JP4182191B2 - Ultrasound imaging of tissue perfusion by contrast energy pulse energy interference - Google Patents
Ultrasound imaging of tissue perfusion by contrast energy pulse energy interference Download PDFInfo
- Publication number
- JP4182191B2 JP4182191B2 JP54530998A JP54530998A JP4182191B2 JP 4182191 B2 JP4182191 B2 JP 4182191B2 JP 54530998 A JP54530998 A JP 54530998A JP 54530998 A JP54530998 A JP 54530998A JP 4182191 B2 JP4182191 B2 JP 4182191B2
- Authority
- JP
- Japan
- Prior art keywords
- contrast agent
- ultrasound
- ultrasound contrast
- ultrasonic
- agent according
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Expired - Fee Related
Links
- 230000010412 perfusion Effects 0.000 title claims abstract description 28
- 238000012285 ultrasound imaging Methods 0.000 title description 10
- 239000002872 contrast media Substances 0.000 claims abstract description 56
- 239000002961 echo contrast media Substances 0.000 claims abstract description 20
- 238000002604 ultrasonography Methods 0.000 claims abstract description 20
- 230000001678 irradiating effect Effects 0.000 claims abstract description 3
- 238000003384 imaging method Methods 0.000 claims description 19
- 238000001514 detection method Methods 0.000 claims description 12
- 150000002632 lipids Chemical class 0.000 claims description 9
- 239000000463 material Substances 0.000 claims description 8
- 150000003904 phospholipids Chemical class 0.000 claims description 8
- KAVGMUDTWQVPDF-UHFFFAOYSA-N perflubutane Chemical compound FC(F)(F)C(F)(F)C(F)(F)C(F)(F)F KAVGMUDTWQVPDF-UHFFFAOYSA-N 0.000 claims description 6
- 230000008859 change Effects 0.000 claims description 5
- 229950003332 perflubutane Drugs 0.000 claims description 5
- TZCPCKNHXULUIY-RGULYWFUSA-N 1,2-distearoyl-sn-glycero-3-phosphoserine Chemical compound CCCCCCCCCCCCCCCCCC(=O)OC[C@H](COP(O)(=O)OC[C@H](N)C(O)=O)OC(=O)CCCCCCCCCCCCCCCCC TZCPCKNHXULUIY-RGULYWFUSA-N 0.000 claims description 3
- ZWZWYGMENQVNFU-UHFFFAOYSA-N Glycerophosphorylserin Natural products OC(=O)C(N)COP(O)(=O)OCC(O)CO ZWZWYGMENQVNFU-UHFFFAOYSA-N 0.000 claims description 3
- 238000005259 measurement Methods 0.000 claims description 3
- -1 sulfur halide Chemical class 0.000 claims description 3
- TXEYQDLBPFQVAA-UHFFFAOYSA-N tetrafluoromethane Chemical compound FC(F)(F)F TXEYQDLBPFQVAA-UHFFFAOYSA-N 0.000 claims description 3
- 238000011002 quantification Methods 0.000 claims description 2
- 241001465754 Metazoa Species 0.000 claims 1
- 238000000691 measurement method Methods 0.000 claims 1
- 229910052717 sulfur Inorganic materials 0.000 claims 1
- 239000011593 sulfur Substances 0.000 claims 1
- 238000000034 method Methods 0.000 abstract description 23
- 239000007789 gas Substances 0.000 description 33
- 239000006185 dispersion Substances 0.000 description 10
- 239000000203 mixture Substances 0.000 description 8
- 210000004165 myocardium Anatomy 0.000 description 8
- 210000002216 heart Anatomy 0.000 description 6
- 239000008280 blood Substances 0.000 description 5
- 210000004369 blood Anatomy 0.000 description 5
- 230000006378 damage Effects 0.000 description 5
- 238000002347 injection Methods 0.000 description 5
- 239000007924 injection Substances 0.000 description 5
- RTZKZFJDLAIYFH-UHFFFAOYSA-N Diethyl ether Chemical compound CCOCC RTZKZFJDLAIYFH-UHFFFAOYSA-N 0.000 description 4
- QWTDNUCVQCZILF-UHFFFAOYSA-N isopentane Chemical compound CCC(C)C QWTDNUCVQCZILF-UHFFFAOYSA-N 0.000 description 4
- 229920000642 polymer Polymers 0.000 description 4
- 238000000527 sonication Methods 0.000 description 4
- 239000004094 surface-active agent Substances 0.000 description 4
- 206010058558 Hypoperfusion Diseases 0.000 description 3
- DNIAPMSPPWPWGF-UHFFFAOYSA-N Propylene glycol Chemical compound CC(O)CO DNIAPMSPPWPWGF-UHFFFAOYSA-N 0.000 description 3
- 230000005540 biological transmission Effects 0.000 description 3
- 239000012530 fluid Substances 0.000 description 3
- 239000000787 lecithin Substances 0.000 description 3
- 235000010445 lecithin Nutrition 0.000 description 3
- OFBQJSOFQDEBGM-UHFFFAOYSA-N n-pentane Natural products CCCCC OFBQJSOFQDEBGM-UHFFFAOYSA-N 0.000 description 3
- QYSGYZVSCZSLHT-UHFFFAOYSA-N octafluoropropane Chemical compound FC(F)(F)C(F)(F)C(F)(F)F QYSGYZVSCZSLHT-UHFFFAOYSA-N 0.000 description 3
- 239000002243 precursor Substances 0.000 description 3
- 230000002792 vascular Effects 0.000 description 3
- XLYOFNOQVPJJNP-UHFFFAOYSA-N water Substances O XLYOFNOQVPJJNP-UHFFFAOYSA-N 0.000 description 3
- PORPENFLTBBHSG-MGBGTMOVSA-N 1,2-dihexadecanoyl-sn-glycerol-3-phosphate Chemical compound CCCCCCCCCCCCCCCC(=O)OC[C@H](COP(O)(O)=O)OC(=O)CCCCCCCCCCCCCCC PORPENFLTBBHSG-MGBGTMOVSA-N 0.000 description 2
- XKRFYHLGVUSROY-UHFFFAOYSA-N Argon Chemical compound [Ar] XKRFYHLGVUSROY-UHFFFAOYSA-N 0.000 description 2
- IJGRMHOSHXDMSA-UHFFFAOYSA-N Atomic nitrogen Chemical compound N#N IJGRMHOSHXDMSA-UHFFFAOYSA-N 0.000 description 2
- CURLTUGMZLYLDI-UHFFFAOYSA-N Carbon dioxide Chemical compound O=C=O CURLTUGMZLYLDI-UHFFFAOYSA-N 0.000 description 2
- RGSFGYAAUTVSQA-UHFFFAOYSA-N Cyclopentane Chemical compound C1CCCC1 RGSFGYAAUTVSQA-UHFFFAOYSA-N 0.000 description 2
- LCGLNKUTAGEVQW-UHFFFAOYSA-N Dimethyl ether Chemical compound COC LCGLNKUTAGEVQW-UHFFFAOYSA-N 0.000 description 2
- 206010028980 Neoplasm Diseases 0.000 description 2
- ATUOYWHBWRKTHZ-UHFFFAOYSA-N Propane Chemical compound CCC ATUOYWHBWRKTHZ-UHFFFAOYSA-N 0.000 description 2
- QQONPFPTGQHPMA-UHFFFAOYSA-N Propene Chemical compound CC=C QQONPFPTGQHPMA-UHFFFAOYSA-N 0.000 description 2
- ATBOMIWRCZXYSZ-XZBBILGWSA-N [1-[2,3-dihydroxypropoxy(hydroxy)phosphoryl]oxy-3-hexadecanoyloxypropan-2-yl] (9e,12e)-octadeca-9,12-dienoate Chemical compound CCCCCCCCCCCCCCCC(=O)OCC(COP(O)(=O)OCC(O)CO)OC(=O)CCCCCCC\C=C\C\C=C\CCCCC ATBOMIWRCZXYSZ-XZBBILGWSA-N 0.000 description 2
- 230000004913 activation Effects 0.000 description 2
- AWUCVROLDVIAJX-UHFFFAOYSA-N alpha-glycerophosphate Natural products OCC(O)COP(O)(O)=O AWUCVROLDVIAJX-UHFFFAOYSA-N 0.000 description 2
- 238000004458 analytical method Methods 0.000 description 2
- 239000007900 aqueous suspension Substances 0.000 description 2
- 230000017531 blood circulation Effects 0.000 description 2
- 125000004432 carbon atom Chemical group C* 0.000 description 2
- 239000003795 chemical substances by application Substances 0.000 description 2
- HVYWMOMLDIMFJA-DPAQBDIFSA-N cholesterol Chemical compound C1C=C2C[C@@H](O)CC[C@]2(C)[C@@H]2[C@@H]1[C@@H]1CC[C@H]([C@H](C)CCCC(C)C)[C@@]1(C)CC2 HVYWMOMLDIMFJA-DPAQBDIFSA-N 0.000 description 2
- 230000007423 decrease Effects 0.000 description 2
- 238000002592 echocardiography Methods 0.000 description 2
- 230000000694 effects Effects 0.000 description 2
- 239000000839 emulsion Substances 0.000 description 2
- 150000002170 ethers Chemical class 0.000 description 2
- 239000010419 fine particle Substances 0.000 description 2
- 239000012634 fragment Substances 0.000 description 2
- 229930195733 hydrocarbon Natural products 0.000 description 2
- 150000002430 hydrocarbons Chemical class 0.000 description 2
- 150000002576 ketones Chemical class 0.000 description 2
- 239000012528 membrane Substances 0.000 description 2
- VNWKTOKETHGBQD-UHFFFAOYSA-N methane Chemical compound C VNWKTOKETHGBQD-UHFFFAOYSA-N 0.000 description 2
- 239000011859 microparticle Substances 0.000 description 2
- 230000002107 myocardial effect Effects 0.000 description 2
