FR2831055A1 - Oxidation dye composition, useful for dyeing keratinic fibers, comprises 1-(2-hydroxyethyl)-4-hydroxyindole as a coupler and a heterocyclic developer - Google Patents
Oxidation dye composition, useful for dyeing keratinic fibers, comprises 1-(2-hydroxyethyl)-4-hydroxyindole as a coupler and a heterocyclic developer Download PDFInfo
- Publication number
- FR2831055A1 FR2831055A1 FR0113764A FR0113764A FR2831055A1 FR 2831055 A1 FR2831055 A1 FR 2831055A1 FR 0113764 A FR0113764 A FR 0113764A FR 0113764 A FR0113764 A FR 0113764A FR 2831055 A1 FR2831055 A1 FR 2831055A1
- Authority
- FR
- France
- Prior art keywords
- amino
- radical
- pyrazolo
- sep
- hydroxyethyl
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Granted
Links
Classifications
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61Q—SPECIFIC USE OF COSMETICS OR SIMILAR TOILETRY PREPARATIONS
- A61Q5/00—Preparations for care of the hair
- A61Q5/10—Preparations for permanently dyeing the hair
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K8/00—Cosmetics or similar toiletry preparations
- A61K8/18—Cosmetics or similar toiletry preparations characterised by the composition
- A61K8/30—Cosmetics or similar toiletry preparations characterised by the composition containing organic compounds
- A61K8/49—Cosmetics or similar toiletry preparations characterised by the composition containing organic compounds containing heterocyclic compounds
- A61K8/4906—Cosmetics or similar toiletry preparations characterised by the composition containing organic compounds containing heterocyclic compounds with one nitrogen as the only hetero atom
- A61K8/4913—Cosmetics or similar toiletry preparations characterised by the composition containing organic compounds containing heterocyclic compounds with one nitrogen as the only hetero atom having five membered rings, e.g. pyrrolidone carboxylic acid
- A61K8/492—Cosmetics or similar toiletry preparations characterised by the composition containing organic compounds containing heterocyclic compounds with one nitrogen as the only hetero atom having five membered rings, e.g. pyrrolidone carboxylic acid having condensed rings, e.g. indol
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K8/00—Cosmetics or similar toiletry preparations
- A61K8/18—Cosmetics or similar toiletry preparations characterised by the composition
- A61K8/30—Cosmetics or similar toiletry preparations characterised by the composition containing organic compounds
- A61K8/49—Cosmetics or similar toiletry preparations characterised by the composition containing organic compounds containing heterocyclic compounds
- A61K8/494—Cosmetics or similar toiletry preparations characterised by the composition containing organic compounds containing heterocyclic compounds with more than one nitrogen as the only hetero atom
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K8/00—Cosmetics or similar toiletry preparations
- A61K8/18—Cosmetics or similar toiletry preparations characterised by the composition
- A61K8/30—Cosmetics or similar toiletry preparations characterised by the composition containing organic compounds
- A61K8/49—Cosmetics or similar toiletry preparations characterised by the composition containing organic compounds containing heterocyclic compounds
- A61K8/494—Cosmetics or similar toiletry preparations characterised by the composition containing organic compounds containing heterocyclic compounds with more than one nitrogen as the only hetero atom
- A61K8/4953—Cosmetics or similar toiletry preparations characterised by the composition containing organic compounds containing heterocyclic compounds with more than one nitrogen as the only hetero atom containing pyrimidine ring derivatives, e.g. minoxidil
Abstract
Description
<Desc/Clms Page number 1> <Desc / Clms Page number 1>
COMPOSITION TINCTORIALE COMPRENANT AU MOINS UNE BASE HETEROCYCLIQUE ET LE t-N- (p-HYDROXYETHYL) 4-HYDROXY INDOLE A TITRE DE COUPLEUR ; PROCEDES DE TEINTURE L'invention a pour objet une composition tinctoriale comprenant dans un milieu approprié pour la teinture : - au moins une base hétérocyclique choisie parmi : (i) le 4,5-diamino 1- (2'hydroxyéthyl) pyrazole et ses sels d'addition ; (ii) les paraphénylènediamines à groupement amino cyclique et leurs sels d'addition ; (iii) les pyrazolo-[1, 5-a]-pyrimidines et leurs sels d'addition ; - au moins ! e t-N- (p-hydroxyéthyi) 4-hydroxy indole et ses sels d'addition comme coupleur. TINCTORIAL COMPOSITION COMPRISING AT LEAST ONE HETEROCYCLIC BASE AND T-N- (p-HYDROXYETHYL) 4-HYDROXY INDOLE AS A COUPLER; The subject of the invention is a dyeing composition comprising, in a medium which is suitable for dyeing: at least one heterocyclic base chosen from: (i) 4,5-diamino-1- (2-hydroxyethyl) pyrazole and its salts addition; (ii) cyclic amino paraphenylenediamines and their addition salts; (iii) pyrazolo [1,5-a] pyrimidines and their addition salts; - at least ! t-N- (p-hydroxyethyl) 4-hydroxyindole and its addition salts as coupler.
L'invention a aussi pour objet l'utilisation de cette composition pour la teinture des fibres kératiniques ainsi que le procédé de teinture mettant en oeuvre cette composition. The subject of the invention is also the use of this composition for dyeing keratinous fibers as well as the dyeing process using this composition.
Il est connu de teindre les fibres kératiniques et en particulier les cheveux humains avec des compositions tinctoriales contenant des précurseurs de colorant d'oxydation, appelés généralement bases d'oxydation, tels que des ortho ou paraphénylènediamines, des ortho ou paraaminophénols et des composés hétérocycliques. Ces bases d'oxydation sont des composés incolores ou faiblement colorés qui, associés à des produits oxydants, peuvent donner naissance par un processus de condensation oxydative à des composés colorés. It is known to dye keratinous fibers and in particular human hair with dye compositions containing oxidation dye precursors, generally known as oxidation bases, such as ortho or para-phenylenediamines, ortho or para-aminophenols and heterocyclic compounds. These oxidation bases are colorless or weakly colored compounds which, when combined with oxidizing products, can give rise to colored compounds by a process of oxidative condensation.
On sait également que l'on peut faire varier les nuances obtenues avec ces bases d'oxydation en les associant à des coupleurs ou modificateurs de coloration, ces derniers étant choisis notamment parmi les métadiamines aromatiques, les métaaminophénols, les métadiphénols et certains composés hétérocycliques tels que des composés indoliques. It is also known that the shades obtained with these oxidation bases can be varied by combining them with couplers or color modifiers, the latter being chosen in particular from aromatic meta-diamines, meta-aminophenols, meta-diphenols and certain heterocyclic compounds such as than indolic compounds.
<Desc/Clms Page number 2> <Desc / Clms Page number 2>
La variété des molécules mises en jeu au niveau des bases d'oxydation et des coupleurs, permet l'obtention d'une riche palette de couleurs. The variety of molecules involved in oxidation bases and couplers, allows to obtain a rich palette of colors.
La coloration dite"permanente"obtenue grâce à ces colorants d'oxydation, doit par ailleurs satisfaire un certain nombre d'exigences. Ainsi, elle doit être sans inconvénient sur le plan toxicologique, elle doit permettre d'obtenir des nuances dans l'intensité souhaitée et présenter une bonne tenue face aux agents extérieurs tels que la lumière, les intempéries, le lavage, les ondulations permanentes, la transpiration et les frottements. The so-called "permanent" coloration obtained with these oxidation dyes must also meet a certain number of requirements. Thus, it must be harmless from the toxicological point of view, it must make it possible to obtain shades in the desired intensity and to have good resistance to external agents such as light, bad weather, washing, permanent undulations, sweating and friction.
Les colorants doivent également permettre de couvrir les cheveux blancs, et être enfin les moins sélectifs possibles, c'est-à-dire permettre d'obtenir des écarts de coloration les plus faibles possibles tout au long d'une même fibre kératinique, qui est en général différemment sensibilisée (i. e. abîmée) entre sa pointe et sa racine. The dyes must also make it possible to cover the white hair, and finally be the least selective possible, that is to say, to obtain the lowest possible color differences throughout the same keratin fiber, which is in general differently sensitized (ie damaged) between its point and its root.
Il a déjà été proposé dans la demande de brevet FR 2 736 640 des compositions de teinture d'oxydation des fibres kératiniques contenant à titre de coupleur un dérivé Nsubstitué de 4-hydroxyindol associé à des bases d'oxydation. Patent application FR 2,736,640 has already proposed compositions for the oxidation dyeing of keratin fibers containing as couplers an N-substituted derivative of 4-hydroxyindol associated with oxidation bases.
On connaît également dans les demandes de brevet FR-A-2 750 048 et FR 2 791 563 des compositions pour la teinture d'oxydation des fibres kératiniques contenant, à titre de base d'oxydation, des pyrazolo-[1, 5-a]-pyrimidines associées avec d'autres bases d'oxydation et un ou plusieurs coupleurs On connaît en particulier dans la demande FR 2 791 563 un exemple de composition comprenant comme bases d'oxydation : l'association paraphénylènediamine (1 % en poids), 3-amino-5-méthyl-7- imidazolylpropylamino pyrazolo-[1, 5-a]-pyrimidine, 2HCI (3% en poids et comme coupleurs : le 1-N- (ss-hydroxyéthyl) 4-hydroxy indole) (1,2% en poids) et la 3,6- diméthylpyrazolo[3, 2-c]-1, 2, 4-triazole (1,2% en poids). La présence de la 3,6- diméthylpyrazolo[3, 2-c]-1, 2, 4-triazole nuit à la stabilité de la composition et induit par ailleurs un tâchage des vêtements. Patent applications FR-A-2 750 048 and FR 2 791 563 also disclose compositions for the oxidation dyeing of keratinous fibers containing, as oxidation base, pyrazolo [1,5-a] ] -pyrimidines Associated with Other Oxidation Bases and One or More Couplers An example of a composition comprising, as the oxidation bases, the paraphenylenediamine combination (1% by weight), is particularly known in patent application FR 2 791 563, 3-amino-5-methyl-7-imidazolylpropylamino pyrazolo [1,5-a] pyrimidine, 2HCl (3% by weight and as couplers: 1-N- (ss-hydroxyethyl) 4-hydroxyindole) (1) 2% by weight) and 3,6-dimethylpyrazolo [3,2-c] -1,2,4-triazole (1.2% by weight). The presence of 3,6-dimethylpyrazolo [3,2-c] -1,2,4-triazole impairs the stability of the composition and also induces staining of the clothing.
Des compositions tinctoriales des fibres kératiniques comprenant des dérivés 4,5diaminopyrazoles pouvant être substitués en position 2 par des radicaux alkyles ou hydroxyalkyles ont été proposées dans les demandes de brevet DE 3843892. La Keratinous fiber dye compositions comprising 4,5-diaminopyrazole derivatives which may be substituted in the 2-position by alkyl or hydroxyalkyl radicals have been proposed in DE 3843892 patent applications.
<Desc/Clms Page number 3><Desc / Clms Page number 3>
demande de brevet EP 692 245 décrit des compositions tinctoriales comprenant des dérivés 4, 5-diaminopyrazoles associés à des métaphénylènediamines particulières. La demande de brevet DE 19643059 décrit des compositions tinctoriales associant des dérivés 4, 5-diaminopyrazoles avec des coupleurs métaaminophénols et métaphénylènediamine. La demande de brevet DE 19646609 décrit des compositions tinctoriales associant des dérivés 4, 5-diaminopyrazoles avec des coupleurs benzoxazines. Patent Application EP 692 245 discloses dye compositions comprising 4,5-diaminopyrazole derivatives associated with particular metaphenylenediamines. Patent Application DE 19643059 describes dyeing compositions combining 4,5-diaminopyrazole derivatives with meta-aminophenol and metaphenylenediamine couplers. Patent Application DE 19646609 describes dyeing compositions combining 4,5-diaminopyrazole derivatives with benzoxazine couplers.
Il est de plus connu dans la demande de brevet JP 11158048 d'utiliser des compositions tinctoriales comprenant des dérivés de paraphénylènediamine dont un des atomes d'azote est compris dans un cycle de 5 à 7 chaînons. Le brevet US 5,851, 237 propose l'utilisation de dérivés 1- (4-aminophényl) pyrrolidine éventuellement substitués sur le noyau benzénique afin de remplacer la paraphénylènediamine. Le brevet US 5,993, 491 propose l'utilisation de dérivés N- (4-aminophényl) -2- (hydroxyméthyl) -pyrrolidines éventuellement substituées sur le noyau benzénique afin de remplacer la paraphénylènediamine. It is moreover known in the patent application JP 11158048 to use dye compositions comprising paraphenylenediamine derivatives, one of the nitrogen atoms is included in a 5- to 7-membered ring. US Pat. No. 5,851,237 proposes the use of optionally substituted 1- (4-aminophenyl) pyrrolidine derivatives on the benzene ring to replace para-phenylenediamine. No. 5,993,491 proposes the use of N- (4-aminophenyl) -2- (hydroxymethyl) -pyrrolidine derivatives optionally substituted on the benzene ring to replace para-phenylenediamine.
Cependant, les colorations obtenues en mettant en oeuvre ces compositions tinctoriales ne sont pas toujours assez puissantes, esthétiques, chromatiques ou suffisamment résistantes aux différentes agressions que peuvent subir les cheveux. However, the colorations obtained by using these dyeing compositions are not always powerful enough, aesthetic, chromatic or sufficiently resistant to the various attacks that can suffer the hair.
