US20060258655A1 - Crystalline forms of linezolid intermediate - Google Patents
Crystalline forms of linezolid intermediate Download PDFInfo
- Publication number
- US20060258655A1 US20060258655A1 US11/362,312 US36231206A US2006258655A1 US 20060258655 A1 US20060258655 A1 US 20060258655A1 US 36231206 A US36231206 A US 36231206A US 2006258655 A1 US2006258655 A1 US 2006258655A1
- Authority
- US
- United States
- Prior art keywords
- oxazolidinyl
- morpholinyl
- oxo
- fluorophenyl
- crystalline
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Abandoned
Links
- TYZROVQLWOKYKF-ZDUSSCGKSA-N linezolid Chemical compound O=C1O[C@@H](CNC(=O)C)CN1C(C=C1F)=CC=C1N1CCOCC1 TYZROVQLWOKYKF-ZDUSSCGKSA-N 0.000 title abstract description 25
- 229960003907 linezolid Drugs 0.000 title abstract description 21
- 238000000634 powder X-ray diffraction Methods 0.000 claims description 14
- 238000001144 powder X-ray diffraction data Methods 0.000 claims description 14
- 150000001412 amines Chemical class 0.000 description 14
- 239000007787 solid Substances 0.000 description 11
- 238000000034 method Methods 0.000 description 9
- 238000004128 high performance liquid chromatography Methods 0.000 description 8
- 238000002360 preparation method Methods 0.000 description 7
- XEKOWRVHYACXOJ-UHFFFAOYSA-N Ethyl acetate Chemical compound CCOC(C)=O XEKOWRVHYACXOJ-UHFFFAOYSA-N 0.000 description 6
- 238000006243 chemical reaction Methods 0.000 description 6
- 239000011541 reaction mixture Substances 0.000 description 6
- UFHFLCQGNIYNRP-UHFFFAOYSA-N Hydrogen Chemical compound [H][H] UFHFLCQGNIYNRP-UHFFFAOYSA-N 0.000 description 5
- 239000013078 crystal Substances 0.000 description 5
- 239000000843 powder Substances 0.000 description 5
- FQUBFDDPWLBKIZ-NSHDSACASA-N (5r)-5-(azidomethyl)-3-(3-fluoro-4-morpholin-4-ylphenyl)-1,3-oxazolidin-2-one Chemical compound FC1=CC(N2C(O[C@@H](CN=[N+]=[N-])C2)=O)=CC=C1N1CCOCC1 FQUBFDDPWLBKIZ-NSHDSACASA-N 0.000 description 4
- DLFVBJFMPXGRIB-UHFFFAOYSA-N Acetamide Chemical compound CC(N)=O DLFVBJFMPXGRIB-UHFFFAOYSA-N 0.000 description 4
- IJGRMHOSHXDMSA-UHFFFAOYSA-N Atomic nitrogen Chemical compound N#N IJGRMHOSHXDMSA-UHFFFAOYSA-N 0.000 description 4
- KDLHZDBZIXYQEI-UHFFFAOYSA-N Palladium Chemical compound [Pd] KDLHZDBZIXYQEI-UHFFFAOYSA-N 0.000 description 4
- 239000001257 hydrogen Substances 0.000 description 4
- 229910052739 hydrogen Inorganic materials 0.000 description 4
- 239000000243 solution Substances 0.000 description 4
- WFDIJRYMOXRFFG-UHFFFAOYSA-N Acetic anhydride Chemical compound CC(=O)OC(C)=O WFDIJRYMOXRFFG-UHFFFAOYSA-N 0.000 description 3
- OKKJLVBELUTLKV-UHFFFAOYSA-N Methanol Chemical compound OC OKKJLVBELUTLKV-UHFFFAOYSA-N 0.000 description 3
- YXFVVABEGXRONW-UHFFFAOYSA-N Toluene Chemical compound CC1=CC=CC=C1 YXFVVABEGXRONW-UHFFFAOYSA-N 0.000 description 3
- 239000000463 material Substances 0.000 description 3
- VYPSYNLAJGMNEJ-UHFFFAOYSA-N silicon dioxide Inorganic materials O=[Si]=O VYPSYNLAJGMNEJ-UHFFFAOYSA-N 0.000 description 3
- IZXIZTKNFFYFOF-UHFFFAOYSA-N 2-Oxazolidone Chemical compound O=C1NCCO1 IZXIZTKNFFYFOF-UHFFFAOYSA-N 0.000 description 2
- CSCPPACGZOOCGX-UHFFFAOYSA-N Acetone Chemical compound CC(C)=O CSCPPACGZOOCGX-UHFFFAOYSA-N 0.000 description 2
- LFQSCWFLJHTTHZ-UHFFFAOYSA-N Ethanol Chemical compound CCO LFQSCWFLJHTTHZ-UHFFFAOYSA-N 0.000 description 2
- BAVYZALUXZFZLV-UHFFFAOYSA-N Methylamine Chemical compound NC BAVYZALUXZFZLV-UHFFFAOYSA-N 0.000 description 2
- PXIPVTKHYLBLMZ-UHFFFAOYSA-N Sodium azide Chemical compound [Na+].[N-]=[N+]=[N-] PXIPVTKHYLBLMZ-UHFFFAOYSA-N 0.000 description 2
- 238000001704 evaporation Methods 0.000 description 2
- 230000008020 evaporation Effects 0.000 description 2
- 208000015181 infectious disease Diseases 0.000 description 2
- 238000002844 melting Methods 0.000 description 2
- 230000008018 melting Effects 0.000 description 2
- 229910052757 nitrogen Inorganic materials 0.000 description 2
- 239000000047 product Substances 0.000 description 2
- 238000003786 synthesis reaction Methods 0.000 description 2
- 238000002411 thermogravimetry Methods 0.000 description 2
- 238000001644 13C nuclear magnetic resonance spectroscopy Methods 0.000 description 1
- VHUUQVKOLVNVRT-UHFFFAOYSA-N Ammonium hydroxide Chemical compound [NH4+].[OH-] VHUUQVKOLVNVRT-UHFFFAOYSA-N 0.000 description 1
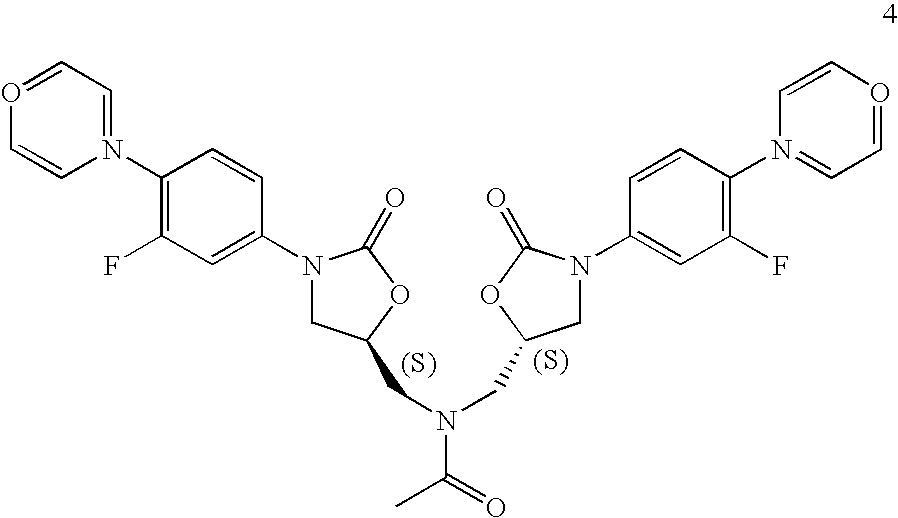
- FJSYXFWBZGUETD-OVEPDTBMSA-L C.C.C#CC#CC.C1COCCN1.CC(=O)NC[C@H]1CN(C2=CC(F)=C(N3CCOCC3)C=C2)C(=O)O1.CC(=O)OC(C)=O.CCCC(=O)OC[C@H]1CO1.CCCCOOC[C@H]1CN(C2=CC(F)=C(N3CCOCC3)C=C2)C(=O)O1.CCN(C(C)C)C(C)C.CCN(CC)CC.CS(=O)(=O)Cl.CS(=O)(=O)OC[C@H]1CN(C2=CC(F)=C(N3CCOCC3)C=C2)C(=O)O1.CS(=O)(=O)OC[C@H]1CN(C2=CC(F)=C(N3CCOCC3)C=C2)C(=O)O1.N.NC[C@H]1CN(C2=CC(F)=C(N3CCOCC3)C=C2)C(=O)O1.O=C(Cl)OCC1=CC=CC=C1.O=C(NC1=CC(F)=C(N2CCOCC2)C=C1)OCC1=CC=CC=C1.O=C1O[C@@H](CO)CN1C1=CC(F)=C(N2CCOCC2)C=C1.O=COO[Na].O=C[O-].O=[N+]([O-])C1=CC(F)=C(F)C=C1.O=[N+]([O-])C1=CC(F)=C(N2CCOCC2)C=C1.O=[N+]([O-])C1=CC(F)=C(N2CCOCC2)C=C1.S.S.[HH].[Li]CCCC.[N-]=[N+]=N[Na].[NH4+] Chemical compound C.C.C#CC#CC.C1COCCN1.CC(=O)NC[C@H]1CN(C2=CC(F)=C(N3CCOCC3)C=C2)C(=O)O1.CC(=O)OC(C)=O.CCCC(=O)OC[C@H]1CO1.CCCCOOC[C@H]1CN(C2=CC(F)=C(N3CCOCC3)C=C2)C(=O)O1.CCN(C(C)C)C(C)C.CCN(CC)CC.CS(=O)(=O)Cl.CS(=O)(=O)OC[C@H]1CN(C2=CC(F)=C(N3CCOCC3)C=C2)C(=O)O1.CS(=O)(=O)OC[C@H]1CN(C2=CC(F)=C(N3CCOCC3)C=C2)C(=O)O1.N.NC[C@H]1CN(C2=CC(F)=C(N3CCOCC3)C=C2)C(=O)O1.O=C(Cl)OCC1=CC=CC=C1.O=C(NC1=CC(F)=C(N2CCOCC2)C=C1)OCC1=CC=CC=C1.O=C1O[C@@H](CO)CN1C1=CC(F)=C(N2CCOCC2)C=C1.O=COO[Na].O=C[O-].O=[N+]([O-])C1=CC(F)=C(F)C=C1.O=[N+]([O-])C1=CC(F)=C(N2CCOCC2)C=C1.O=[N+]([O-])C1=CC(F)=C(N2CCOCC2)C=C1.S.S.[HH].[Li]CCCC.[N-]=[N+]=N[Na].[NH4+] FJSYXFWBZGUETD-OVEPDTBMSA-L 0.000 description 1
