(CCOc4ccncn4)c(cc3NC5=C2C(=O)CCC5)C(=O)c6ccccc6 CCC 1 CC2=C(CNc3cc(Oc4ccccc4)c(cc3'N2)N(C)C(=O)Oc5ccccc5)C(=O)C 1
CC(C)Ocl ccc(cclC(F)(F)F)C2Nc3cc(CS(=O)(=O)c4ccccc4)c(cc3"NC5=C2C(=O)CC(C5)c6ccccc6)N(C
)C(=O)c7ccccc7
OcI CCC(CCl C#N)C2Nc3ccc(cc3NC4=C2C(=O)COC4)S(=O)(=O)Nc5ccccc5 O=C 1 CSCC2-C1 CfNc3ccc(OCc4cncs4)cc3N2)c5oc(nn5)C6CC6
CN(c 1 cccccl )c2cc3NC(C4=C(CC(O)CC4=O)Nc3cc2S(=O)(=O)c5ccccc5)c6ccc7CCCc7c6 COClCC2=C(C(Nc3cc(Oc4ccccc4)c(cc3N2)C(=O)Nc5ccccc5)c6cncc7ncccc67)C(=O)Cl COc i CC(Cc(OC)C 10)C2Nc3cc(N(C)c4ccccc4)c(OCc5ccccc5)cc3NC6=C2C(=O)CNC6 O=C 1 CNCC2=C 1 C(Nc3cc(CCOc4ccncn4)c(OCc5cncs5 )cc3N2)c6cncc7ncccc67 O=CCNcI ccccc 1)C2CC3NC4--=C(CNC3CC2CS(=O)C=O)C5CCCCC5)C(=O)CNC4
CCOC(=O)clcc(ccn l)C2Nc3cc(N(CC4CCC4)C(=O)Oc5ccccc5)c(cc3NC6=C2C(=O)CN(C6)C=O)C(= O)Oc7ccccc7
CC(=O)Cl=C(NC(C)(C)C)Nc2cc(Oc3ccccc3)ccc2NC1 c4cccc(C!)c4 CC(=O)Cl-C(NC(CχC)C)Nc2cc(OCc3cncs3)c(CS(=O)(=O)c4ccccc4)cc2NCl CCOclcc(OCC)cc(cl)C2"Nc3ccc(cc3NC(=C2C(=O)C)OC(C)(C)c4ccccc4)C(=O)Nc5ccccc5
CCOCl =C(C(Nc2cc(CS(=O)(=O)c3ccccc3)c(cc2N l)C(=O)c4ccccc4)c5oc(nn5)C6CC6)C(=O)C
CCOCl=C(CNc2cc(N(C)c3ccccc3)c(cc2Nl)C(=O)OC(C)c4ccccc4)C(=O)C
CC(=O)Cl=C(Nc2cc(c(CS(=O)(=O)c3ccccc3)cc2NClc4ccc(C)c(Cl)c4)S(=O)(=O)c5ccccc5)C#N
CC(=O)C 1 =C(Nc2cc(OCc3ccccc3)c(cc2NC I c4ccc(C)c(Cl)c4)N(CC5CCC5)C(=O)Oc6ccccc6)Sc7cccc c7
CCOc 1 cc(ccc 10)C2Nc3cc(CS(=OX=Ojc4ccccc4)c(cc3NC=C2C(=O)C)C(=O)0C(C)c5ccccc5 CC(=O)C 1 -=CNc2cc(ccc2NC 1 )C(-=O)c3ccccc3
CCOC(=O)C I =C(C)Nc2cc(COc3ccccc3)c(Oc4ccccc4)cc2NCl c5ccc6CCCc6c5
CCOC(=O)Cl=C(NC(C)(C)C)Nc2cc(N(C)c3ccccc3)c(cc2NClc4oc(τm4)C5CC5)Η(CC6CCC6)C(=O)O c7ccccc7
CCOC(=O)Cl=C(NC(C)(C)C)Nc2cc(C(=O)OC(C)c3ccccc3)c(CS(=O)(=O)c4ccccc4)cc2NClc5cncc6n cccc56
CCOC(=O)Cl=C(Nc2cc(OCc3cncs3)c(cc2]vJClc4ccc(O)c(c4)C#N)N(C)c5ccccc5)OC(C)(C)c6ccccc6 CCOC(^O)C 1 =C(Nc2cc(ccc2NC 1 c3ccnc(c3)C(=O)OCC)S(=O)(=O)»c4ccccc4)OCC CCOC(=O)Cl=C(Nc2cc(ccc2NCl)N(C)C(=O)c3ccccc3)C#N CCOC(=O)Cl=C(Nc2cc(N(C)CC=O)Oc3ccccc3)c(CCOc4ccncn4)cc2NCl)Sc5ccccc5 CCCCS(=O)(=O)C 1 =C(C(Nc2ccc(cc2N 1 )C(=O)OC(C)c3ccccc3)c4ccc(C)c(Cl)c4)C(=O)OCC CCOC(«O)Cl=CNc2cc(c(cc2-NClc3ccc(Cl)c(OCC)c3)N(C)c4ccccc4)S(=O)(=O)Nc5ccccc5 CC(C)C(=O)Cl=C(Nc2cc(c(Oc3ccccc3)cc2NCl)S(=O)(=O)Nc4ccccc4)Sc5ccccc5 CC(C)C(=O)C 1 =CNc2cc(OCc3cncs3)c(Oc4ccccc4)cc2NC 1 c5cncc(Br)c5
CCI=C(C(Nc2cc(CS(=0)(=0)c3ccccc3)c(cc2N l)S(=O)(=O)Nc4ccccc4)c5cncc(Br)c5)C(=0)TM6CCCC 6
CN(C(=O)Oc 1 ccccc 1 )c2cc3NC(=C(CCNc3cc2CS(=O)(=O)c4ccccc4)c5ccc(O)c(c5)C#N)C(=O)N6CCC C6)ΗC(C)(C)C
CC(C)(OC 1=C(CNC2CC(CS(=O)(=O)C3CCCCC3)C(CC2N 1 )S(=O)(=O)c4ccccc4)C(=O)N5CCCC5)c6ccccc 6 CCOC 1 ^C(C(Nc2ccccc2N 1 )c3cc(OC)c(O)c(OC)c3)C(=O)N4CCCC4
0=C(N] CCCCl)C2=C(Nc3cc(c(Oc4ccccc4)cc3NC2c5oc(nn5)C6CC6)S(=0)(=0)c7ccccc7)C#N O=C(OcI ccccc 1 )N(CC2CCC2)c3cc4NCC(=C(Nc4cc3OCc5cncs5)C#N)C(=O)N6CCCC6 CCCCS(=O)(=O)Cl=C(CNc2cc(N(C)c3ccccc3)c(cc2N l)C(=O)Nc4ccccc4)C(=O)N5CCCC5
From the foregoing description, one skilled in the art can easily ascertain the essential characteristics of this invention, and without departing from the spirit and scope thereof, can make various changes and modifications of the invention to adapt it to various usages and conditions.
Claims
What is claimed is:
L A method oftreatment of a NPC-I Ll -mediated disease comprising the adininistration of a therapeutically effective amount of a compound of structural Formula I:
R'-N(R2)-SO2-R3
(I)
or a salt, ester, or prodrug thereof, wherein:
R1 and R2 are independently selected from the group consisting of hydrogen, lower alkyl, lower alkoxy, lower alkylamino, lower alkenyl, lower alkynyl, lower alkoxyalkyl, lower alkylaminoalkyl, lower alkylcarbonyl, lower alkoxycarbonylalkyl, lower aminocarbonylalkyl, amido, lower aminoalkyl, aryl, arylalkyl, arylalkenyl, arylalkynyl, lower cycloalkyl, lower cycloalkylalkyl, lower haloalkyl, lower perhaloalkyl, lower alkylthioalkyl, lower alkylsulfonylalkyl, lower alkylsulfonyl, lower perhaloalkylsulfonyl, lower cycloalkylsulfonyl, heteroaryl, heteroarylalkyl, heteroarylalkenyl, and heterocycloalkyl, any of which may be optionally substituted; or R1 and R2, together with the atoms to which they are attached, may be joined to form an optionally substituted heterocycloalkyl moiety; and
R3 is selected form the group consisting of lower alkyl, lower alkenyl, lower alkynyl, lower alkoxyalkyl, lower alkylaminoalkyl, lower aminoalkyl, aryl, arylalkyl, arylalkenyl, arylalkynyl, lower cycloalkyl, lower cycloalkylalkyl, lower haloalkyl, lower perhaloalkyl, heteroaryl, heteroarylalkyl, heteroarylalkenyl, and heterocycloalkyl, any of which may be optionally substituted; or R1 and R3, together with the atoms to which they are attached, may be joined to form an optionally substituted heterocycloalkyl moiety.
2. A method of treatment of a NPC-I Ll -mediated disease comprising the administration of a therapeutically effective amount of a compound of structural Formula II:
00
or a salt, ester, or prodrug thereof, wherein:
X1 is selected from the group consisting of C(R4) and N; X2 is selected from the group consisting of C(R5) and N; X3 is selected from the group consisting of C(R6) and N; X4 is selected from the group consisting of C(R7) and N; and
R4, R5, R6, R7, R8, and R9 are independently absent or selected from the group consisting of hydrogen, lower acyl, lower alkenyl, lower alkoxy, lower alkoxyalkyl, lower alkoxycarbonyl, lower alkyl, lower alkylamidoamino, lower alkylamino, lower alkylaminosulfonyl, lower alkylene, lower alkylsulfinyl, lower alkyl sulfonyl, lower alkylsulfonylamino, lower alkylthio, lower alkylthioamido, amido, amino, lower aminoalkyl, aminocarbonyl, aryl, arylalkoxy, arylalkyl, arylalkylamino, arylalkylthio, arylamino, arylaminosulfonyl, aryloxy, arylsulfinyl, arylsulfonyl, arylsulfonylamino, arylthio, carboxy, cyano, lower cycloalkyl, lower cycloalkylalkyl, halo, lower haloalkoxy, lower haloalkyl, lower haloalkylcarbonyl, heteroaryl, heteroarylalkoxy, heteroarylalkyl, heteroarylamino, heteroarylaminosulfonyl, heteroaryloxy, heteroarylsulfϊnyl, heteroarylsulfonyl, heteroarylsulfonylamino, heterocyclo, heterocycloalkoxy, heterocycloalkyl, hydroxy, hydroxyalkyl, nitro, sulfonate, thio, and trisubstituted silyl; alternatively, R4, R5, R6, R7, R8, and R9 may be linked with any of the other R4, R5, R6, R7, R8, and R9 sites to form an optionally-substituted polycyclic cycloalkyl, aryl, heteroaryl, or heterocyclic ring independent of any other non-adjacent site; and provided that the number of X1"4 that are nitrogen is 2 or 3.
