WO2006014136A1 - Piperidine derivatives as histamine h3 receptor ligands - Google Patents
Piperidine derivatives as histamine h3 receptor ligands Download PDFInfo
- Publication number
- WO2006014136A1 WO2006014136A1 PCT/SE2005/001189 SE2005001189W WO2006014136A1 WO 2006014136 A1 WO2006014136 A1 WO 2006014136A1 SE 2005001189 W SE2005001189 W SE 2005001189W WO 2006014136 A1 WO2006014136 A1 WO 2006014136A1
- Authority
- WO
- WIPO (PCT)
- Prior art keywords
- benzo
- dihydro
- phenyl
- tetrahydro
- compound
- Prior art date
Links
- KUNMVMYJIPDXNX-UHFFFAOYSA-N O=C(NC1CCNCC1)N(CC1)CCN1c(c(Cl)cnc1)c1Cl Chemical compound O=C(NC1CCNCC1)N(CC1)CCN1c(c(Cl)cnc1)c1Cl KUNMVMYJIPDXNX-UHFFFAOYSA-N 0.000 description 1
- HBILLERTJBCBBW-UHFFFAOYSA-N O=C(NC1CCNCC1)N(CC1)CCN1c(cc1)ccc1Cl Chemical compound O=C(NC1CCNCC1)N(CC1)CCN1c(cc1)ccc1Cl HBILLERTJBCBBW-UHFFFAOYSA-N 0.000 description 1
- UPVYQMZJYMLSQG-UHFFFAOYSA-N O=C(NC1CCNCC1)N(CC1)CCN1c(cccc1)c1Cl Chemical compound O=C(NC1CCNCC1)N(CC1)CCN1c(cccc1)c1Cl UPVYQMZJYMLSQG-UHFFFAOYSA-N 0.000 description 1
Classifications
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D401/00—Heterocyclic compounds containing two or more hetero rings, having nitrogen atoms as the only ring hetero atoms, at least one ring being a six-membered ring with only one nitrogen atom
- C07D401/14—Heterocyclic compounds containing two or more hetero rings, having nitrogen atoms as the only ring hetero atoms, at least one ring being a six-membered ring with only one nitrogen atom containing three or more hetero rings
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P25/00—Drugs for disorders of the nervous system
- A61P25/24—Antidepressants
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D211/00—Heterocyclic compounds containing hydrogenated pyridine rings, not condensed with other rings
- C07D211/04—Heterocyclic compounds containing hydrogenated pyridine rings, not condensed with other rings with only hydrogen or carbon atoms directly attached to the ring nitrogen atom
- C07D211/06—Heterocyclic compounds containing hydrogenated pyridine rings, not condensed with other rings with only hydrogen or carbon atoms directly attached to the ring nitrogen atom having no double bonds between ring members or between ring members and non-ring members
- C07D211/36—Heterocyclic compounds containing hydrogenated pyridine rings, not condensed with other rings with only hydrogen or carbon atoms directly attached to the ring nitrogen atom having no double bonds between ring members or between ring members and non-ring members with hetero atoms or with carbon atoms having three bonds to hetero atoms with at the most one bond to halogen, e.g. ester or nitrile radicals, directly attached to ring carbon atoms
- C07D211/56—Nitrogen atoms
- C07D211/58—Nitrogen atoms attached in position 4
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D401/00—Heterocyclic compounds containing two or more hetero rings, having nitrogen atoms as the only ring hetero atoms, at least one ring being a six-membered ring with only one nitrogen atom
- C07D401/02—Heterocyclic compounds containing two or more hetero rings, having nitrogen atoms as the only ring hetero atoms, at least one ring being a six-membered ring with only one nitrogen atom containing two hetero rings
- C07D401/12—Heterocyclic compounds containing two or more hetero rings, having nitrogen atoms as the only ring hetero atoms, at least one ring being a six-membered ring with only one nitrogen atom containing two hetero rings linked by a chain containing hetero atoms as chain links
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D405/00—Heterocyclic compounds containing both one or more hetero rings having oxygen atoms as the only ring hetero atoms, and one or more rings having nitrogen as the only ring hetero atom
- C07D405/02—Heterocyclic compounds containing both one or more hetero rings having oxygen atoms as the only ring hetero atoms, and one or more rings having nitrogen as the only ring hetero atom containing two hetero rings
- C07D405/12—Heterocyclic compounds containing both one or more hetero rings having oxygen atoms as the only ring hetero atoms, and one or more rings having nitrogen as the only ring hetero atom containing two hetero rings linked by a chain containing hetero atoms as chain links
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D413/00—Heterocyclic compounds containing two or more hetero rings, at least one ring having nitrogen and oxygen atoms as the only ring hetero atoms
- C07D413/02—Heterocyclic compounds containing two or more hetero rings, at least one ring having nitrogen and oxygen atoms as the only ring hetero atoms containing two hetero rings
- C07D413/12—Heterocyclic compounds containing two or more hetero rings, at least one ring having nitrogen and oxygen atoms as the only ring hetero atoms containing two hetero rings linked by a chain containing hetero atoms as chain links
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D417/00—Heterocyclic compounds containing two or more hetero rings, at least one ring having nitrogen and sulfur atoms as the only ring hetero atoms, not provided for by group C07D415/00
- C07D417/02—Heterocyclic compounds containing two or more hetero rings, at least one ring having nitrogen and sulfur atoms as the only ring hetero atoms, not provided for by group C07D415/00 containing two hetero rings
- C07D417/12—Heterocyclic compounds containing two or more hetero rings, at least one ring having nitrogen and sulfur atoms as the only ring hetero atoms, not provided for by group C07D415/00 containing two hetero rings linked by a chain containing hetero atoms as chain links
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D471/00—Heterocyclic compounds containing nitrogen atoms as the only ring hetero atoms in the condensed system, at least one ring being a six-membered ring with one nitrogen atom, not provided for by groups C07D451/00 - C07D463/00
- C07D471/02—Heterocyclic compounds containing nitrogen atoms as the only ring hetero atoms in the condensed system, at least one ring being a six-membered ring with one nitrogen atom, not provided for by groups C07D451/00 - C07D463/00 in which the condensed system contains two hetero rings
- C07D471/04—Ortho-condensed systems
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D471/00—Heterocyclic compounds containing nitrogen atoms as the only ring hetero atoms in the condensed system, at least one ring being a six-membered ring with one nitrogen atom, not provided for by groups C07D451/00 - C07D463/00
- C07D471/02—Heterocyclic compounds containing nitrogen atoms as the only ring hetero atoms in the condensed system, at least one ring being a six-membered ring with one nitrogen atom, not provided for by groups C07D451/00 - C07D463/00 in which the condensed system contains two hetero rings
- C07D471/10—Spiro-condensed systems
Definitions
- This invention relates histamine receptor ligands. More specifically, the invention relates to histamine H3 receptor ligands, preparation thereof and uses thereof.
- the histamine H3 receptor is of current interest for the development of new medicaments.
- This receptor is a presynaptic autoreceptor located both in the central and the peripheral nervous system, the skin and in organs such as the lung, the intestine, probably the spleen and the gastrointestinal tract.
- the H3 receptor shows intrinsic, constitutive activity, in vitro as well as in vivo (i.e., it is active in the absence of an agonist. Compounds acting as inverse agonists can inhibit this activity.
- the histamine H3 receptor has been demonstrated to regulate the release of histamine and also of other neurotransmitters such as serotonin and acetylcholine.
- histamine H3 ligands such as histamine H3 receptor antagonists or inverse agonists may increase the release of these neurotransmitters in the brain whereas other histamine H3 ligands such as histamine H3 receptor agonists may lead to an inhibition of the biosynthesis of histamine and an inhibition of the release of histamine and also of other neurotransmitters.
- histamine H3 receptor agonists, inverse agonists and antagonists could be mediators of neuronal activity. Accordingly, the histamine H3 receptor may be a target for new therapeutics.
- C m-n or "C m-n group” used alone or as a prefix, refers to any group having m to n carbon atoms.
- hydrocarbon used alone or as a suffix or prefix, refers to any structure comprising only carbon and hydrogen atoms up to 14 carbon atoms.
- hydrocarbon radical or “hydrocarbyl” used alone or as a suffix or prefix, refers to any structure as a result of removing one or more hydrogens from a hydrocarbon.
- alkyl used alone or as a suffix or prefix, refers to monovalent straight or branched chain hydrocarbon radicals comprising 1 to about 12 carbon atoms.
- alkylene used alone or as suffix or prefix, refers to divalent straight or branched chain hydrocarbon radicals comprising 1 to about 12 carbon atoms, which serves to links two structures together.
- alkenyl used alone or as suffix or prefix, refers to a monovalent straight or branched chain hydrocarbon radical having at least one carbon-carbon double bond and comprising at least 2 up to about 12 carbon atoms.
