WO2006001046A1 - Hydrogels of hyaluronic acid and alpha, beta-polyaspartylhydrazide and their biomedical and pharmaceutical uses - Google Patents
Hydrogels of hyaluronic acid and alpha, beta-polyaspartylhydrazide and their biomedical and pharmaceutical uses Download PDFInfo
- Publication number
- WO2006001046A1 WO2006001046A1 PCT/IT2005/000364 IT2005000364W WO2006001046A1 WO 2006001046 A1 WO2006001046 A1 WO 2006001046A1 IT 2005000364 W IT2005000364 W IT 2005000364W WO 2006001046 A1 WO2006001046 A1 WO 2006001046A1
- Authority
- WO
- WIPO (PCT)
- Prior art keywords
- hyaluronic acid
- hydrogel
- polymer
- beta
- alpha
- Prior art date
Links
Classifications
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K47/00—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient
- A61K47/30—Macromolecular organic or inorganic compounds, e.g. inorganic polyphosphates
- A61K47/36—Polysaccharides; Derivatives thereof, e.g. gums, starch, alginate, dextrin, hyaluronic acid, chitosan, inulin, agar or pectin
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61L—METHODS OR APPARATUS FOR STERILISING MATERIALS OR OBJECTS IN GENERAL; DISINFECTION, STERILISATION OR DEODORISATION OF AIR; CHEMICAL ASPECTS OF BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES; MATERIALS FOR BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES
- A61L27/00—Materials for grafts or prostheses or for coating grafts or prostheses
- A61L27/14—Macromolecular materials
- A61L27/20—Polysaccharides
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K47/00—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient
- A61K47/50—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient the non-active ingredient being chemically bound to the active ingredient, e.g. polymer-drug conjugates
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K9/00—Medicinal preparations characterised by special physical form
- A61K9/70—Web, sheet or filament bases ; Films; Fibres of the matrix type containing drug
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B82—NANOTECHNOLOGY
- B82Y—SPECIFIC USES OR APPLICATIONS OF NANOSTRUCTURES; MEASUREMENT OR ANALYSIS OF NANOSTRUCTURES; MANUFACTURE OR TREATMENT OF NANOSTRUCTURES
- B82Y5/00—Nanobiotechnology or nanomedicine, e.g. protein engineering or drug delivery
Definitions
- the present invention relates to new hydrogels of hyaluronic acid and alpha, beta-polyaspartylhydrazide and their applications in the biomedical and pharmaceutical field.
- this invention concerns products and com- positions based on chemical crosslinking of hyaluronic acid with a multifunc ⁇ tional biocompatible polymer with a protein-like structure bearing hydrazido pendent groups along the polymeric chain. Following this crosslinking it is possible to obtain materials characterized by a strong resistance to chemical and enzymatic degradation, unlike the starting hyaluronic acid, that can be utilized to prepare systems for biomedical and pharmaceutical applications.
- hydrogels consist of natural polymers or their deriva ⁇ tives, synthetic polymers or combinations of natural and synthetic polymers, whose molecules, interacting by electrostatic forces or chemical linkages, form hydrophilic crosslinked polymers, able to take up water in an amount ranging from 10-20% to several hundreds of times their dry weight. Due to their hydro ⁇ philic properties, together with their potential biocompatibility, hydrogels attract an increasing interest in the pharmaceutical and biomedical field. In particular, hydrogels are ideal candidates in the preparation of tis ⁇ sue engineering matrices, with the aim to heal or reconstruct ex novo dam- aged, diseased or deteriorated human tissues or organs.
- Tissue engineering is actually a new science dealing with the development of technologies useful to obtain a regeneration of human damaged tissues or their complete repro ⁇ duction.
- tissue cells e.g. fibroblasts for cutaneous tissue, chondrocites for cartilaginous tissue, osteo- blasts for bone tissue, etc.
- ECM extracellular matrix
- hydrogels are par ⁇ ticularly suitable to constitute similar matrices for tissue engineering, consid- ering their several advantages, for an example, compared to such hydropho ⁇ bic structures.
- these advantages include the ability of hydrogels to allow a good fluxing of nutrients to cells and refluxing of products outside the cells, their usual biocompatibility and progressive bio-reabsorption and their ability to easily incorporate peptide ligands for cellular adhesion by co- valent or physical linkages, in order to stimulate adhesion, proliferation and growing of the cells inside the hydrogel matrix.
- hydrogels different, for example, from hydrophobic polymers applied for the same purpose, such as PLGA (polylactic-co-glycolic acid).
- hydrogels can suffer the disadvantage of a low mechanical resistance that can reduce handling, or they may be also difficult sterilize.
- the tissue engineering applications include the opportunity to use biodegradable sponges or biodegradable films for articular cartilage regenera ⁇ tion, or to protect and support healing of wounds caused by trauma (i.e. burns) or diseases (diabetes, AIDS). In this case the application on wounds can sup- port a faster regenerative activity of fibroblasts that, adhering on the scaffold, will synthesize more rapidly new ECM and heal the wound.
- the scaffold can be at first utilized in vitro to create a real artificial derma that can be subsequently used to cover the wound and to perform its function. Similar skin substitutes perform a temporary cover able to reduce the exu- dates loss from the wound and the infection risk.