3. A method of treatment of a NPC-I Ll -mediated disease comprising the administration of a therapeutically effective amount of a compound of structural Formula III:
(III) or a salt, ester, or prodrug thereof, wherein:
R10 is selected from the group consisting of aryl, cycloalkyl, heteroaryl, and heterocycloalkyl, any of which may be optionally substituted;
R1 1 and R12 are independently selected from the group consisting of hydrogen, lower acyl, lower alkyl, aroyl, and heteroaroyl , any of which may be optionally substituted;
R13 and R14 are independently selected from the group consisting of hydrogen, hydroxy, halogen, amido, amino, aminocarbonyl, carboxy, cyano, nitro, sulfonate, thiol, lower acyl, lower alkenyl, lower alkoxy, lower alkoxyalkyl, lower alkoxycarbonyl, lower alkyl, lower alkylamidoamino, lower alkylamino, lower alkylaminosulfonyl, lower alkylene,lower alkylsulfinyl, lower alkylsulfonyl, lower alkylsulfonylamino, lower alkylthio, lower alkylthioamido, lower cycloalkyl, lower cycloalkylalkyl, lower haloalkoxy, lower haloalkyl, lower haloalkylcarbonyl, lower hydroxyalkyl, and trisubstituted silyl, any of which may be optionally substituted; and
R15 is selected from the group consisting of hydrogen, amido, aminocarbonyl, hydroxy, lower acyl, lower alkenyl, lower alkoxyalkyl, lower alkoxycarbonyl, lower alkyl, lower alkylamido, lower alkylamino, lower alkylaminosulfonyl, lower alkylene, lower alkylsulfinyl, lower alkylsulfonyl, lower aminoalkyl, aryl, arylalkoxy, arylalkyl, arylalkylamino, arylamido, arylamino, arylaminosulfonyl, arylsulfinyl, arylsulfonyl, lower cycloalkyl, lower cycloalkylalkyl, haloalkylcarbonyl, heteroaryl, heteroarylalkyl, heteroarylamido, heteroarylaminosulfonyl, heteroarylsulfonyl, heterocyclo, heterocycloalkoxy, heterocycloalkyl, heterocycloamido, and lower hydroxyalkyl, any of which may be optionally substituted.
4. A method of treatment of a NPC-I Ll -mediated disease comprising the administration of a therapeutically effective amount of a compound of structural Formula IV:
(IV)
or a salt, ester, or prodrug thereof, wherein:
RIδ is selected from the group consisting of optionally substituted aryl, optionally substituted cycloalkyl, optionally substituted heteroaryl, and optionally substituted heterocycloalkyl;
R17 is selected from the group consisting of hydrogen, amido, aminocarbonyl, hydroxy, lower acyl, lower alkenyl, lower alkoxyalkyl, lower alkoxycarbonyl, lower alkyl, lower alkylamido, lower alkylamino, lower alkylaminosulfonyl, lower alkylene, lower alkylsulfinyl, lower alkylsulfonyl, lower aminoalkyl, aryl, arylalkoxy, arylalkyl, arylalkylamino, arylamido, arylamino, arylaminosulfonyl, arylsulfinyl, arylsulfonyl, lower cycloalkyl, lower cycloalkylalkyl, lower haloalkylcarbonyl, heteroaryl, heteroarylalkyl, heteroarylamido, heteroarylaminosulfonyl, heteroarylsulfonyl, heterocycloalkyl, heterocycloalkoxy, heterocycloamido, and lower hydroxyalkyl, any of which may be optionally substituted;
R18 and R19 are independently selected from the group consisting of hydrogen, amido, amino, carboxy, cyano, halo, hydroxy, nitro, sulfonate, -SH, lower acyl, lower alkenyl, lower alkoxy, lower alkoxyalkyl, lower alkoxycarbonyl, lower alkyl, lower alkylamidoamino, lower alkylamino, lower alkylaminosulfonyl, lower alkylene, lower alkylsulfinyl, lower alkylsulfonyl, lower alkylsulfonylamino, lower alkylthio, lower alkylthioamidoaminoalkyl, aminocarbonyl, aryl, arylalkoxy, arylalkyl, arylalkylamino, arylalkylthio, arylamino, arylaminosulfonyl, aryloxy, arylsulfinyl, arylsulfonyl, arylsulfonylamino, arylthio, lower cycloalkyl, lower cycloalkylalkyl, lower haloalkoxy, lower haloalkyl, lower perhaloalkyl, lower haloalkylcarbonyl, heteroaryl, heteroarylalkoxy, heteroarylalkyl, heteroarylamino, heteroarylaminosulfonyl, heteroaryloxy, heteroarylsulfinyl, heteroarylsulfonyl, heteroarylsulfonylamino, heterocycloalkyl, heterocycloalkoxy, lower hydroxyalkyl, and trisubstituted silyl, any of which may be optionally substituted; or both R16 groups may be joined in a optionally substituted lower cycloalkyl, optionally substituted heteroaryl, or optionally substituted heterocycloalkyl ring;
R20 and R21 are independently selected from the group consisting of hydrogen, lower acyl, lower alkyl, lower alkylamido, aryl, arylalkyl, arylamido, lower cycloalkyl, lower cycloalkylalkyl, lower cycloalkylamido, heteroaryl, heteroarylalkyl, heteroarylamido, heterocycloalkyl, heterocycloalkylalkyl, and heterocycloalkylamido, any of which may be optionally substituted; and
R22, R23, R24 and R25 are independently selected from the group consisting of hydrogen, amido, amino, carboxy, cyano, hydroxy, nitro, sulfonate, thio, halogen, aminocarbonyl, lower acyl, lower alkenyl, lower alkoxy, lower alkoxyalkyl, lower alkoxycarbonyl, lower alkyl, lower alkylamidoamino, lower alkylamino, lower alkylaminosulfonyl, lower alkylene, lower alkylsulfϊnyl, lower alkylsulfonyl, lower alkylsulfonylamino, lower alkylthio, lower alkylthioamido, lower aminoalkyl, aryl, arylalkoxy, arylalkyl, arylalkylamino, arylalkylthio, arylamino, arylaminosulfonyl, aryloxy, arylsulfinyl, arylsulfonyl, arylsulfonylamino, arylthio, lower cycloalkyl, lower cycloalkylalkyl, lower haloalkoxy, lower haloalkyl, lower perhaloalkyl, lower haloalkylcarbonyl, heteroaryl, heteroarylalkoxy, heteroarylalkyl, heteroarylamino, heteroarylaminosulfonyl, heteroaryloxy, heteroarylsulfinyl, heteroarylsulfonyl, heteroarylsulfonylamino, heterocycloalkyl, heterocycloalkoxy, lower hydroxyalkyl, and trisubstituted silyl, any of which may be optionally substituted; or R18 and R19, together with the atoms to which they are attached, may be joined to form an optionally substituted aryl, optionally substituted heteroaryl, optionally substituted heterocycloalkyl or optionally substituted cycloalkyl moiety; wherein at least one of R20 and R21 is hydrogen.
5. The method as recited in Claims 1 to 4, selected from the group consisting of Examples 1 to 740.
6. The method as recited in Claims 1 to 4, wherein said disease is a vascular disease.
Applications Claiming Priority (10)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US69781405P | 2005-07-08 | 2005-07-08 | |
| US69765905P | 2005-07-08 | 2005-07-08 | |
| US69768605P | 2005-07-08 | 2005-07-08 | |
| US60/697,659 | 2005-07-08 | ||
| US60/697,814 | 2005-07-08 | ||
| US60/697,686 | 2005-07-08 | ||
| US72764605P | 2005-10-17 | 2005-10-17 | |
| US60/727,646 | 2005-10-17 | ||
| US78230306P | 2006-03-13 | 2006-03-13 | |
| US60/782,303 | 2006-03-13 |
Publications (2)
| Publication Number | Publication Date |
|---|---|
| WO2007008541A2 true WO2007008541A2 (en) | 2007-01-18 |
| WO2007008541A3 WO2007008541A3 (en) | 2007-07-26 |
Family
ID=37637726
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| PCT/US2006/026242 WO2007008541A2 (en) | 2005-07-08 | 2006-07-05 | Cellular cholesterol absorption modifiers |
Country Status (1)
| Country | Link |
|---|---|
| WO (1) | WO2007008541A2 (en) |
Cited By (130)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2007008529A2 (en) * | 2005-07-08 | 2007-01-18 | Kalypsys, Inc | Celullar cholesterol absorption modifiers |
| WO2008003753A1 (en) * | 2006-07-07 | 2008-01-10 | Biovitrum Ab (Publ) | Pyrazolo [1,5-a] pyrimidine analogs for use as inhibitors of stearoyl-coa desaturase (scd) activity |
| WO2008045484A1 (en) * | 2006-10-10 | 2008-04-17 | Amgen Inc. | N-aryl pyrazole compounds for use against diabetes |
| WO2008055945A1 (en) * | 2006-11-09 | 2008-05-15 | Probiodrug Ag | 3-hydr0xy-1,5-dihydr0-pyrr0l-2-one derivatives as inhibitors of glutaminyl cyclase for the treatment of ulcer, cancer and other diseases |
| WO2008061016A1 (en) * | 2006-11-10 | 2008-05-22 | Wyeth | N-substituted piperidinyl 4 -arylsulfonamides as modulators of the secreted frizzled related protein- 1 |
| WO2008149311A1 (en) * | 2007-06-05 | 2008-12-11 | Institut National De La Sante Et De La Recherche Medicale (Inserm) | Use of derivatives of 3-oxo-2,3-dihydro-5h-thiazolof[3,2-a]pyrimidine or of 3-oxo-2,3-dihydro-5h-selenazolo[3,2-a]pyrimidine or of 3-oxo-1,2,3-trihydro-5h-imidazolo[1,2-a]pyrimidine for the preparation of pharmaceutical compositions intended for the treatment of cancer |
| WO2009037247A1 (en) * | 2007-09-17 | 2009-03-26 | Neurosearch A/S | Pyrazine derivatives and their use as potassium channel modulators |
| US7514566B2 (en) | 2006-01-18 | 2009-04-07 | Amgen, Inc. | Thiazole compounds and methods of use |
| WO2009051909A1 (en) * | 2007-10-16 | 2009-04-23 | The Regents Of The University Of California | Compounds having activity in correcting mutant-cftr cellular processing and uses thereof |
| US7528143B2 (en) * | 2005-11-01 | 2009-05-05 | Targegen, Inc. | Bi-aryl meta-pyrimidine inhibitors of kinases |
| WO2009105675A1 (en) | 2008-02-22 | 2009-08-27 | Rigel Pharmaceuticals, Inc. | Use of 2,4-pyrimidinediamines for the treatment of atherosclerosis |
| DE102008020113A1 (en) | 2008-04-23 | 2009-10-29 | Bayer Schering Pharma Aktiengesellschaft | Substituted dihydropyrazolones and their use |
| WO2010011537A1 (en) * | 2008-07-23 | 2010-01-28 | The United States Of America, As Represented By The Secretary, Department Of Health And Human Services | Inhibitors of the plasmodial surface anion channel as antimalarials |
| WO2010017355A2 (en) * | 2008-08-08 | 2010-02-11 | New York Blood Center | Small molecule inhibitors of retroviral assembly and maturation |
| US20100041760A1 (en) * | 2008-08-15 | 2010-02-18 | Jean-Baptiste Blanc | Monoaryl aminotetralines |
| US20100113782A1 (en) * | 2008-10-30 | 2010-05-06 | David Robert Bolin | Diacylglycerol Acyltransferase Inhibitors |
