WO2003014107A1 - Process for the preparation of heterocyclic pentalene derivatives - Google Patents
Process for the preparation of heterocyclic pentalene derivatives Download PDFInfo
- Publication number
- WO2003014107A1 WO2003014107A1 PCT/EP2002/007680 EP0207680W WO03014107A1 WO 2003014107 A1 WO2003014107 A1 WO 2003014107A1 EP 0207680 W EP0207680 W EP 0207680W WO 03014107 A1 WO03014107 A1 WO 03014107A1
- Authority
- WO
- WIPO (PCT)
- Prior art keywords
- formula
- compound
- acid
- process according
- phenyl
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Ceased
Links
- 0 C=CC(c1cc(-c2ccccc2)c(*P)[s]1)=O Chemical compound C=CC(c1cc(-c2ccccc2)c(*P)[s]1)=O 0.000 description 2
- IPBZEJZUAZXNOS-UHFFFAOYSA-N CC(C(c(cc1)ccc1Cl)C#N)=O Chemical compound CC(C(c(cc1)ccc1Cl)C#N)=O IPBZEJZUAZXNOS-UHFFFAOYSA-N 0.000 description 1
- IVYMIRMKXZAHRV-UHFFFAOYSA-N N#CCc(cc1)ccc1Cl Chemical compound N#CCc(cc1)ccc1Cl IVYMIRMKXZAHRV-UHFFFAOYSA-N 0.000 description 1
Classifications
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D333/00—Heterocyclic compounds containing five-membered rings having one sulfur atom as the only ring hetero atom
- C07D333/50—Heterocyclic compounds containing five-membered rings having one sulfur atom as the only ring hetero atom condensed with carbocyclic rings or ring systems
- C07D333/78—Heterocyclic compounds containing five-membered rings having one sulfur atom as the only ring hetero atom condensed with carbocyclic rings or ring systems condensed with rings other than six-membered or with ring systems containing such rings
Definitions
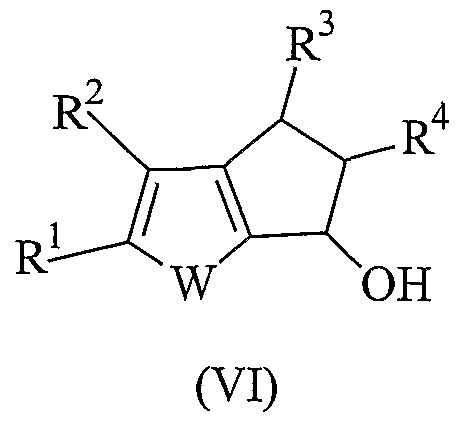
- the present invention relates to a new process for preparing heterocyclic pentalenes derivatives of formula:
- R 1 , R 2 , R 3 and R 4 represent hydrogen or hydrocarbon rests and W is an oxygen atom, a sulfur atom or a NR or PR group and R is an hydrocarbon rest.
- Heterocyclic pentalenes are well known in the art for various uses.
- substituted thiophenes and b, d-ortho-fused thiophenes are used as reference materials in the analysis of sulphur-containing substances of fossil raw materials, such as mineral oils, coal, carbonaceous oils, shale oils and tar sands, as model systems for studying the desulphurisation of the aforementioned fossil raw materials, also on a technical scale, as oxidation inhibitors, for example in lubricants and as active substances in the fields involving biocides.
- Ewen et al. describe metallocene compounds containing thiopentalene and azapentalene derivatives. Also PCT/EP00/12406 describes metallocene compounds containing thiopentalenes ligands. Catalyst based on these compounds produce polypropylene having a high degree of isotacticity. However the synthesis of these compounds involves several steps with low yields and, moreover, some derivatives are not accessible according to the routes proposed in these documents.
