6- PHENYLDIHYDR0PYRR0 0PYRIMIDINEDI0NE DERIVATIVES
The present invention relates to antagonists of A2 adenosine receptors and in particular to antagonists of the A2b adenosine receptor subtype. Such antagonists are useful in preventing mast cell degranulation and are therefore useful in the treatment, prevention or suppression of disease states induced by activation of the A2b receptor and mast cell activation. These disease states include but are not limited to asthma, myocardial reperfusion injury, allergic reactions including but not limited to rhinitis, poison ivy induced responses, urticaria, scleroderm arthritis, other autoimmune diseases and inflammatory bowel diseases.
Adenosine regulates several physiological functions through specific cell membrane receptors. Four distinct adenosine receptors have been identified and classified as Al, A2a, A2b and A3, which are members of the G-protein coupled receptor family. The A2b adenosine receptor subtype (see review Feoktistov, L, Biaggioni, I. Pharmacol. Rev. 1997, 49, 381-402) has been identified in a variety of human and murine tissues and appears to be involved in the control of vascular tone, regulation of vascular smooth muscle growth, regulation of the hepatic glucose production, modulation of intestinal tone as well as intestinal secretion and can also modulate mast cell degranulation mediating the response of human mast cells to adenosine. Adenosine A2a receptors modulate the release of GAB A in the striatum, which possibly regulates the activity of medium spiny neurons. Thus, A2a receptor antagonists may be a useful treatment for Parkinson's disease not only as monotherapy but also in combination with L-DOPA and dopamine agonist drugs.
It has now, surprisingly, been found that certain 6-(substituted)phenyl-l,5- d ydropyττolo[3,2-(i]pvrimidine-2,4-dione derivatives are potent and selective inhibitors of A2 adenosine receptors and in particular the A2b receptor subtype, and have efficacy in treating or preventing asthma, bronchoconstriction, allergic potentiation, inflammation or reperfusion injury, myocardial ischemia, inflammation, diarrheal diseases, brain arteriole diameter constriction, Parkinson's disease, insulin or
non insulin dependent diabetes mellitus, and/or release of allergic mediators. EP 0 480 659 relates to compounds of general formula
wherein each of Z
1, Z
2 and Z
3, independently represents: a nitrogen atom, a group represented by general formula: =C(X
2)- or a group represented by general formula: =C(X
3)-. When Z
2 and Z
3 represent a group of general formula: ^CQi
2)- or a group of general formula: =C(X
3)-, X
2 and X
3 may be combined together to form a group represented by general formula:
X26 O X^ O
I II I II — N-C-N-C — and Y does not represent hydrogen; which possess angiotensin-II receptor antagonizing activity for the prevention or treatment of hyperuricemia.
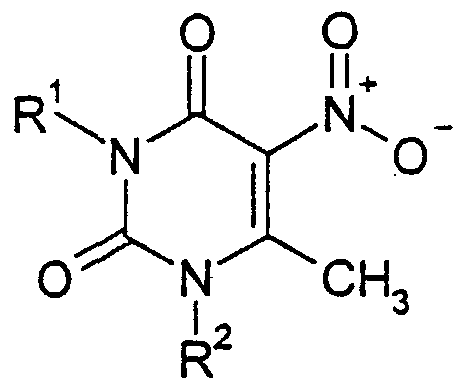
The present invention provides a 6-phenylpyrrolopyrimidinedione derivative of the formula (I), or a pharmaceutically acceptable salt thereof,
wherein: - R1 and R2 are the same or different and each represents hydrogen, a group of formula - CR^ -B , or an alkyl group which is unsubstituted or substituted by one or more, for example 1 or 2, substituents selected from hydroxy, alkoxy, alkylthio,
amino, mono- or αi-alkylamino, hydroxycarbonyl, alkoxycarbonyl, acylamino, carbamoyl, alkylcarbamoyl, dihydroxyphosphoryloxy and dialkoxyphosphoryloxy groups, wherein n is an integer of from 0 to 4 and R7 represents a cycloalkyl group, a phenyl group or a cyclic group which is a 3- to 7-membered, aromatic or non-aromatic ring, which contains from 1 to 4 heteroatoms selected from N, O and S and which is optionally fused to an aromatic or heteroaromatic ring, the phenyl group being unsubstituted or substituted by one or more, for example 1, 2, 3 or 4, substituents selected from halogen, alkyl, aryl, heteroaryl, heterocyclyl, hydroxy, alkylenedioxy, alkoxy, alkylthio, amino, mono- or di-allcylamino, nitro, cyano, hydroxycarbonyl, alkoxycarbonyl, acylamino, carbamoyl, alkylcarbamoyl, dihydrophosphoryloxy, dialkoxyphosphoryloxy and haloalkyl groups and the cyclic group being unsubstituted or substituted by one or more, for example 1, 2, 3 or 4, substituents selected from halogen, hydroxy, alkoxy, phenyl, alkoxycarbonyl, amino, mono-alkylamino, di- alleylamino, hydroxycarbonyl, and alkyl groups, the alkyl substituents being unsubstituted or substituted by one or more, for example 1 or 2, further substituents selected from halogen, hydroxy, alkoxy, alkylthio, acylamino, carbamoyl, alkylcarbamoyl, dihydroxyphosphoryloxy, dialkoxyphosphoryloxy, hydroxyalkoxy, phenyl, alkoxycarbonyl, amino, mono- and di-alkylamino and hydroxycarbonyl groups; - R3 represents hydrogen, halogen, or a nitro, alkoxycarbonyl or alkyl group, the alkyl group being unsubstituted or substituted by one or more, for example 1 or 2, substituents selected from hydroxy, halogen, alkoxy, alkylthio, amino, mono- or di-alkylamino, hydroxycarbonyl, alkoxycarbonyl, acylamino, carbamoyl and alkylcarbamoyl groups; - R4 and R5 are the same or different and each represents hydrogen, halogen, alkyl, hydroxy, alkoxy, alkylthio, dialkylaminoalkoxy, amino, mono- or dialkylamino, nitro, cyano or haloalkyl, or R4 and R5, together with the atoms to which they are attached, form a 5 to 7 membered ring containing from 0 to 4 heteroatoms selected from N, O and S;
L, is a direct bond or is -O-, -S-, -N(Z)-, -S(CR8R9)rn-, -©(CH^-, - O(CR3RV, -CH=CH-, -(CH , -(CR3RV> -(CH^O-, -(CR'R^O-, -(C ^^Z)-, -OiCE β-, -0 0^^ 0-, or -N(ZXCR8RV wherein m is an integer of from 1 to 6, preferably an integer of from 1 to 4, and either Z, R8 and R9 are the same or different and each represent a group selected from hydrogen, C,-C6 alkyl, cycloalkyl, cycloalkyl-C, - Cs alkyl, heterocyclyl, heterocyclyl-C,-C6 alkyl, aryl, aryl- - alkyl, heteroaryl, heteroaryl-Cι-C5 alkyl, hydroxy, C,-C6 alkoxy, halogen, cyano, C,-C3 alkoxycarbonyl, carbamoyl and haloalkyl, the alkyl, cycloalkyl, heterocyclyl, aryl and heteroaryl moieties being unsubstituted or substituted with one to four substituents independently selected from R1, or Z is as defined above and R8 and R9, together with the atom to which they are attached, form a 4 to 8 membered ring; and
R4 represents -C(O)NR10Rπ, -S(O)2NR10Rπ, -ON=CR12R13, or a heterocyclyl, aryl or heteroaryl group, the heterocyclyl, aryl and heteroaryl groups being unsubstituted or substituted with substituents R14 to R17, wherein: R10 and R11 are either
(a) the same or different, each independently representing hydrogen, an alkyl group, a cycloalkyl group or a phenyl group, wherein (i) the alkyl group is unsubstituted or substituted by one or more, for example 1 or 2, substituents selected from hydroxy, halogen, alkoxy, alkylthio, amino and mono- and ( ^alkylamino groups, (ii) the cycloalkyl group is optionally fused to an aromatic ring and (iii) the cycloalkyl group and the phenyl group are unsubstituted or substituted by one or more, for example 1, 2, 3 or 4, substituents selected from (1) groups of formula -(CHj),, R7, -O-(CH,)n R7, -S- (CH^ R7, -COR and -CO HR, wherein R is alkyl or -(CH2)ιrR7 and n and R7 are as defined above, (2) groups of formula -(CH2)n-S(O)2N 'R" wherein n is as defined above and R' and R" are the same or different and are each selected from hydrogen and alkyl or form, together with the nitrogen atom to which they are attached, a 4- to 7- membered heterocyclic ring containing 1, 2 or 3 heteroatoms selected from N, O, and S, (3) groups of formula -(CH2)n-CO2R"' wherein n is as defined above and Rm is hydrogen or alkyl, (4) groups of formula -N* R""3 wherein each R"" is the same or different and is an alkyl
group, and (5) halogen atoms and alkyl, hydroxy, alkylenedioxy, alkoxy, alkylthio, amino, mono- and -alkylamino, nitro, cyano, hydroxycarbonyl, alkoxycarbonyl, acylamino, carbamoyl, dihydroxyphosphoryloxy, dialkoxyphosphoryloxy or haloalkyl groups, the alkyl substituents being unsubstituted or substituted by one or more, for example 1 or 2, further substituents selected from cyano, nitro, amino, hydroxy and halogen,
(b) together with the atom to which they are attached, a 3- to 7-membered ring comprising up to 4 heteroatoms selected from N, O and S, which ring is (i) optionally fused to an aromatic ring or to a heteroaromatic ring which is in turn optionally fused to an aromatic ring and is (ii) unsubstituted or substituted by one or more, for example 1, 2, 3 or 4, substituents independently selected from halogen atoms, groups of formula -X-R7and-CO2-X-R7 wherein X is a direct bond, a C,-C4 alkylene group or a carbonyl group, for example a direct bond or a C,-C4 alkylene group, and R7 is as defined above, and hydroxy, cyano, nitro, oxoalkyl, carbamoyl, hydroxycarbonyl, alkoxycarbonyl, amino, mono- and di-alkylamino, divalent alkylene and alkyl groups, the alkyl substituents being unsubstituted or substituted by one or more, for example 1 or 2, further substituents selected from hydroxy, alkoxy, hydroxyalkoxy, amino and mono- and di-alkylamino groups, and the moiety X being unsubstituted or substituted by one or two further substituents selected from phenyl, alkyl, hydroxy and thio groups and groups of formula -CO,R' and -CONR"R" wherein R' and R" are the same or different and are hydrogen or alkyl or
(c) defined so that R10 represents hydrogen or an alkyl group and R11 represents a group of formula -X-R7 wherein X and R7 are as defined above;
R12 and R13 are defined as R10 and R11 above, except that either or both of R12 and R13 can be an amino, alkylamino or m'alkylamino group; and
R14 to R17 are the same or different and each independently represents hydrogen, a halogen atom, a group of formula -{CH2)n-R7, wherein n and R7 are as defined above or an alkyl group, for example hydrogen, a group of formula -{CH^-R7 or an alkyl group, the alkyl group being unsubstituted or substituted by one or more, for example 1
or 2, substituents selected from hydroxy, alkoxy, alkylthio, amino, mono- or di- alkylamino, hydroxycarbonyl, alkoxycarbonyl, acylamino, carbamoyl, alkylcarbamoyl, dihydroxyphosphoryloxy, dialkoxyphosphoryloxy and haloalkyl groups, or R14 and R15 are as defined above and R16 and R17, together with the atoms to which they are attached, form a 4 to 8 membered aromatic or non-aromatic ring which contains from 0 to 4 heteroatoms selected from N, O and S, and which is unsubstituted or substituted by one or more, for example 1 , 2, 3 or 4, substituents selected from halogen atoms and alkyl, hydroxy, phenyl, alkoxycarbonyl, amino, mono-alkylamino, di-alkylamino and hydroxycarbonyl groups, the alkyl substituents being unsubstituted or substituted by one or more, for example 1 or 2, further substituents selected from halogen atoms and hydroxy, alkoxy, alkylthio, acylamino, carbamoyl, alkylcarbamoyl, dihydroxyphosphoryloxy, dialkoxyphosphoryloxy, hydroxyalkoxy, phenyl, alkoxycarbonyl, amino, mono- or di-alkylamino and hydroxycarbonyl groups.
As used herein, an alkyl group or moiety is typically a linear or branched alkyl group or moiety containing from 1 to 6 carbon atoms, such as a C,-C4 alkyl group or moiety, for example methyl, ethyl, n-propyl, i-propyl, n-butyl, i-butyl and t-butyl. Where a group contains two or more alkyl moities, the alkyl moieties may be the same or different When an alkyl group or moiety carries 2 or more substituents, the substituents may be the same or different As used herein, an alkylenedioxy group or moiety is a linear or branched group or moiety containing from 1 to 6, for example from 1 to 4, carbon atoms. Examples include methylenedioxy, ethylenedioxy, propylenedioxy and butylenedioxy. When an alkylenedioxy group or moiety carries 2 or more substituents, the substituents may be the same or different. As used herein, an alkylene group is a divalent alkyl moiety typically having from 1 to 6, for example from 1 to 4, carbon atoms. Examples of C,-C4 alkylene groups include methylene, ethylene, propylene and butylene groups.
As used herein, an aryl group or moiety is typically a C3-Cw aryl group or moiety such as phenyl or naphthyl. Phenyl is preferred. When an aryl group or moiety carries 2 or more substituents, the substituents may be the same or different.
As used herein, a heteroaryl group or moiety is typically a 5- to 10- membered aromatic ring, such as a 5- or 6- membered ring, containing at least one heteroatom selected from O, S and N. Examples include pyridyl, pyrazinyl, pyrimidinyl, pyridazinyl, furanyl, oxadiazolyl, oxazolyl, imidazolyl, thiazolyl, thiadiazolyl, thienyl, pyrazolidinyl, pyrrolyl and pyrazolyl groups. Oxadiazolyl, oxazolyl, pyridyl, pyrrolyl, imidazolyl, thiazolyl, thiadiazolyl, furanyl, pyrazinyl and pyrimidinyl groups are preferred. When a heteroaryl group or moiety carries 2 or more substituents, the substituents may be the same or different.
As used herein, a halogen is a typically chlorine, fluorine, bromine or iodine and is preferably chlorine, fluorine or bromine.
As used herein, a said alkoxy group or moiety is typically a said alkyl group attached to an oxygen atom. An alkylthio group or moiety is typically a said alkyl group attached to a thio group. A haloalkyl or haloalkoxy group is typically a said alkyl or alkoxy group substituted by one or more said halogen atoms. Typically, it is substituted by 1 , 2 or 3 said halogen atoms. Preferred haloalkyl and haloalkoxy groups include perhaloalkyl and perhaloalkoxy groups such as -CX3 and -OCX3 wherein X is a said halogen atom. Particularly preferred haloalkyl groups are CF3 and CC13. Particularly preferred haloalkoxy groups are -OCF3 and -OCCl3.
As used herein, a cycloalkyl group typically has from 3 to 6 carbon atoms. Examples include cyclopropyl, cyclobutyl, cyclopentyl and cyclohexyl. It is preferably cyclopropyl, cyclopentyl or cyclohexyl. When a cycloalkyl group carries 2 or more substituents, the substituents may be the same or different.
As used herein, a heterocyclyl group is typically a non-aromatic, saturated or unsaturated C3-C10 carbocyclic ring in which one or more, for example 1, 2 or 3, of the carbon atoms are replaced by a heteroatom selected from N, O and S. Saturated heterocyclyl groups are preferred. Examples of suitable heterocyclyl groups include
piperidinyl, piperazinyl, morpholinyL, 4,5-dihydro-oxazolyl, 3-aza-tetrahydrofuranyl, imidazolidinyl and pyrrolidinyl groups. Where a heterocyclyl group carries 2 or more substituents, the substituents may be the same or different
As used herein, an acyl group or moiety typically has from 2 to 7 carbon atoms. Thus, it is typically a group of formula -COR wherein R is a hydrocarbyl group having from 1 to 6 carbon atoms. Preferably, it is a group of formula -COR wherein R is a said C,-Cβ alkyl group.
Compounds of the formula (I) containing one or more chiral centre may be used in enantiomerically or diastereoisomerically pure form, or in the form of a mixture of isomers.
As used herein, a pharmaceutically acceptable salt is a salt with a pharmaceutically acceptable acid or base. Pharmaceutically acceptable acids include both inorganic acids, for example hydrochloric, sulphuric, phosphoric, diphosphoric, hydrobromic and nitric acid and organic acids, for example citric, fumaric, maleic, mahc, ascorbic, succinic, tartaric, benzoic, acetic, methanesulphonic, ethanesulphonic, benzenesulphonic or p-toluenesulphonic acid. Pharmaceutically acceptable bases include alkali metal (e.g. sodium or potassium) and alkali earth metal (e.g. calcium or magnesium) hydroxides and organic bases, for example alkyl amines, aralkyl amines and heterocyclic amines. Typically, at least one of R1 and R2 is hydrogen or a said alkyl group.
Preferably, R1 and R2 are the same or different and each independently represent hydrogen, a group of formula -(CH^-R7 wherein n and R7 are as defined above or a C,-C6 alkyl group which is unsubstituted or substituted by one or more, for example 1 or 2, substituents selected from hydroxy, C,-C3 alkoxy, C,-C3 alkylthio, amino and mono- and di-(Cι-C3 alkyl)amino groups.
When R1 or R2 is a group of formula -(CH^-R7, R7 is preferably a C3-C3 cycloalkyl group or a cyclic group which is a 5- or 6-membered non-aromatic ring containing 1 or 2 heteroatoms selected from N, O and S, for example a morpholino group. In this embodiment, R7 is, for example, a C3-C6 cycloalkyl group.
More preferably, R1 and R2 are the same or different and each independently represent hydrogen, a C,-C4 alkyl group which is unsubsituted or substituted by 1 or 2 substituents selected from C,-C4 alkoxy and C,-C4 alkylthio substituents, a group of formula -(CH2)n-(C3-C3 cycloalkyl) or -(CH^-^orpholino) wherein n is as defined above. Examples of the more preferable R1 and R2 groups are hydrogen, a C,-C4 alkyl group which is unsubsituted or substituted by 1 or 2 substituents selected from C,-C4 alkoxy and C,-C4 alkylthio substituents or a group of formula -(CH2)n-(C3-C6 cycloalkyl) wherein n is as defined above.
More preferably still, R1 and R2 are the same or different and each independently represents a C,-C4 alkyl group, for example methyl, ethyl and n-propyl.
Preferably, R3 represents hydrogen, halogen or a C,-Cβ alkyl group which is unsubstituted or substituted by one or more, for example 1 or 2, substituents selected from halogen atoms and hydroxy groups.
More preferably, R3 represents hydrogen, halogen, for example chlorine and bromine, or C,-C4 haloalkyl, for example -CF3 or -CC13. More preferably still, R3 represents hydrogen or halogen, for example chlorine and bromine.
Typically, when R4 and/or Rs represents a haloalkyl group, the haloalkyl group is a trifluoromethyl group.
Preferably, R4 and R5 are the same or different and each represents hydrogen, halogen, C,-C6 alkyl, C,-C3 haloalkyl, hydroxy, C,-C3 alkoxy, C,-C6 alkylthio, amino or mono- or di-(C,-C3 aJJ yl)amino.
More preferably, R4 and R5 are the same or different and each represents hydrogen, Cj- alkyl, hydroxy, C,-C3 alkoxy, C,-C3 alkylthio, amino or C,-C3 alkylamino. More preferably still, R4 and R5 are the same or different and represent hydrogen, C,-C4 alkyl, C^-C4 alkoxy, for example methoxy, or C,-C4 alkylthio, for example methylthio.
Typically, when Z, R8 and/or R9 contains a cycloalkyl, heterocyclyl, aryl or heteroaryl moiety, the cycloalkyl, heterocyclyl, aryl or heteroaryl moiety is
unsubstituted or substituted by 1 or 2 C,-C4 alkyl groups. Typically, when R8 and/or R9 contains an alkyl moiety, the alkyl moiety is unsubstituted.
When Z, R8 and or R9 is haloalkyl, the haloalkyl group is typically -CFH2, -CF2H or -CF,. Typically, Z, R8 and R9 are the same or different and each represents hydrogen,
C,-C4 alkyl, C3-C6 cycloalkyl, (C3-C6 cycloalkyl)-(C,-C4 alkyl)-, phenyl or phenyl-(C,-C4 alkyl)-. Preferably, Z, R8 and R9 are the same or different and each represents hydrogen, C,-C3 alkyl, for example methyl and ethyl, or phenyl. For example, Z, R8 and R9 are the same or different and each represents - alkyl, for example methyl and ethyl, or phenyl.
Preferably, L, is a direct bond or -©(CH^-, -O(CR
8RV» -S(CR
8RV, -CH-CH-,
or -N(Z)(CR
8R
9)
m-, for example, a direct bond or -OiCE^-, -O(CR
8RV
> -S(CR
8R
9)
m-, -CH-CH-, -(ay.-, -(CR
8RV. -(CH^O-, -(CR^O-, -(C ^^Z)- or -N(Z)(CR
8R
9)
m-, wherein m is from 1 to 4, and is preferably 1, 2 or 3, R
8 and R
9 are as defined above and Z is hydrogen or C,-C
4 alkyl.
More preferably, L, is -CXCHJ,,,-, -C CR"RV. -CH=CH-, -(CH^-, -(CR* \-, -(CH^O-, -C^R^O-, -O(CH2)mO- or -(CR^^Z)-, for example, -C H^ , -O(CR8RV. -CH=CH-, -(CH^-, -(CR8RV, -(CH^O- or -(CRβR9)BlO-, such as -OCCiy,,,-, -O(CR8RV> -CH=CH-, -(CH^-, -(CR8RV or -{CH^O-, wherein m is from 1 to 4, and is preferably 1, 2 or 3, and R8 and R9 are as defined above and are preferably hydrogen, C,-C3 alkyl, for example methyl and ethyl, or phenyl.
More preferably, L, is -O-CH,-, -O^O- or -CHjNH-, for example -O-CHj. The groups L, are herein written such that the left hand end of the group is attached to the phenyl moiety in formula (I) and the right hand end is attached to R6. Thus, for example, when L, represents -CR NH-, the -CH,- moiety is attached to the phenyl ring whilst the -NH- moiety is attached to Rδ. R12 and R13 in the group R4 are either
(a) the same or different, each independently representing amino, alkylamino, dialkylamino, hydrogen, an alkyl group a cycloalkyl group or a phenyl group, wherein (i) the alkyl group is unsubstituted or substituted by one or more, for example 1 or 2, substituents selected from hydroxy, halogen, alkoxy, alkylthio, amino or mono- or di-alkylamino groups, (ii) the cycloalkyl group is optionally fused to an aromatic ring and (iii) the cycloalkyl group and the phenyl group are unsubstituted or substituted by one or more, for example 1, 2, 3 or 4, substituents selected from (1) groups of formula - CΑ ^ , -0-(CR1)ΏR', -S^CHJJl7, -COR and -CONHR, wherein R is alkyl or -(CH^-JR.7 and n and R7 are as defined above, (2) groups of formula -(CH2)n-S(O)2NR,R" wherein n is as defined above and R' and R" are the same or different and are each selected from hydrogen and alkyl or form, together with the nitrogen atom to which they are attached, a 4- to 7- membered heterocyclic ring containing 1, 2 or 3 heteroatoms selected from N, O, and S, (3) groups of formula - (CH2)n-CO2Rm, wherein n is as defined above and R"' is hydrogen or alkyl, (4) groups of formula -N* R""3 wherein each R"" is the same or different and is an alkyl group, and (5) halogen atoms and alkyl, hydroxy, alkylenedioxy, alkoxy, alkylthio, amino, mono- or di-alkylarnino, nitro, cyano, hydroxycarbonyl, alkoxycarbonyl, acylamino, carbamoyl, dihydroxyphosphoryloxy, dialkoxyphosphoryloxy or haloalkyl groups, the alkyl substituents being unsubstituted or substituted by one or more, for example 1 or 2, further substituents selected from cyano, nitro, amino, hydroxy and halogen,
(b) together with the atom to which they are attached, a 3 to 7-membered ring comprising up to 4 heteroatoms selected from N, O and S, which ring is optionally fused to one or two rings selected from aromatic and heterocyclyl rings and is unsubstituted or substituted by one or more, for example 1, 2, 3 or 4, substituents independently selected from halogen atoms, groups of formula -X-R7 and -CO2-X-R7 wherein X is a direct bond or a C,-C4 alkylene group and R7 is as defined above, and hydroxy, cyano, nitro, oxoalkyl, carbamoyl, hydroxycarbonyl, alkoxycarbonyl, amino, mono- and di-alkylamino, divalent alkylene and alkyl groups, the alkyl substituents being unsubstituted or substituted by one or more, for example 1 or 2, further
substituents selected from hydroxy, alkoxy, hydroxyalkoxy, amino or mono- or di- alkylamino groups, and the moiety X being unsubstituted or substituted by one or two further substituents selected from phenyl, alkyl, hydroxy and thio groups and groups of formula -CO2R' and -CONRT ." wherein R' and R" are the same or different and are hydrogen or alkyl, or
(c) defined so that R12 represents hydrogen or an alkyl group and R13 represents a group of formula -X-R7 wherein X and R7 are as defined above.
Preferably, R
12 and R
13 are the same or different and each represents hydrogen, amino, (C,-C
6 alkyl)amino,
alkyl)amino, C,-C
6 alkyl, C
3-C
3 cycloalkyl or phenyl, the alkyl moieties being unsubsituted or substituted by 1 or 2 subsitutents selected from hydroxy groups and halogen atoms and the cycloalkyl group and the phenyl group being unsubstituted or substituted by 1, 2, 3 or 4 substituents selected from halogen atoms and C,-C
4 alkoxy, C,-C
4 alkylthio, C,-C
4 alkyl, hydroxy, C,-C
4 haloalkyl, amino, and mono-and di-(C,-C
4 alkyl)amino groups. More preferably, R
12 and R
13 are the same or different and each represents amino, mono- or di-(C,-C
4 aJl.yl)amino, or phenyl, the phenyl group being unsubstituted or substituted by one or two substituents selected from halogen, for example fluorine, C,-C
4 alkoxy, for example methoxy, C,-C
4 alkyl, for example methyl and ethyl, hydroxy, amino, mono-(C,-C
4 alkyl)-amino and C,-C
4 haloalkyl, for example -CF
3 and -CC1
3.
Most preferably, R12 is amino and R'3 is a phenyl group which is unsubstituted or substituted with a halogen atom, for example a fluorine atom.
When the moiety R7 is a phenyl group which carries one or more haloalkyl substituent, the or each haloalkyl substituent is typically -CF3. When the moiety R7 is a said 3- to 7- membered ring which is fused to an aromatic or heteroaromatic ring, the 3- to 7- membered ring is typically fused to an aromatic ring. Preferably, it is fused to a phenyl group. Preferably, such fused ring moieties are 5- membered heteroaromatic rings containing 1 or 2 heteroatoms selected
from N, O and S, fused to a phenyl group. Examples include benzimidazole and benzothiazole.
Preferably, R7 is: a C3-C6 cycloalkyl group; - a phenyl group which is unsubstituted or substituted with 1, 2 or 3 substituents selected from halogen, C,-C4 alkyl, aryl, for example phenyl, heteroaryl, hydroxy, C,-C4 alkylenedioxy, C,-C4 alkoxy, C,-C4 alkylthio, amino, mono- and di-(C,- C4 alkyl)amino, nitro, cyano, hydroxycarbonyl, (C,-C4 alkoxy)carbonyl, (C2-C7 acyl)arnino, carbamoyl, (C,-C4 alkyl)carbamoyl, dihydrophosphoryloxy, di-(C,-C4 alkoxy)phosphoryloxy and C,-C4 haloalkyl groups; or a cyclic group which is a 3- to 7- membered aromatic or non-aromatic ring containing from 1 to 4, for example 1, 2 or 3, heteroatoms selected from N, O and S which is optionally fused -to an aromatic ring, which group is unsubstituted or substituted by 1, 2 or 3 substituents selected from halogen, hydroxy, C,-C4 alkoxy, phenyl, C,-C4 alkoxycarbonyl, amino, mono-(Ct-C4 alkyl)amino, di-(C,-C4 alkyl)amino, hydroxycarbonyl and C,-C4 alkyl groups, the alkyl substituents being unsubstituted or substituted by 1 or 2 further substituents selected from halogen, hydroxy, C,-C4 alkoxy, C,-C4 alkylthio, C2-C7 acylamino, carbamoyl, C,-C4 alkylcarbamoyl, dihydroxyphosphoryloxy, di-(C,-C4 alkoxy)phosphoryloxy, hydroxy-(C1-C4 alkoxy)-, phenyl, C,-C4 alkoxycarbonyl, amino, mono- and di-{C,-C4 alkyl)amino and hydroxycarbonyl groups.
Preferably, the cyclic group is a 5- or 6- membered aromatic or non-aromatic ring containing 1 or 2 heteroatoms selected from N, O and S, which is optionally fused to a phenyl ring. More preferably, the cyclic group is a pyridinyl, pyrazinyl, pyrimidinyl, imidazolyl, thiazolyl, oxazolyl, isoxazolyl, pyrazolyl, piperidinyl, thiadiazolyl, furanyl, benzimidazolyl, benzothiazolyl, morpholino or thienyl group. For example, the cyclic group is a pyridinyl, pyrazinyl, pyrimidinyl, imidazolyl, thiazolyl, oxazolyl, piperidinyl, thiadiazolyl, furanyl, benzimidazolyl or benzothiazolyl group. Further, the substituents on the cyclic group are preferably selected from halogen, for
example chlorine, hydroxy, phenyl, C,-C4 alkoxy, amino, mono- and di-(C1-C4 alkyl)amino, C,-C4 alkyl, C,-C4 haloalkyl, for example -CF3, hydroxy-(Ct-C4 alkyl)- and phenyl-{C1-C4 alkyl)-, for example benzyl. More preferably, these subsitutents are selected from hydroxy, chlorine, C,-C4 alkyl, -CF3, phenyl and benzyl. Preferably, when R7 is a phenyl group, it is a phenyl group which is unsubstituted or substituted by 1 or 2 subsitutents selected from halogen, for example fluorine and chlorine, C,-C4 alkyl, phenyl, hydroxy, C,-C4 alkoxy, C,-C4 alkylthio, amino, mono- and di-(C,-C4 alkyl)amino and C,-C4 haloalkyl groups. More preferably, these substituents are selected from halogen, for example fluorine and chlorine, C,-C4 alkyl, for example methyl and ethyl, C,-C4 alkoxy, for example methoxy and ethoxy, hydroxy, C,-C4 alkylthio and -CF3.
Typically, when the moiety X is substituted, R7 is a said phenyl group. More typically, when X is substituted, R7 is an unsubstituted phenyl group. Preferred substitutents on the moiety X include phenyl, C,-C4 alkyl, hydroxy, -CO2H and -CO2-(C,-C4 alkyl). More preferably, the substituents on the X moiety are selected from hydroxy, -CO2Me, -CO2H, methyl and phenyl.
When R10 and Rn are defined according to option (a) above, R10 and/or Rn can be a cycloalkyl group which is optionally fused to an aromatic ring. When the cycloalkyl group is fused to an aromatic ring, it is typically fused to a phenyl ring. Examples of such fused rings include a cyclohexyl ring fused to a phenyl ring and a cyclopentyl ring fused to a phenyl ring.
Typically, when R10 and R11 are defined according to option (a) above, at least one of R10 and R" is hydrogen or C,-C3 alkyl.
