WO2005123716A1 - Indole derivatives as histamine receptor antagonists - Google Patents
Indole derivatives as histamine receptor antagonists Download PDFInfo
- Publication number
- WO2005123716A1 WO2005123716A1 PCT/EP2005/006303 EP2005006303W WO2005123716A1 WO 2005123716 A1 WO2005123716 A1 WO 2005123716A1 EP 2005006303 W EP2005006303 W EP 2005006303W WO 2005123716 A1 WO2005123716 A1 WO 2005123716A1
- Authority
- WO
- WIPO (PCT)
- Prior art keywords
- piperidin
- yloxy
- isopropyl
- indol
- methanone
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Ceased
Links
- 0 *Oc(ccc(C=O)c1)c1F Chemical compound *Oc(ccc(C=O)c1)c1F 0.000 description 3
- QSBHJTCAPWOIIE-UHFFFAOYSA-N Oc(ccc(C=O)c1)c1F Chemical compound Oc(ccc(C=O)c1)c1F QSBHJTCAPWOIIE-UHFFFAOYSA-N 0.000 description 1
Classifications
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D405/00—Heterocyclic compounds containing both one or more hetero rings having oxygen atoms as the only ring hetero atoms, and one or more rings having nitrogen as the only ring hetero atom
- C07D405/14—Heterocyclic compounds containing both one or more hetero rings having oxygen atoms as the only ring hetero atoms, and one or more rings having nitrogen as the only ring hetero atom containing three or more hetero rings
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P1/00—Drugs for disorders of the alimentary tract or the digestive system
- A61P1/04—Drugs for disorders of the alimentary tract or the digestive system for ulcers, gastritis or reflux esophagitis, e.g. antacids, inhibitors of acid secretion, mucosal protectants
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P11/00—Drugs for disorders of the respiratory system
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P11/00—Drugs for disorders of the respiratory system
- A61P11/06—Antiasthmatics
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P25/00—Drugs for disorders of the nervous system
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P25/00—Drugs for disorders of the nervous system
- A61P25/02—Drugs for disorders of the nervous system for peripheral neuropathies
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P25/00—Drugs for disorders of the nervous system
- A61P25/04—Centrally acting analgesics, e.g. opioids
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P25/00—Drugs for disorders of the nervous system
- A61P25/06—Antimigraine agents
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P25/00—Drugs for disorders of the nervous system
- A61P25/08—Antiepileptics; Anticonvulsants
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P25/00—Drugs for disorders of the nervous system
- A61P25/08—Antiepileptics; Anticonvulsants
- A61P25/10—Antiepileptics; Anticonvulsants for petit-mal
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P25/00—Drugs for disorders of the nervous system
- A61P25/08—Antiepileptics; Anticonvulsants
- A61P25/12—Antiepileptics; Anticonvulsants for grand-mal
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P25/00—Drugs for disorders of the nervous system
- A61P25/14—Drugs for disorders of the nervous system for treating abnormal movements, e.g. chorea, dyskinesia
- A61P25/16—Anti-Parkinson drugs
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P25/00—Drugs for disorders of the nervous system
- A61P25/18—Antipsychotics, i.e. neuroleptics; Drugs for mania or schizophrenia
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P25/00—Drugs for disorders of the nervous system
- A61P25/20—Hypnotics; Sedatives
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P25/00—Drugs for disorders of the nervous system
- A61P25/24—Antidepressants
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P25/00—Drugs for disorders of the nervous system
- A61P25/28—Drugs for disorders of the nervous system for treating neurodegenerative disorders of the central nervous system, e.g. nootropic agents, cognition enhancers, drugs for treating Alzheimer's disease or other forms of dementia
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P25/00—Drugs for disorders of the nervous system
- A61P25/30—Drugs for disorders of the nervous system for treating abuse or dependence
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P3/00—Drugs for disorders of the metabolism
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P3/00—Drugs for disorders of the metabolism
- A61P3/04—Anorexiants; Antiobesity agents
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P37/00—Drugs for immunological or allergic disorders
- A61P37/08—Antiallergic agents
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P43/00—Drugs for specific purposes, not provided for in groups A61P1/00-A61P41/00
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P9/00—Drugs for disorders of the cardiovascular system
- A61P9/10—Drugs for disorders of the cardiovascular system for treating ischaemic or atherosclerotic diseases, e.g. antianginal drugs, coronary vasodilators, drugs for myocardial infarction, retinopathy, cerebrovascula insufficiency, renal arteriosclerosis
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D209/00—Heterocyclic compounds containing five-membered rings, condensed with other rings, with one nitrogen atom as the only ring hetero atom
- C07D209/02—Heterocyclic compounds containing five-membered rings, condensed with other rings, with one nitrogen atom as the only ring hetero atom condensed with one carbocyclic ring
- C07D209/04—Indoles; Hydrogenated indoles
- C07D209/30—Indoles; Hydrogenated indoles with hetero atoms or with carbon atoms having three bonds to hetero atoms with at the most one bond to halogen, directly attached to carbon atoms of the hetero ring
- C07D209/42—Carbon atoms having three bonds to hetero atoms with at the most one bond to halogen, e.g. ester or nitrile radicals
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D401/00—Heterocyclic compounds containing two or more hetero rings, having nitrogen atoms as the only ring hetero atoms, at least one ring being a six-membered ring with only one nitrogen atom
- C07D401/02—Heterocyclic compounds containing two or more hetero rings, having nitrogen atoms as the only ring hetero atoms, at least one ring being a six-membered ring with only one nitrogen atom containing two hetero rings
- C07D401/12—Heterocyclic compounds containing two or more hetero rings, having nitrogen atoms as the only ring hetero atoms, at least one ring being a six-membered ring with only one nitrogen atom containing two hetero rings linked by a chain containing hetero atoms as chain links
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D401/00—Heterocyclic compounds containing two or more hetero rings, having nitrogen atoms as the only ring hetero atoms, at least one ring being a six-membered ring with only one nitrogen atom
- C07D401/14—Heterocyclic compounds containing two or more hetero rings, having nitrogen atoms as the only ring hetero atoms, at least one ring being a six-membered ring with only one nitrogen atom containing three or more hetero rings
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D403/00—Heterocyclic compounds containing two or more hetero rings, having nitrogen atoms as the only ring hetero atoms, not provided for by group C07D401/00
- C07D403/02—Heterocyclic compounds containing two or more hetero rings, having nitrogen atoms as the only ring hetero atoms, not provided for by group C07D401/00 containing two hetero rings
- C07D403/12—Heterocyclic compounds containing two or more hetero rings, having nitrogen atoms as the only ring hetero atoms, not provided for by group C07D401/00 containing two hetero rings linked by a chain containing hetero atoms as chain links
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D403/00—Heterocyclic compounds containing two or more hetero rings, having nitrogen atoms as the only ring hetero atoms, not provided for by group C07D401/00
- C07D403/14—Heterocyclic compounds containing two or more hetero rings, having nitrogen atoms as the only ring hetero atoms, not provided for by group C07D401/00 containing three or more hetero rings
Definitions
- the present invention is concerned with novel indole derivatives, their manufacture, pharmaceutical compositions containing them and their use as medicaments.
- the active compounds ofthe present invention are useful in treating obesity and other disorders.
- the present invention relates to compounds ofthe general formula
- X is O or S
- R 1 is selected from the group consisting of hydrogen, lower alkyl, lower alkenyl, lower alkinyl, cycloalkyl, lower cycloalkylalkyl, lower hydroxyalkyl, lower alkoxyalkyl, lower alkylsulfanylalkyl, lower dialkylaminoalkyl, lower dialkylcarbamoylalkyl, phenyl unsubstituted or substituted with one or two groups independently selected from lower alkyl, lower halogenalkoxy or lower hydroxyalkyl, lower phenylalkyl wherein the phenyl ring maybe unsubstituted or substituted with one or two groups independently selected from lower alkyl, halogen, lower alkoxy or lower hydroxyalkyl, lower heteroarylalkyl wherein the heteroaryl ring ma be unsubstituted or substituted with one or two groups independently selected from lower alkyl, halogen, lower alkoxy or lower hydroxyalkyl, and lower hetero
- R 1 and R 2 together with the nitrogen atom to which they are attached form a 4-, 5-, 6- or 7-membered saturated or partly unsaturated heterocyclic ring optionally containing a further heteroatom selected from nitrogen, oxygen or sulfur, said saturated heterocyclic ring being unsubstituted or substituted by one, two or three groups independently selected from lower alkyl, halogen, halogenalkyl, hydroxy, lower hydroxyalkyl, lower alkoxy, oxo, phenyl, benzyl, pyridyl and carbamoyl, or being condensed with a phenyl ring, said phenyl ring being unsubstituted or substituted by one, two or three groups independently selected from lower alkyl, lower alkoxy and halogen;
- R 3 is selected from the group consisting of hydrogen, lower alkyl, lower alkoxyalkyl, lower halogenalkyl, lower cycloalkylalkyl, lower alkylsulfonyl and lower alkanoyl;
- R 4 is -O-Het and and R 5 is hydrogen, or R 4 is hydrogen or fluoro and R 5 is -O-Het;
- Het is selected from
- R 6 is selected from lower alkyl, cycloalkyl, lower cycloalkylalkyl and lower phenylalkyl; n is 0, 1 or 2;
- R 7 is lower alkyl; p is 0, 1 or 2; q is 0, 1 or 2; X is selected from CR 10 R 10' , O and S;
- R 8 , R 8' , R 9 , R 9> , R 10 , R 10' , R 11 and R 11' independently from each other are selected from the group consisting of hydrogen, lower alkyl, hydoxy, halogen and dialkylamino, or R 9 and R 10 together form a double bond;
- R 12 is lower alkyl;
- R 13 is C 3 -C 6 -alkyl
- the compounds of formula I are antagonists and/or inverse agonists at the histamine 3 receptor (H3 receptor).
- Histamine (2-(4-imidazolyl) ethylamine) is one ofthe aminergic neurotransmitters which is widely distributed throughout the body, e. g. the gastrointestinal tract (Burks 1994 in Johnson L.R. ed., Physiology ofthe Gastrointestinal Tract, Raven Press, NY, pp. 211 - 242). Histamine regulates a variety of digestive pathophysiological events like gastric acid secretion, intestinal motility (Leurs et al., Br J. Pharmacol.
- histamine is synthesized in histaminergic cell bodies which are found centrally in the tuberomammillary nucleus ofthe posterior basal hypothalamus. From there, the histaminergic cell bodies project to various brain regions (Panula et al., Proc. Natl. Acad. Sci. USA 1984, 81, 2572-2576; Inagaki et al., J. Comp. Neurol 1988, 273, 283 - 300).
- histamine mediates all its actions in both the CNS and the periphery through four distinct histamine receptors, the histamine HI, H2 H3 and H4 receptors.
- H3 receptors are predominantly localized in the central nervous system (CNS).
- CNS central nervous system
- an autoreceptor H3 receptors constitutively inhibit the synthesis and secretion of histamine from histaminergic neurons (Arrang et al., Nature 1983, 302, 832-837; Arrang et al., Neuroscience 1987, 23, 149-157).
- H3 receptors also modulate the release of other neurotransmitters such as acetylcholine, dopamine, serotonin and norepinephrine among others in both the central nervous system and in peripheral organs, such as lungs, cardiovascular system and gastrointestinal tract (Clapham & Kilpatrik, Br. J. Pharmacol. 1982, 107, 919- 923; Blandina et al. in The Histamine H3 Receptor (Leurs RL and Timmermann H eds, 1998, pp 27-40, Elsevier, Amsterdam, The Netherlands). H3 receptors are constitutively active, meaning that even without exogenous histamine, the receptor is tonically activated.
- H3R antagonist In the case of an inhibitory receptor such as the H3 receptor, this inherent activity causes tonic inhibition of neurotransmitter release. Therefore it may be important that a H3R antagonist would also have inverse agonist activity to both block exogenous histamine effects and to shift the receptor from its constitutively active (inhibitory) form to a neutral state.
- the wide distribution of H3 receptors in the mammalian CNS indicates the physiological role of this receptor. Therefore the therapeutic potential as a novel drug development target in various indications has been proposed.
- H3R ligands - as antagonists, inverse agonists, agonists or partial agonists - may influence the histamine levels or the secretion of neurotransmitters in the brain and the periphery and thus may be useful in the treatment of several disorders.
- disorders include obesity, (Masaki et al; Endocrinol. 2003, 144, 2741-2748; Hancock et al., European J.
- cardiovascular disorders such as acute myocardial infarction, dementia and cognitive disorders such as attention deficit hyperactivity disorder (ADHD) and Alzheimer's disease
- ADHD attention deficit hyperactivity disorder
- neurological disorders such as schizophrenia, depression, epilepsy, Parkinson's disease, and seizures or convulsions, sleep disorders, narcolepsy, pain, gastrointestinal disorders, vestibular dysfunction
- ADHD attention deficit hyperactivity disorder
- narcolepsy pain, gastrointestinal disorders, vestibular dysfunction
- H3 receptor antagonists respectively inverse agonists.