Or, la Demanderesse vient maintenant de découvrir qu'il est possible d'obtenir, sans les inconvénients des compositions de teinture de l'art antérieur, de nouvelles teintures, capables de conduire à des colorations aux nuances variées, chromatiques, puissantes, esthétiques, peu sélectives et résistant bien aux diverses agressions que peuvent subir les fibres, en associant au moins une base d'oxydation hétérocyclique choisie parmi (i) le 4,5-diamino 1- (2'hydroxyéthyl) pyrazole et ses sels d'addition ; (ii) les paraphénylènediamines à groupement amino cyclique et leurs sels d'addition ;
(iii) les pyrazolo-[1, 5-a]-pyrimidines et leurs sels d'addition ; au l-N- (p-hydroxyéthyi) 4-hydroxy indole de formule : However, the Applicant has now discovered that it is possible to obtain, without the disadvantages of the dyeing compositions of the prior art, new dyes, capable of leading to colorations with varied shades, chromatic, powerful, aesthetic, not very selective and resistant to the various attacks that the fibers may undergo, by combining at least one heterocyclic oxidation base chosen from (i) 4,5-diamino-1- (2-hydroxyethyl) pyrazole and its addition salts; (ii) cyclic amino paraphenylenediamines and their addition salts;
(iii) pyrazolo [1,5-a] pyrimidines and their addition salts; with N- (p-hydroxyethyl) -4-hydroxyindole of formula:
<Desc/Clms Page number 4> <Desc / Clms Page number 4>
ou l'un des ses sels d'addition comme coupleur ; ladite composition ne contenant pas la 3, 6-diméthylpyrazolo[3, 2-c ]-1, 2, 4-triazole.
or one of its addition salts as a coupler; said composition not containing 3,6-dimethylpyrazolo [3,2-c] -1,2,4-triazole.
L'invention a aussi pour objet un procédé de teinture mettant en oeuvre cette composition. The invention also relates to a dyeing process using this composition.
Un autre objet de l'invention est l'utilisation de la composition de la présente invention pour la teinture des fibres kératiniques, en particulier les fibres kératiniques humaines telles que les cheveux. Another subject of the invention is the use of the composition of the present invention for dyeing keratinous fibers, in particular human keratinous fibers such as the hair.
La composition de la présente invention permet en particulier d'obtenir une coloration de fibres kératiniques chromatique, très puissante, peu sélective et tenace tout en évitant la dégradation de ces fibres. The composition of the present invention makes it possible in particular to obtain a coloring of keratinous chromatic fibers, which is very powerful, not very selective and tenacious while avoiding the degradation of these fibers.
Dans le cadre de la présente invention, on entend par alkyle, des radicaux linéaires ou ramifiés par exemple méthyle, éthyle, n-propyle, iso-propyle, butyle, etc. Un radical alcoxy est un radical alk-O, le radical alkyle ayant la définition donnée ci dessus. In the context of the present invention, alkyl is understood to mean linear or branched radicals, for example methyl, ethyl, n-propyl, iso-propyl or butyl, etc. An alkoxy radical is an alk-O radical, the alkyl radical having the definition given above.
Halogène désigne de préférence CI, Br, l, F. Halogen is preferably CI, Br, I, F.
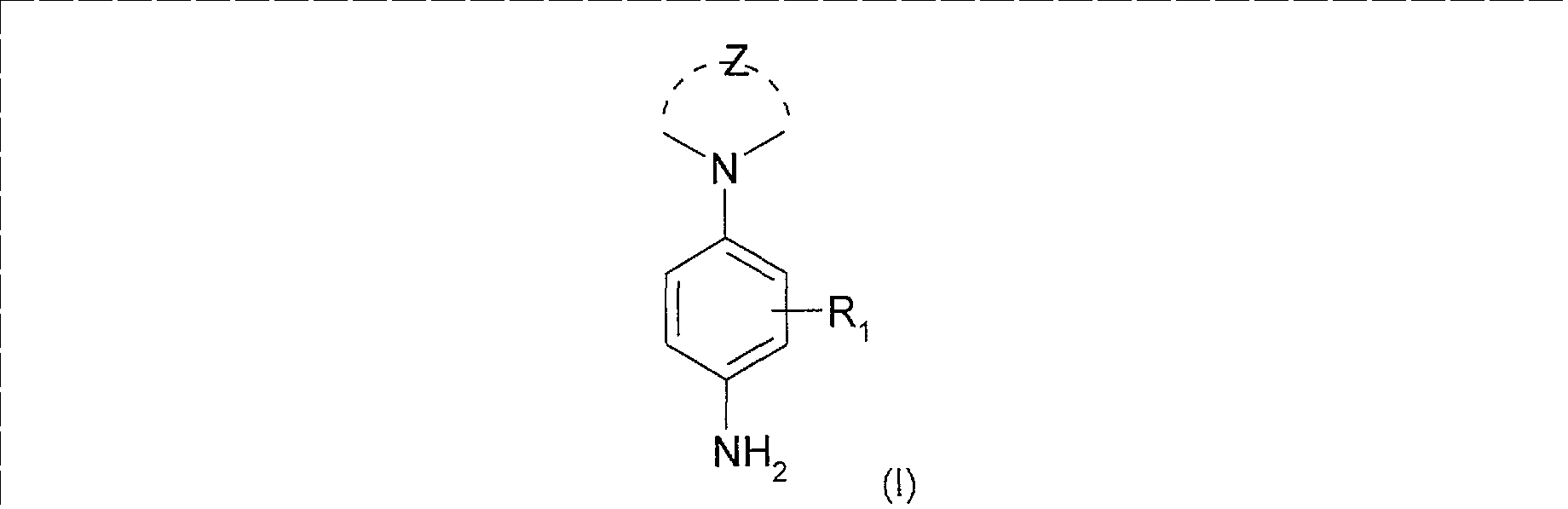
Parmi les paraphénylènediamines à groupement amino cyclique utilisables dans les compositions tinctoriales conformes à l'invention, on peut citer les dérivés de paraphénylènediamine de formule (1) suivante, ou leurs sels d'addition : Among the para-phenylenediamines containing a cyclic amino group that may be used in the dyeing compositions in accordance with the invention, mention may be made of the para-phenylenediamine derivatives of formula (1) below, or their addition salts:
<Desc/Clms Page number 5> <Desc / Clms Page number 5>
dans laquelle - Z représente les atomes nécessaires pour former un cycle saturé de 3 à 8 chaînons, ces atomes pouvant être des atomes de carbone ou d'azote, de préférence uniquement des atomes de carbone, le cycle pouvant être substitué, - R1 représente un atome d'hydrogène ; un atome d'halogène choisi parmi un atome de chlore et de brome ; une chaîne hydrocarbonée en C1-C7 saturée ou insaturée, linéaire ou ramifiée dont un ou plusieurs atomes de carbone peuvent être remplacés par un atome d'oxygène, d'azote ou de soufre ou par un groupement S02, et dont les atomes de carbones peuvent être, indépendamment les uns des autres, substitués par un ou plusieurs atomes d'halogènes ; ledit radical R1 ne comportant pas de liaison peroxyde, ni de radicaux diazo, nitro ou nitroso, Selon la présente invention, le cycle formé avec Z et l'atome d'azote de la paraphénylènediamine peut être un cycle pyrrolidine, pipéridine, homopipéridine, imidazoline, pyrazolidine, piperazine. Le cycle saturé ainsi formé peut être substitué. A titre d'exemple, on peut citer comme substituant les atomes d'halogène, le radical hydroxyle, le radical amino, les radicaux alkyle en C1-C4 éventuellement substitué par un ou plusieurs radicaux hydroxy, amino, (di) alkylamino en C1-C2, carboxy ; le radical carboxy ; les radicaux carbamoyl ou sulfonamido ; les radicaux-OR4 dans lesquels R4 représente un radical alkyle en C1-C4 substitué par un ou plusieurs radicaux choisis parmi un atome d'halogène, les radicaux alcoxy en Cl-C2, amino, aminoalkyl en C1-C2 ou alkyle en C3-C4 substitué par un ou plusieurs radicaux hydroxyle ; un radical méthylcarbonyle ; un radical-NR5R6 dans lequel Rs et Re représentent, indépendamment l'un de l'autre, un atome d'hydrogène, un radical alkyle en Ci-C4 substitué par un ou plusieurs radicaux choisis parmi un atome d'halogène, les radicaux hydroxyle, alcoxy en CI-C2, amino ou aminoalkyl en Cl-C2.
in which - Z represents the atoms necessary to form a saturated ring of 3 to 8 members, these atoms possibly being carbon or nitrogen atoms, preferably only carbon atoms, the ring being able to be substituted, - R1 represents a hydrogen atom; a halogen atom selected from a chlorine and bromine atom; a linear or branched saturated or unsaturated C 1 -C 7 hydrocarbon-based chain of which one or more carbon atoms can be replaced by an oxygen, nitrogen or sulfur atom or by a SO 2 group, and whose carbon atoms can to be, independently of one another, substituted by one or more halogen atoms; said radical R1 having no peroxide bond, or diazo, nitro or nitroso radicals, According to the present invention, the ring formed with Z and the nitrogen atom of para-phenylenediamine may be a pyrrolidine, piperidine, homopiperidine or imidazoline ring. , pyrazolidine, piperazine. The saturated cycle thus formed can be substituted. By way of example, the halogen atoms, the hydroxyl radical, the amino radical or the C 1 -C 4 alkyl radicals may optionally be substituted by one or more hydroxyl, amino or (di) alkylamino radicals. C2, carboxy; the carboxy radical; carbamoyl or sulfonamido radicals; the radicals-OR4 in which R4 represents a C1-C4 alkyl radical substituted with one or more radicals chosen from a halogen atom, C1-C2 alkoxy, amino, C1-C2 aminoalkyl or C3-C4 alkyl radicals; substituted with one or more hydroxyl radicals; a methylcarbonyl radical; a radical-NR5R6 in which Rs and Re represent, independently of one another, a hydrogen atom, a C1-C4 alkyl radical substituted with one or more radicals chosen from a halogen atom, the hydroxyl radicals; , C1-C2 alkoxy, amino or C1-C2 aminoalkyl.
<Desc/Clms Page number 6><Desc / Clms Page number 6>
Selon un mode de réalisation particulier, Z représente les atomes de carbone nécessaire pour former un cycle comprenant de 5 à 8 chaînons, de préférence un cycle pyrrolidine, substitué ou non. According to a particular embodiment, Z represents the carbon atoms necessary to form a 5- to 8-membered ring, preferably a substituted or unsubstituted pyrrolidine ring.
Selon l'invention, une chaîne hydrocarbonée saturée ou insaturée, linéaire ou ramifiée de la formule (1) est une chaîne qui peut comprendre une ou plusieurs liaisons doubles et/ou une ou plusieurs liaisons triples, et peut former un ou plusieurs cycles comportant de 3 à 6 chaînons, les liaisons doubles pouvant éventuellement conduire à des groupements aromatiques. Lorsqu'il est indiqué qu'un ou plusieurs des atomes de carbone de R1 peuvent être remplacés par un atome d'oxygène, d'azote ou de soufre ou par un groupement S02, et/ou que ledit radical R1 peut être insaturé, cela signifie
que l'on peut, à titre d'exemple, faire les transformations suivantes :
1 1 -CH-H peut devenir'-O-H zu : ~O peut devenir : ~O 1 1 peut devenir !'--SON 1 2 1 O peutdevenir eo) < J-O 1 1 1 1 Selon l'invention, R1 représente de préférence un atome d'hydrogène, un radical alkyle, un radical alcényle, un radical alcynyle, un radical alcoxyalkyle, un radical hydroxyalkyl, un radical alcoxy, un radical allyloxy, un radical hydroxyaminoalkyle, un radical hydroxyalcoxy, un radical carboxyalkyl, un radical aminoalkyl. According to the invention, a saturated or unsaturated, linear or branched hydrocarbon chain of the formula (1) is a chain which may comprise one or more double bonds and / or one or more triple bonds, and may form one or more rings comprising 3 to 6 members, the double bonds possibly leading to aromatic groups. When it is stated that one or more of the carbon atoms of R 1 may be replaced by an oxygen, nitrogen or sulfur atom or by a group SO 2, and / or that said radical R 1 may be unsaturated, this means
that we can, by way of example, make the following transformations:
1 -CH-H can become -OH zu: ~ O can become: ~ O 1 1 can become! '- SOUND 1 2 1 O can become eo) <JO 1 1 1 1 According to the invention, R1 represents preferably a hydrogen atom, an alkyl radical, an alkenyl radical, an alkynyl radical, an alkoxyalkyl radical, a hydroxyalkyl radical, an alkoxy radical, an allyloxy radical, a hydroxyaminoalkyl radical, a hydroxyalkoxy radical, a carboxyalkyl radical or an aminoalkyl radical; .
A titre d'exemple, on peut citer pour Ri les radicaux méthyle, éthyle, isopropyle, vinyle, allyle, méthoxyméthyle, hydroxyméthyle, 1-carboxyméthyle, 1-aminométhyle, 2-
carboxyéthyle, 2-hydroxyéthyle, 3-hydroxypropyle, 1, 2-dihydroxyéthyle, 1-hydroxy-2aminoéthyle, méthoxy, éthoxy, allyloxy ou 2-hydroxyéthyloxy. De préférence, R2 représente un atome d'hydrogène, un radical méthyle, hydroxyméthyle, 2- By way of example, mention may be made for R 1 of the radicals methyl, ethyl, isopropyl, vinyl, allyl, methoxymethyl, hydroxymethyl, 1-carboxymethyl, 1-aminomethyl, 2-
carboxyethyl, 2-hydroxyethyl, 3-hydroxypropyl, 1, 2-dihydroxyethyl, 1-hydroxy-2-aminoethyl, methoxy, ethoxy, allyloxy or 2-hydroxyethyloxy. Preferably, R2 represents a hydrogen atom, a methyl radical, hydroxymethyl, 2-
<Desc/Clms Page number 7><Desc / Clms Page number 7>
hydroxyéthyle, 1, 2-dihydroxyéthyle, méthoxy, ou 2-hydroxyéthoxy, préférentiellement un atome d'hydrogène ou un radical méthyle. hydroxyethyl, 1, 2-dihydroxyethyl, methoxy or 2-hydroxyethoxy, preferably a hydrogen atom or a methyl radical.
