WO2007002823A2 - Formulations of conjugated estrogens and bazedoxifene - Google Patents
Formulations of conjugated estrogens and bazedoxifene Download PDFInfo
- Publication number
- WO2007002823A2 WO2007002823A2 PCT/US2006/025348 US2006025348W WO2007002823A2 WO 2007002823 A2 WO2007002823 A2 WO 2007002823A2 US 2006025348 W US2006025348 W US 2006025348W WO 2007002823 A2 WO2007002823 A2 WO 2007002823A2
- Authority
- WO
- WIPO (PCT)
- Prior art keywords
- component
- coating
- weight
- pharmaceutical formulation
- optional
- Prior art date
Links
Classifications
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K9/00—Medicinal preparations characterised by special physical form
- A61K9/20—Pills, tablets, discs, rods
- A61K9/2072—Pills, tablets, discs, rods characterised by shape, structure or size; Tablets with holes, special break lines or identification marks; Partially coated tablets; Disintegrating flat shaped forms
- A61K9/2086—Layered tablets, e.g. bilayer tablets; Tablets of the type inert core-active coat
- A61K9/209—Layered tablets, e.g. bilayer tablets; Tablets of the type inert core-active coat containing drug in at least two layers or in the core and in at least one outer layer
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/33—Heterocyclic compounds
- A61K31/395—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins
- A61K31/55—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins having seven-membered rings, e.g. azelastine, pentylenetetrazole
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/56—Compounds containing cyclopenta[a]hydrophenanthrene ring systems; Derivatives thereof, e.g. steroids
- A61K31/565—Compounds containing cyclopenta[a]hydrophenanthrene ring systems; Derivatives thereof, e.g. steroids not substituted in position 17 beta by a carbon atom, e.g. estrane, estradiol
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K9/00—Medicinal preparations characterised by special physical form
- A61K9/20—Pills, tablets, discs, rods
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P15/00—Drugs for genital or sexual disorders; Contraceptives
- A61P15/12—Drugs for genital or sexual disorders; Contraceptives for climacteric disorders
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P19/00—Drugs for skeletal disorders
- A61P19/08—Drugs for skeletal disorders for bone diseases, e.g. rachitism, Paget's disease
- A61P19/10—Drugs for skeletal disorders for bone diseases, e.g. rachitism, Paget's disease for osteoporosis
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P5/00—Drugs for disorders of the endocrine system
- A61P5/24—Drugs for disorders of the endocrine system of the sex hormones
- A61P5/30—Oestrogens
Definitions
- the present invention relates to solid dosage formulations containing conjugated estrogens and apeledoxifene, or a salt thereof.
- the compositions include a core containing conjugated estrogens, and a coating that includes apeledoxifene, or a salt thereof.
- hormone replacement therapy for bone loss prevention in postmenopausal women is well precedented.
- the normal protocol calls for estrogen supplementation using such formulations containing estrone, estriol, ethynyl estradiol or conjugated estrogens isolated from natural sources (i.e. Premarin® conjugated estrogens from Wyeth-Ayerst).
- therapy may be contraindicated due to the proliferative effects of unopposed estrogens (estrogens not given in combination with progestins) have on uterine tissue.
- This proliferation is associated with increased risk for endometriosis and/or endometrial cancer.
- the effects of unopposed estrogens on breast tissue is less clear, but is of some concern. The need for estrogens which can maintain the bone sparing effect while minimizing the proliferative effects in the uterus and breast is evident.
- indoles as estrogen antagonists has been reported by Von Angerer, Chemical Abstracts, Vol. 99, No. 7 (1983), Abstract No. 53886u. Also, see, J. Med. Chem. 1990, 33, 2635-2640; J. Med. Chem. 1987, 30, 131-136. Also see Ger. Offen., DE 3821148 A1 891228 and WO 96/03375. The majority of the compounds reported in these publications fall into a class of compounds best characterized as being "pure antiestrogens". Additional reports of indole antiestrogens include: WO A 95 17383 (Kar Bio AB), WO A 93 10741 , and WO 93/23374 (Otsuka Pharmaceuticals, Japan).
- U.S. Patent No. 5,998,402 describes 2-phenylindoles that are estrogen agonists/antagonists useful for the treatment of diseases associated with estrogen deficiency.
- the compounds show strong binding to the estrogen receptor.
- In vitro assays including an Ishikawa alkaline phosphatase assay and an ERE transfection assay, show these compounds are antiestrogens with little to no intrinsic estrogenicity and they have proven capable of completely antagonizing the effects of 17 ⁇ -estradiol while showing little or no uterine stimulation in a rat uterine assay when dosed alone. Additionally, some of the compounds are capable of inhibiting bone loss in an ovariectomized rat while showing little or no uterine stimulation.
- Bazedoxifene is a tissue selective estrogen for treatment and prevention of postmenstrual osteoporosis. It has been reported to prevent bone loss and protect the cardiovascular system and reduce or eliminate the negative effects on the uterus and breast (potential risk of uterine and breast cancers).
- Bazedoxifene acetate polymorphs are described in US Provisional App. Ser. Nos. 60/560,582 filed April 7, 2004, and 60/560,584 filed April 7, 2004.
- Bazedoxifene acetate dispersion formulations are described in US Provisional App. Ser. No. 60/560,452, filed April 8, 2004.
- Bazedoxifene ascorbate salt is described in US Provisional App. Ser. No. 60/560,454, filed April 8, 2004.
- the present invention provides pharmaceutical compositions including a core and at least one coating; wherein the core comprises conjugated estrogens; and the coating comprises apeledoxifene, or a pharmaceutically acceptable salt thereof.
- the conjugated estrogens include or consist of Premarin®.
- the pharmaceutical composition is a tablet.
- the pharmaceutical compositions include a core and at least one coating; wherein the core includes conjugated estrogens; and the coating includes apeledoxifene, or a pharmaceutically acceptable salt thereof.
- the coating comprises: a) a filler component comprising from about 5% to about 30% by weight of the pharmaceutical formulation; b) a binder component comprising from about 1% to about 10% by weight of the pharmaceutical formulation; c) a wetting agent component comprising from about 0.01 % to about 2% by weight of the pharmaceutical formulation; d) an optional antioxidant component comprising from 0% to about 2% by weight of the pharmaceutical formulation; e) bazedoxifene acetate, comprising from about 0.1 % to about 20% by weight of the pharmaceutical formulation; and f) an optional chelating component comprising from 0% to about 0.1% by weight of the pharmaceutical formulation.
- the conjugated estrogens is present in an amount of from about 0.10 to about 1.0 mg; or from about 0.3 to about 0.8 mg; or from about 0.4 to about 0.5 mg; or from about 0.5 to about 0.7 mg.
- the apeledoxifene is present in an amount of from about 1 to about 50 mg, based on the weight of the apeledoxifene free base; or from about 5 to about 25 mg, based on the weight of the apeledoxifene free base; or from about 5 to about 15 mg, based on the weight of the apeledoxifene free base; or from about 15 to about 25 mg, based on the weight of the apeledoxifene free base; or from about 35 to about 45 mg, based on the weight of the apeledoxifene free base.
- the conjugated estrogens is present in an amount of from about 0.10 to about 1.0 mg; and the apeledoxifene is present in an amount of from about 5 to about 50 mg, based on the weight of the apeledoxifene free base.
- the conjugated estrogens is Premarin®
- the apeledoxifene is apeledoxifene acetate.
- the filler component of the coating comprises sucrose; the binder component of the coating comprises hydroxypropylmethylcellulose; the wetting agent component of the coating comprises sucrose palmitate; the optional antioxidant component of the coating, when present, comprises ascorbic acid, or a salt thereof; and the optional chelating component, when present, comprises EDTA.
- the pharmaceutical compositions further include color coating.
- the color coating includes: a) an optional filler component comprising from about 0.01% to about 8% by weight of the pharmaceutical formulation; b) an optional binder component comprising from about 0.01% to about 2% by weight of the pharmaceutical formulation; and c) a coloring agent component comprising from about 0.01% to about 6% by weight of the pharmaceutical formulation.