- the scaffold when oppor ⁇ tunely loaded with a drug, can also perform a drug delivery function, support ⁇ ing as an example the prolonged release of antibiotics or growth factors, de ⁇ pending on type of wound.
- skin substitutes in particu- lar a cellular bilayer obtained by growing of fibroblasts and keratinocites on collagen scaffolds, marketed with the trade mark ApligraftTM ).
- hydrogels having great interest for its potentialities, is the use in prevention of post-surgical adhesion.
- the post-surgical adhesions consist in the formation of fibrous sutures between two opposite tissue surfaces, resulting from the trauma that tissues suffer during surgical activities.
- These post-surgical adhesions are a promi- nent problem not only in cardiovascular surgery but also in gastrointestinal and gynaecological surgery, where they can produce intestinal obstruction, infertility and pelvic pain.
- the most used method to prevent this bothersome problem is the positioning of biocompatible materials as physical barriers between tissues in touch, suitable to favour their complete separation and able to remain in place during the whole critical post-surgical period.
- the barrier realized using expanded polytetrafluoroethylene (PrecludeTM, W. L. Gore, Flagstaff, AZ), proposed as pericardial substitute, has a good clinical efficacy but is not completely bioreabsorbable, and therefore a second surgical operation is necessary for its removal.
- Regenerated cellulose-based barriers (IntercedeTM, Johnson & Johnson Medical Inc., Arlington, TX), are also used but they have shown a good efficiency only if used avoiding blood contact.
- Seprafilm ® Genzyme, Cambridge, MA
- hyaluronic acid a linear poly ⁇ saccharide with a high molecular weight composed of alternating units of D- glucuronic acid (GIcUA) and N-acetyl-D-glucosamine (GIcNAc), whose chemical structure can be represented by the following formula:
- Hyaluronic acid is extensively diffused in animal tissues, being a fundamental component of the extracellular matrix, where it acts regulating the cellular proliferation and differentiation. It takes part in several important bio- logical processes, such as cellular mobility and tissue healing; it regulates the inflammatory response and acts as a "scavenger" of free radicals.
- HA is involved in tumour growth by interacting with specific receptors placed on the cell surface: this may explain the recent interest that this polymer has caused for a possible application as a soluble carrier in the production of new macromolecular prodrugs with an antitumoral activity.
- hyaluronic acid is applied in viscosupplementation - both as pharmaceutical agent and as surgical aid - in the ophthalmic field, and it is widely tested in viscosupplementation to relieve articular pains caused by osteoarthritis of different nature, as a lubricating agent administered by intra- articular injections, it is applied as a "drug delivery system", i.e. as a carrier for the prolonged or controlled release of drugs, and not least, as a cosmetic agent.
- HA is reported to facilitate, by injection, the nerves regeneration, and when it is placed on wounds it facilitates tissue regeneration.
- its characteristics of fast cutaneous permeability and epidermic retention can extend the half-life of drugs administered with it, for example in pharmaceutical devices applied as transdermal administration.
- biomaterials based on hyaluronic acid due to its biocompatibility property, are highly suitable as support materi ⁇ als for tissue engineering, useful to facilitate cellular growth processes both in vivo and in vitro, as well as barriers in the prevention of post-surgical adhe ⁇ sions.
- HA re ⁇ sults in scaffolds not very elastic and brittle.
- said scaffolds are pro ⁇ vided with surfaces too hydrophilic to favour adhesion and cellular differentia ⁇ tion.
- biocompatible polymers as collagen or gelatine, or with synthetic polymers such as polylisine, or chemically modifying HA with hydrophobic groups.
- the chemical modification of the polysaccharide molecule of HA by introducing pendent functional groups has as a further target, i.e.
- hyaluronic acid used as here considered is its low residence time in vivo, due to its fast chemical and enzymatic degra ⁇ dation. In fact, it is degraded by hyaluronidases (HAase), ubiquitous enzymes distributed in human cells and serum, as well as it undergoes a chemical hy ⁇ drolysis even in the absence of enzymatic activity.
- HAase hyaluronidases
- the studied materials must have the capability to entrap water and swell in contact with an aqueous medium.
- a suitable crosslinking agent having a polyaminoacid structure, that is substan ⁇ tially linear and with a protein-like structure, whose biocompatible characteris ⁇ tics have been already ascertained.
