US20070232622A1 - Small molecule toll-like receptor (TLR) antagonists - Google Patents
Small molecule toll-like receptor (TLR) antagonists Download PDFInfo
- Publication number
- US20070232622A1 US20070232622A1 US11/543,314 US54331406A US2007232622A1 US 20070232622 A1 US20070232622 A1 US 20070232622A1 US 54331406 A US54331406 A US 54331406A US 2007232622 A1 US2007232622 A1 US 2007232622A1
- Authority
- US
- United States
- Prior art keywords
- tlr
- group
- subject
- signaling
- compounds
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Abandoned
Links
- 102000002689 Toll-like receptor Human genes 0.000 title claims abstract description 375
- 108020000411 Toll-like receptor Proteins 0.000 title claims abstract description 375
- 150000003384 small molecules Chemical class 0.000 title abstract description 62
- 239000005557 antagonist Substances 0.000 title description 13
- 238000000034 method Methods 0.000 claims abstract description 212
- 150000001875 compounds Chemical class 0.000 claims abstract description 192
- 239000000203 mixture Substances 0.000 claims abstract description 146
- 230000011664 signaling Effects 0.000 claims abstract description 116
- 230000002401 inhibitory effect Effects 0.000 claims abstract description 65
- 102000008235 Toll-Like Receptor 9 Human genes 0.000 claims abstract description 46
- 108010060818 Toll-Like Receptor 9 Proteins 0.000 claims abstract description 46
- 125000004435 hydrogen atom Chemical group [H]* 0.000 claims description 104
- 125000003118 aryl group Chemical group 0.000 claims description 88
- 208000023275 Autoimmune disease Diseases 0.000 claims description 49
- 150000003839 salts Chemical class 0.000 claims description 36
- 125000003545 alkoxy group Chemical group 0.000 claims description 30
- 241000282414 Homo sapiens Species 0.000 claims description 25
- 125000000956 methoxy group Chemical group [H]C([H])([H])O* 0.000 claims description 20
- 125000004573 morpholin-4-yl group Chemical group N1(CCOCC1)* 0.000 claims description 20
- 206010028980 Neoplasm Diseases 0.000 abstract description 48
- 101000831496 Homo sapiens Toll-like receptor 3 Proteins 0.000 abstract description 31
- 101000669402 Homo sapiens Toll-like receptor 7 Proteins 0.000 abstract description 31
- 101000800483 Homo sapiens Toll-like receptor 8 Proteins 0.000 abstract description 30
- 201000011510 cancer Diseases 0.000 abstract description 30
- 208000015181 infectious disease Diseases 0.000 abstract description 16
- 102100039390 Toll-like receptor 7 Human genes 0.000 abstract description 13
- 102100024324 Toll-like receptor 3 Human genes 0.000 abstract description 12
- 102100033110 Toll-like receptor 8 Human genes 0.000 abstract description 12
- 208000006673 asthma Diseases 0.000 abstract description 12
- 230000000638 stimulation Effects 0.000 abstract description 12
- 206010020751 Hypersensitivity Diseases 0.000 abstract description 11
- 208000026935 allergic disease Diseases 0.000 abstract description 11
- 208000009329 Graft vs Host Disease Diseases 0.000 abstract description 8
- 206010061598 Immunodeficiency Diseases 0.000 abstract description 8
- 208000029462 Immunodeficiency disease Diseases 0.000 abstract description 8
- 206010061218 Inflammation Diseases 0.000 abstract description 8
- 206010052779 Transplant rejections Diseases 0.000 abstract description 8
- 230000007815 allergy Effects 0.000 abstract description 8
- 230000005784 autoimmunity Effects 0.000 abstract description 8
- 208000024908 graft versus host disease Diseases 0.000 abstract description 8
- 230000007813 immunodeficiency Effects 0.000 abstract description 8
- 230000004054 inflammatory process Effects 0.000 abstract description 8
- 206010040047 Sepsis Diseases 0.000 abstract description 4
- 229940058934 aminoquinoline antimalarials Drugs 0.000 abstract description 4
- 210000004027 cell Anatomy 0.000 description 128
- 230000003308 immunostimulating effect Effects 0.000 description 127
- 230000001404 mediated effect Effects 0.000 description 118
- 102000039446 nucleic acids Human genes 0.000 description 115
- 108020004707 nucleic acids Proteins 0.000 description 115
- 230000004044 response Effects 0.000 description 92
- 150000007523 nucleic acids Chemical class 0.000 description 89
- 125000000217 alkyl group Chemical group 0.000 description 86
- YMWUJEATGCHHMB-UHFFFAOYSA-N Dichloromethane Chemical compound ClCCl YMWUJEATGCHHMB-UHFFFAOYSA-N 0.000 description 84
- 239000000126 substance Substances 0.000 description 83
- 239000000243 solution Substances 0.000 description 78
- 125000000623 heterocyclic group Chemical group 0.000 description 77
- 229940046168 CpG oligodeoxynucleotide Drugs 0.000 description 75
- 239000003446 ligand Substances 0.000 description 74
- -1 CpG nucleic acids Chemical class 0.000 description 73
- 230000000890 antigenic effect Effects 0.000 description 61
- SMWDFEZZVXVKRB-UHFFFAOYSA-N anhydrous quinoline Natural products N1=CC=CC2=CC=CC=C21 SMWDFEZZVXVKRB-UHFFFAOYSA-N 0.000 description 59
- 239000000556 agonist Substances 0.000 description 58
- LFQSCWFLJHTTHZ-UHFFFAOYSA-N Ethanol Chemical compound CCO LFQSCWFLJHTTHZ-UHFFFAOYSA-N 0.000 description 57
- 230000028993 immune response Effects 0.000 description 55
- 239000007787 solid Substances 0.000 description 50
- 125000002496 methyl group Chemical group [H]C([H])([H])* 0.000 description 47
- 125000005843 halogen group Chemical group 0.000 description 43
- JWVCLYRUEFBMGU-UHFFFAOYSA-N quinazoline Chemical compound N1=CN=CC2=CC=CC=C21 JWVCLYRUEFBMGU-UHFFFAOYSA-N 0.000 description 43
- OKKJLVBELUTLKV-UHFFFAOYSA-N Methanol Chemical compound OC OKKJLVBELUTLKV-UHFFFAOYSA-N 0.000 description 41
- 239000000047 product Substances 0.000 description 41
- 239000000427 antigen Substances 0.000 description 39
- 108091007433 antigens Proteins 0.000 description 39
- 102000036639 antigens Human genes 0.000 description 39
- 230000000694 effects Effects 0.000 description 39
- 125000003342 alkenyl group Chemical group 0.000 description 36
- HEMHJVSKTPXQMS-UHFFFAOYSA-M Sodium hydroxide Chemical compound [OH-].[Na+] HEMHJVSKTPXQMS-UHFFFAOYSA-M 0.000 description 33
- 101000800479 Homo sapiens Toll-like receptor 9 Proteins 0.000 description 31
- 102000045710 human TLR9 Human genes 0.000 description 31
- 208000037265 diseases, disorders, signs and symptoms Diseases 0.000 description 29
- 229910052760 oxygen Inorganic materials 0.000 description 29
- XLYOFNOQVPJJNP-UHFFFAOYSA-N water Substances O XLYOFNOQVPJJNP-UHFFFAOYSA-N 0.000 description 29
- SECXISVLQFMRJM-UHFFFAOYSA-N N-Methylpyrrolidone Chemical compound CN1CCCC1=O SECXISVLQFMRJM-UHFFFAOYSA-N 0.000 description 28
- 230000014509 gene expression Effects 0.000 description 28
- 0 CC.CC.[2*]C[Y]([3*])C([4*])C(C)*(C)c1cc([8*])c([7*])c([6*])c1[5*] Chemical compound CC.CC.[2*]C[Y]([3*])C([4*])C(C)*(C)c1cc([8*])c([7*])c([6*])c1[5*] 0.000 description 27
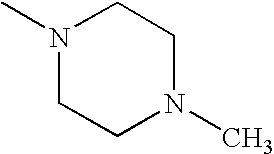
- RXYPXQSKLGGKOL-UHFFFAOYSA-N CN1CCN(C)CC1 Chemical compound CN1CCN(C)CC1 RXYPXQSKLGGKOL-UHFFFAOYSA-N 0.000 description 27
- 210000002865 immune cell Anatomy 0.000 description 26
- 108090000623 proteins and genes Proteins 0.000 description 26
- 125000003710 aryl alkyl group Chemical group 0.000 description 25
- 238000001914 filtration Methods 0.000 description 25
- 230000006698 induction Effects 0.000 description 25
- 229910052757 nitrogen Inorganic materials 0.000 description 25
- 102000004127 Cytokines Human genes 0.000 description 24
- 108090000695 Cytokines Proteins 0.000 description 24
- IJGRMHOSHXDMSA-UHFFFAOYSA-N Atomic nitrogen Chemical compound N#N IJGRMHOSHXDMSA-UHFFFAOYSA-N 0.000 description 23
- 241000699670 Mus sp. Species 0.000 description 23
- 108091026890 Coding region Proteins 0.000 description 22
- 108060008682 Tumor Necrosis Factor Proteins 0.000 description 22
- 102000000852 Tumor Necrosis Factor-alpha Human genes 0.000 description 22
- 229910052717 sulfur Inorganic materials 0.000 description 22
- 201000010099 disease Diseases 0.000 description 21
- URLKBWYHVLBVBO-UHFFFAOYSA-N CC1=CC=C(C)C=C1 Chemical compound CC1=CC=C(C)C=C1 URLKBWYHVLBVBO-UHFFFAOYSA-N 0.000 description 20
- CSNNHWWHGAXBCP-UHFFFAOYSA-L Magnesium sulfate Chemical compound [Mg+2].[O-][S+2]([O-])([O-])[O-] CSNNHWWHGAXBCP-UHFFFAOYSA-L 0.000 description 20
- LRHPLDYGYMQRHN-UHFFFAOYSA-N N-Butanol Chemical compound CCCCO LRHPLDYGYMQRHN-UHFFFAOYSA-N 0.000 description 20
- 238000001727 in vivo Methods 0.000 description 20
- KDWFDOFTPHDNJL-TUBOTVQJSA-N odn-2006 Chemical compound O=C1NC(=O)C(C)=CN1[C@@H]1O[C@H](COP(O)(=O)O[C@@H]2[C@H](O[C@H](C2)N2C(NC(=O)C(C)=C2)=O)COP(O)(=O)O[C@H]2[C@H]([C@@H](O[C@@H]2COP(O)(=S)O[C@H]2[C@H]([C@@H](O[C@@H]2COP(O)(=O)O[C@@H]2[C@H](O[C@H](C2)N2C(NC(=O)C(C)=C2)=O)COP(O)(=O)O[C@@H]2[C@H](O[C@H](C2)N2C(NC(=O)C(C)=C2)=O)COP(O)(=O)O[C@@H]2[C@H](O[C@H](C2)N2C(NC(=O)C(C)=C2)=O)COP(O)(=O)O[C@@H]2[C@H](O[C@H](C2)N2C(NC(=O)C(C)=C2)=O)COP(O)(=O)O[C@H]2[C@H]([C@@H](O[C@@H]2COP(O)(=O)O[C@@H]2[C@H](O[C@H](C2)N2C(NC(=O)C(C)=C2)=O)COP(O)(=O)O[C@H]2[C@H]([C@@H](O[C@@H]2COP(O)(=S)O[C@H]2[C@H]([C@@H](O[C@@H]2COP(O)(=O)OC[C@@H]2[C@H](C[C@@H](O2)N2C(NC(=O)C(C)=C2)=O)OP(O)(=O)OC[C@@H]2[C@H]([C@@H](O)[C@@H](O2)N2C3=C(C(NC(N)=N3)=O)N=C2)OP(O)(=O)OC[C@@H]2[C@H](C[C@@H](O2)N2C(NC(=O)C(C)=C2)=O)OP(O)(=O)OC[C@@H]2[C@H](C[C@@H](O2)N2C(NC(=O)C(C)=C2)=O)OP(O)(=O)OC[C@@H]2[C@H](C[C@@H](O2)N2C(NC(=O)C(C)=C2)=O)OP(O)(=O)OC[C@@H]2[C@H](C[C@@H](O2)N2C(NC(=O)C(C)=C2)=O)OP(O)(=O)OC[C@@H]2[C@H]([C@@H](O)[C@@H](O2)N2C3=C(C(NC(N)=N3)=O)N=C2)OP(S)(=O)OC[C@@H]2[C@H]([C@@H](O)[C@@H](O2)N2C(N=C(N)C=C2)=O)OP(O)(=O)OC[C@@H]2[C@H](C[C@@H](O2)N2C(NC(=O)C(C)=C2)=O)OP(O)(=O)OC[C@@H]2[C@H](C[C@@H](O2)N2C(NC(=O)C(C)=C2)=O)O)N2C3=C(C(NC(N)=N3)=O)N=C2)O)N2C(N=C(N)C=C2)=O)O)N2C3=C(C(NC(N)=N3)=O)N=C2)O)N2C3=C(C(NC(N)=N3)=O)N=C2)O)N2C(N=C(N)C=C2)=O)O)[C@@H](O)C1 KDWFDOFTPHDNJL-TUBOTVQJSA-N 0.000 description 20
- MZOFCQQQCNRIBI-VMXHOPILSA-N (3s)-4-[[(2s)-1-[[(2s)-1-[[(1s)-1-carboxy-2-hydroxyethyl]amino]-4-methyl-1-oxopentan-2-yl]amino]-5-(diaminomethylideneamino)-1-oxopentan-2-yl]amino]-3-[[2-[[(2s)-2,6-diaminohexanoyl]amino]acetyl]amino]-4-oxobutanoic acid Chemical compound OC[C@@H](C(O)=O)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CCCN=C(N)N)NC(=O)[C@H](CC(O)=O)NC(=O)CNC(=O)[C@@H](N)CCCCN MZOFCQQQCNRIBI-VMXHOPILSA-N 0.000 description 19
- 239000005089 Luciferase Substances 0.000 description 19
- 102000045716 human TLR3 Human genes 0.000 description 19
- 238000012216 screening Methods 0.000 description 19
- 238000003786 synthesis reaction Methods 0.000 description 19
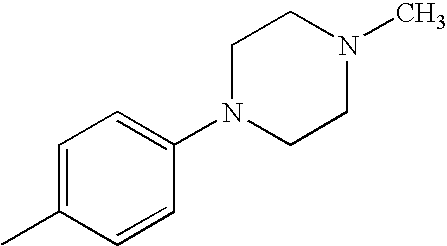
- GYBMIIMARPGPOJ-UHFFFAOYSA-N CC1=CC=C(N2CCN(C)CC2)C=C1 Chemical compound CC1=CC=C(N2CCN(C)CC2)C=C1 GYBMIIMARPGPOJ-UHFFFAOYSA-N 0.000 description 18
- 102000045715 human TLR7 Human genes 0.000 description 18
- 102000045720 human TLR8 Human genes 0.000 description 18
- 238000000338 in vitro Methods 0.000 description 18
- VLKZOEOYAKHREP-UHFFFAOYSA-N n-Hexane Chemical compound CCCCCC VLKZOEOYAKHREP-UHFFFAOYSA-N 0.000 description 18
- 125000003729 nucleotide group Chemical group 0.000 description 18
- 238000003556 assay Methods 0.000 description 17
- 230000015572 biosynthetic process Effects 0.000 description 17
- 229910052799 carbon Inorganic materials 0.000 description 17
- 239000002773 nucleotide Substances 0.000 description 17
- 108020004414 DNA Proteins 0.000 description 16
- 108010065805 Interleukin-12 Proteins 0.000 description 16
- 102000013462 Interleukin-12 Human genes 0.000 description 16
- CDBYLPFSWZWCQE-UHFFFAOYSA-L Sodium Carbonate Chemical compound [Na+].[Na+].[O-]C([O-])=O CDBYLPFSWZWCQE-UHFFFAOYSA-L 0.000 description 16
- 239000003795 chemical substances by application Substances 0.000 description 16
- 125000001997 phenyl group Chemical group [H]C1=C([H])C([H])=C(*)C([H])=C1[H] 0.000 description 16
- XEKOWRVHYACXOJ-UHFFFAOYSA-N Ethyl acetate Chemical compound CCOC(C)=O XEKOWRVHYACXOJ-UHFFFAOYSA-N 0.000 description 15
- 108010057466 NF-kappa B Proteins 0.000 description 15
- 102000003945 NF-kappa B Human genes 0.000 description 15
- 239000000284 extract Substances 0.000 description 15
- 230000015788 innate immune response Effects 0.000 description 15
- 150000003246 quinazolines Chemical class 0.000 description 15
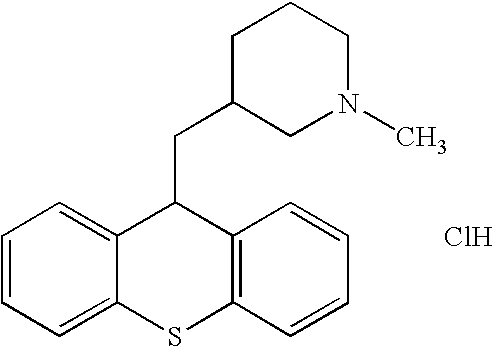
- WHTVZRBIWZFKQO-AWEZNQCLSA-N (S)-chloroquine Chemical compound ClC1=CC=C2C(N[C@@H](C)CCCN(CC)CC)=CC=NC2=C1 WHTVZRBIWZFKQO-AWEZNQCLSA-N 0.000 description 14
- PVOAHINGSUIXLS-UHFFFAOYSA-N 1-Methylpiperazine Chemical group CN1CCNCC1 PVOAHINGSUIXLS-UHFFFAOYSA-N 0.000 description 14
- 102000004889 Interleukin-6 Human genes 0.000 description 14
- 108090001005 Interleukin-6 Proteins 0.000 description 14
- 108060001084 Luciferase Proteins 0.000 description 14
- VYPSYNLAJGMNEJ-UHFFFAOYSA-N Silicium dioxide Chemical compound O=[Si]=O VYPSYNLAJGMNEJ-UHFFFAOYSA-N 0.000 description 14
- 239000013604 expression vector Substances 0.000 description 14
- 239000007924 injection Substances 0.000 description 14
- 238000002347 injection Methods 0.000 description 14
- BXNMTOQRYBFHNZ-UHFFFAOYSA-N resiquimod Chemical group C1=CC=CC2=C(N(C(COCC)=N3)CC(C)(C)O)C3=C(N)N=C21 BXNMTOQRYBFHNZ-UHFFFAOYSA-N 0.000 description 14
- 125000006528 (C2-C6) alkyl group Chemical group 0.000 description 13
- 241001465754 Metazoa Species 0.000 description 13
- BZLVMXJERCGZMT-UHFFFAOYSA-N Methyl tert-butyl ether Chemical compound COC(C)(C)C BZLVMXJERCGZMT-UHFFFAOYSA-N 0.000 description 13
- 230000004913 activation Effects 0.000 description 13
- 230000001965 increasing effect Effects 0.000 description 13
- 102000004169 proteins and genes Human genes 0.000 description 13
- 125000004434 sulfur atom Chemical group 0.000 description 13
- 201000000596 systemic lupus erythematosus Diseases 0.000 description 13
- QTBSBXVTEAMEQO-UHFFFAOYSA-N Acetic acid Chemical compound CC(O)=O QTBSBXVTEAMEQO-UHFFFAOYSA-N 0.000 description 12
- CSCPPACGZOOCGX-UHFFFAOYSA-N Acetone Chemical compound CC(C)=O CSCPPACGZOOCGX-UHFFFAOYSA-N 0.000 description 12
- LTGICOWYNWCIJX-UHFFFAOYSA-N SSS(=O)SS Chemical compound SSS(=O)SS LTGICOWYNWCIJX-UHFFFAOYSA-N 0.000 description 12
- 241000700605 Viruses Species 0.000 description 12
- 125000003368 amide group Chemical group 0.000 description 12
- 150000001413 amino acids Chemical group 0.000 description 12
- 230000008485 antagonism Effects 0.000 description 12
- 150000002148 esters Chemical class 0.000 description 12
- 125000004356 hydroxy functional group Chemical group O* 0.000 description 12
- 229940100601 interleukin-6 Drugs 0.000 description 12
- 150000002576 ketones Chemical class 0.000 description 12
- 125000000449 nitro group Chemical group [O-][N+](*)=O 0.000 description 12
- 125000002924 primary amino group Chemical group [H]N([H])* 0.000 description 12
- 102000005962 receptors Human genes 0.000 description 12
- 108020003175 receptors Proteins 0.000 description 12
- 229950010550 resiquimod Drugs 0.000 description 12
- 125000005420 sulfonamido group Chemical group S(=O)(=O)(N*)* 0.000 description 12
- 150000003457 sulfones Chemical class 0.000 description 12
- 239000013566 allergen Substances 0.000 description 11
- 229960003677 chloroquine Drugs 0.000 description 11
- WHTVZRBIWZFKQO-UHFFFAOYSA-N chloroquine Natural products ClC1=CC=C2C(NC(C)CCCN(CC)CC)=CC=NC2=C1 WHTVZRBIWZFKQO-UHFFFAOYSA-N 0.000 description 11
- 238000001816 cooling Methods 0.000 description 11
- 239000003112 inhibitor Substances 0.000 description 11
- 239000003921 oil Substances 0.000 description 11
- 230000002829 reductive effect Effects 0.000 description 11
- 206010039073 rheumatoid arthritis Diseases 0.000 description 11
- 125000005504 styryl group Chemical group 0.000 description 11
- AVRPFRMDMNDIDH-UHFFFAOYSA-N 1h-quinazolin-2-one Chemical compound C1=CC=CC2=NC(O)=NC=C21 AVRPFRMDMNDIDH-UHFFFAOYSA-N 0.000 description 10
- 102100025248 C-X-C motif chemokine 10 Human genes 0.000 description 10
- 101710098275 C-X-C motif chemokine 10 Proteins 0.000 description 10
- 108010047761 Interferon-alpha Proteins 0.000 description 10
- 102000006992 Interferon-alpha Human genes 0.000 description 10
- 210000003719 b-lymphocyte Anatomy 0.000 description 10
- 229910052943 magnesium sulfate Inorganic materials 0.000 description 10
- 235000019341 magnesium sulphate Nutrition 0.000 description 10
- 239000003550 marker Substances 0.000 description 10
- OHDXDNUPVVYWOV-UHFFFAOYSA-N n-methyl-1-(2-naphthalen-1-ylsulfanylphenyl)methanamine Chemical compound CNCC1=CC=CC=C1SC1=CC=CC2=CC=CC=C12 OHDXDNUPVVYWOV-UHFFFAOYSA-N 0.000 description 10
- 229940046166 oligodeoxynucleotide Drugs 0.000 description 10
- 210000003819 peripheral blood mononuclear cell Anatomy 0.000 description 10
- 238000010992 reflux Methods 0.000 description 10
- 238000003756 stirring Methods 0.000 description 10
- 108091032973 (ribonucleotides)n+m Proteins 0.000 description 9
- WMPTYRGXBUYONY-UHFFFAOYSA-N 2-chloroquinazoline Chemical compound C1=CC=CC2=NC(Cl)=NC=C21 WMPTYRGXBUYONY-UHFFFAOYSA-N 0.000 description 9
- 102000019034 Chemokines Human genes 0.000 description 9
- 108010012236 Chemokines Proteins 0.000 description 9
- 101150029707 ERBB2 gene Proteins 0.000 description 9
- 108090001007 Interleukin-8 Proteins 0.000 description 9
- 102000004890 Interleukin-8 Human genes 0.000 description 9
- 101710100968 Receptor tyrosine-protein kinase erbB-2 Proteins 0.000 description 9
- 102100030086 Receptor tyrosine-protein kinase erbB-2 Human genes 0.000 description 9
- LOKCTEFSRHRXRJ-UHFFFAOYSA-I dipotassium trisodium dihydrogen phosphate hydrogen phosphate dichloride Chemical compound P(=O)(O)(O)[O-].[K+].P(=O)(O)([O-])[O-].[Na+].[Na+].[Cl-].[K+].[Cl-].[Na+] LOKCTEFSRHRXRJ-UHFFFAOYSA-I 0.000 description 9
- 125000001495 ethyl group Chemical group [H]C([H])([H])C([H])([H])* 0.000 description 9
- 125000004430 oxygen atom Chemical group O* 0.000 description 9
- 239000002953 phosphate buffered saline Substances 0.000 description 9
- 125000001424 substituent group Chemical group 0.000 description 9
- PXBFMLJZNCDSMP-UHFFFAOYSA-N 2-Aminobenzamide Chemical compound NC(=O)C1=CC=CC=C1N PXBFMLJZNCDSMP-UHFFFAOYSA-N 0.000 description 8
- CTQNGGLPUBDAKN-UHFFFAOYSA-N CC1=C(C)C=CC=C1 Chemical compound CC1=C(C)C=CC=C1 CTQNGGLPUBDAKN-UHFFFAOYSA-N 0.000 description 8
- 238000002965 ELISA Methods 0.000 description 8
- 108060003951 Immunoglobulin Proteins 0.000 description 8
- 108010074328 Interferon-gamma Proteins 0.000 description 8
- 108091027981 Response element Proteins 0.000 description 8
- 208000035475 disorder Diseases 0.000 description 8
- 230000003828 downregulation Effects 0.000 description 8
- 238000001035 drying Methods 0.000 description 8
- 150000004677 hydrates Chemical class 0.000 description 8
- 102000018358 immunoglobulin Human genes 0.000 description 8
- KJIFKLIQANRMOU-UHFFFAOYSA-N oxidanium;4-methylbenzenesulfonate Chemical compound O.CC1=CC=C(S(O)(=O)=O)C=C1 KJIFKLIQANRMOU-UHFFFAOYSA-N 0.000 description 8
- 239000002002 slurry Substances 0.000 description 8
- 229910000029 sodium carbonate Inorganic materials 0.000 description 8
- 208000024891 symptom Diseases 0.000 description 8
- 101710091881 GTPase HRas Proteins 0.000 description 7
- 102100029974 GTPase HRas Human genes 0.000 description 7
- 102100037850 Interferon gamma Human genes 0.000 description 7
- 108090000978 Interleukin-4 Proteins 0.000 description 7
- 102000004388 Interleukin-4 Human genes 0.000 description 7
- 108700008625 Reporter Genes Proteins 0.000 description 7
- 239000006146 Roswell Park Memorial Institute medium Substances 0.000 description 7
- 125000003277 amino group Chemical group 0.000 description 7
- 238000010171 animal model Methods 0.000 description 7
- 239000012043 crude product Substances 0.000 description 7
- 125000004122 cyclic group Chemical group 0.000 description 7
- 210000000987 immune system Anatomy 0.000 description 7
- 230000002458 infectious effect Effects 0.000 description 7
- 230000005764 inhibitory process Effects 0.000 description 7
- 239000013642 negative control Substances 0.000 description 7
- 239000008194 pharmaceutical composition Substances 0.000 description 7
- XHXFXVLFKHQFAL-UHFFFAOYSA-N phosphoryl trichloride Chemical compound ClP(Cl)(Cl)=O XHXFXVLFKHQFAL-UHFFFAOYSA-N 0.000 description 7
- 108090000765 processed proteins & peptides Proteins 0.000 description 7
- 239000000377 silicon dioxide Substances 0.000 description 7
- QAOWNCQODCNURD-UHFFFAOYSA-N sulfuric acid Substances OS(O)(=O)=O QAOWNCQODCNURD-UHFFFAOYSA-N 0.000 description 7
- 231100000419 toxicity Toxicity 0.000 description 7
- 230000001988 toxicity Effects 0.000 description 7
- 101100504181 Arabidopsis thaliana GCS1 gene Proteins 0.000 description 6
- 208000027496 Behcet disease Diseases 0.000 description 6
- QIMMUPPBPVKWKM-UHFFFAOYSA-N CC1=CC=C2C=CC=CC2=C1 Chemical compound CC1=CC=C2C=CC=CC2=C1 QIMMUPPBPVKWKM-UHFFFAOYSA-N 0.000 description 6
- 108010002350 Interleukin-2 Proteins 0.000 description 6
- 102000000588 Interleukin-2 Human genes 0.000 description 6
- 102000010168 Myeloid Differentiation Factor 88 Human genes 0.000 description 6
- 108010077432 Myeloid Differentiation Factor 88 Proteins 0.000 description 6
- 230000037396 body weight Effects 0.000 description 6
- 238000004113 cell culture Methods 0.000 description 6
- 239000000460 chlorine Substances 0.000 description 6
- 239000003814 drug Substances 0.000 description 6
- 230000036039 immunity Effects 0.000 description 6
- DILRJUIACXKSQE-UHFFFAOYSA-N n',n'-dimethylethane-1,2-diamine Chemical compound CN(C)CCN DILRJUIACXKSQE-UHFFFAOYSA-N 0.000 description 6
- 229940115272 polyinosinic:polycytidylic acid Drugs 0.000 description 6
- 238000002360 preparation method Methods 0.000 description 6
- 229940044655 toll-like receptor 9 agonist Drugs 0.000 description 6
- 238000013518 transcription Methods 0.000 description 6
- 230000035897 transcription Effects 0.000 description 6
- 206010002556 Ankylosing Spondylitis Diseases 0.000 description 5
- PVFIDFYACCGDBG-UHFFFAOYSA-N CC1=CC=CC(N2CCN(C)CC2)=C1 Chemical compound CC1=CC=CC(N2CCN(C)CC2)=C1 PVFIDFYACCGDBG-UHFFFAOYSA-N 0.000 description 5
- OKTJSMMVPCPJKN-UHFFFAOYSA-N Carbon Chemical compound [C] OKTJSMMVPCPJKN-UHFFFAOYSA-N 0.000 description 5
- IAZDPXIOMUYVGZ-UHFFFAOYSA-N Dimethylsulphoxide Chemical compound CS(C)=O IAZDPXIOMUYVGZ-UHFFFAOYSA-N 0.000 description 5
- 102000004190 Enzymes Human genes 0.000 description 5
- 108090000790 Enzymes Proteins 0.000 description 5
- 108010017213 Granulocyte-Macrophage Colony-Stimulating Factor Proteins 0.000 description 5
- 102100039620 Granulocyte-macrophage colony-stimulating factor Human genes 0.000 description 5
- 206010072579 Granulomatosis with polyangiitis Diseases 0.000 description 5
- 208000022559 Inflammatory bowel disease Diseases 0.000 description 5
- 102000003814 Interleukin-10 Human genes 0.000 description 5
- 108090000174 Interleukin-10 Proteins 0.000 description 5
- 208000021386 Sjogren Syndrome Diseases 0.000 description 5
- HVOYZOQVDYHUPF-UHFFFAOYSA-N [H]N(C)CCN(C)C Chemical compound [H]N(C)CCN(C)C HVOYZOQVDYHUPF-UHFFFAOYSA-N 0.000 description 5
- MEGFLXWECAHAGH-UHFFFAOYSA-N [H]N(CCN(C)C)C1=C(C)C=CC=C1 Chemical compound [H]N(CCN(C)C)C1=C(C)C=CC=C1 MEGFLXWECAHAGH-UHFFFAOYSA-N 0.000 description 5
- 230000033289 adaptive immune response Effects 0.000 description 5
- 125000004429 atom Chemical group 0.000 description 5
- 208000010668 atopic eczema Diseases 0.000 description 5
- 210000004369 blood Anatomy 0.000 description 5
- 239000008280 blood Substances 0.000 description 5
- VNFPBHJOKIVQEB-UHFFFAOYSA-N clotrimazole Chemical compound ClC1=CC=CC=C1C(N1C=NC=C1)(C=1C=CC=CC=1)C1=CC=CC=C1 VNFPBHJOKIVQEB-UHFFFAOYSA-N 0.000 description 5
- 229940076144 interleukin-10 Drugs 0.000 description 5
- 238000007912 intraperitoneal administration Methods 0.000 description 5
- 238000004519 manufacturing process Methods 0.000 description 5
- 229960000901 mepacrine Drugs 0.000 description 5
- QJGQUHMNIGDVPM-UHFFFAOYSA-N nitrogen group Chemical group [N] QJGQUHMNIGDVPM-UHFFFAOYSA-N 0.000 description 5
- 239000002777 nucleoside Substances 0.000 description 5
- 125000003835 nucleoside group Chemical group 0.000 description 5
- 229920000642 polymer Polymers 0.000 description 5
- 208000005987 polymyositis Diseases 0.000 description 5
- GPKJTRJOBQGKQK-UHFFFAOYSA-N quinacrine Chemical compound C1=C(OC)C=C2C(NC(C)CCCN(CC)CC)=C(C=CC(Cl)=C3)C3=NC2=C1 GPKJTRJOBQGKQK-UHFFFAOYSA-N 0.000 description 5
- 201000000306 sarcoidosis Diseases 0.000 description 5
- 230000028327 secretion Effects 0.000 description 5
- 210000002966 serum Anatomy 0.000 description 5
- 210000001519 tissue Anatomy 0.000 description 5
- AIFRHYZBTHREPW-UHFFFAOYSA-N β-carboline Chemical compound N1=CC=C2C3=CC=CC=C3NC2=C1 AIFRHYZBTHREPW-UHFFFAOYSA-N 0.000 description 5
- 102100032814 ATP-dependent zinc metalloprotease YME1L1 Human genes 0.000 description 4
- 102000010735 Adenomatous polyposis coli protein Human genes 0.000 description 4
- 108010038310 Adenomatous polyposis coli protein Proteins 0.000 description 4
- 241000894006 Bacteria Species 0.000 description 4
- 102100032367 C-C motif chemokine 5 Human genes 0.000 description 4
- YNQLUTRBYVCPMQ-UHFFFAOYSA-N CCC1=CC=CC=C1 Chemical compound CCC1=CC=CC=C1 YNQLUTRBYVCPMQ-UHFFFAOYSA-N 0.000 description 4
- 108010022366 Carcinoembryonic Antigen Proteins 0.000 description 4
- 102100025475 Carcinoembryonic antigen-related cell adhesion molecule 5 Human genes 0.000 description 4
- 108010035563 Chloramphenicol O-acetyltransferase Proteins 0.000 description 4
- MYMOFIZGZYHOMD-UHFFFAOYSA-N Dioxygen Chemical compound O=O MYMOFIZGZYHOMD-UHFFFAOYSA-N 0.000 description 4
- 241000233866 Fungi Species 0.000 description 4
- 102100041003 Glutamate carboxypeptidase 2 Human genes 0.000 description 4
- 101000892862 Homo sapiens Glutamate carboxypeptidase 2 Proteins 0.000 description 4
- VEXZGXHMUGYJMC-UHFFFAOYSA-N Hydrochloric acid Chemical compound Cl VEXZGXHMUGYJMC-UHFFFAOYSA-N 0.000 description 4
- 102000003816 Interleukin-13 Human genes 0.000 description 4
- 108090000176 Interleukin-13 Proteins 0.000 description 4
- 108010002386 Interleukin-3 Proteins 0.000 description 4
- 102000000646 Interleukin-3 Human genes 0.000 description 4
- 108010002616 Interleukin-5 Proteins 0.000 description 4
- 102000000743 Interleukin-5 Human genes 0.000 description 4
- 102100028389 Melanoma antigen recognized by T-cells 1 Human genes 0.000 description 4
- 241001529936 Murinae Species 0.000 description 4
- UEEJHVSXFDXPFK-UHFFFAOYSA-N N-dimethylaminoethanol Chemical compound CN(C)CCO UEEJHVSXFDXPFK-UHFFFAOYSA-N 0.000 description 4
- 108091028043 Nucleic acid sequence Proteins 0.000 description 4
- 102100040283 Peptidyl-prolyl cis-trans isomerase B Human genes 0.000 description 4
- 101800000795 Proadrenomedullin N-20 terminal peptide Proteins 0.000 description 4
- 102000007066 Prostate-Specific Antigen Human genes 0.000 description 4
- 108010072866 Prostate-Specific Antigen Proteins 0.000 description 4
- 201000001263 Psoriatic Arthritis Diseases 0.000 description 4
- 208000036824 Psoriatic arthropathy Diseases 0.000 description 4
- 208000033464 Reiter syndrome Diseases 0.000 description 4
- KEAYESYHFKHZAL-UHFFFAOYSA-N Sodium Chemical compound [Na] KEAYESYHFKHZAL-UHFFFAOYSA-N 0.000 description 4
- 210000001744 T-lymphocyte Anatomy 0.000 description 4
- IQFYYKKMVGJFEH-XLPZGREQSA-N Thymidine Chemical compound O=C1NC(=O)C(C)=CN1[C@@H]1O[C@H](CO)[C@@H](O)C1 IQFYYKKMVGJFEH-XLPZGREQSA-N 0.000 description 4
- 208000024780 Urticaria Diseases 0.000 description 4
- 206010047115 Vasculitis Diseases 0.000 description 4
- 125000003282 alkyl amino group Chemical group 0.000 description 4
- 230000001413 cellular effect Effects 0.000 description 4
- 229910052801 chlorine Inorganic materials 0.000 description 4
- 125000001309 chloro group Chemical group Cl* 0.000 description 4
- 238000004587 chromatography analysis Methods 0.000 description 4
- 239000012228 culture supernatant Substances 0.000 description 4
- 108010048032 cyclophilin B Proteins 0.000 description 4
- 238000001514 detection method Methods 0.000 description 4
- 125000004663 dialkyl amino group Chemical group 0.000 description 4
- HPNMFZURTQLUMO-UHFFFAOYSA-N diethylamine Chemical compound CCNCC HPNMFZURTQLUMO-UHFFFAOYSA-N 0.000 description 4
- 229960001760 dimethyl sulfoxide Drugs 0.000 description 4
- 229940079593 drug Drugs 0.000 description 4
- 238000001943 fluorescence-activated cell sorting Methods 0.000 description 4
- 230000006870 function Effects 0.000 description 4
- 239000005090 green fluorescent protein Substances 0.000 description 4
- XXSMGPRMXLTPCZ-UHFFFAOYSA-N hydroxychloroquine Chemical class ClC1=CC=C2C(NC(C)CCCN(CCO)CC)=CC=NC2=C1 XXSMGPRMXLTPCZ-UHFFFAOYSA-N 0.000 description 4
- 230000002163 immunogen Effects 0.000 description 4
- 230000002757 inflammatory effect Effects 0.000 description 4
- 230000003993 interaction Effects 0.000 description 4
- 230000004068 intracellular signaling Effects 0.000 description 4
- 238000007918 intramuscular administration Methods 0.000 description 4
- 238000001990 intravenous administration Methods 0.000 description 4
- 150000002611 lead compounds Chemical class 0.000 description 4
- 230000000670 limiting effect Effects 0.000 description 4
- 239000007788 liquid Substances 0.000 description 4
- 239000012139 lysis buffer Substances 0.000 description 4
- BDAGIHXWWSANSR-UHFFFAOYSA-N methanoic acid Natural products OC=O BDAGIHXWWSANSR-UHFFFAOYSA-N 0.000 description 4
- 125000004433 nitrogen atom Chemical group N* 0.000 description 4
- UIRLPEMNFBJPIT-UHFFFAOYSA-N odn 2395 Chemical compound O=C1NC(=O)C(C)=CN1C1OC(COP(O)(O)=O)C(OP(O)(=O)OCC2C(CC(O2)N2C(N=C(N)C=C2)=O)OP(O)(=O)OCC2C(CC(O2)N2C3=C(C(NC(N)=N3)=O)N=C2)OP(O)(=O)OCC2C(CC(O2)N2C(NC(=O)C(C)=C2)=O)OP(O)(=O)OCC2C(CC(O2)N2C(N=C(N)C=C2)=O)OP(O)(=O)OCC2C(CC(O2)N2C3=C(C(NC(N)=N3)=O)N=C2)OP(O)(=O)OCC2C(CC(O2)N2C(NC(=O)C(C)=C2)=O)OP(O)(=O)OCC2C(CC(O2)N2C(NC(=O)C(C)=C2)=O)OP(O)(=O)OCC2C(CC(O2)N2C(NC(=O)C(C)=C2)=O)OP(O)(=O)OCC2C(CC(O2)N2C(NC(=O)C(C)=C2)=O)OP(O)(=O)OCC2C(CC(O2)N2C(N=C(N)C=C2)=O)OP(O)(=O)OCC2C(CC(O2)N2C3=C(C(NC(N)=N3)=O)N=C2)OP(O)(=O)OCC2C(CC(O2)N2C3=C(C(NC(N)=N3)=O)N=C2)OP(O)(=O)OCC2C(CC(O2)N2C(N=C(N)C=C2)=O)OP(O)(=O)OCC2C(CC(O2)N2C3=C(C(NC(N)=N3)=O)N=C2)OP(O)(=O)OCC2C(CC(O2)N2C(N=C(N)C=C2)=O)OP(O)(=O)OCC2C(CC(O2)N2C3=C(C(NC(N)=N3)=O)N=C2)OP(O)(=O)OCC2C(CC(O2)N2C(N=C(N)C=C2)=O)OP(O)(=O)OCC2C(CC(O2)N2C3=C(C(NC(N)=N3)=O)N=C2)OP(O)(=O)OCC2C(CC(O2)N2C(N=C(N)C=C2)=O)OP(O)(=O)OCC2C(CC(O2)N2C(N=C(N)C=C2)=O)OP(O)(=O)OCC2C(CC(O2)N2C3=C(C(NC(N)=N3)=O)N=C2)O)C1 UIRLPEMNFBJPIT-UHFFFAOYSA-N 0.000 description 4
- 239000001301 oxygen Substances 0.000 description 4
- PIRWNASAJNPKHT-SHZATDIYSA-N pamp Chemical compound C([C@@H](C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CC=1C2=CC=CC=C2NC=1)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CC=1C2=CC=CC=C2NC=1)C(=O)N[C@@H](C)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CO)C(=O)N[C@@H](CCCNC(N)=N)C(N)=O)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](CO)NC(=O)[C@H](C)NC(=O)[C@@H](NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](C)N)C(C)C)C1=CC=CC=C1 PIRWNASAJNPKHT-SHZATDIYSA-N 0.000 description 4
- 230000001717 pathogenic effect Effects 0.000 description 4
- 108010044156 peptidyl-prolyl cis-trans isomerase b Proteins 0.000 description 4
- YZTJYBJCZXZGCT-UHFFFAOYSA-N phenylpiperazine Chemical group C1CNCCN1C1=CC=CC=C1 YZTJYBJCZXZGCT-UHFFFAOYSA-N 0.000 description 4
- 150000004713 phosphodiesters Chemical group 0.000 description 4
- 230000003389 potentiating effect Effects 0.000 description 4
- 102000004196 processed proteins & peptides Human genes 0.000 description 4
- 208000002574 reactive arthritis Diseases 0.000 description 4
- 239000012312 sodium hydride Substances 0.000 description 4
- 229910000104 sodium hydride Inorganic materials 0.000 description 4
- UGNWTBMOAKPKBL-UHFFFAOYSA-N tetrachloro-1,4-benzoquinone Chemical compound ClC1=C(Cl)C(=O)C(Cl)=C(Cl)C1=O UGNWTBMOAKPKBL-UHFFFAOYSA-N 0.000 description 4
- 230000001225 therapeutic effect Effects 0.000 description 4
- FYSNRJHAOHDILO-UHFFFAOYSA-N thionyl chloride Chemical compound ClS(Cl)=O FYSNRJHAOHDILO-UHFFFAOYSA-N 0.000 description 4
- 102000040650 (ribonucleotides)n+m Human genes 0.000 description 3
- QBWITSXUUOJHHG-UHFFFAOYSA-N 1,6-dimethyl-2,3-dihydropyrrolo[2,3-b]quinolin-4-amine Chemical compound N1=C2C=CC(C)=CC2=C(N)C2=C1N(C)CC2 QBWITSXUUOJHHG-UHFFFAOYSA-N 0.000 description 3
- ZCZQCKJQIGWLFR-UHFFFAOYSA-N 1-benzyl-2,3-dihydropyrrolo[2,3-b]quinolin-4-amine Chemical compound C12=NC3=CC=CC=C3C(N)=C2CCN1CC1=CC=CC=C1 ZCZQCKJQIGWLFR-UHFFFAOYSA-N 0.000 description 3
- UUAVYXKRUSVCDJ-UHFFFAOYSA-N 1-cycloheptylazepane Chemical group C1CCCCCC1N1CCCCCC1 UUAVYXKRUSVCDJ-UHFFFAOYSA-N 0.000 description 3
- QUIJMHDIAFOPRK-UHFFFAOYSA-N 1-cyclooctylazocane Chemical compound C1CCCCCCC1N1CCCCCCC1 QUIJMHDIAFOPRK-UHFFFAOYSA-N 0.000 description 3
- OQXXXDZZPGCHKC-UHFFFAOYSA-N 1-methyl-2,3,4,5-tetrahydroazepino[2,3-b]quinolin-6-amine Chemical compound CN1CCCCC2=C(N)C3=CC=CC=C3N=C12 OQXXXDZZPGCHKC-UHFFFAOYSA-N 0.000 description 3
- TZNYLDDNIITWPM-UHFFFAOYSA-N 2,4-dimethylbenzo[b][1,8]naphthyridin-5-amine Chemical compound C1=CC=CC2=NC3=NC(C)=CC(C)=C3C(N)=C21 TZNYLDDNIITWPM-UHFFFAOYSA-N 0.000 description 3
- PFODEVGLOVUVHS-UHFFFAOYSA-N 4-(4-methylpiperazin-1-yl)benzaldehyde Chemical compound C1CN(C)CCN1C1=CC=C(C=O)C=C1 PFODEVGLOVUVHS-UHFFFAOYSA-N 0.000 description 3
- GVRRXASZZAKBMN-UHFFFAOYSA-N 4-chloroquinazoline Chemical compound C1=CC=C2C(Cl)=NC=NC2=C1 GVRRXASZZAKBMN-UHFFFAOYSA-N 0.000 description 3
- SGUXXMZBFKDFAD-UHFFFAOYSA-N 6-bromo-1-methyl-2,3-dihydropyrrolo[2,3-b]quinolin-4-amine Chemical compound N1=C2C=CC(Br)=CC2=C(N)C2=C1N(C)CC2 SGUXXMZBFKDFAD-UHFFFAOYSA-N 0.000 description 3
- PRLZBYKCDLTUIG-UHFFFAOYSA-N 9-amino-3,3-dimethyl-2,4-dihydro-1h-acridin-1-ol Chemical compound N1=C2C=CC=CC2=C(N)C2=C1CC(C)(C)CC2O PRLZBYKCDLTUIG-UHFFFAOYSA-N 0.000 description 3
- CPBYHTDUBNSBQM-UHFFFAOYSA-N 9H-pyrido[3,4-b]indol-3-ylmethanol Chemical compound N1C2=CC=CC=C2C2=C1C=NC(CO)=C2 CPBYHTDUBNSBQM-UHFFFAOYSA-N 0.000 description 3
- 102000002260 Alkaline Phosphatase Human genes 0.000 description 3
- 108020004774 Alkaline Phosphatase Proteins 0.000 description 3
- 206010003645 Atopy Diseases 0.000 description 3
- 238000011725 BALB/c mouse Methods 0.000 description 3
- UHOVQNZJYSORNB-UHFFFAOYSA-N Benzene Chemical compound C1=CC=CC=C1 UHOVQNZJYSORNB-UHFFFAOYSA-N 0.000 description 3
- LZJRNLRASBVRRX-ZDUSSCGKSA-N Boldine Chemical compound CN1CCC2=CC(O)=C(OC)C3=C2[C@@H]1CC1=C3C=C(OC)C(O)=C1 LZJRNLRASBVRRX-ZDUSSCGKSA-N 0.000 description 3
- 102100025279 C-X-C motif chemokine 11 Human genes 0.000 description 3
- GSDPDUAEOGGTQE-UHFFFAOYSA-N CC1=CC=C(CN2CCN(C)CC2)C=C1 Chemical compound CC1=CC=C(CN2CCN(C)CC2)C=C1 GSDPDUAEOGGTQE-UHFFFAOYSA-N 0.000 description 3
- JRLPEMVDPFPYPJ-UHFFFAOYSA-N CCC1=CC=C(C)C=C1 Chemical compound CCC1=CC=C(C)C=C1 JRLPEMVDPFPYPJ-UHFFFAOYSA-N 0.000 description 3
- 102100025570 Cancer/testis antigen 1 Human genes 0.000 description 3
- 102100025064 Cellular tumor antigen p53 Human genes 0.000 description 3
- 108010055166 Chemokine CCL5 Proteins 0.000 description 3
- 206010009944 Colon cancer Diseases 0.000 description 3
- 102000003886 Glycoproteins Human genes 0.000 description 3
- 108090000288 Glycoproteins Proteins 0.000 description 3
- 108010017080 Granulocyte Colony-Stimulating Factor Proteins 0.000 description 3
- 102000004269 Granulocyte Colony-Stimulating Factor Human genes 0.000 description 3
- BXNJHAXVSOCGBA-UHFFFAOYSA-N Harmine hydrochloride Natural products N1=CC=C2C3=CC=C(OC)C=C3NC2=C1C BXNJHAXVSOCGBA-UHFFFAOYSA-N 0.000 description 3
- 101000856237 Homo sapiens Cancer/testis antigen 1 Proteins 0.000 description 3
- 101000721661 Homo sapiens Cellular tumor antigen p53 Proteins 0.000 description 3
- 102000000589 Interleukin-1 Human genes 0.000 description 3
- 108010002352 Interleukin-1 Proteins 0.000 description 3
- 102000019223 Interleukin-1 receptor Human genes 0.000 description 3
- 108050006617 Interleukin-1 receptor Proteins 0.000 description 3
- KFZMGEQAYNKOFK-UHFFFAOYSA-N Isopropanol Chemical compound CC(C)O KFZMGEQAYNKOFK-UHFFFAOYSA-N 0.000 description 3
- 241000721662 Juniperus Species 0.000 description 3
- 108091034117 Oligonucleotide Proteins 0.000 description 3
- RGCVKNLCSQQDEP-UHFFFAOYSA-N Perphenazine Chemical compound C1CN(CCO)CCN1CCCN1C2=CC(Cl)=CC=C2SC2=CC=CC=C21 RGCVKNLCSQQDEP-UHFFFAOYSA-N 0.000 description 3
- 108091000080 Phosphotransferase Proteins 0.000 description 3
- 206010035226 Plasma cell myeloma Diseases 0.000 description 3
- 206010039491 Sarcoma Diseases 0.000 description 3
- GUGOEEXESWIERI-UHFFFAOYSA-N Terfenadine Chemical compound C1=CC(C(C)(C)C)=CC=C1C(O)CCCN1CCC(C(O)(C=2C=CC=CC=2)C=2C=CC=CC=2)CC1 GUGOEEXESWIERI-UHFFFAOYSA-N 0.000 description 3
- YXFVVABEGXRONW-UHFFFAOYSA-N Toluene Chemical compound CC1=CC=CC=C1 YXFVVABEGXRONW-UHFFFAOYSA-N 0.000 description 3
- 108010018242 Transcription Factor AP-1 Proteins 0.000 description 3
- 102100023132 Transcription factor Jun Human genes 0.000 description 3
- HCHKCACWOHOZIP-UHFFFAOYSA-N Zinc Chemical compound [Zn] HCHKCACWOHOZIP-UHFFFAOYSA-N 0.000 description 3
- JLCPHMBAVCMARE-UHFFFAOYSA-N [3-[[3-[[3-[[3-[[3-[[3-[[3-[[3-[[3-[[3-[[3-[[5-(2-amino-6-oxo-1H-purin-9-yl)-3-[[3-[[3-[[3-[[3-[[3-[[5-(2-amino-6-oxo-1H-purin-9-yl)-3-[[5-(2-amino-6-oxo-1H-purin-9-yl)-3-hydroxyoxolan-2-yl]methoxy-hydroxyphosphoryl]oxyoxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(5-methyl-2,4-dioxopyrimidin-1-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(6-aminopurin-9-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(6-aminopurin-9-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(6-aminopurin-9-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(6-aminopurin-9-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxyoxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(5-methyl-2,4-dioxopyrimidin-1-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(4-amino-2-oxopyrimidin-1-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(5-methyl-2,4-dioxopyrimidin-1-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(5-methyl-2,4-dioxopyrimidin-1-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(6-aminopurin-9-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(6-aminopurin-9-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(4-amino-2-oxopyrimidin-1-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(4-amino-2-oxopyrimidin-1-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(4-amino-2-oxopyrimidin-1-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(6-aminopurin-9-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(4-amino-2-oxopyrimidin-1-yl)oxolan-2-yl]methyl [5-(6-aminopurin-9-yl)-2-(hydroxymethyl)oxolan-3-yl] hydrogen phosphate Polymers Cc1cn(C2CC(OP(O)(=O)OCC3OC(CC3OP(O)(=O)OCC3OC(CC3O)n3cnc4c3nc(N)[nH]c4=O)n3cnc4c3nc(N)[nH]c4=O)C(COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3CO)n3cnc4c(N)ncnc34)n3ccc(N)nc3=O)n3cnc4c(N)ncnc34)n3ccc(N)nc3=O)n3ccc(N)nc3=O)n3ccc(N)nc3=O)n3cnc4c(N)ncnc34)n3cnc4c(N)ncnc34)n3cc(C)c(=O)[nH]c3=O)n3cc(C)c(=O)[nH]c3=O)n3ccc(N)nc3=O)n3cc(C)c(=O)[nH]c3=O)n3cnc4c3nc(N)[nH]c4=O)n3cnc4c(N)ncnc34)n3cnc4c(N)ncnc34)n3cnc4c(N)ncnc34)n3cnc4c(N)ncnc34)O2)c(=O)[nH]c1=O JLCPHMBAVCMARE-UHFFFAOYSA-N 0.000 description 3
- 239000002253 acid Substances 0.000 description 3
- 230000000172 allergic effect Effects 0.000 description 3
- 210000000612 antigen-presenting cell Anatomy 0.000 description 3
- HMBHRMFLDKKSCT-UHFFFAOYSA-N chembl338115 Chemical compound C12=CC(OC)=CC=C2NC2=C1CCN=C2C HMBHRMFLDKKSCT-UHFFFAOYSA-N 0.000 description 3
- 238000006243 chemical reaction Methods 0.000 description 3
- KRKNYBCHXYNGOX-UHFFFAOYSA-N citric acid Chemical compound OC(=O)CC(O)(C(O)=O)CC(O)=O KRKNYBCHXYNGOX-UHFFFAOYSA-N 0.000 description 3
- 210000004443 dendritic cell Anatomy 0.000 description 3
- 238000010790 dilution Methods 0.000 description 3
- 239000012895 dilution Substances 0.000 description 3
- ZMISODWVFHHWNR-UHFFFAOYSA-N diphenyl(4-piperidinyl)methanol Chemical compound C=1C=CC=CC=1C(C=1C=CC=CC=1)(O)C1CCNCC1 ZMISODWVFHHWNR-UHFFFAOYSA-N 0.000 description 3
- CTSPAMFJBXKSOY-UHFFFAOYSA-N ellipticine Chemical compound N1=CC=C2C(C)=C(NC=3C4=CC=CC=3)C4=C(C)C2=C1 CTSPAMFJBXKSOY-UHFFFAOYSA-N 0.000 description 3
- 239000000706 filtrate Substances 0.000 description 3
- 239000012634 fragment Substances 0.000 description 3
- 229960004171 hydroxychloroquine Drugs 0.000 description 3
- 125000002883 imidazolyl group Chemical group 0.000 description 3
- 230000005934 immune activation Effects 0.000 description 3
- 239000012535 impurity Substances 0.000 description 3
- 230000001939 inductive effect Effects 0.000 description 3
- 239000004615 ingredient Substances 0.000 description 3
- 210000004901 leucine-rich repeat Anatomy 0.000 description 3
- 210000000265 leukocyte Anatomy 0.000 description 3
- 201000004792 malaria Diseases 0.000 description 3
- 238000005259 measurement Methods 0.000 description 3
- 230000007246 mechanism Effects 0.000 description 3
- 201000001441 melanoma Diseases 0.000 description 3
- 108020004999 messenger RNA Proteins 0.000 description 3
- 230000000813 microbial effect Effects 0.000 description 3
- 210000001616 monocyte Anatomy 0.000 description 3
- RWIVICVCHVMHMU-UHFFFAOYSA-N n-aminoethylmorpholine Chemical compound NCCN1CCOCC1 RWIVICVCHVMHMU-UHFFFAOYSA-N 0.000 description 3
- 125000001624 naphthyl group Chemical group 0.000 description 3
- 210000000822 natural killer cell Anatomy 0.000 description 3
- 210000000440 neutrophil Anatomy 0.000 description 3
- OGIAAULPRXAQEV-UHFFFAOYSA-N odn 2216 Chemical compound O=C1NC(=O)C(C)=CN1C1OC(COP(O)(=O)OC2C(OC(C2)N2C3=NC=NC(N)=C3N=C2)COP(O)(=O)OC2C(OC(C2)N2C3=C(C(NC(N)=N3)=O)N=C2)COP(O)(=O)OC2C(OC(C2)N2C(N=C(N)C=C2)=O)COP(O)(=O)OC2C(OC(C2)N2C3=NC=NC(N)=C3N=C2)COP(O)(=O)OC2C(OC(C2)N2C3=C(C(NC(N)=N3)=O)N=C2)COP(O)(=O)OC2C(OC(C2)N2C3=C(C(NC(N)=N3)=O)N=C2)COP(O)(=O)OC2C(OC(C2)N2C3=C(C(NC(N)=N3)=O)N=C2)COP(O)(=O)OC2C(OC(C2)N2C3=C(C(NC(N)=N3)=O)N=C2)COP(O)(=O)OC2C(OC(C2)N2C3=C(C(NC(N)=N3)=O)N=C2)COP(O)(O)=O)C(OP(O)(=O)OCC2C(CC(O2)N2C(N=C(N)C=C2)=O)OP(O)(=O)OCC2C(CC(O2)N2C3=C(C(NC(N)=N3)=O)N=C2)OP(O)(=O)OCC2C(CC(O2)N2C(NC(=O)C(C)=C2)=O)OP(O)(=O)OCC2C(CC(O2)N2C(N=C(N)C=C2)=O)OP(O)(=O)OCC2C(CC(O2)N2C3=C(C(NC(N)=N3)=O)N=C2)OP(O)(=O)OCC2C(CC(O2)N2C3=C(C(NC(N)=N3)=O)N=C2)OP(O)(=O)OCC2C(CC(O2)N2C3=C(C(NC(N)=N3)=O)N=C2)OP(O)(=O)OCC2C(CC(O2)N2C3=C(C(NC(N)=N3)=O)N=C2)OP(O)(=O)OCC2C(CC(O2)N2C3=C(C(NC(N)=N3)=O)N=C2)OP(O)(=O)OCC2C(CC(O2)N2C3=C(C(NC(N)=N3)=O)N=C2)O)C1 OGIAAULPRXAQEV-UHFFFAOYSA-N 0.000 description 3
- 210000000056 organ Anatomy 0.000 description 3
- 244000045947 parasite Species 0.000 description 3
- 244000052769 pathogen Species 0.000 description 3
- 125000005561 phenanthryl group Chemical group 0.000 description 3
- 102000020233 phosphotransferase Human genes 0.000 description 3
- 125000000587 piperidin-1-yl group Chemical group [H]C1([H])N(*)C([H])([H])C([H])([H])C([H])([H])C1([H])[H] 0.000 description 3
- 210000005134 plasmacytoid dendritic cell Anatomy 0.000 description 3
- 229920001184 polypeptide Polymers 0.000 description 3
- 125000004076 pyridyl group Chemical group 0.000 description 3
- 125000002112 pyrrolidino group Chemical group [*]N1C([H])([H])C([H])([H])C([H])([H])C1([H])[H] 0.000 description 3
- 150000003248 quinolines Chemical class 0.000 description 3
- 239000011541 reaction mixture Substances 0.000 description 3
- 230000019491 signal transduction Effects 0.000 description 3
- 239000002904 solvent Substances 0.000 description 3
- 238000007920 subcutaneous administration Methods 0.000 description 3
- 239000000725 suspension Substances 0.000 description 3
- 239000003826 tablet Substances 0.000 description 3
- VWBVCOPVKXNMMZ-UHFFFAOYSA-N 1,5-diaminoanthracene-9,10-dione Chemical group O=C1C2=C(N)C=CC=C2C(=O)C2=C1C=CC=C2N VWBVCOPVKXNMMZ-UHFFFAOYSA-N 0.000 description 2
- FLNXBVJLPJNOSI-UHFFFAOYSA-N 1-[2-[(4-chlorophenyl)-phenylmethoxy]ethyl]piperidine Chemical compound C1=CC(Cl)=CC=C1C(C=1C=CC=CC=1)OCCN1CCCCC1 FLNXBVJLPJNOSI-UHFFFAOYSA-N 0.000 description 2
- RXPRRQLKFXBCSJ-HLAWJBBLSA-N 16-epivincamine Chemical compound C1=CC=C2C(CCN3CCC4)=C5[C@@H]3[C@]4(CC)C[C@@](O)(C(=O)OC)N5C2=C1 RXPRRQLKFXBCSJ-HLAWJBBLSA-N 0.000 description 2
- GYSCQDBTSDBCGY-UHFFFAOYSA-N 2,3-dihydro-1h-cyclopenta[b]quinolin-9-amine Chemical compound C1=CC=C2C(N)=C(CCC3)C3=NC2=C1 GYSCQDBTSDBCGY-UHFFFAOYSA-N 0.000 description 2
- WRUVTYDQVPMYDZ-UHFFFAOYSA-N 2,4,9-trimethylbenzo[b][1,8]naphthyridin-5-amine Chemical compound C1=CC=C(C)C2=NC3=NC(C)=CC(C)=C3C(N)=C21 WRUVTYDQVPMYDZ-UHFFFAOYSA-N 0.000 description 2
- GZONZEUVQKWERU-UHFFFAOYSA-N 2,7-dimethylquinolino[2,3-b]quinolin-11-amine Chemical compound CC1=CC=CC2=C(N)C3=CC4=CC(C)=CC=C4N=C3N=C21 GZONZEUVQKWERU-UHFFFAOYSA-N 0.000 description 2
- ZBIAKUMOEKILTF-UHFFFAOYSA-N 2-[4-[4,4-bis(4-fluorophenyl)butyl]-1-piperazinyl]-N-(2,6-dimethylphenyl)acetamide Chemical compound CC1=CC=CC(C)=C1NC(=O)CN1CCN(CCCC(C=2C=CC(F)=CC=2)C=2C=CC(F)=CC=2)CC1 ZBIAKUMOEKILTF-UHFFFAOYSA-N 0.000 description 2
- WQMAANNAZKNUDL-UHFFFAOYSA-N 2-dimethylamino-1-chloro-ethane hydrochloride Natural products CN(C)CCCl WQMAANNAZKNUDL-UHFFFAOYSA-N 0.000 description 2
- LQLJZSJKRYTKTP-UHFFFAOYSA-N 2-dimethylaminoethyl chloride hydrochloride Chemical compound Cl.CN(C)CCCl LQLJZSJKRYTKTP-UHFFFAOYSA-N 0.000 description 2
- LXEOASMACBAUBO-UHFFFAOYSA-N 3,3-dimethyl-4h-acridin-9-amine Chemical compound C1=CC=C2C(N)=C(C=CC(C)(C)C3)C3=NC2=C1 LXEOASMACBAUBO-UHFFFAOYSA-N 0.000 description 2
- XKCXIUDPGSNQIQ-UHFFFAOYSA-N 3-hydroxymethyl-beta-carboline Natural products C12=CC=CC=C2NC2=C1C=CN=C2CO XKCXIUDPGSNQIQ-UHFFFAOYSA-N 0.000 description 2
- 101800000504 3C-like protease Proteins 0.000 description 2
- OSWFIVFLDKOXQC-UHFFFAOYSA-N 4-(3-methoxyphenyl)aniline Chemical compound COC1=CC=CC(C=2C=CC(N)=CC=2)=C1 OSWFIVFLDKOXQC-UHFFFAOYSA-N 0.000 description 2
- FQYRLEXKXQRZDH-UHFFFAOYSA-N 4-aminoquinoline Chemical group C1=CC=C2C(N)=CC=NC2=C1 FQYRLEXKXQRZDH-UHFFFAOYSA-N 0.000 description 2
- OBHKONRNYCDRKM-UHFFFAOYSA-N 4-chloro-2-phenylquinazoline Chemical compound N=1C2=CC=CC=C2C(Cl)=NC=1C1=CC=CC=C1 OBHKONRNYCDRKM-UHFFFAOYSA-N 0.000 description 2
- UOQXIWFBQSVDPP-UHFFFAOYSA-N 4-fluorobenzaldehyde Chemical compound FC1=CC=C(C=O)C=C1 UOQXIWFBQSVDPP-UHFFFAOYSA-N 0.000 description 2
- RMFDFWIUWQZPBH-UHFFFAOYSA-N 6-methyl-1-phenyl-2,3-dihydropyrrolo[2,3-b]quinolin-4-amine Chemical compound C1CC2=C(N)C3=CC(C)=CC=C3N=C2N1C1=CC=CC=C1 RMFDFWIUWQZPBH-UHFFFAOYSA-N 0.000 description 2
- GOXLVSZAUDIXPN-UHFFFAOYSA-N 7,8,9,10-tetrahydro-6h-cyclohepta[b]quinolin-11-amine Chemical compound C1CCCCC2=NC3=CC=CC=C3C(N)=C21 GOXLVSZAUDIXPN-UHFFFAOYSA-N 0.000 description 2
- IONUJCUELBLOCX-UHFFFAOYSA-N 7-fluoro-2,4-dimethylbenzo[b][1,8]naphthyridin-5-amine Chemical compound C1=C(F)C=CC2=NC3=NC(C)=CC(C)=C3C(N)=C21 IONUJCUELBLOCX-UHFFFAOYSA-N 0.000 description 2
- 102100036464 Activated RNA polymerase II transcriptional coactivator p15 Human genes 0.000 description 2
- 208000035285 Allergic Seasonal Rhinitis Diseases 0.000 description 2
- 206010027654 Allergic conditions Diseases 0.000 description 2
- 241000219496 Alnus Species 0.000 description 2
- 108090000020 Alpha-catenin Proteins 0.000 description 2
- 102000003730 Alpha-catenin Human genes 0.000 description 2
- QGZKDVFQNNGYKY-UHFFFAOYSA-N Ammonia Chemical compound N QGZKDVFQNNGYKY-UHFFFAOYSA-N 0.000 description 2
- 235000003261 Artemisia vulgaris Nutrition 0.000 description 2
- 240000006891 Artemisia vulgaris Species 0.000 description 2
- 244000075850 Avena orientalis Species 0.000 description 2
- 108020000946 Bacterial DNA Proteins 0.000 description 2
- DWRXFEITVBNRMK-UHFFFAOYSA-N Beta-D-1-Arabinofuranosylthymine Natural products O=C1NC(=O)C(C)=CN1C1C(O)C(O)C(CO)O1 DWRXFEITVBNRMK-UHFFFAOYSA-N 0.000 description 2
- 102000015735 Beta-catenin Human genes 0.000 description 2
- 108060000903 Beta-catenin Proteins 0.000 description 2
- 102100026189 Beta-galactosidase Human genes 0.000 description 2
- 108091003079 Bovine Serum Albumin Proteins 0.000 description 2
- 206010006187 Breast cancer Diseases 0.000 description 2
- 208000026310 Breast neoplasm Diseases 0.000 description 2
- 102100021943 C-C motif chemokine 2 Human genes 0.000 description 2
- 101710155857 C-C motif chemokine 2 Proteins 0.000 description 2
- 101710098272 C-X-C motif chemokine 11 Proteins 0.000 description 2
- OCJYIGYOJCODJL-UHFFFAOYSA-N CC1=CC(CN2CCN(C(C3=CC=CC=C3)C3=CC=C(Cl)C=C3)CC2)=CC=C1.Cl.Cl Chemical compound CC1=CC(CN2CCN(C(C3=CC=CC=C3)C3=CC=C(Cl)C=C3)CC2)=CC=C1.Cl.Cl OCJYIGYOJCODJL-UHFFFAOYSA-N 0.000 description 2
- IVSZLXZYQVIEFR-UHFFFAOYSA-N CC1=CC=CC(C)=C1 Chemical compound CC1=CC=CC(C)=C1 IVSZLXZYQVIEFR-UHFFFAOYSA-N 0.000 description 2
- JXFVMNFKABWTHD-UHFFFAOYSA-N CCCC1=CC=C(C)C=C1 Chemical compound CCCC1=CC=C(C)C=C1 JXFVMNFKABWTHD-UHFFFAOYSA-N 0.000 description 2
- XSCGXQMFQXDFCW-UHFFFAOYSA-N CN(C)CCCN1C2=CC=CC=C2SC2=C1C=C(C(F)(F)F)C=C2.Cl Chemical compound CN(C)CCCN1C2=CC=CC=C2SC2=C1C=C(C(F)(F)F)C=C2.Cl XSCGXQMFQXDFCW-UHFFFAOYSA-N 0.000 description 2
- HQMPUWYVYYFFSK-UHFFFAOYSA-N CN1CCC2=C1N=C1C=CC=CC1=C2N Chemical compound CN1CCC2=C1N=C1C=CC=CC1=C2N HQMPUWYVYYFFSK-UHFFFAOYSA-N 0.000 description 2
- UVKZSORBKUEBAZ-UHFFFAOYSA-N CN1CCN(C(C2=CC=CC=C2)C2=CC=CC=C2)CC1.Cl Chemical compound CN1CCN(C(C2=CC=CC=C2)C2=CC=CC=C2)CC1.Cl UVKZSORBKUEBAZ-UHFFFAOYSA-N 0.000 description 2
- 102000000905 Cadherin Human genes 0.000 description 2
- 108050007957 Cadherin Proteins 0.000 description 2
- 201000009030 Carcinoma Diseases 0.000 description 2
- 102100028906 Catenin delta-1 Human genes 0.000 description 2
- 244000281762 Chenopodium ambrosioides Species 0.000 description 2
- 235000000509 Chenopodium ambrosioides Nutrition 0.000 description 2
- 235000005490 Chenopodium botrys Nutrition 0.000 description 2
- JWBOIMRXGHLCPP-UHFFFAOYSA-N Chloditan Chemical compound C=1C=CC=C(Cl)C=1C(C(Cl)Cl)C1=CC=C(Cl)C=C1 JWBOIMRXGHLCPP-UHFFFAOYSA-N 0.000 description 2
- HEDRZPFGACZZDS-UHFFFAOYSA-N Chloroform Chemical compound ClC(Cl)Cl HEDRZPFGACZZDS-UHFFFAOYSA-N 0.000 description 2
- 208000006332 Choriocarcinoma Diseases 0.000 description 2
- 108010077544 Chromatin Proteins 0.000 description 2
- 102000007644 Colony-Stimulating Factors Human genes 0.000 description 2
- 108010071942 Colony-Stimulating Factors Proteins 0.000 description 2
- 108020004635 Complementary DNA Proteins 0.000 description 2
- 241000711573 Coronaviridae Species 0.000 description 2
- 240000005109 Cryptomeria japonica Species 0.000 description 2
- 102100023033 Cyclic AMP-dependent transcription factor ATF-2 Human genes 0.000 description 2
- 241000701022 Cytomegalovirus Species 0.000 description 2
- 201000004624 Dermatitis Diseases 0.000 description 2
- 101100216227 Dictyostelium discoideum anapc3 gene Proteins 0.000 description 2
- ROSDSFDQCJNGOL-UHFFFAOYSA-N Dimethylamine Chemical compound CNC ROSDSFDQCJNGOL-UHFFFAOYSA-N 0.000 description 2
- 102100025012 Dipeptidyl peptidase 4 Human genes 0.000 description 2
- 108010031111 EBV-encoded nuclear antigen 1 Proteins 0.000 description 2
- 241000709661 Enterovirus Species 0.000 description 2
- 208000004262 Food Hypersensitivity Diseases 0.000 description 2
- 102100040578 G antigen 7 Human genes 0.000 description 2
- 101000930822 Giardia intestinalis Dipeptidyl-peptidase 4 Proteins 0.000 description 2
- 108010043121 Green Fluorescent Proteins Proteins 0.000 description 2
- 102000004144 Green Fluorescent Proteins Human genes 0.000 description 2
- 241000700721 Hepatitis B virus Species 0.000 description 2
- 208000005176 Hepatitis C Diseases 0.000 description 2
- 102000006947 Histones Human genes 0.000 description 2
- 108010033040 Histones Proteins 0.000 description 2
- 241000282412 Homo Species 0.000 description 2
- 101000974934 Homo sapiens Cyclic AMP-dependent transcription factor ATF-2 Proteins 0.000 description 2
- 101000893968 Homo sapiens G antigen 7 Proteins 0.000 description 2
- 101000997829 Homo sapiens Glial cell line-derived neurotrophic factor Proteins 0.000 description 2
- 101000959820 Homo sapiens Interferon alpha-1/13 Proteins 0.000 description 2
- 101001011382 Homo sapiens Interferon regulatory factor 3 Proteins 0.000 description 2
- 101001032342 Homo sapiens Interferon regulatory factor 7 Proteins 0.000 description 2
- 101001057504 Homo sapiens Interferon-stimulated gene 20 kDa protein Proteins 0.000 description 2
- 101001055144 Homo sapiens Interleukin-2 receptor subunit alpha Proteins 0.000 description 2
- 101000578784 Homo sapiens Melanoma antigen recognized by T-cells 1 Proteins 0.000 description 2
- 101000914484 Homo sapiens T-lymphocyte activation antigen CD80 Proteins 0.000 description 2
- 101000831567 Homo sapiens Toll-like receptor 2 Proteins 0.000 description 2
- 241000725303 Human immunodeficiency virus Species 0.000 description 2
- 241000701806 Human papillomavirus Species 0.000 description 2
- WTDHULULXKLSOZ-UHFFFAOYSA-N Hydroxylamine hydrochloride Chemical compound Cl.ON WTDHULULXKLSOZ-UHFFFAOYSA-N 0.000 description 2
- 108060006678 I-kappa-B kinase Proteins 0.000 description 2
- 102000001284 I-kappa-B kinase Human genes 0.000 description 2
- 108010043496 Immunoglobulin Idiotypes Proteins 0.000 description 2
- 102100040019 Interferon alpha-1/13 Human genes 0.000 description 2
- 102100026720 Interferon beta Human genes 0.000 description 2
- 102100029843 Interferon regulatory factor 3 Human genes 0.000 description 2
- 102100038070 Interferon regulatory factor 7 Human genes 0.000 description 2
- 108090000467 Interferon-beta Proteins 0.000 description 2
- 102000014150 Interferons Human genes 0.000 description 2
- 108010050904 Interferons Proteins 0.000 description 2
- 102000003810 Interleukin-18 Human genes 0.000 description 2
- 108090000171 Interleukin-18 Proteins 0.000 description 2
- 102100026878 Interleukin-2 receptor subunit alpha Human genes 0.000 description 2
- 108010002335 Interleukin-9 Proteins 0.000 description 2
- 102000000585 Interleukin-9 Human genes 0.000 description 2
- 108010055717 JNK Mitogen-Activated Protein Kinases Proteins 0.000 description 2
- 206010058467 Lung neoplasm malignant Diseases 0.000 description 2
- 108010010995 MART-1 Antigen Proteins 0.000 description 2
- 108700018351 Major Histocompatibility Complex Proteins 0.000 description 2
- CJUOSBUQOWKEKJ-UHFFFAOYSA-N Mebhydrolin napadisilate Chemical compound C1=CC=C2C(S(=O)(=O)O)=CC=CC2=C1S(O)(=O)=O.C1N(C)CCC2=C1C1=CC=CC=C1N2CC1=CC=CC=C1.C1N(C)CCC2=C1C1=CC=CC=C1N2CC1=CC=CC=C1 CJUOSBUQOWKEKJ-UHFFFAOYSA-N 0.000 description 2
- UEQUQVLFIPOEMF-UHFFFAOYSA-N Mianserin Chemical compound C1C2=CC=CC=C2N2CCN(C)CC2C2=CC=CC=C21 UEQUQVLFIPOEMF-UHFFFAOYSA-N 0.000 description 2
- 108090000744 Mitogen-Activated Protein Kinase Kinases Proteins 0.000 description 2
- 102000004232 Mitogen-Activated Protein Kinase Kinases Human genes 0.000 description 2
- 208000034578 Multiple myelomas Diseases 0.000 description 2
- MSFSPUZXLOGKHJ-UHFFFAOYSA-N Muraminsaeure Natural products OC(=O)C(C)OC1C(N)C(O)OC(CO)C1O MSFSPUZXLOGKHJ-UHFFFAOYSA-N 0.000 description 2
- WGQKYBSKWIADBV-UHFFFAOYSA-N NCC1=CC=CC=C1 Chemical compound NCC1=CC=CC=C1 WGQKYBSKWIADBV-UHFFFAOYSA-N 0.000 description 2
- DDRPCXLAQZKBJP-UHFFFAOYSA-N NCC1=CC=CO1 Chemical compound NCC1=CC=CO1 DDRPCXLAQZKBJP-UHFFFAOYSA-N 0.000 description 2
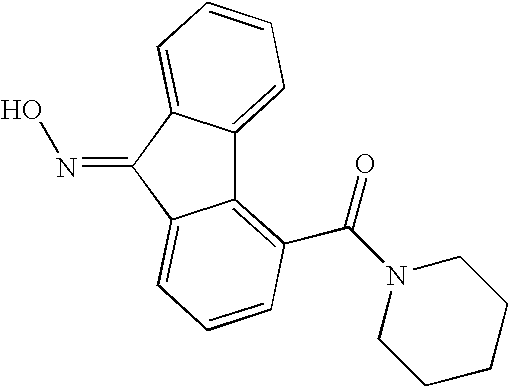
- AWGHPWGLEOCPRZ-UHFFFAOYSA-N NNC1=NC2=C(C(=O)N1C1=CC=CC=C1)C1(CCCC1)CC1=C2C=CC=C1 Chemical compound NNC1=NC2=C(C(=O)N1C1=CC=CC=C1)C1(CCCC1)CC1=C2C=CC=C1 AWGHPWGLEOCPRZ-UHFFFAOYSA-N 0.000 description 2
- 102000007999 Nuclear Proteins Human genes 0.000 description 2
- 108010089610 Nuclear Proteins Proteins 0.000 description 2
- 108010047956 Nucleosomes Proteins 0.000 description 2
- XDJIQPKRIDXXSP-UHFFFAOYSA-N O=C1C2=CC=C3C(=O)N(C4=CC=CC=C4)NC3=C2C(=O)C2=C1C=CC=C2 Chemical compound O=C1C2=CC=C3C(=O)N(C4=CC=CC=C4)NC3=C2C(=O)C2=C1C=CC=C2 XDJIQPKRIDXXSP-UHFFFAOYSA-N 0.000 description 2
- 241000795633 Olea <sea slug> Species 0.000 description 2
- 206010033128 Ovarian cancer Diseases 0.000 description 2
- 206010061535 Ovarian neoplasm Diseases 0.000 description 2
- KDLHZDBZIXYQEI-UHFFFAOYSA-N Palladium Chemical compound [Pd] KDLHZDBZIXYQEI-UHFFFAOYSA-N 0.000 description 2
- 206010061902 Pancreatic neoplasm Diseases 0.000 description 2
- 229930182555 Penicillin Natural products 0.000 description 2
- JGSARLDLIJGVTE-MBNYWOFBSA-N Penicillin G Chemical compound N([C@H]1[C@H]2SC([C@@H](N2C1=O)C(O)=O)(C)C)C(=O)CC1=CC=CC=C1 JGSARLDLIJGVTE-MBNYWOFBSA-N 0.000 description 2
- 108010013639 Peptidoglycan Proteins 0.000 description 2
- NBIIXXVUZAFLBC-UHFFFAOYSA-N Phosphoric acid Chemical compound OP(O)(O)=O NBIIXXVUZAFLBC-UHFFFAOYSA-N 0.000 description 2
- 206010060862 Prostate cancer Diseases 0.000 description 2
- 208000000236 Prostatic Neoplasms Diseases 0.000 description 2
- JUJWROOIHBZHMG-UHFFFAOYSA-N Pyridine Chemical compound C1=CC=NC=C1 JUJWROOIHBZHMG-UHFFFAOYSA-N 0.000 description 2
- 206010039085 Rhinitis allergic Diseases 0.000 description 2
- UIIMBOGNXHQVGW-UHFFFAOYSA-M Sodium bicarbonate Chemical compound [Na+].OC([O-])=O UIIMBOGNXHQVGW-UHFFFAOYSA-M 0.000 description 2
- FAPWRFPIFSIZLT-UHFFFAOYSA-M Sodium chloride Chemical compound [Na+].[Cl-] FAPWRFPIFSIZLT-UHFFFAOYSA-M 0.000 description 2
- 235000011684 Sorghum saccharatum Nutrition 0.000 description 2
- 244000062793 Sorghum vulgare Species 0.000 description 2
- 241000194017 Streptococcus Species 0.000 description 2
- ZCDNRPPFBQDQHR-SSYATKPKSA-N Syrosingopine Chemical compound C1=C(OC)C(OC(=O)OCC)=C(OC)C=C1C(=O)O[C@H]1[C@H](OC)[C@@H](C(=O)OC)[C@H]2C[C@@H]3C(NC=4C5=CC=C(OC)C=4)=C5CCN3C[C@H]2C1 ZCDNRPPFBQDQHR-SSYATKPKSA-N 0.000 description 2
- 201000009594 Systemic Scleroderma Diseases 0.000 description 2
- 206010042953 Systemic sclerosis Diseases 0.000 description 2
- 102000016266 T-Cell Antigen Receptors Human genes 0.000 description 2
- 102100027222 T-lymphocyte activation antigen CD80 Human genes 0.000 description 2
- 208000024313 Testicular Neoplasms Diseases 0.000 description 2
- 206010057644 Testis cancer Diseases 0.000 description 2
- 241000218636 Thuja Species 0.000 description 2
- 102100024333 Toll-like receptor 2 Human genes 0.000 description 2
- 102000040945 Transcription factor Human genes 0.000 description 2
- 108091023040 Transcription factor Proteins 0.000 description 2
- 108090001012 Transforming Growth Factor beta Proteins 0.000 description 2
- 102000004887 Transforming Growth Factor beta Human genes 0.000 description 2
- ISAKRJDGNUQOIC-UHFFFAOYSA-N Uracil Chemical compound O=C1C=CNC(=O)N1 ISAKRJDGNUQOIC-UHFFFAOYSA-N 0.000 description 2
- 241000251539 Vertebrata <Metazoa> Species 0.000 description 2
- SORARJZLMNRBAQ-UHFFFAOYSA-N [H]N(C)CCCN(C)C Chemical compound [H]N(C)CCCN(C)C SORARJZLMNRBAQ-UHFFFAOYSA-N 0.000 description 2
- ZOGWRTUVHRNZEG-UHFFFAOYSA-N [H]N(CCCN(C)C)C1=C(C)C=CC=C1 Chemical compound [H]N(CCCN(C)C)C1=C(C)C=CC=C1 ZOGWRTUVHRNZEG-UHFFFAOYSA-N 0.000 description 2
- SXFMDMJOWMUETN-UHFFFAOYSA-N [H]N(CCN(C)C)C1=NC(N2CCN(C)CC2)=NC2=CC(OC)=C(OC)C=C21 Chemical compound [H]N(CCN(C)C)C1=NC(N2CCN(C)CC2)=NC2=CC(OC)=C(OC)C=C21 SXFMDMJOWMUETN-UHFFFAOYSA-N 0.000 description 2
- 230000003213 activating effect Effects 0.000 description 2
- 239000004480 active ingredient Substances 0.000 description 2
- 230000009692 acute damage Effects 0.000 description 2
- 230000001154 acute effect Effects 0.000 description 2
- 230000004721 adaptive immunity Effects 0.000 description 2
- OIRDTQYFTABQOQ-KQYNXXCUSA-N adenosine Chemical compound C1=NC=2C(N)=NC=NC=2N1[C@@H]1O[C@H](CO)[C@@H](O)[C@H]1O OIRDTQYFTABQOQ-KQYNXXCUSA-N 0.000 description 2