- the optional filler component comprises sucrose.
- the optional binder component comprises hydroxypropylmethylcellulose.
- the coloring agent component comprises titanium dioxide.
- the pharmaceutical compositions further include a clear coating.
- the clear coating forms from about 0.01% to about 2% by weight of the pharmaceutical formulation.
- the optional antioxidant component, the optional chelating component, or both the optional antioxidant component and the optional chelating component, additionally being optionally present in the coating as described above, also can each independently and optionally be present in one or more of the color coating and the clear coating.
- the invention further provides processes for the preparation of pharmaceutical compositions of the invention, and products of the processes.
- the present invention provides pharmaceutical compositions including conjugated estrogens and apeledoxifene, or a salt thereof.
- the conjugated estrogens include or consist of Premarin®.
- the pharmaceutical compositions of the invention include capsules and tablet in capsule (TIC) formulations.
- the pharmaceutical compositions of the invention are tablets.
- the pharmaceutical compositions of the invention include a core and at least one coating; wherein the core includes conjugated estrogens, preferably Premarin®, and the coating includes apeledoxifene, or a pharmaceutically acceptable salt thereof, preferably apeledoxifene acetate.
- the coating comprises: a) a filler component comprising from about 5% to about 30% by weight of the pharmaceutical formulation; b) a binder component comprising from about 1% to about 10% by weight of the pharmaceutical formulation;
- a wetting agent component comprising from about 0.01 % to about 2% by weight of the pharmaceutical formulation
- an optional antioxidant component comprising from 0% to about 2% by weight of the pharmaceutical formulation
- e) a chelating component comprising from 0% to about 0.1 % by weight of the pharmaceutical formulation.
- the coating comprises: a) a filler component comprising from about 6% to about 12% by weight of the pharmaceutical formulation; b) a binder component comprising from about 1 % to about 6% by weight of the pharmaceutical formulation; c) a wetting agent component comprising from about 0.1% to about 3% by weight of the pharmaceutical formulation; d) an optional antioxidant component comprising from 0% to about 0.5% by weight of the pharmaceutical formulation; e) bazedoxifene acetate, comprising from about 2% to about 6% by weight of the pharmaceutical formulation; and f) an optional chelating component comprising from 0% to about 0.1 % by weight of the pharmaceutical formulation.
- the coating comprises: a) a filler component comprising from about 12% to about 18% by weight of the pharmaceutical formulation; b) a binder component comprising from about 4% to about 8% by weight of the pharmaceutical formulation; c) a wetting agent component comprising from about 0.2% to about 0.5% by weight of the pharmaceutical formulation; d) an optional antioxidant component comprising from 0% to about 0.8% by weight of the pharmaceutical formulation; e) bazedoxifene acetate, comprising from about 4% to about 9% by weight of the pharmaceutical formulation; and f) an optional chelating component comprising from 0% to about 0.1 % by weight of the pharmaceutical formulation.
- the coating comprises: a) a filler component comprising from about 20% to about 30% by weight of the pharmaceutical formulation; b) a binder component comprising from about 6% to about 10% by weight of the pharmaceutical formulation; c) a wetting agent component comprising from about 0.4% to about 0.8% by weight of the pharmaceutical formulation; d) an optional antioxidant component comprising from 0% to about 1.2% by weight of the pharmaceutical formulation; e) bazedoxifene acetate, comprising from about 7% to about 14% by weight of the pharmaceutical formulation; and f) an optional chelating component comprising from 0% to about 0.1 % by weight of the pharmaceutical formulation.
- the core of the composition includes conjugated estrogens and one or more fillers and/or binding agents.
- the conjugated estrogens include or consist of Premarin®.
- the core includes conjugated estrogens in an amount of from about 0.10 to about 1.0 mg, and forms from about 45% to about 80% by weight of the pharmaceutical formulation.
- the core includes conjugated estrogens in an amount of from about 0.3 to about 0.8 mg.
- the core includes conjugated estrogens in an amount of from about 0.4 to about 0.5 mg; for example about 0.45 mg, or from about 0.5 to about 0.7 mg, for example about 0.625 mg.
- the core is a filled tablet containing conjugated estrogens, preferably Premarin®.
- the pharmaceutical compositions of the invention include at least one coating that contains apeledoxifene, or a pharmaceutically acceptable salt thereof.
- the apeledoxifene, or pharmaceutically acceptable salt thereof is present in an amount of from about 1 to about 50 mg, based on the weight of the apeledoxifene free base.
- the term "based on the weight of the apeledoxifene free base” is intended to mean that amount of apeledoxifene or salt thereof that provides the same number of molecules of apeledoxifene as the indicated mass of apeledoxifene free base.
- the phrase "10 mg of apeledoxifene acetate, based on the weight of the apeledoxifene free base" would indicate a mass of apeledoxifene acetate sufficient to provide a number of molecules of apeledoxifene acetate that is the same as the number of apeledoxifene molecules present in 10 mg of the free base form of apeledoxifene.
- the apeledoxifene, or pharmaceutically acceptable salt thereof is present in an amount of from about 5 to about 25 mg, or from about 5 to about 15 mg, or from about 15 to about 25 mg, or from about 35 to about 45 mg, based on the weight of the apeledoxifene free base.
- the apeledoxifene is present in the pharmaceutical formulation as the acetate salt.
- the apeledoxifene acetate present in the coating forms from about 0.1% to about 20% by weight of the pharmaceutical formulation; or from about 2% to about 6% by weight of the pharmaceutical formulation; or from about 4% to about 9% by weight of the pharmaceutical formulation; or from about 7% to about 14% by weight of the pharmaceutical formulation.
- the coating can contain one or more of fillers, diluents, binders, wetting agents, and / or antioxidants.
- the filler component of the coating forms from about 5% to about 30% by weight of the pharmaceutical formulation; or from about 6% to about 12% by weight of the pharmaceutical formulation; or from about 12% to about 18% by weight of the pharmaceutical formulation; or from about 20% to about 30% by weight of the pharmaceutical formulation.
- the filler component can include one or more fillers known to be useful in the art, for example one or more of sugars, for example sucrose, mannitol, lactose, and the like, and/or other fillers such as powdered cellulose, microcrystalline cellulose, malodextrin, sorbitol, starch, xylitol, carboxymethyl cellulose, carboxyethyl cellulose, hydroxyethyl celluloses, microcrystalline celluloses, starches, anhydrous dicalcium phosphate, sodium starch glycolates, and metal aluminosilicates.
- the filler component of the coating includes one or more sugars.
- sucrose refers to any type of simple carbohydrate, such as a mono or disaccharide, either naturally obtained, refined from a natural source, or artificially produced, and includes, without limitation, sucrose, dextrose, maltose, glucose, fructose, galactose, mannose, lactose, trehalose, lactulose, levulose, raffinose, ribose, and xylose.
- sucrose also includes various "sugar substitutes" widely known to those of ordinary skill in the art of preparing solid dosage forms, such as the polyhydric alcohols (sometimes referred to as “sugar alcohols” or hydrogenated saccharides), for example sorbitol, mannitol, xylitol, and erythritol, and the sugar derivatives of polyhydric alcohols, such as maltitol, lactitol, isomalt, and polyalditol. Accordingly, the recitation of the term “sugar” generically should be interpreted to include such specific compounds, as well as others not expressly recited.
- the sugar is a mono- or disaccharide, for example, sucrose, dextrose, maltose, glucose, fructose, galactose, mannose, or lactose.
- the filler component of the coating includes or consists of sucrose.
- the binder component of the coating forms from about 1% to about 10% by weight of the pharmaceutical formulation; or from about 1% to about 6% by weight of the pharmaceutical formulation; or from about 4% to about 8% by weight of the pharmaceutical formulation; or from about 6% to about 10% by weight of the pharmaceutical formulation.