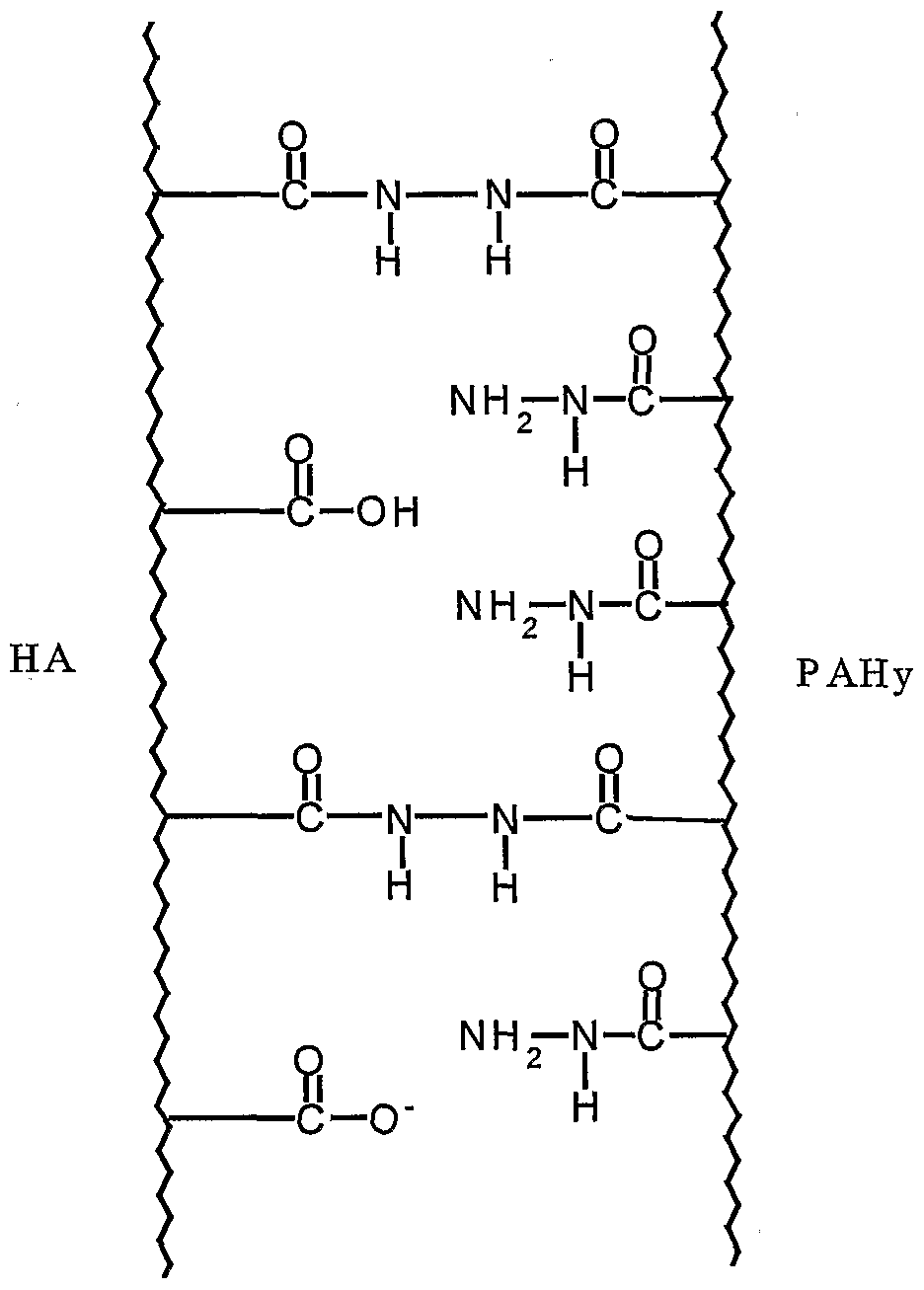
- the crosslinking agent pro- posed according to this invention has a polyhydrazide structure since it shows, for each repeating unit, a pendent hydrazido group (-CO-NH-NH 2 ), poten ⁇ tially available to covalently link the carboxy group of the repeating disaccha- ride unit of hyaluronic acid.
- a pendent hydrazido group (-CO-NH-NH 2 )
- poten ⁇ tially available to covalently link the carboxy group of the repeating disaccha- ride unit of hyaluronic acid The chemical modification of hyaluronic acid by functionalization with bis-hydrazido groups has already been described, for example in the US pat ⁇ ents US 5616568 and US 5652347, both to Pouyani et al. (assignee The Re ⁇ search Foundation of State University of New York) and in the corresponding scientific article (T. Pouyani, G. D.
- the document describes the production of materials having characteristics and structures different from those considered in this invention, firstly because it does not obtain the HA crosslinking by chemical bond with another linear polymer having a different nature, but by using mul ⁇ tifunctional reagents with relatively small molecular size.
- the present invention proposes to employ as crosslinking agent for HA a polydrazide polymer having a polypeptide back ⁇ bone, where each repeating unit contains one hydrazido pendent group.
- the preferred polymer of the type described is alpha,beta-poly- aspartylhydrazide (PAHy), a water-soluble and biocompatible polymeric mate- rial, already synthesized and studied by the research group proposing this invention (G. Giammona, B. Carlisi, G. Cavallaro, G. Pitarresi, S. Spampi- nato, A new water-soluble synthetic polymer, ⁇ , ⁇ -polyasparthydrazide, J. Control. ReI., 1994, 29, 63-72). This material has been obtained, as reported in the mentioned literature, by aminolysis of a high molecular weight polysuc- cinimide with hydrazine.
- PAHy alpha,beta-poly- aspartylhydrazide
- polysuccinimide (PSI) has been obtained by polycondensation of D,L-aspartic acid, and it has been reacted subsequently with hydrazine ( 2 HN-NH 2 ), to obtain a polymer represented by the following chemical formula:
- the repeating unit (the above formula shows five repeating units) can have a structure slightly different in the first or in the second case, but its molecular weight is the same.