Selon un mode de réalisation particulier, la base d'oxydation choisie parmi les dérivés de paraphénylènediamine est un dérivé de paraphénylèdiamine à groupemement pyrrolidine correspondant à la formule (la) suivante
dans laquelle - R1 est tel que définie précédemment, - R2 représente un atome d'hydrogène ; un radical hydroxyle ; un radical amino ; un radical -OR4 dans lequel R4 représente un radical alkyle en Ci-C4 substitué par un ou plusieurs radicaux choisis parmi un atome d'halogène, les radicaux alcoxy en Cl-C2, amino, aminoalkyl en Ci-C2 ou mono-ou poly-hydroxyalkyle en Ci-C4 ; un radical méthylcarbonyle ; un radical -NRsR6 dans lequel R5 et R6 représentent, indépendamment l'un de l'autre, un atome d'hydrogène, un radical alkyle en Ci-C4 pouvant être substitué par un ou plusieurs radicaux choisis parmi un atome d'halogène, les radicaux hydroxyle, alcoxy en CI-C2, amino ou aminoalkyl en Ci-Cz ; - R3 représente un atome d'hydrogène ; un radical carbamoyle, un radical amido ; un radical mono-ou poly-hydroxyalkyle en Cl-C5. According to a particular embodiment, the oxidation base chosen from paraphenylenediamine derivatives is a paraphenylediamine derivative with a pyrrolidine group corresponding to the following formula (Ia)
in which - R 1 is as defined above, - R2 represents a hydrogen atom; a hydroxyl radical; an amino radical; a radical -OR4 in which R4 represents a C1-C4 alkyl radical substituted with one or more radicals chosen from a halogen atom, C1-C2 alkoxy, amino, C1-C2 aminoalkyl or mono-or poly-alkyl radicals; C1-C4 hydroxyalkyl; a methylcarbonyl radical; a radical -NRsR6 in which R5 and R6 represent, independently of one another, a hydrogen atom, a C1-C4 alkyl radical which may be substituted by one or more radicals chosen from a halogen atom, and hydroxyl, C 1 -C 2 alkoxy, amino or C 1 -C 20 aminoalkyl radicals; - R3 represents a hydrogen atom; a carbamoyl radical, an amido radical; a mono-or poly-hydroxy-C 1 -C 5 radical.
Selon un mode de réalisation particulier, R2 représente un atome d'hydrogène, un radical hydroxyle, acétoxy, amino, alkylamino, hydroxyalkylamino. According to a particular embodiment, R2 represents a hydrogen atom, a hydroxyl, acetoxy, amino, alkylamino or hydroxyalkylamino radical.
A titre d'exemple, on peut citer pour R2 un atome d'hydrogène, un radical hydroxyle, acétoxy, amino, méthylamino, diméthylamino ou 2-hydroxyéthylamino. De préférence, R2 représente un atome d'hydrogène, un radical hydroxyle ou un radical amino. By way of example, mention may be made for R 2 of a hydrogen atom, a hydroxyl, acetoxy, amino, methylamino, dimethylamino or 2-hydroxyethylamino radical. Preferably, R2 represents a hydrogen atom, a hydroxyl radical or an amino radical.
<Desc/Clms Page number 8> <Desc / Clms Page number 8>
Dans la formule (la), R3 représente de préférence un atome d'hydrogène, un radical carbamoyl, un radical hydroxy, un radical hydroxyalkyle en C1-C4, un radical méthyle. In formula (Ia), R3 preferably represents a hydrogen atom, a carbamoyl radical, a hydroxyl radical, a C1-C4 hydroxyalkyl radical or a methyl radical.
A titre d'exemple, les dérivés de paraphénylènediamine de formule (1) sont choisis parmi la N- (4-aminophényl)-3-hydroxy-pyrrolidine, la N- (4-amino-2-méthylphényl)-3hydroxypyrrolidine, la N- (4-amino-2-éthylphényl) -3-hydroxypyrrolidine, la N- (4-amino-2méthoxyp h ényl) -3-hyd roxy-pyrrolidi ne, la N- (4-am i n 0-2 - (2-hydroxyéthyl) phényl) -3hydroxypyrrolidine, la N- (4-amino-2- (1-hydroxyéthyl) phényl)-3-hydroxypyrrolidine, la N- (4-amino-2- (1, 2-dihydroxy-éthyl) phényl) -3-hydroxypyrrolidine, la N- (4-amino-3-méthylphényl) -3-hydroxypyrrolidine, la N- (4-amino-3-éthylphényl) -3-hydroxypyrrolidine, la N- (4-amino-3-méthoxyphényl) -3-hydroxypyrrolidine, la N- (4-amino-3- (2-hydroxy- éthyl) phényl) -3-hydroxypyrrolidine, la N- (4-amino-3- ( 1-hydroxyéthyl) phényl) - 3-hydroxypyrrolidine, la N- (4-amino-3- ( 1, 2-dihydroxyéthyl) phényl) -3-hydroxy-pyrrolidine, la N- (4-amino-phényl)-3-aminopyrrolidine, la N- (4-amino-2-méthyl-phényl)-3aminopyrrolidine, la N- (4-amino-2-éthylphényl) -3-aminopyrrolidine, la N- (4-amino-2méthoxyphényl)-3-aminopyrrolidine, la N- (4-amino-2- (2-hydroxy-éthyl) phényl)-3aminopyrrolidine, la N-(4-amino-2-(1-hydroxyéthyl) phényl)-3-aminopyrrolidine, la N-(4amino-2- (1, 2-dihydroxyéthyl) phényl)-3-hydroxy-pyrrolidine, la N- (4-amino-3méthylphényl) -3-aminopyrrolidine, la N- (4-amino-3-éthylphényl) -3-aminopyrrolídine, la N- (4-amino-3-méthoxyphény))-3-am ! no-pyrro ! idine,) a N- (4-amino-3- (2-hydroxy- éthyl) phényl)-3-aminopyrrolidine, la N- (4-amino-3- (1-hydroxyéthyl) phéhyl)-3aminopyrrolidine, la N- (4-amino-3- (1, 2-dihydroxyéthyl) phényl) -3-aminopyrrolídine, la 1- (4-aminophényl) -pyrrolidine, le 1- (4-aminophényl) -2-pyrrolidineméthanol, le 1- (4aminophényl) -4-hydroxy-2-pyrrolidineméthanol, la N- (4-aminophényl) -prolineamide et leurs sels d'addition avec un acide. De préférence, les dérivés de paraphénylènediamine de formule (lia) sont choisis parmi la 3-hydroxy 1- (4'aminophényl) pyrro ! idine, la 3-amino 1- (4'aminophényl) pyrrolidine, la 1- (4-aminophényl) pyrrolidine, le 1- (4-aminophény !)-2-pyrroiidineméthanoi, te 1- (4-aminophény !)-4hydroxy-2-pyrrolidineméthanol, la N- (4-aminophényl)-prolineamide et leurs sels d'addition. By way of example, the paraphenylenediamine derivatives of formula (1) are chosen from N- (4-aminophenyl) -3-hydroxy-pyrrolidine, N- (4-amino-2-methylphenyl) -3-hydroxypyrrolidine, N - (4-Amino-2-ethylphenyl) -3-hydroxypyrrolidine, N- (4-amino-2-methoxypenyl) -3-hydoxy-pyrrolidine, N- (4-amin) -2- (2) hydroxyethyl) phenyl) -3-hydroxypyrrolidine, N- (4-amino-2- (1-hydroxyethyl) phenyl) -3-hydroxypyrrolidine, N- (4-amino-2- (1, 2-dihydroxyethyl) phenyl) ) -3-hydroxypyrrolidine, N- (4-amino-3-methylphenyl) -3-hydroxypyrrolidine, N- (4-amino-3-ethylphenyl) -3-hydroxypyrrolidine, N- (4-amino-3- methoxyphenyl) -3-hydroxypyrrolidine, N- (4-amino-3- (2-hydroxyethyl) phenyl) -3-hydroxypyrrolidine, N- (4-amino-3- (1-hydroxyethyl) phenyl) -3- hydroxypyrrolidine, N- (4-amino-3- (1,2-dihydroxyethyl) phenyl) -3-hydroxy-pyrrolidine, N- (4-amino-phenyl) -3-aminopyrrolidine, N- (4- amino-2-methyl-phenyl) -3aminopyrrolidine, N- (4-amino-2-ethylphenyl) -3-aminopyrrolidine, N- (4-amino-2-methoxyphenyl) -3-aminopyrrolidine, N- (4-amino-2- (2-hydroxy-ethyl) phenyl) 3-aminopyrrolidine, N- (4-amino-2- (1-hydroxyethyl) phenyl) -3-aminopyrrolidine, N- (4-amino-2- (1,2-dihydroxyethyl) phenyl) -3-hydroxy-pyrrolidine, N- (4-amino-3-methylphenyl) -3-aminopyrrolidine, N- (4-amino-3-ethylphenyl) -3-aminopyrrolidine, N- (4-amino-3-methoxyphenyl) -3-am! no-pyrro! N- (4-amino-3- (2-hydroxyethyl) phenyl) -3-aminopyrrolidine, N- (4-amino-3- (1-hydroxyethyl) phenyl) -aminopyrrolidine, N- (4-amino-3- (2-hydroxyethyl) phenyl) -3-aminopyrrolidine; (4-amino-3- (1, 2-dihydroxyethyl) phenyl) -3-aminopyrroline, 1- (4-aminophenyl) -pyrrolidine, 1- (4-aminophenyl) -2-pyrrolidinemethanol, 1- (4-aminophenyl) 4-hydroxy-2-pyrrolidinemethanol, N- (4-aminophenyl) -prolineamide and their addition salts with an acid. Preferably, the paraphenylenediamine derivatives of formula (IIa) are chosen from 3-hydroxy-1- (4'-aminophenyl) pyrrole. idine, 3-amino 1- (4'-aminophenyl) pyrrolidine, 1- (4-aminophenyl) pyrrolidine, 1- (4-aminophenyl) -2-pyrrolidinemethanol, 1- (4-aminophenyl) -4-hydroxy -2-pyrrolidinemethanol, N- (4-aminophenyl) -prolineamide and their addition salts.
<Desc/Clms Page number 9><Desc / Clms Page number 9>
Parmi les pyrazolo-[1, 5-a]-pyrimidines utilisables à titre de base d'oxydation dans la composition tinctoriale conforme à l'invention, on peut notamment citer les composés de formule (II) suivante, et leurs sels d'addition avec un acide ou avec une base :
dans laquelle : - R7 Ra, Rg et R10 désignent, identiques ou différents, un atome d'hydrogène ; un radical alkyle en Ci-C4 ; un radical aryle ; un radical hydroxyalkyle en Ci-C4 ; un radical polyhydroxyalkyle en C2-C4 ; un radical (C1-C4)alcoxy alkyle en C1-C4 ; un radical amino alkyle en C1-C4 ; un radical amino alkyle en Ci-C4 dont l'amine est protégée par un radical acétyle, uréido, ou sulfonyl ; un radical (C1-C4)alkyl amino alkyle en C1-C4 ; un radical di-[ (CrC4) alkyl] amino alkyle en Ci-C4 dans lequel les dialkyles peuvent former un cycle aliphatique ou hétérocyclique à 5 ou 6 chaînons ; un radical hydroxy (C1-C4) alkyl- ou di-[hydroxy(C1-C4)alkyl]-amino alkyle en CI-C4 - les radicaux X désignent, identiques ou différents, un atome d'hydrogène, un radical alkyle en C1-C4, un radical aryle, un radical hydroxyalkyle en Ci-C4, un radical polyhydroxyalkyle en C2-C4, un radical amino alkyle en Ci-C4, un radical (C1-C4)alkyl amino alkyle en C1-C4, un radical di-[ (C1-C4) alkyl] amino alkyle en Ci-C4 dans lequel les dialkyles peuvent former un cycle aliphatique ou hétérocyclique à 5 ou 6 chaînons, un radical hydroxy (C1-C4) alkyl- ou di-[hydroxy(C1-C4)alkyl]amino alkyle en Ci-C4, un radical amino, un radical (C1-C4)alkyl- ou di-[(C1-C4)alkyl]-amino ; un atome d'halogène, un groupe acide carboxylique, ou un groupe acide suifonique ; - i vaut 0, 1, 2 ou 3 ; - p vaut 0 ou 1 ; - q vaut 0 ou 1 ; - n vaut 0 ou 1 ; sous réserve que : - (i) la somme p + q est différente de 0 ; - (ii) lorsque p + q est égal à 2, alors n vaut 0 et les groupes NR6R7 et NRsRg occupent les positions (2,3) ; (5,6) ; (6,7) ; (3,5) ou (3,7) ; Among the pyrazolo [1, 5-a] -pyrimidines which can be used as oxidation base in the dyeing composition in accordance with the invention, mention may especially be made of the compounds of the following formula (II), and their addition salts with an acid or with a base:
in which: R7 Ra, Rg and R10 denote, identical or different, a hydrogen atom; a C1-C4 alkyl radical; an aryl radical; a C1-C4 hydroxyalkyl radical; a C2-C4 polyhydroxyalkyl radical; a (C1-C4) alkoxy C1-C4 alkyl radical; a C1-C4 amino alkyl radical; a C 1 -C 4 alkylamino radical whose amine is protected by an acetyl, ureido or sulfonyl radical; a (C1-C4) alkylamino (C1-C4) alkyl radical; a di- [(CrC4) alkyl] amino C1-C4 alkyl radical in which the dialkyls can form a 5- or 6-membered aliphatic or heterocyclic ring; a hydroxy (C1-C4) alkyl- or di- [hydroxy (C1-C4) alkyl] -amino (C1-C4) alkyl radical - the radicals X denote, identical or different, a hydrogen atom or a C1-alkyl radical; -C4, an aryl radical, a C1-C4 hydroxyalkyl radical, a C2-C4 polyhydroxyalkyl radical, a C1-C4 aminoalkyl radical, a (C1-C4) alkylamino (C1-C4) alkyl radical, a di - [(C1-C4) alkyl] C1-C4 alkyl in which the dialkyls can form a 5- or 6-membered aliphatic or heterocyclic ring, a hydroxy (C1-C4) alkyl- or di- [hydroxy (C1- C4) alkyl] C1-C4 alkyl, amino, (C1-C4) alkyl- or di - [(C1-C4) alkyl] amino; a halogen atom, a carboxylic acid group, or a sulfonic acid group; i is 0, 1, 2 or 3; p is 0 or 1; q is 0 or 1; n is 0 or 1; provided that: - (i) the sum p + q is different from 0; - (ii) when p + q is 2, then n is 0 and the groups NR6R7 and NRsRg occupy the positions (2,3); (5.6); (6.7); (3,5) or (3,7);
<Desc/Clms Page number 10><Desc / Clms Page number 10>
- (iii) lorsque p + q est égal à 1 alors n vaut 1 et le groupe NR6R7 (ou NRgRg) et le groupe OH occupent les positions (2,3) ; (5, 6) ; (6,7) ; (3, 5) ou (3,7). - (iii) when p + q is equal to 1 then n is 1 and the NR6R7 (or NRgRg) group and the OH group occupy the positions (2,3); (5, 6); (6.7); (3, 5) or (3,7).
