EP0222557A2 - Liquid detergent composition - Google Patents
Liquid detergent composition Download PDFInfo
- Publication number
- EP0222557A2 EP0222557A2 EP86308453A EP86308453A EP0222557A2 EP 0222557 A2 EP0222557 A2 EP 0222557A2 EP 86308453 A EP86308453 A EP 86308453A EP 86308453 A EP86308453 A EP 86308453A EP 0222557 A2 EP0222557 A2 EP 0222557A2
- Authority
- EP
- European Patent Office
- Prior art keywords
- groups
- carbon atoms
- composition
- surfactant
- polymeric surfactant
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Granted
Links
- 239000000203 mixture Substances 0.000 title claims abstract description 111
- 239000003599 detergent Substances 0.000 title claims abstract description 30
- 239000007788 liquid Substances 0.000 title claims abstract description 11
- 239000004094 surface-active agent Substances 0.000 claims abstract description 98
- 239000003945 anionic surfactant Substances 0.000 claims abstract description 40
- RTZKZFJDLAIYFH-UHFFFAOYSA-N Diethyl ether Chemical compound CCOCC RTZKZFJDLAIYFH-UHFFFAOYSA-N 0.000 claims abstract description 31
- 125000004432 carbon atom Chemical group C* 0.000 claims description 65
- 125000000217 alkyl group Chemical group 0.000 claims description 39
- IAYPIBMASNFSPL-UHFFFAOYSA-N Ethylene oxide Chemical group C1CO1 IAYPIBMASNFSPL-UHFFFAOYSA-N 0.000 claims description 34
- -1 2-hydroxypropyl Chemical group 0.000 claims description 33
- 150000001875 compounds Chemical class 0.000 claims description 33
- LFQSCWFLJHTTHZ-UHFFFAOYSA-N Ethanol Chemical compound CCO LFQSCWFLJHTTHZ-UHFFFAOYSA-N 0.000 claims description 27
- QAOWNCQODCNURD-UHFFFAOYSA-L Sulfate Chemical compound [O-]S([O-])(=O)=O QAOWNCQODCNURD-UHFFFAOYSA-L 0.000 claims description 23
- XLYOFNOQVPJJNP-UHFFFAOYSA-N water Substances O XLYOFNOQVPJJNP-UHFFFAOYSA-N 0.000 claims description 17
- GOOHAUXETOMSMM-UHFFFAOYSA-N Propylene oxide Chemical group CC1CO1 GOOHAUXETOMSMM-UHFFFAOYSA-N 0.000 claims description 16
- 125000002947 alkylene group Chemical group 0.000 claims description 16
- 229920000642 polymer Polymers 0.000 claims description 16
- 239000002736 nonionic surfactant Substances 0.000 claims description 14
- 150000008051 alkyl sulfates Chemical class 0.000 claims description 13
- 235000014113 dietary fatty acids Nutrition 0.000 claims description 13
- 239000000194 fatty acid Substances 0.000 claims description 13
- 229930195729 fatty acid Natural products 0.000 claims description 13
- DGAQECJNVWCQMB-PUAWFVPOSA-M Ilexoside XXIX Chemical compound C[C@@H]1CC[C@@]2(CC[C@@]3(C(=CC[C@H]4[C@]3(CC[C@@H]5[C@@]4(CC[C@@H](C5(C)C)OS(=O)(=O)[O-])C)C)[C@@H]2[C@]1(C)O)C)C(=O)O[C@H]6[C@@H]([C@H]([C@@H]([C@H](O6)CO)O)O)O.[Na+] DGAQECJNVWCQMB-PUAWFVPOSA-M 0.000 claims description 11
- 125000001165 hydrophobic group Chemical group 0.000 claims description 11
- 229910052708 sodium Inorganic materials 0.000 claims description 11
- 125000002887 hydroxy group Chemical group [H]O* 0.000 claims description 10
- 239000011734 sodium Substances 0.000 claims description 10
- 230000000087 stabilizing effect Effects 0.000 claims description 10
- 150000004665 fatty acids Chemical class 0.000 claims description 9
- 229910001425 magnesium ion Inorganic materials 0.000 claims description 9
- 150000003467 sulfuric acid derivatives Chemical class 0.000 claims description 9
- JLVVSXFLKOJNIY-UHFFFAOYSA-N Magnesium ion Chemical compound [Mg+2] JLVVSXFLKOJNIY-UHFFFAOYSA-N 0.000 claims description 8
- 125000002496 methyl group Chemical group [H]C([H])([H])* 0.000 claims description 8
- QGZKDVFQNNGYKY-UHFFFAOYSA-O Ammonium Chemical compound [NH4+] QGZKDVFQNNGYKY-UHFFFAOYSA-O 0.000 claims description 7