- alkynyl used alone or as suffix or prefix, refers to a monovalent straight or branched chain hydrocarbon radical having at least one carbon-carbon triple bond and comprising at least 2 up to about 12 carbon atoms.
- cycloalkyl used alone or as suffix or prefix, refers to a monovalent ring- containing hydrocarbon radical comprising at least 3 up to about 12 carbon atoms.
- cycloalkenyl used alone or as suffix or prefix, refers to a monovalent ring- containing hydrocarbon radical having at least one carbon-carbon double bond and comprising at least 3 up to about 12 carbon atoms.
- cycloalkynyl used alone or as suffix or prefix, refers to a monovalent ring- containing hydrocarbon radical having at least one carbon-carbon triple bond and comprising about 7 up to about 12 carbon atoms.
- aryl used alone or as suffix or prefix, refers to a monovalent hydrocarbon radical having one or more polyunsaturated carbon rings having aromatic character, (e.g., 4n + 2 delocalized electrons) and comprising 5 up to about 14 carbon atoms.
- arylene used alone or as suffix or prefix, refers to a divalent hydrocarbon radical having one or more polyunsaturated carbon rings having aromatic character, (e.g., 4n + 2 delocalized electrons) and comprising 5 up to about 14 carbon atoms, which serves to link two structures together.
- heterocycle used alone or as a suffix or prefix, refers to a ring-containing structure or molecule having one or more multivalent heteroatoms, independently selected from N, O 3 P and S, as a part of the ring structure and including at least 3 and up to about 20 atoms in the ring(s). Heterocycle may be saturated or unsaturated, containing one or more double bonds, and heterocycle may contain more than one ring. When a heterocycle contains more than one ring, the rings may be fused or unfused. Fused rings generally refer to at least two rings share two atoms therebetween. Heterocycle may have aromatic character or may not have aromatic character.
- heteromatic used alone or as a suffix or prefix, refers to a ring- containing structure or molecule having one or more multivalent heteroatoms, independently selected from N, O, P and S, as a part of the ring structure and including at least 3 and up to about 20 atoms in the ring(s), wherein the ring-containing structure or molecule has an aromatic character (e.g., 4n + 2 delocalized electrons).
- heterocyclic group refers to a radical derived from a heterocycle by removing one or more hydrogens therefrom.
- heterocyclyl used alone or as a suffix or prefix, refers a monovalent radical derived from a heterocycle by removing one hydrogen therefrom.
- heterocyclylene used alone or as a suffix or prefix, refers to a divalent radical derived from a heterocycle by removing two hydrogens therefrom, which serves to links two structures together.
- heteroaryl used alone or as a suffix or prefix, refers to a heterocyclyl having aromatic character.
- heterocyclylcoalkyl used alone or as a suffix or prefix, refers to a heterocyclyl that does not have aromatic character.
- heteroarylene used alone or as a suffix or prefix, refers to a heterocyclylene having aromatic character.
- heterocycloalkylene used alone or as a suffix or prefix, refers to a heterocyclylene that does not have aromatic character.
- suffix or prefix refers to a heterocyclylene that does not have aromatic character.
- ix-membered used as prefix refers to a group having a ring that contains six ring atoms.
- a f ⁇ ve-membered ring heteroaryl is a heteroaryl with a ring having five ring atoms wherein 1, 2 or 3 ring atoms are independently selected from N, O and S.
- Exemplary five-membered ring heteroaryls are thienyl, furyl, pyrrolyl, imidazolyl, thiazolyl, oxazolyl, pyrazolyl, isothiazolyl, isoxazolyl, 1,2,3-triazolyl, tetrazolyl, 1,2,3- thiadiazolyl, 1,2,3-oxadiazolyl, 1,2,4-triazolyl, 1,2,4-thiadiazolyl, 1,2,4-oxadiazolyl, 1,3,4- triazolyl, 1,3,4-thiadiazolyl, and 1,3,4- oxadiazolyl.
- a six-membered ring heteroaryl is a heteroaryl with a ring having six ring atoms wherein 1, 2 or 3 ring atoms are independently selected from N, O and S.
- Exemplary six-membered ring heteroaryls are pyridyl, pyrazinyl, pyrimidinyl, triazinyl and pyridazinyl.
- substituted refers to a structure, molecule or group, wherein one or more hydrogens are replaced with one or more C 1-6 hydrocarbon groups, or one or more chemical groups containing one or more heteroatoms selected from N, O, S, F, Cl, Br, I, and P.
- substituted phenyl may refer to nitrophenyl, methoxyphenyl, chlorophenyl, aminophenyl, etc., wherein the nitro, methoxy, chloro, and amino groups may replace any suitable hydrogen on the phenyl ring.
- substituted used as a suffix of a first structure, molecule or group, followed by one or more names of chemical groups refers to a second structure, molecule or group, which is a result of replacing one or more hydrogens of the first structure, molecule or group with the one or more named chemical groups.
- a "phenyl substituted by nitro” refers to nitrophenyl.
- Heterocycle includes, for example, monocyclic heterocycles such as: aziridine, oxirane, thiirane, azetidine, oxetane, thietane, pyrrolidine, pyrroline, imidazolidine, pyrazolidine, pyrazoline, dioxolane, sulfolane 2,3-dihydroruran, 2,5-dihydrofuran tetrahydrofuran, thiophane, piperidine, 1,2,3,6-tetrahydro-pyridine, piperazine, morpholine, thiomorpholine, pyran, thiopyran, 2,3-dihydropyran, tetrahydropyran, 1,4-dihydropyridine, 1,4-dioxane, 1,3-dioxane, dioxane, homopiperidine, 2,3,4,7-tetrahydro-l//-azepine homopiperazine
- heterocycle includes aromatic heterocycles, for example, pyridine, pyrazine, pyrimidine, pyridazine, thiophene, furan, furazan, pyrrole, imidazole, thiazole, oxazole, pyrazole, isothiazole, isoxazole, 1,2,3-triazole, tetrazole, 1,2,3-thiadiazoIe, 1,2,3- oxadiazole, 1,2,4-triazole, 1,2,4-thiadiazole, 1,2,4-oxadiazole, 1,3,4-triazole, 1,3,4- thiadiazole, and 1,3,4- oxadiazole.
- aromatic heterocycles for example, pyridine, pyrazine, pyrimidine, pyridazine, thiophene, furan, furazan, pyrrole, imidazole, thiazole, oxazole, pyrazole, isothiazo
- heterocycle encompass polycyclic heterocycles, for example, indole, indoline, isoindoline, quinoline, tetrahydroquinoline, isoquinoline, tetrahydroisoquinoline, 1,4-benzodioxan, coumarin, dihydrocoumarin, benzofuran, 2,3-dihydrobenzofuran, isobenzofuran, chromene, chroman, iso ⁇ hroman, xanthene, phenoxathiin, thianthrene, indolizine, isoindole, indazole, purine, phthalazine, naphthyridine, quinoxaline, quinazoline, cinnoline, pteridine, phenanthridine, perimidine, phenanthroline, phenazine, phenothiazine, phenoxazine, 1,2-benzisoxazole, benzothiophene, benzox
- heterocycle includes polycyclic heterocycles wherein the ring fusion between two or more rings includes more than one bond common to both rings and more than two atoms common to both rings.
- bridged heterocycles include quinuclidine, diazabicyclo[2.2.1]heptane and 7-oxabicy clo [2.2.1 jheptane.
- Heterocyclyl includes, for example, monocyclic heterocyclyls, such as: aziridinyl, oxiranyl, thiiranyl, azetidinyl, oxetanyl, thietanyl, pyrrolidinyl, pyrrolinyl, imidazolidinyl, pyrazolidinyl, pyrazolinyl, dioxolanyl, sulfolanyl, 2,3-dihydrofuranyl, 2,5-dihydrofuranyl, tetrahydrofuranyl, thiophanyl, piperidinyl, 1,2,3,6-tetrahydro-pyridinyl, piperazinyl, morpholinyl, thiomorpholinyl, pyranyl, thiopyranyl, 2,3-dihydropyranyl, tetrahydropyranyl, 1,4-dihydro ⁇ yridinyl, 1,4-d
- heterocyclyl includes aromatic heterocyclyls or heteroaryl, for example, pyridinyl, pyrazinyl, pyrimidinyl, pyridazinyl, thienyl, furyl, furazanyl, pyrrolyl, imidazolyl, thiazolyl, oxazolyl, pyrazolyl, isothiazolyl, isoxazolyl, 1,2,3-triazolyl, tetrazolyl, 1,2,3- ) thiadiazolyl, 1,2,3-oxadiazolyl, 1,2,4-triazolyl, 1,2,4-thiadiazolyl, 1,2,4-oxadiazolyl, 1,3,4- triazolyl, 1,3,4-thiadiazolyl, and 1,3,4 oxadiazolyl.