| WO2010098866A1 (en) * | 2009-02-27 | 2010-09-02 | Supergen, Inc. | Cyclopentathiophene/cyclohexathiophene dna methyltransferase inhibitors |
| WO2010030757A3 (en) * | 2008-09-10 | 2010-09-02 | Kalypsys Inc. | Aminopyrimidine inhibitors of histamine receptors for the treatment of disease |
| JP2010535712A (en) * | 2007-08-03 | 2010-11-25 | ロマーク ラボラトリース,エル.シー. | Alkylsulfonyl-substituted thiazolide compounds |
| CN101899051A (en) * | 2010-05-17 | 2010-12-01 | 中国人民解放军第二军医大学 | 1-azaxanthone-3-formamide compounds as well as preparation method and antitumor application thereof |
| WO2010150927A1 (en) * | 2009-06-25 | 2010-12-29 | Sk Holdings Co., Ltd. | Pharmaceutical composition for prevention and treatment of cancer diseases comprising benzamide derivatives |
| US7863265B2 (en) | 2005-06-20 | 2011-01-04 | Astrazeneca Ab | 2-azetidinone derivatives and their use as cholesterol absorption inhibitors for the treatment of hyperlipidaemia |
| WO2010138828A3 (en) * | 2009-05-29 | 2011-02-17 | Abbott Laboratories | Potassium channel modulators |
| US7897619B2 (en) | 2007-07-17 | 2011-03-01 | Amgen Inc. | Heterocyclic modulators of PKB |
| US7906502B2 (en) | 2005-06-22 | 2011-03-15 | Astrazeneca Ab | 2-azetidinone derivatives as cholesterol absorption inhibitors for the treatment of hyperlipidaemic conditions |
| US7915260B2 (en) * | 2006-01-10 | 2011-03-29 | Maryanoff Bruce E | Urotensin II receptor antagonists |
| US7919504B2 (en) | 2007-07-17 | 2011-04-05 | Amgen Inc. | Thiadiazole modulators of PKB |
| WO2010072805A3 (en) * | 2008-12-24 | 2011-04-28 | Unilever Plc | Method and composition for skin color modulation |
| US20110130397A1 (en) * | 2006-09-22 | 2011-06-02 | Soongyu Choi | Pyrrolinone compounds as inhibitors of bacterial peptidyl trna hydrolase and uses thereof |
| US7977358B2 (en) | 2007-07-26 | 2011-07-12 | Hoffmann-La Roche Inc. | Pyrazol derivatives |
| US8030354B2 (en) | 2007-10-10 | 2011-10-04 | Amgen Inc. | Substituted biphenyl GPR40 modulators |
| US20110257184A1 (en) * | 2009-11-13 | 2011-10-20 | Cheng-Kui Qu | Shp-2 phosphatase inhibitor |
| EP2383269A2 (en) | 2006-10-26 | 2011-11-02 | Bayer Pharma AG | Substituted Dihydropyrazolones for the treatment of cardiovascular diseases |
| WO2011136192A1 (en) * | 2010-04-27 | 2011-11-03 | アステラス製薬株式会社 | IMIDAZO[1,2-a]PYRIDINE DERIVATIVE |
| JP2012504632A (en) * | 2008-10-01 | 2012-02-23 | ブリストル−マイヤーズ スクイブ カンパニー | Hepatitis C virus inhibitor |
| US20120046250A1 (en) * | 2009-03-02 | 2012-02-23 | Stemsynergy Therapeutics, Inc | Methods and compositions useful in treating cancer and reducing wnt mediated effects in a cell |
| EP2451453A1 (en) * | 2009-07-10 | 2012-05-16 | Vivalis | Substituted pyrrolidinone as inhibitors of hepatitis c ns5b polymerase, the pharmaceutical composition thereof and their therapeutic use |
| US20120149715A1 (en) * | 2010-05-28 | 2012-06-14 | Yi Tsun Richard Kao | Compounds and methods for the treatment of viral infections |
| US8207167B2 (en) | 2008-09-19 | 2012-06-26 | Pimco 2664 Limited | Aryl-phenyl-sulfonamide-phenylene compounds and their use |
| US20120172374A1 (en) * | 2010-12-29 | 2012-07-05 | Development Center For Biotechnology | Novel tubulin inhibitors and methods of using the same |
| WO2012097196A1 (en) * | 2011-01-12 | 2012-07-19 | Acea Biosciences Inc. | Pyrazolopyrimidine derivatives and uses as anticancer agents |
| US8252817B2 (en) | 2005-04-28 | 2012-08-28 | Bayer Intellectual Property Gmbh | Dipyridyl-dihydropyrazolones and their use |
| CN102670625A (en) * | 2011-03-18 | 2012-09-19 | 中国人民解放军第二军医大学 | Application of urea compound |
| JP2012525336A (en) * | 2009-04-27 | 2012-10-22 | エラン ファーマシューティカルズ,インコーポレイテッド | Alpha-4 integrin pyridinone antagonist |
| WO2012162468A1 (en) * | 2011-05-25 | 2012-11-29 | Janssen Pharmaceutica Nv | Thiazol derivatives as pro -matrix metalloproteinase inhibitors |
| WO2013004332A1 (en) * | 2011-07-07 | 2013-01-10 | Merck Patent Gmbh | Substituted azaheterocycles for the treatment of cancer |
| US20130023522A1 (en) * | 2011-07-18 | 2013-01-24 | Merck Patent Gmbh | Benzamides |
| US8362277B2 (en) | 2009-01-09 | 2013-01-29 | Board Of Regents Of The University Of Texas System | Pro-neurogenic compounds |
| US8372971B2 (en) | 2004-08-25 | 2013-02-12 | Targegen, Inc. | Heterocyclic compounds and methods of use |
| US8435968B2 (en) | 2008-09-19 | 2013-05-07 | Pimco 2664 Limited | Aryl-phenyl-sulfonamido-cycloalkyl compounds and their use |
| US8450522B2 (en) | 2008-03-06 | 2013-05-28 | Amgen Inc. | Conformationally constrained carboxylic acid derivatives useful for treating metabolic disorders |
| JP2013525479A (en) * | 2010-05-07 | 2013-06-20 | エスケー ケミカルズ カンパニー リミテッド | Picolinamide and pyrimidine-4-carboxamide compounds, process for producing the same and pharmaceutical compositions containing the same |
| US8481536B2 (en) | 2004-04-08 | 2013-07-09 | Targegen, Inc. | Benzotriazine inhibitors of kinases |
| CN103254151A (en) * | 2012-02-20 | 2013-08-21 | 北京大学 | Benzisoxa thiazoles 5-LOX and mPGES-1 inhibitor and application |
| US8524699B2 (en) | 2006-10-26 | 2013-09-03 | Bayer Intellectual Property Gmbh | Substituted dihydropyrazolones and use thereof as HIF-prolyl-4-hydroxylase inhibitors |
| US8524778B2 (en) | 2007-03-21 | 2013-09-03 | Pimco 2664 Limited | Biphenyl-4-yl-sulfonic acid arylamides and their use as therapeutic agents |
| EP2632466A2 (en) * | 2010-10-29 | 2013-09-04 | Emory University | Quinazoline derivatives, compositions, and uses related thereto |
| ITMI20120786A1 (en) * | 2012-05-09 | 2013-11-10 | Fond Italiana Sclerosi M Ultipla Fism Onlu | MODULATORS OF THE GPR17 RECEPTOR |
| US8604042B2 (en) | 2005-11-01 | 2013-12-10 | Targegen, Inc. | Bi-aryl meta-pyrimidine inhibitors of kinases |
| US8604074B2 (en) | 2009-01-09 | 2013-12-10 | Board Of Regents Of The University Of Texas System | Pro-neurogenic compounds |
| US8609698B2 (en) | 2006-10-26 | 2013-12-17 | Bayer Intellectual Property Gmbh | Substituted dipyridyl-dihydropyrazolones and use thereof |
| US8609674B2 (en) | 2010-11-16 | 2013-12-17 | Abbvie Inc. | Potassium channel modulators |
| JP2014501763A (en) * | 2010-12-22 | 2014-01-23 | ザ トラスティーズ オブ コロンビア ユニヴァーシティ イン ザ シティ オブ ニューヨーク | Histone acetyltransferase modulators and uses thereof |
| US8653074B2 (en) | 2010-11-18 | 2014-02-18 | Bayer Intellectual Property Gmbh | Substituted sodium 1H-pyrazol-5-olate |
| EP2699566A1 (en) * | 2011-04-19 | 2014-02-26 | Il-Yang Pharm. Co., Ltd. | Phenyl-isoxazol derivatives and preparation process thereof |
| WO2014050134A1 (en) | 2012-09-27 | 2014-04-03 | 興和株式会社 | Therapeutic agent for dyslipidemia |
| CN103709096A (en) * | 2013-12-24 | 2014-04-09 | 中国人民解放军第二军医大学 | Urea type derivative used as nicotinamide ribose phosphate transferase inhibitor, as well as preparation method and application thereof |
| US8735440B2 (en) | 2009-01-09 | 2014-05-27 | Board Of Regents Of The University Of Texas System | Methods for treating amyotrophic lateral sclerosis using pro-neurogenic compounds |
| US8748462B2 (en) | 2008-10-15 | 2014-06-10 | Amgen Inc. | Spirocyclic GPR40 modulators |
| US8759342B2 (en) | 2006-07-31 | 2014-06-24 | Janssen Pharmaceutica Nv | Benzo[1,4]oxazin-3-one, benzo[1,4]thiazin-3-one and quinolin-2-one urotensin II receptor antagonists |
| US8846727B2 (en) | 2009-05-12 | 2014-09-30 | Romark Laboratories, L.C. | Haloalkyl heteroaryl benzamide compounds |
| CN104254534A (en) * | 2011-11-29 | 2014-12-31 | 泰纬生命科技股份有限公司 | Improved modulators of hec1 activity and methods therefor |
| US8946268B2 (en) * | 2010-03-17 | 2015-02-03 | Taivex Therapeutics, Inc. | Modulators of HEC1 activity and methods therefor |
| KR101488583B1 (en) * | 2013-02-06 | 2015-02-03 | 부산대학교 산학협력단 | New compounds having skin whitening activity and medical use thereof |
| WO2015046193A1 (en) * | 2013-09-25 | 2015-04-02 | 塩野義製薬株式会社 | Aromatic heterocyclic amine derivative having trpv4 inhibiting activity |
| US9056877B2 (en) | 2011-07-19 | 2015-06-16 | Infinity Pharmaceuticals, Inc. | Heterocyclic compounds and uses thereof |
| US9067922B2 (en) | 2013-04-19 | 2015-06-30 | Pfizer Limited | Chemical compounds |
| US9079896B2 (en) | 2008-08-02 | 2015-07-14 | Janssen Pharmaceutica Nv | Urotensin II receptor antagonists |
| US9095572B2 (en) | 2009-01-09 | 2015-08-04 | Board Of Regents Of The University Of Texas System | Pro-neurogenic compounds |
| US9115127B2 (en) | 2010-08-05 | 2015-08-25 | Amgen Inc. | Benzimidazole and azabenzimidazole compounds that inhibit anaplastic lymphoma kinase |
| US9126999B2 (en) | 2012-05-31 | 2015-09-08 | Eisai R&D Management Co., Ltd. | Tetrahydropyrazolopyrimidine compounds |
| US9243281B2 (en) | 2013-11-11 | 2016-01-26 | Board Of Regents Of The University Of Texas System | Neuroprotective chemicals and methods for identifying and using same |
| US9320786B2 (en) | 2011-04-12 | 2016-04-26 | The United States Of America, As Represented By The Secretary, Department Of Health And Human Services | Plasmodial surface anion channel inhibitors for the treatment or prevention of malaria |
| US9353117B2 (en) | 2010-12-08 | 2016-05-31 | The United States Of America As Represented By The Secretary, Dept. Of Health And Human Services | Substituted pyrazolopyrimidines as glucocerebrosidase activators |
| US20160208002A1 (en) * | 2013-09-30 | 2016-07-21 | Richard H. Gomer | Compositions Associated with and Methods of Managing Neutrophil Movement |
| US9464035B2 (en) | 2012-03-28 | 2016-10-11 | The United States Of America, As Represented By The Secretary, Department Of Health And Human Services | Salicylic acid derivatives useful as glucocerebrosidase activators |