- 239000002671 adjuvant Substances 0.000 description 2
- 230000002411 adverse Effects 0.000 description 2
- 201000010105 allergic rhinitis Diseases 0.000 description 2
- 108010026331 alpha-Fetoproteins Proteins 0.000 description 2
- 102000013529 alpha-Fetoproteins Human genes 0.000 description 2
- 150000001412 amines Chemical class 0.000 description 2
- KRMDCWKBEZIMAB-UHFFFAOYSA-N amitriptyline Chemical compound C1CC2=CC=CC=C2C(=CCCN(C)C)C2=CC=CC=C21 KRMDCWKBEZIMAB-UHFFFAOYSA-N 0.000 description 2
- 238000004458 analytical method Methods 0.000 description 2
- NUZWLKWWNNJHPT-UHFFFAOYSA-N anthralin Chemical compound C1C2=CC=CC(O)=C2C(=O)C2=C1C=CC=C2O NUZWLKWWNNJHPT-UHFFFAOYSA-N 0.000 description 2
- 239000003430 antimalarial agent Substances 0.000 description 2
- 238000013459 approach Methods 0.000 description 2
- 230000001363 autoimmune Effects 0.000 description 2
- RWCCWEUUXYIKHB-UHFFFAOYSA-N benzophenone Chemical compound C=1C=CC=CC=1C(=O)C1=CC=CC=C1 RWCCWEUUXYIKHB-UHFFFAOYSA-N 0.000 description 2
- 239000012965 benzophenone Substances 0.000 description 2
- 108010005774 beta-Galactosidase Proteins 0.000 description 2
- IQFYYKKMVGJFEH-UHFFFAOYSA-N beta-L-thymidine Natural products O=C1NC(=O)C(C)=CN1C1OC(CO)C(O)C1 IQFYYKKMVGJFEH-UHFFFAOYSA-N 0.000 description 2
- 239000011230 binding agent Substances 0.000 description 2
- 230000029918 bioluminescence Effects 0.000 description 2
- 238000005415 bioluminescence Methods 0.000 description 2
- 238000009835 boiling Methods 0.000 description 2
- 210000004556 brain Anatomy 0.000 description 2
- 239000012267 brine Substances 0.000 description 2
- 239000006172 buffering agent Substances 0.000 description 2
- 238000010804 cDNA synthesis Methods 0.000 description 2
- 239000002775 capsule Substances 0.000 description 2
- 230000008859 change Effects 0.000 description 2
- OSASVXMJTNOKOY-UHFFFAOYSA-N chlorobutanol Chemical compound CC(C)(O)C(Cl)(Cl)Cl OSASVXMJTNOKOY-UHFFFAOYSA-N 0.000 description 2
- ZPEIMTDSQAKGNT-UHFFFAOYSA-N chlorpromazine Chemical compound C1=C(Cl)C=C2N(CCCN(C)C)C3=CC=CC=C3SC2=C1 ZPEIMTDSQAKGNT-UHFFFAOYSA-N 0.000 description 2
- HVYWMOMLDIMFJA-DPAQBDIFSA-N cholesterol Chemical compound C1C=C2C[C@@H](O)CC[C@]2(C)[C@@H]2[C@@H]1[C@@H]1CC[C@H]([C@H](C)CCCC(C)C)[C@@]1(C)CC2 HVYWMOMLDIMFJA-DPAQBDIFSA-N 0.000 description 2
- 210000003483 chromatin Anatomy 0.000 description 2
- 230000009693 chronic damage Effects 0.000 description 2
- 229960004022 clotrimazole Drugs 0.000 description 2
- 229940047120 colony stimulating factors Drugs 0.000 description 2
- 239000002299 complementary DNA Substances 0.000 description 2
- 201000003278 cryoglobulinemia Diseases 0.000 description 2
- 230000016396 cytokine production Effects 0.000 description 2
- 230000001086 cytosolic effect Effects 0.000 description 2
- 210000001151 cytotoxic T lymphocyte Anatomy 0.000 description 2
- 108010031971 delta catenin Proteins 0.000 description 2
- 239000005547 deoxyribonucleotide Substances 0.000 description 2
- 125000002637 deoxyribonucleotide group Chemical group 0.000 description 2
- 239000002274 desiccant Substances 0.000 description 2
- 238000011161 development Methods 0.000 description 2
- 150000004985 diamines Chemical group 0.000 description 2
- 235000014113 dietary fatty acids Nutrition 0.000 description 2
- 230000004069 differentiation Effects 0.000 description 2
- 239000006185 dispersion Substances 0.000 description 2
- 239000003937 drug carrier Substances 0.000 description 2
- 238000012377 drug delivery Methods 0.000 description 2
- 239000002158 endotoxin Substances 0.000 description 2
- LIWAQLJGPBVORC-UHFFFAOYSA-N ethylmethylamine Chemical compound CCNC LIWAQLJGPBVORC-UHFFFAOYSA-N 0.000 description 2
- 239000000194 fatty acid Substances 0.000 description 2
- 229930195729 fatty acid Natural products 0.000 description 2
- 150000004665 fatty acids Chemical class 0.000 description 2
- 239000012894 fetal calf serum Substances 0.000 description 2
- 238000000684 flow cytometry Methods 0.000 description 2
- SMANXXCATUTDDT-QPJJXVBHSA-N flunarizine Chemical compound C1=CC(F)=CC=C1C(C=1C=CC(F)=CC=1)N1CCN(C\C=C\C=2C=CC=CC=2)CC1 SMANXXCATUTDDT-QPJJXVBHSA-N 0.000 description 2
- 235000020932 food allergy Nutrition 0.000 description 2
- 230000037406 food intake Effects 0.000 description 2
- 235000019253 formic acid Nutrition 0.000 description 2
- 238000009472 formulation Methods 0.000 description 2
- 125000002541 furyl group Chemical group 0.000 description 2
- 108010084448 gamma Catenin Proteins 0.000 description 2
- 102000054078 gamma Catenin Human genes 0.000 description 2
- 150000002270 gangliosides Chemical class 0.000 description 2
- 210000003714 granulocyte Anatomy 0.000 description 2
- 230000004727 humoral immunity Effects 0.000 description 2
- 239000001257 hydrogen Substances 0.000 description 2
- 229910052739 hydrogen Inorganic materials 0.000 description 2
- VNPLYCKZIUTKJM-UHFFFAOYSA-N hydron;7-methoxy-1-methyl-9h-pyrido[3,4-b]indole;chloride Chemical compound [Cl-].C1=CN=C(C)C2=C1C1=CC=C(OC)C=C1[NH2+]2 VNPLYCKZIUTKJM-UHFFFAOYSA-N 0.000 description 2
- ZQDWXGKKHFNSQK-UHFFFAOYSA-N hydroxyzine Chemical compound C1CN(CCOCCO)CCN1C(C=1C=CC(Cl)=CC=1)C1=CC=CC=C1 ZQDWXGKKHFNSQK-UHFFFAOYSA-N 0.000 description 2
- 230000009610 hypersensitivity Effects 0.000 description 2
- 230000002519 immonomodulatory effect Effects 0.000 description 2
- 230000005965 immune activity Effects 0.000 description 2
- 230000005931 immune cell recruitment Effects 0.000 description 2
- 230000008102 immune modulation Effects 0.000 description 2
- 239000007943 implant Substances 0.000 description 2
- 238000002513 implantation Methods 0.000 description 2
- 238000012750 in vivo screening Methods 0.000 description 2
- 208000027866 inflammatory disease Diseases 0.000 description 2
- 230000031146 intracellular signal transduction Effects 0.000 description 2
- 210000003734 kidney Anatomy 0.000 description 2
- 208000032839 leukemia Diseases 0.000 description 2
- 150000002632 lipids Chemical class 0.000 description 2
- 229920006008 lipopolysaccharide Polymers 0.000 description 2
- 208000014018 liver neoplasm Diseases 0.000 description 2
- 201000005202 lung cancer Diseases 0.000 description 2
- 208000020816 lung neoplasm Diseases 0.000 description 2
- 210000002540 macrophage Anatomy 0.000 description 2
- 230000003211 malignant effect Effects 0.000 description 2
- 208000015486 malignant pancreatic neoplasm Diseases 0.000 description 2
- 239000000463 material Substances 0.000 description 2
- CRJHBCPQHRVYBS-UHFFFAOYSA-N mesoridazine besylate Chemical compound OS(=O)(=O)C1=CC=CC=C1.CN1CCCCC1CCN1C2=CC(S(C)=O)=CC=C2SC2=CC=CC=C21 CRJHBCPQHRVYBS-UHFFFAOYSA-N 0.000 description 2
- VNWKTOKETHGBQD-UHFFFAOYSA-N methane Chemical compound C VNWKTOKETHGBQD-UHFFFAOYSA-N 0.000 description 2
- AKRXOIHOWVDUBX-UHFFFAOYSA-N n',n'-dimethyl-n-(2-phenylquinazolin-4-yl)ethane-1,2-diamine Chemical compound N=1C2=CC=CC=C2C(NCCN(C)C)=NC=1C1=CC=CC=C1 AKRXOIHOWVDUBX-UHFFFAOYSA-N 0.000 description 2
- NBTOWFLEICHOOL-UHFFFAOYSA-N n',n'-dimethyl-n-[2-(4-phenylphenyl)quinazolin-4-yl]ethane-1,2-diamine Chemical compound N=1C2=CC=CC=C2C(NCCN(C)C)=NC=1C(C=C1)=CC=C1C1=CC=CC=C1 NBTOWFLEICHOOL-UHFFFAOYSA-N 0.000 description 2
- JBGOJKSJKCDVJB-UHFFFAOYSA-N n,n-dimethyl-2-(2-phenylquinazolin-4-yl)oxyethanamine Chemical compound N=1C2=CC=CC=C2C(OCCN(C)C)=NC=1C1=CC=CC=C1 JBGOJKSJKCDVJB-UHFFFAOYSA-N 0.000 description 2
- AVZWCYTVUMJYQE-UHFFFAOYSA-N n,n-dimethyl-2-[2-[4-(4-methylpiperazin-1-yl)phenyl]quinazolin-4-yl]oxyethanamine Chemical compound N=1C2=CC=CC=C2C(OCCN(C)C)=NC=1C(C=C1)=CC=C1N1CCN(C)CC1 AVZWCYTVUMJYQE-UHFFFAOYSA-N 0.000 description 2
- 150000003833 nucleoside derivatives Chemical group 0.000 description 2
- 210000001623 nucleosome Anatomy 0.000 description 2
- 101800000607 p15 Proteins 0.000 description 2
- 201000002528 pancreatic cancer Diseases 0.000 description 2
- 208000008443 pancreatic carcinoma Diseases 0.000 description 2
- 238000007911 parenteral administration Methods 0.000 description 2
- 230000037361 pathway Effects 0.000 description 2
- 102000007863 pattern recognition receptors Human genes 0.000 description 2
- 108010089193 pattern recognition receptors Proteins 0.000 description 2
- 229940049954 penicillin Drugs 0.000 description 2
- 210000005259 peripheral blood Anatomy 0.000 description 2
- 239000011886 peripheral blood Substances 0.000 description 2
- 239000000546 pharmaceutical excipient Substances 0.000 description 2
- YVUQSNJEYSNKRX-UHFFFAOYSA-N pimozide Chemical compound C1=CC(F)=CC=C1C(C=1C=CC(F)=CC=1)CCCN1CCC(N2C(NC3=CC=CC=C32)=O)CC1 YVUQSNJEYSNKRX-UHFFFAOYSA-N 0.000 description 2
- 201000006292 polyarteritis nodosa Diseases 0.000 description 2
- 230000034190 positive regulation of NF-kappaB transcription factor activity Effects 0.000 description 2
- 239000000843 powder Substances 0.000 description 2
- 239000003755 preservative agent Substances 0.000 description 2
- 230000002265 prevention Effects 0.000 description 2
- RQXCLMGKHJWMOA-UHFFFAOYSA-N pridinol Chemical compound C=1C=CC=CC=1C(C=1C=CC=CC=1)(O)CCN1CCCCC1 RQXCLMGKHJWMOA-UHFFFAOYSA-N 0.000 description 2
- 230000001737 promoting effect Effects 0.000 description 2
- 125000004546 quinazolin-4-yl group Chemical group N1=CN=C(C2=CC=CC=C12)* 0.000 description 2
- 125000002294 quinazolinyl group Chemical group N1=C(N=CC2=CC=CC=C12)* 0.000 description 2
- 238000012552 review Methods 0.000 description 2
- 206010039083 rhinitis Diseases 0.000 description 2
- 108020004418 ribosomal RNA Proteins 0.000 description 2
- 210000003705 ribosome Anatomy 0.000 description 2
- 210000003491 skin Anatomy 0.000 description 2
- 159000000000 sodium salts Chemical class 0.000 description 2
- HPALAKNZSZLMCH-UHFFFAOYSA-M sodium;chloride;hydrate Chemical compound O.[Na+].[Cl-] HPALAKNZSZLMCH-UHFFFAOYSA-M 0.000 description 2
- UCSJYZPVAKXKNQ-HZYVHMACSA-N streptomycin Chemical compound CN[C@H]1[C@H](O)[C@@H](O)[C@H](CO)O[C@H]1O[C@@H]1[C@](C=O)(O)[C@H](C)O[C@H]1O[C@@H]1[C@@H](NC(N)=N)[C@H](O)[C@@H](NC(N)=N)[C@H](O)[C@H]1O UCSJYZPVAKXKNQ-HZYVHMACSA-N 0.000 description 2
- 125000000547 substituted alkyl group Chemical group 0.000 description 2
- 150000003467 sulfuric acid derivatives Chemical class 0.000 description 2
- 239000006228 supernatant Substances 0.000 description 2
- 239000000829 suppository Substances 0.000 description 2
- 230000020382 suppression by virus of host antigen processing and presentation of peptide antigen via MHC class I Effects 0.000 description 2
- 230000002195 synergetic effect Effects 0.000 description 2
- 230000009885 systemic effect Effects 0.000 description 2
- 201000003120 testicular cancer Diseases 0.000 description 2
- 238000012360 testing method Methods 0.000 description 2
- GRTOGORTSDXSFK-DLLGKBFGSA-N tetrahydroalstonine Chemical compound C1=CC=C2C(CCN3C[C@H]4[C@H](C)OC=C([C@H]4C[C@H]33)C(=O)OC)=C3NC2=C1 GRTOGORTSDXSFK-DLLGKBFGSA-N 0.000 description 2
- ZRKFYGHZFMAOKI-QMGMOQQFSA-N tgfbeta Chemical compound C([C@H](NC(=O)[C@H](C(C)C)NC(=O)CNC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CC(N)=O)NC(=O)[C@H](CC(C)C)NC(=O)[C@H]([C@@H](C)O)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H]([C@@H](C)O)NC(=O)[C@H](CC(C)C)NC(=O)CNC(=O)[C@H](C)NC(=O)[C@H](CO)NC(=O)[C@H](CCC(N)=O)NC(=O)[C@@H](NC(=O)[C@H](C)NC(=O)[C@H](C)NC(=O)[C@@H](NC(=O)[C@H](CC(C)C)NC(=O)[C@@H](N)CCSC)C(C)C)[C@@H](C)CC)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CC=1C=CC=CC=1)C(=O)N[C@@H](C)C(=O)N1[C@@H](CCC1)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](C)C(=O)N[C@@H](CC=1C=CC=CC=1)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](C)C(=O)N[C@@H](CC(C)C)C(=O)N1[C@@H](CCC1)C(=O)N1[C@@H](CCC1)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CO)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CC(C)C)C(O)=O)C1=CC=C(O)C=C1 ZRKFYGHZFMAOKI-QMGMOQQFSA-N 0.000 description 2
- 125000001544 thienyl group Chemical group 0.000 description 2
- RYYWUUFWQRZTIU-UHFFFAOYSA-K thiophosphate Chemical compound [O-]P([O-])([O-])=S RYYWUUFWQRZTIU-UHFFFAOYSA-K 0.000 description 2
- 229940104230 thymidine Drugs 0.000 description 2
- 238000011200 topical administration Methods 0.000 description 2
- 231100000331 toxic Toxicity 0.000 description 2
- ZEWQUBUPAILYHI-UHFFFAOYSA-N trifluoperazine Chemical compound C1CN(C)CCN1CCCN1C2=CC(C(F)(F)F)=CC=C2SC2=CC=CC=C21 ZEWQUBUPAILYHI-UHFFFAOYSA-N 0.000 description 2
- 238000011144 upstream manufacturing Methods 0.000 description 2
- 230000003612 virological effect Effects 0.000 description 2
- 238000005406 washing Methods 0.000 description 2
- 229910052725 zinc Inorganic materials 0.000 description 2
- 239000011701 zinc Substances 0.000 description 2
- KYVJVURXKAZJRK-UHFFFAOYSA-N (+)-laurolitsine Natural products N1CCC2=CC(O)=C(OC)C3=C2C1CC1=C3C=C(OC)C(O)=C1 KYVJVURXKAZJRK-UHFFFAOYSA-N 0.000 description 1
- MAOBFOXLCJIFLV-UHFFFAOYSA-N (2-aminophenyl)-phenylmethanone Chemical compound NC1=CC=CC=C1C(=O)C1=CC=CC=C1 MAOBFOXLCJIFLV-UHFFFAOYSA-N 0.000 description 1
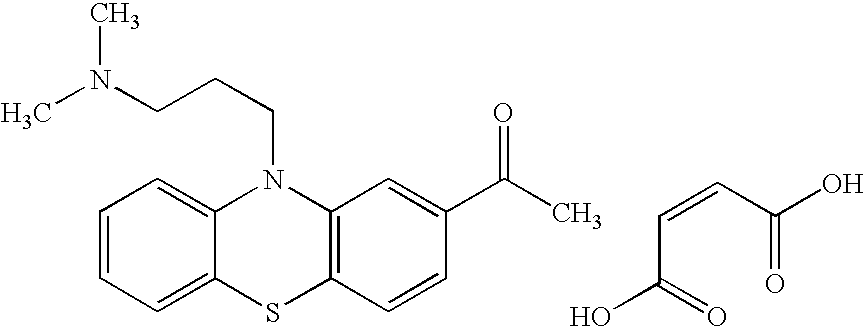
- AJZJIYUOOJLBAU-ZYIUJVGZSA-N (2r,3s)-2,3-dihydroxybutanedioic acid;(2s)-n,n,2-trimethyl-3-phenothiazin-10-ylpropan-1-amine Chemical compound OC(=O)[C@@H](O)[C@@H](O)C(O)=O.C1=CC=C2N(C[C@H](CN(C)C)C)C3=CC=CC=C3SC2=C1.C1=CC=C2N(C[C@H](CN(C)C)C)C3=CC=CC=C3SC2=C1 AJZJIYUOOJLBAU-ZYIUJVGZSA-N 0.000 description 1
- DNXIKVLOVZVMQF-UHFFFAOYSA-N (3beta,16beta,17alpha,18beta,20alpha)-17-hydroxy-11-methoxy-18-[(3,4,5-trimethoxybenzoyl)oxy]-yohimban-16-carboxylic acid, methyl ester Natural products C1C2CN3CCC(C4=CC=C(OC)C=C4N4)=C4C3CC2C(C(=O)OC)C(O)C1OC(=O)C1=CC(OC)=C(OC)C(OC)=C1 DNXIKVLOVZVMQF-UHFFFAOYSA-N 0.000 description 1
- YWKRLOSRDGPEJR-KIUKIJHYSA-N (3z)-3-(2-chlorothioxanthen-9-ylidene)-n,n-dimethylpropan-1-amine;hydron;chloride Chemical compound Cl.C1=C(Cl)C=C2C(=C/CCN(C)C)\C3=CC=CC=C3SC2=C1 YWKRLOSRDGPEJR-KIUKIJHYSA-N 0.000 description 1
- OGTSHGYHILFRHD-UHFFFAOYSA-N (4-fluorophenyl)-phenylmethanone Chemical compound C1=CC(F)=CC=C1C(=O)C1=CC=CC=C1 OGTSHGYHILFRHD-UHFFFAOYSA-N 0.000 description 1
- SSOORFWOBGFTHL-OTEJMHTDSA-N (4S)-5-[[(2S)-1-[[(2S)-1-[[(2S)-1-[[(2S)-1-[[(2S)-1-[[(2S)-1-[[(2S)-1-[[(2S)-6-amino-1-[[(2S)-1-[[(2S)-1-[[(2S)-1-[[(2S)-1-[[2-[(2S)-2-[[(2S)-1-[[(2S)-1-[[(2S)-1-[[(2S)-1-[[(2S)-1-[[(2S)-1-[[(2S)-6-amino-1-[[(2S)-1-[[(2S)-1-[[(2S,3S)-1-[[(2S)-1-[[(2S)-1-[[(2S)-6-amino-1-[[(2S)-1-[[(2S)-1-[[(2S)-1-[[(2S)-1-[[(2S)-1-[[(2S)-5-amino-1-[[(2S)-1-[[(2S)-1-[[(2S)-6-amino-1-[[(2S)-6-amino-1-[[(2S)-1-[[(2S)-1-[[(2S)-5-amino-1-[[(2S)-5-carbamimidamido-1-[[(2S)-5-carbamimidamido-1-[[(1S)-4-carbamimidamido-1-carboxybutyl]amino]-1-oxopentan-2-yl]amino]-1-oxopentan-2-yl]amino]-1,5-dioxopentan-2-yl]amino]-5-carbamimidamido-1-oxopentan-2-yl]amino]-5-carbamimidamido-1-oxopentan-2-yl]amino]-1-oxohexan-2-yl]amino]-1-oxohexan-2-yl]amino]-5-carbamimidamido-1-oxopentan-2-yl]amino]-4-methyl-1-oxopentan-2-yl]amino]-1,5-dioxopentan-2-yl]amino]-4-methyl-1-oxopentan-2-yl]amino]-3-hydroxy-1-oxopropan-2-yl]amino]-3-hydroxy-1-oxopropan-2-yl]amino]-3-hydroxy-1-oxopropan-2-yl]amino]-1-oxopropan-2-yl]amino]-1-oxohexan-2-yl]amino]-3-hydroxy-1-oxopropan-2-yl]amino]-1-oxo-3-phenylpropan-2-yl]amino]-3-methyl-1-oxopentan-2-yl]amino]-3-methyl-1-oxobutan-2-yl]amino]-5-carbamimidamido-1-oxopentan-2-yl]amino]-1-oxohexan-2-yl]amino]-3-methyl-1-oxobutan-2-yl]amino]-5-carbamimidamido-1-oxopentan-2-yl]amino]-3-methyl-1-oxobutan-2-yl]amino]-4-methyl-1-oxopentan-2-yl]amino]-1-oxopropan-2-yl]amino]-5-carbamimidamido-1-oxopentan-2-yl]carbamoyl]pyrrolidin-1-yl]-2-oxoethyl]amino]-3-(1H-indol-3-yl)-1-oxopropan-2-yl]amino]-4-methyl-1-oxopentan-2-yl]amino]-1-oxo-3-phenylpropan-2-yl]amino]-5-carbamimidamido-1-oxopentan-2-yl]amino]-1-oxohexan-2-yl]amino]-3-methyl-1-oxobutan-2-yl]amino]-5-carbamimidamido-1-oxopentan-2-yl]amino]-4-methyl-1-oxopentan-2-yl]amino]-1-oxo-3-phenylpropan-2-yl]amino]-3-(1H-imidazol-4-yl)-1-oxopropan-2-yl]amino]-3-methyl-1-oxobutan-2-yl]amino]-4-methyl-1-oxopentan-2-yl]amino]-4-[[(2S)-2-[[(2S)-2-[[(2S)-2,6-diaminohexanoyl]amino]-3-methylbutanoyl]amino]propanoyl]amino]-5-oxopentanoic acid Chemical compound CC[C@H](C)[C@H](NC(=O)[C@@H](NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CCCCN)NC(=O)[C@@H](NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@@H](NC(=O)[C@H](CC(C)C)NC(=O)[C@H](C)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@@H]1CCCN1C(=O)CNC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CCCCN)NC(=O)[C@@H](NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](Cc1c[nH]cn1)NC(=O)[C@@H](NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](C)NC(=O)[C@@H](NC(=O)[C@@H](N)CCCCN)C(C)C)C(C)C)C(C)C)C(C)C)C(C)C)C(C)C)C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](CO)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](C)C(=O)N[C@@H](CO)C(=O)N[C@@H](CO)C(=O)N[C@@H](CO)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CCCNC(N)=N)C(O)=O SSOORFWOBGFTHL-OTEJMHTDSA-N 0.000 description 1
- SKYZYDSNJIOXRL-BTQNPOSSSA-N (6ar)-6-methyl-5,6,6a,7-tetrahydro-4h-dibenzo[de,g]quinoline-10,11-diol;hydrochloride Chemical compound Cl.C([C@H]1N(C)CC2)C3=CC=C(O)C(O)=C3C3=C1C2=CC=C3 SKYZYDSNJIOXRL-BTQNPOSSSA-N 0.000 description 1
- WRIDQFICGBMAFQ-UHFFFAOYSA-N (E)-8-Octadecenoic acid Natural products CCCCCCCCCC=CCCCCCCC(O)=O WRIDQFICGBMAFQ-UHFFFAOYSA-N 0.000 description 1
- PMGQWSIVQFOFOQ-BDUVBVHRSA-N (e)-but-2-enedioic acid;(2r)-2-[2-[1-(4-chlorophenyl)-1-phenylethoxy]ethyl]-1-methylpyrrolidine Chemical compound OC(=O)\C=C\C(O)=O.CN1CCC[C@@H]1CCOC(C)(C=1C=CC(Cl)=CC=1)C1=CC=CC=C1 PMGQWSIVQFOFOQ-BDUVBVHRSA-N 0.000 description 1
- IPANUAHQWFDVAG-BTJKTKAUSA-N (z)-4-hydroxy-4-oxobut-2-enoate;1-methyl-4-thioxanthen-9-ylidenepiperidin-1-ium Chemical compound OC(=O)\C=C/C(O)=O.C1CN(C)CCC1=C1C2=CC=CC=C2SC2=CC=CC=C21 IPANUAHQWFDVAG-BTJKTKAUSA-N 0.000 description 1
- IFLZPECPTYCEBR-VIEYUMQNSA-N (z)-but-2-enedioic acid;(2r)-3-(2-methoxyphenothiazin-10-yl)-n,n,2-trimethylpropan-1-amine Chemical compound OC(=O)\C=C/C(O)=O.C1=CC=C2N(C[C@H](C)CN(C)C)C3=CC(OC)=CC=C3SC2=C1 IFLZPECPTYCEBR-VIEYUMQNSA-N 0.000 description 1
- BEQLOSVHRBTANS-UHFFFAOYSA-N 1,1,1,2,2,3,3,4,4,5,5,6,6-tridecafluoro-8-iodododecane Chemical compound CCCCC(I)CC(F)(F)C(F)(F)C(F)(F)C(F)(F)C(F)(F)C(F)(F)F BEQLOSVHRBTANS-UHFFFAOYSA-N 0.000 description 1
- PXGRMZYJAOQPNZ-UHFFFAOYSA-N 1,2,3,4-tetrahydroacridin-9-ylazanium;chloride;hydrate Chemical compound O.Cl.C1=CC=C2C(N)=C(CCCC3)C3=NC2=C1 PXGRMZYJAOQPNZ-UHFFFAOYSA-N 0.000 description 1
- WEYNBWVKOYCCQT-UHFFFAOYSA-N 1-(3-chloro-4-methylphenyl)-3-{2-[({5-[(dimethylamino)methyl]-2-furyl}methyl)thio]ethyl}urea Chemical compound O1C(CN(C)C)=CC=C1CSCCNC(=O)NC1=CC=C(C)C(Cl)=C1 WEYNBWVKOYCCQT-UHFFFAOYSA-N 0.000 description 1
- UPUDVKWQBVIKBG-UHFFFAOYSA-N 1-[(3,4-diethoxyphenyl)methyl]-6,7-diethoxyisoquinolin-2-ium;chloride Chemical compound [Cl-].C1=C(OCC)C(OCC)=CC=C1CC1=[NH+]C=CC2=CC(OCC)=C(OCC)C=C12 UPUDVKWQBVIKBG-UHFFFAOYSA-N 0.000 description 1
- UOTMYNBWXDUBNX-UHFFFAOYSA-N 1-[(3,4-dimethoxyphenyl)methyl]-6,7-dimethoxyisoquinolin-2-ium;chloride Chemical compound Cl.C1=C(OC)C(OC)=CC=C1CC1=NC=CC2=CC(OC)=C(OC)C=C12 UOTMYNBWXDUBNX-UHFFFAOYSA-N 0.000 description 1
- CWQORCGWGWEVEL-UHFFFAOYSA-N 1-methyl-2,3,4,9-tetrahydro-1h-pyrido[3,4-b]indole;hydrochloride Chemical compound Cl.N1C2=CC=CC=C2C2=C1C(C)NCC2 CWQORCGWGWEVEL-UHFFFAOYSA-N 0.000 description 1
- VYQGBOFPYBNHBX-UHFFFAOYSA-N 1-methyl-2,9-dihydropyrido[3,4-b]indol-7-one;hydrate;hydrochloride Chemical compound O.Cl.O=C1C=CC2=C3C=CNC(C)=C3NC2=C1 VYQGBOFPYBNHBX-UHFFFAOYSA-N 0.000 description 1
- WJFKNYWRSNBZNX-UHFFFAOYSA-N 10H-phenothiazine Chemical compound C1=CC=C2NC3=CC=CC=C3SC2=C1 WJFKNYWRSNBZNX-UHFFFAOYSA-N 0.000 description 1
- YKBGVTZYEHREMT-KVQBGUIXSA-N 2'-deoxyguanosine Chemical compound C1=NC=2C(=O)NC(N)=NC=2N1[C@H]1C[C@H](O)[C@@H](CO)O1 YKBGVTZYEHREMT-KVQBGUIXSA-N 0.000 description 1
- FJIKEFFDENQLIH-UHFFFAOYSA-M 2,3,10,11-tetramethoxy-8-methylisoquinolino[2,1-b]isoquinolin-7-ium;chloride;hydrate Chemical compound O.[Cl-].C1=C(OC)C(OC)=CC2=CC3=C(C=C(C(OC)=C4)OC)C4=CC=[N+]3C(C)=C21 FJIKEFFDENQLIH-UHFFFAOYSA-M 0.000 description 1
- DGHKCBSVAZXEPP-UHFFFAOYSA-N 2,4-dichloro-6,7-dimethoxyquinazoline Chemical compound ClC1=NC(Cl)=C2C=C(OC)C(OC)=CC2=N1 DGHKCBSVAZXEPP-UHFFFAOYSA-N 0.000 description 1
- CIVCELMLGDGMKZ-UHFFFAOYSA-N 2,4-dichloro-6-methylpyridine-3-carboxylic acid Chemical compound CC1=CC(Cl)=C(C(O)=O)C(Cl)=N1 CIVCELMLGDGMKZ-UHFFFAOYSA-N 0.000 description 1
- FHIKZROVIDCMJA-UHFFFAOYSA-N 2-(2,2-diphenylpentanoyloxy)ethyl-diethylazanium;chloride Chemical compound Cl.C=1C=CC=CC=1C(C(=O)OCCN(CC)CC)(CCC)C1=CC=CC=C1 FHIKZROVIDCMJA-UHFFFAOYSA-N 0.000 description 1
- SCYJKVSESBGURH-UHFFFAOYSA-N 2-chloro-5-nitrobenzoic acid;1-methyl-2,3-dihydropyrrolo[2,3-b]quinolin-4-amine Chemical compound OC(=O)C1=CC([N+]([O-])=O)=CC=C1Cl.N1=C2C=CC=CC2=C(N)C2=C1N(C)CC2 SCYJKVSESBGURH-UHFFFAOYSA-N 0.000 description 1
- CSDSSGBPEUDDEE-UHFFFAOYSA-N 2-formylpyridine Chemical compound O=CC1=CC=CC=N1 CSDSSGBPEUDDEE-UHFFFAOYSA-N 0.000 description 1
- VNJOEUSYAMPBAK-UHFFFAOYSA-N 2-methylbenzenesulfonic acid;hydrate Chemical compound O.CC1=CC=CC=C1S(O)(=O)=O VNJOEUSYAMPBAK-UHFFFAOYSA-N 0.000 description 1
- LCRCBXLHWTVPEQ-UHFFFAOYSA-N 2-phenylbenzaldehyde Chemical compound O=CC1=CC=CC=C1C1=CC=CC=C1 LCRCBXLHWTVPEQ-UHFFFAOYSA-N 0.000 description 1
- LQJBNNIYVWPHFW-UHFFFAOYSA-N 20:1omega9c fatty acid Natural products CCCCCCCCCCC=CCCCCCCCC(O)=O LQJBNNIYVWPHFW-UHFFFAOYSA-N 0.000 description 1
- GLNZEGGVIIKOAL-UHFFFAOYSA-N 3,3-diphenyl-n-(1-phenylpropan-2-yl)propan-1-amine;3-hydroxybutan-2-one Chemical compound CC(O)C(C)=O.C=1C=CC=CC=1C(C=1C=CC=CC=1)CCNC(C)CC1=CC=CC=C1 GLNZEGGVIIKOAL-UHFFFAOYSA-N 0.000 description 1
- NJXWZWXCHBNOOG-UHFFFAOYSA-N 3,3-diphenylpropyl(1-phenylethyl)azanium;chloride Chemical compound [Cl-].C=1C=CC=CC=1C(C)[NH2+]CCC(C=1C=CC=CC=1)C1=CC=CC=C1 NJXWZWXCHBNOOG-UHFFFAOYSA-N 0.000 description 1
- FQRHOOHLUYHMGG-BTJKTKAUSA-N 3-(2-acetylphenothiazin-10-yl)propyl-dimethylazanium;(z)-4-hydroxy-4-oxobut-2-enoate Chemical compound OC(=O)\C=C/C(O)=O.C1=C(C(C)=O)C=C2N(CCCN(C)C)C3=CC=CC=C3SC2=C1 FQRHOOHLUYHMGG-BTJKTKAUSA-N 0.000 description 1
- AKUVRZKNLXYTJX-UHFFFAOYSA-N 3-benzylazetidine Chemical compound C=1C=CC=CC=1CC1CNC1 AKUVRZKNLXYTJX-UHFFFAOYSA-N 0.000 description 1
- RHKWIGHJGOEUSM-UHFFFAOYSA-N 3h-imidazo[4,5-h]quinoline Chemical class C1=CN=C2C(N=CN3)=C3C=CC2=C1 RHKWIGHJGOEUSM-UHFFFAOYSA-N 0.000 description 1
- CKTSBUTUHBMZGZ-ULQXZJNLSA-N 4-amino-1-[(2r,4s,5r)-4-hydroxy-5-(hydroxymethyl)oxolan-2-yl]-5-tritiopyrimidin-2-one Chemical compound O=C1N=C(N)C([3H])=CN1[C@@H]1O[C@H](CO)[C@@H](O)C1 CKTSBUTUHBMZGZ-ULQXZJNLSA-N 0.000 description 1
- 150000005011 4-aminoquinolines Chemical class 0.000 description 1
- 101710169336 5'-deoxyadenosine deaminase Proteins 0.000 description 1
- ZAYHVCMSTBRABG-UHFFFAOYSA-N 5-Methylcytidine Natural products O=C1N=C(N)C(C)=CN1C1C(O)C(O)C(CO)O1 ZAYHVCMSTBRABG-UHFFFAOYSA-N 0.000 description 1
- ZAYHVCMSTBRABG-JXOAFFINSA-N 5-methylcytidine Chemical compound O=C1N=C(N)C(C)=CN1[C@H]1[C@H](O)[C@H](O)[C@@H](CO)O1 ZAYHVCMSTBRABG-JXOAFFINSA-N 0.000 description 1
- QSBYPNXLFMSGKH-UHFFFAOYSA-N 9-Heptadecensaeure Natural products CCCCCCCC=CCCCCCCCC(O)=O QSBYPNXLFMSGKH-UHFFFAOYSA-N 0.000 description 1
- XJGFWWJLMVZSIG-UHFFFAOYSA-N 9-aminoacridine Chemical group C1=CC=C2C(N)=C(C=CC=C3)C3=NC2=C1 XJGFWWJLMVZSIG-UHFFFAOYSA-N 0.000 description 1
- 241000186041 Actinomyces israelii Species 0.000 description 1
- 208000024893 Acute lymphoblastic leukemia Diseases 0.000 description 1
- 208000014697 Acute lymphocytic leukaemia Diseases 0.000 description 1
- 241000701242 Adenoviridae Species 0.000 description 1
- 101710137115 Adenylyl cyclase-associated protein 1 Proteins 0.000 description 1
- 102100021879 Adenylyl cyclase-associated protein 2 Human genes 0.000 description 1
- 101710137132 Adenylyl cyclase-associated protein 2 Proteins 0.000 description 1
- 241000701386 African swine fever virus Species 0.000 description 1
- 241000209136 Agropyron Species 0.000 description 1
- 241000743339 Agrostis Species 0.000 description 1
- 240000005611 Agrostis gigantea Species 0.000 description 1
- 241000223600 Alternaria Species 0.000 description 1
- 241000223602 Alternaria alternata Species 0.000 description 1
- VHUUQVKOLVNVRT-UHFFFAOYSA-N Ammonium hydroxide Chemical compound [NH4+].[OH-] VHUUQVKOLVNVRT-UHFFFAOYSA-N 0.000 description 1
- 241000743857 Anthoxanthum Species 0.000 description 1
- 240000004178 Anthoxanthum odoratum Species 0.000 description 1
- 235000014251 Anthoxanthum odoratum Nutrition 0.000 description 1
- 241000712892 Arenaviridae Species 0.000 description 1
- 241000508787 Arrhenatherum Species 0.000 description 1
- 241000508786 Arrhenatherum elatius Species 0.000 description 1
- 235000003826 Artemisia Nutrition 0.000 description 1
- 235000004355 Artemisia lactiflora Nutrition 0.000 description 1
- 201000001320 Atherosclerosis Diseases 0.000 description 1
- 235000005781 Avena Nutrition 0.000 description 1
- 235000007319 Avena orientalis Nutrition 0.000 description 1
- 102100035526 B melanoma antigen 1 Human genes 0.000 description 1
- 102000019260 B-Cell Antigen Receptors Human genes 0.000 description 1
- 108010012919 B-Cell Antigen Receptors Proteins 0.000 description 1
- 208000003950 B-cell lymphoma Diseases 0.000 description 1
- 241000193738 Bacillus anthracis Species 0.000 description 1
- 241001148536 Bacteroides sp. Species 0.000 description 1
- 208000023328 Basedow disease Diseases 0.000 description 1
- VKJGBAJNNALVAV-UHFFFAOYSA-M Berberine chloride (TN) Chemical compound [Cl-].C1=C2CC[N+]3=CC4=C(OC)C(OC)=CC=C4C=C3C2=CC2=C1OCO2 VKJGBAJNNALVAV-UHFFFAOYSA-M 0.000 description 1
- 241000219429 Betula Species 0.000 description 1
- 235000003932 Betula Nutrition 0.000 description 1
- 241000219430 Betula pendula Species 0.000 description 1
- 235000009109 Betula pendula Nutrition 0.000 description 1
- 241000219495 Betulaceae Species 0.000 description 1
- 206010004593 Bile duct cancer Diseases 0.000 description 1
- 241000702628 Birnaviridae Species 0.000 description 1
- 206010005003 Bladder cancer Diseases 0.000 description 1
- 241000228405 Blastomyces dermatitidis Species 0.000 description 1
- 241000238658 Blattella Species 0.000 description 1
- 241000238657 Blattella germanica Species 0.000 description 1
- 241000589969 Borreliella burgdorferi Species 0.000 description 1
- 208000003174 Brain Neoplasms Diseases 0.000 description 1
- 241000209200 Bromus Species 0.000 description 1
- 241000743756 Bromus inermis Species 0.000 description 1
- 102100032366 C-C motif chemokine 7 Human genes 0.000 description 1
- 101710155834 C-C motif chemokine 7 Proteins 0.000 description 1
- 102100034871 C-C motif chemokine 8 Human genes 0.000 description 1
- 101710155833 C-C motif chemokine 8 Proteins 0.000 description 1
- 102100039398 C-X-C motif chemokine 2 Human genes 0.000 description 1
- 102100036170 C-X-C motif chemokine 9 Human genes 0.000 description 1
- FRAWXKZZBPVVJO-UHFFFAOYSA-N C.C.C.CN(C)CCN.CN1CCN(C2=CC=C(C3=NC4=CC=CC=C4C(Cl)=N3)C=C2)CC1.O=P(Cl)(Cl)Cl.[H]N(CCN(C)C)C1=NC(C2=CC=C(N3CCN(C)CC3)C=C2)=NC2=CC=CC=C21.[H]N1C(=O)C2=CC=CC=C2N=C1C1=CC=C(N2CCN(C)CC2)C=C1 Chemical compound C.C.C.CN(C)CCN.CN1CCN(C2=CC=C(C3=NC4=CC=CC=C4C(Cl)=N3)C=C2)CC1.O=P(Cl)(Cl)Cl.[H]N(CCN(C)C)C1=NC(C2=CC=C(N3CCN(C)CC3)C=C2)=NC2=CC=CC=C21.[H]N1C(=O)C2=CC=CC=C2N=C1C1=CC=C(N2CCN(C)CC2)C=C1 FRAWXKZZBPVVJO-UHFFFAOYSA-N 0.000 description 1
- ULPLZPROIDZVIW-UHFFFAOYSA-N C.CN(C)CCN.[H]C(NCCN(C)C)(C1=CC=CC=C1)C1=CC=C(N2CCN(C)CC2)C=C1.[H]C(O)(C1=CC=CC=C1)C1=CC=C(N2CCN(C)CC2)C=C1 Chemical compound C.CN(C)CCN.[H]C(NCCN(C)C)(C1=CC=CC=C1)C1=CC=C(N2CCN(C)CC2)C=C1.[H]C(O)(C1=CC=CC=C1)C1=CC=C(N2CCN(C)CC2)C=C1 ULPLZPROIDZVIW-UHFFFAOYSA-N 0.000 description 1
- FMEHPDCCZLYPQU-QLCKKJBHSA-N C.CN1CCN(C2=CC=C(/C(=N\O)C3=CC=CC=C3)C=C2)CC1.O=CO.[H]C([H])(C1=CC=CC=C1)C1=CC=C(N2CCN(C)CC2)C=C1 Chemical compound C.CN1CCN(C2=CC=C(/C(=N\O)C3=CC=CC=C3)C=C2)CC1.O=CO.[H]C([H])(C1=CC=CC=C1)C1=CC=C(N2CCN(C)CC2)C=C1 FMEHPDCCZLYPQU-QLCKKJBHSA-N 0.000 description 1
- VFQHMZFBNADYCK-UHFFFAOYSA-N C.CNCCOC(C1=CC=CC=C1)C1=CC=CC=C1.Cl Chemical compound C.CNCCOC(C1=CC=CC=C1)C1=CC=CC=C1.Cl VFQHMZFBNADYCK-UHFFFAOYSA-N 0.000 description 1
- CCSPGBQJFXNHFT-UHFFFAOYSA-N C.COC1=CC2=CC3=C4C=C(OC)C(OC)=CC4=CC=[N+]3C(C)=C2C=C1OC.[Cl-] Chemical compound C.COC1=CC2=CC3=C4C=C(OC)C(OC)=CC4=CC=[N+]3C(C)=C2C=C1OC.[Cl-] CCSPGBQJFXNHFT-UHFFFAOYSA-N 0.000 description 1
- NMZXJTHBOCCVQS-UHFFFAOYSA-N C.Cl.Cl.[H]N(CCN(C)C)C1=NC(C2=CC=C(C3=CC=CC=C3)C=C2)=NC2=CC=CC=C21.[H]N1C(=O)C2=CC=CC=C2N=C1C1=CC=C(C2=CC=CC=C2)C=C1 Chemical compound C.Cl.Cl.[H]N(CCN(C)C)C1=NC(C2=CC=C(C3=CC=CC=C3)C=C2)=NC2=CC=CC=C21.[H]N1C(=O)C2=CC=CC=C2N=C1C1=CC=C(C2=CC=CC=C2)C=C1 NMZXJTHBOCCVQS-UHFFFAOYSA-N 0.000 description 1
- YBVZVRCSVAFKRC-UHFFFAOYSA-N C.Cl.NC1=C2CCCCC2=NC2=CC=CC=C21 Chemical compound C.Cl.NC1=C2CCCCC2=NC2=CC=CC=C21 YBVZVRCSVAFKRC-UHFFFAOYSA-N 0.000 description 1
- ANFWYNHWYUPXMG-UHFFFAOYSA-N C.NC(=O)C1=CC=CC=C1N.O=CC1=CN=CC=C1.[H]N1C(=O)C2=CC=CC=C2N=C1C1=CC=CN=C1 Chemical compound C.NC(=O)C1=CC=CC=C1N.O=CC1=CN=CC=C1.[H]N1C(=O)C2=CC=CC=C2N=C1C1=CC=CN=C1 ANFWYNHWYUPXMG-UHFFFAOYSA-N 0.000 description 1
- AMOHDNIXPZSHCC-UHFFFAOYSA-N C.NC(=O)C1=CC=CC=C1N.O=CC1=NC=CC=C1.[H]N1C(=O)C2=CC=CC=C2N=C1C1=CC=CC=N1 Chemical compound C.NC(=O)C1=CC=CC=C1N.O=CC1=NC=CC=C1.[H]N1C(=O)C2=CC=CC=C2N=C1C1=CC=CC=N1 AMOHDNIXPZSHCC-UHFFFAOYSA-N 0.000 description 1
- PMUCFHHWIAZNDO-UHFFFAOYSA-N C.[H]C(C1=CC=CC=C1)(C1=CC=C(N2CCN(C)CC2)C=C1)N1CCN(C)CC1.[H]C(O)(C1=CC=CC=C1)C1=CC=C(N2CCN(C)CC2)C=C1.[H]N1CCN(C)CC1 Chemical compound C.[H]C(C1=CC=CC=C1)(C1=CC=C(N2CCN(C)CC2)C=C1)N1CCN(C)CC1.[H]C(O)(C1=CC=CC=C1)C1=CC=C(N2CCN(C)CC2)C=C1.[H]N1CCN(C)CC1 PMUCFHHWIAZNDO-UHFFFAOYSA-N 0.000 description 1
- QROGIFZRVHSFLM-QHHAFSJGSA-N C/C=C/C1=CC=CC=C1 Chemical compound C/C=C/C1=CC=CC=C1 QROGIFZRVHSFLM-QHHAFSJGSA-N 0.000 description 1
- SBGPASZOVGSOFJ-UHFFFAOYSA-N C1=CC2=C(C=C1)C1=C(N2)C2C3CCC(C4CCCNC43)N2CC1.Cl.Cl Chemical compound C1=CC2=C(C=C1)C1=C(N2)C2C3CCC(C4CCCNC43)N2CC1.Cl.Cl SBGPASZOVGSOFJ-UHFFFAOYSA-N 0.000 description 1
- APYNUQWABJIFAH-UHFFFAOYSA-N C1=CC=C(C2=NC3=CC=CC=C3C(NCCN3CCOCC3)=N2)N=C1 Chemical compound C1=CC=C(C2=NC3=CC=CC=C3C(NCCN3CCOCC3)=N2)N=C1 APYNUQWABJIFAH-UHFFFAOYSA-N 0.000 description 1
- NIJZBVHSIDPOSH-UHFFFAOYSA-N C1=CC=C2C(=C1)N=C(C1=CC=CN=C1)N=C2NCCN1CCOCC1 Chemical compound C1=CC=C2C(=C1)N=C(C1=CC=CN=C1)N=C2NCCN1CCOCC1 NIJZBVHSIDPOSH-UHFFFAOYSA-N 0.000 description 1
- IVOAPOQXYQRGDG-UHFFFAOYSA-N C=CCC1=C(OC2(C3=CC=CC=C3)OC(=O)C3=CC=CC=C32)C=CC=C1 Chemical compound C=CCC1=C(OC2(C3=CC=CC=C3)OC(=O)C3=CC=CC=C32)C=CC=C1 IVOAPOQXYQRGDG-UHFFFAOYSA-N 0.000 description 1
- NOSIYYJFMPDDSA-UHFFFAOYSA-N CC(=O)C1=CC2=C(C=C1)SC1=CC=CC=C1N2CCCN(C)C.O=C(O)/C=C\C(=O)O Chemical compound CC(=O)C1=CC2=C(C=C1)SC1=CC=CC=C1N2CCCN(C)C.O=C(O)/C=C\C(=O)O NOSIYYJFMPDDSA-UHFFFAOYSA-N 0.000 description 1
- NIUCXEUPLFSISH-UHFFFAOYSA-N CC(C)c(cc(C(c1ccccc11)(c(cc2C(C)C)c(C)cc2OC(C2C(C)CCCC2)=O)OC1=O)c(C)c1)c1OC(C1C(C)CCCC1)=O Chemical compound CC(C)c(cc(C(c1ccccc11)(c(cc2C(C)C)c(C)cc2OC(C2C(C)CCCC2)=O)OC1=O)c(C)c1)c1OC(C1C(C)CCCC1)=O NIUCXEUPLFSISH-UHFFFAOYSA-N 0.000 description 1
- QPOFIDVRLWJICD-UHFFFAOYSA-N CC(CC1=CC=CC=C1)NCCC(C1=CC=CC=C1)C1=CC=CC=C1.CC(O)C(=O)O Chemical compound CC(CC1=CC=CC=C1)NCCC(C1=CC=CC=C1)C1=CC=CC=C1.CC(O)C(=O)O QPOFIDVRLWJICD-UHFFFAOYSA-N 0.000 description 1
- ZZHLYYDVIOPZBE-UHFFFAOYSA-N CC(CN(C)C)CN1C2=C(C=CC=C2)SC2=C1C=CC=C2.O=C(O)C(O)C(O)C(=O)O Chemical compound CC(CN(C)C)CN1C2=C(C=CC=C2)SC2=C1C=CC=C2.O=C(O)C(O)C(O)C(=O)O ZZHLYYDVIOPZBE-UHFFFAOYSA-N 0.000 description 1
- NMKSAYKQLCHXDK-UHFFFAOYSA-N CC(NCCC(C1=CC=CC=C1)C1=CC=CC=C1)C1=CC=CC=C1.Cl Chemical compound CC(NCCC(C1=CC=CC=C1)C1=CC=CC=C1)C1=CC=CC=C1.Cl NMKSAYKQLCHXDK-UHFFFAOYSA-N 0.000 description 1
- MMZYCBHLNZVROM-UHFFFAOYSA-N CC1=C(F)C=CC=C1 Chemical compound CC1=C(F)C=CC=C1 MMZYCBHLNZVROM-UHFFFAOYSA-N 0.000 description 1
- SATMZMMKDDTOSQ-UHFFFAOYSA-N CC1=C2\NC3=C(C=CC(O)=C3)\C2=C\C=N\1.Cl.O Chemical compound CC1=C2\NC3=C(C=CC(O)=C3)\C2=C\C=N\1.Cl.O SATMZMMKDDTOSQ-UHFFFAOYSA-N 0.000 description 1
- PSFDQSOCUJVVGF-UHFFFAOYSA-N CC1=C2\NC3=C(C=CC=C3)\C2=C\C=N\1.Cl Chemical compound CC1=C2\NC3=C(C=CC=C3)\C2=C\C=N\1.Cl PSFDQSOCUJVVGF-UHFFFAOYSA-N 0.000 description 1
- GPUNOVJFCWZTGV-UHFFFAOYSA-N CC1=CC(CN2CCN(C)CC2)=CC=C1 Chemical compound CC1=CC(CN2CCN(C)CC2)=CC=C1 GPUNOVJFCWZTGV-UHFFFAOYSA-N 0.000 description 1
- GQUCODPQLUPVCR-UHFFFAOYSA-N CC1=CC(OC(=O)C2C3CCCCC32)=C(C(C)C)C=C1C1(C2=CC(C(C)C)=C(OC(=O)C3C4CCCCC43)C=C2C)OC(=O)C2=C1C=CC=C2 Chemical compound CC1=CC(OC(=O)C2C3CCCCC32)=C(C(C)C)C=C1C1(C2=CC(C(C)C)=C(OC(=O)C3C4CCCCC43)C=C2C)OC(=O)C2=C1C=CC=C2 GQUCODPQLUPVCR-UHFFFAOYSA-N 0.000 description 1
- VIDFVWRZQFVYPF-UHFFFAOYSA-N CC1=CC2=C(C=C1C)C(N)=NC(N1CCN(C(=O)C3CCCO3)CC1)=N2.Cl Chemical compound CC1=CC2=C(C=C1C)C(N)=NC(N1CCN(C(=O)C3CCCO3)CC1)=N2.Cl VIDFVWRZQFVYPF-UHFFFAOYSA-N 0.000 description 1
- BQJZIRKMFYTCEO-UHFFFAOYSA-N CC1=CC2=C(C=C1C)C(N)=NC(N1CCN(C(=O)C3COC4=C(C=CC=C4)O3)CC1)=N2.CS(=O)(=O)O Chemical compound CC1=CC2=C(C=C1C)C(N)=NC(N1CCN(C(=O)C3COC4=C(C=CC=C4)O3)CC1)=N2.CS(=O)(=O)O BQJZIRKMFYTCEO-UHFFFAOYSA-N 0.000 description 1
- WRWPPGUCZBJXKX-UHFFFAOYSA-N CC1=CC=C(F)C=C1 Chemical compound CC1=CC=C(F)C=C1 WRWPPGUCZBJXKX-UHFFFAOYSA-N 0.000 description 1
- LGSKZTVGGDDIOM-UHFFFAOYSA-N CC1=CC=C(N2C(=O)C3=C(N=C2NCCO)C2=CC=CC=C2CC32CCCCC2)C=C1 Chemical compound CC1=CC=C(N2C(=O)C3=C(N=C2NCCO)C2=CC=CC=C2CC32CCCCC2)C=C1 LGSKZTVGGDDIOM-UHFFFAOYSA-N 0.000 description 1
- SEKVDGJLBQJSTO-UHFFFAOYSA-N CC1=CC=C(N2CCCCC2)C=C1 Chemical compound CC1=CC=C(N2CCCCC2)C=C1 SEKVDGJLBQJSTO-UHFFFAOYSA-N 0.000 description 1
- MEMSYAUTOBKDJW-UHFFFAOYSA-N CC1=CC=C(S(=O)(=O)O)C=C1.NC(=O)C1=CC=CC=C1N.O.O=CC1=CC=C(C2=CC=CC=C2)C=C1.[H]N1C(=O)C2=CC=CC=C2N=C1C1=CC=C(C2=CC=CC=C2)C=C1 Chemical compound CC1=CC=C(S(=O)(=O)O)C=C1.NC(=O)C1=CC=CC=C1N.O.O=CC1=CC=C(C2=CC=CC=C2)C=C1.[H]N1C(=O)C2=CC=CC=C2N=C1C1=CC=C(C2=CC=CC=C2)C=C1 MEMSYAUTOBKDJW-UHFFFAOYSA-N 0.000 description 1
- SHWKZEFERHFBTQ-UHFFFAOYSA-N CC1=CC=C2C=C(O)C=CC2=C1 Chemical compound CC1=CC=C2C=C(O)C=CC2=C1 SHWKZEFERHFBTQ-UHFFFAOYSA-N 0.000 description 1
- KROILPPVVWCRKD-UHFFFAOYSA-N CC1=CC=CC(C2(C3=CC(C)=CC=C3)OC(=O)C3=C2C=CC=C3)=C1 Chemical compound CC1=CC=CC(C2(C3=CC(C)=CC=C3)OC(=O)C3=C2C=CC=C3)=C1 KROILPPVVWCRKD-UHFFFAOYSA-N 0.000 description 1
- QVYRGXJJSLMXQH-UHFFFAOYSA-N CC1=CC=CC=C1C(OCCN(C)C)C1=CC=CC=C1.Cl Chemical compound CC1=CC=CC=C1C(OCCN(C)C)C1=CC=CC=C1.Cl QVYRGXJJSLMXQH-UHFFFAOYSA-N 0.000 description 1
- IJOCLPMYJPNAEI-UHFFFAOYSA-N CC1CCN(C(=O)C2=CC=CC3=C2C2=C(C=CC=C2)C3=O)CC1 Chemical compound CC1CCN(C(=O)C2=CC=CC3=C2C2=C(C=CC=C2)C3=O)CC1 IJOCLPMYJPNAEI-UHFFFAOYSA-N 0.000 description 1
- LPIJOZBIVDCQTE-UHFFFAOYSA-N CC1NCCC2=C1NC1=C2/C=C\C=C/1.Cl Chemical compound CC1NCCC2=C1NC1=C2/C=C\C=C/1.Cl LPIJOZBIVDCQTE-UHFFFAOYSA-N 0.000 description 1
- ZLCSFXXPPANWQY-UHFFFAOYSA-N CCC1=CC=CC(C)=C1 Chemical compound CCC1=CC=CC(C)=C1 ZLCSFXXPPANWQY-UHFFFAOYSA-N 0.000 description 1
- SNTQPLDRUZOSDP-UHFFFAOYSA-N CCCC(C(=O)OCCN(CC)CC)(C1=CC=CC=C1)C1=CC=CC=C1.Cl Chemical compound CCCC(C(=O)OCCN(CC)CC)(C1=CC=CC=C1)C1=CC=CC=C1.Cl SNTQPLDRUZOSDP-UHFFFAOYSA-N 0.000 description 1
- ROOHQUSNNJXYKW-UHFFFAOYSA-N CCCCCCCCCCCC(=O)N/C1=C2\C=CC=C\C2=N\C2=C1CCN2C Chemical compound CCCCCCCCCCCC(=O)N/C1=C2\C=CC=C\C2=N\C2=C1CCN2C ROOHQUSNNJXYKW-UHFFFAOYSA-N 0.000 description 1
- RIZPBTBZLCPMSF-UHFFFAOYSA-N CCCCCCN(CCCCCC)CC(=O)NC1=C2C=CC=CC2=NC2=C1CCN2C Chemical compound CCCCCCN(CCCCCC)CC(=O)NC1=C2C=CC=CC2=NC2=C1CCN2C RIZPBTBZLCPMSF-UHFFFAOYSA-N 0.000 description 1
- LPVWZQYOCIAYQJ-UHFFFAOYSA-N CCCCOC(=O)C1=CC([N+](=O)[O-])=CC2=C1C1=C(/C=C([N+](=O)[O-])\C=C/1)C2=C(C#N)C#N Chemical compound CCCCOC(=O)C1=CC([N+](=O)[O-])=CC2=C1C1=C(/C=C([N+](=O)[O-])\C=C/1)C2=C(C#N)C#N LPVWZQYOCIAYQJ-UHFFFAOYSA-N 0.000 description 1
- 108700012434 CCL3 Proteins 0.000 description 1
- GGVHTCZHCYJEFL-UHFFFAOYSA-N CCN(CC)C(C)CN1C2=CC=CC=C2SC2CCCCC21.Cl Chemical compound CCN(CC)C(C)CN1C2=CC=CC=C2SC2CCCCC21.Cl GGVHTCZHCYJEFL-UHFFFAOYSA-N 0.000 description 1
- JWLITWATDRVAKE-SAPNQHFASA-N CCN(CC)CCCC(C)NC1=NC(/C=C/C2=CC=C(Cl)C=C2)=NC2=CC3=C(C=CC=C3)C=C21 Chemical compound CCN(CC)CCCC(C)NC1=NC(/C=C/C2=CC=C(Cl)C=C2)=NC2=CC3=C(C=CC=C3)C=C21 JWLITWATDRVAKE-SAPNQHFASA-N 0.000 description 1
- ICSZZKJAACPKQV-UHFFFAOYSA-N CCN(CC)CCCNC1=NC(C2=CC=CC=C2)=NC2=C1C=CC=C2 Chemical compound CCN(CC)CCCNC1=NC(C2=CC=CC=C2)=NC2=C1C=CC=C2 ICSZZKJAACPKQV-UHFFFAOYSA-N 0.000 description 1
- LNPBJDZTGPGMQK-UHFFFAOYSA-M CCOC(=O)C1=CC([N+](=O)[O-])=CC2=C1C1=C(C=C(C(=O)[O-])C=C1)C2=C(C#N)C#N Chemical compound CCOC(=O)C1=CC([N+](=O)[O-])=CC2=C1C1=C(C=C(C(=O)[O-])C=C1)C2=C(C#N)C#N LNPBJDZTGPGMQK-UHFFFAOYSA-M 0.000 description 1
- ZOWYFYXTIWQBEP-UHFFFAOYSA-N CCOC1=CC2=C(C=C1OCC)C(CC1=CC(OCC)=C(OCC)C=C1)=NC=C2.Cl Chemical compound CCOC1=CC2=C(C=C1OCC)C(CC1=CC(OCC)=C(OCC)C=C1)=NC=C2.Cl ZOWYFYXTIWQBEP-UHFFFAOYSA-N 0.000 description 1
- PGNWQNJXXLPZEL-UHFFFAOYSA-N CCOC1=CC2=C(N)C3=C(C=C(NCC4=CC=CO4)C=C3)N=C2C=C1 Chemical compound CCOC1=CC2=C(N)C3=C(C=C(NCC4=CC=CO4)C=C3)N=C2C=C1 PGNWQNJXXLPZEL-UHFFFAOYSA-N 0.000 description 1
- WHOBMRMYOZFLCO-UHFFFAOYSA-N CCOCCOC(=O)C1=C2C(=CC([N+](=O)[O-])=C1)C(=C(C#N)C#N)C1=CC([N+](=O)[O-])=CC([N+](=O)[O-])=C12 Chemical compound CCOCCOC(=O)C1=C2C(=CC([N+](=O)[O-])=C1)C(=C(C#N)C#N)C1=CC([N+](=O)[O-])=CC([N+](=O)[O-])=C12 WHOBMRMYOZFLCO-UHFFFAOYSA-N 0.000 description 1
- ZDWVWKDAWBGPDN-UHFFFAOYSA-O CC[N+](C)(CC)CCC[N+]1=C(C2=CC=CC=C2)C2=C(C=CC(N)=C2)C2=C1C=C(N)C=C2.[I-].[I-] Chemical compound CC[N+](C)(CC)CCC[N+]1=C(C2=CC=CC=C2)C2=C(C=CC(N)=C2)C2=C1C=C(N)C=C2.[I-].[I-] ZDWVWKDAWBGPDN-UHFFFAOYSA-O 0.000 description 1
- RDOIQAHITMMDAJ-UHFFFAOYSA-N CN(C)C(=O)C(CCN1CCC(O)(C2=CC=C(Cl)C=C2)CC1)(C1=CC=CC=C1)C1=CC=CC=C1.Cl Chemical compound CN(C)C(=O)C(CCN1CCC(O)(C2=CC=C(Cl)C=C2)CC1)(C1=CC=CC=C1)C1=CC=CC=C1.Cl RDOIQAHITMMDAJ-UHFFFAOYSA-N 0.000 description 1
- OXLDRPPQCHUJAO-UHFFFAOYSA-N CN(C)C1=CC(N)=C2C=CC=CC2=N1 Chemical compound CN(C)C1=CC(N)=C2C=CC=CC2=N1 OXLDRPPQCHUJAO-UHFFFAOYSA-N 0.000 description 1
- PHTUQLWOUWZIMZ-BOPFTXTBSA-N CN(C)CC/C=C1/C2=CC=CC=C2CSC2=C1C=CC=C2.Cl Chemical compound CN(C)CC/C=C1/C2=CC=CC=C2CSC2=C1C=CC=C2.Cl PHTUQLWOUWZIMZ-BOPFTXTBSA-N 0.000 description 1
- WSPOMRSOLSGNFJ-AUWJEWJLSA-N CN(C)CC/C=C1/C2=CC=CC=C2SC2=C1C=C(Cl)C=C2.Cl Chemical compound CN(C)CC/C=C1/C2=CC=CC=C2SC2=C1C=C(Cl)C=C2.Cl WSPOMRSOLSGNFJ-AUWJEWJLSA-N 0.000 description 1
- ZGUGWUXLJSTTMA-UHFFFAOYSA-N CN(C)CCCN1C2=C(C=CC=C2)SC2=C1C=CC=C2.Cl Chemical compound CN(C)CCCN1C2=C(C=CC=C2)SC2=C1C=CC=C2.Cl ZGUGWUXLJSTTMA-UHFFFAOYSA-N 0.000 description 1
- BCGWQEUPMDMJNV-UHFFFAOYSA-N CN(C)CCCN1C2=CC=CC=C2CCC2=C1C=CC=C2.Cl Chemical compound CN(C)CCCN1C2=CC=CC=C2CCC2=C1C=CC=C2.Cl BCGWQEUPMDMJNV-UHFFFAOYSA-N 0.000 description 1
- YIXVZHOIGIAJNX-UHFFFAOYSA-N CN(C)CCCl.[H]C(O)(C1=CC=CC=C1)C1=CC=C(N2CCN(C)CC2)C=C1.[H]C(OCCN(C)C)(C1=CC=CC=C1)C1=CC=C(N2CCN(C)CC2)C=C1.[NaH] Chemical compound CN(C)CCCl.[H]C(O)(C1=CC=CC=C1)C1=CC=C(N2CCN(C)CC2)C=C1.[H]C(OCCN(C)C)(C1=CC=CC=C1)C1=CC=C(N2CCN(C)CC2)C=C1.[NaH] YIXVZHOIGIAJNX-UHFFFAOYSA-N 0.000 description 1
- ICKFFNBDFNZJSX-UHFFFAOYSA-N CN(C)CCN(CC1=CC=C(Cl)C=C1)C1=NC=CC=C1.Cl Chemical compound CN(C)CCN(CC1=CC=C(Cl)C=C1)C1=NC=CC=C1.Cl ICKFFNBDFNZJSX-UHFFFAOYSA-N 0.000 description 1
- UNSJMSSQXJETHB-UHFFFAOYSA-N CN(C)CCN.COC1=C(OC)C=C2C(=C1)N=C(Cl)N=C2Cl.[H]N(CCN(C)C)C1=NC(Cl)=NC2=CC(OC)=C(OC)C=C21 Chemical compound CN(C)CCN.COC1=C(OC)C=C2C(=C1)N=C(Cl)N=C2Cl.[H]N(CCN(C)C)C1=NC(Cl)=NC2=CC(OC)=C(OC)C=C21 UNSJMSSQXJETHB-UHFFFAOYSA-N 0.000 description 1
- IDGRLLNLQSQIMU-UHFFFAOYSA-N CN(C)CCN.ClC1=NC(C2=CC=CC=C2)=NC2=CC=CC=C21.[H]N(CCN(C)C)C1=NC(C2=CC=CC=C2)=NC2=CC=CC=C21.[H]OS(=O)(=O)O Chemical compound CN(C)CCN.ClC1=NC(C2=CC=CC=C2)=NC2=CC=CC=C21.[H]N(CCN(C)C)C1=NC(C2=CC=CC=C2)=NC2=CC=CC=C21.[H]OS(=O)(=O)O IDGRLLNLQSQIMU-UHFFFAOYSA-N 0.000 description 1
- PTRFMNITGPZLOE-UHFFFAOYSA-N CN(C)CCNC1NC(C2=CC=C(N3CCN(C)CC3)C=C2)=NC2=CC=CC=C21 Chemical compound CN(C)CCNC1NC(C2=CC=C(N3CCN(C)CC3)C=C2)=NC2=CC=CC=C21 PTRFMNITGPZLOE-UHFFFAOYSA-N 0.000 description 1
- GXCGMSSOANBKLQ-UHFFFAOYSA-N CN(C)CCO.CN(C)CCOC1=NC(C2=CC=CC=C2)=NC2=CC=CC=C21.ClC1=NC(C2=CC=CC=C2)=NC2=CC=CC=C21.[H]OS(=O)(=O)O Chemical compound CN(C)CCO.CN(C)CCOC1=NC(C2=CC=CC=C2)=NC2=CC=CC=C21.ClC1=NC(C2=CC=CC=C2)=NC2=CC=CC=C21.[H]OS(=O)(=O)O GXCGMSSOANBKLQ-UHFFFAOYSA-N 0.000 description 1
- WMNJPKXOFWWBSV-UHFFFAOYSA-N CN(C)CCOC1=NC(C2=CC=C(N3CCN(C)CC3)C=C2)=NC2=CC=CC=C21.[H]N1C(=O)C2=CC=CC=C2N=C1C1=CC=C(N2CCN(C)CC2)C=C1.[H]OS(=O)(=O)O Chemical compound CN(C)CCOC1=NC(C2=CC=C(N3CCN(C)CC3)C=C2)=NC2=CC=CC=C21.[H]N1C(=O)C2=CC=CC=C2N=C1C1=CC=C(N2CCN(C)CC2)C=C1.[H]OS(=O)(=O)O WMNJPKXOFWWBSV-UHFFFAOYSA-N 0.000 description 1
- XAWVLTHWMRDYCC-UHFFFAOYSA-N CN(CCO)C(=O)C1=CC=CC2=C1C1=C(C=CC=C1)C2=O Chemical compound CN(CCO)C(=O)C1=CC=CC2=C1C1=C(C=CC=C1)C2=O XAWVLTHWMRDYCC-UHFFFAOYSA-N 0.000 description 1
- KHAIKFIWHPKEEP-UHFFFAOYSA-N CN1C(=O)C2=C(N=C1NN)C1=C(C=CC=C1)CC21CCCC1 Chemical compound CN1C(=O)C2=C(N=C1NN)C1=C(C=CC=C1)CC21CCCC1 KHAIKFIWHPKEEP-UHFFFAOYSA-N 0.000 description 1
- XXPANQJNYNUNES-UHFFFAOYSA-N CN1CC2=C(N)C=CC=C2C(C2=CC=CC=C2)C1.O=C(O)/C=C\C(=O)O Chemical compound CN1CC2=C(N)C=CC=C2C(C2=CC=CC=C2)C1.O=C(O)/C=C\C(=O)O XXPANQJNYNUNES-UHFFFAOYSA-N 0.000 description 1
- NZLVRVYNQYGMAB-UHFFFAOYSA-N CN1CCC(=C2C3=C(C=CC=C3)SC3=C2C=CC=C3)CC1.O=C(O)/C=C\C(=O)O Chemical compound CN1CCC(=C2C3=C(C=CC=C3)SC3=C2C=CC=C3)CC1.O=C(O)/C=C\C(=O)O NZLVRVYNQYGMAB-UHFFFAOYSA-N 0.000 description 1
- OWQUZNMMYNAXSL-UHFFFAOYSA-N CN1CCC(OC(C2=CC=CC=C2)C2=CC=CC=C2)CC1.Cl Chemical compound CN1CCC(OC(C2=CC=CC=C2)C2=CC=CC=C2)CC1.Cl OWQUZNMMYNAXSL-UHFFFAOYSA-N 0.000 description 1
- MFVMNUFCTINRIV-UHFFFAOYSA-N CN1CCC2=C(NC(=O)CN3CCN(C4=CC=CC=C4)CC3)C3=C(C=CC=C3)N=C21 Chemical compound CN1CCC2=C(NC(=O)CN3CCN(C4=CC=CC=C4)CC3)C3=C(C=CC=C3)N=C21 MFVMNUFCTINRIV-UHFFFAOYSA-N 0.000 description 1
- UVIAJVAJCNLZJL-UHFFFAOYSA-N CN1CCC2=C1/N=C1/C=CC=C/C1=C/2NC(=O)CN1CCCCCC1 Chemical compound CN1CCC2=C1/N=C1/C=CC=C/C1=C/2NC(=O)CN1CCCCCC1 UVIAJVAJCNLZJL-UHFFFAOYSA-N 0.000 description 1
- MJFJKKXQDNNUJF-UHFFFAOYSA-N CN1CCCC(CC2C3=CC=CC=C3SC3=C2C=CC=C3)C1.Cl Chemical compound CN1CCCC(CC2C3=CC=CC=C3SC3=C2C=CC=C3)C1.Cl MJFJKKXQDNNUJF-UHFFFAOYSA-N 0.000 description 1
- PAMIQIKDUOTOBW-UHFFFAOYSA-N CN1CCCCC1 Chemical compound CN1CCCCC1 PAMIQIKDUOTOBW-UHFFFAOYSA-N 0.000 description 1
- YNNUSGIPVFPVBX-NHCUHLMSSA-N CN1CCC[C@@H]1CCO[C@](C)(C1=CC=CC=C1)C1=CC=C(Cl)C=C1.O=C(O)/C=C/C(=O)O Chemical compound CN1CCC[C@@H]1CCO[C@](C)(C1=CC=CC=C1)C1=CC=C(Cl)C=C1.O=C(O)/C=C/C(=O)O YNNUSGIPVFPVBX-NHCUHLMSSA-N 0.000 description 1
- ZSHARPJAQXGCMB-UHFFFAOYSA-N CN1CCN(C(=O)C2CCCO2)CC1 Chemical compound CN1CCN(C(=O)C2CCCO2)CC1 ZSHARPJAQXGCMB-UHFFFAOYSA-N 0.000 description 1
- OROBEQMQVZFJHA-UHFFFAOYSA-N CN1CCN(C(=O)C2COC3=C(C=CC=C3)O2)CC1 Chemical compound CN1CCN(C(=O)C2COC3=C(C=CC=C3)O2)CC1 OROBEQMQVZFJHA-UHFFFAOYSA-N 0.000 description 1
- WWLXYYFBVIVCRF-HNENSFHCSA-N CN1CCN(C2=CC=C(/C(=N\O)C3=CC=CC=C3)C=C2)CC1 Chemical compound CN1CCN(C2=CC=C(/C(=N\O)C3=CC=CC=C3)C=C2)CC1 WWLXYYFBVIVCRF-HNENSFHCSA-N 0.000 description 1
- WANMDIKRIRQLBF-UAVWNXLESA-M CN1CCN(C2=CC=C(/C(=N\O)C3=CC=CC=C3)C=C2)CC1.CN1CCN(C2=CC=C(C(=O)C3=CC=CC=C3)C=C2)CC1.Cl.NO.O[Na] Chemical compound CN1CCN(C2=CC=C(/C(=N\O)C3=CC=CC=C3)C=C2)CC1.CN1CCN(C2=CC=C(C(=O)C3=CC=CC=C3)C=C2)CC1.Cl.NO.O[Na] WANMDIKRIRQLBF-UAVWNXLESA-M 0.000 description 1
- KOMTVXCAGDGDHE-UHFFFAOYSA-N CN1CCN(C2=CC=C(C(=O)C3=CC=CC=C3)C=C2)CC1.CN1CCNCC1.O=C(C1=CC=CC=C1)C1=CC=C(F)C=C1 Chemical compound CN1CCN(C2=CC=C(C(=O)C3=CC=CC=C3)C=C2)CC1.CN1CCNCC1.O=C(C1=CC=CC=C1)C1=CC=C(F)C=C1 KOMTVXCAGDGDHE-UHFFFAOYSA-N 0.000 description 1
- GGOZCXYLAORXIS-UHFFFAOYSA-M CN1CCN(C2=CC=C(C(=O)C3=CC=CC=C3)C=C2)CC1.O[Na].[H]C(O)(C1=CC=CC=C1)C1=CC=C(N2CCN(C)CC2)C=C1.[Zn] Chemical compound CN1CCN(C2=CC=C(C(=O)C3=CC=CC=C3)C=C2)CC1.O[Na].[H]C(O)(C1=CC=CC=C1)C1=CC=C(N2CCN(C)CC2)C=C1.[Zn] GGOZCXYLAORXIS-UHFFFAOYSA-M 0.000 description 1
- VMSRKPBBVYNKKK-UHFFFAOYSA-N CN1CCN(C2=CC=C(C3=NC4=CC=CC=C4C(Cl)=N3)C=C2)CC1.NCCN1CCOCC1.[H]N(CCN1CCOCC1)C1=NC(C2=CC=C(N3CCN(C)CC3)C=C2)=NC2=CC=CC=C21 Chemical compound CN1CCN(C2=CC=C(C3=NC4=CC=CC=C4C(Cl)=N3)C=C2)CC1.NCCN1CCOCC1.[H]N(CCN1CCOCC1)C1=NC(C2=CC=C(N3CCN(C)CC3)C=C2)=NC2=CC=CC=C21 VMSRKPBBVYNKKK-UHFFFAOYSA-N 0.000 description 1
- WQDDXVGJRSTLED-UHFFFAOYSA-N CN1CCN(C2=CC=CC=C2)CC1 Chemical compound CN1CCN(C2=CC=CC=C2)CC1 WQDDXVGJRSTLED-UHFFFAOYSA-N 0.000 description 1
- WIKYUJGCLQQFNW-UHFFFAOYSA-N CN1CCN(CCCN2C3=C(C=CC=C3)SC3=C2C=C(Cl)C=C3)CC1.O=C(O)/C=C\C(=O)O.O=C(O)/C=C\C(=O)O Chemical compound CN1CCN(CCCN2C3=C(C=CC=C3)SC3=C2C=C(Cl)C=C3)CC1.O=C(O)/C=C\C(=O)O.O=C(O)/C=C\C(=O)O WIKYUJGCLQQFNW-UHFFFAOYSA-N 0.000 description 1
- VZYCZNZBPPHOFY-UHFFFAOYSA-N CN1CCN(CCCN2C3=CC=CC=C3SC3=C2C=C(S(=O)(=O)N(C)C)C=C3)CC1.CS(=O)(=O)O.CS(=O)(=O)O Chemical compound CN1CCN(CCCN2C3=CC=CC=C3SC3=C2C=C(S(=O)(=O)N(C)C)C=C3)CC1.CS(=O)(=O)O.CS(=O)(=O)O VZYCZNZBPPHOFY-UHFFFAOYSA-N 0.000 description 1
- QHTUMQYGZQYEOZ-UHFFFAOYSA-N CN1CCN(CCO)CC1 Chemical compound CN1CCN(CCO)CC1 QHTUMQYGZQYEOZ-UHFFFAOYSA-N 0.000 description 1
- RGPDEAGGEXEMMM-UHFFFAOYSA-N CN1CCOC(C2=CC=CC=C2)C2=CC=CC=C2C1.Cl Chemical compound CN1CCOC(C2=CC=CC=C2)C2=CC=CC=C2C1.Cl RGPDEAGGEXEMMM-UHFFFAOYSA-N 0.000 description 1
- SJRJJKPEHAURKC-UHFFFAOYSA-N CN1CCOCC1 Chemical compound CN1CCOCC1 SJRJJKPEHAURKC-UHFFFAOYSA-N 0.000 description 1
- QSLMDECMDJKHMQ-UHFFFAOYSA-N CNCCCC12CCC(C3=CC=CC=C31)C1=C2C=CC=C1.Cl Chemical compound CNCCCC12CCC(C3=CC=CC=C31)C1=C2C=CC=C1.Cl QSLMDECMDJKHMQ-UHFFFAOYSA-N 0.000 description 1
- HCYAFALTSJYZDH-UHFFFAOYSA-N CNCCCN1C2=C(C=CC=C2)CCC2=C1C=CC=C2.Cl Chemical compound CNCCCN1C2=C(C=CC=C2)CCC2=C1C=CC=C2.Cl HCYAFALTSJYZDH-UHFFFAOYSA-N 0.000 description 1
- IUIHUZWBQBXGCI-UHFFFAOYSA-N COC(=O)C1=C(S(=O)(=O)C(C)(C)C)C(C(O)(C2=CC=CC=C2)C2=CC=CC=C2)=CC=C1 Chemical compound COC(=O)C1=C(S(=O)(=O)C(C)(C)C)C(C(O)(C2=CC=CC=C2)C2=CC=CC=C2)=CC=C1 IUIHUZWBQBXGCI-UHFFFAOYSA-N 0.000 description 1
- NGVLXRBIJYFIFM-PEZBUJJGSA-N COC(=O)C1=C2/C3=CC=CC=C3C(=N/O)/C2=C/C=C\1 Chemical compound COC(=O)C1=C2/C3=CC=CC=C3C(=N/O)/C2=C/C=C\1 NGVLXRBIJYFIFM-PEZBUJJGSA-N 0.000 description 1
- YWFQNWUOAUGGJY-UHFFFAOYSA-N COC(=O)C1=CC=C(C2CC(=O)OC3=C2C=CC2=CC=CC=C23)C=C1 Chemical compound COC(=O)C1=CC=C(C2CC(=O)OC3=C2C=CC2=CC=CC=C23)C=C1 YWFQNWUOAUGGJY-UHFFFAOYSA-N 0.000 description 1
- YBHILYKTIRIUTE-UHFFFAOYSA-N COC1=C(OC)C2=C[N+]3=C(C=C2C=C1)C1=CC2=C(C=C1CC3)OCO2.[Cl-] Chemical compound COC1=C(OC)C2=C[N+]3=C(C=C2C=C1)C1=CC2=C(C=C1CC3)OCO2.[Cl-] YBHILYKTIRIUTE-UHFFFAOYSA-N 0.000 description 1
- MFIDHISLMOJQRL-UHFFFAOYSA-N COC1=C(OC)C=C2C(=C1)N=C(N1CCN(C3=CC=CC=C3)CC1)N=C2N Chemical compound COC1=C(OC)C=C2C(=C1)N=C(N1CCN(C3=CC=CC=C3)CC1)N=C2N MFIDHISLMOJQRL-UHFFFAOYSA-N 0.000 description 1
- VRQVVMDWGGWHTJ-UHFFFAOYSA-N COC1=CC2=C(C=C1)SC1=CC=CC=C1N2CC(C)CN(C)C.O=C(O)/C=C\C(=O)O Chemical compound COC1=CC2=C(C=C1)SC1=CC=CC=C1N2CC(C)CN(C)C.O=C(O)/C=C\C(=O)O VRQVVMDWGGWHTJ-UHFFFAOYSA-N 0.000 description 1
- XQYZDYMELSJDRZ-UHFFFAOYSA-N COC1=CC=C(CC2=C3C=C(OC)C(OC)=CC3=CC=N2)C=C1OC.Cl Chemical compound COC1=CC=C(CC2=C3C=C(OC)C(OC)=CC3=CC=N2)C=C1OC.Cl XQYZDYMELSJDRZ-UHFFFAOYSA-N 0.000 description 1
- OJHFBJYRILITTG-UHFFFAOYSA-N COCCCNC(=O)C1=C(N)C2=C(C=C1)C(=O)C1=C(C=CC=C1)C2=O Chemical compound COCCCNC(=O)C1=C(N)C2=C(C=C1)C(=O)C1=C(C=CC=C1)C2=O OJHFBJYRILITTG-UHFFFAOYSA-N 0.000 description 1
- 201000002829 CREST Syndrome Diseases 0.000 description 1
- LBOJYSIDWZQNJS-CVEARBPZSA-N C[C@]12N[C@H](CC3=C1C=CC=C3)C1=C2C=CC=C1.O=C(O)/C=C\C(=O)O Chemical compound C[C@]12N[C@H](CC3=C1C=CC=C3)C1=C2C=CC=C1.O=C(O)/C=C\C(=O)O LBOJYSIDWZQNJS-CVEARBPZSA-N 0.000 description 1
- HOOSGZJRQIVJSZ-UHFFFAOYSA-N C[N+]12CCC(CC1)C(OC(=O)C(O)(C1=CC=CC=C1)C1=CC=CC=C1)C2.[Br-] Chemical compound C[N+]12CCC(CC1)C(OC(=O)C(O)(C1=CC=CC=C1)C1=CC=CC=C1)C2.[Br-] HOOSGZJRQIVJSZ-UHFFFAOYSA-N 0.000 description 1
- 101100455063 Caenorhabditis elegans lmp-1 gene Proteins 0.000 description 1
- 241000589994 Campylobacter sp. Species 0.000 description 1
- 102100039510 Cancer/testis antigen 2 Human genes 0.000 description 1
- 241000222122 Candida albicans Species 0.000 description 1
- 241000282465 Canis Species 0.000 description 1
- 241000282472 Canis lupus familiaris Species 0.000 description 1
- 208000009458 Carcinoma in Situ Diseases 0.000 description 1
- 206010008342 Cervix carcinoma Diseases 0.000 description 1
- 241000723437 Chamaecyparis Species 0.000 description 1
- 241000723436 Chamaecyparis obtusa Species 0.000 description 1
- 102000000013 Chemokine CCL3 Human genes 0.000 description 1
- 102000001326 Chemokine CCL4 Human genes 0.000 description 1
- 108010055165 Chemokine CCL4 Proteins 0.000 description 1
- 102000016950 Chemokine CXCL1 Human genes 0.000 description 1
- 108010014419 Chemokine CXCL1 Proteins 0.000 description 1
- 241000606153 Chlamydia trachomatis Species 0.000 description 1
- 206010008609 Cholangitis sclerosing Diseases 0.000 description 1
- NAUWTFJOPJWYOT-UHFFFAOYSA-N Cl.Cl.FC1=CC=C(C(OCCN2CCN(CCCC3=CC=CC=C3)CC2)C2=CC=C(F)C=C2)C=C1 Chemical compound Cl.Cl.FC1=CC=C(C(OCCN2CCN(CCCC3=CC=CC=C3)CC2)C2=CC=C(F)C=C2)C=C1 NAUWTFJOPJWYOT-UHFFFAOYSA-N 0.000 description 1
- NJMYODHXAKYRHW-DVZOWYKESA-N Cl.Cl.OCCN1CCN(CC/C=C2/C3=C(C=CC=C3)SC3=C2C=C(C(F)(F)F)C=C3)CC1 Chemical compound Cl.Cl.OCCN1CCN(CC/C=C2/C3=C(C=CC=C3)SC3=C2C=C(C(F)(F)F)C=C3)CC1 NJMYODHXAKYRHW-DVZOWYKESA-N 0.000 description 1
- JCTYWRARKVGOBK-UHFFFAOYSA-N Cl.[H]C12CC3=C(C=CC=C3)C3=C4OCOC4=CC(=C31)CCN2C Chemical compound Cl.[H]C12CC3=C(C=CC=C3)C3=C4OCOC4=CC(=C31)CCN2C JCTYWRARKVGOBK-UHFFFAOYSA-N 0.000 description 1
- VMWNQDUVQKEIOC-CYBMUJFWSA-N Cl.[H][C@@]12CC3=CC=C(O)C(O)=C3C3=CC=CC(=C31)CCN2C Chemical compound Cl.[H][C@@]12CC3=CC=C(O)C(O)=C3C3=CC=CC(=C31)CCN2C VMWNQDUVQKEIOC-CYBMUJFWSA-N 0.000 description 1
- UCRMNAQJMODSAH-UHFFFAOYSA-N ClC1=NC(C2=CC=CC=N2)=NC2=CC=CC=C21.NCCN1CCOCC1.O=P(Cl)(Cl)Cl.[H]N(CCN1CCOCC1)C1=NC(C2=CC=CC=N2)=NC2=CC=CC=C21.[H]N1C(=O)C2=CC=CC=C2N=C1C1=CC=CC=N1 Chemical compound ClC1=NC(C2=CC=CC=N2)=NC2=CC=CC=C21.NCCN1CCOCC1.O=P(Cl)(Cl)Cl.[H]N(CCN1CCOCC1)C1=NC(C2=CC=CC=N2)=NC2=CC=CC=C21.[H]N1C(=O)C2=CC=CC=C2N=C1C1=CC=CC=N1 UCRMNAQJMODSAH-UHFFFAOYSA-N 0.000 description 1
- ZBTKESWLSHPTLU-UHFFFAOYSA-N ClC1=NC(C2=CC=CN=C2)=NC2=CC=CC=C21.NCCN1CCOCC1.O=P(Cl)(Cl)Cl.[H]N(CCN1CCOCC1)C1=NC(C2=CC=CN=C2)=NC2=CC=CC=C21.[H]N1C(=O)C2=CC=CC=C2N=C1C1=CC=CN=C1 Chemical compound ClC1=NC(C2=CC=CN=C2)=NC2=CC=CC=C21.NCCN1CCOCC1.O=P(Cl)(Cl)Cl.[H]N(CCN1CCOCC1)C1=NC(C2=CC=CN=C2)=NC2=CC=CC=C21.[H]N1C(=O)C2=CC=CC=C2N=C1C1=CC=CN=C1 ZBTKESWLSHPTLU-UHFFFAOYSA-N 0.000 description 1
- 241000193468 Clostridium perfringens Species 0.000 description 1
- 241000193449 Clostridium tetani Species 0.000 description 1
- 241000223205 Coccidioides immitis Species 0.000 description 1
- 208000001333 Colorectal Neoplasms Diseases 0.000 description 1
- 208000035473 Communicable disease Diseases 0.000 description 1
- 206010010356 Congenital anomaly Diseases 0.000 description 1
- 241000186227 Corynebacterium diphtheriae Species 0.000 description 1
- 241000186249 Corynebacterium sp. Species 0.000 description 1
- 241000709687 Coxsackievirus Species 0.000 description 1
- 201000007336 Cryptococcosis Diseases 0.000 description 1
- 241000221204 Cryptococcus neoformans Species 0.000 description 1
- 241000723198 Cupressus Species 0.000 description 1
- 244000301850 Cupressus sempervirens Species 0.000 description 1
- 108010025464 Cyclin-Dependent Kinase 4 Proteins 0.000 description 1
- 102100036252 Cyclin-dependent kinase 4 Human genes 0.000 description 1
- 102000053602 DNA Human genes 0.000 description 1
- 241000209210 Dactylis Species 0.000 description 1
- 240000004585 Dactylis glomerata Species 0.000 description 1
- 241000710829 Dengue virus group Species 0.000 description 1
- 241000238710 Dermatophagoides Species 0.000 description 1
- 241000238713 Dermatophagoides farinae Species 0.000 description 1
- NTURVSFTOYPGON-UHFFFAOYSA-N Dihydroquinazoline Chemical compound C1=CC=C2C=NCNC2=C1 NTURVSFTOYPGON-UHFFFAOYSA-N 0.000 description 1
- 206010059866 Drug resistance Diseases 0.000 description 1
- 238000008157 ELISA kit Methods 0.000 description 1
- 241001115402 Ebolavirus Species 0.000 description 1
- 241001466953 Echovirus Species 0.000 description 1
- 241000508725 Elymus repens Species 0.000 description 1
- 241000196324 Embryophyta Species 0.000 description 1
- 206010014733 Endometrial cancer Diseases 0.000 description 1
- 206010014759 Endometrial neoplasm Diseases 0.000 description 1
- 241000194032 Enterococcus faecalis Species 0.000 description 1
- 241001495410 Enterococcus sp. Species 0.000 description 1
- 241000991587 Enterovirus C Species 0.000 description 1
- 208000000832 Equine Encephalomyelitis Diseases 0.000 description 1
- 241000186810 Erysipelothrix rhusiopathiae Species 0.000 description 1
- 208000000461 Esophageal Neoplasms Diseases 0.000 description 1
- 241000282324 Felis Species 0.000 description 1
- 241000282326 Felis catus Species 0.000 description 1
- 241000234642 Festuca Species 0.000 description 1
- 241000234645 Festuca pratensis Species 0.000 description 1
- 241000711950 Filoviridae Species 0.000 description 1
- 241000710781 Flaviviridae Species 0.000 description 1
- 241000605986 Fusobacterium nucleatum Species 0.000 description 1
- 102100039717 G antigen 1 Human genes 0.000 description 1
- 102100039699 G antigen 4 Human genes 0.000 description 1
- 102100039698 G antigen 5 Human genes 0.000 description 1
- 101710092267 G antigen 5 Proteins 0.000 description 1
- 102100039713 G antigen 6 Human genes 0.000 description 1
- 101710092269 G antigen 6 Proteins 0.000 description 1
- 102000040452 GAGE family Human genes 0.000 description 1
- 108091072337 GAGE family Proteins 0.000 description 1
- 102100030525 Gap junction alpha-4 protein Human genes 0.000 description 1
- 208000005577 Gastroenteritis Diseases 0.000 description 1
- 108700028146 Genetic Enhancer Elements Proteins 0.000 description 1
- 108700039691 Genetic Promoter Regions Proteins 0.000 description 1
- 208000034951 Genetic Translocation Diseases 0.000 description 1
- 208000007465 Giant cell arteritis Diseases 0.000 description 1
- 208000032612 Glial tumor Diseases 0.000 description 1
- 206010018338 Glioma Diseases 0.000 description 1
- 206010018364 Glomerulonephritis Diseases 0.000 description 1
- 102000007390 Glycogen Phosphorylase Human genes 0.000 description 1
- 108010046163 Glycogen Phosphorylase Proteins 0.000 description 1
- 208000024869 Goodpasture syndrome Diseases 0.000 description 1
- 208000015023 Graves' disease Diseases 0.000 description 1
- ZIXGXMMUKPLXBB-UHFFFAOYSA-N Guatambuinine Natural products N1C2=CC=CC=C2C2=C1C(C)=C1C=CN=C(C)C1=C2 ZIXGXMMUKPLXBB-UHFFFAOYSA-N 0.000 description 1
- 208000035895 Guillain-Barré syndrome Diseases 0.000 description 1
- 241000606768 Haemophilus influenzae Species 0.000 description 1
- 206010061192 Haemorrhagic fever Diseases 0.000 description 1
- 241000150562 Hantaan orthohantavirus Species 0.000 description 1
- 208000030836 Hashimoto thyroiditis Diseases 0.000 description 1
- 241000590002 Helicobacter pylori Species 0.000 description 1
- 208000035186 Hemolytic Autoimmune Anemia Diseases 0.000 description 1
- 241000700739 Hepadnaviridae Species 0.000 description 1
- 208000005331 Hepatitis D Diseases 0.000 description 1
- 241000709721 Hepatovirus A Species 0.000 description 1
- 241000700586 Herpesviridae Species 0.000 description 1
- 241000226709 Hesperocyparis arizonica Species 0.000 description 1
- 241001290232 Hesperocyparis macrocarpa Species 0.000 description 1
- 241000228404 Histoplasma capsulatum Species 0.000 description 1
- 208000017604 Hodgkin disease Diseases 0.000 description 1
- 208000021519 Hodgkin lymphoma Diseases 0.000 description 1
- 208000010747 Hodgkins lymphoma Diseases 0.000 description 1
- 241000744855 Holcus Species 0.000 description 1
- 240000003857 Holcus lanatus Species 0.000 description 1
- 101000874316 Homo sapiens B melanoma antigen 1 Proteins 0.000 description 1
- 101000797762 Homo sapiens C-C motif chemokine 5 Proteins 0.000 description 1
- 101000858060 Homo sapiens C-X-C motif chemokine 11 Proteins 0.000 description 1
- 101000889128 Homo sapiens C-X-C motif chemokine 2 Proteins 0.000 description 1
- 101000947172 Homo sapiens C-X-C motif chemokine 9 Proteins 0.000 description 1
- 101000889345 Homo sapiens Cancer/testis antigen 2 Proteins 0.000 description 1
- 101000886137 Homo sapiens G antigen 1 Proteins 0.000 description 1
- 101000886678 Homo sapiens G antigen 2D Proteins 0.000 description 1
- 101000886136 Homo sapiens G antigen 4 Proteins 0.000 description 1
- 101000972485 Homo sapiens Lupus La protein Proteins 0.000 description 1
- 101001036406 Homo sapiens Melanoma-associated antigen C1 Proteins 0.000 description 1
- 101001057156 Homo sapiens Melanoma-associated antigen C2 Proteins 0.000 description 1
- 101001057159 Homo sapiens Melanoma-associated antigen C3 Proteins 0.000 description 1
- 101000950669 Homo sapiens Mitogen-activated protein kinase 9 Proteins 0.000 description 1
- 101001114057 Homo sapiens P antigen family member 1 Proteins 0.000 description 1
- 101000880770 Homo sapiens Protein SSX2 Proteins 0.000 description 1
- 101000763579 Homo sapiens Toll-like receptor 1 Proteins 0.000 description 1
- 101000763537 Homo sapiens Toll-like receptor 10 Proteins 0.000 description 1
- 101000669447 Homo sapiens Toll-like receptor 4 Proteins 0.000 description 1
- 101000669460 Homo sapiens Toll-like receptor 5 Proteins 0.000 description 1
- 101000669406 Homo sapiens Toll-like receptor 6 Proteins 0.000 description 1
- 241000714260 Human T-lymphotropic virus 1 Species 0.000 description 1
- 241000701074 Human alphaherpesvirus 2 Species 0.000 description 1
- 241000701085 Human alphaherpesvirus 3 Species 0.000 description 1
- UFHFLCQGNIYNRP-UHFFFAOYSA-N Hydrogen Chemical compound [H][H] UFHFLCQGNIYNRP-UHFFFAOYSA-N 0.000 description 1
- 230000005353 IP-10 production Effects 0.000 description 1
- 206010021245 Idiopathic thrombocytopenic purpura Diseases 0.000 description 1
- DGAQECJNVWCQMB-PUAWFVPOSA-M Ilexoside XXIX Chemical compound C[C@@H]1CC[C@@]2(CC[C@@]3(C(=CC[C@H]4[C@]3(CC[C@@H]5[C@@]4(CC[C@@H](C5(C)C)OS(=O)(=O)[O-])C)C)[C@@H]2[C@]1(C)O)C)C(=O)O[C@H]6[C@@H]([C@H]([C@@H]([C@H](O6)CO)O)O)O.[Na+] DGAQECJNVWCQMB-PUAWFVPOSA-M 0.000 description 1
- 208000024781 Immune Complex disease Diseases 0.000 description 1
- 102000002227 Interferon Type I Human genes 0.000 description 1
- 108010014726 Interferon Type I Proteins 0.000 description 1
- 102000008070 Interferon-gamma Human genes 0.000 description 1
- 108010072621 Interleukin-1 Receptor-Associated Kinases Proteins 0.000 description 1
- 102000006940 Interleukin-1 Receptor-Associated Kinases Human genes 0.000 description 1
- 108090000177 Interleukin-11 Proteins 0.000 description 1
- 102000003815 Interleukin-11 Human genes 0.000 description 1
- 102000003812 Interleukin-15 Human genes 0.000 description 1
- 108090000172 Interleukin-15 Proteins 0.000 description 1
- 108010002586 Interleukin-7 Proteins 0.000 description 1
- 102000000704 Interleukin-7 Human genes 0.000 description 1
- 102000015696 Interleukins Human genes 0.000 description 1
- 108010063738 Interleukins Proteins 0.000 description 1
- 241000701377 Iridoviridae Species 0.000 description 1
- 102000019145 JUN kinase activity proteins Human genes 0.000 description 1
- 241000592238 Juniperus communis Species 0.000 description 1
- 208000008839 Kidney Neoplasms Diseases 0.000 description 1
- 241000588915 Klebsiella aerogenes Species 0.000 description 1
- 241000588747 Klebsiella pneumoniae Species 0.000 description 1
- ZDXPYRJPNDTMRX-VKHMYHEASA-N L-glutamine Chemical compound OC(=O)[C@@H](N)CCC(N)=O ZDXPYRJPNDTMRX-VKHMYHEASA-N 0.000 description 1