- heterocyclyl encompasses polycyclic heterocyclyls (including both aromatic or non-aromatic), for example, indolyl, indolinyl, isoindolinyl, quinolinyl, tetrahydroquinolinyl, isoquinolinyl, tetrahydroisoquinolinyl, 1,4-benzodioxanyl, coumaritiyl, dihydrocoumarinyl, benzofuranyl, 2,3-dihydrobenzofuranyl, isobenzofuranyl, chromenyl, chromanyl, isochromanyl, xanthenyl, phenoxathiinyl, thianthrenyl, indolizinyl, isoindolyl, indazolyl, purinyl, phthalazinyl, naphthyridinyl, quinoxalinyl, quinazolinyl, cinnolinyl, pter
- heterocyclyl includes polycyclic heterocyclyls wherein the ring fusion between two or more rings includes more than one bond common to both rings and more than two atoms common to both rings.
- bridged heterocycles include quinuclidinyl, diazabicyclo[2.2.1]heptyl; and 7-oxabicyclo[2.2. ljheptyl.
- alkoxy used alone or as a suffix or prefix, refers to radicals of the general formula -O-R, wherein R is selected from a hydrocarbon radical.
- exemplary alkoxy includes methoxy, ethoxy, propoxy, isopropoxy, butoxy, t-butoxy, isobutoxy, cyclopropylmethoxy, allyloxy, and propargyloxy.
- amine or “amino” used alone or as a suffix or prefix, refers to radicals of the general formula -NRR', wherein R and R' are independently selected from hydrogen or a hydrocarbon radical.
- Halogen includes fluorine, chlorine, bromine and iodine.
- Halogenated used as a prefix of a group, means one or more hydrogens on the group is replaced with one or more halogens.
- RT room temperature
- the invention provides a compound of formula I, a pharmaceutically acceptable salt thereof, diastereomers thereof, enantiomers thereof, and mixtures thereof:
- Ar 1 is selected from C 6-1 oaryl and C 2-9 heteroaryl, wherein said C 6- ioaryl and
- C 2-9 heteroaryl are optionally substituted with one or more groups selected from -R, -NO 2 , - OR, -Cl, -Br, -I, -F, -CF 3 , -OCF 3 , -C(O)R, -C(O)OH, -NH 2 , -SH, -NHR, -NR 2 , -SR, - SO 3 H, -SO 2 R, -SO 2 NR, -S(O)R, -CN, -OH, -C(O)OR, -C(O)NR 2 , -NRC(O)R, and -NRC(O)-OR, wherein R is, independently, a hydrogen, C 3 .
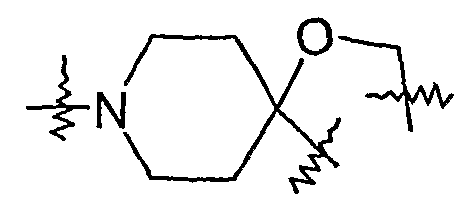
- the compound of the present invention may be a compound of formula I, wherein Ar 1 is represented by
- Ar is selected from phenyl, pyridyl, naphthyl, 1,2,3,4-tetrahydro-naphthyl; thienyl, furyl, thiazolyl, benzo[l,3]dioxolyl, 4,5,6,7-tetrahydro-thieno[2,3-c]pyridinyl; 2,3- dihydro-benzo[l,4]dioxinyl; quinolyl; isoquinolyl; indolyl; pyrroyl, benzotriazolyl; benzoimidazolyl, 2,3 -dihy dro-benzoforanyl; 2,3 -dihy dro-isoindol- 1 -on-y 1; benzo[l,2,3]thiadiazolyl, benzothiazolyl, imidazo[l,2-a]pyridinyl, pyrazinyl,and 4H- benzo [
- R is, independently, a hydrogen, C 5-6 cycloalkyl, C 3-5 heterocyclyl, phenyl, benzyl, Ci -4 alkyl or C 2-
- R is further optionally substituted with one or more groups selected from methyl, cyano, methoxy, hydroxy and halogen;
- Q is selected from:
- Q may be a trivalent group such as , which is fused with Ar 1 , wherein Ar 1 is a divalent aromatic group such as 1,2-phenylene.
- the compounds of the present invention are represented by formula I, wherein Ar 1 is selected from phenyl, 2-pyridyl, 3-pyridyl, 4-pyridyl; 1-na ⁇ hthyl, 2- naphthyl, 1,2,3,4-tetrahydro-naphth-l-yl; l,2,3,4-tetrahydro-na ⁇ hth-5-yl; 2-thienyl, 3-thienyl, 2-furyl, 2-thiazolyl, benzo[l,3]dioxol-5-yl, 4,5,6,7-tetrahydro-thieno[2,3-c]pyridin-2-yl; 2,3- dihydro-benzo[l,4]dioxin-6-yl; 2,3-dihydro-benzo[l,4]dioxin-2-yl; quinol-2-yl, isoquinol-5- yl; lH-indol-4-
- Ar 1 is further optionally substituted with one or more groups selected from Q ⁇ alkyl, C 2-4 alkenyl, Ci -4 alkoxy 3 Ci -4 alkenyloxy, phenoxy, 4-methoxyphenoxy, benzyl, acetoamino, methylsulfonyl, methoxycarbonyl, nitro, chloro, fluoro, bromo, iodo, 1- ⁇ yrroyl, 2 -methyl- pyrro-1-yl, amino, phenylsulfonyl, aceto,l-piperidinyl, [l,2,3]thiadiazol-4-yl, 4-morpholinyl, methoxy, ethoxy, isopropyloxy, methythio, cyano, dimethylamino,
- the compounds of the present invention are selected from salts thereof.
- the compounds of the invention may exist in, and be isolated as, enantiomeric or diastereomeric forms, or as a racemic mixture.
- the present invention includes any possible enantiomers, diastereomers, racemates or mixtures thereof, of a compound of Formula I.
- the optically active forms of the compound of the invention may be prepared, for example, by chiral chromatographic separation of a racemate, by synthesis from optically active starting materials or by asymmetric synthesis based on the procedures described thereafter. It will also be appreciated that certain compounds of the present invention may exist as geometrical isomers, for example E and Z isomers of alkenes.
- the present invention includes any geometrical isomer of a compound of Formula I. It will further be understood that the present invention encompasses tautomers of the compounds of the formula I. It will also be understood that certain compounds of the present invention may exist in solvated, for example hydrated, as well as unsolvated forms. It will further be understood that the present invention encompasses all such solvated forms of the compounds of the formula I.
- salts of the compounds of the formula I are also salts of the compounds of the formula I.
- pharmaceutically acceptable salts of compounds of the present invention may be obtained using standard procedures well known in the art, for example by reacting a sufficiently basic compound, for example an alkyl amine with a suitable acid, for example, HCl or acetic acid, to afford a physiologically acceptable anion.
- a corresponding alkali metal such as sodium, potassium, or lithium
- an alkaline earth metal such as a calcium
- a compound of the present invention having a suitably acidic proton, such as a carboxylic acid or a phenol with one equivalent of an alkali metal or alkaline earth metal hydroxide or alkoxide (such as the ethoxide or methoxide), or a suitably basic organic amine (such as choline or meglumine) in an aqueous medium, followed by conventional purification techniques.
- a suitably acidic proton such as a carboxylic acid or a phenol
- an alkali metal or alkaline earth metal hydroxide or alkoxide such as the ethoxide or methoxide
- a suitably basic organic amine such as choline or meglumine
- the compound of formula I above may be converted to a pharmaceutically acceptable salt or solvate thereof, particularly, an acid addition salt such as a hydrochloride, hydrobromide, phosphate, acetate, fumarate, maleate, tartrate, citrate, methanesulphonate or j ⁇ -toluenesulphonate.
- an acid addition salt such as a hydrochloride, hydrobromide, phosphate, acetate, fumarate, maleate, tartrate, citrate, methanesulphonate or j ⁇ -toluenesulphonate.
- the compounds of the present invention are useful in the treatment of a wide range of conditions and disorders in which an interaction with the histamine H3 receptor is beneficial.
- the compounds may find use e.g. in the treatment of diseases of the central nervous system, the peripheral nervous system, the cardiovascular system, the pulmonary system, the gastrointestinal system and the endocrinological system.
- the compounds of the present invention are useful in therapy, espcially for the treatment of various depression conditions.
- Compounds of the invention are useful as immunomodulators, especially for autoimmune diseases, such as arthritis, for skin grafts, organ transplants and similar surgical needs, for collagen diseases, various allergies, for use as anti-tumour agents and anti viral agents.
- Compounds of the invention are useful for the treatment of obesity, epilepsy, Alzheimer's disease, dementia, schizophrenia, cognitive defect, rhinitis, cognition disorders, central nervous system disease, neurological disorder, epilepsy, attention deficit hyperactivity disorder, eating disorder, allergic rhinitis, allergy, inflammation, migraine, sleep disorder, narcolepsy, anxiety disorder, psychiatric conditions, depression, multiple sclerosis, anxiety, bipolar disorder, stroke, sleep disorder, mental disorder, cognitive disorder and non-insulin dependent diabetes.