- 229960004692 perflenapent Drugs 0.000 description 2
- NJCBUSHGCBERSK-UHFFFAOYSA-N perfluoropentane Chemical compound FC(F)(F)C(F)(F)C(F)(F)C(F)(F)C(F)(F)F NJCBUSHGCBERSK-UHFFFAOYSA-N 0.000 description 2
- 229960004065 perflutren Drugs 0.000 description 2
- 150000003905 phosphatidylinositols Chemical class 0.000 description 2
- 150000008106 phosphatidylserines Chemical class 0.000 description 2
- 102000004169 proteins and genes Human genes 0.000 description 2
- 108090000623 proteins and genes Proteins 0.000 description 2
- 239000002356 single layer Substances 0.000 description 2
- 239000000243 solution Substances 0.000 description 2
- 230000000087 stabilizing effect Effects 0.000 description 2
- JQWAHKMIYCERGA-UHFFFAOYSA-N (2-nonanoyloxy-3-octadeca-9,12-dienoyloxypropoxy)-[2-(trimethylazaniumyl)ethyl]phosphinate Chemical compound CCCCCCCCC(=O)OC(COP([O-])(=O)CC[N+](C)(C)C)COC(=O)CCCCCCCC=CCC=CCCCCC JQWAHKMIYCERGA-UHFFFAOYSA-N 0.000 description 1
- WSJULBMCKQTTIG-OWOJBTEDSA-N (e)-1,1,1,2,3,4,4,4-octafluorobut-2-ene Chemical compound FC(F)(F)C(/F)=C(\F)C(F)(F)F WSJULBMCKQTTIG-OWOJBTEDSA-N 0.000 description 1
- COQIQRBKEGPRSG-UHFFFAOYSA-N 1,1,1,2,3,3,3-heptafluoro-2-(trifluoromethyl)propane Chemical compound FC(F)(F)C(F)(C(F)(F)F)C(F)(F)F COQIQRBKEGPRSG-UHFFFAOYSA-N 0.000 description 1
- FNVLGCVAWPSVSK-UHFFFAOYSA-N 1,1,2,2,3,3,4,4,5,5,6,6,7,7-tetradecafluorocycloheptane Chemical compound FC1(F)C(F)(F)C(F)(F)C(F)(F)C(F)(F)C(F)(F)C1(F)F FNVLGCVAWPSVSK-UHFFFAOYSA-N 0.000 description 1
- RKIMETXDACNTIE-UHFFFAOYSA-N 1,1,2,2,3,3,4,4,5,5,6,6-dodecafluorocyclohexane Chemical compound FC1(F)C(F)(F)C(F)(F)C(F)(F)C(F)(F)C1(F)F RKIMETXDACNTIE-UHFFFAOYSA-N 0.000 description 1
- QIROQPWSJUXOJC-UHFFFAOYSA-N 1,1,2,2,3,3,4,4,5,5,6-undecafluoro-6-(trifluoromethyl)cyclohexane Chemical compound FC(F)(F)C1(F)C(F)(F)C(F)(F)C(F)(F)C(F)(F)C1(F)F QIROQPWSJUXOJC-UHFFFAOYSA-N 0.000 description 1
- PWMJXZJISGDARB-UHFFFAOYSA-N 1,1,2,2,3,3,4,4,5,5-decafluorocyclopentane Chemical compound FC1(F)C(F)(F)C(F)(F)C(F)(F)C1(F)F PWMJXZJISGDARB-UHFFFAOYSA-N 0.000 description 1
- BCNXQFASJTYKDJ-UHFFFAOYSA-N 1,1,2,2,3,3,4,4,5-nonafluoro-5-(trifluoromethyl)cyclopentane Chemical compound FC(F)(F)C1(F)C(F)(F)C(F)(F)C(F)(F)C1(F)F BCNXQFASJTYKDJ-UHFFFAOYSA-N 0.000 description 1
- CIWUYWQUYMZILR-UHFFFAOYSA-N 1,1,2,2,3,3,4,4-octafluoro-5,5-bis(trifluoromethyl)cyclopentane Chemical compound FC(F)(F)C1(C(F)(F)F)C(F)(F)C(F)(F)C(F)(F)C1(F)F CIWUYWQUYMZILR-UHFFFAOYSA-N 0.000 description 1
- ZVXOHSHODRJTCP-UHFFFAOYSA-N 1,1,2,2,3,3,4-heptafluoro-4-(trifluoromethyl)cyclobutane Chemical compound FC(F)(F)C1(F)C(F)(F)C(F)(F)C1(F)F ZVXOHSHODRJTCP-UHFFFAOYSA-N 0.000 description 1
- TXGPGHBYAPBDAG-UHFFFAOYSA-N 1,1,2,2,3,3-hexafluoro-4,4-bis(trifluoromethyl)cyclobutane Chemical compound FC(F)(F)C1(C(F)(F)F)C(F)(F)C(F)(F)C1(F)F TXGPGHBYAPBDAG-UHFFFAOYSA-N 0.000 description 1
- YUFJLVUCHXMKKM-UHFFFAOYSA-N 1,1,2,2,3-pentafluoro-3,4,4-tris(trifluoromethyl)cyclobutane Chemical compound FC(F)(F)C1(F)C(F)(F)C(F)(F)C1(C(F)(F)F)C(F)(F)F YUFJLVUCHXMKKM-UHFFFAOYSA-N 0.000 description 1
- ZVJOQYFQSQJDDX-UHFFFAOYSA-N 1,1,2,3,3,4,4,4-octafluorobut-1-ene Chemical compound FC(F)=C(F)C(F)(F)C(F)(F)F ZVJOQYFQSQJDDX-UHFFFAOYSA-N 0.000 description 1
- LGPPATCNSOSOQH-UHFFFAOYSA-N 1,1,2,3,4,4-hexafluorobuta-1,3-diene Chemical compound FC(F)=C(F)C(F)=C(F)F LGPPATCNSOSOQH-UHFFFAOYSA-N 0.000 description 1
- NPNPZTNLOVBDOC-UHFFFAOYSA-N 1,1-difluoroethane Chemical compound CC(F)F NPNPZTNLOVBDOC-UHFFFAOYSA-N 0.000 description 1
- CITHEXJVPOWHKC-UUWRZZSWSA-N 1,2-di-O-myristoyl-sn-glycero-3-phosphocholine Chemical compound CCCCCCCCCCCCCC(=O)OC[C@H](COP([O-])(=O)OCC[N+](C)(C)C)OC(=O)CCCCCCCCCCCCC CITHEXJVPOWHKC-UUWRZZSWSA-N 0.000 description 1
- DDMOUSALMHHKOS-UHFFFAOYSA-N 1,2-dichloro-1,1,2,2-tetrafluoroethane Chemical compound FC(F)(Cl)C(F)(F)Cl DDMOUSALMHHKOS-UHFFFAOYSA-N 0.000 description 1
- KILNVBDSWZSGLL-KXQOOQHDSA-N 1,2-dihexadecanoyl-sn-glycero-3-phosphocholine Chemical compound CCCCCCCCCCCCCCCC(=O)OC[C@H](COP([O-])(=O)OCC[N+](C)(C)C)OC(=O)CCCCCCCCCCCCCCC KILNVBDSWZSGLL-KXQOOQHDSA-N 0.000 description 1
- NRJAVPSFFCBXDT-HUESYALOSA-N 1,2-distearoyl-sn-glycero-3-phosphocholine Chemical compound CCCCCCCCCCCCCCCCCC(=O)OC[C@H](COP([O-])(=O)OCC[N+](C)(C)C)OC(=O)CCCCCCCCCCCCCCCCC NRJAVPSFFCBXDT-HUESYALOSA-N 0.000 description 1
- VXNZUUAINFGPBY-UHFFFAOYSA-N 1-Butene Chemical compound CCC=C VXNZUUAINFGPBY-UHFFFAOYSA-N 0.000 description 1
- RFCAUADVODFSLZ-UHFFFAOYSA-N 1-Chloro-1,1,2,2,2-pentafluoroethane Chemical compound FC(F)(F)C(F)(F)Cl RFCAUADVODFSLZ-UHFFFAOYSA-N 0.000 description 1
- PZNPLUBHRSSFHT-RRHRGVEJSA-N 1-hexadecanoyl-2-octadecanoyl-sn-glycero-3-phosphocholine Chemical compound CCCCCCCCCCCCCCCCCC(=O)O[C@@H](COP([O-])(=O)OCC[N+](C)(C)C)COC(=O)CCCCCCCCCCCCCCC PZNPLUBHRSSFHT-RRHRGVEJSA-N 0.000 description 1
- IIZPXYDJLKNOIY-JXPKJXOSSA-N 1-palmitoyl-2-arachidonoyl-sn-glycero-3-phosphocholine Chemical compound CCCCCCCCCCCCCCCC(=O)OC[C@H](COP([O-])(=O)OCC[N+](C)(C)C)OC(=O)CCC\C=C/C\C=C/C\C=C/C\C=C/CCCCC IIZPXYDJLKNOIY-JXPKJXOSSA-N 0.000 description 1
- ABQLAMJAQZFPJI-UHFFFAOYSA-N 3-heptyloxolan-2-one Chemical compound CCCCCCCC1CCOC1=O ABQLAMJAQZFPJI-UHFFFAOYSA-N 0.000 description 1
- JBVJKCHENYIOLW-UHFFFAOYSA-N 4-chloro-1,1,1,2,2,6-hexafluorohexane Chemical compound FCCC(Cl)CC(F)(F)C(F)(F)F JBVJKCHENYIOLW-UHFFFAOYSA-N 0.000 description 1
- 102000009027 Albumins Human genes 0.000 description 1
- 108010088751 Albumins Proteins 0.000 description 1
- 239000004215 Carbon black (E152) Substances 0.000 description 1
- VOPWNXZWBYDODV-UHFFFAOYSA-N Chlorodifluoromethane Chemical compound FC(F)Cl VOPWNXZWBYDODV-UHFFFAOYSA-N 0.000 description 1
- 239000004340 Chloropentafluoroethane Substances 0.000 description 1
- 201000000057 Coronary Stenosis Diseases 0.000 description 1
- 206010011089 Coronary artery stenosis Diseases 0.000 description 1
- PMPVIKIVABFJJI-UHFFFAOYSA-N Cyclobutane Chemical compound C1CCC1 PMPVIKIVABFJJI-UHFFFAOYSA-N 0.000 description 1
- LVZWSLJZHVFIQJ-UHFFFAOYSA-N Cyclopropane Chemical compound C1CC1 LVZWSLJZHVFIQJ-UHFFFAOYSA-N 0.000 description 1
- 239000004338 Dichlorodifluoromethane Substances 0.000 description 1
- 102000002322 Egg Proteins Human genes 0.000 description 1
- 108010000912 Egg Proteins Proteins 0.000 description 1
- OTMSDBZUPAUEDD-UHFFFAOYSA-N Ethane Chemical compound CC OTMSDBZUPAUEDD-UHFFFAOYSA-N 0.000 description 1
- VGGSQFUCUMXWEO-UHFFFAOYSA-N Ethene Chemical compound C=C VGGSQFUCUMXWEO-UHFFFAOYSA-N 0.000 description 1
- 239000005977 Ethylene Substances 0.000 description 1
- 108010010803 Gelatin Proteins 0.000 description 1
- JZNWSCPGTDBMEW-UHFFFAOYSA-N Glycerophosphorylethanolamin Natural products NCCOP(O)(=O)OCC(O)CO JZNWSCPGTDBMEW-UHFFFAOYSA-N 0.000 description 1
- 102000008100 Human Serum Albumin Human genes 0.000 description 1
- 108091006905 Human Serum Albumin Proteins 0.000 description 1
- UFHFLCQGNIYNRP-UHFFFAOYSA-N Hydrogen Chemical compound [H][H] UFHFLCQGNIYNRP-UHFFFAOYSA-N 0.000 description 1
- NIPNSKYNPDTRPC-UHFFFAOYSA-N N-[2-oxo-2-(2,4,6,7-tetrahydrotriazolo[4,5-c]pyridin-5-yl)ethyl]-2-[[3-(trifluoromethoxy)phenyl]methylamino]pyrimidine-5-carboxamide Chemical compound O=C(CNC(=O)C=1C=NC(=NC=1)NCC1=CC(=CC=C1)OC(F)(F)F)N1CC2=C(CC1)NN=N2 NIPNSKYNPDTRPC-UHFFFAOYSA-N 0.000 description 1
- 229920001744 Polyaldehyde Polymers 0.000 description 1
- 241000183024 Populus tremula Species 0.000 description 1