- An object of the present invention is a process for preparing heterocyclic pentalene derivatives having formula (I):
- W is a sulfur atom, an oxygen atom or a NR or PR group wherein R is a linear or branched saturated or unsaturated CrC 20 -alkyl, C 3 -C 2 o ⁇ cycloalkyl, C 6 -C 20 -aryl, C 7 -C o ⁇ alkylaryl or C 7 -C o-arylalkyl radical, optionally containing heteroatoms belonging to groups 13-17 of the Periodic Table of the Elements; preferably the group NR is N-methyl or N-phenyl; and the group PR is P-methyl or P-phenyl; more preferably W is a sulfur atom; R 1 , R 2 , R 3 and R 4 , equal to or different from each other, are hydrogen atoms or a linear or branched saturated or unsaturated C 1 -C 0 -alkyl, C -C 20 -cycloalkyl, C 6 -C 2 o-aryl, C 7 -C 20 -alkyla
- R 1 and R 2 are defined as above and T is a OR, NR 2 , OH, CC1 3 , CF 3 , Cl, Br, I, imidazolyl or pirazolyl radical; with at least one molar equivalent of a vinyl compound of formula (III):
- Compounds of formula (HI) can be easily prepared starting from the correspondent vinyl bromide or they can be purchased as such.
- Step a) is carried out at a temperature range of from -78 C to 100°C, preferably from -20°C to 20°C.
- aprotic solvents such as toluene, diethyl ether, hexane, tetrahydrofuran, dimethyl formamide, etc.
- the product obtained from step a) is purified by process known in the art such as filtration, crystallization, chromatography, distillation; otherwise it is used as such.
- T is a NR 2 group; more preferably T is a N(Me) 2 or a N(Et) 2 radical.
- the group M is MgBr or Li.
- Br ⁇ nsted acid used in step b) are methanesulphonic acid, sulfuric acid, phosphoric acid, polyphosphoric acid or P 2 ⁇ 5 /methansulfuric acid.
- methanesulphonic acid or sulfuric acid are used.
- the reaction is preferably carried out in water or in an organic solvent such as dichloromethane, diethyl ether, tetrahydrofuran, dimethyl formamide, or in mixtures of water and organic solvents optionally in the present of a phase transfer agent. The reaction is carried out at a temperature range from 0°C to 100°C.
- the amount of acid in step b) depends from the acid, usually a large excess of acid is used for example from 10 to 10000 equivalents or more.
- step b) The product obtained from step b) is purified by processes known in the art such as filtration, crystallization, chromatography, distillation; otherwise it is used as such.
- A1H 3 , ⁇ aBH or LiHAl(OtBu) 3 can be used.
- LiAlH is used.
- step c) The type of solvent used in step c) depends from the reducing agent used. In the case of
- Step c) the reaction is carried out in an aprotic solvent such as toluene, diethyl ether, hexane, tetrahydrofuran, dimethyl formamide, at a temperature range of from -78 C to 100°C, preferably from 0°C to 80°C.
- the product obtained from step c) is purified by processes known in the art such as filtration, crystallization, chromatography, distillation; otherwise it is used as such.
- Step d) is carried out by treating the alcohol of formula (VI) with a dehydrating agent.
- dehydrating agent examples include p-toluenesulfonic acid, sulfuric acid, hydrochloric acid and iodine. Preferably p-toulensulfonic acid and iodine are used.
- the amount of dehydrating agent depends from the dehydrating agent used. It can vary from one equivalent to a large excess such as 1000 equivalents and more.
- the type of solvent used in step d) depends from the dehydrating agent used. In the case of p- toulensulfonic acid the reaction is carried out in an aprotic solvent such as toluene, diethyl ether, hexane, tetrahydrofuran, dimethyl formamide, at a temperature range of from 0°C to
- step d) The product obtained from step d) is purified by processes known in the art such as filtration, crystallization, chromatography, distillation.
- a further method for purifying compounds obtained in step d) is treating the crude reaction product with at least one equivalent of an organolithium compound such as butylithium, methyllithium, tertbuthylithium and phenyllithium and filtering the obtained salt.
- Steps a), b, c) and d) of the process of the present invention may be carried out in sequence without purification of the intermediate products.
- steps c) and d) are carried out "one pot", i.e. without purification of the alcohol of formula (VI).