- Compounds of the invention are useful as an anti-depression agent. Combinations of agents with different properties may be used to achieve a balance of effects needed to treat depression.
- a further aspect of the invention is a method for the treatment of a subject suffering from any of the conditions discussed above, whereby an effective amount of a compound according to the formula I above, is administered to a patient in need of such treatment.
- the invention provides a compound of formula I, or pharmaceutically acceptable salt or solvate thereof, as hereinbefore defined for use in therapy.
- the present invention provides the use of a compound of formula I, or a pharmaceutically acceptable salt or solvate thereof, as hereinbefore defined in the manufacture of a medicament for use in therapy.
- the term “therapy” also includes “prophylaxis” unless there are specific indications to the contrary.
- the term “therapeutic” and “therapeutically” should be contrued accordingly.
- the term “therapy” within the context of the present invention further encompasses to administer an effective amount of a compound of the present invention, to mitigate either a pre-existing disease state, acute or chronic, or a recurring condition. This definition also encompasses prophylactic therapies for prevention of recurring conditions and continued therapy for chronic disorders.
- the compound of the invention may be administered in the form of a conventional pharmaceutical composition by any route including orally, intramuscularly, subcutaneously, topically, intranasally, intraperitoneally, intrathoracially, intravenously, epidurally, intrathecally, intracerebroventricularly and by injection into the joints.
- the route of administration may be orally, intravenously or intramuscularly.
- the dosage will depend on the route of administration, the severity of the disease, age and weight of the patient and other factors normally considered by the attending physician, when determining the individual regimen and dosage level at the most appropriate for a particular patient.
- inert, pharmaceutically acceptable carriers can be either solid and liquid.
- Solid form preparations include powders, tablets, dispersible granules, capsules, cachets, and suppositories.
- a solid carrier can be one or more substances, which may also act as diluents, flavoring agents, solubilizers, lubricants, suspending agents, binders, or table disintegrating agents; it can also be an encapsulating material.
- the carrier is a finely divided solid, which is in a mixture with the finely divided compound of the invention, or the active component.
- the active component is mixed with the carrier having the necessary binding properties in suitable proportions and compacted in the shape and size desired.
- a low-melting wax such as a mixture of fatty acid glycerides and cocoa butter is first melted and the active ingredient is dispersed therein by, for example, stirring. The molten homogeneous mixture in then poured into convenient sized moulds and allowed to cool and solidify.
- Suitable carriers are magnesium carbonate, magnesium stearate, talc, lactose, sugar, pectin, dextrin, starch, tragacanth, methyl cellulose, sodium carboxymethyl cellulose, a low- melting wax, cocoa butter, and the like.
- the term composition is also intended to include the formulation of the active component with encapsulating material as a carrier providing a capsule in which the active component (with or without other carriers) is surrounded by a carrier which is thus in association with it. Similarly, cachets are included.
- Tablets, powders, cachets, and capsules can be used as solid dosage forms suitable for oral administration.
- Liquid form compositions include solutions, suspensions, and emulsions.
- sterile water or water propylene glycol solutions of the active compounds may be liquid preparations suitable for parenteral administration.
- Liquid compositions can also be formulated in solution in aqueous polyethylene glycol solution.
- Aqueous solutions for oral administration can be prepared by dissolving the active component in water and adding suitable colorants, flavoring agents, stabilizers, and thickening agents as desired.
- Aqueous suspensions for oral use can be made by dispersing the finely divided active component in water together with a viscous material such as natural synthetic gums, resins, methyl cellulose, sodium carboxymethyl cellulose, and other suspending agents known to the pharmaceutical formulation art.
- the pharmaceutical composition will preferably include from 0.05% to 99%w (per cent by weight), more preferably from 0.10 to 50% w, of the compound of the invention, all percentages by weight being based on total composition.
- a therapeutically effective amount for the practice of the present invention may be determined, by the use of known criteria including the age, weight and response of the individual patient, and interpreted within the context of the disease which is being treated or which is being prevented, by one of ordinary skills in the art.
- a further aspect of the invention is a method for therapy of a subject suffering from any of the conditions discussed above, whereby an effective amount of a compound according to the formula I above, is administered to a patient in need of such therapy.
- composition comprising a compound of Formula I, or a pharmaceutically acceptable salt thereof, in association with a pharmaceutically acceptable carrier.
- a pharmaceutical composition comprising a compound of Formula I, or a pharmaceutically acceptable salt thereof, in association with a pharmaceutically acceptable carrier for therapy, more particularly for therapy of depression.
- a pharmaceutical composition comprising a compound of Formula I, or a pharmaceutically acceptable salt thereof, in association with a pharmaceutically acceptable carrier use in any of the conditions discussed above.
- the invention provides a process for preparing a compound of formula I, comprising:
- C 2-9 heteroaryl are optionally substituted with one or more groups selected from -R 3 -NO 2 , - OR 5 -Cl, -Br, -I, -F, -CF 3 , -OCF 3 , -C(O)R, -C(O)OH, -NH 2 , -SH, -NHR, -NR 2 , -SR, - SO 3 H, -SO 2 R, -SO 2 NR, -S(O)R, -CN, -OH, -C(O)OR, -C(O)NR 2 , -NRC(O)R, and -NRC(O)-OR, wherein R is, independently, a hydrogen, C 3 .
- Q is a divalent or trivalent group that connects the carbonyl with Ar 1 , wherein said divalent or trivalent group contains at least one nitrogen, wherein said nitrogen of Q is connected to the H in Ar ⁇ Q-H to form an amino, and said trivalent group is fused with Ar 1 ; and said Q-H of Ar ⁇ Q-H forms an amino group.
- Ar 1 is selected from phenyl, 2-pyridyl, 3-pyridyl, 4-pyridyl; 1-naphthyl, 2- naphthyl, 1,2,3,4-tetrahydro-naphth-l-yl; l,2,3,4-tetrahydro-naphth-5-yl; 2-thienyl, 3-thienyl, 2-furyl, 2-thiazolyl, benzo[l,3]dioxol-5-yl, 4,5,6,7-tetrahydro-thieno[2,3-c]pyridin-2-yl; 2,3- dihydro-benzo[l,4]dioxm-6-yl; 2,3-dihydro-benzo[l,4]dioxin-2-yl; quinol-2-yl, isoquino
- the step of combining Ar 1 -Q-H with 4-amino-l -methyl piperidine and a halofo ⁇ nate may be carried out at ambient temperature and in the presence of organic base such as diisopropylethylamine.
- the haloformate may be 4-nitrophenyl chloroformate.
- the compounds of the invention are found to be active towards H3 receptors in warm ⁇ blooded animal, e.g., human. Particularly the compounds of the invention are found to be effective H3 receptor ligands.
- H3 receptor ligands In vitro assays, infra, demonstrate these surprising activities. These activities may be related to in vivo activity and may not be linearly correlated with binding affinity.
- a compound In these in vitro assays, a compound is tested for their activity toward H3 receptors and pIC 50 is obtained to determine the activity for a particular compound towards H3 receptors.
- H3 receptor activation in response to histamine mediates intracellular Ca 2+ mobilization in human H3 receptor transfected CHO-Kl cells.
- This increase in Ca 2+ can be measured using the fluorometric imaging plate reader (FLIPR) employing Fluo-3 AM loaded H3 receptor transfected cells.
- FLIPR fluorometric imaging plate reader
- CHO-H3-G ⁇ l6 transfected cells were cultured in T225 cm 2 tissue culture flasks as monolayers in NUT Hams (with 1% (v/v) Glutamine) supplemented with 10% (v/v) heat inactivated fetal bovine serum and grown under 1 mg/ml. Geneticin antibiotic selection and 1 mg/ml Zeocin selection.
- Assay Buffer To 1000 mL of Hanks Balanced Salt solution, add 4.8g of HEPES and 0.714g probenecid (which is dissolved in 5 mL 1 M NaOH and added to the solution). This buffer is pH adjusted to 7.4 with NaOH. Assay Buffer contains 10% DMSO (v/v) was prepared for the compound preparation plates. Usually 200ml (containing 20ml neat DMSO) will be sufficient for 12 x 384 plates. Loading Buffer:
- Histamine EC50 determination Cells were harvested using Ix dissociation solution and plated onto poly-D-lysine coated FLIPR plates at 1.OxIO 4 cells per well 18-24 hours prior to experiment. Media was removed from the cells by tipping and the plates gently blotted onto tissue to remove any excess medium. 30 ⁇ L loading buffer was added to all wells for 90 min at 37 0 C.
- 96 well histamine EC50 plate was made and then 40 ⁇ L was indexed into 4 quadrants in a 384 well plate.