- the synthesis and characterization of PAHy are reported as well as the proposal to use this protein-like polymer as a plasma substitute. For this aim, toxicity studies, immunogenic ability and platelet aggregation tests have been reported and they have demonstrated the total biocompatibility of this polyhydrazido polymer.
- the present invention specifically provides a composition comprising hyaluronic acid chemically crosslinked with a polyhydrazide poly ⁇ mer, wherein one or more carboxy groups of the disaccharide units of hyalu ⁇ ronic acid are chemically linked respectively to one or more hydrazido groups of the polyhydrazide polymer.
- said polyhy ⁇ drazide polymer is alpha, beta-polyaspartylhydrazide (PAHy), that has been exhaustively investigated as concerns its water-solubility, biocompatibility and non-immunogenic properties.
- hyaluronic acid has a molecular weight from 50,000 to 1 ,500,000 dalton
- PAHy when the polyhydrazide polymer is PAHy, this has a molecular weight from 2,000 to 40,000 dalton.
- this invention provides a hydrogel composed of hyaluronic acid chemically crosslinked with a polyhydrazide polymer, as previously defined.
- the polyhy- drazido polymer is alpha, beta-polyaspartylhydrazide and, preferably, the hya ⁇ luronic acid employed for the hydrogel production has a molecular weight from 50,000 to 1 ,500,000 dalton, and the alpha, beta-polyaspartylhydrazide has a molecular weight from 2,000 to 40,000 dalton, the most preferred range being from 10,000 to 30,000 dalton.
- the ratio between moles of repeating unit of alpha, beta-polyaspartylhydrazide and moles of repeating unit of hyaluronic acid is from 0.01 to 5, the most preferred range being from 0,5 to 3.
- the preferred activating product is N-ethyl-N'-(3-dimethylaminopropyl)-carbodiimide (EDC), but other simi- laragents could be employed in its place, such as, for instance, N,N'-dicyclo- exylcarbodiimide, cyclohexyl- ⁇ -(N-methylmorpholine)ethyl carbodiimide p- toluensulphonate (CMC) or N-allyl-N'( ⁇ -hydroxyethyl)carbodiimide.
- EDC N-ethyl-N'-(3-dimethylaminopropyl)-carbodiimide
- CMC p- toluensulphonate
- N-allyl-N'( ⁇ -hydroxyethyl)carbodiimide N-allyl-N'( ⁇ -hydroxyethyl)carbodiimide.
- the ratio between moles of N-ethyl-N'-(3- dimethylaminopropyl)-carbodiimide and moles of repeating unit of hyaluronic acid is from 0.01 to 10.
- another activating agent such as a N-hydroxysuccin- imide (NHS), specifically N-hydroxysulfosuccinimide (NHSS).
- NHSS is preferably present in the same molar amount as the said N-etil-N'-3- (3-dimethtylaminopropyl)-carbodiimide.
- the invention provides a manufacturing process for producing a hydrogel composed of hyaluronic acid chemically crosslinked with a polyhydrazide polymer, wherein the hyaluronic acid, obtained from animal or vegetable sources or by biotechnological proc ⁇ esses and having the molecular weight mentioned above, and the polyhy ⁇ drazide polymer, preferably PAHy, with the molecular weight above reported, are dissolved in double-distilled water in a prefixed molar ratio and a fixed amount of carbodiimide, preferably EDC, is added to them.
- the reaction mix ⁇ ture is kept from 0°C to 6O 0 C for a time ranging from 1 hour to 5 days, and subsequently the product is recovered as a hydrogel.
- the pH is maintained in the range between 3 and 8, in particular by using a 0.1 N HCI solution or a solution of bis (2-hydroxyethyl) aminotris (hy- droxymethyl)metane hydrochloride with a concentration ranging from 1 to 500 mM.
- the process according to this invention can include the addition, besides the said carbodiimide, of a prefixed amount of a N-hydroxysuccinimide, preferably NHSS, as a further agent acti- vating the reaction.
- each product is purified by several washings with double-distilled water and then dried by lyophilization (to obtain nanopar- ticles, microparticles or sponges) or dried for some days at 25 0 C at a pressure of 1 atm, inside suitable moulds, to obtain films, membranes or rods.
- the systems prepared have a wide applicative versatility in the biomedical and pharmaceutical field, and are suitable, as an example, to heal wounds, to prevent post- surgical adhesion, to lubricate joints, to allow the in vitro and in vivo cell growth, to realize drug delivery systems.
- both the HA and the polyhydrazide polymer solutions could be singly sterilized.
- the gelation time that is always greater than 10 minutes, could allow to apply the gel forming solution in situ so as to obtain, after a few minutes, the formation of a gel directly on the tissue.
- PEG polyethylenegly- col
- the present invention further specifically provides a kit for the in situ production of a hydrogel composed of hyaluronic acid chemically crosslinked with a polyhydrazide polymer, preferably alpha, beta-polyaspartyl- hydrazide, comprising a container with a first hyaluronic acid-based compo ⁇ nent and a container with a second polyhydrazide polymer-based component, being said two components able to form the hydrogel after their mutual con ⁇ tact, directly on the application site.