When R10 and R" are defined according to option (a) above, preferably they are the same or different and each independently represent hydrogen, a C,-C3 alkyl group, a Cj-Cj cycloalkyl group optionally fused to a phenyl ring or a phenyl group, the alkyl group being unsubstituted or substituted by 1 or 2 substituents selected from hydroxy, halogen, C,-C4 alkoxy and amino groups and the phenyl and cycloalkyl groups being unsusbtituted or substituted by 1, 2, 3 or 4 substituents selected from (1) groups of
formula -(CH-). R7, -O-(CH2)n-R7, -S^CH^-R7 and -COR and -CONHR wherein R is C,-C3 alkyl or -(CHj),, R7 and n and R7 are as defined above, (2) groups of formula -(CH2)n-S(O)2NR'R" wherein n is as defined above and R' and R" are the same or different and are each selected from hydrogen and C,-C6 alkyl or form, together with the N atom to which they are attached, a 4- or 5-membered saturated heterocyclic ring containing 1 or 2 heteroatoms selected from N, O and S, (3) groups of formula -(CBj)n-CO2R'" wherein n is as defined above and R"* is hydrogen or C,-Cβ alkyl, (4) groups of formula -N*R""3 wherein each R"" is the same or different and is a C,-C6 alkyl group, and (5) halogen atoms and C,-Cβ alkyl, hydroxy, C,-C4 alkylenedioxy, C,-C3 alkoxy, C,-C6 alkythio, a ino, mono- and di-(C,-Cβ alkyl)amino, nitro, cyano, hydroxycarbonyl, (C,-C6 alkoxy)carbonyl, (C2-C7 acyl)arnino, carbamoyl, and C,-Cβ haloalkyl groups, the alkyl substituents being unsubstituted or substituted by one or more, for example 1 or 2,- further substituents selected from cyano, nitro, amino, hydroxy and halogen. More preferably, when R10 and R" are defined according to option (a) above, they are the same or different and each represent hydrogen, a C C6 alkyl group, for example methyl and ethyl, a phenyl group or a Cj-C3 cycloalkyl group optionally fused to a phenyl ring, the alkyl group being unsubstituted or substituted by 1 or 2 substituents selected from hydroxy, halogen and amino groups and the phenyl and cycloalkyl groups being unsubstituted or substituted by 1, 2 or 3 substituents selected from (1) groups of formula -(CRJj , -O- CH^-R7, -COR and -CONHR wherein R is C,-C4 alkyl or -(CHJ R7, n is 0, 1 or 2 and R7 is as defined above, (2) groups of formula -(CH2)n-S(O)2-NRrR" wherein n is 0 or 1 and R' and R" are the same or different and are hydrogen or C,-C4 alkyl or, together with the N atom to which they are attached, form a pyrrolidinyl or piperidyl ring, (3) groups of formula -(CEnX-C02Rm , wherein n is 1 or 2 and R"* is hydrogen or C,-C4 alkyl, (4) groups of formula -NR""3 wherein each R"" is the same or different and is a C,-C alkyl group, and (5) halogen atoms and C,-C4 alkyl, hydroxy, C,-C4 alkoxy, amino, mono- and di(C,-C4 alkyl)amino, nitro, cyano, hydroxycarbonyl, C,-C4 alkoxycarbonyl, (C C5 acyl)amino, carbamoyl and C,-C4
haloalkyl groups, the alkyl substituents being unsubstituted or substituted by a further substituent selected from cyano, nitro, amino, hydroxy and halogen.
Typically, when R10 and Ru are as defined in the preceding paragraph, R7 is a phenyl group or a 5- or 6- membered aromatic or non-aromatic heterocycle having 1 or 2 heteroatoms selected from N, O and S, for example 4,5-dihydroxazolyl, the heterocycle being unsubstituted or substituted by 1 or 2 substituents selected from C,-C, alkyl groups and the phenyl group being unsubstituted or substituted by 1 or 2 substituents selected from halogen atoms and C,-C4 alkyl and C,-C4 alkoxy groups.
Most preferably, when R10 and Rn are defined according to option (a) above, R 10 is hydrogen and Ru is a phenyl group which is unsubstituted or substituted by one or two substituents selected from halogen atoms, for example fluorine and bromine, and phenyl and benzyloxy groups.
When R10 and Rπ-are defined according to option (b) above, R10 and Rn form a 3- to 7- membered heterocycle which is optionally fused to an aromatic ring or to a heteroaromatic ring which is in turn optionally fused to an aromatic ring. When the 3- to 7- membered heterocycle is fused to another ring, it is typically fused to a phenyl ring and/or to a 5- or 6- membered heterocyclic ring which is in turn optionally fused to a phenyl ring. Preferably, when the 3- to 7- membered ring is fused to another ring it is fused to a phenyl ring or to an indole group. Examples of such fused rings include 1,2,3,4-tetrahydroqumoline, 1,2,3,4-tetrahydroisoqu oline, 5,6,7,8-tetrahydro-8-aza- carbazole and 1,3,4,9-tetrahydro-beta-carbolinyl rings, for example 1,2,3,4- tetrahydroquinoline, 1^,3,4-tetrahydroisoquinoline and 5,6,7,8-tetrahydro-8-aza- carbazole rings.
When R10 and Rn are defined according to option (b) above, they typically form, together with the N atom to which they are attached, a 3- to 7- membered ring containing from 1 to 4 heteroatoms selected from N, O and S, which ring is (i) optionally fused to an aromatic ring or to a heteroaromatic ring which is in turn optionally fused to an aromatic ring and is (ii) substituted or unsubstituted by 1, 2 or 3 substituents independently selected from halogen atoms, groups of formula -X-R7 and
-CO2-X-R7 wherein X and R7 are as defined above, and hydroxy, cyano, nitro, carbamoyl, hydroxycarbonyl, C,-C6 alkoxycarbonyl, a ino, mono- and di-(C,-C6 alkyl)amino, divalent alkylene and C,-C3 alkyl groups, the alkyl substituents being unsubtituted or substituted by 1 or 2 further substituents selected from hydroxy and amino groups.
More preferably, when R10 and R" are defined according to option (b) above, they form, together with the nitrogen atom to which they are attached, an aromatic or non-aromatic, for example non-aromatic, 5- or 6- membered ring containing 1 or 2 heteroatoms selected from N, O and S, which ring is optionally fused to a phenyl ring or to an indole group, and is unsubstituted or substituted by 1, 2 or 3 substituents independently selected from halogen atoms, groups of formula -X-R7 and -CO2-X-R7 wherein X and R7 are as defined above, and hydroxy, cyano, nitro, C,-C4 alkoxycarbonyl, amino, G,-C2 divalent alkylene, for example methylene and C,-C4 alkyl groups. The aromatic or non-aromatic ring is, for example, unsubstituted or substituted by 1 , 2 or 3 substituents independently selected from halogen atoms, groups of formula -X-R7 and -CO2-X-R7 wherein X and R7 are as defined above, and hydroxy, cyano, nitro, amino, C,-C2 divalent alkylene, for example methylene and C,-C4 alkyl groups.
Typically, when R10 and R11 are as denned in the preceding paragraph, the said aromatic or non-aromatic 5- or 6- membered ring is a piperidinyl, piperazinyl, pyrazolyl or morpholino ring, for example a piperidinyl, piperazinyl or morpholino ring. It can be fused to a phenyl ring to form, for example, a tetrahydroquinoline or tetrahydroisoquinoline group, or to an indole group to form, for example a 5,6,7,8- tetrahydro-8-aza-carbazole ring or a 1,3,4,9-tetrahydro-beta-carbolinyl ring. Further, when R10 and Rπ are as defined in the preceding paragraph, typically, X is a direct bond, a C,-C4 alkylene group or a carbonyl group, for example a direct bond or a C,-C4 alkylene group, wherein the C,-C4 alkylene group is unsubstituted or substituted by a phenyl group, and R7 is a phenyl group or a cyclic group which is a 5- or 6- membered heteroaryl group containing 1 or 2 heteroatoms selected from N, O and S, which is optionally fused to a phenyl ring, the phenyl group and the cyclic group being
unsubstituted or substituted by 1 or 2 substituents selected from halogen atoms and C,- C4 alkyl, C,-C4 alkoxy and C,-C4 haloalkyl groups. Preferably, when R10 and Ru are as defined in the preceding paragraph, X is a direct bond, -CH2-, -CH-Ph- or a carbonyl group, for example a direct bond, -CH2- or -CH-Ph-, and R7 is a pyridinyl, pyrimidyl, pyrazinyl, benzimidazoyl, benzothiazolyl or phenyl group, which group is unsubstituted or substituted by 1 or 2 substitutents selected from halogen atoms, and C -C alkyl, C,- C4 alkoxy and -CF3 groups.
Most preferably, when R
10 and R
11 are defined according to option (b) above they form, together with the N atom to which they are attached, a
a 1,3,4,9-tetrahydro-beta-carbolinyl group, a piperidine group or a piperazine group, for example, a
1 ,2,3 ,4-tefrahydroisoquino line group, a piperidine group or a piperazine group, the piperidine and piperazine-groups being" unsubstituted or subtituted by 1 or 2 substituents selected from phenyl, pyridinyl and hydroxy groups, the phenyl and pyridinyl groups being optionally further substituted by one or two halogen atoms, for example chlorine atoms. The piperidine and piperazine groups are, for example, substituted by one or two phenyl groups.
When R10 and Rn are defined according to option (c) above, typically, R10 represents hydrogen or a C, to C6 alkyl group and R" represents a group of formula -X-R7, wherein X and R7 are as defined above.
Typically, when R10 and Ru are defined according to option (c) above, R10 is hydrogen or a C,-C4 alkyl group and R11 is a group of formula -X-R7 wherein:
X is a direct bond, a C,-C4 alkylene group or a carbonyl group, for example, a direct bond or a C,-C alkylene group, wherein the C,-C4 alkylene group is unsubstituted or substituted by 1 or 2 substituents selected from phenyl, C,-C4 alkyl, hydroxy, -CO2H and -CO2-(C,-C4 alkyl) groups; and
R7 is a C3-C3 cycloalkyl group, a phenyl group or a cyclic group which is a 5- or 6- membered aromatic or non-aromatic ring which contains 1 or 2 heteroatoms selected from N, O and S and which is optionally fused to a phenyl ring, the phenyl
group being unsubstituted or substituted by 1 or 2 substituents selected from halogen atoms and C,-C4 alkyl, phenyl, hydroxy, C,-C4 alkoxy, C,-C4 alkythio, amino, mono- and di-(C1-C4 alkyl)amino and C,-C4 haloalkyl groups, and the cyclic group being unsubstituted or substituted by 1 or 2 substituents selected from halogen atoms and C,-C4 alkyl, phenyl, phenyH -Q-alkyl)-, hydroxy, C,-C4 alkoxy, C,-C4 alkylthio, amino, mono- and di-(C,-C4 alkyl)amino and C,-C4 haloalkyl groups, provided that when X is substituted, R7 is a said unsubstituted or substituted phenyl group.
Preferably, when R10 and R" are as defined in option (c) above, R10 is hydrogen or a C,-C4 alkyl group and Rπ is a group of formula -X-R7 wherein:
- X is a direct bond, a C,-C4 alkylene group or a carbonyl group, for example, a direct bond or a C,-C4 alkylene group, wherein the C,-C4 alkylene group is unsubstituted or substituted by 1 or 2 substituents selected from C,-C4 alky hydroxy, -CO2H and -CO2-(C1-C4 alkyl) groups; and -R7 is a cyclopentyl, cyclohexyl, benzimidazolyl, benzothiazolyl, thiadiazolyl, furanyl, thienyl, pyrimidinyl, pyrazinyl, isoxazolyl, pyrazolyl, pyridyl, phenyl or piperidinyl group, for example a cyclopentyl, cyclohexyl, benzimidazolyl, benzothiazolyl, thiadiazolyl, furanyl, pyridyl, phenyl or piperidinyl group, the pyridyl, pyrimidinyl, piperidinyl, thiadiazolyl and furanyl groups being unsubstituted or substituted by 1 or 2 substituents selected from halogen atoms and hydroxy, C,-C4 alkoxy, phenyl, phenyl-C,-C4 alkyl- and Ct-C4 alkyl groups, and the phenyl, benzothiazolyl and benzimidazolyl groups being unsubstituted or substituted by 1 or 2 substituents selected from halogen atoms and hydroxy, C,-C4 alkoxy, and C,-C4 alkyl groups, provided that when X is substituted, R7 is an unsubstituted phenyl group.
Most preferably, when R10 and R" are as defined in option (c) above, R10 is hydrogen or a C,-C4 alkyl group and Rπ is a phenyl, pyridyl, thiadiazolyl, thienyl or phenylcarbonyl group, which is unsubstituted or substituted by one or two halogen atoms. In this embodiment, R11 is, for example, a phenyl, pyridyl or thiadiazolyl group.
Typically, when the substituents R16 and R17 form a said 4 to 8 membered ring, R16 and R17 are either on adjacent atoms or on the same atom. When R16 and R17 are on adjacent atoms, the said 4 to 8 membered ring is typically a phenyl ring. When R1<s and R17 are on the same atom, the said 4 to 8 membered ring is typically a saturated 5- or 6- membered ring, for example a cyclohexyl ring or a piperidyl ring.
Typically, R14 to R17 are the same or different and each independently represents hydrogen, a halogen atom, a group of formula -(CH^-R7 wherein n and R7 are as defined above, or a Cx-C6 alkyl group, for example hydrogen, a group of formula -(CHJ R7 or a C,- alkyl group or R14 and R15 are as defined above and R16 and R17, together with the atoms to which they are attached, form a 4 to 8 membered aromatic or non-aromatic ring which contains from 0 to 4 heteroatoms selected from N, O and S and which is unsubstituted or substituted by 1 or 2 substituents selected from halogen atoms and C,-C6 alkyl, C,-Cβ haloalkyl, hydroxy, phenyl, phenyl-(C,-C3 alkyl)-, amino and mono- and di-(C,-C6 alkyl)amino groups. Preferably, R14 to R17 are the same or different and each independently represents hydrogen, a halogen atom, a 5- or 6- membered heteroaryl group having 1 or 2 heteroatoms selected from N, O and S, for example pyridyl, a C,-C4 alkyl group or a phenyl group which is unsubstituted or substituted by 1 or 2 substituents selected from halogen atoms, C,-C4 alkyl groups and C^C^ haloalkyl groups. In this embodiment R14 to R17 are, for example, the same or different and each independently represents hydrogen, a 5- or 6-membered heteroaryl group, a Cw alkyl group or a phenyl group, which is unsubstituted or substituted as described above. Alternatively, R14 and R1S are as defined above and R16 and R17, together with the atoms to which they are attached, form a 5- or 6- membered aromatic or non-aromatic ring which contains 0, 1 or 2 heteroatoms selected from N, O and S and which is unsubstituted or substituted by 1 or 2 substituents selected from C,-C4 alkyl, phenyl and phenyl-(C,-C4 alkyl)- substituents. More preferably, the 5- or 6- membered ring is a phenyl ring or a piperidylidene ring.
Typically, R6 represents -C(O)NR10 R", wherein R10 and R" are as defined above, -ON=CR12R13, wherein R12 and R13 are as defined above, or a phenyl,
heterocyclyl or heteroaryl group, for example a heterocyclyl or heteroaryl group, the phenyl, heterocyclyl and heteroaryl groups being unsubstituted or subsituted with substituents R14 to R17, as defined above.
Typically, when R6 is phenyl, it is unsubstituted or substituted by one halogen atom.
Typically, when R6 is a heterocyclyl or heteroaryl group it is a 5- or 6- membered heterocyclyl or heteroaryl group, which group contains 1, 2 or 3 heteroatoms selected from N, O and S and is unsubstituted or substituted with substituents R14 to R17, as defined above. Preferably, the heterocyclyl or heteroaryl group is a 6- membered heteroaryl group having 1 or 2 heteroatoms selected from N, O and S, for example pyridyl, pyrimidinyl and pyrazinyl groups, or a group of formula (H)
wherein X represents O, S or N, and the " * 2" moiety represents
-N=C(R18)-, -C(R18)=N-, -C(RI8)=C(R19)- or -CH(R13)-CH(R19)-, wherein
R18 and R19 are the same or different and each represents hydrogen, a group of formula -(CH^-R7 wherein n and R7 are as defined above, or an alkyl group, the alkyl group being unsubstituted or substituted by one or more, for example 1 or 2, substituents selected from hydroxy, alkoxy, alkylthio, amino, mono- and di-alkylamino, hydroxycarbonyl, alkoxycarbonyl, acylamino, carbamoyl, alkylcarbamoyl, dihydroxyphosphoryloxy, dialkoxyphosphoryloxy and haloalkyl groups, or R18 and R19, together with the atoms to which they are attached, form a 4 to 8 membered, aromatic or non-aromatic ring, which contains from 0 to 4 heteroatoms selected from N, O and S and which is unsubstituted or substituted by one or more, for example 1 or 2, substituents selected from halogen atoms and alkyl, hydroxy, phenyl, alkoxycarbonyl,
amino, mono-alkylamino, di-alkylamino and hydroxycarbonyl groups, the alkyl substituents being unsubstituted or substituted by one or more, for example 1 or 2, further substituents selected from halogen atoms and hydroxy, alkoxy, alkylthio, acylamino, carbamoyl, alkylcarbamoyl, dihydroxyphosphoryloxy, dialkoxyphosphoryloxy, hydroxyalkoxy, phenyl, alkoxycarbonyl, amino, mono- and di-alkylamino and hydroxycarbonyl groups.
Typically, when R18 and R19 form a said 4 to 8 membered ring, R18 and R19 are either on adjacent atoms or on the same atom. When R18 and R19 are on adjacent atoms, the said 4 to 8 membered ring is typically a phenyl ring. When R18 and R19 are on the same atom, the said 4 to 8 membered ring is typically a saturated 5- or 6- membered ring, for example a cyclohexyl ring or a piperidyl ring.
Typically, R18 and R19 are the same or different and each independently represents hydrogen, a group of formula -(CH^-R7 wherein n and R7 are as defined above, or a C,-C3 alkyl group, or R18 or R19, together with the atoms to which they are attached, form a 4 to 8 membered aromatic or non-aromatic ring which contains from 0 to 4 heteroatoms selected from N, O and S and which is unsubstituted or substituted by 1 or 2 substituents selected from halogen atoms and C,-C6 alkyl, C,-C3 haloalkyl, hydroxy, phenyl, phenyl-C,-C3 alkyl, amino and mono- and ch - alkyl)amino groups. . Preferably, R18 and R19 are the same or different and each independently represent hydrogen, a 5- or 6- membered heteroaryl group having 1 or 2 heteroatoms selected from N, O and S, for example pyridyl, a C,-C4 alkyl group or a phenyl group which is unsubstituted or substituted by 1 or 2 substituents selected from halogen atoms, C,-C4 alkyl groups and -C4 haloalkyl groups, or R18 and R19, together with the atoms to which they are attached, form a 5- or 6- membered aromatic or non aromatic ring which contains 0, 1 or 2 heteroatoms selected from N, O and S and which is unsubstituted or substituted by 1 or 2 substitutents selected from C,-C4 alkyl, phenyl and phenyl-{C,-C4 alkyl)- substituents.
Preferably, R6 represents -C(O)NRl0Rn, wherein R10 and Ru are as defined above, -ON=CRl2R13 wherein R12 and R13 are as defined above, a phenyl group which is optionally substituted by a halogen atom, or a 5- or 6- membered heteroaryl or heterocyclyl group which is optionally fused to a phenyl ring and which is unsubstituted or substituted by 1 or 2 substituents selected from phenyl, pyridyl, phenyl-(C,-C4 alkyl)-, C,-C4 alkyl and piperidylidene substituents, the phenyl subsitutents being unsubstiuted or substituted by 1 or 2 further substituents selected from halogen atoms and C,-C4 alkyl groups and the piperidylidene substituents being unsubstituted or substituted by 1 or 2 further substituents selected from phenyl, phenyl-(C,-C4 alkyl)- and C,-C4 alkyl groups.
More preferably, Rδ represents -C(O)NR10RU, a phenyl group or an oxadiazolyl group, for example a group -C(O)NRl0Rπ or an oxadiazolyl group, wherein the oxadiazolyl group is unsubstituted or substituted by a phenyl group wherein either R10 is hydrogen and Ru is a thiadiazolyl group, a pyridyl group, a phenyl group, a thienyl group or a phenylcarbonyl group, for example a thiadiazolyl group, a pyridyl group or a phenyl group, the thiadiazolyl, pyridyl, phenyl, thienyl and phenylcarbonyl groups being unsubstituted or substituted by 1 or 2 substituents selected from halogen atoms and phenyl and benzyloxy groups or R10 and Rn form, together with the N atom to which they are attached, a 1, 2, 3, 4-tetrahydroisoquinoline group, a 1,3,4,9-tetrahydro- beta-carbolinyl group, a piperidine group or a piperazine group, for example a 1, 2, 3, 4- tefc^ydroisoqu oline group, a piperidine group or a piperazine group, the piperidine and piperazine groups being unsubstituted or substituted by 1 or 2 substituents selected from phenyl, pyridyl and hydroxy groups, the phenyl and pyridyl groups being optionally further substituted by one or two halogen atoms, for example chlorine atoms. The piperidine and piperazine groups are, for example, substituted by one or two phenyl groups.
Preferred compounds of formula I include the compounds of formula la described hereinbelow, and pharmaceutically acceptable salts thereof:
la wherein R
1, R
2, R
3, R
4, R
5, R
8, R
9, R
10 and R
π are as defined above. Preferably, in the formulae (I) and (IA), - R
1 and R
2 are the same or different and each independently represent hydrogen, a group of formula -(CH^-R
7 wherein n and R
7 are as defined above or a C,-C
3 alkyl group which is unsubstituted or substituted by one or more, for example 1 or 2, substituents selected from hydroxy, C,-C
3 alkoxy, C,-C
3 alkylthio, amino, and mono- and di-(C,-C
6alkyl)amino.groups. R
3 represents hydrogen, halogen or a C,-C
3 alkyl group which is unsubstituted or substituted by one or more, for example 1 or 2, substituents selected from halogen atoms and hydroxy groups;
R4 and R5 are the same or different and each represent hydrogen, halogen, C,-C6 alkyl, C,-C3 haloalkyl, hydroxy, C,-C6 alkoxy, C,-C3 alkylthio, amino or mono- or όi-(C^-C6 allcyl)amino.
- Preferably, , is a direct bond or ^ CH^-, -O(CR8RV, -S(CRSRV>
-CH=CH-, -(CH,.),,,-, -(CR8RV, -(CH^O-, -(CR'R^O-, -0(01^0-, -(C^ ^Z)- or -N(Z)(CR8RV, for example, a direct bond or -0(CKJm-, -0 0^^-, -S(CR8R9)ra-, -CH=CH-, -(CH,_)m-, -(CR8RV. -(CH^O-, -(C ^O-, -(CR^^Z)- or - N(Z)(CR8R9)m-, wherein m is from 1 to 4, Z is hydrogen or C,-C4 alkyl and R8 and R9 are the same or different and each represent hydrogen, C,-C4 alkyl, C3-C6 cycloalkyl, (C3- cycloalkylH C,-C4 alkyl)-, phenyl or phenyl-(C,-C4 alkyl)-; and
Rδ represents -C(O)NR10R' ' , -ON=CR12R13, or a phenyl, heterocyclyl or heteroaryl group, for example a heterocyclyl or heteroaryl group, the phenyl, heterocyclyl and heteroaryl groups being unsubstituted or substituted with substituents R14 to R17, wherein:
R10 and Ru are either:
(a) the same or different, each independently representing hydrogen, a - alkyl group, a C5-C3 cycloalkyl group optionally fused to a phenyl ring, or a phenyl group, the alkyl group being unsubstituted or substituted by 1 or 2 substituents selected from hydroxy, halogen, C,-C4 alkoxy and amino groups and the phenyl and cycloalkyl groups being unsubstituted or substituted by 1, 2, 3 or 4 substitutents selected from (1) groups of formula -(CK j , -O^CH^-R7, -S-(CH,Jn-R7 and -COR and -CONHR wherein R is -Cg alkyl or -(CH^Jl7 and n and R7 are as defined above, (2) groups of formula -(CH2)n-S(O)2NR'R" wherein n is as defined above and R' and R" are the same or different and are each selected from hydrogen and C,-C6 alkyl or form, together with the N atom to which they are attached, a 4- or 5- membered saturated heterocyclic ring containing 1 or 2 heteroatoms selected from N, O and S, (3) groups of formula - CΑ n- CO2R wherein n is as defined above and R'" is hydrogen or C,-C3 alkyl, (4) groups of formula -N*R""3 wherein each R"" is the same or different and is a C,-C6 alkyl group, and (5) halogen atoms and C,-C3 alkyl, hydroxy, C,-C4 alkylenedioxy, C,-C6 alkoxy, C,- Cs alkylthio, amino, mono- and di-(C,-C6 alkyl)amino, nitro, cyano, hydroxycarbonyl, (C,-C(i alkoxy)carbonyl, (Cx-Cη acyl)amino, carbamoyl and C,-C3 haloalkyl groups,
(b) together with the N atom to which they are attached, a 3- to 7- membered ring containing from 1 to 4 heteroatoms selected from N, O and S which ring is (i) optionally fused to an aromatic ring or to a heteroaromatic ring which is in turn optionally fused to an aromatic ring and is (ii) substituted or unsubstituted by 1, 2 or 3 substituents independently selected from halogen atoms, groups of formula -X-R7 and -CO2-X-R7 wherein X is a direct bond, a C,-C4 alkylene group or a carbonyl group, for example a direct bond or a C,-C4 alkylene group and R7 is as defined above, and hydroxy, cyano, nitro, carbamoyl, hydroxycarbonyl, C,-C6 alkoxycarbonyl, mono- and di^Cj-Cβ alkyl)amino, amino, divalent alkylene and C,-C6 alkyl groups, the alkyl substituents being unsubstituted or substituted by 1 or 2 further substituents selected from hydroxy and amino groups and the moiety X being unsubstituted or substituted by
one or two substituents selected from phenyl, C,-C4 alkyl, hydroxy, -CO2H and -CO2- (C,-C4 alkyl), or
(c) defined so that R10 is hydrogen or a C,-C4 alkyl group and Rn is a group of formula -X-R7' wherein: - X is a direct bond, a C^-C^ alkylene group or a carbonyl group, for example a direct bond or a C,-C4 alkylene group, wherein the C^C^ alkylene group is unsubstituted or substituted by 1 or 2 substituents selected from phenyl, C,-C4 alkyl, hydroxy, -CO2H and -CO2-(C1-C4 alkyl) groups; and
R7' is a C3-C6 cycloalkyl group, a phenyl group or a cyclic group which is a 5- or 6- membered aromatic or non-aromatic ring which contains 1 or 2 heteroatoms selected from N, O and S and which is optionally fused to a phenyl ring, the phenyl group being unsubstituted or substituted by 1 or 2 substituents selected from halogen atoms and C,-C4 alkyl, phenyl, hydroxy, C,-C4 alkoxy, C,-C4 alkylthio, amino, mono- and di-(C!-C4 alkyl) amino and C,-C4 haloalkyl groups, and the cyclic group being unsubstituted or substituted by 1 or 2 substituents selected from halogen atoms and C,-C4 alkyl, phenyl, phenyl-(C,-C4-aIkyl)-, hydroxy, C,-C4 alkoxy, C,-C4 alkylthio, amino, mono- and di-(C,-C4 alkyl)amino and C,-C4 haloalkyl groups, provided that when X' is substituted, R7' is a said unsubstituted or substituted phenyl group, R12 and R13 are the same or different and each represent hydrogen, amino, (C,-C6 alkyl)amino, di-(C,-C3 alkyl)amino, C,-C6 alkyl, C3-C6 cycloalkyl or phenyl, the alkyl moieties being unsubstituted or substituted by 1 or 2 substitutents selected from hydroxy groups and halogen atoms and the cycloalkyl group and the phenyl group being unsubstituted or substituted by 1, 2, 3 or 4 substituents selected from halogen atoms and C,-C4 alkoxy, C,-C4 alkylthio, C,-C4 alkyl, hydroxy, C,-C4 haloalkyl, amino, and mon- and di-(Cι-C4 alkyl)amino groups, and
R14 to R17 are the same or different and each independently represent hydrogen, a halogen atom, a group of formula -(CH^-R7 wherein n and R7 are as defined above, or a C,-C3 alkyl group, for example hydrogen, a group of formula -(CH^-R7 or a -Cg
alkyl group, or R14 and R15 are as defined above and R1<s and R17, together with the atoms to which they are attached, form a 4 to 8 membered aromatic or non-aromatic ring which contains from 0 to 4 heteroatoms selected from N, O and S and which is unsubstituted or substituted by 1 or 2 substituents selected from halogen atoms and C,- C6 alkyl, C,-C3 haloalkyl, hydroxy, phenyl, phenyHCj- alkyl)-, amino and mono- and di-(C,-C6 alkyl)amino groups.