- CZMRCDWAGMRECN-UGDNZRGBSA-N Sucrose Chemical compound O[C@H]1[C@H](O)[C@@H](CO)O[C@@]1(CO)O[C@@H]1[C@H](O)[C@@H](O)[C@H](O)[C@@H](CO)O1 CZMRCDWAGMRECN-UGDNZRGBSA-N 0.000 description 1
- 229930006000 Sucrose Natural products 0.000 description 1
- 229920001963 Synthetic biodegradable polymer Polymers 0.000 description 1
- 230000003213 activating effect Effects 0.000 description 1
- 239000012190 activator Substances 0.000 description 1
- 150000001335 aliphatic alkanes Chemical class 0.000 description 1
- 150000001336 alkenes Chemical class 0.000 description 1
- 150000001345 alkine derivatives Chemical class 0.000 description 1
- IYABWNGZIDDRAK-UHFFFAOYSA-N allene Chemical compound C=C=C IYABWNGZIDDRAK-UHFFFAOYSA-N 0.000 description 1
- HSFWRNGVRCDJHI-UHFFFAOYSA-N alpha-acetylene Natural products C#C HSFWRNGVRCDJHI-UHFFFAOYSA-N 0.000 description 1
- 230000003321 amplification Effects 0.000 description 1
- 230000002547 anomalous effect Effects 0.000 description 1
- 230000002744 anti-aggregatory effect Effects 0.000 description 1
- 229910052786 argon Inorganic materials 0.000 description 1
- QVGXLLKOCUKJST-UHFFFAOYSA-N atomic oxygen Chemical compound [O] QVGXLLKOCUKJST-UHFFFAOYSA-N 0.000 description 1
- 210000003323 beak Anatomy 0.000 description 1
- 230000009286 beneficial effect Effects 0.000 description 1
- 230000008901 benefit Effects 0.000 description 1
- 229920002988 biodegradable polymer Polymers 0.000 description 1
- 239000004621 biodegradable polymer Substances 0.000 description 1
- 230000015572 biosynthetic process Effects 0.000 description 1
- 229920001400 block copolymer Polymers 0.000 description 1
- 230000036770 blood supply Effects 0.000 description 1
- 210000004204 blood vessel Anatomy 0.000 description 1
- 230000036760 body temperature Effects 0.000 description 1
- 238000009835 boiling Methods 0.000 description 1
- MEXUFEQDCXZEON-UHFFFAOYSA-N bromochlorodifluoromethane Chemical compound FC(F)(Cl)Br MEXUFEQDCXZEON-UHFFFAOYSA-N 0.000 description 1
- RJCQBQGAPKAMLL-UHFFFAOYSA-N bromotrifluoromethane Chemical compound FC(F)(F)Br RJCQBQGAPKAMLL-UHFFFAOYSA-N 0.000 description 1
- 239000001273 butane Substances 0.000 description 1
- IAQRGUVFOMOMEM-UHFFFAOYSA-N butene Natural products CC=CC IAQRGUVFOMOMEM-UHFFFAOYSA-N 0.000 description 1
- 230000008822 capillary blood flow Effects 0.000 description 1
- 229910002092 carbon dioxide Inorganic materials 0.000 description 1
- 239000001569 carbon dioxide Substances 0.000 description 1
- 238000005119 centrifugation Methods 0.000 description 1
- NEHMKBQYUWJMIP-NJFSPNSNSA-N chloro(114C)methane Chemical compound [14CH3]Cl NEHMKBQYUWJMIP-NJFSPNSNSA-N 0.000 description 1
- 235000019406 chloropentafluoroethane Nutrition 0.000 description 1
- AFYPFACVUDMOHA-UHFFFAOYSA-N chlorotrifluoromethane Chemical compound FC(F)(F)Cl AFYPFACVUDMOHA-UHFFFAOYSA-N 0.000 description 1
- 235000012000 cholesterol Nutrition 0.000 description 1
- 238000012790 confirmation Methods 0.000 description 1
- 210000004351 coronary vessel Anatomy 0.000 description 1
- 150000001924 cycloalkanes Chemical class 0.000 description 1
- 239000005548 dental material Substances 0.000 description 1
- PXBRQCKWGAHEHS-UHFFFAOYSA-N dichlorodifluoromethane Chemical compound FC(F)(Cl)Cl PXBRQCKWGAHEHS-UHFFFAOYSA-N 0.000 description 1
- 235000019404 dichlorodifluoromethane Nutrition 0.000 description 1
- 229940087091 dichlorotetrafluoroethane Drugs 0.000 description 1
- 238000009792 diffusion process Methods 0.000 description 1
- UBHZUDXTHNMNLD-UHFFFAOYSA-N dimethylsilane Chemical compound C[SiH2]C UBHZUDXTHNMNLD-UHFFFAOYSA-N 0.000 description 1
- 229960003724 dimyristoylphosphatidylcholine Drugs 0.000 description 1
- ZGSPNIOCEDOHGS-UHFFFAOYSA-L disodium [3-[2,3-di(octadeca-9,12-dienoyloxy)propoxy-oxidophosphoryl]oxy-2-hydroxypropyl] 2,3-di(octadeca-9,12-dienoyloxy)propyl phosphate Chemical compound [Na+].[Na+].CCCCCC=CCC=CCCCCCCCC(=O)OCC(OC(=O)CCCCCCCC=CCC=CCCCCC)COP([O-])(=O)OCC(O)COP([O-])(=O)OCC(OC(=O)CCCCCCCC=CCC=CCCCCC)COC(=O)CCCCCCCC=CCC=CCCCCC ZGSPNIOCEDOHGS-UHFFFAOYSA-L 0.000 description 1
- 238000004090 dissolution Methods 0.000 description 1
- 239000012153 distilled water Substances 0.000 description 1
- 235000013345 egg yolk Nutrition 0.000 description 1
- 210000002969 egg yolk Anatomy 0.000 description 1
- 125000002534 ethynyl group Chemical group [H]C#C* 0.000 description 1
- 125000001153 fluoro group Chemical group F* 0.000 description 1
- 238000009472 formulation Methods 0.000 description 1
- 238000005194 fractionation Methods 0.000 description 1
- 229920000159 gelatin Polymers 0.000 description 1
- 239000008273 gelatin Substances 0.000 description 1
- 235000019322 gelatine Nutrition 0.000 description 1
- 235000011852 gelatine desserts Nutrition 0.000 description 1
- 150000008282 halocarbons Chemical class 0.000 description 1
- 125000005843 halogen group Chemical group 0.000 description 1
- 239000001307 helium Substances 0.000 description 1
- 229910052734 helium Inorganic materials 0.000 description 1
- SWQJXJOGLNCZEY-UHFFFAOYSA-N helium atom Chemical compound [He] SWQJXJOGLNCZEY-UHFFFAOYSA-N 0.000 description 1
- DMEGYFMYUHOHGS-UHFFFAOYSA-N heptamethylene Natural products C1CCCCCC1 DMEGYFMYUHOHGS-UHFFFAOYSA-N 0.000 description 1
- WBCLXFIDEDJGCC-UHFFFAOYSA-N hexafluoro-2-butyne Chemical compound FC(F)(F)C#CC(F)(F)F WBCLXFIDEDJGCC-UHFFFAOYSA-N 0.000 description 1
- VBZWSGALLODQNC-UHFFFAOYSA-N hexafluoroacetone Chemical compound FC(F)(F)C(=O)C(F)(F)F VBZWSGALLODQNC-UHFFFAOYSA-N 0.000 description 1
- WMIYKQLTONQJES-UHFFFAOYSA-N hexafluoroethane Chemical compound FC(F)(F)C(F)(F)F WMIYKQLTONQJES-UHFFFAOYSA-N 0.000 description 1
- HCDGVLDPFQMKDK-UHFFFAOYSA-N hexafluoropropylene Chemical compound FC(F)=C(F)C(F)(F)F HCDGVLDPFQMKDK-UHFFFAOYSA-N 0.000 description 1
- 239000001257 hydrogen Substances 0.000 description 1
- 229910052739 hydrogen Inorganic materials 0.000 description 1
- 238000001727 in vivo Methods 0.000 description 1
- 239000011261 inert gas Substances 0.000 description 1
- 239000004615 ingredient Substances 0.000 description 1
- 229910052743 krypton Inorganic materials 0.000 description 1
- DNNSSWSSYDEUBZ-UHFFFAOYSA-N krypton atom Chemical compound [Kr] DNNSSWSSYDEUBZ-UHFFFAOYSA-N 0.000 description 1
- 229940067606 lecithin Drugs 0.000 description 1
- 210000005240 left ventricle Anatomy 0.000 description 1
- 210000004072 lung Anatomy 0.000 description 1
- 238000004519 manufacturing process Methods 0.000 description 1
- UIUXUFNYAYAMOE-UHFFFAOYSA-N methylsilane Chemical compound [SiH3]C UIUXUFNYAYAMOE-UHFFFAOYSA-N 0.000 description 1
- 239000003094 microcapsule Substances 0.000 description 1
- 238000012986 modification Methods 0.000 description 1
- 230000004048 modification Effects 0.000 description 1
- IJDNQMDRQITEOD-UHFFFAOYSA-N n-butane Chemical compound CCCC IJDNQMDRQITEOD-UHFFFAOYSA-N 0.000 description 1
- 229910052757 nitrogen Inorganic materials 0.000 description 1
- 238000003199 nucleic acid amplification method Methods 0.000 description 1
- BCCOBQSFUDVTJQ-UHFFFAOYSA-N octafluorocyclobutane Chemical compound FC1(F)C(F)(F)C(F)(F)C1(F)F BCCOBQSFUDVTJQ-UHFFFAOYSA-N 0.000 description 1
- 235000019407 octafluorocyclobutane Nutrition 0.000 description 1
- 210000000056 organ Anatomy 0.000 description 1
- 239000001301 oxygen Substances 0.000 description 1
- 229910052760 oxygen Inorganic materials 0.000 description 1