- Such antagonists / inverse agonists are useful as therapeutically active substances, particularly in the treatment and/or prevention of diseases which are associated with the modulation of H3 receptors.
- alkyl refers to a branched or straight-chain monovalent saturated aliphatic hydrocarbon radical of one to twenty carbon atoms, preferably one to sixteen carbon atoms, more preferably one to ten carbon atoms.
- lower alkyl or "CrCs-alkyl”, alone or in combination, signifies a straight- chain or branched-chain alkyl group with 1 to 8 carbon atoms, preferably a straight or branched-chain alkyl group with 1 to 6 carbon atoms and particularly preferred a straight or branched-chain alkyl group with 1 to 4 carbon atoms
- straight-chain and branched CrC 8 alkyl groups are methyl, ethyl, propyl, isopropyl, butyl, isobutyl, tert.- butyl, the isomeric pentyls, the isomeric hexyls, the isomeric heptyls and the isomeric octyls, preferably methyl and ethyl and most preferred methyl.
- lower alkenyl or "C 2 . 8 -alkenyl”, alone or in combination, signifies a straight-chain or branched hydrocarbon radical comprising an olefinic bond and up to 8, preferably up to 6, particularly preferred up to 4 carbon atoms.
- alkenyl groups are ethenyl, 1-propenyl, 2-propenyl, isopropenyl, 1-butenyl, 2-butenyl, 3-butenyl and isobutenyl.
- a preferred example is 2-propenyl.
- lower alkinyl or "C 2-8 -alkinyP, alone or in combination, signifies a straight-chain or branched hydrocarbon residue comprising a triple bond and up to 8, preferably up to 6, particularly preferred up to 4 carbon atoms.
- alkinyl groups are ethinyl, 1-propinyl, or 2-propinyl. A preferred example is 2-propinyl.
- cycloalkyl or "C3. 7 -cycloalkyl” denotes a saturated carbocyclic group containing from 3 to 7 carbon atoms, such as cyclopropyl, cyclobutyl, cyclopentyl, cyclohexyl or cycloheptyl. Especially preferred are cyclopropyl, cyclopentyl and cyclohexyl.
- lower cycloalkylalkyl or refers to lower alkyl groups as defined above wherein at least one ofthe hydrogen atoms ofthe lower alkyl group is replaced by cycloalkyl. A preferred example is cyclopropylmethyl.
- alkoxy refers to the group R'-O-, wherein R' is lower alkyl and the term “lower alkyl” has the previously given significance.
- lower alkoxy groups are e.g. methoxy, ethoxy, n-propoxy, isopropoxy, n-butoxy, isobutoxy, sec. butoxy and tertbutoxy, preferably methoxy and ethoxy and most preferred methoxy.
- lower alkoxyalkyl or " -s-alkoxy-Ci-s-alkyl” refers to lower alkyl groups as defined above wherein at least one ofthe hydrogen atoms ofthe lower alkyl groups is replaced by an alkoxy group, preferably methoxy or ethoxy.
- alkoxy group preferably methoxy or ethoxy.
- preferred lower alkoxyalkyl groups are 2-methoxyethyl or 3-methoxypropyl.
- alkylsulfanyl or "C ⁇ s-alkylsulfanyl” refers to the group R'-S-, wherein R' is lower alkyl and the term “lower alkyl” has the previously given significance.
- alkylsulfanyl groups are e.g. methylsulfanyl or ethylsulfanyl.
- lower alkylsulfanylalkyl or "Ci-s-alkylsulfanyl-Ci.g-alkyr refers to lower alkyl groups as defined above wherein at least one ofthe hydrogen atoms ofthe lower alkyl groups is replaced by an alkylsulfanyl group, preferably methylsulfanyl.
- An example for a preferred lower alkylsulfanylalkyl group is 2-methylsulfanylethyl.
- alkylsulfonyl or “lower alkylsulfanyl” refers to the group R'-S(O) 2 -, wherein R' is lower alkyl and the term “lower alkyl” has the previously given significance.
- alkylsulfonyl groups are e.g. methylsulfonyl or ethylsulfonyl.
- halogen refers to fluorine, chlorine, bromine and iodine, with fluorine, chlorine and bromine being preferred.
- lower halogenalkyl or "halogen-Ci-s-alkyl” refers to lower alkyl groups as defined above wherein at least one ofthe hydrogen atoms ofthe lower alkyl group is replaced by a halogen atom, preferably fluoro or chloro, most preferably fluoro.
- halogen atom preferably fluoro or chloro, most preferably fluoro.
- preferred halogenated lower alkyl groups are trifluoromethyl, difluoromethyl, fluoromethyl and chloromethyl, with trifluoromethyl being especially preferred.
- lower halogenalkoxy or "halogen- -s-alkoxy” refers to lower alkoxy groups as defined above wherein at least one ofthe hydrogen atoms ofthe lower alkoxy group is replaced by a halogen atom, preferably fluoro or chloro, most preferably fluoro.
- halogenated lower alkyl groups are trifluoromethoxy, difluoromethoxy, fluormethoxy and chloromethoxy, with trifluoromethoxy being especially preferred.
- lower hydroxyalkyl or "hydroxy- -g-alkyl” refers to lower alkyl groups as defined above wherein at least one ofthe hydrogen atoms ofthe lower alkyl group is replaced by a hydroxy group.
- lower hydroxyalkyl groups are hydroxymethyl or hydroxyethyl.
- dialkylamino refers to the group -NR'R", wherein R' and R" are lower alkyl and the term “lower alkyl” has the previously given significance.
- a preferred dialkylamino group is dimethylamino.
- lower dialkylaminoalkyl or " -s-dialkylamino-Ci-s-alkyl” refers to lower alkyl groups as defined above wherein at least one ofthe hydrogen atoms ofthe lower alkyl group is replaced by a dialkylamino group, preferably dimethylamino.
- a preferred lower dialkylaminoalkyl group is 3-dimethylaminopropyl.
- lower alkanoyl refers to the group -CO-R', wherein R' is lower alkyl and the term “lower alkyl” has the previously given significance.
- Preferred is a group -CO-R', wherein R' is methyl, meaning an acetyl group.
- carbamoyl refers to the group -CO-NH 2 .
- dialkylcarbamoyl or "Ci-s-dialkylcarbamoyl” refers to the group -CO-NR'R" wherein R' and R" are lower alkyl and the term “lower alkyl” has the previously given significance.
- a preferred dialkylcarbamoyl group is dimethylcarbamoyl.
- lower dialkylcarbamoylalkyl or "C 1-8 -dialkylcarbamoyl-C 1-8 -alkyr' refers to lower alkyl groups as defined above wherein at least one ofthe hydrogen atoms ofthe lower alkyl group is replaced by a dialkylcarbamoyl group as defined herein before.
- a preferred lower dialkylcarbamoylalkyl groups is dimethylcarbamoylmethyl.
- lower phenylalkyl or "phenyl-C 1-8 -alkyl” to lower alkyl groups as defined above wherein at least one of the hydrogen atoms of the lower alkyl group is replaced by a phenyl group.
- Preferred lower phenylalkyl groups are benzyl or phenethyl.
- heteroaryl refers to an aromatic 5- or 6-membered ring which can comprise one, two or three atoms selected from nitrogen, oxygen and/or sulphur.
- heteroaryl groups are e.g. furyl, pyridyl, pyrazinyl, pyrimidinyl, pyridazinyl, thienyl, isoxazolyl, thiazolyl, isothiazolyl, oxazolyl, imidazolyl, or pyrrolyl.
- furyl and pyridyl are especially preferred.
- lower heteroarylalkyl or “heteroaryl- -s-alkyl” refers to lower alkyl groups as defined above wherein at least one ofthe hydrogen atoms ofthe lower alkyl group is replaced by a heteroaryl group as defined above.
- heterocyclyl refers to a saturated or partly unsaturated 5- or 6-membered ring which can comprise one, two or three atoms selected from nitrogen, oxygen and/or sulphur.
- heterocyclyl rings include piperidinyl, piperazinyl, azepinyl, pyrrolidinyl, pyrazolidinyl, imidazolinyl, imidazolidinyl, pyridinyl, pyridazinyl, pyrimidinyl, oxazolidinyl, isoxazolidinyl, morpholinyl, thiazolidinyl, isothiazolidinyl, thiadiazolylidinyl, dihydrofuryl, tetrahydrofuryl, dihydropyranyl, tetrahydropyranyl, and thiamorpholinyl.
- a preferred heterocyclyl group is piperidinyl.
- the term "lower heterocyclylalkyl” or “heterocydyl- .s-alkyl” refers to lower alkyl groups as defined above wherein at least one ofthe hydrogen atoms ofthe lower alkyl group is replaced by a heterocyclyl group as defined above.
- form a 4-, 5-, 6- or 7-membered saturated heterocyclic ring optionally containing a further heteroatom selected from nitrogen, oxygen or sulfur refers to a saturated N-heterocyclic ring, which may optionally contain a further nitrogen, oxygen or sulfur atom, such as azetidinyl, pyrrolidinyl, imidazolidinyl, pyrazolidinyl, oxazolidinyl, isoxazolidinyl, thiazolidinyl, isothiazolidinyl, piperidinyl, piperazinyl, morpholinyl, thiomorpholinyl, or azepanyl.
- a "4-, 5-, 6- or 7-membered partly unsaturated heterocyclic ring” means a heterocyclic ring as defined above which contains a double bond, for example 2,5-dihydropyrrolyl or 3,6-dihydro-2H-pyridinyl.
- the heteroyclic ring may be unsubstituted or substituted by one, two or three groups independently selected from lower alkyl, lower alkoxy and oxo.
- the heterocyclic ring may also be condensed with a phenyl ring, said phenyl ring being unsubstituted or substituted by one, two or three groups independently selected from lower alkyl, lower alkoxy and halogen.
- An example for such a condensed heterocyclic ring is 3,4-dihydro-lH-isoquinoline.
- salts refers to those salts which retain the biological effectiveness and properties ofthe free bases or free acids, which are not biologically or otherwise undesirable.
- the salts are formed with inorganic acids such as hydrochloric acid, hydrobromic acid, sulfuric acid, nitric acid, phosphoric acid and the like, preferably hydrochloric acid, and organic acids such as acetic acid, propionic acid, glycolic acid, pyruvic acid, oxylic acid, maleic acid, malonic acid, salicylic acid, succinic acid, fumaric acid, tartaric acid, citric acid, benzoic acid, cinnamic acid, mandelic acid, methanesulfonic acid, ethanesulfonic acid, p-toluenesulfonic acid, salicylic acid, N- acetylcystein and the like.
- salts derived from an inorganic base include, but are not limited to, the sodium, potassium, lithium, ammonium, calcium, magnesium salts and the like.
- Salts derived from organic bases include, but are not limited to salts of primary, secondary, and tertiary amines, substituted amines including naturally occurring substituted amines, cyclic amines and basic ion exchange resins, such as isopropylamine, trimethylamine, diethylamine, triethylamine, tripropylamine, ethanolamine, lysine, arginine, N-ethylpiperidine, piperidine, polymine resins and the like.
- the compound of formula I can also be present in the form of zwitterions. Particularly preferred pharmaceutically acceptable salts of compounds of formula I are the hydrochloride salts.
- the compounds of formula I can also be solvated, e.g. hydrated.
- the solvation can be effected in the course ofthe manufacturing process or can take place e.g. as a consequence of hygroscopic properties of an initially anhydrous compound of formula I (hydration).
- pharmaceutically acceptable salts also includes physiologically acceptable solvates.
- “Isomers” are compounds that have identical molecular formulae but that differ in the nature or the sequence of bonding of their atoms or in the arrangement of their atoms in space. Isomers that differ in the arrangement of their atoms in space are termed “stereoisomers”. Stereoisomers that are not mirror images of one another are termed “diastereoisomers”, and stereoisomers that are non-superimposable mirror images are termed “enantiomers”, or sometimes optical isomers. A carbon atom bonded to four nonidentical substituents is termed a "chiral center”.