- a kit for the in situ production of a hydrogel composed of hyaluronic acid chemically crosslinked with a polyhydrazide polymer, preferably alpha, beta-polyaspartyl- hydrazide comprising a container with a first hyaluronic acid-based compo ⁇ nent and a container with a second polyhydrazide polymer-based component, being said two components able to form the hydrogel after their mutual con ⁇ tact, directly on the
- Each product obtained according to this invention has been charac ⁇ terized by spectophotometric techniques and swelling measurements in dou ⁇ ble- distilled water and in media that simulate some biological fluids (extracel ⁇ lular fluid, gastric fluid, intestinal fluid, synovial fluid, aqueous humour or vitre ⁇ ous humour) in a temperature range from 20°C to 40 0 C.
- the swelling values as reported below, have shown a high affinity of the hydrogels prepared ac ⁇ cording to this invention towards an aqueous medium. The extent of this affin ⁇ ity resulted to be dependent on the crosslinking degree, and on the composi ⁇ tion and pH of the swelling medium (investigated pH range from 1 to 9).
- Each product of this invention has been also subjected to chemical hydrolysis studies at 37°C, by using media with various saline composition and pH values mimicking some biological fluids (range of investigated pH from 1 to 8) with incubation times from 1 to 30 days.
- the obtained results, partially reported below, have demonstrated that the proposed products are resistant to chemical hydrolysis as a function of the hydrolysis medium (composition and pH), of the incubation time and the crosslinking degree of the hydrogel.
- the products according to this invention have been subjected to enzymatic hydrolysis studies by using aqueous solutions containing HAase at various concentrations (ranging from 1 to 1000 U/ml), at 37°C and for incu ⁇ bation times ranging from 30 minutes to 30 days.
- Figure 1 shows the swelling behaviour, in aqueous solution with cit ⁇ rate buffer pH 6.3, of a series of sponges based on HA-PAHy hydrogels ac ⁇ cording to the invention
- Figure 2 shows the swelling behaviour, in aqueous solution with DuI- becco phosphate buffer solution (DPBS) pH 7.4, of a series of sponges similar to those of Figure 1
- Figure 3 shows the results of degradation studies, in the absence of HAase, of a series of sponges based on HA-PAHy hydrogels according to the invention
- Figure 4 shows the results of degradation studies by enzymatic hy ⁇ drolysis, of a similar series of sponges, wherein the concentration of the em ⁇ ployed HAase enzyme is 75U/ml
- Figure 5 shows the results of degradation studies by enzymatic hy- drolysis of a similar series of sponges, wherein the concentration of the em ⁇
- EXAMPLE 1 An aqueous solution containing hyaluronic acid (0.5 % w/v) has been added to an amount of alpha.beta-polyaspartylhydrazide (PAHy) such as to have a ratio between the moles of repeating unit of PAHy and the moles of repeating unit of hyaluronic acid (ratio indicated as "X") equal to 2.
- PAHy alpha.beta-polyaspartylhydrazide
- N-ethyl- N'-(3-dimethylaminopropyl)-carbodiimide alone was employed, in an amount such as to have a ratio between moles of EDC and moles of repeating unit of hyaluronic acid (ratio indicated as "Y") equal to 1.8.
- the reaction mixture was kept at 37°C for 4 hours.
- the pH value was maintained at a constant value of 4.75 by using a solu ⁇ tion of bis (2-hydroxyethyl)aminotris(hydroxymethyl)metane hydrochloride with a concentration of 30OmM.
- the tests are also assembled in series as a function of the molar ratios X and Y employed in the hydrogel preparation.