- Compounds of formula (I) can be used as ligands for the synthesis of metallocene complexes, such as those described in WO 01/44318. These complexes are useful as catalyst components for polymerizing alpha-olefins.
- the syntheses of the metallocene compounds starting from the compounds of the present invention are described in the above mentioned application.
- the compounds of formula (I) can be treated with a base and then contacted with a compound of formula YL'Cp wherein Y is halogen, preferably chlorine, L' is a suitable bridge and Cp is a substituted or unsubstituted cyclopentadienyl radical.
- Y is halogen, preferably chlorine
- L' is a suitable bridge
- Cp is a substituted or unsubstituted cyclopentadienyl radical.
- the obtained bridged ligand is then treated with two equivalents of a base and contacted with the compound of formula ML" 4 wherein M is titanium, zirconium or hafnium and L is generally halogen, preferably chlorine.
- M titanium, zirconium or hafnium
- L is generally halogen, preferably chlorine.
- the compound of formula (I) is treated with a base and then the correspondent anion is contacted with a compound of formula
- 2,3-diphenyl-thienylcarbonic acid dimethylamide was prepared in analogous manner to 2- Methyl-3-phenyl-thienylcarbonyc dimethylamide with the exception that the starting compound was dibenzyl ketone instead of phenylacetone.
- Step a) l-(4,5-DiphenyI-2-thienyl)-2-phenyl-2-propen-l-on
- reaction mixture was cooled to 0°C, was treated quickly with 250ml water and was stirred at 100°C in 3 hours.
- the resulting mixture was cooled to r.t., the organic layer was separated and distilled at 108-110/lOtorr to give 24.5g (65% from p-Cl-phenylacetone).
- Ethyl-2-mercaptoacetate (12g, O.lmol) was added at 0°C to a solution of sodium ethoxide (6.8g, O.lmol) in 150 mL of ethanol and the resulting mixture was stirred at the same temperature for 30 min. Then chloroaldehyde from the previous experiment (21.5g, O.lmol) was added and stirring was continued overnight. The resulting product was refluxed for 2 h, cooled to room temperature and then was treated with solution of 12g (0.3mol) NaOH in 20ml water. The resulting mixture was refluxed in 1 hour, then it was cooled to r.t. and finally was poured into 500ml of water.
- Step a) 1 - [5-Methyl-4-(4-chloro-phenyl)-2-thienyl] -2-methyl-2-propen-l -on
- Viscous liquid so-obtained was dissolved in 15ml THF and the resulting solution was added dropwise to solution of 2-propenylmagnesium bromide prepared from 2g Mg (42mmo ⁇ ) and 7.4g 2-bromopropene (62mmol) in 40ml THF at 0°C. The mixture warmed to r.t. and was stirred in 4h. The resulting solution was poured into 200ml of 5% aqueous HC1. The organic layer was collected, washed with water, dried over MgSO 4 and evaporated to give yellow- reddish oil of vinil-ketone.