- ILDZYFUTZMUMFI-WLKYSPGFSA-N CC(=O)N(C[C@H]1CN(C2=CC(F)=C(N3=CC=OC=C3)C=C2)C(=O)O1)C[C@H]1CN(C2=CC(F)=C(N3=CC=OC=C3)C=C2)C(=O)O1.S.S Chemical compound CC(=O)N(C[C@H]1CN(C2=CC(F)=C(N3=CC=OC=C3)C=C2)C(=O)O1)C[C@H]1CN(C2=CC(F)=C(N3=CC=OC=C3)C=C2)C(=O)O1.S.S ILDZYFUTZMUMFI-WLKYSPGFSA-N 0.000 description 1
- KXDHJXZQYSOELW-UHFFFAOYSA-M Carbamate Chemical compound NC([O-])=O KXDHJXZQYSOELW-UHFFFAOYSA-M 0.000 description 1
- RYGMFSIKBFXOCR-UHFFFAOYSA-N Copper Chemical compound [Cu] RYGMFSIKBFXOCR-UHFFFAOYSA-N 0.000 description 1
- 206010014889 Enterococcal infections Diseases 0.000 description 1
- 208000008745 Healthcare-Associated Pneumonia Diseases 0.000 description 1
- 238000004566 IR spectroscopy Methods 0.000 description 1
- NZWKUHSARXKEFC-MERQFXBCSA-N NC[C@H]1CN(C2=CC(F)=C(N3CCOCC3)C=C2)C(=O)O1.S Chemical compound NC[C@H]1CN(C2=CC(F)=C(N3CCOCC3)C=C2)C(=O)O1.S NZWKUHSARXKEFC-MERQFXBCSA-N 0.000 description 1
- YGYAWVDWMABLBF-UHFFFAOYSA-N Phosgene Chemical compound ClC(Cl)=O YGYAWVDWMABLBF-UHFFFAOYSA-N 0.000 description 1
- 206010035664 Pneumonia Diseases 0.000 description 1
- 108010059993 Vancomycin Proteins 0.000 description 1
- HCHKCACWOHOZIP-UHFFFAOYSA-N Zinc Chemical compound [Zn] HCHKCACWOHOZIP-UHFFFAOYSA-N 0.000 description 1
- 230000021736 acetylation Effects 0.000 description 1
- 238000006640 acetylation reaction Methods 0.000 description 1
- XAGFODPZIPBFFR-UHFFFAOYSA-N aluminium Chemical compound [Al] XAGFODPZIPBFFR-UHFFFAOYSA-N 0.000 description 1
- 229910052782 aluminium Inorganic materials 0.000 description 1
- VZTDIZULWFCMLS-UHFFFAOYSA-N ammonium formate Chemical compound [NH4+].[O-]C=O VZTDIZULWFCMLS-UHFFFAOYSA-N 0.000 description 1
- 239000000908 ammonium hydroxide Substances 0.000 description 1
- 239000004599 antimicrobial Substances 0.000 description 1
- -1 azide R—N-(4-morpholinyl-3-fluorophenyl)-2-oxo-5-oxazolidinyl-methyl azide Chemical class 0.000 description 1
- 150000001540 azides Chemical class 0.000 description 1
- 230000015572 biosynthetic process Effects 0.000 description 1
- 239000006227 byproduct Substances 0.000 description 1
- 239000003054 catalyst Substances 0.000 description 1
- 238000004587 chromatography analysis Methods 0.000 description 1
- 238000005056 compaction Methods 0.000 description 1
- 238000001816 cooling Methods 0.000 description 1
- 229910052802 copper Inorganic materials 0.000 description 1
- 239000010949 copper Substances 0.000 description 1
- 238000001514 detection method Methods 0.000 description 1
- 238000000113 differential scanning calorimetry Methods 0.000 description 1
- ZPWVASYFFYYZEW-UHFFFAOYSA-L dipotassium hydrogen phosphate Chemical compound [K+].[K+].OP([O-])([O-])=O ZPWVASYFFYYZEW-UHFFFAOYSA-L 0.000 description 1
- 229910000396 dipotassium phosphate Inorganic materials 0.000 description 1
- 238000004090 dissolution Methods 0.000 description 1
- 239000003480 eluent Substances 0.000 description 1
- 239000012530 fluid Substances 0.000 description 1
- PCHJSUWPFVWCPO-UHFFFAOYSA-N gold Chemical compound [Au] PCHJSUWPFVWCPO-UHFFFAOYSA-N 0.000 description 1
- 229910052737 gold Inorganic materials 0.000 description 1
- 239000010931 gold Substances 0.000 description 1
- 238000005984 hydrogenation reaction Methods 0.000 description 1
- 239000012535 impurity Substances 0.000 description 1
- 238000002347 injection Methods 0.000 description 1
- 239000007924 injection Substances 0.000 description 1
- 238000002955 isolation Methods 0.000 description 1
- 238000004519 manufacturing process Methods 0.000 description 1
- 125000002496 methyl group Chemical group [H]C([H])([H])* 0.000 description 1
- 230000000813 microbial effect Effects 0.000 description 1
- 239000000203 mixture Substances 0.000 description 1
- 230000004048 modification Effects 0.000 description 1
- 238000012986 modification Methods 0.000 description 1
- KZYMHNDGSFJVMU-ZEQRLZLVSA-N n,n-bis[[(5s)-3-(3-fluoro-4-morpholin-4-ylphenyl)-2-oxo-1,3-oxazolidin-5-yl]methyl]acetamide Chemical compound C([C@@H](OC1=O)CN(C(=O)C)C[C@@H]2OC(=O)N(C2)C=2C=C(F)C(N3CCOCC3)=CC=2)N1C(C=C1F)=CC=C1N1CCOCC1 KZYMHNDGSFJVMU-ZEQRLZLVSA-N 0.000 description 1
- 229940100692 oral suspension Drugs 0.000 description 1
- 125000001997 phenyl group Chemical group [H]C1=C([H])C([H])=C(*)C([H])=C1[H] 0.000 description 1
- XKJCHHZQLQNZHY-UHFFFAOYSA-N phthalimide Chemical compound C1=CC=C2C(=O)NC(=O)C2=C1 XKJCHHZQLQNZHY-UHFFFAOYSA-N 0.000 description 1
- 230000000704 physical effect Effects 0.000 description 1
- FYRHIOVKTDQVFC-UHFFFAOYSA-M potassium phthalimide Chemical compound [K+].C1=CC=C2C(=O)[N-]C(=O)C2=C1 FYRHIOVKTDQVFC-UHFFFAOYSA-M 0.000 description 1
- 239000002243 precursor Substances 0.000 description 1
- 239000010453 quartz Substances 0.000 description 1
- 230000005855 radiation Effects 0.000 description 1