Landscapes
- Health & Medical Sciences (AREA)
- Chemical & Material Sciences (AREA)
- Public Health (AREA)
- Medicinal Chemistry (AREA)
- Veterinary Medicine (AREA)
- Epidemiology (AREA)
- Life Sciences & Earth Sciences (AREA)
- Animal Behavior & Ethology (AREA)
- General Health & Medical Sciences (AREA)
- Pharmacology & Pharmacy (AREA)
- Transplantation (AREA)
- Oral & Maxillofacial Surgery (AREA)
- Engineering & Computer Science (AREA)
- Bioinformatics & Cheminformatics (AREA)
- Dermatology (AREA)
- Inorganic Chemistry (AREA)
- Polysaccharides And Polysaccharide Derivatives (AREA)
- Materials For Medical Uses (AREA)
- Medicinal Preparation (AREA)
- Pharmaceuticals Containing Other Organic And Inorganic Compounds (AREA)
- Thermotherapy And Cooling Therapy Devices (AREA)
Abstract
Description
Claims
Priority Applications (7)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US11/630,985 US20080292664A1 (en) | 2004-06-28 | 2005-06-23 | Hydrogels and Hyaluronic Acid and Alpha, Beta-Polyaspartyl-Hydrazide and Their Biomedical and Pharmaceutical Uses |
| JP2007518813A JP2008504100A (en) | 2004-06-28 | 2005-06-23 | Hydrogels of hyaluronic acid and alpha, beta-polyaspartic acid hydrazide and their biomedical and pharmaceutical uses |
| DE602005005062T DE602005005062T2 (en) | 2004-06-28 | 2005-06-23 | HYALURONIC ACID AND ALPHA, BETA-POLYASPARTYLHYDRAZIDHYDROGELE AND THEIR BIOMEDICAL AND PHARMACEUTICAL USES |
| CA2572127A CA2572127C (en) | 2004-06-28 | 2005-06-23 | Hydrogels of hyaluronic acid and alpha, beta-polyaspartylhydrazide and their biomedical and pharmaceutical uses |
| AU2005257078A AU2005257078B2 (en) | 2004-06-28 | 2005-06-23 | Hydrogels of hyaluronic acid and alpha, beta-polyaspartylhydrazide and their biomedical and pharmaceutical uses |
| DK05760579T DK1773399T3 (en) | 2004-06-28 | 2005-06-23 | Hydrogels of hyaluronic acid and alpha, beta-polyaspartyl hydrazide and their biomedical and pharmaceutical applications |
| EP05760579A EP1773399B1 (en) | 2004-06-28 | 2005-06-23 | Hydrogels of hyaluronic acid and alpha, beta-polyaspartylhydrazide and their biomedical and pharmaceutical uses |
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| IT000318A ITRM20040318A1 (en) | 2004-06-28 | 2004-06-28 | HYALURONIC AND ALPHA HYDROGEL, BETA-POLYASPARTYL HYDRAZIDE AND THEIR BIOMEDICAL AND PHARMACEUTICAL APPLICATIONS. |
| ITRM2004A000318 | 2004-06-28 |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| WO2006001046A1 true WO2006001046A1 (en) | 2006-01-05 |
Family
ID=34981530
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| PCT/IT2005/000364 WO2006001046A1 (en) | 2004-06-28 | 2005-06-23 | Hydrogels of hyaluronic acid and alpha, beta-polyaspartylhydrazide and their biomedical and pharmaceutical uses |
Country Status (13)
| Country | Link |
|---|---|
| US (1) | US20080292664A1 (en) |
| EP (1) | EP1773399B1 (en) |
| JP (1) | JP2008504100A (en) |
| KR (1) | KR20070043724A (en) |
| AT (1) | ATE387218T1 (en) |
| AU (1) | AU2005257078B2 (en) |
| CA (1) | CA2572127C (en) |
| DE (1) | DE602005005062T2 (en) |
| DK (1) | DK1773399T3 (en) |
| ES (1) | ES2303258T3 (en) |
| IT (1) | ITRM20040318A1 (en) |
| PT (1) | PT1773399E (en) |
| WO (1) | WO2006001046A1 (en) |
Cited By (5)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2007102149A2 (en) | 2006-03-07 | 2007-09-13 | Prochon Biotech Ltd. | Hydrazido derivatives of hyaluronic acid |
| WO2010051783A1 (en) | 2008-11-06 | 2010-05-14 | Cpn S.R.O. | Method of preparation of dtpa crosslinked hyaluronic acid derivatives and modification of said derivatives |
| US8790701B2 (en) | 2008-04-28 | 2014-07-29 | Surmodics, Inc. | Poly-α(1→4)glucopyranose-based matrices with hydrazide crosslinking |
| US9701940B2 (en) | 2005-09-19 | 2017-07-11 | Histogenics Corporation | Cell-support matrix having narrowly defined uniformly vertically and non-randomly organized porosity and pore density and a method for preparation thereof |
| US10077420B2 (en) | 2014-12-02 | 2018-09-18 | Histogenics Corporation | Cell and tissue culture container |
Families Citing this family (10)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2009108760A2 (en) | 2008-02-26 | 2009-09-03 | Board Of Regents, The University Of Texas System | Dendritic macroporous hydrogels prepared by crystal templating |
| EP2213315A1 (en) | 2009-01-30 | 2010-08-04 | Mero S.r.L. | Antibacterial hydrogel and use thereof in orthopedics |
| IT1401498B1 (en) | 2010-07-30 | 2013-07-26 | Mero Srl | HYDROGEL BASED ON HYALURONIC ACID AND ITS USE IN ORTHOPEDICS |
| US9095558B2 (en) | 2010-10-08 | 2015-08-04 | Board Of Regents, The University Of Texas System | Anti-adhesive barrier membrane using alginate and hyaluronic acid for biomedical applications |