Landscapes
- Chemical & Material Sciences (AREA)
- Organic Chemistry (AREA)
- Plural Heterocyclic Compounds (AREA)
- Nitrogen And Oxygen Or Sulfur-Condensed Heterocyclic Ring Systems (AREA)
- Low-Molecular Organic Synthesis Reactions Using Catalysts (AREA)
- Nitrogen Condensed Heterocyclic Rings (AREA)
- Heterocyclic Carbon Compounds Containing A Hetero Ring Having Nitrogen And Oxygen As The Only Ring Hetero Atoms (AREA)
- Heterocyclic Carbon Compounds Containing A Hetero Ring Having Oxygen Or Sulfur (AREA)
Abstract
Description
Claims
Priority Applications (4)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| EP02748851A EP1412347B1 (en) | 2001-08-01 | 2002-07-10 | Process for the preparation of heterocyclic pentalene derivatives |
| DE60208683T DE60208683T2 (en) | 2001-08-01 | 2002-07-10 | PROCESS FOR PREPARING HETEROCYCLIC PENTAL DERIVATIVES |
| US10/485,497 US6930190B2 (en) | 2001-08-01 | 2002-07-10 | Process for the preparation of heterocyclic pentalene derivatives |
| JP2003519057A JP2005501841A (en) | 2001-08-01 | 2002-07-10 | Method for producing heterocyclic pentalene derivative |
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| EP01202930 | 2001-08-01 | ||
| EP01202930.2 | 2001-08-01 |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| WO2003014107A1 true WO2003014107A1 (en) | 2003-02-20 |
Family
ID=8180742
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| PCT/EP2002/007680 Ceased WO2003014107A1 (en) | 2001-08-01 | 2002-07-10 | Process for the preparation of heterocyclic pentalene derivatives |
Country Status (6)
| Country | Link |
|---|---|
| US (1) | US6930190B2 (en) |
| EP (1) | EP1412347B1 (en) |
| JP (1) | JP2005501841A (en) |
| AT (1) | ATE315559T1 (en) |
| DE (1) | DE60208683T2 (en) |
| WO (1) | WO2003014107A1 (en) |
Cited By (22)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| FR2860792A1 (en) * | 2003-10-10 | 2005-04-15 | Sanofi Synthelabo | THIOPHENE-2-CARBOXAMIDE DERIVATIVES, THEIR PREPARATION AND THEIR THERAPEUTIC USE |
| US7141637B2 (en) | 2001-11-30 | 2006-11-28 | Basell Polyolefine Gmbh | Metallocene compounds and process for the preparation of propylene polymers |
| US7476717B2 (en) | 2001-06-12 | 2009-01-13 | Basell Polyolefine Gmbh | Butene-1 homopolymer |
| US7534841B2 (en) | 2004-10-21 | 2009-05-19 | Basell Polyolefine Gmbh | 1-butene polymer and process for the preparation thereof |
| US7569647B2 (en) | 2002-10-10 | 2009-08-04 | Basell Polyolefine Gmbh | Process for the copolymerization of ethylene |
| US7615597B2 (en) | 2002-09-06 | 2009-11-10 | Basell Polyolefine Gmbh | Process for the copolymerization of ethylene |
| US7619090B2 (en) | 2001-09-14 | 2009-11-17 | Basell Polyolefine Gmbh | Monocyclopentadienyl complexes comprising a condensed heterocycle |
| DE102008062863A1 (en) | 2008-12-17 | 2010-06-24 | Aicuris Gmbh & Co. Kg | Substituted (thiophenyl-carbonyl) imidazolidinones and their use |
| US7750040B2 (en) | 2004-07-29 | 2010-07-06 | Actelion Pharmaceuticals Ltd | Thiophene derivatives |
| US7776978B2 (en) | 2004-07-22 | 2010-08-17 | Basell Polyolefine Gmbh | Process for producing fractionable 1-butene polymers |
| US7776986B2 (en) | 2004-10-18 | 2010-08-17 | Basell Poliolefine Italia S.R.L. | Butene-1 (Co)Polymers having low isotacticity |
| US7799871B2 (en) | 2004-07-22 | 2010-09-21 | Basell Polyolefine Gmbh | 1-butene polymers composition |