- the present invention relates to compounds ofthe general formula
- X is O or S
- R 1 is selected from the group consisting of hydrogen, lower alkyl, lower alkenyl, lower alkinyl, cycloalkyl, lower cycloalkylalkyl, lower hydroxyalkyl, lower alkoxyalkyl, lower alkylsulfanylalkyl, lower dialkylaminoalkyl, lower dialkylcarbamoylalkyl, phenyl unsubstituted or substituted with one or two groups independently selected from lower alkyl, lower halogenalkoxy or lower hydroxyalkyl, lower phenylalkyl wherein the phenyl ring may be unsubstituted or substituted with one or two groups independently selected from lower alkyl, halogen, lower alkoxy or lower hydroxyalkyl, lower heteroarylalkyl wherein the heteroaryl ring may be unsubstituted or substituted with one or two groups independently selected from lower alkyl, halogen, lower alkoxy or lower hydroxyalkyl, and lower
- R 2 is selected from the group consisting of hydrogen, lower alkyl, lower alkenyl, lower alkinyl, cycloalkyl, lower cycloalkylalkyl, lower hydroxyalkyl, lower alkoxyalkyl, lower alkylsulfanylalkyl, lower dialkylaminoalkyl, lower dialkylcarbamoylalkyl, phenyl unsubstituted or substituted with one or two groups independently selected from lower alkyl, lower halogenalkoxy or lower hydroxyalkyl, lower phenylalkyl wherein the phenyl ring may be unsubstituted or substituted with one or two groups independently selected from lower alkyl, halogen, lower alkoxy or lower hydroxyalkyl, lower heteroarylalkyl wherein the heteroaryl ring may be unsubstituted or substituted with one or two groups independently selected from lower alkyl, halogen, lower alkoxy or lower hydroxyalkyl, and lower
- R 1 and R 2 together with the nitrogen atom to which they are attached form a 4-, 5-, 6- or 7-membered saturated or partly unsaturated heterocyclic ring optionally containing a further heteroatom selected from nitrogen, oxygen or sulfur, said saturated heterocyclic ring being unsubstituted or substituted by one, two or three groups independently selected from lower alkyl, halogen, halogenalkyl, hydroxy, lower hydroxyalkyl, lower alkoxy, oxo, phenyl, benzyl, pyridyl and carbamoyl, or being condensed with a phenyl ring, said phenyl ring being unsubstituted or substituted by one, two or three groups independently selected from lower alkyl, lower alkoxy and halogen;
- R 3 is selected from the group consisting of hydrogen, lower alkyl, lower alkoxyalkyl, lower halogenalkyl, lower cycloalkylalkyl, lower alkylsulfonyl and lower alkanoyl;
- R 4 is -O-Het and and R 5 is hydrogen, or R 4 is hydrogen or fluoro and R 5 is -O-Het;
- Het is selected from
- R 6 is selected from lower alkyl, cycloalkyl, lower cycloalkylalkyl and lower phenylalkyl; n is 0, 1 or 2;
- R 7 is lower alkyl; p is 0, 1 or 2; q is 0, 1 or 2;
- X is selected from CR 10 R 10' , O and S;
- R 8 , R 8' , R 9 , R 9' , R 10 , R 10' , R 11 and R 11' independently from each other are selected from the group consisting of hydrogen, lower alkyl, hydoxy, halogen and dialkylamino, or R 9 and R 10 together form a double bond;
- R 12 is lower alkyl
- R 13 is C 3 -C 6 -alkyl
- the invention relates to compounds of formula I, wherein
- X is O or S
- R 1 is selected from the group consisting of hydrogen, lower alkyl, lower alkenyl, lower alkinyl, cycloalkyl, lower cycloalkylalkyl, lower hydroxyalkyl, lower alkoxyalkyl, lower alkylsulfanylalkyl, lower dialkylaminoalkyl, lower dialkylcarbamoylalkyl, phenyl unsubstituted or substituted with one or two groups independently selected from lower alkyl, lower halogenalkoxy or lower hydroxyalkyl, lower phenylalkyl wherein the phenyl ring may be unsubstituted or substituted with one or two groups independently selected from lower alkyl, halogen, lower alkoxy or lower hydroxyalkyl, lower heteroarylalkyl wherein the heteroaryl ring may be unsubstituted or substituted with one or two groups independently selected from lower alkyl, halogen, lower alkoxy or lower hydroxyalkyl, and lower
- R 2 is selected from the group consisting of hydrogen, lower alkyl, lower alkenyl, lower alkinyl, cycloalkyl, lower cycloalkylalkyl, lower hydroxyalkyl, lower alkoxyalkyl, lower alkylsulfanylalkyl, lower dialkylaminoalkyl, lower dialkylcarbamoylalkyl, phenyl unsubstituted or substituted with one or two groups independently selected from lower alkyl, lower halogenalkoxy or lower hydroxyalkyl, lower phenylalkyl wherein the phenyl ring may be unsubstituted or substituted with one or two groups independently selected from lower alkyl, halogen, lower alkoxy or lower hydroxyalkyl, lower heteroarylalkyl wherein the heteroaryl ring ma be unsubstituted or substituted with one or two groups independently selected from lower alkyl, halogen, lower alkoxy or lower hydroxyalkyl, and lower
- R 1 and R 2 together with the nitrogen atom to which they are attached form a 4-, 5-, 6- or 7-membered saturated or partly unsaturated heterocyclic ring optionally containing a further heteroatom selected from nitrogen, oxygen or sulfur, said saturated heterocyclic ring being unsubstituted or substituted by one, two or three groups independently selected from lower alkyl, halogen, halogenalkyl, hydroxy, lower alkoxy, oxo, phenyl, benzyl, pyridyl and carbamoyl, or being condensed with a phenyl ring, said phenyl ring being unsubstituted or substituted by one, two or three groups independently selected from lower alkyl, lower alkoxy and halogen;
- R 3 is hydrogen or lower alkyl
- R 4 is -O-Het and and R 5 is hydrogen, or R 4 is hydrogen or fluoro and R 5 is -O-Het;
- Het is selected from
- Het l Het 2 Het 3' wherein m is 0, 1 or 2; R 6 is lower alkyl; n is 0, 1 or 2; R 7 is lower alkyl; p is 0, 1 or 2; q is 0, 1 or 2;
- R 8 is hydrogen or lower alkyl
- Preferred compounds offormula I ofthe present invention are compounds of formula I, wherein
- R 1 is selected from the group consisting of lower alkyl, lower alkenyl, lower alkinyl, cycloalkyl, lower cycloalkylalkyl, lower hydroxyalkyl, lower alkoxyalkyl, lower alkylsulfanylalkyl, lower dialkylaminoalkyl, lower dialkylcarbamoylalkyl, phenyl unsubstituted or substituted with one or two groups independently selected from lower alkyl, lower halogenalkoxy or lower hydroxyalkyl, lower phenylalkyl wherein the phenyl ring may be unsubstituted or substituted with one or two groups independently selected from lower alkyl, halogen, lower alkoxy or lower hydroxyalkyl, lower heteroarylalkyl wherein the heteroaryl ring may be unsubstituted or substituted with one or two groups independently selected from lower alkyl, halogen, lower alkoxy or lower hydroxyalkyl, and lower heterocycl
- R 1 is selected from the group consisting of lower alkyl, cycloalkyl, lower cycloalkylalkyl, lower alkoxyalkyl, lower phenylalkyl, lower heteroarylalkyl, and lower heterocyclylalkyl wherein the heterocyclyl ring may be unsubstituted or substituted with one or two lower alkyl groups, and R 2 is hydrogen or lower alkyl.
- R and R 2 are lower alkyl.
- compounds offormula I according to the present invention are preferred, wherein R 1 and R 2 together with the nitrogen atom to which they are attached form a 4-, 5-, 6- or 7-membered saturated or partly unsaturated heterocyclic ring optionally containing a further heteroatom selected from nitrogen, oxygen or sulfur, said saturated heterocyclic ring being unsubstituted or substituted by one, two or three groups independently selected from lower alkyl, halogen, halogenalkyl, hydroxy, lower alkoxy, oxo, phenyl, benzyl, pyridyl and carbamoyl, or being condensed with a phenyl ring, said phenyl ring being unsubstituted or substituted by one, two or three groups independently selected from lower alkyl, lower alkoxy and halogen.
- R 1 and R 2 together with the nitrogen atom to which they are attached form a heterocyclic ring selected from the group consisting of morpholine, piperidine, 2,5-dihydropyrrole, pyrrolidine, azepane, piperazine, azetidine, thiomorpholine and 3,6-dihydro-2H-pyridine, said saturated heterocyclic ring being unsubstituted or substituted by one, two or three groups independently selected from lower alkyl, halogen, halogenalkyl, hydroxy, lower alkoxy, oxo, phenyl, benzyl, pyridyl and carbamoyl, or being condensed with a phenyl ring, said phenyl ring being unsubstituted or substituted by one, two or three groups independently selected from lower alkyl, lower alkoxy and halogen.
- R 1 and R 2 together with the nitrogen atom to which they are attached form a heterocyclic ring selected from the group consisting of morpholine, piperidine, azepane, pyrrolidine and azetidine, wherein the ring is unsubstituted or substituted by lower alkyl.
- R is selected from the group consisting of lower alkyl, lower alkoxyalkyl, lower halogenalkyl, lower cycloalkylalkyl, lower alkylsulfonyl and lower alkanoyl
- R 4 is -O-Het and R 5 is hydrogen
- Especially preferred compounds offormula I according to the present invention are those, wherein Het signifies
- R 6 is selected from lower alkyl, cycloalkyl, lower cycloalkylalkyl and lower phenylalkyl, with those compounds, wherein R 6 is lower alkyl, being especially preferred.
- those compounds of formula I are preferred, wherein m is 0, thus meaning pyrrolidine groups are preferred.
- a further preferred group includes those compounds offormula I, wherein m is 1, thus meaning piperidine groups are also preferred.
- Another preferred group of compounds are those compounds offormula I, wherein Het signifies
- n 0, 1 or 2; and R 7 is lower alkyl, with those compounds, wherein n is 0, thus meaning pyrrolidine derivatives, being more preferred.
- Another group of preferred compounds offormula I are those, wherein Het signifies
- R 8 , R 8' , R 9 , R 9> , R 10 , R 10* , R 11 and R 11' independently from each other are selected from the group consisting of hydrogen, lower alkyl, hydoxy, halogen and dialkylamino.
- R 9 and R 10 together may also form a double bond, meaning a compound ofthe formula
- R 12 is lower alkyl and R 13 is C 3 -C6-al yl.
- Examples of preferred compounds offormula I are the following: morpholin-4-yl-[5-(3-piperidin-l-yl-propoxy)-lH-indol-2-yl]-methanone, [5-(l-isopropyl-pyrrolidin-3-yloxy)-lH-indol-2-yl]-morpholin-4-yl-methanone,
- Particularly preferred compounds offormula I ofthe present invention are the following: morpholin-4-yl-[5-(3-piperidin-l-yl-propoxy)-lH-indol-2-yl]-methanone, [5-( l-isopropyl-pyrrolidin-3-yloxy)-lH-indol-2-yl] -morpholin-4-yl-methanone,
- Compounds offormula I may form acid addition salts with acids, such as conventional pharmaceutically acceptable acids, for example hydrochloride, hydrobromide, phosphate, acetate, fumarate, maleate, salicylate, sulphate, pyruvate, citrate, lactate, mandelate, tartarate, and methanesulphonate.
- acids such as conventional pharmaceutically acceptable acids, for example hydrochloride, hydrobromide, phosphate, acetate, fumarate, maleate, salicylate, sulphate, pyruvate, citrate, lactate, mandelate, tartarate, and methanesulphonate.
- acids such as conventional pharmaceutically acceptable acids, for example hydrochloride, hydrobromide, phosphate, acetate, fumarate, maleate, salicylate, sulphate, pyruvate, citrate, lactate, mandelate, tartarate, and methanesulphonate.
- hydrochloride salts solvates and
- Compounds offormula I can have one or more asymmetric carbon atoms and can exist in the form of optically pure enantiomers, mixtures of enantiomers such as, for example, racemates, optically pure diastereoisomers, mixtures of diastereoisomers, diastereoisomeric racemates or mixtures of diastereoisomeric racemates.
- the optically active forms can be obtained for example by resolution ofthe racemates, by asymmetric synthesis or asymmetric chromatography (chromatography with a chiral adsorbens or eluant). The invention embraces all of these forms.
- the compounds of general formula I in this invention may be derivatised at functional groups to provide derivatives which are capable of conversion back to the parent compound in vivo.
- Physiologically acceptable and metabolically labile derivatives, which are capable of producing the parent compounds of general formula I in vivo are also within the scope of this invention.
- a further aspect ofthe present invention is the process for the manufacture of compounds offormula I as defined above, which process comprises a) reacting a compound ofthe formula II
- R 3 is hydrogen, and optionally alkylating this compound to obtain a compound offormula la' wherein R 3 is lower alkyl, and if desired, converting the compound obtained into a pharmaceutically acceptable acid addition salt, or alternatively, b) coupling a compound offormula IV
- R 4 and R 5 are -O-Het as defined herein before and the other one is H, with an amine ofthe formula V H-NR 1 R 2 V wherein R 1 and R 2 are as defined herein before, under basic conditions to obtain a compound ofthe formula lb
- R 3 is hydrogen, and optionally alkylating this compound to obtain a compound of formula lb'
- R 3 is lower alkyl, and if desired, converting the compound obtained into a pharmaceutically acceptable acid addition salt.