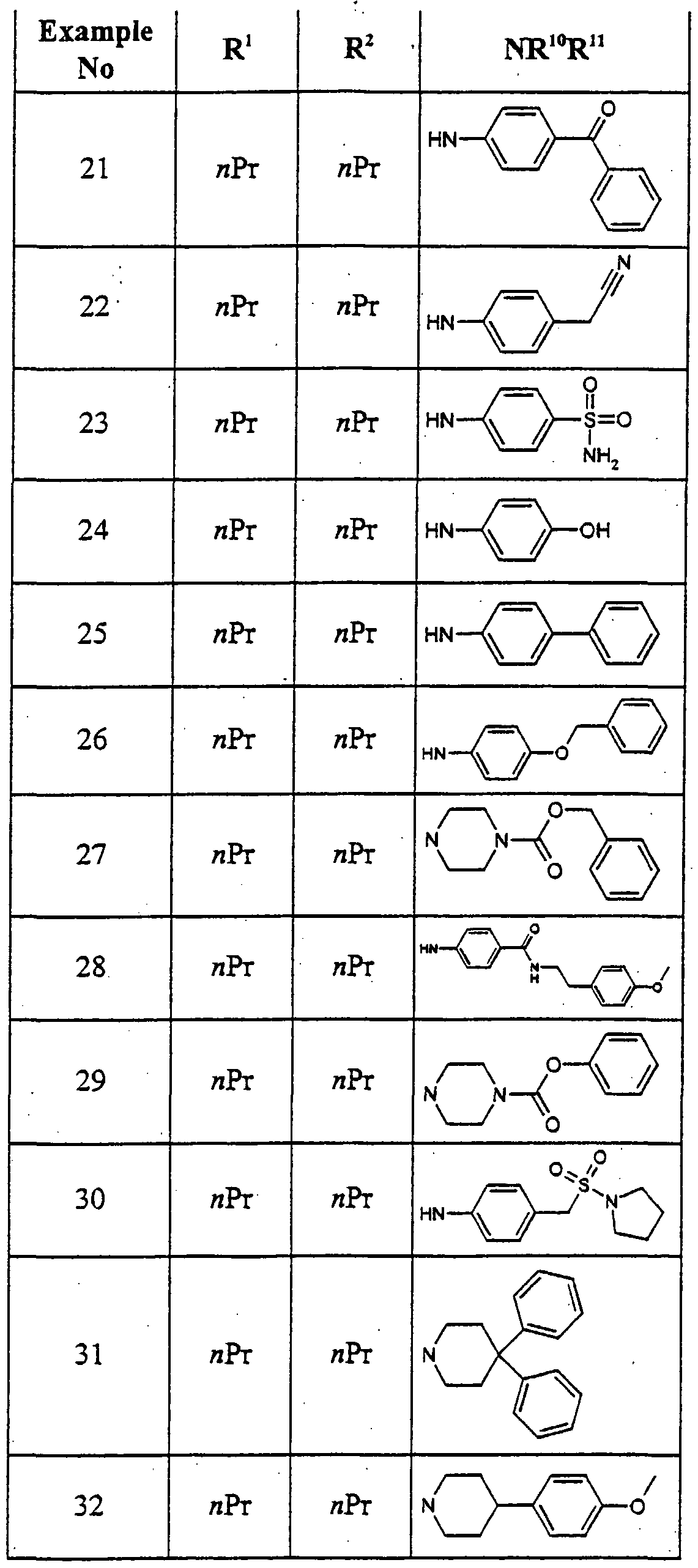
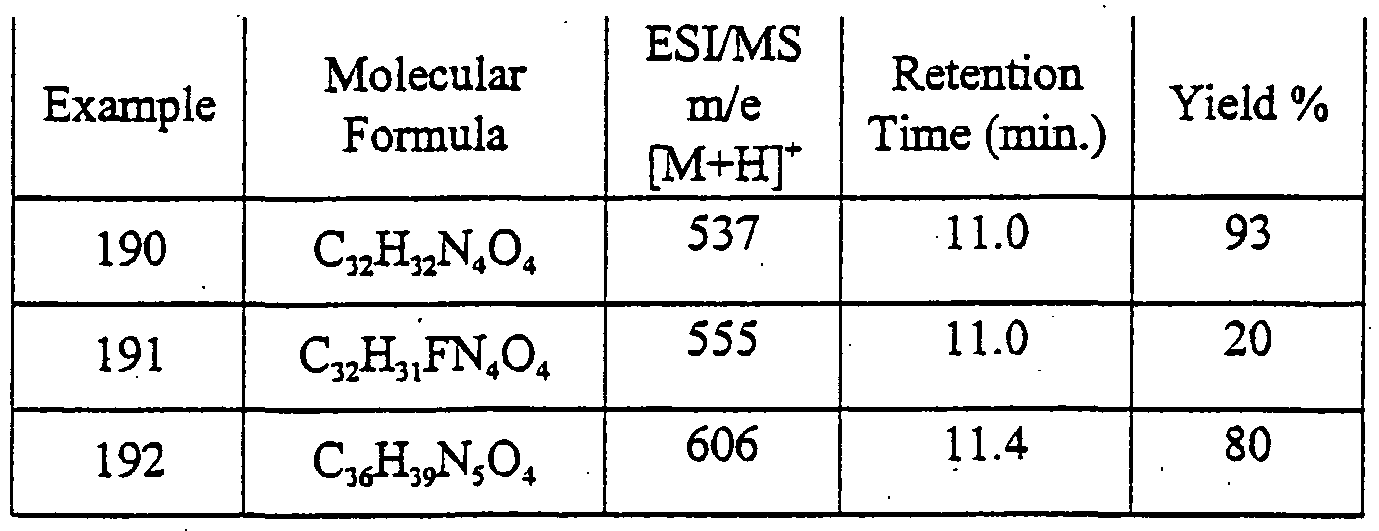
Particular individual compounds of the invention include:
2-[4-<2,4-rΛoxo-l,3-dipropyl-2,3,4,5-tetrahyd^ yl)phenoxy]-N-phenylacetamide 6- {4-[2-Oxo-2-(4-phenylpiperazin- 1 -yl)ethoxy]phenyl} - 1 ,3-dipropyl- 1 ,5-
<i ydropyrrolo(3,2-( ]pyrimidine-2,4-dione
2-[4-<2,4-Dioxo-l,3-dipropyl-2,3,4,5-tetrahydro-lH^^ yl)phenoxy]-N-(4-fluorophenyl) acetamide
6- {4-[2-(3,4-Dihydro- lH-isoquinolin-2-yl)-2-oxoethoxy] phenyl} - 1 ,3-dipropyl- l,5-d y<iropyιτolo[3^-<i]pyrimidine-2,4-dione
N-(4-CMorophenyl)-2-[4-(2,4-dioxo-l,3-dipropyl-2,3,4,5-tetrahydro-lH- pyτrolo[3^-<f]pyτinn'din-6-yl)phenoxy] acetamide
2-[4-(2,4-Dioxo-l,3-dipropyl-2,3,4,5-tetrahydro-lH-pyrrolo[3^-d]pyrimidin-6- yl)phenoxy]-N-(4-trifluoro methoxyphenyl)acetamide N-(4-Cyanophenyl)-2-[4-(2,4-dioxo-l,3-dipropyl-2,3,4,5-tetrahydro-lH- pyrrolo[3,2-<φyrirnidin-6-yl)pb.enoxy] acetamide
4- {2-[4-(2,4-Dioxo- 1 ,3-dipropyl-2,3,4,5-tetrahydro- lH-pyrrolo[3^-<i]pyrirmdin- 6^yl)phenoxy]acetylamino}benzamide
6- {4-[2-Oxo-2-(2,3 ,5,6-tetrahydro-[ 1 ,2']bipyrazinyl-4-yl)ethoxy]phenyl} - 1 ,3- <npropyl-l,5-d ydropyrrolo[3,2-< ] pyrimidine-2,4-dione
2-[4-(2,4-Dioxo-l,3-dipropyl-2,3,4,5-tetrahydro-lH-pyτrolo[3^-<i]pyrinndin-6- yl)phenoxy]-N-(4-methoxyphenyl) acetamide
2-{4-(2,4-Dioxo- 1 ,3-dipropyl-2,3,4,5-tetrahydro- lH-pyιrolo[3,2-<φyrinήdin-6- yl)phenoxy]-N- -tolylacetamide
N-(4-Acetylphenyl)-2-[4-(2,4-dioxo- 1 ,3-dipropyl-2,3,4,5-tetrahydro-lH- pyrrolo[3,2-^pyriιnidin-6-yl)phenoxy] acetamide
4-{2-[4-(2,4-Dioxo-l,3-dipropyl-2,3,4,5-tetrahydro-lH-pyrrolo[3^-J]pyrimidin- 6-yl)phenoxy]acetylamino} benzoic acid ethyl ester 2-[4-(2,4-Dioxo-l,3-diρropyl-2,3,4,5-tetrahydro-lH-pyιτolo[3^- ]pyrinn^in-6- yl)phenoxy]-N-(4-trifluoromethyl phenyl)acetamide
6-(4- {2-[4-(2-Chlorophenyl)piperazin- 1 -ylj-2-oxo-ethoxy } phenyl)- 1 ,3-dipropyl- l,5-dmydropyrrolo[3,2-cf] pyrimidine-2,4-dione
N-(4-tert-Butylphenyl)-2-[4-(2,4-dioxo- 1 ,3-dipropyl-2,3 ,4,5-tetrahydro- 1H- pyrrolo[3,2-ft pyrirnidin-6-yl)phenoxy] acetamide
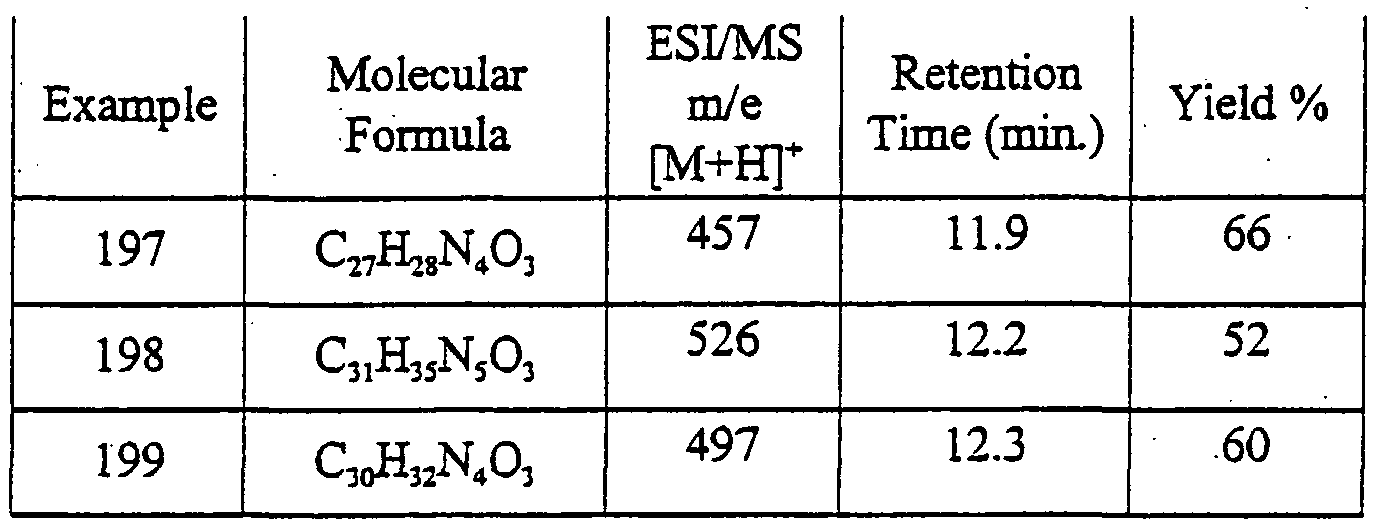
1 - {2-[4-(2,4-Dioxo- 1 ,3-dipropyl-2,3,4,5-tetrahydro- lH-pyrrolo[3,2- jpyrimidin- 6-yl)phenoxy]acetyl}-4-phenyl-piperidine-4-carbonitrile
6- {4-[2-(4-Benzhydrylpiperazin- 1 -yl)-2-oxoethoxy] phenyl} - 1 ,3-dipropyl- 1 ,5- dihydropyrrolo[3 ^-^pyrirnidine-2,4-dione 2-[4-(2,4-Dioxo-l ,3-dipropyl-2,3,4,5-tetrahydro-lH-pyrrolo[3^-ci]pyrimidin-6- yl)phenoxy]-N-(2-hydroxy- 1 -phenylethyl)acetamide
N-(2-(^oro-l-ρhenylethyl>2-[4-(2,4- oxo-l,3-diρropyl-2,3,4,5-tetrahydro-lH- pyrrolo[3,2-d]pyrinn'din-6-yl)phenoxy]acetamide
N-(4-Benzoylphenyl)-2-[4-(2,4-dioxo- 1 ,3-dipropyl-2,3 ,4,5-tetrahydro- 1H- pyrrolo[3,2-<φyrinήd -6-yl)phenoxy] acetamide
N-(4-Cyanomethylphenyl)-2-[4-(2,4- oxo-l,3-dipropyl-2,3,4,5-tetrahydro-lH- pyrrolo[3,2-cflpyτimidin-6-yl)phenoxy] acetamide
2-(4-(2,4-Dioxo- 1 ,3-dipropyl-2,3 ,4,5-tetrahydro- lH-pyrrolo[3 ,2-.φyrirmdin-6- yl)phenoxy]-N-(4-sulfamoylphenyl) acetamide 2-[4-{2,4-Dioxo- 1 ,3-dipropyl-2,3 ,4,5-tetrahydro- lH-pyτrolo[3 ,2-d]j>yτiπήάw.-6- yl)phenoxy]-N-(4-hydroxy-phenyl)acetamide
N-Biphenyl-4-yl-2-[4-(2,4-dioxo- 1 ,3-diρropyl-2,3,4,5-tetrahydro-lH- pyrrolo[3,2-<i]pyrimidin-6-yl)phenoxy] acetamide
N-(4-Benzyloxyphenyl)-2-[4-(2,4-dioxo- 1 ,3-dipropyl-2,3 ,4,5-tetrahydro- 1H- pyrrolo[3,2-< ]pyrimidin-6-yl)phenoxy] acetamide
4- {2-[4-(2,4-Dioxo- 1 ,3-dipropyl-2,3 ,4,5-tetrahydro- lH-pyτrolo[3,2-<i]pyrimidin- 6-yl)phenoxy]acetyl}piperazine-l-carboxylic acid benzyl ester 4-{2-[4-(2,4-Dioxo-l,3-diproρyl-2,3,4,5-tetι^ydro-lH-pyιτolo[3,2- yrimidin-
6-yl)phenoxy]acetylamino } -N-[2-(4-methoxypheny l)ethyl]benzamide
4- {2-[4-(2,4-Dioxo- 1 ,3-dipropyl-2,3 ,4,5-tetrahydro- lH-pyrrolo[3,2-< ]pyrirnidin- 6-yl)phenoxy]acetyl}ρiρerazine-l-carboxylic acid phenyl ester
2-[4-(2,4-Dioxo-l,3-<hpropyl-2,3,4,5-tetrahydro-lH-pyrrolo[3^-^pyrimidin-6- yl)phenoxy]-N-[4-(pyrroIidine-l -sulfonyimethyl)phenyl]acetamide
6- {4-[2-(4,4-Diphenyl-piperidin- 1 -yl)-2-oxo-ethoxy] phenyl} - 1 ,3-dipropyl- 1 ,5- dmydropyrrolo[3,2-ct pyrirmdine-2,4-<iione
6-(4-{2-[4-(4-Methoxyphenyl)piperidin-l-yl]-2-oxo-ethoxy}phenyl)-l,3- dipropyl- 1 ,5-dihydro-pyrτolo[32-d] pyriιnidine-2,4-dione (4-{2-[4-(2,4-Dioxo-l,3-diproρyl-2,3,4,5-tetrahydro-lH-pyrrolo[3^-
< ]pyrinn^m-6-yl)phenoxy]acetylamino}phenyl) acetic acid ethyl ester
6-(4- {2-[4-( 1 -Methyl- lH-benzoinn
'dazol-2-ylmethyl)piperazin- 1 -yl]-2-
6- {4-[2-(3,3-Diphenylpiperazin- 1 -yl)-2-oxo-ethoxy]phenyl} - 1 ,3-dipropyl- 1 ,5- dih.ydro-pyrrolo[3,2-<f] pyrimidine-2,4-dione
N-[4-(4,4_r imethyl-4,5-dmydrooxazol-2-yl)phenyl]-2-[4-(2,4-dioxo- 1 ,3- dipropyl-2,3,4,5-tetrahydro-lH-pyn-olo[3^-if]pyrinήo n-6-yl)phenoxy]acetamide
(4-{2-[4-(2,4-Dioxo-l,3-dipropyl-2,3,4,5-tetrahydro-lH-pyrrolo[3r2- ^pyrimidm-6-yl)phenoxy]acetylamino} phenyl)trimethyl ammonium 6-(4- {2-[4-(3 ,5-DicWoropyridin-4-yl)piperazin- 1 -yl]-2-oxo-ethoxy} -phenyl)- l,3-dipropyl-l,5-d y<iropyrrolo[3^- ]pyrirmdine-2,4-dione
6-(4- {2-[4-(6-CMorobenzoti-iazol-2-yl)piperazin- 1 -yl]-2-oxo-ethoxy}phenyl)- 1 ,3-dipropyl- 1 ,5-dihydropyrrolo{32-d] pyrimidine-2,4-dione
N-(4-Acerylam ophenyl)-2-[4-(2,4-dioxo-l,3-dipropyl-2,3,4,5-tetrahydro-lH- pyrrolo[3 ,2-<f]pyrinήdin-6-yl)phenoxy] acetamide
6-{4-[2-Oxo-2-(l,3,4,9-tetrahydro-β-carbolm-2-yl)ethoxy]phenyl}-l,3-dipropyl- l,5-dihydropyrrolo[3,2-< ] pyrirm'dine-2,4-dione 2-[4-(2,4-Dioxo-l,3-diproρyl-2,3,4,5-tefrahydro-lH-pyrrolo[3,2- ]pyrirmdin-6- yl)phenoxy]-N-(4-iodophenyl) acetamide
2-[4-(2,4-Dioxo-l,3-diproρyl-2,3,4,5-tetrahydro-lH-pyrrolo[3^-<i]pyrirnidin-6- yl)phenoxy]-N-(2-hydroxy-2-phenylethyl)acetamide
2-[4-(2,4-Dioxo-l,3-dipropyl-2,3,4,5-te1xahydro-lH-pyrrolo[3,2-d]pyrinήdin-6- yl)phenoxy]-N-(2-hydroxy- 1 -methyl-2-phenylethyl)acetamide
N-(7-Cyano-3-hydroxy-2^-dimethylchroman-4-yl)-2-[4-(2,4-dioxo- 1 ,3- dipropyl-2,3,4,5-tetrahydro-lH-pyrrolo[3^-d]pyrimidm-6-yl)phenoxy]acetamide
N-(l-Berιzyl-3-hydroxypiperidm-3-ylmethyl)-2-[4-(2,4-dioxo-l,3-dipropyl- 2,3 ,4,5-tetrahydro- lH-pyrrolo[3 ^-d]pyrimidin-6-yl)phenoxy]acetamide
yl)phenoxy]-N-[2-hydroxy-2-(4-hydroxyphenyl)ethyl]acetamide
2-[4-{2,4-Dioxo- 1 ,3-dipropy 1-2,3 ,4,5-tetrahydro- lH-pyrrolo[3 ,2-d]pyrimidin-6- yl)phenoxy]-N-[2-hydroxy-2-(4-hydroxy-3-hydroxymethylphenyl)ethyl]acetamide
2-{4-(2,4-Dioxo- 1 ,3-diproρyl-2,3 ,4,5-tetrahydro- lH-pyrrolo[3 ,2-d]pyrirnidin-6- yl)phenoxy]-N-(2-hydroxyindan- 1 -yl)acetamide
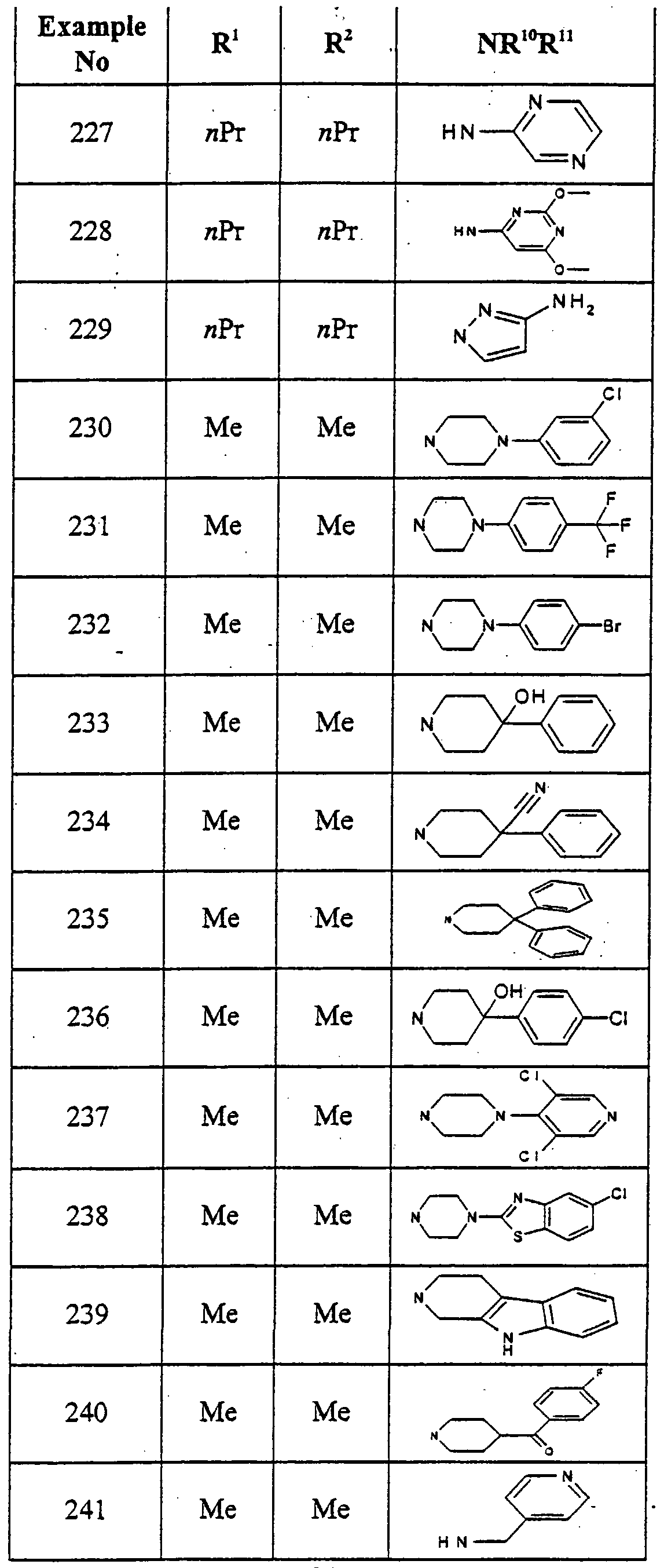
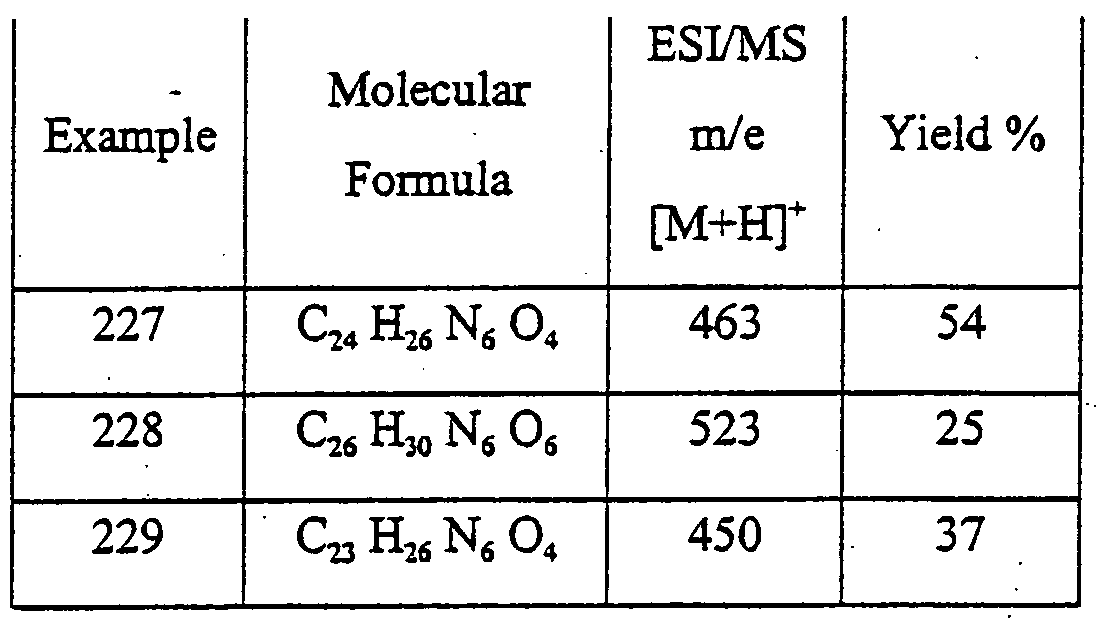
6-{4-{2-Oxo-2-(6- -tolyl-2,6-diazabicyclo[2.2.1]hept-2-yl)ethoxy]phenyl}-l,3- o propyl-l,5-d ydropyιτolo[3,2-d]pyrinndine-2,4-dione
2-[4-(2,4-Dioxo- 1 ,3-dipropyl-2,3 ,4,5-tetrahydro- lH-pyrrolo[3 ,2-d]pyrirnidin-6- yl)phenoxy]-N-(2-hydroxyphenyl) acetamide {2-{4-(2,4-Dioxo- 1 ,3-dipropyl-2,3 ,4,5-tetrahydro- lH-pyrrolo[3 ,2-d]ρyrimidin-6- yl)phenoxy]acetylaπιino} phenylacetic acid methyl ester
{2-[4-{2,4-Dioxo- L,3-dipropyl-2,3 ,4,5-tetrahydro- lH-pyrrolo[3 ,2-d]pyrinridin-6- yl)phenoxy]acetylamino} phenylacetic acid
(4- {2-[4-(2,4-Dioxo- 1 ,3-dipropyl-2,3 ,4,5-tetrahydro- lH-pyrrolo(3 ,2- d]pyrinήdm-6-yl)phenoxy]acerylamino} phenyl)acetic acid
N-(2- Arninoethyl)-2-[4-( 1 ,3-dimethyl-2,4-dioxo-2,3 ,4,5-tetrahydro- IH- pyrrolo[3^-^pyτimidin-6-yl)phenoxy] acetamide N-(4-Bromoρhenyl)-2-[4-( 1 ,3-dimethyl-2,4-dioxo-2,3,4,5-tetrahydro-lH- pyrrolo(3,2- ]pyrimidin-6-yl)phenoxy] acetamide
2-[4-(l ,3-Dimethyl-2,4-dioxo-2,3J4,5-tetrahydro- lH-pyrroloP^-^ yr-n ^iin-ό- yl)phenoxy]-N-phenylacetamide
2-[4-(l,3-Dmethyl-2,4-moxo-2,3,4,5-tetra yl)phenoxy]-N-(4- fluorophenyl) acetamide
1 ,3-Dimethyl-6- {4-[2-(4-methylpiperazin-l -yl)-2-oxo-ethoxy]phenyl} - 1 ,5- dihydropyrrolo[3 ^-c^pyrimidine-2,4-dione
1 ,3-Dimethyl-6-[4-(2-morpholin-4-yl-2-oxoethoxy)phenyl]- 1 ,5-
6- {4-[2-(3 ,4-Dihydro- lH-isoquinoIin-2-yl)-2-oxoethoxy] phenyl} - 1 ,3-dimethyl-
1 ,5-dihydropyrrolo[3 ,2- ]pyrimidine-2,4-dione
N-Cyclopentyl-2-[4-( 1 ,3-dimethyl-2,4-dioxo-2,3 ,4,5-tetrahydro- lH-pyrrolo[3 ,2- ^yrimidin-6-yl)phenoxy] acetamide
N-(4-Acetylphenyl)-2-[4-(l,3-<iimemyl-2,4-dioxo-2,3,4,5-tetrahydro-lH- pyrjolo(3,2-<f]pyrimidin-6-yl)phenoxy] acetamide
N-(lH-Benzoimidazol-2-yl)-2-[4-(l,3-dimethyl-2,4-dioxo-2,3,4,5-tetrahydro- lH-pyrrolo[3 ,2-<^pyrimidin-6-yl) phenoxyjacetamide
N-(4-Cyanoρhenyl)-2-[4-(l,3-dimemyl-2,4-dioxo-2,3,4,5-tetrahydro-lH- pyrrolo[3^- ]pyrimidin-6-yl)phenoxy] acetamide 6-{4-[2-(3,4-D ydro-2H-qumolin-l-yl)-2-oxoethoxy] phenyl} -1,3-dimethyl-
1 ,5-dmydropyιrolo[3,2-(i]pyrinήciine-2,4-dione
2-[4-( 1 ,3-Dimethy l-2,4-dioxo-2,3 ,4,5-tetrahydro- lH-pyσolo[3,2-ci]pyrimidin-6- yl) henoxy]-N-[l,3,4]thiadiazol-2-ylacetamide
1 ,3-Dimethyl-6- {4-[2-oxo-2-(4-phenylpiperazin- 1 -yl)ethoxy]phenyl} - 1 ,5-
dihydropyrrolo[3 ,2-^pyrimidine-2,4-dione
2-[4-( 1 ,3-Dimethyl-2,4-dioxo-2,3 ,4,5-tetrahydro- lH-pyrrolo[3 ,2- Iρyriιmdin-6- yl)phenoxy]-N-(4-nitrophenyl) acetamide
6-(4- {2-[4-(4-Fluorophenyl)piperazin- 1 -yl]-2-oxoethoxy} phenyl)- 1 ,3-dimethyl- l,5-dihydropyrrolo(3,2-<i] pyrirnidine-2,4-dione
6- {4-[2-(4-Benzylpiperazin- 1 -yl)-2-oxoethoxy]phenyl} - 1 ,3-dimethyl- 1 ,5- dmydropyrrolo[3 ^-c2 pyrimidine-2,4-dione
6-<4- {2-[4-(2-Methoxyphenyl)piperazin- 1 -yl]-2-oxoethoxy} phenyl)- 1 ,3- dimethyl-l,5-dihydro-pyrrolo[3,2-c l pyrimidine-2,4-dione 6-(4- {2-[4-(4-Methoxyphenyl)piperazin-l -yl]-2-oxo ethoxy} phenyl)- 1 ,3- dimethyl- 1 ,5-dihydropyrro lo [32-d] pyrimidine-2,4-dione
1 ,3-Dimethyl-6-(4- {2-oxo-2-[4-(3-trifluoromethylphenyl)piperazin-l- yl]ethoxy}phenyl)-l,5-d y(lropyιrolo {3^-(i]pyrimidine-2,4-dione
1 ,3-Dimethyl-6- {4-[2-oxo-2-(4-pyridin-2-yl-piperazin- 1 -yl)ethoxy]phenyl} -1 ,5- dmydropyιτolo[3^- ]pyrimidine-2,4-dione
1 ,3-Dimethyl-6- {4-[2-oxo-2-(4-pyrimidin-2-ylpiperazin- 1 -yl)ethoxy]phenyl} - l,5-d ydropyιτolo[3^-(i]pyrimidine-2,4-dione
N-Benzyl-2-[4-(l,3-dimethyl-2,4-dioxo-2,3,4,5-tetrahydro-lH-pyrrolo[3,2- ^pyrimidm-6-yl)phenoxy]-N-methylacetamide N-Benzyl-2-(4-(l,3-dime yl-2,4-dioxo-2,3,4,5-te^
J]pyrirni(im-6-yl)phenoxy]-N-ethylacetamide
2-[4-( 1 ,3-Dimethyl-2,4-dioxo-2,3 ,4,5-tetrahydro- lH-pyrrolo[3 ,2-d]pyrimidin-6- yl)phenoxy]-N-indan- 1 -yl-acetamide
2-(4-( 1 ,3-Dimethy l-2,4-dioxo-2,3 ,4,5-tetrahydro- lH-pyττolo(3 ,2-d]pyrimidin-6- yl)phenoxy]-N-(4-fluorobenzyl) acetamide
2-[4-( 1 ,3-Dimethyl-2,4-dioxo-2,3 ,4,5-tetrahydro- lH-ρyrrolo[3 ,2-d]pyrin idin-6- yl)phenoxy]-N-furan-2-ylmethyl acetamide
Ν-(4-Cωorobenzyl)-2-[4-(l,3-dimemyl-2,4-<noxo-2,3,4,5-tetrahydro-lH- pyτrolo[3,2-d]pyrimidin-6-yl)phenoxy] acetamide
2-[4-( 1 ,3-Dmethyl-2,4-dioxo-2,3 ,4,5-tetrahydro- 1H-^ yl)-phenoxy]-N-( 1 -phenylethyl) acetamide
2-[4-(l,3-Dimemyl-2,4-dioxo-2,3,4,5-tetrahydro-lH-pvrrolo[3^-d]pyrimidm yl)phenoxy]-N-(3-methoxybenzyl) acetamide N-Benzyl-2-[4-( 1 ,3-dimethyl-2,4-dioxo-2,3 ,4,5-tetrahydro- lH-pyrrolo[3 ,2- d]pyιimidin-6-yl)phenoxy] acetamide
1 ,3-Dimethyl-6- {4-[4-oxo-4-(6-ø-tolyl-2,6-diazabicyclo[2.2.1 ]hept-2- yl)butoxy]pheny 1} - 1 ,5-dihydropyrrolo [3 ,2-d] pyrimidine-2,4-dione
2-[4-(l,3-Diemyl-2,4-dioxo-2,3,4,5-tetrahydro-lH-pyrrolo[3,2- vrinndm-6 yl)phenoxy]-N-phenylacetamide
1 ,3-Diethyl-6- {4-[2-oxo-2-(4-phenylpiperazin- 1 -yl)ethoxy]phenyl}- 1 ,5- d ydropyrrolo[3,2-d]pyrimidme-2,4-dione
N-(4-Cyanophenyl)-2-[4-(l,3-diethyl-2,4-dioxo-2,3,4,5-tetrahydro-lH- pyπ-olo[3^-d]pyrirm'din-6-yl)phenoxy] acetamide 2-[4-(l-Memyl-2,4-dioxo-3-propyl-2,3,4,5-tetrahydro-lH-pyrrolo[3 2- ct pyrirmdin-6-yl)phenoxy]-N-phenylacetamide
1 N-(4-Fluorophenyl)-2-[4-( 1 -methy l-2,4-dioxo-3-propyl-2,3 ,4,5-tetrahydro- 1H- pyrrolo[3,2-cf]pyrinridin-6-yι)phenoxy] acetamide
N-(4-CΗorobenzyl)-2-[4-(l-methyl-2,4-<hoxo-3-propyl-2,3,4,5-tetrahydro-lH- pyrrolo[3^-J]pyrimidin-6-yl)phenoxy] acetamide
6- {4-[2-(3 ,4-Dihydro- lH-isoquinolin-2-yl)-2-oxo-ethoxy]phenyl} - 1 -methyl-3- propyl-l,5-dihydropyrrolo[3,2-rf] pyrimidine-2,4-dione
1 -Methyl-6- {4-{2-oxo-2-(4-phenyl-piperazin- 1 -yl) ethoxy jphenyl} -3-propyl- 1 ,5- dmydropyrrolo[3 ^-<i]pyrirnidine-2,4-dione 6-(4-{2-[4-(4-Fluorophenyl)piperazin-l-yl]-2-oxo-ethoxy}phenyl)-l-methyl-3- propyl- 1 ,5-dihydropyrrolo[3 ,2-d] pyrimidine-2,4-dione
4- {2-[4-{ 1 -Methyl-2,4-dioxo-3-propyl-2,3 ,4,5-tetrahydro- lH-pyrrolo[3 ,2- ^pyrimidin-6-yl)phenoxy] acetylamino} benzoic acid ethyl ester
6- {4-[2-(4-Ηycfroxy-4-phenylpiperidin- 1 -yl)-2-oxo ethoxy ]phenyl} - 1 -methyl-3-
propyl- 1 ,5-d ydropyrrolo[3,2--/] pyrimidine-2,4-dione
1 - {2-(4-( 1 -Methyl-2,4-dioxo-3-propyl-2,3 ,4,5-tetrahydro- lH-pyrrolo[3 ,2- ^pyrimid -c^yl)phenoxy]acetyl}-4-phenylpiperidme-4-carbomtrile
. N-Biphenyl-4-yl-2-[4-( 1 -methyl-2,4-dioxo-3-propyl-2,3 ,4,5-tetrahydro- 1H- pyrrolo[3,2-<^pvrimidin-6-yl)phenoxy] acetamide
6- {4-[2-(4,4-Dipheny piperidin- 1 -yl)-2-oxo-ethoxy] phenyl} - 1 -methyl-3-propyl- l,5-dihydropyττolo[3,2-^] pyriπύdine-2,4-dione
6-(4- {2-[4-(4-Methoxyphenyl)piperidin- 1 -yl]-2-oxo-ethoxy } phenyl)-l -methyl- 3-propyl-l,5-dihydropyrrolo[3,2-< ] pyrimidine-2,4-dione (4- {2-[4-( 1 -Methyl-2,4-dioxo-3-propyl-2,3 ,4,5-tetrahydro- lH-ρyrrolo[3 ,2- i]pyrinridin-6-yl)phenoxy] acetylamino}phenyl)acetic acid ethyl ester
6-{4-{2-(3,3-Diphenylpiperazin-l-yl)-2-oxoethoxy] phenyl}-l-methyl-3-propyl- l,5-d ydropyrrolo[3,2--/] pyrimidine-2,4-dione
6-(4- {2-[4-(6-Cnlorobenzothiazol-2-yl)-piperazin- 1 -yl]-2-oxoethoxy } phenyl)-! - methyl-3-propyl-l,5-dihydro pyrrolo[3,2- t pyrimidine-2,4-dione
1 -Methyl-6- {4-[2-oxo-2-( 1 ,3 ,4,9-tetrahydro-β-carbolin-2-yl)ethoxy]phenyl} -3- propy 1- 1 ,5-dihydropyrrolo [3 ,2 -rf]pyrimidine-2,4-dione
N-(4-Iodophenyl)-2-[4-( 1 -methyl-2,4-dioxo-3-propyl-2,3 ,4,5-tetrahydro- 1H- pyrrolo[3 ,2- ]pyrimidin-6-yl)phenoxy] acetamide l-Methyl-6-{4-[4-oxo-4-(6- -tolyl-2,6-diazabicyclo[2.2.1]hept-2- yl)butoxy]phenyl}-3-propyl-l,5-d ydropyrrolo[3^-d]pyrinήdine-2,4-dione
N-(4-Fluoroρhenyl)-2-[4-(3-me yl-2,4-dioxo-l-propyl-2,3,4,5-tetrahydro-lH- pyrrolo[3,2-£t pyrimidin-6-yl)phenoxy] acetamide
2-{4-(3-Methyl-2,4-dioxo- 1 -proρyl-2,3 ,4,5-tetrahydro- lH-ρyrrolo[3,2- f]pyrimidin-6-yl)phenoxy]-N-phenylacetaιnide
N-(4-Bromoρhenyl)-2-[4-(3-methyl-2,4-dioxo- 1 -propy 1-2,3 ,4,5-tetrahydro- 1H- pynτ>lo(3^-<φyrimidin-6-yι)phenoxy] acetamide
6- {4-[2-(3,4-Dihydro- lH-isoquinolin-2-yl)-2-oxoethoxy] phenyl} -3-methyl- 1 - propyl- l,5-dihydropyrrolo[3,2-<i] pyrimidine-2,4-dione
N-Benzyl-2-[4-(3-memyl-2,4-dioxo-l-propyl-2,3,4,5-tetrahydro-lH-pyrrolo[3,2- d]pyrimidin-6-yl)phenoxy] acetamide
N-Benzyl-N-methyl-2-[4-(3-methyl-2,4-dioxo- 1 -propyl-2,3 ,4,5-tetrahydro- 1H- pyrrolo[3 ,2-£t pyrimidin-6-yl)phenoxy] acetamide 3-Methyl-6- {4-[2-oxo-2-(4-phenylpiperazin- 1 -yl)-ethoxy]phenyl} - 1 -propyl- 1 ,5- dihydropyrrolo[3,2--f] pyrinήdine-2,4-dione
6- {4-[2-(4-Benzylpiperazin- 1 -yl)-2-oxoethoxy Jphenyl} -3-methyl- 1 -propyl- 1 ,5-
3-Methyl-6- {4-[4-oxo-4-(6-o-tolyl-2,6-diazabicyclo[2.2.1 ]hept-2- yl)butoxy]phenyl} - 1 -propyl- 1 ,5-dihydropyrτolo[3 ,2- ]pyrimidine-2,4-dione
N-Cyclopentyl-2- {4-[ 1 -(3-methoxypropyl)-3-methyl-2,4-dioxo-2,3 ,4,5- tetτahydro-lH-pyrrolo[3^--/]pyrimidin-6-yl] phenoxy} acetamide
2- {4-[ 1 -(3-Methoxypropyl)-3-methy l-2,4-dioxo-2,3 ,4,5-tetrahydro- 1H-
2-[4-(3-Isobutyl-l-metnyl-2,4- oxo-2,3,4,5-tetrahydro-lH-pyrrolo[3^-
^pyrirm'din-6-yl)phenoxy]-N-phenylacetamide
3-Isobutyl- 1 -methyl-6- {4-[2-oxo-2-(4-pheny Ipiperazin- 1 -yl)ethoxy]phenyl} - 1 ,5- dmydropyrrolo[3^-c/]pyrimidine-2,4-dione
4- {2-[4-(2,4-Dioxo- 1 -propyl-2,3 ,4,5-tetrahydro- lH-pyrrolo(3,2-J]pyrimidin-6- yl)phenoxy]acetylamino} benzoic acid ethyl ester
6-(4- {2-[4-(4-Methoxyphenyl)piperidin- 1 -yl]-2-oxo-ethoxy } phenyl)- 1 -propyl- l,5-dihydropyrrolo[3^-< ] pyrimidine-2,4-dione
6-(4- {2-[4-(4-Methoxyphenyl)piperazin- 1 -yl]-2-oxo-ethoxy }phenyl)- 1 -propyl- l,5-dmydropyrrolo[3,2--f] pyr-nήdine-2,4-dione N-(4-Bromophenyl)-2-[4-(2,4-dioxo-l-propyl-2,3,4,5-tetrahydro-lH- pyrrolo[3 ,2-^pyrimidin-6-yl)phenoxy] acetamide
2-[4-(2,4-Dioxo- 1 -propyl-2,3 ,4,5-tetrahydro- lH-pyrrolo[3 ^- ]pyrimidin-6- yl)phenoxy]-N-(4-fluorophenyι) acetamide
2-{4-[l,3-Bis(2-memoxyemyl)-2,4-<hoxo-2,3,4,5-tetrahydro-lH-pyrrolo[3,2-
^pyrinndin-6-yl]phenoxy}-N-phenylacetamide
2- {4-[ 1 ,3-Bis(2-methoxyethyl)-2,4-dioxo-2,3 ,4,5-tetrahydro- lH-pyrrolo[3^- d]pyτinήdin-6-yl]phenoxy}-N-(4-fluorophenyl)acetaπιide
2- {4-[ 1 ,3-Bis(2-methoxyethyl 2,4-dioxo-2,3 ,4,5-tetrahydro- lH-pyrrolo[3,2- c )pyrimidin-6-yl]phenoxy } -N-(4-bromopheny l)acetamide
1 ,3-Bis(2-methoxyethyl)-6- {4-[2-oxo-2-(4-phenylpiperazin- 1 - yl)ethoxy Jphenyl} - 1 ,5-dihydropyrrolo[3 ,2-<iJpyrimidine-2,4-dione
6- {4-[2-(3 ,4-Dihydro- lH-isoqumolin-2-yl)-2-oxoethoxyJphenyl} - 1 ,3-bis(2- methoxyethyl)- 1 ,5-dihydropyrrolo[3 ,2-d] pyrimidine-2,4-dione 2-[4-(l,3-Bis(cyclopropylmemyl 2,4-dioxo-2,3,4,5-tefrahydro-lH-pyrrolo[3^-
^pyrirnidm-6-yl)phenoxy]-N-phenylacetamide
2-[4-(l,3-Bis(cycloρropylme yl)-2,4-dioxo-2,3,4,5-tetrahydro-lH-pyrrolo[3^- ]pyrirmdm-6-yl)phenoxyJ-N-(4-fluorophenyl)acetamide
2-[4-(l,3-Bis(cyclopropylmethyl)-2,4-dioxo-2,3,4,5-tetrahydro-lH-pyrrolo[3^- Jpyrimidin-6-yl)phenoxyJ-N-(4-bromophenyl)acetamide
1 ,3-Bis(cyclopropylmethyl)-6- {4-[2-oxo-2-(4-phenylpiperazin- 1 - - l)ethoxyJphenyl}-l,5-dihydropyrrolo[3^-cf] pyrinridine-2,4-dione
1 ,3-Bis(cyclopropylmethyl)-6- {4-[2-(3,4-dihydro- lH-isoquinolin-2-yl)-2- oxoethoxy Jphenyl} - 1 ,5-dihydropyrrolo [3 ,2-</]pyrimidine-2,4-dione 2-[4-(7-Cmoro-l,3-dime yl-2,4- oxo-2,3,4,5-teϋ^y(lro-lH-pyrrolo[3r2- ci]pyriιmdin-6-yl)phenoxyJ-N-{4-cyanophenyl)acetamide
2-[4-(7-Bromo-2,4-dioxo- 1 ,3-dipropyl-2,3 ,4,5-tetrahydro- lH-pyrrolo[3 ,2- <φyrimidm-6-yl)phenoxyJ-N-phenylacetamide
2-[4-(7-Bromo-2,4-dioxo- 1 ,3-diproρyl-2,3,4,5-tetrahydro- lH-pyrrolo[3,2- d ]pyrimidm-6-yl)phenoxy]-N-(4-fluorophenyl)acetamide
2-{4-{7-Chloro-2,4-dioxo- 1 ,3-dipropyl-2,3 ,4,5-tetrahydro- lH-pyrrolo[3 ^« ]pyrimidin-6-yl)phenoxy]-N-(4-fluorophenyl)acetamide
2-[4-(7-Chloro-2,4-dioxo- 1 ,3-diρropyl-2,3 ,4,5-tetrahydro- lH-pyrrolo[3 ,2- ^pyrirmdm-6-yl)phenoxy]-N-phenylacetamide