| US7834112B2 (en) | 2001-09-14 | 2010-11-16 | Basell Polyolefine Gmbh | Method of polymerization of olefins |
| US7951794B2 (en) | 2005-06-24 | 2011-05-31 | Actelion Pharmaceuticals Ltd. | Thiophene derivatives |
| US8003743B2 (en) | 2004-07-13 | 2011-08-23 | Basell Poliolefine Italia, s.r.l. | Metallocene compounds, ligands used in their preparation, preparation of 1-butene polymers and 1-butene polymers therefrom |
| US8097682B2 (en) | 2006-08-30 | 2012-01-17 | Basell Polyolefine Gmbh | 1-butene propylene copolymer compositions |
| US8097681B2 (en) | 2006-08-30 | 2012-01-17 | Basell Polyolefine Gmbh | 1-butene propylene copolymer compositions |
| US8178562B2 (en) | 2006-01-24 | 2012-05-15 | Actelion Pharmaceuticals, Ltd. | Pyridine derivatives |
| US8314089B2 (en) | 2008-03-17 | 2012-11-20 | Aicuris Gmbh & Co. Kg | Substituted pyrazolamides and their use |
| US8324268B2 (en) | 2008-12-17 | 2012-12-04 | Aicuris Gmbh & Co. Kg | Substituted furancarboxamides, and use thereof |
| US8399682B2 (en) | 2008-03-17 | 2013-03-19 | Aicuris Gmbh & Co. Kg | Substituted (pyrazolylcarbonyl)imidazolidinones and their use |
| CN110372662A (en) * | 2019-08-07 | 2019-10-25 | 西安近代化学研究所 | Liquid-crystal compounds and its synthetic method based on pentamethylene bithiophene skeleton |
Families Citing this family (8)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| DE10260095A1 (en) * | 2002-12-19 | 2004-07-01 | Basell Polyolefine Gmbh | Preparation of cyclopenta-heterocycles, useful for preparing metallocene catalysts for olefin polymerization, by reacting heterocycle with unsaturated carboxylic acid in presence of strong acid and dehydrating agent |
| US7559969B2 (en) * | 2003-09-19 | 2009-07-14 | Sri International | Methods and apparatuses for producing metallic compositions via reduction of metal halides |
| US8051611B2 (en) * | 2005-06-24 | 2011-11-08 | Dryvit Systems, Inc. | Exterior insulation and finish system and method and tool for installing same |
| DE102005061326A1 (en) * | 2005-12-20 | 2007-06-21 | Basell Polyolefine Gmbh | Preparation of metallocene compound, useful e.g. in the preparation of isotactic polypropylene, comprises using a cyclopentadienyl derivative that is recycled from the filtrate obtained from the preparation of e.g. metallocene compound |
| BRPI0908965A2 (en) * | 2008-03-20 | 2016-04-26 | Basell Poliolefine Srl | 1-butene copolymers |
| JP2011515519A (en) * | 2008-03-20 | 2011-05-19 | バーゼル・ポリオレフィン・イタリア・ソチエタ・ア・レスポンサビリタ・リミタータ | 1-butene terpolymer |
| BRPI0910237A2 (en) * | 2008-03-20 | 2015-09-29 | Basell Poliolefine Srl | 1-butene copolymers |
| EP2254946B1 (en) * | 2008-03-20 | 2012-02-01 | Basell Poliolefine Italia S.r.l. | Compositions of 1-butene based polymers |
Citations (2)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US5252749A (en) * | 1991-09-25 | 1993-10-12 | Elf Sanofi | Ethers of thienocyclopentanone oximes, their preparation and pharmaceutical compositions containing them |
| WO2001044318A1 (en) * | 1999-12-15 | 2001-06-21 | Basell Technology Company B.V. | Metallocene compounds, process for their preparation and their use in catalytic systems for the polymerization of olefins |
Family Cites Families (8)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US4496742A (en) * | 1981-10-13 | 1985-01-29 | The Upjohn Company | Analogs of 5,6-dihydro PGI2 |