| US9616048B2 (en) | 2009-01-09 | 2017-04-11 | Board Of Regents Of The University Of Texas System | Anti-depression compounds |
| US9624167B2 (en) | 2013-06-26 | 2017-04-18 | Pimco 2664 Limited | N-(4-hydroxy-4-methyl-cyclohexyl)-4-phenyl-benzenesulfonamides and N-(4-hydroxy-4-methyl-cyclohexyl)-4-(2-pyridyl)benzenesulfonamides and their therapeutic use |
| US9701676B2 (en) | 2012-08-24 | 2017-07-11 | Board Of Regents Of The University Of Texas System | Pro-neurogenic compounds |
| US9708348B2 (en) | 2014-10-03 | 2017-07-18 | Infinity Pharmaceuticals, Inc. | Trisubstituted bicyclic heterocyclic compounds with kinase activities and uses thereof |
| US9751888B2 (en) | 2013-10-04 | 2017-09-05 | Infinity Pharmaceuticals, Inc. | Heterocyclic compounds and uses thereof |
| US9775844B2 (en) | 2014-03-19 | 2017-10-03 | Infinity Pharmaceuticals, Inc. | Heterocyclic compounds and uses thereof |
| US9820975B2 (en) | 2009-06-26 | 2017-11-21 | Romark Laboratories L.C. | Compounds and methods for treating influenza |
| US9828377B2 (en) | 2013-10-04 | 2017-11-28 | Infinity Pharmaceuticals, Inc. | Heterocyclic compounds and uses thereof |
| US9902713B2 (en) | 2013-11-11 | 2018-02-27 | Board Of Regents Of The University Of Texas System | Neuroprotective compounds and use thereof |
| US9951069B1 (en) | 2017-01-11 | 2018-04-24 | Rodin Therapeutics, Inc. | Bicyclic inhibitors of histone deacetylase |
| US10005733B2 (en) | 2014-12-17 | 2018-06-26 | Pimco 2664 Limited | N-(4-hydroxy-4-methyl-cyclohexyl)-4-phenyl-benzenesulfonamide and N-(4-hydroxy-4-methyl-cyclohexyl)-4-(2-pyridyl)-benzenesulfonamide compounds and their therapeutic use |
| WO2018181892A1 (en) * | 2017-03-31 | 2018-10-04 | 国立大学法人 長崎大学 | Quinolinone compound and anti-rna virus drug |
| US10092583B2 (en) | 2010-03-11 | 2018-10-09 | Gilead Connecticut, Inc. | Imidazopyridines Syk inhibitors |
| US10093684B2 (en) | 2008-12-08 | 2018-10-09 | Gilead Connecticut, Inc. | Substituted imidazo[1,2-a]pyrazines as Syk inhibitors |
| US10106507B2 (en) * | 2014-08-03 | 2018-10-23 | H. Lee Moffitt Cancer Center and Research Insitute, Inc. | Potent dual BRD4-kinase inhibitors as cancer therapeutics |
| WO2018217757A1 (en) * | 2017-05-22 | 2018-11-29 | University Of Virginia Patent Foundation | Compositions and methods for preparing and using mitochondrial uncouplers |
| US10160761B2 (en) | 2015-09-14 | 2018-12-25 | Infinity Pharmaceuticals, Inc. | Solid forms of isoquinolinones, and process of making, composition comprising, and methods of using the same |
| CN109574920A (en) * | 2018-12-25 | 2019-04-05 | 药大制药有限公司 | - 6 cyclopropyl pyridine class IDO1 inhibitor of 3- itrile group and application thereof |
| CN109627205A (en) * | 2019-01-11 | 2019-04-16 | 贵州大学 | The preparation method and applications of a kind of carbazyl Isopropanolamine derivatives |
| CN109627206A (en) * | 2019-01-11 | 2019-04-16 | 贵州大学 | One kind has the preparation method and application of the carbazyl Isopropanolamine derivatives of chiral centre |
| US10287290B2 (en) * | 2013-04-16 | 2019-05-14 | Drexel University | Compositions useful in treating brain-related diseases or disorders and methods using same |
| EP2850068B1 (en) * | 2012-05-09 | 2019-05-29 | Universita' degli Studi di Milano | N-(phenyl)-2-[[3-(phenyl)-1H-1,2,4-triazol-5-yl]thio]-acetamide derivatives and related compounds as G protein coupled receptor 17 (GPCR17) modulators for use in the treatment of neuro-degenerative diseases |
| US10391094B2 (en) | 2010-11-07 | 2019-08-27 | Impact Biomedicines, Inc. | Compositions and methods for treating myelofibrosis |
| US10421756B2 (en) | 2015-07-06 | 2019-09-24 | Rodin Therapeutics, Inc. | Heterobicyclic N-aminophenyl-amides as inhibitors of histone deacetylase |
| US10640457B2 (en) | 2009-12-10 | 2020-05-05 | The Trustees Of Columbia University In The City Of New York | Histone acetyltransferase activators and uses thereof |
| US10738016B2 (en) | 2015-10-13 | 2020-08-11 | H. Lee Moffitt Cancer Center And Research Institute, Inc. | BRD4-kinase inhibitors as cancer therapeutics |
| US10759806B2 (en) | 2016-03-17 | 2020-09-01 | Infinity Pharmaceuticals, Inc. | Isotopologues of isoquinolinone and quinazolinone compounds and uses thereof as PI3K kinase inhibitors |
| US10919914B2 (en) | 2016-06-08 | 2021-02-16 | Infinity Pharmaceuticals, Inc. | Heterocyclic compounds and uses thereof |
| US10919902B2 (en) | 2015-07-06 | 2021-02-16 | Alkermes, Inc. | Hetero-halo inhibitors of histone deacetylase |
| US20210047316A1 (en) * | 2018-04-12 | 2021-02-18 | Purdue Research Foundation | Benzamide antibacterial agents |
| US11071736B2 (en) | 2011-11-21 | 2021-07-27 | Taivex Therapeutics Corporation | Modulators of HEC1 activity and methods therefor |
| US11084807B2 (en) | 2016-08-18 | 2021-08-10 | Vidac Pharama Ltd. | Piperazine derivatives, pharmaceutical compositions and methods of use thereof |
| US11168068B2 (en) | 2016-07-18 | 2021-11-09 | Janssen Pharmaceutica Nv | Tau PET imaging ligands |
| US11225475B2 (en) | 2017-08-07 | 2022-01-18 | Alkermes, Inc. | Substituted pyridines as inhibitors of histone deacetylase |
| WO2022055926A1 (en) * | 2020-09-11 | 2022-03-17 | The Regents Of The University Of California | Compositions and methods for treating muscular dystrophies |
| WO2022159445A1 (en) * | 2021-01-19 | 2022-07-28 | Metrea Biosciences, Inc. | Compounds and methods of activating lipoprotein lipase |
| US11555012B2 (en) * | 2018-11-09 | 2023-01-17 | Nimbus Artemis, Inc. | ACLY inhibitors and uses thereof |
| US11591327B2 (en) | 2016-02-25 | 2023-02-28 | Asceneuron Sa | Acid addition salts of piperazine derivatives |
| US11612599B2 (en) | 2016-02-25 | 2023-03-28 | Asceneuron Sa | Glycosidase inhibitors |
| US11795165B2 (en) | 2018-08-22 | 2023-10-24 | Asceneuron Sa | Tetrahydro-benzoazepine glycosidase inhibitors |
| WO2023209368A1 (en) * | 2022-04-26 | 2023-11-02 | Cerevance, Inc. | Nitrogen comprising heterocyclic derivatives for the treatment of disorders associated with gpr55 receptor |
| US12016852B2 (en) | 2018-08-22 | 2024-06-25 | Asceneuron Sa | Pyrrolidine glycosidase inhibitors |
| US12060330B2 (en) | 2017-08-29 | 2024-08-13 | Rutgers, The State University Of New Jersey | Therapeutic indazoles |
| WO2024211823A1 (en) * | 2023-04-06 | 2024-10-10 | Integrated Biosciences, Inc. | Compounds and uses thereof |
Families Citing this family (4)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| AU2004303742B2 (en) | 2003-12-23 | 2008-06-19 | Astrazeneca Ab | Diphenylazetidinone derivatives possessing cholesterol absorption inhibitory activity |
| SA06270191B1 (en) | 2005-06-22 | 2010-03-29 | استرازينيكا ايه بي | Novel 2-Azetidinone Derivatives as Cholesterol Absorption Inhibitors for the Treatment of Hyperlipidaemic Conditions |
| AR060623A1 (en) | 2006-04-27 | 2008-07-02 | Astrazeneca Ab | COMPOUNDS DERIVED FROM 2-AZETIDINONE AND A PREPARATION METHOD |
| US8940742B2 (en) | 2012-04-10 | 2015-01-27 | Infinity Pharmaceuticals, Inc. | Heterocyclic compounds and uses thereof |
Citations (4)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| EP0223593A2 (en) * | 1985-11-19 | 1987-05-27 | Glaxo Group Limited | Phenoxyalkylcarboxylic acids and esters and their preparation and pharmaceutical formulation |
| EP0472449A2 (en) * | 1990-07-31 | 1992-02-26 | Lipha, Lyonnaise Industrielle Pharmaceutique | Substituted sulfonamides, processes for their preparation and medicines containing them |
| WO2003043985A1 (en) * | 2001-11-21 | 2003-05-30 | Novartis Ag | Heterocyclic compounds and methods of use |
| WO2005015988A1 (en) * | 2003-07-17 | 2005-02-24 | Schering Corporation | Npc1l1 (npc3) and methods of use thereof |
-
2006
- 2006-07-05 WO PCT/US2006/026242 patent/WO2007008541A2/en active Application Filing
Patent Citations (5)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| EP0223593A2 (en) * | 1985-11-19 | 1987-05-27 | Glaxo Group Limited | Phenoxyalkylcarboxylic acids and esters and their preparation and pharmaceutical formulation |
| EP0472449A2 (en) * | 1990-07-31 | 1992-02-26 | Lipha, Lyonnaise Industrielle Pharmaceutique | Substituted sulfonamides, processes for their preparation and medicines containing them |
| US5476846A (en) * | 1990-07-31 | 1995-12-19 | Lipha, Lyonnaise Industrielle Pharmaceutique | Substituted sulfonamides, process of preparation and medicines containing same |
| WO2003043985A1 (en) * | 2001-11-21 | 2003-05-30 | Novartis Ag | Heterocyclic compounds and methods of use |
| WO2005015988A1 (en) * | 2003-07-17 | 2005-02-24 | Schering Corporation | Npc1l1 (npc3) and methods of use thereof |
Cited By (260)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US8481536B2 (en) | 2004-04-08 | 2013-07-09 | Targegen, Inc. | Benzotriazine inhibitors of kinases |
| US8372971B2 (en) | 2004-08-25 | 2013-02-12 | Targegen, Inc. | Heterocyclic compounds and methods of use |
| US8252817B2 (en) | 2005-04-28 | 2012-08-28 | Bayer Intellectual Property Gmbh | Dipyridyl-dihydropyrazolones and their use |
| US9085572B2 (en) | 2005-04-28 | 2015-07-21 | Bayer Intellectual Property Gmbh | 4-(pyridin-3-yl)-2(pyridin-2yl)-1,2-dihydro-3H-pyrazol-3-one derivatives as specific HIF-pyrolyl-4-hydroxylase inhibitors for treating cardiovascular and haematological diseases |
| US7863265B2 (en) | 2005-06-20 | 2011-01-04 | Astrazeneca Ab | 2-azetidinone derivatives and their use as cholesterol absorption inhibitors for the treatment of hyperlipidaemia |
| US7906502B2 (en) | 2005-06-22 | 2011-03-15 | Astrazeneca Ab | 2-azetidinone derivatives as cholesterol absorption inhibitors for the treatment of hyperlipidaemic conditions |
| WO2007008529A2 (en) * | 2005-07-08 | 2007-01-18 | Kalypsys, Inc | Celullar cholesterol absorption modifiers |