- the compounds offormula I can be manufactured by the methods given below, by the methods given in the examples or by analogous methods. Appropriate reaction conditions for the individual reaction steps are known to a person skilled in the art. Starting materials are either commercially available or can be prepared by methods analogous to the methods given below, by methods described in references cited in the text or in the examples, or by methods known in the art.
- GDI N,N'-carbonyldiimidazole
- DCC N,N'- dicyclohexylcarbodiimide
- EDCI l-(3-(iimethylaminopropyl)-3-ethylcarbodiimide hydrochloride
- HATU 1- [bis(dimethylamino)methylene] - 1H- 1,2,3-triazolo [4,5- b]pyridinium-3-oxide hexafluorophosphate
- HOBT l-hydroxy-l,2,3-benzotriazole
- TBTU 0-benzotriazol-l-yl-N,N,N',N'-tetramethyluronium tetrafluoroborate
- reaction can take place over a wide range of temperatures, and the precise reaction temperature is not critical to the invention. We find it convenient to carry out the reaction with heating from ambient temperature to reflux.
- the time required for the reaction may also vary widely, depending on many factors, notably the reaction temperature and the nature ofthe reagents. However, a period of from 0.5 h to several days will usually suffice to yield amide derivatives VIII.
- the solvent there is no particular restriction on the nature ofthe solvent to be employed, provided that it has no adverse effect on the reaction or the reagents involved and that it can dissolve the reagents, at least to some extent.
- the reaction can take place over a wide range of temperatures, and the precise reaction temperature is not critical to the invention. We find it convenient to carry out the reaction with heating from ambient temperature to reflux.
- the time required for the reaction may also vary widely, depending on many factors, notably the reaction temperature and the nature ofthe reagents. However, a period of from 0.5 h to several days will usually suffice to yield the title compounds lb.
- the reaction is carried out in the presence of a base. Suitable bases include NaH, DIPEA, Na 2 CO 3 and the like.
- Suitable bases include NaH, DIPEA, Na 2 CO 3 and the like.
- the reaction can take place over a wide range of temperatures, and the precise reaction temperature is not critical to the invention. We find it convenient to carry out the reaction with heating from ambient temperature to reflux.
- the time required for the reaction may also vary widely, depending on many factors, notably the reaction temperature and the nature ofthe reagents. However, a period of from 0.5 h to several days will usually suffice to yield the title compounds Ic.
- the indoles lb might be the desired products, however, they might optionally be subjected to a subsequent alkylating reaction as described above under point c) to furnish the desired compounds Ic.
- compounds offormula I can be prepared according to scheme 3 below. Exemplified is a sterespecific synthetic passway optionally starting from enantiomerically pure starting materials synonymous shown for N-protected-3- pyrrolidinol.
- PG benzyl the protecting group is removed under hydrogenolytical conditions widely described in literature.
- Those intermediates are conveniently alkylated with a suitable alkylating reagent under basic conditions to give access to the indole derivatives Id.
- Aldehyde XV is conveniently transformed to the respective indole derivative XVI through reaction with methyl 2-azidoacetate (commercially available) under basic conditions and elevated temperatures (Synthesis 1985, 186-188).
- the compounds offormula I ofthe present invention can be used as medicaments for the treatment and/or prevention of diseases which are associated with the modulation of H3 receptors.
- diseases are obesity, metabolic syndrome (syndrome X), neurological diseases including Alzheimer's disease, dementia, age-related memory dysfunction, mild cognitive impairment, cognitive deficit, attention deficit hyperactivity disorder, epilepsy, neuropathic pain, inflammatory pain, migraine, Parkinson's disease, multiple sclerosis, stroke, dizziness, schizophrenia, depression, addiction, motion sickness and sleep disorders including narcolepsy, and other diseases including asthma, allergy, allergy-induced airway responses, congestion, chronic obstructive pulmonary disease and gastro-intestinal disorders.
- the use as medicament for the treatment and/or prevention of obesity is preferred.
- the invention therefore also relates to pharmaceutical compositions comprising a compound as defined above and a pharmaceutically acceptable carrier and/or adjuvant.
- the invention relates to compounds as defined above for use as therapeutically active substances, particularly as therapeutic active substances for the treatment and/or prevention of diseases which are associated with the modulation of H3 receptors.
- diseases are obesity, metabolic syndrome (syndrome X), neurological diseases including Alzheimer's disease, dementia, age-related memory dysfunction, mild cognitive impairment, cognitive deficit, attention deficit hyperactivity disorder, epilepsy, neuropathic pain, inflammatory pain, migraine, Parkinson's disease, multiple sclerosis, stroke, dizziness, schizophrenia, depression, addiction, motion sickness and sleep disorders including narcolepsy, and other diseases including asthma, allergy, allergy-induced airway responses, congestion, chronic obstructive pulmonary disease and gastro-intestinal disorders.
- the invention relates to a method for the treatment and/or prevention of diseases which are associated with the modulation of H3 receptors.
- diseases are obesity, metabolic syndrome (syndrome X), neurological diseases including Alzheimer's disease, dementia, age-related memory dysfunction, mild cognitive impairment, cognitive deficit, attention deficit hyperactivity disorder, epilepsy, neuropathic pain, inflammatory pain, migraine, Parkinson's disease, multiple sclerosis, stroke, dizziness, schizophrenia, depression, addiction, motion sickness and sleep disorders including narcolepsy, and other diseases including asthma, allergy, allergy- induced airway responses, congestion, chronic obstructive pulmonary disease and gastrointestinal disorders.
- a method for the treatment and/or prevention of obesity is preferred.
- the invention further relates to the use of compounds of formula I as defined above for the treatment and/or prevention of diseases which are associated with the modulation of H3 receptors.
- diseases are obesity, metabolic syndrome (syndrome X), neurological diseases including Alzheimer's disease, dementia, age-related memory dysfunction, mild cognitive impairment, cognitive deficit, attention deficit hyperactivity disorder, epilepsy, neuropathic pain, inflammatory pain, migraine, Parkinson's disease, multiple sclerosis, stroke, dizziness, schizophrenia, depression, addiction, motion sickness and sleep disorders including narcolepsy, and other diseases including asthma, allergy, allergy-induced airway responses, congestion, chronic obstructive pulmonary disease and gastro-intestinal disorders.
- the use of compounds offormula I as defined above for the treatment and/or prevention of obesity is preferred.
- the invention relates to the use of compounds offormula I as defined above for the preparation of medicaments for the treatment and/or prevention of diseases which are associated with the modulation of H3 receptors.
- diseases are obesity, metabolic syndrome (syndrome X), neurological diseases including Alzheimer's disease, dementia, age-related memory dysfunction, mild cognitive impairment, cognitive deficit, attention deficit hyperactivity disorder, epilepsy, neuropathic pain, inflammatory pain, migraine, Parkinson's disease, multiple sclerosis, stroke, dizziness, schizophrenia, depression, addiction, motion sickness and sleep disorders including narcolepsy, and other diseases including asthma, allergy, allergy-induced airway responses, congestion, chronic obstructive pulmonary disease and gastro-intestinal disorders.
- the use of compounds of formula I as defined above for the preparation of medicaments for the treatment and/or prevention of obesity is preferred.
- the compounds offormula I and their pharmaceutically acceptable salts possess valuable pharmacological properties. Specifically, it has been found that the compounds of the present invention are good histamine 3 receptor (H3R) antagonists and/or inverse agonists.
- H3R histamine 3 receptor
- Binding Buffer 50 mM Tris-HCl pH 7.4 and 5 mM MgCl 2 x 6H 2 O pH 7.4.
- Washing Buffer 50 mM Tris-HCl pH 7.4 and 5 mM MgCl 2 x6H 2 O and 0.5 M NaCI pH 7.4.
- Indirect measurement of affinity of H3R inverse agonists twelve increasing concentrations (ranging from 10 ⁇ M to 0.3 nM) ofthe selected compounds were always tested in competition binding experiments using membrane ofthe human HR3-CHO cell line. An appropriate amount of protein, e.g.
- the compounds ofthe present invention exhibit Ki values within the range of about 1 nM to about 1000 nM, preferably of about 1 nM to about 100 nM, and more preferably of about 1 nM to about 30 nM.
- the following table shows measured values for some selected compounds ofthe present invention.
- the compounds offormula (I) and their pharmaceutically acceptable salts and esters can be used as medicaments, e.g. in the form of pharmaceutical preparations for enteral, parenteral or topical administration. They can be administered, for example, perorally, e.g. in the form of tablets, coated tablets, dragees, hard and soft gelatine capsules, solutions, emulsions or suspensions, rectally, e.g. in the form of suppositories, parenteraily, e.g. in the form of injection solutions or infusion solutions, or topically, e.g. in the form of ointments, creams or oils.
- perorally e.g. in the form of tablets, coated tablets, dragees, hard and soft gelatine capsules, solutions, emulsions or suspensions, rectally, e.g. in the form of suppositories, parenteraily, e.g. in the form of injection solutions or infusion solutions, or topically,
- the production ofthe pharmaceutical preparations can be effected in a manner which will be familiar to any person skilled in the art by bringing the described compounds offormula (I) and their pharmaceutically acceptable, into a galenical administration form together with suitable, non-toxic, inert, therapeutically compatible solid or liquid carrier materials and, if desired, usual pharmaceutical adjuvants.
- Suitable carrier materials are not only inorganic carrier materials, but also organic carrier materials.
- lactose, corn starch or derivatives thereof, talc, stearic acid or its salts can be used as carrier materials for tablets, coated tablets, drag ⁇ es and hard gelatine capsules.
- Suitable carrier materials for soft gelatine capsules are, for example, vegetable oils, waxes, fats and semi-solid and liquid polyols (depending on the nature of the active ingredient no carriers are, however, required in the case of soft gelatine capsules).
- Suitable carrier materials for the production of solutions and syrups are, for example, water, polyols, sucrose, invert sugar and the like.
- Suitable carrier materials for injection solutions are, for example, water, alcohols, polyols, glycerol and vegetable oils.
- Suitable carrier materials for suppositories are, for example, natural or hardened oils, waxes, fats and semi-liquid or liquid polyols.
- Suitable carrier materials for topical preparations are glycerides, semi-synthetic and synthetic glycerides, hydrogenated oils, liquid waxes, liquid paraffins, liquid fatty alcohols, sterols, polyethylene glycols and cellulose derivatives.
- Usual stabilizers preservatives, wetting and emulsifying agents, consistency- improving agents, flavour-improving agents, salts for varying the osmotic pressure, buffer substances, solubilizers, colorants and masking agents and antioxidants come into consideration as pharmaceutical adjuvants.
- the dosage ofthe compounds offormula (I) can vary within wide limits depending on the disease to be controlled, the age and the individual condition ofthe patient and the mode of administration, and will, of course, be fitted to the individual requirements in each particular case. For adult patients a daily dosage of about 1 mg to about 1000 mg, especially about 1 mg to about 100 mg, comes into consideration. Depending on the dosage it is convenient to administer the daily dosage in several dosage units.
- the pharmaceutical preparations conveniently contain about 0.1-500 mg, preferably 0.5-100 mg, of a compound offormula (I).
- Example 1 According to the procedure described for the synthesis of Example 1 the title compound was synthesized from (5-hydroxy-lH-indol-2-yl)-morpholin-4-yl- methanone and 1-isopropyl-pyrrolidinol which was yielded in 8 % as white solid. MS (m/e): 358.1 (MH + , 100%).
- Step 1 (3,4-Dihydro-lH-isoquinolin-2-yl)-(5-hydroxy-lH-indol-2-yl)-methanone
- Example 1 According to the procedure described for the synthesis of Example 1 / stepl (3,4- dihydro- lH-isoquinolin-2-yl) - ( 5-hydroxy- lH-indol-2-yl) -methanone was synthesized from 5-hydroxy-indole-2-carboxylic acid and 1,2,3,4-tetrahydro-isoquinoline which was yielded in 72 % as white solid. MS (m/e): 293.0 (MH + , 100%).
- Step 2 (3,4-dihydro-lH-isoquinolin-2-yl)-[5-(l-isopropyl-pyrrolidin-3-yloxy)-lH- indol-2-yl] -methanone
- Example 4 According to the procedure described for the synthesis of Example 1 / step 2 the title compound was synthesized from (3,4-dihydro-lH-isoquinolin-2-yl)-(5-hydroxy-lH- indol-2-yl) -methanone and 1-isopropyl-pyrrolidinol which was yielded in 28 % as white solid. MS (m/e): 404.5 (MH + , 100%).
- Example 4 Example 4
- Example 1 According to the procedure described for the synthesis of Example 1 the title compound was synthesized from (3,4-dihydro-lH-isoquinolin-2-yl)-(5-hydroxy-lH- indol-2-yl) -methanone and l-methyl-2-pyrrolidineethanol (commercially available) which was yielded in 3 % as white solid. MS (m/e): 404.5 (MH + , 100%).