- ZLMJMSJWJFRBEC-UHFFFAOYSA-N Potassium Chemical compound [K] ZLMJMSJWJFRBEC-UHFFFAOYSA-N 0.000 claims description 7
- 229910052700 potassium Inorganic materials 0.000 claims description 7
- 239000011591 potassium Substances 0.000 claims description 7
- 125000003277 amino group Chemical group 0.000 claims description 6
- 229920001400 block copolymer Polymers 0.000 claims description 6
- 150000001768 cations Chemical class 0.000 claims description 6
- 125000001301 ethoxy group Chemical group [H]C([H])([H])C([H])([H])O* 0.000 claims description 6
- 125000002252 acyl group Chemical group 0.000 claims description 5
- 150000004996 alkyl benzenes Chemical class 0.000 claims description 5
- 229910052739 hydrogen Inorganic materials 0.000 claims description 5
- 239000001257 hydrogen Substances 0.000 claims description 5
- 125000004356 hydroxy functional group Chemical group O* 0.000 claims description 5
- 159000000003 magnesium salts Chemical class 0.000 claims description 5
- 239000003752 hydrotrope Substances 0.000 claims description 4
- 229920006395 saturated elastomer Polymers 0.000 claims description 4
- QUCDWLYKDRVKMI-UHFFFAOYSA-M sodium;3,4-dimethylbenzenesulfonate Chemical compound [Na+].CC1=CC=C(S([O-])(=O)=O)C=C1C QUCDWLYKDRVKMI-UHFFFAOYSA-M 0.000 claims description 4
- 125000005270 trialkylamine group Chemical group 0.000 claims description 4
- 229920000388 Polyphosphate Polymers 0.000 claims description 3
- 125000003368 amide group Chemical group 0.000 claims description 3
- 150000007942 carboxylates Chemical class 0.000 claims description 3
- 150000002148 esters Chemical class 0.000 claims description 3
- 125000001495 ethyl group Chemical group [H]C([H])([H])C([H])([H])* 0.000 claims description 3
- 239000001205 polyphosphate Substances 0.000 claims description 3
- 235000011176 polyphosphates Nutrition 0.000 claims description 3
- 150000003254 radicals Chemical class 0.000 claims description 3
- GSEJCLTVZPLZKY-UHFFFAOYSA-O triethanolammonium Chemical compound OCC[NH+](CCO)CCO GSEJCLTVZPLZKY-UHFFFAOYSA-O 0.000 claims description 3
- 125000000954 2-hydroxyethyl group Chemical group [H]C([*])([H])C([H])([H])O[H] 0.000 claims description 2
- QOXOZONBQWIKDA-UHFFFAOYSA-N 3-hydroxypropyl Chemical compound [CH2]CCO QOXOZONBQWIKDA-UHFFFAOYSA-N 0.000 claims description 2
- UFHFLCQGNIYNRP-UHFFFAOYSA-N Hydrogen Chemical compound [H][H] UFHFLCQGNIYNRP-UHFFFAOYSA-N 0.000 claims description 2
- ABLZXFCXXLZCGV-UHFFFAOYSA-N Phosphorous acid Chemical class OP(O)=O ABLZXFCXXLZCGV-UHFFFAOYSA-N 0.000 claims description 2
- 125000003545 alkoxy group Chemical group 0.000 claims description 2
- 125000000837 carbohydrate group Chemical group 0.000 claims description 2
- 125000004185 ester group Chemical group 0.000 claims description 2
- 125000000816 ethylene group Chemical group [H]C([H])([*:1])C([H])([H])[*:2] 0.000 claims description 2
- 125000004435 hydrogen atom Chemical group [H]* 0.000 claims description 2
- 125000002768 hydroxyalkyl group Chemical group 0.000 claims description 2
- 229910052806 inorganic carbonate Inorganic materials 0.000 claims description 2
- 229910052816 inorganic phosphate Inorganic materials 0.000 claims description 2
- 229910052909 inorganic silicate Inorganic materials 0.000 claims description 2
- 125000001449 isopropyl group Chemical group [H]C([H])([H])C([H])(*)C([H])([H])[H] 0.000 claims description 2
- 125000000325 methylidene group Chemical group [H]C([H])=* 0.000 claims description 2
- 229920000728 polyester Polymers 0.000 claims description 2