- QEVHRUUCFGRFIF-MDEJGZGSSA-N reserpine Chemical compound O([C@H]1[C@@H]([C@H]([C@H]2C[C@@H]3C4=C(C5=CC=C(OC)C=C5N4)CCN3C[C@H]2C1)C(=O)OC)OC)C(=O)C1=CC(OC)=C(OC)C(OC)=C1 QEVHRUUCFGRFIF-MDEJGZGSSA-N 0.000 description 1
- 238000000926 separation method Methods 0.000 description 1
- 239000002904 solvent Substances 0.000 description 1
- 238000003860 storage Methods 0.000 description 1
- 239000000126 substance Substances 0.000 description 1
- 238000001665 trituration Methods 0.000 description 1
- MYPYJXKWCTUITO-LYRMYLQWSA-N vancomycin Chemical compound O([C@@H]1[C@@H](O)[C@H](O)[C@@H](CO)O[C@H]1OC1=C2C=C3C=C1OC1=CC=C(C=C1Cl)[C@@H](O)[C@H](C(N[C@@H](CC(N)=O)C(=O)N[C@H]3C(=O)N[C@H]1C(=O)N[C@H](C(N[C@@H](C3=CC(O)=CC(O)=C3C=3C(O)=CC=C1C=3)C(O)=O)=O)[C@H](O)C1=CC=C(C(=C1)Cl)O2)=O)NC(=O)[C@@H](CC(C)C)NC)[C@H]1C[C@](C)(N)[C@H](O)[C@H](C)O1 MYPYJXKWCTUITO-LYRMYLQWSA-N 0.000 description 1
- 229960003165 vancomycin Drugs 0.000 description 1
- MYPYJXKWCTUITO-UHFFFAOYSA-N vancomycin Natural products O1C(C(=C2)Cl)=CC=C2C(O)C(C(NC(C2=CC(O)=CC(O)=C2C=2C(O)=CC=C3C=2)C(O)=O)=O)NC(=O)C3NC(=O)C2NC(=O)C(CC(N)=O)NC(=O)C(NC(=O)C(CC(C)C)NC)C(O)C(C=C3Cl)=CC=C3OC3=CC2=CC1=C3OC1OC(CO)C(O)C(O)C1OC1CC(C)(N)C(O)C(C)O1 MYPYJXKWCTUITO-UHFFFAOYSA-N 0.000 description 1
- 238000002424 x-ray crystallography Methods 0.000 description 1
- 229940061740 zyvox Drugs 0.000 description 1
Images
Classifications
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D263/00—Heterocyclic compounds containing 1,3-oxazole or hydrogenated 1,3-oxazole rings
- C07D263/02—Heterocyclic compounds containing 1,3-oxazole or hydrogenated 1,3-oxazole rings not condensed with other rings
- C07D263/08—Heterocyclic compounds containing 1,3-oxazole or hydrogenated 1,3-oxazole rings not condensed with other rings having one double bond between ring members or between a ring member and a non-ring member
- C07D263/16—Heterocyclic compounds containing 1,3-oxazole or hydrogenated 1,3-oxazole rings not condensed with other rings having one double bond between ring members or between a ring member and a non-ring member with hetero atoms or with carbon atoms having three bonds to hetero atoms with at the most one bond to halogen, e.g. ester or nitrile radicals, directly attached to ring carbon atoms
- C07D263/18—Oxygen atoms
- C07D263/20—Oxygen atoms attached in position 2
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/33—Heterocyclic compounds
- A61K31/395—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins
- A61K31/535—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins having six-membered rings with at least one nitrogen and one oxygen as the ring hetero atoms, e.g. 1,2-oxazines
- A61K31/5375—1,4-Oxazines, e.g. morpholine
- A61K31/5377—1,4-Oxazines, e.g. morpholine not condensed and containing further heterocyclic rings, e.g. timolol
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P31/00—Antiinfectives, i.e. antibiotics, antiseptics, chemotherapeutics
- A61P31/12—Antivirals
- A61P31/14—Antivirals for RNA viruses
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D413/00—Heterocyclic compounds containing two or more hetero rings, at least one ring having nitrogen and oxygen atoms as the only ring hetero atoms
- C07D413/02—Heterocyclic compounds containing two or more hetero rings, at least one ring having nitrogen and oxygen atoms as the only ring hetero atoms containing two hetero rings
- C07D413/10—Heterocyclic compounds containing two or more hetero rings, at least one ring having nitrogen and oxygen atoms as the only ring hetero atoms containing two hetero rings linked by a carbon chain containing aromatic rings
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D413/00—Heterocyclic compounds containing two or more hetero rings, at least one ring having nitrogen and oxygen atoms as the only ring hetero atoms
- C07D413/14—Heterocyclic compounds containing two or more hetero rings, at least one ring having nitrogen and oxygen atoms as the only ring hetero atoms containing three or more hetero rings
Definitions
- the present invention relates to the solid state chemistry of the linezolid intermediate S—N-(4-morpholinyl-3-fluorophenyl)-2-oxo-5-oxazolidinyl-methyl amine.
- Linezolid [(S)—N-[[3-(3-Fluoro-4-morpholinyl)phenyl]-2-oxo-5-oxazolidinyl]methyl]acetamide] is an antimicrobial agent.
- Linezolid is an oxazolidinone, having the empirical formula C 16 H 20 FN 3 O 4 and the following structure (I):
- Linezolid is described in The Merck Index (13th edition, Monograph number: 05526, CAS Registry Number: 165800-03-3) as white crystals, with a melting point of 181.5-182.5°.
- Linezolid, as well as a process for its preparation, is disclosed in U.S. Pat. No. 5,688,792 (Example 5), European Patent No. 717738, Israeli Patent No. 110,802, Canadian Patent No. 2,168,560, and International Patent Publication WO 95/07271.
- Crystalline Form III linezolid is disclosed in U.S. Pat. No. 6,559,305.
- Linezolid is marketed in the United States by Pfizer, Inc. as an injection, tablets, and oral suspension under the name ZYVOX®. Its main indications are nosocomial pneumonia, skin and skin-structure infections, and vancomycin-resistant Enterococcus faecium infections.