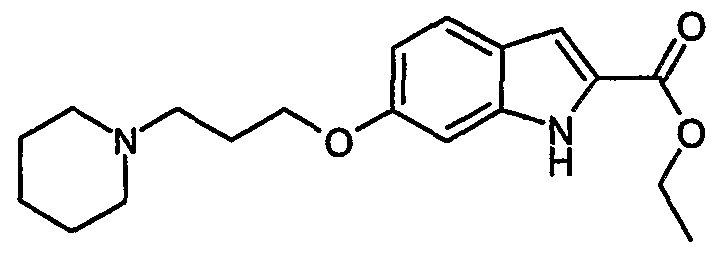
- Step 1 5-(l-isopropyl-piperidin-4-yloxy)-lH-indole-2-carboxylic acid ethyl ester
- 5-(l- isopropyl-piperidin-4-yloxy)-lH-indole-2-carboxylic acid ethyl ester was synthesized from 5-hydroxy-lH-indole-2-carboxylic acid ethyl ester (commercially available) and 1- isopropyl-piperidin-4-ol (commercially available). The title compound was yielded in 33 % as off-white solid. MS (m/e): 331.1 (MH + , 100%).
- Example 5 / step 25-(l- isopropyl-piperidin-4-yloxy)-lH-indole-2-carboxylic acid was synthesized from 5-(l- isopropyl- ⁇ iperidin-4-yloxy)-lH-indole-2-carboxylic acid ethyl ester with lithium hydroxide monohydrate.
- Step 1 5-[2-(l-methyl-pyrrolidin-2-yl)-ethoxy]-lH-indole-2-carboxylic acid ethyl ester
- 5-[2-(l- methyl-pyrrolidin-2-yl)-ethoxy]-lH-indole-2-carboxylic acid ethyl ester was synthesized from 5-hydroxy-lH-indole-2-carboxylic acid ethyl ester (commercially available) and 2- (l-methyl-pyrrolidin-2-yl) -ethanol (commercially available). The title compound was yielded in 38 % as light brown foam. MS (m/e): 317.1 (MH + , 100%).
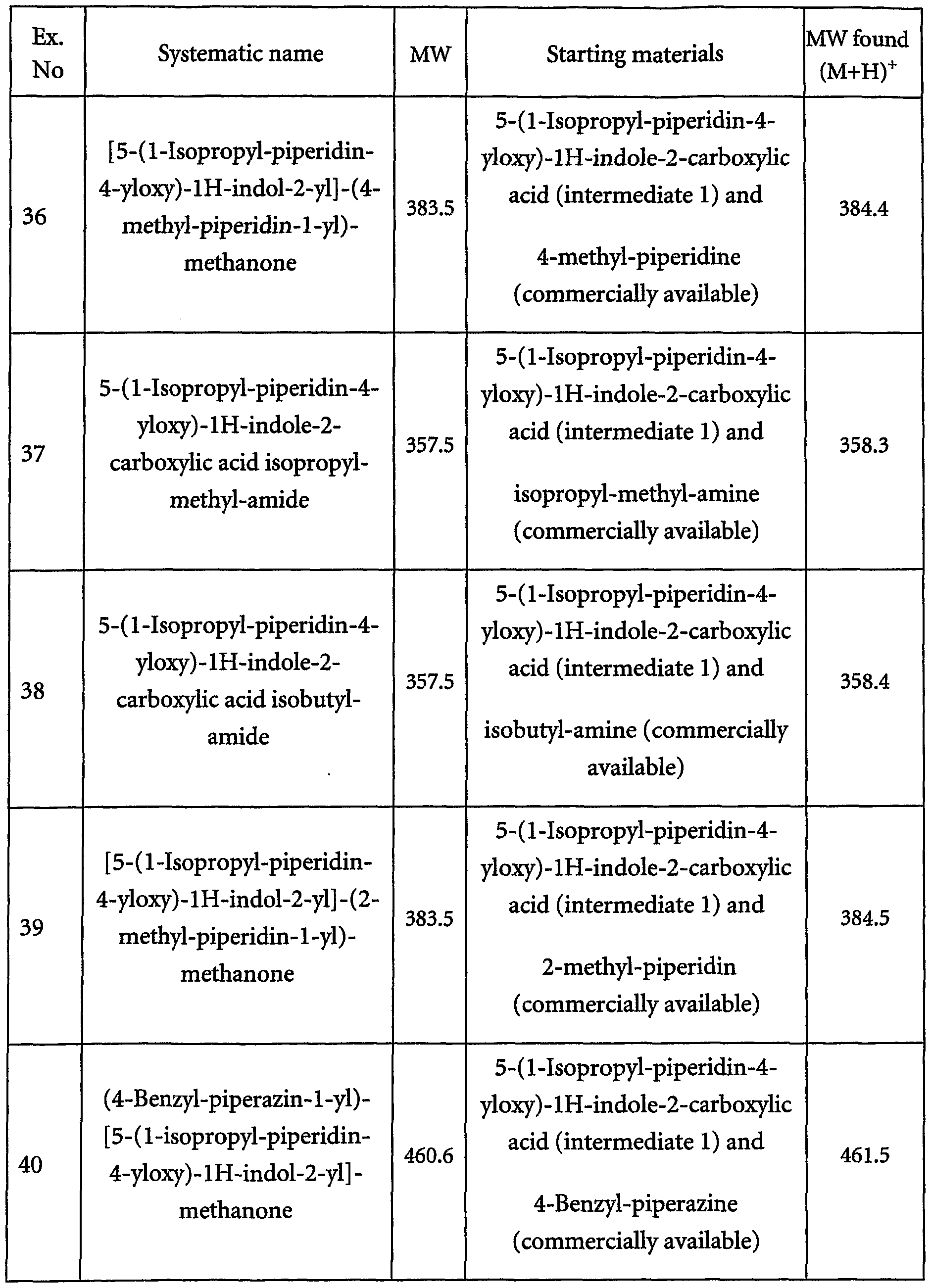
- Example 5 further indole derivatives have been synthesized from 5-[2-(l-methyl-pyrrolidin-2-yl)-ethoxy]-lH- indole-2-carboxylic acid, 5-(l-isopropyl-piperidin-4-yloxy)-lH-indole-2-carboxylic acid or 5-(l-isopropyl-pyrrolidin-3-yloxy)-lH-indole-2-carboxylic acid respectively through the coupling procedure described for Example5 / step 3 with the respective amine mentioned in table 1.
- the purification procedure has been adapted due to precipitation ofthe respective compound from the respective mixture. In those cases the title compound was filtered off, washed with methanol (containing HCL in case of example 85) and diethyl ether and dried. The results are shown in Table 1 and comprise Example 6 to Example 134. Table 1
- Step 1 5-((S)-l-Benzyl-pyrrolidin-3-yloxy)-lH-indole-2-carboxylic acid ethyl ester A mixture of 20.5 g (0.1 mol) ethyl-5-hydroxyindole-2-carboxylate, 23 g ( 0.13 mol)
- Step 4 [5-((S)-l-Isopropyl-pyrrolidin-3-yloxy)-lH-indol-2-yl]-morpholin-4-yl- methanone
- a mixture of 315 mg (1 mmol) Morpholin-4-yl-[5-((S)-pyrrolidin-3-yloxy)-lH- indol-2-yl] -methanone 615 mg (5 mmol) 2-bromopropane and 173 mg (1.25 mmol) K 2 CO 3 in 3 ml DMF was heated to 50 °C for 16 h.
- Step 1 6-(l-Isopropyl-pyrrolidin-3-yloxy)-lH-indole-2-carboxylic acid ethyl ester
- 6-(l- isopropyl-piperidin-4-yloxy)-lH-indole-2-carboxylic acid ethyl ester was synthesized from 6-hydroxy-lH-indole-2-carboxylic acid ethyl ester and l-isopropyl-piperidin-4-ol (commercially available). The title compound was yielded in 15 % as light brown solid. MS (m/e): 331.1 (MH + , 100%).
- Example 138 / step 1 6- [2- ( l-methyl-pyrrolidin-2-yl)-ethoxy] - lH-indole-2-carboxylic acid ethyl ester was synthesized from 6-hydroxy-lH-indole-2-carboxylic acid ethyl ester and 2-(l-methyl- pyrrolidin-2-yl) -ethanol (commercially available). The titie compound was yielded in 77 % as light brown oil. MS (m/e): 317.3 (MH + , 100%).
- Example 138 / step 2 further indole derivatives have been synthesized from 6-[2-(l-methyl-pyrrolidin-2-yl)- ethoxy]-lH-indole-2-carboxylic acid ethyl ester, 6-(l-isopropyl-piperidin-4-yloxy)-lH- indole-2-carboxylic acid ethyl ester, 6-(l-isopropyl-pyrrolidin-3-yloxy)-lH-indole-2- carboxylic acid ethyl ester or 6-(3-Piperidin-l-yl-propoxy)-lH-indole-2-carboxylic acid ethyl ester, respectively, with the respective amine mentioned in Table 2.
- Table 2 The results are shown in Table 2 and comprise Example 139 to Example 162.
- Step 2 6-Benzyloxy-5-fluoro-lH-indole-2-carboxylic acid methyl ester
- a mixture of methyl 2-azidoacetate, 4-benzyloxy-3-fluoro-benzaldehyde and sodium methanolate (in methanol) in toluene was reacted for 3 h at 0 °C.
- the residue after filtration ofthe suspension was washed with methanol, partitioned between ethyl acetate and ammonium chloride solution and extracted with ethyl acetate .
- the combined organic layers were dried with Na 2 SO , evaporated to dryness and the residue taken up in p-xylene and brought to reflux temperature for 2 h.
- Step 3 5-Fluoro-6-hydroxy-lH-indole-2-carboxylic acid methyl ester
- a solution of 20.2 g (0.067 mol) 6-benzyloxy-5-fluoro-lH-indole-2-carboxylic acid methyl ester in 800 ml ethyl acetate was treated with 2 g (10%) Pd/C and hydrogenated at 1 bar for 2 h. After filtration and evaporation the residue was re-crystallised from ethyl acetate. The crystals were filtered off washed with diethyl ether and dried at 40 °C under vacuum to yield 10.9 g (74 %) ofthe titie compound as white crystals. MS (m/e): 208.1 (MH " , 100%).
- Example 5 According to the procedure described for the synthesis of Example 5 (step 2) the titie compound was synthesized starting from 5-fluoro-6-hydroxy-lH-indole-2-carboxylic acid methyl ester and l-isopropyl-piperidin-4-ol (commercially available) in 48 % yield as white crystals. MS (m/e):335.4 (MH + , 100%).
- the titie compound was synthesized starting from 5-fluoro-6-(l-isopropyl-piperidin-4-yloxy)-lH- indole-2-carboxylic acid methyl ester and lithium hydroxide and used without further purification in the consecutive step.
- Example 5 According to the procedure described for the synthesis of Example 5 (step 3) the titie compound was synthesized starting from 5-fluoro-6-(l-isopropyl-piperidin-4-yloxy)-lH- indole-2-carboxylic acid and morpholine (commercially available) in 68 % yield. MS (m/e): 390.4(MH + , 100%).
- Example 164 According to the procedure described above for the synthesis of [5-fluoro-6-(l- isopropyl-piperidin-4-yloxy)-lH-indol-2-yl]-morpholin-4-yl-methanone (Example 164) the title compound was synthesized from 5-fluoro-6-(l-isopropyl-piperidin-4-yloxy)-lH- indole-2-carboxylic acid and thiomorpholine (commercially available). MS (m/e): 406.3 (MH + , 100%).
- Example 166 According to the procedure described above for the synthesis of [5-fluoro-6-(l- isopropyl-piperidin-4-yloxy)-lH-indol-2-yl]-morpholin-4-yl-methanone (Example 164) the title compound was synthesized from 5-fluoro-6-(l-isopropyl-piperidin-4-yloxy)-lH- in
- Example 5/ step 3 the titie compound was synthesized from [5-(l-isopropyl-piperidin-4-yloxy)-lH-indole-2- carboxylic acid and 3,3'-difluoropiperidine (commercially available). MS (m/e): 406.6 (MH + , 100%).
- Step 1 [5-((S)-l-Benzyl-pyrrolidin-3-yloxy)-lH-indol-2-yl]-(4,4-difluoro-piperidin-l- yl) -methanone
- Example 135 According to the procedure described for Example 135 the title compound was synthesised from 5-((S)-l-benzyl-pyrrolidin-3-yloxy)-lH-indole-2-carboxylic acid ethyl ester and 4,4-difluoropiperidine. MS (m/e): 440.4 (MH + , 100%).
- the titie compound was synthesised from (4,4-difluoro-piperidin-l-yl)-[5-((S)-pyrrolidin-3-yloxy)-lH-indol-2- yl] -methanone and 2-iodopropane.