- the binder component can include one or more binders known to be useful in the art, for example one or more of hydroxypropylmethylcellulose, carboxymethyl cellulose, carboxyethyl cellulose, hydroxyethyl celluloses, microcrystalline celluloses, starches and polyvinyl pyrrolidine (PVP).
- the filler component includes or consists of hydroxypropyl methylcellulose.
- sucrose although not a typical film forming agent like hydroxypropyl methylcellulose, is particularly advantageous when used with a lower viscosity grade of hydroxypropyl methylcellulose, for example 3 cps. While not wishing to be bound by any particular theory, the sucrose is believed to add body to the coating, and to act as a soluble- filler in an active overcoat process. Generally, it is beneficial to employ a ratio of hydroxypropyl methylcellulose to sucrose of from about 1 :2 to about 1 :5, or from about 1 :2 to about 1 :4; or from about 1 :2.5 to about 1 :3.5; or about 1 :3. Such a ratio is believed to provide the most acceptable viscosity and sprayability characteristics of the filler suspension at 20% w/w solids level for a continuous coating process.
- the wetting agent component of the coating is selected to increase wettability of the components of the filler coating, and in particular, apeledoxifene, and to therefore aid in dispersing ' the apeledoxifene.
- the wetting agent possesses low foaming characteristics, and preferably has antimicrobial activity.
- the wetting agent component of the coating forms from about 0.01% to about 2% by weight of the pharmaceutical formulation; or from about 0.1% to about 3% by weight of the pharmaceutical formulation; or from about 0.2% to about 0.5% by weight of the pharmaceutical formulation; or from about 0.4% to about 0.8% by weight of the pharmaceutical formulation.
- the wetting agent component can include one or more wetting agents known to be useful in the art, for example one or more of sucrose fatty acid esters, such as sucrose palmitate, and Poloxamer 188, metal alkyl sulfates, sodium lauryl sulfate, polyoxyethylene sorbitan fatty acid esters, polyethylene glycols, polyoxyethylene castor oil derivatives, docusate sodium, quaternary ammonium amine compounds, sugar esters of fatty acids and glycerides of fatty acids.
- the wetting agent component includes or consists of sucrose palmitate.
- the optional antioxidant component of the coating forms up to about 15% by weight, e.g., from 0% to about 15% by weight of the pharmaceutical formulation, from about 0.01% to about 5% by weight of the pharmaceutical formulation; from about 0.01% to about 2% by weight of the pharmaceutical formulation; or from about 0.1% to about 0.5% by weight of the pharmaceutical formulation; or from about 0.3% to about 0.8% by weight of the pharmaceutical formulation; or from about 0.6% to about 1.2% by weight of the pharmaceutical formulation.
- the antioxidant component when present, can include one or more antioxidants known to be useful in the art, for example one or more of ascorbic acid or a salt thereof such as sodium ascorbate, ascorbyl palmitate, nicotinamide ascorbate, propyl gallate, tocopherol (alpha, beta and gamma), BHA/BHT, citric acid and salts thereof, for example sodium citrate.
- the antioxidant component includes or consists of ascorbic acid.
- fillers for example, Remington's Pharmaceutical Sciences, 17th ed., Mack Publishing Company, Easton, Pa., 1985, which is incorporated herein by reference in its entirety.
- the optional chelating component forms up to about 0.1% by weight of the pharmaceutical formulation. In some embodiments, the chelating component is present in an amount of from about 0.01% to about 0.10% by weight of the coating.
- the chelating component can include one or more chelating agents as are known in the art for use in pharmaceutical formulations.
- One preferred chelating agent is ethylenediaminetetraacetic acid (EDTA).
- EDTA ethylenediaminetetraacetic acid
- Other suitable chelating agents can be found in, for example, Remington's Pharmaceutical Sciences, supra.
- the optional antioxidant component, the optional chelating component, or both the optional antioxidant component and the optional chelating component, additionally being optionally present in the coating as described above also can each independently and optionally be present in one or more of the color coating and the clear coating, as described below.
- the antioxidant component and the chelating component are both present together in one or more of the coatings.
- the antioxidant component and the chelating component are both present in the coating containing the active agent, e.g., apeledoxifene; or in the color coating; or in the clear coating; or in any two of the coatings, or in all three of the coatings.
- the pharmaceutical composition further includes a color coating.
- the color coating is formed over the first coating described above, and contains at least one coloring agent.
- the color coating includes: a) an optional filler component including from about 0.01% to about 8% by weight of the pharmaceutical formulation; b) an optional binder component including from about 0.01% to about 2% by weight of the pharmaceutical formulation; and c) a coloring agent component including from about 0.01 % to about 6% by weight of the pharmaceutical formulation.
- the color coating can also optionally include the aforementioned antioxidant component, or the aforementioned chelating component, or both, as described above.
- the optional filler component of the color coating can include one or more fillers as described above for the first coating.
- the filler component of the color coating when present, includes or consists of sucrose.
- the optional binder component of the color coating can include one or more binders as described above for the first coating.
- the binder component of the color coating when present, includes or consists of hydroxypropylmethylcellulose.
- the coloring agent component can include one or more of any of the variety of coloring agents known to be useful in the pharmaceutical arts.
- the coloring agent component includes or consists of titanium dioxide.
- Further preferred coloring agents include, for example, Opadry® agents, for example Opadry® White YS-1 -18202 A
- the pharmaceutical compositions of the invention further include a clear coating.
- the clear coating includes from about 0.01% to about 2% by weight of the pharmaceutical formulation.
- the clear coating is formed over the color coating, when present, as described above, or, alternatively, directly on the first coating as described above.
- Any of the numerous clear coatings known in the art are suitable for use in the pharmaceutical compositions of the invention, for example Opadry® coatings, for example Opadry® Clear YS-2-19114 A.
- the clear coating can also optionally include the aforementioned antioxidant component, or the aforementioned chelating component, or both, as described above.
- the invention provides processes for preparing pharmaceutical compositions of the invention.
- the processes are used to prepare pharmaceutical compositions of the invention that include: a core including conjugated estrogens; and a first coating including apeledoxifene or a pharmaceutically acceptable salt thereof.
- the processes include: i) providing a core including conjugated estrogens; and ii) coating the core with a coating composition including apeledoxifene or a pharmaceutically acceptable salt thereof to form a coated core.
- the processes further include the step of: iii) coating the coated core with a color coating composition to form a color coated composition.
- the processes further include the step of: iv) coating the color coating composition with a clear coating composition to form a clear coat thereon.
- sugar coatings are applied to tablet formulations using an intermittent process.
- intermittent active sugar coating process discrete amounts of the active sugar coat suspension are applied to the surface of a dosage form, for example a tablet, followed by a distribution phase and drying phase, which is repeated several hundred times until the desired weight gain is achieved.
- a dosage form for example a tablet
- distribution phase and drying phase which is repeated several hundred times until the desired weight gain is achieved.
- Several products currently sold are manufactured using this technique. This process, however, has some limitations. Examples of such limitations are the limited drug loading capacity to keep a reasonable tablet size and process times, and the limitation of the available excipients that can be used to modify the release rate and inherent variability of the process.
- sugar coatings can be applied to tablets or other coatable dosage forms by a continuous process.
- active suspension is applied in a "continuous manner" which is similar to traditional film coating process from the process stand- point. It is known that sugar, as such in a solution, can not be sprayed continuously on to tablets because of its inherent physico-chemical properties, such as solubility, viscosity, and its crystallization kinetics when dried by spray application on to tablets.
- use of the formulations described herein, with appropriate control of process variables produces a product of acceptable quality and stability, and addresses the limitations of the intermittent sugar coating process described above.
- the coating of the core with the coating composition including apeledoxifene or a pharmaceutically acceptable salt thereof is performed by a continuous process.
- the coating composition of step ii) includes: a) a filler component; b) a binder component; c) a wetting agent component; d) an optional antioxidant component; e) bazedoxifene acetate; and f) an optional chelating component.
- the filler component, the binder component, the wetting agent component, the optional antioxidant component, and the optional chelating component are as described above.