- the intermediate azide R—N-(4-morpholinyl-3-fluorophenyl)-2-oxo-5-oxazolidinyl-methyl azide (III) is reduced to its corresponding amine, S—N-(4-morpholinyl-3-fluorophenyl)-2-oxo-5-oxazolidinyl-methyl amine (II) in the solvent ethyl acetate by hydrogenation using hydrogen gas and a palladium/carbon catalyst.
- These reaction conditions lead to the production of an undesirable level of reaction by-products, and, following the acetylation of the intermediate amine (II) to linezolid (I), to undesirably high levels of bis-linezolid (IV).
- the present invention relates to the solid state physical properties of an intermediate of linezolid, S—N-(4-morpholinyl-3-fluorophenyl)-2-oxo-5-oxazolidinyl-methyl amine (II). These properties can be influenced by controlling the conditions under which S—N-(4-morpholinyl-3-fluorophenyl)-2-oxo-5-oxazolidinyl-methyl amine (II) is obtained in solid form.
- One important solid state property is its rate of dissolution in aqueous fluid or its behavior on compaction and its storage stability.
- polymorphic form may give rise to thermal behavior different from that of the amorphous material or another polymorphic form. Thermal behavior is measured in the laboratory by such techniques as capillary melting point, thermogravimetric analysis (TGA) and differential scanning calorimetry (DSC) and can be used to distinguish some polymorphic forms from others.
- TGA thermogravimetric analysis
- DSC differential scanning calorimetry
- a particular polymorphic form may also give rise to distinct spectroscopic properties that may be detectable by powder X-ray crystallography, solid state 13 C NMR spectrometry and infrared spectrometry.
- the present invention is based on the finding that the linezolid intermediate S—N-(4-morpholinyl-3-fluorophenyl)-2-oxo-5-oxazolidinyl-methyl amine (II) can be obtained in at least three different crystalline forms: Form A, Form B, and Form C.
- the present invention provides a crystalline S—N-(4-morpholinyl-3-fluorophenyl)-2-oxo-5-oxazolidinyl-methyl amine (II) referred to herein as Form A, characterized by a powder X-ray diffraction (PXRD) pattern with peaks at 13.2 ⁇ 0.2, 14.8 ⁇ 0.2, 15.1 ⁇ 0.2, and 25.0 ⁇ 0.2 degrees 2 theta or substantially as indicated in FIG. 1 .
- PXRD powder X-ray diffraction
- the present invention provides a crystalline S—N-(4-morpholinyl-3-fluorophenyl)-2-oxo-5-oxazolidinyl-methyl amine (II) referred to herein as Form B, characterized by a powder X-ray diffraction (PXRD) pattern with peaks at 15.6 ⁇ 0.2, 19.2 ⁇ 0.2, 22.5 ⁇ 0.2, and 24.3 ⁇ 0.2 degrees 2 theta or substantially as indicated in FIG. 2 .
- PXRD powder X-ray diffraction
- the present invention provides a crystalline S—N-(4-morpholinyl-3-fluorophenyl)-2-oxo-5-oxazolidinyl-methyl amine (II) referred to herein as Form C, characterized by a powder X-ray diffraction (PXRD) pattern with peaks at 5.8 ⁇ 0.2, 11.5 ⁇ 0.2, 19.6 ⁇ 0.2, and 26.3 ⁇ 0.2 degrees 2 theta or substantially as indicated in FIG. 3 .
- PXRD powder X-ray diffraction
- FIG. 1 shows the powder X-ray diffractogram of linezolid intermediate amine (II) Form A.
- FIG. 2 shows the powder X-ray diffractogram of linezolid intermediate amine (II) Form B.
- FIG. 3 shows the powder X-ray diffractogram of linezolid intermediate amine (II) Form C.
- the present invention is based on the finding that the linezolid intermediate S—N-(4-morpholinyl-3-fluorophenyl)-2-oxo-5-oxazolidinyl-methyl amine (II) can be obtained in at least three different crystalline forms: Form A, Form B, and Form C.
- the present invention provides novel solid crystalline forms of S—N-(4-morpholinyl-3-fluorophenyl)-2-oxo-5-oxazolidinyl-methyl amine (II), referred to herein as Form A, Form B, and Form C.
- the crystalline Forms A, B, and C may be distinguished by their respective powder X-ray diffraction (PXRD) patterns.
- the crystalline forms have characteristic PXRD peak positions in the range of 2-40 degrees two theta. Crystalline Forms A, B, and C can be identified by these characteristic peak positions and the identity and quantify of their crystalline impurities can also be determined.
- the present invention provides a crystalline S—N-(4-morpholinyl-3-fluorophenyl)-2-oxo-5-oxazolidinyl-methyl amine (II) referred to herein as Form A, characterized by a powder X-ray diffraction (PXRD) pattern with peaks at 13.2 ⁇ 0.2, 14.8 ⁇ 0.2, 15.1 ⁇ 0.2, and 25.0 ⁇ 0.2 degrees 2 theta.
- Form A maybe further characterized by PXRD peaks at 3.05 +0.2, 16.1 ⁇ 0.2, 17.9 ⁇ 0.2, 19.3 ⁇ 0.2, and 23.0 ⁇ 0.2 degrees 2 theta, substantially as depicted in FIG. 1 .
- the present invention provides a crystalline S—N-(4-morpholinyl-3-fluorophenyl)-2-oxo-5-oxazolidinyl-methyl amine (II) referred to herein as Form B, characterized by a powder X-ray diffraction (PXRD) pattern with peaks at 15.6 ⁇ 0.2, 19.2 ⁇ 0.2, 22.5 ⁇ 0.2, and 24.3 ⁇ 0.2 degrees 2 theta.
- Form B may be further characterized by PXRD peaks at 7.2 ⁇ 0.2, 14.6 ⁇ 0.2, 16.5 ⁇ 0.2, 20.1 ⁇ 0.2, and 23.0 ⁇ 0.2 degrees 2 theta, substantially as depicted in FIG. 2 .
- the present invention provides a crystalline S—N-(4-morpholinyl-3-fluorophenyl)-2-oxo-5-oxazolidinyl-methyl amine (II) referred to herein as Form C, characterized by a powder X-ray diffraction (PXRD) pattern with peaks at 5.8 ⁇ 0.2, 11.5 ⁇ 0.2, 19.6 ⁇ 0.2, and 26.3 ⁇ 0.2 degrees 2 theta.
- Form C may be further characterized by PXRD peaks at 13.2 ⁇ 0.2, 20.4 ⁇ 0.2, 21.6 ⁇ 0.2, 22.3 ⁇ 0.2, 23.0 ⁇ 0.2, and 23.8 ⁇ 0.2 degrees 2 theta, substantially as depicted in FIG. 3 .
- the crystalline Forms A, B, and C of S—N-(4-morpholinyl-3-fluorophenyl)-2-oxo-5-oxazolidinyl-methyl amine (II) of the present invention may be substantially pure with respect to other crystalline forms, i.e., the novel forms contain less than about 10%, preferably less than about 5%, and even more preferably less than about 1% (by weight) of other crystalline forms of S—N-(4-morpholinyl-3-fluorophenyl)-2-oxo-5-oxazolidinyl-methyl amine (II).
- the novel crystalline forms contain less than about 10%, preferably less than about 5%, and even more preferably less than about 1% (by weight) of amorphous S—N-(4-morpholinyl-3-fluorophenyl)-2-oxo-5-oxazolidinyl-methyl amine (II).
- the present invention is not intended to encompass true solutions of S—N-(4-morpholinyl-3-fluorophenyl)-2-oxo-5-oxazolidinyl-methyl amine (II) wherein the crystal structure of the novel crystalline Forms A, B, and C and the properties that distinguish the novel crystalline forms of the present invention are lost.