- 229960004624 perflexane Drugs 0.000 description 1
- ZJIJAJXFLBMLCK-UHFFFAOYSA-N perfluorohexane Chemical compound FC(F)(F)C(F)(F)C(F)(F)C(F)(F)C(F)(F)C(F)(F)F ZJIJAJXFLBMLCK-UHFFFAOYSA-N 0.000 description 1
- 230000002688 persistence Effects 0.000 description 1
- WTJKGGKOPKCXLL-RRHRGVEJSA-N phosphatidylcholine Chemical compound CCCCCCCCCCCCCCCC(=O)OC[C@H](COP([O-])(=O)OCC[N+](C)(C)C)OC(=O)CCCCCCCC=CCCCCCCCC WTJKGGKOPKCXLL-RRHRGVEJSA-N 0.000 description 1
- 150000008104 phosphatidylethanolamines Chemical class 0.000 description 1
- 229920001983 poloxamer Polymers 0.000 description 1
- 229920002503 polyoxyethylene-polyoxypropylene Polymers 0.000 description 1
- 239000011148 porous material Substances 0.000 description 1
- 230000001737 promoting effect Effects 0.000 description 1
- 239000001294 propane Substances 0.000 description 1
- 238000011321 prophylaxis Methods 0.000 description 1
- MWWATHDPGQKSAR-UHFFFAOYSA-N propyne Chemical compound CC#C MWWATHDPGQKSAR-UHFFFAOYSA-N 0.000 description 1
- 239000012857 radioactive material Substances 0.000 description 1
- 230000009467 reduction Effects 0.000 description 1
- 238000011160 research Methods 0.000 description 1
- 230000003307 reticuloendothelial effect Effects 0.000 description 1
- 239000011257 shell material Substances 0.000 description 1
- 150000004756 silanes Chemical class 0.000 description 1
- 238000002603 single-photon emission computed tomography Methods 0.000 description 1
- 239000007787 solid Substances 0.000 description 1
- 239000008347 soybean phospholipid Substances 0.000 description 1
- 239000000126 substance Substances 0.000 description 1
- 239000005720 sucrose Substances 0.000 description 1
- QTJXVIKNLHZIKL-UHFFFAOYSA-N sulfur difluoride Chemical class FSF QTJXVIKNLHZIKL-UHFFFAOYSA-N 0.000 description 1
- 239000006228 supernatant Substances 0.000 description 1
- 208000024891 symptom Diseases 0.000 description 1
- 229920001059 synthetic polymer Polymers 0.000 description 1
- 238000012360 testing method Methods 0.000 description 1
- 238000003325 tomography Methods 0.000 description 1
- 238000012546 transfer Methods 0.000 description 1
- 238000011282 treatment Methods 0.000 description 1
- 210000005166 vasculature Anatomy 0.000 description 1
- 210000003462 vein Anatomy 0.000 description 1
- 230000002861 ventricular Effects 0.000 description 1
- 238000012800 visualization Methods 0.000 description 1
- 229910052724 xenon Inorganic materials 0.000 description 1
- FHNFHKCVQCLJFQ-UHFFFAOYSA-N xenon atom Chemical compound [Xe] FHNFHKCVQCLJFQ-UHFFFAOYSA-N 0.000 description 1
Images
Classifications
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61B—DIAGNOSIS; SURGERY; IDENTIFICATION
- A61B8/00—Diagnosis using ultrasonic, sonic or infrasonic waves
- A61B8/48—Diagnostic techniques
- A61B8/481—Diagnostic techniques involving the use of contrast agent, e.g. microbubbles introduced into the bloodstream
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K41/00—Medicinal preparations obtained by treating materials with wave energy or particle radiation ; Therapies using these preparations
- A61K41/0028—Disruption, e.g. by heat or ultrasounds, sonophysical or sonochemical activation, e.g. thermosensitive or heat-sensitive liposomes, disruption of calculi with a medicinal preparation and ultrasounds
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K49/00—Preparations for testing in vivo
- A61K49/22—Echographic preparations; Ultrasound imaging preparations ; Optoacoustic imaging preparations
- A61K49/222—Echographic preparations; Ultrasound imaging preparations ; Optoacoustic imaging preparations characterised by a special physical form, e.g. emulsions, liposomes
- A61K49/223—Microbubbles, hollow microspheres, free gas bubbles, gas microspheres
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61B—DIAGNOSIS; SURGERY; IDENTIFICATION
- A61B8/00—Diagnosis using ultrasonic, sonic or infrasonic waves
- A61B8/06—Measuring blood flow
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61B—DIAGNOSIS; SURGERY; IDENTIFICATION
- A61B8/00—Diagnosis using ultrasonic, sonic or infrasonic waves
- A61B8/13—Tomography
Abstract
Description
発明の技術分野
本発明は超音波画像形成に関し、更に詳しくは組織灌流の測定への超音波画像形成の使用に関する。
従来の技術
ガス微小気泡の分散体を含む造影剤は、微小気泡が低密度で且つ圧縮が容易であるために、超音波の特に効率的な後方散乱物であることがよく知られている。かかる微小気泡分散体は適当に安定化されると、例えば血管系および組織の微細血管系を、しばしば有利には低い用量で、非常に効率的に超音波視覚化を可能にし得る。
発明が解決しようとする課題
組織灌流の測定は、例えば腫瘍検出、典型的には健康な組織と違った血管を有する腫瘍組織の検出と、心筋層、例えばそこへの血液供給を評価するための心筋層の研究に重要である。現在の超音波画像形成技術を使用した造影剤検出法は、特定の器官又はその領域が灌流されたか否かについて情報を与えるかもしれないが、灌流の定量を容易にしない。かかる情報は、患者が低灌流のために危険状態にあるか否かを評価するのに有用であり、従って予防法および/又は治療に有益であるかもしれないが、現在はシンチグラフィー、陽電子放出トモグラフィー又は単一光子放出計算トモグラフィーのような放射性同位体画像形成技術を用いて得なければならない。かかる技術は全て放射性活性物質の注入を含み、患者および医療部員の両者に安全性の危険を潜在的に伴い、そして高価な画像形成設備を使用する。このことは、それらの普及を妨げる。
本発明は造影剤の超音波誘導破壊又は変更を含む超音波画像形成が組織灌流の尺度を与えるために使用し得ること、従って超音波画像形成に感受性のあらゆる組織における組織灌流の相対速度を容易に且つ安価に測定させることの知見に基づく。
現在のところ、造影剤破壊を含む超音波画像形成に関係する従来技術には限界がある。US-A-5425366には、ある種の微粒子超音波造影剤、例えばガス含有ポリマーミクロカプセルは、カラードップラー技法により、例えば細網内皮組織系による摂取の結果として本質的に動きが少ないにかかわらず、視覚化され得ることが述べられている。カラードップラー検査に伴う比較的高い照射エネルギー量は微粒子を破裂させ、“音響的に刺激された音響放出”と記載されるドップラー−感受性シグナルを発生することが提案される。しかし実際には、検査者は後方散乱されたシグナルの不連続性を動きの結果と解釈しそうであり、そして適当な表示を発生する。この技法はもっぱら本質的に動きがない造影剤微粒子の検出に関するので、灌流の速度の測定に本来適用できないことが理解されるであろう。
US-A-5456257は、患者の体内の被覆された微小気泡造影剤を、被覆された微小気泡を破壊するのに充分なエネルギー量の超音波パルス照射を適用しそして微小気泡破壊事象を相非反応性検出法(例えば封入検出法)および引き続く超音波伝送から受信したエコーの分別を用いて確認することにより検出することを記載する。最初の伝送の後に、微小気泡破壊部位から出る音響エネルギーを超音波変換器で受信し、生じたシグナル波形を増幅検出に付す。引き続く伝送から受信したエコーを同様の方法で検出し、そして二つの受信期間からのシグナルを空間基準で区別する。典型的には、2番目の受信期間から誘導されたシグナルを1番目の受信期間から誘導されたシグナルから引いて、微小気泡破壊事象から出るシグナルを他のシグナルを除外して発生させる。組織の移動および流れる流体から生じる変動を除くために、閾値を適用し得る。シグナルを、持続系を用いそして/又は体内の所定の領域内の事象をある時間にわたって数えることにより処理し、これにより造影剤−含有血液の例えば心室内へのバルク(正味)流れの指示を与えることができるが、この技法は組織内の毛細血管血液流の定量、灌流の測定に使用し得ることは示唆されていない。
課題を解決するための手段
本発明は同様に、1番目の検出系列から差し引く背景シグナルを検出するために引き続くパルスを用いるのではなく、標的領域内の認識可能な量の造影剤を破壊又は見分けられるほど変更するために1番目の高エネルギー超音波のパルス又は一連のパルスを使用し、本発明は標的領域への“新たな”又は未変更の造影剤の流れ(従って血液の流れ)を検出するために、引き続くパルスを使用する。これは、標的領域内の局部血管状態に関する血管血液容量フラクション、平均通過時間および組織灌流のようなパラメータの決定を可能にする。初めの高エネルギーパルスを例えば、検出可能な造影剤の綿密に規定された標的領域を明らかにするために使用し得るので、超音波画像形成により容易に検出可能で且つ定量可能である追加の造影剤の鋭い(シャープな)フロントは、次にこの領域に流入する。興味の対象である標的領域内の移動する造影剤の鋭いフロントを生成する能力により、該方法は造影剤の注入直後に造影剤が組織に流入する速度を見積もる以前の試みよりも実質的に有利な方法となる。何故なら、注入した造影剤のフロントは、肺および心臓を通過することにより不可避的に滑らかにされるか又は不鮮明にされるからである。或いは、初めのパルスを使用して、造影剤のエコー源性(echogenicity)を、例えば前駆体の形態で活性化して標的領域にエコー源性の上昇を生じさせることにより変更し得る。超音波暴露中およびその後のエコー源性変更の時間経過は、局部的血管状態、例えば局部の血液容量および灌流、についての情報を与えるであろう。例えば、超音波暴露により活性化された造影剤の流出速度を決定し得、従って灌流マップ(地図)の作成に使用し得る。
従って、本発明の一つの側面によると、ヒト又はヒト以外の動物対象体における組織灌流の測定方法が提供され、該方法は超音波造影剤の有効量を該対象体に投与し、標的領域の組織に、該標的領域内の認識可能量の造影剤のエコー源性を破壊又は見分け可能に変更するのに充分なエネルギーを有する超音波の少なくとも一つのパルスを照射し、そして該標的領域に入る追加の造影剤又は該標的領域から出る変更された造影剤の流速を超音波で検出しそして定量することを含む。
別の側面から見ると、本発明はヒト又はヒト以外の動物対象体における組織灌流の測定方法に使用するための診断材料の製造における超音波造影剤の使用を提供し、該方法は超音波造影剤の有効量を該対象体に投与し、標的領域の組織に、該標的領域内の認識可能な量の造影剤のエコー源性を破壊又は見分け可能に変更するのに充分なエネルギーを有する超音波の少なくとも一つのパルスを照射し、そして該標的領域に入る追加の造影剤又は該標的領域から出る変更された造影剤の流速を超音波で検出しそして定量することを含む。
本発明によると、広範囲の超音波造影剤が使用でき、最も普通に使用される造影剤はガス含有性又はガス発生性であろう。かかる造影剤の代表的例には、耐凝集性表面膜(例えばWO-A-8002365に記載されたようなゼラチン)、フィルモゲン性(filmogenic)タンパク質(例えばUS-A-4718433、US-A-4774958、US-A-4844882、EP-A-0359246、WO-A-9112823、WO-A-9205806、WO-A-9217213、WO-A-9406477又はWO-A-9501187に記載されたような、例えばヒト血清アルブミンのようなアルブミン)、ポリマー材料(例えばEP-A-0398935に記載された合成生物分解性ポリマー、EP-A-0458745に記載された弾性界面合成ポリマー膜、EP-A-0441468に記載された微粒子状生物分解性ポリアルデヒド、EP-A-0458079に記載されたポリアミノ酸−多環式イミドの微粒子状N−ジカルボン酸誘導体、又はWO-A-9317718又はWO-A-9607434に記載された生物分解性ポリマー)、非ポリマーおよび非重合性壁形成性材料(例えばWO-A-9521631に記載されたもの)、又は表面活性剤(例えば、Pluronicのようなポリオキシエチレン−ポリオキシプロピレンブロックコポリマー表面活性剤、WO-A-9506518に記載されたポリマー表面活性剤、例えばWO-A-9211873、WO-A-9217212、WO-A-9222247、WO-A-9428780、WO-A-9503835、WO-A-9640275又はWO-A-9729783に記載されたリン脂質のようなフィルム形成性表面活性剤)によって安定化された(例えば、少なくとも部分的に封入された)ガスの微小気泡が含まれる。