- 229930182816 L-glutamine Natural products 0.000 description 1
- 241000589248 Legionella Species 0.000 description 1
- 208000007764 Legionnaires' Disease Diseases 0.000 description 1
- 241000589902 Leptospira Species 0.000 description 1
- 108010028921 Lipopeptides Proteins 0.000 description 1
- 241000186779 Listeria monocytogenes Species 0.000 description 1
- 241000209082 Lolium Species 0.000 description 1
- 244000100545 Lolium multiflorum Species 0.000 description 1
- 240000004296 Lolium perenne Species 0.000 description 1
- 102100022742 Lupus La protein Human genes 0.000 description 1
- 206010025323 Lymphomas Diseases 0.000 description 1
- 108091054455 MAP kinase family Proteins 0.000 description 1
- 102000043136 MAP kinase family Human genes 0.000 description 1
- 108010046938 Macrophage Colony-Stimulating Factor Proteins 0.000 description 1
- 102000007651 Macrophage Colony-Stimulating Factor Human genes 0.000 description 1
- 241000124008 Mammalia Species 0.000 description 1
- 241000712079 Measles morbillivirus Species 0.000 description 1
- KDLHYOMCWBWLMM-UHFFFAOYSA-N Meclizine hydrochloride Chemical compound O.Cl.Cl.CC1=CC=CC(CN2CCN(CC2)C(C=2C=CC=CC=2)C=2C=CC(Cl)=CC=2)=C1 KDLHYOMCWBWLMM-UHFFFAOYSA-N 0.000 description 1
- 102000000440 Melanoma-associated antigen Human genes 0.000 description 1
- 108050008953 Melanoma-associated antigen Proteins 0.000 description 1
- 102100039447 Melanoma-associated antigen C1 Human genes 0.000 description 1
- 102100027252 Melanoma-associated antigen C2 Human genes 0.000 description 1
- 102100027248 Melanoma-associated antigen C3 Human genes 0.000 description 1
- 206010049567 Miller Fisher syndrome Diseases 0.000 description 1
- 102100037809 Mitogen-activated protein kinase 9 Human genes 0.000 description 1
- 208000003445 Mouth Neoplasms Diseases 0.000 description 1
- 241000711386 Mumps virus Species 0.000 description 1
- 101100260758 Mus musculus Tlr9 gene Proteins 0.000 description 1
- 241000186367 Mycobacterium avium Species 0.000 description 1
- 241000187484 Mycobacterium gordonae Species 0.000 description 1
- 241000186364 Mycobacterium intracellulare Species 0.000 description 1
- 241000186363 Mycobacterium kansasii Species 0.000 description 1
- ODAPLLWDLHTCPH-UHFFFAOYSA-N NC(=O)C1=C(N)C2=C(C(=O)C3=C(C=CC=C3)C2=O)C(N)=C1 Chemical compound NC(=O)C1=C(N)C2=C(C(=O)C3=C(C=CC=C3)C2=O)C(N)=C1 ODAPLLWDLHTCPH-UHFFFAOYSA-N 0.000 description 1
- FTFHZWLNIADXOA-UHFFFAOYSA-N NC(=O)C1=CC=CC2=C1C1=C(C=CC=C1)C2 Chemical compound NC(=O)C1=CC=CC2=C1C1=C(C=CC=C1)C2 FTFHZWLNIADXOA-UHFFFAOYSA-N 0.000 description 1
- QVVXJEYIVXVWHH-UHFFFAOYSA-N NC(=O)C1=CC=CC=C1N.O.[H]C(=O)C1=CC=C(N2CCN(C)CC2)C=C1.[H]N1C(=O)C2=CC=CC=C2N([H])C1([H])C1=CC=C(N2CCN(C)CC2)C=C1.[H]N1C(=O)C2=CC=CC=C2N=C1C1=CC=C(N2CCN(C)CC2)C=C1 Chemical compound NC(=O)C1=CC=CC=C1N.O.[H]C(=O)C1=CC=C(N2CCN(C)CC2)C=C1.[H]N1C(=O)C2=CC=CC=C2N([H])C1([H])C1=CC=C(N2CCN(C)CC2)C=C1.[H]N1C(=O)C2=CC=CC=C2N=C1C1=CC=C(N2CCN(C)CC2)C=C1 QVVXJEYIVXVWHH-UHFFFAOYSA-N 0.000 description 1
- LFKLTGPCMCEKPE-UHFFFAOYSA-N NC1=C(C(=O)O)C=C(Br)C2=C1C(=O)C1=C(C=CC=C1)C2=O Chemical compound NC1=C(C(=O)O)C=C(Br)C2=C1C(=O)C1=C(C=CC=C1)C2=O LFKLTGPCMCEKPE-UHFFFAOYSA-N 0.000 description 1
- PAYRUJLWNCNPSJ-UHFFFAOYSA-N NC1=CC=CC=C1 Chemical compound NC1=CC=CC=C1 PAYRUJLWNCNPSJ-UHFFFAOYSA-N 0.000 description 1
- YXVLHVQLYTURGI-UHFFFAOYSA-N NNC1=NC2=C(C(=O)N1C1CCCC1)C1(CCCC1)CC1=C2C=CC=C1 Chemical compound NNC1=NC2=C(C(=O)N1C1CCCC1)C1(CCCC1)CC1=C2C=CC=C1 YXVLHVQLYTURGI-UHFFFAOYSA-N 0.000 description 1
- 208000001894 Nasopharyngeal Neoplasms Diseases 0.000 description 1
- 206010061306 Nasopharyngeal cancer Diseases 0.000 description 1
- 238000011887 Necropsy Methods 0.000 description 1
- 241000588652 Neisseria gonorrhoeae Species 0.000 description 1
- 241000588650 Neisseria meningitidis Species 0.000 description 1
- 206010029260 Neuroblastoma Diseases 0.000 description 1
- 206010029379 Neutrophilia Diseases 0.000 description 1
- 108010051791 Nuclear Antigens Proteins 0.000 description 1
- 102000019040 Nuclear Antigens Human genes 0.000 description 1
- MBWOTAXKUVDVNQ-CZIZESTLSA-N O=C(C1=C2/C3=CC=CC=C3C(=N\O)/C2=C/C=C\1)N1CCCCC1 Chemical compound O=C(C1=C2/C3=CC=CC=C3C(=N\O)/C2=C/C=C\1)N1CCCCC1 MBWOTAXKUVDVNQ-CZIZESTLSA-N 0.000 description 1
- NEGLTAOBUVVVFF-UHFFFAOYSA-N O=C(CN1CCCC1)NC1=C2C=CC=CC2=NC2=C1CCN2C1=CC=CC=C1 Chemical compound O=C(CN1CCCC1)NC1=C2C=CC=CC2=NC2=C1CCN2C1=CC=CC=C1 NEGLTAOBUVVVFF-UHFFFAOYSA-N 0.000 description 1
- QHLBJLZMJWFUSH-QOCHGBHMSA-N O=C(O)/C1=C/C=C\C2=C1C1=C(C=CC=C1)/C2=N/NC1=C([N+](=O)[O-])C=C([N+](=O)[O-])C=C1 Chemical compound O=C(O)/C1=C/C=C\C2=C1C1=C(C=CC=C1)/C2=N/NC1=C([N+](=O)[O-])C=C([N+](=O)[O-])C=C1 QHLBJLZMJWFUSH-QOCHGBHMSA-N 0.000 description 1
- UQMFETDKXJWZDS-FYWRMAATSA-N O=C(O)C1=C2/C(=C\C=C/1)/C1=CC=CC=C1/C2=N\O Chemical compound O=C(O)C1=C2/C(=C\C=C/1)/C1=CC=CC=C1/C2=N\O UQMFETDKXJWZDS-FYWRMAATSA-N 0.000 description 1
- JKVBVCSXZSPKTC-UHFFFAOYSA-N O=C(O)C1=CC(C(C2=CC(C(=O)O)=C(O)C=C2)C(Cl)(Cl)Cl)=CC=C1O Chemical compound O=C(O)C1=CC(C(C2=CC(C(=O)O)=C(O)C=C2)C(Cl)(Cl)Cl)=CC=C1O JKVBVCSXZSPKTC-UHFFFAOYSA-N 0.000 description 1
- CEPYVIYDCXDLEE-UHFFFAOYSA-N O=C(O)C1=CC=C(C2(C3=CC=C(C(=O)O)C=C3)OC(=O)C3=C2C=CC=C3)C=C1 Chemical compound O=C(O)C1=CC=C(C2(C3=CC=C(C(=O)O)C=C3)OC(=O)C3=C2C=CC=C3)C=C1 CEPYVIYDCXDLEE-UHFFFAOYSA-N 0.000 description 1
- BXSCARJCEFJTJZ-UHFFFAOYSA-N O=C(O)C1=CC=CC=C1NC1=CC=CC2=C1C(=O)C1=C(C=CC=C1)C2=O Chemical compound O=C(O)C1=CC=CC=C1NC1=CC=CC2=C1C(=O)C1=C(C=CC=C1)C2=O BXSCARJCEFJTJZ-UHFFFAOYSA-N 0.000 description 1
- MSJCBSNEBHMHCA-UHFFFAOYSA-N O=C1C2=C(C=C(OC3=CC=C(S(=O)(=O)C4=CC=C(OC5=C/C6=C(\C=C/5)C(=O)N(C5=CC=CC=N5)C6=O)C=C4)C=C3)C=C2)C(=O)N1C1=CC=CC=N1 Chemical compound O=C1C2=C(C=C(OC3=CC=C(S(=O)(=O)C4=CC=C(OC5=C/C6=C(\C=C/5)C(=O)N(C5=CC=CC=N5)C6=O)C=C4)C=C3)C=C2)C(=O)N1C1=CC=CC=N1 MSJCBSNEBHMHCA-UHFFFAOYSA-N 0.000 description 1
- OMXSQRCTHHUYMR-UHFFFAOYSA-N O=C1C2=C(C=C(S(=O)(=O)C3=CC4=C(C=C3)C(=O)N(C3=CC=CC=N3)C4=O)C=C2)C(=O)N1C1=CC=CC=N1 Chemical compound O=C1C2=C(C=C(S(=O)(=O)C3=CC4=C(C=C3)C(=O)N(C3=CC=CC=N3)C4=O)C=C2)C(=O)N1C1=CC=CC=N1 OMXSQRCTHHUYMR-UHFFFAOYSA-N 0.000 description 1
- PGBGUZRKFRBNBT-UHFFFAOYSA-N O=C1C2=C(C=CC=C2)C(=O)C2=C1C=CC1=C2NN(C2CCCCC2)C1=O Chemical compound O=C1C2=C(C=CC=C2)C(=O)C2=C1C=CC1=C2NN(C2CCCCC2)C1=O PGBGUZRKFRBNBT-UHFFFAOYSA-N 0.000 description 1
- YFYLSIWJEUCFCV-UHFFFAOYSA-N O=C1C2=C(N=C(NCCO)N1C1=CC=CC=C1)C1=C(C=CC=C1)CC21CCCC1 Chemical compound O=C1C2=C(N=C(NCCO)N1C1=CC=CC=C1)C1=C(C=CC=C1)CC21CCCC1 YFYLSIWJEUCFCV-UHFFFAOYSA-N 0.000 description 1
- CDTIDPQDTLFZKS-UHFFFAOYSA-N O=C1C2=C(N=C(NCCO)N1CC1=CC=CC=C1)C1=CC=CC=C1CC21CCCC1 Chemical compound O=C1C2=C(N=C(NCCO)N1CC1=CC=CC=C1)C1=CC=CC=C1CC21CCCC1 CDTIDPQDTLFZKS-UHFFFAOYSA-N 0.000 description 1
- XUTYIDAGCCGARZ-UHFFFAOYSA-N O=C1NC(NCCO)=NC2=C1C1(CCCC1)CC1=C2C=CC=C1 Chemical compound O=C1NC(NCCO)=NC2=C1C1(CCCC1)CC1=C2C=CC=C1 XUTYIDAGCCGARZ-UHFFFAOYSA-N 0.000 description 1
- AKXKFNUVGGYMJP-UHFFFAOYSA-N O=C1NC2=C(C=CC=C2)OC2=CC=C([N+](=O)[O-])C=C12 Chemical compound O=C1NC2=C(C=CC=C2)OC2=CC=C([N+](=O)[O-])C=C12 AKXKFNUVGGYMJP-UHFFFAOYSA-N 0.000 description 1
- XCMYSSWGBOKNQS-UHFFFAOYSA-M O=COO[Na].[H]C(=O)C1=CC=C(F)C=C1.[H]C(=O)C1=CC=C(N2CCN(C)CC2)C=C1.[H]N1CCN(C)CC1.[NaH] Chemical compound O=COO[Na].[H]C(=O)C1=CC=C(F)C=C1.[H]C(=O)C1=CC=C(N2CCN(C)CC2)C=C1.[H]N1CCN(C)CC1.[NaH] XCMYSSWGBOKNQS-UHFFFAOYSA-M 0.000 description 1
- PUUROYYEIKBGJJ-UHFFFAOYSA-N O=S(OO)OS(=O)OOO.[H]N(CCN(C)C)C1=NC(Cl)=NC2=CC(OC)=C(OC)C=C21.[H]N(CCN(C)C)C1=NC(N2CCN(C)CC2)=NC2=CC(OC)=C(OC)C=C21.[H]N1CCN(C)CC1 Chemical compound O=S(OO)OS(=O)OOO.[H]N(CCN(C)C)C1=NC(Cl)=NC2=CC(OC)=C(OC)C=C21.[H]N(CCN(C)C)C1=NC(N2CCN(C)CC2)=NC2=CC(OC)=C(OC)C=C21.[H]N1CCN(C)CC1 PUUROYYEIKBGJJ-UHFFFAOYSA-N 0.000 description 1
- 206010030155 Oesophageal carcinoma Diseases 0.000 description 1
- 239000005642 Oleic acid Substances 0.000 description 1
- ZQPPMHVWECSIRJ-UHFFFAOYSA-N Oleic acid Natural products CCCCCCCCC=CCCCCCCCC(O)=O ZQPPMHVWECSIRJ-UHFFFAOYSA-N 0.000 description 1
- 102000043276 Oncogene Human genes 0.000 description 1
- 108700020796 Oncogene Proteins 0.000 description 1
- 241000713112 Orthobunyavirus Species 0.000 description 1
- 241000712464 Orthomyxoviridae Species 0.000 description 1
- 241000150218 Orthonairovirus Species 0.000 description 1
- 241000702244 Orthoreovirus Species 0.000 description 1
- 102100023219 P antigen family member 1 Human genes 0.000 description 1
- 108060006580 PRAME Proteins 0.000 description 1
- 102000036673 PRAME Human genes 0.000 description 1
- 102100034640 PWWP domain-containing DNA repair factor 3A Human genes 0.000 description 1
- 108050007154 PWWP domain-containing DNA repair factor 3A Proteins 0.000 description 1
- 241001631646 Papillomaviridae Species 0.000 description 1
- 241000711504 Paramyxoviridae Species 0.000 description 1
- 208000002606 Paramyxoviridae Infections Diseases 0.000 description 1
- 241001465379 Parietaria judaica Species 0.000 description 1
- 241000721464 Parietaria officinalis Species 0.000 description 1
- 241000701945 Parvoviridae Species 0.000 description 1
- 241001330453 Paspalum Species 0.000 description 1
- 241001330451 Paspalum notatum Species 0.000 description 1
- 241000150350 Peribunyaviridae Species 0.000 description 1
- 241000238661 Periplaneta Species 0.000 description 1
- 241000238675 Periplaneta americana Species 0.000 description 1
- 208000031845 Pernicious anaemia Diseases 0.000 description 1
- 208000037581 Persistent Infection Diseases 0.000 description 1
- 206010057249 Phagocytosis Diseases 0.000 description 1
- 241000745991 Phalaris Species 0.000 description 1
- 244000081757 Phalaris arundinacea Species 0.000 description 1
- 241000713137 Phlebovirus Species 0.000 description 1
- 241000746981 Phleum Species 0.000 description 1
- 241000746983 Phleum pratense Species 0.000 description 1
- 241000709664 Picornaviridae Species 0.000 description 1
- 241001127637 Plantago Species 0.000 description 1
- 244000239204 Plantago lanceolata Species 0.000 description 1
- 235000010503 Plantago lanceolata Nutrition 0.000 description 1
- 241000223960 Plasmodium falciparum Species 0.000 description 1
- 241000209048 Poa Species 0.000 description 1
- 241000136254 Poa compressa Species 0.000 description 1
- 241000209049 Poa pratensis Species 0.000 description 1
- 229920002732 Polyanhydride Polymers 0.000 description 1
- 241001505332 Polyomavirus sp. Species 0.000 description 1
- 229920001710 Polyorthoester Polymers 0.000 description 1
- 206010036303 Post streptococcal glomerulonephritis Diseases 0.000 description 1
- ZLMJMSJWJFRBEC-UHFFFAOYSA-N Potassium Chemical compound [K] ZLMJMSJWJFRBEC-UHFFFAOYSA-N 0.000 description 1
- 208000006664 Precursor Cell Lymphoblastic Leukemia-Lymphoma Diseases 0.000 description 1
- 208000024777 Prion disease Diseases 0.000 description 1
- 102100037686 Protein SSX2 Human genes 0.000 description 1
- 108010076504 Protein Sorting Signals Proteins 0.000 description 1
- 241000125945 Protoparvovirus Species 0.000 description 1
- 201000004681 Psoriasis Diseases 0.000 description 1
- 241000219492 Quercus Species 0.000 description 1
- 244000274906 Quercus alba Species 0.000 description 1
- 235000009137 Quercus alba Nutrition 0.000 description 1
- 239000012980 RPMI-1640 medium Substances 0.000 description 1
- 241000711798 Rabies lyssavirus Species 0.000 description 1
- 208000015634 Rectal Neoplasms Diseases 0.000 description 1
- 206010038389 Renal cancer Diseases 0.000 description 1
- 241000702247 Reoviridae Species 0.000 description 1
- SZLZWPPUNLXJEA-PYUPNBKESA-N Rescinnamin Natural products CO[C@@H]1[C@H](C[C@@H]2CN3CCc4c([nH]c5cc(OC)ccc45)[C@@H]3C[C@@H]2[C@H]1C(=O)OC)OC(=O)C=Cc6cc(OC)c(OC)c(OC)c6 SZLZWPPUNLXJEA-PYUPNBKESA-N 0.000 description 1
- LCQMZZCPPSWADO-UHFFFAOYSA-N Reserpilin Natural products COC(=O)C1COCC2CN3CCc4c([nH]c5cc(OC)c(OC)cc45)C3CC12 LCQMZZCPPSWADO-UHFFFAOYSA-N 0.000 description 1
- QEVHRUUCFGRFIF-SFWBKIHZSA-N Reserpine Natural products O=C(OC)[C@@H]1[C@H](OC)[C@H](OC(=O)c2cc(OC)c(OC)c(OC)c2)C[C@H]2[C@@H]1C[C@H]1N(C2)CCc2c3c([nH]c12)cc(OC)cc3 QEVHRUUCFGRFIF-SFWBKIHZSA-N 0.000 description 1
- 241000725643 Respiratory syncytial virus Species 0.000 description 1
- 206010057190 Respiratory tract infections Diseases 0.000 description 1
- 241000712907 Retroviridae Species 0.000 description 1
- 241000711931 Rhabdoviridae Species 0.000 description 1
- 108091028664 Ribonucleotide Proteins 0.000 description 1
- 241000702670 Rotavirus Species 0.000 description 1
- 241000710799 Rubella virus Species 0.000 description 1
- SUYXJDLXGFPMCQ-INIZCTEOSA-N SJ000287331 Natural products CC1=c2cnccc2=C(C)C2=Nc3ccccc3[C@H]12 SUYXJDLXGFPMCQ-INIZCTEOSA-N 0.000 description 1
- 244000004774 Sabina virginiana Species 0.000 description 1
- 235000008691 Sabina virginiana Nutrition 0.000 description 1
- 208000034189 Sclerosis Diseases 0.000 description 1
- 241000209056 Secale Species 0.000 description 1
- 244000082988 Secale cereale Species 0.000 description 1
- 235000007238 Secale cereale Nutrition 0.000 description 1
- 241000700584 Simplexvirus Species 0.000 description 1
- 208000000453 Skin Neoplasms Diseases 0.000 description 1
- 241000191967 Staphylococcus aureus Species 0.000 description 1
- 240000006694 Stellaria media Species 0.000 description 1
- 229930182558 Sterol Natural products 0.000 description 1
- 208000005718 Stomach Neoplasms Diseases 0.000 description 1
- 241001478880 Streptobacillus moniliformis Species 0.000 description 1
- 241000193985 Streptococcus agalactiae Species 0.000 description 1
- 241000194049 Streptococcus equinus Species 0.000 description 1
- 241000193998 Streptococcus pneumoniae Species 0.000 description 1
- 241000193996 Streptococcus pyogenes Species 0.000 description 1
- 241001505901 Streptococcus sp. 'group A' Species 0.000 description 1
- 241000193990 Streptococcus sp. 'group B' Species 0.000 description 1
- 108700025695 Suppressor Genes Proteins 0.000 description 1
- 101710143177 Synaptonemal complex protein 1 Proteins 0.000 description 1
- 102100036234 Synaptonemal complex protein 1 Human genes 0.000 description 1
- 230000024932 T cell mediated immunity Effects 0.000 description 1
- 108091008874 T cell receptors Proteins 0.000 description 1
- 108010092262 T-Cell Antigen Receptors Proteins 0.000 description 1
- 208000000389 T-cell leukemia Diseases 0.000 description 1
- 208000028530 T-cell lymphoblastic leukemia/lymphoma Diseases 0.000 description 1
- 108700026226 TATA Box Proteins 0.000 description 1
- 102100033082 TNF receptor-associated factor 3 Human genes 0.000 description 1
- 208000001106 Takayasu Arteritis Diseases 0.000 description 1
- 210000000447 Th1 cell Anatomy 0.000 description 1
- 210000004241 Th2 cell Anatomy 0.000 description 1
- RYYWUUFWQRZTIU-UHFFFAOYSA-N Thiophosphoric acid Chemical group OP(O)(S)=O RYYWUUFWQRZTIU-UHFFFAOYSA-N 0.000 description 1
- QPMDKXBBAVQDCB-UHFFFAOYSA-N Thioproperazine mesilate Chemical compound CS(O)(=O)=O.CS(O)(=O)=O.C12=CC(S(=O)(=O)N(C)C)=CC=C2SC2=CC=CC=C2N1CCCN1CCN(C)CC1 QPMDKXBBAVQDCB-UHFFFAOYSA-N 0.000 description 1
- 208000024770 Thyroid neoplasm Diseases 0.000 description 1
- 241000710924 Togaviridae Species 0.000 description 1
- 102100027010 Toll-like receptor 1 Human genes 0.000 description 1
- 102100027009 Toll-like receptor 10 Human genes 0.000 description 1
- 102100039360 Toll-like receptor 4 Human genes 0.000 description 1
- 102100039357 Toll-like receptor 5 Human genes 0.000 description 1
- 102100039387 Toll-like receptor 6 Human genes 0.000 description 1
- 241000589884 Treponema pallidum Species 0.000 description 1
- 241000589904 Treponema pallidum subsp. pertenue Species 0.000 description 1
- 235000021307 Triticum Nutrition 0.000 description 1
- 241000209140 Triticum Species 0.000 description 1
- 244000098338 Triticum aestivum Species 0.000 description 1
- 206010067584 Type 1 diabetes mellitus Diseases 0.000 description 1
- 206010053613 Type IV hypersensitivity reaction Diseases 0.000 description 1
- 102100039094 Tyrosinase Human genes 0.000 description 1
- 108060008724 Tyrosinase Proteins 0.000 description 1
- 208000007097 Urinary Bladder Neoplasms Diseases 0.000 description 1
- 208000006105 Uterine Cervical Neoplasms Diseases 0.000 description 1
- 241000700618 Vaccinia virus Species 0.000 description 1
- 241000700647 Variola virus Species 0.000 description 1
- 241000711975 Vesicular stomatitis virus Species 0.000 description 1
- 108700005077 Viral Genes Proteins 0.000 description 1
- 241000120645 Yellow fever virus group Species 0.000 description 1
- NQMFDMWJBWEDBD-UHFFFAOYSA-N [H]C(C1=CC=CC=C1)(C1=CC=C(N2CCN(C)CC2)C=C1)N1CCN(C)CC1 Chemical compound [H]C(C1=CC=CC=C1)(C1=CC=C(N2CCN(C)CC2)C=C1)N1CCN(C)CC1 NQMFDMWJBWEDBD-UHFFFAOYSA-N 0.000 description 1
- MDSWJGZCZJXLCT-UHFFFAOYSA-N [H]C(NCCN(C)C)(C1=CC=CC=C1)C1=CC=C(N2CCN(C)CC2)C=C1 Chemical compound [H]C(NCCN(C)C)(C1=CC=CC=C1)C1=CC=C(N2CCN(C)CC2)C=C1 MDSWJGZCZJXLCT-UHFFFAOYSA-N 0.000 description 1
- OJOFTYQIOPIQMP-UHFFFAOYSA-N [H]C(O)(C1=CC=CC=C1)C1=CC=C(N2CCN(C)CC2)C=C1 Chemical compound [H]C(O)(C1=CC=CC=C1)C1=CC=C(N2CCN(C)CC2)C=C1 OJOFTYQIOPIQMP-UHFFFAOYSA-N 0.000 description 1
- IRHNMMMEOSEUPZ-UHFFFAOYSA-N [H]C(OCCN(C)C)(C1=CC=CC=C1)C1=CC=C(N2CCN(C)CC2)C=C1 Chemical compound [H]C(OCCN(C)C)(C1=CC=CC=C1)C1=CC=C(N2CCN(C)CC2)C=C1 IRHNMMMEOSEUPZ-UHFFFAOYSA-N 0.000 description 1
- UDPPHFGUOMVFNH-UHFFFAOYSA-N [H]C([H])(C1=CC=CC=C1)C1=CC=C(N2CCN(C)CC2)C=C1 Chemical compound [H]C([H])(C1=CC=CC=C1)C1=CC=C(N2CCN(C)CC2)C=C1 UDPPHFGUOMVFNH-UHFFFAOYSA-N 0.000 description 1
- ROVHLTPFQBRCFZ-UHFFFAOYSA-N [H]N(C1=CC=NC2=CC(Cl)=CC=C21)C(C)CCCN(CC)CC.[H]N(CC)CCCC(C)N([H])C1=CC=NC2=CC(Cl)=CC=C21.[H]N([H])CCCC(C)N([H])C1=CC=NC2=CC(Cl)=CC=C21 Chemical compound [H]N(C1=CC=NC2=CC(Cl)=CC=C21)C(C)CCCN(CC)CC.[H]N(CC)CCCC(C)N([H])C1=CC=NC2=CC(Cl)=CC=C21.[H]N([H])CCCC(C)N([H])C1=CC=NC2=CC(Cl)=CC=C21 ROVHLTPFQBRCFZ-UHFFFAOYSA-N 0.000 description 1
- VLRDLNVGQBSZFM-UHFFFAOYSA-N [H]N(CCN(C)C)C1=CC(C2=CC=C(N3CCN(C)CC3)C=C2)=NC2=CC=CC=C21 Chemical compound [H]N(CCN(C)C)C1=CC(C2=CC=C(N3CCN(C)CC3)C=C2)=NC2=CC=CC=C21 VLRDLNVGQBSZFM-UHFFFAOYSA-N 0.000 description 1
- DLRTUNCSRHYUAM-UHFFFAOYSA-N [H]N(CCN(C)C)C1=NC(Cl)=NC2=CC(OC)=C(OC)C=C21.[H]N(CCN(C)C)C1=NC(N2CCN(C3=CC=CC=C3)CC2)=NC2=CC(OC)=C(OC)C=C21.[H]N1CCN(C2=CC=CC=C2)CC1 Chemical compound [H]N(CCN(C)C)C1=NC(Cl)=NC2=CC(OC)=C(OC)C=C21.[H]N(CCN(C)C)C1=NC(N2CCN(C3=CC=CC=C3)CC2)=NC2=CC(OC)=C(OC)C=C21.[H]N1CCN(C2=CC=CC=C2)CC1 DLRTUNCSRHYUAM-UHFFFAOYSA-N 0.000 description 1
- NRDOWTUUBQCUMC-UHFFFAOYSA-N [H]N(CCN(C)C)C1=NC(N2CCN(C3=CC=CC=C3)CC2)=NC2=CC(OC)=C(OC)C=C21 Chemical compound [H]N(CCN(C)C)C1=NC(N2CCN(C3=CC=CC=C3)CC2)=NC2=CC(OC)=C(OC)C=C21 NRDOWTUUBQCUMC-UHFFFAOYSA-N 0.000 description 1
- DPBUIYRVIADVFD-UHFFFAOYSA-N [H]N(CCN1CCOCC1)C1=NC(C2=CC=C(N3CCN(C)CC3)C=C2)=NC2=CC=CC=C21 Chemical compound [H]N(CCN1CCOCC1)C1=NC(C2=CC=C(N3CCN(C)CC3)C=C2)=NC2=CC=CC=C21 DPBUIYRVIADVFD-UHFFFAOYSA-N 0.000 description 1
- SZLZWPPUNLXJEA-YALYAIOQSA-N [H][C@@]12C[C@]3([H])C4=C(CCN3C[C@]1([H])C[C@@H](OC(=O)/C=C/C1=CC(OC)=C(OC)C(OC)=C1)[C@H](OC)[C@H]2C(=O)OC)C1=CC=C(OC)C=C1N4 Chemical compound [H][C@@]12C[C@]3([H])C4=C(CCN3C[C@]1([H])C[C@@H](OC(=O)/C=C/C1=CC(OC)=C(OC)C(OC)=C1)[C@H](OC)[C@H]2C(=O)OC)C1=CC=C(OC)C=C1N4 SZLZWPPUNLXJEA-YALYAIOQSA-N 0.000 description 1
- QEVHRUUCFGRFIF-WJIPZCLTSA-N [H][C@@]12C[C@]3([H])C4=C(CCN3C[C@]1([H])C[C@@H](OC(=O)C1=CC(OC)=C(OC)C(OC)=C1)[C@H](OC)[C@H]2C(=O)OC)C1=C(C=C(OC)C=C1)N4 Chemical compound [H][C@@]12C[C@]3([H])C4=C(CCN3C[C@]1([H])C[C@@H](OC(=O)C1=CC(OC)=C(OC)C(OC)=C1)[C@H](OC)[C@H]2C(=O)OC)C1=C(C=C(OC)C=C1)N4 QEVHRUUCFGRFIF-WJIPZCLTSA-N 0.000 description 1
- WYJAPUKIYAZSEM-MOPGFXCFSA-N [H][C@]12C3=C4CCN1CCC[C@@]2(CC)CC(=O)N3C1=CC=CC=C14 Chemical compound [H][C@]12C3=C4CCN1CCC[C@@]2(CC)CC(=O)N3C1=CC=CC=C14 WYJAPUKIYAZSEM-MOPGFXCFSA-N 0.000 description 1
- GRTOGORTSDXSFK-WADGIJBFSA-N [H][C@]12CC3C4=C(CCN3C[C@@]1([H])[C@H](C)OC=C2C(=O)OC)C1=C(C=CC=C1)N4 Chemical compound [H][C@]12CC3C4=C(CCN3C[C@@]1([H])[C@H](C)OC=C2C(=O)OC)C1=C(C=CC=C1)N4 GRTOGORTSDXSFK-WADGIJBFSA-N 0.000 description 1
- 229960005054 acepromazine Drugs 0.000 description 1
- 150000007513 acids Chemical class 0.000 description 1
- 230000009471 action Effects 0.000 description 1
- 201000005638 acute proliferative glomerulonephritis Diseases 0.000 description 1
- 230000003044 adaptive effect Effects 0.000 description 1
- 102000035181 adaptor proteins Human genes 0.000 description 1
- 108091005764 adaptor proteins Proteins 0.000 description 1
- 239000000654 additive Substances 0.000 description 1
- 108700010877 adenoviridae proteins Proteins 0.000 description 1
- 230000001464 adherent effect Effects 0.000 description 1
- 239000000443 aerosol Substances 0.000 description 1
- 230000001270 agonistic effect Effects 0.000 description 1
- 125000004183 alkoxy alkyl group Chemical group 0.000 description 1
- 208000030961 allergic reaction Diseases 0.000 description 1
- 229910000147 aluminium phosphate Inorganic materials 0.000 description 1
- 229960000836 amitriptyline Drugs 0.000 description 1
- 229910021529 ammonia Inorganic materials 0.000 description 1
- 239000000908 ammonium hydroxide Substances 0.000 description 1
- 230000003042 antagnostic effect Effects 0.000 description 1
- 230000002583 anti-histone Effects 0.000 description 1
- 230000000078 anti-malarial effect Effects 0.000 description 1
- 230000003460 anti-nuclear Effects 0.000 description 1
- 229940111121 antirheumatic drug quinolines Drugs 0.000 description 1
- 235000009052 artemisia Nutrition 0.000 description 1
- 244000309743 astrovirus Species 0.000 description 1
- 201000000448 autoimmune hemolytic anemia Diseases 0.000 description 1
- 201000003710 autoimmune thrombocytopenic purpura Diseases 0.000 description 1
- 229950008193 azacyclonol Drugs 0.000 description 1
- 229940065181 bacillus anthracis Drugs 0.000 description 1
- 230000008901 benefit Effects 0.000 description 1
- 229960000686 benzalkonium chloride Drugs 0.000 description 1
- 125000001797 benzyl group Chemical group [H]C1=C([H])C([H])=C(C([H])=C1[H])C([H])([H])* 0.000 description 1
- CADWTSSKOVRVJC-UHFFFAOYSA-N benzyl(dimethyl)azanium;chloride Chemical compound [Cl-].C[NH+](C)CC1=CC=CC=C1 CADWTSSKOVRVJC-UHFFFAOYSA-N 0.000 description 1
- 201000009036 biliary tract cancer Diseases 0.000 description 1
- 208000020790 biliary tract neoplasm Diseases 0.000 description 1
- 238000004166 bioassay Methods 0.000 description 1
- 230000004071 biological effect Effects 0.000 description 1
- 230000008512 biological response Effects 0.000 description 1
- 238000004820 blood count Methods 0.000 description 1
- 108091005948 blue fluorescent proteins Proteins 0.000 description 1
- MHVCSDLBQKSFQV-HNNXBMFYSA-N boldine Natural products COc1cc2c(C[C@@H]3N(C)CCc4cc(C)c(OC)c2c34)cc1O MHVCSDLBQKSFQV-HNNXBMFYSA-N 0.000 description 1
- LZJRNLRASBVRRX-UHFFFAOYSA-N boldine trifluoroacetic acid salt Natural products CN1CCC2=CC(O)=C(OC)C3=C2C1CC1=C3C=C(OC)C(O)=C1 LZJRNLRASBVRRX-UHFFFAOYSA-N 0.000 description 1
- KGBXLFKZBHKPEV-UHFFFAOYSA-N boric acid Chemical compound OB(O)O KGBXLFKZBHKPEV-UHFFFAOYSA-N 0.000 description 1
- 239000004327 boric acid Substances 0.000 description 1
- 210000000481 breast Anatomy 0.000 description 1
- 159000000007 calcium salts Chemical class 0.000 description 1
- 229940095731 candida albicans Drugs 0.000 description 1
- 150000001720 carbohydrates Chemical class 0.000 description 1
- 235000014633 carbohydrates Nutrition 0.000 description 1
- 150000001721 carbon Chemical group 0.000 description 1
- 125000004432 carbon atom Chemical group C* 0.000 description 1
- 125000002843 carboxylic acid group Chemical group 0.000 description 1
- 238000012754 cardiac puncture Methods 0.000 description 1
- 239000000969 carrier Substances 0.000 description 1
- 230000015556 catabolic process Effects 0.000 description 1
- 239000003054 catalyst Substances 0.000 description 1
- 239000002458 cell surface marker Substances 0.000 description 1
- 230000036755 cellular response Effects 0.000 description 1
- 238000005119 centrifugation Methods 0.000 description 1
- 201000010881 cervical cancer Diseases 0.000 description 1
- 208000019065 cervical carcinoma Diseases 0.000 description 1
- 210000003679 cervix uteri Anatomy 0.000 description 1
- SQQXRXKYTKFFSM-UHFFFAOYSA-N chembl1992147 Chemical compound OC1=C(OC)C(OC)=CC=C1C1=C(C)C(C(O)=O)=NC(C=2N=C3C4=NC(C)(C)N=C4C(OC)=C(O)C3=CC=2)=C1N SQQXRXKYTKFFSM-UHFFFAOYSA-N 0.000 description 1
- 230000014564 chemokine production Effects 0.000 description 1
- 229940038705 chlamydia trachomatis Drugs 0.000 description 1
- 229960004926 chlorobutanol Drugs 0.000 description 1
- 229960002347 chloropyramine hydrochloride Drugs 0.000 description 1
- 229960001076 chlorpromazine Drugs 0.000 description 1
- 229960001657 chlorpromazine hydrochloride Drugs 0.000 description 1
- 229960000322 chlorprothixene hydrochloride Drugs 0.000 description 1
- 235000012000 cholesterol Nutrition 0.000 description 1
- 150000001840 cholesterol esters Chemical class 0.000 description 1
- 230000001684 chronic effect Effects 0.000 description 1
- IOVDQEIIMOZNNA-MHKBYHAFSA-N cis-flupenthixol dihydrochloride Chemical compound Cl.Cl.C1CN(CCO)CCN1CC\C=C\1C2=CC(C(F)(F)F)=CC=C2SC2=CC=CC=C2/1 IOVDQEIIMOZNNA-MHKBYHAFSA-N 0.000 description 1
- 229960002689 clemastine fumarate Drugs 0.000 description 1
- GKEGFOKQMZHVOW-KUTGSRRKSA-M clidinium bromide Chemical compound [Br-].C1([C@H]2CC[N@+](CC2)(C1)C)OC(=O)C(O)(C=1C=CC=CC=1)C1=CC=CC=C1 GKEGFOKQMZHVOW-KUTGSRRKSA-M 0.000 description 1
- 229960005098 clidinium bromide Drugs 0.000 description 1
- 229960002544 cloperastine Drugs 0.000 description 1
- 239000011248 coating agent Substances 0.000 description 1
- 238000000576 coating method Methods 0.000 description 1
- 210000001072 colon Anatomy 0.000 description 1
- 208000029742 colonic neoplasm Diseases 0.000 description 1
- 230000024203 complement activation Effects 0.000 description 1
- 239000007891 compressed tablet Substances 0.000 description 1
- 208000018631 connective tissue disease Diseases 0.000 description 1
- 108010015408 connexin 37 Proteins 0.000 description 1
- 230000000139 costimulatory effect Effects 0.000 description 1
- 239000006071 cream Substances 0.000 description 1
- 239000000287 crude extract Substances 0.000 description 1
- 239000013078 crystal Substances 0.000 description 1
- UKPBEPCQTDRZSE-UHFFFAOYSA-N cyclizine hydrochloride Chemical compound Cl.C1CN(C)CCN1C(C=1C=CC=CC=1)C1=CC=CC=C1 UKPBEPCQTDRZSE-UHFFFAOYSA-N 0.000 description 1
- 229960001677 cyclizine hydrochloride Drugs 0.000 description 1
- 125000000753 cycloalkyl group Chemical group 0.000 description 1
- 230000001461 cytolytic effect Effects 0.000 description 1
- GDPJWJXLKPPEKK-SJAYXVESSA-N dT4 Chemical compound O=C1NC(=O)C(C)=CN1[C@@H]1O[C@H](COP(O)(=O)O[C@@H]2[C@H](O[C@H](C2)N2C(NC(=O)C(C)=C2)=O)COP(O)(=O)O[C@@H]2[C@H](O[C@H](C2)N2C(NC(=O)C(C)=C2)=O)COP(O)(=O)O[C@@H]2[C@H](O[C@H](C2)N2C(NC(=O)C(C)=C2)=O)CO)[C@@H](O)C1 GDPJWJXLKPPEKK-SJAYXVESSA-N 0.000 description 1
- 238000010908 decantation Methods 0.000 description 1
- 230000007123 defense Effects 0.000 description 1
- 230000002950 deficient Effects 0.000 description 1
- 238000006731 degradation reaction Methods 0.000 description 1
- 230000003111 delayed effect Effects 0.000 description 1
- 238000012217 deletion Methods 0.000 description 1
- 230000037430 deletion Effects 0.000 description 1
- 239000005549 deoxyribonucleoside Substances 0.000 description 1
- 230000001419 dependent effect Effects 0.000 description 1
- 230000008021 deposition Effects 0.000 description 1
- 201000001981 dermatomyositis Diseases 0.000 description 1
- XAEWZDYWZHIUCT-UHFFFAOYSA-N desipramine hydrochloride Chemical compound [H+].[Cl-].C1CC2=CC=CC=C2N(CCCNC)C2=CC=CC=C21 XAEWZDYWZHIUCT-UHFFFAOYSA-N 0.000 description 1
- 229960003829 desipramine hydrochloride Drugs 0.000 description 1
- 238000003745 diagnosis Methods 0.000 description 1
- 239000010432 diamond Substances 0.000 description 1
- NAGJZTKCGNOGPW-UHFFFAOYSA-K dioxido-sulfanylidene-sulfido-$l^{5}-phosphane Chemical compound [O-]P([O-])([S-])=S NAGJZTKCGNOGPW-UHFFFAOYSA-K 0.000 description 1
- 229960000525 diphenhydramine hydrochloride Drugs 0.000 description 1
- LPRLDRXGWKXRMQ-UHFFFAOYSA-N diphenylpyraline hydrochloride Chemical compound [Cl-].C1C[NH+](C)CCC1OC(C=1C=CC=CC=1)C1=CC=CC=C1 LPRLDRXGWKXRMQ-UHFFFAOYSA-N 0.000 description 1
- 229960002392 diphenylpyraline hydrochloride Drugs 0.000 description 1
- 230000009266 disease activity Effects 0.000 description 1
- 239000007884 disintegrant Substances 0.000 description 1
- 238000004090 dissolution Methods 0.000 description 1
- 238000004821 distillation Methods 0.000 description 1
- 229960002311 dithranol Drugs 0.000 description 1
- QLTXKCWMEZIHBJ-PJGJYSAQSA-N dizocilpine maleate Chemical compound OC(=O)\C=C/C(O)=O.C12=CC=CC=C2[C@]2(C)C3=CC=CC=C3C[C@H]1N2 QLTXKCWMEZIHBJ-PJGJYSAQSA-N 0.000 description 1
- 239000002552 dosage form Substances 0.000 description 1
- VJECBOKJABCYMF-UHFFFAOYSA-N doxazosin mesylate Chemical compound [H+].CS([O-])(=O)=O.C1OC2=CC=CC=C2OC1C(=O)N(CC1)CCN1C1=NC(N)=C(C=C(C(OC)=C2)OC)C2=N1 VJECBOKJABCYMF-UHFFFAOYSA-N 0.000 description 1
- 229960000220 doxazosin mesylate Drugs 0.000 description 1
- 239000006196 drop Substances 0.000 description 1
- 239000000428 dust Substances 0.000 description 1
- WYJAPUKIYAZSEM-RBUKOAKNSA-N eburnamonine(-) Chemical compound C1=CC=C2C(CCN3CCC4)=C5[C@H]3[C@@]4(CC)CC(=O)N5C2=C1 WYJAPUKIYAZSEM-RBUKOAKNSA-N 0.000 description 1
- 239000003480 eluent Substances 0.000 description 1
- 239000000839 emulsion Substances 0.000 description 1
- 206010014599 encephalitis Diseases 0.000 description 1
- 238000005516 engineering process Methods 0.000 description 1
- 229940092559 enterobacter aerogenes Drugs 0.000 description 1
- 210000002615 epidermis Anatomy 0.000 description 1
- 210000002919 epithelial cell Anatomy 0.000 description 1
- 201000004101 esophageal cancer Diseases 0.000 description 1
- 210000003238 esophagus Anatomy 0.000 description 1
- 125000004185 ester group Chemical group 0.000 description 1
- 229960004886 ethaverine hydrochloride Drugs 0.000 description 1
- 229960002077 ethopropazine hydrochloride Drugs 0.000 description 1
- 239000003925 fat Substances 0.000 description 1
- 229960002602 fendiline Drugs 0.000 description 1
- 230000001605 fetal effect Effects 0.000 description 1
- 210000002950 fibroblast Anatomy 0.000 description 1
- 239000000945 filler Substances 0.000 description 1
- 229960000326 flunarizine Drugs 0.000 description 1
- 108010006620 fodrin Proteins 0.000 description 1
- 235000003599 food sweetener Nutrition 0.000 description 1
- 230000037433 frameshift Effects 0.000 description 1
- 238000002825 functional assay Methods 0.000 description 1
- 230000002538 fungal effect Effects 0.000 description 1
- 108020001507 fusion proteins Proteins 0.000 description 1
- 102000037865 fusion proteins Human genes 0.000 description 1
- 229940044627 gamma-interferon Drugs 0.000 description 1
- 206010017758 gastric cancer Diseases 0.000 description 1
- 230000002496 gastric effect Effects 0.000 description 1
- 239000011521 glass Substances 0.000 description 1
- PCHJSUWPFVWCPO-UHFFFAOYSA-N gold Chemical compound [Au] PCHJSUWPFVWCPO-UHFFFAOYSA-N 0.000 description 1
- 239000010931 gold Substances 0.000 description 1
- 229910052737 gold Inorganic materials 0.000 description 1
- 239000008187 granular material Substances 0.000 description 1
- 244000144993 groups of animals Species 0.000 description 1
- 230000012010 growth Effects 0.000 description 1
- 239000003102 growth factor Substances 0.000 description 1
- 239000001963 growth medium Substances 0.000 description 1
- 229940047650 haemophilus influenzae Drugs 0.000 description 1
- 230000003862 health status Effects 0.000 description 1
- 210000002443 helper t lymphocyte Anatomy 0.000 description 1
- 230000002489 hematologic effect Effects 0.000 description 1
- 208000006454 hepatitis Diseases 0.000 description 1
- 231100000283 hepatitis Toxicity 0.000 description 1
- 208000002672 hepatitis B Diseases 0.000 description 1
- 208000029570 hepatitis D virus infection Diseases 0.000 description 1
- 206010073071 hepatocellular carcinoma Diseases 0.000 description 1
- 229940088597 hormone Drugs 0.000 description 1
- 239000005556 hormone Substances 0.000 description 1
- 239000000017 hydrogel Substances 0.000 description 1
- COVNOIRYDKHLJD-UHFFFAOYSA-N hydron;1-methyl-9h-pyrido[3,4-b]indole;chloride Chemical compound Cl.C12=CC=CC=C2NC2=C1C=CN=C2C COVNOIRYDKHLJD-UHFFFAOYSA-N 0.000 description 1
- CNNVSINJDJNHQK-UHFFFAOYSA-N hydron;5-methyl-1-phenyl-1,3,4,6-tetrahydro-2,5-benzoxazocine;chloride Chemical compound [Cl-].C12=CC=CC=C2C[NH+](C)CCOC1C1=CC=CC=C1 CNNVSINJDJNHQK-UHFFFAOYSA-N 0.000 description 1
- 125000002887 hydroxy group Chemical group [H]O* 0.000 description 1
- 229960000930 hydroxyzine Drugs 0.000 description 1
- 208000024326 hypersensitivity reaction type III disease Diseases 0.000 description 1
- 229940124669 imidazoquinoline Drugs 0.000 description 1
- XZZXIYZZBJDEEP-UHFFFAOYSA-N imipramine hydrochloride Chemical compound [Cl-].C1CC2=CC=CC=C2N(CCC[NH+](C)C)C2=CC=CC=C21 XZZXIYZZBJDEEP-UHFFFAOYSA-N 0.000 description 1
- 229960002102 imipramine hydrochloride Drugs 0.000 description 1
- 230000036737 immune function Effects 0.000 description 1
- 230000008629 immune suppression Effects 0.000 description 1
- 230000003259 immunoinhibitory effect Effects 0.000 description 1
- 230000001771 impaired effect Effects 0.000 description 1
- 230000001976 improved effect Effects 0.000 description 1
- 238000000099 in vitro assay Methods 0.000 description 1
- 238000010348 incorporation Methods 0.000 description 1
- 238000011534 incubation Methods 0.000 description 1
- 238000001802 infusion Methods 0.000 description 1
- 208000030603 inherited susceptibility to asthma Diseases 0.000 description 1
- 229910052500 inorganic mineral Inorganic materials 0.000 description 1
- 239000002919 insect venom Substances 0.000 description 1
- 229940079322 interferon Drugs 0.000 description 1
- 229940047124 interferons Drugs 0.000 description 1
- 230000031261 interleukin-10 production Effects 0.000 description 1
- 230000017306 interleukin-6 production Effects 0.000 description 1
- 230000021995 interleukin-8 production Effects 0.000 description 1
- 229940047122 interleukins Drugs 0.000 description 1
- 208000003243 intestinal obstruction Diseases 0.000 description 1
- 230000003834 intracellular effect Effects 0.000 description 1
- 239000007928 intraperitoneal injection Substances 0.000 description 1
- 238000007913 intrathecal administration Methods 0.000 description 1
- QXJSBBXBKPUZAA-UHFFFAOYSA-N isooleic acid Natural products CCCCCCCC=CCCCCCCCCC(O)=O QXJSBBXBKPUZAA-UHFFFAOYSA-N 0.000 description 1
- 238000005304 joining Methods 0.000 description 1
- 201000010982 kidney cancer Diseases 0.000 description 1
- 150000002596 lactones Chemical class 0.000 description 1
- 229960001941 lidoflazine Drugs 0.000 description 1
- 208000012987 lip and oral cavity carcinoma Diseases 0.000 description 1
- 239000002502 liposome Substances 0.000 description 1
- 201000007270 liver cancer Diseases 0.000 description 1
- 244000144972 livestock Species 0.000 description 1
- PGYPOBZJRVSMDS-UHFFFAOYSA-N loperamide hydrochloride Chemical compound Cl.C=1C=CC=CC=1C(C=1C=CC=CC=1)(C(=O)N(C)C)CCN(CC1)CCC1(O)C1=CC=C(Cl)C=C1 PGYPOBZJRVSMDS-UHFFFAOYSA-N 0.000 description 1
- 229960002983 loperamide hydrochloride Drugs 0.000 description 1
- YQZBAXDVDZTKEQ-UHFFFAOYSA-N loxapine succinate Chemical compound [H+].[H+].[O-]C(=O)CCC([O-])=O.C1CN(C)CCN1C1=NC2=CC=CC=C2OC2=CC=C(Cl)C=C12 YQZBAXDVDZTKEQ-UHFFFAOYSA-N 0.000 description 1
- 239000000314 lubricant Substances 0.000 description 1
- 238000003670 luciferase enzyme activity assay Methods 0.000 description 1
- 210000004072 lung Anatomy 0.000 description 1
- 210000004698 lymphocyte Anatomy 0.000 description 1
- 210000003563 lymphoid tissue Anatomy 0.000 description 1
- 229960004992 maprotiline hydrochloride Drugs 0.000 description 1
- 239000011159 matrix material Substances 0.000 description 1
- AEUKDPKXTPNBNY-XEYRWQBLSA-N mcp 2 Chemical compound C([C@@H](C(=O)N[C@@H](CS)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@@H](CCCNC(N)=N)C(=O)NCC(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@@H](CC=1NC=NC=1)C(=O)N1[C@@H](CCC1)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CS)C(=O)N[C@@H](CS)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CCCNC(N)=N)C(O)=O)NC(=O)CNC(=O)[C@H](C)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](CC(C)C)NC(=O)[C@H]1N(CCC1)C(=O)[C@H](CC(C)C)NC(=O)[C@H](CS)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](C)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CS)NC(=O)[C@H](C)NC(=O)[C@H](CS)NC(=O)[C@@H](NC(=O)[C@@H](N)C(C)C)C(C)C)C1=CC=CC=C1 AEUKDPKXTPNBNY-XEYRWQBLSA-N 0.000 description 1
- 229960001474 meclozine Drugs 0.000 description 1
- 239000002609 medium Substances 0.000 description 1
- 229960003664 mesoridazine besylate Drugs 0.000 description 1
- 239000002207 metabolite Substances 0.000 description 1
- 229910052751 metal Inorganic materials 0.000 description 1
- 239000002184 metal Substances 0.000 description 1
- 230000001394 metastastic effect Effects 0.000 description 1
- 206010061289 metastatic neoplasm Diseases 0.000 description 1
- RAOHHYUBMJLHNC-UHFFFAOYSA-N methixene hydrochloride Chemical compound [H+].O.[Cl-].C1N(C)CCCC1CC1C2=CC=CC=C2SC2=CC=CC=C21 RAOHHYUBMJLHNC-UHFFFAOYSA-N 0.000 description 1
- 230000011987 methylation Effects 0.000 description 1
- 238000007069 methylation reaction Methods 0.000 description 1
- 229960003955 mianserin Drugs 0.000 description 1
- 238000012737 microarray-based gene expression Methods 0.000 description 1
- 239000003094 microcapsule Substances 0.000 description 1
- 230000005012 migration Effects 0.000 description 1
- 238000013508 migration Methods 0.000 description 1
- 235000010755 mineral Nutrition 0.000 description 1
- 239000011707 mineral Substances 0.000 description 1
- 239000002480 mineral oil Substances 0.000 description 1
- 235000010446 mineral oil Nutrition 0.000 description 1
- 229960000350 mitotane Drugs 0.000 description 1
- 230000004048 modification Effects 0.000 description 1
- 238000012986 modification Methods 0.000 description 1
- SFAVZRNMTSBKPO-PFEQFJNWSA-N molport-000-716-723 Chemical compound Cl.C12=C3C4=CC=CC=C4C[C@H]1N(C)CCC2=CC1=C3OCO1 SFAVZRNMTSBKPO-PFEQFJNWSA-N 0.000 description 1
- 210000002864 mononuclear phagocyte Anatomy 0.000 description 1
- 201000006417 multiple sclerosis Diseases 0.000 description 1
- 238000012243 multiplex automated genomic engineering Methods 0.000 description 1
- 206010028417 myasthenia gravis Diseases 0.000 description 1
- 201000000050 myeloid neoplasm Diseases 0.000 description 1
- VEYWWAGBHABATA-UHFFFAOYSA-N n'-[(4-chlorophenyl)methyl]-n,n-dimethyl-n'-pyridin-2-ylethane-1,2-diamine;hydron;chloride Chemical compound [Cl-].C=1C=CC=NC=1N(CC[NH+](C)C)CC1=CC=C(Cl)C=C1 VEYWWAGBHABATA-UHFFFAOYSA-N 0.000 description 1
- PUPNJSIFIXXJCH-UHFFFAOYSA-N n-(4-hydroxyphenyl)-2-(1,1,3-trioxo-1,2-benzothiazol-2-yl)acetamide Chemical compound C1=CC(O)=CC=C1NC(=O)CN1S(=O)(=O)C2=CC=CC=C2C1=O PUPNJSIFIXXJCH-UHFFFAOYSA-N 0.000 description 1
- DAZSWUUAFHBCGE-KRWDZBQOSA-N n-[(2s)-3-methyl-1-oxo-1-pyrrolidin-1-ylbutan-2-yl]-3-phenylpropanamide Chemical compound N([C@@H](C(C)C)C(=O)N1CCCC1)C(=O)CCC1=CC=CC=C1 DAZSWUUAFHBCGE-KRWDZBQOSA-N 0.000 description 1
- 210000000581 natural killer T-cell Anatomy 0.000 description 1
- 229960004925 nefopam hydrochloride Drugs 0.000 description 1
- 230000007935 neutral effect Effects 0.000 description 1
- 230000003472 neutralizing effect Effects 0.000 description 1
- AJFJKGLXKPEJMU-UHFFFAOYSA-N nitrarine dihydrochloride Chemical compound Cl.Cl.N1C2=CC=CC=C2C(CCN23)=C1C3C1CCC2C2C1NCCC2 AJFJKGLXKPEJMU-UHFFFAOYSA-N 0.000 description 1
- 229960002498 nomifensine maleate Drugs 0.000 description 1
- 208000002154 non-small cell lung carcinoma Diseases 0.000 description 1
- 239000000346 nonvolatile oil Substances 0.000 description 1
- 230000005937 nuclear translocation Effects 0.000 description 1
- 238000010899 nucleation Methods 0.000 description 1
- 210000004940 nucleus Anatomy 0.000 description 1
- ZQPPMHVWECSIRJ-KTKRTIGZSA-N oleic acid Chemical compound CCCCCCCC\C=C/CCCCCCCC(O)=O ZQPPMHVWECSIRJ-KTKRTIGZSA-N 0.000 description 1
- 230000014207 opsonization Effects 0.000 description 1
- 230000003571 opsonizing effect Effects 0.000 description 1
- 239000012074 organic phase Substances 0.000 description 1
- 229960002400 orphenadrine hydrochloride Drugs 0.000 description 1
- 210000001672 ovary Anatomy 0.000 description 1
- 150000002923 oximes Chemical class 0.000 description 1
- 229960003207 papaverine hydrochloride Drugs 0.000 description 1
- 239000002245 particle Substances 0.000 description 1
- 239000008188 pellet Substances 0.000 description 1
- 239000012466 permeate Substances 0.000 description 1
- 229960000762 perphenazine Drugs 0.000 description 1
- 210000001539 phagocyte Anatomy 0.000 description 1
- 230000008782 phagocytosis Effects 0.000 description 1
- 239000008177 pharmaceutical agent Substances 0.000 description 1
- 239000000825 pharmaceutical preparation Substances 0.000 description 1
- 229950000688 phenothiazine Drugs 0.000 description 1
- 150000003904 phospholipids Chemical class 0.000 description 1
- PTMHPRAIXMAOOB-UHFFFAOYSA-L phosphoramidate Chemical compound NP([O-])([O-])=O PTMHPRAIXMAOOB-UHFFFAOYSA-L 0.000 description 1
- 229960000399 pimethixene Drugs 0.000 description 1
- 229960003634 pimozide Drugs 0.000 description 1
- 239000013612 plasmid Substances 0.000 description 1
- 210000004180 plasmocyte Anatomy 0.000 description 1
- 238000007747 plating Methods 0.000 description 1
- 230000008488 polyadenylation Effects 0.000 description 1
- 229920001610 polycaprolactone Polymers 0.000 description 1
- 229920006149 polyester-amide block copolymer Polymers 0.000 description 1
- 239000013641 positive control Substances 0.000 description 1
- 239000011591 potassium Substances 0.000 description 1
- 229910052700 potassium Inorganic materials 0.000 description 1
- 229940094472 prenylamine lactate Drugs 0.000 description 1
- 229960003195 pridinol Drugs 0.000 description 1
- 229960003111 prochlorperazine Drugs 0.000 description 1
- DSKIOWHQLUWFLG-SPIKMXEPSA-N prochlorperazine maleate Chemical compound [H+].[H+].[H+].[H+].[O-]C(=O)\C=C/C([O-])=O.[O-]C(=O)\C=C/C([O-])=O.C1CN(C)CCN1CCCN1C2=CC(Cl)=CC=C2SC2=CC=CC=C21 DSKIOWHQLUWFLG-SPIKMXEPSA-N 0.000 description 1
- VXPCQISYVPFYRK-UHFFFAOYSA-N profenamine hydrochloride Chemical compound Cl.C1=CC=C2N(CC(C)N(CC)CC)C3=CC=CC=C3SC2=C1 VXPCQISYVPFYRK-UHFFFAOYSA-N 0.000 description 1
- 230000035755 proliferation Effects 0.000 description 1
- 230000009696 proliferative response Effects 0.000 description 1
- JIVSXRLRGOICGA-UHFFFAOYSA-N promazine hydrochloride Chemical compound [H+].[Cl-].C1=CC=C2N(CCCN(C)C)C3=CC=CC=C3SC2=C1 JIVSXRLRGOICGA-UHFFFAOYSA-N 0.000 description 1
- 229960001836 promazine hydrochloride Drugs 0.000 description 1
- 230000000069 prophylactic effect Effects 0.000 description 1
- XJMOSONTPMZWPB-UHFFFAOYSA-M propidium iodide Chemical compound [I-].[I-].C12=CC(N)=CC=C2C2=CC=C(N)C=C2[N+](CCC[N+](C)(CC)CC)=C1C1=CC=CC=C1 XJMOSONTPMZWPB-UHFFFAOYSA-M 0.000 description 1
- 210000002307 prostate Anatomy 0.000 description 1
- 230000001681 protective effect Effects 0.000 description 1
- 239000012264 purified product Substances 0.000 description 1
- UMJSCPRVCHMLSP-UHFFFAOYSA-N pyridine Natural products COC1=CC=CN=C1 UMJSCPRVCHMLSP-UHFFFAOYSA-N 0.000 description 1
- QJZUKDFHGGYHMC-UHFFFAOYSA-N pyridine-3-carbaldehyde Chemical compound O=CC1=CC=CN=C1 QJZUKDFHGGYHMC-UHFFFAOYSA-N 0.000 description 1
- 230000002285 radioactive effect Effects 0.000 description 1
- 108700042226 ras Genes Proteins 0.000 description 1
- 102000016914 ras Proteins Human genes 0.000 description 1
- 108010014186 ras Proteins Proteins 0.000 description 1
- ZLQMRLSBXKQKMG-UHFFFAOYSA-N rauniticine Natural products COC(=O)C1=CC2CC3N(CCc4c3[nH]c5ccccc45)CC2C(C)O1 ZLQMRLSBXKQKMG-UHFFFAOYSA-N 0.000 description 1
- 230000009257 reactivity Effects 0.000 description 1
- 206010038038 rectal cancer Diseases 0.000 description 1
- 201000001275 rectum cancer Diseases 0.000 description 1
- 230000003362 replicative effect Effects 0.000 description 1
- SZLZWPPUNLXJEA-QEGASFHISA-N rescinnamine Chemical compound O([C@H]1[C@@H]([C@H]([C@H]2C[C@@H]3C4=C(C5=CC=C(OC)C=C5N4)CCN3C[C@H]2C1)C(=O)OC)OC)C(=O)\C=C\C1=CC(OC)=C(OC)C(OC)=C1 SZLZWPPUNLXJEA-QEGASFHISA-N 0.000 description 1
- 229960001965 rescinnamine Drugs 0.000 description 1
- BJOIZNZVOZKDIG-MDEJGZGSSA-N reserpine Chemical compound O([C@H]1[C@@H]([C@H]([C@H]2C[C@@H]3C4=C([C]5C=CC(OC)=CC5=N4)CCN3C[C@H]2C1)C(=O)OC)OC)C(=O)C1=CC(OC)=C(OC)C(OC)=C1 BJOIZNZVOZKDIG-MDEJGZGSSA-N 0.000 description 1
- 229960003147 reserpine Drugs 0.000 description 1
- 210000002345 respiratory system Anatomy 0.000 description 1
- 239000002342 ribonucleoside Substances 0.000 description 1
- 239000002336 ribonucleotide Substances 0.000 description 1
- 125000002652 ribonucleotide group Chemical group 0.000 description 1
- ULFRLSNUDGIQQP-UHFFFAOYSA-N rizatriptan Chemical compound C1=C2C(CCN(C)C)=CNC2=CC=C1CN1C=NC=N1 ULFRLSNUDGIQQP-UHFFFAOYSA-N 0.000 description 1
- MDMGHDFNKNZPAU-UHFFFAOYSA-N roserpine Natural products C1C2CN3CCC(C4=CC=C(OC)C=C4N4)=C4C3CC2C(OC(C)=O)C(OC)C1OC(=O)C1=CC(OC)=C(OC)C(OC)=C1 MDMGHDFNKNZPAU-UHFFFAOYSA-N 0.000 description 1
- 208000010157 sclerosing cholangitis Diseases 0.000 description 1
- 206010040400 serum sickness Diseases 0.000 description 1
- 238000007493 shaping process Methods 0.000 description 1
- 230000007781 signaling event Effects 0.000 description 1
- 229920000260 silastic Polymers 0.000 description 1
- 201000000849 skin cancer Diseases 0.000 description 1
- 239000011734 sodium Substances 0.000 description 1
- 229910052708 sodium Inorganic materials 0.000 description 1
- 235000017557 sodium bicarbonate Nutrition 0.000 description 1
- 229910000030 sodium bicarbonate Inorganic materials 0.000 description 1
- 239000011780 sodium chloride Substances 0.000 description 1
- 239000012265 solid product Substances 0.000 description 1
- 125000003003 spiro group Chemical group 0.000 description 1
- 230000002269 spontaneous effect Effects 0.000 description 1
- 208000017572 squamous cell neoplasm Diseases 0.000 description 1
- 239000007858 starting material Substances 0.000 description 1
- 235000003702 sterols Nutrition 0.000 description 1
- 150000003432 sterols Chemical class 0.000 description 1
- 210000002784 stomach Anatomy 0.000 description 1
- 201000011549 stomach cancer Diseases 0.000 description 1
- 229940031000 streptococcus pneumoniae Drugs 0.000 description 1
- 229960005322 streptomycin Drugs 0.000 description 1
- 239000000758 substrate Substances 0.000 description 1
- 238000013268 sustained release Methods 0.000 description 1
- 239000012730 sustained-release form Substances 0.000 description 1
- 239000003765 sweetening agent Substances 0.000 description 1
- 230000008961 swelling Effects 0.000 description 1
- 229950006534 syrosingopine Drugs 0.000 description 1
- 239000006188 syrup Substances 0.000 description 1
- 235000020357 syrup Nutrition 0.000 description 1
- YLJREFDVOIBQDA-UHFFFAOYSA-N tacrine Chemical compound C1=CC=C2C(N)=C(CCCC3)C3=NC2=C1 YLJREFDVOIBQDA-UHFFFAOYSA-N 0.000 description 1
- 206010043207 temporal arteritis Diseases 0.000 description 1
- VCKUSRYTPJJLNI-UHFFFAOYSA-N terazosin Chemical compound N=1C(N)=C2C=C(OC)C(OC)=CC2=NC=1N(CC1)CCN1C(=O)C1CCCO1 VCKUSRYTPJJLNI-UHFFFAOYSA-N 0.000 description 1
- 229960001909 terazosin hydrochloride Drugs 0.000 description 1
- 229960000351 terfenadine Drugs 0.000 description 1
- 150000003512 tertiary amines Chemical class 0.000 description 1
- STOSPPMGXZPHKP-UHFFFAOYSA-N tetrachlorohydroquinone Chemical compound OC1=C(Cl)C(Cl)=C(O)C(Cl)=C1Cl STOSPPMGXZPHKP-UHFFFAOYSA-N 0.000 description 1
- 238000002560 therapeutic procedure Methods 0.000 description 1
- RTKIYNMVFMVABJ-UHFFFAOYSA-L thimerosal Chemical compound [Na+].CC[Hg]SC1=CC=CC=C1C([O-])=O RTKIYNMVFMVABJ-UHFFFAOYSA-L 0.000 description 1
- 229940033663 thimerosal Drugs 0.000 description 1
- 201000002510 thyroid cancer Diseases 0.000 description 1
- 230000000699 topical effect Effects 0.000 description 1
- 230000002588 toxic effect Effects 0.000 description 1
- 238000002723 toxicity assay Methods 0.000 description 1
- 231100000440 toxicity profile Toxicity 0.000 description 1
- XUPZAARQDNSRJB-SJDTYFKWSA-N trans-dothiepin hydrochloride Chemical compound [Cl-].C1SC2=CC=CC=C2C(=C/CC[NH+](C)C)/C2=CC=CC=C21 XUPZAARQDNSRJB-SJDTYFKWSA-N 0.000 description 1
- 238000013519 translation Methods 0.000 description 1
- 230000005945 translocation Effects 0.000 description 1
- 150000003626 triacylglycerols Chemical class 0.000 description 1
- 229960002324 trifluoperazine Drugs 0.000 description 1
- FTNWXGFYRHWUKG-UHFFFAOYSA-N triflupromazine hydrochloride Chemical compound [H+].[Cl-].C1=C(C(F)(F)F)C=C2N(CCCN(C)C)C3=CC=CC=C3SC2=C1 FTNWXGFYRHWUKG-UHFFFAOYSA-N 0.000 description 1
- 229960004312 triflupromazine hydrochloride Drugs 0.000 description 1
- 229940076784 trimeprazine tartrate Drugs 0.000 description 1
- 201000008827 tuberculosis Diseases 0.000 description 1
- 230000037455 tumor specific immune response Effects 0.000 description 1
- 208000035408 type 1 diabetes mellitus 1 Diseases 0.000 description 1
- 230000014567 type I interferon production Effects 0.000 description 1
- 208000025883 type III hypersensitivity disease Diseases 0.000 description 1
- 230000005951 type IV hypersensitivity Effects 0.000 description 1
- 208000027930 type IV hypersensitivity disease Diseases 0.000 description 1
- 241000724775 unclassified viruses Species 0.000 description 1
- 241000701161 unidentified adenovirus Species 0.000 description 1
- 241001529453 unidentified herpesvirus Species 0.000 description 1
- 241000712461 unidentified influenza virus Species 0.000 description 1
- 229940035893 uracil Drugs 0.000 description 1
- 201000005112 urinary bladder cancer Diseases 0.000 description 1
- MIBSKSYCRFWIRU-UHFFFAOYSA-N vanoxerine dihydrochloride Chemical compound Cl.Cl.C1=CC(F)=CC=C1C(C=1C=CC(F)=CC=1)OCCN1CCN(CCCC=2C=CC=CC=2)CC1 MIBSKSYCRFWIRU-UHFFFAOYSA-N 0.000 description 1
- 239000013598 vector Substances 0.000 description 1
- 239000003981 vehicle Substances 0.000 description 1
- 231100000611 venom Toxicity 0.000 description 1
- 238000010792 warming Methods 0.000 description 1
- 230000004580 weight loss Effects 0.000 description 1
Images
Classifications
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/33—Heterocyclic compounds
- A61K31/395—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins
- A61K31/435—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins having six-membered rings with one nitrogen as the only ring hetero atom
- A61K31/4353—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins having six-membered rings with one nitrogen as the only ring hetero atom ortho- or peri-condensed with heterocyclic ring systems
- A61K31/437—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins having six-membered rings with one nitrogen as the only ring hetero atom ortho- or peri-condensed with heterocyclic ring systems the heterocyclic ring system containing a five-membered ring having nitrogen as a ring hetero atom, e.g. indolizine, beta-carboline
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/12—Ketones
- A61K31/122—Ketones having the oxygen directly attached to a ring, e.g. quinones, vitamin K1, anthralin
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/33—Heterocyclic compounds
- A61K31/38—Heterocyclic compounds having sulfur as a ring hetero atom
- A61K31/382—Heterocyclic compounds having sulfur as a ring hetero atom having six-membered rings, e.g. thioxanthenes
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/33—Heterocyclic compounds
- A61K31/395—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins
- A61K31/435—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins having six-membered rings with one nitrogen as the only ring hetero atom
- A61K31/44—Non condensed pyridines; Hydrogenated derivatives thereof
- A61K31/445—Non condensed piperidines, e.g. piperocaine
- A61K31/4462—Non condensed piperidines, e.g. piperocaine only substituted in position 3
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/33—Heterocyclic compounds
- A61K31/395—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins
- A61K31/435—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins having six-membered rings with one nitrogen as the only ring hetero atom
- A61K31/47—Quinolines; Isoquinolines
- A61K31/4709—Non-condensed quinolines and containing further heterocyclic rings
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/33—Heterocyclic compounds
- A61K31/395—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins
- A61K31/495—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins having six-membered rings with two or more nitrogen atoms as the only ring heteroatoms, e.g. piperazine or tetrazines
- A61K31/496—Non-condensed piperazines containing further heterocyclic rings, e.g. rifampin, thiothixene
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/33—Heterocyclic compounds
- A61K31/395—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins
- A61K31/495—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins having six-membered rings with two or more nitrogen atoms as the only ring heteroatoms, e.g. piperazine or tetrazines
- A61K31/505—Pyrimidines; Hydrogenated pyrimidines, e.g. trimethoprim
- A61K31/517—Pyrimidines; Hydrogenated pyrimidines, e.g. trimethoprim ortho- or peri-condensed with carbocyclic ring systems, e.g. quinazoline, perimidine
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/33—Heterocyclic compounds
- A61K31/395—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins
- A61K31/54—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins having six-membered rings with at least one nitrogen and one sulfur as the ring hetero atoms, e.g. sulthiame
- A61K31/5415—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins having six-membered rings with at least one nitrogen and one sulfur as the ring hetero atoms, e.g. sulthiame ortho- or peri-condensed with carbocyclic ring systems, e.g. phenothiazine, chlorpromazine, piroxicam
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/33—Heterocyclic compounds
- A61K31/395—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins
- A61K31/55—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins having seven-membered rings, e.g. azelastine, pentylenetetrazole
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/33—Heterocyclic compounds
- A61K31/395—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins
- A61K31/55—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins having seven-membered rings, e.g. azelastine, pentylenetetrazole
- A61K31/553—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins having seven-membered rings, e.g. azelastine, pentylenetetrazole having at least one nitrogen and one oxygen as ring hetero atoms, e.g. loxapine, staurosporine
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P1/00—Drugs for disorders of the alimentary tract or the digestive system
- A61P1/04—Drugs for disorders of the alimentary tract or the digestive system for ulcers, gastritis or reflux esophagitis, e.g. antacids, inhibitors of acid secretion, mucosal protectants
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P13/00—Drugs for disorders of the urinary system
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P17/00—Drugs for dermatological disorders
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P17/00—Drugs for dermatological disorders
- A61P17/06—Antipsoriatics
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P19/00—Drugs for skeletal disorders
- A61P19/02—Drugs for skeletal disorders for joint disorders, e.g. arthritis, arthrosis
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P21/00—Drugs for disorders of the muscular or neuromuscular system
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P25/00—Drugs for disorders of the nervous system
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P35/00—Antineoplastic agents
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P37/00—Drugs for immunological or allergic disorders
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P37/00—Drugs for immunological or allergic disorders
- A61P37/02—Immunomodulators
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P37/00—Drugs for immunological or allergic disorders
- A61P37/02—Immunomodulators
- A61P37/06—Immunosuppressants, e.g. drugs for graft rejection
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P43/00—Drugs for specific purposes, not provided for in groups A61P1/00-A61P41/00
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P9/00—Drugs for disorders of the cardiovascular system
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D221/00—Heterocyclic compounds containing six-membered rings having one nitrogen atom as the only ring hetero atom, not provided for by groups C07D211/00 - C07D219/00
- C07D221/02—Heterocyclic compounds containing six-membered rings having one nitrogen atom as the only ring hetero atom, not provided for by groups C07D211/00 - C07D219/00 condensed with carbocyclic rings or ring systems
- C07D221/04—Ortho- or peri-condensed ring systems
- C07D221/06—Ring systems of three rings
- C07D221/10—Aza-phenanthrenes
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D239/00—Heterocyclic compounds containing 1,3-diazine or hydrogenated 1,3-diazine rings
- C07D239/70—Heterocyclic compounds containing 1,3-diazine or hydrogenated 1,3-diazine rings condensed with carbocyclic rings or ring systems
- C07D239/72—Quinazolines; Hydrogenated quinazolines
- C07D239/86—Quinazolines; Hydrogenated quinazolines with hetero atoms directly attached in position 4
- C07D239/94—Nitrogen atoms
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D239/00—Heterocyclic compounds containing 1,3-diazine or hydrogenated 1,3-diazine rings
- C07D239/70—Heterocyclic compounds containing 1,3-diazine or hydrogenated 1,3-diazine rings condensed with carbocyclic rings or ring systems
- C07D239/72—Quinazolines; Hydrogenated quinazolines
- C07D239/95—Quinazolines; Hydrogenated quinazolines with hetero atoms directly attached in positions 2 and 4
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D405/00—Heterocyclic compounds containing both one or more hetero rings having oxygen atoms as the only ring hetero atoms, and one or more rings having nitrogen as the only ring hetero atom
- C07D405/02—Heterocyclic compounds containing both one or more hetero rings having oxygen atoms as the only ring hetero atoms, and one or more rings having nitrogen as the only ring hetero atom containing two hetero rings
- C07D405/12—Heterocyclic compounds containing both one or more hetero rings having oxygen atoms as the only ring hetero atoms, and one or more rings having nitrogen as the only ring hetero atom containing two hetero rings linked by a chain containing hetero atoms as chain links
Definitions
- the invention relates to generally to the field of immunology. More particularly, the invention relates to compositions and methods for altering immune function. More specifically, the invention relates to compositions and methods for affecting immune stimulation mediated through Toll-like receptor (TLR) molecules.