| DE3147714A1 (en) * | 1981-11-27 | 1983-06-01 | Schering Ag, 1000 Berlin Und 4619 Bergkamen | NEW PROSTACYCLINE AND METHOD FOR THEIR PRODUCTION |
| JPS63122669A (en) * | 1986-10-25 | 1988-05-26 | メルク・パテント・ゲゼルシヤフト・ミツト・ベシユレンクテル・ハフツング | Heterocyclic liquid crystal compound |
| CA1333612C (en) * | 1988-09-23 | 1994-12-20 | Paul Howard Briner | Process for the preparation of cyclopentene, cyclopentane, and cyclohexane derivatives |
| ES2116630T3 (en) * | 1994-02-17 | 1998-07-16 | Asahi Glass Co Ltd | DIFLUORPROSTACICLINAS; INTERMEDIARY PRODUCTS AND PROCEDURES FOR ITS PREPARATION. |
| FR2773798B1 (en) * | 1998-01-16 | 2001-02-02 | Adir | NOVEL TRICYCLIC COMPOUNDS, PROCESS FOR THEIR PREPARATION AND THE PHARMACEUTICAL COMPOSITIONS CONTAINING THEM |
| US6057316A (en) * | 1998-05-12 | 2000-05-02 | American Home Products Corporation | 4-aryl-1-oxa-9-thia-cyclopenta[b]fluorenes |
| US6762316B1 (en) * | 1999-06-28 | 2004-07-13 | Biocryst Pharmaceuticals, Inc. | Preparation of substituted cyclopentane and cyclopentene compounds and certain intermediates |
-
2002
- 2002-07-10 WO PCT/EP2002/007680 patent/WO2003014107A1/en not_active Ceased
- 2002-07-10 DE DE60208683T patent/DE60208683T2/en not_active Expired - Lifetime
- 2002-07-10 AT AT02748851T patent/ATE315559T1/en not_active IP Right Cessation
- 2002-07-10 EP EP02748851A patent/EP1412347B1/en not_active Expired - Lifetime
- 2002-07-10 US US10/485,497 patent/US6930190B2/en not_active Expired - Fee Related
- 2002-07-10 JP JP2003519057A patent/JP2005501841A/en not_active Ceased
Patent Citations (2)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US5252749A (en) * | 1991-09-25 | 1993-10-12 | Elf Sanofi | Ethers of thienocyclopentanone oximes, their preparation and pharmaceutical compositions containing them |
| WO2001044318A1 (en) * | 1999-12-15 | 2001-06-21 | Basell Technology Company B.V. | Metallocene compounds, process for their preparation and their use in catalytic systems for the polymerization of olefins |
Non-Patent Citations (5)
| Title |
|---|
| ACTA CHEM. SCAND. (1966), 20(7), 1733-42 * |
| DATABASE CA [online] CHEMICAL ABSTRACTS SERVICE, COLUMBUS, OHIO, US; METH-COHN, OTTO ET AL: "Thiophene analogs of indenes. II. Synthesis, tautomerism, and metalation of the thiophene analogs of 2-methylindene", XP002215127, retrieved from STN Database accession no. 67:21765 * |
| DATABASE CROSSFIRE BEILSTEIN BEILSTEIN INSTITUT ZUR FOERDERUNG DER WISSENSCHAFTEN, FRANKFURT AM MAIN, DE; XP002215128 * |
| MAXIM ET AL, BULLETIN DE LA SOCIETE CHIMIQUE FRANCAIS, vol. 5, no. 6, 1939, pages 1339 - 1345 * |
| MINORU ISHIKURA ET AL: "A Concise Preparation of Yuehchukene And Its Analogues", HETEROCYCLES, ELSEVIER SCIENCE PUBLISHERS B.V. AMSTERDAM, NL, vol. 53, no. 10, 2000, pages 2201 - 2220, XP001093781, ISSN: 0385-5414 * |
Cited By (27)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US7476717B2 (en) | 2001-06-12 | 2009-01-13 | Basell Polyolefine Gmbh | Butene-1 homopolymer |
| US7619051B2 (en) | 2001-06-12 | 2009-11-17 | Basell Polyolefine Gmbh | Butene-1/ethylene copolymer |
| US7619090B2 (en) | 2001-09-14 | 2009-11-17 | Basell Polyolefine Gmbh | Monocyclopentadienyl complexes comprising a condensed heterocycle |
| US7834112B2 (en) | 2001-09-14 | 2010-11-16 | Basell Polyolefine Gmbh | Method of polymerization of olefins |