他の有用なガス含有造影剤には、ガス含有固体系、例えば内部にガスを含有した又はガスと結合した(例えばEP-A-0122624、EP-A-123235、EP-A-0365467、WO-A-9221382、WO-A-9300930、WO-A-9313802、WO-A-9313808又はWO-A-9313809に記載された、例えば表面にガスを吸着したおよび/又はガスを空隙、空洞又は孔の内部に含む)微粒子(特に微粒子の集合体)が含まれる。
例えば分散気相−含有組成物およびインビボで例えば拡散により該分散気相に移動可能な揮発性成分を含む組成物を含む多成分造影剤配合物もまた有用であろう。かかる造影剤は、本出願人の未公開の国際特許出願No.PCT/GB97/02898の明細書に記載されている。それらは、所望により前駆体の形態で製造でき、造影剤は高エネルギー超音波処理により活性化した後にのみ、著しいエコー源性を示す。
ガス含有造影剤に関する上記の文献の全ての開示を、参照用に本願に含める。
リン脂質含有組成物は本発明に従って、例えばリン脂質安定化ガス微小気泡の形態で使用されるが、有用なリン脂質の代表的例には、レシチン(即ち、ホスファチジルコリン)、例えば卵黄レシチン又は大豆レシチンのような天然レシチン、およびジミリストイルホスファチジルコリン、ジパルミトイルホスファチジルコリン又はジステアロイルホスファチジルコリンのような合成又は半合成レシチン;ホスファチジン酸;ホスファチジルエタノールアミン;ホスファチジルセリン;ホスファチジルグリセロール;ホスファチジルイノシトール;カルジオリピン;スフィンゴミエリン(sphingomyelins);前記のいずれかのフッ素化類似体;前記のいずれかとコレステロールのような他の脂質との混合物が含まれる。各々が正味の全体電荷、例えば負の電荷、を帯びた分子を優勢的に(例えば少なくとも75%)含むリン脂質、例えば天然の(例えば大豆又は卵黄誘導の)、半合成の(例えば部分的に又は完全に水素添加された)および合成のホスファチジルセリン、ホスファチジルグリセロール、ホスファチジルイノシトール、ホスファチジン酸および/又はカルジオリピン、の使用は特に有利であろう。
あらゆる生物両立性ガスが本発明による微小気泡に存在してもよい。本願で使用される“ガス”の用語は、37℃の通常のヒトの体温で実質的に又は完全に気体(蒸気を含む)の形態にあるあらゆる物質(混合物を含む)を含む。該ガスは従って、例えば空気;窒素;酸素;二酸化炭素;水素;ヘリウム、アルゴン、キセノン又はクリプトンのような不活性ガス;サルファヘキサフルオリド、ジサルファデカフルオリド又はトリフルオロメチルサルファペンタフルオリドのようなイオウフッ素化物;セレンヘキサフルオリド;メチルシラン又はジメチルシランのような任意に水素添加されたシラン;低分子量炭化水素(例えば7個までの炭素原子を含む)、例えばメタン、エタン、プロパン、ブタン又はペンタンのようなアルカン、シクロプロパン、シクロブタン又はシクロペンタンのようなシクロアルカン、エチレン、プロペン、プロパジエン又はブテンのようなアルケン、アセチレン又はプロピンのようなアルキン;ジメチルエーテルのようなエーテル;ケトン;エステル;ハロゲン化低分子量炭化水素(例えば7個までの炭素原子を含む);又は前記のいずれかの混合物が含まれる。ハロゲン化ガス内の少なくとも幾つかのハロゲン原子はフッ素原子であるのが有利である;従って生物両立性ハロゲン化炭化水素ガスは、例えばブロモクロロジフルオロメタン、クロロジフルオロメタン、ジクロロジフルオロメタン、ブロモトリフルオロメタン、クロロトリフルオロメタン、クロロペンタフルオロエタン、ジクロロテトラフルオロエタン、クロロトリフルオロエチレン、フルオロエチレン、エチルフルオリド、1,1−ジフルオロエタンおよびペルフルオロカーボン、例えばペルフルオロメタン、ペルフルオロエタン、ペルフルオロプロパン、ペルフルオロブタン(例えば任意にペルフルオロ−イソ−ブタンのような異性体と混合したペルフルオロ−n−ブタン)、ペルフルオロペンタン、ペルフルオロヘキサンおよびペルフルオロヘプタンのようなペルフルオロアルカン;例えばペルフルオロプロペン、ペルフルオロブテン(例えばペルフルオロブト−2−エン)およびペルフルオロブタジエンのようなペルフルオロアルケン;ペルフルオロブト−2−インのようなペルフルオロアルキン;およびペルフルオロシクロブタン、ペルフルオロメチルシクロブタン、ペルフルオロジメチルシクロブタン、ペルフルオロトリメチルシクロブタン、ペルフルオロシクロペンタン、ペルフルオロメチルシクロペンタン、ペルフルオロジメチルシクロペンタン、ペルフルオロシクロヘキサン、ペルフルオロメチルシクロヘキサンおよびペルフルオロシクロヘプタンのようなペルフルオロシクロアルカンから選ぶことができる。その他のハロゲン化ガスには、メチルクロリド、ペルフルオロアセトンのようなフッ素化(例えばペルフルオロ化)ケトン、およびペルフルオロジエチルエーテルのようなフッ素化(例えばペルフルオロ化)エーテルが含まれる。ペルフルオロ化ガス、例えばサルファヘキサフルオリドおよびペルフルオロプロパン、ペルフルオロブタンおよびペルフルオロペンタンのようなペルフルオロカーボン、の使用は、かかるガスを含む微小気泡の血液流における高い安定性が認められたことから、特に有利であろう。
初期の高エネルギー超音波パルスは、例えばガス含有造影剤の破壊を、例えばタンパク質、ポリマー又はフィルム形成性表面活性剤のような安定化表面膜を破壊することにより、および/又はガス含有物の周囲の組織流体への溶解を促進することにより引き起こし得る。しかしながら、標的領域内の造影剤のエコー源性を完全に破壊する必要はない。その“音響サイン”を、続いて流入する“新たな”造影剤から超音波的に区別できるように変更すれば充分である。従って、例えばカプセル封入用殻材料の部分的又は完全な超音波誘導崩壊は、造影剤カプセル封入ガスよりも更に自由に振動するガス微小気泡を発生し、そして見分けられるように変更された音響的性質を示し得る。或いは、初めの超音波照射は、造影剤部分の組成、寸法および/又は機械的性質のようなパラメータにその他のエコー源性を変更する変化を引き起こし得る。これは、例えば前駆体の形態の造影剤の活性化を導くために使用し得る。
所望により、造影剤は、初めの超音波パルスによる崩壊に特に敏感になるように設計し得、これにより、初めの超音波照射に必要な強度を制限する。これは例えば、単層の安定化用両親媒性脂質材料を使用することにより達成し得る;荷電両親媒性脂質材料、例えば負に荷電したリン脂質は、荷電した脂質膜の間の電子的反発の結果、単層の形成を奨励し得る。
多様な超音波画像形成技術を、初めの超音波照射の後に流入する別の造影剤を検出しそして定量するために使用して、例えば標的領域内に流入する造影剤の時間に関連する分量を表示する灌流関連画像を発生させ、それにより異なる灌流領域間の区別を可能にする。望ましい画像は、個々の走査線の分析から又はフレーム基準でフレームの分析から得られる;前者は、異なる灌流を有する領域を区別するために充分な数のサンプルを得るために、高い灌流速度の領域に有利であり、後者は灌流速度が低い領域に好ましいであろう。
使用し得る画像形成モードには、B−モードおよびドップラーに基づく画像形成、例えば電力ドップラー画像形成のようなパルス波ドップラー技術が含まれる。例えばUS-A-5410516に記載されたような(例えば画像形成周波数の2、3、4....倍の)高調波、(例えば画像形成周波数の1/2、1/3、2/3、3/4...の)分周波、(例えば画像形成周波数の3/2、5/4...倍の)超調波(ultraharmonics)、および(例えばかかる調波および画像形成周波数から誘導する)周波数の和又は差の効果に基づく非線形画像形成技術を、所望により例えば公開された米国特許出願第08/440,266に記載された、異なる周波数の二つの入射超音波シグナルにより生成した周波数の和又は差の検出に基づく技術のように使用し得る。二番目の調波画像形成は特に有利であろう。例えば二番目の調波電力ドップラー画像形成における上記の技術の組み合わせもまた有用であろう。一般に、得られる画像は、例えば灌流速度を表すカラーマップとして表示し得、そして所望により、標的領域の慣用のB−モード画像上に重ねてもよい。
本発明の方法は、初めの超音波パルスのエネルギーレベル(従って破壊された又は変更された造影剤を含む調査された領域のサイズ)および引き続く超音波画像形成パラメータ、特にフレーム速度又は個々のパルスの間の時間、を適当に選ぶことにより、希望する灌流速度に仕立てることができ、適当な場合はECG−トリガー法を使用できる。所望により、個々の結果を、例えばそれ自体公知の方法で空間的に平均してもよい。
本方法は、従来のドップラー技術の検出限界以下の低い灌流速度の測定を可能にし、組織の動きが従来のドップラー画像形成を有効でなくする心筋層における相対的灌流速度を見積もるのにも使用し得る。心筋層の画像形成は、造影剤をそのピーク濃度で左心室を通過するように注入してから充分時間が経過した後に行うのが有利であり、それにより、望ましくない造影剤含有左心室の血液により弱められるのを最小にし、造影剤の心筋層組織への流入速度が適当な定常状態に達するようにする。毛細管レベルでの血液および造影剤により示される本質的にランダムな流れパターンは、本発明の方法をこのレベルで使用した場合、優勢な流れパターンが走査系に実質的に垂直であり従って相対的に検出不可能になり得る、変則的な結果となる可能性を回避する。該方法はまた、その結果が心筋層自体からの後方散乱と実質的に独立しているという利点を有する。かかる後方散乱のレベルが、心筋層のいろいろな領域のエコー源性が相違しそして、かかる領域と超音波変換器との間の隣接する組織、流体等の減衰性が相違する結果、心筋層のいろいろな領域でかなり変動し得るので、このことは有益である。更に、該方法により得られる結果は、初めの超音波照射が造影剤のエコー源性効果を見分けられるほどに変更するか破壊するのに充分であれば、投与した造影剤の用量には影響されない。
下記の非限定的実施例により本発明を説明する。
【図面の簡単な説明】
添付の図面において、図1−3は、それぞれ実施例1−3の手順に従って得られた標準化時間−強度曲線である。強度は線状単位(LU)で表されている。
実施例1
麻酔した犬に、WO-A-9317718に記載されたガス含有微粒子状造影剤の水性懸濁液2mlを含む静脈内注射をする。注射の1分後に、3.7MHzおよび高音響電力で伝送するビングメド サンド システム(Vingmed Sound System)5スキャナーを用いて心臓を約10秒走査し、画像形成した断片内の全ての造影剤を破壊する。次に出力を急速に12dBだけ減少させて12dBの補償利得増加を得、引き続く数回の心臓鼓動についてのB−モードフレームを走査−変換しそしてデジタル貯蔵する。線状スケール上の時間に対する標準化シグナル強度のプロットを図1に示すように作成する。各時間点は5×5画素領域の平均を表す。このプロットは5秒で始まる高電力超音波照射を示す。流入曲線は出力電力が減少する15秒の時点で始まり、そして約0.7秒のハーフピーク時間(ハーフタイム)を示し、画像形成領域が正常に灌流されたことを示す。ハーフタイム画像は、流入ハーフタイムを単調指数曲線に合わせることにより生成される。灌流画像を表すこの画像を、偽着色しそしてB−モード画像に重ねる。灌流画像は心筋層の全ての領域について正常な灌流を表示する。
実施例2
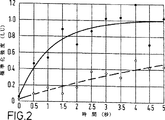
麻酔した犬の左冠動脈を部分的に閉鎖して心筋層の一つの領域への冠状血液流を減少させ、これにより冠動脈狭窄症の症状を惹起した。次に犬に、WO-A-9317718に記載されたガス含有微粒子状造影剤の水性懸濁液2m1を含む静脈内注射をした。注射の1分後に、2.7MHzおよび中音響電力で伝送するP5-3変換器付HDI 3000スキャナーを用いて心臓を約10秒走査し、画像形成した断片内の造影剤のかなりのフラクションを破壊した。スキャナーを急速に45.4MHzで受信するECG−トリガーモードに設定し(即ち、第2の調波)、そして数回の心臓鼓動についての終点−心収縮期フレーム(end-systolic frame)をデジタルで貯蔵した。全体的超音波暴露の減少の結果として、ECG−トリガーモードでの操作中に最小の造影剤破壊が起きたので、新たな造影剤の流入を示す流入曲線は、第2の調波シグナル強度から容易に導かれた。図2は、得られた線状スケール上の時間に対する標準化シグナル強度のプロットを示し、各点は5×5画素領域の平均を表す。白丸は低灌流領域からの結果を示し、黒丸は正常に灌流された領域からとった。時間軸上のゼロはECG−トリガーが開始した時点に対応する。引いた線は、これらの二つのデータセットについての最小平均平方適合(fit)を示す。実線は、正常に灌流された領域についての曲線を表し、そして約0.7秒のハーフピーク時間を示す。破線は、低灌流領域についての曲線を表し、そして約6秒のハーフピーク時間、即ち正常の約8倍の時間、を示す。流入ハーフタイムを表す偽着色された画像を作成しそしてB−モード画像に重ねた。
実施例3
(a) 純水(20ml)中のプロピレングリコール2%溶液の中の水素添加ホスファチジルセリン(100mg)を80℃に5分間加熱し、得られた分散体を室温に一晩放冷する。1ml部分を2mlの小壜に移し、各部分の上のヘッドスペースをペルフルオロブタンガスでフラッシし、そして小壜を歯科材料用のEspe CapMix(商標)ミキサーを用いて45秒間振盪して、乳状白色微小気泡分散体を得る。
(b) 上記(a)で調製した乳状白色分散体のサンプルを遠心分離により3回洗浄し、下澄液を除去し、その後同量の10%蔗糖溶液を加える。得られた分散体を凍結乾燥しそして次に蒸留水中に再分散させ、クールター(Coulter)カウンターを用いて測定して中間直径3.5μmの容積の乳状白色微小気泡分散体を得る。
(c) 純水(20ml)中の水素添加ホスファチジルセリン(100mg)を80℃に5分間加熱し、得られた分散体を0℃に一晩冷却する。該分散体1mlを2mlの小壜に移し、それに200μlの2−メチルブタン(沸点28℃)を加える。該小壜をCapMix(商標)を用いて45秒間振盪して、拡散性成分のエマルションを得、それを使用しない場合は0℃で貯蔵する。
上記(b)で調製したペルフルオロブタンガス分散体のガス含有量2μlに相当する量を含む注射器を、上記(c)で調製した2−メチルブタンエマルションのガス含有量2μlに相当する量を含む注射器と共に調製し、そして内容物を同時にY−型連結器および上足静脈に挿入したカテーテルを使用して犬に注射する。
注射の1分後に、2.7MHzおよび高音響電力で伝送しそして5.4MHzで受信するATL HDI 3000スキャナーを用いて心臓を約10秒走査する。この高エネルギー超音波照射は半安定の遊離ガス微小気泡を発生し、心筋層からの後方散乱の強度を著しく高めることになる。次に出力電力を急速に12dBだけ減少させて12dBの補償利得増加を得、引き続く数回の心臓鼓動についての第2の調波フレームをデジタル貯蔵する。線状スケール上の時間に対する標準化シグナル強度のプロットを図3に示すように作成する。各時間点は5×5画素領域の平均を表す。このプロットは、5秒で始まりそして後方散乱の急速な立ち上がりを生じる高電力超音波照射を示す。流入曲線は出力電力が減少する15秒の時点で始まり、そして約3.5秒の半減期を示し、画像形成領域が不全灌流されたことを示す。流出ハーフタイムの偽着色画像を作成し、第2の調波B−モード画像に重ねる。低灌流の領域は容易に確認されそして不充分な灌流として等級分けされる。 TECHNICAL FIELD OF THE INVENTION The present invention relates to ultrasound imaging, and more particularly to the use of ultrasound imaging to measure tissue perfusion.