| WO2007008529A3 (en) * | 2005-07-08 | 2007-08-23 | Kalypsys Inc | Celullar cholesterol absorption modifiers |
| US7825246B2 (en) * | 2005-11-01 | 2010-11-02 | Targegen, Inc. | Bi-aryl meta-pyrimidine inhibitors of kinases |
| US7528143B2 (en) * | 2005-11-01 | 2009-05-05 | Targegen, Inc. | Bi-aryl meta-pyrimidine inhibitors of kinases |
| US8604042B2 (en) | 2005-11-01 | 2013-12-10 | Targegen, Inc. | Bi-aryl meta-pyrimidine inhibitors of kinases |
| US8193191B2 (en) | 2006-01-10 | 2012-06-05 | Janssen Pharmaceutica N.V. | Urotensin II receptor antagonists |
| US7915260B2 (en) * | 2006-01-10 | 2011-03-29 | Maryanoff Bruce E | Urotensin II receptor antagonists |
| US8536174B2 (en) | 2006-01-10 | 2013-09-17 | Janssen Pharmaceutica Nv | Urotensin II receptor antagonists |
| US7514566B2 (en) | 2006-01-18 | 2009-04-07 | Amgen, Inc. | Thiazole compounds and methods of use |
| US8084479B2 (en) | 2006-01-18 | 2011-12-27 | Amgen Inc. | Thiazole compounds and methods of use |
| WO2008003753A1 (en) * | 2006-07-07 | 2008-01-10 | Biovitrum Ab (Publ) | Pyrazolo [1,5-a] pyrimidine analogs for use as inhibitors of stearoyl-coa desaturase (scd) activity |
| US8759342B2 (en) | 2006-07-31 | 2014-06-24 | Janssen Pharmaceutica Nv | Benzo[1,4]oxazin-3-one, benzo[1,4]thiazin-3-one and quinolin-2-one urotensin II receptor antagonists |
| US20110130397A1 (en) * | 2006-09-22 | 2011-06-02 | Soongyu Choi | Pyrrolinone compounds as inhibitors of bacterial peptidyl trna hydrolase and uses thereof |
| WO2008045484A1 (en) * | 2006-10-10 | 2008-04-17 | Amgen Inc. | N-aryl pyrazole compounds for use against diabetes |
| US8022061B2 (en) | 2006-10-10 | 2011-09-20 | Amgen Inc. | N-aryl pyrazole compounds, compositions, and methods for their use |
| US8987261B2 (en) | 2006-10-26 | 2015-03-24 | Bayer Intellectual Property Gmbh | Substituted dihydropyrazolones for treating cardiovascular and hematological diseases |
| US8653111B2 (en) | 2006-10-26 | 2014-02-18 | Bayer Intellectual Property Gmbh | Substituted dihydropyrazolones for treating cardiovascular and hematological diseases |
| EP2546253A1 (en) | 2006-10-26 | 2013-01-16 | Bayer Intellectual Property GmbH | Substituted dihydropyrazolones for the treatment of cardiovascular and hematological diseases |
| US8389520B2 (en) | 2006-10-26 | 2013-03-05 | Bayer Intellectual Property Gmbh | Substituted dihydropyrazolones for treating cardiovascular and hematological diseases |
| US8609698B2 (en) | 2006-10-26 | 2013-12-17 | Bayer Intellectual Property Gmbh | Substituted dipyridyl-dihydropyrazolones and use thereof |
| EP2383269A2 (en) | 2006-10-26 | 2011-11-02 | Bayer Pharma AG | Substituted Dihydropyrazolones for the treatment of cardiovascular diseases |
| US8524699B2 (en) | 2006-10-26 | 2013-09-03 | Bayer Intellectual Property Gmbh | Substituted dihydropyrazolones and use thereof as HIF-prolyl-4-hydroxylase inhibitors |
| US8278345B2 (en) | 2006-11-09 | 2012-10-02 | Probiodrug Ag | Inhibitors of glutaminyl cyclase |
| WO2008055945A1 (en) * | 2006-11-09 | 2008-05-15 | Probiodrug Ag | 3-hydr0xy-1,5-dihydr0-pyrr0l-2-one derivatives as inhibitors of glutaminyl cyclase for the treatment of ulcer, cancer and other diseases |
| WO2008061016A1 (en) * | 2006-11-10 | 2008-05-22 | Wyeth | N-substituted piperidinyl 4 -arylsulfonamides as modulators of the secreted frizzled related protein- 1 |
| US8524778B2 (en) | 2007-03-21 | 2013-09-03 | Pimco 2664 Limited | Biphenyl-4-yl-sulfonic acid arylamides and their use as therapeutic agents |
| WO2008149311A1 (en) * | 2007-06-05 | 2008-12-11 | Institut National De La Sante Et De La Recherche Medicale (Inserm) | Use of derivatives of 3-oxo-2,3-dihydro-5h-thiazolof[3,2-a]pyrimidine or of 3-oxo-2,3-dihydro-5h-selenazolo[3,2-a]pyrimidine or of 3-oxo-1,2,3-trihydro-5h-imidazolo[1,2-a]pyrimidine for the preparation of pharmaceutical compositions intended for the treatment of cancer |
| US7897619B2 (en) | 2007-07-17 | 2011-03-01 | Amgen Inc. | Heterocyclic modulators of PKB |
| US7919504B2 (en) | 2007-07-17 | 2011-04-05 | Amgen Inc. | Thiadiazole modulators of PKB |
| US7977358B2 (en) | 2007-07-26 | 2011-07-12 | Hoffmann-La Roche Inc. | Pyrazol derivatives |
| JP2010535712A (en) * | 2007-08-03 | 2010-11-25 | ロマーク ラボラトリース,エル.シー. | Alkylsulfonyl-substituted thiazolide compounds |
| US8895752B2 (en) | 2007-08-03 | 2014-11-25 | Romark Laboratories L.C. | Alkylsulfonyl-substituted thiazolide compounds |
| WO2009037247A1 (en) * | 2007-09-17 | 2009-03-26 | Neurosearch A/S | Pyrazine derivatives and their use as potassium channel modulators |
| US8415358B2 (en) | 2007-09-17 | 2013-04-09 | Neurosearch A/S | Pyrazine derivatives and their use as potassium channel modulators |
| US8030354B2 (en) | 2007-10-10 | 2011-10-04 | Amgen Inc. | Substituted biphenyl GPR40 modulators |
| US8389736B2 (en) | 2007-10-16 | 2013-03-05 | The Regents Of The University Of California | Compounds having activity in correcting mutant-CFTR processing and uses thereof |
| WO2009051909A1 (en) * | 2007-10-16 | 2009-04-23 | The Regents Of The University Of California | Compounds having activity in correcting mutant-cftr cellular processing and uses thereof |
| US8530466B2 (en) | 2008-02-22 | 2013-09-10 | Rigel Pharmaceuticals, Inc. | Use of 2,4-pyrimidinediamines for the treatment of atherosclerosis |
| WO2009105675A1 (en) | 2008-02-22 | 2009-08-27 | Rigel Pharmaceuticals, Inc. | Use of 2,4-pyrimidinediamines for the treatment of atherosclerosis |
| AU2009215430B2 (en) * | 2008-02-22 | 2015-02-12 | Rigel Pharmaceuticals, Inc. | Use of 2,4-pyrimidinediamines for the treatment of atherosclerosis |
| JP2011513230A (en) * | 2008-02-22 | 2011-04-28 | ライジェル ファーマシューティカルズ, インコーポレイテッド | Use of 2,4-pyrimidinediamine for the treatment of atherosclerosis |
| US8450522B2 (en) | 2008-03-06 | 2013-05-28 | Amgen Inc. | Conformationally constrained carboxylic acid derivatives useful for treating metabolic disorders |
| US8067407B2 (en) | 2008-04-23 | 2011-11-29 | Bayer Pharma Aktiengesellschaft | Substituted dihydropyrazolones and their use |
| DE102008020113A1 (en) | 2008-04-23 | 2009-10-29 | Bayer Schering Pharma Aktiengesellschaft | Substituted dihydropyrazolones and their use |
| US9394316B2 (en) | 2008-07-23 | 2016-07-19 | The United States Of America, As Represented By The Secretary, Department Of Health And Human Services | Inhibitors of the plasmodial surface anion channel as antimalarials |
| US10881669B2 (en) | 2008-07-23 | 2021-01-05 | The United States Of America, As Represented By The Secretary, Department Of Health And Human Services | Inhibitors of the plasmodial surface anion channel as antimalarials |
| WO2010011537A1 (en) * | 2008-07-23 | 2010-01-28 | The United States Of America, As Represented By The Secretary, Department Of Health And Human Services | Inhibitors of the plasmodial surface anion channel as antimalarials |
| US8618090B2 (en) | 2008-07-23 | 2013-12-31 | The United States Of America, As Represented By The Secretary, Department Of Health And Human Services | Inhibitors of the plasmodial surface anion channel as antimalarials |
| US9974796B2 (en) | 2008-07-23 | 2018-05-22 | The United States Of America, As Represented By The Secretary, Department Of Health And Human Services | Inhibitors of the plasmodial surface anion channel as antimalarials |
| US9079896B2 (en) | 2008-08-02 | 2015-07-14 | Janssen Pharmaceutica Nv | Urotensin II receptor antagonists |
| JP2013216693A (en) * | 2008-08-08 | 2013-10-24 | New York Blood Center Inc | Small-molecule inhibitor of assembly and maturation of retrovirus |
| US8546439B2 (en) | 2008-08-08 | 2013-10-01 | New York Blood Center, Inc. | Small molecule inhibitors of retroviral assembly and maturation |
| US8299093B2 (en) | 2008-08-08 | 2012-10-30 | New York Blood Center, Inc. | Small molecule inhibitors of retroviral assembly and maturation |
| WO2010017355A2 (en) * | 2008-08-08 | 2010-02-11 | New York Blood Center | Small molecule inhibitors of retroviral assembly and maturation |
| WO2010017355A3 (en) * | 2008-08-08 | 2010-05-27 | New York Blood Center | Small molecule inhibitors of retroviral assembly and maturation |
| JP2011530528A (en) * | 2008-08-08 | 2011-12-22 | ニューヨーク ブラッド センター, インコーポレイテッド | Small molecule inhibitors of retrovirus assembly and maturation |
| US20100041760A1 (en) * | 2008-08-15 | 2010-02-18 | Jean-Baptiste Blanc | Monoaryl aminotetralines |
| WO2010030757A3 (en) * | 2008-09-10 | 2010-09-02 | Kalypsys Inc. | Aminopyrimidine inhibitors of histamine receptors for the treatment of disease |
| US9050329B1 (en) | 2008-09-19 | 2015-06-09 | Pimco 2664 Limited | Aryl-phenyl-sulfonamido-cycloalkyl compounds and their use |
| US8435968B2 (en) | 2008-09-19 | 2013-05-07 | Pimco 2664 Limited | Aryl-phenyl-sulfonamido-cycloalkyl compounds and their use |
| US9302984B2 (en) | 2008-09-19 | 2016-04-05 | Pimco 2664 Limited | Aryl-phenyl-sulfonamido-cycloalkyl compounds and their use |
| US8207167B2 (en) | 2008-09-19 | 2012-06-26 | Pimco 2664 Limited | Aryl-phenyl-sulfonamide-phenylene compounds and their use |
| US9616037B2 (en) | 2008-09-19 | 2017-04-11 | Pimco 2664 Limited | Aryl-phenyl-sulfonamido-cycloalkyl compounds and their use |
| US8822507B2 (en) | 2008-09-19 | 2014-09-02 | Pimco 2664 Limited | Aryl-phenyl-sulfonamido-cycloalkyl compounds and their use |
| JP2012504632A (en) * | 2008-10-01 | 2012-02-23 | ブリストル−マイヤーズ スクイブ カンパニー | Hepatitis C virus inhibitor |
| US8748462B2 (en) | 2008-10-15 | 2014-06-10 | Amgen Inc. | Spirocyclic GPR40 modulators |
| US8324385B2 (en) * | 2008-10-30 | 2012-12-04 | Madrigal Pharmaceuticals, Inc. | Diacylglycerol acyltransferase inhibitors |