- TLR Toll-like receptor
- TLRs Toll-like receptors
- compositions and methods useful for modulating innate immunity are therefore of great interest, as they may affect therapeutic approaches to conditions involving autoimmunity, inflammation, allergy, asthma, graft rejection, graft versus host disease (GvHD), infection, cancer, and immunodeficiency.
- TLR9 Toll-like receptor 9
- IgG and nucleic acid can stimulate TLR9 and participate in B-cell activation in certain autoimmune diseases. Leadbetter E A et al. (2002) Nature 416:595-8.
- Chloroquines have been recognized as useful not only as anti-malarial agents but also as anti-inflammatory agents. Although its mechanism of action is not well understood, chloroquine has been used effectively in the treatment of various autoimmune diseases, including rheumatoid arthritis (RA) and systemic lupus erythematosus (SLE). For a review, see Wallace D J (1996) Lupus 5 Suppl 1:S59-64. Recently Macfarlane and colleagues described a number of small molecule analogs and derivatives of chloroquine (4-aminoquinoline) and quinacrine (9-aminoacridine) which reportedly inhibit stimulation of the immune system. U.S. Pat. No. 6,221,882; U.S. Pat. No.
- the present invention is based, in part, on the discovery by the applicants that a number of small molecules, some previously known but distinct from those described by Macfarlane et al. and by Tobe et al., can alter TLR-mediated immunostimulatory signaling. Many of the compounds inhibit TLR signaling and are useful as inhibitors of immune stimulation.
- Compositions and methods described herein are useful for inhibiting immune stimulation in vitro and in vivo. Such compositions and methods thus will find use in a number of clinical applications, including as pharmaceutical agents and methods for treating conditions involving unwanted immune activity, including inflammatory and autoimmune disorders.
- the compositions of the invention can also be used in methods for the preparation of medicaments for use in the treatment of conditions involving unwanted immune activity, including a variety of inflammatory and autoimmune disorders.
- molecules having similar substituents but different core structures compared to those related to chloroquine and quinacrine described by Macfarlane et al. are potent immunomodulatory small molecules.
- the small molecules described by the present invention affect immune stimulation via interaction with a TLR. More particularly, it is believed that many of the small molecules described by the present invention inhibit immune stimulation via TLR antagonism. In particular, it is believed that many of the small molecules described by the present invention inhibit immune stimulation via TLR9 antagonism.
- the invention in certain aspects provides methods useful for altering TLR-mediated signaling.
- the methods of the invention are useful whenever it is desirable to alter TLR-mediated signaling in response to a suitable TLR ligand or TLR signaling agonist.
- the methods can be used to treat any of a variety of conditions involving autoimmunity, inflammation, allergy, asthma, graft rejection, graft-versus-host disease (GvHD), infection, sepsis, cancer, and immunodeficiency.
- GvHD graft-versus-host disease
- methods useful in the treatment of conditions involving autoimmunity, inflammation, allergy, asthma, graft rejection, and GvHD will employ small molecules that inhibit TLR-mediated signaling in response to a suitable TLR ligand or TLR signaling agonist.
- methods useful in the treatment of conditions involving infection, cancer, and immunodeficiency will employ small molecules that augment TLR-mediated signaling in response to a suitable TLR ligand.
- the methods can be used to inhibit or promote TLR-mediated signaling in response to a TLR ligand or TLR signaling agonist.
- the methods can be used to inhibit TLR-mediated immunostimulatory signaling in response to a TLR ligand or TLR signaling agonist.
- the methods can be used to inhibit or promote TLR-mediated immunostimulation in a subject.
- the methods can be used to inhibit TLR-mediated immunostimulation in a subject.
- the methods can be used to inhibit an immunostimulatory nucleic acid-associated response in a subject.
- the invention in certain aspects provides novel small molecule compositions useful for altering TLR-mediated signaling.
- the compositions of the invention are useful whenever it is desirable to alter TLR-mediated signaling in response to a suitable TLR ligand or TLR signaling agonist.
- the small molecules can be used in methods to treat any of a variety of conditions involving autoimmunity, inflammation, allergy, asthma, graft rejection, GvHD, infection, sepsis, cancer, and immunodeficiency.
- methods useful in the treatment of conditions involving autoimmunity, inflammation, allergy, asthma, graft rejection, and GvHD will employ small molecules that inhibit TLR-mediated signaling in response to a suitable TLR ligand or TLR signaling agonist.
- methods useful in the treatment of conditions involving infection, cancer, and immunodeficiency will employ small molecules that augment TLR-mediated signaling in response to a suitable TLR ligand.
- the molecules can be used in a method to inhibit or promote TLR-mediated signaling in response to a TLR ligand or TLR signaling agonist.
- the small molecules can be used in a method to inhibit TLR-mediated immunostimulatory signaling in response to a TLR ligand or TLR signaling agonist.
- the small molecules can be used in a method to inhibit or promote TLR-mediated immunostimulation in a subject.
- the small molecules can be used in a method to inhibit TLR-mediated immunostimulation in a subject.
- the small molecules can be used to inhibit an immunostimulatory nucleic acid-associated response in a subject.
- the methods of the invention can be combined with administration of additional agents to achieve synergistic effect on TLR-mediated immunostimulation.
- agents described herein have been discovered to affect TLRs directly and thus directly affect TLR-bearing cells, e.g., antigen-presenting cells (APCs)
- such agents can be used in conjunction with additional agents which affect non-APC immune cells, e.g., T lymphocytes (T cells).
- T cells T lymphocytes
- a method of affecting TLR-mediated signaling in response to a TLR ligand involves contacting a cell expressing a TLR with an effective amount of a compound of Formula I wherein 2 is a five- to seven-membered homocyclic or heterocyclic ring, wherein each of X, Y, and Z is independently chosen from a carbon atom (C), a nitrogen atom (N), an oxygen atom (O), and a sulfur atom (S), and wherein B2 optionally includes at least one atom selected from C, N, O, and S; wherein 1 and 2 are optionally bridged by B3 to form a five- to seven-membered ring (3), wherein B3 optionally includes at least one atom selected from C, N, O, and S, and wherein when A is carbon, (3) is not pyridine; wherein 2 optionally includes an unsaturated bond; wherein (3) optionally includes an unsaturated bond; wherein R2, when present,
- a method of inhibiting TLR-mediated immunostimulatory signaling involves contacting a cell expressing a TLR with an effective amount of a compound of Formula I, as provided above, to inhibit TLR-mediated immunostimulatory signaling in response to a ligand for the TLR.
- the invention provides a method of affecting TLR-mediated immunostimulation in a subject.
- the method according to this aspect of the invention involves administering to a subject having or at risk of developing TLR-mediated immunostimulation an effective amount of a compound of Formula I, as provided above, to inhibit or promote TLR-mediated immunostimulation in the subject.
- the invention in one aspect provides a method of inhibiting TLR-mediated immunostimulation in a subject.
- the method according to this aspect of the invention involves administering to a subject having or at risk of developing TLR-mediated immunostimulation an effective amount of a compound of Formula I, as provided above, to inhibit TLR-mediated immunostimulation in the subject.
- the invention provides a method of inhibiting an immunostimulatory nucleic acid-associated response in a subject.
- the method according to this aspect of the invention involves administering to a subject in need of such treatment an effective amount of a compound of Formula I, as provided above, to inhibit an immunostimulatory nucleic acid-associated response in the subject.
- the subject is otherwise free of symptoms calling for treatment with a compound of Formula I.
- A is nitrogen and (3) is a five-membered ring.
- the compound is Formula II wherein A is chosen from C and N; and R1 is a hydrogen atom, a halogen atom, a substituted or unsubstituted lower alkyl, aryl, aralkyl, heterocyclic, or alkylheterocyclic group, each optionally linked via N, O, or S.
- the compound is any one of compounds 455, 470, 564, 568, 593, 607, 612-614, 619-621, 636, 685, 875, 878, 890, 904, 918, 939, 944, 1039, 1050, 1132, 1241, and 1243 listed in Table 5 below.
- A is nitrogen
- (3) is a six-membered ring
- B3 is one atom selected from C, N, O, and S.
- A is nitrogen
- 3 is a six-membered ring
- B3 is S.
- the compound is Formula III wherein R1 is a hydrogen atom, a halogen atom, a substituted or unsubstituted lower alkyl, aryl, aralkyl, heterocyclic, or alkylheterocyclic group, each optionally linked via N, O, or S, and wherein X is chosen from N, O, and S. In certain embodiments X is S.
- the compound is any one of compounds 53, 64, 125, 149, 313, 399, 529, 576, 693, 797, 840, and 842 listed in Table 6 below.
- A is carbon
- (3) is a six-membered ring
- B3 is C or S.
- the compound is Formula IV wherein X is C or S and R1 is a hydrogen atom, a halogen atom, a substituted or unsubstituted lower alkyl, aryl, aralkyl, heterocyclic, or alkylheterocyclic group, each optionally linked via N, O, or S.
- the compound is any one of compounds 43, 294, 340, 346, 348, 413, 491, 917, 1015, 1042, 1158, 1287, and 1337 listed in Table 7 below.
- A is nitrogen
- (3) is a seven-membered ring
- B3 includes two carbon atoms.
- the compound is Formula V wherein X and Y are each independently C, N, O, or S; and R1 is a hydrogen atom, a halogen atom, a substituted or unsubstituted lower alkyl, aryl, aralkyl, heterocyclic, or alkylheterocyclic group, each optionally linked via N, O, or S.
- the compound is any one of compounds 72, 74, 99, 109, 343, 488, 1013, and 1352 listed in Table 8 below.
- 1 and 2 are unbridged by B3, A is carbon, and A′ is —OR9.
- the compound is Formula VI wherein R1 is a hydrogen atom, a halogen atom, a substituted or unsubstituted lower alkyl, aryl, aralkyl, heterocyclic, or alkylheterocyclic group, each optionally linked via N, O, or S; and R9 is a substituted or unsubstituted lower alkyl, aryl, aralkyl, heterocyclic, or alkylheterocyclic group.
- R9 and R4 are united to form a lactone.
- the compound is any one of compounds 65, 229, 239, 306, 386, 707, 793, 957, 970, 974, 1161, and 1308 listed in Table 9 below.
- 1 and 2 are unbridged by B3, A is carbon, and A′ is —NR9R10.
- the compound is Formula VII wherein R1 is a hydrogen atom, a halogen atom, a substituted or unsubstituted lower alkyl, aryl, aralkyl, heterocyclic, or alkylheterocyclic group, each optionally linked via N, O, or S.
- R1 is a hydrogen atom, a halogen atom, a substituted or unsubstituted lower alkyl, aryl, aralkyl, heterocyclic, or alkylheterocyclic group, each optionally linked via N, O, or S.
- the compound is any one of compounds 133, 267, 312, 457, and 510 listed in Table 10 below.
- 1 and 2 are unbridged by B3, A is carbon, and A′ is —CR9R10.
- the compound is Formula VIII wherein R1 is a hydrogen atom, a halogen atom, a substituted or unsubstituted lower alkyl, aryl, aralkyl, heterocyclic, or alkylheterocyclic group, each optionally linked via N, O, or S.
- the compound is any one of compounds 93, 108, 138, 144, 267, 270, 308, 381, 560, 778, 799, 822, 833, 884, 965, and 1187 listed in Table 11 below.
- the invention provides a method of affecting TLR-mediated signaling in response to a TLR ligand.
- the method according to this aspect of the invention involves contacting a cell expressing a TLR with an effective amount of a compound of Formula IX wherein R2 is a substituted or unsubstituted lower alkyl, aryl, aralkyl, heterocyclic, or alkylheterocyclic group, each optionally linked via N, O, or S; wherein R5, R6, R7, and R8 are each independently a hydrogen atom, a halogen atom, a substituted or unsubstituted lower alkyl, aryl, aralkyl, heterocyclic, or alkylheterocyclic group, each optionally linked via N, O, or S; wherein R9 is a hydrogen atom, substituted or unsubstituted lower alkyl, aryl, aralkyl, heterocyclic, or alkylheterocyclic group; and wherein
- a method of inhibiting TLR-mediated immunostimulatory signaling involves contacting a cell expressing a TLR with an effective amount of a compound of Formula IX, as provided above, to inhibit TLR-mediated immunostimulatory signaling in response to a ligand for the TLR.
- the invention provides a method of affecting TLR-mediated immunostimulation in a subject.
- the method according to this aspect of the invention involves administering to a subject having or at risk of developing TLR-mediated immunostimulation an effective amount of a compound of Formula IX, as provided above, to inhibit or promote TLR-mediated immunostimulation in the subject.
- the invention in one aspect provides a method of inhibiting TLR-mediated immunostimulation in a subject.
- the method according to this aspect of the invention involves administering to a subject having or at risk of developing TLR-mediated immunostimulation an effective amount of a compound of Formula IX, as provided above, to inhibit TLR-mediated immunostimulation in the subject.
- the invention provides a method of inhibiting an immunostimulatory nucleic acid-associated response in a subject.
- the method according to this aspect of the invention involves administering to a subject in need of such treatment an effective amount of a compound of Formula IX, as provided above, to inhibit an immunostimulatory nucleic acid-associated response in the subject.
- the subject is otherwise free of symptoms calling for treatment with a compound of Formula IX.
- the invention provides a method of affecting TLR-mediated signaling in response to a TLR ligand.
- the method according to this aspect of the invention involves contacting a cell expressing a TLR with an effective amount of a compound of Formula X wherein each of R1, R2, R3, R4, R5, R6, R7, and R8 is independently a hydrogen atom, a halogen atom, a substituted or unsubstituted lower alkyl, aryl, aralkyl, heterocyclic, or alkylheterocyclic group, each optionally linked via N, O, or S, to inhibit or promote TLR-mediated signaling in response to a ligand for the TLR.
- the compound is any one of compounds 431, 583, 586, 792, and 830 listed in Table 13 below.
- a method of inhibiting TLR-mediated immunostimulatory signaling involves contacting a cell expressing a TLR with an effective amount of a compound of Formula X, as provided above, to inhibit TLR-mediated immunostimulatory signaling in response to a ligand for the TLR.
- the invention provides a method of affecting TLR-mediated immunostimulation in a subject.
- the method according to this aspect of the invention involves administering to a subject having or at risk of developing TLR-mediated immunostimulation an effective amount of a compound of Formula X, as provided above, to inhibit or promote TLR-mediated immunostimulation in the subject.
- the invention in one aspect provides a method of inhibiting TLR-mediated immunostimulation in a subject.
- the method according to this aspect of the invention involves administering to a subject having or at risk of developing TLR-mediated immunostimulation an effective amount of a compound of Formula X, as provided above, to inhibit TLR-mediated immunostimulation in the subject.
- the invention provides a method of inhibiting an immunostimulatory nucleic acid-associated response in a subject.
- the method according to this aspect of the invention involves administering to a subject in need of such treatment an effective amount of a compound of Formula X, as provided above, to inhibit an immunostimulatory nucleic acid-associated response in the subject.
- the subject is otherwise free of symptoms calling for treatment with a compound of Formula X.
- the invention provides a method of affecting TLR-mediated signaling in response to a TLR ligand.
- the method according to this aspect of the invention involves contacting a cell expressing a TLR with an effective amount of a compound of Formula XI wherein each of R1, R2, R5, R6, R7, R8, R11, and R12 is a hydrogen atom, a halogen atom, a substituted or unsubstituted lower alkyl, aryl, aralkyl, heterocyclic, or alkylheterocyclic group, each optionally linked via N, O, or S; and X is C, N, O, or S, to inhibit or promote TLR-mediated signaling in response to a ligand for the TLR.
- R2 includes a cycloalkyl, benzyl or phenyl group.
- X is N.
- R11 and R12 are linked as a spiro group in a five- or six-membered ring.
- the compound is any one of compounds 891, 926, 1137, 1213, 1248, 1320, and 1322 listed in Table 14 below.
- a method of inhibiting TLR-mediated immunostimulatory signaling involves contacting a cell expressing a TLR with an effective amount of a compound of Formula XI, as provided above, to inhibit TLR-mediated immunostimulatory signaling in response to a ligand for the TLR.
- the invention provides a method of affecting TLR-mediated immunostimulation in a subject.
- the method according to this aspect of the invention involves administering to a subject having or at risk of developing TLR-mediated immunostimulation an effective amount of a compound of Formula XI, as provided above, to inhibit or promote TLR-mediated immunostimulation in the subject.
- the invention in one aspect provides a method of inhibiting TLR-mediated immunostimulation in a subject.
- the method according to this aspect of the invention involves administering to a subject having or at risk of developing TLR-mediated immunostimulation an effective amount of a compound of Formula XI, as provided above, to inhibit TLR-mediated immunostimulation in the subject.
- the invention provides a method of inhibiting an immunostimulatory nucleic acid-associated response in a subject.
- the method according to this aspect of the invention involves administering to a subject in need of such treatment an effective amount of a compound of Formula XI, as provided above, to inhibit an immunostimulatory nucleic acid-associated response in the subject.
- the subject is otherwise free of symptoms calling for treatment with a compound of Formula XI.
- the invention provides a method of affecting TLR-mediated signaling in response to a TLR ligand.
- the method according to this aspect of the invention involves contacting a cell expressing a TLR with an effective amount of a compound of Formula XII wherein R1, R2, R3, R4, R5, R6, R7, R8, R9, R10, and R11 are each independently a hydrogen atom, a halogen atom, a substituted or unsubstituted lower alkyl, aryl, aralkyl, heterocyclic, or alkylheterocyclic group, each optionally linked via N, O, or S, to inhibit or promote TLR-mediated signaling in response to a ligand for the TLR.
- the compound is any one of compounds 101, 600, and 609 listed in Table 15 below.
- a method of inhibiting TLR-mediated immunostimulatory signaling involves contacting a cell expressing a TLR with an effective amount of a compound of Formula XII, as provided above, to inhibit TLR-mediated immunostimulatory signaling in response to a ligand for the TLR.
- the invention provides a method of affecting TLR-mediated immunostimulation in a subject.
- the method according to this aspect of the invention involves administering to a subject having or at risk of developing TLR-mediated immunostimulation an effective amount of a compound of Formula XII, as provided above, to inhibit or promote TLR-mediated immunostimulation in the subject.
- the invention in one aspect provides a method of inhibiting TLR-mediated immunostimulation in a subject.
- the method according to this aspect of the invention involves administering to a subject having or at risk of developing TLR-mediated immunostimulation an effective amount of a compound of Formula XII, as provided above, to inhibit TLR-mediated immunostimulation in the subject.
- the invention provides a method of inhibiting an immunostimulatory nucleic acid-associated response in a subject.
- the method according to this aspect of the invention involves administering to a subject in need of such treatment an effective amount of a compound of Formula XII, as provided above, to inhibit an immunostimulatory nucleic acid-associated response in the subject.
- the subject is otherwise free of symptoms calling for treatment with a compound of Formula XII.
- the invention provides a method of affecting TLR-mediated signaling in response to a TLR ligand.
- the method according to this aspect of the invention involves contacting a cell expressing a TLR with an effective amount of a compound of Formula XIII wherein 2′ is a five- to seven-membered heterocyclic ring, wherein each of X, Y, and Z is independently chosen from C, N, O, and S, and wherein B2′ optionally includes at least one atom selected from C, N, O, and S; wherein 2′ optionally includes an unsaturated bond; wherein R4, R5, R6, R7, and R8 are each independently a hydrogen atom, a halogen atom, a substituted or unsubstituted lower alkyl, aryl, aralkyl, heterocyclic, or alkylheterocyclic group, each optionally linked via N, O, or S; wherein R9 is a hydrogen atom, substituted or unsubstituted lower alkyl
- a method of inhibiting TLR-mediated immunostimulatory signaling involves contacting a cell expressing a TLR with an effective amount of a compound of Formula XIII, as provided above, to inhibit TLR-mediated immunostimulatory signaling in response to a ligand for the TLR.
- the invention provides a method of affecting TLR-mediated immunostimulation in a subject.
- the method according to this aspect of the invention involves administering to a subject having or at risk of developing TLR-mediated immunostimulation an effective amount of a compound of Formula XIII, as provided above, to inhibit or promote TLR-mediated immunostimulation in the subject.
- the invention in one aspect provides a method of inhibiting TLR-mediated immunostimulation in a subject.
- the method according to this aspect of the invention involves administering to a subject having or at risk of developing TLR-mediated immunostimulation an effective amount of a compound of Formula XIII, as provided above, to inhibit TLR-mediated immunostimulation in the subject.
- the invention provides a method of inhibiting an immunostimulatory nucleic acid-associated response in a subject.
- the method according to this aspect of the invention involves administering to a subject in need of such treatment an effective amount of a compound of Formula XIII, as provided above, to inhibit an immunostimulatory nucleic acid-associated response in the subject.
- A′′ is a substituted alkyl group selected from the group consisting of a cyclic amino group, an alkylamino group, a dialkylamino group, furyl, phenyl, thienyl, azabicyclooctyl, azabicycloheptyl, and any combination thereof.
- the cyclic amino group is a piperazino group, a piperidino group, a pyrrolidino group, an imidazolyl group, a pyridyl group, or a morpholino group.
- the subject is otherwise free of symptoms calling for treatment with a compound of Formula XIII.
- the invention provides a method of affecting TLR-mediated signaling in response to a TLR ligand.
- the method according to this aspect of the invention involves contacting a cell expressing a TLR with an effective amount of a compound of Formula XIV or Formula XV wherein R1, R2, R3, R5, R6, R7, and R8 are each independently a hydrogen atom, a halogen atom, a substituted or unsubstituted lower alkyl, aryl, aralkyl, heterocyclic, or alkylheterocyclic group, each optionally linked via N, O, or S; and wherein R9 is a hydrogen atom, substituted or unsubstituted lower alkyl, aryl, aralkyl, heterocyclic, or alkylheterocyclic group, to inhibit or promote TLR-mediated signaling in response to a ligand for the TLR.
- the compound is any one of compounds 807, 1163, and 1367 listed in Table 17
- a method of inhibiting TLR-mediated immunostimulatory signaling involves contacting a cell expressing a TLR with an effective amount of a compound of Formula XIV or Formula XV, as provided above, to inhibit TLR-mediated immunostimulatory signaling in response to a ligand for the TLR.
- the invention provides a method of affecting TLR-mediated immunostimulation in a subject.
- the method according to this aspect of the invention involves administering to a subject having or at risk of developing TLR-mediated immunostimulation an effective amount of a compound of Formula XIV or Formula XV, as provided above, to inhibit or promote TLR-mediated immunostimulation in the subject.
- the invention in one aspect provides a method of inhibiting TLR-mediated immunostimulation in a subject.
- the method according to this aspect of the invention involves administering to a subject having or at risk of developing TLR-mediated immunostimulation an effective amount of a compound of Formula XIV or Formula XV, as provided above, to inhibit TLR-mediated immunostimulation in the subject.
- the invention provides a method of inhibiting an immunostimulatory nucleic acid-associated response in a subject.
- the method according to this aspect of the invention involves administering to a subject in need of such treatment an effective amount of a compound of Formula XIV or Formula XV, as provided above, to inhibit an immunostimulatory nucleic acid-associated response in the subject.
- the subject is otherwise free of symptoms calling for treatment with a compound of Formula XIV or Formula XV.
- R2 is a substituted or unsubstituted phenyl, naphthyl, phenanthryl, styryl, azabicyclooctane, or azabicycloheptane group.
- R2 is a phenyl, naphthyl, phenanthryl, styryl, azabicyclooctane, or azabicycloheptane group substituted with one or more substituent groups selected from the group consisting of an alkyl group, an alkoxy group, an alkoxyalkyl group, an ester group, an alkylamino group, a dialkylamino group, a cyclic amino group, a halogen atom, and any combination thereof.
- R2 is a phenyl, naphthyl, phenanthryl, styryl, azabicyclooctane, or azabicycloheptane group substituted with one or more substituent groups selected from the group consisting of an alkylamino group, a dialkylamino group, and a cyclic amino group.
- the cyclic amino group is a piperazino group, a piperidino group, a pyrrolidino group, an imidazolyl group, a pyridyl group, or a morpholino group.
- R9 is a substituted alkyl group selected from the group consisting of a cyclic amino group, an alkylamino group, a dialkylamino group, furyl, phenyl, thienyl, azabicyclooctyl, azabicycloheptyl, and any combination thereof.
- the cyclic amino group is a piperazino group, a piperidino group, a pyrrolidino group, an imidazolyl group, a pyridyl group, or a morpholino group.
- each of R4 and R5 is hydrogen atom. In some embodiments each of R5 and R8 is a hydrogen atom. In some embodiments each of R4, R5, and R8 is a hydrogen atom. In some embodiments R10 is a hydrogen atom.
- the TLR is TLR9.
- the ligand for the TLR is an immunostimulatory nucleic acid.
- the immunostimulatory nucleic acid is a CpG nucleic acid.
- the TLR is TLR8.
- the ligand for the TLR is a natural ligand for TLR8.
- the ligand for the TLR is resiquimod (R848).
- the TLR is TLR7.
- the ligand for the TLR is a natural ligand for TLR7.
- the ligand for the TLR is R848.
- the TLR is TLR3.
- the ligand for the TLR is a double stranded RNA.
- the ligand for the TLR is poly(I:C).
- the present invention is based, in part, on the appreciation by the applicants of a potential connection between the antimalarial activity of known metabolites of chloroquine and the biological activity of related small molecules with respect to inhibition of TLR9.
- the major metabolites are mono de-ethylated chloroquine and bis de-ethylated chloroquine.
- the half life of mono de-ethylchloroquine in humans is 1.5 times longer than that of chloroquine (649 hrs versus 432 hrs).
- chloroquine, hydroxychloroquine, mono de-ethylchloroquine, and bis de-ethylchloroquine have been examined by Macfarlane.
- the IC50 of these compounds by his assay were: chloroquine, 1.1 ⁇ 10 ⁇ 7 M; hydroxychloroquine, 4.1 ⁇ 10 ⁇ 7 M; mono de-ethylchloroquine, 7.08 ⁇ 10 ⁇ 7 M; and bis de-ethylchloroquine, 18.6 ⁇ 10 ⁇ 7 M. So by this data the loss of one ethyl group from chloroquine reduced the potency by about 7-fold and the loss of both ethyl groups reduced the potency by about 18-fold.
- the present invention in certain aspects is related to compositions and methods involving certain 4-primary amino quinoline compounds which are useful for inhibiting TLR9 signaling and immune activation.
- the present invention is also related to compositions and methods involving certain quinazoline compounds, some of which are structurally related to the 4-primary amino quinoline compounds of the invention, which are also useful for inhibiting TLR9 signaling and immune activation.
- the 4-primary amino quinoline compounds and the quinazoline compounds of the invention are highly and similarly active as inhibitors of TLR9 signaling activity. It was also surprisingly discovered according to the instant invention that despite their high degree of structural and biological similarity, the quinazoline molecules exhibit a significantly improved toxicity profile in vivo compared with the quinoline compounds.
- the invention provides novel substituted 4-primary amino quinoline compositions having a structural Formula XVI wherein
- X is absent or is an aryl, alkyl, heterocyclic, or styryl group
- R 1 and R 2 are each independently a hydrogen atom or a substituted or unsubstituted alkyl, alkenyl, or aryl group, wherein R 1 and R 2 optionally are combined to form a heterocycle;
- R 3 is a hydrogen atom, a halogen atom, or an alkyl, alkenyl, aryl, heterocyclic, nitro, cyano, carboxy, ester, ketone, amino, amido, hydroxy, alkoxy, mercapto, thio, sulfoxide, sulfone, or sulfonamido group, wherein R 1 and R 3 optionally are combined to form a heterocycle;
- Y is absent or is an oxygen atom, a sulfur atom, CR 8 R 9 , or NR 10 , where R 8 , R 9 , and R 10 are each independently a hydrogen atom or a substituted or unsubstituted alkyl, alkenyl, or aryl group;
- L is an alkyl or alkenyl group containing from 1 to 10 carbons or is an aryl group
- R 4 , R 5 , R 6 , and R 7 are each independently a hydrogen atom, a halogen atom, or an alkyl, alkenyl, aryl, heterocyclic, nitro, cyano, carboxy, ester, ketone, amino, amido, hydroxy, alkoxy, mercapto, thio, sulfoxide, sulfone, or sulfonamido group, wherein any pair of R 4 , R 5 , R 6 , and R 7 which are adjacent one another optionally are combined to form a heterocycle or a carbocycle,
- R 5 and R 6 are each independently a halogen atom or an alkoxy group. In one embodiment R 5 and R 6 are each independently a chlorine atom or a methoxy group.
- X is absent or is an aryl group
- NR 1 R 2 is a heterocyclic amine or is NR 8 (CH 2 ) n NR 9 R 10 , wherein n is an integer from 2 to 6, inclusive, and R 8 , R 9 , and R 10 are each independently a hydrogen atom or an alkyl group;
- R 3 is a hydrogen atom
- Y is an aryl group or is NR 11 where R 11 is a hydrogen atom or an aryl or alkyl group;
- L is absent or is a C 2 -C 6 alkyl group
- R 4 , R 5 , R 6 , and R 7 are each independently a hydrogen atom, a halogen atom, or an alkoxy group.
- X is absent or is an aryl group
- NR 1 R 2 is a substituted or unsubstituted piperazino or morpholino group or is NR 8 (CH 2 ) n NR 9 R 10 , wherein n is an integer from 2 to 6, inclusive, R 8 is a hydrogen atom, and R 9 and R 10 are each independently an alkyl group;
- R 3 is a hydrogen atom
- Y is NH
- L is a C 2 -C 6 alkyl group
- R 4 , R 5 , R 6 , and R 7 are each independently a hydrogen atom, a halogen atom, or an alkoxy group.
- X is a phenyl group
- NR 1 R 2 is attached to a para position of the phenyl group X;
- Y is NH
- L is —(CH 2 ) n — where n is an integer between 2 and 6, inclusive;
- each of R 3 , R 4 , R 5 , R 6 , and R 7 is a hydrogen atom.
- composition is optionally in the form of a pharmaceutically acceptable hydrate or salt.
- the invention provides a method for inhibiting signaling by a TLR.
- the method according to this aspect of the invention involves contacting a cell expressing a functional TLR with an effective amount of a substituted 4-primary amino quinoline composition having structural Formula XVII wherein
- X is absent or is a nitrogen, oxygen, or sulfur atom or an SO or SO 2 group
- R 1 is a hydrogen atom or a substituted or unsubstituted aryl, alkyl, heterocyclic or styryl group
- R 2 is a hydrogen atom, a halogen atom, or an alkyl, alkenyl, aryl, heterocyclic, nitro, cyano, carboxy, ester, ketone, amino, amido, hydroxy, alkoxy, mercapto, thio, sulfoxide, sulfone, or sulfonamido group, wherein R 1 and R 2 optionally are combined to form a heterocycle or carbocycle;
- Y is absent or is an oxygen atom, a sulfur atom, CR 7 R 8 , or NR 9 , where R 7 , R 8 , and R 9 are each independently a hydrogen atom or a substituted or unsubstituted alkyl, alkenyl, or aryl group;
- L is absent or is an alkyl or alkenyl group containing from 1 to 10 carbons or is an aryl group;
- R 3 , R 4 , R 5 , and R 6 are each independently a hydrogen atom, a halogen atom, or an alkyl, alkenyl, aryl, heterocyclic, nitro, cyano, carboxy, ester, ketone, amino, amido, hydroxy, alkoxy, mercapto, thio, sulfoxide, sulfone, or sulfonamido group, wherein any pair of R 3 , R 4 , R 5 , and R 6 which are adjacent one another optionally are combined to form a heterocycle or a carbocycle, and
- the invention provides a method for inhibiting signaling by a TLR.
- the method according to this aspect of the invention involves contacting an immune cell expressing a functional TLR with
- the TLR is TLR9 and the TLR signal agonist is a TLR9 signal agonist.
- the TLR signal agonist in one embodiment is CpG DNA, which can be an oligodeoxynucleotide (ODN).
- the TLR signal agonist is an immune complex. In one embodiment the TLR signal agonist is an immune complex that includes a nucleic acid. In one embodiment the TLR signal agonist is an immune complex that includes a nucleic acid that is self-DNA. In one embodiment the TLR signal agonist is an immune complex that includes a nucleic acid that is self-RNA.
- the invention provides a method for inhibiting an immune response to an antigenic substance.
- the method according to this aspect of the invention involves
- the immune response is an innate immune response. In one embodiment the immune response includes an adaptive immune response.
- a method of treating an autoimmune disorder in a subject involves the step of administering to a subject having an autoimmune disorder an effective amount of a substituted 4-primary amino quinoline composition having structural Formula XVII, as defined above, to treat the autoimmune disorder.
- the autoimmune disorder is chosen from systemic lupus erythematosus, rheumatoid arthritis, inflammatory bowel disease, Sjögren's syndrome, polymyositis, vasculitis, Wegener's granulomatosis, sarcoidosis, ankylosing spondylitis, Reiter's syndrome, psoriatic arthritis, and Behçet's syndrome.
- the autoimmune disease is systemic lupus erythematosus.
- the autoimmune disease is rheumatoid arthritis.
- the subject is a human.
- the autoimmune disorder is an immune complex associated disease.
- the invention provides novel quinazoline compositions of the invention having a structural Formula XVIII wherein
- X is absent or is an aryl, alkyl, heterocyclic or styryl group, provided that if X is a phenyl group, NR 1 R 2 is part if a heterocycle or is a diamine;
- R 1 and R 2 are each independently a hydrogen atom or an alkyl, alkenyl, or aryl group, wherein R 1 and R 2 optionally are combined to form a heterocycle;
- Y is an oxygen atom, a sulfur atom, CR 9 R 10 , or NR 11 , where R 9 , R 10 , and R 11 are each independently a hydrogen atom or an alkyl, alkenyl, or aryl group, wherein any one of R 9 , R 10 , and R 11 optionally is combined with R 3 or R 4 to form a substituted or unsubstituted heterocycle;
- L is an alkyl or alkenyl group containing from 1 to 10 carbons or is an aryl group
- R 3 and R 4 are each independently a hydrogen atom or an alkyl, alkenyl, or aryl group, wherein R 3 and R 4 optionally are combined to form a heterocycle;
- R 5 , R 6 , R 7 , and R 8 are each independently a hydrogen atom, a halogen atom, or an alkyl, alkenyl, aryl, heterocyclic, nitro, cyano, carboxy, ester, ketone, amino, amido, hydroxy, alkoxy, mercapto, thio, sulfoxide, sulfone, or sulfonamido group, wherein any pair of R 5 , R 6 , R 7 , and R 8 which are adjacent one another optionally are combined to form a heterocycle or a carbocycle,
- R 6 and R 7 are each independently a halogen atom or an alkoxy group.
- R 6 and R 7 are each independently a chlorine atom or a methoxy group.
- X is absent or is an aryl group
- NR 1 R 2 is a heterocyclic amine or NR 9 (CH 2 ) n NR 10 R 11 , wherein n is an integer from 2 to 6, inclusive, and R 9 , R 10 , and R 11 are each independently a hydrogen atom or an alkyl group;
- Y is an aryl group or is NR 12 where R 12 is a hydrogen atom or an aryl or alkyl group;
- L is absent or is a C 2 -C 6 alkyl group
- R 3 and R 4 are each independently a hydrogen atom or an alkyl group, wherein R 3 and R 4 optionally are combined to form a heterocycle;
- R 5 , R 6 , R 7 , and R 8 are each independently a hydrogen atom, a halogen atom, or an alkoxy group.
- X is absent or is an aryl group
- NR 1 R 2 is a substituted or unsubstituted piperazino or morpholino group or is NR 9 (CH 2 ) n NR 10 R 11 , wherein n is an integer from 2 to 6, inclusive, R 9 is a hydrogen atom, and
- R 10 and R 11 are each independently an alkyl group
- Y is NH
- L is a C 2 -C 6 alkyl group
- R 3 and R 4 are each independently a hydrogen atom or an alkyl group, wherein R 3 and R 4 optionally are combined to form a heterocycle;
- R 5 , R 6 , R 7 , and R 8 are each independently a hydrogen atom, a halogen atom, or an alkoxy group.
- X is an aryl group
- NR 1 R 2 is substituted or unsubstituted piperazino or morpholino group
- Y is NH
- L is a C 2 -C 6 alkyl group
- R 3 and R 4 are each independently a methyl or ethyl group or R 3 and R 4 optionally are combined to form a morpholino group;
- R 5 , R 6 , R 7 , and R 8 are each independently a hydrogen atom, a halogen atom, or an alkoxy group.
- X is a phenyl group
- NR 1 R 2 is N-methylpiperazine
- Y is NH
- L is —CH 2 CH 2 —
- R 3 and R 4 are each a methyl group
- R 5 , R 6 , R 7 , and R 8 are each a hydrogen atom.
- X is a phenyl group
- NR 1 R 2 is N-methylpiperazine
- Y is NH
- L is —CH 2 CH 2 —
- R 3 and R 4 are combined as a morpholino group
- R 5 , R 6 , R 7 , and R 8 are each a hydrogen atom.
- X is absent
- NR 1 R 2 is a substituted or unsubstituted piperazino or morpholino group
- Y is NH
- L is a C 2 -C 6 alkyl group
- R 3 and R 4 are each independently a methyl or ethyl group or R 3 and R 4 optionally are combined to form a morpholino group;
- R 5 , R 6 , R 7 , and R 8 are each independently a hydrogen atom, a halogen atom, or an alkoxy group.
- X is absent
- NR 1 R 2 is N-methylpiperazine
- Y is NH
- L is —CH 2 CH 2 —
- R 3 and R 4 are each a methyl group
- R 6 and R 7 are each a methoxy group
- R 5 and R 8 are each a hydrogen atom.
- X is absent
- NR 1 R 2 is N-phenylpiperazine
- Y is NH
- L is —CH 2 CH 2 —
- R 3 and R 4 are each a methyl group
- R 6 and R 7 are each a methoxy group
- R 5 and R 8 are each a hydrogen atom.
- X is absent
- NR 1 R 2 is N-methylpiperazine
- Y is NH
- L is —CH 2 CH 2 ;
- R 3 and R 4 are combined as a morpholino group
- R 6 and R 7 are each a methoxy group
- R 5 and R 8 are each a hydrogen atom.
- composition is optionally in the form of a pharmaceutically acceptable hydrate or salt.
- the invention provides a method for inhibiting signaling by a TLR.
- the method according to this aspect of the invention involves contacting a cell expressing a functional TLR with an effective amount of quinazoline composition having structural wherein
- X is a substituted or unsubstituted aryl, alkyl, heterocyclic or styryl group, optionally attached to the quinazoline by a nitrogen, oxygen, or sulfur atom or by a SO or SO 2 group;
- Y is absent or is an oxygen atom, a sulfur atom, CR 9 R 10 , or NR 11 , where R 9 , R 10 , and R 11 are each independently a hydrogen atom or an alkyl, alkenyl, or aryl group, wherein any one of R 9 , R 10 , and R 11 optionally is combined with R 3 or R 4 to form a heterocycle;
- L is absent or is a hydrogen atom, an alkyl or alkenyl group containing from 1 to 10 carbons, or an aryl group;
- R 3 and R 4 are each independently a hydrogen atom or an alkyl, alkenyl, or aryl group, wherein R 3 and R 4 optionally are combined to form a heterocycle;
- R 5 , R 6 , R 7 , and R 8 are each independently a hydrogen atom, a halogen atom, or an alkyl, alkenyl, aryl, heterocyclic, nitro, cyano, carboxy, ester, ketone, amino, amido, hydroxy, alkoxy, mercapto, thio, sulfoxide, sulfone, or sulfonamido group, wherein any pair of R 5 , R 6 , R 7 , and R 9 which are adjacent one another optionally are combined to form a heterocycle or a carbocycle,
- the invention provides a method for inhibiting signaling by a TLR.
- the method according to this aspect of the invention involves contacting an immune cell expressing a functional TLR with
- the TLR is TLR9 and the TLR signal agonist is a TLR9 signal agonist.
- the TLR signal agonist in one embodiment is CpG DNA, which can be an oligodeoxynucleotide (ODN).
- the TLR signal agonist is an immune complex that includes a nucleic acid.
- the invention provides a method for inhibiting an immune response to an antigenic substance.
- the method according to this aspect of the invention involves contacting an immune cell expressing a functional Toll-like receptor with
- the immune response is an innate immune response. In one embodiment the immune response includes an adaptive immune response.
- a method of treating an autoimmune disorder in a subject involves the step of administering to a subject having an autoimmune disorder an effective amount of a quinazoline composition having structural Formula XIX, as defined above, to treat the autoimmune disorder.
- the autoimmune disorder is chosen from systemic lupus erythematosus, rheumatoid arthritis, inflammatory bowel disease, Sjögren's syndrome, polymyositis, vasculitis, Wegener's granulomatosis, sarcoidosis, ankylosing spondylitis, Reiter's syndrome, psoriatic arthritis, and Behçet's syndrome.
- the autoimmune disease is systemic lupus erythematosus. In one particular embodiment the autoimmune disease is rheumatoid arthritis. In one embodiment the subject is a human. In one embodiment the autoimmune disorder is an immune complex associated disease, as described above.
- FIG. 1 is a graph depicting the concentration-dependent inhibition of TLR9 signaling in response to CpG ODN 2006 (5′-TCGTCGTTTTGTCGTTTTGTCGTT-3′; SEQ ID NO:1) by compounds 613 (harmine hydrochloride), 878 (3-hydroxymethyl-beta-carboline, and 470 (norharman), molecules representative of compounds of Formula II.
- FIG. 2 is a pair of graphs depicting results of an in vivo toxicity assay in mice for specific quinoline and quinazoline compounds.
- FIG. 2A shows change in mean body weight (grams) and
- FIG. 2B shows mean body weight (grams) over 5 days for mice treated with dimethysulfoxide (DMSO) control (closed circles), compound CMZ 203-43 (also referred to herein as 203-43) administered at 1 mg and 4 mg (open and closed triangles, respectively), compound CMZ 203-34 at 1 mg and 4 mg (open and closed diamonds, respectively), and compound CMZ 203-49 at 1 mg and 4 mg (open and closed inverted triangles, respectively).
- N 3 for each treatment group.
- the group receiving CMZ 203-43 at 4 mg were sick and had to be sacrificed on day 3.
- the present invention provides novel compositions and methods for inhibiting immune responses, including immune responses involved in clinical disorders characterized as autoimmune disorders and immune complex associated diseases.
- novel compositions disclosed herein which include 4-primary amino quinolines and structurally similar quinazolines
- the invention also provides methods for use of previously known compositions within these and other classes of compounds in inhibiting immune responses, including immune responses involved in clinical disorders characterized as autoimmune disorders and immune complex associated diseases.
- the present invention is based in part on discoveries made by the applicants arising from screening a library of small molecule compounds in an in vitro assay of TLR9 signaling in response to immunostimulatory CpG oligodeoxynucleotides (ODN). It was observed that certain of the small molecules in the library were effective in altering TLR9 signaling in response to CpG ODN. Based on the initial screening results, additional compounds were selected for additional screening. Importantly, the small molecules so identified were distinct from those described by Macfarlane and colleagues.
- certain small molecules characterized by certain minimum features are able to modulate TLR signaling, e.g., in the presence of or in response to a PAMP or other TLR ligand.
- Some of the small molecules are potent inhibitors of TLR signaling and thus can be used to inhibit TLR-mediated immunostimulation.
- the minimal features for many, but not all, of the small molecules can be summarized as follows: a core structure including at least two rings, which can be fused or unfused, wherein at least one of the rings contains a nitrogen atom and/or has a side chain that contains a nitrogen atom. Certain of the molecules are effective even without presence of a nitrogen atom.
- fused three-ring structures such as quinacrine are typically an order of magnitude more potent as inhibitors of TLR-mediated immunostimulation than are fused two-ring structures such as chloroquine.
- quinacrine is reported to have an EC 50 of about 8 nM, where EC 50 is the concentration required for half-maximal inhibition of CpG-ODN effect on thymidine uptake by WEHI 231 B-cells in the presence of anti-surface IgM.
- U.S. Pat. No. 6,479,504 B1 It has now also been discovered according to the present invention that fused two-ring core structures to which are attached a nitrogen-containing ring substituent can be as potent as fused three-ring structures such as quinacrine.
- the nitrogen-containing ring substituent is a C 2 -C 6 alkyl group terminated by a tertiary amine, such as methylethylamine, diethyl amine, dimethyl amine, or a ring containing at least one nitrogen.
- IL-2 interleukin 2
- TNF- ⁇ tumor necrosis factor alpha
- IFN- ⁇ gamma interferon
- Histamine is a widely recognized mediator of immediate hypersensitivity and inflammation, which are principally mast cell- and basophil-mediated immune phenomena.
- the pleiotropic effects of histamine are mediated through three types of membrane-associated histamine receptor, histamine H1 receptor (H1R), histamine H2 receptor (H2R), and histamine H3 receptor (H3R).
- Pharmacologic inhibitors for these receptors are known and include pyrilamine and tripelennamine (H1R); cimetidine, famotidine, and ranitidine (H2R); and thioperamide and clobenpropit (H3R). Recently it was reported that signaling through H1R augments T-cell and B-cell antigen receptor-mediated responses.
- adaptive immune response refers to any type of antigen-specific immune response. Adaptive immune responses, which are also known in the art as specific immune responses, involve lymphocytes are also characterized by immunological memory, whereby response to a second or subsequent exposure to antigen is more vigorous than the response to a first exposure to the antigen.
- adaptive immune response encompasses both humoral (antibody) immunity and cell-mediated (cellular) immunity.
- allergy refers to acquired hypersensitivity to a substance (allergen). Allergic conditions include eczema, allergic rhinitis or coryza, hay fever, asthma, urticaria (hives) and food allergies, and other atopic conditions.
- antigenic substance refers to any substance that induces an adaptive (specific) immune response.
- An antigen typically is any substance that can be specifically bound by a T-cell antigen receptor, antibody, or B-cell antigen receptor.
- Antigenic substances include, without limitation, peptides, proteins, carbohydrates, lipids, phospholipids, nucleic acids, autacoids, and hormones. Antigenic substances further specifically include antigens that are classified as allergens, cancer antigens, and microbial antigens.
- asthma refers to a disorder of the respiratory system characterized by inflammation, narrowing of the airways and increased reactivity of the airways to inhaled agents. Asthma is frequently, although not exclusively associated with atopic or allergic symptoms. For example, asthma can be precipitated by exposure to an allergen, exposure to cold air, respiratory infection, and exertion.
- autoimmune disease and, equivalently, “autoimmune disorder” and “autoimmunity”, refer to immunologically mediated acute or chronic injury to a tissue or organ derived from the host. The terms encompass both cellular and antibody-mediated autoimmune phenomena, as well as organ-specific and organ-nonspecific autoimmunity. Autoimmune diseases include insulin-dependent diabetes mellitus, rheumatoid arthritis, systemic lupus erythematosus, multiple sclerosis, atherosclerosis, and inflammatory bowel disease.
- Autoimmune diseases also include, without limitation, ankylosing spondylitis, autoimmune hemolytic anemia, Behçet's syndrome, Goodpasture's syndrome, Graves' disease, Guillain-Barré syndrome, Hashimoto's thyroiditis, idiopathic thrombocytopenia, myasthenia gravis, pernicious anemia, polyarteritis nodosa, polymyositis/dermatomyositis, primary biliary sclerosis, psoriasis, sarcoidosis, sclerosing cholangitis, Sjögren's syndrome, systemic sclerosis (scleroderma and CREST syndrome), Takayasu's arteritis, temporal arteritis, and Wegener's granulomatosis. Autoimmune diseases also include certain immune complex-associated diseases.
- cancer and, equivalently, “tumor” refer to a condition in which abnormally replicating cells of host origin are present in a detectable amount in a subject.
- the cancer can be a malignant or non-malignant cancer.
- Cancers or tumors include but are not limited to biliary tract cancer; brain cancer; breast cancer; cervical cancer; choriocarcinoma; colon cancer; endometrial cancer; esophageal cancer; gastric (stomach) cancer; intraepithelial neoplasms; leukemias; lymphomas; liver cancer; lung cancer (e.g., small cell and non-small cell); melanoma; neuroblastomas; oral cancer; ovarian cancer; pancreatic cancer; prostate cancer; rectal cancer; renal (kidney) cancer; sarcomas; skin cancer; testicular cancer; thyroid cancer; as well as other carcinomas and sarcomas. Cancers can be primary or metastatic.
- CpG DNA refers to an immunostimulatory nucleic acid which contains a cytosine-guanine (CG) dinucleotide, the C residue of which is unmethylated.
- CG cytosine-guanine
- the effects of CpG nucleic acids on immune modulation have been described extensively in U.S. patents such as U.S. Pat. Nos. 6,194,388; 6,207,646; 6,239,116; and 6,218,371, and published international patent applications, such as WO98/37919, WO98/40100, WO98/52581, and WO99/56755. The entire contents of each of these patents and published patent applications is hereby incorporated by reference.
- the entire immunostimulatory nucleic acid can be unmethylated or portions may be unmethylated but at least the C of the 5′-CG-3′ must be unmethylated.
- CpG DNA includes both naturally occurring immunostimulatory nucleic acids, as found in bacterial DNA and plasmids, as well as synthetic oligodeoxynucleotides (ODN).
- the CpG DNA is a CpG ODN that has a base sequence provided by 5′-TCGTCGTTTTGTCGTTTTGTCGTT-3′ (ODN 2006; SEQ ID NO:1).
- B-class CpG ODN such as ODN 2006 include the originally described immunostimulatory CpG ODN and characteristically activate B cells and NK cells but do not induce or only weakly induce expression of type I interferon (e.g., IFN- ⁇ ).
- A-class CpG ODN described in published PCT international application WO 01/22990, incorporate a CpG motif, include a chimeric phosphodiester/phosphorothioate backbone, and characteristically activate NK cells and induce plasmacytoid dendritic cells to express large amounts of IFN- ⁇ but do not activate or only weakly activate B cells.
- An example of an A-class CpG ODN is 5′-G*G*G_G_G_A_C_G_A_T_C_G_T_C_G*G*G*G*G-3′ (ODN 2216, SEQ ID NO:2), wherein “*” represents phosphorothioate and “_” represents phosphodiester.
- C-class CpG ODN incorporate a CpG, include a wholly phosphorothioate backbone, include a GC-rich palindromic or nearly-palindromic region, and are capable of both activating B cells and inducing expression of IFN- ⁇ .
- C-class CpG ODN have been described, for example, in published U.S. patent application 2003/0148976.
- An example of a C-class CpG ODN is 5′-TCGTCGTTTTCGGCGCGCGCCG-3′ (ODN 2395; SEQ ID NO:3).
- cytokine refers to any of a number of soluble proteins or glycoproteins that act on immune cells through specific receptors to affect the state of activation and function of the immune cells. Cytokines include interferons, interleukins, tumor necrosis factor, transforming growth factor beta, colony-stimulating factors (CSFs), chemokines, as well as others. Various cytokines affect innate immunity, acquired immunity, or both.
- Cytokines specifically include, without limitation, IFN- ⁇ , IFN- ⁇ , IFN- ⁇ , IL-1, IL-2, IL-3, IL-4, IL-5, IL-6, IL-9, IL-10, IL-12, IL-13, IL-18, TNF- ⁇ , TGF- ⁇ , granulocyte colony-stimulating factor (G-CSF), and granulocyte-macrophage colony-stimulating factor (GM-CSF).
- Chemokines specifically include, without limitation, IL-8, IP-10, I-TAC, RANTES, MIP-1 ⁇ , MIP-1 ⁇ , Gro- ⁇ , Gro- ⁇ , Gro- ⁇ , MCP-1, MCP-2, and MCP-3.
- Th1 cells are associated with IL-2, IL-3, IFN, GM-CSF and high levels of TNF- ⁇ .
- Th2 cells are associated with IL-3, IL-4, IL-5, IL-6, IL-9, IL-10, IL-13, GM-CSF and low levels of TNF- ⁇ .
- the Th1 subset promotes both cell-mediated immunity and humoral immunity that is characterized by immunoglobulin class switching to IgG2a in mice.
- Th1 responses can also be associated with delayed-type hypersensitivity and autoimmune disease.
- the Th2 subset induces primarily humoral immunity and induces immunoglobulin class switching to IgE and IgG1 in mice.
- the antibody isotypes associated with Th1 responses generally have good neutralizing and opsonizing capabilities, whereas those associated with Th2 responses are associated more with allergic responses.
- IL-12 and IFN- ⁇ are positive Th1 and negative Th2 regulators.
- IL-12 promotes IFN- ⁇ production, and IFN- ⁇ provides positive feedback for IL-12.
- IL-4 and IL-10 appear to be required for the establishment of the Th2 cytokine profile and to down-regulate Th1 cytokine production; the effects of IL-4 are in some cases dominant over those of IL-12.
- IL-13 was shown to inhibit expression of inflammatory cytokines, including IL-12 and TNF- ⁇ by LPS-induced monocytes, in a way similar to IL-4.
- an effective amount refers to any amount that is necessary or sufficient for achieving or promoting a desired outcome. In some instances an effective amount is a therapeutically effective amount. A therapeutically effective amount is any amount that is necessary or sufficient for promoting or achieving a desired biological response in a subject.
- the effective amount for any particular application can vary depending on such factors as the disease or condition being treated, the particular agent being administered, the size of the subject, or the severity of the disease or condition. One of ordinary skill in the art can empirically determine the effective amount of a particular agent without necessitating undue experimentation.
- graft rejection refers to immunologically mediated hyperacute, acute, or chronic injury to a tissue or organ derived from a source other than the host. The term thus encompasses both cellular and antibody-mediated rejection, as well as rejection of both allografts and xenografts.
- Immune cell refers to a cell belonging to the immune system.
- Immune cells include T lymphocytes (T cells), B lymphocytes (B cells), natural killer (NK) cells, granulocytes, neutrophils, macrophages, monocytes, dendritic cells, and specialized forms of any of the foregoing, e.g., plasmacytoid dendritic cells, plasma cells, NKT, T helper, and cytotoxic T lymphocytes (CTL).
- immune complex refers to any conjugate including an antibody and an antigen specifically bound by the antibody.
- the antigen is an autoantigen.
- the term “immune complex comprising a nucleic acid” refers to any conjugate including an antibody and a nucleic acid-containing antigen specifically bound by the antibody.
- the nucleic acid-containing antigen can include chromatin, ribosomes, small nuclear proteins, histones, nucleosomes, DNA, RNA, or any combination thereof.
- the antibody can but need not necessarily bind specifically to a nucleic acid component of the nucleic acid-containing antigen.
- immune complex-associated disease refers to any disease characterized by the production and/or tissue deposition of immune complexes, including, but not limited to systemic lupus erythematosus (SLE) and related connective tissue diseases, rheumatoid arthritis, hepatitis C— and hepatitis B-related immune complex disease (e.g., cryoglobulinemia), Behçet's syndrome, autoimmune glomerulonephritides, and vasculopathy associated with the presence of LDL/anti-LDL immune complexes.
- SLE systemic lupus erythematosus
- connective tissue diseases e.g., rheumatoid arthritis
- hepatitis C— hepatitis B-related immune complex disease
- Behçet's syndrome e.g., Behçet's syndrome
- autoimmune glomerulonephritides e.g., autoimmune glomerulonephritides
- immunodeficiency refers to a disease or disorder in which the subject's immune system is not functioning in normal capacity or in which it would be useful to boost a subject's immune response for example to eliminate a tumor or cancer (e.g., tumors of the brain, lung (e.g., small cell and non-small cell), ovary, breast, prostate, colon, as well as other carcinomas and sarcomas) or an infection in a subject.
- the immunodeficiency can be acquired or it can be congenital.
- immunostimulatory nucleic acid-associated response in a subject refers to a measurable response in a subject associated with administration to the subject of an immunostimulatory nucleic acid.
- Such responses include, without limitation, elaboration of cytokines, chemokines, growth factors, or immunoglobulin; expression of immune cell surface activation markers; Th1/Th2 skewing; and clinical disease activity.
- infection and, equivalently, “infectious disease” refer to a condition in which an infectious organism or agent is present in a detectable amount in the blood or in a normally sterile tissue or normally sterile compartment of a subject. Infectious organisms and agents include viruses, bacteria, fungi, and parasites. The terms encompass both acute and chronic infections, as well as sepsis.
- innate immune response refers to any type of immune response to certain pathogen-associated molecular patterns (PAMPs).
- PAMPs pathogen-associated molecular patterns
- Innate immunity which is also known in the art as natural or native immunity, involves principally neutrophils, granulocytes, mononuclear phagocytes, dendritic cells, NKT cells, and NK cells.
- Innate immune responses can include, without limitation, type I interferon production (e.g., IFN- ⁇ ), neutrophil activation, macrophage activation, phagocytosis, opsonization, complement activation, and any combination thereof.
- self-DNA refers to any DNA derived from the genome of a host subject.
- self-DNA includes complementary DNA (cDNA) derived from a host subject.
- Self-DNA includes intact and degraded DNA.
- self-RNA refers to any RNA derived from the genome of a host subject.
- self-RNA is a messenger RNA (mRNA) derived from a host subject.
- self-RNA includes ribosomal RNA (rRNA) derived from a host subject.
- Self-RNA includes intact and degraded RNA.
- the term “subject” refers to a vertebrate animal. In one embodiment the subject is a mammal. In one embodiment the subject is a human. In other embodiments the subject is a non-human vertebrate animal, including, without limitation, non-human primates, laboratory animals, livestock, domesticated animals, and non-domesticated animals.
- subject having or at risk of developing TLR-mediated immunostimulation refers to a subject exposed to or at risk of exposure to a PAMP or other TLR ligand.
- TLR Toll-like receptor
- TLR1-TLR10 highly conserved mammalian pattern recognition receptor proteins
- PAMPs pathogen-associated molecular patterns
- TLR polypeptides share a characteristic structure that includes an extracellular (extracytoplasmic) domain that has leucine-rich repeats, a transmembrane domain, and an intracellular (cytoplasmic) domain that is involved in TLR signaling.
- TLRs include but are not limited to human TLRs.
- Nucleic acid and amino acid sequences for all ten currently known human TLRs are available from public databases such as GenBank.
- nucleic acid and amino acid sequences for various TLRs from numerous non-human species are also available from public databases including GenBank.
- nucleic acid and amino acid sequences for human TLR9 can be found as GenBank accession numbers AF245704 (coding region spanning nucleotides 145-3243) and AAF78037, respectively.
- Nucleic acid and amino acid sequences for murine TLR9 (mTLR9) can be found as GenBank accession numbers AF348140 (coding region spanning nucleotides 40-3138) and AAK29625, respectively.
- human TLR9 contains 1,032 amino acids and shares an overall amino acid identity of 75.5% with mouse TLR9.
- human TLR9 contains extracellular leucine-rich repeats (LRRs) and a cytoplasmic Toll/interleukin-1R (TIR) domain. It also has a signal peptide (residues 1-25) and a transmembrane domain (residues 819-836).
- Nucleic acid and amino acid sequences for human TLR8 can be found as GenBank accession numbers AF245703 (coding region spanning nucleotides 49-3174) and AAF78036, respectively.
- Nucleic acid and amino acid sequences for murine TLR8 can be found as GenBank accession numbers AY035890 (coding region spanning nucleotides 59-3157) and AAK62677, respectively.
- Nucleic acid and amino acid sequences for human TLR7 can be found as GenBank accession numbers AF240467 (coding region spanning nucleotides 135-3285) and AAF60188, respectively.
- Nucleic acid and amino acid sequences for murine TLR7 can be found as GenBank accession numbers AY035889 (coding region spanning nucleotides 49-3201) and AAK62676, respectively.
- Nucleic acid and amino acid sequences for human TLR3 can be found as GenBank accession numbers NM — 003265 (coding region spanning nucleotides 102-2816) and NP — 003256, respectively.
- Nucleic acid and amino acid sequences for murine TLR3 can be found as GenBank accession numbers AF355152 (coding region spanning nucleotides 44-2761) and AAK26117, respectively.
- hTLR1 is ubiquitously expressed
- hTLR2, hTLR4 and hTLR5 are present in monocytes, polymorphonuclear phagocytes, and dendritic cells. Muzio M et al. (2000) J Leukoc Biol 67:450-6.
- hTLR1, hTLR6, hTLR7, hTLR9 and hTLR10 are present in human B cells.
- Human TLR7 and hTLR9 are present in plasmacytoid dendritic cells (pDCs), while myeloid dendritic cells express hTLR7 and hTLR8 but not hTLR9. Human TLR8, however, appears not to be expressed in pDCs.
- TLRs As members of the pro-inflammatory interleukin-1 receptor (IL-1R) family, TLRs share homologies in their cytoplasmic domains called Toll/IL-1R homology (TIR) domains.
- TIR Toll/IL-1R homology
- Intracellular signaling mechanisms mediated by TLRs appear generally similar, with MyD88 and tumor necrosis factor receptor-associated factor 6 (TRAF6) believed to have critical roles.
- TLRs Wesche H et al. (1997) Immunity 7:837-47; Medzhitov R et al. (1998) Mol Cell 2:253-8; Adachi O et al. (1998) Immunity 9:143-50; Kawai T et al.
- MyD88 is believed to act as an adapter molecule between the TIR domain of a TLR or IL-1R and IRAK (which includes at least any one of IRAK-1, IRAK-2, IRAK-4, and IRAK-M).
- MyD88 includes a C-terminal Toll homology domain and an N-terminal death domain.
- the Toll homology domain of MyD88 binds the TIR domain of TLR or IL-1R, and the death domain of MyD88 binds the death domain of the serine kinase IRAK.
- IRAK interacts with TRAF6, which acts as an entryway into at least two pathways, one leading to activation of the transcription factor NF- ⁇ B and another leading to activation of Jun and Fos, members of the activator protein-1 (AP-1) transcription factor family.
- Activation of NF- ⁇ B involves the activation of TAK-1, a member of the MAP 3 kinase (MAPK) family, and I ⁇ B kinases.
- the I ⁇ B kinases phosphorylate I ⁇ B, leading to its degradation and the translocation of NF- ⁇ B to the nucleus.
- MAPKKs MAP kinase kinases
- ERK p38
- JNK/SAPK MAP kinase kinases
- TLR ligand and, equivalently, “ligand for a TLR” and “TLR signaling agonist”, refer to a molecule, other than a small molecule according to Formula I-XIX described herein or a 4-primary amino quinoline or quinazoline molecule according to the invention, that interacts, directly or indirectly, with a TLR through a TLR domain other than a TIR domain and induces TLR-mediated signaling.
- a TLR ligand is a natural ligand, i.e., a TLR ligand that is found in nature.
- a TLR ligand refers to a molecule other than a natural ligand of a TLR, e.g., a molecule prepared by human activity.
- the TLR is TLR9 and the TLR signal agonist is a CpG nucleic acid.
- TLR2 signals in response to peptidoglycan and lipopeptides.
- TLR4 has been reported to signal in response to lipopolysaccharide (LPS). Hoshino K et al.
- TLR9 is a receptor for CpG DNA. Hemmi H et al. (2000) Nature 408:740-5; Bauer S et al. (2001) Proc Natl Acad Sci USA 98:9237-42.
- CpG DNA which includes bacterial DNA and synthetic DNA with CG dinucleotides in which cytosine is unmethylated, is described in greater detail elsewhere herein. Marshak-Rothstein and colleagues also recently reported their finding that TLR9 signaling can occur in certain autoimmune diseases in response to immune complexes containing IgG and chromatin. Leadbetter E A et al. (2002) Nature 416:595-8. Thus, in a broader sense it appears that TLR9 can signal in response to self or non-self nucleic acid, either DNA or RNA, when the nucleic acid is presented in a suitable context, e.g., as part of an immune complex.
- Imidazoquinolines are potent synthetic activators of immune cells with antiviral and antitumor properties.
- macrophages from wildtype and MyD88-deficient mice Hemmi et al. recently reported that two imidazoquinolines, imiquimod and resiquimod (R848), induce tumor necrosis factor (TNF) and interleukin-12 (IL-112) and activate NF- ⁇ B only in wildtype cells, consistent with activation through a TLR.
- TNF tumor necrosis factor
- IL-112 interleukin-12
- ligands of TLR3 include poly(I:C) and double-stranded RNA (dsRNA).
- poly(I:C) and double-stranded RNA (dsRNA) are classified as oligonucleotide molecules.
- Alexopoulou et al. By stimulating kidney cells expressing one of a range of TLRs with poly(I:C), Alexopoulou et al. reported that only cells expressing TLR3 respond by activating NF- ⁇ B. Alexopoulou L et al. (2001) Nature 413: 732-8. Alexopoulou et al.
- TLR3 ⁇ / ⁇ cells responded equivalently to wildtype cells in response to lipopolysaccharide, peptidoglycan, and CpG dinucleotides.
- MyD88 ⁇ / ⁇ cells indicated that this adaptor protein is involved in dsRNA-induced production of cytokines and proliferative responses, although activation of NF- ⁇ B and MAP kinases are not affected, indicating distinct pathways for these cellular responses.
- Alexopoulou et al. proposed that TLR3 may have a role in host defense against viruses.
- a “cell expressing a TLR” refers to any cell which expresses, either naturally or artificially, a functional TLR.
- a functional TLR is a full-length TLR protein or a fragment thereof capable of inducing a signal in response to interaction with its ligand.
- the functional TLR will include at least a TLR ligand-binding fragment of the extracellular domain of the full-length TLR and at least a fragment of a TIR domain capable of interacting with another Toll homology domain-containing polypeptide, e.g., MyD88.
- the functional TLR is a full-length TLR selected from TLR1, TLR2, TLR3, TLR4, TLR5, TLR6, TLR7, TLR8, TLR9, and TLR10.
- the functional TLR is naturally expressed by a cell.
- the cell naturally expresses functional TLR and is an isolated cell from human multiple myeloma cell line RPMI 8226 (ATCC CCL-155). This cell line was established from the peripheral blood of a 61 year old man at the time of diagnosis of multiple myeloma (IgG lambda type). Matsuoka Y et al. (1967) Proc Soc Exp Biol Med 125:1246-50. RPMI 8226 was previously reported as responsive to CpG nucleic acids as evidenced by the induction of IL-6 protein and IL-12p40 mRNA. Takeshita F et al. (2000) Eur J Immunol 30:108-16; Takeshita F et al.
- the RPMI 8226 cell line Similar to peripheral blood mononuclear cells (PBMCs), the RPMI 8226 cell line has been observed to upregulate its cell surface expression of markers such as CD71, CD86 and HLA-DR in response to CpG nucleic acid exposure. This has been observed by flow cytometric analysis of the cell line. Accordingly, the methods provided herein can be structured to use appropriately selected cell surface marker expression as a readout, in addition to or in place of chemokine or cytokine production or other readouts described elsewhere herein.
- the RPMI 8226 cell line has also been found to respond to certain small molecules including imidazoquinoline compounds. For example, incubation of RPMI 8226 cells with the imidazoquinoline compound R848 (resiquimod) induces IL-8, IL-10, and IP-10 production. It has recently been reported that R848 mediates its immunostimulatory effects through TLR7 and TLR8. The ability of RPMI 8226 to respond to R848 suggests that the RPMI 8226 cell line also expresses TLR7, as previously reported for normal human B cells.
- R848 resiquimod
- the RPMI cell line can be used in unmodified form or in a modified form.
- the RPMI 8226 cell is transfected with a reporter construct.
- the cell is stably transfected with the reporter construct.
- the reporter construct generally includes a promoter, a coding sequence and a polyadenylation signal.
- the coding sequence can include a reporter sequence selected from the group consisting of an enzyme (e.g., luciferase, alkaline phosphatase, beta-galactosidase, chloramphenicol acetyltransferase (CAT), secreted alkaline phosphatase, etc.), a bioluminescence marker (e.g., green fluorescent protein (GFP, U.S. Pat. No. 5,491,084), etc.), a surface-expressed molecule (e.g., CD25), a secreted molecule (e.g., IL-8, IL-12 p40, TNF- ⁇ , etc.), and other detectable protein products known to those of skill in the art.
- the coding sequence encodes a protein having a level or an activity that is quantifiable.
- the functional TLR is artificially expressed (including over-expressed) by a cell, for example by introduction into the cell of an expression vector bearing a coding sequence for the functional TLR wherein the coding sequence is operably linked to a gene expression sequence.
- a coding sequence and the gene expression sequence are said to be operably linked when they are covalently linked in such a way as to place the expression or transcription and/or translation of the coding sequence under the influence or control of the gene expression sequence.
- Two DNA sequences are said to be operably linked if induction of a promoter in the 5′ gene expression sequence results in the transcription of the coding sequence and if the nature of the linkage between the two DNA sequences does not (1) result in the introduction of a frame-shift mutation, (2) interfere with the ability of the promoter region to direct the transcription of the coding sequence, or (3) interfere with the ability of the corresponding RNA transcript to be translated into a protein.
- a gene expression sequence would be operably linked to a coding sequence if the gene expression sequence were capable of effecting transcription of that coding sequence such that the resulting transcript is translated into the desired protein or polypeptide.
- a coding sequence refers to a nucleic acid sequence coding for a functional TLR. In some embodiments a coding sequence refers to a nucleic acid sequence coding for a reporter.
- a cell that artificially expresses a functional TLR can be a cell that does not express the functional TLR but for the TLR expression vector.
- human 293 fibroblasts (ATCC CRL-1573) do not express TLR3, TLR7, TLR8, or TLR9.
- suitable expression vector or vectors
- a cell that artificially expresses a functional TLR can be a cell that expresses the functional TLR at a significantly higher level with the TLR expression vector than it does without the TLR expression vector.
- a cell that artificially expresses a functional TLR is preferably a stably transfected cell that expresses the functional TLR.
- a cell can also be stably transfected with a suitable reporter construct.
- TLR-mediated signaling refers to any portion of the intracellular signal transduction pathway involving interaction of a TLR with a suitable TLR ligand and engagement by the TLR of MyD88 or any aspect downstream of MyD88 engagement, described above in relation to the structure and function of the TIR domain of a TLR.
- TLR-mediated immunostimulatory signaling refers to TLR-mediated signaling that results in a measurable immunostimulatory response. This term applies to such signaling both in vitro and in vivo.
- TLR-mediated immunostimulation in a subject refers to TLR-mediated immunostimulatory signaling as it applies in vivo.
- treat as used in reference to a disorder, disease, or condition means to intervene in such disorder, disease, or condition so as to prevent or slow the development of, to prevent, slow or halt the progression of, or to eliminate the disorder, disease, or condition.
- immunological nucleic acid refers to a nucleic acid molecule that, when contacted with a cell of the immune system, induces the cell of the immune system to become activated.
- Immune cell activation can be determined using any suitable means known to one of skill in the art, including but not limited to measurement of stimulated growth, proliferation, differentiation, migration, transcription or expression of gene products that are expressed or secreted in association with immune activation, activity of intracellular signaling pathways, and the like.
- markers of immune cell activation include, without limitation, secretion of cytokines (e.g., IL-6), secretion of immunoglobulin (e.g., IgG), expression of cell surface antigens (e.g., CD86), induction of cytolytic activity, and induction and nuclear translocation of transcription factors (e.g., NF- ⁇ B).
- cytokines e.g., IL-6
- immunoglobulin e.g., IgG
- CD86 cell surface antigens
- induction of cytolytic activity e.g., CD86
- nucleic acid as used herein with respect to the methods of the invention, shall refer to any polymer of two or more individual nucleoside or nucleotide units. Nucleic acids can be single- or double-stranded. Typically individual nucleoside or nucleotide units will include any one or combination of deoxyribonucleosides, ribonucleosides, deoxyribonucleotides, and ribonucleotides. The individual nucleotide or nucleoside units of the nucleic acid can be naturally occurring or not naturally occurring.
- the individual nucleotide units can include deoxyadenosine, deoxycytidine, deoxyguanosine, thymidine, and uracil.
- individual nucleosides also include synthetic nucleosides having modified base moieties and/or modified sugar moieties, e.g., as described in Uhlmann E et al. (1990) Chem Rev 90:543-84.
- the linkages between individual nucleotide or nucleoside units can be naturally occurring or not naturally occurring.
- the linkages can be phosphodiester, phosphorothioate, phosphorodithioate, phosphoramidate, as well as peptide linkages and other covalent linkages, known in the art, suitable for joining adjacent nucleoside or nucleotide units.
- Immunostimulatory nucleic acids typically range in size from 3-4 units to a few tens of units, e.g., 18-40 units, although longer nucleic acids are also contemplated by the invention.
- the nucleic acids are oligonucleotides made up of 2 to about 100 nucleotides, and more typically 4 to about 40 nucleotides. Oligonucleotides composed exclusively of deoxyribonucleotides are termed oligodeoriboxynucleotides or, equivalently, oligodeoxynucleotides (ODN).
- ODN oligodeoxynucleotides
- An immunostimulatory nucleic acid includes any of a number of different types of immunostimulatory nucleic acids, including specifically immunostimulatory CpG nucleic acids (CpG nucleic acids), including but not limited to types A, B, and C; and immunostimulatory non-CpG nucleic acids, including without limitation methylated CpG nucleic acids, T-rich nucleic acids, and poly-G nucleic acids. Certain of these various classes of immunostimulatory nucleic acids can coexist in a given nucleic acid molecule.
- CpG nucleic acid and, equivalently, “CpG ODN” refer to an immunostimulatory nucleic acid which contains a cytosine-guanine (CG) dinucleotide, the C residue of which is unmethylated.
- CG cytosine-guanine
- the effects of CpG nucleic acids on immune modulation have been described extensively in U.S. patents such as U.S. Pat. Nos. 6,194,388; 6,207,646; 6,218,371; and 6,239,116, and published international patent applications, such as WO 98/37919, WO 98/40100, WO 98/52581, and WO 99/56755.
- the entire contents of each of these patents and published patent applications is hereby incorporated by reference.
- the entire immunostimulatory nucleic acid can be unmethylated or portions may be unmethylated but at least the C of the 5′-CG-3′ must be unmethylated.
- the CpG nucleic acid sequences of the invention include those broadly described above as well as disclosed in U.S. Pat. Nos. 6,207,646 B1 and 6,239,116 B1.
- the CpG nucleic acid has a base sequence provided by 5′-TCGTCGTTTTGTCGTTTTGTCGTT-3′ (ODN 2006; SEQ ID NO:1).
- Type B CpG nucleic acids such as ODN 2006 include the earliest described CpG nucleic acids and characteristically activate B cells but do not induce or only weakly induce expression of IFN- ⁇ .
- Type A CpG nucleic acids described in published international application PCT/US00/26527 (WO 01/22990), incorporate a CpG motif, include a hybrid phosphodiester/phosphorothioate backbone, and characteristically induce plasmacytoid dendritic cells to express large amounts of IFN- ⁇ but do not activate or only weakly activate B cells.
- Type C oligonucleotides incorporate a CpG, include a chimeric backbone, include a GC-rich palindromic or nearly-palindromic region, and are capable of both activating B cells and inducing expression of IFN- ⁇ . These have been described, for example, in published U.S. patent application 2003/0148976.
- a non-CpG nucleic acid is used.
- a non-CpG nucleic acid is an immunostimulatory nucleic acid which either does not have a CpG motif in its sequence, or has a CpG motif which contains a methylated C residue.
- the non-CpG nucleic acid may still be immunostimulatory by virtue of its having other immunostimulatory motifs such as those described herein and known in the art.
- the non-CpG nucleic acid is a methylated CpG nucleic acid.
- the non-CpG nucleic acid is still immunostimulatory despite methylation of the C of the CpG motif, even without having another non-CpG immunostimulatory motif described herein and known in the art.
- the non-CpG nucleic acid is a methylated CpG nucleic acid having a base sequence provided by 5′-TZGTZGTTTTGTZGTTTTGTZGTT-3′ (ODN 2117; SEQ ID NO:4, wherein Z represents 5-methylcytidine).
- Non-CpG nucleic acids include T-rich immunostimulatory nucleic acids.
- the T-rich immunostimulatory nucleic acids include those disclosed in published PCT patent application PCT/US00/26383, the entire contents of which are incorporated herein by reference.
- T-rich nucleic acids 24 bases in length are used.
- a T-rich nucleic acid is a nucleic acid which includes at least one poly T sequence and/or which has a nucleotide composition of greater than 25% T nucleotide residues.
- a nucleic acid having a poly-T sequence includes at least four Ts in a row, such as 5′-TTTT-3′.
- the T-rich nucleic acid includes more than one poly T sequence.
- the T-rich nucleic acid may have 2, 3, 4, or more poly T sequences, such as ODN 2006.
- Non-CpG nucleic acids also include poly-G immunostimulatory nucleic acids.
- a variety of references describe the immunostimulatory properties of poly-G nucleic acids. Pisetsky D S et al. (1993) Mol Biol Reports 18:217-221; Krieger M et al. (1994) Ann Rev Biochem 63:601-637; Macaya R F et al. (1993) Proc Natl Acad Sci USA 90:3745-3749; Wyatt J R et al. (1994) Proc Natl Acad Sci USA 91:1356-1360; Rando and Hogan, 1998, In Applied Antisense Oligonucleotide Technology, Krieg and Stein, eds., pp. 335-352; Kimura Y et al. (1994) J Biochem ( Tokyo ) 116:991-994.
- the immunostimulatory nucleic acids of the invention can also be those which do not possess CpG, methylated CpG, T-rich, or poly-G motifs.
- immunostimulatory nucleic acid sequences further include but are not limited to those immunostimulatory sequences described and listed in published PCT patent application WO 01/22972.
- the invention provides novel compounds that fall within Formula VI and Formula VII as disclosed herein.
- These compounds include various diarylmethane TLR9 antagonists denoted herein as CMZ 203-84, CMZ 203-85, CMZ 203-88, CMZ 203-88-1, CMZ 203-89, and CMZ 203-91. Syntheses for each of these compounds are provided in Examples 12-17, respectively.
- CMZ 203-84, CMZ 203-85, CMZ 203-88, CMZ 203-88-1, CMZ 203-89, and CMZ 203-91 are believed to be useful in the methods of the invention. More specifically, in one aspect the invention provides a method of affecting TLR-mediated signaling in response to a TLR ligand.
- the method includes the step of contacting a cell expressing a TLR with an effective amount of any one or combination of compounds CMZ 203-84, CMZ 203-85, CMZ 203-88, CMZ 203-88-1, CMZ 203-89, and CMZ 203-91, to inhibit or promote TLR-mediated signaling in response to a ligand for the TLR.
- the invention provides a method of inhibiting TLR-mediated signaling in response to a TLR ligand.
- the method includes the step of contacting a cell expressing a TLR with an effective amount of any one or combination of compounds CMZ 203-84, CMZ 203-85, CMZ 203-88, CMZ 203-88-1, CMZ 203-89, and CMZ 203-91, to inhibit TLR-mediated immunostimulatory signaling in response to a ligand for the TLR.
- the invention provides a method of affecting TLR-mediated immunostimulation in a subject.
- the method includes the step of administering to a subject having or at risk of developing TLR-mediated immunostimulation an effective amount of any one or combination of compounds CMZ 203-84, CMZ 203-85, CMZ 203-88, CMZ 203-88-1, CMZ 203-89, and CMZ 203-91, to inhibit or promote TLR-mediated immunostimulation in the subject.
- the invention provides a method of inhibiting TLR-mediated immunostimulation in a subject.
- the method includes the step of administering to a subject having or at risk of developing TLR-mediated immunostimulation an effective amount of any one or combination of compounds CMZ 203-84, CMZ 203-85, CMZ 203-88, CMZ 203-88-1, CMZ 203-89, and CMZ 203-91, to inhibit TLR-mediated immunostimulation in the subject.
- the invention provides a method of inhibiting an immunostimulatory nucleic acid-associated response in a subject.
- the method according to this aspect includes the step of administering to a subject in need of such treatment an effective amount of any one or combination of compounds CMZ 203-84, CMZ 203-85, CMZ 203-88, CMZ 203-88-1, CMZ 203-89, and CMZ 203-91, to inhibit an immunostimulatory nucleic acid-associated response in the subject.
- the invention provides novel substituted 4-primary amino quinoline compositions.
- these compositions and other substituted 4-primary amino quinoline compositions have been discovered to be useful in methods for inhibiting an immune response, both in vitro and in vivo, including methods for treating immune complex associated diseases and autoimmune disorders. Due to their similarity to certain known antimalarial agents, it is also believed that the novel substituted 4-primary amino quinoline compositions of the invention will also be useful for prevention and treatment of malaria, as well as for treatment of other diseases which have been described to be responsive to treatment with chloroquines.
- novel substituted 4-primary amino quinoline compositions of the invention have a structural Formula XVI wherein
- X is absent or is an aryl, alkyl, heterocyclic, or styryl group
- R 1 and R 2 are each independently a hydrogen atom or a substituted or unsubstituted alkyl, alkenyl, or aryl group, wherein R 1 and R 2 optionally are combined to form a heterocycle;
- R 3 is a hydrogen atom, a halogen atom, or an alkyl, alkenyl, aryl, heterocyclic, nitro, cyano, carboxy, ester, ketone, amino, amido, hydroxy, alkoxy, mercapto, thio, sulfoxide, sulfone, or sulfonamido group, wherein R 1 and R 3 optionally are combined to form a heterocycle or a carbocycle;
- Y is absent or is an oxygen atom, a sulfur atom, CR 8 R 9 , or NR 10 , where R 8 , R 9 , and R 10 are each independently a hydrogen atom or a substituted or unsubstituted alkyl, alkenyl, or aryl group;
- L is an alkyl or alkenyl group containing from 1 to 10 carbons or is an aryl group
- R 4 , R 5 , R 6 , and R 7 are each independently a hydrogen atom, a halogen atom, or an alkyl, alkenyl, aryl, heterocyclic, nitro, cyano, carboxy, ester, ketone, amino, amido, hydroxy, alkoxy, mercapto, thio, sulfoxide, sulfone, or sulfonamido group, wherein any pair of R 4 , R 5 , R 6 , and R 7 which are adjacent one another optionally are combined to form a heterocycle or a carbocycle,
- R 5 and R 6 are each independently a halogen atom or an alkoxy group. In one embodiment R 5 and R 6 are each independently a chlorine atom or a methoxy group.
- X is absent or is an aryl group
- NR 1 R 2 is a heterocyclic amine or is NR 8 (CH 2 ) n NR 9 R 10 , wherein n is an integer from 2 to 6, inclusive, and R 8 , R 9 , and R 10 are each independently a hydrogen atom or an alkyl group;
- R 3 is a hydrogen atom
- Y is an aryl group or is NR 11 where R 11 is a hydrogen atom or an aryl or alkyl group;
- L is absent or is a C 2 -C 6 alkyl group
- R 4 , R 5 , R 6 , and R 7 are each independently a hydrogen atom, a halogen atom, or an alkoxy group.
- X is absent or is an aryl group
- NR 1 R 2 is a substituted or unsubstituted piperazino or morpholino group or is NR 8 (CH 2 ) n NR 9 R 10 , wherein n is an integer from 2 to 6, inclusive, R 8 is a hydrogen atom, and R 9 and R 10 are each independently an alkyl group;
- R 3 is a hydrogen atom
- Y is NH
- L is a C 2 -C 6 alkyl group
- R 4 , R 5 , R 6 , and R 7 are each independently a hydrogen atom, a halogen atom, or an alkoxy group.