- the preferred form of the present invention is that of solid forms of crystalline Forms A, B, and C.
- the use of the novel forms to prepare solutions is considered to be within the contemplation of the invention.
- Powder X-ray diffraction data were obtained by methods known in the art using a SCINTAG® powder X-ray diffractometer model X'TRA® equipped with a solid state detector. Copper radiation of 1.5418 ⁇ was used. A round aluminum sample holder with round zero background quartz plate was used, with cavity of 25(diameter)* 0.5(depth) mm. The obtained characteristic peaks were in the range of 2-40 degrees two theta.
Landscapes
- Chemical & Material Sciences (AREA)
- Organic Chemistry (AREA)
- Health & Medical Sciences (AREA)
- Life Sciences & Earth Sciences (AREA)
- Medicinal Chemistry (AREA)
- Pharmacology & Pharmacy (AREA)
- Animal Behavior & Ethology (AREA)
- General Health & Medical Sciences (AREA)
- Public Health (AREA)
- Veterinary Medicine (AREA)
- Virology (AREA)
- Epidemiology (AREA)
- Nuclear Medicine, Radiotherapy & Molecular Imaging (AREA)
- Communicable Diseases (AREA)
- Oncology (AREA)
- Chemical Kinetics & Catalysis (AREA)
- General Chemical & Material Sciences (AREA)
- Molecular Biology (AREA)
- Heterocyclic Carbon Compounds Containing A Hetero Ring Having Nitrogen And Oxygen As The Only Ring Hetero Atoms (AREA)
- Pharmaceuticals Containing Other Organic And Inorganic Compounds (AREA)
- Plural Heterocyclic Compounds (AREA)
- Sampling And Sample Adjustment (AREA)
- Investigating Or Analysing Biological Materials (AREA)
- Medicines That Contain Protein Lipid Enzymes And Other Medicines (AREA)
- Low-Molecular Organic Synthesis Reactions Using Catalysts (AREA)
Abstract
Description
- This application claims the benefit of provisional application Ser. Nos. 60/656,778, filed Feb. 24, 2005, 60/656,646, filed Feb. 24, 2005, and 60/690,822, filed Jun. 14, 2005, which are incorporated herein by reference.
- The present invention relates to the solid state chemistry of the linezolid intermediate S—N-(4-morpholinyl-3-fluorophenyl)-2-oxo-5-oxazolidinyl-methyl amine.
-
- Linezolid is described in The Merck Index (13th edition, Monograph number: 05526, CAS Registry Number: 165800-03-3) as white crystals, with a melting point of 181.5-182.5°. Linezolid, as well as a process for its preparation, is disclosed in U.S. Pat. No. 5,688,792 (Example 5), European Patent No. 717738, Israeli Patent No. 110,802, Canadian Patent No. 2,168,560, and International Patent Publication WO 95/07271.
- Crystalline Form III linezolid is disclosed in U.S. Pat. No. 6,559,305.
- Linezolid is marketed in the United States by Pfizer, Inc. as an injection, tablets, and oral suspension under the name ZYVOX®. Its main indications are nosocomial pneumonia, skin and skin-structure infections, and vancomycin-resistant Enterococcus faecium infections.
-
- This method of preparation was also disclosed in Bricker, et al., J. Med. Chem., 39 673-679 (1996), where it was stated that the above route avoids the use of phosgene to make the carbamate precursor of the oxazolidinone ring. The authors also disclose that the use of NaN3 can be avoided by using potassium phthalimide, followed by deblocking of the phthalimide with aqueous methyl amine.
- In the above-described synthesis, the intermediate amine, S—N-(4-morpholinyl-3-fluorophenyl)-2-oxo-5-oxazolidinyl-methyl amine (II)
is reacted without isolation with acetic anhydride as an oily product, or in solution, to produce the acetamide, linezolid (I). This is followed by procedures for isolating the linezolid such as those described in U.S. Pat. No. 5,688,792, at col. 15, 11. 22-28 (chromatography and separation of the desired fraction, followed by evaporation and trituration of the product to obtain pure linezolid). - In the above-described syntheses, the intermediate azide R—N-(4-morpholinyl-3-fluorophenyl)-2-oxo-5-oxazolidinyl-methyl azide (III)
is reduced to its corresponding amine, S—N-(4-morpholinyl-3-fluorophenyl)-2-oxo-5-oxazolidinyl-methyl amine (II) in the solvent ethyl acetate by hydrogenation using hydrogen gas and a palladium/carbon catalyst. These reaction conditions lead to the production of an undesirable level of reaction by-products, and, following the acetylation of the intermediate amine (II) to linezolid (I), to undesirably high levels of bis-linezolid (IV). - The present invention relates to the solid state physical properties of an intermediate of linezolid, S—N-(4-morpholinyl-3-fluorophenyl)-2-oxo-5-oxazolidinyl-methyl amine (II). These properties can be influenced by controlling the conditions under which S—N-(4-morpholinyl-3-fluorophenyl)-2-oxo-5-oxazolidinyl-methyl amine (II) is obtained in solid form. One important solid state property is its rate of dissolution in aqueous fluid or its behavior on compaction and its storage stability.
- These practical physical characteristics are influenced by the conformation and orientation of molecules in the unit cell, which defines a particular polymorphic form of a substance. The polymorphic form may give rise to thermal behavior different from that of the amorphous material or another polymorphic form. Thermal behavior is measured in the laboratory by such techniques as capillary melting point, thermogravimetric analysis (TGA) and differential scanning calorimetry (DSC) and can be used to distinguish some polymorphic forms from others. A particular polymorphic form may also give rise to distinct spectroscopic properties that may be detectable by powder X-ray crystallography, solid state 13C NMR spectrometry and infrared spectrometry.
- The present invention is based on the finding that the linezolid intermediate S—N-(4-morpholinyl-3-fluorophenyl)-2-oxo-5-oxazolidinyl-methyl amine (II) can be obtained in at least three different crystalline forms: Form A, Form B, and Form C.
- In one embodiment, the present invention provides a crystalline S—N-(4-morpholinyl-3-fluorophenyl)-2-oxo-5-oxazolidinyl-methyl amine (II) referred to herein as Form A, characterized by a powder X-ray diffraction (PXRD) pattern with peaks at 13.2±0.2, 14.8±0.2, 15.1±0.2, and 25.0±0.2 degrees 2 theta or substantially as indicated in
FIG. 1 . - In one embodiment, the present invention provides a crystalline S—N-(4-morpholinyl-3-fluorophenyl)-2-oxo-5-oxazolidinyl-methyl amine (II) referred to herein as Form B, characterized by a powder X-ray diffraction (PXRD) pattern with peaks at 15.6±0.2, 19.2±0.2, 22.5±0.2, and 24.3±0.2 degrees 2 theta or substantially as indicated in
FIG. 2 . - In one embodiment, the present invention provides a crystalline S—N-(4-morpholinyl-3-fluorophenyl)-2-oxo-5-oxazolidinyl-methyl amine (II) referred to herein as Form C, characterized by a powder X-ray diffraction (PXRD) pattern with peaks at 5.8±0.2, 11.5±0.2, 19.6±0.2, and 26.3±0.2 degrees 2 theta or substantially as indicated in
FIG. 3 . - These new crystalline forms were found to be chemically pure (by HPLC) and found to be a single crystalline form (by PXRD). Each form contains less than about 5% of other crystalline forms of intermediate amine (II) and contains less than about 5% of the intermediate azide (III).
-
FIG. 1 shows the powder X-ray diffractogram of linezolid intermediate amine (II) Form A. -
FIG. 2 shows the powder X-ray diffractogram of linezolid intermediate amine (II) Form B. -
FIG. 3 shows the powder X-ray diffractogram of linezolid intermediate amine (II) Form C. - The present invention is based on the finding that the linezolid intermediate S—N-(4-morpholinyl-3-fluorophenyl)-2-oxo-5-oxazolidinyl-methyl amine (II) can be obtained in at least three different crystalline forms: Form A, Form B, and Form C. Thus, the present invention provides novel solid crystalline forms of S—N-(4-morpholinyl-3-fluorophenyl)-2-oxo-5-oxazolidinyl-methyl amine (II), referred to herein as Form A, Form B, and Form C.