- Step 1 5-((S)-l-Isopropyl-pyrrolidin-3-yloxy)-lH-indole-2-carboxylic acid ethyl ester
- Step 3 [5-((S)-l-Isopropyl-pyrrolidin-3-yloxy)-lH-indol-2-yl]-pyrrolidin-l-yl- methanone
- the titie compound was synthesised from 5-((S)-l-isopropyl-pyrrolidin-3-yloxy)-lH-indole-2- carboxylic acid 1:1 hydrochloride and pyrrolidine under coupling conditions employing TBTU and DIPEA in DMF.
- the crude product was purified over silica eluting with a gradient formed from DCM/MeOH(2 N NH 3 ) 98/2 to 94/6.
- the product fractions were evaporated to yield the title compound as off-white solid, (m/e): 342.3 (MH + , 100%).
- the titie compound was synthesized starting from 5-fluoro-6-(3-piperidin-l-yl-propoxy)-lH- indole-2-carboxylic acid methyl ester and lithium hydroxide and used without further purification in the consecutive step.
- Step 3 (4,4-Difluoro-piperidin-l-yl)-[5-fluoro-6-(3-piperidin-l-yl-propoxy)-lH- indol-2-yl] -methanone
- Example 179 According to the procedure described for the synthesis of Example 5 (step 3) the titie compound was synthesized starting from 5-fluoro-6-(3-piperidin-l-yl-propoxy)-lH- indole-2-carboxylic acid and 4,4'-difluoropiperidine (commercially available). MS (m/e): 424.5 (MH + , 100%).
- Example 179
- Example 5 According to the procedure described for the synthesis of Example 5 (step 3) the titie compound was synthesized starting from 5-fluoro-6-(3-piperidin-l-yl-propoxy)-lH- indole-2-carboxylic acid and morpholine (commercially available). MS (m/e): 390.5 (MH + , 100%).
- Example 172 (4,4-Difluoro-piperidin-l-yl)-ri-isopropyl-5-(l-isopropyl-piperidin-4-yloxy)-li : j r -indol- 2-yll -methanone
- the titie compound was synthesized starting from (4,4-difluoro-piperidin- 1-yl) -[5- (1 -isopropyl- piperidin-4-yloxy)-lH-indol-2-yl] -methanone (Example 102) and 2-bromopropane (commercially available).
- Example 172 According to the procedure described for the synthesis of Example 172 the titie compound was synthesized starting from (4,4-difluoro-piperidin- 1-yl) -[5- (1 -isopropyl- piperidin-4-yloxy)-lH-indol-2-yl] -methanone (Example 102) and 2-bromoethyl methyl ether (commercially available). MS (m/e): 464.6 (MH + , 100%).
- Example 172 According to the procedure described for the synthesis of Example 172 the titie compound was synthesized starting from (4,4-difluoro-piperidin-l-yl)-[5-(l-isopropyl- piperidin-4-yloxy)-lH-indol-2-yl] -methanone (Example 102) and bromoethane (commercially available). MS (m/e): 434.5 (MH + , 100%).
- Example 183 Example 183
- Example 172 According to the procedure described for the synthesis of Example 172 the titie compound was synthesized starting from (4,4-difluoro-piperidin-l-yl)-[5-(l-isopropyl- piperidin-4-yloxy)-lH-indol-2-yl] -methanone (Example 102) and 2,2,2 -trifluoroethyl trifluoromethanesulfonate (commercially available). MS (m/e): 434.5 (MH + , 100%).
- Example 172 According to the procedure described for the synthesis of Example 172 the titie compound was synthesized starting from (4,4-difluoro-piperidin-l-yl)-[5-(l-isopropyl- piperidin-4-yloxy)-lH-indol-2-yl] -methanone (Example 102) and bromomethyl cyclopropane (commercially available). MS (m/e): 434.5 (MH + , 100%).
- Example 185 Example 185
- Example 172 According to the procedure described for the synthesis of Example 172 the titie compound was synthesized starting from [5-(l-isopropyl-piperidin-4-yloxy)-lH-indol-2- yl] -pyrrolidin- 1 -yl-methanone (Example 90) and 2,2,2 -trifluoroethyl trifluoromethanesulfonate (commercially available). MS (m/e): 437.5 (MH + , 100%).
- Example 172 [5-(l-Isopropyl-piperidin-4-yloxy)-l-(2,2,2-trifluoro-ethyl)-lJ ⁇ : J r -indol-2-yl1-morpholin- 4-yl-methanone According to the procedure described for the synthesis of Example 172 the titie compound was synthesized starting from [5-(l-isopropyl-piperidin-4-yloxy)-lH-indol-2- yl]-morpholin-4-yl-methanone (Example 92) and 2,2,2-trifluoroethyl trifluoromethanesulfonate (commercially available). MS (m/e): 454.5 (MH + , 100%).
- Example 187 Example 187
- Example 172 According to the procedure described for the synthesis of Example 172 the title compound was synthesized starting from (3,3-difluoro-piperidin-l-yl)-[5-(l-isopropyl- piperidin-4-yloxy)-lH-indol-2-yl] -methanone (Example 174) and 2,2,2-trifluoroethyl trifluoromethanesulfonate (commercially available). MS (m/e): 488.5 (MH + , 100%).
- Example 172 According to the procedure described for the synthesis of Example 172 the titie compound was synthesized starting from (4,4-difluoro-piperidin-l-yl)-[5-(l-isopropyl- piperidin-4-yloxy)-lH-indol-2-yl]-methanone (Example 102) and 1,3,2-dioxathiolane- 2,2-dioxide (commercially available). MS (m/e): 488.5 (MH + , 100%).
- Example 189 Example 189
- Example 172 According to the procedure described for the synthesis of Example 172 the titie compound was synthesized starting from (4,4-difluoro-piperidin-l-yl)-[5-(l-isopropyl- piperidin-4-yloxy)-lH-indol-2-yl] -methanone (Example 102) and methanesulfonyl chloride (commercially available). MS (m/e): 484.5 (MH + , 100%).
- Example 172 1- [2-(4,4-Difluoro-piperidine-l-carbonyl)-5-( l-isopropyl-piperidin-4-yloxy) -indol- 1-yll ⁇ ethanone
- the titie compound was synthesized starting from (4,4-difluoro-piperidin-l-yl)-[5-(l-isopropyl- piperidin-4-yloxy)-lH-indol-2-yl] -methanone (Example 102) and acetyl chloride (commercially available).
- Step 1 4-[2-(4,4-Difluoro-piperidine-l-carbonyl)-l-methyl-lH-indol-5-yloxy]-l- isopropyl-1-methyl-piperidinium as monomethylsulfate salt
- Step 2 (4,4-Difluoro-piperidin-l-yl)-[5-(l-isopropyl-piperidin-4-yloxy)-l-methyl-lH- indol-2-yl] -methanone
- 4-[2-(4,4-difluoro-piperidine-l-carbonyl)-l-methyl-lH-indol-5- yloxy]-l-isopropyl-l-methyl-piperidinium as monomethylsulfate salt 120 mg, 0.2 mmol, 1.0 eq.
- lithium hydride 5 mg, 0.5 mmol, 2.5 eq.
- N,N-dimethylformamide 0.5 mL
- ethanethiol 0.05 mL, 0.6 mmol, 2.7 eq.
- Step 1 l-Cyclopropylmethyl-piperidin-4-one
- Example 1/ Step 2 the titie compound was synthesized from (5-hydroxy-lH-indol-2-yl)-morpholin-4-yl-methanone (Example 1, Step 1) and l-cyclopropylmethyl-piperidin-4-ol (Example 192, Step 2). (m/e): 384.4 (MH + , 100%).
- Example 5 According to the procedure described for the synthesis of Example 5 / step 2 [5-(3- chloro-propoxy)-lH-indole-2-carboxylic acid was synthesized from 5-(3-chloro- propoxy)-lH-indole-2-carboxylic acid ethyl ester. The titie compound was yielded in 98 % as an off-white solid. MS (m/e): 253.1 (M, 100%).
- Step 4 (4,4-Difluoro-piperidin-l-yl)- ⁇ 5-[3-(methyl-propyl-amino)-propoxy]-lH- indol-2-yl ⁇ methanone as formic acid salt
- Example 194 / Step 4 further indole derivatives have been synthesized from [5-(3-chloro-propoxy)-lH-indol-2- yl]- (4,4-difluoro-piperidin- 1-yl) -methanone with the respective amine mentioned in Table 3. The results are shown in Table 3 and comprise Example 195 to Example 208.
- Step 1 [5-(3-Chloro-propoxy)-lH-indol-2-yl]-morpholin-4-yl-methanone
- [5-(3- chloro-propoxy)-lH-indol-2-yl]-morpholin-4-yl-methanone was synthesized from 5-(3- chloro-propoxy)-lH-indole-2-carboxylic acid and morpholine (commercially available). The title compound was yielded in 92 % as an off-white solid. MS (m/e): 323.9 (MH + , 100%).
- Step 2 ⁇ 5-[3-(4,4-Difluoro-piperidin-l-yl)-propoxy]-lH-indol-2-yl ⁇ -morpholin-4-yl- methanone
- Example 194 / step 4 ⁇ 5- [ 3- ( 4,4-difluoro-piperidin- 1 -yl) -propoxy] - lH-indol-2-yl ⁇ -morpholin-4-yl-methanone was synthesized from [5-(3-chloro-propoxy)-lH-indol-2-yl]-morpholin-4-yl-methanone and 4,4'-difluoropiperidine hydrochloride (commercially available). The titie compound was yielded in 54 % as a brown solid. MS (m/e): 408.5 (MH + , 100%).
- Example 210 f5- ( l-Cyclopropyl-piperidin-4-yloxy)-lH-indol-2-yn-morpholin-4-yl-methanone
- Step 1 3-[Cyclopropyl-(2-ethoxycarbonyl-ethyl)-amino] -propionic acid ethyl ester
- Step 2 l-Cyclopropyl-piperidin-4-one
- 3- [cyclopropyl-(2-ethoxycarbonyl-ethyl)-amino] -propionic acid ethyl ester (10.0 g, 39 mmol, 1.0 eq.) in anhydrous tetiahydrofuran (65 mL) was added dropwise to a solution of sodium hydride (60% oil dispersion , 2.33 g, 58 mmol, 1.5 eq.) in anhydrous tetrahydrofuran (65 mL). Absolute ethanol (1.79 g, 39 mmol, 1.0 eq.) was then added.
- Example 1 According to the procedure described for the synthesis of Example 1 / step 2 [5-(l- cyclopropyl-piperidin-4-yloxy)-lH-indol-2-yl]-morpholin-4-yl-methanone was synthesized from (5-hydroxy-lH-indol-2-yl)-morpholin-4-yl-methanone (Example 1, Step 1) and l-cyclopropyl-piperidin-4-ol (Example 201, Step 3). The titie compound was yielded in 14 % as a white solid. MS (m/e): 370.5 (MH + , 100%).
- Film coated tablets containing the following ingredients can be manufactured in a conventional manner:
- the active ingredient is sieved and mixed with microcristalline cellulose and the mixture is granulated with a solution of polyvinylpyrrolidon in water.
- the granulate is mixed with sodium starch glycolate and magesiumstearate and compressed to yield kernels of 120 or 350 mg respectively.
- the kernels are lacquered with an aqueous solution / suspension ofthe above mentioned film coat.
- Capsules containing the following ingredients can be manufactured in a conventional manner:
- the components are sieved and mixed and filled into capsules of size 2.
- Injection solutions can have the following composition:
- Soft gelatin capsules containing the following ingredients can be manufactured in a conventional manner:
- Example E The active ingredient is dissolved in a warm melting ofthe other ingredients and the mixture is filled into soft gelatin capsules of appropriate size.
- the filled soft gelatin capsules are treated according to the usual procedures.
- Sachets containing the following ingredients can be manufactured in a conventional manner:
- Microcristalline cellulose (ANICEL PH 102) 1400.0 mg
- Flavoring additives 1.0 mg
- the active ingredient is mixed with lactose, microcristalline cellulose and sodium carboxymethyl cellulose and granulated with a mixture of polyvinylpyrrolidone in water.
- the granulate is mixed with magnesiumstearate and the flavouring additives and filled into sachets.