Les pyrazolo-[1, S-a]-pyrimidines de formule (II) utilisables à titre de base d'oxydation dans la composition tinctoriale conforme à l'invention sont des composés connus et décrits dans la demande de brevet FR-A-2 750 048 dont le contenu fait partie intégrante de la présente demande. The pyrazolo [1, Sa] -pyrimidines of formula (II) used as oxidation base in the dye composition according to the invention are known compounds and described in the patent application FR-A-2 750 048 the content of which forms an integral part of this application.
Parmi les pyrazolo-[1, S-a]-pyrimidines de formule (II) ci-dessus, on peut notamment citer :
- la pyrazolo- [1, 5-a]-pyrimidine-3, 7-diamine ; - la 2-méthyl pyrazolo- [1, 5-a]-pyrimidine-3, 7-diamine ; - la 2, 5-dimethyl pyrazolo-[1, S-a]-pyrimidine-3, 7-diamine ; - la pyrazolo-[1, S-a]-pyrimidine-3, S-diamine ; - la 2, 7-diméthyl pyrazolo- [1, 5-a]-pyrimidine-3, 5-diamine ; - le 3-amino pyrazolo-[1, S-a]-pyrimidin-7 -01 ; - le 3-amino 5-méthyle pyrazolo- [1, 5-a]-pyrimidin-7-oi ; - le 3-amino pyrazolo-[1, S-a]-pyrimidin-S-ol ; - le 2-(3-amino pyrazolo-[1,5-a]-pyrimidin-7-ylamino)-éthanol ; - la 3-amino-7-ss-hyhdroxyéthylamino-5-méthyl-pyrazolo-[1,5-a]-pyrimidine; - le 2-(7-amino pyrazolo-[1,5-a]-pyrimidin-3-ylamino)-éthanol ; - le 2-[(3-amino-pyrazolo-[1,5-a]-pyrimidin-7-yl)-(2-hydroxyéthyl)-amino]- éthanol ; - le 2-[(7-amino-pyrazolo-[1,5-a]-pyrimidin-3-yl)-(2-hydroxyéthyl)-amino]- éthanol ;
- la S, 6-diméthyl pyrazolo-[1, S-a]-pyrimidine-3, 7-diamine ; - la 2, 6-diméthyl pyrazolo-[1, 5-a]-pyrimidine-3, 7-diamine ; - la 2, 5, N-7, N-7-tetraméthyl pyrazolo-[1,5-a]-pyrimidine-3,7-diamine ; - la 3-amino-5-méthyl-7-imidazolylpropylamino pyrazolo-[1,5-a]-pyrimidine ; et leurs sels d'addition. Among the pyrazolo- [1, Sa] -pyrimidines of formula (II) above, mention may be made in particular of:
pyrazolo [1,5-a] pyrimidine-3,7-diamine; 2-methyl pyrazolo [1,5-a] pyrimidine-3,7-diamine; 2,5-dimethyl pyrazolo [1,1'-Sa] pyrimidine-3,7-diamine; pyrazolo [1,1'-Sa] pyrimidine-3,5-diamine; 2,7-dimethyl pyrazolo [1,5-a] pyrimidine-3,5-diamine; 3-amino pyrazolo [1, Sa] -pyrimidin-7-01; 3-amino-5-methyl pyrazolo [1,5-a] pyrimidin-7-ol; 3-amino pyrazolo- [1, sa] pyrimidin-5-ol; 2- (3-amino pyrazolo [1,5-a] pyrimidin-7-ylamino) ethanol; 3-amino-7-ss-hydroxyethylamino-5-methyl-pyrazolo [1,5-a] pyrimidine; 2- (7-amino pyrazolo [1,5-a] pyrimidin-3-ylamino) ethanol; 2 - [(3-amino-pyrazolo [1,5-a] pyrimidin-7-yl) - (2-hydroxyethyl) amino] ethanol; 2 - [(7-amino-pyrazolo [1,5-a] pyrimidin-3-yl) - (2-hydroxyethyl) amino] ethanol;
S, 6-dimethyl pyrazolo [1,1'-Sa] pyrimidine-3,7-diamine; 2,6-dimethyl pyrazolo [1,5-a] pyrimidine-3,7-diamine; 2,5, N-7, N-7-tetramethyl pyrazolo [1,5-a] pyrimidine-3,7-diamine; 3-amino-5-methyl-7-imidazolylpropylamino pyrazolo [1,5-a] pyrimidine; and their addition salts.
La composition de la présente invention peut en outre comprendre une ou plusieurs bases d'oxydation additionnelles classiquement utilisées en teinture d'oxydation autres que celles décrites précédemment. A titre d'exemple, ces bases d'oxydation The composition of the present invention may further comprise one or more additional oxidation bases conventionally used in oxidation dyeing other than those described above. By way of example, these oxidation bases
<Desc/Clms Page number 11> <Desc / Clms Page number 11>
additionnelles sont choisies parmi les paraphénylènediamines, les bisphénylalkylènediamines, les para-aminophénols, les ortho-aminophénols, les bases hétérocycliques autres que celles décrites précédemment et leurs sels d'addition.
additional are selected from para-phenylenediamines, bisphenylalkylenediamines, para-aminophenols, ortho-aminophenols, heterocyclic bases other than those previously described and their addition salts.
Parmi les paraphénylènediamines, on peut citer à titre d'exemple, la paraphénylènediamine, la paratoluylènediamine, la 2-chloro paraphénylènediamine, la 2, 3-diméthyl paraphénylènediamine, la 2, 6-diméthyl paraphénylènediamine, la 2, 6diéthyl paraphénylènediamine, la 2, 5-diméthyl paraphénylènediamine, la N, N-diméthyl paraphénylènediamine, la N, N-diéthyl paraphénylènediamine, la N, N-dipropyl paraphénylènediamine, la 4-amino N, N-diéthyl 3-méthyl aniline, fa N, N-bis- (sshydroxyéthyl) paraphénylènediamine, la 4-N, N-bis- (p-hydroxyéthyi) amino 2-méthyi aniline, la 4-N, N-bis- (p-hydroxyéthy)) amino 2-chloro aniline, la 2-ss-hydroxyéthyl paraphénylènediamine, la 2-fluor paraphénylènediamine, la 2-isopropyl paraphénylènediamine, la N- (ss-hydroxypropyl) paraphénylènediamine, la 2-hydroxyméthyl paraphénylènediamine, la N, N-diméthyl 3-méthyl paraphénylènediamine, la N, N- (éthyi, p-hydroxyéthyi) paraphényiènediamine, ! a N- (p, ydihydroxypropyl) paraphénylènediamine, la N- (4'-aminophényl) paraphénylènediamine, la N-phényl paraphénylènediamine, la 2-ss-hydroxyéthyloxy paraphényiènediamine, la 2- ss-acétylaminoéthyloxy paraphénylènediamine, la N- (p-méthoxyéthyi) paraphényiènediamine, la 2-thiényl paraphénylènediamine, le 2-ss hydroxyéthylamino 5-amino toluène et leurs sels d'addition. Among the paraphenylenediamines, there may be mentioned, for example, para-phenylenediamine, paratoluylenediamine, 2-chloro-para-phenylenediamine, 2,3-dimethyl-para-phenylenediamine, 2,6-dimethyl-para-phenylenediamine, 2,6-diisyl-para-phenylenediamine, 2, 5-dimethyl-para-phenylenediamine, N, N-dimethyl-para-phenylenediamine, N, N-diethyl-para-phenylenediamine, N, N-dipropyl-para-phenylenediamine, 4-amino-N, N-diethyl-3-methylaniline, N, N-bis- (sshydroxyethyl) paraphenylenediamine, 4-N, N-bis- (p-hydroxyethyl) amino-2-methyl aniline, 4-N, N-bis- (p-hydroxyethyl) amino 2-chloroaniline, 2-ss hydroxy-para-phenylenediamine, 2-fluoro-para-phenylenediamine, 2-isopropyl-para-phenylenediamine, N- (ss-hydroxypropyl) -paraphenylenediamine, 2-hydroxymethyl-para-phenylenediamine, N, N-dimethyl-3-methyl-para-phenylenediamine, N, N- (ethyl) p-hydroxyethyl) paraphenylenediamine, N- (p, dihydroxypropyl) paraphenylenediamine, N- (4'-aminophenyl) paraphenylenediamine, N-phenyl paraphenylenediamine, 2-ss-hydroxyethyloxy para-phenylenediamine, 2-ss-acetylaminoethyloxy para-phenylenediamine, N- (p-methoxyethyl) ) paraphenylenediamine, 2-thienyl paraphenylenediamine, 2-ss hydroxyethylamino 5-amino toluene and their addition salts.
Parmi les paraphénylènediamines citées ci-dessus, la paraphénylènediamine, la paratoluylènediamine, la 2-isopropyl paraphénylènediamine, la 2-ss-hydroxyéthyl paraphénylènediamine, la 2-ss-hydroxyéthyloxy paraphénylène-diamine, la 2, 6-diméthyl paraphénylènediamine, la 2, 6-diéthyl paraphénylènediamine, la 2, 3-diméthyl paraphénylènediamine, la N, N-bis- (ss-hydroxyéthyl) paraphénylènediamine, la 2-chloro paraphénylènediamine, la 2-ss-acétylaminoéthyloxy paraphénylènediamine, et leurs sels d'addition sont particulièrement préférées. Among the para-phenylenediamines mentioned above, paraphenylenediamine, paratoluylenediamine, 2-isopropyl paraphenylenediamine, 2-ss-hydroxyethyl paraphenylenediamine, 2-ss-hydroxyethyloxy paraphenylenediamine, 2,6-dimethyl paraphenylenediamine, 2,6 ethyl paraphenylenediamine, 2,3-dimethyl paraphenylenediamine, N, N-bis (ss-hydroxyethyl) paraphenylenediamine, 2-chloro-para-phenylenediamine, 2-ss-acetylaminoethyloxy-para-phenylenediamine, and their addition salts are particularly preferred.
Parmi les bis-phénylalkylènediamines, on peut citer à titre d'exemple, le N, N'-bis- (phydroxyéthyl) N, N'-bis- (4'-aminophényl) 1, 3-diamino propanol, la N, N'-bis- (sshydroxyéthyl) N, N'-bis- (4'-aminophényl) éthylènediamine, la N, N'-bis- (4-aminophényl) Among the bis-phenylalkylenediamines, mention may be made, by way of example, of N, N'-bis- (phydroxyethyl) N, N'-bis- (4'-aminophenyl) 1,3-diamino propanol, N, N N, N'-bis- (4'-aminophenyl) ethylenediamine, N, N'-bis- (4-aminophenyl) -bis- (sshydroxyethyl) -N, N'-bis (4'-aminophenyl) ethylenediamine
<Desc/Clms Page number 12><Desc / Clms Page number 12>
tétraméthylènediamine, la N, N'-bis- (P-hydroxyéthyl) N, N'-bis- (4-aminophényl) tétraméthylènediamine, la N, N'-bis- (4-méthyl-aminophényl) tétraméthylènediamine, la N, N'-bis- (éthyl) N, N'-bis- (4'-amino, 3'-méthylphényl) éthylènediamine, le 1, 8-bis- (2, 5diamino phénoxy)-3, 6-dioxaoctane, et leurs sels d'addition. tetramethylenediamine, N, N'-bis- (β-hydroxyethyl) N, N'-bis (4-aminophenyl) tetramethylenediamine, N, N'-bis (4-methylaminophenyl) tetramethylenediamine, N, N Bis (ethyl) N, N'-bis- (4'-amino, 3'-methylphenyl) ethylenediamine, 1,8-bis (2,5-diamino phenoxy) -3,6-dioxaoctane, and salts thereof addition.
Parmi les para-aminophénols, on peut citer à titre d'exemple, le para-aminophénol, le 4amino 3-méthyl phénol, le 4-amino 3-fluoro phénol, le 4-amino 3-hydroxyméthyl phénol, le 4-amino 2-méthyl phénol, le 4-amino 2-hydroxyméthyl phénol, le 4-amino 2méthoxyméthyl phénol, le 4-amino 2-aminométhyl phénol, le 4-amino 2- (ss-hydroxyéthyl aminométhyl) phénol, le 4-amino 2-fluor phénol, et leurs sels d'addition. Among the para-aminophenols, para-aminophenol, 4-amino-3-methylphenol, 4-amino-3-fluoro phenol, 4-amino-3-hydroxymethylphenol, 4-amino-2 are exemplary; methyl phenol, 4-amino-2-hydroxymethylphenol, 4-amino-2-methoxymethylphenol, 4-amino-2-aminomethylphenol, 4-amino-2- (ss-hydroxyethylaminomethyl) phenol, 4-amino-2-fluorine phenol, and their addition salts.