- 96 well compound vehicle plates were made and indexed into a quadrant of a 384 well plate. Plates were transferred to FLIPR and run using a standard protocol. The results were used to calculate an EC50 for histamine.
- Assays were performed in 96 deep well plates containing 0.1-10 ⁇ M compounds or 20 ⁇ M histamine; 0.015 mg protein/well H4 membranes and 3.9 nM of [ 3 H] -histamine in a final volume of 200 ⁇ l. Plates were incubated at room temperature for 1.5 hours. The contents of the wells was captured on filters, washed 2x 1 mL with Tris/EDTA wash buffer. The filters were dried for about 2 hrs at 60 0 C and the [ 3 H] determined by scintillation counting.
- ACN acetonitrile
- DCM dichloromethane
- DMR N,N-dimethylformamide
- DMSO dimethyl sulfoxide
- EDC-HCl l-(3-Dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride; HOBT: 1-hydroxybenzotriazole; MeOH: methanol; min: minutes; MS: mass spectrum; NMR: nuclear magnetic resonance; psi: pounds per square inch; RT: room temperature; sat.: saturated; TEA: triethylamine; TFA: trifluoroacetic acid;
- reaction mixture was concentrated under reduced pressure, diluted with EtOAc (50 mL) and the solution was washed with saturated aqueous sodium bicarbonate (2 x 50 mL) and brine (50 mL). The solvent was removed under reduced pressure and the residue was subjected to supercritical fluid chromatography (21 mm x 150 mm diol-bonded SiO 2 (6 ⁇ m particle size), isocratic method, 25% MeOH (containing 0.5% isopropyl amine) in CO 2 ) to afford the title compound as a white solid (0.0744 g, 22%).
- Example 1 may be used to prepare all the compounds described earlier in the present specification.
Landscapes
- Chemical & Material Sciences (AREA)
- Organic Chemistry (AREA)
- Health & Medical Sciences (AREA)
- Medicinal Chemistry (AREA)
- Pharmacology & Pharmacy (AREA)
- Engineering & Computer Science (AREA)
- Bioinformatics & Cheminformatics (AREA)
- Biomedical Technology (AREA)
- Neurology (AREA)
- Neurosurgery (AREA)
- Chemical Kinetics & Catalysis (AREA)
- General Chemical & Material Sciences (AREA)
- Pain & Pain Management (AREA)
- Nuclear Medicine, Radiotherapy & Molecular Imaging (AREA)
- Psychiatry (AREA)
- Life Sciences & Earth Sciences (AREA)
- Animal Behavior & Ethology (AREA)
- General Health & Medical Sciences (AREA)
- Public Health (AREA)
- Veterinary Medicine (AREA)
- Pharmaceuticals Containing Other Organic And Inorganic Compounds (AREA)
- Plural Heterocyclic Compounds (AREA)
- Hydrogenated Pyridines (AREA)
- Nitrogen Condensed Heterocyclic Rings (AREA)
- Acyclic And Carbocyclic Compounds In Medicinal Compositions (AREA)
- Organic Low-Molecular-Weight Compounds And Preparation Thereof (AREA)
Abstract
Description
Claims
Priority Applications (8)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US11/572,967 US20080064706A1 (en) | 2004-08-02 | 2005-07-27 | Piperidine Derivatives as Histamine H3 Receptor Ligands |
| JP2007524768A JP2008508353A (en) | 2004-08-02 | 2005-07-27 | Piperidine derivatives as histamine H3 receptor ligands |
| MX2007001226A MX2007001226A (en) | 2004-08-02 | 2005-07-27 | Piperidine derivatives as histamine h3 receptor ligands. |
| BRPI0514035-8A BRPI0514035A (en) | 2004-08-02 | 2005-07-27 | compound, pharmaceutically acceptable salts thereof, diastereomers, enantiomers or mixtures thereof, use of a compound, pharmaceutical composition, method for the therapy of depression in a warm-blooded animal, and process for preparing a compound |
| EP05761797A EP1781613A1 (en) | 2004-08-02 | 2005-07-27 | Piperidine derivatives as histamine h3 receptor ligands |
| CA002576112A CA2576112A1 (en) | 2004-08-02 | 2005-07-27 | Piperidine derivatives as histamine h3 receptor ligands |
| AU2005267932A AU2005267932A1 (en) | 2004-08-02 | 2005-07-27 | Piperidine derivatives as histamine H3 receptor ligands |
| IL180548A IL180548A0 (en) | 2004-08-02 | 2007-01-04 | Piperidine derivatives as histamine h3 receptor ligands |
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| SE0401971A SE0401971D0 (en) | 2004-08-02 | 2004-08-02 | Piperidne derivatives |
| SE0401971-7 | 2004-08-02 |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| WO2006014136A1 true WO2006014136A1 (en) | 2006-02-09 |
Family
ID=32906883
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| PCT/SE2005/001189 WO2006014136A1 (en) | 2004-08-02 | 2005-07-27 | Piperidine derivatives as histamine h3 receptor ligands |
Country Status (14)
| Country | Link |
|---|---|
| US (1) | US20080064706A1 (en) |
| EP (1) | EP1781613A1 (en) |
| JP (1) | JP2008508353A (en) |
| KR (1) | KR20070043998A (en) |
| CN (1) | CN1993325A (en) |
| AU (1) | AU2005267932A1 (en) |
| BR (1) | BRPI0514035A (en) |
| CA (1) | CA2576112A1 (en) |
| IL (1) | IL180548A0 (en) |
| MX (1) | MX2007001226A (en) |
| RU (1) | RU2007105970A (en) |
| SE (1) | SE0401971D0 (en) |
| WO (1) | WO2006014136A1 (en) |
| ZA (1) | ZA200700683B (en) |
Cited By (36)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2007106525A1 (en) * | 2006-03-13 | 2007-09-20 | The Regents Of The University Of California | Piperidinyl, indolyl, pirinidyl, morpholinyl and benzimidazolyl urea derivatives as inhibitors of soluble epoxide hydrolase for the treatment of hypertension, inflammations and other diseases |
| WO2009063953A1 (en) | 2007-11-13 | 2009-05-22 | Taisho Pharmaceutical Co., Ltd. | Phenylpyrazole derivatives |
| US7662910B2 (en) | 2004-10-20 | 2010-02-16 | The Regents Of The University Of California | Inhibitors for the soluble epoxide hydrolase |
| WO2010068452A1 (en) * | 2008-11-25 | 2010-06-17 | Janssen Pharmaceutica Nv | Heteroaryl-substituted urea modulators of fatty acid amide hydrolase |
| WO2010090347A1 (en) | 2009-02-06 | 2010-08-12 | Taisho Pharmaceutical Co., Ltd. | Dihydroquinolinone derivatives |
| US7851474B2 (en) | 2005-08-02 | 2010-12-14 | Neurogen Corporation | Dipiperazinyl ketones and related analogues |
| US7947709B2 (en) * | 2007-06-22 | 2011-05-24 | Roche Palo Alto Llc | Non-nucleoside reverse transcriptase inhibitors |
| US8455652B2 (en) | 2003-04-03 | 2013-06-04 | The United States Of America As Represented By The Secretary Of The Department Of Health And Human Services | Inhibitors for the soluble epoxide hydrolase |
| US8461159B2 (en) | 2008-11-25 | 2013-06-11 | Jannsen Pharmaceutica BV | Heteroaryl-substituted urea modulators of fatty acid amide hydrolase |
| WO2013085018A1 (en) | 2011-12-08 | 2013-06-13 | 大正製薬株式会社 | Phenylpyrrole derivative |
| WO2013100054A1 (en) | 2011-12-27 | 2013-07-04 | 大正製薬株式会社 | Phenyltriazole derivative |
| US8513302B2 (en) | 2003-04-03 | 2013-08-20 | The Regents Of The University Of California | Reducing nephropathy with inhibitors of soluble epoxide hydrolase and epoxyeicosanoids |
| US8658797B2 (en) | 2011-02-25 | 2014-02-25 | Helsinn Healthcare Sa | Asymmetric ureas and medical uses thereof |
| US8940745B2 (en) | 2010-05-03 | 2015-01-27 | Janssen Pharmaceutica Nv | Modulators of fatty acid amide hydrolase |
| US9034874B2 (en) | 2012-07-20 | 2015-05-19 | Novartis Ag | Carbamate/urea derivatives |
| WO2015134839A1 (en) * | 2014-03-07 | 2015-09-11 | Helsinn Healthcare Sa | P-substituted asymmetric ureas and medical uses thereof |
| US9169224B2 (en) | 2004-12-30 | 2015-10-27 | Janssen Pharmaceutica Nv | Piperazinyl and piperidinyl ureas as modulators of fatty acid amide hydrolase |