- the filler component of the coating comprises sucrose; the binder component of the coating comprises hydroxypropylmethylcellulose; the wetting agent component of the coating comprises sucrose palmitate; the optional antioxidant component of the coating, when present, comprises ascorbic acid, or a salt thereof; and the optional chelating component, when present, comprises EDTA.
- the color coating composition includes: an optional filler component; an optional binder component; and a coloring agent component; wherein the optional filler component, the optional binder component, and the coloring agent component are as described above.
- the color coating can also optionally include the aforementioned antioxidant component, or the aforementioned chelating component, or both, as described above.
- the optional filler component of the color coating composition includes sucrose; the optional binder component of the color coating composition includes hydroxypropylmethylcellulose; and the coloring agent component of the color coating composition includes titanium dioxide.
- the optional antioxidant component of the color coating when present, comprises ascorbic acid, or a salt thereof; and the optional chelating component of the color coating, when present, comprises EDTA.
- the filler component of the coating composition of step ii) forms from about 5% to about 30% by weight of the pharmaceutical formulation; the binder component of the coating composition of step ii) forms from about 1 % to about 10% by weight of the pharmaceutical formulation; the wetting agent component of the coating composition of step ii) forms from about 0.01% to about 2% by weight of the pharmaceutical formulation; the optional antioxidant component of the coating composition of step ii) forms from about 0% to about 2% by weight of the pharmaceutical formulation; the apeledoxifene acetate includes forms from about 0.1% to about 20% by weight of the pharmaceutical formulation; and the optional chelating component of the coating forms from 0% to about 0.1% by weight of the pharmaceutical formulation.
- the conjugated estrogens is present in an amount of from about 0.10 to about 1.0 mg. In some further embodiments, the apeledoxifene is present in an amount of from about 1 to about 50 mg, based on the weight of the apeledoxifene free base. In some further embodiments, the conjugated estrogens is present in an amount of from about 0.10 to about 1.0 mg; and the apeledoxifene is present in an amount of from about 5 to about 50 mg, based on the weight of the apeledoxifene free base.
- the conjugated estrogens include or consist of Premarin®.
- the conjugated estrogens is present in the composition in an amount of from about 0.10 to about 1.0 mg; or from about 0.3 to about 0.8 mg; or from about 0.4 to about 0.5 mg; or from about 0.5 to about 0.7 mg.
- the apeledoxifene is present in an amount of from about 1 to about 50 mg, or from about 5 to about 25 mg, or from about 5 to about 15 mg, or from about 15 to about 25 mg, or from about 35 to about 45 mg, based on the weight of the apeledoxifene free base.
- the conjugated estrogens is present in an amount of from about 0.10 to about 1.0 mg; and the apeledoxifene is present in an amount of from about 5 to about 50 mg, based on the weight of the apeledoxifene free base.
- the conjugated estrogens is present in the composition in an amount of from about 0.4 to about 0.5 mg; or from about 0.5 to about 0.7 mg; and the apeledoxifene is present in an amount of from about 5 to about 15 mg, or from about 15 to about 25 mg, or from about 35 to about 45 mg, based on the weight of the apeledoxifene free base.
- the present invention also provides products of the processes described herein.
- weight percentages set forth for each of the core, filler components, a binder components, wetting agent components, optional antioxidant components, optional chelating components, optional filler components, optional binder components, coloring agent components and clear coatings of the compositions disclosed herein are the percentages that each component will comprise of a final pharmaceutical composition, including, if present, the clear and color coatings.
- Oral formulations containing the present solid dispersions can comprise a variety of conventionally used oral forms, for example tablets and tablet-in-capsule forms. Generally, tablets and tablet-in-capsule forms are preferred. Capsules or tablets of containing the present solid dispersion can also be combined with mixtures of other active compounds or inert fillers and/or diluents such as the pharmaceutically acceptable starches (e.g. corn, potato or tapioca starch), sugars, artificial sweetening agents, powdered celluloses, such as crystalline and microcrystalline celluloses, flours, gelatins, gums, etc. In some preferred embodiments, the formulations are tablets.
- the pharmaceutically acceptable starches e.g. corn, potato or tapioca starch
- sugars e.g. corn, potato or tapioca starch
- artificial sweetening agents ed celluloses, such as crystalline and microcrystalline celluloses, flours, gelatins, gums, etc.
- the formulations are tablets
- Tablet formulations can be made by conventional compression, wet granulation, or dry granulation methods and utilize pharmaceutically acceptable diluents (fillers), binding agents, lubricants, disintegrants, suspending or stabilizing agents, including those described above, as well as, without limitation, magnesium stearate, stearic acid, talc, sodium lauryl sulfate, microcrystalline cellulose, carboxymethylcellulose calcium, polyvinylpyrrolidone, gelatin, alginic acid, acacia gum, xanthan gum, sodium citrate, complex silicates, calcium carbonate, glycine, dextrin, sucrose, sorbitol, dicalcium phosphate, calcium sulfate, lactose, kaolin, mannitol, sodium chloride, talc, dry starches and powdered sugar.
- pharmaceutically acceptable diluents fillers
- binding agents including those described above, as well as, without limitation, magnesium stearate, stearic acid
- Oral formulations used herein may utilize standard delay or time release formulations or spansules.
- Suppository formulations may be made from traditional materials, including cocoa butter, with or without the addition of waxes to alter the suppositories melting point, and glycerin.
- Water soluble suppository bases such as polyethylene glycols of various molecular weights, may also be used.
- Film coatings useful with the present formulations are known in the art and generally consist of a polymer (usually a cellulosic type of polymer), a colorant and a plasticizer. Additional ingredients such as wetting agents, sugars, flavors, oils and lubricants can be included in film coating formulations to impart certain characteristics to the film coat.
- the compositions and formulations herein may also be combined and processed as a solid, then placed in a capsule form, such as a gelatin capsule.
- a given component can act as both a binder and a filler.
- the function of a given component can be considered singular, even though its properties may allow multiple functionality.
- the present invention provides processes for preparing a pharmaceutical composition, the composition comprising: a core comprising a therapeutic agent; and a coating optionally comprising a second therapeutic agent and at least one sugar; the process comprising: i) providing a core comprising the first therapeutic agent; and ii) coating the core with a coating composition comprising: a) a filler component that comprises at least one sugar; b) a binder component; c) a wetting agent component; d) an optional antioxidant component; e) optionally, a second therapeutic agent; and f) an optional chelating component; wherein the coating composition of step ii) is applied by a continuous sugar coating technique.
- the filler component comprises one or more of sucrose, mannitol, lactose, powdered cellulose, microcrystalline cellulose, malodextrin, sorbitol, starch, xylitol, carboxymethyl cellulose, carboxyethyl cellulose, hydroxyethyl celluloses, microcrystalline celluloses, starches, anhydrous dicalcium phosphate, sodium starch glycolates, and metal aluminosilicates;
- the binder component comprises one or more of hydroxypropylmethylcellulose, carboxymethyl cellulose, carboxyethyl cellulose, hydroxyethyl celluloses, microcrystalline celluloses, starches;
- the wetting agent component comprises one or more of sucrose palmitate, Poloxamer 188, metal alkyl sulfates, sodium lauryl sulfate, polyoxyethylene sorbitan fatty acid esters, polyethylene glycols, polyoxyethylene castor oil derivatives, docusate sodium, quatern
- the filler component of the coating comprises sucrose; the binder component of the coating comprises hydroxypropylmethylcellulose; the wetting agent component of the coating comprises sucrose palmitate; the optional antioxidant component of the coating comprises ascorbic acid, or a salt thereof; and the optional chelatin component, when present, comprises EDTA.
- the processes further comprising the step of iii) coating the coated core with a color coating composition to form a color coated composition; and in some further embodiments, the processes further comprising the step of iv) coating the color coated composition with a clear coating composition to form a clear coat thereon.
- the first therapeutic agent can be any of a wide variety of therapeutic agents.
- therapeutic agent also refers to a substance which is capable of exerting a therapeutic biological effect in vivo.
- the therapeutic agents may be neutral or positively or negatively charged.