Landscapes
- Health & Medical Sciences (AREA)
- Chemical & Material Sciences (AREA)
- Organic Chemistry (AREA)
- Engineering & Computer Science (AREA)
- Bioinformatics & Cheminformatics (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Public Health (AREA)
- General Chemical & Material Sciences (AREA)
- Medicinal Chemistry (AREA)
- Nuclear Medicine, Radiotherapy & Molecular Imaging (AREA)
- Veterinary Medicine (AREA)
- Pharmacology & Pharmacy (AREA)
- Life Sciences & Earth Sciences (AREA)
- Animal Behavior & Ethology (AREA)
- General Health & Medical Sciences (AREA)
- Neurology (AREA)
- Neurosurgery (AREA)
- Biomedical Technology (AREA)
- Pain & Pain Management (AREA)
- Pulmonology (AREA)
- Psychiatry (AREA)
- Diabetes (AREA)
- Hematology (AREA)
- Obesity (AREA)
- Urology & Nephrology (AREA)
- Heart & Thoracic Surgery (AREA)
- Cardiology (AREA)
- Vascular Medicine (AREA)
- Immunology (AREA)
- Addiction (AREA)
- Hospice & Palliative Care (AREA)
- Child & Adolescent Psychology (AREA)
- Psychology (AREA)
- Anesthesiology (AREA)
- Pharmaceuticals Containing Other Organic And Inorganic Compounds (AREA)
- Plural Heterocyclic Compounds (AREA)
- Low-Molecular Organic Synthesis Reactions Using Catalysts (AREA)
Abstract
Description
Claims
Priority Applications (14)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| BRPI0512335-6A BRPI0512335A (en) | 2004-06-21 | 2005-06-13 | compounds, process for their manufacture, pharmaceutical compositions comprising them, method for treating and / or preventing diseases and their use |
| AU2005254658A AU2005254658B2 (en) | 2004-06-21 | 2005-06-13 | Indole derivatives as histamine receptor antagonists |
| PL05756581T PL1761519T3 (en) | 2004-06-21 | 2005-06-13 | Indole derivatives as histamine receptor antagonists |
| CA2569611A CA2569611C (en) | 2004-06-21 | 2005-06-13 | Indole derivatives as histamine receptor antagonists |
| NZ551663A NZ551663A (en) | 2004-06-21 | 2005-06-13 | Indole derivatives as histamine receptor antagonists |
| ES05756581T ES2306170T3 (en) | 2004-06-21 | 2005-06-13 | DERIVATIVES OF INDOL AS REDISTING ANTAGONISTS OF HISTAMINE. |
| DK05756581T DK1761519T3 (en) | 2004-06-21 | 2005-06-13 | Indole derivatives as histamine receptor antagonists |
| DE602005006567T DE602005006567D1 (en) | 2004-06-21 | 2005-06-13 | INDOLE DERIVATIVES AS HISTAMINE RECEPTOR ANTAGONISTS |
| CN2005800204455A CN1972926B (en) | 2004-06-21 | 2005-06-13 | Indole derivatives as histamine receptor antagonists |
| MXPA06014810A MXPA06014810A (en) | 2004-06-21 | 2005-06-13 | Indole derivatives as histamine receptor antagonists. |
| JP2007517135A JP4652404B2 (en) | 2004-06-21 | 2005-06-13 | Indole derivatives as histamine receptor antagonists |
| EP05756581A EP1761519B1 (en) | 2004-06-21 | 2005-06-13 | Indole derivatives as histamine receptor antagonists |
| IL179737A IL179737A (en) | 2004-06-21 | 2006-11-30 | Indole derivatives, pharmaceutical compositions comprising them and use thereof in the preparation of medicaments for treating or preventing diseases associated with modulation of h3 receptors |
| NO20070336A NO20070336L (en) | 2004-06-21 | 2007-01-18 | Indole derivatives as histamine receptor antagonists |
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| EP04102839.0 | 2004-06-21 | ||
| EP04102839 | 2004-06-21 |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| WO2005123716A1 true WO2005123716A1 (en) | 2005-12-29 |
Family
ID=34982342
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| PCT/EP2005/006303 Ceased WO2005123716A1 (en) | 2004-06-21 | 2005-06-13 | Indole derivatives as histamine receptor antagonists |
Country Status (23)
| Country | Link |
|---|---|
| US (1) | US7361682B2 (en) |
| EP (1) | EP1761519B1 (en) |
| JP (1) | JP4652404B2 (en) |
| CN (1) | CN1972926B (en) |
| AR (1) | AR049696A1 (en) |
| AT (1) | ATE394391T1 (en) |
| AU (1) | AU2005254658B2 (en) |
| BR (1) | BRPI0512335A (en) |
| CA (1) | CA2569611C (en) |
| DE (1) | DE602005006567D1 (en) |
| DK (1) | DK1761519T3 (en) |
| ES (1) | ES2306170T3 (en) |
| IL (1) | IL179737A (en) |
| MX (1) | MXPA06014810A (en) |
| MY (1) | MY139941A (en) |
| NO (1) | NO20070336L (en) |
| NZ (1) | NZ551663A (en) |
| PL (1) | PL1761519T3 (en) |
| PT (1) | PT1761519E (en) |
| RU (1) | RU2382778C2 (en) |
| TW (1) | TW200612936A (en) |
| WO (1) | WO2005123716A1 (en) |
| ZA (1) | ZA200610416B (en) |
Cited By (12)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2006077024A3 (en) * | 2005-01-19 | 2006-09-08 | Hoffmann La Roche | 5-aminoindole derivatives |
| WO2007062997A1 (en) * | 2005-11-30 | 2007-06-07 | F. Hoffmann-La Roche Ag | 1,1-dioxo-thiomorpholinyl indolyl methanone derivatives for use as h3 modulators |
| WO2007063000A1 (en) * | 2005-11-30 | 2007-06-07 | F. Hoffmann-La Roche Ag | 5-substituted indole-2-carboxamide derivatives |
| WO2007062999A3 (en) * | 2005-11-30 | 2007-07-26 | Hoffmann La Roche | 1,5-substituted indol-2-yl amide derivatives |
| WO2007115938A1 (en) * | 2006-04-12 | 2007-10-18 | F. Hoffmann-La Roche Ag | 5-amido-2-carboxamide indoles |
| WO2007131907A3 (en) * | 2006-05-16 | 2008-01-24 | Hoffmann La Roche | 1h-indol-5-yl-piperazin-1-yl-methanone derivatives |
| WO2008095821A1 (en) * | 2007-02-07 | 2008-08-14 | F. Hoffmann-La Roche Ag | Indol-2-yl-piperazin-1-yl-methanone derivatives |
| WO2009158375A1 (en) * | 2008-06-25 | 2009-12-30 | Abbott Laboratories | Aza-cylic indole- 2 -carboxamides and methods of use thereof |
| JP2010534217A (en) * | 2007-07-25 | 2010-11-04 | エフ.ホフマン−ラ ロシュ アーゲー | Benzofuran- and benzo [B] thiophene-2-carboxylic acid amide derivatives and their use as histamine 3 receptor modulators |
| JP2011503210A (en) * | 2007-11-16 | 2011-01-27 | ライジェル ファーマシューティカルズ, インコーポレイテッド | Carboxamide, sulfonamide, and amine compounds for metabolic disorders |
| US8084623B2 (en) | 2006-12-19 | 2011-12-27 | Roche Palo Alto Llc | Pyrrolidinyl and piperidinyl ketone derivatives and uses thereof |
| US9328092B2 (en) | 2012-08-23 | 2016-05-03 | Suven Life Sciences Limited | Acrylamide compounds as histamine H3 receptor ligands |
Families Citing this family (14)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US7514433B2 (en) * | 2006-08-03 | 2009-04-07 | Hoffmann-La Roche Inc. | 1H-indole-6-yl-piperazin-1-yl-methanone derivatives |
| EP2079694B1 (en) | 2006-12-28 | 2017-03-01 | Rigel Pharmaceuticals, Inc. | N-substituted-heterocycloalkyloxybenzamide compounds and methods of use |
| US7557108B2 (en) * | 2007-02-07 | 2009-07-07 | Hoffmann-La Roche Inc. | (Indol-4-yl) or (indol-5-yl)-piperazinylmethanones |
| US7648979B2 (en) * | 2007-02-07 | 2010-01-19 | Hoffmann-La Roche Inc. | 5-amido-(1H-indol-2-yl)-piperazin-1-yl-methanone derivatives |
| AU2008223142A1 (en) * | 2007-03-01 | 2008-09-12 | Janssen Pharmaceutica N.V. | Indole and benzothiophene compounds as modulators of the histamine H3 receptor |
| CN101821253B (en) * | 2007-08-31 | 2013-07-17 | 安斯泰来制药有限公司 | Piperidine derivative |
| EP2231666B1 (en) * | 2007-12-12 | 2015-07-29 | Rigel Pharmaceuticals, Inc. | Carboxamide, sulfonamide and amine compounds for metabolic disorders |
| CA2722139C (en) * | 2008-04-23 | 2017-04-11 | Rigel Pharmaceuticals, Inc. | Carboxamide compounds for the treatment of metabolic disorders |
| FR2950053B1 (en) * | 2009-09-11 | 2014-08-01 | Fournier Lab Sa | USE OF BENZOIC INDOLE DERIVATIVES AS NURR-1 ACTIVATORS FOR THE TREATMENT OF PARKINSON'S DISEASE |
| EP2722329A1 (en) * | 2012-10-18 | 2014-04-23 | F. Hoffmann-La Roche AG | Indol-amide compounds as beta-amyloid inhbitors |
| KR101551313B1 (en) | 2014-07-28 | 2015-09-09 | 충남대학교산학협력단 | Novel indene derivatives, preparation method thereof, and pharmaceutical composition for use in preventing or treating blindness related diseases containing the same as an active ingredient |
| CN106065008B (en) * | 2016-06-16 | 2018-06-05 | 黑龙江中医药大学 | It is a kind of that there is the indoles -2- amides compounds for improving sleep effect and its application |
| WO2018152286A1 (en) | 2017-02-17 | 2018-08-23 | Trevena, Inc. | 7-membered aza-heterocyclic containing delta-opioid receptor modulating compounds, methods of using and making the same |
| KR20190129867A (en) * | 2017-02-17 | 2019-11-20 | 트레베나, 인코포레이티드. | 5-membered aza-heterocycle-containing delta-opioid receptor modulating compounds, and methods of using and preparing the same |
Citations (5)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| EP0624575A1 (en) * | 1993-05-12 | 1994-11-17 | Adir Et Compagnie | Substituted indoles and pharmaceutical compositions containing them |
| EP0978512A1 (en) * | 1998-07-29 | 2000-02-09 | Societe Civile Bioprojet | Non-imidazole aryloxy (or arylthio) alkylamines as histamine H3-receptor antagonists and their therapeutic applications |
| WO2001074773A2 (en) * | 2000-03-31 | 2001-10-11 | Ortho Mcneil Pharmaceutical, Inc. | Phenyl-substituted indoles as histamine h3-receptor antagonists |
| WO2002072548A2 (en) * | 2001-03-09 | 2002-09-19 | Ortho-Mcneil Pharmaceutical, Inc. | Heterocyclic compounds and their use as histamine h4 ligands. |
| WO2004000831A1 (en) * | 2002-06-24 | 2003-12-31 | Schering Corporation | Indole derivatives useful as histamine h3 antagonists |
Family Cites Families (3)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| ID27044A (en) * | 1997-12-24 | 2001-02-22 | Aventis Pharma Gmbh | INDOLE DERIVATIVES AS OBJECTORS IN FACTOR XA |
| HU227197B1 (en) * | 2000-10-24 | 2010-10-28 | Richter Gedeon Nyrt | Nmda receptor antagonist carboxylic acid amide derivatives and pharmaceutical compositions containing them |
| US6673829B2 (en) * | 2001-09-14 | 2004-01-06 | Novo Nordisk A/S | Aminoazetidine,-pyrrolidine and -piperidine derivatives |
-
2005
- 2005-06-13 CN CN2005800204455A patent/CN1972926B/en not_active Expired - Fee Related
- 2005-06-13 WO PCT/EP2005/006303 patent/WO2005123716A1/en not_active Ceased
- 2005-06-13 CA CA2569611A patent/CA2569611C/en not_active Expired - Fee Related
- 2005-06-13 RU RU2007102224/04A patent/RU2382778C2/en not_active IP Right Cessation
- 2005-06-13 DE DE602005006567T patent/DE602005006567D1/en not_active Expired - Lifetime
- 2005-06-13 DK DK05756581T patent/DK1761519T3/en active
- 2005-06-13 BR BRPI0512335-6A patent/BRPI0512335A/en not_active IP Right Cessation
- 2005-06-13 MX MXPA06014810A patent/MXPA06014810A/en active IP Right Grant
- 2005-06-13 EP EP05756581A patent/EP1761519B1/en not_active Expired - Lifetime
- 2005-06-13 PT PT05756581T patent/PT1761519E/en unknown