| WO2012048283A1 (en) | 2010-10-08 | 2012-04-12 | Board Of Regents, The University Of Texas System | One-step processing of hydrogels for mechanically robust and chemically desired features |
| US11565027B2 (en) | 2012-12-11 | 2023-01-31 | Board Of Regents, The University Of Texas System | Hydrogel membrane for adhesion prevention |
| US20180291123A1 (en) * | 2015-09-04 | 2018-10-11 | The Broad Of Regents Of The University Of Texas System | Di-functionalized hyaluronic acid derivatives and hydrogels thereof |
| KR101872222B1 (en) * | 2016-08-02 | 2018-06-28 | 한림대학교 산학협력단 | A membrane spacer and membrane spacer-antiadhesive agent mixture |
| WO2018165327A1 (en) | 2017-03-08 | 2018-09-13 | Alafair Biosciences, Inc. | Hydrogel medium for the storage and preservation of tissue |
| CN115957373A (en) * | 2022-12-29 | 2023-04-14 | 陕西科技大学 | Autolysis degradable injectable polyamino acid hydrogel and preparation method thereof |
Citations (2)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US5616568A (en) * | 1993-11-30 | 1997-04-01 | The Research Foundation Of State University Of New York | Functionalized derivatives of hyaluronic acid |
| WO2002041877A1 (en) * | 2000-10-24 | 2002-05-30 | Clear Solutions Biotech, Inc. | Sodium hyaluronate microspheres |
Family Cites Families (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2005056608A1 (en) * | 2003-12-04 | 2005-06-23 | University Of Utah Research Foundation | Modified macromolecules and methods of making and using thereof |
-
2004
- 2004-06-28 IT IT000318A patent/ITRM20040318A1/en unknown
-
2005
- 2005-06-23 ES ES05760579T patent/ES2303258T3/en active Active
- 2005-06-23 CA CA2572127A patent/CA2572127C/en not_active Expired - Fee Related
- 2005-06-23 AU AU2005257078A patent/AU2005257078B2/en not_active Ceased
- 2005-06-23 PT PT05760579T patent/PT1773399E/en unknown
- 2005-06-23 DK DK05760579T patent/DK1773399T3/en active
- 2005-06-23 WO PCT/IT2005/000364 patent/WO2006001046A1/en active Application Filing
- 2005-06-23 US US11/630,985 patent/US20080292664A1/en not_active Abandoned
- 2005-06-23 KR KR1020067027380A patent/KR20070043724A/en not_active Application Discontinuation
- 2005-06-23 DE DE602005005062T patent/DE602005005062T2/en active Active
- 2005-06-23 EP EP05760579A patent/EP1773399B1/en not_active Not-in-force
- 2005-06-23 JP JP2007518813A patent/JP2008504100A/en active Pending
- 2005-06-23 AT AT05760579T patent/ATE387218T1/en active
Patent Citations (3)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US5616568A (en) * | 1993-11-30 | 1997-04-01 | The Research Foundation Of State University Of New York | Functionalized derivatives of hyaluronic acid |
| US5874417A (en) * | 1993-11-30 | 1999-02-23 | The Research Foundation Of State University Of New York | Functionalized derivatives of hyaluronic acid |
| WO2002041877A1 (en) * | 2000-10-24 | 2002-05-30 | Clear Solutions Biotech, Inc. | Sodium hyaluronate microspheres |
Non-Patent Citations (2)
| Title |
|---|
| GIAMMONA G ET AL: "A NEW WATER-SOLUBLE SYNTHETIC POLYMER, , -POLYASPARTHYDRAZIDE, AS POTENTIAL PLASMA EXPANDER AND DRUG CARRIER", JOURNAL OF CONTROLLED RELEASE, ELSEVIER SCIENCE PUBLISHERS B.V. AMSTERDAM, NL, vol. 29, no. 1/2, 1 February 1994 (1994-02-01), pages 63 - 72, XP000433652, ISSN: 0168-3659 * |
| PRESTWICH G D ET AL: "Controlled chemical modification of hyaluronic acid: synthesis, applications, and biodegradation of hydrazide derivatives", JOURNAL OF CONTROLLED RELEASE, ELSEVIER SCIENCE PUBLISHERS B.V. AMSTERDAM, NL, vol. 53, no. 1-3, 30 April 1998 (1998-04-30), pages 93 - 103, XP004121260, ISSN: 0168-3659 * |
Cited By (11)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US9701940B2 (en) | 2005-09-19 | 2017-07-11 | Histogenics Corporation | Cell-support matrix having narrowly defined uniformly vertically and non-randomly organized porosity and pore density and a method for preparation thereof |
| WO2007102149A2 (en) | 2006-03-07 | 2007-09-13 | Prochon Biotech Ltd. | Hydrazido derivatives of hyaluronic acid |
| WO2007102149A3 (en) * | 2006-03-07 | 2008-03-13 | Prochon Biotech Ltd | Hydrazido derivatives of hyaluronic acid |
| JP2009529086A (en) * | 2006-03-07 | 2009-08-13 | プロコン バイオテック リミテッド | Hydrazide derivatives of hyaluronic acid |
| EP2492285A1 (en) * | 2006-03-07 | 2012-08-29 | ProChon Biotech Ltd. | Hydrazido derivatives of hyaluronic acid |
| US8524885B2 (en) | 2006-03-07 | 2013-09-03 | Prochon Biotech Ltd. | Hydrazido derivatives of hyaluronic acid |
| US8940888B2 (en) | 2006-03-07 | 2015-01-27 | Prochon Biotech Ltd. | Hydrazido derivatives of hyaluronic acid |
| US8790701B2 (en) | 2008-04-28 | 2014-07-29 | Surmodics, Inc. | Poly-α(1→4)glucopyranose-based matrices with hydrazide crosslinking |