| US20100113782A1 (en) * | 2008-10-30 | 2010-05-06 | David Robert Bolin | Diacylglycerol Acyltransferase Inhibitors |
| US10093684B2 (en) | 2008-12-08 | 2018-10-09 | Gilead Connecticut, Inc. | Substituted imidazo[1,2-a]pyrazines as Syk inhibitors |
| WO2010072805A3 (en) * | 2008-12-24 | 2011-04-28 | Unilever Plc | Method and composition for skin color modulation |
| US10172827B2 (en) | 2009-01-09 | 2019-01-08 | Board Of Regents Of The University Of Texas System | Pro-neurogenic compounds |
| US8791149B2 (en) | 2009-01-09 | 2014-07-29 | Board Of Regents Of The University Of Texas System | Methods of treating traumatic brain injury using pro-neurogenic compounds |
| US8877797B2 (en) | 2009-01-09 | 2014-11-04 | Board Of Regents Of The University Of Texas System | Methods for treating Parkinson's disease using pro-neurogenic compounds |
| US9962368B2 (en) | 2009-01-09 | 2018-05-08 | Board Of Regents Of The University Of Texas System | Pro-neurogenic compounds |
| US9884820B2 (en) | 2009-01-09 | 2018-02-06 | Board Of Regents Of The University Of Texas System | Pro-neurogenic compounds |
| US8604074B2 (en) | 2009-01-09 | 2013-12-10 | Board Of Regents Of The University Of Texas System | Pro-neurogenic compounds |
| US8362277B2 (en) | 2009-01-09 | 2013-01-29 | Board Of Regents Of The University Of Texas System | Pro-neurogenic compounds |
| US9156787B2 (en) | 2009-01-09 | 2015-10-13 | Board Of Regents Of The University Of Texas System | Pro-neurogenic compounds |
| US9446022B2 (en) | 2009-01-09 | 2016-09-20 | Board Of Regents Of The University Of Texas System | Pro-neurogenic compounds |
| US10183011B2 (en) | 2009-01-09 | 2019-01-22 | Board Of Regents Of The University Of Texas System | Anti-depression compounds |
| US8748473B2 (en) | 2009-01-09 | 2014-06-10 | Board Of The Regents Of The University Of Texas System | Methods of treating post-traumatic stress disorder using pro-neurogenic compounds |
| US9095572B2 (en) | 2009-01-09 | 2015-08-04 | Board Of Regents Of The University Of Texas System | Pro-neurogenic compounds |
| US8735440B2 (en) | 2009-01-09 | 2014-05-27 | Board Of Regents Of The University Of Texas System | Methods for treating amyotrophic lateral sclerosis using pro-neurogenic compounds |
| US9095571B2 (en) | 2009-01-09 | 2015-08-04 | Board Of Regents Of The University Of Texas System | Pro-neurogenic compounds |
| US9278923B2 (en) | 2009-01-09 | 2016-03-08 | Board Of Regents Of The University Of Texas System | Pro-neurogenic compounds |
| US9616048B2 (en) | 2009-01-09 | 2017-04-11 | Board Of Regents Of The University Of Texas System | Anti-depression compounds |
| US9446042B2 (en) | 2009-01-09 | 2016-09-20 | Board Of Regents Of The University Of Texas System | Pro-neurogenic compounds |
| WO2010098866A1 (en) * | 2009-02-27 | 2010-09-02 | Supergen, Inc. | Cyclopentathiophene/cyclohexathiophene dna methyltransferase inhibitors |
| US11512081B2 (en) | 2009-03-02 | 2022-11-29 | Stemsynergy Therapeutics, Inc. | Methods and compositions useful in treating cancer and reducing WNT mediated effects in a cell |
| US20120046250A1 (en) * | 2009-03-02 | 2012-02-23 | Stemsynergy Therapeutics, Inc | Methods and compositions useful in treating cancer and reducing wnt mediated effects in a cell |
| US10975067B2 (en) | 2009-03-02 | 2021-04-13 | Stemsynergy Therapeutics, Inc. | Methods and compositions useful in treating cancer and reducing Wnt mediated effects in a cell |
| US11834446B2 (en) | 2009-03-02 | 2023-12-05 | Stemsynergy Therapeutics, Inc. | Methods and compositions useful in treating cancer and reducing Wnt mediated effects in a cell |
| US9862714B2 (en) | 2009-03-02 | 2018-01-09 | Stemsynergy Therapeutics, Inc. | Methods and compositions useful in treating cancer and reducing Wnt mediated effects in a cell |
| US8901306B2 (en) * | 2009-03-02 | 2014-12-02 | Stemsynergy Therapeutics, Inc. | Methods and compositions useful in treating cancer and reducing Wnt mediated effects in a cell |
| JP2012525336A (en) * | 2009-04-27 | 2012-10-22 | エラン ファーマシューティカルズ,インコーポレイテッド | Alpha-4 integrin pyridinone antagonist |
| USRE47786E1 (en) | 2009-05-12 | 2019-12-31 | Romark Laboratories L.C. | Haloalkyl heteroaryl benzamide compounds |
| US9126992B2 (en) | 2009-05-12 | 2015-09-08 | Romark Laboratories, L.C. | Haloalkyl heteroaryl benzamide compounds |
| USRE46724E1 (en) | 2009-05-12 | 2018-02-20 | Romark Laboratories, L.C. | Haloalkyl heteroaryl benzamide compounds |
| US8846727B2 (en) | 2009-05-12 | 2014-09-30 | Romark Laboratories, L.C. | Haloalkyl heteroaryl benzamide compounds |
| US8962639B2 (en) | 2009-05-29 | 2015-02-24 | Abbvie Inc. | Potassium channel modulators |
| WO2010138828A3 (en) * | 2009-05-29 | 2011-02-17 | Abbott Laboratories | Potassium channel modulators |
| WO2010150927A1 (en) * | 2009-06-25 | 2010-12-29 | Sk Holdings Co., Ltd. | Pharmaceutical composition for prevention and treatment of cancer diseases comprising benzamide derivatives |
| US10363243B2 (en) | 2009-06-26 | 2019-07-30 | Romark Laboratories L.C. | Compounds and methods for treating influenza |
| US9820975B2 (en) | 2009-06-26 | 2017-11-21 | Romark Laboratories L.C. | Compounds and methods for treating influenza |
| US11850237B2 (en) | 2009-06-26 | 2023-12-26 | Romark Laboratories L.C. | Compounds and methods for treating influenza |
| US10912768B2 (en) | 2009-06-26 | 2021-02-09 | Romark Laboratories L.C. | Compounds and methods for treating influenza |
| EP2451453A1 (en) * | 2009-07-10 | 2012-05-16 | Vivalis | Substituted pyrrolidinone as inhibitors of hepatitis c ns5b polymerase, the pharmaceutical composition thereof and their therapeutic use |
| US20110257184A1 (en) * | 2009-11-13 | 2011-10-20 | Cheng-Kui Qu | Shp-2 phosphatase inhibitor |
| US8673913B2 (en) * | 2009-11-13 | 2014-03-18 | Case Western Reserve University | SHP-2 phosphatase inhibitor |
| US10640457B2 (en) | 2009-12-10 | 2020-05-05 | The Trustees Of Columbia University In The City Of New York | Histone acetyltransferase activators and uses thereof |
| US11034647B2 (en) | 2009-12-10 | 2021-06-15 | The Trustees Of Columbia University In The City Of New York | Histone acetyltransferase activators and uses thereof |
| US10842803B2 (en) | 2010-03-11 | 2020-11-24 | Kronos Bio, Inc. | Imidazopyridines Syk inhibitors |
| US10092583B2 (en) | 2010-03-11 | 2018-10-09 | Gilead Connecticut, Inc. | Imidazopyridines Syk inhibitors |
| US9409902B2 (en) | 2010-03-17 | 2016-08-09 | Taivex Therapeutics Corporation | Modulators of HEC1 activity and methods therefor |
| US8946268B2 (en) * | 2010-03-17 | 2015-02-03 | Taivex Therapeutics, Inc. | Modulators of HEC1 activity and methods therefor |
| US8822688B2 (en) | 2010-04-27 | 2014-09-02 | Astellas Pharma Inc. | Imidazo[1,2-a]pyridine derivative |
| CN102858772A (en) * | 2010-04-27 | 2013-01-02 | 安斯泰来制药株式会社 | Imidazo[1,2-a]pyridine derivative |
| JP5673676B2 (en) * | 2010-04-27 | 2015-02-18 | アステラス製薬株式会社 | Imidazo [1,2-a] pyridine derivatives |
| WO2011136192A1 (en) * | 2010-04-27 | 2011-11-03 | アステラス製薬株式会社 | IMIDAZO[1,2-a]PYRIDINE DERIVATIVE |
| JP2013525479A (en) * | 2010-05-07 | 2013-06-20 | エスケー ケミカルズ カンパニー リミテッド | Picolinamide and pyrimidine-4-carboxamide compounds, process for producing the same and pharmaceutical compositions containing the same |
| CN101899051A (en) * | 2010-05-17 | 2010-12-01 | 中国人民解放军第二军医大学 | 1-azaxanthone-3-formamide compounds as well as preparation method and antitumor application thereof |
| CN101899051B (en) * | 2010-05-17 | 2013-03-20 | 中国人民解放军第二军医大学 | 1-azaxanthone-3-formamide compounds as well as preparation method and antitumor application thereof |
| US20120149715A1 (en) * | 2010-05-28 | 2012-06-14 | Yi Tsun Richard Kao | Compounds and methods for the treatment of viral infections |
| US9115127B2 (en) | 2010-08-05 | 2015-08-25 | Amgen Inc. | Benzimidazole and azabenzimidazole compounds that inhibit anaplastic lymphoma kinase |
| EP2632466A4 (en) * | 2010-10-29 | 2014-03-19 | Univ Emory | Quinazoline derivatives, compositions, and uses related thereto |
| EP2632466A2 (en) * | 2010-10-29 | 2013-09-04 | Emory University | Quinazoline derivatives, compositions, and uses related thereto |
| US9868728B2 (en) | 2010-10-29 | 2018-01-16 | Emory University | Quinazoline derivatives, compositions, and uses related thereto |
| US10391094B2 (en) | 2010-11-07 | 2019-08-27 | Impact Biomedicines, Inc. | Compositions and methods for treating myelofibrosis |
| US8609674B2 (en) | 2010-11-16 | 2013-12-17 | Abbvie Inc. | Potassium channel modulators |
| US9533972B2 (en) | 2010-11-18 | 2017-01-03 | Bayer Intellectual Property Gmbh | Substituted sodium-1H-pyrazole-5-olate |
| US8653074B2 (en) | 2010-11-18 | 2014-02-18 | Bayer Intellectual Property Gmbh | Substituted sodium 1H-pyrazol-5-olate |
| US10925874B2 (en) | 2010-12-08 | 2021-02-23 | The United States Of America, As Represented By The Secretary, Department Of Health And Human Services | Substituted pyrazolopyrimidines as glucocerebrosidase activators |
| US9353117B2 (en) | 2010-12-08 | 2016-05-31 | The United States Of America As Represented By The Secretary, Dept. Of Health And Human Services | Substituted pyrazolopyrimidines as glucocerebrosidase activators |
| US9974789B2 (en) | 2010-12-08 | 2018-05-22 | The United States Of America, As Represented By The Secretary, Department Of Health And Human Services | Substituted pyrazolopyrimidines as glucocerebrosidase activators |
| US9969677B2 (en) | 2010-12-22 | 2018-05-15 | The Trustees Of Columbia University In The City Of New York | Histone acetyltransferase modulators and uses thereof |
| JP2014501763A (en) * | 2010-12-22 | 2014-01-23 | ザ トラスティーズ オブ コロンビア ユニヴァーシティ イン ザ シティ オブ ニューヨーク | Histone acetyltransferase modulators and uses thereof |