- 2005-06-13 ES ES05756581T patent/ES2306170T3/en not_active Expired - Lifetime
- 2005-06-13 PL PL05756581T patent/PL1761519T3/en unknown
- 2005-06-13 AT AT05756581T patent/ATE394391T1/en active
- 2005-06-13 NZ NZ551663A patent/NZ551663A/en unknown
- 2005-06-13 AU AU2005254658A patent/AU2005254658B2/en not_active Ceased
- 2005-06-13 JP JP2007517135A patent/JP4652404B2/en not_active Expired - Fee Related
- 2005-06-17 AR ARP050102510A patent/AR049696A1/en not_active Application Discontinuation
- 2005-06-20 MY MYPI20052800A patent/MY139941A/en unknown
- 2005-06-20 US US11/157,093 patent/US7361682B2/en not_active Expired - Fee Related
- 2005-06-20 TW TW094120464A patent/TW200612936A/en unknown
-
2006
- 2006-11-30 IL IL179737A patent/IL179737A/en not_active IP Right Cessation
- 2006-12-12 ZA ZA200610416A patent/ZA200610416B/en unknown
-
2007
- 2007-01-18 NO NO20070336A patent/NO20070336L/en not_active Application Discontinuation
Patent Citations (5)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| EP0624575A1 (en) * | 1993-05-12 | 1994-11-17 | Adir Et Compagnie | Substituted indoles and pharmaceutical compositions containing them |
| EP0978512A1 (en) * | 1998-07-29 | 2000-02-09 | Societe Civile Bioprojet | Non-imidazole aryloxy (or arylthio) alkylamines as histamine H3-receptor antagonists and their therapeutic applications |
| WO2001074773A2 (en) * | 2000-03-31 | 2001-10-11 | Ortho Mcneil Pharmaceutical, Inc. | Phenyl-substituted indoles as histamine h3-receptor antagonists |
| WO2002072548A2 (en) * | 2001-03-09 | 2002-09-19 | Ortho-Mcneil Pharmaceutical, Inc. | Heterocyclic compounds and their use as histamine h4 ligands. |
| WO2004000831A1 (en) * | 2002-06-24 | 2003-12-31 | Schering Corporation | Indole derivatives useful as histamine h3 antagonists |
Non-Patent Citations (1)
| Title |
|---|
| K. M. BRASHEAR ET. AL.: "Nonpeptide Glycoprotein IIB/IIIA Inhibitors. 18. Indole Alpha Sulfonamide Acids are Potent Inhibitors of Platelet Aggregation.", BIOORGANIC AND MEDICINAL CHEMISTRY LETTERS, vol. 7, no. 21, 1997, pages 2793 - 8, XP002347978 * |
Cited By (27)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US7456174B2 (en) | 2005-01-19 | 2008-11-25 | Hoffmann-La Roche Inc. | 5-aminoindole derivatives as H3 inverse agonists |
| WO2006077024A3 (en) * | 2005-01-19 | 2006-09-08 | Hoffmann La Roche | 5-aminoindole derivatives |
| US7745479B2 (en) | 2005-11-30 | 2010-06-29 | Hoffmann-La Roche Inc. | 1,5-substituted indol-2-yl amide derivatives |
| KR101124156B1 (en) | 2005-11-30 | 2012-03-23 | 에프. 호프만-라 로슈 아게 | 1,5-substituted indol-2-yl amide derivatives |
| AU2006319232B2 (en) * | 2005-11-30 | 2012-09-13 | F. Hoffmann-La Roche Ag | 1,1-dioxo-thiomorpholinyl indolyl methanone derivatives for use as H3 modulators |
| CN101316840B (en) * | 2005-11-30 | 2012-08-08 | 霍夫曼-拉罗奇有限公司 | 1,5-substituted indol-2-ylamide derivatives |
| US7388095B2 (en) | 2005-11-30 | 2008-06-17 | Hoffmann-La Roche Inc. | 5-substituted indole-2-carboxamide derivatives |
| AU2006319234B2 (en) * | 2005-11-30 | 2012-01-19 | F. Hoffmann-La Roche Ag | 1,5-substituted indol-2-yl amide derivatives |
| WO2007062997A1 (en) * | 2005-11-30 | 2007-06-07 | F. Hoffmann-La Roche Ag | 1,1-dioxo-thiomorpholinyl indolyl methanone derivatives for use as h3 modulators |
| WO2007063000A1 (en) * | 2005-11-30 | 2007-06-07 | F. Hoffmann-La Roche Ag | 5-substituted indole-2-carboxamide derivatives |
| WO2007062999A3 (en) * | 2005-11-30 | 2007-07-26 | Hoffmann La Roche | 1,5-substituted indol-2-yl amide derivatives |
| US7538101B2 (en) | 2005-11-30 | 2009-05-26 | Hoffmann-La Roche Inc. | 1,1-dioxo-thiomorpholinyl indolyl methanone derivatives |
| US7601711B2 (en) | 2006-04-12 | 2009-10-13 | Hoffmann-La Roche Inc. | 5-amido-indole-2-carboxamide derivatives |
| WO2007115938A1 (en) * | 2006-04-12 | 2007-10-18 | F. Hoffmann-La Roche Ag | 5-amido-2-carboxamide indoles |
| US7723325B2 (en) | 2006-04-12 | 2010-05-25 | Hoffman-La Roche Inc. | 5-amido-indole-2-carboxamide derivatives |
| JP2009533375A (en) * | 2006-04-12 | 2009-09-17 | エフ.ホフマン−ラ ロシュ アーゲー | 5-Amido-2-carboxamide indoles |
| US7432255B2 (en) | 2006-05-16 | 2008-10-07 | Hoffmann-La Roche Inc. | 1H-indol-5-yl-piperazin-1-yl-methanone derivatives |
| JP2009537472A (en) * | 2006-05-16 | 2009-10-29 | エフ.ホフマン−ラ ロシュ アーゲー | 1H-Indol-5-yl-piperazin-1-yl-methanone derivative |
| WO2007131907A3 (en) * | 2006-05-16 | 2008-01-24 | Hoffmann La Roche | 1h-indol-5-yl-piperazin-1-yl-methanone derivatives |
| US8084623B2 (en) | 2006-12-19 | 2011-12-27 | Roche Palo Alto Llc | Pyrrolidinyl and piperidinyl ketone derivatives and uses thereof |
| JP2010518043A (en) * | 2007-02-07 | 2010-05-27 | エフ.ホフマン−ラ ロシュ アーゲー | Indol-2-yl-piperazin-1-yl-methanone derivative |
| WO2008095821A1 (en) * | 2007-02-07 | 2008-08-14 | F. Hoffmann-La Roche Ag | Indol-2-yl-piperazin-1-yl-methanone derivatives |
| US7507736B2 (en) | 2007-02-07 | 2009-03-24 | Hoffmann-La Roche Inc. | Indol-2-yl-piperazin-1-yl-methanone derivatives |
| JP2010534217A (en) * | 2007-07-25 | 2010-11-04 | エフ.ホフマン−ラ ロシュ アーゲー | Benzofuran- and benzo [B] thiophene-2-carboxylic acid amide derivatives and their use as histamine 3 receptor modulators |
| JP2011503210A (en) * | 2007-11-16 | 2011-01-27 | ライジェル ファーマシューティカルズ, インコーポレイテッド | Carboxamide, sulfonamide, and amine compounds for metabolic disorders |
| WO2009158375A1 (en) * | 2008-06-25 | 2009-12-30 | Abbott Laboratories | Aza-cylic indole- 2 -carboxamides and methods of use thereof |
| US9328092B2 (en) | 2012-08-23 | 2016-05-03 | Suven Life Sciences Limited | Acrylamide compounds as histamine H3 receptor ligands |
Also Published As
| Publication number | Publication date |
|---|---|
| DK1761519T3 (en) | 2008-07-21 |
| RU2007102224A (en) | 2008-07-27 |
| BRPI0512335A (en) | 2008-03-04 |
| AU2005254658A1 (en) | 2005-12-29 |
| ATE394391T1 (en) | 2008-05-15 |
| TW200612936A (en) | 2006-05-01 |
| PT1761519E (en) | 2008-07-02 |
| CA2569611A1 (en) | 2005-12-29 |
| MXPA06014810A (en) | 2007-02-12 |
| JP2008503511A (en) | 2008-02-07 |
| EP1761519A1 (en) | 2007-03-14 |
| CA2569611C (en) | 2012-12-11 |
| IL179737A (en) | 2011-12-29 |
| CN1972926A (en) | 2007-05-30 |
| ZA200610416B (en) | 2008-09-25 |
| EP1761519B1 (en) | 2008-05-07 |
| US20050282864A1 (en) | 2005-12-22 |
| AU2005254658B2 (en) | 2011-06-16 |
| RU2382778C2 (en) | 2010-02-27 |
| MY139941A (en) | 2009-11-30 |
| JP4652404B2 (en) | 2011-03-16 |
| NO20070336L (en) | 2007-02-13 |
| CN1972926B (en) | 2011-02-16 |
| NZ551663A (en) | 2009-09-25 |
| PL1761519T3 (en) | 2008-09-30 |
| IL179737A0 (en) | 2007-05-15 |
| DE602005006567D1 (en) | 2008-06-19 |
| AR049696A1 (en) | 2006-08-30 |
| US7361682B2 (en) | 2008-04-22 |
| ES2306170T3 (en) | 2008-11-01 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| EP1761519B1 (en) | Indole derivatives as histamine receptor antagonists | |
| US7534891B2 (en) | Quinoline derivatives as H3R inverse agonists | |
| US7608617B2 (en) | Naphthaline derivatives as H3 inverse agonists | |
| KR100841838B1 (en) | Indole Derivatives as Histamine Receptor Antagonists | |
| HK1104554A (en) | Indole derivatives as histamine receptor antagonists |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| AK | Designated states |
Kind code of ref document: A1 Designated state(s): AE AG AL AM AT AU AZ BA BB BG BR BW BY BZ CA CH CN CO CR CU CZ DE DK DM DZ EC EE EG ES FI GB GD GE GH GM HR HU ID IL IN IS JP KE KG KM KP KR KZ LC LK LR LS LT LU LV MA MD MG MK MN MW MX MZ NA NG NI NO NZ OM PG PH PL PT RO RU SC SD SE SG SK SL SM SY TJ TM TN TR TT TZ UA UG US UZ VC VN YU ZA ZM ZW |
|
| AL | Designated countries for regional patents |
Kind code of ref document: A1 Designated state(s): GM KE LS MW MZ NA SD SL SZ TZ UG ZM ZW AM AZ BY KG KZ MD RU TJ TM AT BE BG CH CY CZ DE DK EE ES FI FR GB GR HU IE IS IT LT LU MC NL PL PT RO SE SI SK TR BF BJ CF CG CI CM GA GN GQ GW ML MR NE SN TD TG |
|
| 121 | Ep: the epo has been informed by wipo that ep was designated in this application | ||
| DPEN | Request for preliminary examination filed prior to expiration of 19th month from priority date (pct application filed from 20040101) | ||
| WWE | Wipo information: entry into national phase |
Ref document number: 2005756581 Country of ref document: EP |
|
| WWE | Wipo information: entry into national phase |
Ref document number: 551663 Country of ref document: NZ |
|
| WWE | Wipo information: entry into national phase |
Ref document number: 179737 Country of ref document: IL |
|
| WWE | Wipo information: entry into national phase |
Ref document number: 2569611 Country of ref document: CA |
|
| WWE | Wipo information: entry into national phase |
Ref document number: 2005254658 Country of ref document: AU |
|
| WWE | Wipo information: entry into national phase |
Ref document number: 200610416 Country of ref document: ZA |
|
| WWE | Wipo information: entry into national phase |
Ref document number: 12006502536 Country of ref document: PH Ref document number: PA/a/2006/014810 Country of ref document: MX |
|
| WWE | Wipo information: entry into national phase |
Ref document number: 1020067026875 Country of ref document: KR Ref document number: 06127694 Country of ref document: CO |
|
| WWE | Wipo information: entry into national phase |
Ref document number: 200580020445.5 Country of ref document: CN Ref document number: 2007517135 Country of ref document: JP |
|
| NENP | Non-entry into the national phase |
Ref country code: DE |
|
| WWW | Wipo information: withdrawn in national office |
Ref document number: DE |
|
| WWE | Wipo information: entry into national phase |
Ref document number: 7854/DELNP/2006 Country of ref document: IN |
|
| ENP | Entry into the national phase |
Ref document number: 2005254658 Country of ref document: AU Date of ref document: 20050613 Kind code of ref document: A |
|
| WWP | Wipo information: published in national office |
Ref document number: 2005254658 Country of ref document: AU |
|
| WWE | Wipo information: entry into national phase |
Ref document number: 2007102224 Country of ref document: RU |
|
| WWP | Wipo information: published in national office |
Ref document number: 2005756581 Country of ref document: EP |
|
| WWP | Wipo information: published in national office |
Ref document number: 1020067026875 Country of ref document: KR |
|
| ENP | Entry into the national phase |
Ref document number: PI0512335 Country of ref document: BR |
|
| WWG | Wipo information: grant in national office |
Ref document number: 2005756581 Country of ref document: EP |