Parmi les ortho-aminophénols, on peut citer à titre d'exemple, le 2-amino phénol, le 2amino 5-méthyl phénol, le 2-amino 6-méthyl phénol, le 5-acétamido 2-amino phénol, et leurs sels d'addition. Among the ortho-aminophenols, mention may be made, by way of example, of 2-amino phenol, 2-amino-5-methylphenol, 2-amino-6-methylphenol, 5-acetamido-2-amino phenol, and their salts. 'addition.
Parmi les bases hétérocycliques, on peut citer à titre d'exemple, les dérivés pyridiniques, les dérivés pyrimidiniques et les dérivés pyrazoliques autres que ceux cités précédemment. Among the heterocyclic bases, mention may be made, by way of example, of pyridine derivatives, pyrimidine derivatives and pyrazole derivatives other than those mentioned above.
Parmi les dérivés pyridiniques, on peut citer les composés décrits par exemple dans les brevets GB 1 026 978 et GB 1 153 196, comme la 2, 5-diamino pyridine, la 2- (4méthoxyphényl) amino 3-amino pyridine, la 2,3-diamino 6-méthoxy pyridine, la 2-(|3- méthoxyéthyl) amino 3-amino 6-méthoxy pyridine, la 3,4-diamino pyridine, et leurs sels d'addition. Among the pyridine derivatives, mention may be made of the compounds described for example in patents GB 1 026 978 and GB 1 153 196, such as 2,5-diaminopyridine, 2- (4-methoxyphenyl) amino-3-amino pyridine, 2, 3-diamino 6-methoxy pyridine, 2- (3-methoxyethyl) amino-3-amino-6-methoxy pyridine, 3,4-diamino pyridine, and their addition salts.
D'autres bases d'oxydation pyridiniques utiles dans la présente invention sont les bases d'oxydation 3-amino pyrazolo-[1, 5-a]-pyridines ou leurs sels d'addition décrits par exemple dans la demande de brevet FR 2801308. A titre d'exemple, on peut citer
la pyrazol 5-a]pyridin-3-ylamine ; la 2-acétylamino pyrazolo-[1, 5-a] pyridin-3ylamine ; la 2-morpholin-4-yl-pyrazolo 5-a]pyridin-3-ylamine ; t'acide 3-aminopyrazol 5-a]pyridin-2-carboxylique, la 2-méthoxy-pyrazolo[1, 5-a]pyridine-3- ylamino ; le (3-amino-pyrazolo[1,5-a]pyridine-7-yl)-méthanol ; le 2- (3-amino- pyrazolo[1, 5-a]pyridine-5-yl) -éthanol ; le 2-(3-amino-pyrazolo[1,5-a]pyridine-7-yl)- éthanol ; le (3-amino-pyrazolo[1,5-a]pyridine-2-yl)-méthanol ; la 3,6-diamino- Other pyridinic oxidation bases useful in the present invention are the 3-amino pyrazolo [1, 5-a] -pyridines oxidation bases or their addition salts described for example in the patent application FR 2801308. By way of example, mention may be made
pyrazol 5-a] pyridin-3-ylamine; 2-acetylamino pyrazolo [1,5-a] pyridin-3-ylamine; 2-morpholin-4-yl-pyrazolo 5-a] pyridin-3-ylamine; 3-aminopyrazol 5-a] pyridin-2-carboxylic acid, 2-methoxy-pyrazolo [1,5-a] pyridin-3-ylamino; (3-amino-pyrazolo [1,5-a] pyridin-7-yl) -methanol; 2- (3-Amino-pyrazolo [1,5-a] pyridin-5-yl) -ethanol; 2- (3-Amino-pyrazolo [1,5-a] pyridin-7-yl) ethanol; (3-Amino-pyrazolo [1,5-a] pyridin-2-yl) -methanol; 3,6-diamino
<Desc/Clms Page number 13><Desc / Clms Page number 13>
pyrazolo[1, 5-a]pyridine ; la 3, 4-diamino-pyrazolo[1, 5-a]pyridine ; la pyrazolo[1, 5- a]pyridine-3, 7 -diamine ; la 7 -morpholin-4-yl-pyrazolo[1, 5-a]pyridin-3-ylamine ; la pyrazolo[1, 5-a]pyridine-3, 5-diamine ; la 5-morpholin-4-yl-pyrazolo[1, 5-a]pyridin-3- ylamine ; le 2-[(3-amino-pyrazolo[1,5-a]pyridin-5-yl)-(2-hydroxyéthyl)-amino]-éthanol ; le 2-[(3-amino-pyrazolo[1,5-a]pyridin-7-yl)-(2-hydroxyéthyl)-amino]-éthanol ; la 3-
amino-pyrazol 5-a]pyridine-5-01 ; 3-amino-pyrazolo [1, 5-a] pyridine-4-ol la 3amino-pyrazolo [1, 5-a] pyridine-6-ol ; la 3-amino-pyrazolo [1, 5-a] pyridine-7-ol ainsi que leurs d'addition. pyrazolo [1,5-a] pyridine; 3,4-diamino-pyrazolo [1,5-a] pyridine; pyrazolo [1,5-a] pyridine-3,7-diamine; 7-morpholin-4-yl-pyrazolo [1,5-a] pyridin-3-ylamine; pyrazolo [1,5-a] pyridine-3,5-diamine; 5-morpholin-4-yl-pyrazolo [1,5-a] pyridin-3-ylamine; 2 - [(3-amino-pyrazolo [1,5-a] pyridin-5-yl) - (2-hydroxyethyl) amino] ethanol; 2 - [(3-Amino-pyrazolo [1,5-a] pyridin-7-yl) - (2-hydroxyethyl) amino] ethanol; 3-
amino-pyrazol 5-a] pyridine-5-01; 3-amino-pyrazolo [1,5-a] pyridin-4-ol; 3-amino-pyrazolo [1,5-a] pyridin-6-ol; 3-amino-pyrazolo [1,5-a] pyridin-7-ol as well as their addition.
Parmi les dérivés pyrimidiniques, on peut citer les composés décrits par exemple dans les brevets DE 2359399 ; JP 88-169571 ; JP 05-63124 ; EP 0770375 ou demande de brevet WO 96/15765 comme la 2, 4,5, 6-tétra-aminopyrimidine, la 4-hydroxy 2,5, 6triaminopyrimidine, la 2-hydroxy 4,5, 6-triaminopyrimidine, la 2,4-dihydroxy 5,6diaminopyrimidine, la 2,5, 6-triaminopyrimidine et leurs sels d'addition et leurs formes tautomères, lorsqu'il existe un équilibre tautomérique. Among the pyrimidine derivatives, mention may be made of the compounds described, for example, in DE 2359399; JP 88-169571; JP 05-63124; EP 0770375 or patent application WO 96/15765 such as 2, 4,5,6-tetraaminopyrimidine, 4-hydroxy 2,5,6triaminopyrimidine, 2-hydroxy 4,5,6-triaminopyrimidine 2,4 -dihydroxy-5,6-diaminopyrimidine, 2,5,6-triaminopyrimidine and their addition salts and their tautomeric forms, when tautomeric equilibrium exists.
Parmi les dérivés pyrazoliques, on peut citer les composés décrits dans les brevets DE 3843892, DE 4133957 et demandes de brevet WO 94/08969, WO 94/08970, FR-A-2 733 749 et DE 195 43 988 comme le 4,5-diamino 1-méthyl pyrazole, le 4,5-diamino 1- (ss-hydroxyéthyl) pyrazole, le 3,4-diamino pyrazole, le 4,5-diamino 1- (4'-chlorobenzyl) pyrazole, le 4,5-diamino 1, 3-diméthyl pyrazole, le 4,5-diamino 3-méthyl 1-phényl pyrazole, le 4,5-diamino 1-méthyl 3-phényl pyrazole, le 4-amino 1, 3-diméthyl 5hydrazino pyrazole, le 1-benzyl 4,5-diamino 3-méthyl pyrazole, le 4,5-diamino 3-tert- but yi 1-méthyl pyrazole, le 4, 5-diamino 1-tert-butyl 3-méthyl pyrazole, le 4,5-diamino 1-éthyl 3-méthyl pyrazole, le 4,5-diamino 1-éthyl 3- (4'-méthoxyphényl) pyrazole, le 4, 5diamino 1-éthyl 3-hydroxyméthyl pyrazole, le 4, 5-diamino 3-hydroxyméthyl 1-méthyl pyrazole, le 4,5-diamino 3-hydroxyméthyl 1-isopropyl pyrazole, le 4,5-diamino 3-méthyl 1-isopropyl pyrazole, le 4-amino 5- (2'-aminoéthyl) amino 1, 3-diméthyl pyrazole, le 3,4, 5-triamino pyrazole, le 1-méthyl 3,4, 5-triamino pyrazole, le 3,5-diamino 1-méthyl 4méthylamino pyrazole, le 3,5-diamino 4- (ss-hydroxyéthyl) amino 1-méthyl pyrazole, et leurs sels d'addition. Among the pyrazole derivatives, mention may be made of the compounds described in DE 3843892, DE 4133957 and patent applications WO 94/08969, WO 94/08970, FR-A-2 733 749 and DE 195 43 988 as the 4.5 1-methyl pyrazole, 4,5-diamino 1- (ss-hydroxyethyl) pyrazole, 3,4-diamino pyrazole, 4,5-diamino-1- (4'-chlorobenzyl) pyrazole, 4,5 1-diamino-1,3-dimethyl pyrazole, 4,5-diamino-3-methyl-1-phenylpyrazole, 4,5-diamino-1-methyl-3-phenylpyrazole, 4-amino-1,3-dimethylhydrazino pyrazole, 1-benzyl 4,5-diamino-3-methylpyrazole, 4,5-diamino-3-tert-butyl-1-methyl-pyrazole, 4,5-diamino-1-tert-butyl-3-methyl-pyrazole, 4,5 1-ethyl-3-methyl pyrazole, 4,5-diamino-1-ethyl-3- (4'-methoxyphenyl) pyrazole, 4,5-diamino-1-ethyl-3-hydroxymethyl-pyrazole, 4,5-diamino-3-hydroxymethyl 1-methyl pyrazole, 4,5-diamino-3-hydroxymethyl-1-isopropyl pyrazole, 4,5-diamino-3-methyl-1-isopropyl pyrazole, 4-amino-5- (2'-amin) oethyl) amino, 1,3-dimethyl pyrazole, 3,4,5-triamino pyrazole, 1-methyl-3,4,5-triamino pyrazole, 3,5-diamino-1-methyl-4-methylamino pyrazole, diamino 4- (5-hydroxyethyl) amino-1-methyl pyrazole, and their addition salts.
<Desc/Clms Page number 14><Desc / Clms Page number 14>
La ou les bases d'oxydation présentes dans la composition de l'invention sont en général présentent chacune en quantité comprise entre 0,001 à 10 % en poids environ du poids total de la composition tinctoriale, de préférence entre 0,005 et 6 %. The oxidation base (s) present in the composition of the invention are in general each present in an amount of between 0.001 to 10% by weight approximately of the total weight of the dyeing composition, preferably between 0.005 and 6%.
La composition selon l'invention peut contenir en plus du 4-hydroxy N-hydroxyéthyl indole un ou plusieurs coupleurs additionnels conventionnellement utilisés pour la teinture de fibres kératiniques. Parmi ces coupleurs, on peut notamment citer les métaphénylènediamines, les méta-aminophénols, les métadiphénols, les coupleurs naphtaléniques, les coupleurs hétérocycliques autres que le 4-hydroxy N-hydroxyéthyl indole ainsi que leur sels d'addition. The composition according to the invention may contain in addition to 4-hydroxy-N-hydroxyethylindole one or more additional couplers conventionally used for dyeing keratinous fibers. Among these couplers, mention may in particular be made of meta-phenylenediamines, meta-aminophenols, meta-diphenols, naphthalenic couplers, heterocyclic couplers other than 4-hydroxy-N-hydroxyethylindole and their addition salts.
A titre d'exemple, on peut citer le 2-méthyl 5-aminophénol, le 5-N- (ss- hydroxyéthyl) amino 2-méthyl phénol, le 6-chloro-2-méthyl-5-aminophénol, le 3-amino phénol, le 1,3-dihydroxy benzène, le 1,3-dihydroxy 2-méthyl benzène, le 4-chloro 1,3dihydroxy benzène, le 2,4-diamino l- (S-hydroxyéthytoxy) benzène, le 2-amino 4- (sshydroxyéthylamino) 1-méthoxybenzène, le 1,3-diamino benzène, le 1, 3-bis- (2, 4diaminophénoxy) propane, la 3-uréido aniline, le 3-uréido 1-diméthylamino benzène, le sésamol, le 1-ss-hydroxéthylamino-3,4-méthylènedioxybenzène, l'α-naphtol, le 2 méthyl-1-naphtol, le 6-hydroxy indole, le 4-hydroxy indole, le 4-hydroxy N-méthyl indole, la 2-amino-3-hydroxy pyridine, la 6-hydroxy benzomorpholine la 3,5-diamino- 2,6-diméthoxypyridine, le 1-N- (ss-hydroxyéthyl) amino-3, 4-méthylène dioxybenzène, le 2, 6-bis- (ss-hydroxyéthylamino) toluène et leurs sels d'addition. By way of example, mention may be made of 2-methyl-5-aminophenol, 5-N- (ss-hydroxyethyl) amino-2-methylphenol, 6-chloro-2-methyl-5-aminophenol and 3-amino. phenol, 1,3-dihydroxybenzene, 1,3-dihydroxy-2-methylbenzene, 4-chloro-1,3-dihydroxybenzene, 2,4-diamino-1- (S-hydroxyethoxy) benzene, 2-amino-4 - (sshydroxyethylamino) 1-methoxybenzene, 1,3-diamino benzene, 1,3-bis (2,4-diaminophenoxy) propane, 3-ureido aniline, 3-ureido 1-dimethylamino benzene, sesamol, -ss-hydroxethylamino-3,4-methylenedioxybenzene, α-naphthol, 2-methyl-1-naphthol, 6-hydroxyindole, 4-hydroxyindole, 4-hydroxy N -methylindole, 2- amino-3-hydroxy pyridine, 6-hydroxy benzomorpholine 3,5-diamino-2,6-dimethoxypyridine, 1-N- (ss-hydroxyethyl) amino-3,4-methylene dioxybenzene, 2,6-bis - (5-hydroxyethylamino) toluene and their addition salts.