- suitable pharmaceutical agents include, inter alia, diagnostic agents, pharmaceuticals, drugs, synthetic organic molecules, proteins, peptides, vitamins, and steroids.
- the composition may include one or more hormonal steroids, such as medroxyprogesterone acetate, levonorgestrel, gestodene, medrogestone, estradiol, estriol, ethinylestradiol, mestranol, estrone, dienestrol, hexestrol, diethylstilbestrol, progesterone, desogestrel, norgestimate, hydroxyprogesterone, norethindrone, norethindone acetate, norgestrel, megestrol acetate, methyltestosterone, ethylestrenol, methandienone, oxandrolone, trimegestone, dionogest, and the like.
- hormonal steroids such as medroxyprogesterone acetate, levonorgestrel, gestodene, medrogestone, estradiol, estriol, ethinylestradiol, mestranol, estrone,
- tissue selective progesterones and/or progesterone antagonists which may or may not have the typical steroidal functionality, may be present in the composition.
- tissue selective progesterones and/or progesterone antagonists which may or may not have the typical steroidal functionality, may be present in the composition.
- These include, but are not limited to: RU-486 (mifepristone), ZK 98 299 (onapristone), ZK-137316 (Schering AG, Berlin), ZK-230211 (Schering AG, Berlin), and HRP-2000 (17-acetoxy-[11 ⁇ -(4-N,N-dimethylaminophenyl)]-19-norpregna-4,9- diene-3,20-dione).
- estrogenic steroids and progestogenic steroids may be used in combination.
- core material refers to any tablet, caplet, particle, micronized particle, particulate, pellet, pill, core, granule, granulate, small mass, seed, specks, spheres, crystals, beads, agglomerates, mixtures thereof and the like that are sufficiently stable physically and chemically to be effectively coated in a continuous sugar coating process.
- the core material is present in the form of a tablet.
- tablette refers to a solid pharmaceutical dosage form containing a therapeutic agent with or without suitable diluents and prepared by either compression or molding methods, such as are well known to those of ordinary skill in the art. Suitable methods of forming tablets are described, for example, in Edward M Rudnick, et al., "Oral Solid Dosage Forms," in Remington: The Science and Practice of Pharmacy, 20 th Ed., Chap. 45, Alfonso R. Gennaro, ed., Philadelphia College of Pharmacy and Science, Philadelphia, PA (2000), herein incorporated by reference in its entirety.
- the core material is a tablet formed by compression methods.
- the core material will comprise at least one therapeutic agent, as defined previously, and at least one pharmaceutically acceptable excipient.
- pharmaceutically acceptable refers to materials that are generally not toxic or injurious to a patient when used in the compositions of the present invention, including when the compositions are administered by the oral route.
- patient refers to animals, including mammals, preferably humans.
- Excipients refers to ingredients that provide bulk, impart satisfactory processing and compression characteristics, help control the dissolution rate, and/or otherwise give additional desirable physical characteristics to the core material.
- therapeutic agents may be utilized in either the core material i.e., as the first therapeutic agent), or in the coating (i.e., as the second therapeutic agent).
- therapeutic agents include, but are not limited to: acetazolamide, acetohexamide, acrivastine, alatrofloxacin, albuterol, alclofenac, aloxiprin, alprostadil, amodiaquine, amphotericin, amylobarbital, aspirin, atorvastatin, atovaquone, baclofen, barbital, benazepril, bezafibrate, bromfenac, bumetanide, butobarbital, candesartan, capsaicin, captopril, cefazolin, celecoxib, cephadrine, cephalexin, cerivastatin, cetrizine, chlorambucil, chlorothiazide, chlorpropamide, chlorthalidone, cinoxacin, ciproflox
- Additional therapeutic agents include abacavir, acebutolol, acrivastine, alatrofloxacin, albuterol, albendazole, alfentanil, alprazolam, alprenolol, amantadine, amiloride, aminoglutethimide, amiodarone, amitriptyline, amlodipine, amodiaquine, amoxapine, amphetamine, amphotericin, amprenavir, amrinone, amsacrine, apomorphine, astemizole, atenolol, atropine, azathioprine, azelastine, azithromycin, baclofen, benethamine, benidipine, benzhexol, benznidazole, benztropine, biperiden, bisacodyl, bisanthrene, bromazepam, bromocriptine, bromperidol, brompheniramine, brotizol
- the core material may be designed for delivering therapeutic agents intended to be delivered over a sustained period of time.
- therapeutic agents anti-inflammatory, antipyretic, antispasmodics or analgesics such as indomethacin, diclofenac, diclofenac sodium, codeine, ibuprofen, phenylbutazone, oxyphenbutazone, mepirizole, aspirin, ethenzamide, acetaminophen, aminopyrine, phenacetin, butylscopolamine bromide, morphine, etomidoline, pentazocine, fenoprofen calcium, naproxen, selecxip, valdecxip, and tolamadol, anti-rheumatism drugs such as etodolac, anti-tuberculoses drugs such as isoniazide and ethambutol hydrochloride, cardiovascular drugs such as isosorbide dinitrate, nitroglycer
- the therapeutic agent in the core material includes conjugated estrogens.
- Conjugated estrogens CE as used herein includes both natural and synthetic conjugated estrogens, such as the compounds described in the United States Pharmacopia (USP 23), as well as other estrogens so considered by those skilled in the art.
- conjugated estrogens refers to esters of such compounds, such as the sulfate esters, salts of such compounds, such as sodium salts, and esters of the salts of such compounds, such as sodium salts of a sulfate ester, as well as other derivatives known in the art.
- Some specific examples include: 17-alpha and beta-dihydroequilin, equilenin, 17-alpha and beta-dihydroequilenin, estrone, 17-beta-estradiol, and their sodium sulfate esters.
- CE are typically a mixture of estrogenic components, such as estrone and equilin
- the core material may be formulated to either utilize such a mixture, or to include only selected or individual estrogenic components.
- These CE may be of synthetic or natural origin. Examples of synthetically produced estrogens include, inter alia, sodium estrone sulfate, sodium equilin sulfate, sodium 17 ⁇ - dihydroequilin sulfate, sodium 17 ⁇ -dihydroequilin sulfate, sodium 17 ⁇ -estradiol sulfate, sodium 17 ⁇ -estradiol sulfate, sodium equilenin sulfate, sodium 17 ⁇ - dihydroequilenin sulfate, sodium 17 ⁇ -dihydroequilenin sulfate, estropipate and ethinyl estradiol.
- alkali metal salts of 8,9-dehydroestrone and the alkali metal salts of 8,9-dehydroestrone sulfate ester as described in U.S. Patent No. 5,210,081 , which is herein incorporated by reference, also may be used.
- Naturally occurring CE are usually obtained from pregnant mare urine and then are processed and may be stabilized. Examples of such processes are set forth in U.S. Pat. Nos. 2,565,115 and 2,720,483, each of which are herein incorporated by reference.
- CE products are commercially available. Preferred among these is the naturally occurring CE product known as Premarin ® (Wyeth, Madison, NJ). Another commercially available CE product prepared from synthetic estrogens is Cenestin ® (Duramed Pharmaceuticals, Inc., Cincinnati, Ohio).
- the specific CE dose included in the core material may be any dosage required to achieve a specific therapeutic effect, and may vary depending on the specific treatment indicated, and on the specific CE included in the tablet.
- the first active agent comprises conjugated estrogens
- the second active agent comprises apeldoxifene or a salt thereof.
- Methylcellulose, USP, 2910, 3 cps was added to the vortex and reheated to 55-65° C (target 60° C).
- the high shear mixer could be used during and after the addition to aid the wetting of the Hydroxypropyl Methylcellulose, USP, 2910, 3 cps.
- the dispersion was mixed for approximately 15 minutes to disperse Hydroxypropyl Methylcellulose, USP, 2910, 3 cps, with the speed of the mixer being adjusted to obtain adequate mixing.
- the temperature was held at 60° C for approximately 15 minutes to ensure all the Hydroxypropyl Methylcellulose, USP, 2910, 3 cps was well dispersed without forming any clumps.