- X is a phenyl group
- NR 1 R 2 is attached to a para position of the phenyl group X;
- Y is NH
- L is —(CH 2 ) n — where n is an integer between 2 and 6, inclusive;
- each of R 3 , R 4 , R 5 , R 6 , and R 7 is a hydrogen atom.
- composition is optionally in the form of a pharmaceutically acceptable hydrate or salt.
- substituted 4-primary amino quinoline compositions of Formula XVI of the invention are compounds 101-104, 106-109, 111, 113-116, and 118-119, presented in Table 1.
- Table 1 Substituted 4-Primary Amino Quinoline Compositions of the Invention ID X NR 1 R 2 Y L R 3 R 4 R 5 R 6 R 7 101 NH —(CH 2 ) 2 — H H H H H H 102 NH —(CH 2 ) 3 — H H H H H 103 NH —(CH 2 ) 2 — H H H H H H 104 NH —(CH 2 ) 4 — H H H H H 106 NH —(CH 2 ) 5 — H H H H H H 107 NH —(CH 2 ) 2 — H H Cl H H H 108 NH —(CH 2 ) 3 — H H H H H 109 NH —(CH 2 ) 6 — H H H H H 111
- the invention provides a method for inhibiting signaling by a TLR.
- the method according to this aspect of the invention involves contacting a cell expressing a functional TLR with an effective amount of a substituted 4-primary amino quinoline composition having structural Formula XVII wherein
- X is absent or is a nitrogen, oxygen, or sulfur atom or an SO or SO 2 group
- R 1 is a hydrogen atom or a substituted or unsubstituted aryl, alkyl, heterocyclic or styryl group
- R 2 is a hydrogen atom, a halogen atom, or an alkyl, alkenyl, aryl, heterocyclic, nitro, cyano, carboxy, ester, ketone, amino, amido, hydroxy, alkoxy, mercapto, thio, sulfoxide, sulfone, or sulfonamido group, wherein R 1 and R 2 optionally are combined to form a heterocycle or carbocycle;
- Y is absent or is an oxygen atom, a sulfur atom, CR 7 R 8 , or NR 9 , where R 7 , R 8 , and R 9 are each independently a hydrogen atom or a substituted or unsubstituted alkyl, alkenyl, or aryl group;
- L is absent or is an alkyl or alkenyl group containing from 1 to 10 carbons or is an aryl group;
- R 3 , R 4 , R 5 , and R 6 are each independently a hydrogen atom, a halogen atom, or an alkyl, alkenyl, aryl, heterocyclic, nitro, cyano, carboxy, ester, ketone, amino, amido, hydroxy, alkoxy, mercapto, thio, sulfoxide, sulfone, or sulfonamido group, wherein any pair of R 3 , R 4 , R 5 , and R 6 which are adjacent one another optionally are combined to form a heterocycle or a carbocycle, and
- the substituted 4-primary amino quinoline composition in this and all other aspects of the invention involving the use of a substituted 4-primary amino quinoline composition can be in the form a hydrate or pharmaceutically acceptable salt.
- the method according to this aspect of the invention can be performed in vitro or it can be performed in vivo.
- the cell expressing the functional TLR can, but need not necessarily, be an immune cell.
- the cell expressing the functional TLR can be a cell transfected with an expression vector that directs expression of the TLR by the cell.
- the TLR is TLR9 and the method is thus a method for inhibiting intracellular signaling by TLR9.
- the invention provides a method for inhibiting signaling by a TLR.
- the method according to this aspect of the invention involves contacting an immune cell expressing a functional TLR with
- a substituted 4-primary amino quinoline composition having structural Formula XVII, as defined above to inhibit signaling by the TLR in response to the TLR signal agonist compared with the signaling by the TLR in response to the TLR signal agonist in absence of the substituted 4-primary amino quinoline composition.
- the substituted 4-primary amino quinoline composition in this aspect of the invention can be in the form a hydrate or pharmaceutically acceptable salt.
- the method according to this aspect of the invention can be performed in vitro or it can be performed in vivo.
- the TLR is TLR9 and the TLR signal agonist is a TLR9 signal agonist, and the method in one embodiment is thus a method of inhibiting intracellular signaling by TLR9 in response to a TLR9 signal agonist.
- the TLR signal agonist in one embodiment is CpG DNA, for example a CpG ODN such as ODN 2006.
- the CpG ODN can belong to any class of CpG ODN, including A-class (e.g., ODN 2216), B-class (e.g., ODN 2006), or C-class (e.g., ODN 2395).
- the TLR signal agonist is an immune complex that includes a nucleic acid.
- Immune complexes that include a nucleic acid are known by those of skill in the art to include immunoglobulin complexed with nucleic acids that are either naked or, more commonly, that are associated with proteins or other non-nucleic acid components.
- the nucleic acids that are associated with proteins or other non-nucleic acid components can include, for example, chromatin, ribosomes, small nuclear proteins, ribonuclear proteins (RNP), histones, nucleosomal protein-DNA complexes, and nucleosomes.
- Examples of clinically important antibodies specific for nucleic acids and for nucleic acid-containing material include antinuclear antibodies (ANA), anti-dsDNA, anti-ssDNA, anti-Sm, anti-RNP, anti-Ro (SS-A), anti-La (SS-B), and antihistone antibodies.
- Immune complexes that include a nucleic acid include, without limitation, immunoglobulin complexed with DNA, including specifically double-stranded DNA, and immunoglobulin complexed with nucleosomal material, both characteristic of systemic lupus erythematosus, and immunoglobulin complexed with RNA.
- the invention provides a method for inhibiting an immune response to an antigenic substance.
- the method according to this aspect of the invention involves contacting an immune cell expressing a functional Toll-like receptor with
- a substituted 4-primary amino quinoline composition having structural Formula XVII, as defined above to inhibit an immune response to the antigenic substance compared with the immune response to the antigenic substance in absence of the substituted 4-primary amino quinoline composition.
- the substituted 4-primary amino quinoline composition in this aspect of the invention can be in the form a hydrate or pharmaceutically acceptable salt.
- the method according to this aspect of the invention can be performed in vitro or it can be performed in vivo.
- the immune response is an innate immune response.
- the immune response includes an adaptive immune response.
- the method involves contacting, in a subject, an immune cell expressing a functional Toll-like receptor with
- the step of contacting an immune cell expressing a functional Toll-like receptor with an effective amount of an antigenic substance to stimulate an immune response to the antigenic substance in absence of a substituted 4-primary amino quinoline composition involves the active step of administering an effective amount of an antigenic substance to stimulate an immune response to the antigenic substance in absence of a substituted 4-primary amino quinoline composition.
- the antigenic substance can be administered using any effective route or means for administering the antigenic substance to effect the contacting.
- the administering can be by local or systemic injection, inhalation, oral ingestion, topical administration, mucosal administration, or any combination thereof.
- the step of contacting an immune cell expressing a functional Toll-like receptor with an effective amount of an antigenic substance to stimulate an immune response to the antigenic substance in absence of a substituted 4-primary amino quinoline composition is passive.
- the immune cell is already in contact or has already been in contact with an effective amount of an antigenic substance to stimulate an immune response to the antigenic substance, at the time of or prior to the step of contacting the immune cell expressing a functional Toll-like receptor with an effective amount of a substituted 4-primary amino quinoline composition having structural Formula XVII, as defined above, to inhibit an immune response to the antigenic substance compared with the immune response to the antigenic substance in absence of the substituted 4-primary amino quinoline composition.
- the antigenic substance can be an allergen.
- An allergen is a substance that can induce an allergic or asthmatic response in a susceptible subject.
- the list of allergens is enormous and can include pollens, insect venoms, animal dander, dust, fungal spores and drugs (e.g., penicillin).
- Examples of natural animal and plant allergens include proteins specific to the following genera: Canis ( Canis familiaris ); Dermatophagoides (e.g., Dermatophagoides farinae ); Felis ( Felis domesticus ); Ambrosia ( Ambrosia artemiisfolia ); Lolium (e.g., Lolium perenne or Lolium multiflorum ); Cryptomeria ( Cryptomeria japonica ); Alternaria ( Alternaria alternata ); Alder; Alnus ( Alnus gultinosa ); Betula ( Betula verrucosa ); Quercus ( Quercus alba ); Olea ( Olea europa ); Artemisia ( Artemisia vulgaris ); Plantago (e.g., Plantago lanceolata ); Parietaria (e.g., Parietaria officinalis or Parietaria judaica ); Blattella (e.g., Blattella german
- allergy refers to acquired hypersensitivity to a substance (allergen).
- An “allergic reaction” is the response of an immune system to an allergen in a subject allergic to the allergen. Allergic conditions include eczema, allergic rhinitis or coryza, hay fever, bronchial asthma, urticaria (hives) and food allergies, and other atopic conditions.
- the antigenic substance can be an antigen that is or is derived from an infectious microbial agent, including a bacterium, a virus, a fungus, or a parasite.
- infectious bacteria include: Helicobacter pyloris, Borrelia burgdorferi, Legionella pneumophilia, Mycobacteria sps (such as. M. tuberculosis, M. avium, M. intracellulare, M. kansasii , and M.
- Retroviridae including but not limited to human immunodeficiency virus (HIV)
- Picornaviridae for example, polio viruses, hepatitis A virus; enteroviruses, human coxsackie viruses, rhinoviruses, echoviruses); Calciviridae (such as strains that cause gastroenteritis); Togaviridae (for example, equine encephalitis viruses, rubella viruses); Flaviviridae (for example, dengue viruses, encephalitis viruses, yellow fever viruses); Coronaviridae (for example, coronaviruses); Rhabdoviridae (for example, vesicular stomatitis viruses, rabies viruses); Filoviridae (for example, ebola viruses); Paramyxoviridae (for example, parainfluenza viruses, mumps virus, measles virus, respiratory syncytial virus); Orthomyxoviridae (for example, influenza viruses); Buny
- infectious fungi examples include, but are not limited to, Cryptococcus neoformans, Histoplasma capsulatum, Coccidioides immitis, Blastomyces dermatitidis, Chlamydia trachomatis , and Candida albicans.
- the antigenic substance can be a cancer antigen.
- a cancer antigen as used herein is a compound, such as a peptide or protein, associated with a tumor or cancer cell surface and which is capable of provoking an immune response when expressed on the surface of an antigen-presenting cell in the context of a major histocompatibility complex (MHC) molecule.
- MHC major histocompatibility complex
- Cancer antigens can be prepared from cancer cells either by preparing crude extracts of cancer cells, for example, as described in Cohen P A et al. (1994) Cancer Res 54:1055-8, by partially purifying the antigens, by recombinant technology, or by de novo synthesis of known antigens.
- Cancer antigens include but are not limited to antigens that are recombinantly expressed, an immunogenic portion thereof, or a whole tumor or cancer cell. Such antigens can be isolated or prepared recombinantly or by any other means known in the art.
- cancer antigen and “tumor antigen” are used interchangeably and refer to antigens which are differentially expressed by cancer cells and can thereby be exploited in order to target cancer cells.
- Cancer antigens are antigens which can potentially stimulate apparently tumor-specific immune responses. Some of these antigens are encoded, although not necessarily expressed, by normal cells. These antigens can be characterized as those which are normally silent (i.e., not expressed) in normal cells, those that are expressed only at certain stages of differentiation and those that are temporally expressed such as embryonic and fetal antigens.
- cancer antigens are encoded by mutant cellular genes, such as oncogenes (e.g., activated ras oncogene), suppressor genes (e.g., mutant p53), fusion proteins resulting from internal deletions or chromosomal translocations. Still other cancer antigens can be encoded by viral genes such as those carried on RNA and DNA tumor viruses.
- tumor antigens examples include MAGE, MART-1/Melan-A, gp 100, Dipeptidyl peptidase IV (DPPIV), adenosine deaminase-binding protein (ADAbp), cyclophilin b, Colorectal associated antigen (CRC)—C017-1A/GA733, Carcinoembryonic Antigen (CEA) and its immunogenic epitopes CAP-1 and CAP-2, etv6, aml1, Prostate Specific Antigen (PSA) and its immunogenic epitopes PSA-1, PSA-2, and PSA-3, prostate-specific membrane antigen (PSMA), T-cell receptor/CD3-zeta chain, MAGE-family of tumor antigens (e.g., MAGE-A1, MAGE-A2, MAGE-A3, MAGE-A4, MAGE-A5, MAGE-A6, MAGE-A7, MAGE-A8, MAGE-A9, MAGE-A10, MAGE-A11, MAGE-
- Cancers or tumors and tumor antigens associated with such tumors include acute lymphoblastic leukemia (etv6; aml1; cyclophilin b), B cell lymphoma (Ig-idiotype), glioma (E-cadherin; ⁇ -catenin; ⁇ -catenin; ⁇ -catenin; p120ctn), bladder cancer (p21ras), biliary cancer (p21ras), breast cancer (MUC family; HER2/neu; c-erbB-2), cervical carcinoma (p53; p21ras), colon carcinoma (p21ras; HER2/neu; c-erbB-2; MUC family), colorectal cancer (Colorectal associated antigen (CRC)—C017-1A/GA733; APC), choriocarcinoma (CEA), epithelial cell cancer (cyclophilin b), gastric cancer (HER2/neu; c-erbB-2; ga733
- CRC
- a method of treating an autoimmune disorder in a subject involves the step of administering to a subject having an autoimmune disorder an effective amount of a substituted 4-primary amino quinoline composition having structural Formula XVII, as defined above, to treat the autoimmune disorder.
- the substituted 4-primary amino quinoline composition in this aspect of the invention can be in the form a hydrate or pharmaceutically acceptable salt.
- the autoimmune disorder is chosen from systemic lupus erythematosus, rheumatoid arthritis, inflammatory bowel disease, Sjögren's syndrome, polymyositis, vasculitis, Wegener's granulomatosis, sarcoidosis, ankylosing spondylitis, Reiter's syndrome, psoriatic arthritis, and Behçet's syndrome.
- the autoimmune disease is systemic lupus erythematosus.
- the autoimmune disease is rheumatoid arthritis.
- the subject is a human. The method can also be applied to animal models of any of the autoimmune disorders listed above.
- the autoimmune disorder is an immune complex associated disease.
- Immune complex associated diseases specifically include, without limitation, systemic lupus erythematosus, rheumatoid arthritis, polyarteritis nodosa, poststreptococcal glomerulonephritis, cryoglobulinemia, and acute and chronic serum sickness.
- the substituted 4-primary amino quinoline composition can be administered to the subject by any suitable route of administration, including, without limitation, oral and parenteral.
- Parenteral routes of administration include, without limitation, intravenous, intramuscular, intraperitoneal, subcutaneous, intranasal, intrapulmonary, transdermal, topical, and mucosal.
- Parenteral routes of administration also include direct injection into a specific tissue or other site of injection, including, for example, lymphoid tissue and a site of inflammation.
- quinzoline compounds structurally similar to quinolines of the invention are unexpectedly useful in methods for inhibiting an immune response, both in vitro and in vivo, including methods for treating immune complex associated diseases and autoimmune disorders.
- the quinazoline compounds and their methods of use according to the invention were unexpectedly found to retain much or all of the immunoinhibitory features of corresponding quinoline compounds and their methods of use, but with the additional feature of having reduced toxicity, at least in vivo.
- the invention provides novel quinazoline compositions.
- these compositions and other quinazoline compositions have been discovered to be useful in methods for inhibiting an immune response, both in vitro and in vivo, including methods for treating immune complex associated diseases and autoimmune disorders. Due to their similarity to certain known antimalarial agents, it is also believed that the novel quinazoline compositions of the invention will also be useful for prevention and treatment of malaria, as well as for treatment of other diseases which have been described to be responsive to treatment with chloroquines.
- novel quinazoline compositions of the invention have a structural Formula XVIII wherein
- X is absent or is an aryl, alkyl, heterocyclic or styryl group, provided that if X is a phenyl group, NR 1 R 2 is part if a heterocycle or is a diamine;
- R 1 and R 2 are each independently a hydrogen atom or an alkyl, alkenyl, or aryl group, wherein R 1 and R 2 optionally are combined to form a heterocycle;
- Y is an oxygen atom, a sulfur atom, CR 9 R 10 , or NR 11 , where R 9 , R 10 , and R 11 are each independently a hydrogen atom or an alkyl, alkenyl, or aryl group, wherein any one of R 9 , R 10 , and R 11 optionally is combined with R 3 or R 4 to form a substituted or unsubstituted heterocycle;
- L is an alkyl or alkenyl group containing from 1 to 10 carbons or is an aryl group
- R 3 and R 4 are each independently a hydrogen atom or an alkyl, alkenyl, or aryl group, wherein R 3 and R 4 optionally are combined to form a heterocycle;
- R 5 , R 6 , R 7 , and R 8 are each independently a hydrogen atom, a halogen atom, or an alkyl, alkenyl, aryl, heterocyclic, nitro, cyano, carboxy, ester, ketone, amino, amido, hydroxy, alkoxy, mercapto, thio, sulfoxide, sulfone, or sulfonamido group, wherein any pair of R 5 , R 6 , R 7 , and R 8 which are adjacent one another optionally are combined to form a heterocycle or a carbocycle,
- R 6 and R 7 are each independently a halogen atom or an alkoxy group.
- R 6 and R 7 are each independently a chlorine atom or a methoxy group.
- X is absent or is an aryl group
- NR 1 R 2 is a heterocyclic amine or NR 9 (CH 2 ) n NR 10 R 11 , wherein n is an integer from 2 to 6, inclusive, and R 9 , R 10 , and R 11 are each independently a hydrogen atom or an alkyl group;
- Y is an aryl group or is NR 12 where R 12 is a hydrogen atom or an aryl or alkyl group;
- L is absent or is a C 2 -C 6 alkyl group
- R 3 and R 4 are each independently a hydrogen atom or an alkyl group, wherein R 3 and R 4 optionally are combined to form a heterocycle;
- R 5 , R 6 , R 7 , and R 8 are each independently a hydrogen atom, a halogen atom, or an alkoxy group.
- X is absent or is an aryl group
- NR 1 R 2 is a substituted or unsubstituted piperazino or morpholino group or is NR 9 (CH 2 ) n NR 10 R 11 , wherein n is an integer from 2 to 6, inclusive, R 9 is a hydrogen atom, and R 10 and R 11 are each independently an alkyl group;
- Y is NH
- L is a C 2 -C 6 alkyl group
- R 3 and R 4 are each independently a hydrogen atom or an alkyl group, wherein R 3 and R 4 optionally are combined to form a heterocycle;
- R 5 , R 6 , R 7 , and R 8 are each independently a hydrogen atom, a halogen atom, or an alkoxy group.
- X is an aryl group
- NR 1 R 2 is substituted or unsubstituted piperazino or morpholino group
- Y is NH
- L is a C 2 -C 6 alkyl group
- R 3 and R 4 are each independently a methyl or ethyl group or R 3 and R 4 optionally are combined to form a morpholino group;
- R 5 , R 6 , R 7 , and R 8 are each independently a hydrogen atom, a halogen atom, or an alkoxy group.
- X is a phenyl group
- NR 1 R 2 is N-methylpiperazine
- Y is NH
- L is —CH 2 CH 2 —
- R 3 and R 4 are each a methyl group
- R 5 , R 6 , R 7 , and R 8 are each a hydrogen atom.
- X is a phenyl group
- NR 1 R 2 is N-methylpiperazine
- Y is NH
- L is —CH 2 CH 2 —
- R 3 and R 4 are combined as a morpholino group
- R 5 , R 6 , R 7 , and R 8 are each a hydrogen atom.
- X is absent
- NR 1 R 2 is a substituted or unsubstituted piperazino or morpholino group
- Y is NH
- L is a C 2 -C 6 alkyl group
- R 3 and R 4 are each independently a methyl or ethyl group or R 3 and R 4 optionally are combined to form a morpholino group;
- R 5 , R 6 , R 7 , and R 8 are each independently a hydrogen atom, a halogen atom, or an alkoxy group.
- X is absent
- NR 1 R 2 is N-methylpiperazine
- Y is NH
- L is —CH 2 CH 2 —
- R 3 and R 4 are each a methyl group
- R 6 and R 7 are each a methoxy group
- R 5 and R 9 are each a hydrogen atom.
- X is absent
- NR 1 R 2 is N-phenylpiperazine
- Y is NH
- L is —CH 2 CH 2 —
- R 3 and R 4 are each a methyl group
- R 6 and R 7 are each a methoxy group
- R 5 and R 8 are each a hydrogen atom.
- X is absent
- NR 1 R 2 is N-methylpiperazine
- Y is NH
- L is —CH 2 CH 2 ;
- R 3 and R 4 are combined as a morpholino group
- R 6 and R 7 are each a methoxy group
- R 5 and R 8 are each a hydrogen atom.
- composition is optionally in the form of a pharmaceutically acceptable hydrate or salt.
- quinazoline compositions of Formula XVIII of the invention are compounds 201-214, presented in Table 3. TABLE 3 Quinazoline Compositions of the Invention ID X NR 1 R 2 Y L R 3 R 4 R 5 R 6 R 7 R 8 201 NH —(CH 2 ) 2 — CH 3 CH 3 H H H H H 202 NH —(CH 2 ) 3 — CH 3 CH 3 H H H H H 203 NH —(CH 2 ) 4 — CH 3 CH 3 H H H H H H 204 NH —(CH 2 ) 5 — CH 3 CH 3 H H H H H H 205 NH —(CH 2 ) 2 — CH 3 CH 3 H H H H H H 206 NH —(CH 2 ) 2 — CH 2 CH 3 CH 2 CH 3 H H H H H 207 NH —(CH 2 ) 2 — CH 3 CH 3 H Cl H H H 208 NH —(CH 2 ) 2 — CH 3 CH 3 H H H H H 209
- the invention provides additional novel quinazoline compounds useful in the methods of the invention.
- Such compounds are referred to herein as CMZ 203-34, CMZ 203-44, CMZ 203-45, CMZ 203-49, CMZ 203-51, CMZ 203-76, CMZ 203-78, CMZ 203-87, CMZ 203-93, and CMZ 203-95. Syntheses for each of these novel compounds are provided in Examples 19-28, respectively.
- CMZ 203-34, CMZ 203-44, CMZ 203-45, CMZ 203-49, CMZ 203-51, CMZ 203-76, CMZ 203-78, CMZ 203-87, CMZ 202-93, and CMZ 203-95 are believed to be useful in the methods of the invention.
- the invention provides a method for inhibiting signaling by a TLR.
- the method according to this aspect of the invention involves contacting a cell expressing a functional TLR with an effective amount of quinazoline composition having structural Formula XIX wherein
- X is a substituted or unsubstituted aryl, alkyl, heterocyclic or styryl group, optionally attached to the quinazoline by a nitrogen, oxygen, or sulfur atom or by a SO or SO 2 group;
- Y is absent or is an oxygen atom, a sulfur atom, CR 9 R 10 , or NR 11 , where R 9 , R 10 , and R 11 are each independently a hydrogen atom or an alkyl, alkenyl, or aryl group, wherein any one of R 9 , R 10 , and R 11 optionally is combined with R 3 or R 4 to form a heterocycle;
- L is absent or is a hydrogen atom, an alkyl or alkenyl group containing from 1 to 10 carbons, or an aryl group;
- R 3 and R 4 are each independently a hydrogen atom or an alkyl, alkenyl, or aryl group, wherein R 3 and R 4 optionally are combined to form a heterocycle;
- R 5 , R 6 , R 7 , and R 8 are each independently a hydrogen atom, a halogen atom, or an alkyl, alkenyl, aryl, heterocyclic, nitro, cyano, carboxy, ester, ketone, amino, amido, hydroxy, alkoxy, mercapto, thio, sulfoxide, sulfone, or sulfonamido group, wherein any pair of R 5 , R 6 , R 7 , and R 8 which are adjacent one another optionally are combined to form a heterocycle or a carbocycle,
- the cell expressing the functional TLR can, but need not necessarily, be an immune cell.
- the cell expressing the functional TLR can be a cell transfected with an expression vector that directs expression of the TLR by the cell.
- the TLR is TLR9 and the method is thus a method for inhibiting intracellular signaling by TLR9.
- the invention provides a method for inhibiting signaling by a TLR.
- the method according to this aspect of the invention involves contacting an immune cell expressing a functional TLR with
- the quinazoline composition in this aspect of the invention can be in the form a hydrate or pharmaceutically acceptable salt.
- the method according to this aspect of the invention can be performed in vitro or it can be performed in vivo.
- the TLR is TLR9 and the TLR signal agonist is a TLR9 signal agonist, and the method in one embodiment is thus a method of inhibiting intracellular signaling by TLR9 in response to a TLR9 signal agonist.
- the TLR signal agonist in one embodiment is CpG DNA, for example a CpG ODN such as ODN 2006.
- the CpG ODN can belong to any class of CpG ODN, including A-class (e.g., ODN 2216), B-class (e.g., ODN 2006), or C-class (e.g., ODN 2395).
- the TLR signal agonist is an immune complex that includes a nucleic acid, as described above.
- the invention provides a method for inhibiting an immune response to an antigenic substance.
- the method according to this aspect of the invention involves contacting an immune cell expressing a functional Toll-like receptor with
- a quinazoline composition having structural Formula XIX, as defined above to inhibit an immune response to the antigenic substance compared with the immune response to the antigenic substance in absence of the quinazoline composition.
- the quinazoline composition in this aspect of the invention can be in the form a hydrate or pharmaceutically acceptable salt.
- the method according to this aspect of the invention can be performed in vitro or it can be performed in vivo.
- the immune response is an innate immune response.
- the immune response includes an adaptive immune response.
- the step of contacting an immune cell expressing a functional Toll-like receptor with an effective amount of an antigenic substance to stimulate an immune response to the antigenic substance in absence of a quinazoline composition involves the active step of administering an effective amount of an antigenic substance to stimulate an immune response to the antigenic substance in absence of a quinazoline composition.
- the antigenic substance can be administered using any effective route or means for administering the antigenic substance to effect the contacting.
- the administering can be by local or systemic injection, inhalation, oral ingestion, topical administration, mucosal administration, or any combination thereof.
- the method involves contacting, in a subject, an immune cell expressing a functional Toll-like receptor with
- the step of contacting an immune cell expressing a functional Toll-like receptor with an effective amount of an antigenic substance to stimulate an immune response to the antigenic substance in absence of a quinazoline composition is passive.
- the immune cell is already in contact or has already been in contact with an effective amount of an antigenic substance to stimulate an immune response to the antigenic substance, at the time of or prior to the step of contacting the immune cell expressing a functional Toll-like receptor with an effective amount of a quinazoline composition having structural Formula XVII, as defined above, to inhibit an immune response to the antigenic substance compared with the immune response to the antigenic substance in absence of the quinazoline composition.
- the antigenic substance can be an allergen, as described above.
- the antigenic substance can be an antigen that is or is derived from an infectious microbial agent, including a bacterium, a virus, a fungus, or a parasite, as described above.
- the antigenic substance can be a cancer antigen, as described above.
- a method of treating an autoimmune disorder in a subject involves the step of administering to a subject having an autoimmune disorder an effective amount of a quinazoline composition having structural Formula XIX, as defined above, to treat the autoimmune disorder.
- the quinazoline composition in this aspect of the invention can be in the form a hydrate or pharmaceutically acceptable salt.
- the autoimmune disorder is chosen from systemic lupus erythematosus, rheumatoid arthritis, inflammatory bowel disease, Sjögren's syndrome, polymyositis, vasculitis, Wegener's granulomatosis, sarcoidosis, ankylosing spondylitis, Reiter's syndrome, psoriatic arthritis, and Behçet's syndrome.
- the autoimmune disease is systemic lupus erythematosus.
- the autoimmune disease is rheumatoid arthritis.
- the subject is a human.
- the method can also be applied to animal models of any of the autoimmune disorders listed above.
- the autoimmune disorder is an immune complex associated disease, as described above.
- the quinazoline composition can be administered to the subject by any suitable route of administration, including, without limitation, oral and parenteral.
- Parenteral routes of administration are as described above with respect to substituted 4-primary amino quinolines.
- the methods of the invention can be assessed using any of a number of possible readout systems based upon a TLR/IL-1R signal transduction pathway.
- the readout for the method is based on the use of native genes or, alternatively, transfected or otherwise artificially introduced reporter gene constructs which are responsive to the TLR/IL-1R signal transduction pathway involving MyD88, TRAF, p38, and/or ERK. Häcker H et al. (1999) EMBO J 18:6973-82. These pathways activate kinases including KB kinase complex and c-Jun N-terminal kinases.
- reporter genes and reporter gene constructs particularly useful for the assays include, e.g., a reporter gene operatively linked to a promoter sensitive to NF- ⁇ B.
- promoters include, without limitation, those for NF- ⁇ B, IL-1 ⁇ , IL-6, IL-8, IL-12 p40, IP-10, CD80, CD86, and TNF- ⁇ .
- the reporter gene operatively linked to the TLR-sensitive promoter can include, without limitation, an enzyme (e.g., luciferase, alkaline phosphatase, ⁇ -galactosidase, chloramphenicol acetyltransferase (CAT), etc.), a bioluminescence marker (e.g., green-fluorescent protein (GFP, e.g., U.S. Pat. No. 5,491,084), blue fluorescent protein (BFP, e.g., U.S. Pat. No.
- an enzyme e.g., luciferase, alkaline phosphatase, ⁇ -galactosidase, chloramphenicol acetyltransferase (CAT), etc.
- CAT chloramphenicol acetyltransferase
- bioluminescence marker e.g., green-fluorescent protein (GFP, e.g., U.S. Pat. No.
- the reporter is selected from IL-8, TNF- ⁇ , NF- ⁇ B-luciferase (NF- ⁇ B-luc; hacker H et al. (1999) EMBO J 18:6973-82), IL-12 p40-luc (Murphy T L et al. (1995) Mol Cell Biol 15:5258-67), and TNF-luc (Humbleer H et al. (1999) EMBO J 18:6973-82).
- substrate can be supplied as part of the assay, and detection can involve measurement of chemiluminescence, fluorescence, color development, incorporation of radioactive label, drug resistance, or other marker of enzyme activity.
- detection can be accomplished using flow cytometry (FACS) analysis or functional assays.
- Secreted molecules can be assayed using enzyme-linked immunosorbent assay (ELISA) or bioassays. Many of these and other suitable readout systems are well known in the art and are commercially available.
- a cell expressing a functional TLR and useful for the methods of the invention has, in some embodiments, an expression vector including an isolated nucleic acid which encodes a reporter construct useful for detecting TLR signaling.
- the expression vector including an isolated nucleic acid which encodes a reporter construct useful for detecting TLR signaling can include a reporter gene under control of a promoter response element (enhancer element).
- the promoter response element is associated with a minimal promoter responsive to a transcription factor believed by the applicant to be activated as a consequence of TLR signaling. Examples of such minimal promoters include, without limitation, promoters for the following genes: AP-1, NF- ⁇ B, ATF2, IRF3, and IRF7.
- the expression vector including an isolated nucleic acid which encodes a reporter construct useful for detecting TLR signaling can include a gene under control of a promoter response element selected from response elements sensitive to IL-6, IL-8, IL-12 p40 subunit, a type I IFN, RANTES, TNF, IP-10, I-TAC, and interferon-stimulated response element (ISRE).
- the promoter response element generally will be present in multiple copies, e.g., as tandem repeats.
- coding sequence for luciferase is under control of an upstream 6 ⁇ tandem repeat of NF- ⁇ B response element.
- an ISRE-luciferase reporter construct useful in the invention is available from Stratagene (catalog no. 219092) and includes a 5 ⁇ ISRE tandem repeat joined to a TATA box upstream of a luciferase reporter gene.
- the reporter itself can be any gene product suitable for detection by methods recognized in the art. Such methods for detection can include, for example, measurement of spontaneous or stimulated light emission, enzyme activity, expression of a soluble molecule, expression of a cell surface molecule, etc.
- Readouts typically involve usual elements of Toll/IL-1R signaling, e.g., MyD88, TRAF, and IRAK molecules, although in the case of TLR3 the role of MyD88 is less clear than for other TLR family members.
- Such responses include the induction of a gene under control of a specific promoter such as a NF- ⁇ B promoter, increases in particular cytokine levels, increases in particular chemokine levels, etc.
- the gene under the control of the NF- ⁇ B promoter can be a gene which naturally includes an NF- ⁇ B promoter or it can be a gene in a construct in which an NF- ⁇ B promoter has been inserted.
- Genes and constructs which include the NF- ⁇ B promoter include but are not limited to IL-8, IL-12 p40, NF- ⁇ B-luc, IL-12 p40-luc, and TNF-luc.
- Cytokines generally include, without limitation, IL-1, IL-2, IL-3, IL-4, IL-5, IL-6, IL-7, IL-10, IL-11, IL-12, IL-13, IL-15, IL-18, IFN- ⁇ , IFN- ⁇ , IFN- ⁇ , TNF- ⁇ , GM-CSF, G-CSF, M-CSF.
- Th1 cytokines include but are not limited to IL-2, IFN- ⁇ , and IL-12.
- Th2 cytokines include but are not limited to IL-4, IL-5, and IL-10.
- Chemokines of particular significance in the invention include but are not limited to CCL5 (RANTES), CXCL9 (Mig), CXCL 10 (IP-10), and CXCL11 (1-TAC), IL-8, and MCP-1.
- compositions useful according to the methods of the present invention thus can be formulated in any manner suitable for pharmaceutical use.
- compositions of the invention are administered in pharmaceutically acceptable solutions, which may routinely contain pharmaceutically acceptable concentrations of salt, buffering agents, preservatives, compatible carriers, adjuvants, and optionally other therapeutic ingredients.
- an effective amount of the compound can be administered to a subject by any mode allowing the compound to be taken up by the appropriate target cells.
- administering the pharmaceutical composition of the present invention can be accomplished by any means known to the skilled artisan. Specific routes of administration include but are not limited to oral, transdermal (e.g., via a patch), parenteral injection (subcutaneous, intradermal, intramuscular, intravenous, intraperitoneal, intrathecal, etc.), or mucosal (intranasal, intratracheal, inhalation, intrarectal, intravaginal, etc.). An injection can be in a bolus or a continuous infusion.
- compositions according to the invention are often administered by intravenous, intramuscular, or other parenteral means, or by biolistic “gene-gun” application to the epidermis. They can also be administered by intranasal application, inhalation, topically, orally, or as implants, and even rectal or vaginal use is possible.
- Suitable liquid or solid pharmaceutical preparation forms are, for example, aqueous or saline solutions for injection or inhalation, microencapsulated, encochleated, coated onto microscopic gold particles, contained in liposomes, nebulized, aerosols, pellets for implantation into the skin, or dried onto a sharp object to be scratched into the skin.
- the pharmaceutical compositions also include granules, powders, tablets, coated tablets, (micro)capsules, suppositories, syrups, emulsions, suspensions, creams, drops or preparations with protracted release of active compounds, in whose preparation excipients and additives and/or auxiliaries such as disintegrants, binders, coating agents, swelling agents, lubricants, flavorings, sweeteners or solubilizers are customarily used as described above.
- the pharmaceutical compositions are suitable for use in a variety of drug delivery systems. For a brief review of present methods for drug delivery, see Langer R (1990) Science 249:1527-33, which is incorporated herein by reference.
- concentration of compounds included in compositions used in the methods of the invention can range from about 1 nM to about 100 ⁇ M. Effective doses are believed to range from about 10 picomole/kg to about 100 micromole/kg.
- the pharmaceutical compositions are preferably prepared and administered in dose units.
- Liquid dose units are vials or ampoules for injection or other parenteral administration.
- Solid dose units are tablets, capsules, powders, and suppositories.
- purpose of the administration i.e., prophylactic or therapeutic
- nature and severity of the disorder age and body weight of the patient, different doses may be necessary.
- the administration of a given dose can be carried out both by single administration in the form of an individual dose unit or else several smaller dose units. Repeated and multiple administration of doses at specific intervals of days, weeks, or months apart are also contemplated by the invention.
- compositions can be administered per se (neat) or in the form of a pharmaceutically acceptable salt.
- the salts should be pharmaceutically acceptable, but non-pharmaceutically acceptable salts can conveniently be used to prepare pharmaceutically acceptable salts thereof.
- Such salts include, but are not limited to, those prepared from the following acids: hydrochloric, hydrobromic, sulphuric, nitric, phosphoric, maleic, acetic, salicylic, p-toluene sulphonic, tartaric, citric, methane sulphonic, formic, malonic, succinic, naphthalene-2-sulphonic, and benzene sulphonic.
- such salts can be prepared as alkaline metal or alkaline earth salts, such as sodium, potassium or calcium salts of the carboxylic acid group.
- Suitable buffering agents include: acetic acid and a salt (1-2% w/v); citric acid and a salt (1-3% w/v); boric acid and a salt (0.5-2.5% w/v); and phosphoric acid and a salt (0.8-2% w/v).
- Suitable preservatives include benzalkonium chloride (0.003-0.03% w/v); chlorobutanol (0.3-0.9% w/v); parabens (0.01-0.25% w/v) and thimerosal (0.004-0.02% w/v).
- compositions of the invention optionally include an active ingredient and optionally a pharmaceutically-acceptable carrier.
- pharmaceutically-acceptable carrier means one or more compatible solid or liquid fillers, dilutants or encapsulating substances which are suitable for administration to a human or other vertebrate animal.
- carrier denotes an organic or inorganic ingredient, natural or synthetic, with which the active ingredient is combined to facilitate the application.
- the components of the pharmaceutical compositions also are capable of being comingled with the compounds of the present invention, and with each other, in a manner such that there is no interaction which would substantially impair the desired pharmaceutical efficiency.
- compositions suitable for parenteral administration conveniently include sterile aqueous preparations, which can be isotonic with the blood of the recipient.
- acceptable vehicles and solvents are water, Ringer's solution, phosphate buffered saline, and isotonic sodium chloride solution.
- sterile, fixed oils are conventionally employed as a solvent or suspending medium.
- any bland fixed mineral or non-mineral oil may be employed including synthetic mono- or diglycerides.
- fatty acids such as oleic acid find use in the preparation of injectables.
- Carrier formulations suitable for subcutaneous, intramuscular, intraperitoneal, intravenous, etc. administrations can be found in Remington's Pharmaceutical Sciences , Mack Publishing Company, Easton, Pa.
- the compounds useful in the invention can be delivered in mixtures of more than two such compounds.
- a mixture can further include one or more adjuvants in addition to the combination of compounds.
- a variety of administration routes is available. The particular mode selected will depend, of course, upon the particular compound selected, the age and general health status of the subject, the particular condition being treated, and the dosage required for therapeutic efficacy.
- the methods of this invention can be practiced using any mode of administration that is medically acceptable, meaning any mode that produces effective levels of response without causing clinically unacceptable adverse effects. Preferred modes of administration are discussed above.
- compositions can conveniently be presented in unit dosage form and can be prepared by any of the methods well known in the art of pharmacy. All methods include the step of bringing the compounds into association with a carrier which constitutes one or more accessory ingredients. In general, the compositions are prepared by uniformly and intimately bringing the compounds into association with a liquid carrier, a finely divided solid carrier, or both, and then, if necessary, shaping the product.
- Other delivery systems can include time-release, delayed release or sustained release delivery systems. Such systems can avoid repeated administrations of the compounds, increasing convenience to the subject and the physician.
- Many types of release delivery systems are available and known to those of ordinary skill in the art. They include polymer base systems such as poly(lactide-glycolide), copolyoxalates, polycaprolactones, polyesteramides, polyorthoesters, polyhydroxybutyric acid, and polyanhydrides. Microcapsules of the foregoing polymers containing drugs are described in, for example, U.S. Pat. No. 5,075,109.
- Delivery systems also include non-polymer systems that are: lipids including sterols such as cholesterol, cholesterol esters and fatty acids or neutral fats such as mono-di- and tri-glycerides; hydrogel release systems; silastic systems; peptide based systems; wax coatings; compressed tablets using conventional binders and excipients; partially fused implants; and the like.
- Specific examples include, but are not limited to: (a) erosional systems in which an agent of the invention is contained in a form within a matrix such as those described in U.S. Pat. Nos. 4,452,775, 4,675,189, and 5,736,152, and (b) diffusional systems in which an active component permeates at a controlled rate from a polymer such as described in U.S. Pat. Nos. 3,854,480, 5,133,974 and 5,407,686.
- pump-based hardware delivery systems can be used, some of which are adapted for implantation.
- Candidate small molecules for initial screening were obtained from a commercially available library of 880 off-patent small molecules selected for structural diversity and proven bioavailability in humans (Prestwick Chemical Library).
- Human embryonic kidney HEK293 cells stably transfected with a human TLR9 (hTLR9) expression vector were incubated overnight in the presence of 50 nM CpG ODN 2006 (i.e., the EC 50 concentration of ODN 2006) and selected small molecule candidate compounds at different concentrations ranging from 5 ⁇ 10 ⁇ 7 M to 5 ⁇ 10 ⁇ 5 M.
- TLR9 activity was assayed in terms of induction of a 6 ⁇ NF- ⁇ B-luciferase reporter construct cotransfected in the cells.
- Results were measured as fold induction over baseline luciferase activity measured in the absence of ODN 2006 and compared to fold induction over baseline in the presence of the EC 50 concentration of ODN 2006 alone. From this initial screening a number of small molecules were identified as lead compounds and were categorized by structure and activity. Additional screening was performed in like manner using additional small molecules selected from another library or specifically prepared for this purpose. Results of some of these assays are presented in FIG. 1 and in Tables 5-16.
- the method is described in greater detail as follows.
- the cells for each individual cell clone (hTLR3-NF ⁇ B-293, hTLR7-NF ⁇ B-293, hTLR8-NF ⁇ B-293 and hTLR9-NF ⁇ B-293) were counted and seeded one day before the stimulation at 1.5 ⁇ 10 4 cells per well in 96-well cell culture plates. To become adherent the cells were incubated after seeding overnight at 37° C. in 5% CO 2 humidified air.
- test compounds each assayed in duplicate at each of three different final concentrations (0.5 ⁇ g/ml, 5 ⁇ g/ml, and 50 ⁇ g/ml), were used for 16 h cell stimulation on a single multiwell culture plate.
- a 4 ⁇ final concentration master plate was created to prepare the different test compounds before they were added to the cells.
- Controls were added as duplicates individually for each cell clone on the cell culture plate. Positive controls were used as follows (final concentrations): 50 ⁇ g/ml poly(I:C) for hTLR3-NF ⁇ B-293; 8 ⁇ M resiquimod (R848) for hTLR7-NF ⁇ B-293; 30 ⁇ M R848 for hTLR8-NF ⁇ B-293; and 2.5 ⁇ M CpG-ODN 2006 for hTLR9-NF ⁇ B-293. Media alone was used as negative control. After addition of controls and small molecules, the cell clones were additionally stimulated with the EC 50 concentration of the appropriate receptor-specific ligand.
- wells H3 and H6 did not receive receptor-specific ligand.
- the mean value of wells H2 and H5 (each with cells and EC 50 concentration of the appropriate receptor-specific ligand) divided by the mean of wells H3 and H6 (each with cells alone) yielded the baseline response (i.e., fold induction of luciferase activity) to EC 50 concentration of the appropriate receptor-specific ligand.
- An agonist screen was performed in parallel with the antagonist screen just described. The procedure was comparable to the antagonist screen except there was no addition of EC 50 concentration of receptor-specific ligand. The outcome of this screen reflected agonistic and toxicity effects.
- the baseline result (measured with addition of the EC 50 concentration of the receptor-specific ligand) for each cell culture plate was measured as described above. Antagonistic effects were measured by down regulation compared to the baseline. Antagonists were scored as follows:
- the cells were treated in the above described manner but without adding the EC 50 concentration of receptor-specific ligand after treatment of the cells with small molecules.
- the baseline in the agonist screen is taken to be 1 because these cells have not been additionally stimulated with the EC 50 concentration of the receptor-specific ligand.
- Agonist activity was present when there was above-baseline activity. Agonists were scored as follows:
- ⁇ 2 compounds for which there was a 2- to 5-fold induction compared to baseline
- ⁇ 3 compounds for which there was a less than 2-fold induction compared to baseline.
- toxicity of the small molecules was performed by analysing below-baseline activity within the agonist screen.
- “Toxicity” can have different explanations—either differences between the cell clones, the compound are effecting the signal transduction pathway or the TLR within the cell clone, or the compounds do really have a toxic effect on the cells.
- each compound was screened for “toxicity” on each of the four cell lines. Usually the baseline in the agonist screen is taken to be 1 because these cells have not been additionally stimulated with the EC 50 concentration of the receptor-specific ligand. Apparent toxicity was scored as follows:
- T3 compounds for which there was clear down regulation at 0.5 ⁇ g/ml, 5 ⁇ g/ml, and 50 ⁇ g/ml;
- T2 compounds for which there was clear down regulation at 5 ⁇ g/ml and 50 ⁇ g/ml;
- T1 compounds for which there was clear down regulation only at 50 ⁇ g/ml.
- Candidate small molecules for initial screening were identified in an assay similar to that in Example 1 except HEK293 cells were stably transfected with a human TLR8 (hTLR8) expression vector and incubated overnight in the presence of 500 nM R848 (EC 50 of R848 for TLR8) and selected small molecule candidate compounds at different concentrations ranging from 5 ⁇ 10 ⁇ 7 M to 5 ⁇ 10 ⁇ 5 M. TLR8 activity was assayed in terms of induction of a 6 ⁇ NF- ⁇ B-luciferase reporter construct cotransfected in the cells. Results were expressed as fold induction over baseline luciferase activity measured in the absence of R848.
- hTLR8 human TLR8
- Candidate small molecules for initial screening were identified in an assay similar to that in Example 1 except HEK293 cells were stably transfected with a human TLR7 (hTLR7) expression vector and incubated overnight in the presence of 2 ⁇ M R848 (EC 50 of R848 for TLR7) and selected small molecule candidate compounds at different concentrations ranging from 5 ⁇ 10 ⁇ 7 M to 5 ⁇ 10 ⁇ 5 M. TLR7 activity was assayed in terms of induction of a 6 ⁇ NF- ⁇ B-luciferase reporter construct cotransfected in the cells. Results were expressed as fold induction over baseline luciferase activity measured in the absence of R848.
- Candidate small molecules for initial screening were identified in an assay similar to that in Example 1 except HEK293 cells were stably transfected with a human TLR3 (hTLR3) expression vector and incubated overnight in the presence of 2.5 ⁇ g/ml poly(I:C) (EC 50 of poly(I:C) for TLR3) and selected small molecule candidate compounds at different concentrations ranging from 5 ⁇ 10 ⁇ 7 M to 5 ⁇ 10 ⁇ 5 M. TLR3 activity was assayed in terms of induction of a 6 ⁇ NF- ⁇ B-luciferase reporter construct cotransfected in the cells. Results were expressed as fold induction over baseline luciferase activity measured in the absence of poly(I:C).
- mice are administered known amounts of candidate small molecules and a source of PAMP or other suitable TLR ligand, e.g., CpG nucleic acid.
- Negative control mice receive the source of PAMP or other suitable TLR ligand, e.g., CpG nucleic acid, alone.
- blood samples are obtained from control and experimental mice and evaluated for serum concentration of cytokine using a suitable method, e.g., enzyme-linked immunosorbent assay (ELISA).
- ELISA enzyme-linked immunosorbent assay
- PBMC peripheral blood mononuclear cells
- FACS fluorescence activated cell sorting
- Reduced expression of activation marker or reduced concentration of cytokine in an experimental animal compared to a negative control animal indicates the small molecule inhibits TLR-mediated signaling in response to a ligand for the TLR.
- Increased expression of activation marker or increased concentration of cytokine in an experimental animal compared to a negative control animal indicates the small molecule promotes TLR-mediated signaling in response to a ligand for the TLR.
- mice are administered candidate small molecules. Negative control mice are administered carrier alone. After administration or small molecule or carrier alone, PBMC are isolated and then exposed in vitro to CpG nucleic acid under conditions in which the PBMC, in the absence of small molecule, are stimulated to express an activation marker such as CD86 or secrete a product such as cytokine (e.g., IFN- ⁇ , IL-6, TNF- ⁇ ) or chemokine (e.g., IP-10). The expression of the activation marker or the secretion of the product is quantified using FACS, ELISA, or other suitable method, and comparison is made between results obtained with and without the small molecule.
- an activation marker such as CD86 or secrete a product
- cytokine e.g., IFN- ⁇ , IL-6, TNF- ⁇
- chemokine e.g., IP-10
- Reduced expression of activation marker or reduced concentration of cytokine in an experimental animal compared to a negative control animal indicates the small molecule inhibits TLR-mediated signaling in response to a ligand for the TLR.
- Increased expression of activation marker or increased concentration of cytokine in an experimental animal compared to a negative control animal indicates the small molecule promotes TLR-mediated signaling in response to a ligand for the TLR.
- hTLR9-NFkB-293 cells Stably co-transfecting the human TLR9 receptor (hTLR9) and an NF- ⁇ B promoter-driven luciferase gene into HEK-293 cells created the cell line hTLR9-NFkB-293.
- hTLR9-NFkB-293 cells were counted and seeded at 1.5 ⁇ 04 cells per well in 96-well cell culture plates one day before assaying and cultured at 37° C./5% CO 2 . The day of the assay, antagonist was added at 50 ⁇ M, 5.0 ⁇ M or 0.5 ⁇ M. The cells were then stimulated with the EC50 dose of the TLR9 agonist, CpG-ODN 2006, and cultured for 16 h in a humidified incubator at 37° C.
- the culture supernatant was removed and the cells were treated with lysis buffer and stored at ⁇ 80° C. before measuring luciferase activity.
- the luciferase readout was measured according to manufacturer's instructions using a luciferase assay system available from Promega, USA. Results are summarized in Table 18.
- the antagonist dose listed in Table 18 is the minimum effective dose ( ⁇ M) for blockade of the TLR9 agonist.
- the minimum effective dose ( ⁇ M) for blockade of the TLR9 agonist was 0.5 ⁇ M or 5 ⁇ M for all of the compounds tested, as measured by this three-point assay.
- hTLR9-NFkB-293 cells were counted and seeded at 1.5 ⁇ 10 4 cells per well in 96-well cell culture plates one day before assaying and cultured at 37° C./5% CO 2 , as described in Example 7.
- the day of the assay, antagonist was added at varying concentrations starting at 50 ⁇ M with a 1 ⁇ 5 to 1 ⁇ 6 dilution for 7 steps.
- the cells were then stimulated with the EC50 dose of the TLR9 agonist, CpG-ODN 2006, and cultured for 16 h in a humidified incubator at 37° C.
- the culture supernatant was removed and the cells were treated with lysis buffer and stored as ⁇ 80° C. before measuring luciferase activity as described in Example 7.
- Sigmoidal antagonism curves were generated and the inhibitor concentration at which 50% of the agonist response was blocked (IC50) calculated.
- Results shown in Table 19 under the heading hTLR9 are the IC50 dose ( ⁇ M) for blockade of a CpG ODN-generated signal in hTLR9-NFkB-293 cells.
- PBMC peripheral blood mononuclear cells
- the purified PBMC were resuspended in RPMI 1640 culture medium supplemented with 5% (v/v) heat-inactivated human AB serum (BioWhittaker, Belgium) or 10% (v/v) heat-inactivated fetal calf serum (FCS), 1.5 mM L-glutamine, 100 U/ml penicillin and 100 ⁇ g/ml streptomycin (all from Sigma).
- Fresh PBMC at a concentration of 3 ⁇ 10 6 /ml to 5 ⁇ 10 6 /ml were added to 96-well round-bottomed plates (150 ⁇ l/well). After cell plating, antagonist was added at varying concentrations starting at 50 ⁇ M with a 1 ⁇ 5 to 1 ⁇ 6 dilution for 7 steps.
- the cells were then stimulated with the TLR9 agonist, CpG-ODN 2006, and cultured for 16 h in a humidified incubator at 37° C. Culture supernatants were collected and, if not used immediately, frozen at ⁇ 20° C. until required. Interleukin 6 (IL-6) in the supernatants was quantitatively assessed using commercially available ELISA Kits (IL6, Diaclone, USA). Sigmoidal antagonism curves were generated and IC50 calculated. Results shown in Table 19 under the heading IL-6 are the IC50 dose ( ⁇ M) for blockade of CpG ODN-generated IL-6 production by human PBMC.
- CMZ 203-34 (201) N,N-dimethyl-N′- ⁇ 2-[4-(4-methyl- piperazin-1-yl)-phenyl]-3,4-dihydro- quinazoline-4-yl ⁇ -ethane-1,2,-diamine 0.003 0.05
- CMZ 203-78 N′-[6,7-Dimethoxy-2-(4-phenyl-piperzin- 1-yl)quinazolin-4-yl]-N,N-dimethyl- ethane-1,2-diamine 0.003 0.008
- CMZ 203-76 N′-[6,7-Dimethoxy-2-(4-methyl-piperzin- 1-yl)quinazolin-4-yl]-N,N-dimethyl- ethane-1,2-diamine 0.003 0.221
- Compound CMZ 203-43 has the structural formula
- Other mice received 10% dimethyl sulfoxide (DMSO) in an identical manner. Animals were weighed immediately prior to injection (day 1) and then daily until day 5. On day 5, blood was collected by cardiac puncture and analyzed for hematological and biochemical parameters. Animals were monitored daily for morbidity and mortality. Results are shown in FIG. 2 and FIG. 3 .
- FIG. 2A shows change in body weight (relative to body weight prior to first injection) ⁇ standard error of the means for different groups.
- FIG. 2B shows mean body weight ⁇ standard error of the means for different groups.
- Mice receiving 1.0 mg CMZ 203-43 had significant weight loss, while mice receiving quinazoline at either dose did not. All of the mice receiving 4.0 mg CMZ 203-43 group were dead on day 4. Necropsy of mice in the 4.0 mg CMZ 203-43 group revealed bowel obstruction.
- FIG. 3 shows white blood cell (WBC) differential as mean percentage of total white cells ⁇ standard error of the means for different groups.
- PBS sterile phosphate buffered saline
- mice were injected subcutaneously with 100 ⁇ g CpG ODN 2006.
- Animals were bled at 3 hr post injection with CpG ODN 2006 and IP-10 levels in plasma measured by IP-10-specific enzyme-linked immunosorbent assay (ELISA). Results are presented in FIG. 4 .
- the solid aminobenzophenone was extracted into ethyl acetate (200 mL) and the extracts were washed with brine. The ethyl acetate was evaporated under vacuum to give the product as a white solid in a yield of 7.0 gm, 33%.
- CMZ 203-91 The product was purified by chromatography on silica using 20% methanol in methylene chloride to elute the impurities. The product was then eluted with 20% methanol in methylene chloride containing 1% diethylamine. The yield of CMZ 203-91 was 0.58 gm, 77% as an oil which crystallized upon standing.
- hTLR9-NFkB-293 cells were counted and seeded at 1.5 ⁇ 10 4 cells per well in 96-well cell culture plates one day before assaying and cultured at 37° C./5% CO 2 , as described in Example 7.
- the day of the assay, antagonist was added at varying concentrations starting at 50 ⁇ M with a 1 ⁇ 5 to 1 ⁇ 6 dilution for 7 steps.
- the cells were then stimulated with the EC50 dose of the TLR9 agonist, CpG-ODN 2006, and cultured for 16 h in a humidified incubator at 37° C.
- the culture supernatant was removed and the cells were treated with lysis buffer and stored at ⁇ 80° C. before measuring luciferase activity as described in Example 7.
- the quinazolinone (2.4 gm, 7.5 ⁇ 10 ⁇ 3 moles) was added in portions with stirring to phosphorous oxychloride (10 mL). This mixture was warmed to 80° C. to give a red solution from which solid began to precipitate. The mixture was stirred at 80° C. for 15 minutes and was then heated briefly to reflux. Upon cooling the slurry was slowly added to a cold, stirred solution of 10% sodium carbonate (650 mL). After stirring for 15 minutes, the solid chloroquinazoline was extracted into chloroform (2 ⁇ 150 mL). The combined extracts were washed with brine and then dried over magnesium sulfate. The solution was filtered to remove the drying agent and the filtrates were stripped under vacuum to give a yellow solid.
- NMP N-methylpyrrolidinone
- 20 mL N-methylpyrrolidinone
- N,N-dimethylethylenediamine 1.32 gm, 0.015 moles
- the NMP solution was diluted with water (200 mL) and concentrated ammonium hydroxide (50 mL).
- the product, which had precipitated, was extracted into methylene chloride (2 ⁇ 100 mL).
- the extracts were combined, dried over magnesium sulfate and then, after filtration, were stripped to give the product as a light brown oil. This was purified by chromatography on silica using 10% methanol in methylene chloride as eluent.
- N,N-dimethylethylenediamine (3.5 gm, 0.04 moles) and 2-phenyl-4-chloroquinazoline (2.4 gm, 0.01 moles) were combined in n-butanol (50 mL) and were brought to reflux. Once reflux had been achieved, TLC (silica, 10% methanol in dichloromethane) showed that the reaction was complete. The solution was cooled and stripped. The residue was dissolved in t-butylmethyl ether (TBME) and the solution was washed with water. After drying over magnesium sulfate the solution was filtered and stripped to give 1.37 gm (0.0047 moles, 47%) of the crude product as an oil.
- TBME t-butylmethyl ether
- the product was extracted into methylene chloride (3 ⁇ 50 mL). After drying over magnesium sulfate the solution was filtered and stripped to give the crude product as an oil. This was dissolved in ethanol (20 mL) and was treated with a solution of concentrated sulfuric acid (0.85 gm, 0.0087 moles) dissolved in ethanol (10 mL). The crystalline sulfate salt separated slowly and after 60 minutes at room temperature was isolated by filtration. The salt washed with ethanol and dried. The yield was 1.37 gm, (40% based on the sulfuric acid).
- the quinazolinone (3.2 gm, 0.01 moles) was added in portions with stirring to phosphorous oxychloride (10 mL). This mixture was refluxed for 30 minutes. The orange slurry was cooled and TBME (100 mL) was added. After stirring for 10 minutes the chloroquinazoline was isolated by filtration and added to a solution of the sodium salt of N,N-dimethylaminoethanol in NMP (50 mL). The solution of the sodium salt of N,N-dimethylaminoethanol in NMP was prepared as follows.
- Phosphorous oxychloride (12 mL) and the biphenylquinazoline (2.98 gm, 0.01 moles) were stirred together and refluxed for one hour.
- the excess phosphorous oxychloride was removed by distillation and the oily yellow residue was placed under aspirator vacuum to remove traces of phosphorous oxychloride.
- the resulting yellow solid was triturated in hexane which was removed by decantation.
- n-butanol 50 mL
- N,N-dimethylethylenediamine 6 mL
- the 4-chloroquinazoline (3.7 gm, 0.015 moles) was refluxed in ethanol with N-2-aminoethylmorpholine (3.99 gm, 0.031 moles) for one hour. Once cooled, the solution was stripped and the residue was dissolved in water (400 mL). The solution was made basic by the addition of sodium carbonate and the product was extracted into methylene chloride (2 ⁇ 100 mL). The combined extracts were washed with water (50 mL) and were then dried over magnesium sulfate. After filtration, the extracts were stripped to give the product as a tan solid. The solid was recrystallized from ethyl acetate (25 mL) and hexane (50 mL). The product, 203-93 was isolated as an off white solid in a yield of 2.86 gm, 56.0%.
Abstract
The invention provides methods and compositions useful for modulating signaling through Toll-like receptors. The methods involve contacting a TLR-expressing cell with a small molecule having a core structure including at least two rings. Certain of the compounds are 4-primary amino quinolines. Many of the compounds and methods are useful specifically for inhibiting immune stimulation involving at least one of TLR9, TLR8, TLR7, and TLR3. The methods may have use in the treatment of autoimmunity, inflammation, allergy, asthma, graft rejection, graft versus host disease, infection, sepsis, cancer, and immunodeficiency.
Description
- This application claims priority under 35 U.S.C. § 119(e) to U.S. provisional patent application Ser. No. 60/556,007, filed Mar. 23, 2004, and U.S. provisional patent application Ser. No. 60/480,588, filed Jun. 20, 2003, the entire contents of both of which are incorporated herein by reference.
- The invention relates to generally to the field of immunology. More particularly, the invention relates to compositions and methods for altering immune function. More specifically, the invention relates to compositions and methods for affecting immune stimulation mediated through Toll-like receptor (TLR) molecules.
- Stimulation of the immune system, which includes stimulation of either or both innate immunity and adaptive immunity, is a complex phenomenon that can result in either protective or adverse physiologic outcomes for the host. In recent years there has been increased interest in the mechanisms underlying innate immunity, which is believed to initiate and support adaptive immunity. This interest has been fueled in part by the recent discovery of a family of highly conserved pattern recognition receptor proteins known as Toll-like receptors (TLRs) believed to be involved in innate immunity as receptors for pathogen-associated molecular patterns (PAMPs). Compositions and methods useful for modulating innate immunity are therefore of great interest, as they may affect therapeutic approaches to conditions involving autoimmunity, inflammation, allergy, asthma, graft rejection, graft versus host disease (GvHD), infection, cancer, and immunodeficiency.
- Recently there have been a number of reports describing the immunostimulatory effect of certain types of nucleic acid molecules, including CpG nucleic acids and double-stranded RNA. Of note, it was recently reported that Toll-like receptor 9 (TLR9) recognizes bacterial DNA and CpG DNA. Hemmi H et al. (2000) Nature 408:740-5; Bauer S et al. (2001) Proc Natl Acad Sci USA 98:9237-42. It was also recently reported that immune complexes containing IgG and nucleic acid can stimulate TLR9 and participate in B-cell activation in certain autoimmune diseases. Leadbetter E A et al. (2002) Nature 416:595-8.
- Chloroquines have been recognized as useful not only as anti-malarial agents but also as anti-inflammatory agents. Although its mechanism of action is not well understood, chloroquine has been used effectively in the treatment of various autoimmune diseases, including rheumatoid arthritis (RA) and systemic lupus erythematosus (SLE). For a review, see Wallace D J (1996) Lupus 5 Suppl 1:S59-64. Recently Macfarlane and colleagues described a number of small molecule analogs and derivatives of chloroquine (4-aminoquinoline) and quinacrine (9-aminoacridine) which reportedly inhibit stimulation of the immune system. U.S. Pat. No. 6,221,882; U.S. Pat. No. 6,479,504; U.S. Pat. No. 6,521,637; PCT published application PCT/US00/16723 (WO 00/76982); and PCT published application PCT/US98/13820 (WO 99/01154). Macfarlane and colleagues report these small molecule inhibitors of the immune response, even when used at nanomolar concentrations, can block the action of immunostimulatory DNA. U.S. Pat. No. 6,221,882 B1. Macfarlane and coworkers studied a large number of compounds by varying substituents on a limited number of 4-aminoquinoline and 9-aminoacridine core structures related to chloroquine and quinacrine.
- The present invention is based, in part, on the discovery by the applicants that a number of small molecules, some previously known but distinct from those described by Macfarlane et al. and by Tobe et al., can alter TLR-mediated immunostimulatory signaling. Many of the compounds inhibit TLR signaling and are useful as inhibitors of immune stimulation. Compositions and methods described herein are useful for inhibiting immune stimulation in vitro and in vivo. Such compositions and methods thus will find use in a number of clinical applications, including as pharmaceutical agents and methods for treating conditions involving unwanted immune activity, including inflammatory and autoimmune disorders. The compositions of the invention can also be used in methods for the preparation of medicaments for use in the treatment of conditions involving unwanted immune activity, including a variety of inflammatory and autoimmune disorders.
- It was surprisingly discovered that molecules having similar substituents but different core structures compared to those related to chloroquine and quinacrine described by Macfarlane et al., are potent immunomodulatory small molecules. Without being bound to any theory or mechanism, it is believed that the small molecules described by the present invention affect immune stimulation via interaction with a TLR. More particularly, it is believed that many of the small molecules described by the present invention inhibit immune stimulation via TLR antagonism. In particular, it is believed that many of the small molecules described by the present invention inhibit immune stimulation via TLR9 antagonism.
- The invention in certain aspects provides methods useful for altering TLR-mediated signaling. The methods of the invention are useful whenever it is desirable to alter TLR-mediated signaling in response to a suitable TLR ligand or TLR signaling agonist. For example, the methods can be used to treat any of a variety of conditions involving autoimmunity, inflammation, allergy, asthma, graft rejection, graft-versus-host disease (GvHD), infection, sepsis, cancer, and immunodeficiency. Generally, methods useful in the treatment of conditions involving autoimmunity, inflammation, allergy, asthma, graft rejection, and GvHD will employ small molecules that inhibit TLR-mediated signaling in response to a suitable TLR ligand or TLR signaling agonist. Generally, methods useful in the treatment of conditions involving infection, cancer, and immunodeficiency will employ small molecules that augment TLR-mediated signaling in response to a suitable TLR ligand. In some instances the methods can be used to inhibit or promote TLR-mediated signaling in response to a TLR ligand or TLR signaling agonist. In some instances the methods can be used to inhibit TLR-mediated immunostimulatory signaling in response to a TLR ligand or TLR signaling agonist. In some instances the methods can be used to inhibit or promote TLR-mediated immunostimulation in a subject. In some instances the methods can be used to inhibit TLR-mediated immunostimulation in a subject. In some instances the methods can be used to inhibit an immunostimulatory nucleic acid-associated response in a subject.
- The invention in certain aspects provides novel small molecule compositions useful for altering TLR-mediated signaling. The compositions of the invention are useful whenever it is desirable to alter TLR-mediated signaling in response to a suitable TLR ligand or TLR signaling agonist. For example, the small molecules can be used in methods to treat any of a variety of conditions involving autoimmunity, inflammation, allergy, asthma, graft rejection, GvHD, infection, sepsis, cancer, and immunodeficiency. Generally, methods useful in the treatment of conditions involving autoimmunity, inflammation, allergy, asthma, graft rejection, and GvHD will employ small molecules that inhibit TLR-mediated signaling in response to a suitable TLR ligand or TLR signaling agonist. Generally, methods useful in the treatment of conditions involving infection, cancer, and immunodeficiency will employ small molecules that augment TLR-mediated signaling in response to a suitable TLR ligand. In some instances the molecules can be used in a method to inhibit or promote TLR-mediated signaling in response to a TLR ligand or TLR signaling agonist. In some instances the small molecules can be used in a method to inhibit TLR-mediated immunostimulatory signaling in response to a TLR ligand or TLR signaling agonist. In some instances the small molecules can be used in a method to inhibit or promote TLR-mediated immunostimulation in a subject. In some instances the small molecules can be used in a method to inhibit TLR-mediated immunostimulation in a subject. In some instances the small molecules can be used to inhibit an immunostimulatory nucleic acid-associated response in a subject.
- As a feature of the present invention, the methods of the invention can be combined with administration of additional agents to achieve synergistic effect on TLR-mediated immunostimulation. More specifically, whereas the agents described herein have been discovered to affect TLRs directly and thus directly affect TLR-bearing cells, e.g., antigen-presenting cells (APCs), such agents can be used in conjunction with additional agents which affect non-APC immune cells, e.g., T lymphocytes (T cells). Such an approach effectively introduces an immunomodulatory intervention at two levels: innate immunity and acquired immunity. Since innate immunity is believed to initiate and support acquired immunity, the combination intervention is synergistic.
- In one aspect of the invention, a method of affecting TLR-mediated signaling in response to a TLR ligand is provided. The method according to this aspect involves contacting a cell expressing a TLR with an effective amount of a compound of Formula I
wherein 2 is a five- to seven-membered homocyclic or heterocyclic ring, wherein each of X, Y, and Z is independently chosen from a carbon atom (C), a nitrogen atom (N), an oxygen atom (O), and a sulfur atom (S), and wherein B2 optionally includes at least one atom selected from C, N, O, and S; wherein 1 and 2 are optionally bridged by B3 to form a five- to seven-membered ring (3), wherein B3 optionally includes at least one atom selected from C, N, O, and S, and wherein when A is carbon, (3) is not pyridine; wherein 2 optionally includes an unsaturated bond; wherein (3) optionally includes an unsaturated bond; wherein R2, when present, is a hydrogen atom, a substituted or unsubstituted lower alkyl, aryl, aralkyl, heterocyclic, or alkylheterocyclic group, optionally linked to Z via N, O, or S; wherein R3, R4, R5, R6, R7, and R8, when present, are each independently a hydrogen atom, a halogen atom, a substituted or unsubstituted lower alkyl, aryl, aralkyl, heterocyclic, or alkylheterocyclic group, each optionally linked via N, O, or S; wherein A is an atom selected from C, N, O, and S; wherein each of A′, A″, and A′″ independently is R9, —NR9R10, —OR9, or —CR9R10, wherein R9 is a hydrogen atom, hydroxyl, substituted or unsubstituted lower alkyl, aryl, aralkyl, heterocyclic, or alkylheterocyclic group, and wherein R10 is optionally absent or is a hydrogen atom, lower alkyl, aryl, aralkyl, heterocyclic, or alkylheterocyclic group, to inhibit or promote TLR-mediated signaling in response to a ligand for the TLR. - In one aspect of the invention, a method of inhibiting TLR-mediated immunostimulatory signaling is provided. The method according to this aspect of the invention involves contacting a cell expressing a TLR with an effective amount of a compound of Formula I, as provided above, to inhibit TLR-mediated immunostimulatory signaling in response to a ligand for the TLR.
- In one aspect the invention provides a method of affecting TLR-mediated immunostimulation in a subject. The method according to this aspect of the invention involves administering to a subject having or at risk of developing TLR-mediated immunostimulation an effective amount of a compound of Formula I, as provided above, to inhibit or promote TLR-mediated immunostimulation in the subject.
- The invention in one aspect provides a method of inhibiting TLR-mediated immunostimulation in a subject. The method according to this aspect of the invention involves administering to a subject having or at risk of developing TLR-mediated immunostimulation an effective amount of a compound of Formula I, as provided above, to inhibit TLR-mediated immunostimulation in the subject.
- In one aspect the invention provides a method of inhibiting an immunostimulatory nucleic acid-associated response in a subject. The method according to this aspect of the invention involves administering to a subject in need of such treatment an effective amount of a compound of Formula I, as provided above, to inhibit an immunostimulatory nucleic acid-associated response in the subject.
- In one embodiment the subject is otherwise free of symptoms calling for treatment with a compound of Formula I.
- In one embodiment according to any of the foregoing aspects of the invention, A is nitrogen and (3) is a five-membered ring. In one embodiment the compound is Formula II
wherein A is chosen from C and N; and R1 is a hydrogen atom, a halogen atom, a substituted or unsubstituted lower alkyl, aryl, aralkyl, heterocyclic, or alkylheterocyclic group, each optionally linked via N, O, or S. In various specific embodiments the compound is any one of compounds 455, 470, 564, 568, 593, 607, 612-614, 619-621, 636, 685, 875, 878, 890, 904, 918, 939, 944, 1039, 1050, 1132, 1241, and 1243 listed in Table 5 below. - In one embodiment according to any of the foregoing aspects of the invention, A is nitrogen, (3) is a six-membered ring, and B3 is one atom selected from C, N, O, and S. In one embodiment A is nitrogen, 3 is a six-membered ring, and B3 is S. In one embodiment the compound is Formula III
wherein R1 is a hydrogen atom, a halogen atom, a substituted or unsubstituted lower alkyl, aryl, aralkyl, heterocyclic, or alkylheterocyclic group, each optionally linked via N, O, or S, and wherein X is chosen from N, O, and S. In certain embodiments X is S. In various specific embodiments the compound is any one of compounds 53, 64, 125, 149, 313, 399, 529, 576, 693, 797, 840, and 842 listed in Table 6 below. - In one embodiment according to any of the foregoing aspects of the invention, A is carbon, (3) is a six-membered ring, and B3 is C or S. In one embodiment the compound is Formula IV
wherein X is C or S and R1 is a hydrogen atom, a halogen atom, a substituted or unsubstituted lower alkyl, aryl, aralkyl, heterocyclic, or alkylheterocyclic group, each optionally linked via N, O, or S. In various specific embodiments the compound is any one of compounds 43, 294, 340, 346, 348, 413, 491, 917, 1015, 1042, 1158, 1287, and 1337 listed in Table 7 below. - In one embodiment according to any of the foregoing aspects of the invention, A is nitrogen, (3) is a seven-membered ring, and B3 includes two carbon atoms. In one embodiment the compound is Formula V
wherein X and Y are each independently C, N, O, or S; and R1 is a hydrogen atom, a halogen atom, a substituted or unsubstituted lower alkyl, aryl, aralkyl, heterocyclic, or alkylheterocyclic group, each optionally linked via N, O, or S. In various specific embodiments the compound is any one of compounds 72, 74, 99, 109, 343, 488, 1013, and 1352 listed in Table 8 below. - In one embodiment according to any of the foregoing aspects of the invention, 1 and 2 are unbridged by B3, A is carbon, and A′ is —OR9. In one embodiment the compound is Formula VI
wherein R1 is a hydrogen atom, a halogen atom, a substituted or unsubstituted lower alkyl, aryl, aralkyl, heterocyclic, or alkylheterocyclic group, each optionally linked via N, O, or S; and R9 is a substituted or unsubstituted lower alkyl, aryl, aralkyl, heterocyclic, or alkylheterocyclic group. In some embodiments R9 and R4 are united to form a lactone. In various specific embodiments the compound is any one of compounds 65, 229, 239, 306, 386, 707, 793, 957, 970, 974, 1161, and 1308 listed in Table 9 below. - In one embodiment according to any of the foregoing aspects of the invention, 1 and 2 are unbridged by B3, A is carbon, and A′ is —NR9R10. In one embodiment the compound is Formula VII
wherein R1 is a hydrogen atom, a halogen atom, a substituted or unsubstituted lower alkyl, aryl, aralkyl, heterocyclic, or alkylheterocyclic group, each optionally linked via N, O, or S. In various specific embodiments the compound is any one of compounds 133, 267, 312, 457, and 510 listed in Table 10 below. - In one embodiment according to any of the foregoing aspects of the invention, 1 and 2 are unbridged by B3, A is carbon, and A′ is —CR9R10. In one embodiment the compound is Formula VIII
wherein R1 is a hydrogen atom, a halogen atom, a substituted or unsubstituted lower alkyl, aryl, aralkyl, heterocyclic, or alkylheterocyclic group, each optionally linked via N, O, or S. In various specific embodiments the compound is any one of compounds 93, 108, 138, 144, 267, 270, 308, 381, 560, 778, 799, 822, 833, 884, 965, and 1187 listed in Table 11 below. - In one aspect the invention provides a method of affecting TLR-mediated signaling in response to a TLR ligand. The method according to this aspect of the invention involves contacting a cell expressing a TLR with an effective amount of a compound of Formula IX
wherein R2 is a substituted or unsubstituted lower alkyl, aryl, aralkyl, heterocyclic, or alkylheterocyclic group, each optionally linked via N, O, or S; wherein R5, R6, R7, and R8 are each independently a hydrogen atom, a halogen atom, a substituted or unsubstituted lower alkyl, aryl, aralkyl, heterocyclic, or alkylheterocyclic group, each optionally linked via N, O, or S; wherein R9 is a hydrogen atom, substituted or unsubstituted lower alkyl, aryl, aralkyl, heterocyclic, or alkylheterocyclic group; and wherein R10 is a hydrogen atom, lower alkyl, aryl, aralkyl, heterocyclic, or alkylheterocyclic group, to inhibit or promote TLR-mediated signaling in response to a ligand for the TLR. In various specific embodiments the compound is any one of compounds 751, 858, 2000, and 2001 listed in Table 12 below. - In one aspect of the invention, a method of inhibiting TLR-mediated immunostimulatory signaling is provided. The method according to this aspect of the invention involves contacting a cell expressing a TLR with an effective amount of a compound of Formula IX, as provided above, to inhibit TLR-mediated immunostimulatory signaling in response to a ligand for the TLR.
- In one aspect the invention provides a method of affecting TLR-mediated immunostimulation in a subject. The method according to this aspect of the invention involves administering to a subject having or at risk of developing TLR-mediated immunostimulation an effective amount of a compound of Formula IX, as provided above, to inhibit or promote TLR-mediated immunostimulation in the subject.
- The invention in one aspect provides a method of inhibiting TLR-mediated immunostimulation in a subject. The method according to this aspect of the invention involves administering to a subject having or at risk of developing TLR-mediated immunostimulation an effective amount of a compound of Formula IX, as provided above, to inhibit TLR-mediated immunostimulation in the subject.
- In one aspect the invention provides a method of inhibiting an immunostimulatory nucleic acid-associated response in a subject. The method according to this aspect of the invention involves administering to a subject in need of such treatment an effective amount of a compound of Formula IX, as provided above, to inhibit an immunostimulatory nucleic acid-associated response in the subject.
- In one embodiment the subject is otherwise free of symptoms calling for treatment with a compound of Formula IX.
- In one aspect the invention provides a method of affecting TLR-mediated signaling in response to a TLR ligand. The method according to this aspect of the invention involves contacting a cell expressing a TLR with an effective amount of a compound of Formula X
wherein each of R1, R2, R3, R4, R5, R6, R7, and R8 is independently a hydrogen atom, a halogen atom, a substituted or unsubstituted lower alkyl, aryl, aralkyl, heterocyclic, or alkylheterocyclic group, each optionally linked via N, O, or S, to inhibit or promote TLR-mediated signaling in response to a ligand for the TLR. In various specific embodiments the compound is any one of compounds 431, 583, 586, 792, and 830 listed in Table 13 below. - In one aspect of the invention, a method of inhibiting TLR-mediated immunostimulatory signaling is provided. The method according to this aspect of the invention involves contacting a cell expressing a TLR with an effective amount of a compound of Formula X, as provided above, to inhibit TLR-mediated immunostimulatory signaling in response to a ligand for the TLR.
- In one aspect the invention provides a method of affecting TLR-mediated immunostimulation in a subject. The method according to this aspect of the invention involves administering to a subject having or at risk of developing TLR-mediated immunostimulation an effective amount of a compound of Formula X, as provided above, to inhibit or promote TLR-mediated immunostimulation in the subject.
- The invention in one aspect provides a method of inhibiting TLR-mediated immunostimulation in a subject. The method according to this aspect of the invention involves administering to a subject having or at risk of developing TLR-mediated immunostimulation an effective amount of a compound of Formula X, as provided above, to inhibit TLR-mediated immunostimulation in the subject.
- In one aspect the invention provides a method of inhibiting an immunostimulatory nucleic acid-associated response in a subject. The method according to this aspect of the invention involves administering to a subject in need of such treatment an effective amount of a compound of Formula X, as provided above, to inhibit an immunostimulatory nucleic acid-associated response in the subject.
- In one embodiment the subject is otherwise free of symptoms calling for treatment with a compound of Formula X.
- In one aspect the invention provides a method of affecting TLR-mediated signaling in response to a TLR ligand. The method according to this aspect of the invention involves contacting a cell expressing a TLR with an effective amount of a compound of Formula XI
wherein each of R1, R2, R5, R6, R7, R8, R11, and R12 is a hydrogen atom, a halogen atom, a substituted or unsubstituted lower alkyl, aryl, aralkyl, heterocyclic, or alkylheterocyclic group, each optionally linked via N, O, or S; and X is C, N, O, or S, to inhibit or promote TLR-mediated signaling in response to a ligand for the TLR. In some embodiments R2 includes a cycloalkyl, benzyl or phenyl group. In some embodiments X is N. In some embodiments R11 and R12 are linked as a spiro group in a five- or six-membered ring. In various specific embodiments the compound is any one of compounds 891, 926, 1137, 1213, 1248, 1320, and 1322 listed in Table 14 below. - In one aspect of the invention, a method of inhibiting TLR-mediated immunostimulatory signaling is provided. The method according to this aspect of the invention involves contacting a cell expressing a TLR with an effective amount of a compound of Formula XI, as provided above, to inhibit TLR-mediated immunostimulatory signaling in response to a ligand for the TLR.
- In one aspect the invention provides a method of affecting TLR-mediated immunostimulation in a subject. The method according to this aspect of the invention involves administering to a subject having or at risk of developing TLR-mediated immunostimulation an effective amount of a compound of Formula XI, as provided above, to inhibit or promote TLR-mediated immunostimulation in the subject.
- The invention in one aspect provides a method of inhibiting TLR-mediated immunostimulation in a subject. The method according to this aspect of the invention involves administering to a subject having or at risk of developing TLR-mediated immunostimulation an effective amount of a compound of Formula XI, as provided above, to inhibit TLR-mediated immunostimulation in the subject.
- In one aspect the invention provides a method of inhibiting an immunostimulatory nucleic acid-associated response in a subject. The method according to this aspect of the invention involves administering to a subject in need of such treatment an effective amount of a compound of Formula XI, as provided above, to inhibit an immunostimulatory nucleic acid-associated response in the subject.
- In one embodiment the subject is otherwise free of symptoms calling for treatment with a compound of Formula XI.
- In one aspect the invention provides a method of affecting TLR-mediated signaling in response to a TLR ligand. The method according to this aspect of the invention involves contacting a cell expressing a TLR with an effective amount of a compound of Formula XII
wherein R1, R2, R3, R4, R5, R6, R7, R8, R9, R10, and R11 are each independently a hydrogen atom, a halogen atom, a substituted or unsubstituted lower alkyl, aryl, aralkyl, heterocyclic, or alkylheterocyclic group, each optionally linked via N, O, or S, to inhibit or promote TLR-mediated signaling in response to a ligand for the TLR. In various specific embodiments the compound is any one ofcompounds 101, 600, and 609 listed in Table 15 below. - In one aspect of the invention, a method of inhibiting TLR-mediated immunostimulatory signaling is provided. The method according to this aspect of the invention involves contacting a cell expressing a TLR with an effective amount of a compound of Formula XII, as provided above, to inhibit TLR-mediated immunostimulatory signaling in response to a ligand for the TLR.
- In one aspect the invention provides a method of affecting TLR-mediated immunostimulation in a subject. The method according to this aspect of the invention involves administering to a subject having or at risk of developing TLR-mediated immunostimulation an effective amount of a compound of Formula XII, as provided above, to inhibit or promote TLR-mediated immunostimulation in the subject.
- The invention in one aspect provides a method of inhibiting TLR-mediated immunostimulation in a subject. The method according to this aspect of the invention involves administering to a subject having or at risk of developing TLR-mediated immunostimulation an effective amount of a compound of Formula XII, as provided above, to inhibit TLR-mediated immunostimulation in the subject.
- In one aspect the invention provides a method of inhibiting an immunostimulatory nucleic acid-associated response in a subject. The method according to this aspect of the invention involves administering to a subject in need of such treatment an effective amount of a compound of Formula XII, as provided above, to inhibit an immunostimulatory nucleic acid-associated response in the subject.
- In one embodiment the subject is otherwise free of symptoms calling for treatment with a compound of Formula XII.
- In one aspect the invention provides a method of affecting TLR-mediated signaling in response to a TLR ligand. The method according to this aspect of the invention involves contacting a cell expressing a TLR with an effective amount of a compound of Formula XIII
wherein 2′ is a five- to seven-membered heterocyclic ring, wherein each of X, Y, and Z is independently chosen from C, N, O, and S, and wherein B2′ optionally includes at least one atom selected from C, N, O, and S; wherein 2′ optionally includes an unsaturated bond; wherein R4, R5, R6, R7, and R8 are each independently a hydrogen atom, a halogen atom, a substituted or unsubstituted lower alkyl, aryl, aralkyl, heterocyclic, or alkylheterocyclic group, each optionally linked via N, O, or S; wherein R9 is a hydrogen atom, substituted or unsubstituted lower alkyl, aryl, aralkyl, heterocyclic, or alkylheterocyclic group; wherein R10 is a hydrogen atom, lower alkyl, aryl, aralkyl, heterocyclic, or alkylheterocyclic group; and wherein A″ is hydrogen, a substituted or unsubstituted lower alkyl, aryl, aralkyl, heterocyclic, or alkylheterocyclic group, to inhibit or promote TLR-mediated signaling in response to a ligand for the TLR. In various specific embodiments the compound is any one of compounds 990, 1003, 1091, 1142, 1185, 1212, 1217, 1224, 1244, and 1334 listed in Table 16 below. - In one aspect of the invention, a method of inhibiting TLR-mediated immunostimulatory signaling is provided. The method according to this aspect of the invention involves contacting a cell expressing a TLR with an effective amount of a compound of Formula XIII, as provided above, to inhibit TLR-mediated immunostimulatory signaling in response to a ligand for the TLR.
- In one aspect the invention provides a method of affecting TLR-mediated immunostimulation in a subject. The method according to this aspect of the invention involves administering to a subject having or at risk of developing TLR-mediated immunostimulation an effective amount of a compound of Formula XIII, as provided above, to inhibit or promote TLR-mediated immunostimulation in the subject.
- The invention in one aspect provides a method of inhibiting TLR-mediated immunostimulation in a subject. The method according to this aspect of the invention involves administering to a subject having or at risk of developing TLR-mediated immunostimulation an effective amount of a compound of Formula XIII, as provided above, to inhibit TLR-mediated immunostimulation in the subject.
- In one aspect the invention provides a method of inhibiting an immunostimulatory nucleic acid-associated response in a subject. The method according to this aspect of the invention involves administering to a subject in need of such treatment an effective amount of a compound of Formula XIII, as provided above, to inhibit an immunostimulatory nucleic acid-associated response in the subject.
- In each of the foregoing aspects involving a compound of Formula XIII, in one embodiment A″ is a substituted alkyl group selected from the group consisting of a cyclic amino group, an alkylamino group, a dialkylamino group, furyl, phenyl, thienyl, azabicyclooctyl, azabicycloheptyl, and any combination thereof. In one embodiment the cyclic amino group is a piperazino group, a piperidino group, a pyrrolidino group, an imidazolyl group, a pyridyl group, or a morpholino group.
- In one embodiment the subject is otherwise free of symptoms calling for treatment with a compound of Formula XIII.