| TWI450891B (en) * | 2010-12-29 | 2014-09-01 | Dev Center Biotechnology | Novel tubulin inhibitors |
| US8883793B2 (en) * | 2010-12-29 | 2014-11-11 | Development Center For Biotechnology | Tubulin inhibitors and methods of using the same |
| US20120172374A1 (en) * | 2010-12-29 | 2012-07-05 | Development Center For Biotechnology | Novel tubulin inhibitors and methods of using the same |
| WO2012097196A1 (en) * | 2011-01-12 | 2012-07-19 | Acea Biosciences Inc. | Pyrazolopyrimidine derivatives and uses as anticancer agents |
| CN102670625A (en) * | 2011-03-18 | 2012-09-19 | 中国人民解放军第二军医大学 | Application of urea compound |
| CN102670625B (en) * | 2011-03-18 | 2014-05-28 | 中国人民解放军第二军医大学 | Application of urea compound |
| US9808458B2 (en) | 2011-04-12 | 2017-11-07 | The United States Of America, As Represented By The Secretary, Department Of Health And Human Services | Plasmodial surface anion channel inhibitors for the treatment or prevention of malaria |
| US12059418B2 (en) | 2011-04-12 | 2024-08-13 | The United States Of America, As Represented By The Secretary, Department Of Health And Human Services | Plasmodial surface anion channel inhibitors for the treatment or prevention of malaria |
| US10869865B2 (en) | 2011-04-12 | 2020-12-22 | The United States Of America, As Represented By The Secretary, Department Of Health And Human Serv | Plasmodial surface anion channel inhibitors for the treatment or prevention of malaria |
| US9320786B2 (en) | 2011-04-12 | 2016-04-26 | The United States Of America, As Represented By The Secretary, Department Of Health And Human Services | Plasmodial surface anion channel inhibitors for the treatment or prevention of malaria |
| US10265313B2 (en) | 2011-04-12 | 2019-04-23 | The United States Of America, As Represented By The Secretary, Department Of Health And Human Services | Plasmodial surface anion channel inhibitors for the treatment or prevention of malaria |
| US9132126B2 (en) | 2011-04-19 | 2015-09-15 | Il-Yang Pharm. Co., Ltd. | Phenyl-isoxazole derivatives and preparation process thereof |
| EP2699566A4 (en) * | 2011-04-19 | 2014-09-03 | Il Yang Pharm Co Ltd | Phenyl-isoxazol derivatives and preparation process thereof |
| EP2699566A1 (en) * | 2011-04-19 | 2014-02-26 | Il-Yang Pharm. Co., Ltd. | Phenyl-isoxazol derivatives and preparation process thereof |
| WO2012162468A1 (en) * | 2011-05-25 | 2012-11-29 | Janssen Pharmaceutica Nv | Thiazol derivatives as pro -matrix metalloproteinase inhibitors |
| AU2012280725B2 (en) * | 2011-07-07 | 2017-02-02 | Merck Patent Gmbh | Substituted azaheterocycles for the treatment of cancer |
| JP2014520767A (en) * | 2011-07-07 | 2014-08-25 | メルク パテント ゲゼルシャフト ミット ベシュレンクテル ハフツング | Substituted azaheterocycles for the treatment of cancer |
| US9199962B2 (en) | 2011-07-07 | 2015-12-01 | Merck Patent Gmbh | Substituted azaheterocycles for the treatment of cancer |
| WO2013004332A1 (en) * | 2011-07-07 | 2013-01-10 | Merck Patent Gmbh | Substituted azaheterocycles for the treatment of cancer |
| US20130023522A1 (en) * | 2011-07-18 | 2013-01-24 | Merck Patent Gmbh | Benzamides |
| US9938262B2 (en) | 2011-07-18 | 2018-04-10 | Merck Patent Gmbh | Benzamides |
| US9181226B2 (en) * | 2011-07-18 | 2015-11-10 | Merck Patent Gmbh | Benzamides |
| US9498475B2 (en) | 2011-07-18 | 2016-11-22 | Merck Patent Gmbh | Benzamides |
| US9605003B2 (en) | 2011-07-19 | 2017-03-28 | Infinity Pharmaceuticals, Inc. | Heterocyclic compounds and uses thereof |
| US9056877B2 (en) | 2011-07-19 | 2015-06-16 | Infinity Pharmaceuticals, Inc. | Heterocyclic compounds and uses thereof |
| US11071736B2 (en) | 2011-11-21 | 2021-07-27 | Taivex Therapeutics Corporation | Modulators of HEC1 activity and methods therefor |
| AU2012345843B2 (en) * | 2011-11-29 | 2017-11-23 | Taivex Therapeutics Corporation | Improved modulators of Hec1 activity and methods therefor |
| TWI640519B (en) * | 2011-11-29 | 2018-11-11 | 泰緯生命科技股份有限公司 | Modulators of hec1 activity and methods therefor |
| CN104254534A (en) * | 2011-11-29 | 2014-12-31 | 泰纬生命科技股份有限公司 | Improved modulators of hec1 activity and methods therefor |
| EP2785714A4 (en) * | 2011-11-29 | 2015-09-09 | Taivex Therapeutics Corp | Improved modulators of hec1 activity and methods therefor |
| CN104254534B (en) * | 2011-11-29 | 2017-07-14 | 泰纬生命科技股份有限公司 | The improved adjusting control agent and its method of HEC1 activity |
| CN103254151B (en) * | 2012-02-20 | 2015-10-28 | 北京大学 | Benzo isothiazole 5-LOX and mPGES-1 inhibitor and application |
| CN103254151A (en) * | 2012-02-20 | 2013-08-21 | 北京大学 | Benzisoxa thiazoles 5-LOX and mPGES-1 inhibitor and application |
| US9464035B2 (en) | 2012-03-28 | 2016-10-11 | The United States Of America, As Represented By The Secretary, Department Of Health And Human Services | Salicylic acid derivatives useful as glucocerebrosidase activators |
| EP2850068B1 (en) * | 2012-05-09 | 2019-05-29 | Universita' degli Studi di Milano | N-(phenyl)-2-[[3-(phenyl)-1H-1,2,4-triazol-5-yl]thio]-acetamide derivatives and related compounds as G protein coupled receptor 17 (GPCR17) modulators for use in the treatment of neuro-degenerative diseases |
| ITMI20120786A1 (en) * | 2012-05-09 | 2013-11-10 | Fond Italiana Sclerosi M Ultipla Fism Onlu | MODULATORS OF THE GPR17 RECEPTOR |
| US9126999B2 (en) | 2012-05-31 | 2015-09-08 | Eisai R&D Management Co., Ltd. | Tetrahydropyrazolopyrimidine compounds |
| US9850242B2 (en) | 2012-05-31 | 2017-12-26 | Eisai R&D Management Co., Ltd | Tetrahydropyrazolopyrimidine compounds |
| US9446046B2 (en) | 2012-05-31 | 2016-09-20 | Eisai R&D Management Co., Ltd. | Tetrahydropyrazolopyrimidine compounds |
| US11130758B2 (en) | 2012-05-31 | 2021-09-28 | Eisai R&D Management Co., Ltd. | Tetrahydropyrazolopyrimidine compounds |
| US10640500B2 (en) | 2012-05-31 | 2020-05-05 | Eisai R&D Management Co., Ltd. | Tetrahydropyrazolopyrimidine compounds |
| US9701676B2 (en) | 2012-08-24 | 2017-07-11 | Board Of Regents Of The University Of Texas System | Pro-neurogenic compounds |
| US9572798B2 (en) | 2012-09-27 | 2017-02-21 | Kowa Company, Ltd. | Therapeutic agent for dyslipidemia |
| US11013722B2 (en) | 2012-09-27 | 2021-05-25 | Kowa Company, Ltd. | Therapeutic agent for dyslipidemia |
| US10258609B2 (en) | 2012-09-27 | 2019-04-16 | Kowa Company, Ltd. | Therapeutic agent for dyslipidemia |
| WO2014050134A1 (en) | 2012-09-27 | 2014-04-03 | 興和株式会社 | Therapeutic agent for dyslipidemia |
| US9931321B2 (en) | 2012-09-27 | 2018-04-03 | Kowa Company, Ltd. | Therapeutic agent for dyslipidemia |
| KR20150063035A (en) | 2012-09-27 | 2015-06-08 | 교와 가부시키가이샤 | Therapeutic agent for dyslipidemia |
| KR101488583B1 (en) * | 2013-02-06 | 2015-02-03 | 부산대학교 산학협력단 | New compounds having skin whitening activity and medical use thereof |
| US10287290B2 (en) * | 2013-04-16 | 2019-05-14 | Drexel University | Compositions useful in treating brain-related diseases or disorders and methods using same |
| US9067922B2 (en) | 2013-04-19 | 2015-06-30 | Pfizer Limited | Chemical compounds |
| US10233147B2 (en) | 2013-06-26 | 2019-03-19 | Pimco 2664 Limited | N-(4-hydroxy-4-methyl-cyclohexyl)-4-phenyl-benzenesulfonamides and N-(4-hydroxy-4-methyl-cyclohexyl)-4-(2-pyridyl)benzenesulfonamides and their therapeutic use |
| US9796670B2 (en) | 2013-06-26 | 2017-10-24 | Pimco 2664 Limited | N-(4-hydroxy-4-methyl-cyclohexyl)-4-phenyl-benzenesulfonamides and N-(4-hydroxy-4-methyl-cyclohexyl)-4-(2-pyridyl)benzenesulfonamides and their therapeutic use |
| US9624167B2 (en) | 2013-06-26 | 2017-04-18 | Pimco 2664 Limited | N-(4-hydroxy-4-methyl-cyclohexyl)-4-phenyl-benzenesulfonamides and N-(4-hydroxy-4-methyl-cyclohexyl)-4-(2-pyridyl)benzenesulfonamides and their therapeutic use |
| US10029979B2 (en) | 2013-06-26 | 2018-07-24 | Pimco 2664 Limited | N-(4-hydroxy-4-methyl-cyclohexyl)-4-phenyl-benzenesulfonamides and N-(4-hydroxy-4-methyl-cyclohexyl)-4-(2-pyridyl)benzenesulfonamides and their therapeutic use |
| WO2015046193A1 (en) * | 2013-09-25 | 2015-04-02 | 塩野義製薬株式会社 | Aromatic heterocyclic amine derivative having trpv4 inhibiting activity |
| US9708338B2 (en) | 2013-09-25 | 2017-07-18 | Shionogi & Co., Ltd. | Aromatic heterocyclylamine derivative having TRPV4-inhibiting activity |
| US20160208002A1 (en) * | 2013-09-30 | 2016-07-21 | Richard H. Gomer | Compositions Associated with and Methods of Managing Neutrophil Movement |
| US9828377B2 (en) | 2013-10-04 | 2017-11-28 | Infinity Pharmaceuticals, Inc. | Heterocyclic compounds and uses thereof |
| US10329299B2 (en) | 2013-10-04 | 2019-06-25 | Infinity Pharmaceuticals, Inc. | Heterocyclic compounds and uses thereof |
| US9751888B2 (en) | 2013-10-04 | 2017-09-05 | Infinity Pharmaceuticals, Inc. | Heterocyclic compounds and uses thereof |
| US9645139B2 (en) | 2013-11-11 | 2017-05-09 | Board Of Regents Of The University Of Texas System | Neuroprotective chemicals and methods for identifying and using same |
| US9902713B2 (en) | 2013-11-11 | 2018-02-27 | Board Of Regents Of The University Of Texas System | Neuroprotective compounds and use thereof |
| US9243281B2 (en) | 2013-11-11 | 2016-01-26 | Board Of Regents Of The University Of Texas System | Neuroprotective chemicals and methods for identifying and using same |
| CN103709096A (en) * | 2013-12-24 | 2014-04-09 | 中国人民解放军第二军医大学 | Urea type derivative used as nicotinamide ribose phosphate transferase inhibitor, as well as preparation method and application thereof |
| US9775844B2 (en) | 2014-03-19 | 2017-10-03 | Infinity Pharmaceuticals, Inc. | Heterocyclic compounds and uses thereof |
| US10675286B2 (en) | 2014-03-19 | 2020-06-09 | Infinity Pharmaceuticals, Inc. | Heterocyclic compounds and uses thereof |