| US7141637B2 (en) | 2001-11-30 | 2006-11-28 | Basell Polyolefine Gmbh | Metallocene compounds and process for the preparation of propylene polymers |
| US7615597B2 (en) | 2002-09-06 | 2009-11-10 | Basell Polyolefine Gmbh | Process for the copolymerization of ethylene |
| US7569647B2 (en) | 2002-10-10 | 2009-08-04 | Basell Polyolefine Gmbh | Process for the copolymerization of ethylene |
| FR2860792A1 (en) * | 2003-10-10 | 2005-04-15 | Sanofi Synthelabo | THIOPHENE-2-CARBOXAMIDE DERIVATIVES, THEIR PREPARATION AND THEIR THERAPEUTIC USE |
| US8003743B2 (en) | 2004-07-13 | 2011-08-23 | Basell Poliolefine Italia, s.r.l. | Metallocene compounds, ligands used in their preparation, preparation of 1-butene polymers and 1-butene polymers therefrom |
| US7799871B2 (en) | 2004-07-22 | 2010-09-21 | Basell Polyolefine Gmbh | 1-butene polymers composition |
| US7776978B2 (en) | 2004-07-22 | 2010-08-17 | Basell Polyolefine Gmbh | Process for producing fractionable 1-butene polymers |
| US7750040B2 (en) | 2004-07-29 | 2010-07-06 | Actelion Pharmaceuticals Ltd | Thiophene derivatives |
| US7776986B2 (en) | 2004-10-18 | 2010-08-17 | Basell Poliolefine Italia S.R.L. | Butene-1 (Co)Polymers having low isotacticity |
| US7534841B2 (en) | 2004-10-21 | 2009-05-19 | Basell Polyolefine Gmbh | 1-butene polymer and process for the preparation thereof |
| US7951794B2 (en) | 2005-06-24 | 2011-05-31 | Actelion Pharmaceuticals Ltd. | Thiophene derivatives |
| US8178562B2 (en) | 2006-01-24 | 2012-05-15 | Actelion Pharmaceuticals, Ltd. | Pyridine derivatives |
| US8697732B2 (en) | 2006-01-24 | 2014-04-15 | Actelion Pharmaceuticals Ltd. | Pyridine derivatives |
| US8097682B2 (en) | 2006-08-30 | 2012-01-17 | Basell Polyolefine Gmbh | 1-butene propylene copolymer compositions |
| US8097681B2 (en) | 2006-08-30 | 2012-01-17 | Basell Polyolefine Gmbh | 1-butene propylene copolymer compositions |
| US8314089B2 (en) | 2008-03-17 | 2012-11-20 | Aicuris Gmbh & Co. Kg | Substituted pyrazolamides and their use |
| US8399682B2 (en) | 2008-03-17 | 2013-03-19 | Aicuris Gmbh & Co. Kg | Substituted (pyrazolylcarbonyl)imidazolidinones and their use |
| DE102008062863A1 (en) | 2008-12-17 | 2010-06-24 | Aicuris Gmbh & Co. Kg | Substituted (thiophenyl-carbonyl) imidazolidinones and their use |
| WO2010075962A1 (en) | 2008-12-17 | 2010-07-08 | Aicuris Gmbh & Co. Kg | Substituted (thiophenyl-carbonyl)imidazolidinones, and use thereof |
| US8324268B2 (en) | 2008-12-17 | 2012-12-04 | Aicuris Gmbh & Co. Kg | Substituted furancarboxamides, and use thereof |
| US8546438B2 (en) | 2008-12-17 | 2013-10-01 | Kai Thede | Substituted (thiophenyl-carbonyl)imidazolidinones, and use thereof |
| CN110372662A (en) * | 2019-08-07 | 2019-10-25 | 西安近代化学研究所 | Liquid-crystal compounds and its synthetic method based on pentamethylene bithiophene skeleton |
| CN110372662B (en) * | 2019-08-07 | 2021-12-21 | 西安近代化学研究所 | Liquid crystal compound based on cyclopentanothiophene skeleton and synthetic method thereof |
Also Published As
| Publication number | Publication date |
|---|---|
| EP1412347B1 (en) | 2006-01-11 |
| DE60208683T2 (en) | 2006-11-02 |
| JP2005501841A (en) | 2005-01-20 |
| US6930190B2 (en) | 2005-08-16 |
| ATE315559T1 (en) | 2006-02-15 |
| EP1412347A1 (en) | 2004-04-28 |
| US20040192931A1 (en) | 2004-09-30 |