Dans la composition de la présente invention, le ou les coupleurs sont chacun généralement présents en quantité comprise entre 0,001 et 10 % en poids environ du poids total de la composition tinctoriale, de préférence entre 0, 005 et 6 %. In the composition of the present invention, the coupler or couplers are each generally present in an amount of between 0.001 and 10% by weight of the total weight of the dyeing composition, preferably between 0.005 and 6%.
D'une manière générale, les sels d'addition des bases d'oxydation et des coupleurs utilisables dans le cadre de l'invention sont notamment choisis parmi les sels d'addition avec un acide tels que les chlorhydrates, les bromhydrates, les sulfates, les citrates, les succinates, les tartrates, les lactates, les tosylates, les benzènesulfonates, les phosphates et les acétates et les sels d'addition avec une base telles que la soude, la potasse, l'ammoniaque, les amines ou les alcanolamines. In general, the addition salts of the oxidation bases and couplers that can be used in the context of the invention are chosen especially from the addition salts with an acid such as hydrochlorides, hydrobromides, sulphates, citrates, succinates, tartrates, lactates, tosylates, benzenesulfonates, phosphates and acetates and addition salts with a base such as sodium hydroxide, potassium hydroxide, ammonia, amines or alkanolamines.
<Desc/Clms Page number 15> <Desc / Clms Page number 15>
La composition tinctoriale conforme à l'invention peut en outre contenir un ou plusieurs colorants directs pouvant notamment être choisis parmi les colorants nitrés de la série benzénique, les colorants directs azoïques, les colorants directs méthiniques. Ces colorants directs peuvent être de nature non ionique, anionique ou cationique. The dye composition in accordance with the invention may also contain one or more direct dyes that may be chosen in particular from nitro dyes of the benzene series, azo direct dyes and methine direct dyes. These direct dyes may be nonionic, anionic or cationic in nature.
Le milieu approprié pour la teinture appelé aussi support de teinture est généralement constitué par de l'eau ou par un mélange d'eau et d'au moins un solvant organique pour solubiliser les composés qui ne seraient pas suffisamment solubles dans l'eau. A titre de solvant organique, on peut par exemple citer les alcanols inférieurs en C1-C4, tels que l'methanol et l'isopropanol ; les polyols et éthers de polyols comme le 2butoxyéthanol, le propylèneglycol, le monométhyléther de propylèneglycol, le monoéthyléther et le monométhyléther du diéthytènegtycot, ainsi que les alcools aromatiques comme l'alcool benzylique ou le phénoxyéthanol, et leurs mélanges. The medium suitable for dyeing, also known as dyeing medium, generally consists of water or a mixture of water and at least one organic solvent for solubilizing compounds that are not sufficiently soluble in water. As organic solvent, there may be mentioned, for example lower C1-C4 alkanols, such as methanol and isopropanol; polyols and polyol ethers such as 2-butoxyethanol, propylene glycol, propylene glycol monomethyl ether, diethylene glycol monoethyl ether and monomethyl ether, as well as aromatic alcohols such as benzyl alcohol or phenoxyethanol, and mixtures thereof.
Les solvants sont, de préférence, présents dans des proportions de préférence comprises entre 1 et 40 % en poids environ par rapport au poids total de la composition tinctoriale, et encore plus préférentiellement entre 5 et 30 % en poids environ. The solvents are preferably present in proportions preferably of between 1 and 40% by weight approximately relative to the total weight of the dye composition, and even more preferably between 5 and 30% by weight approximately.
La composition tinctoriale conforme à l'invention peut également renfermer divers adjuvants utilisés classiquement dans les compositions pour la teinture des cheveux, tels que des agents tensio-actifs anioniques, cationiques, non-ioniques, amphotères, zwittérioniques ou leurs mélanges, des polymères anioniques, cationiques, non-ioniques, amphotères, zwittérioniques ou leurs mélanges, des agents épaississants minéraux ou organiques, et en particulier les épaississants associatifs polymères anioniques, cationiques, non ioniques et amphotères, des agents antioxydants, des agents de pénétration, des agents séquestrants, des parfums, des tampons, des agents dispersants, des agents de conditionnement tels que par exemple des silicones volatiles ou non volatiles, modifiées ou non modifiées, des agents filmogènes, des céramides, des agents conservateurs, des agents opacifiants. The dye composition in accordance with the invention may also contain various adjuvants conventionally used in compositions for dyeing hair, such as anionic, cationic, nonionic, amphoteric, zwitterionic surfactants or mixtures thereof, anionic polymers, cationic, nonionic, amphoteric, zwitterionic or their mixtures, inorganic or organic thickeners, and in particular anionic, cationic, nonionic and amphoteric polymeric associative thickeners, antioxidants, penetrating agents, sequestering agents, perfumes, buffers, dispersing agents, conditioning agents such as, for example, volatile or non-volatile silicones, modified or unmodified, film-forming agents, ceramides, preserving agents, opacifying agents.
Les adjuvants ci dessus sont en général présents en quantité comprise pour chacun d'eux entre 0, 01 et 20 % en poids par rapport au poids de la composition. The adjuvants above are generally present in an amount for each of them between 0.01 and 20% by weight relative to the weight of the composition.
<Desc/Clms Page number 16> <Desc / Clms Page number 16>
Bien entendu, l'homme de l'art veillera à choisir ce ou ces éventuels composés complémentaires de manière telle que les propriétés avantageuses attachées intrinsèquement à la composition de teinture d'oxydation conforme à l'invention ne soient pas, ou substantiellement pas, altérées par la ou les adjonctions envisagées. Of course, one skilled in the art will take care to choose this or these optional additional compounds such that the advantageous properties intrinsically attached to the oxidation dyeing composition according to the invention are not, or not substantially impaired by the addition or additions envisaged.
Le pH de la composition tinctoriale conforme à l'invention est généralement compris entre 3 et 12 environ, et de préférence entre 5 et 11 environ. Il peut être ajusté à la valeur désirée au moyen d'agents acidifiants ou alcalinisant habituellement utilisés en teinture des fibres kératiniques ou bien encore à l'aide de systèmes tampons classiques. The pH of the dye composition according to the invention is generally between 3 and 12 approximately, and preferably between 5 and 11 approximately. It can be adjusted to the desired value by means of acidifying or basifying agents usually used for dyeing keratinous fibers or else using conventional buffer systems.
Parmi les agents acidifiants, on peut citer, à titre d'exemple, les acides minéraux ou organiques comme l'acide chlorhydrique, l'acide orthophosphorique, l'acide sulfurique, les acides carboxyliques comme l'acide acétique, l'acide tartrique, l'acide citrique, l'acide lactique, les acides sulfoniques. Among the acidifying agents, mention may be made, by way of example, of mineral or organic acids such as hydrochloric acid, orthophosphoric acid, sulfuric acid, carboxylic acids such as acetic acid, tartaric acid, citric acid, lactic acid, sulphonic acids.
Parmi les agents alcalinisant on peut citer, à titre d'exemple, l'ammoniaque, les carbonates alcalins, les alcanolamines telles que les mono-, di-et triéthanolamines ainsi que leurs dérivés, les hydroxydes de sodium ou de potassium et les composés de formule (III) suivante :
dans laquelle West un reste propylène éventuellement substitué par un groupement hydroxyle ou un radical alkyle en C1-C4 ; Ra, Rb, Re et Rd, identiques ou différents, représentent un atome d'hydrogène, un radical alkyle en Ci-C4 ou hydroxyalkyl en C1-C4. Among the alkalinizing agents, mention may be made, for example, of ammonia, alkaline carbonates, alkanolamines such as mono-, di- and triethanolamines and their derivatives, sodium or potassium hydroxides and following formula (III):
wherein West a propylene residue optionally substituted with a hydroxyl group or a C1-C4 alkyl radical; Ra, Rb, Re and Rd, which may be identical or different, represent a hydrogen atom, a C1-C4 alkyl radical or a C1-C4 hydroxyalkyl radical.
La composition tinctoriale selon l'invention peut se présenter sous des formes diverses, telles que sous forme de liquides, de crèmes, de gels, ou sous toute autre forme appropriée pour réaliser une teinture des fibres kératiniques, et notamment des cheveux humains. The dye composition according to the invention may be in various forms, such as in the form of liquids, creams, gels, or in any other form suitable for dyeing keratinous fibers, and especially human hair.
<Desc/Clms Page number 17> <Desc / Clms Page number 17>
Le procédé de la présente invention est un procédé dans lequel on applique sur les fibres la composition selon la présente invention telle que définie précédemment, et qu'on révèle la couleur à l'aide d'un agent oxydant. La couleur peut être révélée à pH acide, neutre ou alcalin et l'agent oxydant peut être ajouté à la composition de l'invention juste au moment de l'emploi ou il peut être mis en oeuvre à partir d'une composition oxydante le contenant, appliquée simultanément ou séquentiellement à la composition de l'invention Selon un mode de réalisation particulier, la composition selon la présente invention est mélangée, de préférence au moment de l'emploi, à une composition contenant, dans un milieu approprié pour la teinture, au moins un agent oxydant, cet agent oxydant étant présent en une quantité suffisante pour développer une coloration. Le mélange obtenu est ensuite appliqué sur les fibres kératiniques. Après un temps de pose de 3 à 50 minutes environ, de préférence 5 à 30 minutes environ, les fibres kératiniques sont rincées, lavées au shampooing, rincées à nouveau puis séchées. The process of the present invention is a process in which the composition according to the present invention as defined above is applied to the fibers, and the color is revealed using an oxidizing agent. The color can be revealed at acidic, neutral or alkaline pH and the oxidizing agent can be added to the composition of the invention just at the time of use or it can be used from an oxidizing composition containing it , applied simultaneously or sequentially to the composition of the invention According to a particular embodiment, the composition according to the present invention is mixed, preferably at the time of use, with a composition containing, in a medium suitable for dyeing, at least one oxidizing agent, this oxidizing agent being present in an amount sufficient to develop a coloration. The mixture obtained is then applied to the keratinous fibers. After a residence time of about 3 to 50 minutes, preferably about 5 to 30 minutes, the keratinous fibers are rinsed, washed with shampoo, rinsed again and then dried.
Les agents oxydants classiquement utilisés pour la teinture d'oxydation des fibres kératiniques sont par exemple le peroxyde d'hydrogène, le peroxyde d'urée, les bromates de métaux alcalins, les persels tels que les perborates et persulfates, les peracides et les enzymes oxydases parmi lesquelles on peut citer les peroxydases, les oxydo-réductases à 2 électrons telles que les uricases et les oxygénases à 4 électrons comme les laccases. Le peroxyde d'hydrogène est particulièrement préféré. The oxidizing agents conventionally used for the oxidation dyeing of keratin fibers are, for example, hydrogen peroxide, urea peroxide, alkali metal bromates, persalts such as perborates and persulfates, peracids and oxidase enzymes. among which there may be mentioned peroxidases, 2-electron oxidoreductases such as uricases and 4-electron oxygenases such as laccases. Hydrogen peroxide is particularly preferred.
La composition oxydante peut également renfermer divers adjuvants utilisés classiquement dans les compositions pour la teinture des cheveux et tels que définis précédemment. The oxidizing composition may also contain various adjuvants conventionally used in compositions for dyeing hair and as defined above.
Le pH de la composition oxydante renfermant l'agent oxydant est tel qu'après mélange avec la composition tinctoriale, le pH de la composition résultante appliquée sur les fibres kératiniques varie de préférence entre 3 et 12 environ, et encore plus préférentiellement entre 5 et 11. Il peut être ajusté à la valeur désirée au moyen d'agents acidifiants ou alcalinisant habituellement utilisés en teinture des fibres kératiniques et tels que définis précédemment. The pH of the oxidizing composition containing the oxidizing agent is such that, after mixing with the dyeing composition, the pH of the resulting composition applied to the keratinous fibers preferably varies between 3 and 12 approximately, and even more preferably between 5 and 11. It can be adjusted to the desired value by means of acidifying or basifying agents usually used for dyeing keratin fibers and as defined above.
<Desc/Clms Page number 18> <Desc / Clms Page number 18>
La composition prête à l'emploi qui est finalement appliquée sur les fibres kératiniques peut se présenter sous des formes diverses, telles que sous forme de liquides, de crèmes, de gels ou sous toute autre forme appropriée pour réaliser une teinture des fibres kératiniques, et notamment des cheveux humains. The ready-to-use composition which is finally applied to the keratin fibers may be in various forms, such as in the form of liquids, creams, gels or in any other form suitable for dyeing keratinous fibers, and including human hair.
L'invention a aussi pour objet un dispositif à plusieurs compartiments ou"kit"de teinture dans lequel un premier compartiment renferme la composition tinctoriale définie ci-dessus et un deuxième compartiment renferme une composition oxydante. Ce dispositif peut être équipé d'un moyen permettant de délivrer sur les cheveux le mélange souhaité, tel que les dispositifs décrits dans le brevet FR-2 586 913 au nom de la demanderesse. The invention also relates to a multi-compartment device or "kit" of dyeing in which a first compartment contains the dye composition defined above and a second compartment contains an oxidizing composition. This device may be equipped with means for delivering the desired mixture to the hair, such as the devices described in patent FR-2 586 913 in the name of the applicant.
A partir de ce dispositif, il est possible de teindre les fibres kératiniques à partir d'un procédé qui comprend le mélange d'une composition tinctoriale conforme à l'invention avec un agent oxydant tel que défini précédemment, et l'application du mélange obtenu sur les fibres kératiniques pendant un temps suffisant pour développer la coloration désirée.
i Les exemples qui suivent servent à illustrer l'invention sans toutefois présenter un caractère limitatif. From this device, it is possible to dye the keratinous fibers from a process which comprises mixing a dye composition according to the invention with an oxidizing agent as defined above, and the application of the mixture obtained. on the keratinous fibers for a time sufficient to develop the desired coloration.