| WO2015179414A1 (en) * | 2014-05-19 | 2015-11-26 | Merial, Inc. | Anthelmintic compounds |
| US9216182B2 (en) | 2011-10-08 | 2015-12-22 | Novartis Ag | Carbamate/urea derivatives containing piperidin and piperazin rings as H3 receptor inhibitors |
| US9296693B2 (en) | 2010-01-29 | 2016-03-29 | The Regents Of The University Of California | Acyl piperidine inhibitors of soluble epoxide hydrolase |
| US9351954B2 (en) | 2009-12-04 | 2016-05-31 | Sunovion Pharmaceuticals Inc. | Multicyclic compounds and methods of use thereof |
| WO2017095758A1 (en) * | 2015-12-01 | 2017-06-08 | Merck Sharp & Dohme Corp. | Homobispiperidinyl derivatives as liver x receptor beta agonists, compositions, and their use |
| US9975886B1 (en) | 2017-01-23 | 2018-05-22 | Cadent Therapeutics, Inc. | Potassium channel modulators |
| US10196403B2 (en) | 2016-07-29 | 2019-02-05 | Sunovion Pharmaceuticals Inc. | Compounds and compositions and uses thereof |
| US10501479B2 (en) | 2016-03-22 | 2019-12-10 | Helsinn Healthcare Sa | Benzenesulfonyl-asymmetric ureas and medical uses thereof |
| US10689371B2 (en) | 2018-04-18 | 2020-06-23 | Constellation Pharmaceuticals, Inc. | Modulators of methyl modifying enzymes, compositions and uses thereof |
| US10774064B2 (en) | 2016-06-02 | 2020-09-15 | Cadent Therapeutics, Inc. | Potassium channel modulators |
| US10780074B2 (en) | 2017-08-02 | 2020-09-22 | Sunovion Pharmaceuticals Inc. | Compounds and uses thereof |
| US10815249B2 (en) | 2018-02-16 | 2020-10-27 | Sunovion Pharmaceuticals Inc. | Salts, crystal forms, and production methods thereof |
| ES2819309A1 (en) * | 2019-10-14 | 2021-04-15 | Fundacion Para La Investigacion Biomedica Del Hospital Univ De La Princesa | Nicotinic agonist and antioxidant compounds for the treatment of neurodegenerative diseases (Machine-translation by Google Translate, not legally binding) |
| US11077090B2 (en) | 2016-07-29 | 2021-08-03 | Sunovion Pharmaceuticals Inc. | Compounds and compositions and uses thereof |
| US11129807B2 (en) | 2017-02-16 | 2021-09-28 | Sunovion Pharmaceuticals Inc. | Methods of treating schizophrenia |
| US11136304B2 (en) | 2019-03-14 | 2021-10-05 | Sunovion Pharmaceuticals Inc. | Salts of a heterocyclic compound and crystalline forms, processes for preparing, therapeutic uses, and pharmaceutical compositions thereof |
| US11738002B2 (en) | 2020-04-14 | 2023-08-29 | Sunovion Pharmaceuticals Inc. | Methods of treating neurological and psychiatric disorders |
| US11919912B2 (en) | 2018-05-21 | 2024-03-05 | Constellation Pharmaceuticals, Inc. | Modulators of methyl modifying enzymes, compositions and uses thereof |
| US11993586B2 (en) | 2018-10-22 | 2024-05-28 | Novartis Ag | Crystalline forms of potassium channel modulators |
Families Citing this family (3)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| AU2011261375B2 (en) | 2010-06-04 | 2016-09-22 | Albany Molecular Research, Inc. | Glycine transporter-1 inhibitors, methods of making them, and uses thereof |
| CN111349609A (en) * | 2018-12-21 | 2020-06-30 | 泰州医药城国科化物生物医药科技有限公司 | Cell screening model of unmarked histamine receptor H3 |
| CN113549006B (en) * | 2020-04-26 | 2023-07-21 | 江苏恩华药业股份有限公司 | Amide derivative and application thereof |
Citations (7)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| EP0343307A1 (en) * | 1988-05-26 | 1989-11-29 | Fabrica Espanola De Productos Quimicos Y Farmaceuticos, S.A. | 4-Piperidinealkanamine derivatives |
| WO2003024929A1 (en) * | 2001-09-14 | 2003-03-27 | Novo Nordisk A/S | Substituted piperidines with selective binding to histamine h3-receptor |
| US20030130253A1 (en) * | 2001-09-14 | 2003-07-10 | Dorwald Florencio Zaragoza | Novel aminoazetidine,-pyrrolidine and -piperidine derivatives |
| US20030191112A1 (en) * | 2001-10-12 | 2003-10-09 | Dorwald Florencio Zaragoza | Novel substituted piperidines |
| WO2004037800A1 (en) * | 2002-10-22 | 2004-05-06 | Glaxo Group Limited | Aryloxyalkylamine derivates as h3 receptor ligands |
| WO2004054973A2 (en) * | 2002-12-18 | 2004-07-01 | Novo Nordisk A/S | Piperidine and pyrrolidine derivatives as antagonists of histamine h3 receptor |
| WO2005028438A1 (en) * | 2003-09-22 | 2005-03-31 | Banyu Pharmaceutical Co., Ltd. | Novel piperidine derivative |
Family Cites Families (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| DE19614204A1 (en) * | 1996-04-10 | 1997-10-16 | Thomae Gmbh Dr K | Carboxylic acid derivatives, medicaments containing these compounds, their use and processes for their preparation |
-
2004
- 2004-08-02 SE SE0401971A patent/SE0401971D0/en unknown
-
2005
- 2005-07-27 MX MX2007001226A patent/MX2007001226A/en not_active Application Discontinuation
- 2005-07-27 EP EP05761797A patent/EP1781613A1/en not_active Withdrawn
- 2005-07-27 WO PCT/SE2005/001189 patent/WO2006014136A1/en active Application Filing
- 2005-07-27 BR BRPI0514035-8A patent/BRPI0514035A/en not_active Application Discontinuation
- 2005-07-27 JP JP2007524768A patent/JP2008508353A/en not_active Abandoned
- 2005-07-27 US US11/572,967 patent/US20080064706A1/en not_active Abandoned
- 2005-07-27 AU AU2005267932A patent/AU2005267932A1/en not_active Abandoned
- 2005-07-27 CA CA002576112A patent/CA2576112A1/en not_active Abandoned
- 2005-07-27 CN CNA2005800262732A patent/CN1993325A/en active Pending
- 2005-07-27 RU RU2007105970/04A patent/RU2007105970A/en not_active Application Discontinuation
- 2005-07-27 KR KR1020077002643A patent/KR20070043998A/en not_active Application Discontinuation
-
2007
- 2007-01-04 IL IL180548A patent/IL180548A0/en unknown
- 2007-01-24 ZA ZA200700683A patent/ZA200700683B/en unknown
Patent Citations (7)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| EP0343307A1 (en) * | 1988-05-26 | 1989-11-29 | Fabrica Espanola De Productos Quimicos Y Farmaceuticos, S.A. | 4-Piperidinealkanamine derivatives |
| WO2003024929A1 (en) * | 2001-09-14 | 2003-03-27 | Novo Nordisk A/S | Substituted piperidines with selective binding to histamine h3-receptor |
| US20030130253A1 (en) * | 2001-09-14 | 2003-07-10 | Dorwald Florencio Zaragoza | Novel aminoazetidine,-pyrrolidine and -piperidine derivatives |
| US20030191112A1 (en) * | 2001-10-12 | 2003-10-09 | Dorwald Florencio Zaragoza | Novel substituted piperidines |
| WO2004037800A1 (en) * | 2002-10-22 | 2004-05-06 | Glaxo Group Limited | Aryloxyalkylamine derivates as h3 receptor ligands |
| WO2004054973A2 (en) * | 2002-12-18 | 2004-07-01 | Novo Nordisk A/S | Piperidine and pyrrolidine derivatives as antagonists of histamine h3 receptor |
| WO2005028438A1 (en) * | 2003-09-22 | 2005-03-31 | Banyu Pharmaceutical Co., Ltd. | Novel piperidine derivative |
Cited By (73)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US8513302B2 (en) | 2003-04-03 | 2013-08-20 | The Regents Of The University Of California | Reducing nephropathy with inhibitors of soluble epoxide hydrolase and epoxyeicosanoids |
| US8455652B2 (en) | 2003-04-03 | 2013-06-04 | The United States Of America As Represented By The Secretary Of The Department Of Health And Human Services | Inhibitors for the soluble epoxide hydrolase |
| US8476043B2 (en) | 2004-10-20 | 2013-07-02 | The Regents Of The University Of California | Inhibitors for the soluble epoxide hydrolase |
| US7662910B2 (en) | 2004-10-20 | 2010-02-16 | The Regents Of The University Of California | Inhibitors for the soluble epoxide hydrolase |