- The crystalline Forms A, B, and C may be distinguished by their respective powder X-ray diffraction (PXRD) patterns. The crystalline forms have characteristic PXRD peak positions in the range of 2-40 degrees two theta. Crystalline Forms A, B, and C can be identified by these characteristic peak positions and the identity and quantify of their crystalline impurities can also be determined.
- In one embodiment, the present invention provides a crystalline S—N-(4-morpholinyl-3-fluorophenyl)-2-oxo-5-oxazolidinyl-methyl amine (II) referred to herein as Form A, characterized by a powder X-ray diffraction (PXRD) pattern with peaks at 13.2±0.2, 14.8±0.2, 15.1±0.2, and 25.0±0.2 degrees 2 theta. Form A maybe further characterized by PXRD peaks at 3.05 +0.2, 16.1±0.2, 17.9±0.2, 19.3±0.2, and 23.0±0.2 degrees 2 theta, substantially as depicted in
FIG. 1 . - In one embodiment, the present invention provides a crystalline S—N-(4-morpholinyl-3-fluorophenyl)-2-oxo-5-oxazolidinyl-methyl amine (II) referred to herein as Form B, characterized by a powder X-ray diffraction (PXRD) pattern with peaks at 15.6±0.2, 19.2±0.2, 22.5±0.2, and 24.3±0.2 degrees 2 theta. Form B may be further characterized by PXRD peaks at 7.2±0.2, 14.6±0.2, 16.5±0.2, 20.1±0.2, and 23.0±0.2 degrees 2 theta, substantially as depicted in
FIG. 2 . - In one embodiment, the present invention provides a crystalline S—N-(4-morpholinyl-3-fluorophenyl)-2-oxo-5-oxazolidinyl-methyl amine (II) referred to herein as Form C, characterized by a powder X-ray diffraction (PXRD) pattern with peaks at 5.8±0.2, 11.5±0.2, 19.6±0.2, and 26.3±0.2 degrees 2 theta. Form C may be further characterized by PXRD peaks at 13.2±0.2, 20.4±0.2, 21.6±0.2, 22.3±0.2, 23.0±0.2, and 23.8±0.2 degrees 2 theta, substantially as depicted in
FIG. 3 . - The characteristic PXRD peaks of the novel crystalline forms of the intermediate amine (II) are shown in Table 1, with the most characteristic peaks indicated in bold.
TABLE 1 Characteristic PXRD peaks in degrees 2 theta Form A Form B Form C 3.0 7.2 5.8 13.2 14.6 11.5 14.8 15.6 13.2 15.1 16.5 19.6 16.1 19.2 20.4 17.9 20.1 21.6 19.3 22.5 22.3 23.0 23.0 23.0 25.0 24.3 23.8 26.3 - The crystalline Forms A, B, and C of S—N-(4-morpholinyl-3-fluorophenyl)-2-oxo-5-oxazolidinyl-methyl amine (II) of the present invention may be substantially pure with respect to other crystalline forms, i.e., the novel forms contain less than about 10%, preferably less than about 5%, and even more preferably less than about 1% (by weight) of other crystalline forms of S—N-(4-morpholinyl-3-fluorophenyl)-2-oxo-5-oxazolidinyl-methyl amine (II). In certain embodiments, the novel crystalline forms contain less than about 10%, preferably less than about 5%, and even more preferably less than about 1% (by weight) of amorphous S—N-(4-morpholinyl-3-fluorophenyl)-2-oxo-5-oxazolidinyl-methyl amine (II).
- The present invention is not intended to encompass true solutions of S—N-(4-morpholinyl-3-fluorophenyl)-2-oxo-5-oxazolidinyl-methyl amine (II) wherein the crystal structure of the novel crystalline Forms A, B, and C and the properties that distinguish the novel crystalline forms of the present invention are lost. Thus, the preferred form of the present invention is that of solid forms of crystalline Forms A, B, and C. However, the use of the novel forms to prepare solutions (e.g., so as to provide a material for conversion into linezolid (I)) is considered to be within the contemplation of the invention.
- Having described the invention with reference to certain preferred embodiments, other embodiments will become apparent to one skilled in the art from consideration of the specification. The invention is further defined by reference to the following examples describing in detail the preparation of the composition and methods of use of the invention. It will be apparent to those skilled in the art that many modifications, both to materials and methods, may be practiced without departing from the scope of the invention.
- HPLC Method
- Column Hypersil Gold 150×4.6, 5μ
- Detection limit: 0.1%
- Eluents: K2HPO4 0.01M: MeOH A: 80:20 B: 50:50
TABLE 2 Time A B Flow 0 100 0 1.5 15 57 43 2 25 35 65 2
Powder X-Ray Diffraction - Powder X-ray diffraction data were obtained by methods known in the art using a SCINTAG® powder X-ray diffractometer model X'TRA® equipped with a solid state detector. Copper radiation of 1.5418 Å was used. A round aluminum sample holder with round zero background quartz plate was used, with cavity of 25(diameter)* 0.5(depth) mm. The obtained characteristic peaks were in the range of 2-40 degrees two theta.
- In a 1L reactor, 6 g R—N-(4-morpholinyl-3-fluorophenyl)-2-oxo-5-oxazolidinyl-methyl azide (III) was charged with 150 ml ethyl acetate, followed by 0.6 g 10% Pd/C. The system was flushed 3 times with nitrogen and 3 times with hydrogen. The pressure of hydrogen was set to 1.5 atm. The reaction mixture was stirred at RT and the reaction followed by TLC or HPLC until completion. The reaction mixture was filtered through celite and the solution was evaporated to obtain 4.6 g S—N-(4-morpholinyl-3-fluorophenyl)-2-oxo-5-oxazolidinyl-methyl amine (II) as a white solid. The crystals were analyzed by PXRD and showed a novel form of) the intermediate amine (II) (Form B, 91.7% total purity by HPLC).
- In a 10L reactor, 150 g R—N-(4-morpholinyl-3-fluorophenyl)-2-oxo-5-oxazolidinyl-methyl azide (III) was charged, followed by 15 g Pd/C in 5L toluene. Finally 500 ml ammonium hydroxide was added. The system was flushed 3 times with nitrogen and 3 times with hydrogen. The pressure of hydrogen was set to 1.5 atm. The reaction mixture was stirred at RT and the reaction followed by TLC or HPLC until completion. The reaction mixture was filtered through celite. S—N-(4-morpholinyl-3-fluorophenyl)-2-oxo-5-oxazolidinyl-methyl amine (II) precipitated on standing and/or cooling as a white solid, was filtered, and dried at 50° C. overnight. The crystals obtained were analyzed by PXRD and showed a novel form of the intermediate amine (II) (Form C, 98.6% total purity by HPLC).
- In a three necked flask, 6.4 g R—N-(4-morpholinyl-3-fluorophenyl)-2-oxo-5-oxazolidinyl-methyl azide (III) was charged, followed by 2.5 g ammonium formate, 23 ml ethanol, and 2.6 g zinc powder. The reaction mixture was stirred at RT and the reaction followed by TLC or HPLC until completion. 60 ml acetone were then added. The reaction mixture was filtered and by evaporation S—N-(4-morpholinyl-3-fluorophenyl)-2-oxo-5-oxazolidinyl-methyl amine (II) was obtained as a solid. The crystals obtained were analyzed by PXRD and showed a novel form of the intermediate amine (II) (Form A, 96.5% total purity by HPLC).