- the speed of the Lightnin type mixer was adjusted to create a vortex without drawing air into the water. 300 g of sucrose, NF was added to the vortex, and the mixture was reheated to 60-70°C (target 65°C), mixed approximately 15 minutes to dissolve the sucrose, NF, and then held at that temperature for 15 minutes, making sure that all the sucrose was dissolved.
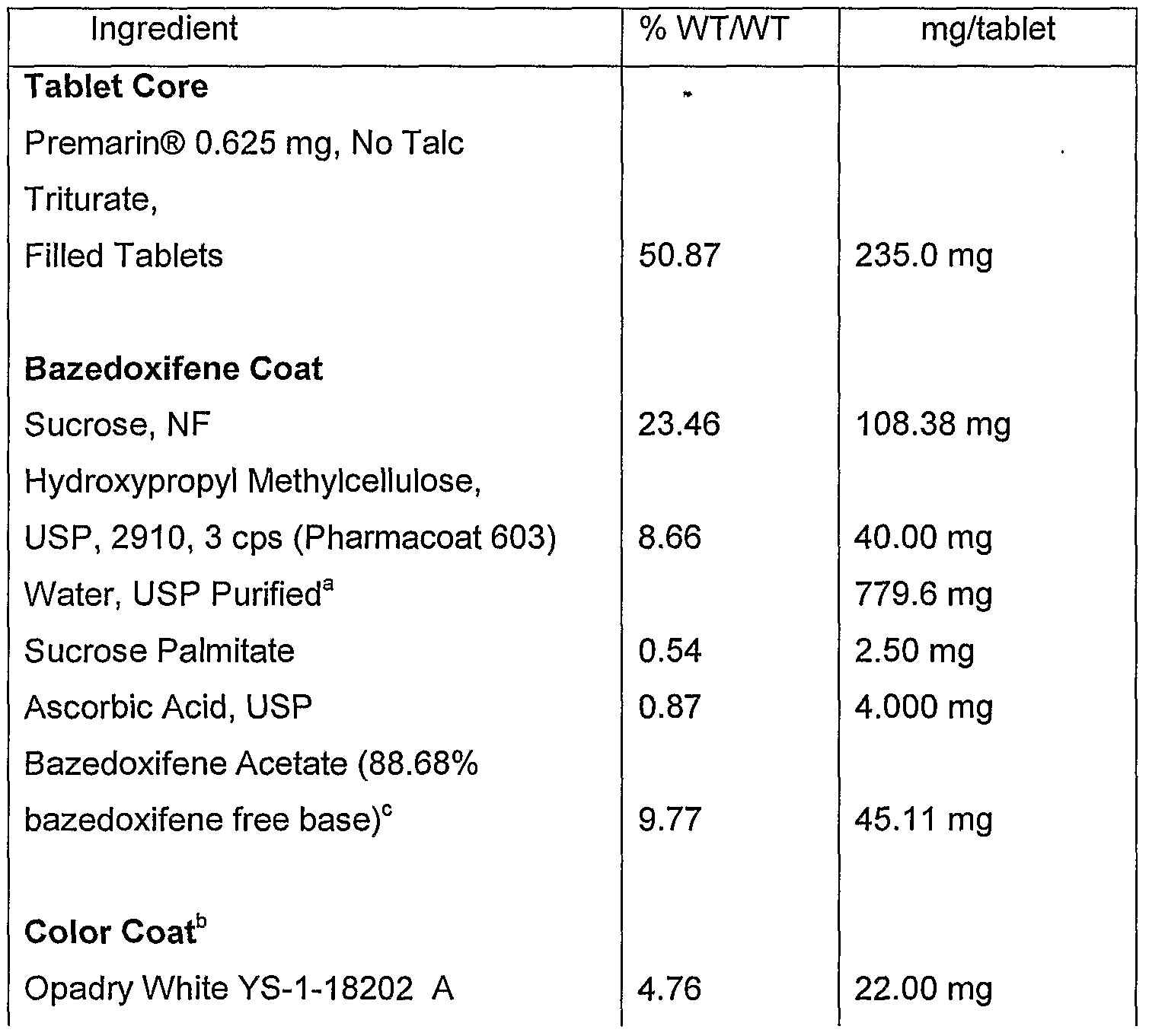
- composition of the tablets is shown in the Table below. a Removed during processing. b The quantities of the ingredients per tablet represent theoretical amounts of coating solids applied. For elegance, the amount of water and coating ingredients used may vary. These should not exceed +/- 10% of the theoretical values. 0 The potency of apeledoxifene acetate may vary, and the amount in the formula must be adjusted accordingly with a corresponding adjustment in the amount of Sucrose.
- composition of the tablets is shown in the Table below.
- Example 2 The procedure is essentially as described for Example 1 , except that: a) the Color Coat Suspension contained: 125.00 g of Opadry White YS-1 -18202 A; 875.00 g of water, USP, purified; and b) the Clear Coat Suspension contained:
- composition of the tablets is shown in the Table below.
- the potency of apeline acetate may vary, and the amount in the formula must be adjusted accordingly with a corresponding adjustment in the amount of Sucrose.
- Example 2 The procedure is essentially as described for Example 1 , except that tablets to which the coatings were applied were Premarin® 0.625 mg, No Talc Triturate, Filled Tablets.
- composition of the tablets is shown in the Table below.
- the potency of apeline acetate may vary, and the amount in the formula must be adjusted accordingly with a corresponding adjustment in the amount of Sucrose.
- Example 2 The procedure is essentially as described for Example 2, except that tablets to which the coatings were applied were Premarin® 0.625 mg, No Talc Triturate, Filled Tablets.
- composition of the tablets is shown in the Table below.
- the potency of apeline acetate may vary, and the amount in the formula must be adjusted accordingly with a corresponding adjustment in the amount of Sucrose.
- Example 3 The procedure is essentially as described for Example 3, except that tablets to which the coatings were applied were Premarin® 0.625 mg, No Talc Triturate, Filled Tablets.
- composition of the tablets is shown in the Table below.