The following examples serve to illustrate the invention without being limiting in nature.
<Desc/Clms Page number 19> <Desc / Clms Page number 19>
EXEMPLES DE FORMULATION On a préparé les compositions tinctoriales suivantes (teneurs en grammes) :
EXAMPLES OF FORMULATION The following dye compositions were prepared (contents in grams):
<tb>
<tb> Compositions1234
<tb> 1,075
<tb> 4, <SEP> 5-diamino-1-2'hydroxyéthyl)pyrazole, <SEP> dichlorhydrate
<tb> (base <SEP> d'oxydation)
<tb> pyrazolo-[1,5-a]-pyrimidine-3,7- <SEP> - <SEP> 0,311
<tb> diamine, <SEP> dichlorhydrate
<tb> (base <SEP> d'oxydation)
<tb> 3-amino-7-méthyamino-pyrazolo- <SEP> 1, <SEP> 180
<tb> [1,5-a]-pyrimidine-3, <SEP> 7-diamine,
<tb> dichlorhydrate <SEP> (base <SEP> d'oxydation)
<tb> N-4-aminophényl <SEP> 3-hydroxy- <SEP> - <SEP> - <SEP> - <SEP> 1,255
<tb> pyrrolidine <SEP> (base <SEP> d'oxydation)
<tb> 4-hydroxy <SEP> 1-N-(ss-hydroxyéthyl) <SEP> indole <SEP> 0,885 <SEP> 0,248 <SEP> 0,885 <SEP> 0,885
<tb> (coupleur)
<tb> Support <SEP> de <SEP> teinture <SEP> commun
<tb> Eau <SEP> déminéralisée <SEP> q. <SEP> s. <SEP> p. <SEP> 100g <SEP> 100g <SEP> 100g <SEP> 100g
<tb> <Tb>
<tb> Compositions1234
<tb> 1,075
<tb> 4, <SEP> 5-diamino-1-2 hydroxyethyl) pyrazole, <SEP> dihydrochloride
<tb> (oxidation base <SEP>)
<tb> pyrazolo [1,5-a] pyrimidine-3,7- <SEP> - <SEP> 0.311
<tb> diamine, <SEP> dihydrochloride
<tb> (oxidation base <SEP>)
<tb> 3-Amino-7-methylamino-pyrazolo- <SEP> 1, <SEP> 180
<tb> [1,5-a] -pyrimidine-3, <SEP> 7-diamine,
<tb> dihydrochloride <SEP> (base <SEP> oxidation)
<tb> N-4-aminophenyl <SEP> 3-hydroxy- <SEP> - <SEP> - <SEP> - <SEP> 1.255
<tb> pyrrolidine <SEP> (oxidation base <SEP>)
<tb> 4-hydroxy <SEP> 1-N- (ss-hydroxyethyl) <SEP> indole <SEP> 0.885 <SEP> 0.248 <SEP> 0.885 <SEP> 0.885
<tb> (coupler)
<tb> Support <SEP> of <SEP> common <SEP> dyeing
<tb> Demineralized <SEP> Water <SEP> q. <SEP> s. <SEP> p. <SEP> 100g <SEP> 100g <SEP> 100g <SEP> 100g
<Tb>
<tb>
<tb> (**) <SEP> : <SEP> support <SEP> de <SEP> teinture <SEP> commun <SEP> :
<tb> - <SEP> Alcool <SEP> oléique <SEP> polyglycérolé <SEP> à <SEP> 2 <SEP> moles <SEP> de <SEP> glycérol <SEP> 4, <SEP> 0g
<tb> - <SEP> Alcool <SEP> oléique <SEP> polyglycérolé <SEP> à <SEP> 4 <SEP> moles <SEP> de <SEP> glycérol <SEP> à <SEP> 78 <SEP> % <SEP> de
<tb> matières <SEP> actives <SEP> (M. <SEP> A. <SEP> ) <SEP> 5,69g <SEP> M. <SEP> A.
<tb> <Tb>
<tb> (**) <SEP>: <SEP> support <SEP> of <SEP> dye <SEP> common <SEP>:
<tb> - <SEP> Alcohol <SEP> Oleic <SEP> polyglycerolated <SEP> to <SEP> 2 <SEP> moles <SEP> of <SEP> glycerol <SEP> 4, <SEP> 0g
<tb> - <SEP> Alcohol <SEP> Oleic <SEP> polyglycerolated <SEP> at <SEP> 4 <SEP> moles <SEP> of <SEP> glycerol <SEP> at <SEP> 78 <SEP>% <SEP > from
<tb> active <SEP> materials <SEP> (M. <SEP> A. <SEP>) <SEP> 5,69g <SEP> M. <SEP> A.
<Tb>
- <SEP> Acide <SEP> oléique <SEP> 3, <SEP> 0g
<tb> - <SEP> Amine <SEP> oléique <SEP> à <SEP> 2 <SEP> moles <SEP> d'oxyde <SEP> d'éthylène <SEP> vendue <SEP> sous <SEP> la
<tb> dénomination <SEP> commerciale <SEP> ETHOMEEN <SEP> 012 <SEP> par <SEP> la <SEP> société <SEP> AKZO <SEP> 7, <SEP> 0g
<tb> - <SEP> Laurylamino <SEP> succinamate <SEP> de <SEP> diéthylaminopropyle, <SEP> sel <SEP> de <SEP> sodium
<tb> - <SEP> Oleic <SEP> acid <SEP> 3, <SEP> 0g
<tb> - <SEP> Amine <SEP> oleic <SEP> to <SEP> 2 <SEP> moles <SEP> of oxide <SEP> of ethylene <SEP> sold <SEP> under <SEP>
<tb> denomination <SEP> commercial <SEP> ETHOMEEN <SEP> 012 <SEP> by <SEP> the <SEP> company <SEP> AKZO <SEP> 7, <SEP> 0g
<tb> - <SEP> Laurylamino <SEP> succinamate <SEP> of <SEP> diethylaminopropyl, <SEP> salt <SEP> of <SEP> sodium
<Tb>
<Desc/Clms Page number 20> <Desc / Clms Page number 20>
<tb>
<tb> à <SEP> 55 <SEP> % <SEP> de <SEP> M. <SEP> A. <SEP> 3, <SEP> 0g <SEP> M. <SEP> A.
<tb> <Tb>
<tb> to <SEP> 55 <SEP>% <SEP> from <SEP> M. <SEP> A. <SEP> 3, <SEP> 0g <SEP> M. <SEP> A.
<Tb>
Alcool <SEP> oléique <SEP> 5, <SEP> Og
<tb> diethanolamide <SEP> d'acide <SEP> oléique <SEP> 12, <SEP> 0g
<tb> - <SEP> Propylèneglycol <SEP> 3,5g
<tb> - <SEP> Alcool <SEP> éthylique <SEP> 7, <SEP> 0g
<tb> - <SEP> Dipropylèneglycol <SEP> 0,5g
<tb> - <SEP> Monométhyléther <SEP> de <SEP> propylèneglycol <SEP> 9, <SEP> 0g
<tb> - <SEP> Métabisuifite <SEP> de <SEP> sodium <SEP> à <SEP> en <SEP> solution <SEP> aqueuse <SEP> à <SEP> 35 <SEP> % <SEP> de <SEP> M. <SEP> A. <SEP> 0,455g <SEP> M. <SEP> A.
<tb> Alcohol <SEP> Oleic <SEP> 5, <SEP> Og
<tb> diethanolamide <SEP> of <SEP> oleic acid <SEP> 12, <SEP> 0g
<tb> - <SEP> Propylene Glycol <SEP> 3.5g
<tb> - <SEP> Alcohol <SEP> ethyl <SEP> 7, <SEP> 0g
<tb> - <SEP> Dipropylene Glycol <SEP> 0.5g
<tb> - <SEP> Monomethyl Ether <SEP> of <SEP> Propylene Glycol <SEP> 9, <SEP> 0g
<tb> - <SEP> Metabisuifite <SEP> from <SEP> sodium <SEP> to <SEP> in <SEP> solution <SEP> aqueous <SEP> to <SEP> 35 <SEP>% <SEP> from <SEP > M. <SEP> A. <SEP> 0.455g <SEP> M. <SEP> A.
<Tb>
- <SEP> Acétate <SEP> d'ammonium <SEP> 0,8g
<tb> - <SEP> Antioxydant, <SEP> séquestrant <SEP> q. <SEP> s.
<tb> - <SEP> Acetate <SEP> Ammonium <SEP> 0.8g
<tb> - <SEP> Antioxidant, <SEP> sequestering <SEP> q. <SEP> s.
<Tb>
- <SEP> Parfum, <SEP> conservateur <SEP> q. <SEP> s.
<tb> - <SEP> Fragrance, <SEP> preservative <SEP> q. <SEP> s.
<Tb>
- <SEP> Ammoniaque <SEP> à <SEP> 20 <SEP> % <SEP> de <SEP> NH3 <SEP> 10, <SEP> 0g
<tb>
Au moment de l'emploi, chaque composition tinctoriale 1 à 4 a été mélangée avec un poids égal d'eau oxygénée à 20 volumes de pH 3 (6% en poids). - <SEP> Ammonia <SEP> to <SEP> 20 <SEP>% <SEP> of <SEP> NH3 <SEP> 10, <SEP> 0g
<Tb>
At the time of use, each dye composition 1 to 4 was mixed with an equal weight of hydrogen peroxide at 20 volumes of pH 3 (6% by weight).
Chaque mélange obtenu a été appliqué pendant 30 minutes, sur des mèches de cheveux gris naturels à 90 % de blancs permanentés à raison de 30g pour 3g de cheveux. Après rinçage, lavage avec un shampooing standard et séchage, les mèches ont été teintes dans les nuances figurant dans le tableau 1 ci-dessous ; elles ont été évaluées visuellement.
Each mixture obtained was applied for 30 minutes, on strands of gray hair natural to 90% of white permed at a rate of 30g for 3g of hair. After rinsing, washing with standard shampoo and drying, the locks were dyed in the shades shown in Table 1 below; they were assessed visually.