N-(4-Bromophenyl)-2-[4-(7-cUoro-2,4-dioxo-l,3-dipropyl-2,3,4,5-tetrahydro- lH-pyrrolo[3^-<ι]pyrinndin-6-yl)phenoxyJacetamide
2-[4-(7-C oro-2,4-dioxo-l,3-diproρyl-2,3,4,5-tetrahydro-lH-pyrrolo[3r2- (iJpyrirmdin-6-yl)phenoxy]-N-(2-chlorophenyl)acetamide 2-[4-(7-Chloro-2,4-dioxo- 1 ,3-dipropyl-2,3 ,4,5-tetrahydro- lH-pyrrolo[3 ^2-
^pyrimidm-6-yl)phenoxyJ-N-(4-cUorophenyl)acetamide
2-[4-(7-C loro-2,4- oxo-l,3-diproρyl-2,3,4,5-tetrahydro-lH-pyrrolo[3^- ^pyrimidm-6-yl)phenoxyJ-N-(2-fluorophenyl)acetamide
2-{4-{7-Chloro-2,4-dioxo- 1 ,3-diρropyl-2,3 ,4,5-tetrahydro- lH-pyrrolo[3 ,2- dJpyrinήdin-6-yl)phenoxyJ-N-(4-fluorobenzyl)acetamide
2-[4-(7-CWoro-2,4-dioxo-l,3-dipropyl-2,3,4,5-tetrahydro-lH-pyrrolo[3^- ^pyrimidm-6-yl)phenoxyJ-N-(4-metnoxyphenyl)acetamide
N-Benzyl-2-[4-(7^Woro-2,4-dibxo-l,3-dipropyl-2,3,4,5-tetrahydro-lH- pyrrolo[3^-^pyrirnidin-6-yl)phenoxyJ acetamide 2-{4-(7-C oro-2,4-dioxo-l,3-diproρyl-2,3,4,5-tetrahydro-lH-pyrrolo[3^- ]pyrinndin-6-yl)phenoxyJ-N-p-tolylacetamide
2-[4-(7-Chloro-2,4-dioxo- 1 ,3-diproρyl-2,3 ,4,5-tetrahydro- lH-pyrrolo[3r2- cz]pyrinnα -6-yl)phenoxyJ-N-(3-fluorophenyl)acetamide
2-{4-( 1 ,3-Dimethyl-2,4-dioxo-2,3 ,4,5-tetrahydro- lH-pyrrolo[3 ,2- dJpyrinndin-6- yl)-3-methoxyphenoxy]-N-phenyl-acetamide
yl)-3-methoxy-phenoxyJ-N-(4-fluorophenyl)acetamide
N-(4-Chlorobenzyl)-2-[4-( 1 ,3-dimethyl-2,4-dioxo-2,3 ,4,5-tetrahydro- 1H- pyrrolo[3^-<fJpyrinήdin-6-yl)-3-methoxyphenoxyJacetamide 6-{4-[2-{3,4-D ydro-lH-isoqumolm-2-yl)-2-oxo-ethoxyJ-2-methoxyphenyl}-
1 ,3-dimethyl- 1 ,5-dihydropyrrolo [3 ,2-^pyrirmdine-2,4-dione
6- {2-Methoxy-4-[2-oxo-2-(4-phenylpiperazin- 1 -yl)ethoxy]pheny 1} - 1 ,3- dimethyl- 1 ,5-dihydropyrrolo[3 ,2-d] pyrimidine-2,4-dione
N-(4-Cyanoρhenyl)-2-[4-(l,3-dime l-2,4-<noxo-2,3,4,5-tetrahydro-lH-
pyrrolo(3,2-(-]pyrirm'dm-6-yl)-3-methoxyphenoxyJacεtamide
N-(4-Bromophenyl)-2-[4-(l,3-dimethyl-2,4-dioxo-2,3,4,5-tetrahydro-lH- pyrrolo[3,2-(f]pyrirnid -6-yl)-3-metnoxyphenoxyJacetamide
6-(2-Methoxy-4- {2-[4-(4-methoxyphenyl)-piperidin- 1 -ylJ-2 -oxoethoxy } phenyl)- l,3-dimethyl-l,5-dihydropyrrolo [3^-<iJpyrimidine-2,4-dione
6-(2-Methoxy-4- {2-{4-(4-methoxyphenyl)-piperazin- 1 -ylJ-2-
2-[4-( 1 ,3-Dimethyl-2,4-dioxo-2,3 ,4,5-tetrahydro- lH-pyrrolo[3 ^-<f]pyrirnidin-6- y l)-2 -methoxyphenoxyJ-N-pheny 1 acetamide 2-[4-( 1 ,3-Dimethyl-2,4-dioxo-2,3 ,4,5-tetrahydro- lH-pyrrolo[3,2-<iJpyrirnidin-6- yl)-2-methoxyphenoxyJ-N-(4-fluorophenyl)acetamide
N-(4-CMorobenzyl)-2-{4-(l,3-dime l-2,4-dioxo-2,3,4,5-tetrahydro-lH- pyrrolo[3^-c(]pyrimidm-6-yl)-2-methoxyphenoxy-acetamide
6-{4-[2-(3,4-D ydro-lH-isoqumol -2-yl)-2-oxoetnoxyJ -3-methoxyphenyl}- l,3-dimethyl-l,5-<iihydropyrrolo[3r2- ] pyrimidine-2,4-dione
6- {3-Methoxy-4-[2 -oxo-2 -(4-phenylpiperazin- 1 -yl) ethoxy Jphenyl} -1 ,3- dimethyl- 1 ,5-dihydropyrrolo [3 ,2- d] pyrimidine-2,4-dione
N-(4-Cyanoρhenyl)-2-[4-(l,3-dimemyl-2,4-dioxo-2,3,4,5-tetrahydro-lH- pyrrolo[3,2- Jpyrirnid -6^yl)-2-metnoxyphenoxy]acetamide N-(4-Bromophenyl)-2-[4-(l,3-<iimemyl-2,4-dioxo-2,3,4,5-tetrahydro-lH- pyrrolo[3^-c pyrimidm-6^yl)-2-methoxyphenoxy]acetamide
4- {2-[4-(l ,3-Dimethyl-2,4-dioxo-2,3,4,5-tetτahydro- lH-pyrrolo[3,2- Jpyrimidin-6-yl)-2-methoxyphenoxyJ acetylamino} benzoic acid ethyl ester
6-{3-Methoxy-4- {2-[4-(4-methoxyphenyl)piperidin- 1 -ylJ-2-oxoethoxy}phenyl)- l,3-dimethyl-l,5-dihydropyrrolo[3^- ] pyrimidine-2,4-dione
6-(3-Methoxy-4- {2-{4-(4-methoxyphenyl)piperazin- 1 -ylJ-2-oxoethoxy}phenyl)- 1 ,3-dimethyl-l ,5-dmydropyιτolo[3,2-^pyrimidine-2,4-dione
2-[4-(2,4-Dioxo- 1 ,3-dipropyl-2,3 ,4,5-tetrahydro- lH-pyrrolo(3 ,2-dJpyrimidin-6- yl)phenoxyJ-N-phenylpropionamide
6- {4-[2-(3 ,4-Dihydro- lH-isoqumolin-2-yl)- 1 -methyl-2-oxoethoxyJphenyl} - 1 ,3- dipropyl- 1 ,5-dihydropyrrolo[3 ,2-d] pyrirnidine-2,4-dione
6- {4-[ 1 -Methyl-2-oxo-2-(4-phenylpiperazin- 1 -yl)ethoxyJphenyl} - 1 ,3-dipropyl- 1 ,5-dihydfopyrrolo[3 ,2- JJpyrimidine-2,4-dione N-(4-Chlorobenzyl)-2-[4-(2,4-dioxo- 1 ,3-dipropyl-2,3 ,4,5-tetrahydro- 1H- pyrrolo[3,2-cT]pyrirmdin-6-yl) phenoxy]propionamide
2-[4-(2,4-Dioxo-l,3-dipropyl-2,3,4,5-tefrahydro-lH-pyrrolo[3^-<i]pyrirm
'din-6- yl)phenoxyJ-N-(4-fluorophenyl) propionamide
yl)phenoxyJ-N-(4-methoxyphenyl) propionamide
2-[4-(l,3-Dmethyl-2,4-dioxo-2,3,4,5-tetτahy^ yl)phenoxyJ-N-phenylpropionamide
2-[4-( 1 ,3-Dimethyl-2,4-dioxo-2,3,4,5-tetrahydro- lH-pyrrolo[3 ,2- ]pvrimidin-6- yl)phenoxyJ-N-(4-fluorophenyl) propionamide N-(4-Bromophenyl)-2-[4-(l ,3-dimethyi-2,4-dioxo-2,3 ,4,5-tetrahydro- 1H- pyrrolo[3^-^pyrimidin-6-yl)phenoxyJ propionamide
1 ,3-Dimethyl-6- {4-[ 1 -methyl-2-oxo-2-(4-phenyl piperazin- 1 -yl)ethoxy Jphenyl} - l,5-dihydropyrrolo[3^- J pyrimidine-2,4-dione
6^{4-[2-(3,4-D ydro-lH-isoqumolin-2-yl)-l-methyl-2 -oxoethoxy Jphenyl}-1,3- dimethyl- l,5-d ydropyτrolo[3 ,2 -<ϊj pyrirm'dine-2,4-dione
2-[4-(2,4-Dioxo-l,3-dipropyl-2,3,4,5-tetι^ydro-lH-pyrrolo[3^- Jpyrirmdih-6- yl)phenoxy]-N-phenylbut ramide
2-[4-(2,4-Dioxo- 1 ,3-dipropyl-2,3 ,4,5-tetrahydro- 1^ yl)phenoxyJ-N-(4-fluorophenyl) butyramide N-(4-Bromophenyl)-2-[4-(2,4-dioxo-l ,3-diρropyl-2,3,4,5-tetτahydro-lH- pyrrolo[3 ,2-<f]pyriιnidin-6-yι)phenoxyJ butyramide
6- {4-[ 1 -(4-Phenylpiperazine- 1 -carbony l)propoxy Jphenyl} - 1 ,3-dipropyl- 1 ,5- d ydropyrrolo(3^-<-^pyrinndine-2,4-dione
6- {4-[ 1 -(3 ,4-Dihydro- lH-isoquinoline-2-carbony I) propoxy Jphenyl} - 1 ,3-
pyrimidine-2
i)4-dione
2-[4-(2,4-Dioxo- 1 ,3-dipropyl-2,3 ,4,5-tetrahydro- lH-pyrrolo[3 ,2-< Jpvrimidin-6- yl)phenoxy]-2-methyl-N-phenyl propionamide
2-[4-(2,4-Dioxo- 1 ,3-dipropyl-2,3 ,4,5-tetrahydro- lH-pyrrolo[3 ,2--φyrimidin-6- yl)phenoxyJ-N-(4-fluorophenyl)-2-methylpropionamide
N-(4-Bromophenyl)-2-[4-(2,4-dioxo- 1 ,3-dipropyl-2,3 ,4,5-tetrahydro- 1H- pyrrolo(3 ,2- JJpyrimidin-6-yl)phenoxyJ -2-methylpropionamide
6- {4-( 1 , 1 -Dimethyl-2-oxo-2-(4-phenylpiperazin- 1 -yl)ethoxy Jphenyl} - 1 ,3- < propyl-l,5-d ydropyrrolo[3,2-fiJ pyriinidine-2,4-dione 6- {4-[2-(3 ,4-Dihydro- lH-isoquinolin-2-yl)- 1 , 1 -dimethyl -2 -oxoethoxy Jphenyl} - l,3-dipropyl-l,5-d ydropyιτolo[3,2- iJ pyrinndine-2,4-dione
2-[4-(2,4-Dioxo- 1 ,3-dipropyl-2,3 ,4,5-tetrahydro- lH-pyrrolo[3 ,2-d]pyrirnidin-6- yl)phenoxyJ-2,N-diphenylacetamide
yl)phenoxy]-N-(4-fluorophenyl)-2-phenylacetamide
6- {4-[2-Oxo- 1 -phenyl-2-(4-phenylpiperazin-l -yl)ethoxyJ phenyl} - 1 ,3-dipropyl- 1 ,5-dihydropyrrolo[3 ,2- ]pyrimidine-2,4-dione
3-[4-(2,4-Dioxo-l,3-dipropyl-2,3,4,5-tetι hydro-lH-ρyrrolo[3^- lpvrirnidin-6- yl)phenylJ-N-phenylpropionamide 3-[4-(2,4-Dioxo- 1 ,3-dipropyl-2,3 ,4,5-tetrahydro- lH-ρyrrolo[3 ^-c Jpvrimidin-6- yl)phenylJ-N-(4- fluorophenyl) propionamide
6- {4-[3-Oxo-3-(4-phenylpiperazin- 1 -yl)propylJphenyl} - 1 ,3-dipropyl- 1 ,5- dmydropyrrolo[3 ^-^pyrimidine-2,4-dione
6-{4-[3-(3,4-D ydro-lH-isoquinolin-2-yl)-3-oxopropyl] phenyl}-l,3-dipropyl- 1 ,5-dihydropyrrolo[3 ,2- Jpyrimidine-2,4-dione
3-[4-(2,4-Dioxo- 1 ,3-dipropyl-2,3 ,4,5-tetrahydro- lH-pyrrolo(3 ^-<f]pyrirnidin-6- yl)phenylJ-N-phenylacrylamide
6- {4-[3-Oxo-3-(4-phenylpiperazin- 1 -yl)propenylJphenyl} - 1 ,3-dipropyl- 1 ,5- dmydropyrrolo[3^-^pyrimidine-2,4-dione
6- {4-[3-(3 ,4-Dihydro- lH-isoquinolin-2-yl)-3-oxo propenyljphenyl} - 1 ,3- dipropyl-l,5-d ydropyrrolo[3,2-(iJ pyrimidine-2,4-dione
4-[4-(2,4-Dioxo-l,3-dipropyl-2,3,4,5-tetτahydro-lH-pyro^ yl)phenoxyJ-N-phenylbutyramide 4-[4-(2,4-Dioxo- 1 ,3-diproρyl-2,3 ,4,5-tetrahydro- lH-pyrrolo[3 ,2-<iJpyrimidin-6- yl)phenoxyJ-N-(4-fluorophenyl) butyramide
6- {4-[4-Oxo-4-(4-phenylpiperazin- 1 -yl)butoxyJphenyl} - 1 ,3-dipropyl- 1 ,5- dihydropyrrolo[3 ,2- ]pyrimidine-2,4-dione
6- {4-[4-(3 ,4-D ydro-lH-isoqumolin-2-yl)-4-oxobutoxyJ phenyl} - 1 ,3-dipropyl- l,5-dmydropyrrolo[3,2-(iJpyrimidine-2,4-dione
6- {4-[4-Oxo-4-(6-otolyl-2,5-diazabicyclo[2.2.1 Jhept-2-yl)butoxyJphenyl}- 1 ,3- dipropyl-l,5-dihydropyrrolo[3^2-f ] pyrimidine-2,4-dione
4-(2,4-Dioxo- 1 ,3→iipropyl-2,3 ,4,5-tetrahydro- lH-pyrrolo[3 ,2-<f]pyrimidin-6-yl)- N-phenylbenzamide 4-(2,4-Dioxo-l,3-dipropyl-2,3,4,5-tetrahyc o-lH-pyrroto^
N-(4-fluorophenyl)benzamide
Ν-(4-Bromophenyl)-4-(2,4-dioxo- 1 ,3-dipropyl-2,3 ,4,5-tetrahydro- 1H- pyrrolo [3 ,2- ]pyrimidin-6-yl)benzamide
6-[4-(4-Pheny lpiperazine- 1 -carbonyl)pheny 1J- 1 ,3 -dipropy 1- 1 ,5- dihydropyrrolo[3^-</]pyrirnidine-2,4-dione
6-[4-(3 ,4-Dihydro- lH-isoqumoline-2-carbonyl)phenylJ- 1 ,3-dipropyl- 1 ,5- d ydropyrrolo[3^- z pyrirnidine-2,4-dione
6-[4-(3-Phenyl-[ 1 ,2,4Joxadiazol-5-ylmethoxy)phenylJ- 1 ,3-dipropyl- 1 ,5- <i y(fropyrrolo[3,2- ]pyrirmdine-2,4-dione 6- {4-[2-oxo-2- {[amino(4-fluorophenyi)methylene diamino Joxy } ethoxy Jphenyl} - 1 ,3-dipropyl- 1 ,5-dihydropyrrolo [3 ,2-(φyrimidine-2,4- dione
6- {4-[3-(4-Fluorophenyl)-[ 1 ,2,4]oxadiazol-5-ylmethoxyJ phenyl} - 1 ,3-dipropyl- l,5-dmydropyrrolo[3^-<fJpyrir dine-2,4-dione
l,3-Dipropyl-6-[4-(3-pyridin-4-yl-{l,2,4Joxadiazol-5-ylmethoxy)phenyl]-l,5- dihydropyrrolo[3 ,2- Jpyrimidine-2,4-dione
6-[4-(Benzooxazol-2-ylmethoxy)phenylJ- 1 ,3-dipropyl- 1 ,5-dihydropyrrolo [3 ,2- ]pyrimidine-2,4-dione 6-[4-(5-Phenyl-4,5-dihydrooxazol-2-ylmethoxy)phenylJ- 1 ,3-dipropyl- 1 ,5-
6-[4-(4-Methyl-5-phenyl-4,5-dihydrooxazol-2-ylmethoxy)phenylJ- 1 ,3-diproρyl- 1 ,5-dmy<iropyrrolo[3^- Jpyrirnidine-2,4-dione
6-[4-(7-Benzyl-l-oxa-3,7-diazaspiro[4.5Jdec-2-en-2-ylmethoxy)phenylJ-l,3- dipropyl-1 ,5-d ydropyτrolo[3,2-^pyrimidine-2,4-dione
1 ,3-DipropyI-6-[4-(qumolin-2-ylmethoxy)pheny 1J- 1 ,5-dihydropyrrolo[3 ,2-
2-[4-(2,4-Dioxo-t,3-dipropyl-2",3,4,5-tetrahydro-lH-pyπ-olo[3,2-dJpyrinήdin-6- yl)phenoxyJ-N-pyridin-2-ylacetamide 2-[4-(2,4-Dioxo-l,3-dipropyl-2,3,4,5-tefrahydro-lH-pyrrolo[3,2-dJρyrimidin-6- yl)phenoxy]-N-{3-hydroxypyridin-2-yl)acetamide
2-[4-(2,4-Dioxo-l^-dipropyl-2,3,4,5-tetι^ydro-lH-pyrrolo[3^-dJpyrinήdin-6- yl)-phenoxyJ-N-(5-methylpyridin-2-yl)acetarnide
2-[4-(2,4-Dioxo-l,3-dipropyl-2,3,4,5-tetrahydro-lH-pyrrolo[3,2-dJpyrinn^in-6- yl) phenoxyJ-N-pyridin-3-ylacetamide
2-[4-(2,4-Dioxo-l,3-dipropyl-2,3,4,5-tefrahydro-lH-pyrrolo[3^-dJpyrimidin-6- yl)-phenoxyJ-N-(6-methoxypyridin-3-yl)acetamide
2-[4-(2,4-Dioxo-l,3-dipropyl-2,3,4,5-tetrahydro-lH-pyιτolo[3^-dJpyrirmdin-6- yl)phenoxyJ-N-pyridm-4-ylmethylacetamide 6-(4- {2-Oxo-2-[4-(4-trifluoromethylphenyl)piperazin- 1 -yl Jethoxy Jphenyl)- 1,3- dipropyl-l,5-dmydropyrrolo[3^-dJpyrimidine-2,4-dione
6-{4- {2-[4-(3-Chlorophenyl)piperazin- 1 -ylJ-2-oxoethoxy } phenyl)- 1 ,3-dipropyl- 1 ,5-<imydropyrrolo[3,2-dJpyrirnidine-2,4-dione
2-[4-(2,4-Dioxo- 1 ,3-dipropyl-2,3 ,4,5-tetrahydro- lH-pyrrolo[3 ,2-dJpyrirnidin-o'- yl)-phenoxy]-N-pyrazin-2-ylacetamide
N-(2,6-Dimethoxypyrirnidin-4-yl)-2-[4-(2,4-dioxo- 1 ,3-dipropyl-2,3,4,5- tetrahydro-lH-pyrrolo[3^-dJpyrimidm-6-yl)phenoxyJacetamide 6- {4-[2 -(3- Aminopyrazol- 1 -yl)-2 -oxoethoxy Jphenyl} - 1 ,3-dipropyl- 1 ,5-dihydro- pyrrolo[3^-dJpyrirnidine-2,4-dione
6-(4- {2-[4-(3-Chlorophenyl)piperazin- 1 -ylJ-2-oxoethoxy } phenyl)- 1 ,3-dimethyl- 1 ,5-dihydropyrrolo [3 ,2-dJpyrirnidine-2,4-dione
1 ,3-Dimethyl-6-(4- {2-oxc>-2-[4-(4-trifluoromethylphenyl)piperazin- 1 -
6-(4- {2-[4-(4-Bromophenyl)piperazin- 1 -ylJ-2-oxoethoxy } phenyl)- 1 ,3-dimethyl- 1 ,5-d ydropyrrolo[3^-dJpyrinήdine-2,4-dione
6- {4-[2-(4-Hydroxy-4-phenylpiperidin- 1 -yl)-2 -oxoethoxy Jphenyl} - 1 ,3- dimethyl-l,5-dmydropyιτolo[3^-dJpyrimidine-2,4-dione 1 - {2-[4-( 1 ,3-Dimethyl-2,4-dioxo-2,3 ,4,5-tetrahydro- lH-pyrrolo[3 >2- dJpyrir dm-6-yl)phenoxyJacetyl}-4-phenylpiperidme-4-carbonitrile
6- {4-[2-(4,4-Diphenylpiperidin- 1 -yl)-2-oxoethoxyJphenyl} - 1 ,3-dimethyl- 1 ,5- d ydropyι olo(3^-dJpyrirnidine-2,4-dione
6-(4- {2-{4-(4-C orophenyl)-4-hydroxypiperidin- 1 -ylJ-2 -oxoethoxy} phenyl)- 1 ,3rdimethyl- 1 ,5-<imydropyιτolo[3^-d]pyrimidine-2,4-dione
6-{4- {2-[4-(3 ,5-Dichloropyridin-4-yl)piperazin- 1 -ylJ-2 -oxoethoxy Jphenyl)- 1 ,3- dimethyl- 1 ,5-dihydropyrrolo[3 ,2-dJpyrimidine-2,4-dione
6-(4- {2-[4-(5-C orobenzotbiazol-2-yl)piperazin- 1 -ylJ-2-oxoethoxy}phenyl)-
1 ,3-Dimethyl-6-{4-[2-oxo-2-(l,3,4,9-tetrahydro-b-carbolin-2- yl)ethoxyJphenyl}-l,5-dmydropyιτolo[3,2-dJpyrirmdine-2,4-dione
6-[4-(2- {4-( 1 -(4-Fluorophenyl)methanoylJpiperidin- 1 -yl}-2-oxo- ethoxy)pheny IJ- 1 ,3-dimethyl- 1 ,5-dihydropyrrolo[3 ,2-dJpyrirmdine-2,4-dione
2-[4-( 1 ,3-Dmethyl-2,4-dioxo-2,3 ,4,5-tetrahydro^ yl)-phenoxyJ-N-pyridin-4-ylmethylacetamide
4- {2-[4-(l ,3-Dimethyl-2,4-dioxo-2,3 ,4,5-tetrahydro- lH-pyrrolo(3 ,2- dJpyrimidin-6-yi)phenoxy]ethanoyl} piperazine- 1 -carboxy lie acid ethyl ester 6-(4- {2-[4-(2-Methoxyphenyl)piperidin- 1 -ylJ-2 -oxoethoxy } phenyl)- 1 -methyl-3- propyl-l,5-d ydropyrroki[3,2-dJpyrimidine-2,4-dione
6-(4- {2-[4-(3 ,5-DicUoropyridm-4-yl)piperazin- 1 -y lJ-2-oxoethoxy } phenyl)- 1 - memyl-3-propyl-l,5-d ydropyττolo[3,2-dJpvrirm'dine-2,4-dione
N-(6-Methoxypyridin-3-yl)-2-[4-(l-methyl-2,4-dioxo-3-propyl-2,3,4,5- tetrahydro- 1 H-pyrrolo [3 ,2 -dJpyrimidin-6-y l)phenoxyJacetamide
1 -Methyl-6-(4- {2-oxo-2-[4-(4-trifluoromethylphenyl)piperazin- 1 - ylJethoxy}phenyl)-3-propyl-l,5-dmydropyrrolo[3^-dJpyrinndine-2,4-dione
6-[4-(2-Mθ holώ-4-yl-2-oxoethoxy)phenylJ-3-propyl-l,5-<imydropyrrolo[3,2 dJpyrirmdine-2,4-dione 6- {4-[2-(4-Methylpiperazin- 1 -yl)-2 -oxoethoxy Jphenyl} -3-propyl- 1 ,5- dihydropyrrolo[3 ,2-d]pyrirmdine-2,4-dione
2-[4-(2,4-DioxcH3-propyl-2,3,4,5-tetrahydro-lH-pyrrolo[3,2-dJpyrimidin-6- yl)phenoxy]-N-(2-hydroxyethyl)acetamide
6-(4- {2-{4-(2-Methoxypheny piperazin- 1 -ylJ-2-oxoethoxy } phenyl)-3-propyl- 1 ,5τdmydropyrrolo[3 ^-dJpyrimidine-2,4-dione
6- {4-[2-{4-Benzylpiperazin- 1 -yl)-2-oxoethoxy Jphenyl} -3-propyl-l ,5- dihydropyrrolo[3 ^-dJpyrirmdine-2,4-dione
2-[4-(2,4-Dioxo-3-propyl-2,3,4,5-tetrahyoi-o-lH-pyrrolo[3^-dJpyrirnidin-6-yl)- phenoxyJ-N-(4-fluorophenyl)acetamide N-(4-Bromoρhenyl)-2-[4-(2,4-dioxo-3-propyl-2,3 ,4,5-tetrahydro- 1H- pyιτolo(3,2-dJpyrimidm-6-yl)phenoxy]acetamide
6- {4-[2-Oxo-2-(4-phenylpiperazin- 1 -yl)-ethoxy Jphenyl} - 1 -propyl- 1 ,5- dihydropyrrolo[3 ^-dJpyrimidine-2,4-dione
6-(4- {2-[4-(4-Fluorophenyl)piperazin- 1 -ylJ-2-oxo-ethoxy } phenyl)-3-methyl- 1 - (3-morpholm-4-ylpropyl)- 1 ,5-<iihy(iro^
3-Methy 1- 1 -(3-morpholin-4-yl-propyl)-6- {4-[2-oxo-2-{4-phenyl-piperazin- 1 - yl)ethoxy Jphenyl} - 1 ,5-dihydropyrrolo(3 ,2-dJpyrimidine-2,4-dione 3-Methyl- 1 -(3-morpholin-4-yl-propyl)-6-(4- {2-oxo-2-[4-(4-trifluoromethyl- phenyl)-piperazin- 1 -ylj-ethoxy } phenyl)- 1 ,5-dihydro-pyrrolo[3 ,2-d]pyrirnidine-2,4- dione
Pyrazin-2-yl-carbarnic acid 4-(2,4-dioxo-l,3-dipropyl-2,3,4,5-tetrahydro-lH- pyrrolo[3,2-dJpyrimidin-6-yl)benzyl ester (2,6-Dimemoxy-pyrirnidm-4-yl)-carbamic acid 4-(2,4-dioxo- 1 ,3-dipropyl-
2,3,4,5-tetτahydro-lH-pyrrolo[3^-dJpyrimidin-6-yl)benzyl ester
Pyridin-4-ylmethyl carbamic acid 4-(2,4-dioxo-l,3-dipropyl-2,3,4,5-tetrahydro- lH-pyrrolo[3,2-d]pyriιmdin-6-yl)benzyl ester
4-(3-Chlorophenyl)piperazine-l -carbox he acid 4-(2,4-dioxo-l,3-dipropyl- 2,3,4,5-tetrahydrc>-lH-pyτrolo[3,2-dJpyrimidin-6-yl)benzyl ester
(lH-Pyrazol-3-yl)carbamic acid 4-(2,4-dioxo-l,3-dipropyl-2,3,4,5-tetrahydro- lH-pyrrolo[3,2-dJpyrinndin-6-yl)benzyl ester
4-(3-Trifluoromethylphenyl)piperazine-l -carboxylic acid 4-(2,4-dioxo-l,3- dipropyl-2,3,4,5-tetrahydro-lH-pyrτolo[3^-dJpyrirnidin-6-yl)benzyl ester Isoxazol-3-yl-carbamic acid 4-(2,4-dioxo-l,3-dipropyl-2,3,4,5-tetrahydro-lH- pyrrolo[3,2-dJpyrimidin-6-yl)benzyl ester
(4-Fluorophenyl)-carbamic acid 4-(l,3-dimethyl-2,4-dioxo-2,3,4,5-tetrahydro- lH-pyιτolo[3,2-d]pyrinndin-6-yl)benzyl ester
Benzylcarbamic acid 4-(l,3-dimethyl-2,4-dioxo-2,3,4,5-tetrahydro-lH- pyrrolo[3,2-dJpyrinridin-6-yi)benzyl ester
Phenylcarbamic acid 4-(l,3-dimethyl-2,4-dioxo-2,3 ,4,5-tetrahydro- 1H- pyπOlo[3^-d]pyrirm'din-6-yl)benzyl ester
Pyridm-2-yl-carbamic acid 4-(l,3-dimethyl-2,4-dioxo-2,3,4,5-tetrahydro-lH- pyrrolo[3,2-dJpyrimidin-6-yl)benzyl ester
(5-Methylpyridin-2-yl)-carbamic acid 4-(l,3-dimethyl-2,4-dioxo-2,3 ,4,5- tetrahydro- lH-pyrrolo[3 ^-dJpyrirnidin-6-yl)-benzyl ester
Thiophen-2-yl-carbamic acid 4-(l,3-dimethyl-2,4-dioxo-2,3,4,5-tetrahydro-lH- pyrrolo[3^-dJpyrimidin-6-yl)-benzyl ester Thiophen-3-yl-carbamic acid 4-(l,3-dimethyl-2,4-<lioxo-2,3,4,5-tetrahydro-lH- pyrrolo[3,2-dJpyrirnidin-6-yl)-benzyl ester
Furan-2-yl-carbamic acid 4-(l,3-dimethyl-2,4-dioxo-2,3 ,4,5-tetrahydro- 1H- pyrrolo[3^-dJpyrirnidin-6-yl)-benzyl ester
4-Phenylpiperazine-l -carboxy Uc acid 4-(l,3-dimethyl-2,4-dioxo-2,3,4,5- tetrahydro- 1 H-pyrrolo[3 ,2-dJpyrimidin-6-yl)-benzyl ester
3,4-Dmydro-lH-isoqu oline-2-carboxyUc acid 4-(l,3-dimethyl-2,4-dioxo- 2,3,4,5-tetτahydro-lH-pyrrolo[3^-dJpyrimidin-6-yl)benzyl ester
Thiophen-2-yl-carbamic acid 2 [4-(2,4-dioxo-l,3-dipropyl-2,3,4,5-tetrahydro- lH-pyrrolo[3^-dJpyrirrudin-6-yl)phenoxyJethyl ester (4-Bromophenyl)carbamic acid 2-[4-(2,4-dioxo- 1 ,3-dipropyl-2,3,4,5-tetrahydro- lH-pyrrolo[3,2-dJpyrimidin-6-yl)phenoxyJethyl ester
1 -[ 1 -(2,6-Difluoro-phenyl)methanoylJ-3-[4-(2,4-dioxo- 1 ,3-diproρyl-2,3 ,4,5- tetrahydro- 1 H-pyirolo [3 ^-dJpyrirnidin-6-yl)benzylJurea
6-[4-(5-Fluorobenzooxazol-2-ylmethoxy)phenylJ- 1 ,3-dipropyl- 1 ,5- dihydropyrrolo[3^-dJpyrimidine-2,4-dione
6-[4-( lH-Benzoirnidazol-2-ylmethoxy)phenylJ- 1 ,3-dipropyl- 1 ,5- dihydropyrrolo(3 ^-dJpyrimidine-2,4-dione
1 ,3-Dimethyl-6-[4-(qumolin-2-ylmethoxy)phenylJ- 1 ,5-dihydropyrrolo[3 ,2- dJpyrimidine-2,4-dione 1 ,3-Dimethyl-6-[4-(3-phenyl[ 1 ^,4Joxadiazol-5-ylmethoxy)phenyl]- 1 ,5- dώydropyrrolo[3^-dJpyrimidine-2,4-dione
1 -Methyl-6-[4-(3-phenyl[ 1 ,2,4Joxadiazol-5-ylmethoxy)phenylJ-3-propyl- 1 ,5- dihydropyrrolo[3 ,2-dJpyrimidine-2,4-dione
6- {4-{3-(4-Fluorophenyl)[ 1 ,2,4]oxadiazol-5-ylmethoxy Jphenyl} - 1 -methyl-3- propyl- 1 ,5-d ydropyrrolo[3,2-dJpyrirnidine-2,4-dione
6-[4-(5-Chlorobenzooxazol-2-ylmethoxy)phenyl]-l-methyl-3-propyl-l,5- dihydropyrrolo[3 ,2-dJpyrir dine-2,4-dione 6- {4-{3-(4-Bromophenyl)-[ 1 ,2,4Joxadiazol-5-ylmethoxy]-phenyl} -3-propyl- 1 ,5- dihydropyrrolo[3 ^-dJpyrύnidine-2,4-dione