Landscapes
- Health & Medical Sciences (AREA)
- Life Sciences & Earth Sciences (AREA)
- Veterinary Medicine (AREA)
- Chemical & Material Sciences (AREA)
- Public Health (AREA)
- Medicinal Chemistry (AREA)
- General Health & Medical Sciences (AREA)
- Animal Behavior & Ethology (AREA)
- Pharmacology & Pharmacy (AREA)
- Epidemiology (AREA)
- Engineering & Computer Science (AREA)
- Bioinformatics & Cheminformatics (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Organic Chemistry (AREA)
- Nuclear Medicine, Radiotherapy & Molecular Imaging (AREA)
- General Chemical & Material Sciences (AREA)
- Physical Education & Sports Medicine (AREA)
- Endocrinology (AREA)
- Orthopedic Medicine & Surgery (AREA)
- Rheumatology (AREA)
- Reproductive Health (AREA)
- Diabetes (AREA)
- Medicinal Preparation (AREA)
- Pharmaceuticals Containing Other Organic And Inorganic Compounds (AREA)
- Medical Preparation Storing Or Oral Administration Devices (AREA)
Abstract
Description
Claims
Priority Applications (7)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| EP06785833A EP1898888A2 (en) | 2005-06-29 | 2006-06-28 | Formulations of conjugated estrogens and bazedoxifene |
| BRPI0612586-7A BRPI0612586A2 (en) | 2005-06-29 | 2006-06-28 | conjugated estrogen and bazedoxifene formulations |
| AU2006263638A AU2006263638A1 (en) | 2005-06-29 | 2006-06-28 | Formulations of conjugated estrogens and bazedoxifene |
| JP2008520286A JP2008545012A (en) | 2005-06-29 | 2006-06-28 | Pharmaceutical formulations of conjugated estrogens and bazedoxifene |
| CA002613102A CA2613102A1 (en) | 2005-06-29 | 2006-06-28 | Formulations of conjugated estrogens and bazedoxifene |
| IL188223A IL188223A0 (en) | 2005-06-29 | 2007-12-18 | Formulations of conjugated estrogens and bazedoxifene |
| NO20080002A NO20080002L (en) | 2005-06-29 | 2008-01-02 | Formulations of conjugated estrogens and bazedoxifene |
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US69488905P | 2005-06-29 | 2005-06-29 | |
| US60/694,889 | 2005-06-29 |
Publications (2)
| Publication Number | Publication Date |
|---|---|
| WO2007002823A2 true WO2007002823A2 (en) | 2007-01-04 |
| WO2007002823A3 WO2007002823A3 (en) | 2007-08-09 |
Family
ID=37401610
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| PCT/US2006/025348 WO2007002823A2 (en) | 2005-06-29 | 2006-06-28 | Formulations of conjugated estrogens and bazedoxifene |
Country Status (19)
| Country | Link |
|---|---|
| US (1) | US20070003623A1 (en) |
| EP (1) | EP1898888A2 (en) |
| JP (1) | JP2008545012A (en) |
| KR (1) | KR20080031037A (en) |
| CN (1) | CN101252921A (en) |
| AR (1) | AR054806A1 (en) |
| AU (1) | AU2006263638A1 (en) |
| BR (1) | BRPI0612586A2 (en) |
| CA (1) | CA2613102A1 (en) |
| CR (1) | CR9597A (en) |
| EC (1) | ECSP078057A (en) |
| IL (1) | IL188223A0 (en) |
| NI (1) | NI200700331A (en) |
| NO (1) | NO20080002L (en) |
| PA (1) | PA8684501A1 (en) |
| PE (1) | PE20070188A1 (en) |
| RU (1) | RU2395286C2 (en) |
| TW (1) | TW200738283A (en) |
| WO (1) | WO2007002823A2 (en) |
Cited By (7)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2008067387A2 (en) * | 2006-11-29 | 2008-06-05 | Wyeth | Estrogen/ serm and estrogen/ progestin bi-layer tablets |
| WO2008089087A2 (en) * | 2007-01-12 | 2008-07-24 | Wyeth | Tablet-in-tablet compositions |
| WO2011056532A3 (en) * | 2009-10-27 | 2011-12-15 | Wyeth Llc | Bazedoxifene formulations with antioxidants |
| WO2011131943A3 (en) * | 2010-04-20 | 2011-12-29 | Cipla Limited | Pharmaceutical compositions |
| US11666585B2 (en) | 2018-04-19 | 2023-06-06 | Estetra Srl | Compounds and their uses for alleviating menopause-associated symptoms |
| US11793760B2 (en) | 2015-06-18 | 2023-10-24 | Estetra Srl | Orodispersible dosage unit containing an estetrol component |
| US11896602B2 (en) | 2016-08-05 | 2024-02-13 | Estetra Srl | Method for preventing pregnancy |
Families Citing this family (7)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CN1942177B (en) * | 2004-04-08 | 2011-05-25 | 惠氏公司 | Bazedoxifene acetate solid dispersion formulations |
| PE20090100A1 (en) * | 2007-03-30 | 2009-02-26 | Wyeth Corp | METHODS OF SEPARATION AND DETECTION OF BACEDOXIFEN ACETATE IN PHARMACEUTICAL COMPOSITIONS |
| CN103119878B (en) * | 2010-07-29 | 2016-11-16 | 熵通信有限公司 | For eliminating cross polarization interference and the method and apparatus of intersection satellite interference |
| SG10201606751XA (en) * | 2011-05-13 | 2016-10-28 | Eb Ip Hybritabs B V | Drug Delivery System |
| CN104013630B (en) * | 2014-05-23 | 2018-08-21 | 合肥九研医药科技开发有限公司 | A kind of compound bazedoxifene acetate estrogen compositions |
| WO2018182205A1 (en) * | 2017-03-30 | 2018-10-04 | 한미약품 주식회사 | Stabilized pharmaceutical composition containing bazedoxifene acetate |
| JOP20200260A1 (en) * | 2018-04-19 | 2019-10-19 | Estetra Sprl | Compounds and their uses for alleviating menopause-associated symptoms |
Citations (3)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| EP0803250A1 (en) * | 1996-04-24 | 1997-10-29 | American Home Food Products, Inc. | Controlled release of steroids from sugar coatings |
| US6710059B1 (en) * | 1999-07-06 | 2004-03-23 | Endorecherche, Inc. | Methods of treating and/or suppressing weight gain |
| US20040063692A1 (en) * | 2002-06-13 | 2004-04-01 | Wyeth | Bazedoxifene treatment regimens |
Family Cites Families (11)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US2565115A (en) * | 1948-10-28 | 1951-08-21 | Squibb & Sons Inc | Method of obtaining a conjugated estrogen preparation |
| US2720483A (en) * | 1951-02-21 | 1955-10-11 | Olin Mathieson | Method of obtaining a conjugatedestrogen preparation |
| US5210081A (en) * | 1992-02-26 | 1993-05-11 | American Home Products Corporation | Alkali metal 8,9-dehydroestrone sulfate esters |
| US5547948A (en) * | 1995-01-17 | 1996-08-20 | American Home Products Corporation | Controlled release of steroids from sugar coatings |
| US5998402A (en) * | 1996-04-19 | 1999-12-07 | American Home Products Corporation | 2-phenyl-1-[4-(2-aminoethoxy)-benzyl]-indoles as estrogenic agents |
| US6479535B1 (en) * | 1998-05-15 | 2002-11-12 | Wyeth | 2-phenyl-1-[4-(2-aminoethoxy)-benzyl]-indole and estrogen formulations |
| AR029538A1 (en) * | 2000-07-06 | 2003-07-02 | Wyeth Corp | PHARMACEUTICAL COMPOSITIONS OF ESTROGEN AGENTS |
| PE20060173A1 (en) * | 2004-04-07 | 2006-04-12 | Wyeth Corp | CRYSTALLINE POLYMORPH OF BAZEDOXIFEN ACETATE |
| PA8629101A1 (en) * | 2004-04-07 | 2005-11-25 | Wyeth Corp | BAZEDOXIFEN ACETATE CRYSTAL POLYMORPH |
| CN1942177B (en) * | 2004-04-08 | 2011-05-25 | 惠氏公司 | Bazedoxifene acetate solid dispersion formulations |
| RU2006132181A (en) * | 2004-04-08 | 2008-05-20 | Вайет (Us) | BASEDOXIFEN ASCORBATE AS A SELECTIVE MODULATOR OF AN ESTROGEN RECEPTOR |
-
2006
- 2006-06-28 CA CA002613102A patent/CA2613102A1/en not_active Abandoned
- 2006-06-28 TW TW095123252A patent/TW200738283A/en unknown
- 2006-06-28 US US11/478,400 patent/US20070003623A1/en not_active Abandoned
- 2006-06-28 RU RU2007148071/15A patent/RU2395286C2/en not_active IP Right Cessation
- 2006-06-28 JP JP2008520286A patent/JP2008545012A/en not_active Withdrawn
- 2006-06-28 BR BRPI0612586-7A patent/BRPI0612586A2/en not_active IP Right Cessation
- 2006-06-28 CN CNA2006800313156A patent/CN101252921A/en active Pending
- 2006-06-28 AU AU2006263638A patent/AU2006263638A1/en not_active Abandoned
- 2006-06-28 EP EP06785833A patent/EP1898888A2/en not_active Withdrawn