| US11541059B2 (en) | 2014-03-19 | 2023-01-03 | Infinity Pharmaceuticals, Inc. | Heterocyclic compounds and uses thereof |
| US10106507B2 (en) * | 2014-08-03 | 2018-10-23 | H. Lee Moffitt Cancer Center and Research Insitute, Inc. | Potent dual BRD4-kinase inhibitors as cancer therapeutics |
| US10526291B2 (en) | 2014-08-03 | 2020-01-07 | H. Lee Moffitt Cancer Center And Research Institute, Inc. | Potent dual BRD4-kinase inhibitors as cancer therapeutics |
| US9708348B2 (en) | 2014-10-03 | 2017-07-18 | Infinity Pharmaceuticals, Inc. | Trisubstituted bicyclic heterocyclic compounds with kinase activities and uses thereof |
| US10941162B2 (en) | 2014-10-03 | 2021-03-09 | Infinity Pharmaceuticals, Inc. | Heterocyclic compounds and uses thereof |
| US10253047B2 (en) | 2014-10-03 | 2019-04-09 | Infinity Pharmaceuticals, Inc. | Heterocyclic compounds and uses thereof |
| US10005733B2 (en) | 2014-12-17 | 2018-06-26 | Pimco 2664 Limited | N-(4-hydroxy-4-methyl-cyclohexyl)-4-phenyl-benzenesulfonamide and N-(4-hydroxy-4-methyl-cyclohexyl)-4-(2-pyridyl)-benzenesulfonamide compounds and their therapeutic use |
| US10421756B2 (en) | 2015-07-06 | 2019-09-24 | Rodin Therapeutics, Inc. | Heterobicyclic N-aminophenyl-amides as inhibitors of histone deacetylase |
| US11858939B2 (en) | 2015-07-06 | 2024-01-02 | Alkermes, Inc. | Hetero-halo inhibitors of histone deacetylase |
| US10919902B2 (en) | 2015-07-06 | 2021-02-16 | Alkermes, Inc. | Hetero-halo inhibitors of histone deacetylase |
| US11939333B2 (en) | 2015-09-14 | 2024-03-26 | Infinity Pharmaceuticals, Inc. | Solid forms of isoquinolinones, and process of making, composition comprising, and methods of using the same |
| US10160761B2 (en) | 2015-09-14 | 2018-12-25 | Infinity Pharmaceuticals, Inc. | Solid forms of isoquinolinones, and process of making, composition comprising, and methods of using the same |
| US11247995B2 (en) | 2015-09-14 | 2022-02-15 | Infinity Pharmaceuticals, Inc. | Solid forms of isoquinolinones, and process of making, composition comprising, and methods of using the same |
| US10738016B2 (en) | 2015-10-13 | 2020-08-11 | H. Lee Moffitt Cancer Center And Research Institute, Inc. | BRD4-kinase inhibitors as cancer therapeutics |
| US11643396B2 (en) | 2015-10-13 | 2023-05-09 | H. Lee Moffitt Cancer Center And Research Institute, Inc. | BRD4-kinase inhibitors as cancer therapeutics |
| US11612599B2 (en) | 2016-02-25 | 2023-03-28 | Asceneuron Sa | Glycosidase inhibitors |
| US11591327B2 (en) | 2016-02-25 | 2023-02-28 | Asceneuron Sa | Acid addition salts of piperazine derivatives |
| US10759806B2 (en) | 2016-03-17 | 2020-09-01 | Infinity Pharmaceuticals, Inc. | Isotopologues of isoquinolinone and quinazolinone compounds and uses thereof as PI3K kinase inhibitors |
| US10919914B2 (en) | 2016-06-08 | 2021-02-16 | Infinity Pharmaceuticals, Inc. | Heterocyclic compounds and uses thereof |
| US11168068B2 (en) | 2016-07-18 | 2021-11-09 | Janssen Pharmaceutica Nv | Tau PET imaging ligands |
| US12006302B2 (en) | 2016-07-18 | 2024-06-11 | Janssen Pharmaceutica Nv | Tau PET imaging ligands |
| US11084807B2 (en) | 2016-08-18 | 2021-08-10 | Vidac Pharama Ltd. | Piperazine derivatives, pharmaceutical compositions and methods of use thereof |
| US11286256B2 (en) | 2017-01-11 | 2022-03-29 | Alkermes, Inc. | Bicyclic inhibitors of histone deacetylase |
| US10793567B2 (en) | 2017-01-11 | 2020-10-06 | Rodin Therapeutics, Inc. | Bicyclic inhibitors of histone deacetylase |
| US11225479B2 (en) | 2017-01-11 | 2022-01-18 | Alkermes, Inc. | Bicyclic inhibitors of histone deacetylase |
| US10696673B2 (en) | 2017-01-11 | 2020-06-30 | Rodin Therapeutics, Inc. | Bicyclic inhibitors of histone deacetylase |
| US9951069B1 (en) | 2017-01-11 | 2018-04-24 | Rodin Therapeutics, Inc. | Bicyclic inhibitors of histone deacetylase |
| US10519149B2 (en) | 2017-01-11 | 2019-12-31 | Rodin Therapeutics, Inc. | Bicyclic inhibitors of histone deacetylase |
| US11987580B2 (en) | 2017-01-11 | 2024-05-21 | Alkermes, Inc. | Bicyclic inhibitors of histone deacetylase |
| JPWO2018181892A1 (en) * | 2017-03-31 | 2020-04-16 | 国立大学法人 長崎大学 | Quinolinone compounds and anti-RNA virus drugs |
| WO2018181892A1 (en) * | 2017-03-31 | 2018-10-04 | 国立大学法人 長崎大学 | Quinolinone compound and anti-rna virus drug |
| JP2020520949A (en) * | 2017-05-22 | 2020-07-16 | ユニバーシティ オブ ヴァージニア パテント ファウンデーションUniversity Of Verginia Patent Foundation | Compositions and methods of preparing and using mitochondrial uncouplers |
| US11896591B2 (en) | 2017-05-22 | 2024-02-13 | University Of Virginia Patent Foundation | Compositions and methods for preparing and using mitochondrial uncouplers |
| WO2018217757A1 (en) * | 2017-05-22 | 2018-11-29 | University Of Virginia Patent Foundation | Compositions and methods for preparing and using mitochondrial uncouplers |
| US11225475B2 (en) | 2017-08-07 | 2022-01-18 | Alkermes, Inc. | Substituted pyridines as inhibitors of histone deacetylase |
| US11912702B2 (en) | 2017-08-07 | 2024-02-27 | Alkermes, Inc. | Substituted pyridines as inhibitors of histone deacetylase |
| US12060330B2 (en) | 2017-08-29 | 2024-08-13 | Rutgers, The State University Of New Jersey | Therapeutic indazoles |
| JP2021521175A (en) * | 2018-04-12 | 2021-08-26 | パーデュー・リサーチ・ファウンデーションPurdue Research Foundation | Benzamide antibacterial agent |
| US20210047316A1 (en) * | 2018-04-12 | 2021-02-18 | Purdue Research Foundation | Benzamide antibacterial agents |
| US11731964B2 (en) * | 2018-04-12 | 2023-08-22 | Purdue Research Foundation | Benzamide antibacterial agents |
| US11795165B2 (en) | 2018-08-22 | 2023-10-24 | Asceneuron Sa | Tetrahydro-benzoazepine glycosidase inhibitors |
| US12016852B2 (en) | 2018-08-22 | 2024-06-25 | Asceneuron Sa | Pyrrolidine glycosidase inhibitors |
| US11555012B2 (en) * | 2018-11-09 | 2023-01-17 | Nimbus Artemis, Inc. | ACLY inhibitors and uses thereof |
| CN109574920A (en) * | 2018-12-25 | 2019-04-05 | 药大制药有限公司 | - 6 cyclopropyl pyridine class IDO1 inhibitor of 3- itrile group and application thereof |
| CN109574920B (en) * | 2018-12-25 | 2022-03-04 | 药大制药有限公司 | 3-nitrile-6-cyclopropyl pyridine IDO1 inhibitor and application thereof |
| CN109627206A (en) * | 2019-01-11 | 2019-04-16 | 贵州大学 | One kind has the preparation method and application of the carbazyl Isopropanolamine derivatives of chiral centre |
| CN109627205A (en) * | 2019-01-11 | 2019-04-16 | 贵州大学 | The preparation method and applications of a kind of carbazyl Isopropanolamine derivatives |
| WO2022055926A1 (en) * | 2020-09-11 | 2022-03-17 | The Regents Of The University Of California | Compositions and methods for treating muscular dystrophies |
| WO2022159445A1 (en) * | 2021-01-19 | 2022-07-28 | Metrea Biosciences, Inc. | Compounds and methods of activating lipoprotein lipase |
| WO2023209368A1 (en) * | 2022-04-26 | 2023-11-02 | Cerevance, Inc. | Nitrogen comprising heterocyclic derivatives for the treatment of disorders associated with gpr55 receptor |
| WO2024211823A1 (en) * | 2023-04-06 | 2024-10-10 | Integrated Biosciences, Inc. | Compounds and uses thereof |
Also Published As
| Publication number | Publication date |
|---|---|
| WO2007008541A3 (en) | 2007-07-26 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| WO2007008541A2 (en) | Cellular cholesterol absorption modifiers | |
| WO2007008529A2 (en) | Celullar cholesterol absorption modifiers | |
| US10988475B2 (en) | Agonists of the apelin receptor and methods of use thereof | |
| AU2016202439B2 (en) | Haloalkyl heteroaryl benzamide compounds | |
| ES2977656T3 (en) | KDM1A inhibitors for the treatment of diseases | |
| AU2007269070B2 (en) | Bicyclic heteroaryl inhibitors of PDE4 | |
| US20110312992A1 (en) | Inhibitors of C-Kit and Uses Thereof | |
| WO2008067219A2 (en) | Quinazolinone modulators of tgr5 | |
| US11191765B2 (en) | Heterocyclic compounds for the inhibition of PASK | |
| EP2681207B1 (en) | Heterocyclic compounds for the inhibition of pask | |
| WO2008091863A1 (en) | Sulfonyl-substituted bicyclic compounds as ppar modulators for the treatment of non-alcoholic steatohepatitis | |
| WO2006124874A2 (en) | Inhibitors of b-raf kinase | |
| WO2006124780A2 (en) | Ih-benzo [d] imidazole compounds as inhibitors of b-raf kinase | |
| WO2008097976A1 (en) | Heterocyclic modulators of tgr5 for treatment of disease | |
| WO2007015866A2 (en) | Inhibitors of p38 kinase and methods of treating inflammatory disorders | |
| JP7346425B2 (en) | Dihydroceramide desaturase inhibitors for treating diseases | |
| AU2012204353B2 (en) | Heterocyclic compounds for the inhibition of PASK | |
| WO2010016846A1 (en) | Heterocyclic modulators of tgr5 for treatment of disease | |
| AU2012204353A1 (en) | Heterocyclic compounds for the inhibition of PASK | |
| WO2007137098A2 (en) | Sulfonyl-substituted bicyclic compounds as modulators of ppar | |
| WO2008045663A2 (en) | Aryl-substituted heterocyclic pde4 inhibitors as antiinflammatory agents | |
| US9675584B2 (en) | Substituted indoles for the inhibition of PASK | |
| WO2010014739A2 (en) | Heterocyclic modulators of tgr5 | |
| EP1996198A1 (en) | Alkylamine-substituted bicyclic aryl compounds useful as modulators of ppar | |
| WO2007011647A2 (en) | Inhibitors of mitotic kinesin ksp |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| NENP | Non-entry into the national phase in: |
Ref country code: DE |
|
| 121 | Ep: the epo has been informed by wipo that ep was designated in this application |
Ref document number: 06786403 Country of ref document: EP Kind code of ref document: A2 |
|
| 122 | Ep: pct application non-entry in european phase |
Ref document number: 06786403 Country of ref document: EP Kind code of ref document: A2 |