| US9169224B2 (en) | 2004-12-30 | 2015-10-27 | Janssen Pharmaceutica Nv | Piperazinyl and piperidinyl ureas as modulators of fatty acid amide hydrolase |
| US7851474B2 (en) | 2005-08-02 | 2010-12-14 | Neurogen Corporation | Dipiperazinyl ketones and related analogues |
| AU2007225170B2 (en) * | 2006-03-13 | 2012-11-01 | The Regents Of The University Of California | Piperidinyl, indolyl, pirinidyl, morpholinyl and benzimidazolyl urea derivatives as inhibitors of soluble epoxide hydrolase for the treatment of hypertension, inflammations and other diseases |
| US8188289B2 (en) | 2006-03-13 | 2012-05-29 | The Regents Of The University Of California | Conformationally restricted urea inhibitors of soluble epoxide hydrolase |
| US8501783B2 (en) | 2006-03-13 | 2013-08-06 | The Regents Of The University Of California | Conformationally restricted urea inhibitors of soluble epoxide hydrolase |
| WO2007106525A1 (en) * | 2006-03-13 | 2007-09-20 | The Regents Of The University Of California | Piperidinyl, indolyl, pirinidyl, morpholinyl and benzimidazolyl urea derivatives as inhibitors of soluble epoxide hydrolase for the treatment of hypertension, inflammations and other diseases |
| US9029550B2 (en) | 2006-03-13 | 2015-05-12 | The Regents Of The University Of California | Conformationally restricted urea inhibitors of soluble epoxide hydrolase |
| JP2009530287A (en) * | 2006-03-13 | 2009-08-27 | ザ リージェンツ オブ ザ ユニバーシティ オブ カリフォルニア | Piperidinyl, indolyl, pyrinidyl, morpholinyl and benzimidazolyl urea derivatives as inhibitors of soluble epoxide hydrolase for the treatment of hypertension, inflammation and other diseases |
| US7947709B2 (en) * | 2007-06-22 | 2011-05-24 | Roche Palo Alto Llc | Non-nucleoside reverse transcriptase inhibitors |
| US7888354B2 (en) | 2007-11-13 | 2011-02-15 | Taisho Pharmaceutical Co., Ltd | Phenylpyrazole derivatives |
| US8183387B2 (en) | 2007-11-13 | 2012-05-22 | Taisho Pharmaceutical Co., Ltd | Phenylpyrazole derivatives |
| US8193176B2 (en) | 2007-11-13 | 2012-06-05 | Taisho Pharmaceutical Co., Ltd | Phenylpyrazole derivatives |
| WO2009063953A1 (en) | 2007-11-13 | 2009-05-22 | Taisho Pharmaceutical Co., Ltd. | Phenylpyrazole derivatives |
| US8598356B2 (en) | 2008-11-25 | 2013-12-03 | Janssen Pharmaceutica Nv | Heteroaryl-substituted urea modulators of fatty acid amide hydrolase |
| US8877769B2 (en) | 2008-11-25 | 2014-11-04 | Janseen Pharmaceutica Nv | Heteroaryl-substituted urea modulators of fatty acid amide hydrolase |
| WO2010068452A1 (en) * | 2008-11-25 | 2010-06-17 | Janssen Pharmaceutica Nv | Heteroaryl-substituted urea modulators of fatty acid amide hydrolase |
| US8461159B2 (en) | 2008-11-25 | 2013-06-11 | Jannsen Pharmaceutica BV | Heteroaryl-substituted urea modulators of fatty acid amide hydrolase |
| WO2010090347A1 (en) | 2009-02-06 | 2010-08-12 | Taisho Pharmaceutical Co., Ltd. | Dihydroquinolinone derivatives |
| US8609847B2 (en) | 2009-02-06 | 2013-12-17 | Taisho Pharmaceutical Co., Ltd | Dihydroquinolinone derivatives |
| US10894033B2 (en) | 2009-12-04 | 2021-01-19 | Sunovion Pharmaceuticals Inc. | Multicyclic compounds and methods of use thereof |
| US10085968B2 (en) | 2009-12-04 | 2018-10-02 | Sunovion Pharmaceuticals Inc. | Multicyclic compounds and methods of use thereof |
| US9351954B2 (en) | 2009-12-04 | 2016-05-31 | Sunovion Pharmaceuticals Inc. | Multicyclic compounds and methods of use thereof |
| US9296693B2 (en) | 2010-01-29 | 2016-03-29 | The Regents Of The University Of California | Acyl piperidine inhibitors of soluble epoxide hydrolase |
| US8940745B2 (en) | 2010-05-03 | 2015-01-27 | Janssen Pharmaceutica Nv | Modulators of fatty acid amide hydrolase |
| US9688664B2 (en) | 2010-05-03 | 2017-06-27 | Janssen Pharmaceutica Nv | Modulators of fatty acid amide hydrolase |
| US9751836B2 (en) | 2011-02-25 | 2017-09-05 | Helsinn Healthcare Sa | Asymmetric ureas and medical uses thereof |
| AU2012220531B2 (en) * | 2011-02-25 | 2017-02-23 | Helsinn Healthcare Sa | Asymmetric ureas and medical uses thereof |
| US10407390B2 (en) | 2011-02-25 | 2019-09-10 | Helsinn Healthcare Sa | Asymmetric ureas and medical uses thereof |
| US8658797B2 (en) | 2011-02-25 | 2014-02-25 | Helsinn Healthcare Sa | Asymmetric ureas and medical uses thereof |
| US9216182B2 (en) | 2011-10-08 | 2015-12-22 | Novartis Ag | Carbamate/urea derivatives containing piperidin and piperazin rings as H3 receptor inhibitors |
| US9284324B2 (en) | 2011-12-08 | 2016-03-15 | Taisho Pharmaceutical Co., Ltd | Phenylpyrrole derivative |
| WO2013085018A1 (en) | 2011-12-08 | 2013-06-13 | 大正製薬株式会社 | Phenylpyrrole derivative |
| WO2013100054A1 (en) | 2011-12-27 | 2013-07-04 | 大正製薬株式会社 | Phenyltriazole derivative |
| US9273026B2 (en) | 2012-07-20 | 2016-03-01 | Novartis Ag | Carbamate/urea derivatives |
| US9624192B2 (en) | 2012-07-20 | 2017-04-18 | Novartis Ag | Carbamate/urea derivatives |
| US9034874B2 (en) | 2012-07-20 | 2015-05-19 | Novartis Ag | Carbamate/urea derivatives |
| KR20160130218A (en) * | 2014-03-07 | 2016-11-10 | 헬신 헬쓰케어 에스.에이. | P-substituted asymmetric ureas and medical uses thereof |
| US10577384B2 (en) | 2014-03-07 | 2020-03-03 | Helsinn Healthcare Sa | Substituted asymmetric ureas as modulators of ghrelin receptor activity |
| US9926337B2 (en) | 2014-03-07 | 2018-03-27 | Helsinn Healthcare Sa | Substituted asymmetric ureas as modulators of ghrelin receptor activity |
| WO2015134839A1 (en) * | 2014-03-07 | 2015-09-11 | Helsinn Healthcare Sa | P-substituted asymmetric ureas and medical uses thereof |
| US9546157B2 (en) | 2014-03-07 | 2017-01-17 | Helsinn Healthcare Sa | Asymmetric piperidinyl-substituted ureas as medicines |
| KR102354790B1 (en) * | 2014-03-07 | 2022-01-21 | 헬신 헬쓰케어 에스.에이. | P-substituted asymmetric ureas and medical uses thereof |
| AU2015227011B2 (en) * | 2014-03-07 | 2019-05-02 | Helsinn Healthcare Sa | P-substituted asymmetric ureas and medical uses thereof |
| EA032847B1 (en) * | 2014-03-07 | 2019-07-31 | Хелсинн Хелскеа Са | P-substituted asymmetric ureas and medical uses thereof |
| WO2015179414A1 (en) * | 2014-05-19 | 2015-11-26 | Merial, Inc. | Anthelmintic compounds |
| WO2017095758A1 (en) * | 2015-12-01 | 2017-06-08 | Merck Sharp & Dohme Corp. | Homobispiperidinyl derivatives as liver x receptor beta agonists, compositions, and their use |
| US10752587B2 (en) | 2015-12-01 | 2020-08-25 | Merck Sharp & Dohme Corp. | Homobispiperidinyl derivatives as liver X receptor beta agonists, compositions and their use |
| US10501479B2 (en) | 2016-03-22 | 2019-12-10 | Helsinn Healthcare Sa | Benzenesulfonyl-asymmetric ureas and medical uses thereof |
| US10774064B2 (en) | 2016-06-02 | 2020-09-15 | Cadent Therapeutics, Inc. | Potassium channel modulators |
| US10927124B2 (en) | 2016-07-29 | 2021-02-23 | Sunovion Pharmaceuticals Inc. | Compounds and compositions and uses thereof |
| US10196403B2 (en) | 2016-07-29 | 2019-02-05 | Sunovion Pharmaceuticals Inc. | Compounds and compositions and uses thereof |
| US11958862B2 (en) | 2016-07-29 | 2024-04-16 | Sumitomo Pharma America, Inc. | Compounds and compositions and uses thereof |
| US11077090B2 (en) | 2016-07-29 | 2021-08-03 | Sunovion Pharmaceuticals Inc. | Compounds and compositions and uses thereof |
| US10717728B2 (en) | 2017-01-23 | 2020-07-21 | Cadent Therapeutics, Inc. | Potassium channel modulators |