1 ,3-Dimethyl-6- {4-[ 1 -(3-phenyl[ 1 ,2,4Joxadiazol-5-yl)ethoxyJpheny 1} - 1 ,5- dihydropyrrolo[3 ^-dJpyrimidine-2,4-dione
6-(4- { 1 -[3-(4-Fluorophenyl)[ 1 ,2,4]oxadiazol-5-ylJethoxy Jphenyl)- 1 ,3-dimethyl- l,5-d ydropyτrolo[3^-dJpyrimidine-2,4-dione l,3-Dimethyl-6-{4-[l-(3-thiophen-3-yl[l^,4Joxadiazol-5-yl)ethoxy]phenyl}- 1 ,5-dmydropyrrolo[3^-dJpyrinndine-2,4-dione
1 ,3-Dimethyl-6-(4- { 1 -[3-(4-methylsulfanylphenyi)[l ,2,4]oxadiazol-5- yljethoxy } phenyl)- 1 ,5-dihydropyrrolo[3 ,2-dJpyrinήdme-2,4-dione 6- {4-[(4-Bromophenylamino)methylJphenyl} -1 ,3-dipropyl- 1 ,5- dihydroρyιτolo[3^-d]pyriπύdine-2,4-dione
6-(4-Phenylaminomethylphenyl)- 1 ,3-dipropyl- 1 ,5-dihydropyrrolo[3 ,2- dJpyrimidine-2,4-dione
and pharmaceutically acceptable salts thereof.
Of outstanding interest are 6-phenylpyrrolopyrimidinedione derivatives of formula (I), and pharmaceutically acceptable salts thereof, wherein:
R1 and R2 are the same or different and each independently represent a C -C4 alkyl group;
R3 represents hydrogen or halogen;
R4 and R5 are the same or different and each independently represent hydrogen, C,-C4 alkyl, C,-C4 alkoxy or C,-C4 alkylthio;
L, is -O-CH,-, -CH2-O- or -CH2NH-, for example -O-CH2-; and
R6 represents a phenyl group; an oxadiazolyl group which is unsubstituted or substituted by a phenyl group; or a group of formula -C(O)NR10R", wherein either R10 is hydrogen and R" is a thienyl group, a thiadiazolyl group, a pyridyl group, an optionally substituted phenylcarbonyl group or a phenyl group which is unsubstituted or substituted by 1 or 2 substituents selected from halogen atoms and phenyl and benzyloxy groups or R10 and R" form, together with the N atom to which they are attached, a 1, 2, 3, 4-tetrahydroisoqumoline group, a 1,3,4,9-tetrahydro-beta- carbolinyl group, a piperidinyl group or a piperazinyl group, the piperidinyl and piperazinyl groups being unsubstituted or substituted by 1 or 2 groups selected from hydroxy, optionally substituted phenyl and optionally substituted pyridyl.
In this embodiment, R6 may represent, for example, -C(O)NRI0Rπ or an oxadiazolyl group which is unsubstituted or substituted by a phenyl group, wherein either R10 is hydrogen and R11 is a thiadiazolyl group, a pyridyl group or a phenyl group which is unsubstituted or substituted by 1 or 2 substituents selected from halogen atoms and phenyl and benzyloxy groups or R10 and Rn form, together with the N atom to which they are attached, a 1, 2, 3, 4-tetrahydroisoqumoline group, a piperidinyl group or a piperazinyl group, the piperidinyl and piperazinyl groups being unsubstituted or substituted by 1 or 2 phenyl groups.
Examples of such compounds include:
6- {4-[2-Oxo-2-(4-pheny Ipiperazin- 1 -yl)ethoxy Jphenyl} - 1 ,3-dipropyl- 1 ,5- dihydropyrrolo[3 ,2- Jpyrimidine-2,4-dione
6- {4-[2-(3,4-Dihydro- lH-isoquinolin-2-yl)-2-oxoethoxyJ phenyl} - 1 ,3-dipropyl- l,5-d ydropyrrolo[3^-^pyrinndine-2,4-dione
yl)phenoxy]-N-(4-fluorophenyl) acetamide
1 ,3-Dimethyl-6- {4-{2-oxo-2-(4-ρhenylpiperazin- 1 -yl)ethoxy Jphenyl} -1 ,5-
N-Biphenyl-4-yl-2-[4-(l-methyl-2,4-dioxo-3-proρyl-2,3,4,5-tetrahydro-lH-
pyrrolo[3,2-^pyrirnidin-6-yl)phenoxyJ acetamide
6- {4-[2-(4,4-Diphenylpiperidin- 1 -yl)-2-oxo-ethoxy] phenyl} - 1 -methyl-3-propyl- l,5-d ydropyrrolo[3,2-e ] pyrirnidine-2,4-dione
6-[4-(3-Phenyl-[ 1 ,2,4Joxadiazol-5-ylmethoxy)phenyl]- 1 ,3-dipropyl- 1 ,5- d ydropyιτolo[3,2- t pyriπιidine-2,4-dione
N-(4-Bromophenyl)-2-[4-(l,3-dimemyl-2,4-dioxo-2,3,4,5-tetrahydro-lH- pyrrolo[3^- t pyrimidm-6-yl)-3-methoxyphenoxyJacetarriide
2-[4-(2,4-Dioxo- 1 ,3-dipropyl-2,3 ,4,5-tetrahydro- lH-pyττolo[3^- Jpyrirnidin-6- yl)phenoxyJ-N-(4-fluorophenyl) acetamide 2-[4-( 1 ,3-Dimethyl-2,4-dioxo-2,3 ,4,5-tetrahydro- lH-pyrrolo[3,2-<f]pyrinιidin-6- yl)phenoxyJ-N-[ 1 ,3 ,4Jthiadiazol-2-y lacetamide
2-[4-(7-Bromo-2,4-dioxo- 1 ,3-dipropyl-2,3 ,4,5-tetrahydro- lH-pyrrolo(3 ^2- ^pyrimidm-6-yl)phenoxyJ-N-(4-fiuorophenyl)acetarnide
N-(4-Berizyloxyphenyl)-2-[4-(2,4-dioxo-l,3-diρroρyl-2,3,4,5-tetrahydro-lH- pyrrolo[3,2- Jpyrimidin-6-yl)phenoxyJ acetamide
2-[4-(l,3-Dime yl-2,4-dioxo-2,3,4,5-tetrahydro-lH-^^ yl)-2-methoxyphenoxyJ-N-(4-fluorophenyl)acetamide
Thiophen-3-yl-carbamic acid 4-(l,3-dimethyl-2,4-dioxo-2,3,4,5-tetrahydro-lΗ- pyrrolo[3,2-dJpyrimidin-6-yl)benzyl ester 6-(4-{2-[4-(4-Chlorophenyl)-4-hydroxypiperidin-l-ylJ-2 -oxoethoxy} phenyl)- l,3-dimemyl-l,5-dmydropyrrolo[3^-dJpyrimid e-2,4-dione
6-(4- {2-[4-(3 ,5-Dichloropyridin-4-yl)piperazin- 1 -ylJ-2 -oxoethoxy Jphenyl)- 1 - methyl-3-propyl- 1 ,5-dmydropyrrolo[3^-dJpyrimidine-2,4-dione
1 ,3-Dimethyl-6- {4-[2-oxo-2-( 1 ,3 ,4,9-tetrahydro-b-carbolin-2- yl)ethoxyJphenyl}-l,5-d ydropyrrolo[3,2-dJpyrinndine-2,4-dione
1 -{ 1 -(2,6-Difluoroρhenyl)methanoylJ-3-[4-(2,4-dioxo- 1 ,3-dipropyl-2,3 ,4,5- tetrahydro-lH-pyrrolo[3^-dJpyrirmdm-€-yl)benzylJurea
6-(4-Phenylarnmomethylphenyl)-l,3- propyl-l,5-dihydropyrrolo(3^- dJpyriιmdine-2,4-dione
and pharmaceutically acceptable salts thereof.
According to a further feature of the present invention, the 6-phenyl-l,5- dihydropyrrolo[3^-c ] pyrimidine-2,4-dione derivatives of general formula (I) in which R* is -CONR10Ru can be prepared by reaction of the corresponding carboxylic acids of formula (H):
(π)
(wherein Rl, R2, R3 , R4, Rs, and L, are as hereinbefore defined) and the corresponding amines (III):
,11
HN
V°
(m)
(wherein R10 and Ru are as hereinbefore defined). The reaction is carried out in an organic solvent, preferably a polar aprotic organic solvent such as dichloromethane, N^-dimethylformamide or tetrahydrofuran, at a temperature from 10°C to 60°C and in the presence of an organic base, preferably an amine base such as trie ylamine or polymer supported morpholine, and in the presence of standard coupling agents such as 1-hydroxybenzotriazole and l-{3-(dimethylammo)propylJ-3-ethylcarbodiimide hydrochloride.
The thus obtained compound of formula (T) can be converted to a further compound of formula (I) by standard functional group interconversions known to those of skill in the art. Thus, for example, in the case that R3 is chlorine or bromine, the carboxylic acid of formula (H is obtained from the compound of formula (IT) where R3 is hydrogen by chlorination or bromination using methods known er se.
The 6-phenylpyrrolopyrimidinedione derivatives of general formula (I) are also prepared from vinyl derivatives (TV) (wherein R\ R2, R4, R5, and L, are as hereinbefore defined) and amines (HI) using the coupling procedure described below and subsequent reductive cyclization mediated by triethyl phosphite or sodium dithionite in formic acid both at reflux temperature.
(TV) When R* is a said group of formula (H), wherein X, Y
1 and Y
2 are as hereinbefore defined, the ring of R
6 is prepared from carboxylic acid (H) and amines (V) or amide derivatives (VI) by amide type coupling followed by cyclodehydration typically performed in toluene with catalytic amounts of acid or in dichloromethane or tetrahydrofuran using dehydration agents (such as SOClj, POCl
3, Burgess reagent or polyphosphoric acid) and in the products derived from amine (V) a further oxidation can be done, typically performed by NiO
2 or MnO
2.
(V) (VI) (vn)
The 6-phenylpyrrolopyrimidinedione derivatives of general formula (H) are prepared from vinyl derivatives (TV) by reductive cychzation using the methods described hereinbefore.
The vinyl derivatives of general formula (TV) are prepared by reaction of the corresponding 6,-methyl-5-nitrouracils (VTfl):
(vm)
(wherein R1 and R2 are as hereinbefore defined), and the corresponding benzaldehydes (TX):
(TX)
(wherein L,, R4 and R5 are as hereinbefore defined) by methods known per se, e.g. C. E. Mϋller et al., J. Med. Chem. 1994, 37, 1526-1534 and references cited therein.
When R6 is -ON=CR12R13, the products of general formula (I) are prepared by reacting a carboxyhc acid of formula (H) with a compound of formula R12-C(R13)=N- OH using standard coupling procedures known in the art.
When R4 is -S(O)2-NR10Rn , aryl, heterocyclyl or heteroaryl the products of general formula (J) are prepared by condensation of the 6-methyl-5-nitrouracils (VET) with the corresponding benzaldehydes (X) to give the vinyl derivatives, followed by reductive cychzation as in the preparation of compounds of general formula (II).
(X)
When L, is -(C ^O-, -O(CR
8R
9)
πιO or -(CR' - the products of general formula (I) are prepared by condensation of the alcohols (XI), (XB) or amine (Xπi) with the corresponding isocianates to give the carbamate or urea derivatives.
(xπo
Compounds (XI) and (XH) are prepared by reduction of the carboxyhc acid of general formula (H) wherein L, is -(CR3R9)m.1- or -©(CR'R9),^- using standard reductive agents such as borane or aluminium hydrides in common organic solvents such as tetrahydrofuran at a temperature from 0°C to 100°C.
Compounds of general formula (XTf!) can be obtained from alcohols (XT) by using standard procedures known in the art.
The 6-methyl-5-nitrouracils (VTfl) can be prepared from the corresponding _V,_V- disubstituted ureas by methods known per se, e.g. S. Senda et al., J. Med. Chem. 1972, 15, 471-476 or H. Egg Synthesis 1982, 1071-1072 and references cited therein. The compounds of formulae (IH), (V), (VT), (VII), CVTJI), (IX) and (X) are known compounds or may be prepared by analogy with known methods. The compounds of formula R12-C(R13)=N-OH are commercially available or may be prepared by analogy with known methods.
The 6-phenyl-l,5-d ydropyττolo[3,2-<f]pyτirmdine-2,4-dione derivatives of formula (I) in which there is the presence of a basic group can be converted by methods known er se into pharmaceutically acceptable salts, preferably acid addition salts by
treatment with organic or inorganic acids such as fumaric, tartaric, succinic or hydrochloric acid. Also 6-phenyl-l,5-d ydropyrrolo[3,2- Jpyrirmdine-2,4-dione derivatives of formula (I) in which there is the presence of an acidic group, may be converted into pharmacologically acceptable salts by reaction with an alkali metal hydroxide such as sodium or potassium hydroxide or an organic base such as diethanolamine. The acid or alkali addition salts so formed may be interchanged with suitable pharmaceutically acceptable counter ions using processes known r se. Adenosine 2b receptor subtype competition radioligand bmding
Human membranes from recombinant A2b receptors were purchased from Receptor Biology, Inc.(USA).
Competition assays were carried out by incubation of membranes from hA2b receptors transfected to HE 293 cells,. [3HJDPCPX as radioligand, buffer (50mM Tris- HCI (pH 6.5), lOmM MgClj, ImM EDTA, O.lmM benzanήdine, 2units/ml adenosine deaminase), and unlabelled hgand in a total volume of 0.1 ml for 30 min at 25°C. NECA was used to determinate non-specific binding. Filter over Schleicher&Schuell GF/52 niters (pre-soaked 0.5% polyethylenyimine) in a Brandel cell harvester. Unbound radioligand was removed with 4x2 ml ice-cold 50 mM Tris-Hcl 50 mM (pH 6.5).
Adenosine 2a receptor subtype competition radioligand binding
Human membranes from recombinant A2a receptors were purchased from Receptor Biology, Inc.(USA).
Competition assays were carried out by incubation of membranes from hA2a receptors transfected to HEK293 cells, [3HJZM241385 as radioligand, buffer (50mM Tris-HCI (pH 7.4), lOmM MgC^, ImM EDTA, 2units/ml adenosine deaminase), and unlabelled hgand in a total volume of 0.2 ml for 90 min at 25°C. NECA was used to determinate non-specific binding. Filter over Schleicher&Schuell GF/52 filters (pre- soaked 0.5% polyethylenyimine) in a Brandel cell harvester. Unbound radioligand was removed with 3x3 ml ice-cold 50 mM Tris-Hcl 50 mM (pH 7.4), 0.9% NaCl.
The results are shown in Table 1 and Table 2.
TABLE 1
It can be seen from Table 1 that the compounds of formula (I) are potent inhibitors of the A2b adenosine receptor subtype. Preferred 6-phenyl-l,5-dihydropyrrolo
derivatives of the invention possess an IC
S0 value for the inhibition of A2b (determined as defined above) of less than 50 nM, preferably less than 10 nM and most preferably less than 5 nM.
TABLE 2
It can be seen from Table 2 that the compounds of formula (I) are potent inhibitors of the A2a adenosine receptor subtype. Some preferred 6-phenyl-l,5-dihydro pyrrolo(3,2-< Jpyrinndine-2,4-dione derivatives of the invention possess an IC
S0 value for the inhibition of A2a (determined as defined above) of less than 100 nM, preferably less than 50 nM and most preferably less than 10 nM.
The 6-phenyl-l,5-dihydropyrrolo[3,2-c Jpyrinιidine-2,4-dione derivatives of the invention are useful in the treatment or prevention of asthma, bronchoconstriction, allergic potentiation, inflamation or reperfusion injury, myocardial ischemia, inflammation, diarrheal diseases, brain arteriole diameter constriction, Parkinson's disease, non insulin dependent diabetes melhtus, release of allergic mediators, and/or treatment of an autoimmune diseases. Examples of autoimmune diseases which can be treated or prevented using the compounds of the invention are Addison's disease, autoimmune hemolytic anemia, Crohn's disease, Goodpasture's syndrome, Grave's disease, Hashimoto's thyroiditis, idiopathic thrombocytopinic purpura, insulin- dependent diabetes melhtus, multiple sclerosis, myasthenia gravis, pemphigus vulgaris, pernicious anemia, poststreptococcal glomerulonephritis, psoriasis, rheumatoid arthritis, scleroderma, Sjogren's syndrome, spontaneous infertility, and syntemic lupus erythematosus. Accordingly, the 6-phenyl-l,5-dihydropyrrolo[3,2- J pyrimidine-2,4-dione derivatives of the invention and pharmaceutically acceptable salts thereof, and pharmaceutical compositions comprising such compound and/or salts thereof, may be used in a method of treatment of disorders of the human body which comprises administering to a patient requiring such treatment an effective amount of a 6-phenyl- l,5-dmydropyrrolo[3^-<f]pyrinήdine-2,4-dione derivative of the invention or a pharmaceutically acceptable salt thereof.
The present invention also provides pharmaceutical compositions which comprise, as an active ingredient, at least a 6-phenyl-l,5-dihydropyrrolo[3^- Jpyrirnidine-2,4-dione derivative of formula (I) or a pharmaceutically acceptable salt
thereof in association with a pharmaceutically acceptable excipient such as a carrier or diluent. The active ingredient may comprise 0.001% to 99% by weight, preferably 0.01% to 90% by weight of the composition depending upon the nature of the formulation and whether further dilution is to be made prior to application. Preferably the compositions are made up in a form suitable for oral, topical, nasal, rectal, percutaneous or injectable administration.
The pharmaceutically acceptable excipients which are admixed with the active compound, or salts of such compound, to form the compositions of this invention are well-known p r se and the actual excipients used depend inter alia on the intended method of administering the compositions.
Compositions of this invention are preferably adapted for injectable and per os administration. In this case, the compositions for oral administration may take the form of tablets, retard tablets, sublingual tablets, capsules, inhalation aerosols, inhalation solutions, dry powder inhalation, or liquid preparations, such as mixtures, elixirs, syrups or suspensions, all containing the compound of the invention; such preparations may be made by methods well-known in the art.
The diluents which may be used in the preparation of the compositions include those liquid and solid diluents which are compatible with the active ingredient, together with colouring or flavouring agents, if desired. Tablets or capsules may conveniently contain between 2 and 500 mg of active ingredient or the equivalent amount of a salt thereof.
The liquid composition adapted for oral use may be in the form of solutions or suspensions. The solutions may be aqueous solutions of a soluble salt or other derivative of the active compound in association with, for example, sucrose to form a syrup. The suspensions may comprise an insoluble active compound of the invention or a pharmaceutically acceptable salt thereof in association with water, together with a suspending agent or flavouring agent.
Compositions for parenteral injection may be prepared from soluble salts, which may or may not be freeze-dried and which may be dissolved in pyrogen free aqueous media or other appropriate parenteral injection fluid.
Effective doses are normally in the range of 2-2000 mg of active ingredient per day. Daily dosage may be administered in one or more treatments, preferably from 1 to 4 treatments, per day.
The syntheses of the compounds of the invention and of the intermediates for use therein are illustrated by the following Examples (including Preparation Examples (Preparations 1-26)) which do not limit the scope of the invention in any way. 'H Nuclear Magnetic Resonance Spectra were recorded on a Varian Gemini 300 spectrometer. Melting points were recorded using a Perkin Elmer DSC-7 apparatus. The chromatographic separations were obtained using a Waters 2690 system equipped with a Symmetry C18 (2.1 x 10 mm, 3.5 μM) column. As detectors a Micromass ZMD mass spectrometer using ES ionization and a Waters 996 Diode Array detector were used. The mobile phase was formic acid (0.46 mL), ammonia (0.115 mL) and water (1000 mL) (A) and formic acid (0.4 mL), ammonia (0.1 mL), methanol (500 mL) and acetonitrile (500 mL) (B): initially from 0% to 95% of B in 20 min, and then 4 min. with 95% of B. The reequilibration time between two injections was 5 min. The flow rate was 0.4 mL/min. The injection volume was 5 μL. Diode array chromatograms were processed at 210 nm.