| DE60208683D1 (en) | 2006-04-06 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| EP1412347B1 (en) | Process for the preparation of heterocyclic pentalene derivatives | |
| KR910002583B1 (en) | Piperazine derivatives or salts thereof, methods for their preparation and pharmaceutical compositions containing them as active ingredients | |
| CA2452881C (en) | Novel bicyclic and tricyclic cannabinoids | |
| KR100634039B1 (en) | New Phenylpiperazine | |
| JP3210023B2 (en) | Synthesis of intermediates useful in preparing bromo-substituted tricyclic compounds | |
| JPS6056143B2 (en) | Amidine derivatives and their production method | |
| IE73223B1 (en) | 2-thienylglycidic acid derivative process for its preparation and its use as synthetic intermediate | |
| EP0133323A1 (en) | Benzhydrylpiperazines | |
| JP2005501841A5 (en) | ||
| EP0249950B1 (en) | Aromatic compounds | |
| KR19990029982A (en) | Novel Piperazine and Piperidine Compounds | |
| DK151251B (en) | METHOD OF ANALOGUE FOR THE PREPARATION OF RACEMIC OR OPTICALLY ACTIVE 2- (4- (CYCLOALKYLIDEENMETHYL) -PHENYL) PROPIONIC ACID DERIVATIVES OR PHARMACEUTICAL ACCEPTABLE SALTS THEREOF | |
| EP0093521B1 (en) | Quinoline derivatives | |
| CA1244436A (en) | 4-quinolone derivatives | |
| CN102070514A (en) | Method for preparing halofuginone intermediate | |
| CS214806B2 (en) | Method of making the derivatives of the auron | |
| NO890803L (en) | PROCEDURE FOR THE PREPARATION OF TRISUBSTITUTED AMINES. | |
| CZ294957B6 (en) | Process for preparing a substituted imidazopyridine compound | |
| CA1064038A (en) | 3-(.alpha.-IMINOBENZYL)-4-HYDROXY-6-PHENYL-2(1H)PYRIDONE COMPOUNDS | |
| US4487931A (en) | Process for the preparation of 2-(thien-2-yl)- and 2-(thien-3-yl)-ethylamine derivatives | |
| JPS6031823B2 (en) | Method for producing carbazole derivatives | |
| US4383995A (en) | [4,5] 4H-Benzo [1,2-b] cyclohepta furan derivatives and application thereof as anti-fibrillating agents | |
| CA2026274A1 (en) | Imidazoles | |
| IE64209B1 (en) | New indole derivatives process for preparing them and pharmaceutical compositions containing them | |
| CN110845511B (en) | Preparation method of 3-benzimidazole-4-arylpyranocoumarin derivative |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| AK | Designated states |
Kind code of ref document: A1 Designated state(s): JP Kind code of ref document: A1 Designated state(s): JP US |
|
| AL | Designated countries for regional patents |
Kind code of ref document: A1 Designated state(s): AT BE BG CH CY CZ DE DK EE ES FI FR GB GR IE IT LU MC NL PT SE SK TR Kind code of ref document: A1 Designated state(s): AT BE BG CH CY CZ DE DK EE ES FR GB GR IE IT LU MC NL PT SE SK TR |
|
| 121 | Ep: the epo has been informed by wipo that ep was designated in this application | ||
| WWE | Wipo information: entry into national phase |
Ref document number: 2002748851 Country of ref document: EP |
|
| WWE | Wipo information: entry into national phase |
Ref document number: 10485497 Country of ref document: US |
|
| WWE | Wipo information: entry into national phase |
Ref document number: 2003519057 Country of ref document: JP |
|
| WWP | Wipo information: published in national office |
Ref document number: 2002748851 Country of ref document: EP |
|
| WWG | Wipo information: grant in national office |
Ref document number: 2002748851 Country of ref document: EP |