<tb>
<tb> <Tb>
<Tb>
Exempte <SEP> 1 <SEP> 2 <SEP> 3 <SEP> 4
<tb> Hauteur <SEP> de <SEP> ton <SEP> Châtain <SEP> Châtain <SEP> Châtain <SEP> Châtain
<tb> fonçé <SEP> clair <SEP> fonçé
<tb> Reflet <SEP> Rose <SEP> Violine <SEP> Acajou <SEP> Bleu
<tb> fuchsia <SEP> irisé <SEP> irisé <SEP> profond
<tb> Exempt <SEP> 1 <SEP> 2 <SEP> 3 <SEP> 4
<tb> Height <SEP> of <SEP> tone <SEP> Chestnut <SEP> Chestnut <SEP> Chestnut <SEP> Chestnut
<tb> dark <SEP> clear <SEP> dark
<tb> Reflection <SEP> Pink <SEP> Violine <SEP> Mahogany <SEP> Blue
<tb> fuchsia <SEP> iridescent <SEP> iridescent <SEP> deep
<Tb>
Claims (26)
Priority Applications (1)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| FR0113764A FR2831055B1 (en) | 2001-10-24 | 2001-10-24 | TINCTORIAL COMPOSITION COMPRISING AT LEAST ONE HETEROCYCLIC BASE AND 1-N- (B-HYDROXYETHYL) 4-HYDROXY INDOLE AS A COUPLER; DYEING PROCESSES |
Applications Claiming Priority (1)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| FR0113764A FR2831055B1 (en) | 2001-10-24 | 2001-10-24 | TINCTORIAL COMPOSITION COMPRISING AT LEAST ONE HETEROCYCLIC BASE AND 1-N- (B-HYDROXYETHYL) 4-HYDROXY INDOLE AS A COUPLER; DYEING PROCESSES |
Publications (2)
| Publication Number | Publication Date |
|---|---|
| FR2831055A1 true FR2831055A1 (en) | 2003-04-25 |
| FR2831055B1 FR2831055B1 (en) | 2004-05-28 |
Family
ID=8868679
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| FR0113764A Expired - Fee Related FR2831055B1 (en) | 2001-10-24 | 2001-10-24 | TINCTORIAL COMPOSITION COMPRISING AT LEAST ONE HETEROCYCLIC BASE AND 1-N- (B-HYDROXYETHYL) 4-HYDROXY INDOLE AS A COUPLER; DYEING PROCESSES |
Country Status (1)
| Country | Link |
|---|---|
| FR (1) | FR2831055B1 (en) |
Cited By (10)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| FR2864957A1 (en) * | 2004-01-09 | 2005-07-15 | Oreal | Dyeing composition for keratin fibers, especially human hair, containing new or known p-phenylene diamine derivative with one amine group in heptamethyleneimine form as oxidation base |
| WO2005068431A1 (en) * | 2004-01-09 | 2005-07-28 | L'oreal | Composition for dyeing keratin fibres, comprising at least one para-phenylenediamine derivative |
| US8444714B2 (en) | 2011-02-22 | 2013-05-21 | The Procter & Gamble Company | Oxidative dyeing compositions comprising an 1-Hexy1/Hepty1-4,5-diaminopyrazole and a benzene-1,3-diol and derivatives thereof |
| US8444709B2 (en) | 2011-02-22 | 2013-05-21 | The Procter & Gamble Company | Oxidative dyeing compositions comprising an 1-hexyl/heptyl-4,5-diaminopyrazole and a 2-aminophenol and derivatives thereof |
| US8444710B2 (en) | 2011-02-22 | 2013-05-21 | The Procter & Gamble Company | Oxidative dyeing compositions comprising an 1-hexyl/heptyl-4,5-diaminopyrazole and a m-aminophenol and derivatives thereof |
| US8444711B2 (en) | 2011-02-22 | 2013-05-21 | The Procter & Gamble Company | Oxidative dyeing compositions comprising an 1-hexyl/heptyl-4,5-diaminopyrazole and a benzene-1,3-diamine and derivatives thereof |
| US8444712B2 (en) | 2011-02-22 | 2013-05-21 | The Procter & Gamble Company | Oxidative dyeing compositions comprising an 1-hexyl/heptyl-4,5-diaminopyrazole and a benzo[1,3]dioxol-5-ylamine and derivatives thereof |
| US8444713B2 (en) | 2011-02-22 | 2013-05-21 | The Procter & Gamble Company | Oxidative dyeing compositions comprising an 1-hexyl/heptyl-4,5-diaminopyrazole and a naphthalen-1-ol and derivatives thereof |
| US8460397B2 (en) | 2011-02-22 | 2013-06-11 | The Procter & Gamble Company | Oxidative dyeing compositions comprising an 1-hexyl/heptyl-4,5-diaminopyrazole and a pyridine and derivatives thereof |
| US10800782B2 (en) | 2016-08-31 | 2020-10-13 | Agios Pharmaceutical, Inc. | Inhibitors of cellular metabolic processes |
Families Citing this family (2)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| EP2628731B1 (en) | 2012-02-16 | 2014-04-23 | The Procter and Gamble Company | 1-Hexyl-1H-pyrazole-4,5-diamine hemisulfate, and its use in dyeing compositions |
| EP2628730B1 (en) | 2012-02-16 | 2017-12-06 | Noxell Corporation | Telescoping synthesis of 5-amino-4-nitroso-1-alkyl-1h-pyrazole salts |
Citations (7)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| DE3843892A1 (en) * | 1988-12-24 | 1990-06-28 | Wella Ag | OXIDATION HAIR AGENTS CONTAINING DIAMINOPYRAZOL DERIVATIVES AND NEW DIAMINOPYRAZOLE DERIVATIVES |
| US5609649A (en) * | 1989-11-10 | 1997-03-11 | L'oreal | Tinctorial composition for keratinous fibers containing oxidation dye precursors and couplers derived from 4-hydroxyindole, and dyeing method using them |
| FR2750048A1 (en) * | 1996-06-21 | 1997-12-26 | Oreal | KERATIN FIBER DYEING COMPOSITIONS CONTAINING PYRAZOLO- (1,5-A) -PYRIMIDINE DERIVATIVES, DYEING PROCESS, NOVEL PYRAZOLO- (1,5-A) -PYRIMIDINE DERIVATIVES AND PROCESS FOR PREPARING THE SAME |
| US5704948A (en) * | 1995-07-13 | 1998-01-06 | L'oreal | Compositions for dyeing keratinous fibers and keratinous fiber dyeing processes with derivatives of 4-hydroxyindole and oxidation bases |
| US5785717A (en) * | 1995-02-27 | 1998-07-28 | L'oreal | Composition for the oxidation dyeing of keratin fibers, comprising a diaminopyrazole derivative and a heterocyclic coupler, and dyeing process |
| FR2791563A1 (en) * | 1999-03-29 | 2000-10-06 | Oreal | KERATIN FIBER OXIDATION DYEING COMPOSITION AND DYEING METHOD USING THE SAME |
| FR2805737A1 (en) * | 2000-03-06 | 2001-09-07 | Oreal | Composition for oxidation dyeing of keratinic fibers, especially human hair, comprises a substituted p-phenylenediamine developer and a coupler |
-
2001
- 2001-10-24 FR FR0113764A patent/FR2831055B1/en not_active Expired - Fee Related
Patent Citations (7)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| DE3843892A1 (en) * | 1988-12-24 | 1990-06-28 | Wella Ag | OXIDATION HAIR AGENTS CONTAINING DIAMINOPYRAZOL DERIVATIVES AND NEW DIAMINOPYRAZOLE DERIVATIVES |
| US5609649A (en) * | 1989-11-10 | 1997-03-11 | L'oreal | Tinctorial composition for keratinous fibers containing oxidation dye precursors and couplers derived from 4-hydroxyindole, and dyeing method using them |
| US5785717A (en) * | 1995-02-27 | 1998-07-28 | L'oreal | Composition for the oxidation dyeing of keratin fibers, comprising a diaminopyrazole derivative and a heterocyclic coupler, and dyeing process |
| US5704948A (en) * | 1995-07-13 | 1998-01-06 | L'oreal | Compositions for dyeing keratinous fibers and keratinous fiber dyeing processes with derivatives of 4-hydroxyindole and oxidation bases |
| FR2750048A1 (en) * | 1996-06-21 | 1997-12-26 | Oreal | KERATIN FIBER DYEING COMPOSITIONS CONTAINING PYRAZOLO- (1,5-A) -PYRIMIDINE DERIVATIVES, DYEING PROCESS, NOVEL PYRAZOLO- (1,5-A) -PYRIMIDINE DERIVATIVES AND PROCESS FOR PREPARING THE SAME |
| FR2791563A1 (en) * | 1999-03-29 | 2000-10-06 | Oreal | KERATIN FIBER OXIDATION DYEING COMPOSITION AND DYEING METHOD USING THE SAME |
| FR2805737A1 (en) * | 2000-03-06 | 2001-09-07 | Oreal | Composition for oxidation dyeing of keratinic fibers, especially human hair, comprises a substituted p-phenylenediamine developer and a coupler |
Cited By (11)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| FR2864957A1 (en) * | 2004-01-09 | 2005-07-15 | Oreal | Dyeing composition for keratin fibers, especially human hair, containing new or known p-phenylene diamine derivative with one amine group in heptamethyleneimine form as oxidation base |
| WO2005068431A1 (en) * | 2004-01-09 | 2005-07-28 | L'oreal | Composition for dyeing keratin fibres, comprising at least one para-phenylenediamine derivative |
| US8444714B2 (en) | 2011-02-22 | 2013-05-21 | The Procter & Gamble Company | Oxidative dyeing compositions comprising an 1-Hexy1/Hepty1-4,5-diaminopyrazole and a benzene-1,3-diol and derivatives thereof |
| US8444709B2 (en) | 2011-02-22 | 2013-05-21 | The Procter & Gamble Company | Oxidative dyeing compositions comprising an 1-hexyl/heptyl-4,5-diaminopyrazole and a 2-aminophenol and derivatives thereof |
| US8444710B2 (en) | 2011-02-22 | 2013-05-21 | The Procter & Gamble Company | Oxidative dyeing compositions comprising an 1-hexyl/heptyl-4,5-diaminopyrazole and a m-aminophenol and derivatives thereof |
| US8444711B2 (en) | 2011-02-22 | 2013-05-21 | The Procter & Gamble Company | Oxidative dyeing compositions comprising an 1-hexyl/heptyl-4,5-diaminopyrazole and a benzene-1,3-diamine and derivatives thereof |
| US8444712B2 (en) | 2011-02-22 | 2013-05-21 | The Procter & Gamble Company | Oxidative dyeing compositions comprising an 1-hexyl/heptyl-4,5-diaminopyrazole and a benzo[1,3]dioxol-5-ylamine and derivatives thereof |
| US8444713B2 (en) | 2011-02-22 | 2013-05-21 | The Procter & Gamble Company | Oxidative dyeing compositions comprising an 1-hexyl/heptyl-4,5-diaminopyrazole and a naphthalen-1-ol and derivatives thereof |
| US8460397B2 (en) | 2011-02-22 | 2013-06-11 | The Procter & Gamble Company | Oxidative dyeing compositions comprising an 1-hexyl/heptyl-4,5-diaminopyrazole and a pyridine and derivatives thereof |
| US10800782B2 (en) | 2016-08-31 | 2020-10-13 | Agios Pharmaceutical, Inc. | Inhibitors of cellular metabolic processes |
| US11325914B1 (en) | 2016-08-31 | 2022-05-10 | Servier Pharmaceuticals Llc | Inhibitors of cellular metabolic processes |
Also Published As
| Publication number | Publication date |
|---|---|
| FR2831055B1 (en) | 2004-05-28 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| EP1927377B1 (en) | Dye composition of acidic pH comprising 2,3-diamino-6,7-dihydro-1H,5H-pyrazolo[1,2-a]pyrazol-1-one, a para- phenylenediamine, a meta-aminophenol and an oxidizing agent | |
| FR2920090A1 (en) | Composition for coloring keratin fibers, preferably human hair, comprises amino pyrazolopyridine oxidation bases, couplers, and surfactants comprising alkyl ether carboxylic acid and alkyl polyglucosides | |
| FR2806299A1 (en) | COMPOSITIONS FOR DYEING KERATIN FIBERS CONTAINING PYRROLIDINYL GROUP PARAPHENYLENEDIAMINE DERIVATIVES | |
| FR2830190A1 (en) | TINCTORIAL COMPOSITION COMPRISING A DIAMINOPYRAZOLE TYPE OXIDATION BASE, A HETEROCYCLIC OXIDATION BASE AND A COUPLER | |
| FR2845283A1 (en) | Composition for dyeing keratinic fibers, e.g. hair, comprises a 2,3-diaminopyridine coupler and a heterocyclic developer | |
| FR2822692A1 (en) | COMPOSITION FOR THE OXIDIZATION DYE CONTAINING AT LEAST ONE 3-AMINO PYRAZOLO- [1,5-A] -PYRIDINE OXIDIZATION BASE AND AT LEAST ONE PARTICULAR AMINOPHENOL COUPLER | |
| FR2831055A1 (en) | Oxidation dye composition, useful for dyeing keratinic fibers, comprises 1-(2-hydroxyethyl)-4-hydroxyindole as a coupler and a heterocyclic developer | |
| FR2822691A1 (en) | COMPOSITION FOR OXIDATION DYE CONTAINING AT LEAST ONE 3-AMINO PYRAZOLO- [1,5-A] -PYRIDINE OXIDATION BASE AND AT LEAST ONE PARTICULAR PYRIDINE COUPLER | |
| FR2830192A1 (en) | TINCTORIAL COMPOSITION COMPRISING A DIAMINOPYRAZOLE TYPE OXIDATION BASE AND A PYRAZOLO-AZOLE COUPLER | |
| FR2822689A1 (en) | COMPOSITION FOR THE OXIDIZING DYE CONTAINING AT LEAST ONE 3-AMINO PYRAZOLO- [1,5-A] PYRIDINE OXIDIZATION BASE AND AT LEAST ONE 2-AMINO 3-HYDROXY PYRIDINE COUPLER | |
| FR2856290A1 (en) | TINCTORIAL COMPOSITION COMPRISING 4,5-DIAMINO-1- (B-HYDROXYETHYL) -1H-PYRAZOLE AS OXIDATION BASE AND 2,6-DIHYDROXY-3,4-DIMETHYL PYRIDINE AS A COUPLER | |
| FR2830191A1 (en) | TINCTORIAL COMPOSITION COMPRISING A DIAMINOPYRAZOLE TYPE OXIDATION BASE, AN AMINO CYCLIC GROUPED PARAPHENYLENEDIAMINE OXIDATION BASE AND A COUPLER | |
| FR2845281A1 (en) | TINCTORIAL COMPOSITION COMPRISING AT LEAST ONE PYRAZOLOPYRIMIDINE OXIDATION BASE AND AT LEAST ONE 6-ALCOXY 2,3-DIAMINOPYRIDIMINE COUPLER | |
| FR2856922A1 (en) | TINCTORIAL COMPOSITION COMPRISING AT LEAST ONE OXIDATION BASE, 2-CHLORO 6-METHYL 3-AMINOPHENOL AND 3-METHYL 1-PHENYL 5-PYRAZOLONE | |
| EP1334713B1 (en) | Dyeing composition comprising diaminopyrazole and cationic compounds as oxidation bases and a coupling agent | |
| FR2844271A1 (en) | BIS-PARAPHENYLENEDIAMINE DERIVATIVES WITH PYRROLIDINYL GROUPING AND USE THEREOF FOR THE COLORING OF KERATIN FIBERS | |
| FR2915882A1 (en) | Coloring composition, useful for coloring keratin fibers, preferably human hair, comprises amino pyrazolopyridine oxidation bases, and one or more dye comprising indole compound or benzene-1,2,4-triol, in a coloring medium | |
| EP1586302A1 (en) | Dye composition comprising at least one pyrazolopyrimidine as oxidation base and at least one 6-alkoxy-2,3-diaminopyridine as coupler | |
| FR2860151A1 (en) | TINCTORIAL COMPOSITION COMPRISING AT LEAST ONE HYDROXYALKYLATED SECONDARY PARAPHENYLENEDIAMINE BASE, AT LEAST ONE COUPLER AND OCTYLDODECANOL | |
| FR2852241A1 (en) | Composition for oxidation dyeing of human hair, comprises a heterocyclic base and, as coupler, a 2,3,5-trisamino-pyridine derivative, provides gray or black shades | |
| FR2860152A1 (en) | TINCTORIAL COMPOSITION COMPRISING AT LEAST ONE HYDROXALKYLATED SECONDARY PARAPHENYLENEDIAMINE BASE, AT LEAST ONE COUPLER AND ETIDRONIC ACID | |
| EP1518539A1 (en) | Dye composition comprising a secondary, hydroxyalkylated p-phenylendiamine, a second base and as coupling agent a m-diphenol and/or a m-aminophenol | |
| EP1586303A1 (en) | Dye composition comprising at least one diaminopyrazole as oxidation base and at least one 6-alkoxy-2,3-diaminopyridine as coupler | |
| FR2845282A1 (en) | Composition useful for dyeing keratinic fibers, e.g. hair, comprises a 2,3-diaminopyridine coupler and a non-fused pyrazole developer | |
| FR2906462A1 (en) | Composition useful for oxidation dyeing of keratinous fibers, comprises an oxidation base comprising diamino-N,N-dihydropyrazolone derivatives and vitamin E and its esters |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| ST | Notification of lapse |
Effective date: 20090630 |