PREPARATION EXAMPLES
PREPARATION 1 {4-{2-{5-Ni ro-2,6-dioxo-l^-dipropyl-l^^,6-tetrahydropyriιιιidin-4- yi)vinyI]phenoxy} acetic acid
To a solution of 6-methyl-5-rn^o-l,3-dipropyl-lH-pyrimidine-2,4-dione (4.1 g, 16.08 mmol) in dry dioxane (52 mL) was added piperidine (1.6 mL, 1835 mmol) and (4-formylphenoxy)acetic acid (2.9 g, 16.08 mmol). The mixture was stirred at reflux
temperature for 6S hours. The resulting solution was concentrated under vacuum and the residue was treated with ethanol (100 mL) until formation of a precipitate was observed. The solid was collected by filtration and dried under vacuum to yield the title product (4.8 g, 72%) as a yellow solid. m.p.(H2O): 72-74 °C. δ 'H NMR (DMSO): 10.10 (bs, IH), 7.61 (d, 2H), 6.99 (m, 4H), 4.76 (s, 2H), 3.84 (m, 4H), 1.61 (m, 4H), 0.87 (m, 6H).
ESI/MS (m/e, %): 418 [(M+l)\ 100].
PREPARATION 2
[4-<2,4-Dioxc-lJ- ipropyI-2J,4^-tetrahydro-lH-pyrroIo[3^-<ι]pyrimidin-6- yl)phenoxy] acetic acid a)A solution of 6-methyl-5-mfro-l,3-dipropyl-lHpyrimidine-2,4-dione (7.72 g, 30.24 mmol), (4-formylphenoxy)acetic acid (6 g, 33.26 mmol) and piperidine (4.5 mL, 45.36 mmol) in ethanol (140 mL) with 3 A molecular sieves (9.8 g) was refluxed for 5 hours. The resulting suspension was diluted with dichloromethane (75 mL), filtrated and the filtrates were evaporated under reduced pressure. The residue was suspended in water (100 mL) and acetic acid was added until pΗ was slightly acidic. The aqueous suspension was partitioned between dichloromethane and brine, then the organic phase was separated, washed with 2N ΗC1, brine, dried (MgSO4) and evaporated under reduced pressure. The residue was triturated with a mixture of ethyl ether and isopropyl ether. The precipitate was collected by filtration and dried under vacuum to yield the compound of Preparation 1 (8.08 g, 64%). b)To a stirred solution of the above compound (8.08 g, 19.36 mmol) in formic acid (180 mL) was slowly added sodium dithionite (19.8 g, 96.8 mmol) and the mixture was refluxed overnight The resulting solution was cooled to room temperature and poured into water (750 mL). The precipitate was collected by filtration and washed with water and ethyl ether, then dried under vacuum to yield [4-(2,4-Dioxo-l,3-dipropyl- 2,3,4,5-tetι^ydro-lH-pyrrolo[3,2-ύt pyrimidin-6-yl)phenoxyJacetic acid(5.6 g, 75%) as
a white solid. m.p.(MeOH/H2O): 280-282 °C. δ Η NMR (DMSO): 7.85 (d, 2H), 6.98 (d, 2H), 6.64 (d, IH), 4.74 (s, 2H), 3.87 (m, 4H), 1.62 (m, 4H), 0.90 (m, 6H). ESLMS (m/e, %): 386 [(M+l)*, 100J.
PREPARATION 3
[4-<lJ-Dimethyl-2,4-dioxo-2J,4^tetrahydro-ljEr-pyrrolo[3^-</]pyriιιιidin-€- yl)phenoxy] acetic acid ethyl ester a)Following the same procedure as in Preparation 1, from l,3,6-trimethyl-5- m^o-lH-pyrimidine-2,4-dione (2.47 g, 12.4 mmol) and (4-formylphenoxy)acetic acid ethyl ester (2.58 g, 12.4 mmol), {4-[2-(l,3-Dimethyl-5-nitro-2,6-dioxo-l^,3,6- tetrahydropyrirm'd -4-yl)vinylJphenoxy} acetic acid ethyl ester was obtained (2.4 g, 50%) as a yellow solid. m.p.(EtOH): 136-138 °C. δ 'H NMR (CDC13):7.43 (d, 2H), 7.00 (d, IH), 6.92 (d, 2H), 6.52 (d, IH), 4.66 (s, 2H), 4.28 (q, 2H), 3.48 (s, 3H), 3.41 (s, 3H), 1.30 (t, 3H). b)A solution of the above ester (1.18 g, 3.025 mmol) in triethyl phosphite (5 mL) was refluxed for 7 hours. The resulting mixture was cooled, the precipitate collected by filtration and washed with ethyl ether to yield [4-(l,3-dimethyl-2,4-dioxo-2,3,4,5- tetrahydro-lH-pyrrolo[3,2-- j pyrimidin-6-yl)phenoxyJacetic acid ethyl ester (0.32 g, 30%) as a white solid. m-p- MeOH/HjO): 243-245 °C. δ Η NMR (DMSO): 12.45 (bs, IH), 7.95 (d, 2H), 7.10 (d, 2H), 6.72 (s, IH), 4.94 (s, 2H), 4.28 (q, 2H), 3.52 (s, 3H), 3.36 (s, 3H), 1.32 (t, 3H). ESLMS (m/e, %): 357 (M+, 80), 270 (100).
PREPARATION 4
[4-(lJ-Dimethyl-2,4-dioxo-2J,4^-tetrahydro-li-r-pyrrolo[3^-rf]pyriιnidin-6- yI)phenoxyJ acetic acid
Obtained as a white solid (44% overall) from l,3,6-trimethyl-5-nitro-lH- pyιimidine-2,4-dione and (4-forrnylphenoxy)acetic acid following the procedure described in Preparation 2. m.p.(MeOH/H2O): 261-263 °C. δ 'H NMR (DMSO): 12.89 (bs, IH), 12.19 (s, IH), 7.76 (d, 2H), 6.89 (d, 2H), 6.54 (d, IH), 4.65 (s, 2H), 3.33 (s, 3H), 3.17 (s, 3H). ESI/MS (m/e, %): 329 (M*, 5).
PREPARATION 5
[4-(1 -Diethyl-2,4-dioxό-2J,4,5-tetrahydro-lif-^ yI)phenoxy] acetic acid Obtained as a white solid (41% overall) from l,3-diethyl-6-methyl-5-nitro-lH- pyrimidine-2,4-dione and (4-formylphenoxy)acetic acid following the procedure described in Preparation 2. δ 'Η NMR (DMSO): 12.38 (bs, 1Η), 7.82 (d, 2Η), 7.01 (d, 2H), 6.62 (s, IH), 4.78 (s, 2H), 3.98 (m, 4H), 1.20 (m, 6H).
PREPARATION 6
[4-<l-Methyl-2,4-dioxo-3-propyl-23,4^tetrahydro-]L^ yl)phenoxy] acetic acid
Obtained as a white solid (60% overall) from l,6-dimethyl-5-nitro-3-propyl-lH- pyrimidine-2,4-dione and (4-formylphenoxy)acetic acid following the procedure described in Preparation 2. m.p.: 300-301 °C. δ 'H NMR (DMSO): 13.5 (bs, IH), 12.2 (bs, IH), 7.9 (d, 2H), 7.1 (d, 2H), 6.8 (s, 2H), 4.8 (s, 2H), 3.9 (t, 2H), 3.4 (s, 3H), 1.6 (m, 2H), 0.9 (t, 3H). -
ESI/MS (m/e, %): 357 [(M+l)+, 91J.
PREPARATION 7
[4-{3-Methyl-2,4-dioxo-l-propyl-2 ,4^tetrahydro-Lff-pyrroIo[3^-< ]pvri idin-^ yi)-phenoxy] acetic acid
Obtained as a yellow solid (48% overall) from 3,6-dimethyl-5-nitro-l-propyl- lH-pyrimidine-2,4-dione and (4-formylphenoxy)acetic acid following the procedure described in Preparation 2. δ 'Η NMR (DMSO): 13.0 (bs, 1Η), 12.2 (bs, 1Η), 7.9 (d, 2Η), 7.0 (d, 2H), 6.7 (s, 2H), 4.7 (s, 2H), 3.9 (t, 2H), 3.3 (s, 3H), 1.7 (m, 2H), 0.9 (t, 3H).
PREPARATION 8
{4- [l-(3-Methoxypropyr)-3-methyl-2,4-dioxo-2 ,4^-tetrahydro-li -pyrrolo [3,2-
< ]pyrimidin-6-yI]phenoxy} acetic acid ethyl ester Obtained as white solid (17% overall) from l-(3-methoxypropyl)-3,6-dimethyl-
5-rn^o-lH-pyrimidine-2,4-dione and (4-formylphenoxy)acetic acid ethyl ester following the procedure described in Preparation 3. m.p.(MeOH/H2O): 177-179 °C. δ 'H NMR (CDC13): 11.7 (s, IH), 7.85 (d, 2H), 6.95 (d, 2H), 6.46 (d, IH), 4.67 (s, 2H), 4.30 (q, 2H), 4.07 (t, 2H), 3.48 (s, 3H), 3.43 (m, 2H), 3.34 (s, 3H), 2.05 (m, 2H), 1.32 (t, 3H).
ESLMS (m e, %): 415 (M+, 65).
PREPARATION 9 [4-(3-IsobutyI-l-methyI-2,4-dioxo-2J,4,5-tetrahydro-l^-pyπ,oIo{3^-<f]pyriιιιidin- 6-yl)phenoxy] acetic acid
Obtained as a white solid (50% overall) from 3-isobutyl-l,6-dimethyl-5-nitro- lH-pyrinndine-2,4-dione and (4-formylphenoxy)acetic acid following the procedure described in Preparation 2.
δ 'H NMR (DMSO): 13.00 (bs, IH), 12.45 (bs, IH), 7.95 (m, 2H), 6.90 (m, 2H), 6.72 (s, IH), 4.74 (s, 2H), 3.72 (d, 2H), 3.26 (s, 3H), 2.10 (m, IH), 0.90 (d, 6H).
PREPARAΗON 10 [4-(2,4-Dioxo-l-propyl-2J,4,5-tetrahydro-lJ7-pyrroIo[3^-rf]pyrimidin-6- yl)phenoxy] acetic acid
Obtained as a yellow solid (45% overall) from 6-methyl -5-nitro-l -propyl- 1H- pyrimidine-2,4-dione and (4-foπnylphenoxy)acetic acid following the procedure described in Preparation 2. m.p.: 306-307 °C. δ Η NMR (DMSO): 11.99 (bs, 1Η), 10.57 (s, 1Η), 7.62 (d, 2Η), 6.75 (d, 2H), 6.40 (s, IH), 4.51 (s, 2H), 3.57 (t, 2H), 1.44 (m, 2H), 0.68 (t, 3H).
PREPARATION 11 {4-[lJ-Bis(2-methoxyethy -2,4-dioxo-2 3,4,5-terrahydro-L9r-pyrrolo[3^- έ/lpyrimidin- -yllphenoxyJacεtic acid
Obtained as a white solid (30% overall) from 5-amino-l,3-bis(2-methoxyethyl)- 6-methyl-lH-pyrimidine-2,4-dione and (4-formylphenoxy)acetic acid following the procedure described in Preparation 2. δ 'H NMR (DMSO): 13.10 (bs, IH), 12.25 (bs, IH), 7.82 (d, 2H), 7.05 (d, 2H),
6.63 (s, IH), 4.78 (s, 2H), 4.05 (m, 4H), 3.58 (m, 4H), 3.38 (s, 3H), 3.24 (s, 3H).
PREPARAΗON 12
{4-[l r3-Bis(cyciopropyImethyl)-2,4-dioxo-2 J,4^-tetrahydro-l Br-pyrrolo[3^- </]pyrimidin-6-yl]phenoxy} acetic acid
Obtained as a white solid (45% overall) from 5-amino-l,3- bis(cyclopropylmethyl)-6-methyl-lH-pyrirmcline-2,4-dione and (4- formylphenoxy)acetic acid following the procedure described in Preparation 2. δ Η NMR (DMSO): 13.10 (bs, IH), 12.28 (bs, IH), 7.88 (d; 2H); 7.02 (d, 2H),
6.72 (s, IH), 4.76 (s, 2H), 3.81 (m, 4H), 1.25 (m,,2H), 0.38 (m, 8H).
PREPARAΗON 13
[4-<7-Chloro-l -dimethyl-2,4-dioxo-2J,4^-tetrahydro-Lfir-pyττolo(3^- ^pyrimidin-^-yI)phenoχy]acetic acid
To a solution of the title compound of Preparation 4 (0.5 g, 1.52 mmol) in glacial acetic acid (3 mL) was slowly added sulfuryl chloride (0.13 mL) and the mixture was stirred at room temperature for 4 hours. The reaction mixture was carefully poured into stirred ice-water and the aqueous suspension was partitioned between dichloromethane and brine, then the organic phase was separated, washed with water, dried (MgSO4) and evaporated under reduced pressure to yield the title product (500 mg, 90%) as an off white solid. δ lH NMR (DMSO): 12.7 (s, IH), 7.6 (d, 2H), 7.0 (d, 2H), 4.8 (s, 2H), 3.7 (s, 3H), 3.3 (s, 3H).
PREPARAΗON 14
[4-{7-Bromo-2,4-dioxo-lr3-dipropyl-2,3,4^-tetrahydro-l-Sr-pvrroIo[3^- <-]pyrimidin-6-yl)phenoxy]acetic acid
To a solution of the title compound of Preparation 2 (1 g, 2.59 mmol) in glacial acetic acid (22 mL) was slowly added bromine (0.187 mL, 3.63 mmol) and the mixture was stirred at room temperature for 1 hour. Then the reaction mixture was poured into ice-water and partitioned between dichloromethane and brine, the organic phase was separated, dried (MgSO4) and evaporated under reduced pressure to yield the title product (0.88 g, 73%) as an orange solid. δ Η NMR (DMSO): 12.7 (s, IH), 7.5 (d, 2H), 6.9 (d, 2H), 4.7 (s, 2H), 4.1 (t,
2H), 3.8 (t, 2H), 1.5 (m, 4H), 0.86 (dt, 6H).
PREPARAΗON 15
[4-(7-Chloro-2,4-dioxo-lJ-dipropyl-2J,4^-tetrahydro-lir-pyrrolo[3^- Jpyrimidin-6-yl)phenoxy]acetic acid
Obtained as a yellow solid (89%) from the title compound of Preparation 2 following the procedure described in Preparation 13. δ 'H NMR (DMSO): 12.7 (s, IH), 7.6 (d, 2H), 7.0 (d, 2H), 4.7 (s, 2H), 4.1 (t, 2H), 3.9 (t, 2H), 1.6 (m, 4H), 0.9 (dt, 6H).
PREPARAΗON 16
methoxyphenoxyjaceric acid a)Following the same procedure as in Preparation 3, from l,3,6-trimethyl-5-nitro-lH- pyrimidine-2,4-dione and
"(4-formyl-3-methoxyphenoxy)acetic acid ethyl ester, [4-(l,3- dimethyl-2,4-dioxo-2,3,4,5-tetrahycho-lH-pyrrolo[3^^ methoxyphenoxyjacetic acid ethyl ester was obtained (50% overall) as a yellow sohd. m.p.(EtOH/H
2O): 234-236 °C. δ "H NMR (DMSO): 11.75 (bs, IH), 7.66 (d, IH), 6.65 (d, IH), 6.54 (dd, IH), 6.48 (s, IH), 4.81 (s, 2H), 4.14 (q, 2H), 3.83 (s, 3H), 3.36 (s, 3H), 3.20 (s, 3H), 1.17 (t, 3H). ; ESI/MS (m/e, %): 387 (M
*. 100). b)A stirred mixture of the above compound (1.43 g, 3.7 mmol) and 10% NaOH (37 mL) in ethanol (37 mL) was heated to reflux temperature for 1 hour. The resulting mixture was concentrated under reduced pressure and the residue was treated with 10% HCI. The precipitate was collected by filtration and washed with EtOH to yield the title product (1.3 g, 99%) as a white sohd. m.p.(MeOH/H
2O): >260 °C (dec). δ Η NMR (DMSO): 11.84 (bs, IH), 7.72 (d, IH), 6.71 (s, IH), 6.54 (m, 2H), 4.77 (s, 2H), 3.89 (s, 3H), 3.43 (s, 3H), 3.27 (s, 3H).
PREPARAΗON 17
[4-(lJ-Dimethyl-2,4-dioxo-2 ,4,5-tetrahydro-L5r-pyττolo [3^-<i]pyriπudin-6-y-)-2- methoxyphenoxyj acetic acid
Obtained as a white sohd (25% overall) from l,3,6-trimethyl-5-nitro-lH- pvrimidine-2,4-dione and (4-formyl-2-methoxyphenoxy)acetic acid ethyl ester following the procedure described in Preparation 16. m.p.(MeOΗ/Η2O): >300 °C (dec.). δ 'H NMR (DMSO): 12.3 (bs, IH), 7.60 (s, IH), 7.42 (d, IH), 6.91 (d, IH), 6.6S (s, IH), 4.73 (s, 2H), 3.88 (s, 3H), 3.43 (s, 3H), 3.27 (s, 3H).
PREPARAΗON 18
2-[4-(2,4-Dioxc>-1 -dipropyl-2J,4^tetrahydro-li -pyrroIo[3^-< ]pyrinaidin^ yl)phenoxy]propionic acid
Obtained as a yellow sohd (41% overall) from 1 J-dipropyl-6-methyl-5-nitro- lH-pyrimidine-2,4-dione and 2-(4-foπnylphenoxy)propionic acid following the procedure described in Preparation 2. δ "Η NMR (DMSO): 11.5 (s, 1Η), 7.5 (d, 2Η), 7.0 (d, 2H), 6.1 (s, IH), 4.9(q, IH), 4.0 (t, 2H), 3.9 (t, 2H), 1.8 (d,3H), 1.7 (m, 4H), 0.9 (t, 6H).
PREPARAΗON 19
2-{4-<lJ-Dimethyl-2,4-dioxo-2J,4,5-tetrahydro-L!Ef-pyrrolo{3^-i/]pyrimidm- - yi)phenoxy]propionic acid
Obtained as a yellow sohd (53% overall) from l,3,6-trimethyl-5-nitro-lH- pyrimidine-2,4-dione and 2-(4-formylphenoxy)propionic acid following the procedure described in Preparation 2. δ Η NMR (DMSO): 12.2 (s, IH), 7.8 (d, 2H), 6.9 (d, 2H), 3.9 ( , IH), 3.4 (s, 3H), 3.2 (s, 3H), 0.9 (dt, 3H).
PREPARAΗON 20
2-[4-(2,4-Dioxo-lJ-dipropyI-2J,4,5-tetrahydro-l^-pyrroIo[3^-<flpyrimidiιι-6- yI)phenoxy]butyric acid
Obtained as white sohd (65% overall) from 6-methyl-5-nitro-l,3-dipropyl-lH pyrimidine-2,4-dione and 2-(4-formylphenoxy)butyric acid following the procedure described in Preparation 2. δ 'Η NMR (CDC13): 11.60 (bs, 1Η), 7.51 (d, 2Η), 7.02 (d, 2H), 4.78 (t, IH), 4.05 ( 2H), 3.94 (t 2H), 2.18 (m, 2H), 1.77 (m, 4H), 1.22 (t, 3H), 0.98 (dt, 6H).
PREPARAΗON 21
2-[4-<2,4-Dioxo-lJ-dipropyl-2J,4^-tetrahydro-l y-pyrrolo{3^-<ilpyrimidiii- - yi)phenoxy]-2-methylpropionic acid
Obtained as white~solid (25% overall) from 6-methyl-5-nitiO-l,3-dipropyl-lH pyrimidine-2,4-dione and 2-(4-formylphenoxy)-2-methylpropionic acid following the procedure described in Preparation 2. δ Η NMR (CDCI3): 11.6 (s, IH), 7.4 (d, 2H), 7.0 (d, 2H), 6.0 (s, IH), 4.0 (t, 2H), 3.9 (t, 2H), 1.7 (m, 10H), 0.9 (t, 6H).
PREPARAΗON 22 [4-(2,4-Dioxo-lJ-dipropyl-2 ,4^tetrahydro-lH-pyrrolo[3^-< ]pyriιιιidin-6- yi)phenoxy]phenylacetic acid
Obtained as white sohd (90% overall) from 6-methyl-5-nitro-l,3-dipropyl-lH- pyrimidine-2,4-dione and (4-formylphenoxy)phenylacetic acid following the procedure described in Preparation 2. δ Η NMR (CDC13): 11.5 (s, IH), 7.7 (d, 2H), 7.5 (d, 2H), 7.1 (d, 2H), 6.1 (s,
IH), 5.8 (s, IH), 3.9 (rn, 4H), 1.7 (m, 4H), 0.9 (dt, 6H).
PREPARAΗON 23
3-{4-(2,4-Dioxo-lJ-dipropyl-2J,4,5-tetrahydro-liT-pyrrolo[3^-<ιlpyriιnidin-6- yi)phenylJpropionic acid
Obt ined as white sohd (22% overall) from 6-methyl-5-mtro-l,3-dipropyl-lH pyrimidine-2,4-dione and 3-(4-formylphenyl)propionic acid following the procedure described in Preparation 2. δ 'Η NMR (CDCI3): 12.3 (s, 1Η), 12.1 (s, 1Η), 7.8 (2Η, d), 7.3 (d, 2H), 6.7 (s, IH), 3.8 (m, 4H), 2.8 (t 2H), 2.5 (t, 2H), 1.6 (m, 4H), 0.9 (dt, 6H).
PREPARAΗON 24
3-[4-(2,4-Dioxo-13-dipropyl-2J,4^tetrahydr(>-lH-pv^ yl)phenyl]acrylic acid
Obtained as white'solid (20% overall) from 6-methyl-5-nitro-l,3-dipropyl-lH pyrimidine-2,4-dione and 3-(4-formylphenyl)acrylic acid following the procedure described in Preparation 2. δ Η NMR (DMSO): 12.3 (s, IH), 7.9 (d, 2H), 7.7 (d, 2H), 7.5 (d, IH), 6.8 (s, IH), 6.5 (d, IH), 3.8 (m, 4H), 1.5 (m, 4H), 0.8 (dt, 6H).
PREPARAΗON 25
4-[4-<2,4-Dioxo-l -dipropyi-2J,4,5-tetrahydro-l-H-pyrrolo{3^-<flpyriπudin-6- yI)ptaenoxy]butyric acid
Obtained as white sohd (45% overall) from 6-methyl-5-nitro-l,3-dipropyl-lH pyrimidine-2,4-dione and 4-(4-formylphenoxy)butyric acid following the procedure described in Preparation 2. δ 'H NMR (CDCI3): 11.7 (s, IH), 7.7 (d, 2H), 6.9 (d, 2H), 6.1 (s, IH), 4.2 (bs,
2H), 3.9 (m, 4H), 2.1 (bs, 2H), 1.7 (m, 4H), 0.9 (m, 6H).
PREPARAΗON 26
4-<2,4-Dioxo-lJ-dipropyl-2J,4,5-tetrahydro-l.H-pyrroIo [3^-</]pyrimidin-6- yl)benzoic acid
Obtained as yellow sohd (36% overall) from 6-methyl-5-nitro-l,3-dipropyl-lH pyrirmdine-2,4-dione and 4-formylbenzoic acid following the procedure described in Preparation 2. δ Η NMR (DMSO): 13.0 (bs, IH), 12.6 (s, IH), 8.0 (dd, 4H), 6.9 (s, IH), 3.9 (m, 4H), 1.6 (m, 4H), 0.9 (dt, 6H).
PREPARAΗON 27
yl)phenoxy] acetic acid
Obtained as a yellow sohd (6% overall) from 6-methyl-5-nitro-3-propyl-lH- pyrimidine-2,4-dione and (4-formylphenoxy)acetic acid following the same procedure described in Preparation 2. δ Η NMR (DMSO): 12.9 (s, 1Η), 11.9 (s, 1Η), 11.0 (s, 1Η), 7.8 (d, 2Η), 6.9 (d, 2H), 6.2 (d, IH), 4.7 (s, 2H), 3.8 (t, 2H), 1.6 (m, 2H), 0.9 (t, 3H).
PREPARAΗON 28 {4-p-Methyl-l-(3-morphoIin-4-ylpropyl)-2,4-dioxo-2J,4^-terr.ahydr(>-l^- pyn-olo[3,2-<ι]pyrimidm-6-yl]phenoxy}acetic acid hydrochloride a) A solution of 3,6-<iimethyl-l-(3-morpholin-4-ylpropyl)-5-nitro-lH- pyrimidine-2,4-dione (0.50 g, 1.60 mmol), (4-formylphenoxy)acetic acid (0.31 g, 1.76 mmol) and piperidine (79 μL, 0.80 mmol) in ethanol (8 mL) with 3A molecular sieves (0.83 g) was refluxed for 3 hours. The resulting suspension was filtrated and the filtrates were evaporated under reduced pressure. The residue was suspended in water (100 mL), extracted with dichloromethane and water was evaporated under reduced pressure to yield (4- {-2-[ 1 -methyl-3-(3-morpholin-4-ylpropy l)-5-nitro-2,6-dioxo- 1 ,2,3 ,6- tetrahydropyrimidm-4-ylJvinyl}phenoxy)acetic acid (0.76 g, 100%) as a yellow sohd.
b) To a stirred solution of the above compound (0.76 g, 1.60 mmol) in formic acid (15 mL) was slowly added sodium dithionite (1.64 g, 8.00 mmol) and the mixture was refluxed overnight The solvent was evaporated under reduced pressure, the residue was redissolved in a mixture of dichloromethane and methanol and the insoluble salts were separated by filtration. The filtrates were acidified until pH 3 by adding dioxane saturated with hydrochloric acid, the solvent was evaporated under reduced pressure and the residue was triturated with a mixture of dichloromethane-diethyl ether, to yield the title compound as a dark yellow sohd (0.70 g, 99%). δ Η NMR (CDC13): 9.8 (s, IH), 7.8 (d, 2H), 6.9 (d, 2H), 6.6 (s, IH), 4.6 (s, 2H), 3.9 (t, 2H), 3.6 (m, 4H), 3.3 (s, 3H), 2.3 (m, 6H), 1.8 (m, 2H).
PREPARATION 29
6-(4-HydroxvmethyIphenyl)-lJ-dimethyi-l^-dihydropyrrolo[3^-</]pyriιnidine-
2,4-dione a) To a solution of 6-methyl-5-nifro-l,3-dimethyl-lH-pyrimidine-2,4-dione
(1.59 g, 7.99 mmol) in dry dioxane (50 mL) was added piperidine (1.18 mL, 11.99 mmol), 4-formylbenzoic acid (1.44 g, 7.99 mmol) and 3 A molecular sieves. The mixture was stirred at 50 °C for 5 hours. The resulting solution was concentrated under vacuum and the residue was treated with ethyl acetate, washed with 10% aqueous hydrochloric acid (3 x 50 mL) and brine (3 x 50 mL), dried (Na,SO4) and evaporated under reduced pressure. The obtained residue was crystalized from ethanol to yield 4-[2- ( 1 ,3-dimethyl-5-nitro-2,6-dioxo- 1 ,2,3 ,6-tetrahydropyrirm^m-4-yl)vinylJbenzoic acid (1.89 g, 70%) as a yellow sohd. b) To a stirred solution of the above compound (0.50 g, 1.51 mmol) in formic acid (15 mL) was slowly added sodium dithionite (1.84 g, 10.56 mmol) and the mixture was refluxed for 24 hours. The resulting solution was cooled to room temperature and poured into water. The resulting precipitate was collected by filtration, washed with water and dried under vacuum to yield 4-(l,3-<iimethyl-2,4-dioxo-2,3,4,5-tetrahydro- lH-pyrrolo[3^-<φyrimidin-6-yl)benzoic acid (0.37 g, 80%) as a white sohd.
c) To a stirred solution of the above compound (0.20 g, 0.66 mmol) in dry tetrahydrofuran (3 mL) at 0°C and under argon atmosphere, was slowly added a 1 M solution of borane in tetrahydrofuran (6.67 mL, 6.67 mmol) and the mixture was refluxed for 24 hours. The resulting solution was cooled to room temperature, methanol was slowly added and the solvent was evaporated under reduced pressure. The residue was suspended in ethyl acetate (100 mL), washed with 10% aqueous sodium hydroxide (2 x 10 mL) and water (10 mL). The organic layer was dried (Na^O and evaporated under reduced pressure. The obtained residue was crystalized from a mixture of dietyl ether and methanol to yield the title compound (0.080 g, 40%) as a white sohd. δ 'H NMR (CDC13): 12.3 (bs, IH), 7.8 (d, 2H), 7.3 (d, 2H), 6.7 (s, IH), 5.2 (t,
IH), 4.5 (d, 2H), 3.4 (s, 3H), 3.2 (s, 3H).
PREPARAΗON 30 -
6-(4-Hydroxymethylphenyl)-l^-dipropyl-l^-dihydropyrrolo[3^-<flpyrimidine-2,4- dione
Obtained as a white sohd (65% overall) from the title compound of Preparation 26 following the procedure described in Preparation 29c. δ Η NMR (CDC13): 12.2 (bs, IH), 7.7 (d, 2H), 7.2 (d, 2H), 6.7 (d, IH), 5.12 (m, IH), 4.4(d, 2H), 3.7 (m, 4H), 1.4-1.55 (m, 4H), 0.65-0.8 (m, 6H).
EXAMPLES
TABLE 3
TABLE 4
TABLE 5
TABLE 9
TABLE 11
TABLE 12
TABLE 14
EXAMPLE 1 2-{4-(2,4-Dioxo-l^-dipropyl-2^,4^terrahydro-Lr7-pyrroIo[3^-</]pyrimidiιι-6- yI)phenoxy]-iV-plienylacetamide a) To a solution of the title compound of Preparation 1 (300 mg, 0.72 mmol) in anhydrous tetrahydrofuran (20 mL) under argon atmosphere was slowly added at -40°C N-memylmorpholine (0.079 mL, 0.72 mmol) and isobutyl chloroformate (0.093 mL, 0.72 mmol). The mixture was stirred at -40°C for 2 hours. Then aniline was added (0.066 mL, 0.72 mmol) and the mixture was stirred 15 minutes at -40°C .and 12 hours at
room temperature. The resulting solution was evaporated under reduced pressure and the residue was partitioned between dichloromethane and a saturated aqueous solution of sodium bicarbonate. The organic phase was separated, washed with water and brine, dried (NajS ,) and evaporated under reduced pressure. The resulting crude was purified by flash column chromatography on silica-gel (dichloromethane) to yield the intermediate amide as a yellow sohd (150 mg, 42%). m.p.(EtOH): 62-64°C δ Η NMR (CDC13): 8.18 (bs, IH), 7.59 (d, 2H), 7.46 (d, 2H), 7.12 (m, 5H), 6.53 (d, IH), 4.66 (s, 2H), 3.91 (m, 4H), 1.68 (m, 4H), 0.97 (m, 6H). ESI/MS (m/e,%): 492 (M*, 46). b) A stirred solution of the above compound (150 mg, 0.305 mmol) in triethylphosphite (2 mL) was refluxed under argon atmosphere for 5 hours. The mixture was cooled to room temperature and the resulting precipitate was collected by filtration, washed with ethyl ether and dried under vacuum to yield the title compound (65 mg, 46%) as a white solid. m.p.tMeOH/HjO): 257-259°C δ Η NMR (DMSO): 12.20 (s, IH), 10.10 (s, IH), 7.87 (d, 2H), 7.63 (d, 2H), 7.32 (m, 2H), 7.07 (m, 3H), 6.65 (s, IH), 4.75 (s, 2H), 3.85 (m, 4H), 1.61 (m, 4H), 0.88 (m, 6H). - ESI/MS (m/e,%): 460 (M+, 100).