Claims (12)
Priority Applications (1)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US11/362,312 US20060258655A1 (en) | 2005-02-24 | 2006-02-24 | Crystalline forms of linezolid intermediate |
Applications Claiming Priority (4)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US65664605P | 2005-02-24 | 2005-02-24 | |
| US65677805P | 2005-02-24 | 2005-02-24 | |
| US69082205P | 2005-06-14 | 2005-06-14 | |
| US11/362,312 US20060258655A1 (en) | 2005-02-24 | 2006-02-24 | Crystalline forms of linezolid intermediate |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| US20060258655A1 true US20060258655A1 (en) | 2006-11-16 |
Family
ID=36498839
Family Applications (4)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| US11/361,457 Expired - Fee Related US7291614B2 (en) | 2005-02-24 | 2006-02-23 | Processes for the preparation of linezolid intermediate |
| US11/361,509 Abandoned US20060252932A1 (en) | 2005-02-24 | 2006-02-24 | Isolated bis-linezolid, preparation thereof, and its use as a reference standard |
| US11/362,312 Abandoned US20060258655A1 (en) | 2005-02-24 | 2006-02-24 | Crystalline forms of linezolid intermediate |
| US11/977,344 Abandoned US20080045707A1 (en) | 2005-02-24 | 2007-10-23 | Processes for the preparation of linezolid intermediate |
Family Applications Before (2)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| US11/361,457 Expired - Fee Related US7291614B2 (en) | 2005-02-24 | 2006-02-23 | Processes for the preparation of linezolid intermediate |
| US11/361,509 Abandoned US20060252932A1 (en) | 2005-02-24 | 2006-02-24 | Isolated bis-linezolid, preparation thereof, and its use as a reference standard |
Family Applications After (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| US11/977,344 Abandoned US20080045707A1 (en) | 2005-02-24 | 2007-10-23 | Processes for the preparation of linezolid intermediate |
Country Status (8)
| Country | Link |
|---|---|
| US (4) | US7291614B2 (en) |
| EP (3) | EP1866295A2 (en) |
| JP (2) | JP2008530028A (en) |
| CA (2) | CA2602073A1 (en) |
| IL (3) | IL183380A0 (en) |
| MX (3) | MX2007010141A (en) |
| TW (1) | TW200640886A (en) |
| WO (3) | WO2006091731A2 (en) |
Cited By (3)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2012019632A1 (en) | 2010-08-11 | 2012-02-16 | Synthon B.V. | Process for making linezolid |
| EP2690100A1 (en) | 2010-08-11 | 2014-01-29 | Synhton B.V. | Process for making linezolid |
| WO2014071990A1 (en) | 2012-11-09 | 2014-05-15 | Synthon Bv | Process for making linezolid |
Families Citing this family (8)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2011114210A2 (en) | 2010-03-15 | 2011-09-22 | Jubilant Life Sciences Limited | Processes for the preparation of linezolid |
| AU2011245302A1 (en) | 2010-04-30 | 2012-11-22 | Indiana University Research And Technology Corporation | Processes for preparing linezolid |
| WO2012094622A2 (en) * | 2011-01-07 | 2012-07-12 | The Regents Of The University Of California | Amination of aryl alcohol derivatives |
| US20150025236A1 (en) | 2012-01-24 | 2015-01-22 | Jubilant Life Sciences Limited | Process for the preparation of stable crystalline form-i of linezolid, substantially free of residual solvent |
| US9586913B2 (en) | 2013-04-25 | 2017-03-07 | Symed Labs Limited | Processes for the preparation of linezolid using novel intermediates |
| MY180626A (en) | 2013-11-15 | 2020-12-03 | Akebia Therapeutics Inc | Solid forms of {[5-(3-chlorophenyl)-3-hydroxypyridine-2-carbonyl]amino}acetic acid, compositions, and uses thereof |
| CN109265407B (en) * | 2018-10-23 | 2020-05-29 | 扬子江药业集团北京海燕药业有限公司 | Synthesis method of bislinezolid |
| CN109444294B (en) * | 2018-12-27 | 2021-06-22 | 苏州莱奥生物技术有限公司 | High performance liquid chromatography method for separating linezolid and chiral isomer thereof |
Citations (4)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US4705799A (en) * | 1983-06-07 | 1987-11-10 | E. I. Du Pont De Nemours And Company | Aminomethyl oxooxazolidinyl benzenes useful as antibacterial agents |
| US5688792A (en) * | 1994-08-16 | 1997-11-18 | Pharmacia & Upjohn Company | Substituted oxazine and thiazine oxazolidinone antimicrobials |
| US6333198B1 (en) * | 1998-06-10 | 2001-12-25 | Glaxo Wellcome, Inc. | Compound and its use |
| US6444813B2 (en) * | 2000-02-02 | 2002-09-03 | Pharmacia & Upjohn Company | Linezolid-crystal form II |
Family Cites Families (17)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US4801600A (en) * | 1987-10-09 | 1989-01-31 | E. I. Du Pont De Nemours And Company | Aminomethyl oxooxazolidinyl cycloalkylbenzene derivatives useful as antibacterial agents |
| US4948801A (en) * | 1988-07-29 | 1990-08-14 | E. I. Du Pont De Nemours And Company | Aminomethyloxooxazolidinyl arylbenzene derivatives useful as antibacterial agents |
| KR100257418B1 (en) | 1991-11-01 | 2000-05-15 | 로렌스 티. 마이젠헬더 | Substituted aryl-and heteroaryl-phenyloxazolidinone |
| SK283420B6 (en) * | 1992-05-08 | 2003-07-01 | Pharmacia & Upjohn Company | Oxazolidinones containing a substituted diazine moiety and their use as antimicrobials |
| MY115155A (en) | 1993-09-09 | 2003-04-30 | Upjohn Co | Substituted oxazine and thiazine oxazolidinone antimicrobials. |
| EP0748801B1 (en) * | 1994-03-02 | 2001-12-19 | Daicel Chemical Industries, Ltd. | 2-isoxazoline derivative and process for producing the same, and process for producing related derivatives from the same |
| JP3831954B2 (en) * | 1995-05-19 | 2006-10-11 | ダイソー株式会社 | Process for producing 4-hydroxy-2-pyrrolidone |
| DE19827282A1 (en) * | 1998-06-19 | 1999-12-23 | Bayer Ag | Process for the preparation of cycloaliphatic amines |
| AR027261A1 (en) | 2000-02-02 | 2003-03-19 | Upjohn Co | LINEZOLID CRYSTAL FORM II |
| US6514529B2 (en) * | 2000-03-22 | 2003-02-04 | Pharmacia & Upjohn Company | Oxazolidinone tablet formulation |
| YU52403A (en) * | 2000-12-26 | 2006-03-03 | Dr.Reddy's Research Foundation | Heterocyclic compounds having antibacterial activity, process for their preparation and pharmaceutical compositions containing them |
| ATE449763T1 (en) * | 2001-04-16 | 2009-12-15 | Eisai R&D Man Co Ltd | 1H-INDAZOLE COMPOUNDS THAT INHIBIT JNK |
| AU2001100437A4 (en) | 2001-10-03 | 2001-11-01 | Pfizer Limited | Reference standards for determining the purity or stability of amlodipine maleate and processes therefor |
| AU2003224345A1 (en) | 2002-04-30 | 2003-11-17 | Orchid Chemicals & Pharmaceuticals | Antibacterial agents |
| WO2004026848A1 (en) | 2002-09-20 | 2004-04-01 | Lupin Limited | Oxazolidinone derivatives, process for their preperation and their use as antimycobacterial agents |