- In one aspect the invention provides a method of affecting TLR-mediated signaling in response to a TLR ligand. The method according to this aspect of the invention involves contacting a cell expressing a TLR with an effective amount of a compound of Formula XIV or Formula XV
wherein R1, R2, R3, R5, R6, R7, and R8 are each independently a hydrogen atom, a halogen atom, a substituted or unsubstituted lower alkyl, aryl, aralkyl, heterocyclic, or alkylheterocyclic group, each optionally linked via N, O, or S; and wherein R9 is a hydrogen atom, substituted or unsubstituted lower alkyl, aryl, aralkyl, heterocyclic, or alkylheterocyclic group, to inhibit or promote TLR-mediated signaling in response to a ligand for the TLR. In various specific embodiments the compound is any one of compounds 807, 1163, and 1367 listed in Table 17 below. - In one aspect of the invention, a method of inhibiting TLR-mediated immunostimulatory signaling is provided. The method according to this aspect of the invention involves contacting a cell expressing a TLR with an effective amount of a compound of Formula XIV or Formula XV, as provided above, to inhibit TLR-mediated immunostimulatory signaling in response to a ligand for the TLR.
- In one aspect the invention provides a method of affecting TLR-mediated immunostimulation in a subject. The method according to this aspect of the invention involves administering to a subject having or at risk of developing TLR-mediated immunostimulation an effective amount of a compound of Formula XIV or Formula XV, as provided above, to inhibit or promote TLR-mediated immunostimulation in the subject.
- The invention in one aspect provides a method of inhibiting TLR-mediated immunostimulation in a subject. The method according to this aspect of the invention involves administering to a subject having or at risk of developing TLR-mediated immunostimulation an effective amount of a compound of Formula XIV or Formula XV, as provided above, to inhibit TLR-mediated immunostimulation in the subject.
- In one aspect the invention provides a method of inhibiting an immunostimulatory nucleic acid-associated response in a subject. The method according to this aspect of the invention involves administering to a subject in need of such treatment an effective amount of a compound of Formula XIV or Formula XV, as provided above, to inhibit an immunostimulatory nucleic acid-associated response in the subject.
- In one embodiment the subject is otherwise free of symptoms calling for treatment with a compound of Formula XIV or Formula XV.
- In each of the foregoing aspects of the invention, in some embodiments R2 is a substituted or unsubstituted phenyl, naphthyl, phenanthryl, styryl, azabicyclooctane, or azabicycloheptane group. In some embodiments R2 is a phenyl, naphthyl, phenanthryl, styryl, azabicyclooctane, or azabicycloheptane group substituted with one or more substituent groups selected from the group consisting of an alkyl group, an alkoxy group, an alkoxyalkyl group, an ester group, an alkylamino group, a dialkylamino group, a cyclic amino group, a halogen atom, and any combination thereof. In some embodiments R2 is a phenyl, naphthyl, phenanthryl, styryl, azabicyclooctane, or azabicycloheptane group substituted with one or more substituent groups selected from the group consisting of an alkylamino group, a dialkylamino group, and a cyclic amino group. In some embodiments the cyclic amino group is a piperazino group, a piperidino group, a pyrrolidino group, an imidazolyl group, a pyridyl group, or a morpholino group.
- In each of the foregoing aspects of the invention, in some embodiments R9 is a substituted alkyl group selected from the group consisting of a cyclic amino group, an alkylamino group, a dialkylamino group, furyl, phenyl, thienyl, azabicyclooctyl, azabicycloheptyl, and any combination thereof. In some embodiments the cyclic amino group is a piperazino group, a piperidino group, a pyrrolidino group, an imidazolyl group, a pyridyl group, or a morpholino group.
- In the foregoing aspects of the invention, in some embodiments each of R4 and R5 is hydrogen atom. In some embodiments each of R5 and R8 is a hydrogen atom. In some embodiments each of R4, R5, and R8 is a hydrogen atom. In some embodiments R10 is a hydrogen atom.
- In each of the foregoing aspects of the invention, in one embodiment the TLR is TLR9. In one embodiment the ligand for the TLR is an immunostimulatory nucleic acid. In one embodiment the immunostimulatory nucleic acid is a CpG nucleic acid.
- In each of the foregoing aspects of the invention, in one embodiment the TLR is TLR8. In one embodiment the ligand for the TLR is a natural ligand for TLR8. In one embodiment the ligand for the TLR is resiquimod (R848).
- In each of the foregoing aspects of the invention, in one embodiment the TLR is TLR7. In one embodiment the ligand for the TLR is a natural ligand for TLR7. In one embodiment the ligand for the TLR is R848.
- In each of the foregoing aspects of the invention, in one embodiment the TLR is TLR3. In one embodiment the ligand for the TLR is a double stranded RNA. In one embodiment the ligand for the TLR is poly(I:C).
- The present invention is based, in part, on the appreciation by the applicants of a potential connection between the antimalarial activity of known metabolites of chloroquine and the biological activity of related small molecules with respect to inhibition of TLR9. For chloroquine, the major metabolites are mono de-ethylated chloroquine and bis de-ethylated chloroquine.
The half life of mono de-ethylchloroquine in humans is 1.5 times longer than that of chloroquine (649 hrs versus 432 hrs). de Vries P J et al. (1994) Drug Invest 8:143-9. Data for bis de-ethylchloroquine is not available. In addition, the major metabolites of hydroxychloroquine are de-ethylhydroxychloroquine, mono de-ethylchloroquine, and bis de-ethylchloroquine. In the treatment of malaria the potency of chloroquine and mono de-ethylchloroquine against Plasmodium falciparum are about equal whereas bis de-ethylchloroquine is about half as active. Aderounmu A F (1984) Ann Trop Med Parasitol. 78(6):581-5. Also since the side chain is chiral, R and S chloroquine have differing activities with the (S)-(+)-mono de-ethylchloroquine being preferentially produced. Ansari A M et al. (1994) J Pharm Sci. 83(7):1040-2. It was thus hypothesized by the applicants that both chloroquine and hydroxychloroquine derive a substantial portion of their efficacy from the long lived de-ethyl metabolite and that this relationship may carry over to the binding to TLR9. - It turns out that chloroquine, hydroxychloroquine, mono de-ethylchloroquine, and bis de-ethylchloroquine have been examined by Macfarlane. The IC50 of these compounds by his assay were: chloroquine, 1.1×10−7M; hydroxychloroquine, 4.1×10−7M; mono de-ethylchloroquine, 7.08×10−7M; and bis de-ethylchloroquine, 18.6×10−7M. So by this data the loss of one ethyl group from chloroquine reduced the potency by about 7-fold and the loss of both ethyl groups reduced the potency by about 18-fold.
- The present invention in certain aspects is related to compositions and methods involving certain 4-primary amino quinoline compounds which are useful for inhibiting TLR9 signaling and immune activation. In certain aspects the present invention is also related to compositions and methods involving certain quinazoline compounds, some of which are structurally related to the 4-primary amino quinoline compounds of the invention, which are also useful for inhibiting TLR9 signaling and immune activation.
- It was surprisingly discovered according to the invention that the 4-primary amino quinoline compounds and the quinazoline compounds of the invention are highly and similarly active as inhibitors of TLR9 signaling activity. It was also surprisingly discovered according to the instant invention that despite their high degree of structural and biological similarity, the quinazoline molecules exhibit a significantly improved toxicity profile in vivo compared with the quinoline compounds.
-
- X is absent or is an aryl, alkyl, heterocyclic, or styryl group;
- R1 and R2 are each independently a hydrogen atom or a substituted or unsubstituted alkyl, alkenyl, or aryl group, wherein R1 and R2 optionally are combined to form a heterocycle;
- R3 is a hydrogen atom, a halogen atom, or an alkyl, alkenyl, aryl, heterocyclic, nitro, cyano, carboxy, ester, ketone, amino, amido, hydroxy, alkoxy, mercapto, thio, sulfoxide, sulfone, or sulfonamido group, wherein R1 and R3 optionally are combined to form a heterocycle;
- Y is absent or is an oxygen atom, a sulfur atom, CR8R9, or NR10, where R8, R9, and R10 are each independently a hydrogen atom or a substituted or unsubstituted alkyl, alkenyl, or aryl group;
- L is an alkyl or alkenyl group containing from 1 to 10 carbons or is an aryl group; and
- R4, R5, R6, and R7 are each independently a hydrogen atom, a halogen atom, or an alkyl, alkenyl, aryl, heterocyclic, nitro, cyano, carboxy, ester, ketone, amino, amido, hydroxy, alkoxy, mercapto, thio, sulfoxide, sulfone, or sulfonamido group, wherein any pair of R4, R5, R6, and R7 which are adjacent one another optionally are combined to form a heterocycle or a carbocycle,
- and pharmaceutically acceptable hydrates and salts thereof.
- In one embodiment R5 and R6 are each independently a halogen atom or an alkoxy group. In one embodiment R5 and R6 are each independently a chlorine atom or a methoxy group.
- In one embodiment X is absent or is an aryl group;
- NR1R2 is a heterocyclic amine or is NR8(CH2)nNR9R10, wherein n is an integer from 2 to 6, inclusive, and R8, R9, and R10 are each independently a hydrogen atom or an alkyl group;
- R3 is a hydrogen atom;
- Y is an aryl group or is NR11 where R11 is a hydrogen atom or an aryl or alkyl group;
- L is absent or is a C2-C6 alkyl group; and
- R4, R5, R6, and R7 are each independently a hydrogen atom, a halogen atom, or an alkoxy group.
- In one embodiment X is absent or is an aryl group;
- NR1R2 is a substituted or unsubstituted piperazino or morpholino group or is NR8(CH2)nNR9R10, wherein n is an integer from 2 to 6, inclusive, R8 is a hydrogen atom, and R9 and R10 are each independently an alkyl group;
- R3 is a hydrogen atom;
- Y is NH;
- L is a C2-C6 alkyl group; and
- R4, R5, R6, and R7 are each independently a hydrogen atom, a halogen atom, or an alkoxy group.
- In one embodiment X is a phenyl group;
-
- Y is NH;
- L is —(CH2)n— where n is an integer between 2 and 6, inclusive; and
- each of R3, R4, R5, R6, and R7 is a hydrogen atom.
- In each of the foregoing embodiments, the composition is optionally in the form of a pharmaceutically acceptable hydrate or salt.
- In one aspect the invention provides a method for inhibiting signaling by a TLR. The method according to this aspect of the invention involves contacting a cell expressing a functional TLR with an effective amount of a substituted 4-primary amino quinoline composition having structural Formula XVII
wherein - X is absent or is a nitrogen, oxygen, or sulfur atom or an SO or SO2 group;
- R1 is a hydrogen atom or a substituted or unsubstituted aryl, alkyl, heterocyclic or styryl group;
- R2 is a hydrogen atom, a halogen atom, or an alkyl, alkenyl, aryl, heterocyclic, nitro, cyano, carboxy, ester, ketone, amino, amido, hydroxy, alkoxy, mercapto, thio, sulfoxide, sulfone, or sulfonamido group, wherein R1 and R2 optionally are combined to form a heterocycle or carbocycle;
- Y is absent or is an oxygen atom, a sulfur atom, CR7R8, or NR9, where R7, R8, and R9 are each independently a hydrogen atom or a substituted or unsubstituted alkyl, alkenyl, or aryl group;
- L is absent or is an alkyl or alkenyl group containing from 1 to 10 carbons or is an aryl group; and
- R3, R4, R5, and R6 are each independently a hydrogen atom, a halogen atom, or an alkyl, alkenyl, aryl, heterocyclic, nitro, cyano, carboxy, ester, ketone, amino, amido, hydroxy, alkoxy, mercapto, thio, sulfoxide, sulfone, or sulfonamido group, wherein any pair of R3, R4, R5, and R6 which are adjacent one another optionally are combined to form a heterocycle or a carbocycle, and
- pharmaceutically acceptable hydrates and salts thereof, to inhibit signaling by the TLR.
- In one aspect the invention provides a method for inhibiting signaling by a TLR. The method according to this aspect of the invention involves contacting an immune cell expressing a functional TLR with
- (a) an effective amount of a TLR signal agonist to stimulate signaling by the TLR in absence of a substituted 4-primary amino quinoline composition, and
- (b) an effective amount of a substituted 4-primary amino quinoline composition having structural Formula XVII, as defined above, to inhibit signaling by the TLR in response to the TLR signal agonist compared with the signaling by the TLR in response to the TLR signal agonist in absence of the substituted 4-primary amino quinoline composition.
- In one embodiment the TLR is TLR9 and the TLR signal agonist is a TLR9 signal agonist. The TLR signal agonist in one embodiment is CpG DNA, which can be an oligodeoxynucleotide (ODN).
- In one embodiment the TLR signal agonist is an immune complex. In one embodiment the TLR signal agonist is an immune complex that includes a nucleic acid. In one embodiment the TLR signal agonist is an immune complex that includes a nucleic acid that is self-DNA. In one embodiment the TLR signal agonist is an immune complex that includes a nucleic acid that is self-RNA.
- In one aspect the invention provides a method for inhibiting an immune response to an antigenic substance. The method according to this aspect of the invention involves
- contacting an immune cell expressing a functional Toll-like receptor with
- (a) an effective amount of an antigenic substance to stimulate an immune response to the antigenic substance in absence of a substituted 4-primary amino quinoline composition, and
- (b) an effective amount of a substituted 4-primary amino quinoline composition having structural Formula XVII, as defined above, to inhibit an immune response to the antigenic substance compared with the immune response to the antigenic substance in absence of the substituted 4-primary amino quinoline composition.
- In one embodiment the immune response is an innate immune response. In one embodiment the immune response includes an adaptive immune response.
- In one aspect of the invention, a method of treating an autoimmune disorder in a subject is provided. The method according to this aspect of the invention involves the step of administering to a subject having an autoimmune disorder an effective amount of a substituted 4-primary amino quinoline composition having structural Formula XVII, as defined above, to treat the autoimmune disorder. In one embodiment the autoimmune disorder is chosen from systemic lupus erythematosus, rheumatoid arthritis, inflammatory bowel disease, Sjögren's syndrome, polymyositis, vasculitis, Wegener's granulomatosis, sarcoidosis, ankylosing spondylitis, Reiter's syndrome, psoriatic arthritis, and Behçet's syndrome. In one particular embodiment the autoimmune disease is systemic lupus erythematosus. In one particular embodiment the autoimmune disease is rheumatoid arthritis. In one embodiment the subject is a human. In one embodiment the autoimmune disorder is an immune complex associated disease.
-
- X is absent or is an aryl, alkyl, heterocyclic or styryl group, provided that if X is a phenyl group, NR1R2 is part if a heterocycle or is a diamine;
- R1 and R2 are each independently a hydrogen atom or an alkyl, alkenyl, or aryl group, wherein R1 and R2 optionally are combined to form a heterocycle;
- Y is an oxygen atom, a sulfur atom, CR9R10, or NR11, where R9, R10, and R11 are each independently a hydrogen atom or an alkyl, alkenyl, or aryl group, wherein any one of R9, R10, and R11 optionally is combined with R3 or R4 to form a substituted or unsubstituted heterocycle;
- L is an alkyl or alkenyl group containing from 1 to 10 carbons or is an aryl group;
- R3 and R4 are each independently a hydrogen atom or an alkyl, alkenyl, or aryl group, wherein R3 and R4 optionally are combined to form a heterocycle; and
- R5, R6, R7, and R8 are each independently a hydrogen atom, a halogen atom, or an alkyl, alkenyl, aryl, heterocyclic, nitro, cyano, carboxy, ester, ketone, amino, amido, hydroxy, alkoxy, mercapto, thio, sulfoxide, sulfone, or sulfonamido group, wherein any pair of R5, R6, R7, and R8 which are adjacent one another optionally are combined to form a heterocycle or a carbocycle,
- and pharmaceutically acceptable hydrates and salts thereof.
- In one embodiment R6 and R7 are each independently a halogen atom or an alkoxy group.
- In one embodiment R6 and R7 are each independently a chlorine atom or a methoxy group.
- In one embodiment X is absent or is an aryl group;
- NR1R2 is a heterocyclic amine or NR9(CH2)nNR10R11, wherein n is an integer from 2 to 6, inclusive, and R9, R10, and R11 are each independently a hydrogen atom or an alkyl group;
- Y is an aryl group or is NR12 where R12 is a hydrogen atom or an aryl or alkyl group;
- L is absent or is a C2-C6 alkyl group;
- R3 and R4 are each independently a hydrogen atom or an alkyl group, wherein R3 and R4 optionally are combined to form a heterocycle; and
- R5, R6, R7, and R8 are each independently a hydrogen atom, a halogen atom, or an alkoxy group.
- In one embodiment X is absent or is an aryl group;
- NR1R2 is a substituted or unsubstituted piperazino or morpholino group or is NR9(CH2)nNR10R11, wherein n is an integer from 2 to 6, inclusive, R9 is a hydrogen atom, and
- R10 and R11 are each independently an alkyl group;
- Y is NH;
- L is a C2-C6 alkyl group;
- R3 and R4 are each independently a hydrogen atom or an alkyl group, wherein R3 and R4 optionally are combined to form a heterocycle; and
- R5, R6, R7, and R8 are each independently a hydrogen atom, a halogen atom, or an alkoxy group.
- In one embodiment X is an aryl group;
- NR1R2 is substituted or unsubstituted piperazino or morpholino group;
- Y is NH;
- L is a C2-C6 alkyl group;
- R3 and R4 are each independently a methyl or ethyl group or R3 and R4 optionally are combined to form a morpholino group; and
- R5, R6, R7, and R8 are each independently a hydrogen atom, a halogen atom, or an alkoxy group.
- In one embodiment X is a phenyl group;
- NR1R2 is N-methylpiperazine;
- Y is NH;
- L is —CH2CH2—;
- R3 and R4 are each a methyl group; and
- R5, R6, R7, and R8 are each a hydrogen atom.
- In one embodiment X is a phenyl group;
- NR1R2 is N-methylpiperazine;
- Y is NH;
- L is —CH2CH2—;
- R3 and R4 are combined as a morpholino group; and
- R5, R6, R7, and R8 are each a hydrogen atom.
- In one embodiment X is absent;
- NR1R2 is a substituted or unsubstituted piperazino or morpholino group;
- Y is NH;
- L is a C2-C6 alkyl group;
- R3 and R4 are each independently a methyl or ethyl group or R3 and R4 optionally are combined to form a morpholino group; and
- R5, R6, R7, and R8 are each independently a hydrogen atom, a halogen atom, or an alkoxy group.
- In one embodiment X is absent;
- NR1R2 is N-methylpiperazine;
- Y is NH;
- L is —CH2CH2—;
- R3 and R4 are each a methyl group;
- R6 and R7 are each a methoxy group; and
- R5 and R8 are each a hydrogen atom.
- In one embodiment X is absent;
- NR1R2 is N-phenylpiperazine;
- Y is NH;
- L is —CH2CH2—;
- R3 and R4 are each a methyl group;
- R6 and R7 are each a methoxy group; and
- R5 and R8 are each a hydrogen atom.
- In one embodiment X is absent;
- NR1R2 is N-methylpiperazine;
- Y is NH;
- L is —CH2CH2;
- R3 and R4 are combined as a morpholino group;
- R6 and R7 are each a methoxy group; and
- R5 and R8 are each a hydrogen atom.
- In each of the foregoing embodiments, the composition is optionally in the form of a pharmaceutically acceptable hydrate or salt.
-
- X is a substituted or unsubstituted aryl, alkyl, heterocyclic or styryl group, optionally attached to the quinazoline by a nitrogen, oxygen, or sulfur atom or by a SO or SO2 group;
- Y is absent or is an oxygen atom, a sulfur atom, CR9R10, or NR11, where R9, R10, and R11 are each independently a hydrogen atom or an alkyl, alkenyl, or aryl group, wherein any one of R9, R10, and R11 optionally is combined with R3 or R4 to form a heterocycle;
- L is absent or is a hydrogen atom, an alkyl or alkenyl group containing from 1 to 10 carbons, or an aryl group;
- R3 and R4 are each independently a hydrogen atom or an alkyl, alkenyl, or aryl group, wherein R3 and R4 optionally are combined to form a heterocycle; and
- R5, R6, R7, and R8 are each independently a hydrogen atom, a halogen atom, or an alkyl, alkenyl, aryl, heterocyclic, nitro, cyano, carboxy, ester, ketone, amino, amido, hydroxy, alkoxy, mercapto, thio, sulfoxide, sulfone, or sulfonamido group, wherein any pair of R5, R6, R7, and R9 which are adjacent one another optionally are combined to form a heterocycle or a carbocycle,
- and pharmaceutically acceptable hydrates and salts thereof, to inhibit signaling by the TLR.
- In one aspect the invention provides a method for inhibiting signaling by a TLR. The method according to this aspect of the invention involves contacting an immune cell expressing a functional TLR with
- (a) an effective amount of a TLR signal agonist to stimulate signaling by the TLR in absence of a quinazoline composition, and
- (b) an effective amount of a quinazoline composition having structural Formula XIX, as defined above, to inhibit signaling by the TLR in response to the TLR signal agonist compared with the signaling by the TLR in response to the TLR signal agonist in absence of the quinazoline composition.
- In one embodiment the TLR is TLR9 and the TLR signal agonist is a TLR9 signal agonist. The TLR signal agonist in one embodiment is CpG DNA, which can be an oligodeoxynucleotide (ODN).
- In one embodiment the TLR signal agonist is an immune complex that includes a nucleic acid.
- In one aspect the invention provides a method for inhibiting an immune response to an antigenic substance. The method according to this aspect of the invention involves contacting an immune cell expressing a functional Toll-like receptor with
- (a) an effective amount of an antigenic substance to stimulate an immune response to the antigenic substance in absence of a quinazoline composition, and
- (b) an effective amount of a quinazoline composition having structural Formula XIX, as defined above, to inhibit an immune response to the antigenic substance compared with the immune response to the antigenic substance in absence of the quinazoline composition.
- In one embodiment the immune response is an innate immune response. In one embodiment the immune response includes an adaptive immune response.
- In one aspect of the invention, a method of treating an autoimmune disorder in a subject is provided. The method according to this aspect of the invention involves the step of administering to a subject having an autoimmune disorder an effective amount of a quinazoline composition having structural Formula XIX, as defined above, to treat the autoimmune disorder. In one embodiment the autoimmune disorder is chosen from systemic lupus erythematosus, rheumatoid arthritis, inflammatory bowel disease, Sjögren's syndrome, polymyositis, vasculitis, Wegener's granulomatosis, sarcoidosis, ankylosing spondylitis, Reiter's syndrome, psoriatic arthritis, and Behçet's syndrome. In one particular embodiment the autoimmune disease is systemic lupus erythematosus. In one particular embodiment the autoimmune disease is rheumatoid arthritis. In one embodiment the subject is a human. In one embodiment the autoimmune disorder is an immune complex associated disease, as described above.
-
FIG. 1 is a graph depicting the concentration-dependent inhibition of TLR9 signaling in response to CpG ODN 2006 (5′-TCGTCGTTTTGTCGTTTTGTCGTT-3′; SEQ ID NO:1) by compounds 613 (harmine hydrochloride), 878 (3-hydroxymethyl-beta-carboline, and 470 (norharman), molecules representative of compounds of Formula II. -
FIG. 2 is a pair of graphs depicting results of an in vivo toxicity assay in mice for specific quinoline and quinazoline compounds.FIG. 2A shows change in mean body weight (grams) andFIG. 2B shows mean body weight (grams) over 5 days for mice treated with dimethysulfoxide (DMSO) control (closed circles), compound CMZ 203-43 (also referred to herein as 203-43) administered at 1 mg and 4 mg (open and closed triangles, respectively), compound CMZ 203-34 at 1 mg and 4 mg (open and closed diamonds, respectively), and compound CMZ 203-49 at 1 mg and 4 mg (open and closed inverted triangles, respectively). N=3 for each treatment group. The group receiving CMZ 203-43 at 4 mg were sick and had to be sacrificed onday 3. -
FIG. 3 is a bar graph depicting total and differential white blood cell counts in mice measured five days after administration of a single intraperitoneal dose of specific quinoline and quinazoline compounds. N=3 for each treatment group. -
FIG. 4 is a bar graph depicting in vivo inhibition of IP-10 induction (pg/ml) by CpG ODN 2006 following pretreatment with PBS control or a quinoline compound (203-43) or various indicated quinazoline compounds (203-34, 203-49, or 203-51). N=5 for each group. -
FIG. 5 is a bar graph depicting in vivo inhibition of TNF-α induction (pg/ml) by CpG ODN 2006 following pretreatment with PBS control or a quinoline compound (203-43) or various indicated quinazoline compounds (203-34, 203-49, or 203-51). N=5 for each group. - The present invention provides novel compositions and methods for inhibiting immune responses, including immune responses involved in clinical disorders characterized as autoimmune disorders and immune complex associated diseases. In addition to the novel compositions disclosed herein, which include 4-primary amino quinolines and structurally similar quinazolines, the invention also provides methods for use of previously known compositions within these and other classes of compounds in inhibiting immune responses, including immune responses involved in clinical disorders characterized as autoimmune disorders and immune complex associated diseases.
- The present invention is based in part on discoveries made by the applicants arising from screening a library of small molecule compounds in an in vitro assay of TLR9 signaling in response to immunostimulatory CpG oligodeoxynucleotides (ODN). It was observed that certain of the small molecules in the library were effective in altering TLR9 signaling in response to CpG ODN. Based on the initial screening results, additional compounds were selected for additional screening. Importantly, the small molecules so identified were distinct from those described by Macfarlane and colleagues.
- It has now been discovered according to the present invention that certain small molecules characterized by certain minimum features are able to modulate TLR signaling, e.g., in the presence of or in response to a PAMP or other TLR ligand. Some of the small molecules are potent inhibitors of TLR signaling and thus can be used to inhibit TLR-mediated immunostimulation. The minimal features for many, but not all, of the small molecules can be summarized as follows: a core structure including at least two rings, which can be fused or unfused, wherein at least one of the rings contains a nitrogen atom and/or has a side chain that contains a nitrogen atom. Certain of the molecules are effective even without presence of a nitrogen atom.
- It has been observed that fused three-ring structures such as quinacrine are typically an order of magnitude more potent as inhibitors of TLR-mediated immunostimulation than are fused two-ring structures such as chloroquine. For example, quinacrine is reported to have an EC50 of about 8 nM, where EC50 is the concentration required for half-maximal inhibition of CpG-ODN effect on thymidine uptake by WEHI 231 B-cells in the presence of anti-surface IgM. U.S. Pat. No. 6,479,504 B1. It has now also been discovered according to the present invention that fused two-ring core structures to which are attached a nitrogen-containing ring substituent can be as potent as fused three-ring structures such as quinacrine. In particular, such compounds can exhibit EC50 in the low nanomolar range. In certain embodiments the nitrogen-containing ring substituent is a C2-C6 alkyl group terminated by a tertiary amine, such as methylethylamine, diethyl amine, dimethyl amine, or a ring containing at least one nitrogen.
- Without meaning to be bound to any particular theory or mechanism, it is the belief of the instant inventors that both the ring-containing core structure and the side chain substituents contribute to affinity and efficacy. It is believed that molecules with high affinity core structure and low affinity substituents can be as effective as molecules with low affinity core structure and high affinity substituents.
- Over many years there have been observations that certain common classes of drugs used for purposes other than affecting the immune system in fact have side effects that include immune suppression. For example, the phenothiazine chlorpromazine has been reported to inhibit messenger RNA expression for interleukin 2 (IL-2), tumor necrosis factor alpha (TNF-α), and gamma interferon (IFN-γ). Schleuning M J et al. (1989) Eur J Immunol 19:1491-6. In a separate study, chlorpromazine was reported to depress contact hypersensitivity to dinitrochlorobenzene in guinea-pigs. Descotes J et al. (1982) J Neuroimmunol 2:21-5. Yet another study reported that acute, high dose administration of the tricyclic antidepressant drug imipramine inhibits lipopolysaccharide (LPS)-induced increases in TNF-α but has little or no effect on LPS-induced
interleukin 1 beta (IL-1β) or interleukin 10 (IL-10). Dredge K et al. (1999) Int J Immunopharm 21:663-73. - Histamine is a widely recognized mediator of immediate hypersensitivity and inflammation, which are principally mast cell- and basophil-mediated immune phenomena. The pleiotropic effects of histamine are mediated through three types of membrane-associated histamine receptor, histamine H1 receptor (H1R), histamine H2 receptor (H2R), and histamine H3 receptor (H3R). Pharmacologic inhibitors for these receptors are known and include pyrilamine and tripelennamine (H1R); cimetidine, famotidine, and ranitidine (H2R); and thioperamide and clobenpropit (H3R). Recently it was reported that signaling through H1R augments T-cell and B-cell antigen receptor-mediated responses. Banu Y et al. (1999) J Exp Med 189:673-82. T cells and B cells derived from H1R-deficient (H1R−/−) mice were reported to have normal responses to LPS. Banu et al. also reported that administration of the H2R-specific antagonist famotidine further depressed T-cell and B-cell antigen receptor-mediated responses in H1R−/− mice. Jutel and coworkers recently reported that in their study of T cells, the Th1 subset of CD4+ T cells preferentially express H1R, while Th2 cells preferentially express H2R. They also reported that histamine enhances Th1-type responses by triggering H1R, and that H1R−/− mice have suppressed levels of the Th1 cytokine IFN-γ and increased levels of the Th2 cytokines IL-4 and IL-13. Jutel M et al. (2001) Nature 413:420-5.
- In contrast, with respect to dendritic cells (DC), Mazzoni and others have reported that histamine inhibits IL-12 production and stimulates IL-10 secretion in LPS-stimulated monocyte-derived DC, resulting in a shift from a Th1- to a Th2-polarized immune response. Mazzoni A et al. (2001) J Clin Invest 108:1865; Caron G et al. (2001) J Immunol 166:6000-6. Recently Mazzoni et al. also reported their observation that histamine, acting through H2R, inhibits release of type I IFN and TNF-α by plasmacytoid DC (pDC), the principal producers of IFN-α, in response to exposure to CpG ODN or live influenza virus. Mazzoni A et al. (2003) J Immunol 170:2269-73. Finally, it was recently reported that histamine, acting through H2R on monocyte/macrophages, suppresses NADPH oxidase, a key enzyme in oxygen radical formation, resulting in protection of natural killer (NK) cells and T cells against oxygen radical-induced dysfunction and apoptosis. Hellstrand K (2002) Semin Oncol 29(3 Suppl 7):35-40.
- Definitions
- Unless otherwise defined, all technical and scientific terms used herein have the same meaning as commonly understood by one of ordinary skill in the art to which this invention belongs.
- As used herein, the term “adaptive immune response” refers to any type of antigen-specific immune response. Adaptive immune responses, which are also known in the art as specific immune responses, involve lymphocytes are also characterized by immunological memory, whereby response to a second or subsequent exposure to antigen is more vigorous than the response to a first exposure to the antigen. The term adaptive immune response encompasses both humoral (antibody) immunity and cell-mediated (cellular) immunity.
- As used herein, “allergy” refers to acquired hypersensitivity to a substance (allergen). Allergic conditions include eczema, allergic rhinitis or coryza, hay fever, asthma, urticaria (hives) and food allergies, and other atopic conditions.
- As used herein, the term “antigenic substance” refers to any substance that induces an adaptive (specific) immune response. An antigen typically is any substance that can be specifically bound by a T-cell antigen receptor, antibody, or B-cell antigen receptor. Antigenic substances include, without limitation, peptides, proteins, carbohydrates, lipids, phospholipids, nucleic acids, autacoids, and hormones. Antigenic substances further specifically include antigens that are classified as allergens, cancer antigens, and microbial antigens.
- As used herein, “asthma” refers to a disorder of the respiratory system characterized by inflammation, narrowing of the airways and increased reactivity of the airways to inhaled agents. Asthma is frequently, although not exclusively associated with atopic or allergic symptoms. For example, asthma can be precipitated by exposure to an allergen, exposure to cold air, respiratory infection, and exertion.
- As used herein, the terms “autoimmune disease” and, equivalently, “autoimmune disorder” and “autoimmunity”, refer to immunologically mediated acute or chronic injury to a tissue or organ derived from the host. The terms encompass both cellular and antibody-mediated autoimmune phenomena, as well as organ-specific and organ-nonspecific autoimmunity. Autoimmune diseases include insulin-dependent diabetes mellitus, rheumatoid arthritis, systemic lupus erythematosus, multiple sclerosis, atherosclerosis, and inflammatory bowel disease. Autoimmune diseases also include, without limitation, ankylosing spondylitis, autoimmune hemolytic anemia, Behçet's syndrome, Goodpasture's syndrome, Graves' disease, Guillain-Barré syndrome, Hashimoto's thyroiditis, idiopathic thrombocytopenia, myasthenia gravis, pernicious anemia, polyarteritis nodosa, polymyositis/dermatomyositis, primary biliary sclerosis, psoriasis, sarcoidosis, sclerosing cholangitis, Sjögren's syndrome, systemic sclerosis (scleroderma and CREST syndrome), Takayasu's arteritis, temporal arteritis, and Wegener's granulomatosis. Autoimmune diseases also include certain immune complex-associated diseases.
- As used herein, the terms “cancer” and, equivalently, “tumor” refer to a condition in which abnormally replicating cells of host origin are present in a detectable amount in a subject. The cancer can be a malignant or non-malignant cancer. Cancers or tumors include but are not limited to biliary tract cancer; brain cancer; breast cancer; cervical cancer; choriocarcinoma; colon cancer; endometrial cancer; esophageal cancer; gastric (stomach) cancer; intraepithelial neoplasms; leukemias; lymphomas; liver cancer; lung cancer (e.g., small cell and non-small cell); melanoma; neuroblastomas; oral cancer; ovarian cancer; pancreatic cancer; prostate cancer; rectal cancer; renal (kidney) cancer; sarcomas; skin cancer; testicular cancer; thyroid cancer; as well as other carcinomas and sarcomas. Cancers can be primary or metastatic.
- As used herein, the term “CpG DNA” refers to an immunostimulatory nucleic acid which contains a cytosine-guanine (CG) dinucleotide, the C residue of which is unmethylated. The effects of CpG nucleic acids on immune modulation have been described extensively in U.S. patents such as U.S. Pat. Nos. 6,194,388; 6,207,646; 6,239,116; and 6,218,371, and published international patent applications, such as WO98/37919, WO98/40100, WO98/52581, and WO99/56755. The entire contents of each of these patents and published patent applications is hereby incorporated by reference. The entire immunostimulatory nucleic acid can be unmethylated or portions may be unmethylated but at least the C of the 5′-CG-3′ must be unmethylated.
- CpG DNA includes both naturally occurring immunostimulatory nucleic acids, as found in bacterial DNA and plasmids, as well as synthetic oligodeoxynucleotides (ODN).
- In one embodiment the CpG DNA is a CpG ODN that has a base sequence provided by 5′-TCGTCGTTTTGTCGTTTTGTCGTT-3′ (ODN 2006; SEQ ID NO:1).
- CpG ODN have been further classified by structure and function into at least the following three classes or types, all of which are intended to be encompassed within the term CpG DNA as used herein: B-class CpG ODN such as ODN 2006 include the originally described immunostimulatory CpG ODN and characteristically activate B cells and NK cells but do not induce or only weakly induce expression of type I interferon (e.g., IFN-α). A-class CpG ODN, described in published PCT international application WO 01/22990, incorporate a CpG motif, include a chimeric phosphodiester/phosphorothioate backbone, and characteristically activate NK cells and induce plasmacytoid dendritic cells to express large amounts of IFN-α but do not activate or only weakly activate B cells. An example of an A-class CpG ODN is 5′-G*G*G_G_G_A_C_G_A_T_C_G_T_C_G*G*G*G*G*G-3′ (ODN 2216, SEQ ID NO:2), wherein “*” represents phosphorothioate and “_” represents phosphodiester. C-class CpG ODN incorporate a CpG, include a wholly phosphorothioate backbone, include a GC-rich palindromic or nearly-palindromic region, and are capable of both activating B cells and inducing expression of IFN-α. C-class CpG ODN have been described, for example, in published U.S. patent application 2003/0148976. An example of a C-class CpG ODN is 5′-TCGTCGTTTTCGGCGCGCGCCG-3′ (ODN 2395; SEQ ID NO:3). For a review of the various classes of CpG ODN, see also Vollmer J et al. (2004) Eur J Immunol 34:251-62.
- As used herein, “cytokine” refers to any of a number of soluble proteins or glycoproteins that act on immune cells through specific receptors to affect the state of activation and function of the immune cells. Cytokines include interferons, interleukins, tumor necrosis factor, transforming growth factor beta, colony-stimulating factors (CSFs), chemokines, as well as others. Various cytokines affect innate immunity, acquired immunity, or both. Cytokines specifically include, without limitation, IFN-α, IFN-β, IFN-γ, IL-1, IL-2, IL-3, IL-4, IL-5, IL-6, IL-9, IL-10, IL-12, IL-13, IL-18, TNF-α, TGF-β, granulocyte colony-stimulating factor (G-CSF), and granulocyte-macrophage colony-stimulating factor (GM-CSF). Chemokines specifically include, without limitation, IL-8, IP-10, I-TAC, RANTES, MIP-1α, MIP-1β, Gro-α, Gro-β, Gro-γ, MCP-1, MCP-2, and MCP-3.
- Most mature CD4+ T helper cells can be categorized into one of two cytokine-associated, cross-regulatory subsets or phenotypes: Th1 or Th2. Th1 cells are associated with IL-2, IL-3, IFN, GM-CSF and high levels of TNF-α. Th2 cells are associated with IL-3, IL-4, IL-5, IL-6, IL-9, IL-10, IL-13, GM-CSF and low levels of TNF-α. The Th1 subset promotes both cell-mediated immunity and humoral immunity that is characterized by immunoglobulin class switching to IgG2a in mice. Th1 responses can also be associated with delayed-type hypersensitivity and autoimmune disease. The Th2 subset induces primarily humoral immunity and induces immunoglobulin class switching to IgE and IgG1 in mice. The antibody isotypes associated with Th1 responses generally have good neutralizing and opsonizing capabilities, whereas those associated with Th2 responses are associated more with allergic responses.
- Several factors have been shown to influence commitment to Th1 or Th2 profiles. The best characterized regulators are cytokines. IL-12 and IFN-γ are positive Th1 and negative Th2 regulators. IL-12 promotes IFN-γ production, and IFN-γ provides positive feedback for IL-12. IL-4 and IL-10 appear to be required for the establishment of the Th2 cytokine profile and to down-regulate Th1 cytokine production; the effects of IL-4 are in some cases dominant over those of IL-12. IL-13 was shown to inhibit expression of inflammatory cytokines, including IL-12 and TNF-α by LPS-induced monocytes, in a way similar to IL-4.
- As used herein, “effective amount” refers to any amount that is necessary or sufficient for achieving or promoting a desired outcome. In some instances an effective amount is a therapeutically effective amount. A therapeutically effective amount is any amount that is necessary or sufficient for promoting or achieving a desired biological response in a subject. The effective amount for any particular application can vary depending on such factors as the disease or condition being treated, the particular agent being administered, the size of the subject, or the severity of the disease or condition. One of ordinary skill in the art can empirically determine the effective amount of a particular agent without necessitating undue experimentation.
- As used herein, “graft rejection” refers to immunologically mediated hyperacute, acute, or chronic injury to a tissue or organ derived from a source other than the host. The term thus encompasses both cellular and antibody-mediated rejection, as well as rejection of both allografts and xenografts.
- As used herein, the term “immune cell” refers to a cell belonging to the immune system. Immune cells include T lymphocytes (T cells), B lymphocytes (B cells), natural killer (NK) cells, granulocytes, neutrophils, macrophages, monocytes, dendritic cells, and specialized forms of any of the foregoing, e.g., plasmacytoid dendritic cells, plasma cells, NKT, T helper, and cytotoxic T lymphocytes (CTL).
- As used herein, the term “immune complex” refers to any conjugate including an antibody and an antigen specifically bound by the antibody. In one embodiment the antigen is an autoantigen.
- As used herein, the term “immune complex comprising a nucleic acid” refers to any conjugate including an antibody and a nucleic acid-containing antigen specifically bound by the antibody. The nucleic acid-containing antigen can include chromatin, ribosomes, small nuclear proteins, histones, nucleosomes, DNA, RNA, or any combination thereof. The antibody can but need not necessarily bind specifically to a nucleic acid component of the nucleic acid-containing antigen.
- As used herein, the term “immune complex-associated disease” refers to any disease characterized by the production and/or tissue deposition of immune complexes, including, but not limited to systemic lupus erythematosus (SLE) and related connective tissue diseases, rheumatoid arthritis, hepatitis C— and hepatitis B-related immune complex disease (e.g., cryoglobulinemia), Behçet's syndrome, autoimmune glomerulonephritides, and vasculopathy associated with the presence of LDL/anti-LDL immune complexes.
- As used herein, “immunodeficiency” refers to a disease or disorder in which the subject's immune system is not functioning in normal capacity or in which it would be useful to boost a subject's immune response for example to eliminate a tumor or cancer (e.g., tumors of the brain, lung (e.g., small cell and non-small cell), ovary, breast, prostate, colon, as well as other carcinomas and sarcomas) or an infection in a subject. The immunodeficiency can be acquired or it can be congenital.
- As used herein, “immunostimulatory nucleic acid-associated response in a subject” refers to a measurable response in a subject associated with administration to the subject of an immunostimulatory nucleic acid. Such responses include, without limitation, elaboration of cytokines, chemokines, growth factors, or immunoglobulin; expression of immune cell surface activation markers; Th1/Th2 skewing; and clinical disease activity.
- As used herein, the terms “infection” and, equivalently, “infectious disease” refer to a condition in which an infectious organism or agent is present in a detectable amount in the blood or in a normally sterile tissue or normally sterile compartment of a subject. Infectious organisms and agents include viruses, bacteria, fungi, and parasites. The terms encompass both acute and chronic infections, as well as sepsis.
- As used herein, the term “innate immune response” refers to any type of immune response to certain pathogen-associated molecular patterns (PAMPs). Innate immunity, which is also known in the art as natural or native immunity, involves principally neutrophils, granulocytes, mononuclear phagocytes, dendritic cells, NKT cells, and NK cells. Innate immune responses can include, without limitation, type I interferon production (e.g., IFN-α), neutrophil activation, macrophage activation, phagocytosis, opsonization, complement activation, and any combination thereof.
- As used herein, the term “self-DNA” refers to any DNA derived from the genome of a host subject. In one embodiment self-DNA includes complementary DNA (cDNA) derived from a host subject. Self-DNA includes intact and degraded DNA.
- As used herein, the term “self-RNA” refers to any RNA derived from the genome of a host subject. In one embodiment self-RNA is a messenger RNA (mRNA) derived from a host subject. In one embodiment self-RNA includes ribosomal RNA (rRNA) derived from a host subject. Self-RNA includes intact and degraded RNA.
- As used herein, the term “subject” refers to a vertebrate animal. In one embodiment the subject is a mammal. In one embodiment the subject is a human. In other embodiments the subject is a non-human vertebrate animal, including, without limitation, non-human primates, laboratory animals, livestock, domesticated animals, and non-domesticated animals.
- As used herein, “subject having or at risk of developing TLR-mediated immunostimulation” refers to a subject exposed to or at risk of exposure to a PAMP or other TLR ligand.
- As used herein, the terms “Toll-like receptor” and, equivalently, “TLR” refer to any member of a family of at least ten highly conserved mammalian pattern recognition receptor proteins (TLR1-TLR10) which recognize pathogen-associated molecular patterns (PAMPs) and act as key signaling elements in innate immunity. TLR polypeptides share a characteristic structure that includes an extracellular (extracytoplasmic) domain that has leucine-rich repeats, a transmembrane domain, and an intracellular (cytoplasmic) domain that is involved in TLR signaling. TLRs include but are not limited to human TLRs.
- Nucleic acid and amino acid sequences for all ten currently known human TLRs are available from public databases such as GenBank. Similarly, nucleic acid and amino acid sequences for various TLRs from numerous non-human species are also available from public databases including GenBank. For example, nucleic acid and amino acid sequences for human TLR9 (hTLR9) can be found as GenBank accession numbers AF245704 (coding region spanning nucleotides 145-3243) and AAF78037, respectively. Nucleic acid and amino acid sequences for murine TLR9 (mTLR9) can be found as GenBank accession numbers AF348140 (coding region spanning nucleotides 40-3138) and AAK29625, respectively. The deduced human TLR9 protein contains 1,032 amino acids and shares an overall amino acid identity of 75.5% with mouse TLR9. Like other TLR proteins, human TLR9 contains extracellular leucine-rich repeats (LRRs) and a cytoplasmic Toll/interleukin-1R (TIR) domain. It also has a signal peptide (residues 1-25) and a transmembrane domain (residues 819-836).
- Nucleic acid and amino acid sequences for human TLR8 (hTLR8) can be found as GenBank accession numbers AF245703 (coding region spanning nucleotides 49-3174) and AAF78036, respectively. Nucleic acid and amino acid sequences for murine TLR8 (mTLR8) can be found as GenBank accession numbers AY035890 (coding region spanning nucleotides 59-3157) and AAK62677, respectively.
- Nucleic acid and amino acid sequences for human TLR7 (hTLR7) can be found as GenBank accession numbers AF240467 (coding region spanning nucleotides 135-3285) and AAF60188, respectively. Nucleic acid and amino acid sequences for murine TLR7 (mTLR7) can be found as GenBank accession numbers AY035889 (coding region spanning nucleotides 49-3201) and AAK62676, respectively.
- Nucleic acid and amino acid sequences for human TLR3 (hTLR3) can be found as GenBank accession numbers NM—003265 (coding region spanning nucleotides 102-2816) and NP—003256, respectively. Nucleic acid and amino acid sequences for murine TLR3 (hTLR3) can be found as GenBank accession numbers AF355152 (coding region spanning nucleotides 44-2761) and AAK26117, respectively.
- While hTLR1 is ubiquitously expressed, hTLR2, hTLR4 and hTLR5 are present in monocytes, polymorphonuclear phagocytes, and dendritic cells. Muzio M et al. (2000) J Leukoc Biol 67:450-6. Recent publications reported that hTLR1, hTLR6, hTLR7, hTLR9 and hTLR10 are present in human B cells. Human TLR7 and hTLR9 are present in plasmacytoid dendritic cells (pDCs), while myeloid dendritic cells express hTLR7 and hTLR8 but not hTLR9. Human TLR8, however, appears not to be expressed in pDCs.
- As members of the pro-inflammatory interleukin-1 receptor (IL-1R) family, TLRs share homologies in their cytoplasmic domains called Toll/IL-1R homology (TIR) domains. PCT published applications PCT/US98/08979 and PCT/US01/16766. Intracellular signaling mechanisms mediated by TLRs appear generally similar, with MyD88 and tumor necrosis factor receptor-associated factor 6 (TRAF6) believed to have critical roles. Wesche H et al. (1997) Immunity 7:837-47; Medzhitov R et al. (1998) Mol Cell 2:253-8; Adachi O et al. (1998) Immunity 9:143-50; Kawai T et al. (1999) Immunity 11:115-22); Cao Z et al. (1996) Nature 383:443-6; Lomaga M A et al. (1999) Genes Dev 13:1015-24. Signal transduction between MyD88 and TRAF6 is known to involve members of the serine-threonine kinase IL-1 receptor-associated kinase (IRAK) family, including at least IRAK-1 and IRAK-2. Muzio M et al. (1997) Science 278:1612-5.
- Briefly, MyD88 is believed to act as an adapter molecule between the TIR domain of a TLR or IL-1R and IRAK (which includes at least any one of IRAK-1, IRAK-2, IRAK-4, and IRAK-M). MyD88 includes a C-terminal Toll homology domain and an N-terminal death domain. The Toll homology domain of MyD88 binds the TIR domain of TLR or IL-1R, and the death domain of MyD88 binds the death domain of the serine kinase IRAK. IRAK interacts with TRAF6, which acts as an entryway into at least two pathways, one leading to activation of the transcription factor NF-κB and another leading to activation of Jun and Fos, members of the activator protein-1 (AP-1) transcription factor family. Activation of NF-κB involves the activation of TAK-1, a member of the
MAP 3 kinase (MAPK) family, and IκB kinases. The IκB kinases phosphorylate IκB, leading to its degradation and the translocation of NF-κB to the nucleus. Activation of Jun and Fos is believed to involve MAP kinase kinases (MAPKKs) and MAP kinases ERK, p38, and JNK/SAPK. Both NF-κB and AP-1 are involved in controlling the transcription of a number of key immune response genes, including genes for various cytokines and costimulatory molecules. See Aderem A et al. (2000) Nature 406:782-7; Häcker H et al. (1999) EMBO J 18:6973-82. - As used herein, the terms “TLR ligand” and, equivalently, “ligand for a TLR” and “TLR signaling agonist”, refer to a molecule, other than a small molecule according to Formula I-XIX described herein or a 4-primary amino quinoline or quinazoline molecule according to the invention, that interacts, directly or indirectly, with a TLR through a TLR domain other than a TIR domain and induces TLR-mediated signaling. In one embodiment a TLR ligand is a natural ligand, i.e., a TLR ligand that is found in nature. In one embodiment a TLR ligand refers to a molecule other than a natural ligand of a TLR, e.g., a molecule prepared by human activity. In one embodiment the TLR is TLR9 and the TLR signal agonist is a CpG nucleic acid.
- Ligands for many but not all of the TLRs have been described. For instance, it has been reported that TLR2 signals in response to peptidoglycan and lipopeptides. Yoshimura A et al. (1999) J Immunol 163:1-5; Brightbill H D et al. (1999) Science 285:732-6; Aliprantis A O et al. (1999) Science 285:736-9; Takeuchi O et al. (1999) Immunity 11:443-51; Underhill D M et al. (1999) Nature 401:811-5. TLR4 has been reported to signal in response to lipopolysaccharide (LPS). Hoshino K et al. (1999) J Immunol 162:3749-52; Poltorak A et al. (1998) Science 282:2085-8; Medzhitov R et al. (1997) Nature 388:394-7. Bacterial flagellin has been reported to be a natural ligand for TLR5. Hayashi F et al. (2001) Nature 410:1099-1103. TLR6, in conjunction with TLR2, has been reported to signal in response to proteoglycan. Ozinsky A et al. (2000) Proc Natl Acad Sci USA 97:13766-71; Takeuchi O et al. (2001) Int Immunol 13:933-40.
- Recently it was reported that TLR9 is a receptor for CpG DNA. Hemmi H et al. (2000) Nature 408:740-5; Bauer S et al. (2001) Proc Natl Acad Sci USA 98:9237-42. CpG DNA, which includes bacterial DNA and synthetic DNA with CG dinucleotides in which cytosine is unmethylated, is described in greater detail elsewhere herein. Marshak-Rothstein and colleagues also recently reported their finding that TLR9 signaling can occur in certain autoimmune diseases in response to immune complexes containing IgG and chromatin. Leadbetter E A et al. (2002) Nature 416:595-8. Thus, in a broader sense it appears that TLR9 can signal in response to self or non-self nucleic acid, either DNA or RNA, when the nucleic acid is presented in a suitable context, e.g., as part of an immune complex.
- Recently it was reported that certain imidazoquinoline compounds having antiviral activity are ligands of TLR7 and TLR8. Hemmi H et al. (2002) Nat Immunol 3:196-200; Jurk M et al. (2002) Nat Immunol 3:499. Imidazoquinolines are potent synthetic activators of immune cells with antiviral and antitumor properties. Using macrophages from wildtype and MyD88-deficient mice, Hemmi et al. recently reported that two imidazoquinolines, imiquimod and resiquimod (R848), induce tumor necrosis factor (TNF) and interleukin-12 (IL-112) and activate NF-κB only in wildtype cells, consistent with activation through a TLR. Hemmi H et al. (2002) Nat Immunol 3:196-200. Macrophages from mice deficient in TLR7 but not other TLRs produced no detectable cytokines in response to these imidazoquinolines. In addition, the imidazoquinolines induced dose-dependent proliferation of splenic B cells and the activation of intracellular signaling cascades in cells from wildtype but not TLR7−/− mice. Luciferase analysis established that expression of human TLR7, but not TLR2 or TLR4, in human embryonic kidney cells results in NF-κB activation in response to resiquimod. The findings of Hemmi et al. thus suggested that these imidazoquinoline compounds are non-natural ligands of TLR7 that can induce signaling through TLR7.
- Recently it was reported that R848 is also a ligand for human TLR8. Jurk M et al. (2002) Nat Immunol 3:499.
- It was recently reported that ligands of TLR3 include poly(I:C) and double-stranded RNA (dsRNA). For purposes of this invention, poly(I:C) and double-stranded RNA (dsRNA) are classified as oligonucleotide molecules. By stimulating kidney cells expressing one of a range of TLRs with poly(I:C), Alexopoulou et al. reported that only cells expressing TLR3 respond by activating NF-κB. Alexopoulou L et al. (2001) Nature 413: 732-8. Alexopoulou et al. also reported that wildtype cells stimulated with poly(I:C) activate NF-κB and produce inflammatory cytokines IL-6, IL-12, and TNF-α, whereas the corresponding responses of TLR3−/− cells were significantly impaired. In contrast, TLR3−/− cells responded equivalently to wildtype cells in response to lipopolysaccharide, peptidoglycan, and CpG dinucleotides. Analysis of MyD88−/− cells indicated that this adaptor protein is involved in dsRNA-induced production of cytokines and proliferative responses, although activation of NF-κB and MAP kinases are not affected, indicating distinct pathways for these cellular responses. Alexopoulou et al. proposed that TLR3 may have a role in host defense against viruses.
- As used herein, a “cell expressing a TLR” refers to any cell which expresses, either naturally or artificially, a functional TLR. A functional TLR is a full-length TLR protein or a fragment thereof capable of inducing a signal in response to interaction with its ligand. Generally the functional TLR will include at least a TLR ligand-binding fragment of the extracellular domain of the full-length TLR and at least a fragment of a TIR domain capable of interacting with another Toll homology domain-containing polypeptide, e.g., MyD88. In various embodiments the functional TLR is a full-length TLR selected from TLR1, TLR2, TLR3, TLR4, TLR5, TLR6, TLR7, TLR8, TLR9, and TLR10.
- In certain embodiments the functional TLR is naturally expressed by a cell.
- In one embodiment the cell naturally expresses functional TLR and is an isolated cell from human multiple myeloma cell line RPMI 8226 (ATCC CCL-155). This cell line was established from the peripheral blood of a 61 year old man at the time of diagnosis of multiple myeloma (IgG lambda type). Matsuoka Y et al. (1967) Proc Soc Exp Biol Med 125:1246-50. RPMI 8226 was previously reported as responsive to CpG nucleic acids as evidenced by the induction of IL-6 protein and IL-12p40 mRNA. Takeshita F et al. (2000) Eur J Immunol 30:108-16; Takeshita F et al. (2000) Eur J Immunol 30:1967-76. Takeshita et al. used the cell line solely to study promoter constructs in order to identify transcription factor binding sites important for CpG nucleic acid signaling. It is now known that RPMI 8226 cells secrete a number of other chemokines and cytokines including IL-8, IL-10 and IP-10 in response to immunostimulatory nucleic acids. Because this cell line expresses TLR9, through which immunostimulatory nucleic acids such as for example CpG nucleic acids mediate their effects, it is a suitable cell line for use in the methods of the invention relating to CpG nucleic acids as reference and test compounds, as well as to other TLR9 ligands.
- Similar to peripheral blood mononuclear cells (PBMCs), the RPMI 8226 cell line has been observed to upregulate its cell surface expression of markers such as CD71, CD86 and HLA-DR in response to CpG nucleic acid exposure. This has been observed by flow cytometric analysis of the cell line. Accordingly, the methods provided herein can be structured to use appropriately selected cell surface marker expression as a readout, in addition to or in place of chemokine or cytokine production or other readouts described elsewhere herein.
- The RPMI 8226 cell line has also been found to respond to certain small molecules including imidazoquinoline compounds. For example, incubation of RPMI 8226 cells with the imidazoquinoline compound R848 (resiquimod) induces IL-8, IL-10, and IP-10 production. It has recently been reported that R848 mediates its immunostimulatory effects through TLR7 and TLR8. The ability of RPMI 8226 to respond to R848 suggests that the RPMI 8226 cell line also expresses TLR7, as previously reported for normal human B cells.
- The RPMI cell line can be used in unmodified form or in a modified form. In one embodiment, the RPMI 8226 cell is transfected with a reporter construct. Preferably, the cell is stably transfected with the reporter construct. The reporter construct generally includes a promoter, a coding sequence and a polyadenylation signal. The coding sequence can include a reporter sequence selected from the group consisting of an enzyme (e.g., luciferase, alkaline phosphatase, beta-galactosidase, chloramphenicol acetyltransferase (CAT), secreted alkaline phosphatase, etc.), a bioluminescence marker (e.g., green fluorescent protein (GFP, U.S. Pat. No. 5,491,084), etc.), a surface-expressed molecule (e.g., CD25), a secreted molecule (e.g., IL-8, IL-12 p40, TNF-α, etc.), and other detectable protein products known to those of skill in the art. Preferably, the coding sequence encodes a protein having a level or an activity that is quantifiable.
- In certain embodiments the functional TLR is artificially expressed (including over-expressed) by a cell, for example by introduction into the cell of an expression vector bearing a coding sequence for the functional TLR wherein the coding sequence is operably linked to a gene expression sequence. As used herein, a coding sequence and the gene expression sequence are said to be operably linked when they are covalently linked in such a way as to place the expression or transcription and/or translation of the coding sequence under the influence or control of the gene expression sequence. Two DNA sequences are said to be operably linked if induction of a promoter in the 5′ gene expression sequence results in the transcription of the coding sequence and if the nature of the linkage between the two DNA sequences does not (1) result in the introduction of a frame-shift mutation, (2) interfere with the ability of the promoter region to direct the transcription of the coding sequence, or (3) interfere with the ability of the corresponding RNA transcript to be translated into a protein. Thus, a gene expression sequence would be operably linked to a coding sequence if the gene expression sequence were capable of effecting transcription of that coding sequence such that the resulting transcript is translated into the desired protein or polypeptide.
- In some embodiments a coding sequence refers to a nucleic acid sequence coding for a functional TLR. In some embodiments a coding sequence refers to a nucleic acid sequence coding for a reporter.
- A cell that artificially expresses a functional TLR can be a cell that does not express the functional TLR but for the TLR expression vector. For example, human 293 fibroblasts (ATCC CRL-1573) do not express TLR3, TLR7, TLR8, or TLR9. As described in the examples below, such cells can be transiently or stably transfected with suitable expression vector (or vectors) so as to yield cells that do express TLR3, TLR7, TLR8, TLR9, or any combination thereof. Alternatively, a cell that artificially expresses a functional TLR can be a cell that expresses the functional TLR at a significantly higher level with the TLR expression vector than it does without the TLR expression vector.
- For use in the methods of the instant invention, a cell that artificially expresses a functional TLR is preferably a stably transfected cell that expresses the functional TLR. Such a cell can also be stably transfected with a suitable reporter construct.
- As used herein, “TLR-mediated signaling” refers to any portion of the intracellular signal transduction pathway involving interaction of a TLR with a suitable TLR ligand and engagement by the TLR of MyD88 or any aspect downstream of MyD88 engagement, described above in relation to the structure and function of the TIR domain of a TLR.
- As used herein, “TLR-mediated immunostimulatory signaling” refers to TLR-mediated signaling that results in a measurable immunostimulatory response. This term applies to such signaling both in vitro and in vivo.
- As used herein, “TLR-mediated immunostimulation in a subject” refers to TLR-mediated immunostimulatory signaling as it applies in vivo.
- As used herein, the term “treat” as used in reference to a disorder, disease, or condition means to intervene in such disorder, disease, or condition so as to prevent or slow the development of, to prevent, slow or halt the progression of, or to eliminate the disorder, disease, or condition.
- Immunostimulatory Nucleic Acids
- As used herein, “immunostimulatory nucleic acid” refers to a nucleic acid molecule that, when contacted with a cell of the immune system, induces the cell of the immune system to become activated. Immune cell activation can be determined using any suitable means known to one of skill in the art, including but not limited to measurement of stimulated growth, proliferation, differentiation, migration, transcription or expression of gene products that are expressed or secreted in association with immune activation, activity of intracellular signaling pathways, and the like. Examples of markers of immune cell activation include, without limitation, secretion of cytokines (e.g., IL-6), secretion of immunoglobulin (e.g., IgG), expression of cell surface antigens (e.g., CD86), induction of cytolytic activity, and induction and nuclear translocation of transcription factors (e.g., NF-κB).
- A “nucleic acid” as used herein with respect to the methods of the invention, shall refer to any polymer of two or more individual nucleoside or nucleotide units. Nucleic acids can be single- or double-stranded. Typically individual nucleoside or nucleotide units will include any one or combination of deoxyribonucleosides, ribonucleosides, deoxyribonucleotides, and ribonucleotides. The individual nucleotide or nucleoside units of the nucleic acid can be naturally occurring or not naturally occurring. For example, the individual nucleotide units can include deoxyadenosine, deoxycytidine, deoxyguanosine, thymidine, and uracil. In addition to naturally occurring 2′-deoxy and 2′-hydroxyl forms, individual nucleosides also include synthetic nucleosides having modified base moieties and/or modified sugar moieties, e.g., as described in Uhlmann E et al. (1990) Chem Rev 90:543-84. The linkages between individual nucleotide or nucleoside units can be naturally occurring or not naturally occurring. For example, the linkages can be phosphodiester, phosphorothioate, phosphorodithioate, phosphoramidate, as well as peptide linkages and other covalent linkages, known in the art, suitable for joining adjacent nucleoside or nucleotide units. Immunostimulatory nucleic acids typically range in size from 3-4 units to a few tens of units, e.g., 18-40 units, although longer nucleic acids are also contemplated by the invention.
- In some embodiments the nucleic acids are oligonucleotides made up of 2 to about 100 nucleotides, and more typically 4 to about 40 nucleotides. Oligonucleotides composed exclusively of deoxyribonucleotides are termed oligodeoriboxynucleotides or, equivalently, oligodeoxynucleotides (ODN).
- An immunostimulatory nucleic acid includes any of a number of different types of immunostimulatory nucleic acids, including specifically immunostimulatory CpG nucleic acids (CpG nucleic acids), including but not limited to types A, B, and C; and immunostimulatory non-CpG nucleic acids, including without limitation methylated CpG nucleic acids, T-rich nucleic acids, and poly-G nucleic acids. Certain of these various classes of immunostimulatory nucleic acids can coexist in a given nucleic acid molecule.
- As used herein, the terms “CpG nucleic acid” and, equivalently, “CpG ODN” refer to an immunostimulatory nucleic acid which contains a cytosine-guanine (CG) dinucleotide, the C residue of which is unmethylated. The effects of CpG nucleic acids on immune modulation have been described extensively in U.S. patents such as U.S. Pat. Nos. 6,194,388; 6,207,646; 6,218,371; and 6,239,116, and published international patent applications, such as WO 98/37919, WO 98/40100, WO 98/52581, and WO 99/56755. The entire contents of each of these patents and published patent applications is hereby incorporated by reference. The entire immunostimulatory nucleic acid can be unmethylated or portions may be unmethylated but at least the C of the 5′-CG-3′ must be unmethylated. The CpG nucleic acid sequences of the invention include those broadly described above as well as disclosed in U.S. Pat. Nos. 6,207,646 B1 and 6,239,116 B1.
- In one embodiment the CpG nucleic acid has a base sequence provided by 5′-TCGTCGTTTTGTCGTTTTGTCGTT-3′ (ODN 2006; SEQ ID NO:1).
- CpG nucleic acids have been further classified by structure and function into at least the following three types, all of which are intended to be encompassed within the methods of the instant invention: Type B CpG nucleic acids such as ODN 2006 include the earliest described CpG nucleic acids and characteristically activate B cells but do not induce or only weakly induce expression of IFN-α. Type A CpG nucleic acids, described in published international application PCT/US00/26527 (WO 01/22990), incorporate a CpG motif, include a hybrid phosphodiester/phosphorothioate backbone, and characteristically induce plasmacytoid dendritic cells to express large amounts of IFN-α but do not activate or only weakly activate B cells. Type C oligonucleotides incorporate a CpG, include a chimeric backbone, include a GC-rich palindromic or nearly-palindromic region, and are capable of both activating B cells and inducing expression of IFN-α. These have been described, for example, in published U.S. patent application 2003/0148976.
- In other embodiments of the invention, a non-CpG nucleic acid is used. A non-CpG nucleic acid is an immunostimulatory nucleic acid which either does not have a CpG motif in its sequence, or has a CpG motif which contains a methylated C residue. In some instances, the non-CpG nucleic acid may still be immunostimulatory by virtue of its having other immunostimulatory motifs such as those described herein and known in the art. In one embodiment the non-CpG nucleic acid is a methylated CpG nucleic acid. In some instances the non-CpG nucleic acid is still immunostimulatory despite methylation of the C of the CpG motif, even without having another non-CpG immunostimulatory motif described herein and known in the art.
- In one embodiment the non-CpG nucleic acid is a methylated CpG nucleic acid having a base sequence provided by 5′-TZGTZGTTTTGTZGTTTTGTZGTT-3′ (ODN 2117; SEQ ID NO:4, wherein Z represents 5-methylcytidine).
- Non-CpG nucleic acids include T-rich immunostimulatory nucleic acids. The T-rich immunostimulatory nucleic acids include those disclosed in published PCT patent application PCT/US00/26383, the entire contents of which are incorporated herein by reference. In some embodiments, T-rich nucleic acids 24 bases in length are used. A T-rich nucleic acid is a nucleic acid which includes at least one poly T sequence and/or which has a nucleotide composition of greater than 25% T nucleotide residues. A nucleic acid having a poly-T sequence includes at least four Ts in a row, such as 5′-TTTT-3′. In some embodiments the T-rich nucleic acid includes more than one poly T sequence. In important embodiments, the T-rich nucleic acid may have 2, 3, 4, or more poly T sequences, such as ODN 2006.
- Non-CpG nucleic acids also include poly-G immunostimulatory nucleic acids. A variety of references describe the immunostimulatory properties of poly-G nucleic acids. Pisetsky D S et al. (1993) Mol Biol Reports 18:217-221; Krieger M et al. (1994) Ann Rev Biochem 63:601-637; Macaya R F et al. (1993) Proc Natl Acad Sci USA 90:3745-3749; Wyatt J R et al. (1994) Proc Natl Acad Sci USA 91:1356-1360; Rando and Hogan, 1998, In Applied Antisense Oligonucleotide Technology, Krieg and Stein, eds., pp. 335-352; Kimura Y et al. (1994) J Biochem (Tokyo) 116:991-994.
- The immunostimulatory nucleic acids of the invention can also be those which do not possess CpG, methylated CpG, T-rich, or poly-G motifs.
- Exemplary immunostimulatory nucleic acid sequences further include but are not limited to those immunostimulatory sequences described and listed in published PCT patent application WO 01/22972.
- In one aspect the invention provides novel compounds that fall within Formula VI and Formula VII as disclosed herein. These compounds include various diarylmethane TLR9 antagonists denoted herein as CMZ 203-84, CMZ 203-85, CMZ 203-88, CMZ 203-88-1, CMZ 203-89, and CMZ 203-91. Syntheses for each of these compounds are provided in Examples 12-17, respectively.
- Similar to other compounds of Formula VI and Formula VII, these specific compounds were tested in vitro and found to inhibit TLR9 signaling. See Example 18. Thus compounds CMZ 203-84, CMZ 203-85, CMZ 203-88, CMZ 203-88-1, CMZ 203-89, and CMZ 203-91 are believed to be useful in the methods of the invention. More specifically, in one aspect the invention provides a method of affecting TLR-mediated signaling in response to a TLR ligand. The method includes the step of contacting a cell expressing a TLR with an effective amount of any one or combination of compounds CMZ 203-84, CMZ 203-85, CMZ 203-88, CMZ 203-88-1, CMZ 203-89, and CMZ 203-91, to inhibit or promote TLR-mediated signaling in response to a ligand for the TLR. In one aspect the invention provides a method of inhibiting TLR-mediated signaling in response to a TLR ligand. The method includes the step of contacting a cell expressing a TLR with an effective amount of any one or combination of compounds CMZ 203-84, CMZ 203-85, CMZ 203-88, CMZ 203-88-1, CMZ 203-89, and CMZ 203-91, to inhibit TLR-mediated immunostimulatory signaling in response to a ligand for the TLR. In one aspect the invention provides a method of affecting TLR-mediated immunostimulation in a subject. The method includes the step of administering to a subject having or at risk of developing TLR-mediated immunostimulation an effective amount of any one or combination of compounds CMZ 203-84, CMZ 203-85, CMZ 203-88, CMZ 203-88-1, CMZ 203-89, and CMZ 203-91, to inhibit or promote TLR-mediated immunostimulation in the subject. In one aspect the invention provides a method of inhibiting TLR-mediated immunostimulation in a subject. The method includes the step of administering to a subject having or at risk of developing TLR-mediated immunostimulation an effective amount of any one or combination of compounds CMZ 203-84, CMZ 203-85, CMZ 203-88, CMZ 203-88-1, CMZ 203-89, and CMZ 203-91, to inhibit TLR-mediated immunostimulation in the subject. In one aspect the invention provides a method of inhibiting an immunostimulatory nucleic acid-associated response in a subject. The method according to this aspect includes the step of administering to a subject in need of such treatment an effective amount of any one or combination of compounds CMZ 203-84, CMZ 203-85, CMZ 203-88, CMZ 203-88-1, CMZ 203-89, and CMZ 203-91, to inhibit an immunostimulatory nucleic acid-associated response in the subject.
- In one aspect the invention provides novel substituted 4-primary amino quinoline compositions. As described further below, these compositions and other substituted 4-primary amino quinoline compositions have been discovered to be useful in methods for inhibiting an immune response, both in vitro and in vivo, including methods for treating immune complex associated diseases and autoimmune disorders. Due to their similarity to certain known antimalarial agents, it is also believed that the novel substituted 4-primary amino quinoline compositions of the invention will also be useful for prevention and treatment of malaria, as well as for treatment of other diseases which have been described to be responsive to treatment with chloroquines.
-
- X is absent or is an aryl, alkyl, heterocyclic, or styryl group;
- R1 and R2 are each independently a hydrogen atom or a substituted or unsubstituted alkyl, alkenyl, or aryl group, wherein R1 and R2 optionally are combined to form a heterocycle;
- R3 is a hydrogen atom, a halogen atom, or an alkyl, alkenyl, aryl, heterocyclic, nitro, cyano, carboxy, ester, ketone, amino, amido, hydroxy, alkoxy, mercapto, thio, sulfoxide, sulfone, or sulfonamido group, wherein R1 and R3 optionally are combined to form a heterocycle or a carbocycle;
- Y is absent or is an oxygen atom, a sulfur atom, CR8R9, or NR10, where R8, R9, and R10 are each independently a hydrogen atom or a substituted or unsubstituted alkyl, alkenyl, or aryl group;
- L is an alkyl or alkenyl group containing from 1 to 10 carbons or is an aryl group; and
- R4, R5, R6, and R7 are each independently a hydrogen atom, a halogen atom, or an alkyl, alkenyl, aryl, heterocyclic, nitro, cyano, carboxy, ester, ketone, amino, amido, hydroxy, alkoxy, mercapto, thio, sulfoxide, sulfone, or sulfonamido group, wherein any pair of R4, R5, R6, and R7 which are adjacent one another optionally are combined to form a heterocycle or a carbocycle,
- and pharmaceutically acceptable hydrates and salts thereof.
- In one embodiment R5 and R6 are each independently a halogen atom or an alkoxy group. In one embodiment R5 and R6 are each independently a chlorine atom or a methoxy group.
- In one embodiment X is absent or is an aryl group;
- NR1R2 is a heterocyclic amine or is NR8(CH2)nNR9R10, wherein n is an integer from 2 to 6, inclusive, and R8, R9, and R10 are each independently a hydrogen atom or an alkyl group;
- R3 is a hydrogen atom;
- Y is an aryl group or is NR11 where R11 is a hydrogen atom or an aryl or alkyl group;
- L is absent or is a C2-C6 alkyl group; and
- R4, R5, R6, and R7 are each independently a hydrogen atom, a halogen atom, or an alkoxy group.
- In one embodiment X is absent or is an aryl group;
- NR1R2 is a substituted or unsubstituted piperazino or morpholino group or is NR8(CH2)nNR9R10, wherein n is an integer from 2 to 6, inclusive, R8 is a hydrogen atom, and R9 and R10 are each independently an alkyl group;
- R3 is a hydrogen atom;
- Y is NH;
- L is a C2-C6 alkyl group; and
- R4, R5, R6, and R7 are each independently a hydrogen atom, a halogen atom, or an alkoxy group.
- In one embodiment X is a phenyl group;
-
- Y is NH;
- L is —(CH2)n— where n is an integer between 2 and 6, inclusive; and
- each of R3, R4, R5, R6, and R7 is a hydrogen atom.
- In each of the foregoing embodiments, the composition is optionally in the form of a pharmaceutically acceptable hydrate or salt.
- Representative, non-limiting examples of substituted 4-primary amino quinoline compositions of Formula XVI of the invention are compounds 101-104, 106-109, 111, 113-116, and 118-119, presented in Table 1.
TABLE 1 Substituted 4-Primary Amino Quinoline Compositions of the Invention ID X NR1R2 Y L R3 R4 R5 R6 R7 101 NH —(CH2)2— H H H H H 102 NH —(CH2)3— H H H H H 103 NH —(CH2)2— H H H H H 104 NH —(CH2)4— H H H H H 106 NH —(CH2)5— H H H H H 107 NH —(CH2)2— H H Cl H H 108 NH —(CH2)3— H H H H H 109 NH —(CH2)6— H H H H H 111 NH —(CH2)3— H H H H H 113 NH —(CH2)2— H H H H H 114 NH —(CH2)3— H H H H H 115 NH —(CH2)4— H H H H H 116 NH —(CH2)2— H H H H H 118 NH —(CH2)3— H H H H H 119 NH —(CH2)2— H H H H H - It has been discovered according to the invention that 4-primary amino quinoline compositions, similar to 4-secondary and 4-tertiary amino quinoline compounds, can be used to inhibit TLR9 signaling.
- In one aspect the invention provides a method for inhibiting signaling by a TLR. The method according to this aspect of the invention involves contacting a cell expressing a functional TLR with an effective amount of a substituted 4-primary amino quinoline composition having structural Formula XVII
wherein - X is absent or is a nitrogen, oxygen, or sulfur atom or an SO or SO2 group;
- R1 is a hydrogen atom or a substituted or unsubstituted aryl, alkyl, heterocyclic or styryl group;
- R2 is a hydrogen atom, a halogen atom, or an alkyl, alkenyl, aryl, heterocyclic, nitro, cyano, carboxy, ester, ketone, amino, amido, hydroxy, alkoxy, mercapto, thio, sulfoxide, sulfone, or sulfonamido group, wherein R1 and R2 optionally are combined to form a heterocycle or carbocycle;
- Y is absent or is an oxygen atom, a sulfur atom, CR7R8, or NR9, where R7, R8, and R9 are each independently a hydrogen atom or a substituted or unsubstituted alkyl, alkenyl, or aryl group;
- L is absent or is an alkyl or alkenyl group containing from 1 to 10 carbons or is an aryl group; and
- R3, R4, R5, and R6 are each independently a hydrogen atom, a halogen atom, or an alkyl, alkenyl, aryl, heterocyclic, nitro, cyano, carboxy, ester, ketone, amino, amido, hydroxy, alkoxy, mercapto, thio, sulfoxide, sulfone, or sulfonamido group, wherein any pair of R3, R4, R5, and R6 which are adjacent one another optionally are combined to form a heterocycle or a carbocycle, and
- pharmaceutically acceptable hydrates and salts thereof, to inhibit signaling by the TLR. The substituted 4-primary amino quinoline composition in this and all other aspects of the invention involving the use of a substituted 4-primary amino quinoline composition can be in the form a hydrate or pharmaceutically acceptable salt. The method according to this aspect of the invention can be performed in vitro or it can be performed in vivo. In addition, the cell expressing the functional TLR can, but need not necessarily, be an immune cell. For example, the cell expressing the functional TLR can be a cell transfected with an expression vector that directs expression of the TLR by the cell. In one embodiment the TLR is TLR9 and the method is thus a method for inhibiting intracellular signaling by TLR9.
- In addition to compounds 101-104, 106-109, 111, 113-116, and 118-119, presented in Table 1, the following additional representative and non-limiting substituted 4-primary amino quinoline compounds, compounds 105, 110, 117, and 120-133, presented in Table 2 with reference to Formula XVII, can be used in the method according to this and all other aspects of the invention directed to methods involving use of substituted 4-primary amino quinoline compounds. Additional examples are presented in the Examples below.
TABLE 2 Additional Substituted 4-Primary Amino Quinoline Compositions for Use in Methods of the Invention ID X R1 Y L R2 R3 R4 R5 R6 101 absent NH —(CH2)2— H H H H H 102 absent NH —(CH2)3— H H H H H 103 absent NH —(CH2)2— H H H H H 104 absent NH —(CH2)4— H H H H H 105 absent NH H H H H H 106 absent NH —(CH2)5— H H H H H 107 absent NH —(CH2)2— H H Cl H H 108 absent NH —(CH2)3— H H H H H 109 absent NH —(CH2)6— H H H H H 110 absent NH —(CH2)2— H H H H H 111 absent NH —(CH2)3— H H H H H 112 absent NH —(CH2)4— H H H H H 113 absent NH —(CH2)2— H H H H H 114 absent NH —(CH2)3— H H H H H 115 absent NH —(CH2)4— H H H H H 116 absent NH —(CH2)2— H H H H H 117 absent NH —(CH2)2— H H H H H 118 absent NH —(CH2)3— H H H H H 119 absent NH —(CH2)2— H H H H H 120 absent NH H H H H H 121 absent —(CH2)2— H H H H H 122 NCH3 —CH2— absent absent CH2 H H H H 123 NCH3 —CH2— absent absent CH2 H CH3 H H 124 NCH3 —CH2— absent absent CH2 H Br H H 125 NCH3 —(CH2)3— absent absent CH2 H H H H 126 —CH2— absent absent CH2 H H H H 127 —CH2— absent absent CH2 H CH3 H H 128 NCH3 CH3 absent absent H H H H H 129 absent NH —(CH2)2— H H Cl H H 130 absent NH —(CH2)2— H H Cl H H 131 absent NCH2CH3 —(CH2)2— H H H H H 132 absent O —(CH2)2— H H H H H 133 absent S —(CH2)2— H H H H H - In one aspect the invention provides a method for inhibiting signaling by a TLR. The method according to this aspect of the invention involves contacting an immune cell expressing a functional TLR with
- (a) an effective amount of a TLR signal agonist to stimulate signaling by the TLR in absence of a substituted 4-primary amino quinoline composition, and
- (b) an effective amount of a substituted 4-primary amino quinoline composition having structural Formula XVII, as defined above, to inhibit signaling by the TLR in response to the TLR signal agonist compared with the signaling by the TLR in response to the TLR signal agonist in absence of the substituted 4-primary amino quinoline composition. The substituted 4-primary amino quinoline composition in this aspect of the invention can be in the form a hydrate or pharmaceutically acceptable salt. The method according to this aspect of the invention can be performed in vitro or it can be performed in vivo.
- Also according to this aspect of the invention, in one embodiment the TLR is TLR9 and the TLR signal agonist is a TLR9 signal agonist, and the method in one embodiment is thus a method of inhibiting intracellular signaling by TLR9 in response to a TLR9 signal agonist. The TLR signal agonist in one embodiment is CpG DNA, for example a CpG ODN such as ODN 2006. The CpG ODN can belong to any class of CpG ODN, including A-class (e.g., ODN 2216), B-class (e.g., ODN 2006), or C-class (e.g., ODN 2395).
- In one embodiment the TLR signal agonist is an immune complex that includes a nucleic acid. Immune complexes that include a nucleic acid are known by those of skill in the art to include immunoglobulin complexed with nucleic acids that are either naked or, more commonly, that are associated with proteins or other non-nucleic acid components. The nucleic acids that are associated with proteins or other non-nucleic acid components can include, for example, chromatin, ribosomes, small nuclear proteins, ribonuclear proteins (RNP), histones, nucleosomal protein-DNA complexes, and nucleosomes. Examples of clinically important antibodies specific for nucleic acids and for nucleic acid-containing material include antinuclear antibodies (ANA), anti-dsDNA, anti-ssDNA, anti-Sm, anti-RNP, anti-Ro (SS-A), anti-La (SS-B), and antihistone antibodies. Immune complexes that include a nucleic acid include, without limitation, immunoglobulin complexed with DNA, including specifically double-stranded DNA, and immunoglobulin complexed with nucleosomal material, both characteristic of systemic lupus erythematosus, and immunoglobulin complexed with RNA.
- In one aspect the invention provides a method for inhibiting an immune response to an antigenic substance. The method according to this aspect of the invention involves contacting an immune cell expressing a functional Toll-like receptor with
- (a) an effective amount of an antigenic substance to stimulate an immune response to the antigenic substance in absence of a substituted 4-primary amino quinoline composition, and
- (b) an effective amount of a substituted 4-primary amino quinoline composition having structural Formula XVII, as defined above, to inhibit an immune response to the antigenic substance compared with the immune response to the antigenic substance in absence of the substituted 4-primary amino quinoline composition. The substituted 4-primary amino quinoline composition in this aspect of the invention can be in the form a hydrate or pharmaceutically acceptable salt. The method according to this aspect of the invention can be performed in vitro or it can be performed in vivo. In one embodiment the immune response is an innate immune response. In one embodiment the immune response includes an adaptive immune response.
- In one embodiment the method involves contacting, in a subject, an immune cell expressing a functional Toll-like receptor with
- (a) an effective amount of an antigenic substance to stimulate an immune response in the subject to the antigenic substance in absence of a substituted 4-primary amino quinoline composition, and
- (b) an effective amount of a substituted 4-primary amino quinoline composition having structural Formula XVII, as defined above, to inhibit an immune response in the subject to the antigenic substance compared with the immune response to the antigenic substance in absence of the substituted 4-primary amino quinoline composition.
- In one embodiment the step of contacting an immune cell expressing a functional Toll-like receptor with an effective amount of an antigenic substance to stimulate an immune response to the antigenic substance in absence of a substituted 4-primary amino quinoline composition involves the active step of administering an effective amount of an antigenic substance to stimulate an immune response to the antigenic substance in absence of a substituted 4-primary amino quinoline composition. The antigenic substance can be administered using any effective route or means for administering the antigenic substance to effect the contacting. By way of example, the administering can be by local or systemic injection, inhalation, oral ingestion, topical administration, mucosal administration, or any combination thereof.
- In one embodiment the step of contacting an immune cell expressing a functional Toll-like receptor with an effective amount of an antigenic substance to stimulate an immune response to the antigenic substance in absence of a substituted 4-primary amino quinoline composition is passive. For example, in one embodiment the immune cell is already in contact or has already been in contact with an effective amount of an antigenic substance to stimulate an immune response to the antigenic substance, at the time of or prior to the step of contacting the immune cell expressing a functional Toll-like receptor with an effective amount of a substituted 4-primary amino quinoline composition having structural Formula XVII, as defined above, to inhibit an immune response to the antigenic substance compared with the immune response to the antigenic substance in absence of the substituted 4-primary amino quinoline composition.
- The antigenic substance can be an allergen. An allergen is a substance that can induce an allergic or asthmatic response in a susceptible subject. The list of allergens is enormous and can include pollens, insect venoms, animal dander, dust, fungal spores and drugs (e.g., penicillin). Examples of natural animal and plant allergens include proteins specific to the following genera: Canis (Canis familiaris); Dermatophagoides (e.g., Dermatophagoides farinae); Felis (Felis domesticus); Ambrosia (Ambrosia artemiisfolia); Lolium (e.g., Lolium perenne or Lolium multiflorum); Cryptomeria (Cryptomeria japonica); Alternaria (Alternaria alternata); Alder; Alnus (Alnus gultinosa); Betula (Betula verrucosa); Quercus (Quercus alba); Olea (Olea europa); Artemisia (Artemisia vulgaris); Plantago (e.g., Plantago lanceolata); Parietaria (e.g., Parietaria officinalis or Parietaria judaica); Blattella (e.g., Blattella germanica); Apis (e.g., Apis multiforum); Cupressus (e.g., Cupressus sempervirens, Cupressus arizonica, and Cupressus macrocarpa); Juniperus (e.g., Juniperus sabinoides, Juniperus virginiana, Juniperus communis, and Juniperus ashel); Thuya (e.g., Thuya orientalis); Chamaecyparis (e.g., Chamaecyparis obtusa); Periplaneta (e.g., Periplaneta americana); Agropyron (e.g., Agropyron repens); Secale (e.g., Secale cereale); Triticum (e.g., Triticum aestivum); Dactylis (e.g., Dactylis glomerata); Festuca (e.g., Festuca elatior); Poa (e.g., Poapratensis or Poa compressa); Avena (e.g., Avena sativa); Holcus (e.g., Holcus lanatus); Anthoxanthum (e.g., Anthoxanthum odoratum); Arrhenatherum (e.g., Arrhenatherum elatius); Agrostis (e.g., Agrostis alba); Phleum (e.g., Phleum pratense); Phalaris (e.g., Phalaris arundinacea); Paspalum (e.g., Paspalum notatum); Sorghum (e.g., Sorghum halepensis); and Bromus (e.g., Bromus inermis). The term “allergy” refers to acquired hypersensitivity to a substance (allergen). An “allergic reaction” is the response of an immune system to an allergen in a subject allergic to the allergen. Allergic conditions include eczema, allergic rhinitis or coryza, hay fever, bronchial asthma, urticaria (hives) and food allergies, and other atopic conditions.