| US9975886B1 (en) | 2017-01-23 | 2018-05-22 | Cadent Therapeutics, Inc. | Potassium channel modulators |
| US10351553B2 (en) | 2017-01-23 | 2019-07-16 | Cadent Therapeutics, Inc. | Potassium channel modulators |
| US11129807B2 (en) | 2017-02-16 | 2021-09-28 | Sunovion Pharmaceuticals Inc. | Methods of treating schizophrenia |
| US11491133B2 (en) | 2017-08-02 | 2022-11-08 | Sunovion Pharmaceuticals Inc. | Heteroaryl-isochroman compounds and uses thereof |
| US10780074B2 (en) | 2017-08-02 | 2020-09-22 | Sunovion Pharmaceuticals Inc. | Compounds and uses thereof |
| US10815249B2 (en) | 2018-02-16 | 2020-10-27 | Sunovion Pharmaceuticals Inc. | Salts, crystal forms, and production methods thereof |
| US11440921B2 (en) | 2018-02-16 | 2022-09-13 | Sunovion Pharmaceuticals Inc. | Salts, crystal forms, and production methods thereof |
| US11987591B2 (en) | 2018-02-16 | 2024-05-21 | Sumitomo Pharma America, Inc. | Salts, crystal forms, and production methods thereof |
| US11274095B2 (en) | 2018-04-18 | 2022-03-15 | Constellation Pharmaceuticals, Inc. | Modulators of methyl modifying enzymes, compositions and uses thereof |
| US10689371B2 (en) | 2018-04-18 | 2020-06-23 | Constellation Pharmaceuticals, Inc. | Modulators of methyl modifying enzymes, compositions and uses thereof |
| US11919912B2 (en) | 2018-05-21 | 2024-03-05 | Constellation Pharmaceuticals, Inc. | Modulators of methyl modifying enzymes, compositions and uses thereof |
| US11993586B2 (en) | 2018-10-22 | 2024-05-28 | Novartis Ag | Crystalline forms of potassium channel modulators |
| US11136304B2 (en) | 2019-03-14 | 2021-10-05 | Sunovion Pharmaceuticals Inc. | Salts of a heterocyclic compound and crystalline forms, processes for preparing, therapeutic uses, and pharmaceutical compositions thereof |
| ES2819309A1 (en) * | 2019-10-14 | 2021-04-15 | Fundacion Para La Investigacion Biomedica Del Hospital Univ De La Princesa | Nicotinic agonist and antioxidant compounds for the treatment of neurodegenerative diseases (Machine-translation by Google Translate, not legally binding) |
| US11738002B2 (en) | 2020-04-14 | 2023-08-29 | Sunovion Pharmaceuticals Inc. | Methods of treating neurological and psychiatric disorders |
Also Published As
| Publication number | Publication date |
|---|---|
| ZA200700683B (en) | 2008-08-27 |
| AU2005267932A1 (en) | 2006-02-09 |
| RU2007105970A (en) | 2008-09-10 |
| BRPI0514035A (en) | 2008-05-27 |
| US20080064706A1 (en) | 2008-03-13 |
| KR20070043998A (en) | 2007-04-26 |
| EP1781613A1 (en) | 2007-05-09 |
| CA2576112A1 (en) | 2006-02-09 |
| CN1993325A (en) | 2007-07-04 |
| SE0401971D0 (en) | 2004-08-02 |
| MX2007001226A (en) | 2007-03-23 |
| IL180548A0 (en) | 2007-06-03 |
| JP2008508353A (en) | 2008-03-21 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| EP1781613A1 (en) | Piperidine derivatives as histamine h3 receptor ligands | |
| WO2006014135A1 (en) | Novel piperidine derivatives as histamine h3 receptor ligands for treatment of depression | |
| WO2006014134A1 (en) | Novel piperidine derivative for the treatment of depression | |
| US7384955B2 (en) | Azaindole derivatives, preparations thereof, uses thereof and compositions containing them | |
| JP2008519833A (en) | Indazolesulfonamide derivatives | |
| ZA200503556B (en) | 4(phenyl-piperazinyl-methyl) benzamide derivatves and their use for the treatment of pain or gastrointestinal disorders | |
| ZA200503553B (en) | 4(Pheny-piperazinyl-methyl) benzamide derivatives and their use for the treatment of pain or gastrointestinal disorders | |
| ZA200505186B (en) | Diarylmethylidene piperidine derivatives, preperations thereof and uses thereof | |
| JP2006527249A (en) | Benzimidazole derivatives, compositions containing them, their production and their use | |
| US20070105893A1 (en) | Novel Compounds | |
| ZA200605442B (en) | Diarylmethyl piperazine derivatives, preparations thereof and uses thereof | |
| AU2004245296A1 (en) | Benzimidazole derivatives, compositions containing them, preparation thereof and uses thereof | |
| ZA200505189B (en) | Diarylmethylidene piperidine derivatives, preperations thereof and uses thereof | |
| EP1458684B1 (en) | Therapeutic heterocycles as bradykinin b2 receptor antagonists | |
| US20070265325A1 (en) | Nitro Indazole Derivatives | |
| US7244850B2 (en) | Benzimidazole derivatives, compositions containing them, preparation thereof and uses thereof |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| AK | Designated states |
Kind code of ref document: A1 Designated state(s): AE AG AL AM AT AU AZ BA BB BG BR BW BY BZ CA CH CN CO CR CU CZ DE DK DM DZ EC EE EG ES FI GB GD GE GH GM HR HU ID IL IN IS JP KE KG KM KP KR KZ LC LK LR LS LT LU LV MA MD MG MK MN MW MX MZ NA NG NI NO NZ OM PG PH PL PT RO RU SC SD SE SG SK SL SM SY TJ TM TN TR TT TZ UA UG US UZ VC VN YU ZA ZM ZW |
|
| AL | Designated countries for regional patents |
Kind code of ref document: A1 Designated state(s): BW GH GM KE LS MW MZ NA SD SL SZ TZ UG ZM ZW AM AZ BY KG KZ MD RU TJ TM AT BE BG CH CY CZ DE DK EE ES FI FR GB GR HU IE IS IT LT LU LV MC NL PL PT RO SE SI SK TR BF BJ CF CG CI CM GA GN GQ GW ML MR NE SN TD TG |
|
| 121 | Ep: the epo has been informed by wipo that ep was designated in this application | ||
| WWE | Wipo information: entry into national phase |
Ref document number: 552402 Country of ref document: NZ |
|
| WWE | Wipo information: entry into national phase |
Ref document number: 180548 Country of ref document: IL |
|
| WWE | Wipo information: entry into national phase |
Ref document number: 225/DELNP/2007 Country of ref document: IN |
|
| WWE | Wipo information: entry into national phase |
Ref document number: 2005267932 Country of ref document: AU |
|
| WWE | Wipo information: entry into national phase |
Ref document number: 200700683 Country of ref document: ZA |
|
| WWE | Wipo information: entry into national phase |
Ref document number: 2005761797 Country of ref document: EP |
|
| WWE | Wipo information: entry into national phase |
Ref document number: 07008181 Country of ref document: CO |
|
| WWE | Wipo information: entry into national phase |
Ref document number: MX/a/2007/001226 Country of ref document: MX Ref document number: 11572967 Country of ref document: US |
|
| WWE | Wipo information: entry into national phase |
Ref document number: 2007524768 Country of ref document: JP Ref document number: 12007500286 Country of ref document: PH Ref document number: 1020077002643 Country of ref document: KR Ref document number: 2576112 Country of ref document: CA |
|
| WWE | Wipo information: entry into national phase |
Ref document number: 200580026273.2 Country of ref document: CN |
|
| NENP | Non-entry into the national phase |
Ref country code: DE |
|
| ENP | Entry into the national phase |
Ref document number: 2005267932 Country of ref document: AU Date of ref document: 20050727 Kind code of ref document: A |
|
| WWP | Wipo information: published in national office |
Ref document number: 2005267932 Country of ref document: AU |
|
| WWE | Wipo information: entry into national phase |
Ref document number: 2007105970 Country of ref document: RU |
|
| WWP | Wipo information: published in national office |
Ref document number: 2005761797 Country of ref document: EP |
|
| ENP | Entry into the national phase |
Ref document number: PI0514035 Country of ref document: BR |