| EP1673370B1 (en) * | 2003-10-16 | 2009-09-09 | Symed Labs Limited | Crystalline form of linezolid |
| WO2006110155A1 (en) * | 2004-06-29 | 2006-10-19 | Teva Pharmaceutical Industries Ltd | Solid forms of linezolid and processes for preparation thereof |
-
2006
- 2006-02-23 MX MX2007010141A patent/MX2007010141A/en not_active Application Discontinuation
- 2006-02-23 WO PCT/US2006/006414 patent/WO2006091731A2/en active Application Filing
- 2006-02-23 EP EP06735895A patent/EP1866295A2/en not_active Withdrawn
- 2006-02-23 US US11/361,457 patent/US7291614B2/en not_active Expired - Fee Related
- 2006-02-23 CA CA002602073A patent/CA2602073A1/en not_active Abandoned
- 2006-02-23 JP JP2007554359A patent/JP2008530028A/en active Pending
- 2006-02-24 WO PCT/US2006/006655 patent/WO2006091848A2/en active Application Filing
- 2006-02-24 MX MX2007010136A patent/MX2007010136A/en not_active Application Discontinuation
- 2006-02-24 MX MX2007010143A patent/MX2007010143A/en not_active Application Discontinuation
- 2006-02-24 JP JP2007555389A patent/JP2008530144A/en active Pending
- 2006-02-24 TW TW095106393A patent/TW200640886A/en unknown
- 2006-02-24 US US11/361,509 patent/US20060252932A1/en not_active Abandoned
- 2006-02-24 EP EP06721050A patent/EP1861383A2/en not_active Withdrawn
- 2006-02-24 CA CA002588876A patent/CA2588876A1/en not_active Abandoned
- 2006-02-24 US US11/362,312 patent/US20060258655A1/en not_active Abandoned
- 2006-02-24 WO PCT/US2006/006529 patent/WO2006091777A1/en active Application Filing
- 2006-02-24 EP EP06735977A patent/EP1853571A1/en not_active Withdrawn
-
2007
- 2007-05-24 IL IL183380A patent/IL183380A0/en unknown
- 2007-05-24 IL IL183379A patent/IL183379A0/en unknown
- 2007-06-19 IL IL184038A patent/IL184038A0/en unknown
- 2007-10-23 US US11/977,344 patent/US20080045707A1/en not_active Abandoned
Patent Citations (5)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US4705799A (en) * | 1983-06-07 | 1987-11-10 | E. I. Du Pont De Nemours And Company | Aminomethyl oxooxazolidinyl benzenes useful as antibacterial agents |
| US5688792A (en) * | 1994-08-16 | 1997-11-18 | Pharmacia & Upjohn Company | Substituted oxazine and thiazine oxazolidinone antimicrobials |
| US6333198B1 (en) * | 1998-06-10 | 2001-12-25 | Glaxo Wellcome, Inc. | Compound and its use |
| US6444813B2 (en) * | 2000-02-02 | 2002-09-03 | Pharmacia & Upjohn Company | Linezolid-crystal form II |
| US6559305B1 (en) * | 2000-02-02 | 2003-05-06 | Pharmacia & Upjohn Company | Linezolid—crystal form II |
Cited By (4)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2012019632A1 (en) | 2010-08-11 | 2012-02-16 | Synthon B.V. | Process for making linezolid |
| WO2012019862A1 (en) | 2010-08-11 | 2012-02-16 | Synthon B.V. | Process for making linezolid |
| EP2690100A1 (en) | 2010-08-11 | 2014-01-29 | Synhton B.V. | Process for making linezolid |
| WO2014071990A1 (en) | 2012-11-09 | 2014-05-15 | Synthon Bv | Process for making linezolid |
Also Published As
| Publication number | Publication date |
|---|---|
| JP2008530144A (en) | 2008-08-07 |
| WO2006091848A2 (en) | 2006-08-31 |
| EP1861383A2 (en) | 2007-12-05 |
| EP1853571A1 (en) | 2007-11-14 |
| IL183379A0 (en) | 2007-09-20 |
| CA2602073A1 (en) | 2006-08-31 |
| TW200640886A (en) | 2006-12-01 |
| MX2007010143A (en) | 2007-09-27 |
| US20080045707A1 (en) | 2008-02-21 |
| WO2006091731A3 (en) | 2006-10-19 |
| IL183380A0 (en) | 2007-09-20 |
| US7291614B2 (en) | 2007-11-06 |
| EP1866295A2 (en) | 2007-12-19 |
| US20060252932A1 (en) | 2006-11-09 |
| WO2006091731A2 (en) | 2006-08-31 |
| CA2588876A1 (en) | 2006-08-31 |
| MX2007010141A (en) | 2007-09-27 |
| WO2006091848A9 (en) | 2006-11-09 |
| WO2006091848A3 (en) | 2006-12-28 |
| IL184038A0 (en) | 2007-10-31 |
| MX2007010136A (en) | 2007-09-27 |
| US20070021417A1 (en) | 2007-01-25 |
| WO2006091777A1 (en) | 2006-08-31 |
| JP2008530028A (en) | 2008-08-07 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| US20060258655A1 (en) | Crystalline forms of linezolid intermediate | |
| US11149017B2 (en) | Solid state forms of apalutamide | |
| US9630952B2 (en) | Crystalline forms of afatinib di-maleate | |
| US8394962B2 (en) | Method for the preparation of dabigatran and its intermediates | |
| US10023566B2 (en) | Dasatinib salts | |
| SK286569B6 (en) | Method for the production of oxazolidinones | |
| US20120101099A1 (en) | Histone deacetylase inhibitors | |
| CN110372562B (en) | Crystal form of key intermediate of BTK kinase inhibitor and preparation method thereof | |
| KR20230021075A (en) | Forms and Compositions of Beta Adrenergic Agonists | |
| JPWO2007148711A1 (en) | N- (3,4-disubstituted phenyl) salicylamide derivatives | |
| US20080269342A1 (en) | Pleuromutilin Derivatives and Its Use | |
| US8962827B2 (en) | Linezolid intermediate and method for synthesizing linezolid | |
| US7728135B2 (en) | Synthesis of CCR5 receptor antagonists | |
| US20230278977A1 (en) | Stable polymorph of r-mdma hcl | |
| JP2014139175A (en) | Therapeutic compounds | |
| US11214547B2 (en) | Crystalline Eltrombopag monoethanolamine salt form D | |
| US20200347027A1 (en) | Crystalline forms of lenalidomide | |
| US20090312351A1 (en) | Processes for the Preparation of Alfuzosin | |
| TWI711612B (en) | Pharmaceutically acceptable salt, crystalline form of azabicyclo substituted triazole derivative and preparation method thereof | |
| JP5077232B2 (en) | Crystals of benzooxadiazole derivatives | |
| US20170066728A1 (en) | Process for the preparation of stable crystalline form-i of linezolid, substantially free of residual solvent | |
| WO2003061552A2 (en) | Novel process for the preparation of substantially pure 5-(3,5-dimethylphenoxy)methyl-2-oxazolidinone | |
| CN112830919B (en) | Benzopiperidine derivatives as pharmaceutical agents and process for preparing them | |
| US20240092768A1 (en) | Crystal form of anti-influenza virus compound, preparation method for crystal form, and use of crystal form | |
| US11059813B2 (en) | Polymorphic forms of Dasatinib |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| AS | Assignment |
Owner name: TEVA PHARMACEUTICAL INDUSTRIES LTD., ISRAEL Free format text: ASSIGNMENT OF ASSIGNORS INTEREST;ASSIGNORS:KOLTAI, TAMAS;NIDAM, TAMAR;BRAUDE, VIVIANA;AND OTHERS;REEL/FRAME:017926/0069;SIGNING DATES FROM 20060423 TO 20060518 Owner name: TEVA PHARMACEUTICALS USA, INC., PENNSYLVANIA Free format text: ASSIGNMENT OF ASSIGNORS INTEREST;ASSIGNOR:TEVA PHARMACEUTICAL INDUSTRIES LTD.;REEL/FRAME:017926/0266 Effective date: 20060604 |
|
| STCB | Information on status: application discontinuation |
Free format text: ABANDONED -- FAILURE TO RESPOND TO AN OFFICE ACTION |