EXAMPLE 2
6-{4-[2-Oxo-2-(4-phenylpiperaziα-l-yl)ethoxy]pIιenyl}-lr3-dipropyl-l^- dihydropyrrolo[3^-rf]pyrimidme-2,4-dione Obtained as a white sohd (20%) from the title compound of Preparation 1 and 1- phenylpiperazine following the procedure of example 1. m.p.(MeOH/H2O): 180-184°C δ Η NMR (DMSO): 12.15 (s, IH), 7.86 (d, 2H), 7.25 (m, 2H), 7.00 (m, 4H), 6.83 (m, IH), 6.66 (s, IH), 4.96 (s, 2H), 3.87 (m, 4H), 3.63 (m, 4H), 3.21 (m, 2H), 3.14
(m, 2H), 2.51 (m, 4H), 0.90 (m, 6H). ESI MS (m/e,%): 529 (M*, 19).
EXAMPLE 3 2-{4-(2,4-Dioxo-l -dipropyl-2^,4^-tetrahydro-l Sr-pyrrolo [3,2-<f]pyriπιidiιHϊ- yl)phenoxy]-Λr-(4-fluorophenyl) acetamide
Obtained as a white sohd (23%) from the title compound of Preparation 1 and 4- fluoroaniline following the procedure of example 1. m.p.(MeOH/H2O): 256-258°C δ lH NMR (DMSO): 12.21 (s, IH), 10.18 (s, IH), 7.89 (m, 2H), 7.63 (m, 2H),
7.12 (m, 4H), 6.67 (s, IH), 4.77 (s, 2H), 3.84 (m, 4H), 1.61 (m, 4H), 0.91 (m, 6H).
ESI MS (m/e,%): 478 (M*, 100).
EXAMPLE 4 6-{4-[2-(3,4-Dihydro-lJ3r-isoqumolin-2-yl)-2-oxoethoxy] phenyl}-l,3-dipropyl-l ,5- dmydropyrrolo[3,2-</]pyrimidine-2,4-dione
To mixture of the title compound of Preparation 2 (480 mg, 1.24 mmol), N-(3- dimemylaminopropyl)-N '-ethyl carbodiimide hydrochloride (285 mg, 1.49 mmol), 1- hydroxybenzotriazole (201 mg, 1.49 mmol) and triemylamine (0.44 mL, 2.48 mmol) in dimethylformamide (20 mL) was added 1^,3,4-tetπώydroisoquinoline (0.205 mL, 1.61 mmol) and the mixture was stirred at room temperature overnight. The resulting solution was evaporated under reduced pressure and the residue was partitioned between dichloromethane and a saturated aqueous solution of sodium bicarbonate. The organic phase was separated, washed with water and brine, dried (ΝajSO and evaporated under reduced pressure. The resulting crude was purified by flash column chromatography on silica-gel (hexanes:ethyl acetate 1:1) to yield the title compound as a white sohd (270 mg, 43%). m.p.: 176.9-177.6°C δ Η ΝMR (DMSO): 12.22 (bs, IH), 7.83 (d, 2H), 7.20 (m, 4H), 7-00 (d, 2H),
6.65 (s, IH), 4.98 (s, 2H), 4.67 (m, 2H), 3.85 (m, 4H), 3.70 (m, 2H), 2.86 (m, 2H), 1.65 (m, 4H), 0.89 (m, 6H).
ESI/MS (m/e,%): 500 (M*, 82).
EXAMPLE 5
N-<4-Chlorophenyl)-2-[4-(2,4-dioxo-lr3-dipropyl-2^,4,5-tetrahydro-LH- pyrrolo [3 -d pyrimidin-6-yl)phenoxy] acetamide
To mixture of the title compound of Preparation 2 (80 mg, 0.21 mmol), N-(3- dimemylammopropyl)-N'-e ylcarbodiimide hydrochloride (44 mg, 0.23 mmol), 1- hydroxybenzotriazole (31 mg, 0.23 mmol) and polymer bound morphohne (280 mg, 2.75 mmol/g based on nitrogen analysis) in dimethylformamide (4 mL) was added 4- chloroaniline (32 mg, 0.25 mmol) and the mixture was stirred at room temperature overnight To the resulting suspension was added macroporous triethylammonium methylpolystyrene carbonate (250 mg, 2.8-3.5 mmol g based on nitrogen elemental analysis) and Amberlyst 15 (650 mg) as scavengers and stirred for 2 hours (in case of acidic or basic final products the corresponding scavenger was not added). The resulting suspension was filtered and evaporated under reduced pressure. The residue was triturated with a mixture of MeOH:ethyl ether and the precipitate collected by filtration to yield the title compound as a white solid (80 mg, 78%). ESI MS m/e: 495 ([M+Hf, C26H27CIΝ4O4).
Retention Time (min.): 11.0
EXAMPLE 6-53
The compounds of this invention were synthesized from the title compound of Preparation 2 following the procedure of example 5 and using the corresponding reactant respectively. The ESI/MS data, HPLC retention times and yields are summarised in the following table.
TABLE 15
EXAMPLE 54
(4-{2-{4-(2,4-Dioxo-lv3-dipropyl-2^,4^tetrahydro-lff-pvrroIo[3^-<flpyriimdm yI)phenoxy]acetyIamino}phenyI) acetic acid
To a suspension of the title compound of Example 33 (33 mg, 0.06 mmol) in methanol (0.3 mL) was added NaOH 2N (0.3 mL) and the mixture was heated at 50°C for 1 hour. The mixture was cooled to room temperature and acetic acid was added until acidic pH was observed. The resulting precipitate was collected by filtration and dried to yield the title compound (13 mg, 42%) as a white sohd. ESI/MS m/e: 519 ([M+H]
+,
Retention Time (min.): 9.7
General procedure for the synthesis of examples 55-76
The reaction took place in a sealed tube under argon atmosphere. Usually 50 mg of the title compound of Preparation 3 were used and 2 mL of those amines that are liquid and 160 equivalents of those amines that are sohd. In all reactions a catalytic amount of sodium cyanide was added. In case of liquid amines the reaction mixture was heated at the boiling temperature of the amine and in the case of sohd amines 2 mL of anhydrous dioxane were added and heated to the boiling point of dioxane. The reactions were followed by TLC and when no more starting material was left, the mixture was cooled to room temperature and usually the final product was isolated by filtration of the corresponding precipitate which was washed with ethyl ether. Occasionally the reaction mixture was concentrated under reduced pressure and the residue chromatographed on silica-gel (dichloromethane:methanol). The title compounds were crystallized in mixtures of MeOH:H2O.
EXAMPLE 55
N-<2-Aj.nmoethyl)-2-[4-(l^-dmιemyl-2,4-dioxo-2^,4^tetrahydro-l^-pvrrolo[3^- </]pyrimidin-6-yl)phenoxy] acetamide
Obtained as a white sohd (33%) from the title compound of Preparation 3 and e ylenediamine following the procedure described above. δ Η NMR (DMSO): 7.85 (d, 2H), 7.01 (d, 2H), 6.63 (s, IH), 4.52 (s, 2H), 3.41 (s, 3H), 3.25 (s, 3H), 3.12 (m, 2H), 2.50 (m, 2H).
EXAMPLE 56 N-(4-Bromophenyl)-2-{4-<lr3-dimethyl-2,4-dioxo-2^,4,5-tetrahydro-li-'- pyrrolo[3,2-</]pyrimidin-6'-yl)phenoxy] acetamide
Obtained as a brown sohd (15%) from the title compound of Preparation 3 and 4-bromoaniline following the procedure described above. δ Η NMR (DMSO): 7.89 (d, 2H), 7.19 (d, 2H), 7.00 (d, 2H), 6.67 (s, IH), 6.57 (m, 2H), 4.61 (s, 2H), 3.49 (s, 3H), 3.33 (s, 3H).
EXAMPLE 57
2-[4-(l^Dimethyl-2,4-dioxo-2^,4^-tetι^ydro-Lff-pyrroIo[3^-<flpyrimidin-6- yl)pαenoxy]-iV-phenylacetamide Obtained as a brown sohd (74%) from the title compound of Preparation 3 and aniline following the procedure described above. m.p.: >300°C δ Η NMR (DMSO): 12.30 (bs, IH), 10.22 (bs, IH), 7.88 (d, 2H), 7.66 (d, 2H), 7.34 (m, 2H), 7.09 (m, 3H), 6.62 (s, IH), 4.78 (s, 2H), 3.42 (s, 3H), 3.27 (s, 3H). ESI/MS (m/e,%): 405 [(M+l)+, 46].
EXAMPLE 58
2-{4-<l^-Dimemyl-2,4-dioxo-2^,4,5-tetrahydro-lH-pyrτoIo{3^-< ]pyriπudin-6- yl)phenoxy]-N-{4-fluorophenyl) acetamide
Obtained as a white sohd (10%) from the title compound of Preparation 3 and 4- fluoroaniline following the procedure described above. m.p.: >300°C δ Η NMR (DMSO): 12.50 (bs, IH), 10.36 (bs, IH), 8.08 (d, 2H), 7.87 (m, 2H), 7.38 (m, 2H), 7.28 (d, 2H), 6.83 (s, IH), 5.00 (s, 2H), 3.53 (s, 3H), 3.46 (s, 3H). ESI/MS (m/e,%): 423 [(M+l)+, 100].
EXAMPLE 59 l^-Dimethyl-6-{4-[2-(4-methylpiperazin-l-yl)-2-oxo-etIιoxy]plιenyl}-l^- dwydropyrrolo[3,2-</]pyrimidine-2,4-dione
Obtained as a brown solid (72%) from the title compound of Preparation 3 and 1-methylpiperazine following the procedure described above. m.p.: >275°C δ Η NMR (DMSO): 7.84 (d, 2H), 6.97 (d, 2H), 6.57 (s, IH), 4.88 (s, 2H), 3.42 (s, 3H), 3.27 (s, 3H), 2.51 (m, 2H), 2.35 (m, 2H), 2.27 (m, 2H), 2.13 (m, 2H). ESI/MS (m/e,%): 412 [(M+l)+, 100].
EXAMPLE 60 l^-Dimethyl-6^{4-(2-morpholin-4-yl-2-oxoethoxy)phenyl]-l^-dihydropyrrolo{3^- </]pyrimidine-2,4-dione
Obtained as a brown sohd (27%) from the title compound of Preparation 3 and morpholine following the procedure described above. m.p.: >300°C δ Η NMR (DMSO): 12.42 (bs, IH), 8.01 (d, 2H), 7.16 (d, 2H), 6.80 (s, IH), 5.07 (s, 2H), 3.76 (m, 4H), 3.63 (m, 4H), 3.59 (s, 3H), 3.43 (s, 3H). ESI MS (m/e,%): 398 (M*, 42).
EXAMPLE 61
6^{4-{2-(3,4-Dmydro-lff-isoqumolm-2-yl)-2-oxoethoxy] phenyl}-1 -<iimet yl-l^- dihydr opyrrolo [3,2-rf] p vrimidine-2,4-dione
Obtained as a white sohd (32%) from the title compound of Preparation 3 and 12,2 ,4-tetrahydro isoquinoline following the procedure described above. m.p.: >280°C δ Η NMR (DMSO): 12.14 (bs, IH), 7.71 (d, 2H), 7.07 (m, 4H), 6.89 (d, 2H), 6.50 (s, IH), 4.86 (s, 2H), 3.56 (m, 2H), 3.35 (m, 2H), 3.29 (s, 3H), 3.13 (s, 3H), 2.72 (m, IH), 2.39 (m, IH). ESI/MS (m/e,%): 444 (M*, 34).
EXAMPLE 62
N-Cyclopentyl-2-[4-(l ^imethyl-2,4^ioxo-2^,4^tetrahydro-lH-pvrrolo[3^- </]pyrimidin-6-yl)phenoxy] acetamide Obtained as a white sohd (81%) from the title compound of Preparation 3 and cyclopentylamine following the procedure described above. m.p.: >270°C δ lH NMR (DMSO): 8.02 (d, 2H), 7.18 (d, 2H), 6.69 (s, IH), 4.68 (s, 2H), 4.27 (m, IH), 3.60 (s, 3H), 3.45 (s, 3H), 2.03-1.60 (m, 8H). ^ - ESI/MS (m/e,%): 396 ^, 18).
EXAMPLE 63
7V-<4-Acerylphenyl)-2-{4-(l^-dimetlιyl-2,4-dioxo-2 3,4^-tetrahydro-lJH- pyrrolo[3^-</]pyrimidin-6-yl)phenoxy] acetamide Obtained as a brown sohd (24%) from the title compound of Preparation 3 and acetanilide following the procedure described above. m.p.: >300°C δ Η NMR (DMSO): 8.03 (d, 2H), 7.93 (d, 2H), 7.85 (d, 2H), 7.15 (d, 2H), M (s, IH), 4.89 (s, 2H), 3.49 (s, 3H), 3.33 (s, 3H), 2.60 (s, 3H).
ESI/MS (m/e,%): 446 ( T, 35).
EXAMPLE 64
N-(l^-Benzoimidazol-2-yI)-2-[4-(l^-dimetlιyl-2,4-dioxo-2^,4,5-tetrahydro-Lff- pyrrolo[3^-rf]pyrimidin-6-yl)phenoxy] acetamide
Obtained as a brown sohd (84%) from the title compound of Preparation 3.and 2-anιmobenzimidazole following the procedure described above. m.p.: >287°C (decomposition) δ 'H NMR (DMSO): 12.12 (bs, IH), 7.83 (d, 2H), 7.40 ( , 2H), 7.04 (m, 4H), 6.80 (bs, IH), 6.58 (s, IH), 6.04 (bs, IH), 4.88 (s, 2H), 3.38 (s, 3H), 3.22 (s, 3H).
EXAMPLE 65
N-(4-Cyanophenyl 2-[4^(l 3-dimethyI-2,4-dioxo-2y3,4^-tetrahydro-lJff- pyrrolo[3^-i/]pvrimidin-6-yl)phenoxy] acetamide Obtained as a brown sohd (13%) from the title compound of Preparation 3 and
4-aminobenzonitrile following the procedure described above. m.p.: 263-265°C δ lH NMR (DMSO): 12.18 (bs, IH), 10.50 (bs, IH), 7.79 (m, 6H), 7.05 ( , 2H), 6.60 (s, IH), 4.77 (s, 2H), 3.37 (s, 3H), 3.24 (s, 3H).
EXAMPLE 66
6^{4-[2-{3,4-D ydro-l-ϊ-qumolm-l-yI)-2-oxoe oxy]phenyI}-l^-dimethyl-l^- dihydropyrrolo{3^-</]pyrimidine-2,4- ione
Obtained as a yellow sohd (47%) from the title compound of Preparation 3 and 1,2,3,4-tetrahydroquώoline following the procedure described above. δ Η NMR (CDC13): 11.60 (s, IH), 7.62 (d, 2H), 7.10 ( , 4H), 6.78 (d, 2H), 5.20 (s, IH), 4.79 (s, 2H), 3.76 ( , 2H), 3.73 (s, 3H), 3.23 (s, 3H), 2.60 (m, 2H), 1.77 (m, 2H).
EXAMPLE 67
2-[4-(1 -DimethyI-2,4-dioxo-2^,4,5-tefrahydro^ y phenoxy]-/V-{l^,4]thiadiazoI-2-ylacetamide
Obtained as a brown sohd (29%) from the title compound of Preparation 3 and 2-amino-l,3,4-thiadiazole following the procedure described above. m.p.: >300°C (decomposition) δ Η NMR (DMSO): 9.23 (s, IH), 8.64 (s, IH), 7.93 (d, 2H), 7.26 (s, IH), 7.12 (d, 2H), 6.70 (s, IH), 5.03 (s, 2H), 3.49 (s, 3H), 3.33 (s, 3H).
EXAMPLE 68 l^-Dimethyl-6-{4-{2-oxo-2-(4-phenylpiperazin-l-yl)ethoxy]phenyl}-l^- dihydropyrrolo [32-d] pyrimidine-2,4-dioαe
Obtained as a white sohd (20%) from the title compound of Preparation 3 and 1- phenylpiperazine following the procedure described above. m.p.: >270°C (decomposition) δ Η NMR (DMSO): 11.25 (s, IH), 7.76 (d, 2H), 7.28 (m, 3H), 7.02 (d, 2H), 6.91 (m, 2H), 6.18 (s, IH), 4.80 (s, 2H), 3.78 (m, 4H), 3.53 (s, 3H), 3.49 (s, 3H), 3.47 (m, 4H).
EXAMPLE 69
2-{4^1^Dimethyl-2,4-dioxo-2^,4^tetrahydro-l^-pvrroIo{3^-<flpvrimidin-6- yl)phenoxy]-N-(4-nitrophenyl) acetamide
Obtained as a yellow sohd (15% overall) from the title compound of Preparation 1 and 4-nitroaniline following the procedure of example 1. m.p.: 228-230°C δ Η NMR (DMSO): 12.30 (bs, IH), 10.80 (bs, IH), 8.31 (d, 2H), 7.95 ( , 4H), 7.13 (d, 2H), 6.69 (s, IH), 4.91 (s, 2H), 3.51 (s, 3H), 3.31 (s, 3H).
EXAMPLE 70
6-(4-{2-[4-(4-Fluorophenyl)piperazm-l-yI]-2-oxoethoxy} pheny.)-l,3-dimethyl-l,5- dmydropyrroIo[3^2-</]pyrimidine-2,4-dione
Obtained as a white sohd (50%) from the title compound of Preparation 3 and 1- (4-fluorophenyl)piperazine following the general procedure described above. m.p.: >265°C (decomposition) δ lH NMR (DMSO): 12.30 (bs, IH), 7.84 (d, 2H), 7.04 (m, 6H), 6.63 (s, IH), 4.95 (s, 2H), 3.62 (m, 4H), 3.42 (s, 3H), 3.26 (s, 3H), 3.14 (m, 2H), 3.07 (m, 2H).
ESI/MS (m/e,%): 491 (M+, 100).
EXAMPLE 71
6^{4-{2-(4-Benzylpiperazm-l-yl)-2-oxoethoxy]phenyl}-l 3-dimetlιyl-l^- dmydropyrrolo [32-d] pyrimidine-2,4-dione
Obtained as an off-white sohd (40%) from the title compound of Preparation 3 and 1-benzylpiperazine following the general procedure described above. m.p.: 170-172°C δ Η NMR (DMSO): 12.05 (bs, IH), 7.64 (d, 2H), 7.14 (s, 5H), 6.78 (d, 2H), 6.38 (s, IH), 4.68 (s, 2H), 3.20 (m, 8H), 2.24 (m, 2H), 2.16 (m, 2H). ESI MS (m/e,%): 487 (M*, 100). •
EXAMPLE 72
6 4-{2-[4-(2-Methoχvphenyl)piperazin-l-yl]-2-oxoethoxy} phenyl)- 1,3-dimethyl-
Obtained as a brown sohd (83%) from the title compound of Preparation 3 and l-(2-methoxyphenyl)piperazine following the general procedure described above. m.p.: >295°C (decomposition) δ Η NMR (DMSO): 12.21 (bs, IH), 7.76 (m, 2H), 6.86 (m, 6H), 6.52 (s, IH), 4.88 (s, 2H), 3.76 (s, 3H), 3.58 (m, 4H), 3.38 (s, 3H), 3.22 (s, 3H), 2.49 (m, 4H). ESI^IS (m/e,%): 503 (MT, 100).
Ill
EXAMPLE 73
6-(4-{2-[4-(4-Methoxvphenyl)piperazin-l -yl]-2-oxoethoxy} phenyl)- 1 ,3-dimethyl- l^-dihydropyrrolo[3^2-< ]pyriιιιidme-2,4-dione Obtained as a white sohd (23%) from the title compound of Preparation 3 and 1-
(4-methoxyphenyl)piperazine following the general procedure described above. m.p.: 269-271°C δ Η NMR (DMSO): 12.38 (bs, IH), 7.96 (d, 2H), 7.09 (d, 2H), 7.05 (d, 2H), 6.96 (d, 2H), 6.74 (s, IH), 5.06 (s, 2H), 3.81 (s, 3H), 3.73 (m, 4H), 3.54 (s, 3H), 3.38 (s, 3H), 3.19 (m, 2H), 3.11 (m, 4H).
ESI/MS (m/e,%): 503 (M+, 100).
EXAMPLE 74 l^Dimethyl-6-(4-{2-oxo-2-{4-(3-triflαoromethylpheny piperazin-l- yl]ethoxy}phenyI)-l^-dihydropyrrolo[3^-</] pyrimidine-2,4-dione
Obtained as a white sohd (50%) from the title compound of Preparation 3 and 1- (3-trifluoromethylphenyl) piperazine following the general procedure described above. m.p.: >275°C (decomposition) δ Η NMR (DMSO): 7.77 (m, 2H), 7.40 (m, IH), 7.28 (m, 2H), 7.15 (d, IH), 6.94 (m, 3H), 4.88 (s, 2H), 4.65 (s, IH), 3.68 (s, 3H), 3.30 (m, 1 IH). ES MS (m/e,%): 541 (M*, 100).
EXAMPLE 75 l^-DimethyI-6-{4-[2-oxo-2-(4-pyridin-2-yl-piperazin-l-yl)ethoxy]phenyl}-l^- dmydropyrrolo[3,2-- ]pyrimidine-2,4-dione
Obtained as a white sohd (42%) from the title compound of Preparation 3 and 1- pyridm-2-ylpiperazine following the general procedure described above. m.p.: >260°C (decomposition) δ Η NMR (DMSO): 8.14 (d, IH), 7.84 (d, 2H), 7.57 (m, IH), 7.01 (d, 2H), 6.88
(m, IH), 6.68 (m, IH), 6.60 (s, IH), 4.91 (s, 2H), 3.60-3.26 (m, 14H). ESI MS (m/e,%): 474 (M*, 100).
EXAMPLE 76
1 ,3-Dimethy l-6-{4- [2-oxo-2-(4-pyrimidin-2-y Ipiper azin-1 -yl)ethoxy] phenyl}-l ,5- dihydrop yrrolo [32-d] pyriιnidine-2,4-dione
Obtained as a white sohd (60%) from the title compound of Preparation 3 and 2- piperazm-l-ylpyrimidine following the general procedure described above. m.p.: >275°C (decomposition) δ Η NMR (DMSO): 12.27 (bs, IH), 8.41 (d, 2H), 7.85 (d, 2H), 7.03 (d, IH), 6.69 (t, IH), 6.63 (s, IH), 4.96 (s, 2H), 3.83 (m, 2H), 3.76 (m, 2H), 3.57 (m, 4H), 3.43 (s, 3H), 3.27 (s, 3H).
EXAMPLES 77-86
The compounds of this invention were synthesized from the title compound of Preparation 4 following the procedure of example 5 and using the corresponding reactant respectively. The ESI/MS data, HPLC retention times and yields are summarised in the following table.
TABLE 16
EXAMPLES 87-89
The compounds of this invention were synthesized from the title compound of Preparation 5 following the procedure of example 5 and using the corresponding reactant respectively. The ESI/MS data, HPLC retention times and yields are summarised in the following table.
TABLE 17
EXAMPLES 90-107
The compounds of this invention were synthesized from the title compound of Preparation 6 following the procedure of example 5 and using the corresponding reactant respectively. The ESI/MS data, HPLC retention times and yields are summarised in the following table.
TABLE 18
EXAMPLES 108-116
The compounds of this invention were synthesized from the title compound of Preparation 7 following the procedure of example 5 and using the corresponding reactant respectively. The ESI/MS data, HPLC retention times and yields are summarised in the following table.
TABLE 19
EXAMPLE 117
N-Cyclopentyl-2-{4-[l-(3-methoxypropyl)-3-methyl-2,4-dioxo-2^,4^-tetrahydro- LE -pyrrolo[3,2-rf]pyrimidin-6-yl]phenoxy} acetamide
The compound of this invention was synthesized from the title compound of Preparation 8 and cyclopentylamine following the general procedure described for examples 55-76. m-p-OfeOH/HA: 234-236°C δ (DMSO): 7.83 (d, 2H), 6.99 (d, 2H), 6.56 (s, IH), 4.48 (s, 2H), 4.05 (m, IH), 3.93 (t, 2H), 3.55 (m, 2H), 3.39 (s, 3H), 3.37 (s, 3H), 1.92-1.07 ( , lOH).
EXAMPLE 118 2-{4-{l-<3- ethoxypropyl)-3-memyl-2,4-dioxo-2^,4^tetrahydro-liSr-pvrrolo{3^- < ]pyriniidin-6-yl]phenoxy}-N-plienylacetainide
The compound of this invention was synthesized from the title compound of
Preparation 8 and aniline following the general procedure described for examples 55-76. m.p.(MeOH/H- ): >251°C (dec.) δ lH NMR (CDC13): 7.71 (d, 2H), 7.60 (d, 2H), 7.38 (m, 3H), 7.15 (m, 2H), 6.34 (s, IH), 4.70 (s, 2H), 4.10 (m, 2H), 3.48 (m, 2H), 3.43 (s, 3H), 3.37 (s, 3H), 2.05 (m, 2H).
ESI/MS (m/e,%):'462 (M*, 100).
EXAMPLES 119-120
The compounds of this invention were synthesized from the title compound of Preparation 9 followmg the procedure of example 5 and using the corresponding reactant respectively. The ESI MS data, HPLC retention times and yields are summarised in the following table.
TABLE 20
EXAMPLES 121-123
The compounds of this invention were synthesized from the title compound of Preparation 10 following the procedure of example 5 and using the corresponding reactant respectively. The ESI MS data, HPLC retention times and yields are summarised in the following table.
TABLE 21
EXAMPLE 124 iV-(4-Bromophenyl)-2-[4-(2,4-dioxo-l-propyl-2 3,4^-tetrahydro-LB:-pyrrolo[3 2- rf]pyrimidin-6-yl)phenoxy] acetamide
Obtained as a white sohd (11%) from the title compound of Preparation 10 and 4-bromoaniline following" the procedure of Example 4. m.p.: 276-278 (dec.) δ Η NMR (DMSO): 12.00 (bs, IH), 10.60 (bs, IH), 10.22 (bs, IH), 7.86 (d, 2H), 7.60 (d, 2H), 7.40 (d, 2H), 7.09 (d, 2H), 6.60 (s, IH), 4.78 (s, 2H), 3.80 (t, 2H), 1.64 (m, 2H), 0.90 (t, 3H).
EXAMPLE 125 2-[4-(2,4-Dioxo-l-propyl-2^,4^tetrahydro-li -pvn-olo[3,2-<i]pyrimidin-6- yl)phenoxy]-iV-(4-fluorophenyI)acetamide
Obtained as a white sohd (56%) from the title compound of Preparation 10 and 4-fluoroaniline following the procedure of Example 4. m.p.: 306-308°C (dec.) δ Η NMR (DMSO): 12.20 (bs, IH), 10.78 (bs, IH), 10.15 (bs, IH), 7.85 (d,
2H), 7.65 (dd, 2H), 7.16 (t, 2H), 7.05 (d, 2H), 6.61 (s, IH), 4.73 (s, 2H), 3.78 (t, 2H), 1.64 (m, 2H), 0.90 (t, 3H).
EXAMPLE 126-130
The compounds of this invention were synthesized from the title compound of Preparation 11 following the procedure of example 5 and using the corresponding reactant respectively. The ESI/MS data, HPLC retention times and yields are summarised in the following table.
TABLE 22
EXAMPLES 131-135
The compounds of this invention were synthesized from the title compound of Preparation 12 following the procedure of example 5 and using the corresponding reactant respectively. The ESI MS data, HPLC retention times and yields are summarised in the following table. TABLE 23
EXAMPLE 136
2-[4-(7-Chloro-l^-dLmιemyl-2,4-dioxo-2^,4^tetrahydro-l^-pvrrolo[3^- rf]pyrimidin-6-yl)phenoxy]-/Y-{4-cyaπophenyl) acetamide Obtained as a white sohd (42%) from the title compound of Preparation 13 and
4-aminobenzonitrile following the procedure of example 5. ESI/MS m/e: 463 flM+HT, C^CINA). Retention Time (min.): 16.7
EXAMPLES 137-138
The compounds of this invention were synthesized from the title compound of Preparation 14 following the procedure of example 5 and using the corresponding reactant respectively. The ESI/MS data, HPLC retention times and yields are summarised in the following table. TABLE 24
EXAMPLES 139-149
The compounds of this invention were synthesized from the title compound of Preparation 15 following the procedure of example 5 and using the corresponding reactant respectively. The ESI/MS data, HPLC retention times and yields are summarised in the following table.
TABLE 25
EXAMPLES 150-158
The compounds of this invention were synthesized from the title compound of Preparation 16 following the procedure of example 5 and using the corresponding reactant respectively. The ESI/MS data, HPLC retention times and yields are summarised in the following table.
TABLE 26
EXAMPLES 159-168
The compounds of this invention were synthesized from the title compound of Preparation 17 following the procedure of example 5 and using the corresponding reactant respectively. The ESI/MS data, HPLC retention times and yields are summarised in the following table.
TABLE 27
EXAMPLES 169-174
The compounds of this invention were synthesized from the title compound of Preparation 18 following the procedure of example 5 and using the corresponding reactant respectively. The ESI/MS data, HPLC retention times and yields are summarised in the following table.
TABLE 28
EXAMPLES 175-179
The compounds of this invention were synthesized from the title compound of Preparation 19 following the procedure of example 5 and using the corresponding reactant respectively. The ESI/MS data, HPLC retention times and yields are summarised in the following table.
TABLE 29
EXAMPLES 180-184
The compounds of this invention were synthesized from the title compound of Preparation 20 following the procedure of example 5 and using the corresponding reactant respectively. The ESI/MS data, HPLC retention times and yields are summarised in the following table.
TABLE 30
EXAMPLES 185-189
The compounds of this invention were synthesized from the title compound of
Preparation 21 following the procedure of example 5 and using the corresponding reactant respectively. The ESI MS data, HPLC retention times and yields are summarised in the followmg table.
TABLE 31
EXAMPLES 190-192
The compounds of this invention were synthesized from the title compound of Preparation 22 following the procedure of example 5 and using the corresponding reactant respectively. The ESI MS data, HPLC retention times and yields are summarised in the following table.
TABLE 32
The compounds of this invention were synthesized from the title compound of Preparation 23 following the procedure of example 5 and using the corresponding reactant respectively. The ESI/MS data, HPLC retention times and yields are summarised in the following table.
TABLE 33
EXAMPLES 197-199
The compounds of this invention were synthesized from the title compound of Preparation 24 following the procedure of example 5 and using the corresponding reactant respectively. The ESI/MS data, HPLC retention times and yields are summarised in the following table.
TABLE 34
The compounds of this invention were synthesized from the title compound of Preparation 25 following the procedure of example 5 and using the corresponding reactant respectively. The ESI/MS data, HPLC retention times and yields are summarised in the following table.
TABLE 35
EXAMPLES 205-209
The compounds of this invention were synthesized from the title compound of Preparation 26 following the procedure of example 5 and using the corresponding reactant respectively. The ESI/MS data, HPLC retention times and yields are summarised in the following table.
TABLE 36
EXAMPLE 210
6-[4-(3-Phenyl[l^,4]oxadiazol-5-ylmethoxy)plιenyl]-l 3-dipropyl-l^- dftydropvrrolo[3,2-<-lpyrimidine-2,4-dione a) To a mixture of the title compound of Preparation 2 (400 mg, 1.03 mmol), N- (3-dimethylammopropyl)-N'-ethyl-carbodiimide hydrochloride (237 mg, 1.24 mmol) and 1 -hydroxybenzotriazole (167 mg, 1.24 mmol) in dimethylformamide (15 mL) was added triethylamine (288 μL, 2.06 mmol) and N-hydroxybenzamidine (168 mg, 1.24 mmol). The mixture was stirred at room temperature overnight.