- 2006-06-28 PE PE2006000757A patent/PE20070188A1/en not_active Application Discontinuation
- 2006-06-28 KR KR1020087002416A patent/KR20080031037A/en not_active Application Discontinuation
- 2006-06-28 AR ARP060102789A patent/AR054806A1/en unknown
- 2006-06-28 WO PCT/US2006/025348 patent/WO2007002823A2/en active Application Filing
- 2006-06-29 PA PA20068684501A patent/PA8684501A1/en unknown
-
2007
- 2007-12-17 CR CR9597A patent/CR9597A/en not_active Application Discontinuation
- 2007-12-18 IL IL188223A patent/IL188223A0/en unknown
- 2007-12-18 NI NI200700331A patent/NI200700331A/en unknown
- 2007-12-27 EC EC2007008057A patent/ECSP078057A/en unknown
-
2008
- 2008-01-02 NO NO20080002A patent/NO20080002L/en not_active Application Discontinuation
Patent Citations (3)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| EP0803250A1 (en) * | 1996-04-24 | 1997-10-29 | American Home Food Products, Inc. | Controlled release of steroids from sugar coatings |
| US6710059B1 (en) * | 1999-07-06 | 2004-03-23 | Endorecherche, Inc. | Methods of treating and/or suppressing weight gain |
| US20040063692A1 (en) * | 2002-06-13 | 2004-04-01 | Wyeth | Bazedoxifene treatment regimens |
Non-Patent Citations (3)
| Title |
|---|
| GRUBER CHRISTIAN ET AL: "Bazedoxifene (Wyeth)." CURRENT OPINION IN INVESTIGATIONAL DRUGS (LONDON, ENGLAND : 2000) OCT 2004, vol. 5, no. 10, October 2004 (2004-10), pages 1086-1093, XP009080474 ISSN: 1472-4472 * |
| LABRIE FERNAND ET AL: "The combination of a novel selective estrogen receptor modulator with an estrogen protects the mammary gland and uterus in a rodent model: The future of postmenopausal women's health?" ENDOCRINOLOGY, vol. 144, no. 11, November 2003 (2003-11), pages 4700-4706, XP002424697 ISSN: 0013-7227 * |
| MARQUSEE E ET AL: "The effect of droloxifene and estrogen on thyroid function in postmenopausal women." THE JOURNAL OF CLINICAL ENDOCRINOLOGY AND METABOLISM NOV 2000, vol. 85, no. 11, November 2000 (2000-11), pages 4407-4410, XP002424698 ISSN: 0021-972X * |
Cited By (10)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2008067387A2 (en) * | 2006-11-29 | 2008-06-05 | Wyeth | Estrogen/ serm and estrogen/ progestin bi-layer tablets |
| WO2008067387A3 (en) * | 2006-11-29 | 2008-08-07 | Wyeth Corp | Estrogen/ serm and estrogen/ progestin bi-layer tablets |
| WO2008089087A2 (en) * | 2007-01-12 | 2008-07-24 | Wyeth | Tablet-in-tablet compositions |
| WO2008089087A3 (en) * | 2007-01-12 | 2009-06-25 | Wyeth Corp | Tablet-in-tablet compositions |
| WO2011056532A3 (en) * | 2009-10-27 | 2011-12-15 | Wyeth Llc | Bazedoxifene formulations with antioxidants |
| WO2011131943A3 (en) * | 2010-04-20 | 2011-12-29 | Cipla Limited | Pharmaceutical compositions |
| US11793760B2 (en) | 2015-06-18 | 2023-10-24 | Estetra Srl | Orodispersible dosage unit containing an estetrol component |
| US11964055B2 (en) | 2015-06-18 | 2024-04-23 | Estetra Srl | Orodispersible dosage unit containing an estetrol component |
| US11896602B2 (en) | 2016-08-05 | 2024-02-13 | Estetra Srl | Method for preventing pregnancy |
| US11666585B2 (en) | 2018-04-19 | 2023-06-06 | Estetra Srl | Compounds and their uses for alleviating menopause-associated symptoms |
Also Published As
| Publication number | Publication date |
|---|---|
| PA8684501A1 (en) | 2007-01-17 |
| RU2395286C2 (en) | 2010-07-27 |
| AR054806A1 (en) | 2007-07-18 |
| JP2008545012A (en) | 2008-12-11 |
| RU2007148071A (en) | 2009-08-10 |
| EP1898888A2 (en) | 2008-03-19 |
| CN101252921A (en) | 2008-08-27 |
| TW200738283A (en) | 2007-10-16 |
| ECSP078057A (en) | 2008-01-23 |
| IL188223A0 (en) | 2008-03-20 |
| NI200700331A (en) | 2009-02-16 |
| NO20080002L (en) | 2008-03-12 |
| AU2006263638A1 (en) | 2007-01-04 |
| US20070003623A1 (en) | 2007-01-04 |
| CA2613102A1 (en) | 2007-01-04 |
| WO2007002823A3 (en) | 2007-08-09 |
| PE20070188A1 (en) | 2007-03-16 |
| CR9597A (en) | 2008-03-06 |
| BRPI0612586A2 (en) | 2010-11-23 |
| KR20080031037A (en) | 2008-04-07 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| US20070003623A1 (en) | Formulations of conjugated estrogens and bazedoxifene | |
| EP1753407B1 (en) | Sugar coatings and methods therefor | |
| US3965256A (en) | Slow release pharmaceutical compositions | |
| JP5105685B2 (en) | Pharmaceuticalization of thyroid hormone | |
| NO138683B (en) | BASIC FOR USE IN THE PREPARATION OF A PHARMACEUTICAL PREPARATION WITH SLOW RELEASE OF THE ACTIVE INGREDIENT | |
| BRPI0713565B1 (en) | process for making a solid oral dosage form | |
| JP2013509403A (en) | Fast dissolving solid dosage form | |
| KR20130048227A (en) | Pharmaceutical composition comprising drospirenone and contraceptive kit | |
| CN107787224A (en) | The orodispersible dosage unit of the component containing E4 | |
| IL256283A (en) | Orodispersible dosage unit containing an estetrol component | |
| JP3261331B2 (en) | Soft capsule for chewing | |
| US20080107780A1 (en) | Sugar coatings and methods therefor | |
| CA1334933C (en) | Pharmaceutical composition and process for its preparation | |
| MX2007016363A (en) | Formulations of conjugated estrogens and bazedoxifene | |
| KR20070022712A (en) | Sugar coatings and methods therefor | |
| JPH0977675A (en) | Crude drug-formulated foaming tablet | |
| KR102210982B1 (en) | Pharmaceutical composition comprising drospirenone and contraceptive kit | |
| KR20060087618A (en) | Pharmaceutical formulations for thyroid hormones |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| WWE | Wipo information: entry into national phase |
Ref document number: 200680031315.6 Country of ref document: CN |
|
| 121 | Ep: the epo has been informed by wipo that ep was designated in this application | ||
| WWE | Wipo information: entry into national phase |
Ref document number: 2006785833 Country of ref document: EP |
|
| WWE | Wipo information: entry into national phase |
Ref document number: 4903/KOLNP/2007 Country of ref document: IN Ref document number: CR2007-009597 Country of ref document: CR |
|
| WWE | Wipo information: entry into national phase |
Ref document number: 188223 Country of ref document: IL Ref document number: MX/a/2007/016363 Country of ref document: MX |
|
| WWE | Wipo information: entry into national phase |
Ref document number: 2006263638 Country of ref document: AU |
|
| ENP | Entry into the national phase |
Ref document number: 2613102 Country of ref document: CA |
|
| WWE | Wipo information: entry into national phase |
Ref document number: 564623 Country of ref document: NZ |
|
| WWE | Wipo information: entry into national phase |
Ref document number: 12007502935 Country of ref document: PH |
|
| WWE | Wipo information: entry into national phase |
Ref document number: 07136799 Country of ref document: CO |
|
| NENP | Non-entry into the national phase |
Ref country code: DE |
|
| ENP | Entry into the national phase |
Ref document number: 2008520286 Country of ref document: JP Kind code of ref document: A |
|
| WWE | Wipo information: entry into national phase |
Ref document number: 2007148071 Country of ref document: RU Ref document number: 1020087002416 Country of ref document: KR |
|
| ENP | Entry into the national phase |
Ref document number: 2006263638 Country of ref document: AU Date of ref document: 20060628 Kind code of ref document: A |
|
| ENP | Entry into the national phase |
Ref document number: PI0612586 Country of ref document: BR Kind code of ref document: A2 Effective date: 20080102 |