Prior art Contrast agents containing a dispersion of gas microbubbles are well known to be particularly efficient backscatterers of ultrasound because the microbubbles are low density and easy to compress. It has been. Such microbubble dispersions, when properly stabilized, can allow for very efficient ultrasound visualization of, for example, vasculature and tissue microvasculature, often at advantageously low doses.
Problems to be solved by the invention The measurement of tissue perfusion involves, for example, tumor detection, typically detection of tumor tissue with blood vessels different from healthy tissue, and blood supply to the myocardium, for example. It is important for myocardial research to evaluate. Contrast agent detection methods using current ultrasound imaging techniques may provide information about whether a particular organ or region thereof has been perfused, but do not facilitate perfusion quantification. Such information is useful for assessing whether a patient is at risk due to hypoperfusion and therefore may be useful for prophylaxis and / or treatment, but now scintigraphy, positron emission Must be obtained using radioisotope imaging techniques such as tomography or single photon emission computed tomography. All such techniques involve injection of radioactive material, potentially posing a safety hazard for both patients and medical personnel, and using expensive imaging equipment. This hinders their spread.
The present invention facilitates the relative velocity of tissue perfusion in any tissue that is sensitive to ultrasound imaging, as ultrasound imaging involving ultrasound-induced destruction or modification of contrast agents can provide a measure of tissue perfusion. And based on the knowledge that it can be measured inexpensively.
Currently, there are limitations to the prior art related to ultrasound imaging including contrast agent destruction. US-A-5425366 states that certain particulate ultrasound contrast agents, such as gas-containing polymer microcapsules, may be used by the color Doppler technique, even though they are essentially less moving as a result of uptake by the reticuloendothelial tissue system. It is stated that it can be visualized. It is proposed that the relatively high amount of irradiation energy associated with the color Doppler test ruptures the microparticles and generates a Doppler-sensitive signal described as “acoustically stimulated acoustic emission”. In practice, however, the examiner is likely to interpret the backscattered signal discontinuity as a result of the movement and produces an appropriate display. It will be appreciated that this technique is inherently inapplicable to measuring the rate of perfusion, as it relates exclusively to the detection of contrast agent microparticles that are essentially stationary.
US-A-5456257 applies a coated microbubble contrast agent in a patient's body by applying ultrasonic pulsed irradiation of an amount of energy sufficient to destroy the coated microbubble and counteracts the microbubble destruction event. The detection is described by confirmation using a reactive detection method (eg, encapsulated detection method) and subsequent fractionation of echoes received from ultrasonic transmission. After the initial transmission, the acoustic energy emitted from the microbubble destruction site is received by an ultrasonic transducer, and the resulting signal waveform is subjected to amplification detection. Echoes received from subsequent transmissions are detected in a similar manner, and signals from the two reception periods are distinguished on a spatial basis. Typically, the signal derived from the second reception period is subtracted from the signal derived from the first reception period to generate a signal exiting the microbubble destruction event, excluding other signals. A threshold may be applied to remove variations resulting from tissue movement and flowing fluid. The signal is processed using a persistence system and / or by counting events within a given area of the body over time, thereby providing an indication of the bulk (net) flow of contrast-containing blood, eg into the ventricle Although not possible, it has not been suggested that this technique can be used to quantify capillary blood flow in tissues and to measure perfusion.
Means for solving the problem The present invention also applies a recognizable amount of contrast agent in the target area, rather than using subsequent pulses to detect the background signal subtracted from the first detection series. The first high energy ultrasound pulse or series of pulses is used to disrupt or alter the appreciably, and the present invention uses a “new” or unaltered contrast agent flow to the target area (and thus blood flow). Subsequent pulses are used to detect (flow). This allows the determination of parameters such as vascular blood volume fraction, average transit time and tissue perfusion for local vascular conditions within the target area. Additional contrast that is easily detectable and quantifiable by ultrasound imaging, since the initial high energy pulse can be used, for example, to reveal a well-defined target region of detectable contrast agent The sharp front of the agent then flows into this area. Due to the ability to generate a sharp front of the moving contrast agent within the target area of interest, the method is substantially advantageous over previous attempts to estimate the rate at which contrast agent flows into the tissue immediately after injection of the contrast agent. Method. This is because the injected contrast agent front is inevitably smoothed or smeared by passing through the lungs and heart. Alternatively, the initial pulse can be used to alter the echogenicity of the contrast agent, for example by activating it in the form of a precursor, causing an increase in echogenicity in the target area. The time course of echogenic changes during and after ultrasound exposure will provide information about local vascular conditions, such as local blood volume and perfusion. For example, the flow rate of contrast agent activated by ultrasound exposure can be determined and thus used to create a perfusion map.
Thus, according to one aspect of the present invention, there is provided a method for measuring tissue perfusion in a human or non-human animal subject comprising administering an effective amount of an ultrasound contrast agent to the subject, Tissue is irradiated with at least one pulse of ultrasound having sufficient energy to disrupt or distinguishably change the echogenicity of a recognizable amount of contrast agent in the target area and enters the target area Detecting and quantifying the flow rate of additional contrast agent or altered contrast agent exiting the target area with ultrasound.
Viewed from another aspect, the present invention provides the use of an ultrasound contrast agent in the manufacture of a diagnostic material for use in a method of measuring tissue perfusion in a human or non-human animal subject, said method comprising ultrasound contrast imaging. An effective amount of an agent is administered to the subject, and the target region tissue has sufficient energy to disrupt or distinguishably change the echogenicity of a recognizable amount of contrast agent in the target region. Irradiating at least one pulse of sound waves and detecting and quantifying the flow rate of additional contrast agent entering or exiting the target area with ultrasound.
According to the present invention, a wide range of ultrasound contrast agents can be used, and the most commonly used contrast agents will be gas-containing or gasogenic. Representative examples of such contrast agents include anti-aggregation surface films (eg gelatin as described in WO-A-8002365), filmogenic proteins (eg US-A-4718433, US-A-4774958). , US-A-4844882, EP-A-0359246, WO-A-9112823, WO-A-9205806, WO-A-9217213, WO-A-9406477 or WO-A-9501187, for example Albumin such as human serum albumin), polymeric materials (eg synthetic biodegradable polymers described in EP-A-0398935, elastic interfacial synthetic polymer membranes described in EP-A-0458745, described in EP-A-0441468 Fine particulate biodegradable polyaldehyde, polyamino acid-polycyclic imide fine particulate N-dicarboxylic acid derivative described in EP-A-0458079, or WO-A-9317718 or WO-A-9607434 Biodegradable polymers), non-polymers and non-polymerizable wall-forming materials (eg those described in WO-A-9521631), or surfaces Activators (eg polyoxyethylene-polyoxypropylene block copolymer surfactants such as Pluronic, polymer surfactants described in WO-A-9506518, eg WO-A-9211873, WO-A-9217212, WO -A-9222247, WO-A-9428780, WO-A-9503835, WO-A-9640275 or WO-A-9729783 film-forming surfactants such as phospholipids) For example, gas microbubbles that are at least partially enclosed) are included.
Other useful gas-containing contrast agents include gas-containing solid systems, such as those containing or bound to gas (eg EP-A-0122624, EP-A-123235, EP-A-0365467, WO- A-9221382, WO-A-9300930, WO-A-9313802, WO-A-9313808 or WO-A-9313809, for example, gas adsorbed on the surface and / or gas is voids, cavities or pores Fine particles (particularly aggregates of fine particles).
Multi-component contrast agent formulations including, for example, a dispersed gas phase-containing composition and a composition comprising a volatile component that can be transferred to the dispersed gas phase in vivo, for example, by diffusion, would also be useful. Such contrast agents are described in the specification of the applicant's unpublished international patent application No. PCT / GB97 / 02898. They can be produced in the form of precursors if desired, and the contrast agents show significant echogenic properties only after activation by high energy sonication.
The entire disclosures of the above documents relating to gas-containing contrast agents are included in this application for reference.
Phospholipid-containing compositions are used according to the present invention, for example in the form of phospholipid-stabilized gas microbubbles, but representative examples of useful phospholipids include lecithin (ie, phosphatidylcholine) such as egg yolk lecithin or soy lecithin Natural lecithins, and synthetic or semi-synthetic lecithins such as dimyristoylphosphatidylcholine, dipalmitoylphosphatidylcholine or distearoylphosphatidylcholine; phosphatidic acid; phosphatidylethanolamine; phosphatidylserine; phosphatidylglycerol; phosphatidylinositol; Any of the foregoing fluorinated analogs; and mixtures of any of the foregoing with other lipids such as cholesterol. Phospholipids, such as natural (eg, soy or egg yolk derived), semi-synthetic (eg, partially synthetic) (eg, partially derived), each containing predominantly (eg, at least 75%) molecules bearing a net overall charge, eg, negative charge The use of (or fully hydrogenated) and synthetic phosphatidylserine, phosphatidylglycerol, phosphatidylinositol, phosphatidic acid and / or cardiolipin will be particularly advantageous.
Any biocompatible gas may be present in the microbubbles according to the present invention. The term “gas” as used herein includes any substance (including mixtures) that is substantially or completely in the form of a gas (including vapor) at a normal human body temperature of 37 ° C. The gas is therefore for example air; nitrogen; oxygen; carbon dioxide; hydrogen; an inert gas such as helium, argon, xenon or krypton; sulfahexafluoride, disulfadecafluoride or trifluoromethylsulfapentafluoride. Sulfur fluorides such as; selenium hexafluoride; optionally hydrogenated silanes such as methylsilane or dimethylsilane; low molecular weight hydrocarbons (eg containing up to 7 carbon atoms) such as methane, ethane, propane, butane Or alkanes such as pentane, cycloalkanes such as cyclopropane, cyclobutane or cyclopentane, alkenes such as ethylene, propene, propadiene or butene, alkynes such as acetylene or propyne; ethers such as dimethyl ether; ketones; Ether; (including, for example, up to 7 carbon atoms) a halogenated low molecular weight hydrocarbon; or mixtures of any of the included. Advantageously, at least some of the halogen atoms in the halogenated gas are fluorine atoms; therefore biocompatible halogenated hydrocarbon gases are, for example, bromochlorodifluoromethane, chlorodifluoromethane, dichlorodifluoromethane, bromotrifluoromethane. Chlorotrifluoromethane, chloropentafluoroethane, dichlorotetrafluoroethane, chlorotrifluoroethylene, fluoroethylene, ethyl fluoride, 1,1-difluoroethane and perfluorocarbons such as perfluoromethane, perfluoroethane, perfluoropropane, perfluorobutane (e.g. Perfluoro-n-butane optionally mixed with isomers such as perfluoro-iso-butane), perfluoropentane, perfluorohexane and perflurane Perfluoroalkanes such as loheptane; perfluoroalkenes such as perfluoropropene, perfluorobutene (eg perfluorobut-2-ene) and perfluorobutadiene; perfluoroalkynes such as perfluorobut-2-yne; and perfluorocyclobutane, perfluoromethylcyclobutane , Perfluorodimethylcyclobutane, perfluorotrimethylcyclobutane, perfluorocyclopentane, perfluoromethylcyclopentane, perfluorodimethylcyclopentane, perfluorocyclohexane, perfluoromethylcyclohexane and perfluorocycloalkanes such as perfluorocycloheptane. Other halogenated gases include methyl chloride, fluorinated (eg, perfluorinated) ketones such as perfluoroacetone, and fluorinated (eg, perfluorinated) ethers such as perfluorodiethyl ether. The use of perfluorinated gases, such as sulfahexafluoride and perfluoropropanes such as perfluoropropane, perfluorobutane and perfluoropentane, is particularly advantageous because of the high stability observed in the flow of microbubbles containing such gases. Will.