The solvent was evaporated under reduced pressure and the residue was partitioned between dichloromethane and a 1 M aqueous solution of citric acid. The organic phase was separated, washed with a saturated aqueous solution of sodium bicarbonate, dried (Νa^SO and evaporated under reduced pressure. The residue was triturated with ethyl ether and the precipitate was collected by filtration to yield the title compound as a yellow sohd (144 mg, 28%). b) A stirred solution of the above compound (140 mg, 0.277 mmol) in toluene (50 mL) was refluxed using a Dean-Stark apparatus for 20 hours. The solvent was evaporated under reduced pressure, the residue was triturated with ethyl ether and the precipitate was collected by filtration to yield the title compound as a yellow sohd (90 mg, 67%). δ Η ΝMR (CDC13): 10.3 (bs, IH), 8.1 (m, 2H), 7.7 (d, 2H), 7.5 (d, 2H), 7.2 (d, IH), 7.1 (d, IH), 6.2 (s, IH), 5.4 (s, 2H), 4.0 (m, 4H), 1.7 (m, 4H), 0.9 (dt, 6H). ESI/MS (m/e,%): 486 (M*, 100). Retention Time (min.): 11.4
EXAMPLE 211
6-{4-[2-oxo-2-{ [amino(4-fluorophenyl)methylenediamino]-oxy}ethoxy] phenyl}-l ,3- dipropyl-l,5-dihydropyπOlo[3-2-d]pyriιnidine-2,4-dione
Obtained as a white sohd (88%) from the title compound of Preparation 2 and 4- fluoro-N-hydroxybenzamidine following the procedure a) of Example 210. δ 'H ΝMR (DMSO): 12.2 (s, IH), 7.8 (dd, 4H), 7.3 (m, 2H), 7.0 (d, 4H), 6.6 (s, IH), 5.0 (s, 2H), 3.8 (m, 4H), 1.6 (m, 4H), 0.9 (m, 6H).
ESI/MS (m/e,%): 522 (M*, 100).
Retention Time (min.): 10.1
EXAMPLE 212
6-{4-[3-(4-Fluorophenyl)[l^,4]oxadiazoI-5-ylmethoxy] phenyl}-l-3-dipropyl-l,5- dihydropyrrolo[3^-d]pyrimidine-2,4-dione
Obtained as a white sohd (83%) from the title compound of Example 211 following the procedure b) of Example 210. δ Η ΝMR (DMSO): 12.2 (s, IH), 8.1 (dd, 2H), 7.9 (d, 2H), 7.4 (t, 2H), 7.1 (d, 2H), 6.7 (s, IH), 5.6 (s, 2H), 3.8 (m, 4H), 1.6 ( , 4H), 0.9 (dt, 6H).
ESI MS (m/e,%): 504 (M*, 100).
Retention Time (min.): 11.4
EXAMPLE 213
1 -Dipropyl-6^[4-(3-pyridin-4-yl[l^,4]oxadiazol-5-ylmethoxy)phenyl]-l^- dihydr opyrrolo [32-d] pyrimidine-2,4-dione
Obtained as a white sohd (87%) from the title compound of Preparation 2 and N- hydroxyisorhcotinamidine following the same procedure of Example 210. δ lH ΝMR (DMSO): 12.2 (bs, IH), 8.8 (d, IH), 8.7 (d, IH), 7.9 ( , 3H), 7.7 (d, IH), 7.2 (d, IH), 7.1 (d, IH), 6.7 (s, IH), 5.7 (s, 2H), 3.9 (m, 4H), 1.6 (m, 4H), 0.9 (dt, 6H).
ESI MS (m/e,%): 487 (M*, 100).
Retention Time (min.): 10.4
EXAMPLE 214
6-[4-(BenzooxazoI-2-yhnethoxy)phenyl]-1 -<iipropyl-l^-dihydropyrrolo[3^- <f]pyrimidine-2,4-dione
A'stirred solution of the title compound of Example 51 (134 mg, 0.28 ,mmol) and -toluensulfonic acid (48 mg, 0.28 mmol) in toluene (10 mL) was refluxed using a Dean-Stark apparatus for 5 hours. The solvent was evaporated under reduced pressure, the residue was partitioned between dichlorometane and a saturated aqueous solution of sodium bicarbonate. The organic phase was separated, washed with brine, dried
(MgSOJ and evaporated under reduced pressure. The residue was triturated with ethyl ether and the precipitate was collected by filtration to yield the title compound as a white sohd (82 mg, 64%}. δ Η NMR (CDC13): 10.9 (s, IH), 7.7 (m, 3H), 7.5 (m, IH), 7.3 (dd, 2H), 7.1 (d, 2H), 6.1 (s, IH), 5.3 (s, 2H), 3.9 (m, 4H), 1.7 (dq, 4H), 0.9 (dt, 6H).
ESI MS (m e,%): 459 (M*, 100).
Retention Time (min.): 10.9
EXAMPLE 215 6-{.4-<5-Phenyl-4^-dihydrooxazol-2-ylmethoxy)phenyl]-l^-dipropyH,5- dmydropyrτolo[3^-d]pyrimidme-2,4-dione
A solution of the title compound of Example 43 (60 mg, 0.119 mmol) in thionyl chloride (173 μL) was stirred at room temperature for 1 hour. The resulting solution was poured into water and a yellow sohd precipitated. A suspension of the above sohd in water was treated with a 2 N aqueous solution of sodium hydroxide until alkaline pH. The sohd was collected by filtration and dried to yield the title compund as a yellow sohd (35 mg, 60%).
ESI MS (m/e,%): 487 (M*. 100).
Retention Time (min.): 10.7
EXAMPLE 216
6-[4-(4-iVfethyl-5-phenyl-4^-dihydrooxazol-2-ylmethoxy>-phenyl]-l 3-dipropyI-l^- dihydropyrrolo[3^-d]pyrimidine-2,4-dione Obtained as a yellow sohd (45%) from the title compound of Example 44 following procedure of Example 215. ESI/MS (m/e,%): 501 (NT, 100). Retention Time (min.): 11.0
EXAMPLE 217
6-[4-(7-Ben--yl-l-oxa-3,7-diazaspiro[4.5]dec-2-en-2-ylmethoxy)phenyl]-l - dipropyl-l,5-dihydropyιτolo{3^-d]pyr-ιιιidine-2,4-dione
Obtained as a white sohd (33%) from the title compound of Example 46 following the procedure described in Example 215. ESI MS (m/e,%): 570 (M*. 100).
Retention Time (min.): 7.3
EXAMPLE 218 l^-Dipropyl-6-[4-{quinolin-2-ylmethoxy)phenyI]-l^-dihydropyrrolo[3^- d]pyrimidine-2,4-dione a) A mixture of -hydroxybenzaldehyde (17.02 g, 0.139 mmol), 2- chloromethylquinoline (24.76 g, 0.139 mmol), potassium carbonate (57.64 g, 0.417 mmol) and potassium iodide (2.17 g, 0.013 mmol) in methyl isobutyl ketone (515 mL) was refluxed for 20 h. After cooling to room temperature, the inorganic salts were filtered and the solvent was evaporated under reduced pressure. The residue was partitioned between dichlorometane and water, the aqueous phase extracted with dichloromethane and the organic phase washed with water and brine, dried (MgSO and evaporated under reduced pressure. The residue was triturated with ethyl ether and the precipitate was collected by filtration to yield the 4-(quinolin-2-
ylmethoxy)benzaldehyde as a yellow sohd (25.62 g, 70%). m.p.: 70.0-72.0°C b) The title compound was obtained as a yellow sohd (560 mg, 61%) from 6- memyl-5-nifro-l,3-<hpropyl-lHpyrirmdine-2,4-dione (1.0- g, 3.92 mmol) and 4- (qumolin-2-ylmethoxy)benzaldehyde (1.13 g, 4.31 mmol) following the same procedure described in Preparation 2. δ Η NMR (CDC13): 10.5 (s, IE), 8.3 (d, 2Η), 7.7 (m, 6H), 7.1 (d, 2H), 6.2 (s, IH), 5.5 (s, 2H), 3.9 (m, 4H), 1.7 (m, 4H), 0.9 (dt, 6H).
ESLMS (m e,%): 469 (M+, 100).
Retention Time (min.): 11.3
EXAMPLES 219-226
The compounds of this invention were synthesized from the title compound of Preparation 2 following the procedure of example 5 and using the corresponding reactant respectively. The ESLMS data, HPLC retention times and yields are summarised in the following table.
TABLE 37
EXAMPLES 227-229
The compounds of this invention were synthesized from the title compound of Preparation 2 following the procedure of example 5a and using the corresponding reactant respectively. The ESI/MS data and yields are summarised in the following table.
TABLE 38
(Example 227) δ Η NMR (DMSO): 12.33 (bs, IH), 11.05 (bs, IH), 9.41 (s, IH), 8.52 (m, 2H), 7.97 (d, 2H), 7.14 (d, 2H), 6.16 (s, IH), 5.01 (s, 2H), 3.95 (m, 4H), 1.70 (m, 4H), 1.00 (m, 6H).
(Example 228) δ Η NMR (DMSO): 12.43 (bs, IH), 11.04 (bs, IH), 8.06 (d, 2H), 7.20 (m, 3H), 6.85 (bs, IH), 5.08 (s, 2H), 4.08 (m, 10H), 1.80 (m, 4H), 1.07 (m, 6H). (Example 229) δ Η NMR (DMSO): 12.27 (bs, IH), 8.97 (d, IH), 8.11 (d, 2H), 7.03 (d, 2H), 6.67 (s, IH), 6.03 (d, IH), 5.84 (s, 2H), 5.44 (s, 2H), 3.90 (m, 4H), 1.60 (m, 4H), 0.90 (m, 6H).
EXAMPLES 230-239
The compounds of this invention were synthesized from the title compound of Preparation 4 following the procedure of example 5 and using the corresponding reactant respectively. The ES MS data, HPLC retention times and yields are summarised in the following table.
TABLE 39
EXAMPLES 240-242
The compounds of this invention were synthesized from the title compound of Preparation 3 following the procedure of example 55 and using the corresponding reactant respectively. The ESI/MS data, HPLC retention times and yields are
summarised in the following table.
TABLE 40
(Example 240) δ Η NMR (DMSO): 12.29 (bs, IH), 8.13 (dd, 2H), 7.85 (d, 2H), 7.40 (m, 2H), 7.00 (d, 2H), 6.65 (s, IH), 4.92 (d, 2H), 4.37 (d, IH), 3.92 (d, IH), 3.76 (m, IH), 3.47 (m, IH), 3.43 (s, 3H), 3.27 (s, 3H), 2.82 (m, IH), 1.84 (m, 2H), 1.61 (m, IH), 1.41 (m, IH).
(Example 241) δ Η NMR (DMSO): 12.30 (bs, IH), 8.79 (m, IH), 8.50 (m, IH), 7.89 (d, 2H), 7.25 (d, 2H), 7.01 (d, 2H), 6.66 (d, IH), 4.68 (s, 2H), 4.38 (d, 2H), 3.43 (s, 3H), 3.27 (s, 3H).
(Example 242) δ lH NMR (DMSO): 12.32 (bs, IH), 7.89 (d, 2H), 7.05 (d, 2H), 6.66 (s, IH), 4.96 (s, 2H), 4.10 (m, 2H), 3.40 (m, 14H), 1.25 (s, 3H).
EXAMPLES 243-246
The compounds of this invention were synthesized from the title compound of Preparation 6 followmg the procedure of example 5 and using the corresponding reactant respectively. The ESLMS data, HPLC retention times and yields are summarised in the following table.
TABLE 41
EXAMPLES 247-253
The compounds of this invention were synthesized from the title compound of Preparation X following the procedure of example 5 and using the corresponding reactant respectively. The ESI/MS data, HPLC retention times and yields are summarised in the following table.
TABLE 42
(Example 252) δ Η NMR (DMSO): 12.15 (bs, IH), 11.18 (bs, IH), 10.27 (bs, IH), 7.91 (d, 2H), 7.76 (m, 2H), 7.54 (m, 4H), 6.29 (s, IH), 4.83 (s, 2H), 3.91 (m, 2H), 1.66 (m, 2H), 0.95 (t, 3H).
(Example 253) δ Η NMR (DMSO): 12.03 (bs, IH), 11.20 (bs, IH), 10.25 (bs, IH), 7.81 (d, 2H), 7.61 (d, 2H), 7.50 (d, 2H), 7.02 (d, 2H), 6.19 (s, IH), 4.74 (s, 2H), 3.80 (m, 2H), 1.55 (m, 2H), 0.86 (t, 3H).
EXAMPLE 254 6^{4-[2-Oxo-2-(4-phenylpipera-αn-l-yI)-ethoxy]phenyl}-l-propyI-l,5- dihydr opyrrolo [3,2-d] pyrimid ine-2,4-dione
Obtained as a white sohd (2%) from the title compound of Preparation 10 and 1- phenyl piperazine followmg the procedure of example 5. m.p.(MeOH/H2O): 280-282°C δ 'H NMR (DMSO): 12.19 (s, IH), 10.78 (s, IH), 7.81 (d, 2H), 7.22 ( , 2H), 6.96 (m, 4H), 6.80 (t, IH), 6.60 (s, IH), 4.92 (s, 2H), 3.78 (m, 2H), 3.60 (m, 4H), 3.15 (m, 4H), 1.66 (m, 2H), 0.90 (t, 3H).
ESLMS (m/e,%): 487 (M+, 33).
EXAMPLES 255-257
The compounds of this invention were synthesized from the title compound of Preparation 28 following the procedure of example 5 and using the corresponding reactant respectively. The ES MS data, HPLC retention times and yields are summarised in the following table.
TABLE 43
EXAMPLE 258
Pyrazin-2-yI-carbamic acid 4-(2,4-dioxo-l J-dipropyl-23,4^5-tetrahydro-lH-
in-6-yl)benzyl ester a)To a solution of triphosgene (87 mg, 0.29 mmol) in anhydrous dioxane (5 mL) under argon atmosphere was slowly added at room temperature a solution of 2- an_rinopyrazine (84 mg, 0.89 mmol) and triethylamine (0.24 mL, 1.76 mmol) in dioxane (5 mL). The mixture was stirred at room temperature for 1 hour. b)Then the title compound of Preparation 30 was added to the above reaction mixture (100 mg, 0.29 mmol) and the solution was stirred 48 hours at room temperature. The mixture was evaporated under reduced pressure and the residue was partitioned between dichloromethane and a saturated aqueous solution of sodium bicarbonate. The organic phase was separated, washed with water and brine, dried (Na
jSO and evaporated under reduced pressure. The resulting crude was purified by flash column chromatography on silica-gel (dichloromethane/MeOH 95:5) to yield the title compound as a white sohd (25 mg, 19%). m.p.(MeOH): 267-270°C δ Η NMR (DMSO): 12.56 (s, IH), 10.83 (s, IH), 9.27 (s, IH), 8.50 (m, 2H), 8.11 (d, 2H), 7.66 (d, 2H), 6.95 (s, IH), 5.40 (s, 2H), 4.02 (m, 4H), 1.75 (m, 4H), 1.05
(m, 6H).
EXAMPLE 259
(2,6-Dimethoxy-pyrimidin-4-yl)-carbamic acid 4-(2,4-dioxo-13-dipropyl-2,3,4,5- tetrahydro-lH-pyrrolo[3^-d]pyrimidin-6-yl)benzyl ester
Obtained as a white sohd (20%) from the title compound of Preparation 30 and 4-ammo-2,6-dimemoxypyrirnidine following the procedure of example 258. m.p.(MeOH): 182-185°C δ Η NMR (DMSO): 12.5 (bs, IH), 10.73 (s, IH), 8.04 (d, 2H), 7.57 (d, 2H),
6.94 (s, IH), 6.89 (s, IH), 5.30 (s, 2H), 3.96 (m, 4H), 1.73 (m, 4H), 0.98 (m, 6H).
ESI/MS (m/e,%): 523; 342 (100).
EXAMPLE 260 Pyridin-4-ylmethyl carbamic acid 4-(2,4-dioxo-l,3-dipropyl-2,3,4,5-tetralιydro-lH- pyrrolo[3,2-d]p yrimidin-6-yI)benzyl ester
To a solution of l.l'-carbonyldiimidazole (48 mg, 0.29 mmol) in pyridine (0.5 mL) under argon atmosphere was slowly added at 0°C a solution of the title compound of Preparation 30 (100 mg, 0.29 mmol) in pyridine (1 mL). The mixture was stirred at room temperature for for 1 hour. Then the title compound of Preparation 30 was added (100 mg, 0.29 mmol) and the mixture was stirred 2 hours at 0°C and 2 hours at room temperature. To the reaction mixture was slowly added 1-phenylpyperacine (162 mg, 0.29 mmol) and the mixture was stirred at room temperature overnight. The resulting solution was cooled to 4°C and the precipitate was collected by filtration to yield the title compound as a white sohd (51 mg, 33%). m.p.(MeOH): 240-242°C δ :H NMR (DMSO): 12.36 (bs, IH), 7.90 (d, 2H), 7.42 (d, 2H), 7.03 (m.2H), 6.95 (m,
2H), 6.73 (s, IH), 5.11 (s, 2H), 3.85 (m, 4H), 3.53 (m, 4H), 3.04 (m, 4H), 1.67 (m, 2H), 1.56 (m, 2H), 0.88 (m, 6H).
EXAMPLE 261 4-(3-Chlorophenyl)piperazine-l-carboxylic acid 4-(2,4-dioxo-l J-dipropyI-23,4,5- terrahydro-lH-pyrrolo[3,2-d]pyrimidiιι-6'-yl)beπzyl ester
Obtained as a white sohd (15%) from the title compound of Preparation 30 and l-(3-Chloro phenyl) piperazine following the procedure of example 260. - m.p.(MeOH): 188-190°C δ Η NMR (DMSO): 12.36 (s, IH), 7.91 (d, 2H), 7.42 (d, 2H), 7.21 (m, IH), 6.88 (m, 2H), 6.74 (m, 2H), 5.11 (s, 2H), 3.86 (m, 4H), 3.18 (m, 4H), 1.40 (m, 4H), 0.88 (m, 6H).
ESLMS (m/e,%): 476; 324 (100).
EXAMPLE 262
(lH-Pvrazol-3-yl)carbamic acid 4-(2,4-dioxo-13- iipropy--23,4,5-tetrab.ydro-lH- pvrrolo[3-2-d] pvrimidm-6-yl)benzyI ester Obtained as a white sohd (60%) from the title compound of Preparation 30 and lH-pyrazol-3-ylamine following the procedure of example 260. m.p.(MeOH): 210-213°C δ 'H NMR (DMSO): 12.39 (bs, IH), 7.93 (m, 4H), 7.49 (d, 2H), 6.76 (s, IH), 5.85 (s, IH), 5.51 (s, IH), 5.33 (s, 2H), 3.86 (m, 4H), 1.75 (m, 4H), 0.88 (m, 6H).
EXAMPLE 263
4-(3-Trifluoromethylphenyl)piperazine-l-carboxylic acid 4-(2,4-dioxo-13- dipropyl-2354^tetrahydro-lH-pyrroIo{3^-d]pyrimidm-6-yl)benzyl ester
Obtained as a white sohd (42%) from the title compound of Preparation 30 and l-(3-trifluoro methylphenyl)piperazine following the procedure of example 260. m.p.(MeOH): 232-233°C δ Η NMR (DMSO): 12.38 (s, IH), 7.91 (m, 2H), 7.43 (m, 3H), 7.20 (m, 2H), 7.08 (m, IH), 6.75 (s, IH), 5.11 (s, 2H), 3.86 (m, 4H), 3.55 (m, 4H), 3.23 (m, 4H), 1.60 (m, 4H), 0.88 (m, 6H):
EXAMPLE 264
Isoxazol-3-yl-carbamic acid 4-(2,4-dioxo-13-dipropyl-23,4,5-tetrahydro-lH- pvrrolo[3,2-d]pyriιni dm-6-yi)benzyl ester
Obtained as a white sohd (41%) from the title compound of Preparation 30 and isoxazol-3-ylamine following the procedure of example 260. m.p.(MeOH): 168-171°C δ lH NMR (DMSO): 12.40 (bs, IH), 8.30 (m, IH), 7.95 (d, 2H), 7.62 (s, IH),
7.55 (d, 2H), 7.07 (s, IH), 6.76 (s, IH), 5.45 (s, 2H), 3.86 (m, 4H), 1.60 (m, 4H), 0.88 (m, 6H).
EXAMPLE 265-272 The compounds of this invention were synthesized from the title compound of
Preparation 29 following the procedure of example 258 and using the corresponding reactant respectively. The ESLMS data, melting points and yields are summarised in the following table.
TABLE 44
(Example 265) δ Η NMR (DMSO): 12.52 (bs, IH), 9.91 (s, IH), 8.00 (d, 2H), 7.55 (m, 2H), 7.20 (m, 2H), 6.83 (s, IH), 5.24 (s, 2H), 3.50 (s, 3H), 3.33 (s, 3H).
(Example 266) δ lH NMR (DMSO): 12.53 (bs, IH), 7.91 (d, 2H), 7.41 (d, 2H), 7.30 (m, 5H), 6.75 (s, IH), 5.07 (s, 2H), 4.21 (d, 2H), 3.42 (s, 3H), 3.26 (s, 3H).
(Example 267) δ 'H NMR (DMSO): 12.50 (bs, IH), 9.85 (s, IH), 8.00 (d, 2H), 7.53 (m, 4H), 7.33 ( , 2H), 7.05 (m, IH), 6.81 (s, IH), 5.23 (s, 2H), 3.49 (s, 3H), 3.32 (s, 3H).
EXAMPLE 273-274
The compounds of this invention were synthesized from the title compound of Preparation 30 following the procedure of example 260 and using the corresponding reactant respectively. The ESI/MS data and yields are summarised in the following table.
TABLE 45
EXAMPLE 275
Thiophen-2-yl-carbamic acid 2-{4-(2,4-dioxo-l 3-dipropyl-23,4-5-tetraliydro-lH- pyrroIo[3^-d]pyrimidm-6-yl)phenoxy]ethyl ester a) From the title compound of Preparation 2 following the procedure of Preparation 29c, 6-[4-(2-hyo^oxyethoxy)phenyl]-l,3-dipropyl-l,5-dihydropyrrolo[3 2- d]pyrimidine-2,4-dione was obtained (70%) as a white sohd. δ Η NMR (DMSO): 12.04 (bs, IH), 7.68 (d, 2H), 6.82 (d, 2H), 6.47 (d, IH), 4.71 (t, IH), 3.86 (m, 2H), 3.68 (m, 4H), 3.54 (m, 2H), 1.45 (m, 4H), 0.72 (m, 6H). b) The title compound was obtained as a white sohd (60%) from the above compound and 2-isocyanatothiophene following the procedure of example 258. : m-p.tMeOH/EtjO): 223-225°C δ lH NMR (DMSO): 12.29 (bs, IH), 10.86 (bs, IH), 7.94 (d, 2H), 7.10 (d, 2H), 6.99 (dd, IH), 6.87 (dd, IH), 6.73 (s, IH), 6.63 (dd, IH), 4.52 (m, 2H), 4.35 (m, 2H), 3.93 (m, 4H), 1.69 (m, 4H), 0.95 (m, 6H).
EXAMPLE 276
(4-Bromophenyl)-carbamic acid 2-[4-(2,4-dioxo-13-dipropyl-23j4 -tetrahydro- lH-pyrrolo[3^-d]pyrimidm-6-y plιenoxy]ethyl ester
Obtained as a brownish sohd (23%) from the title compound of Preparation 2 and 4-bromo phenylisocyanate following the procedure of example"275. '
m.p.(MeOH): 281°C (dec.) δ Η NMR (DMSO): 12.23 (bs, IH), 9.99 (s, IH), 7.88 (d, 2H), 7.46 (m, 4H), 7.04 (d, 2H), 6.66 (s, IH), 4.44 (m, 2H), 4.30 (m, 2H), 3.86 (m, 4H), 1.62 (m, 4H), 0.90 (m, 6H).
EXAMPLE 277 l-[l-(2,6-Difluoro-phenyl)methanoyl]-3-{4-(2,4-dioxo-13-dipropyl-23,4^- tetrahydro-lH-pyrrolo[3^-<i]pyrimidin-6-yl)benzyl]urea a) To a suspension of the title compound of Preparation 30 (0.48 g, 1.40 mmol) in dichloromethane (45 mL) was added methanesulfonyl chloride (545 μL, 7.04 mmol) and triethyl amine (981 μL, 7.04 mmol) and the mixture was stirred at room temperature for 5 hours. The solvent was evaporated under reduced pressure, the residue was triturated with dichloromethane and the resulting sohd was filtered, washed with dichloromethane and dried to yield methanesulfonic acid 4-(2,4-dioxo-l,3-dipropyl- 2,3,4,5-tetrahyα^-lH-pyrrolo[3,2- ]ρyrimidm-6-yl)benzyl ester (0.22 g, 37%) as a yellow sohd. b) To a suspension of the above compound (0.22g, 0.52 mmol) in dimethylformamide (5.5 mL) under argon atmosphere, was added sodium azide (68 mg, 1.05 mmol) and the mixture was heated at 40 °C for 4 hours. The solvent was evaporated under reduced pressure, the residue was triturated with water and the resulting sohd was filtrated, washed with water and diethyl ether and dried to yield 6-(4- azidomethylphenyl)-l,3-dipropyl-l,5-d ychopyro (0.15 g, 79%) as a yellow sohd. c) To a suspension of the above compound (0.15 g, 0.41 mmol) in tetrahydrofuran (2 mL) at 0 °C, was added a solution of 1 M trimethyl phosphine in toluene (656 μL, 0.65 mmol) and the resulting solution was stirred at room temperature for 5 hours. Water (22 μL, 1.23 mmol) was added and the solution was stirred at room temperature for 18 hours. The solvent was evaporated under reduced pressure, the residue was triturated with dichloromethane and the resulting sohd was filtrated, washed
with dichloromethane and dried to yield 6-(4-aminomethylphenyl)-l,3-dipropy 1-1,5- d ydropyrrolo[3^- i]pyrimidine-2,4-dione (96 mg, 69%) as a yellow sohd. d) To a solution of the above compound (25 mg, 0.07 mmol) in dimethylformamide (1 mL) was added 2,6-difluorobenzoyl isocyanate (20 mg, 0.088 mmol) and the mixture was stirred at room temperature for 4 hours. Tris-{2- aminoethyl)amine polystyrene (0.12 g, 0.44 mmol) was added and the mixture was stirred for 1 hour. After filtration, the solvent was evaporated under reduced pressure, the residue was triturated with a mixture of diethyl ether and dichloromethane and the resulting sohd was filtrated, washed with diethyl ether and dried to yield the title compound (53%) as a yellow sohd. ESLMS m/e: 524 ([M+Hf, C* H,, F2 Ns O4). Retention Time (min.): 10.1
EXAMPLE 278-279 The compounds of this invention were synthesized from the title compound of
Preparation 2 following the procedure of example 214 and using the corresponding reactant respectively. The ESLMS data, HPLC retention times and yields are summarised in the following table.
TABLE 46
13-Dimethyl-6^[4-(quinolm-2-ylmethoxy)phenyl]-l^-dihydropyrroIo[3^- d]pvrimidine-2,4-dione
Obtained as a white sohd (50%) from 6-memyl-5-mfro-l,3-o^ethyl-lH-pyrimidine- 2,4-dione and 4-(quinolin-2-ylmethoxy)benzaldehyde following the procedure of example 218.
ESLMS m/e: 413 ([M+Hf, C24 H20 N4 O3). Retention Time (min.): 9.7
EXAMPLE 281
13-Dimethyl-6-[4-(3-phenyl[l^,4]oxadiazol-5-ylmethoxy)phenyl]-l^- dmydropyrrolo[3-2-d]pyriιnidine-2,4-dione
Obtained as a white solidr(60%) from the title compound of Preparation 4 and N- hydroxybenzamidine following the procedure of example 210. ESLMS m/e: 430 ([M+Hf, C^ H19 Νs O4). Retention Time (min.): 10.0
EXAMPLE 282-284
The compounds of this invention were synthesized from the title compound of Preparation 6 following the procedure of example 210 and using the corresponding reactant respectively. The ESLMS data, HPLC retention times and yields are summarised in the following table.
TABLE 47
EXAMPLE 285
6-{4-[3-{4-BromophenyI)[l^,4]oxadiazol-5-ylmethoxy]-phenyl}-3-propyl-l^- dihy dr opyrrolo [3,2-d]p vrimidine-2,4-dione
Obtained as a white sohd (30%) from the title compound of Preparation 27 and 4- bromo-N-hydroxybenzamidine following the procedure of example 210. δ Η ΝMR (DMSO): 12.13 (bs, IH), 11.16 (bs, IH), 7.96 (d, 2H), 7.85 (d, 4H), 7.15 (d, 2H), 6.24 (s, IH), 5.67 (s, 2H), 3.83 (m, 2H), 1.58 (m, 2H), 0.88 (m, 3H).
EXAMPLE 286-289
The compounds of this invention were synthesized from the title compound of Preparation 19 following the procedure of example 210 and using the corresponding reactant respectively. The ESI/MS data, HPLC retention times and yields are summarised in the following table.
TABLE 48
6^{4-[(4-Bromophenylamino)methyl]phenyl}-13-dipropyl-l^-dihydropyrrolo[3^- d] pyrimidine-2,4-dione a) To a solution of the title compound of Preparation 30 (200mg, 0.59 mmol) in DMF (5 mL) was added CBr4 (480 mg, 1.02 mmol) and the mixture was cooled to 0°C.
Then a solution of triphenyl phosphine (270 mg, 1.02 mmol) in DMF (2 mL) was added and the mixture was stirred at room temperature for 14 hours. The precipitate was collected by filtration and used in the next step without further purification. b) To a solution of 4-bromoanihne (43 mg, 0.25 mmol) in ethanol (2 mL) was added K^COj (34 mg, 0.025 mmol) and the above bromide (20 mg, 0.05 mmol). The mixture was refluxed for 1 hour. The solvent was evaporated under reduced pressure, the residue was suspended in chloroform, the organic phase was washed with water, dried (Na-.SO4) and evaporated. Flash column chromatography (chloroform.-petroleum ether 9:1) provided the title compound as a brown sohd (11 mg, 44%). m.p.(MeOH): >250°C δ Η NMR (DMSO): 10.7 (bs, IH), 7.72 (d, 2H), 7.42 (d, 2H), 7.24 (d, 2H), 6.50 (d, 2H), 6.24 (s, IH), 4.36 (s, 2H), 3.95 (m, 4H), 1.75 (m, 4H), 0.95 (m, 6H).
EXAMPLE 291
6 4-Phenylaminomethylphenyl)-13-<lipropyl-1 -dihydropyrrolo[3,2- d]pyrimidine-2,4-dione.
Obtained as a brownish sohd (64%) from the title compound of Preparation 30 and aniline following the procedure of example 290. m.p.(MeOH): 201°C δ Η NMR (DMSO): 10.51 (bs, IH), 7.70 ( , IH), 7.46 (m, IH), 7.24 (m, 4H), 6.75 (m, 3H), 6.24 (s, IH), 4.39 (s, 2H), 3.96 (m, 4H), 1.70 (m, 4H), 1.00 (m, 6H).
The following examples illustrate pharmaceutical compositions according to the present invention and procedures for their preparation.
COMPOSITION EXAMPLE 1 50,000 capsules each containing 100 mg of active ingredient were prepared according to the following formulation:
Active ingredient 5 Kg
Lactose monohydrate 10 Kg
Colloidal silicone dioxide 0.1 Kg Com starch 1 Kg
Magnesium stearate 0.2 Kg
Procedure
The above ingredients were sieved through a 60 mesh sieve, and were loaded into a suitable mixer and filled into 50,000 gelatine capsules.
COMPOSITION EXAMPLE 2
50,000 Tablets each containing 50 mg of active ingredient were prepared from the following formulation:
Active ingredient 2.5 Kg
Microcrystalline cellulose 1.95 Kg
Spray dried lactose 9.95 Kg
Carboxymethyl starch 0.4 Kg Sodium stearyl fumarate 0.1 Kg
Colloidal silicon dioxide 0.1 Kg
Procedure
All the powders were passed through a screen with an aperture of 0.6 mm, then mixed in a suitable mixer for 20 minutes and compressed into 300 mg tablets using 9 mm disc and flat bevelled punches. The disintegration time of the tablets was about 3 minutes.