- The antigenic substance can be an antigen that is or is derived from an infectious microbial agent, including a bacterium, a virus, a fungus, or a parasite. Examples of infectious bacteria include: Helicobacter pyloris, Borrelia burgdorferi, Legionella pneumophilia, Mycobacteria sps (such as. M. tuberculosis, M. avium, M. intracellulare, M. kansasii, and M. gordonae), Staphylococcus aureus, Neisseria gonorrhoeae, Neisseria meningitidis, Listeria monocytogenes, Streptococcus pyogenes (Group A Streptococcus), Streptococcus agalactiae (Group B Streptococcus), Streptococcus (viridans group), Streptococcus faecalis, Streptococcus bovis, Streptococcus (anaerobic sps.), Streptococcus pneumoniae, pathogenic Campylobacter sp., Enterococcus sp., Haemophilus influenzae, Bacillus anthracis, Corynebacterium diphtheriae, Corynebacterium sp., Erysipelothrix rhusiopathiae, Clostridium perfringens, Clostridium tetani, Enterobacter aerogenes, Klebsiella pneumoniae, Pasturella multocida, Bacteroides sp., Fusobacterium nucleatum, Streptobacillus moniliformis, Treponema pallidum, Treponema pertenue, Leptospira, and Actinomyces israelii. Examples of infectious virus include: Retroviridae (including but not limited to human immunodeficiency virus (HIV)); Picornaviridae (for example, polio viruses, hepatitis A virus; enteroviruses, human coxsackie viruses, rhinoviruses, echoviruses); Calciviridae (such as strains that cause gastroenteritis); Togaviridae (for example, equine encephalitis viruses, rubella viruses); Flaviviridae (for example, dengue viruses, encephalitis viruses, yellow fever viruses); Coronaviridae (for example, coronaviruses); Rhabdoviridae (for example, vesicular stomatitis viruses, rabies viruses); Filoviridae (for example, ebola viruses); Paramyxoviridae (for example, parainfluenza viruses, mumps virus, measles virus, respiratory syncytial virus); Orthomyxoviridae (for example, influenza viruses); Bunyaviridae (for example, Hantaan viruses, bunya viruses, phleboviruses, and Nairo viruses); Arena viridae (hemorrhagic fever viruses); Reoviridae (e.g., reoviruses, orbiviurses, and rotaviruses); Birnaviridae; Hepadnaviridae (Hepatitis B virus); Parvoviridae (parvoviruses); Papovaviridae (papilloma viruses, polyoma viruses); Adenoviridae (most adenoviruses); Herpesviridae (herpes simplex virus (HSV) 1 and HSV-2, varicella zoster virus, cytomegalovirus (CMV), herpes viruses); Poxyiridae (variola viruses, vaccinia viruses, pox viruses); and Iridoviridae (such as African swine fever virus); and unclassified viruses (for example, the etiological agents of spongiform encephalopathies, the agent of delta hepatitis (thought to be a defective satellite of hepatitis B virus), the agents of non-A, non-B hepatitis (class 1=internally transmitted; class 2=parenterally transmitted (i.e., Hepatitis C); Norwalk and related viruses, and astroviruses). Examples of infectious fungi include, but are not limited to, Cryptococcus neoformans, Histoplasma capsulatum, Coccidioides immitis, Blastomyces dermatitidis, Chlamydia trachomatis, and Candida albicans.
- The antigenic substance can be a cancer antigen. A cancer antigen as used herein is a compound, such as a peptide or protein, associated with a tumor or cancer cell surface and which is capable of provoking an immune response when expressed on the surface of an antigen-presenting cell in the context of a major histocompatibility complex (MHC) molecule. Cancer antigens can be prepared from cancer cells either by preparing crude extracts of cancer cells, for example, as described in Cohen P A et al. (1994) Cancer Res 54:1055-8, by partially purifying the antigens, by recombinant technology, or by de novo synthesis of known antigens. Cancer antigens include but are not limited to antigens that are recombinantly expressed, an immunogenic portion thereof, or a whole tumor or cancer cell. Such antigens can be isolated or prepared recombinantly or by any other means known in the art.
- The terms “cancer antigen” and “tumor antigen” are used interchangeably and refer to antigens which are differentially expressed by cancer cells and can thereby be exploited in order to target cancer cells. Cancer antigens are antigens which can potentially stimulate apparently tumor-specific immune responses. Some of these antigens are encoded, although not necessarily expressed, by normal cells. These antigens can be characterized as those which are normally silent (i.e., not expressed) in normal cells, those that are expressed only at certain stages of differentiation and those that are temporally expressed such as embryonic and fetal antigens. Other cancer antigens are encoded by mutant cellular genes, such as oncogenes (e.g., activated ras oncogene), suppressor genes (e.g., mutant p53), fusion proteins resulting from internal deletions or chromosomal translocations. Still other cancer antigens can be encoded by viral genes such as those carried on RNA and DNA tumor viruses. Examples of tumor antigens include MAGE, MART-1/Melan-A, gp 100, Dipeptidyl peptidase IV (DPPIV), adenosine deaminase-binding protein (ADAbp), cyclophilin b, Colorectal associated antigen (CRC)—C017-1A/GA733, Carcinoembryonic Antigen (CEA) and its immunogenic epitopes CAP-1 and CAP-2, etv6, aml1, Prostate Specific Antigen (PSA) and its immunogenic epitopes PSA-1, PSA-2, and PSA-3, prostate-specific membrane antigen (PSMA), T-cell receptor/CD3-zeta chain, MAGE-family of tumor antigens (e.g., MAGE-A1, MAGE-A2, MAGE-A3, MAGE-A4, MAGE-A5, MAGE-A6, MAGE-A7, MAGE-A8, MAGE-A9, MAGE-A10, MAGE-A11, MAGE-A12, MAGE-Xp2 (MAGE-B2), MAGE-Xp3 (MAGE-B3), MAGE-Xp4 (MAGE-B4), MAGE-C1, MAGE-C2, MAGE-C3, MAGE-C4, MAGE-C5), GAGE-family of tumor antigens (e.g., GAGE-1, GAGE-2, GAGE-3, GAGE-4, GAGE-5, GAGE-6, GAGE-7, GAGE-8, GAGE-9), BAGE, RAGE, LAGE-1, NAG, GnT-V, MUM-1, CDK4, tyrosinase, p53, MUC family, HER2/neu, p21ras, RCASi, α-fetoprotein, E-cadherin, α-catenin, β-catenin and γ-catenin, p120ctn, gp100Pmel117, PRAME, NY-ESO-1, cdc27, adenomatous polyposis coli protein (APC), fodrin, Connexin 37, Ig-idiotype, p15, gp75, GM2 and GD2 gangliosides, viral products such as human papillomavirus proteins, Smad family of tumor antigens, Imp-1, P1A, EBV-encoded nuclear antigen (EBNA)-1, brain glycogen phosphorylase, SSX-1, SSX-2 (HOM-MEL-40), SSX-1, SSX-4, SSX-5, SCP-1 and CT-7, and c-erbB-2.
- Cancers or tumors and tumor antigens associated with such tumors (but not exclusively), include acute lymphoblastic leukemia (etv6; aml1; cyclophilin b), B cell lymphoma (Ig-idiotype), glioma (E-cadherin; α-catenin; β-catenin; γ-catenin; p120ctn), bladder cancer (p21ras), biliary cancer (p21ras), breast cancer (MUC family; HER2/neu; c-erbB-2), cervical carcinoma (p53; p21ras), colon carcinoma (p21ras; HER2/neu; c-erbB-2; MUC family), colorectal cancer (Colorectal associated antigen (CRC)—C017-1A/GA733; APC), choriocarcinoma (CEA), epithelial cell cancer (cyclophilin b), gastric cancer (HER2/neu; c-erbB-2; ga733 glycoprotein), hepatocellular cancer (α-fetoprotein), Hodgkins lymphoma (lmp-1; EBNA-1), lung cancer (CEA; MAGE-3; NY-ESO-1), lymphoid cell-derived leukemia (cyclophilin b), melanoma (p15 protein, gp75, oncofetal antigen, GM2 and GD2 gangliosides), myeloma (MUC family; p21 ras), non-small cell lung carcinoma (HER2/neu; c-erbB-2), nasopharyngeal cancer (Imp-1; EBNA-1), ovarian cancer (MUC family; HER2/neu; c-erbB-2), prostate cancer (Prostate Specific Antigen (PSA) and its immunogenic epitopes PSA-1, PSA-2, and PSA-3; prostate-specific membrane antigen (PSMA); HER2/neu; c-erbB-2), pancreatic cancer (p21ras; MUC family; HER2/neu; c-erbB-2; ga733 glycoprotein), renal cancer (HER2/neu; c-erbB-2), squamous cell cancers of cervix and esophagus (viral products such as human papillomavirus proteins), testicular cancer (NY-ESO-1), T-cell leukemia (HTLV-1 epitopes), and melanoma (Melan-A/MART-1; cdc27; MAGE-3; p21ras; gp100Pmel117).
- In one aspect of the invention, a method of treating an autoimmune disorder in a subject is provided. The method according to this aspect of the invention involves the step of administering to a subject having an autoimmune disorder an effective amount of a substituted 4-primary amino quinoline composition having structural Formula XVII, as defined above, to treat the autoimmune disorder. The substituted 4-primary amino quinoline composition in this aspect of the invention can be in the form a hydrate or pharmaceutically acceptable salt. In one embodiment the autoimmune disorder is chosen from systemic lupus erythematosus, rheumatoid arthritis, inflammatory bowel disease, Sjögren's syndrome, polymyositis, vasculitis, Wegener's granulomatosis, sarcoidosis, ankylosing spondylitis, Reiter's syndrome, psoriatic arthritis, and Behçet's syndrome. In one particular embodiment the autoimmune disease is systemic lupus erythematosus. In one particular embodiment the autoimmune disease is rheumatoid arthritis. In one embodiment the subject is a human. The method can also be applied to animal models of any of the autoimmune disorders listed above.
- In one embodiment the autoimmune disorder is an immune complex associated disease. Immune complex associated diseases specifically include, without limitation, systemic lupus erythematosus, rheumatoid arthritis, polyarteritis nodosa, poststreptococcal glomerulonephritis, cryoglobulinemia, and acute and chronic serum sickness.
- The substituted 4-primary amino quinoline composition can be administered to the subject by any suitable route of administration, including, without limitation, oral and parenteral. Parenteral routes of administration include, without limitation, intravenous, intramuscular, intraperitoneal, subcutaneous, intranasal, intrapulmonary, transdermal, topical, and mucosal. Parenteral routes of administration also include direct injection into a specific tissue or other site of injection, including, for example, lymphoid tissue and a site of inflammation.
- It has been discovered according to the invention that quinzoline compounds structurally similar to quinolines of the invention are unexpectedly useful in methods for inhibiting an immune response, both in vitro and in vivo, including methods for treating immune complex associated diseases and autoimmune disorders. The quinazoline compounds and their methods of use according to the invention were unexpectedly found to retain much or all of the immunoinhibitory features of corresponding quinoline compounds and their methods of use, but with the additional feature of having reduced toxicity, at least in vivo.
- In one aspect the invention provides novel quinazoline compositions. As described further below, these compositions and other quinazoline compositions have been discovered to be useful in methods for inhibiting an immune response, both in vitro and in vivo, including methods for treating immune complex associated diseases and autoimmune disorders. Due to their similarity to certain known antimalarial agents, it is also believed that the novel quinazoline compositions of the invention will also be useful for prevention and treatment of malaria, as well as for treatment of other diseases which have been described to be responsive to treatment with chloroquines.
-
- X is absent or is an aryl, alkyl, heterocyclic or styryl group, provided that if X is a phenyl group, NR1R2 is part if a heterocycle or is a diamine;
- R1 and R2 are each independently a hydrogen atom or an alkyl, alkenyl, or aryl group, wherein R1 and R2 optionally are combined to form a heterocycle;
- Y is an oxygen atom, a sulfur atom, CR9R10, or NR11, where R9, R10, and R11 are each independently a hydrogen atom or an alkyl, alkenyl, or aryl group, wherein any one of R9, R10, and R11 optionally is combined with R3 or R4 to form a substituted or unsubstituted heterocycle;
- L is an alkyl or alkenyl group containing from 1 to 10 carbons or is an aryl group;
- R3 and R4 are each independently a hydrogen atom or an alkyl, alkenyl, or aryl group, wherein R3 and R4 optionally are combined to form a heterocycle; and
- R5, R6, R7, and R8 are each independently a hydrogen atom, a halogen atom, or an alkyl, alkenyl, aryl, heterocyclic, nitro, cyano, carboxy, ester, ketone, amino, amido, hydroxy, alkoxy, mercapto, thio, sulfoxide, sulfone, or sulfonamido group, wherein any pair of R5, R6, R7, and R8 which are adjacent one another optionally are combined to form a heterocycle or a carbocycle,
- and pharmaceutically acceptable hydrates and salts thereof.
- In one embodiment R6 and R7 are each independently a halogen atom or an alkoxy group.
- In one embodiment R6 and R7 are each independently a chlorine atom or a methoxy group.
- In one embodiment X is absent or is an aryl group;
- NR1R2 is a heterocyclic amine or NR9(CH2)nNR10R11, wherein n is an integer from 2 to 6, inclusive, and R9, R10, and R11 are each independently a hydrogen atom or an alkyl group;
- Y is an aryl group or is NR12 where R12 is a hydrogen atom or an aryl or alkyl group;
- L is absent or is a C2-C6 alkyl group;
- R3 and R4 are each independently a hydrogen atom or an alkyl group, wherein R3 and R4 optionally are combined to form a heterocycle; and
- R5, R6, R7, and R8 are each independently a hydrogen atom, a halogen atom, or an alkoxy group.
- In one embodiment X is absent or is an aryl group;
- NR1R2 is a substituted or unsubstituted piperazino or morpholino group or is NR9(CH2)nNR10R11, wherein n is an integer from 2 to 6, inclusive, R9 is a hydrogen atom, and R10 and R11 are each independently an alkyl group;
- Y is NH;
- L is a C2-C6 alkyl group;
- R3 and R4 are each independently a hydrogen atom or an alkyl group, wherein R3 and R4 optionally are combined to form a heterocycle; and
- R5, R6, R7, and R8 are each independently a hydrogen atom, a halogen atom, or an alkoxy group.
- In one embodiment X is an aryl group;
- NR1R2 is substituted or unsubstituted piperazino or morpholino group;
- Y is NH;
- L is a C2-C6 alkyl group;
- R3 and R4 are each independently a methyl or ethyl group or R3 and R4 optionally are combined to form a morpholino group; and
- R5, R6, R7, and R8 are each independently a hydrogen atom, a halogen atom, or an alkoxy group.
- In one embodiment X is a phenyl group;
- NR1R2 is N-methylpiperazine;
- Y is NH;
- L is —CH2CH2—;
- R3 and R4 are each a methyl group; and
- R5, R6, R7, and R8 are each a hydrogen atom.
- In one embodiment X is a phenyl group;
- NR1R2 is N-methylpiperazine;
- Y is NH;
- L is —CH2CH2—;
- R3 and R4 are combined as a morpholino group; and
- R5, R6, R7, and R8 are each a hydrogen atom.
- In one embodiment X is absent;
- NR1R2 is a substituted or unsubstituted piperazino or morpholino group;
- Y is NH;
- L is a C2-C6 alkyl group;
- R3 and R4 are each independently a methyl or ethyl group or R3 and R4 optionally are combined to form a morpholino group; and
- R5, R6, R7, and R8 are each independently a hydrogen atom, a halogen atom, or an alkoxy group.
- In one embodiment X is absent;
- NR1R2 is N-methylpiperazine;
- Y is NH;
- L is —CH2CH2—;
- R3 and R4 are each a methyl group;
- R6 and R7 are each a methoxy group; and
- R5 and R9 are each a hydrogen atom.
- In one embodiment X is absent;
- NR1R2 is N-phenylpiperazine;
- Y is NH;
- L is —CH2CH2—;
- R3 and R4 are each a methyl group;
- R6 and R7 are each a methoxy group; and
- R5 and R8 are each a hydrogen atom.
- In one embodiment X is absent;
- NR1R2 is N-methylpiperazine;
- Y is NH;
- L is —CH2CH2;
- R3 and R4 are combined as a morpholino group;
- R6 and R7 are each a methoxy group; and
- R5 and R8 are each a hydrogen atom.
- In each of the foregoing embodiments, the composition is optionally in the form of a pharmaceutically acceptable hydrate or salt.
- Representative, non-limiting examples of quinazoline compositions of Formula XVIII of the invention are compounds 201-214, presented in Table 3.
TABLE 3 Quinazoline Compositions of the Invention ID X NR1R2 Y L R3 R4 R5 R6 R7 R8 201 NH —(CH2)2— CH3 CH3 H H H H 202 NH —(CH2)3— CH3 CH3 H H H H 203 NH —(CH2)4— CH3 CH3 H H H H 204 NH —(CH2)5— CH3 CH3 H H H H 205 NH —(CH2)2— CH3 CH3 H H H H 206 NH —(CH2)2— CH2CH3 CH2CH3 H H H H 207 NH —(CH2)2— CH3 CH3 H Cl H H 208 NH —(CH2)2— CH3 CH3 H H H H 209 NH —(CH2)3— CH3 CH3 H H H H 210 NH —(CH2)2— CH3 CH3 H H H H 211 NH —(CH2)3— CH3 CH3 H H H H 212 NH —(CH2)2— CH3 CH3 H OCH3 OCH3 H 213 O —(CH2)2— CH3 CH3 H H H H 214 S —(CH2)2— CH3 CH3 H H H H - It has been discovered according to the invention that certain quinazoline compositions can be used to inhibit TLR9 signaling.
- In one aspect the invention provides additional novel quinazoline compounds useful in the methods of the invention. Such compounds are referred to herein as CMZ 203-34, CMZ 203-44, CMZ 203-45, CMZ 203-49, CMZ 203-51, CMZ 203-76, CMZ 203-78, CMZ 203-87, CMZ 203-93, and CMZ 203-95. Syntheses for each of these novel compounds are provided in Examples 19-28, respectively.
- Certain of these specific compounds have already been tested in vitro and found to inhibit TLR9 signaling. See Example 29. Thus compounds CMZ 203-34, CMZ 203-44, CMZ 203-45, CMZ 203-49, CMZ 203-51, CMZ 203-76, CMZ 203-78, CMZ 203-87, CMZ 202-93, and CMZ 203-95 are believed to be useful in the methods of the invention.
-
- X is a substituted or unsubstituted aryl, alkyl, heterocyclic or styryl group, optionally attached to the quinazoline by a nitrogen, oxygen, or sulfur atom or by a SO or SO2 group;
- Y is absent or is an oxygen atom, a sulfur atom, CR9R10, or NR11, where R9, R10, and R11 are each independently a hydrogen atom or an alkyl, alkenyl, or aryl group, wherein any one of R9, R10, and R11 optionally is combined with R3 or R4 to form a heterocycle;
- L is absent or is a hydrogen atom, an alkyl or alkenyl group containing from 1 to 10 carbons, or an aryl group;
- R3 and R4 are each independently a hydrogen atom or an alkyl, alkenyl, or aryl group, wherein R3 and R4 optionally are combined to form a heterocycle; and
- R5, R6, R7, and R8 are each independently a hydrogen atom, a halogen atom, or an alkyl, alkenyl, aryl, heterocyclic, nitro, cyano, carboxy, ester, ketone, amino, amido, hydroxy, alkoxy, mercapto, thio, sulfoxide, sulfone, or sulfonamido group, wherein any pair of R5, R6, R7, and R8 which are adjacent one another optionally are combined to form a heterocycle or a carbocycle,
- and pharmaceutically acceptable hydrates and salts thereof, to inhibit signaling by the TLR. The method according to this aspect of the invention can be performed in vitro or it can be performed in vivo. In addition, the cell expressing the functional TLR can, but need not necessarily, be an immune cell. For example, the cell expressing the functional TLR can be a cell transfected with an expression vector that directs expression of the TLR by the cell. In one embodiment the TLR is TLR9 and the method is thus a method for inhibiting intracellular signaling by TLR9.
- In addition to compounds 201-214, presented in Table 3, the following additional representative and non-limiting quinazoline compounds, compounds 215-229, presented in Table 4 with reference to Formula XIX, can be used in the method according to this and all other aspects of the invention directed to methods involving use of substituted 4-primary amino quinoline compounds. Additional examples are presented in the Examples below.
TABLE 4 Additional Quinazoline Compositions for Use in Methods of the Invention ID X Y L R3 R4 R5 R6 R7 R8 201 NH —(CH2)2— CH3 CH3 H H H H 202 NH —(CH2)3— CH3 CH3 H H H H 203 NH —(CH2)4— CH3 CH3 H H H H 204 NH —(CH2)5— CH3 CH3 H H H H 205 NH —(CH2)2— CH3 CH3 H H H H 206 NH —(CH2)2— CH2CH3 CH2CH3 H H H H 207 NH —(CH2)2— CH3 CH3 H Cl H H 208 NH —(CH2)2— CH3 CH3 H H H H 209 NH —(CH2)3— CH3 CH3 H H H H 210 NH —(CH2)2— CH3 CH3 H H H H 211 NH —(CH2)3— CH3 CH3 H H H H 212 NH —(CH2)2— CH3 CH3 H OCH3 OCH3 H 213 O —(CH2)2— CH3 CH3 H H H H 214 S —(CH2)2— CH3 CH3 H H H H 215 N(CH3) —(CH2)2— CH3 CH3 H H H H 216 NH —(CH2)2— CH3 CH3 H H H H 217 NH —(CH2)2— CH3 CH3 H H H H 218 NH —(CH2)2— CH3 CH3 H H H H 219 NH —(CH2)2— CH3 CH3 H H H H 220 NH —(CH2)2— CH3 CH3 H H H H 221 NH —(CH2)2— CH3 CH3 H H H H 222 absent absent H H H OCH3 OCH3 H 223 absent absent H H H OCH3 OCH3 H 224 absent —(CH2)3— CH2CH3 CH2CH3 H H H H 225 absent absent H H H H H 226 absent absent H H H H H 227 absent absent H H H H H 228 absent absent H H H H H 229 absent absent H H H H H H - In one aspect the invention provides a method for inhibiting signaling by a TLR. The method according to this aspect of the invention involves contacting an immune cell expressing a functional TLR with
- (a) an effective amount of a TLR signal agonist to stimulate signaling by the TLR in absence of a quinazoline composition, and
- (b) an effective amount of a quinazoline composition having structural Formula XIX, as defined above, to inhibit signaling by the TLR in response to the TLR signal agonist compared with the signaling by the TLR in response to the TLR signal agonist in absence of the quinazoline composition. The quinazoline composition in this aspect of the invention can be in the form a hydrate or pharmaceutically acceptable salt. The method according to this aspect of the invention can be performed in vitro or it can be performed in vivo.
- Also according to this aspect of the invention, in one embodiment the TLR is TLR9 and the TLR signal agonist is a TLR9 signal agonist, and the method in one embodiment is thus a method of inhibiting intracellular signaling by TLR9 in response to a TLR9 signal agonist. The TLR signal agonist in one embodiment is CpG DNA, for example a CpG ODN such as ODN 2006. The CpG ODN can belong to any class of CpG ODN, including A-class (e.g., ODN 2216), B-class (e.g., ODN 2006), or C-class (e.g., ODN 2395).
- In one embodiment the TLR signal agonist is an immune complex that includes a nucleic acid, as described above.
- In one aspect the invention provides a method for inhibiting an immune response to an antigenic substance. The method according to this aspect of the invention involves contacting an immune cell expressing a functional Toll-like receptor with
- (a) an effective amount of an antigenic substance to stimulate an immune response to the antigenic substance in absence of a quinazoline composition, and
- (b) an effective amount of a quinazoline composition having structural Formula XIX, as defined above, to inhibit an immune response to the antigenic substance compared with the immune response to the antigenic substance in absence of the quinazoline composition. The quinazoline composition in this aspect of the invention can be in the form a hydrate or pharmaceutically acceptable salt. The method according to this aspect of the invention can be performed in vitro or it can be performed in vivo. In one embodiment the immune response is an innate immune response. In one embodiment the immune response includes an adaptive immune response.
- In one embodiment the step of contacting an immune cell expressing a functional Toll-like receptor with an effective amount of an antigenic substance to stimulate an immune response to the antigenic substance in absence of a quinazoline composition involves the active step of administering an effective amount of an antigenic substance to stimulate an immune response to the antigenic substance in absence of a quinazoline composition. The antigenic substance can be administered using any effective route or means for administering the antigenic substance to effect the contacting. By way of example, the administering can be by local or systemic injection, inhalation, oral ingestion, topical administration, mucosal administration, or any combination thereof.
- In one embodiment the method involves contacting, in a subject, an immune cell expressing a functional Toll-like receptor with
- (a) an effective amount of an antigenic substance to stimulate an immune response in the subject to the antigenic substance in absence of a quinazoline composition, and
- (b) an effective amount of a quinazoline composition having structural Formula XVII, as defined above, to inhibit an immune response in the subject to the antigenic substance compared with the immune response to the antigenic substance in absence of the quinazoline composition.
- In one embodiment the step of contacting an immune cell expressing a functional Toll-like receptor with an effective amount of an antigenic substance to stimulate an immune response to the antigenic substance in absence of a quinazoline composition is passive. For example, in one embodiment the immune cell is already in contact or has already been in contact with an effective amount of an antigenic substance to stimulate an immune response to the antigenic substance, at the time of or prior to the step of contacting the immune cell expressing a functional Toll-like receptor with an effective amount of a quinazoline composition having structural Formula XVII, as defined above, to inhibit an immune response to the antigenic substance compared with the immune response to the antigenic substance in absence of the quinazoline composition.
- The antigenic substance can be an allergen, as described above.
- The antigenic substance can be an antigen that is or is derived from an infectious microbial agent, including a bacterium, a virus, a fungus, or a parasite, as described above.
- The antigenic substance can be a cancer antigen, as described above.
- In one aspect of the invention, a method of treating an autoimmune disorder in a subject is provided. The method according to this aspect of the invention involves the step of administering to a subject having an autoimmune disorder an effective amount of a quinazoline composition having structural Formula XIX, as defined above, to treat the autoimmune disorder. The quinazoline composition in this aspect of the invention can be in the form a hydrate or pharmaceutically acceptable salt. In one embodiment the autoimmune disorder is chosen from systemic lupus erythematosus, rheumatoid arthritis, inflammatory bowel disease, Sjögren's syndrome, polymyositis, vasculitis, Wegener's granulomatosis, sarcoidosis, ankylosing spondylitis, Reiter's syndrome, psoriatic arthritis, and Behçet's syndrome. In one particular embodiment the autoimmune disease is systemic lupus erythematosus. In one particular embodiment the autoimmune disease is rheumatoid arthritis.
- In one embodiment the subject is a human. The method can also be applied to animal models of any of the autoimmune disorders listed above.
- In one embodiment the autoimmune disorder is an immune complex associated disease, as described above.
- The quinazoline composition can be administered to the subject by any suitable route of administration, including, without limitation, oral and parenteral. Parenteral routes of administration are as described above with respect to substituted 4-primary amino quinolines.
- Assays for Effectiveness
- The methods of the invention can be assessed using any of a number of possible readout systems based upon a TLR/IL-1R signal transduction pathway. In some embodiments, the readout for the method is based on the use of native genes or, alternatively, transfected or otherwise artificially introduced reporter gene constructs which are responsive to the TLR/IL-1R signal transduction pathway involving MyD88, TRAF, p38, and/or ERK. Häcker H et al. (1999) EMBO J 18:6973-82. These pathways activate kinases including KB kinase complex and c-Jun N-terminal kinases. Thus reporter genes and reporter gene constructs particularly useful for the assays include, e.g., a reporter gene operatively linked to a promoter sensitive to NF-κB. Examples of such promoters include, without limitation, those for NF-κB, IL-1β, IL-6, IL-8, IL-12 p40, IP-10, CD80, CD86, and TNF-α. The reporter gene operatively linked to the TLR-sensitive promoter can include, without limitation, an enzyme (e.g., luciferase, alkaline phosphatase, β-galactosidase, chloramphenicol acetyltransferase (CAT), etc.), a bioluminescence marker (e.g., green-fluorescent protein (GFP, e.g., U.S. Pat. No. 5,491,084), blue fluorescent protein (BFP, e.g., U.S. Pat. No. 6,486,382), etc.), a surface-expressed molecule (e.g., CD25, CD80, CD86), and a secreted molecule (e.g., IL-1, IL-6, IL-8, IL-12 p40, TNF-α). In certain embodiments the reporter is selected from IL-8, TNF-α, NF-κB-luciferase (NF-κB-luc; Hacker H et al. (1999) EMBO J 18:6973-82), IL-12 p40-luc (Murphy T L et al. (1995) Mol Cell Biol 15:5258-67), and TNF-luc (Häcker H et al. (1999) EMBO J 18:6973-82). In assays relying on enzyme activity readout, substrate can be supplied as part of the assay, and detection can involve measurement of chemiluminescence, fluorescence, color development, incorporation of radioactive label, drug resistance, or other marker of enzyme activity. For assays relying on surface expression of a molecule, detection can be accomplished using flow cytometry (FACS) analysis or functional assays. Secreted molecules can be assayed using enzyme-linked immunosorbent assay (ELISA) or bioassays. Many of these and other suitable readout systems are well known in the art and are commercially available.
- Reporter Constructs
- A cell expressing a functional TLR and useful for the methods of the invention has, in some embodiments, an expression vector including an isolated nucleic acid which encodes a reporter construct useful for detecting TLR signaling. The expression vector including an isolated nucleic acid which encodes a reporter construct useful for detecting TLR signaling can include a reporter gene under control of a promoter response element (enhancer element). In some embodiments the promoter response element is associated with a minimal promoter responsive to a transcription factor believed by the applicant to be activated as a consequence of TLR signaling. Examples of such minimal promoters include, without limitation, promoters for the following genes: AP-1, NF-κB, ATF2, IRF3, and IRF7. These minimal promoters contain corresponding promoter response elements sensitive to AP-1, NF-κB, ATF2, IRF3, and IRF7, respectively. In other embodiments the expression vector including an isolated nucleic acid which encodes a reporter construct useful for detecting TLR signaling can include a gene under control of a promoter response element selected from response elements sensitive to IL-6, IL-8, IL-12 p40 subunit, a type I IFN, RANTES, TNF, IP-10, I-TAC, and interferon-stimulated response element (ISRE). The promoter response element generally will be present in multiple copies, e.g., as tandem repeats. For example, in one reporter construct, coding sequence for luciferase is under control of an upstream 6× tandem repeat of NF-κB response element. In another example, an ISRE-luciferase reporter construct useful in the invention is available from Stratagene (catalog no. 219092) and includes a 5×ISRE tandem repeat joined to a TATA box upstream of a luciferase reporter gene. As discussed further elsewhere herein, the reporter itself can be any gene product suitable for detection by methods recognized in the art. Such methods for detection can include, for example, measurement of spontaneous or stimulated light emission, enzyme activity, expression of a soluble molecule, expression of a cell surface molecule, etc.
- Readouts typically involve usual elements of Toll/IL-1R signaling, e.g., MyD88, TRAF, and IRAK molecules, although in the case of TLR3 the role of MyD88 is less clear than for other TLR family members. As demonstrated herein such responses include the induction of a gene under control of a specific promoter such as a NF-κB promoter, increases in particular cytokine levels, increases in particular chemokine levels, etc. The gene under the control of the NF-κB promoter can be a gene which naturally includes an NF-κB promoter or it can be a gene in a construct in which an NF-κB promoter has been inserted. Genes and constructs which include the NF-κB promoter include but are not limited to IL-8, IL-12 p40, NF-κB-luc, IL-12 p40-luc, and TNF-luc.
- Increases in cytokine levels can result from increased production, increased stability, increased secretion, or any combination of the forgoing, of the cytokine in response to the TLR-mediated signaling. Cytokines generally include, without limitation, IL-1, IL-2, IL-3, IL-4, IL-5, IL-6, IL-7, IL-10, IL-11, IL-12, IL-13, IL-15, IL-18, IFN-α, IFN-β, IFN-γ, TNF-α, GM-CSF, G-CSF, M-CSF. Th1 cytokines include but are not limited to IL-2, IFN-γ, and IL-12. Th2 cytokines include but are not limited to IL-4, IL-5, and IL-10.
- Increases in chemokine levels can result from increased production, increased stability, increased secretion, or any combination of the forgoing, of the chemokine in response to the TLR-mediated signaling. Chemokines of particular significance in the invention include but are not limited to CCL5 (RANTES), CXCL9 (Mig), CXCL 10 (IP-10), and CXCL11 (1-TAC), IL-8, and MCP-1.
- Administration to a Subject
- Some aspects of the invention involve administering an effective amount of a composition to a subject to achieve a specific outcome. The small molecule compositions useful according to the methods of the present invention thus can be formulated in any manner suitable for pharmaceutical use.
- The formulations of the invention are administered in pharmaceutically acceptable solutions, which may routinely contain pharmaceutically acceptable concentrations of salt, buffering agents, preservatives, compatible carriers, adjuvants, and optionally other therapeutic ingredients.
- For use in therapy, an effective amount of the compound can be administered to a subject by any mode allowing the compound to be taken up by the appropriate target cells. “Administering” the pharmaceutical composition of the present invention can be accomplished by any means known to the skilled artisan. Specific routes of administration include but are not limited to oral, transdermal (e.g., via a patch), parenteral injection (subcutaneous, intradermal, intramuscular, intravenous, intraperitoneal, intrathecal, etc.), or mucosal (intranasal, intratracheal, inhalation, intrarectal, intravaginal, etc.). An injection can be in a bolus or a continuous infusion.
- For example the pharmaceutical compositions according to the invention are often administered by intravenous, intramuscular, or other parenteral means, or by biolistic “gene-gun” application to the epidermis. They can also be administered by intranasal application, inhalation, topically, orally, or as implants, and even rectal or vaginal use is possible. Suitable liquid or solid pharmaceutical preparation forms are, for example, aqueous or saline solutions for injection or inhalation, microencapsulated, encochleated, coated onto microscopic gold particles, contained in liposomes, nebulized, aerosols, pellets for implantation into the skin, or dried onto a sharp object to be scratched into the skin. The pharmaceutical compositions also include granules, powders, tablets, coated tablets, (micro)capsules, suppositories, syrups, emulsions, suspensions, creams, drops or preparations with protracted release of active compounds, in whose preparation excipients and additives and/or auxiliaries such as disintegrants, binders, coating agents, swelling agents, lubricants, flavorings, sweeteners or solubilizers are customarily used as described above. The pharmaceutical compositions are suitable for use in a variety of drug delivery systems. For a brief review of present methods for drug delivery, see Langer R (1990) Science 249:1527-33, which is incorporated herein by reference.
- The concentration of compounds included in compositions used in the methods of the invention can range from about 1 nM to about 100 μM. Effective doses are believed to range from about 10 picomole/kg to about 100 micromole/kg.
- The pharmaceutical compositions are preferably prepared and administered in dose units. Liquid dose units are vials or ampoules for injection or other parenteral administration. Solid dose units are tablets, capsules, powders, and suppositories. For treatment of a patient, depending on activity of the compound, manner of administration, purpose of the administration (i.e., prophylactic or therapeutic), nature and severity of the disorder, age and body weight of the patient, different doses may be necessary. The administration of a given dose can be carried out both by single administration in the form of an individual dose unit or else several smaller dose units. Repeated and multiple administration of doses at specific intervals of days, weeks, or months apart are also contemplated by the invention.
- The compositions can be administered per se (neat) or in the form of a pharmaceutically acceptable salt. When used in medicine the salts should be pharmaceutically acceptable, but non-pharmaceutically acceptable salts can conveniently be used to prepare pharmaceutically acceptable salts thereof. Such salts include, but are not limited to, those prepared from the following acids: hydrochloric, hydrobromic, sulphuric, nitric, phosphoric, maleic, acetic, salicylic, p-toluene sulphonic, tartaric, citric, methane sulphonic, formic, malonic, succinic, naphthalene-2-sulphonic, and benzene sulphonic. Also, such salts can be prepared as alkaline metal or alkaline earth salts, such as sodium, potassium or calcium salts of the carboxylic acid group.
- Suitable buffering agents include: acetic acid and a salt (1-2% w/v); citric acid and a salt (1-3% w/v); boric acid and a salt (0.5-2.5% w/v); and phosphoric acid and a salt (0.8-2% w/v). Suitable preservatives include benzalkonium chloride (0.003-0.03% w/v); chlorobutanol (0.3-0.9% w/v); parabens (0.01-0.25% w/v) and thimerosal (0.004-0.02% w/v).
- The pharmaceutical compositions of the invention optionally include an active ingredient and optionally a pharmaceutically-acceptable carrier. The term “pharmaceutically-acceptable carrier” means one or more compatible solid or liquid fillers, dilutants or encapsulating substances which are suitable for administration to a human or other vertebrate animal. The term “carrier” denotes an organic or inorganic ingredient, natural or synthetic, with which the active ingredient is combined to facilitate the application. The components of the pharmaceutical compositions also are capable of being comingled with the compounds of the present invention, and with each other, in a manner such that there is no interaction which would substantially impair the desired pharmaceutical efficiency.
- Compositions suitable for parenteral administration conveniently include sterile aqueous preparations, which can be isotonic with the blood of the recipient. Among the acceptable vehicles and solvents are water, Ringer's solution, phosphate buffered saline, and isotonic sodium chloride solution. In addition, sterile, fixed oils are conventionally employed as a solvent or suspending medium. For this purpose any bland fixed mineral or non-mineral oil may be employed including synthetic mono- or diglycerides. In addition, fatty acids such as oleic acid find use in the preparation of injectables. Carrier formulations suitable for subcutaneous, intramuscular, intraperitoneal, intravenous, etc. administrations can be found in Remington's Pharmaceutical Sciences, Mack Publishing Company, Easton, Pa.
- The compounds useful in the invention can be delivered in mixtures of more than two such compounds. A mixture can further include one or more adjuvants in addition to the combination of compounds.
- A variety of administration routes is available. The particular mode selected will depend, of course, upon the particular compound selected, the age and general health status of the subject, the particular condition being treated, and the dosage required for therapeutic efficacy. The methods of this invention, generally speaking, can be practiced using any mode of administration that is medically acceptable, meaning any mode that produces effective levels of response without causing clinically unacceptable adverse effects. Preferred modes of administration are discussed above.
- The compositions can conveniently be presented in unit dosage form and can be prepared by any of the methods well known in the art of pharmacy. All methods include the step of bringing the compounds into association with a carrier which constitutes one or more accessory ingredients. In general, the compositions are prepared by uniformly and intimately bringing the compounds into association with a liquid carrier, a finely divided solid carrier, or both, and then, if necessary, shaping the product.
- Other delivery systems can include time-release, delayed release or sustained release delivery systems. Such systems can avoid repeated administrations of the compounds, increasing convenience to the subject and the physician. Many types of release delivery systems are available and known to those of ordinary skill in the art. They include polymer base systems such as poly(lactide-glycolide), copolyoxalates, polycaprolactones, polyesteramides, polyorthoesters, polyhydroxybutyric acid, and polyanhydrides. Microcapsules of the foregoing polymers containing drugs are described in, for example, U.S. Pat. No. 5,075,109. Delivery systems also include non-polymer systems that are: lipids including sterols such as cholesterol, cholesterol esters and fatty acids or neutral fats such as mono-di- and tri-glycerides; hydrogel release systems; silastic systems; peptide based systems; wax coatings; compressed tablets using conventional binders and excipients; partially fused implants; and the like. Specific examples include, but are not limited to: (a) erosional systems in which an agent of the invention is contained in a form within a matrix such as those described in U.S. Pat. Nos. 4,452,775, 4,675,189, and 5,736,152, and (b) diffusional systems in which an active component permeates at a controlled rate from a polymer such as described in U.S. Pat. Nos. 3,854,480, 5,133,974 and 5,407,686. In addition, pump-based hardware delivery systems can be used, some of which are adapted for implantation.
- The invention will be further described in the following examples, which do not limit the scope of the invention described in the claims.
- Candidate small molecules for initial screening were obtained from a commercially available library of 880 off-patent small molecules selected for structural diversity and proven bioavailability in humans (Prestwick Chemical Library). Human embryonic kidney HEK293 cells stably transfected with a human TLR9 (hTLR9) expression vector were incubated overnight in the presence of 50 nM CpG ODN 2006 (i.e., the EC50 concentration of ODN 2006) and selected small molecule candidate compounds at different concentrations ranging from 5×10−7 M to 5×10−5 M. TLR9 activity was assayed in terms of induction of a 6× NF-κB-luciferase reporter construct cotransfected in the cells. Results were measured as fold induction over baseline luciferase activity measured in the absence of ODN 2006 and compared to fold induction over baseline in the presence of the EC50 concentration of ODN 2006 alone. From this initial screening a number of small molecules were identified as lead compounds and were categorized by structure and activity. Additional screening was performed in like manner using additional small molecules selected from another library or specifically prepared for this purpose. Results of some of these assays are presented in
FIG. 1 and in Tables 5-16. - The method is described in greater detail as follows. The cells for each individual cell clone (hTLR3-NFκB-293, hTLR7-NFκB-293, hTLR8-NFκB-293 and hTLR9-NFκB-293) were counted and seeded one day before the stimulation at 1.5×104 cells per well in 96-well cell culture plates. To become adherent the cells were incubated after seeding overnight at 37° C. in 5% CO2 humidified air.
- The screen for each compound was performed at the same time for all of the receptors TLR3, TLR7, TLR8 and TLR9. Up to fifteen test compounds, each assayed in duplicate at each of three different final concentrations (0.5 μg/ml, 5 μg/ml, and 50 μg/ml), were used for 16 h cell stimulation on a single multiwell culture plate. A 4× final concentration master plate was created to prepare the different test compounds before they were added to the cells.
- Controls were added as duplicates individually for each cell clone on the cell culture plate. Positive controls were used as follows (final concentrations): 50 μg/ml poly(I:C) for hTLR3-NFκB-293; 8 μM resiquimod (R848) for hTLR7-NFκB-293; 30 μM R848 for hTLR8-NFκB-293; and 2.5 μM CpG-ODN 2006 for hTLR9-NFκB-293. Media alone was used as negative control. After addition of controls and small molecules, the cell clones were additionally stimulated with the EC50 concentration of the appropriate receptor-specific ligand.
- To calculate the baseline response to the EC50 concentration of the appropriate receptor-specific ligand, wells H3 and H6 did not receive receptor-specific ligand. The mean value of wells H2 and H5 (each with cells and EC50 concentration of the appropriate receptor-specific ligand) divided by the mean of wells H3 and H6 (each with cells alone) yielded the baseline response (i.e., fold induction of luciferase activity) to EC50 concentration of the appropriate receptor-specific ligand.
- After 16 h stimulation the supernatant was removed and the cells treated with lysis buffer and stored at −80° C. before measuring.
- An agonist screen was performed in parallel with the antagonist screen just described. The procedure was comparable to the antagonist screen except there was no addition of EC50 concentration of receptor-specific ligand. The outcome of this screen reflected agonistic and toxicity effects.
- Graphical Ranking System
- For each screen result a graphical documentation was performed. A numerical score, describing the graphical results, was determined for each compound. For positive compounds either for antagonists or agonists or synergists the screen has been repeated at least twice.
- Antagonists
- The baseline result (measured with addition of the EC50 concentration of the receptor-specific ligand) for each cell culture plate was measured as described above. Antagonistic effects were measured by down regulation compared to the baseline. Antagonists were scored as follows:
- 13: compounds for which there was clear down regulation at 0.5 μg/ml, 5 μg/ml, and 50 μg/ml;
- 12: compounds for which there was clear down regulation at 5 μg/ml and 50 μg/ml;
- 11: compounds for which there was clear down regulation only at 50 μg/ml;
- --: compounds for which there was no clear down regulation even at 50 μg/ml.
- Agonists
- To detect agonist activity the cells were treated in the above described manner but without adding the EC50 concentration of receptor-specific ligand after treatment of the cells with small molecules. Usually the baseline in the agonist screen is taken to be 1 because these cells have not been additionally stimulated with the EC50 concentration of the receptor-specific ligand. Agonist activity was present when there was above-baseline activity. Agonists were scored as follows:
- For compounds with highest agonist activity at 0.5 μg/ml,
- −1: compounds for which there was a greater than 5-fold induction compared to baseline;
- −2: compounds for which there was a 2- to 5-fold induction compared to baseline;
- −3: compounds for which there was a less than 2-fold induction compared to baseline.
- For compounds with highest agonist activity at 5 μg/ml,
- 0.25: compounds for which there was a greater than 5-fold induction compared to baseline;
- 0.5: compounds for which there was a 2- to 5-fold induction compared to baseline;
- 0.75: compounds for which there was a less than 2-fold induction compared to baseline.
- For compounds with highest agonist activity at 50 μg/ml,
- 1: compounds for which there was a greater than 5-fold induction compared to baseline;
- 2: compounds for which there was a 2- to 5-fold induction compared to baseline;
- 3: compounds for which there was a less than 2-fold induction compared to baseline.
- Toxicity
- Measuring of “toxicity” of the small molecules was performed by analysing below-baseline activity within the agonist screen. “Toxicity” can have different explanations—either differences between the cell clones, the compound are effecting the signal transduction pathway or the TLR within the cell clone, or the compounds do really have a toxic effect on the cells.
- Each compound was screened for “toxicity” on each of the four cell lines. Usually the baseline in the agonist screen is taken to be 1 because these cells have not been additionally stimulated with the EC50 concentration of the receptor-specific ligand. Apparent toxicity was scored as follows:
- T3: compounds for which there was clear down regulation at 0.5 μg/ml, 5 μg/ml, and 50 μg/ml;
- T2: compounds for which there was clear down regulation at 5 μg/ml and 50 μg/ml;
- T1: compounds for which there was clear down regulation only at 50 μg/ml.
- Candidate small molecules for initial screening were identified in an assay similar to that in Example 1 except HEK293 cells were stably transfected with a human TLR8 (hTLR8) expression vector and incubated overnight in the presence of 500 nM R848 (EC50 of R848 for TLR8) and selected small molecule candidate compounds at different concentrations ranging from 5×10−7 M to 5×10−5 M. TLR8 activity was assayed in terms of induction of a 6×NF-κB-luciferase reporter construct cotransfected in the cells. Results were expressed as fold induction over baseline luciferase activity measured in the absence of R848. From this initial screening a number of small molecules were identified as lead compounds and were categorized by structure and activity. Additional screening was performed in like manner using additional small molecules selected from another library or specifically prepared for this purpose. Results of some of these assays are presented in Tables 5-16. Scoring is reported as above but as applied to TLR8.
- Candidate small molecules for initial screening were identified in an assay similar to that in Example 1 except HEK293 cells were stably transfected with a human TLR7 (hTLR7) expression vector and incubated overnight in the presence of 2 μM R848 (EC50 of R848 for TLR7) and selected small molecule candidate compounds at different concentrations ranging from 5×10−7 M to 5×10−5 M. TLR7 activity was assayed in terms of induction of a 6×NF-κB-luciferase reporter construct cotransfected in the cells. Results were expressed as fold induction over baseline luciferase activity measured in the absence of R848. From this initial screening a number of small molecules were identified as lead compounds and were categorized by structure and activity. Additional screening was performed in like manner using additional small molecules selected from another library or specifically prepared for this purpose. Results of some of these assays are presented in Tables 5-16. Scoring is reported as above but as applied to TLR7.
- Candidate small molecules for initial screening were identified in an assay similar to that in Example 1 except HEK293 cells were stably transfected with a human TLR3 (hTLR3) expression vector and incubated overnight in the presence of 2.5 μg/ml poly(I:C) (EC50 of poly(I:C) for TLR3) and selected small molecule candidate compounds at different concentrations ranging from 5×10−7 M to 5×10−5 M. TLR3 activity was assayed in terms of induction of a 6×NF-κB-luciferase reporter construct cotransfected in the cells. Results were expressed as fold induction over baseline luciferase activity measured in the absence of poly(I:C). From this initial screening a number of small molecules were identified as lead compounds and were categorized by structure and activity. Additional screening was performed in like manner using additional small molecules selected from another library or specifically prepared for this purpose. Results of some of these assays are presented in Tables 5-16. Scoring is reported as above but as applied to TLR3.
- In the data presented in Tables 5-17 below, it should be recognized that at least certain compounds can conform to more than a single Formula.
TABLE 5 Exemplary Compounds of Formula II and Their Inhibitory Effect on Select TLRs ID structure chemical name hTLR3 hTLR7 hTLR8 hTLR9 564 Syrosingopine 13 11 12 13 904 12 12 13 13 614 Ellipticine 12 13 11 13 613 Harmine hydrochloride 12 13 13 13 455 Mebhydroline 1,5- naphtalenedisulfonate— — — 12 568 Rescinnamin — — — 12 619 Eleagnine hydrochloride (R,S) — — — 12 620 Harmane hydrochloride — — — 12 621 Methoxy-6-harmalan — — — 12 875 Reserpine — — — 12 878 3-Hydroxymethyl-beta- carboline — — — 12 612 Harmol hydrochloride monohydrate 11 11 11 12 1050 12 12 11 12 685 Nitrarine dihydrochloride — — — 11 593 Tetrahydroalstonine — 11 — 11 939 11 11 — 11 470 Norharman 11 11 11 11 1039 — 11 11 11 636 Epivincamine 11 — — — 1241 11 — — — 607 Eburnamonine (−) — 11 — — 918 — 11 — — 944 11 11 — — 890 — 11 11 — 1132 11 11 11 — 1243 — 11 11 — -
TABLE 6 Exemplary Compounds of Formula III and Their Inhibitory Effect on Select TLRs ID structure chemical name hTLR3 hTLR7 hTLR8 hTLR9 149 Thioproperasine dimesylate — — — 13 53 Triflupromazine hydrochloride 11 11 11 13 576 Acetopromazine maleate salt 11 — — 12 64 Chlorpromazine hydrochloride 11 11 11 12 693 Promazine hydrochloride — 12 — 12 399 Prochlorperazine dimaleate 12 12 11 12 529 Mesoridazine besylate — — — 11 125 Perphenazine — — 11 11 840 Ethopropazine hydrochloride — — 11 11 797 Methotrimeprazine maleat salt — 11 — 11 313 Trifluoperazine dihydrochloride — 11 11 11 842 Trimeprazine tartrate — — 11 — -
TABLE 7 Exemplary Compounds of Formula IV and Their Inhibitory Effect on Select TLRs ID structure chemical name hTLR3 hTLR7 hTLR8 hTLR9 413 Anthraquinone, 1,5- diamino 11 — 11 13 43 Anthralin — — 12 13 348 Chlorprothixene hydrochloride 11 11 11 12 491 Metixene hydrochloride 12 11 11 12 1337 11 11 11 12 1042 11 12 13 12 1158 — — — 11 1015 — — 11 11 1287 — — 11 11 294 Pimethixene maleate 11 11 11 11 340 Flupentixol dihydrochloride cis-(Z) 11 11 11 11 346 Maprotiline hydrochloride 11 11 11 11 917 12 11 — — -
TABLE 8 Exemplary Compounds of Formula V and Their Inhibitory Effect on Select TLRs ID structure chemical name hTLR3 hTLR7 hTLR8 hTLR9 72 Imipramine hydrochloride — — — 13 109 Dizocilpine maleate — — — 13 488 Dosulepin hydrochloride 11 — — 12 74 Amitryptiline hydrochloride — 11 — 12 343 Desipramine hydrochloride 11 12 11 12 1013 — — — 11 1352 — 13 — 11 99 Mianserine hydrochloride 11 — — — -
TABLE 9 Exemplary Compounds of Formula VI and Their Inhibitory Effect on Select TLRs ID structure chemical name hTLR3 hTLR7 hTLR8 hTLR9 239 Orphenadrine hydrochloride — — — 13 229 Nefopam hydrochloride — — — 12 707 Diphenylpyraline hydrochloride — 11 — 12 793 Cloperastine hydrochloride 11 11 — 12 957 12 11 — 12 1161 12 12 12 12 65 Diphenhydramine hydrochloride — — — 11 970 — — — 11 306 Clemastine fumarate 11 12 11 11 1308 12 12 12 11 974 11 11 11 — 386 GBR 12909 dihydrochloride 11 11 12 — -
TABLE 10 Exemplary Compounds of Formula VII and Their Inhibitory Effect on Select TLRs ID structure chemical name hTLR3 hTLR7 hTLR8 hTLR9 312 Flunarizine dihydrochloride 11 — — 12 457 Meclozine dihydrochloride — — — 11 510 Cyclizine hydrochloride — — — 11 133 Hydroxyzine dihydrochloride 11 11 — 11 267 Clotrimazole — 12 12 — -
TABLE 11 Exemplary Compounds of Formula VIII and Their Inhibitory Effect on Select TLRs ID structure chemical name hTLR3 hTLR7 hTLR8 hTLR9 822 Clidinium bromide — — — 12 138 Terfenadine 11 11 11 12 144 Loperamide hydrochloride 11 11 11 12 308 Pimozide 12 11 11 12 833 Mitotane 11 11 11 12 560 Prenylamine lactate — 12 — 12 93 Azacyclonol — — — 11 108 Nomifensine maleate — — — 11 799 Pridinol methanesulfonate salt — — — 11 381 Lidoflazine 11 11 11 11 778 Proadifen hydrochloride 11 11 11 11 884 — 11 11 — 270 Fendiline hydrochloride 11 11 12 — 965 12 12 — — 1187 12 12 11 — 267 Clotrimazole — 12 12 — -
-
TABLE 13 Exemplary Compounds of Formula X and Their Inhibitory Effect on Select TLRs ID structure chemical name hTLR3 hTLR7 hTLR8 hTLR9 431 Coralyne chloride hydrate — — — 13 792 Propidium iodide 12 — — 12 583 Papaverine hydrochloride — 11 — 12 830 Ethaverine hydrochloride — 11 11 11 586 Berberine chloride 11 — 12 — -
-
-
-
- Experimental mice are administered known amounts of candidate small molecules and a source of PAMP or other suitable TLR ligand, e.g., CpG nucleic acid. Negative control mice receive the source of PAMP or other suitable TLR ligand, e.g., CpG nucleic acid, alone. After an appropriate period, blood samples are obtained from control and experimental mice and evaluated for serum concentration of cytokine using a suitable method, e.g., enzyme-linked immunosorbent assay (ELISA). Alternatively or in addition, peripheral blood mononuclear cells (PBMC) are isolated from both groups of animals and assessed for expression of activation marker using a suitable technique such as fluorescence activated cell sorting (FACS). Control and experimental results are compared in pairwise fashion. Reduced expression of activation marker or reduced concentration of cytokine in an experimental animal compared to a negative control animal indicates the small molecule inhibits TLR-mediated signaling in response to a ligand for the TLR. Increased expression of activation marker or increased concentration of cytokine in an experimental animal compared to a negative control animal indicates the small molecule promotes TLR-mediated signaling in response to a ligand for the TLR.
- Experimental mice are administered candidate small molecules. Negative control mice are administered carrier alone. After administration or small molecule or carrier alone, PBMC are isolated and then exposed in vitro to CpG nucleic acid under conditions in which the PBMC, in the absence of small molecule, are stimulated to express an activation marker such as CD86 or secrete a product such as cytokine (e.g., IFN-α, IL-6, TNF-α) or chemokine (e.g., IP-10). The expression of the activation marker or the secretion of the product is quantified using FACS, ELISA, or other suitable method, and comparison is made between results obtained with and without the small molecule. Reduced expression of activation marker or reduced concentration of cytokine in an experimental animal compared to a negative control animal indicates the small molecule inhibits TLR-mediated signaling in response to a ligand for the TLR. Increased expression of activation marker or increased concentration of cytokine in an experimental animal compared to a negative control animal indicates the small molecule promotes TLR-mediated signaling in response to a ligand for the TLR.
- Stably co-transfecting the human TLR9 receptor (hTLR9) and an NF-κB promoter-driven luciferase gene into HEK-293 cells created the cell line hTLR9-NFkB-293. hTLR9-NFkB-293 cells were counted and seeded at 1.5×04 cells per well in 96-well cell culture plates one day before assaying and cultured at 37° C./5% CO2. The day of the assay, antagonist was added at 50 μM, 5.0 μM or 0.5 μM. The cells were then stimulated with the EC50 dose of the TLR9 agonist, CpG-ODN 2006, and cultured for 16 h in a humidified incubator at 37° C. The culture supernatant was removed and the cells were treated with lysis buffer and stored at −80° C. before measuring luciferase activity. The luciferase readout was measured according to manufacturer's instructions using a luciferase assay system available from Promega, USA. Results are summarized in Table 18. The antagonist dose listed in Table 18 is the minimum effective dose (μM) for blockade of the TLR9 agonist. As evident from the results presented in Table 18, the minimum effective dose (μM) for blockade of the TLR9 agonist was 0.5 μM or 5 μM for all of the compounds tested, as measured by this three-point assay.
TABLE 18 Human TLR9 Antagonism by 4-Amino Quinolines. Compound Number structure chemical name antagonist dose 936 7,8,9,10-Tetrahydro-6H- cyclohepta[b]quinolin-11-ylamine 0.5 μM 990 (122) 1-Methyl-2,3-dihydro-1H- pyrrolo[2,3-b]quinolin-4-ylamine 0.5 μM 1091 (123) 1,6-Dimethyl-2,3-dihydro-1H- pyrrolo[2,3-b]quinolin-4-ylamin 0.5 μM 1142 (124) 6-Bromo-1-methyl-2,3-dihydro-1H- pyrrolo[2,3-b]quinolin-4-ylamin 0.5 μM 1185 (125) 1-Methyl-2,3,4,5-tetrahydro-1H- azepino[2,3-b]quinolin-6-ylamin 0.5 μM 1201 3,3-Dimethyl-3,4-dihydro-acridin-9- ylamin 0.5 μM 1217 (126) 1-Benzyl-2,3-dihydro-1H- pyrrolo[2,3-b]quinolin-4-ylamin 0.5 μM 1552 (127) 6-Methyl-1-phenyl-2,3-dihydro-1H- pyrrolo[2,3-b]quinolin-4-ylamin 0.5 μM 1553 (128) N*2*,N*2*-Dimethyl-quinolin-2,4- diamine 0.5 μM 1595 2,7-Dimethyl- dibenzo[b,g][1,8]naphthyridin-11- ylamine 0.5 μM 1690 2,4-Dimethyl- benzo[b][1,8]naphthyridin-5- ylamine 0.5 μM 1727 7-Fluoro-2,4-dimethyl- benzo[b][1,8]naphthyridin-5- ylamine 0.5 μM 329 1,2,3,4-Tetrahydro-acridin-9- ylamine Tacrine hydrochloride hydrate 5.0 μM 1061 2,3-Dihydro-1H- cyclopenta[b]quinolin-9-ylamin 5.0 μM 1227 2,4,9-Trimethyl- benzo[b][1,8]naphthyridin-5- ylamine 5.0 μM 1286 9-Amino-3,3-dimethyl-1,2,3,4- tetrahydro-acridin-1-ol 5.0 μM 1674 7-Ethoxy-N*3*-furan-2-ylmethyl- acridine-3,9-diamine 5.0 μM - hTLR9-NFkB-293 cells were counted and seeded at 1.5×104 cells per well in 96-well cell culture plates one day before assaying and cultured at 37° C./5% CO2, as described in Example 7. The day of the assay, antagonist was added at varying concentrations starting at 50 μM with a ⅕ to ⅙ dilution for 7 steps. The cells were then stimulated with the EC50 dose of the TLR9 agonist, CpG-ODN 2006, and cultured for 16 h in a humidified incubator at 37° C. The culture supernatant was removed and the cells were treated with lysis buffer and stored as −80° C. before measuring luciferase activity as described in Example 7. Sigmoidal antagonism curves were generated and the inhibitor concentration at which 50% of the agonist response was blocked (IC50) calculated. Results shown in Table 19 under the heading hTLR9 are the IC50 dose (μM) for blockade of a CpG ODN-generated signal in hTLR9-NFkB-293 cells.
- Alternatively, TLR9 ligand antagonism in human peripheral blood mononuclear cells (PBMC) was monitored. Peripheral blood buffy coat preparations from healthy male and female human donors were obtained from the Blood Bank of the University of Düsseldorf (Germany) and from these, PBMC were purified by centrifugation over Ficoll-Hypaque (Sigma). The purified PBMC were resuspended in RPMI 1640 culture medium supplemented with 5% (v/v) heat-inactivated human AB serum (BioWhittaker, Belgium) or 10% (v/v) heat-inactivated fetal calf serum (FCS), 1.5 mM L-glutamine, 100 U/ml penicillin and 100 μg/ml streptomycin (all from Sigma). Fresh PBMC at a concentration of 3×106/ml to 5×106/ml were added to 96-well round-bottomed plates (150 μl/well). After cell plating, antagonist was added at varying concentrations starting at 50 μM with a ⅕ to ⅙ dilution for 7 steps. The cells were then stimulated with the TLR9 agonist, CpG-ODN 2006, and cultured for 16 h in a humidified incubator at 37° C. Culture supernatants were collected and, if not used immediately, frozen at −20° C. until required. Interleukin 6 (IL-6) in the supernatants was quantitatively assessed using commercially available ELISA Kits (IL6, Diaclone, USA). Sigmoidal antagonism curves were generated and IC50 calculated. Results shown in Table 19 under the heading IL-6 are the IC50 dose (μM) for blockade of CpG ODN-generated IL-6 production by human PBMC.
TABLE 19 Antagonism of TLR9 Signal Agonist CpG ODN 2006 by Quinazolines Compound number Structure Chemical name hTLR9 IL-6 CMZ 203-34 (201) N,N-dimethyl-N′-{2-[4-(4-methyl- piperazin-1-yl)-phenyl]-3,4-dihydro- quinazoline-4-yl}-ethane-1,2,-diamine 0.003 0.05 CMZ 203-78 N′-[6,7-Dimethoxy-2-(4-phenyl-piperzin- 1-yl)quinazolin-4-yl]-N,N-dimethyl- ethane-1,2-diamine 0.003 0.008 CMZ 203-76 N′-[6,7-Dimethoxy-2-(4-methyl-piperzin- 1-yl)quinazolin-4-yl]-N,N-dimethyl- ethane-1,2-diamine 0.003 0.221 CMZ 203-87 0.02 ND CMZ 203-44 N,N-Dimethyl-N′-(2-phenyl-quinazolin-4- yl)ethane-1,2-diamine 2.4 4.0 CMZ 203-49 (213) Dimethyl-(2-{2-[4-(4-methyl-piperazin-1- yl)-phenyl]-quinazolin-4-yloxy}-ethyl)- amine 2.4 0.6 CMZ 203-51 N′-(2-Biphenyl-4-yl-quinazolin-4-yl)-N,N- dimethyl-ethane-1,2-diamine 5.2 6.6 CMZ 203-90 5.43 ND CMZ 203-45 Dimethyl-[2-(2-phenyl-quinazolin-4-yloxy)- ethyl]-amine 29 ND CMZ 203-93 ND ND CMZ 203-95 ND ND
ND: no data
- Female BALB/c mice (n=3 per group) were given a single intraperitoneal bolus injection of CMZ 203-43 (1.0 or 4.0 mg), CMZ 203-34 (1.0 or 4.0 mg) or CMZ 203-49 (1.0 or 4.0 mg) in a volume of 0.2 ml. Compound CMZ 203-43 has the structural formula
Other mice received 10% dimethyl sulfoxide (DMSO) in an identical manner. Animals were weighed immediately prior to injection (day 1) and then daily untilday 5. Onday 5, blood was collected by cardiac puncture and analyzed for hematological and biochemical parameters. Animals were monitored daily for morbidity and mortality. Results are shown inFIG. 2 andFIG. 3 . -
FIG. 2A shows change in body weight (relative to body weight prior to first injection)±standard error of the means for different groups.FIG. 2B shows mean body weight±standard error of the means for different groups. Mice receiving 1.0 mg CMZ 203-43 had significant weight loss, while mice receiving quinazoline at either dose did not. All of the mice receiving 4.0 mg CMZ 203-43 group were dead onday 4. Necropsy of mice in the 4.0 mg CMZ 203-43 group revealed bowel obstruction. -
FIG. 3 shows white blood cell (WBC) differential as mean percentage of total white cells±standard error of the means for different groups. Mice treated with 1.0 mg CMZ 203-43 had a significant neutrophilia while mice receiving quinazoline at either dose did not. Data for mice treated with 4.0 mg CMZ 203-43 was not available because all these mice were dead beforeday 5. - BALB/c mice (n=5) were pretreated with intraperitoneal injection of 40 μg quinoline 203-43, quinazoline 203-34, 203-49, or 203-51, or with 100 μl of sterile phosphate buffered saline (PBS) control. One hour after the injection, mice were injected subcutaneously with 100 μg CpG ODN 2006. Animals were bled at 3 hr post injection with CpG ODN 2006 and IP-10 levels in plasma measured by IP-10-specific enzyme-linked immunosorbent assay (ELISA). Results are presented in
FIG. 4 . Serum IP-10 concentration was significantly reduced in mice pretreated with 203-43 (p=0.00013 versus PBS control) or 203-34 (p=0.00044 versus PBS control). - Groups of BALB/c mice treated as in Example 10 were also analyzed for TNF-α. Animals were bled at 1 hr post injection with CpG ODN 2006 and TNF-α levels in plasma measured by TNF-α-specific ELISA. Results are presented in
FIG. 5 . Serum TNF-α concentration was significantly reduced in mice pretreated with 203-43 (p=0.0023 versus PBS control) or 203-34 (p=0.0012 versus PBS control). Mice treated with 203-49 showed a trend toward reduced serum TNF-α concentration. -
- A mixture of 4-fluorobenzophenone (16 gm, 0.08 moles) and N-methylpiperazine (14 gm, 0.14 moles) was stirred and refluxed for 20 hours in water containing sodium carbonate (12.7 gm, 0.12 moles). After cooling, the mixture was extracted with ethyl acetate (200 mL). The organic phase washed with water (100 mL) and was then extracted with 10% hydrochloric acid solution (3×50 mL). The combined acid extracts were washed with ethyl acetate (100 mL) and were then made basic by the addition of 40% sodium hydroxide solution. The solid aminobenzophenone was extracted into ethyl acetate (200 mL) and the extracts were washed with brine. The ethyl acetate was evaporated under vacuum to give the product as a white solid in a yield of 7.0 gm, 33%.
- A mixture of the benzophenone (15 gm, 0.056 moles) and powdered sodium hydroxide (15 gm) in ethanol (150 mL) was stirred and heated to reflux. The heat was removed and zinc dust (15 gm) was added in portions at a rate that kept the mixture at reflux. After the addition was complete, the mixture was heated at reflux for one hour. The reaction mixture was allowed to cool and was then filtered to remove zinc. The zinc washed with ethanol (20 mL) and the combined filtrates were added to water (500 mL). The crystalline white product was isolated by filtration, washed with water and dried. The carbinol was obtained in a yield of 12.1 gm, 80%.
A solution of the carbinol (1.13 gm, 4.18×10−3 moles) in NMP (10 mL) was stirred under nitrogen as a sodium hydride dispersion (0.336 gm of 60%, 8.36×10−3 moles) was added. This mixture was stirred at 65° C. until hydrogen evolution stopped. Then 2-(dimethylamino)ethyl chloride hydrochloride (0.602 gm, 4.18×10−3 moles) was added and stirring at 65° C. was continued for one hour. The addition of identical portions of sodium hydride dispersion and 2-(dimethylamino)ethyl chloride hydrochloride was done twice more at one hour intervals. After the last addition, the mixture was stirred and heated for two hours and then cooled. The mixture was poured into 10% sodium hydroxide solution (200 mL) and this suspension was extracted with methylene chloride (2×100 mL). The combined extracts were washed with 10% sodium hydroxide solution (100 mL) and were then stripped under vacuum to give the crude product contaminated with some NMP as well as a small amount of starting material. The product was purified by chromatography on silica using 20% methanol in methylene chloride to elute the impurities. The product was then eluted with 20% methanol in methylene chloride containing 1% diethylamine. The yield of CMZ 203-85 was 0.540 gm, 37% as a light brown oil. -
- The benzophenone (4.65 gm, 0.0173 moles) and hydroxylamine hydrochloride (1.89 gm, 0.027 moles) were combined in ethanol (25 mL). To this mixture was added 40% sodium hydroxide solution (8.7 gm, 0.087) moles. The resulting mixture was stirred at reflux until TLC (silica, 10% methanol in methylene chloride) showed that the reaction had gone to completion (about 30 minutes). After cooling, a solution of sodium bicarbonate (20 gm) in water (300 mL) was added and the precipitated solid was isolated by filtration. After washing with water and drying, there was obtained 4.85 gm (95%) of CMZ 203-88 as a white solid.
-
- A solution of the oxime (1.0 gm, 3.39×10−3 moles) in ethanol (20 mL) was stirred at reflux in the presence of 10% palladium on carbon (200 mg). To this mixture was added 90% formic acid (0.35 gm, 6.77×10−3 moles) dropwise. Upon complete addition of the formic acid, the reaction mixture was heated at reflux for 30 minutes. The mixture was cooled and filtered free of the catalyst and the filtrates were stripped under vacuum. The residual oil quickly solidified to provide CMZ 203-88-1 in quantitative yield.
-
- A solution of the carbinol (1.4 gm, 5.0×10−3 moles) in DMF (10 mL) and methylene chloride (20 mL) was stirred as thionyl chloride (0.6 gm, 5.0×10−3 moles) was slowly added. After stirring at room temperature for 30 minutes, N,N-dimethylethylenediamine (0.88 gm, 1.0×10−3 moles) was added dropwise to the hazy solution. This mixture was stirred at room temperature for 30 minutes and was then poured into 5% sodium hydroxide solution (400 mL). This mixture was extracted with methylene chloride (2×100 mL) and the combined extracts were washed with 5% sodium hydroxide solution (200 mL). After drying over magnesium sulfate the extracts were filtered and stripped under vacuum to give an oil. The product was purified by chromatography on silica using 20% methanol in methylene chloride to elute the impurities. The product was then eluted with 20% methanol in methylene chloride containing 1% diethylamine. The yield of CMZ 203-89 was 0.588 gm, 33.4% as a light brown oil.
-
- A solution of the carbinol (0.6 gm, 2.03×10−3 moles) in DMF (5 mL) was stirred as thionyl chloride (0.242 gm, 2.03×10−3 moles) was slowly added. After stirring at room temperature for 30 minutes, N-methylpiperazine (0.400 gm, 4.0×10−3 moles) was added dropwise to the solution. This mixture was stirred at room temperature for 30 minutes and was then poured into 5% sodium hydroxide solution (200 mL). This suspension was extracted with methylene chloride (2×100 mL) and the combined extracts were washed with water (100 mL). The extracts were stripped under vacuum to give an oil. The product was purified by chromatography on silica using 20% methanol in methylene chloride to elute the impurities. The product was then eluted with 20% methanol in methylene chloride containing 1% diethylamine. The yield of CMZ 203-91 was 0.58 gm, 77% as an oil which crystallized upon standing.
- hTLR9-NFkB-293 cells were counted and seeded at 1.5×104 cells per well in 96-well cell culture plates one day before assaying and cultured at 37° C./5% CO2, as described in Example 7. The day of the assay, antagonist was added at varying concentrations starting at 50 μM with a ⅕ to ⅙ dilution for 7 steps. The cells were then stimulated with the EC50 dose of the TLR9 agonist, CpG-ODN 2006, and cultured for 16 h in a humidified incubator at 37° C. The culture supernatant was removed and the cells were treated with lysis buffer and stored at −80° C. before measuring luciferase activity as described in Example 7. Sigmoidal antagonism curves were generated and the inhibitor concentration at which 50% of the agonist response was blocked (IC50) calculated. Results shown in Table 20 under the heading hTLR9 are the IC50 dose (μM) for blockade of a CpG ODN-generated signal in hTLR9-NFkB-293 cells.
TABLE 20 Human TLR9 Antagonism by Novel Diarylmethane Compounds of the Invention ID Structure hTLR9 CMZ 203-84 25.56 CMZ 203-85 0.372 CMZ 203-88 11.56 CMZ 203-88-1 12.01 CMZ 203-89 0.241 CMZ 203-91 3.95 -
- A mixture of 4-fluorobenzaldehyde (10 gm, 0.08 moles) and N-methylpiperazine (14 gm, 0.14 moles) in water (80 mL) containing sodium carbonate (12.7 gm, 0.12 moles) was stirred and refluxed for 21 hours. After cooling, the mixture was poured into a separatory funnel containing water (200 mL) and the oily product was extracted into methylene chloride (3×50 mL). The combined extracts were washed with water and were then stripped under vacuum. The product, which was isolated as an oil, solidified into a tan solid upon cooling. The solid product was triturated in hexane and isolated by filtration. After drying, there was obtained 14.9 gm (91%) of N-(4-formylphenyl)-N′-methylpiperazine as an off white solid.
Step 2. - Anthranilamide (9.94 gm, 0.073 moles) and N-(4-formylphenyl)-N′-methylpiperazine (14.9 gm, 0.073 moles) were combined in NMP (100 mL). This mixture was stirred and warmed until dissolution was complete. To the warm solution was added p-toluenesulfonic acid monohydrate (2.0 gm) after which the solution was heated at 100° C. for 30 minutes. Acetic acid (50 mL) was added and the mixture was stirred and heated at 100° C. for an additional 30 minutes. Chloranil (17.9 gm, 0.073 moles) was added in portions over a period of approximately two minutes. The dark solution was heated at 100° C. for 10 minutes. The solution was cooled to room temperature and was diluted with water (500 mL) and tert-butylmethyl ether (TBME, 500 mL). The mixture was stirred and made basic by the addition of solid sodium carbonate. After stirring for 15 minutes the precipitated product was isolated by filtration. The crude product was re-suspended in warm water, stirred for 5 minutes and collected by filtration. After washing with water followed by TBME, the solid was air dried. The dried quinazolinone was recrystallized from n-butanol to give 11.1 gm (50%) of product as colorless needles.
Step 3. - The quinazolinone (2.4 gm, 7.5×10−3 moles) was added in portions with stirring to phosphorous oxychloride (10 mL). This mixture was warmed to 80° C. to give a red solution from which solid began to precipitate. The mixture was stirred at 80° C. for 15 minutes and was then heated briefly to reflux. Upon cooling the slurry was slowly added to a cold, stirred solution of 10% sodium carbonate (650 mL). After stirring for 15 minutes, the solid chloroquinazoline was extracted into chloroform (2×150 mL). The combined extracts were washed with brine and then dried over magnesium sulfate. The solution was filtered to remove the drying agent and the filtrates were stripped under vacuum to give a yellow solid. N-methylpyrrolidinone (NMP, 20 mL) was added and the solid was dissolved by warming. N,N-dimethylethylenediamine (1.32 gm, 0.015 moles) was added and the solution was then warmed at 100° C. for 30 minutes. After cooling, the NMP solution was diluted with water (200 mL) and concentrated ammonium hydroxide (50 mL). The product, which had precipitated, was extracted into methylene chloride (2×100 mL). The extracts were combined, dried over magnesium sulfate and then, after filtration, were stripped to give the product as a light brown oil. This was purified by chromatography on silica using 10% methanol in methylene chloride as eluent. In this
manner 600 mg (1.54×10−3 moles) of pure quinazoline was isolated as an oil. This was dissolved in t-butylmethyl ether (TBME, 50 mL) and was stirred as a solution of toluenesulfonic acid monohydrate (584 mg, 3.1×10−3 moles) dissolved in TBME (50 mL) was added. The solid bis-tosylate salt was isolated by filtration, washed with TBME and dried to give 1.0 gm of the product as a yellow solid. -
- N,N-dimethylethylenediamine (3.5 gm, 0.04 moles) and 2-phenyl-4-chloroquinazoline (2.4 gm, 0.01 moles) were combined in n-butanol (50 mL) and were brought to reflux. Once reflux had been achieved, TLC (silica, 10% methanol in dichloromethane) showed that the reaction was complete. The solution was cooled and stripped. The residue was dissolved in t-butylmethyl ether (TBME) and the solution was washed with water. After drying over magnesium sulfate the solution was filtered and stripped to give 1.37 gm (0.0047 moles, 47%) of the crude product as an oil. This was dissolved in ethanol (25 mL) and was treated with a solution of concentrated sulfuric acid (0.46 gm, 0.0047 moles) dissolved in ethanol (5 mL). The crystalline sulfate salt began to separate within a few seconds and after 30 minutes at room temperature was isolated by filtration. The salt washed with ethanol and dried. The yield was 1.77 gm, (96% from crude).
-
- A slurry of 60% sodium hydride (0.80 gm, 0.02 moles) in NMP (50 mL) was stirred under nitrogen as N,N-dimethylethanolamine (1.96 gm, 0.022 moles, 2.2 mL) was slowly added via syringe. After the amine had been added the solution was stirred at 30° C. for 10 minutes after which 2-phenyl-4-chloroquinazoline (2.4 gm, 0.01 moles) was added in a single portion. Stirring was continued at 40° C. for 30 minutes after which TLC (silica, 10% methanol in methylene chloride) showed that the reaction had gone to completion. The solution was cooled and poured into water (250 mL). The product was extracted into methylene chloride (3×50 mL). After drying over magnesium sulfate the solution was filtered and stripped to give the crude product as an oil. This was dissolved in ethanol (20 mL) and was treated with a solution of concentrated sulfuric acid (0.85 gm, 0.0087 moles) dissolved in ethanol (10 mL). The crystalline sulfate salt separated slowly and after 60 minutes at room temperature was isolated by filtration. The salt washed with ethanol and dried. The yield was 1.37 gm, (40% based on the sulfuric acid).
-
- The quinazolinone (3.2 gm, 0.01 moles) was added in portions with stirring to phosphorous oxychloride (10 mL). This mixture was refluxed for 30 minutes. The orange slurry was cooled and TBME (100 mL) was added. After stirring for 10 minutes the chloroquinazoline was isolated by filtration and added to a solution of the sodium salt of N,N-dimethylaminoethanol in NMP (50 mL). The solution of the sodium salt of N,N-dimethylaminoethanol in NMP was prepared as follows. A slurry of 60% sodium hydride (2.1 gm, 0.05 moles) in NMP (50 mL) was stirred under nitrogen as N,N-dimethylethanolamine (4.9 gm, 0.055 moles) was slowly added via syringe. After the amine had been added the solution was stirred at 30° C. for 10 minutes. This mixture was stirred at 100° C. for 30 minutes. The cooled solution was added to methylene chloride (150 mL) and this solution was extracted with water (3×150 mL). The methylene chloride solution was stripped and the residue was dissolved in ethanol (50 mL). To this solution was added concentrated sulfuric acid (0.8 gm, 0.008 moles) dissolved in ethanol (5 mL). The solid salt which separated was isolated by filtration, washed with ethanol and TBME and dried. The yield was 1.3 gm, 23%.
-
- A solution of anthranilamide (6.8 gm, 0.05 moles) and biphenylcarboxaldehyde (9.1 gm, 0.05 moles) in DMF (100 mL) was stirred as p-toluenesulfonic acid monohydrate (1.0 gm) was added. A yellow solution formed which quickly changed to a thick slurry as the dihydroquinazoline precipitated. The slurry was heated to 100° C. which caused most of the solid to dissolve. To this hot mixture was added chloranil (12.3 gm, 0.05 moles) in portions over a 2 minute period. A dark solution formed from which the product began to crystallize. The mixture was heated to boiling to provide a dark solution. Upon cooling, the quinazolinone crystallized as colorless needles. The product was isolated by filtration, washed well with DMF and acetone, and then dried. The yield was 11.9 gm, 80%.
Step 2. - Phosphorous oxychloride (12 mL) and the biphenylquinazoline (2.98 gm, 0.01 moles) were stirred together and refluxed for one hour. The excess phosphorous oxychloride was removed by distillation and the oily yellow residue was placed under aspirator vacuum to remove traces of phosphorous oxychloride. The resulting yellow solid was triturated in hexane which was removed by decantation. To the yellow solid was added n-butanol (50 mL) and N,N-dimethylethylenediamine (6 mL). This solution was stirred and refluxed for 30 minutes. The butanol solution was cooled and poured into a separatory funnel containing TBME (200 mL) and 10% aqueous hydrochloric acid (200 mL). The funnel was vigorously shaken after which the layers were allowed to separate. The aqueous layer was isolated and the flask in which it was collected was scratched with a glass rod. The product began to immediately separate as a pale yellow solid. After 30 minutes at room temperature the product was isolated by filtration and washed with a small amount of cold water. After drying there was obtained 3.64 gm (82%) of product as a pale yellow solid.
-
- A solution of 2,4-dichloro-6,7-dimethoxyquinazoline (5.2 gm, 0.02 moles) and N,N-dimethylethylenediamine (1.76 gm, 0.02 moles) in methylene chloride (65 mL) was stirred at room temperature for 2 hours. To the slurry that had formed another portion of N,N-dimethylethylenediamine (1.76 gm, 0.02 moles) was added forming a clear solution. This solution was stirred at room temperature overnight. The solution was poured into 10% sodium carbonate solution (200 mL) causing the product to separate as a solid. The solid was isolated by filtration and then washed with water followed by methylene chloride. This solid was recrystallized from 2-propanol to give 2.6 gm (42%) of the purified product as a white crystalline solid.
Step 2. - A mixture of the chloroquinazoline (930 mg, 0.003 moles) and N-methylpiperazine (5 mL) was heated at 100° C. for 3 hours. The solution was cooled and stripped under vacuum. n-Butanol (10 mL) was added and the solution was again stripped under vacuum to remove residual N-methyl piperazine. The residue was dissolved in ethanol (20 mL) and a solution of sulfuric acid (0.59 gm, 0.006 moles) in ethanol (5 mL) was added. The mixture was refluxed for one minute and then cooled. The product was isolated by filtration, washed with ethanol followed by TBME and then dried. The yield was 0.85 gm, (50%).
-
- A mixture of the chloroquinazoline (1.55 gm, 0.005 moles) and N-phenylpiperazine (1.6 gm, 0.01 moles) in n-butanol (5 mL) was heated at 100° C. for 2 hours. The resulting slurry was diluted with n-Butanol (15 mL) and the mixture was heated to boiling to give a clear solution. Upon cooling a solid separated which was isolated by filtration. The solid was stirred in warm water for 15 minutes to remove a small amount of N-phenyl-piperazine. The product was isolated by filtration, washed with warm water and dried to provide 1.0 gm (46%) of the product as a white solid.
-
- A mixture of the 4-chloroquinazoline (2.74 gm, 8.0×10−3 moles) and N-aminoethyl-morpholine (2.10 gm, 1.6×10−2 moles) in ethanol (25 mL) was refluxed for 2 hours. The reaction mixture was cooled and poured into water (200 mL) containing ammonia (50 mL of 28%) and the precipitated product was extracted into methylene chloride (3×100 mL). The combined extracts were washed with water (200 mL) and then dried over magnesium sulfate. After filtering to remove the drying agent the solution was stripped under vacuum to give the crude product as a tan solid. This solid was dissolved in warm methylene chloride (10 mL) and the solution was diluted with warm hexane (20 mL). Upon cooling, the product crystallized as a tan solid. This was isolated by filtration, the solid washed with 30% methylene chloride in hexane and dried. The yield was 1.2 gm, (35%).
-
- To a solution of anthranilamide (13.6 gm, 0.10 moles) and 3-pyridinecarboxaldehyde (10.7 gm, 0.10 moles) in NMP (100 mL) and acetic acid (50 mL) was added p-toluenesulfonic acid monohydrate (2.0 gm). This solution was stirred at 50° C. for 30 minutes at which point chloranil (24.6 gm, 0.10 moles) was added in portions through a funnel during one minute. The dark solution was stirred and cooled to room temperature which caused the quinazolinone to crystallize. The solid was isolated by filtration, washed with acetone/NMP (1:1) followed by acetone. After drying, the crude product was recrystallized from n-butanol (200 mL) and NMP (90 mL). The quinazolinone was isolated as tan fibrous crystals in a yield of 10.6 gm, 47.5%.
Step 2. - A portion of the quinazolinone (4.46 gm, 0.02 moles) was refluxed in phosphorous oxychloride (20 mL) for one hour. After cooling, the solution was carefully poured into cold 20% sodium carbonate solution (500 mL). The solid chloroquinazoline was isolated by filtration, washed with water and dissolved in methylene chloride (400 mL). The solution was dried over magnesium sulfate, filtered and stripped to give the chloroquinazoline as a pale yellow solid in a yield of 3.7 gm, 76.5%. The 4-chloroquinazoline (3.7 gm, 0.015 moles) was refluxed in ethanol with N-2-aminoethylmorpholine (3.99 gm, 0.031 moles) for one hour. Once cooled, the solution was stripped and the residue was dissolved in water (400 mL). The solution was made basic by the addition of sodium carbonate and the product was extracted into methylene chloride (2×100 mL). The combined extracts were washed with water (50 mL) and were then dried over magnesium sulfate. After filtration, the extracts were stripped to give the product as a tan solid. The solid was recrystallized from ethyl acetate (25 mL) and hexane (50 mL). The product, 203-93 was isolated as an off white solid in a yield of 2.86 gm, 56.0%.