The initial high-energy ultrasonic pulse may e.g. destroy the gas-containing contrast agent, e.g. by destroying a stabilizing surface film such as a protein, polymer or film-forming surfactant and / or around the gas-containing material Can be caused by promoting dissolution of tissue into tissue fluid. However, it is not necessary to completely destroy the echogenic nature of the contrast agent in the target area. It is sufficient to change the “acoustic signature” so that it can be ultrasonically distinguished from the “new” contrast agent that subsequently flows in. Thus, for example, partial or complete ultrasound-induced collapse of an encapsulating shell material generates gas microbubbles that vibrate more freely than contrast agent encapsulated gas, and have been modified to distinguish acoustic properties. Can be shown. Alternatively, the initial sonication can cause other echogenic changes to parameters such as the composition, size and / or mechanical properties of the contrast agent portion. This can be used, for example, to guide activation of a contrast agent in the form of a precursor.
If desired, the contrast agent can be designed to be particularly sensitive to disruption by the initial ultrasound pulse, thereby limiting the intensity required for the initial ultrasound irradiation. This can be achieved, for example, by using a monolayer of stabilizing amphiphilic lipid material; charged amphiphilic lipid materials, such as negatively charged phospholipids, can be electronically repelled between charged lipid membranes. As a result, the formation of a single layer can be encouraged.
A variety of ultrasound imaging techniques can be used to detect and quantify another contrast agent that flows after the initial ultrasound exposure, e.g., to determine the amount of time related contrast agent that flows into the target area. Generate perfusion related images to be displayed, thereby allowing discrimination between different perfusion regions. Desired images are obtained from individual scan line analysis or from frame analysis on a frame basis; the former is a region of high perfusion rate to obtain a sufficient number of samples to distinguish regions with different perfusions. The latter may be preferred in areas where the perfusion rate is low.
Imaging modes that may be used include B-mode and Doppler based imaging, for example pulsed wave Doppler techniques such as power Doppler imaging. Harmonics (for example, 2, 3, 4 ... times the imaging frequency), eg as described in US-A-5410516 (for example, 1/2, 1/3, 2/3 of the imaging frequency) , 3/4 ...), subharmonic (eg, 3/2, 5/4 ... times the imaging frequency), and (eg, derived from such harmonics and imaging frequency) Non-linear imaging techniques based on the effects of frequency sum or difference are optionally combined with the frequency sum produced by two incident ultrasound signals of different frequencies, as described, for example, in published US patent application Ser. No. 08 / 440,266. Alternatively, it can be used like a technique based on difference detection. The second harmonic imaging may be particularly advantageous. For example, a combination of the above techniques in second harmonic power Doppler imaging would also be useful. In general, the resulting image can be displayed, for example, as a color map representing the perfusion rate, and optionally overlaid on a conventional B-mode image of the target area.
The method of the present invention provides the energy level of the initial ultrasound pulse (and thus the size of the investigated area containing the destroyed or altered contrast agent) and the subsequent ultrasound imaging parameters, particularly the frame rate or individual pulse By appropriately selecting the time between, the desired perfusion rate can be tailored and, where appropriate, the ECG-trigger method can be used. If desired, the individual results may be spatially averaged, for example in a manner known per se.
The method also allows measurement of perfusion rates below the detection limits of conventional Doppler techniques, and is also used to estimate the relative perfusion rate in the myocardium where tissue movement renders traditional Doppler imaging ineffective. obtain. Imaging of the myocardium is advantageously performed after a sufficient amount of time has elapsed since the contrast agent was injected at its peak concentration to pass through the left ventricle, so that undesired contrast-containing left ventricular blood Is weakened so that the flow rate of the contrast agent into the myocardial tissue reaches an appropriate steady state. The essentially random flow pattern exhibited by blood and contrast agents at the capillary level is such that when the method of the present invention is used at this level, the dominant flow pattern is substantially perpendicular to the scanning system and thus relatively Avoid the possibility of anomalous results that can become undetectable. The method also has the advantage that the result is substantially independent of backscatter from the myocardium itself. This level of backscattering results in different echogenicity of various regions of the myocardium and different attenuation of adjacent tissues, fluids, etc. between such regions and the ultrasonic transducer, resulting in This is beneficial because it can vary considerably in different areas. Furthermore, the results obtained by the method are not affected by the dose of contrast agent administered, provided that the initial ultrasound exposure is sufficient to change or destroy the echogenic effects of the contrast agent. .
The following non-limiting examples illustrate the invention.
[Brief description of the drawings]
In the accompanying drawings, FIGS. 1-3 are standardized time-intensity curves respectively obtained according to the procedure of Example 1-3. Intensity is expressed in linear units (LU).
Example 1
An anesthetized dog is injected intravenously with 2 ml of an aqueous suspension of a gas-containing particulate contrast agent described in WO-A-9317718. One minute after injection, the heart is scanned for approximately 10 seconds using a
Example 2
The left coronary artery of an anesthetized dog was partially closed to reduce coronary blood flow to a region of the myocardium, thereby causing symptoms of coronary artery stenosis. The dog was then intravenously injected with 2 ml of an aqueous suspension of gas-containing particulate contrast agent described in WO-A-9317718. One minute after injection, the heart was scanned for about 10 seconds using an HDI 3000 scanner with a P5-3 transducer transmitting at 2.7 MHz and medium acoustic power, destroying a significant fraction of contrast in the imaged fragment. . Set the ECG-trigger mode to receive the scanner rapidly at 45.4MHz (ie second harmonic) and store the end-systolic frame for several heartbeats digitally did. As a result of the reduction in overall ultrasound exposure, minimal contrast agent disruption occurred during operation in ECG-trigger mode, so the inflow curve showing the inflow of new contrast agent is from the second harmonic signal intensity. Easily led. FIG. 2 shows a plot of normalized signal intensity against time on the resulting linear scale, where each point represents the average of a 5 × 5 pixel area. White circles show results from the hypoperfused area and black circles are taken from the normally perfused area. The zero on the time axis corresponds to the time when the ECG-trigger starts. The drawn line shows the minimum mean square fit for these two data sets. The solid line represents the curve for the normally perfused area and shows a half peak time of about 0.7 seconds. The dashed line represents the curve for the hypoperfusion region and shows a half peak time of about 6 seconds, ie about 8 times the normal time. A false colored image representing the inflow half time was created and overlaid on the B-mode image.
Example 3
(A) Hydrogenated phosphatidylserine (100 mg) in a 2% solution of propylene glycol in pure water (20 ml) is heated to 80 ° C. for 5 minutes and the resulting dispersion is allowed to cool to room temperature overnight.
(B) A sample of the milky white dispersion prepared in (a) above is washed three times by centrifugation, the supernatant is removed, and then the same amount of 10% sucrose solution is added. The resulting dispersion is lyophilized and then redispersed in distilled water to give a milky white microbubble dispersion having a volume of 3.5 μm in median diameter as measured using a Coulter counter.
(C) Hydrogenated phosphatidylserine (100 mg) in pure water (20 ml) is heated to 80 ° C. for 5 minutes and the resulting dispersion is cooled to 0 ° C. overnight. 1 ml of the dispersion is transferred to a 2 ml kettle and 200 μl of 2-methylbutane (boiling point 28 ° C.) is added to it. Shake the gavel with CapMix ™ for 45 seconds to obtain an emulsion of diffusible ingredients, which is stored at 0 ° C. when not in use.
A syringe containing an amount corresponding to 2 μl of the gas content of the perfluorobutane gas dispersion prepared in (b) above and a syringe containing an amount corresponding to 2 μl of the gas content of the 2-methylbutane emulsion prepared in (c) above Prepare and inject the contents into the dog using a Y-connector and a catheter inserted into the superior paw vein at the same time.
One minute after injection, the heart is scanned for approximately 10 seconds using an ATL HDI 3000 scanner that transmits at 2.7 MHz and high acoustic power and receives at 5.4 MHz. This high-energy ultrasonic irradiation generates semi-stable free gas microbubbles and significantly increases the intensity of backscattering from the myocardium. The output power is then rapidly reduced by 12 dB to obtain a 12 dB increase in compensation gain, and a second harmonic frame for several subsequent heart beats is digitally stored. A plot of normalized signal intensity against time on a linear scale is generated as shown in FIG. Each time point represents the average of a 5 × 5 pixel area. This plot shows high power sonication starting at 5 seconds and producing a rapid rise of backscatter. The inflow curve begins at 15 seconds when the output power decreases and shows a half-life of about 3.5 seconds, indicating that the imaging area has been perfused. An outflow half-time pseudo-colored image is created and overlaid on the second harmonic B-mode image. The area of hypoperfusion is easily identified and graded as inadequate perfusion.