| WO2010051783A1 (en) | 2008-11-06 | 2010-05-14 | Cpn S.R.O. | Method of preparation of dtpa crosslinked hyaluronic acid derivatives and modification of said derivatives |
| US10077420B2 (en) | 2014-12-02 | 2018-09-18 | Histogenics Corporation | Cell and tissue culture container |
| US11555172B2 (en) | 2014-12-02 | 2023-01-17 | Ocugen, Inc. | Cell and tissue culture container |
Also Published As
| Publication number | Publication date |
|---|---|
| CA2572127C (en) | 2013-02-05 |
| EP1773399A1 (en) | 2007-04-18 |
| ATE387218T1 (en) | 2008-03-15 |
| PT1773399E (en) | 2008-06-05 |
| DE602005005062T2 (en) | 2008-12-04 |
| CA2572127A1 (en) | 2006-01-05 |
| US20080292664A1 (en) | 2008-11-27 |
| DK1773399T3 (en) | 2008-06-16 |
| ES2303258T3 (en) | 2008-08-01 |
| EP1773399B1 (en) | 2008-02-27 |
| DE602005005062D1 (en) | 2008-04-10 |
| AU2005257078A1 (en) | 2006-01-05 |
| ITRM20040318A1 (en) | 2004-09-28 |
| AU2005257078B2 (en) | 2010-04-22 |
| KR20070043724A (en) | 2007-04-25 |
| JP2008504100A (en) | 2008-02-14 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| AU2005257078B2 (en) | Hydrogels of hyaluronic acid and alpha, beta-polyaspartylhydrazide and their biomedical and pharmaceutical uses | |
| RU2230752C2 (en) | Cross-linked hyaluronic acids and their application in medicine | |
| EP1753787B1 (en) | Method of covalently linking hyaluronan and chitosan | |
| Rinaudo | Main properties and current applications of some polysaccharides as biomaterials | |
| CA2266581C (en) | Polymers containing polysaccharides such as alginates or modified alginates | |
| Berger et al. | Structure and interactions in chitosan hydrogels formed by complexation or aggregation for biomedical applications | |
| US7879818B2 (en) | Hyaluronic acid-based cross-linked nanoparticles | |
| WO2023125689A1 (en) | Hyaluronic acid-collagen copolymer compositions and medical applications thereof | |
| EP2529226A1 (en) | Silylated biomolecules | |
| US9867909B2 (en) | Multilayer implants for delivery of therapeutic agents | |
| EP1806367A2 (en) | Polymers containing polysaccharides such as alginates or modified alginates | |
| KR20020080339A (en) | Reversible cross-linked hydrogels | |
| AU6185801A (en) | Polymers containing polysaccharides such as alginates or modified alginates |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| AK | Designated states |
Kind code of ref document: A1 Designated state(s): AE AG AL AM AT AU AZ BA BB BG BR BW BY BZ CA CH CN CO CR CU CZ DE DK DM DZ EC EE EG ES FI GB GD GE GH GM HR HU ID IL IN IS JP KE KG KM KP KR KZ LC LK LR LS LT LU LV MA MD MG MK MN MW MX MZ NA NG NI NO NZ OM PG PH PL PT RO RU SC SD SE SG SK SL SM SY TJ TM TN TR TT TZ UA UG US UZ VC VN YU ZA ZM ZW |
|
| AL | Designated countries for regional patents |
Kind code of ref document: A1 Designated state(s): BW GH GM KE LS MW MZ NA SD SL SZ TZ UG ZM ZW AM AZ BY KG KZ MD RU TJ TM AT BE BG CH CY CZ DE DK EE ES FI FR GB GR HU IE IS IT LT LU MC NL PL PT RO SE SI SK TR BF BJ CF CG CI CM GA GN GQ GW ML MR NE SN TD TG |
|
| 121 | Ep: the epo has been informed by wipo that ep was designated in this application | ||
| DPEN | Request for preliminary examination filed prior to expiration of 19th month from priority date (pct application filed from 20040101) | ||
| WWE | Wipo information: entry into national phase |
Ref document number: 1020067027380 Country of ref document: KR Ref document number: 2007518813 Country of ref document: JP Ref document number: 2572127 Country of ref document: CA |
|
| NENP | Non-entry into the national phase |
Ref country code: DE |
|
| WWW | Wipo information: withdrawn in national office |
Country of ref document: DE |
|
| WWE | Wipo information: entry into national phase |
Ref document number: 2005760579 Country of ref document: EP |
|
| WWE | Wipo information: entry into national phase |
Ref document number: 2005257078 Country of ref document: AU |
|
| ENP | Entry into the national phase |
Ref document number: 2005257078 Country of ref document: AU Date of ref document: 20050623 Kind code of ref document: A |
|
| WWP | Wipo information: published in national office |
Ref document number: 2005257078 Country of ref document: AU |
|
| WWP | Wipo information: published in national office |
Ref document number: 2005760579 Country of ref document: EP |
|
| ENP | Entry into the national phase |
Ref document number: 2572127 Country of ref document: CA |
|
| WWG | Wipo information: grant in national office |
Ref document number: 2005760579 Country of ref document: EP |
|
| WWE | Wipo information: entry into national phase |
Ref document number: 11630985 Country of ref document: US |