-
- To a solution of anthranilamide (13.6 gm, 0.10 moles) and 2-pyridinecarboxaldehyde (10.7 gm, 0.10 moles) in methanol (250 mL) and acetic acid (25 mL) was added p-toluenesulfonic acid monohydrate (2.0 gm). This solution was stirred at 50° C. for 30 minutes at which point chloranil (24.6 gm, 0.10 moles) was added in portions through a funnel during one minute. The funnel washed with NMP (25 mL). This mixture was heated to reflux for 5 minutes. The dark solution was stirred and cooled to room temperature which caused the quinazolinone to crystallize. The solid was isolated by filtration, washed with acetone. After drying, the crude product was recrystallized twice from n-butanol. A small amount of tetrachlorohydroquinone will co-crystallize under these conditions. It can be removed by stirring the solid in warm 5% sodium carbonate solution. The quinazolinone was isolated as a tan solid in a yield of 9.5 gm, 43%.
Step 2. - A portion of the quinazolinone (4.46 gm, 0.02 moles) was refluxed in phosphorous oxychloride (20 mL) for 30 minutes. After cooling, the solution was carefully poured into cold water (500 mL). This solution was neutralized by the addition of 40% sodium hydroxide solution which caused the chloroquinazoline to separate as a white solid. The solid chloroquinazoline was isolated by filtration, washed with water and dissolved in methylene chloride (300 mL). The solution was dried over magnesium sulfate, filtered and stripped to give the chloroquinazoline as a pale yellow solid in a yield of 2.75 gm, 57%. The 4-chloroquinazoline (2.75 gm, 0.0114 moles) was refluxed in ethanol with N-2-aminoethylmorpholine (2.97 gm, 0.0228 moles) for one 30 minutes. Once cooled, the solution was poured into 10% sodium hydroxide solution (200 mL) and the product was extracted into methylene chloride (3×100 mL). The combined extracts were washed with 10% sodium hydroxide (150 mL) and were then dried over magnesium sulfate. After filtration, the extracts were stripped to give the product as a tan solid. The solid was recrystallized from n-butanol (10 mL) and hexane (20 mL). The product, 203-95 was isolated as an off white solid in a yield of 1.32 gm, 34.5%.
- The foregoing written specification is considered to be sufficient to enable one skilled in the art to practice the invention. The present invention is not to be limited in scope by examples provided, since the examples are intended as a single illustration of one aspect of the invention and other functionally equivalent embodiments are within the scope of the invention. Various modifications of the invention in addition to those shown and described herein will become apparent to those skilled in the art from the foregoing description and fall within the scope of the appended claims. The advantages and objects of the invention are not necessarily encompassed by each embodiment of the invention.
- All references, patents and patent publications that are recited in this application are incorporated in their entirety herein by reference.
Claims (16)
1-142. (canceled)
144. The compound of claim 143 , wherein R6 and R7 are each methoxy, and pharmaceutically acceptable salts thereof.
145. The compound of claim 143 , wherein R6 and R7 are each methoxy.
146. A method of inhibiting signaling by a Toll-like receptor (TLR), comprising
contacting a cell expressing a functional TLR with an effective amount of a composition comprising the compound of claim 143 , to inhibit signaling by the TLR.
147. The method of claim 146 , wherein the TLR is TLR9.
148. A method of inhibiting signaling by a Toll-like receptor (TLR), comprising
contacting a cell expressing a functional TLR with an effective amount of a composition comprising the compound of claim 144 , to inhibit signaling by the TLR.
149. The method of claim 148 , wherein the TLR is TLR9.
150. A method of inhibiting signaling by a Toll-like receptor (TLR), comprising
contacting a cell expressing a functional TLR with an effective amount of a composition comprising the compound of claim 145 , to inhibit signaling by the TLR.
151. The method of claim 150 , wherein the TLR is TLR9.
152. A method of treating an autoimmune disorder in a subject, comprising
administering to the subject an effective amount of a composition comprising the compound of claims 143, to treat the autoimmune disorder.
153. The method of claim 152 , wherein the subject is a human.
154. A method of treating an autoimmune disorder in a subject, comprising
administering to the subject an effective amount of a composition comprising the compound of claims 144, to treat the autoimmune disorder.
155. The method of claim 154 , wherein the subject is a human.
156. A method of treating an autoimmune disorder in a subject, comprising
administering to the subject an effective amount of a composition comprising the compound of claims 145, to treat the autoimmune disorder.
157. The method of claim 156 , wherein the subject is a human.
Priority Applications (1)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US11/543,314 US20070232622A1 (en) | 2003-06-20 | 2006-10-04 | Small molecule toll-like receptor (TLR) antagonists |
Applications Claiming Priority (4)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US48058803P | 2003-06-20 | 2003-06-20 | |
| US55600704P | 2004-03-23 | 2004-03-23 | |
| US10/872,196 US7410975B2 (en) | 2003-06-20 | 2004-06-18 | Small molecule toll-like receptor (TLR) antagonists |
| US11/543,314 US20070232622A1 (en) | 2003-06-20 | 2006-10-04 | Small molecule toll-like receptor (TLR) antagonists |
Related Parent Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| US10/872,196 Continuation US7410975B2 (en) | 2003-06-20 | 2004-06-18 | Small molecule toll-like receptor (TLR) antagonists |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| US20070232622A1 true US20070232622A1 (en) | 2007-10-04 |
Family
ID=34083269
Family Applications (2)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| US10/872,196 Expired - Fee Related US7410975B2 (en) | 2003-06-20 | 2004-06-18 | Small molecule toll-like receptor (TLR) antagonists |
| US11/543,314 Abandoned US20070232622A1 (en) | 2003-06-20 | 2006-10-04 | Small molecule toll-like receptor (TLR) antagonists |
Family Applications Before (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| US10/872,196 Expired - Fee Related US7410975B2 (en) | 2003-06-20 | 2004-06-18 | Small molecule toll-like receptor (TLR) antagonists |
Country Status (11)
| Country | Link |
|---|---|
| US (2) | US7410975B2 (en) |
| EP (1) | EP1635846A4 (en) |
| JP (1) | JP2007524615A (en) |
| KR (1) | KR20060016817A (en) |
| AU (1) | AU2004257149A1 (en) |
| BR (1) | BRPI0411514A (en) |
| CA (1) | CA2528774A1 (en) |
| EA (1) | EA200600069A1 (en) |
| IL (1) | IL172580A0 (en) |
| MX (1) | MXPA05013922A (en) |
| WO (1) | WO2005007672A2 (en) |
Cited By (40)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US20040087534A1 (en) * | 1994-07-15 | 2004-05-06 | University Of Iowa Research Foundation | Immunomodulatory oligonucleotides |
| US20040191270A1 (en) * | 1999-11-19 | 2004-09-30 | Csl Limited And Chiron Corporation | Vaccine compositions |
| US20050169888A1 (en) * | 1999-09-27 | 2005-08-04 | Coley Pharmaceutical Gmbh | Methods related to immunostimulatory nucleic acid-induced interferon |
| US20060089326A1 (en) * | 1994-07-15 | 2006-04-27 | University Of Iowa Research Foundation | Immunostimulatory nucleic acid molecules |
| US20080113929A1 (en) * | 2004-06-08 | 2008-05-15 | Coley Pharmaceutical Gmbh | Abasic Oligonucleotide as Carrier Platform for Antigen and Immunostimulatory Agonist and Antagonist |
| WO2009059048A2 (en) * | 2007-10-30 | 2009-05-07 | The Regents Of The University Of Colorado | (+)-opioids and methods of use |
| WO2009059050A2 (en) * | 2007-10-30 | 2009-05-07 | The Regents Of The University Of Colorado | Tlr modulators and methods for using the same |
| WO2009105260A2 (en) * | 2008-02-21 | 2009-08-27 | University Of Kentucky | Ultra-small rnas as toll-like receptor-3 antagonists |
| US7662949B2 (en) | 2005-11-25 | 2010-02-16 | Coley Pharmaceutical Gmbh | Immunostimulatory oligoribonucleotides |
| US7713529B2 (en) | 1994-07-15 | 2010-05-11 | University Of Iowa Research Foundation | Methods for treating and preventing infectious disease |
| US20100136095A1 (en) * | 2008-12-02 | 2010-06-03 | Searete Llc, A Limited Liability Corporation Of The State Of Delaware | Systems for modulating inflammation |
| US20100136096A1 (en) * | 2008-12-02 | 2010-06-03 | Searete Llc, A Limited Liability Corporation Of The State Of Delaware | Systems for modulating inflammation |
| US20100136097A1 (en) * | 2008-12-02 | 2010-06-03 | Searete Llc, A Limited Liability Corporation Of The State Of Delaware | Systems for modulating inflammation |
| US20100137247A1 (en) * | 2008-12-02 | 2010-06-03 | Searete Llc, A Limited Liability Corporation Of The State Of Delaware | Anti-inflammatory compositions and methods |
| US20100137246A1 (en) * | 2008-12-02 | 2010-06-03 | Searete Llc, A Limited Liability Corporation Of The State Of Delaware | Anti-inflammatory compositions and methods |
| US20100136094A1 (en) * | 2008-12-02 | 2010-06-03 | Searete Llc, A Limited Liability Corporation Of The State Of Delaware | Systems for modulating inflammation |
| US20100135983A1 (en) * | 2008-12-02 | 2010-06-03 | Searete Llc, A Limited Liability Corporation Of The State Of Delaware | Anti-inflammatory compositions and methods |
| US7776343B1 (en) | 1999-02-17 | 2010-08-17 | Csl Limited | Immunogenic complexes and methods relating thereto |
| US7795235B2 (en) | 2004-10-20 | 2010-09-14 | Coley Pharmaceutical Gmbh | Semi-soft c-class immunostimulatory oligonucleotides |
| US7807803B2 (en) | 2002-07-03 | 2010-10-05 | Coley Pharmaceutical Group, Inc. | Nucleic acid compositions for stimulating immune responses |
| US7935675B1 (en) | 1994-07-15 | 2011-05-03 | University Of Iowa Research Foundation | Immunostimulatory nucleic acid molecules |
| US7956043B2 (en) | 2002-12-11 | 2011-06-07 | Coley Pharmaceutical Group, Inc. | 5′ CpG nucleic acids and methods of use |
| US7998492B2 (en) | 2002-10-29 | 2011-08-16 | Coley Pharmaceutical Group, Inc. | Methods and products related to treatment and prevention of hepatitis C virus infection |
| US8114419B2 (en) | 2002-07-03 | 2012-02-14 | Coley Pharmaceutical Group, Inc. | Nucleic acid compositions for stimulating immune responses |
| US8153141B2 (en) | 2002-04-04 | 2012-04-10 | Coley Pharmaceutical Gmbh | Immunostimulatory G, U-containing oligoribonucleotides |
| US8188254B2 (en) | 2003-10-30 | 2012-05-29 | Coley Pharmaceutical Gmbh | C-class oligonucleotide analogs with enhanced immunostimulatory potency |
| US8202688B2 (en) | 1997-03-10 | 2012-06-19 | University Of Iowa Research Foundation | Use of nucleic acids containing unmethylated CpG dinucleotide as an adjuvant |
| US8283328B2 (en) | 2002-08-19 | 2012-10-09 | Coley Pharmaceutical Group, Inc. | Immunostimulatory nucleic acids |
| WO2013116590A1 (en) * | 2012-02-01 | 2013-08-08 | George Miller | Inhibition of pattern recognition receptors in pancreatic cancer treatment using tlr inhibitors |
| US8574599B1 (en) | 1998-05-22 | 2013-11-05 | Ottawa Hospital Research Institute | Methods and products for inducing mucosal immunity |
| US8580268B2 (en) | 2006-09-27 | 2013-11-12 | Coley Pharmaceutical Gmbh | CpG oligonucleotide analogs containing hydrophobic T analogs with enhanced immunostimulatory activity |
| WO2013052550A3 (en) * | 2011-10-04 | 2014-05-15 | Janus Biotherapeutics, Inc. | Novel imidazole quinoline-based immune system modulators |
| US8834900B2 (en) | 2001-08-17 | 2014-09-16 | University Of Iowa Research Foundation | Combination motif immune stimulatory oligonucleotides with improved activity |
| US8883174B2 (en) | 2009-03-25 | 2014-11-11 | The Board Of Regents, The University Of Texas System | Compositions for stimulation of mammalian innate immune resistance to pathogens |
| US9228184B2 (en) | 2012-09-29 | 2016-01-05 | Dynavax Technologies Corporation | Human toll-like receptor inhibitors and methods of use thereof |
| WO2016029077A1 (en) * | 2014-08-22 | 2016-02-25 | Janus Biotherapeutics, Inc. | Novel n2, n4, n7, 6-tetrasubstituted pteridine-2,4,7-triamine and 2, 4, 6, 7-tetrasubstituted pteridine compounds and methods of synthesis and use thereof |
| WO2016176222A1 (en) * | 2015-04-30 | 2016-11-03 | University Of Washington | Cgas in systemic lupus erythematosus (sle) |
| US9868955B2 (en) | 2012-09-29 | 2018-01-16 | Dynavax Technologies Corporation | Human toll-like receptor inhibitors and methods of use thereof |
| WO2018067302A3 (en) * | 2016-09-19 | 2018-06-07 | North Western University | Therapeutic effects of cellular delivery of small molecules and macromolecules with liposomal spherical nucleic acids |
| US10286065B2 (en) | 2014-09-19 | 2019-05-14 | Board Of Regents, The University Of Texas System | Compositions and methods for treating viral infections through stimulated innate immunity in combination with antiviral compounds |
Families Citing this family (188)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US6429199B1 (en) * | 1994-07-15 | 2002-08-06 | University Of Iowa Research Foundation | Immunostimulatory nucleic acid molecules for activating dendritic cells |
| EP0855184A1 (en) * | 1997-01-23 | 1998-07-29 | Grayson B. Dr. Lipford | Pharmaceutical composition comprising a polynucleotide and an antigen especially for vaccination |
| EP1067956B1 (en) * | 1998-04-03 | 2007-03-14 | University Of Iowa Research Foundation | Methods and products for stimulating the immune system using immunotherapeutic oligonucleotides and cytokines |
| DE69927495T2 (en) * | 1998-05-14 | 2006-07-06 | Coley Pharmaceutical Group, Inc., Wellesley | METHOD OF REGULATING HEMATOPOESIS BY USING CPG OLIGONUCLEOTIDES |
| US20030022854A1 (en) | 1998-06-25 | 2003-01-30 | Dow Steven W. | Vaccines using nucleic acid-lipid complexes |
| MXPA02003108A (en) * | 1999-09-25 | 2003-10-14 | Univ Iowa Res Found | Immunostimulatory nucleic acids. |
| US7585847B2 (en) * | 2000-02-03 | 2009-09-08 | Coley Pharmaceutical Group, Inc. | Immunostimulatory nucleic acids for the treatment of asthma and allergy |
| US20040131628A1 (en) * | 2000-03-08 | 2004-07-08 | Bratzler Robert L. | Nucleic acids for the treatment of disorders associated with microorganisms |
| US7534772B2 (en) * | 2000-06-22 | 2009-05-19 | University Of Iowa Research Foundation | Methods for enhancing antibody-induced cell lysis and treating cancer |
| AU2001291096A1 (en) * | 2000-09-15 | 2002-03-26 | Coley Pharmaceutical Gmbh | Process for high throughput screening of CpG-based immuno-agonist/antagonist |
| CA2462203A1 (en) * | 2001-10-12 | 2003-11-20 | University Of Iowa Research Foundation | Methods and products for enhancing immune responses using imidazoquinoline compounds |
| US7576066B2 (en) * | 2002-07-03 | 2009-08-18 | Coley Pharmaceutical Group, Inc. | Nucleic acid compositions for stimulating immune responses |
| US7605138B2 (en) | 2002-07-03 | 2009-10-20 | Coley Pharmaceutical Group, Inc. | Nucleic acid compositions for stimulating immune responses |
| US7569553B2 (en) | 2002-07-03 | 2009-08-04 | Coley Pharmaceutical Group, Inc. | Nucleic acid compositions for stimulating immune responses |
| AU2004257149A1 (en) | 2003-06-20 | 2005-01-27 | Coley Pharmaceutical Gmbh | Small molecule toll-like receptor (TLR) antagonists |
| EP1666059A4 (en) * | 2003-08-11 | 2008-08-27 | Univ Osaka Res Found | Novel vaccine containing adjuvant capable of inducing mucosal immunity |
| AU2004266658A1 (en) | 2003-08-12 | 2005-03-03 | 3M Innovative Properties Company | Hydroxylamine substituted imidazo-containing compounds |
| KR101106812B1 (en) | 2003-08-27 | 2012-01-19 | 쓰리엠 이노베이티브 프로퍼티즈 컴파니 | Aryloxy and Arylalkyleneoxy Substituted Imidazoquinolines |
| WO2005023190A2 (en) | 2003-09-05 | 2005-03-17 | 3M Innovative Properties Company | Treatment for cd5+ b cell lymphoma |
| CA2536139A1 (en) * | 2003-09-25 | 2005-04-07 | Coley Pharmaceutical Group, Inc. | Nucleic acid-lipophilic conjugates |
| AR046046A1 (en) | 2003-10-03 | 2005-11-23 | 3M Innovative Properties Co | IMIDAZOQUINOLINAS ALCOXI SUBSTITUTED. PHARMACEUTICAL COMPOSITIONS. |
| US7544697B2 (en) | 2003-10-03 | 2009-06-09 | Coley Pharmaceutical Group, Inc. | Pyrazolopyridines and analogs thereof |
| US20050239733A1 (en) * | 2003-10-31 | 2005-10-27 | Coley Pharmaceutical Gmbh | Sequence requirements for inhibitory oligonucleotides |
| US20050100983A1 (en) * | 2003-11-06 | 2005-05-12 | Coley Pharmaceutical Gmbh | Cell-free methods for identifying compounds that affect toll-like receptor 9 (TLR9) signaling |
| CA2545774A1 (en) | 2003-11-14 | 2005-06-02 | 3M Innovative Properties Company | Oxime substituted imidazo ring compounds |
| JP2007511535A (en) | 2003-11-14 | 2007-05-10 | スリーエム イノベイティブ プロパティズ カンパニー | Hydroxylamine substituted imidazo ring compounds |
| WO2005051317A2 (en) | 2003-11-25 | 2005-06-09 | 3M Innovative Properties Company | Substituted imidazo ring systems and methods |
| WO2005066170A1 (en) | 2003-12-29 | 2005-07-21 | 3M Innovative Properties Company | Arylalkenyl and arylalkynyl substituted imidazoquinolines |
| EP1699788A2 (en) | 2003-12-30 | 2006-09-13 | 3M Innovative Properties Company | Imidazoquinolinyl, imidazopyridinyl and imidazonaphthyridinyl sulfonamides |
| TW200533750A (en) * | 2004-02-19 | 2005-10-16 | Coley Pharm Group Inc | Immunostimulatory viral RNA oligonucleotides |
| US7767689B2 (en) | 2004-03-15 | 2010-08-03 | Ptc Therapeutics, Inc. | Carboline derivatives useful in the treatment of cancer |
| US7601840B2 (en) | 2004-03-15 | 2009-10-13 | Ptc Therapeutics, Inc. | Carboline derivatives useful in the inhibition of angiogenesis |
| US8076353B2 (en) | 2004-03-15 | 2011-12-13 | Ptc Therapeutics, Inc. | Inhibition of VEGF translation |
| US8076352B2 (en) | 2004-03-15 | 2011-12-13 | Ptc Therapeutics, Inc. | Administration of carboline derivatives useful in the treatment of cancer and other diseases |
| EP1730143A2 (en) | 2004-03-24 | 2006-12-13 | 3M Innovative Properties Company | Amide substituted imidazopyridines, imidazoquinolines, and imidazonaphthyridines |
| NZ549903A (en) | 2004-03-26 | 2010-04-30 | Astrazeneca Ab | 9-substituted 8-oxoadenine compound |
| US8017779B2 (en) | 2004-06-15 | 2011-09-13 | 3M Innovative Properties Company | Nitrogen containing heterocyclyl substituted imidazoquinolines and imidazonaphthyridines |
| WO2006065280A2 (en) | 2004-06-18 | 2006-06-22 | 3M Innovative Properties Company | Isoxazole, dihydroisoxazole, and oxadiazole substituted imidazo ring compounds and methods |
| WO2006009826A1 (en) | 2004-06-18 | 2006-01-26 | 3M Innovative Properties Company | Aryloxy and arylalkyleneoxy substituted thiazoloquinolines and thiazolonaphthyridines |
| US7897609B2 (en) | 2004-06-18 | 2011-03-01 | 3M Innovative Properties Company | Aryl substituted imidazonaphthyridines |
| AU2005335104C1 (en) * | 2004-07-18 | 2010-10-28 | Csl Limited | Immuno stimulating complex and oligonucleotide formulations for inducing enhanced interferon-gamma responses |
| CA2483732A1 (en) * | 2004-09-29 | 2006-03-29 | Ronan Le Goffic | Toll-like receptor 3, its signalling associated molecule trif and their use thereof |
| EP1655027A1 (en) * | 2004-11-04 | 2006-05-10 | Biofrontera Pharmaceuticals GmbH | 9-(N-methyl-piperidyliden-4)-thioxanthene for treatment of pulmonary hypertension and migraine |
| EP2199298A1 (en) * | 2004-11-17 | 2010-06-23 | Protiva Biotherapeutics Inc. | Sirna silencing of Apolipoprotein B |
| US20120189643A1 (en) * | 2004-11-30 | 2012-07-26 | Carton Jill M | Toll Like Receptor 3 Antagonists, Methods and Uses |
| AR051836A1 (en) * | 2004-11-30 | 2007-02-14 | Centocor Inc | RECEIVER ANTAGONISTS 3 SIMIL TOLL METHODS AND USES |
| WO2006071997A2 (en) * | 2004-12-30 | 2006-07-06 | 3M Innovative Properties Company | Treatment for cutaneous metastases |
| EP1831226B1 (en) | 2004-12-30 | 2012-08-08 | 3M Innovative Properties Company | Chiral tetracyclic compounds inducing interferon biosynthesis |
| JP5313502B2 (en) | 2004-12-30 | 2013-10-09 | スリーエム イノベイティブ プロパティズ カンパニー | Substituted chiral condensed [1,2] imidazo [4,5-c] cyclic compounds |
| EP1844201B1 (en) | 2005-02-04 | 2016-08-24 | 3M Innovative Properties Company | Aqueous gel formulations containing immune response modifiers |
| EP1846405A2 (en) | 2005-02-11 | 2007-10-24 | 3M Innovative Properties Company | Oxime and hydroxylamine substituted imidazo 4,5-c ring compounds and methods |
| JP2008531502A (en) | 2005-02-22 | 2008-08-14 | ザ レジェンツ オブ ザ ユニバーシティ オブ カリフォルニア | How to treat gastrointestinal inflammation |
| EP1863814A1 (en) | 2005-04-01 | 2007-12-12 | Coley Pharmaceutical Group, Inc. | 1-substituted pyrazolo (3,4-c) ring compounds as modulators of cytokine biosynthesis for the treatment of viral infections and neoplastic diseases |
| JP2008535832A (en) | 2005-04-01 | 2008-09-04 | コーリー ファーマシューティカル グループ,インコーポレイテッド | Pyrazolopyridine-1,4-diamine and analogs thereof |
| RS20070422A (en) * | 2005-04-08 | 2009-01-22 | Sanofi-Aventis U.S. Llc., | Methods for treating infectious disease exacerbated asthma |
| US20060241076A1 (en) * | 2005-04-26 | 2006-10-26 | Coley Pharmaceutical Gmbh | Modified oligoribonucleotide analogs with enhanced immunostimulatory activity |
| AU2006269555A1 (en) * | 2005-07-07 | 2007-01-18 | Coley Pharmaceutical Group, Inc. | Anti-CTLA-4 antibody and CpG-motif-containing synthetic oligodeoxynucleotide combination therapy for cancer treatment |
| TW201402124A (en) | 2005-08-19 | 2014-01-16 | Array Biopharma Inc | 8-substituted benzoazepines as toll-like receptor modulators |
| WO2007022946A1 (en) | 2005-08-21 | 2007-03-01 | Abbott Gmbh & Co. Kg | Heterocyclic compounds and their use as binding partners for 5-ht5 receptors |
| US20070054873A1 (en) * | 2005-08-26 | 2007-03-08 | Protiva Biotherapeutics, Inc. | Glucocorticoid modulation of nucleic acid-mediated immune stimulation |
| KR20080047463A (en) * | 2005-09-16 | 2008-05-28 | 콜리 파마슈티칼 게엠베하 | Modulation of immunostimulatory properties of short interfering ribonucleic acid (sirna) by nucleotide modification |
| WO2007038720A2 (en) * | 2005-09-27 | 2007-04-05 | Coley Pharmaceutical Gmbh | Modulation of tlr-mediated immune responses using adaptor oligonucleotides |
| US8426375B2 (en) | 2005-10-12 | 2013-04-23 | Idera Pharmaceuticals, Inc. | Immune regulatory oligonucleotide (IRO) compounds to modulate toll-like receptor based immune response |
| KR101455081B1 (en) | 2005-10-12 | 2014-10-27 | 이데라 파마슈티칼즈, 인코포레이티드 | Immune regulatory oligonucleotide (iro) compounds to modulate toll-like receptor based immune response |
| US8383598B2 (en) | 2005-10-12 | 2013-02-26 | Idera Pharmaceuticals, Inc. | Immune regulatory oligonucleotide (IRO) compounds to modulate toll-like receptor based immune response |
| US8399423B2 (en) | 2005-10-12 | 2013-03-19 | Idera Pharmaceuticals, Inc. | Immune regulatory oligonucleotide (IRO) compounds to modulate toll-like receptor based immune response |
| EP2395012B8 (en) * | 2005-11-02 | 2018-06-06 | Arbutus Biopharma Corporation | Modified siRNA molecules and uses thereof |
| CA2636929A1 (en) | 2005-12-21 | 2007-07-12 | Decode Genetics, Ehf | Biaryl nitrogen heterocycle inhibitors of lta4h for treating inflammation |
| EP2260869A3 (en) * | 2006-04-20 | 2011-03-23 | Takeda Pharmaceutical Company Limited | Pharmaceutical product |
| EP2012772A1 (en) * | 2006-04-26 | 2009-01-14 | The Uab Research Foundation | Reducing cancer cell invasion using an inhibitor of toll like receptor signaling |
| CA2652349A1 (en) * | 2006-05-15 | 2007-11-22 | University Of Kentucky Research Foundation | Toll-like receptor (tlr) stimulation for ocular angiogenesis and macular degeneration |
| US7915399B2 (en) * | 2006-06-09 | 2011-03-29 | Protiva Biotherapeutics, Inc. | Modified siRNA molecules and uses thereof |
| JP2009542645A (en) * | 2006-07-05 | 2009-12-03 | アストラゼネカ・アクチエボラーグ | 8-oxoadenine derivatives acting as modulators of TLR7 |
| WO2008008432A2 (en) | 2006-07-12 | 2008-01-17 | Coley Pharmaceutical Group, Inc. | Substituted chiral fused( 1,2) imidazo (4,5-c) ring compounds and methods |
| US20080171716A1 (en) * | 2006-08-16 | 2008-07-17 | Protiva Biotherapeutics, Inc. | Nucleic acid modulation of toll-like receptor-mediated immune stimulation |
| ATE543810T1 (en) | 2006-09-01 | 2012-02-15 | Merck Sharp & Dohme | 5-LIPOXYGENASE ACTIVATE PROTEIN (FLAP) INHIBITORS |
| US8377898B2 (en) | 2006-10-12 | 2013-02-19 | Idera Pharmaceuticals, Inc. | Immune regulatory oligonucleotide (IRO) compounds to modulate toll-like receptor based immune response |
| WO2008057529A2 (en) * | 2006-11-06 | 2008-05-15 | Coley Pharmaceutical Group, Inc. | Peptide-based vaccine compositions to endogenous cholesteryl ester transfer protein (cetp) |
| TW200831105A (en) * | 2006-12-14 | 2008-08-01 | Astrazeneca Ab | Novel compounds |
| US7629135B2 (en) * | 2006-12-22 | 2009-12-08 | The Board Of Trustees Of The University Of Illinois | Toll-like receptor agonists and antagonists and methods of use thereof |
| JP2010522697A (en) | 2007-03-16 | 2010-07-08 | ユニバーシティ オブ フロリダ リサーチ ファウンデーション | Kinase protein binding inhibitor |
| PT2132209E (en) * | 2007-03-19 | 2014-04-15 | Astrazeneca Ab | 9-substituted-8-oxo-adenine compounds as toll-like receptor (tlr7 ) modulators |
| WO2008114006A1 (en) * | 2007-03-19 | 2008-09-25 | Astrazeneca Ab | 9-substituted-8-oxo-adenine compounds as toll-like receptor (tlr7) modulators |
| US8044056B2 (en) * | 2007-03-20 | 2011-10-25 | Dainippon Sumitomo Pharma Co., Ltd. | Adenine compound |
| GB0705566D0 (en) * | 2007-03-23 | 2007-05-02 | Univ Dundee | Method of treating learning impairment in down's syndrome subjects |
| KR20100016289A (en) * | 2007-05-08 | 2010-02-12 | 아스트라제네카 아베 | Imidazoquinolines with immuno-modulating properties |
| WO2008152471A1 (en) * | 2007-06-12 | 2008-12-18 | Coley Pharmaceutical Group, Inc. | Quinazoline derivative useful as toll-like receptor antagonist |
| WO2008155441A1 (en) * | 2007-06-20 | 2008-12-24 | Marikki Laiho | Activators and therapeutic applications thereof |
| US20100190761A1 (en) * | 2007-06-20 | 2010-07-29 | Anthony Ogawa | Diphenyl substituted alkanes |
| CA2703019A1 (en) * | 2007-09-20 | 2009-03-26 | University Of Rochester | Method and compositions for treatment or prevention of inflammatory conditions |
| US20090215908A1 (en) * | 2007-09-24 | 2009-08-27 | Reliance Life Sciences Pvt. Ltd. | Toll like receptor (tlr) signaling antagonist |
| RU2010112771A (en) * | 2007-10-09 | 2011-11-20 | Коули Фармасьютикал ГмбХ (DE) | IMMUNITY MULATING ANALOGUES OF OLIGONUCLEOTIDES CONTAINING MODIFIED SUGAR GROUPS |
| JPWO2009054454A1 (en) | 2007-10-24 | 2011-03-03 | 国立大学法人 東京医科歯科大学 | Modulator of signal transduction of Toll-like receptor containing cathepsin inhibitor as an active ingredient |
| PE20091236A1 (en) * | 2007-11-22 | 2009-09-16 | Astrazeneca Ab | PYRIMIDINE DERIVATIVES AS IMMUNOMODULATORS OF TLR7 |
| UY31531A1 (en) | 2007-12-17 | 2009-08-03 | SALTS DERIVED FROM 8-OXOADENINE PHARMACEUTICAL COMPOSITIONS THAT CONTAIN THEM AND THEIR USE IN THERAPY AS TOLL TYPE RECEIVER MODULATORS (TLR) | |
| WO2010002835A2 (en) * | 2008-07-03 | 2010-01-07 | Osteogenex, Inc. | Vinpocetine and eburnamonine derivatives for promoting bone growth |
| EP2149565A1 (en) * | 2008-07-24 | 2010-02-03 | Bayer Schering Pharma AG | Sulfoximine substituted chinazoline derivatives as immune modulators for the treatment of inflammatory and allergic diseases |
| EP2316032A1 (en) * | 2008-08-20 | 2011-05-04 | INSERM - Institut National de la Santé et de la Recherche Médicale | Methods for predicting the response to anti-cancer treatment with an agonist of tlr7 or an agonist of tlr8 |
| RU2374245C1 (en) * | 2008-08-22 | 2009-11-27 | Андрей Александрович Иващенко | Ligand with wide range of simultaneous receptor activity, pharmaceutical composition, method of preparing said composition and medicinal agent |
| KR20110071108A (en) | 2008-10-06 | 2011-06-28 | 이데라 파마슈티칼즈, 인코포레이티드 | Use of inhibitors of toll-like receptors in the prevention and treatment of hypercholesterolemia and hyperlipidemia and diseases related thereto |
| US8460659B2 (en) | 2008-10-31 | 2013-06-11 | Janssen Biotech, Inc. | Toll-like receptor 3 antagonists for the treatment of metabolic and cardiovascular diseases |
| CA2742014A1 (en) | 2008-10-31 | 2010-05-06 | Centocor Ortho Biotech Inc. | Toll-like receptor 3 antagonists |
| US20100135984A1 (en) * | 2008-12-02 | 2010-06-03 | Searete Llc, A Limited Liability Corporation Of The State Of Delaware | Anti-inflammatory compositions and methods |
| WO2010064146A2 (en) | 2008-12-02 | 2010-06-10 | Chiralgen, Ltd. | Method for the synthesis of phosphorus atom modified nucleic acids |
| US8367316B2 (en) | 2009-05-01 | 2013-02-05 | Alfagene Bioscience, Inc. | Human gastrointestinal stem cell-derived primary intestinal epithelial cell system and methods of use thereof |
| GB0908772D0 (en) * | 2009-05-21 | 2009-07-01 | Astrazeneca Ab | New salts 756 |
| UY32648A (en) | 2009-05-21 | 2010-12-31 | Astrazeneca Ab | NEW DERIVATIVES OF PYRIMIDINE AND ITS USE IN THE TREATMENT OF DISEASES |
| WO2010138685A1 (en) | 2009-05-27 | 2010-12-02 | Ptc Therapeutics, Inc. | Methods for treating prostate conditions |
| CA2763479A1 (en) | 2009-05-27 | 2010-12-02 | Ptc Therapeutics, Inc. | Processes for the preparation of substituted tetrahydro beta-carbolines |
| CA3080983C (en) | 2009-05-27 | 2023-02-28 | Pct Therapeutics, Inc. | Method of inhibiting and reducing a viral infection |
| US8697662B2 (en) | 2009-05-27 | 2014-04-15 | Ptc Therapeutics, Inc. | Methods for treating Kaposi sarcoma |
| IN2012DN00720A (en) | 2009-07-06 | 2015-06-19 | Ontorii Inc | |
| WO2011011594A2 (en) * | 2009-07-23 | 2011-01-27 | Shriners Hospitals For Children | Intracellular toll-like receptors pathways and axonal degeneration |
| EP3124475B1 (en) | 2009-09-03 | 2019-08-07 | Bristol-Myers Squibb Company | Quinazolines as potassium ion channel inhibitors |
| WO2011041582A2 (en) | 2009-09-30 | 2011-04-07 | President And Fellows Of Harvard College | Methods for modulation of autophagy through the modulation of autophagy-inhibiting gene products |
| JP2013512859A (en) * | 2009-12-03 | 2013-04-18 | 大日本住友製薬株式会社 | Imidazoquinoline acting through a toll-like receptor (TLR) |
| JP2013147428A (en) * | 2010-04-27 | 2013-08-01 | Dainippon Sumitomo Pharma Co Ltd | Novel 2-heteroaryl monocyclic pyrimidine derivative |
| JP2013166700A (en) * | 2010-06-02 | 2013-08-29 | Dainippon Sumitomo Pharma Co Ltd | Novel 4, 5-fused pyrimidine derivative |
| ES2648882T3 (en) | 2010-07-19 | 2018-01-08 | Yeda Research And Development Co. Ltd. | Peptides based on the transmembrane domain of a toll-like receptor (TLR) for the treatment of TLR-mediated diseases |
| US20130165474A1 (en) * | 2010-08-17 | 2013-06-27 | Travis Dunckley | Compounds that inhibit tau phosphorylation |
| EP2620428B1 (en) | 2010-09-24 | 2019-05-22 | Wave Life Sciences Ltd. | Asymmetric auxiliary group |
| EP2651937B8 (en) | 2010-12-16 | 2016-07-13 | Sumitomo Dainippon Pharma Co., Ltd. | Imidazo[4,5-c]quinolin-1-yl derivative useful in therapy |
| WO2012080730A1 (en) | 2010-12-17 | 2012-06-21 | Astrazeneca Ab | Purine derivatives |
| US8993587B2 (en) * | 2011-01-11 | 2015-03-31 | Universitat Basel | Combination of syrosingopine and mitochondrial inhibitors for the treatment of cancer and immunosuppression |
| TR201815062T4 (en) | 2011-04-08 | 2018-11-21 | Janssen Sciences Ireland Uc | Pyrimidine derivatives for the treatment of viral infections. |
| SI2709989T1 (en) | 2011-05-18 | 2018-04-30 | Janssen Sciences Ireland Uc | Quinazoline derivatives for the treatment of viral infections and further diseases |
| BR112013032557A2 (en) | 2011-06-17 | 2020-09-24 | Merck Sharp & Dohe Corp. | compound, pharmaceutical formulation, use of a compound, and use of a compound and an additional therapeutic agent |
| US9605019B2 (en) | 2011-07-19 | 2017-03-28 | Wave Life Sciences Ltd. | Methods for the synthesis of functionalized nucleic acids |
| EA036645B1 (en) | 2011-11-09 | 2020-12-03 | Янссен Сайенсиз Айрлэнд Юси | Purine derivatives for the treatment of viral infections |
| ES2639941T3 (en) * | 2011-12-19 | 2017-10-30 | Becton Dickinson And Company | White blood cell labeling with phosphorothioate oligonucleotides |
| EP2850067B1 (en) | 2012-05-18 | 2017-08-16 | Sumitomo Dainippon Pharma Co., Ltd. | Carboxylic acid compounds |
| DK2872485T3 (en) | 2012-07-13 | 2021-03-08 | Wave Life Sciences Ltd | ASYMMETRIC ASSISTANCE GROUP |
| CA2879066C (en) * | 2012-07-13 | 2019-08-13 | Shin Nippon Biomedical Laboratories, Ltd. | Chiral nucleic acid adjuvant |
| DK2872147T3 (en) | 2012-07-13 | 2023-02-20 | Wave Life Sciences Ltd | METHOD FOR PREPARATION OF CHIRAL OLIGONUCLEOTIDES |
| SI2872515T1 (en) | 2012-07-13 | 2016-10-28 | Janssen Sciences Ireland Uc | Macrocyclic purines for the treatment of viral infections |
| JPWO2014034719A1 (en) * | 2012-08-29 | 2016-08-08 | 興和株式会社 | Quinoline derivative having TLR inhibitory action |
| ES2670513T3 (en) | 2012-10-10 | 2018-05-30 | Janssen Sciences Ireland Uc | Pyrrolo [3,2-d] pyrimidine derivatives for the treatment of viral infections and other diseases |
| CA2886635C (en) | 2012-11-16 | 2021-01-05 | Janssen Sciences Ireland Uc | Heterocyclic substituted 2-amino-quinazoline derivatives for the treatment of viral infections |
| SG11201506639XA (en) | 2013-02-21 | 2015-09-29 | Janssen Sciences Ireland Uc | 2-aminopyrimidine derivatives for the treatment of viral infections |
| EP2769723A1 (en) * | 2013-02-22 | 2014-08-27 | Ruprecht-Karls-Universität Heidelberg | Compounds for use in inhibiting HIV capsid assembly |
| CN108434138A (en) * | 2013-03-15 | 2018-08-24 | 小利兰·斯坦福大学托管委员会 | For treating ethyl hydroxychloroquine is gone with inflammation relevant disease |
| PL2978429T3 (en) | 2013-03-29 | 2017-08-31 | Janssen Sciences Ireland Uc | Macrocyclic deaza-purinones for the treatment of viral infections |
| CN105377833B (en) | 2013-05-24 | 2018-11-06 | 爱尔兰詹森科学公司 | Pyridione derivatives for treating virus infection and other disease |
| JP6763769B2 (en) | 2013-06-27 | 2020-09-30 | ヤンセン・サイエンシズ・アイルランド・アンリミテッド・カンパニー | Piroro [3,2-d] pyrimidine derivatives for the treatment of viral infections and other diseases |
| UA117253C2 (en) | 2013-07-30 | 2018-07-10 | ЯНССЕН САЙЄНСЕЗ АЙРЛЕНД ЮСі | THIENO[3,2-d]PYRIMIDINES DERIVATIVES FOR THE TREATMENT OF VIRAL INFECTIONS |
| WO2015057655A1 (en) | 2013-10-14 | 2015-04-23 | Eisai R&D Management Co., Ltd. | Selectively substituted quinoline compounds |
| KR102103256B1 (en) * | 2013-10-14 | 2020-04-23 | 에자이 알앤드디 매니지먼트 가부시키가이샤 | Selectively substituted quinoline compounds |
| EP3080124A1 (en) * | 2013-12-13 | 2016-10-19 | Takeda Pharmaceutical Company Limited | Pyrrolo[3,2-c]pyridine derivatives as tlr inhibitors |
| EP3095459A4 (en) | 2014-01-15 | 2017-08-23 | Shin Nippon Biomedical Laboratories, Ltd. | Chiral nucleic acid adjuvant having antitumor effect and antitumor agent |
| JPWO2015108046A1 (en) | 2014-01-15 | 2017-03-23 | 株式会社新日本科学 | Chiral nucleic acid adjuvant and antiallergic agent having antiallergic action |
| WO2015108047A1 (en) | 2014-01-15 | 2015-07-23 | 株式会社新日本科学 | Chiral nucleic acid adjuvant having immunity induction activity, and immunity induction activator |
| KR20220106232A (en) | 2014-01-16 | 2022-07-28 | 웨이브 라이프 사이언시스 리미티드 | Chiral design |
| EP3200788B1 (en) | 2014-09-30 | 2019-09-18 | Merck Sharp & Dohme Corp. | Inhibitors of irak4 activity |
| WO2016053770A1 (en) * | 2014-09-30 | 2016-04-07 | Merck Sharp & Dohme Corp. | Inhibitors of irak4 activity |
| US9969749B2 (en) | 2014-09-30 | 2018-05-15 | Merck Sharp & Dohme Corp. | Inhibitors of IRAK4 activity |
| EP3200789B1 (en) | 2014-09-30 | 2019-11-06 | Merck Sharp & Dohme Corp. | Inhibitors of irak4 activity |
| CA2969856A1 (en) * | 2014-12-05 | 2016-06-09 | Subramaniam Ananthan | Novel quinazolines as biogenic amine transport modulators |
| US9873702B2 (en) | 2014-12-05 | 2018-01-23 | Southern Research Institute | Heterocyclic compounds as biogenic amine transport modulators |
| EP3072891A1 (en) | 2015-03-24 | 2016-09-28 | Fraunhofer-Gesellschaft zur Förderung der angewandten Forschung e.V. | New Toll-Like Receptor 9 Antagonists |
| JP6750177B2 (en) * | 2015-12-11 | 2020-09-02 | ロート製薬株式会社 | Anthranilamide derivative and therapeutic agent for diseases involving TLR3 containing the same |
| CA3018537A1 (en) | 2016-03-21 | 2017-09-28 | Council Of Scientific & Industrial Research | Blocking toll-like receptor 9 signaling with small molecule antagonist |
| EP3436066A1 (en) | 2016-04-01 | 2019-02-06 | Checkmate Pharmaceuticals, Inc. | Fc receptor-mediated drug delivery |
| US10071079B2 (en) | 2016-06-29 | 2018-09-11 | Bristol-Myers Squibb Company | [1,2,4]triazolo[1,5-a]pyridinyl substituted indole compounds |
| WO2018002319A1 (en) | 2016-07-01 | 2018-01-04 | Janssen Sciences Ireland Uc | Dihydropyranopyrimidines for the treatment of viral infections |
| WO2018026620A1 (en) | 2016-07-30 | 2018-02-08 | Bristol-Myers Squibb Company | Dimethoxyphenyl substituted indole compounds as tlr7, tlr8 or tlr9 inhibitors |
| KR102519535B1 (en) | 2016-09-09 | 2023-04-06 | 브리스톨-마이어스 스큅 컴퍼니 | Pyridyl substituted indole compounds |
| US10968184B2 (en) | 2016-09-29 | 2021-04-06 | Janssen Sciences Ireland Unlimited Company | Pyrimidine prodrugs for the treatment of viral infections and further diseases |
| WO2018146171A1 (en) | 2017-02-09 | 2018-08-16 | INSERM (Institut National de la Santé et de la Recherche Médicale) | Methods for treating mast cell activation diseases |
| US11473145B2 (en) | 2017-07-27 | 2022-10-18 | INSERM (Institut National de la Santé et de la Recherche Médicale) | Methods for determining whether a patient suffering from rhabdomyolysis achieves a response with a TLR9 antagonist |
| EP3664803A4 (en) | 2017-08-01 | 2021-05-05 | PTC Therapeutics, Inc. | Dhodh inhibitor for use in treating hematologic cancers |
| CN110997656B (en) | 2017-08-04 | 2023-04-14 | 百时美施贵宝公司 | Substituted indole compounds useful as TLR7/8/9 inhibitors |
| EP3661934B1 (en) | 2017-08-04 | 2022-06-01 | Bristol-Myers Squibb Company | [1,2,4]triazolo[4,3-a]pyridinyl substituted indole compounds |
| AU2018364807B2 (en) * | 2017-11-08 | 2022-05-19 | Council Of Scientific & Industrial Research | Purine based compounds as toll-like receptor 9 antagonist |
| JP7265554B2 (en) | 2017-11-14 | 2023-04-26 | ブリストル-マイヤーズ スクイブ カンパニー | substituted indole compounds |
| HRP20231244T1 (en) | 2017-12-18 | 2024-02-16 | Bristol-Myers Squibb Company | 4-azaindole compounds |
| CA3085346A1 (en) | 2017-12-19 | 2019-06-27 | Bristol-Myers Squibb Company | Amide substituted indole compounds useful as tlr inhibitors |
| EP3728225B1 (en) | 2017-12-19 | 2022-11-09 | Bristol-Myers Squibb Company | Substituted indole compounds useful as tlr inhibitors |
| EP3728253B1 (en) | 2017-12-19 | 2024-03-27 | Bristol-Myers Squibb Company | 6-azaindole compounds |
| AU2018390544A1 (en) | 2017-12-20 | 2020-08-06 | Bristol-Myers Squibb Company | Diazaindole compounds |
| ES2904676T3 (en) | 2017-12-20 | 2022-04-05 | Bristol Myers Squibb Co | Amino indole compounds useful as TLR inhibitors |
| JP7291707B2 (en) | 2017-12-20 | 2023-06-15 | ブリストル-マイヤーズ スクイブ カンパニー | Aryl- and heteroaryl-substituted indole compounds |
| TW201945003A (en) | 2018-03-01 | 2019-12-01 | 愛爾蘭商健生科學愛爾蘭無限公司 | 2,4-diaminoquinazoline derivatives and medical uses thereof |
| TW202012395A (en) | 2018-04-14 | 2020-04-01 | 德商4Sc製藥公司 | Pharmaceutical combination products comprising a histone deacetylase (hdac) inhibitor and a tlr7 agonist and/or tlr8 agonist for the treatment of cancer |
| WO2019207066A1 (en) | 2018-04-26 | 2019-10-31 | INSERM (Institut National de la Santé et de la Recherche Médicale) | Methods and compositions for the treatment of sjögren's syndrome |
| MX2022001870A (en) | 2019-08-14 | 2022-05-30 | Curevac Ag | Head-up display. |
| US20230265092A1 (en) * | 2020-09-15 | 2023-08-24 | The Johns Hopkins University | Compounds which inhibit rna polymerase |
| EP4119145A1 (en) * | 2021-07-15 | 2023-01-18 | Dompe' Farmaceutici S.P.A. | Compounds for the treatment of covid-19 |
| CN115368300B (en) * | 2022-10-27 | 2023-03-24 | 北京拓领博泰生物科技有限公司 | Compound for TLR8 inhibitor and preparation method and application thereof |
Citations (34)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US4034075A (en) * | 1972-07-03 | 1977-07-05 | Allen & Hanburys Limited | Quinaldic acid derivatives |
| US4716168A (en) * | 1985-01-08 | 1987-12-29 | Norwich Eaton Pharmaceuticals, Inc. | Imidazo(4,5-f)quinolines useful as immunomodulating agents |
| US5436233A (en) * | 1992-07-15 | 1995-07-25 | Ono Pharmaceutical Co., Ltd. | 4-aminoquinazoline derivatives |
| US6221882B1 (en) * | 1997-07-03 | 2001-04-24 | University Of Iowa Research Foundation | Methods for inhibiting immunostimulatory DNA associated responses |
| US6310070B1 (en) * | 1997-07-15 | 2001-10-30 | Japan Energy Corporation | Purine derivatives and medicinal use thereof |
| US6479504B1 (en) * | 1999-06-16 | 2002-11-12 | The University Of Iowa Research Foundation | Antagonism of immunostimulatory CpG-oligonucleotides by 4-aminoquinolines and other weak bases |
| US6613772B1 (en) * | 1997-12-18 | 2003-09-02 | Aventis Pharma Deutschland Gmbh | Substituted 2-aryl-4-amino-chinazolines, method for the production and use thereof as medicaments |
| US20040067902A9 (en) * | 2000-02-03 | 2004-04-08 | Bratzler Robert L. | Immunostimulatory nucleic acids for the treatment of asthma and allergy |
| US20060069110A1 (en) * | 2004-09-27 | 2006-03-30 | Andersen Denise L | Substituted heterocyclic compounds and methods of use |
| US20070041981A1 (en) * | 2005-06-01 | 2007-02-22 | Northwestern University | Compositions and methods for altering immune function |
| US7223741B2 (en) * | 1994-07-15 | 2007-05-29 | University Of Iowa Research Foundation | Immunostimulatory nucleic acid molecules |
| US7271156B2 (en) * | 1999-09-25 | 2007-09-18 | University Of Iowa Research Foundation | Immunostimulatory nucleic acids |
| US20080009455A9 (en) * | 2005-02-24 | 2008-01-10 | Coley Pharmaceutical Group, Inc. | Immunostimulatory oligonucleotides |
| US20080026011A1 (en) * | 1994-07-15 | 2008-01-31 | University Of Iowa Research Foundation | Immunostimulatory nucleic acid molecules |
| US20080045473A1 (en) * | 2006-02-15 | 2008-02-21 | Coley Pharmaceutical Gmbh | Compositions and methods for oligonucleotide formulations |
| US20080113929A1 (en) * | 2004-06-08 | 2008-05-15 | Coley Pharmaceutical Gmbh | Abasic Oligonucleotide as Carrier Platform for Antigen and Immunostimulatory Agonist and Antagonist |
| US7410975B2 (en) * | 2003-06-20 | 2008-08-12 | Coley Pharmaceutical Group, Inc. | Small molecule toll-like receptor (TLR) antagonists |
| US20080226649A1 (en) * | 2000-12-08 | 2008-09-18 | Coley Pharmaceutical Gmbh | CPG-like nucleic acids and methods of use thereof |
| US20090017021A1 (en) * | 2004-07-18 | 2009-01-15 | Coley Pharmaceutical Group, Ltd. | Methods and compositions for inducing innate immune responses |
| US7488490B2 (en) * | 1997-03-10 | 2009-02-10 | University Of Iowa Research Foundation | Method of inducing an antigen-specific immune response by administering a synergistic combination of adjuvants comprising unmethylated CpG-containing nucleic acids and a non-nucleic acid adjuvant |
| US20090060927A1 (en) * | 1997-01-23 | 2009-03-05 | Coley Pharmaceutical Gmbh | Pharmaceutical compositions comprising a polynucleotide and optionally an antigen especially for vaccination |
| US7524828B2 (en) * | 1994-07-15 | 2009-04-28 | University Of Iowa Research Foundation | Immunostimulatory nucleic acid molecules |
| US7534772B2 (en) * | 2000-06-22 | 2009-05-19 | University Of Iowa Research Foundation | Methods for enhancing antibody-induced cell lysis and treating cancer |
| US20090137519A1 (en) * | 2004-10-20 | 2009-05-28 | Coley Pharmaceutical Group, Inc. | Semi-soft c-class immunostimulatory oligonucleotides |
| US20090142362A1 (en) * | 2006-11-06 | 2009-06-04 | Avant Immunotherapeutics, Inc. | Peptide-based vaccine compositions to endogenous cholesteryl ester transfer protein (CETP) |
| US20090155212A1 (en) * | 2000-03-03 | 2009-06-18 | Bratzler Robert L | Immunostimulatory nucleic acids and cancer medicament combination therapy for the treatment of cancer |
| US20090155307A1 (en) * | 2003-04-02 | 2009-06-18 | Coley Pharmaceutical Group, Ltd. | Immunostimulatory nucleic acid oil-in-water formulations and related methods of use |
| US7569553B2 (en) * | 2002-07-03 | 2009-08-04 | Coley Pharmaceutical Group, Inc. | Nucleic acid compositions for stimulating immune responses |
| US7576066B2 (en) * | 2002-07-03 | 2009-08-18 | Coley Pharmaceutical Group, Inc. | Nucleic acid compositions for stimulating immune responses |
| US20090214578A1 (en) * | 2005-09-16 | 2009-08-27 | Coley Pharmaceutical Gmbh | Immunostimulatory Single-Stranded Ribonucleic Acid with Phosphodiester Backbone |
| US7605138B2 (en) * | 2002-07-03 | 2009-10-20 | Coley Pharmaceutical Group, Inc. | Nucleic acid compositions for stimulating immune responses |
| US7615539B2 (en) * | 2003-09-25 | 2009-11-10 | Coley Pharmaceutical Group, Inc. | Nucleic acid-lipophilic conjugates |
| US20090306177A1 (en) * | 2005-09-16 | 2009-12-10 | Coley Pharmaceutical Gmbh | Modulation of Immunostimulatory Properties of Short Interfering Ribonucleic Acid (Sirna) by Nucleotide Modification |
| US20090311277A1 (en) * | 2002-07-03 | 2009-12-17 | Coley Pharmaceutical Group, Inc. | Nucleic acid compositions for stimulating immune responses |
Family Cites Families (45)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CH612432A5 (en) * | 1975-05-12 | 1979-07-31 | Sandoz Ag | |
| US4766211A (en) * | 1985-09-06 | 1988-08-23 | Ciba-Geigy Corporation | Chromogenic quinazolines |
| FR2711670B1 (en) * | 1993-10-22 | 1996-01-12 | Pasteur Institut | Nucleotide vector, composition containing it and vaccine for immunization against hepatitis. |
| WO1995026204A1 (en) * | 1994-03-25 | 1995-10-05 | Isis Pharmaceuticals, Inc. | Immune stimulation by phosphorothioate oligonucleotide analogs |
| US6727230B1 (en) * | 1994-03-25 | 2004-04-27 | Coley Pharmaceutical Group, Inc. | Immune stimulation by phosphorothioate oligonucleotide analogs |
| US6429199B1 (en) * | 1994-07-15 | 2002-08-06 | University Of Iowa Research Foundation | Immunostimulatory nucleic acid molecules for activating dendritic cells |
| US20030050263A1 (en) * | 1994-07-15 | 2003-03-13 | The University Of Iowa Research Foundation | Methods and products for treating HIV infection |
| US20030026782A1 (en) * | 1995-02-07 | 2003-02-06 | Arthur M. Krieg | Immunomodulatory oligonucleotides |
| CA2194761C (en) * | 1994-07-15 | 2006-12-19 | Arthur M. Krieg | Immunomodulatory oligonucleotides |
| AU7890598A (en) * | 1996-12-27 | 1998-07-31 | Yoshitomi Pharmaceutical Industries, Ltd. | Fused pyrimidine compounds and medicinal use thereof |
| US6214806B1 (en) * | 1997-02-28 | 2001-04-10 | University Of Iowa Research Foundation | Use of nucleic acids containing unmethylated CPC dinucleotide in the treatment of LPS-associated disorders |
| AU7690898A (en) | 1997-05-20 | 1998-12-11 | Ottawa Civic Hospital Loeb Research Institute | Vectors and methods for immunization or therapeutic protocols |
| US5939421A (en) * | 1997-07-01 | 1999-08-17 | Signal Pharmaceuticals, Inc. | Quinazoline analogs and related compounds and methods for treating inflammatory conditions |
| EP1067956B1 (en) * | 1998-04-03 | 2007-03-14 | University Of Iowa Research Foundation | Methods and products for stimulating the immune system using immunotherapeutic oligonucleotides and cytokines |
| DE69927495T2 (en) | 1998-05-14 | 2006-07-06 | Coley Pharmaceutical Group, Inc., Wellesley | METHOD OF REGULATING HEMATOPOESIS BY USING CPG OLIGONUCLEOTIDES |
| ATE335510T1 (en) | 1998-05-22 | 2006-09-15 | Ottawa Health Research Inst | METHODS AND PRODUCTS FOR INDUCING MUCOSA IMMUNITY |
| AU5648599A (en) * | 1998-09-11 | 2000-04-03 | Kyorin Pharmaceutical Co. Ltd. | Phosphonic ester derivatives and process for producing the same |
| US6949520B1 (en) | 1999-09-27 | 2005-09-27 | Coley Pharmaceutical Group, Inc. | Methods related to immunostimulatory nucleic acid-induced interferon |
| CA2396871A1 (en) | 2000-01-20 | 2001-12-20 | Ottawa Health Research Institute | Immunostimulatory nucleic acids for inducing a th2 immune response |
| US20040131628A1 (en) | 2000-03-08 | 2004-07-08 | Bratzler Robert L. | Nucleic acids for the treatment of disorders associated with microorganisms |
| US20020165178A1 (en) | 2000-06-28 | 2002-11-07 | Christian Schetter | Immunostimulatory nucleic acids for the treatment of anemia, thrombocytopenia, and neutropenia |
| US20020198165A1 (en) | 2000-08-01 | 2002-12-26 | Bratzler Robert L. | Nucleic acids for the prevention and treatment of gastric ulcers |
| US20020091097A1 (en) * | 2000-09-07 | 2002-07-11 | Bratzler Robert L. | Nucleic acids for the prevention and treatment of sexually transmitted diseases |
| NZ525008A (en) * | 2000-09-15 | 2004-12-24 | Vertex Pharma | Pyrazole compounds useful as protein kinase inhibitors |
| AU2001291096A1 (en) * | 2000-09-15 | 2002-03-26 | Coley Pharmaceutical Gmbh | Process for high throughput screening of CpG-based immuno-agonist/antagonist |
| CA2422488A1 (en) * | 2000-09-20 | 2002-03-28 | Merck Patent Gesellschaft Mit Beschraenkter Haftung | 4-amino-quinazolines |
| WO2002053141A2 (en) * | 2000-12-14 | 2002-07-11 | Coley Pharmaceutical Group, Inc. | Inhibition of angiogenesis by nucleic acids |
| WO2002062767A1 (en) * | 2001-02-07 | 2002-08-15 | Sumitomo Pharmaceuticals Company, Limited | Novel quinazoline derivatives |
| US20030050268A1 (en) * | 2001-03-29 | 2003-03-13 | Krieg Arthur M. | Immunostimulatory nucleic acid for treatment of non-allergic inflammatory diseases |
| AU2002360243A1 (en) * | 2001-07-26 | 2003-05-06 | Tanox, Inc. | Agents that activate or inhibit toll-like receptor 9 |
| WO2003012061A2 (en) * | 2001-08-01 | 2003-02-13 | Coley Pharmaceutical Gmbh | Methods and compositions relating to plasmacytoid dendritic cells |
| EP1446162B1 (en) * | 2001-08-17 | 2008-10-15 | Coley Pharmaceutical GmbH | Combination motif immune stimulatory oligonucleotides with improved activity |
| WO2003031573A2 (en) | 2001-10-05 | 2003-04-17 | Coley Pharmaceutical Gmbh | Toll-like receptor 3 signaling agonists and antagonists |
| CA2462203A1 (en) * | 2001-10-12 | 2003-11-20 | University Of Iowa Research Foundation | Methods and products for enhancing immune responses using imidazoquinoline compounds |
| JP4846200B2 (en) * | 2002-04-04 | 2011-12-28 | コーリー ファーマシューティカル ゲーエムベーハー | Immunostimulatory G and U-containing oligoribonucleotides |
| AU2003243409A1 (en) * | 2002-06-05 | 2003-12-22 | Coley Pharmaceutical Group, Inc. | Method for treating autoimmune or inflammatory diseases with combinations of inhibitory oligonucleotides and small molecule antagonists of immunostimulatory cpg nucleic acids |
| US7807803B2 (en) * | 2002-07-03 | 2010-10-05 | Coley Pharmaceutical Group, Inc. | Nucleic acid compositions for stimulating immune responses |
| AR040996A1 (en) * | 2002-08-19 | 2005-04-27 | Coley Pharm Group Inc | IMMUNE STIMULATING NUCLEIC ACIDS |
| JP3896933B2 (en) * | 2002-09-18 | 2007-03-22 | セイコーエプソン株式会社 | Electro-optical device and electronic apparatus |
| US7998492B2 (en) * | 2002-10-29 | 2011-08-16 | Coley Pharmaceutical Group, Inc. | Methods and products related to treatment and prevention of hepatitis C virus infection |
| AU2003300919A1 (en) | 2002-12-11 | 2004-06-30 | Coley Pharmaceutical Gmbh | 5' cpg nucleic acids and methods of use |
| CL2004000409A1 (en) * | 2003-03-03 | 2005-01-07 | Vertex Pharma | COMPOUNDS DERIVED FROM 2- (REPLACED CILO) -1- (AMINO OR REPLACED OXI) -CHINAZOLINE, INHIBITORS OF IONIC SODIUM AND CALCIUM VOLTAGE DEPENDENTS; PHARMACEUTICAL COMPOSITION; AND USE OF THE COMPOUND IN THE TREATMENT OF ACUTE PAIN, CHRONIC, NEU |
| US20050100983A1 (en) * | 2003-11-06 | 2005-05-12 | Coley Pharmaceutical Gmbh | Cell-free methods for identifying compounds that affect toll-like receptor 9 (TLR9) signaling |
| AU2005243250A1 (en) * | 2004-04-02 | 2005-11-24 | Coley Pharmaceutical Gmbh | Immunostimulatory nucleic acids for inducing IL-10 responses |
| AU2006318464B2 (en) * | 2005-11-25 | 2011-02-17 | Zoetis Belgium Sa | Immunostimulatory oligoribonucleotides |
-
2004
- 2004-06-18 AU AU2004257149A patent/AU2004257149A1/en not_active Abandoned
- 2004-06-18 EP EP04776820A patent/EP1635846A4/en not_active Withdrawn
- 2004-06-18 KR KR1020057024410A patent/KR20060016817A/en not_active Application Discontinuation
- 2004-06-18 BR BRPI0411514-7A patent/BRPI0411514A/en not_active IP Right Cessation
- 2004-06-18 CA CA002528774A patent/CA2528774A1/en not_active Abandoned
- 2004-06-18 EA EA200600069A patent/EA200600069A1/en unknown
- 2004-06-18 WO PCT/US2004/019714 patent/WO2005007672A2/en active Application Filing
- 2004-06-18 US US10/872,196 patent/US7410975B2/en not_active Expired - Fee Related
- 2004-06-18 MX MXPA05013922A patent/MXPA05013922A/en unknown
- 2004-06-18 JP JP2006517471A patent/JP2007524615A/en active Pending
-
2005
- 2005-12-14 IL IL172580A patent/IL172580A0/en unknown
-
2006
- 2006-10-04 US US11/543,314 patent/US20070232622A1/en not_active Abandoned
Patent Citations (44)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US4034075A (en) * | 1972-07-03 | 1977-07-05 | Allen & Hanburys Limited | Quinaldic acid derivatives |
| US4716168A (en) * | 1985-01-08 | 1987-12-29 | Norwich Eaton Pharmaceuticals, Inc. | Imidazo(4,5-f)quinolines useful as immunomodulating agents |
| US5436233A (en) * | 1992-07-15 | 1995-07-25 | Ono Pharmaceutical Co., Ltd. | 4-aminoquinazoline derivatives |
| US7223741B2 (en) * | 1994-07-15 | 2007-05-29 | University Of Iowa Research Foundation | Immunostimulatory nucleic acid molecules |
| US7524828B2 (en) * | 1994-07-15 | 2009-04-28 | University Of Iowa Research Foundation | Immunostimulatory nucleic acid molecules |
| US7517861B2 (en) * | 1994-07-15 | 2009-04-14 | University Of Iowa Research Foundation | Immunostimulatory nucleic acid molecules |
| US7402572B2 (en) * | 1994-07-15 | 2008-07-22 | University Of Iowa Research Foundation | Immunostimulatory nucleic acid molecules |
| US20090202575A1 (en) * | 1994-07-15 | 2009-08-13 | University Of Iowa Research Foundation | Immunostimulatory nucleic acid molecules |
| US20080031936A1 (en) * | 1994-07-15 | 2008-02-07 | University Of Iowa Research Foundation | Immunostimulatory nucleic acid molecules |
| US20080026011A1 (en) * | 1994-07-15 | 2008-01-31 | University Of Iowa Research Foundation | Immunostimulatory nucleic acid molecules |
| US20090060927A1 (en) * | 1997-01-23 | 2009-03-05 | Coley Pharmaceutical Gmbh | Pharmaceutical compositions comprising a polynucleotide and optionally an antigen especially for vaccination |
| US7488490B2 (en) * | 1997-03-10 | 2009-02-10 | University Of Iowa Research Foundation | Method of inducing an antigen-specific immune response by administering a synergistic combination of adjuvants comprising unmethylated CpG-containing nucleic acids and a non-nucleic acid adjuvant |
| US7354711B2 (en) * | 1997-07-03 | 2008-04-08 | University Of Iowa Research Foundation | Methods for inhibiting immunostimulatory DNA associated responses |
| US6521637B2 (en) * | 1997-07-03 | 2003-02-18 | University Of Iowa Research Foundation | Methods for inhibiting immunostimulatory DNA associated responses |
| US6221882B1 (en) * | 1997-07-03 | 2001-04-24 | University Of Iowa Research Foundation | Methods for inhibiting immunostimulatory DNA associated responses |
| US6399630B1 (en) * | 1997-07-03 | 2002-06-04 | University Of Iowa Research Foundation | Methods for inhibiting immunostimulatory DNA associated responses |
| US6310070B1 (en) * | 1997-07-15 | 2001-10-30 | Japan Energy Corporation | Purine derivatives and medicinal use thereof |
| US6613772B1 (en) * | 1997-12-18 | 2003-09-02 | Aventis Pharma Deutschland Gmbh | Substituted 2-aryl-4-amino-chinazolines, method for the production and use thereof as medicaments |
| US6479504B1 (en) * | 1999-06-16 | 2002-11-12 | The University Of Iowa Research Foundation | Antagonism of immunostimulatory CpG-oligonucleotides by 4-aminoquinolines and other weak bases |
| US20090191188A1 (en) * | 1999-09-25 | 2009-07-30 | University Of Iowa Research Foundation | Immunostimulatory nucleic acids |
| US7271156B2 (en) * | 1999-09-25 | 2007-09-18 | University Of Iowa Research Foundation | Immunostimulatory nucleic acids |
| US7585847B2 (en) * | 2000-02-03 | 2009-09-08 | Coley Pharmaceutical Group, Inc. | Immunostimulatory nucleic acids for the treatment of asthma and allergy |
| US20040067902A9 (en) * | 2000-02-03 | 2004-04-08 | Bratzler Robert L. | Immunostimulatory nucleic acids for the treatment of asthma and allergy |
| US20090155212A1 (en) * | 2000-03-03 | 2009-06-18 | Bratzler Robert L | Immunostimulatory nucleic acids and cancer medicament combination therapy for the treatment of cancer |
| US7534772B2 (en) * | 2000-06-22 | 2009-05-19 | University Of Iowa Research Foundation | Methods for enhancing antibody-induced cell lysis and treating cancer |
| US20080226649A1 (en) * | 2000-12-08 | 2008-09-18 | Coley Pharmaceutical Gmbh | CPG-like nucleic acids and methods of use thereof |
| US7605138B2 (en) * | 2002-07-03 | 2009-10-20 | Coley Pharmaceutical Group, Inc. | Nucleic acid compositions for stimulating immune responses |
| US7576066B2 (en) * | 2002-07-03 | 2009-08-18 | Coley Pharmaceutical Group, Inc. | Nucleic acid compositions for stimulating immune responses |
| US20090311277A1 (en) * | 2002-07-03 | 2009-12-17 | Coley Pharmaceutical Group, Inc. | Nucleic acid compositions for stimulating immune responses |
| US7569553B2 (en) * | 2002-07-03 | 2009-08-04 | Coley Pharmaceutical Group, Inc. | Nucleic acid compositions for stimulating immune responses |
| US20090155307A1 (en) * | 2003-04-02 | 2009-06-18 | Coley Pharmaceutical Group, Ltd. | Immunostimulatory nucleic acid oil-in-water formulations and related methods of use |
| US7410975B2 (en) * | 2003-06-20 | 2008-08-12 | Coley Pharmaceutical Group, Inc. | Small molecule toll-like receptor (TLR) antagonists |
| US7615539B2 (en) * | 2003-09-25 | 2009-11-10 | Coley Pharmaceutical Group, Inc. | Nucleic acid-lipophilic conjugates |
| US20080113929A1 (en) * | 2004-06-08 | 2008-05-15 | Coley Pharmaceutical Gmbh | Abasic Oligonucleotide as Carrier Platform for Antigen and Immunostimulatory Agonist and Antagonist |
| US20090017021A1 (en) * | 2004-07-18 | 2009-01-15 | Coley Pharmaceutical Group, Ltd. | Methods and compositions for inducing innate immune responses |
| US20060069110A1 (en) * | 2004-09-27 | 2006-03-30 | Andersen Denise L | Substituted heterocyclic compounds and methods of use |
| US7566703B2 (en) * | 2004-10-20 | 2009-07-28 | Coley Pharmaceutical Group, Inc. | Semi-soft C-class immunostimulatory oligonucleotides |
| US20090137519A1 (en) * | 2004-10-20 | 2009-05-28 | Coley Pharmaceutical Group, Inc. | Semi-soft c-class immunostimulatory oligonucleotides |
| US20080009455A9 (en) * | 2005-02-24 | 2008-01-10 | Coley Pharmaceutical Group, Inc. | Immunostimulatory oligonucleotides |
| US20070041981A1 (en) * | 2005-06-01 | 2007-02-22 | Northwestern University | Compositions and methods for altering immune function |
| US20090214578A1 (en) * | 2005-09-16 | 2009-08-27 | Coley Pharmaceutical Gmbh | Immunostimulatory Single-Stranded Ribonucleic Acid with Phosphodiester Backbone |
| US20090306177A1 (en) * | 2005-09-16 | 2009-12-10 | Coley Pharmaceutical Gmbh | Modulation of Immunostimulatory Properties of Short Interfering Ribonucleic Acid (Sirna) by Nucleotide Modification |
| US20080045473A1 (en) * | 2006-02-15 | 2008-02-21 | Coley Pharmaceutical Gmbh | Compositions and methods for oligonucleotide formulations |
| US20090142362A1 (en) * | 2006-11-06 | 2009-06-04 | Avant Immunotherapeutics, Inc. | Peptide-based vaccine compositions to endogenous cholesteryl ester transfer protein (CETP) |
Cited By (76)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US8058249B2 (en) | 1994-07-15 | 2011-11-15 | University Of Iowa Research Foundation | Immunostimulatory nucleic acid molecules |
| US8309527B2 (en) | 1994-07-15 | 2012-11-13 | University Of Iowa Research Foundation | Immunomodulatory oligonucleotides |
| US20040162262A1 (en) * | 1994-07-15 | 2004-08-19 | University Of Iowa Research Foundation | Immunomodulatory oligonucleotides |
| US8129351B2 (en) | 1994-07-15 | 2012-03-06 | The University Of Iowa Research Foundation | Immunostimulatory nucleic acid molecules |
| US8114848B2 (en) | 1994-07-15 | 2012-02-14 | The United States Of America As Represented By The Department Of Health And Human Services | Immunomodulatory oligonucleotides |
| US20050244380A1 (en) * | 1994-07-15 | 2005-11-03 | University Of Iowa Research Foundation | Immunomodulatory oligonucleotides |
| US20060089326A1 (en) * | 1994-07-15 | 2006-04-27 | University Of Iowa Research Foundation | Immunostimulatory nucleic acid molecules |
| US20070009482A9 (en) * | 1994-07-15 | 2007-01-11 | University Of Iowa Research Foundation | Immunomodulatory oligonucleotides |
| US20040087534A1 (en) * | 1994-07-15 | 2004-05-06 | University Of Iowa Research Foundation | Immunomodulatory oligonucleotides |
| US20040162258A1 (en) * | 1994-07-15 | 2004-08-19 | University Of Iowa Research Foundation | Immunomodulatory oligonucleotides |
| US7935675B1 (en) | 1994-07-15 | 2011-05-03 | University Of Iowa Research Foundation | Immunostimulatory nucleic acid molecules |
| US8008266B2 (en) | 1994-07-15 | 2011-08-30 | University Of Iowa Foundation | Methods of treating cancer using immunostimulatory oligonucleotides |
| US8158592B2 (en) | 1994-07-15 | 2012-04-17 | Coley Pharmaceutical Group, Inc. | Immunostimulatory nucleic acid molecules |
| US7888327B2 (en) | 1994-07-15 | 2011-02-15 | University Of Iowa Research Foundation | Methods of using immunostimulatory nucleic acid molecules to treat allergic conditions |
| US7879810B2 (en) | 1994-07-15 | 2011-02-01 | University Of Iowa Research Foundation | Immunostimulatory nucleic acid molecules |
| US8258106B2 (en) | 1994-07-15 | 2012-09-04 | University Of Iowa Research Foundation | Immunostimulatory nucleic acid molecules |
| US8148340B2 (en) | 1994-07-15 | 2012-04-03 | The United States Of America As Represented By The Department Of Health And Human Services | Immunomodulatory oligonucleotides |
| US7713529B2 (en) | 1994-07-15 | 2010-05-11 | University Of Iowa Research Foundation | Methods for treating and preventing infectious disease |
| US7723500B2 (en) | 1994-07-15 | 2010-05-25 | University Of Iowa Research Foundation | Immunostimulatory nucleic acid molecules |
| US7723022B2 (en) | 1994-07-15 | 2010-05-25 | University Of Iowa Research Foundation | Immunostimulatory nucleic acid molecules |
| US8202688B2 (en) | 1997-03-10 | 2012-06-19 | University Of Iowa Research Foundation | Use of nucleic acids containing unmethylated CpG dinucleotide as an adjuvant |
| US8574599B1 (en) | 1998-05-22 | 2013-11-05 | Ottawa Hospital Research Institute | Methods and products for inducing mucosal immunity |
| US8173141B2 (en) | 1999-02-17 | 2012-05-08 | Csl Limited | Immunogenic complexes and methods relating thereto |
| US7776343B1 (en) | 1999-02-17 | 2010-08-17 | Csl Limited | Immunogenic complexes and methods relating thereto |
| US7776344B2 (en) | 1999-09-27 | 2010-08-17 | University Of Iowa Research Foundation | Methods related to immunostimulatory nucleic acid-induced interferon |
| US20050169888A1 (en) * | 1999-09-27 | 2005-08-04 | Coley Pharmaceutical Gmbh | Methods related to immunostimulatory nucleic acid-induced interferon |
| US20040191270A1 (en) * | 1999-11-19 | 2004-09-30 | Csl Limited And Chiron Corporation | Vaccine compositions |
| US20100047271A1 (en) * | 1999-11-19 | 2010-02-25 | Csl Limited | Vaccine compositions |
| US8834900B2 (en) | 2001-08-17 | 2014-09-16 | University Of Iowa Research Foundation | Combination motif immune stimulatory oligonucleotides with improved activity |
| US8153141B2 (en) | 2002-04-04 | 2012-04-10 | Coley Pharmaceutical Gmbh | Immunostimulatory G, U-containing oligoribonucleotides |
| US8658607B2 (en) | 2002-04-04 | 2014-02-25 | Zoetis Belgium | Immunostimulatory G, U-containing oligoribonucleotides |
| US9428536B2 (en) | 2002-04-04 | 2016-08-30 | Zoetis Belgium Sa | Immunostimulatory G, U-containing oligoribonucleotides |
| US8114419B2 (en) | 2002-07-03 | 2012-02-14 | Coley Pharmaceutical Group, Inc. | Nucleic acid compositions for stimulating immune responses |
| US7807803B2 (en) | 2002-07-03 | 2010-10-05 | Coley Pharmaceutical Group, Inc. | Nucleic acid compositions for stimulating immune responses |
| US8283328B2 (en) | 2002-08-19 | 2012-10-09 | Coley Pharmaceutical Group, Inc. | Immunostimulatory nucleic acids |
| US8304396B2 (en) | 2002-08-19 | 2012-11-06 | Coley Pharmaceutical Group, Inc. | Immunostimulatory nucleic acids |
| US7998492B2 (en) | 2002-10-29 | 2011-08-16 | Coley Pharmaceutical Group, Inc. | Methods and products related to treatment and prevention of hepatitis C virus infection |
| US7956043B2 (en) | 2002-12-11 | 2011-06-07 | Coley Pharmaceutical Group, Inc. | 5′ CpG nucleic acids and methods of use |
| US8188254B2 (en) | 2003-10-30 | 2012-05-29 | Coley Pharmaceutical Gmbh | C-class oligonucleotide analogs with enhanced immunostimulatory potency |
| US20080113929A1 (en) * | 2004-06-08 | 2008-05-15 | Coley Pharmaceutical Gmbh | Abasic Oligonucleotide as Carrier Platform for Antigen and Immunostimulatory Agonist and Antagonist |
| US7795235B2 (en) | 2004-10-20 | 2010-09-14 | Coley Pharmaceutical Gmbh | Semi-soft c-class immunostimulatory oligonucleotides |
| US8354522B2 (en) | 2005-11-25 | 2013-01-15 | Coley Pharmaceutical Gmbh | Immunostimulatory oligoribonucleotides |
| US7662949B2 (en) | 2005-11-25 | 2010-02-16 | Coley Pharmaceutical Gmbh | Immunostimulatory oligoribonucleotides |
| US10260071B2 (en) | 2006-09-27 | 2019-04-16 | Coley Pharmaceutical Gmbh | CpG oligonucleotide analogs containing hydrophobic T analogs with enhanced immunostimulatory activity |
| US9382545B2 (en) | 2006-09-27 | 2016-07-05 | Coley Pharmaceutical Gmbh | CpG oligonucleotide analogs containing hydrophobic T analogs with enhanced immunostimulatory activity |
| US8580268B2 (en) | 2006-09-27 | 2013-11-12 | Coley Pharmaceutical Gmbh | CpG oligonucleotide analogs containing hydrophobic T analogs with enhanced immunostimulatory activity |
| WO2009059048A2 (en) * | 2007-10-30 | 2009-05-07 | The Regents Of The University Of Colorado | (+)-opioids and methods of use |
| WO2009059050A2 (en) * | 2007-10-30 | 2009-05-07 | The Regents Of The University Of Colorado | Tlr modulators and methods for using the same |
| WO2009059048A3 (en) * | 2007-10-30 | 2009-08-20 | Univ Colorado | (+)-opioids and methods of use |
| WO2009059050A3 (en) * | 2007-10-30 | 2009-08-20 | Univ Colorado | Tlr modulators and methods for using the same |
| US8481508B2 (en) * | 2008-02-21 | 2013-07-09 | University Of Kentucky Research Foundation | Ultra-small RNAs as toll-like receptor-3 antagonists |
| US20110097390A1 (en) * | 2008-02-21 | 2011-04-28 | University Of Kentucky Research Foundation | Ultra-Small RNAs as Toll-Like Receptor-3 Antagonists |
| WO2009105260A2 (en) * | 2008-02-21 | 2009-08-27 | University Of Kentucky | Ultra-small rnas as toll-like receptor-3 antagonists |
| WO2009105260A3 (en) * | 2008-02-21 | 2009-12-30 | University Of Kentucky | Ultra-small rnas as toll-like receptor-3 antagonists |
| US20100136096A1 (en) * | 2008-12-02 | 2010-06-03 | Searete Llc, A Limited Liability Corporation Of The State Of Delaware | Systems for modulating inflammation |
| US20100136094A1 (en) * | 2008-12-02 | 2010-06-03 | Searete Llc, A Limited Liability Corporation Of The State Of Delaware | Systems for modulating inflammation |
| US20100136095A1 (en) * | 2008-12-02 | 2010-06-03 | Searete Llc, A Limited Liability Corporation Of The State Of Delaware | Systems for modulating inflammation |
| US20100135983A1 (en) * | 2008-12-02 | 2010-06-03 | Searete Llc, A Limited Liability Corporation Of The State Of Delaware | Anti-inflammatory compositions and methods |
| US20100136097A1 (en) * | 2008-12-02 | 2010-06-03 | Searete Llc, A Limited Liability Corporation Of The State Of Delaware | Systems for modulating inflammation |
| US20100135908A1 (en) * | 2008-12-02 | 2010-06-03 | Searete Llc, A Limited Liability Corporation Of The State Of Delaware | Delivery devices for modulating inflammation |
| US20100137247A1 (en) * | 2008-12-02 | 2010-06-03 | Searete Llc, A Limited Liability Corporation Of The State Of Delaware | Anti-inflammatory compositions and methods |
| US20100137246A1 (en) * | 2008-12-02 | 2010-06-03 | Searete Llc, A Limited Liability Corporation Of The State Of Delaware | Anti-inflammatory compositions and methods |
| US9504742B2 (en) | 2009-03-25 | 2016-11-29 | The Board Of Regents, The University Of Texas System | Compositions for stimulation of mammalian innate immune resistance to pathogens |
| US10722573B2 (en) | 2009-03-25 | 2020-07-28 | The Board Of Regents, The University Of Texas System | Compositions for stimulation of mammalian innate immune resistance to pathogens |
| US8883174B2 (en) | 2009-03-25 | 2014-11-11 | The Board Of Regents, The University Of Texas System | Compositions for stimulation of mammalian innate immune resistance to pathogens |
| US9186400B2 (en) | 2009-03-25 | 2015-11-17 | The Board Of Regents, The University Of Texas System | Compositions for stimulation of mammalian innate immune resistance to pathogens |
| WO2013052550A3 (en) * | 2011-10-04 | 2014-05-15 | Janus Biotherapeutics, Inc. | Novel imidazole quinoline-based immune system modulators |
| US9873694B2 (en) | 2011-10-04 | 2018-01-23 | Janus Biotherapeutics, Inc. | Imidazole quinoline-based immune system modulators |
| WO2013116590A1 (en) * | 2012-02-01 | 2013-08-08 | George Miller | Inhibition of pattern recognition receptors in pancreatic cancer treatment using tlr inhibitors |
| US9868955B2 (en) | 2012-09-29 | 2018-01-16 | Dynavax Technologies Corporation | Human toll-like receptor inhibitors and methods of use thereof |
| US9228184B2 (en) | 2012-09-29 | 2016-01-05 | Dynavax Technologies Corporation | Human toll-like receptor inhibitors and methods of use thereof |
| WO2016029077A1 (en) * | 2014-08-22 | 2016-02-25 | Janus Biotherapeutics, Inc. | Novel n2, n4, n7, 6-tetrasubstituted pteridine-2,4,7-triamine and 2, 4, 6, 7-tetrasubstituted pteridine compounds and methods of synthesis and use thereof |
| US10286065B2 (en) | 2014-09-19 | 2019-05-14 | Board Of Regents, The University Of Texas System | Compositions and methods for treating viral infections through stimulated innate immunity in combination with antiviral compounds |
| WO2016176222A1 (en) * | 2015-04-30 | 2016-11-03 | University Of Washington | Cgas in systemic lupus erythematosus (sle) |
| US10329258B2 (en) | 2015-04-30 | 2019-06-25 | University Of Washington | CGAS in systemic lupus erythematosus (SLE) |
| WO2018067302A3 (en) * | 2016-09-19 | 2018-06-07 | North Western University | Therapeutic effects of cellular delivery of small molecules and macromolecules with liposomal spherical nucleic acids |
Also Published As
| Publication number | Publication date |
|---|---|
| US7410975B2 (en) | 2008-08-12 |
| EP1635846A4 (en) | 2009-01-28 |
| EA200600069A1 (en) | 2006-08-25 |
| US20050119273A1 (en) | 2005-06-02 |
| WO2005007672A8 (en) | 2007-11-01 |
| JP2007524615A (en) | 2007-08-30 |
| EP1635846A2 (en) | 2006-03-22 |
| IL172580A0 (en) | 2006-04-10 |
| KR20060016817A (en) | 2006-02-22 |
| WO2005007672A2 (en) | 2005-01-27 |
| MXPA05013922A (en) | 2006-02-24 |
| BRPI0411514A (en) | 2006-08-01 |
| AU2004257149A1 (en) | 2005-01-27 |
| CA2528774A1 (en) | 2005-01-27 |
| WO2005007672A3 (en) | 2005-09-15 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| US7410975B2 (en) | Small molecule toll-like receptor (TLR) antagonists | |
| US10117875B2 (en) | Immune system modulators | |
| US20100160314A1 (en) | Small Molecule Inhibitors of Toll-Like Receptor 9 | |
| US9873694B2 (en) | Imidazole quinoline-based immune system modulators | |
| US9353115B2 (en) | Immune system modulators | |
| US20170275287A1 (en) | Novel n2, n4, n7, 6-tetrasubstituted pteridine-2,4,7-triamine and 2, 4, 6, 7-tetrasubstituted pteridine compounds and methods of synthesis and use thereof | |
| ZA200510028B (en) | Small molicule toll-like receptor (TLR) antagonists |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| AS | Assignment |
Owner name: COLEY PHARMACEUTICAL GMBH, GERMANY Free format text: ASSIGNMENT OF ASSIGNORS INTEREST;ASSIGNORS:LIPFORD, GRAYSON B.;FORSBACH, ALEXANDRA;REEL/FRAME:018476/0031;SIGNING DATES FROM 20040723 TO 20040802 Owner name: COLEY PHARMACEUTICAL GROUP, INC., MASSACHUSETTS Free format text: ASSIGNMENT OF ASSIGNORS INTEREST;ASSIGNOR:ZEPP, CHARLES;REEL/FRAME:018476/0040 Effective date: 20040727 |
|
| STCB | Information on status: application discontinuation |
Free format text: ABANDONED -- FAILURE TO PAY ISSUE FEE |