Claims (13)
Applications Claiming Priority (4)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| GBGB9708246.5A GB9708246D0 (en) | 1997-04-24 | 1997-04-24 | Improvements in or relating to ultrasound imaging |
| GB9708246.5 | 1997-04-24 | ||
| US4440897P | 1997-04-29 | 1997-04-29 | |
| PCT/GB1998/001217 WO1998047533A1 (en) | 1997-04-24 | 1998-04-24 | Ultrasound imaging of tissue perfusion by pulse energy disruption of contrast agent |
Publications (3)
| Publication Number | Publication Date |
|---|---|
| JP2001523997A JP2001523997A (en) | 2001-11-27 |
| JP2001523997A5 JP2001523997A5 (en) | 2005-12-02 |
| JP4182191B2 true JP4182191B2 (en) | 2008-11-19 |
Family
ID=26311434
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| JP54530998A Expired - Fee Related JP4182191B2 (en) | 1997-04-24 | 1998-04-24 | Ultrasound imaging of tissue perfusion by contrast energy pulse energy interference |
Country Status (10)
| Country | Link |
|---|---|
| US (1) | US6315730B1 (en) |
| EP (1) | EP0977594B1 (en) |
| JP (1) | JP4182191B2 (en) |
| AT (1) | ATE332707T1 (en) |
| AU (1) | AU7219098A (en) |
| DE (1) | DE69835202T2 (en) |
| ES (1) | ES2268770T3 (en) |
| GB (1) | GB9708246D0 (en) |
| NO (1) | NO995148L (en) |
| WO (1) | WO1998047533A1 (en) |
Families Citing this family (33)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO1998053855A1 (en) * | 1997-05-30 | 1998-12-03 | Alliance Pharmaceutical Corp. | Methods and apparatus for monitoring and quantifying the movement of fluid |
| US6553327B2 (en) * | 1998-09-16 | 2003-04-22 | Yeda Research & Development Co., Ltd. | Apparatus for monitoring a system with time in space and method therefor |
| US6353803B1 (en) * | 1996-01-18 | 2002-03-05 | Yeda Research And Development Co., Ltd. At The Welzmann Institute Of Science | Apparatus for monitoring a system in which a fluid flows |
| JP4473981B2 (en) * | 1999-07-21 | 2010-06-02 | 株式会社日立メディコ | Ultrasonic diagnostic equipment |
| US6436045B1 (en) | 2000-04-27 | 2002-08-20 | Koninklijke Phillips Electronics N.V. | User controlled destructive waveform routine for ultrasound systems |
| IL155527A0 (en) * | 2000-10-25 | 2003-11-23 | Robarts John P Res Inst | Method and apparatus for calculating blood flow parameters |
| WO2002056666A2 (en) * | 2001-01-19 | 2002-07-25 | Angelsen Bjoern A J | A method of detecting ultrasound contrast agent in soft tissue, and quantitating blood perfusion through regions of tissue |
| JP2002209898A (en) * | 2001-01-22 | 2002-07-30 | Toshiba Corp | Ultrasonic diagnostic device |
| JP2003061959A (en) * | 2001-08-22 | 2003-03-04 | Toshiba Corp | Ultrasonic diagnostic apparatus |
| US6730036B2 (en) * | 2002-02-28 | 2004-05-04 | Koninklijke Philips Electronics, N.V. | Ultrasonic imaging to detect coronary artery stenosis at rest |
| AUPS214502A0 (en) * | 2002-05-06 | 2002-06-06 | Uscom Pty Ltd | Blood flow oxygen measurement |
| AU2003227122B2 (en) * | 2002-05-06 | 2008-05-01 | Uscom Limited | Blood flow oxygen measurement system and method |
| AUPS335502A0 (en) * | 2002-07-03 | 2002-07-25 | Uscom Pty Ltd | Pacemaker evaluation method and apparatus |
| WO2004049950A1 (en) | 2002-11-29 | 2004-06-17 | Amersham Health As | Ultrasound triggering method |
| JP3683886B2 (en) * | 2002-12-27 | 2005-08-17 | 株式会社ワイディ | Blood volume analysis and display method using Myo Cardial Blood volume map |
| AU2003900261A0 (en) * | 2003-01-22 | 2003-02-06 | Uscom Pty Ltd | Method and system for the determination of blood characteristics |
| US8021303B2 (en) * | 2003-06-12 | 2011-09-20 | Bracco Research Sa | System for extracting morphological information through a perfusion assessment process |
| WO2004110279A1 (en) * | 2003-06-12 | 2004-12-23 | Bracco Research Sa | Blood flow estimates through replenishment curve fitting in ultrasound contrast imaging |
| US7252638B2 (en) * | 2003-06-23 | 2007-08-07 | Siemens Medical Solutions Usa, Inc. | Method and system for simultaneously displaying relationships of measurements of features associated with a medical image |
| WO2005020820A1 (en) * | 2003-08-28 | 2005-03-10 | University Of Bern | Method and system for determining the absolute perfusion rate of a fluid in an analysis region |
| JP2005081073A (en) * | 2003-09-11 | 2005-03-31 | Toshiba Corp | Ultrasonic diagnostic device |
| US7658714B2 (en) * | 2003-10-31 | 2010-02-09 | Siemens Medical Solutions Usa, Inc. | Intelligent ultrasound examination storage system |
| US8047993B2 (en) * | 2004-12-08 | 2011-11-01 | Industrial Technology Research Institute | Quantitative non-invasive method for detecting degree of malignancy in tumors and application thereof |
| JP5308674B2 (en) * | 2004-12-23 | 2013-10-09 | ブラッコ・シュイス・ソシエテ・アノニム | Perfusion assessment method and system based on bolus administration |
| US7415164B2 (en) * | 2005-01-05 | 2008-08-19 | Mitsubishi Electric Research Laboratories, Inc. | Modeling scenes in videos using spectral similarity |
| WO2007070827A2 (en) * | 2005-12-15 | 2007-06-21 | Bristol-Myers Squibb Pharma Company | Contrast agents for myocardium perfusion imaging |
| DE102006057211B3 (en) * | 2006-12-01 | 2008-03-27 | Kompetenzzentrum Medizintechnik Ruhr (Kmr) E.V. | Object e.g. biological tissue, infusion measuring method, involves determining re-enrichment conditions in time period, in which no tomogram series is received, by mathematical approximation technique |
| JP2008245891A (en) * | 2007-03-30 | 2008-10-16 | Ge Medical Systems Global Technology Co Llc | Ultrasonic contrast radiography and ultrasonic diagnostic equipment |
| US20100132731A1 (en) * | 2008-12-01 | 2010-06-03 | Matthew Waitesmith | Ergonomic Cosmetic Brush |
| US8192364B2 (en) * | 2009-06-10 | 2012-06-05 | Mayo Foundation For Medical Education And Research | Method for assessing vascular disease by quantitatively measuring vaso vasorum |
| US20110144495A1 (en) * | 2009-12-14 | 2011-06-16 | Siemens Medical Solutions Usa, Inc. | Perfusion Imaging of a Volume in Medical Diagnostic Ultrasound |
| DE102014103884A1 (en) | 2014-03-21 | 2015-09-24 | Endress + Hauser Flowtec Ag | Ultrasonic transducer and ultrasonic flowmeter |
| EP3638126A4 (en) * | 2017-05-04 | 2021-03-10 | Gynesonics, Inc. | Methods for monitoring ablation progress with doppler ultrasound |
Family Cites Families (21)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US4276885A (en) | 1979-05-04 | 1981-07-07 | Rasor Associates, Inc | Ultrasonic image enhancement |
| US4718433A (en) | 1983-01-27 | 1988-01-12 | Feinstein Steven B | Contrast agents for ultrasonic imaging |
| US5040537A (en) * | 1987-11-24 | 1991-08-20 | Hitachi, Ltd. | Method and apparatus for the measurement and medical treatment using an ultrasonic wave |
| US4844882A (en) | 1987-12-29 | 1989-07-04 | Molecular Biosystems, Inc. | Concentrated stabilized microbubble-type ultrasonic imaging agent |
| US4957656A (en) | 1988-09-14 | 1990-09-18 | Molecular Biosystems, Inc. | Continuous sonication method for preparing protein encapsulated microbubbles |
| GB9003821D0 (en) | 1990-02-20 | 1990-04-18 | Danbiosyst Uk | Diagnostic aid |
| US5556610A (en) * | 1992-01-24 | 1996-09-17 | Bracco Research S.A. | Gas mixtures useful as ultrasound contrast media, contrast agents containing the media and method |
| EP0504340B1 (en) | 1990-10-05 | 1995-06-21 | BRACCO International B.V. | Method for the preparation of stable suspensions of hollow gas-filled microspheres suitable for ultrasonic echography |
| GB9106686D0 (en) | 1991-03-28 | 1991-05-15 | Hafslund Nycomed As | Improvements in or relating to contrast agents |
| US5235984A (en) * | 1992-03-30 | 1993-08-17 | Hewlett-Packard Company | On-line acoustic densitometry tool for use with an ultrasonic imaging system |
| US5678553A (en) * | 1994-11-01 | 1997-10-21 | Schering Aktiengesellschaft | Ultrasonic processes and circuits for carrying out those processes |
| US5456257A (en) * | 1994-11-23 | 1995-10-10 | Advanced Technology Laboratories, Inc. | Ultrasonic detection of contrast agents |
| US5560364A (en) * | 1995-05-12 | 1996-10-01 | The Board Of Regents Of The University Of Nebraska | Suspended ultra-sound induced microbubble cavitation imaging |
| US5601086A (en) * | 1995-05-12 | 1997-02-11 | The United States Of America As Represented By The Administrator Of The National Aeronautics And Space Administration | Beat frequency ultrasonic microsphere contrast agent detection system |
| US5833613A (en) * | 1996-09-27 | 1998-11-10 | Advanced Technology Laboratories, Inc. | Ultrasonic diagnostic imaging with contrast agents |
| EP0770352B1 (en) * | 1995-10-10 | 2004-12-29 | Advanced Technology Laboratories, Inc. | Ultrasonic diagnostic imaging with contrast agents |
| JP3634869B2 (en) * | 1996-08-02 | 2005-03-30 | アメルシャム ヘルス アクスイェ セルスカプ | Improvements in or relating to contrast media |
| US5735281A (en) * | 1996-08-09 | 1998-04-07 | Hewlett-Packard Company | Method of enhancing and prolonging the effect of ultrasound contrast agents |
| CA2263568C (en) * | 1996-09-11 | 2008-12-02 | Imarx Pharmaceutical Corp. | Methods for diagnostic imaging using a contrast agent and a renal vasodilator |
| JP2001502349A (en) * | 1996-10-21 | 2001-02-20 | ニユコメド・イメージング・アクシエセルカペト | Improvements in or related to contrast agents |
| WO1998018501A2 (en) * | 1996-10-28 | 1998-05-07 | Marsden, John, Christopher | Improvements in or relating to diagnostic/therapeutic agents |
-
1997
- 1997-04-24 GB GBGB9708246.5A patent/GB9708246D0/en active Pending
-
1998
- 1998-04-24 AU AU72190/98A patent/AU7219098A/en not_active Abandoned
- 1998-04-24 AT AT98919310T patent/ATE332707T1/en not_active IP Right Cessation
- 1998-04-24 JP JP54530998A patent/JP4182191B2/en not_active Expired - Fee Related
- 1998-04-24 ES ES98919310T patent/ES2268770T3/en not_active Expired - Lifetime
- 1998-04-24 WO PCT/GB1998/001217 patent/WO1998047533A1/en active IP Right Grant
- 1998-04-24 EP EP98919310A patent/EP0977594B1/en not_active Expired - Lifetime
- 1998-04-24 DE DE69835202T patent/DE69835202T2/en not_active Expired - Lifetime
-
1999
- 1999-10-22 NO NO995148A patent/NO995148L/en not_active Application Discontinuation
- 1999-10-25 US US09/425,290 patent/US6315730B1/en not_active Expired - Lifetime
Also Published As
| Publication number | Publication date |
|---|---|
| NO995148D0 (en) | 1999-10-22 |
| ES2268770T3 (en) | 2007-03-16 |
| AU7219098A (en) | 1998-11-13 |
| GB9708246D0 (en) | 1997-06-18 |
| US6315730B1 (en) | 2001-11-13 |
| WO1998047533A1 (en) | 1998-10-29 |
| DE69835202D1 (en) | 2006-08-24 |
| DE69835202T2 (en) | 2007-05-31 |
| JP2001523997A (en) | 2001-11-27 |
| EP0977594B1 (en) | 2006-07-12 |
| NO995148L (en) | 1999-10-22 |
| EP0977594A1 (en) | 2000-02-09 |
| ATE332707T1 (en) | 2006-08-15 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| JP4182191B2 (en) | Ultrasound imaging of tissue perfusion by contrast energy pulse energy interference | |
| CA2575677C (en) | Gas-filled microvesicles composition for contrast imaging | |
| AU726503B2 (en) | Improvements in or relating to contrast agents | |
| US6802813B2 (en) | Methods and apparatus for monitoring and quantifying the movement of fluid | |
| US7892522B2 (en) | Contrast agents | |
| WO2007027584A2 (en) | Deposit contrast agents and related methods thereof | |
| US6409671B1 (en) | Ultrasonography | |
| Gottlieb et al. | Effect of pressure on echocardiographic videodensity from sonicated albumin: an in vitro model. | |
| US20040052728A1 (en) | Diagnostic imaging | |
| US20010021371A1 (en) | Improvements in or relating to cardiac imaging | |
| Unger et al. | Gas fIIIed lipid bilayers as imaging contrast agents | |
| AU784367B2 (en) | Improvements in or relating to contrast agents |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20050414 |
|
| A621 | Written request for application examination |
Free format text: JAPANESE INTERMEDIATE CODE: A621 Effective date: 20050414 |
|
| A131 | Notification of reasons for refusal |
Free format text: JAPANESE INTERMEDIATE CODE: A131 Effective date: 20080311 |
|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20080603 |
|
| TRDD | Decision of grant or rejection written | ||
| A01 | Written decision to grant a patent or to grant a registration (utility model) |
Free format text: JAPANESE INTERMEDIATE CODE: A01 Effective date: 20080729 |
|
| A01 | Written decision to grant a patent or to grant a registration (utility model) |
Free format text: JAPANESE INTERMEDIATE CODE: A01 |
|
| A61 | First payment of annual fees (during grant procedure) |
Free format text: JAPANESE INTERMEDIATE CODE: A61 Effective date: 20080807 |
|
| A72 | Notification of change in name of applicant |
Free format text: JAPANESE INTERMEDIATE CODE: A721 Effective date: 20080807 |
|
| FPAY | Renewal fee payment (event date is renewal date of database) |
Free format text: PAYMENT UNTIL: 20110912 Year of fee payment: 3 |
|
| R150 | Certificate of patent or registration of utility model |
Free format text: JAPANESE INTERMEDIATE CODE: R150 |
|
| FPAY | Renewal fee payment (event date is renewal date of database) |
Free format text: PAYMENT UNTIL: 20110912 Year of fee payment: 3 |
|
| FPAY | Renewal fee payment (event date is renewal date of database) |
Free format text: PAYMENT UNTIL: 20120912 Year of fee payment: 4 |
|
| FPAY | Renewal fee payment (event date is renewal date of database) |
Free format text: PAYMENT UNTIL: 20130912 Year of fee payment: 5 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| LAPS | Cancellation because of no payment of annual fees |