- 125000001436 propyl group Chemical group [H]C([*])([H])C([H])([H])C([H])([H])[H] 0.000 claims description 2
- HNJBEVLQSNELDL-UHFFFAOYSA-N pyrrolidin-2-one Chemical group O=C1CCCN1 HNJBEVLQSNELDL-UHFFFAOYSA-N 0.000 claims description 2
- CNPURSDMOWDNOQ-UHFFFAOYSA-N 4-methoxy-7h-pyrrolo[2,3-d]pyrimidin-2-amine Chemical group COC1=NC(N)=NC2=C1C=CN2 CNPURSDMOWDNOQ-UHFFFAOYSA-N 0.000 claims 4
- 125000005313 fatty acid group Chemical group 0.000 claims 1
- 150000002431 hydrogen Chemical group 0.000 claims 1
- 239000004519 grease Substances 0.000 abstract description 35
- KWIUHFFTVRNATP-UHFFFAOYSA-N glycine betaine Chemical compound C[N+](C)(C)CC([O-])=O KWIUHFFTVRNATP-UHFFFAOYSA-N 0.000 description 28
- 235000019441 ethanol Nutrition 0.000 description 18
- 230000008901 benefit Effects 0.000 description 15
- 229960003237 betaine Drugs 0.000 description 14
- 239000011777 magnesium Substances 0.000 description 12
- 239000000047 product Substances 0.000 description 12
- 238000005520 cutting process Methods 0.000 description 11
- 229910052749 magnesium Inorganic materials 0.000 description 11
- FYYHWMGAXLPEAU-UHFFFAOYSA-N Magnesium Chemical compound [Mg] FYYHWMGAXLPEAU-UHFFFAOYSA-N 0.000 description 10
- 229920001983 poloxamer Polymers 0.000 description 10
- 239000002585 base Substances 0.000 description 8
- 230000009467 reduction Effects 0.000 description 8
- 150000003839 salts Chemical class 0.000 description 8
- PEDCQBHIVMGVHV-UHFFFAOYSA-N Glycerine Chemical compound OCC(O)CO PEDCQBHIVMGVHV-UHFFFAOYSA-N 0.000 description 7
- 238000004851 dishwashing Methods 0.000 description 7
- 239000000243 solution Substances 0.000 description 7
- 150000001298 alcohols Chemical class 0.000 description 6
- 150000001408 amides Chemical class 0.000 description 6
- 239000003240 coconut oil Substances 0.000 description 6
- 235000019864 coconut oil Nutrition 0.000 description 6
- 229960003975 potassium Drugs 0.000 description 6
- 229920002359 Tetronic® Polymers 0.000 description 5
- 150000001412 amines Chemical class 0.000 description 5
- 230000000694 effects Effects 0.000 description 5
- 230000002209 hydrophobic effect Effects 0.000 description 5
- 239000012071 phase Substances 0.000 description 5
- 229920001451 polypropylene glycol Polymers 0.000 description 5
- 239000003760 tallow Substances 0.000 description 5
- 125000000129 anionic group Chemical group 0.000 description 4
- 239000004615 ingredient Substances 0.000 description 4
- 238000000034 method Methods 0.000 description 4
- 239000002689 soil Substances 0.000 description 4
- 150000003871 sulfonates Chemical class 0.000 description 4
- 235000013162 Cocos nucifera Nutrition 0.000 description 3
- 244000060011 Cocos nucifera Species 0.000 description 3
- 229920003171 Poly (ethylene oxide) Polymers 0.000 description 3
- DNIAPMSPPWPWGF-UHFFFAOYSA-N Propylene glycol Chemical compound CC(O)CO DNIAPMSPPWPWGF-UHFFFAOYSA-N 0.000 description 3
- 239000000654 additive Substances 0.000 description 3
- 229910052783 alkali metal Inorganic materials 0.000 description 3
- 150000001340 alkali metals Chemical class 0.000 description 3
- 238000007046 ethoxylation reaction Methods 0.000 description 3
- 238000009472 formulation Methods 0.000 description 3
- 235000011187 glycerol Nutrition 0.000 description 3
- 239000004033 plastic Substances 0.000 description 3
- 229920003023 plastic Polymers 0.000 description 3
- BDHFUVZGWQCTTF-UHFFFAOYSA-M sulfonate Chemical compound [O-]S(=O)=O BDHFUVZGWQCTTF-UHFFFAOYSA-M 0.000 description 3
- QGZKDVFQNNGYKY-UHFFFAOYSA-N Ammonia Chemical compound N QGZKDVFQNNGYKY-UHFFFAOYSA-N 0.000 description 2
- NOWKCMXCCJGMRR-UHFFFAOYSA-N Aziridine Chemical group C1CN1 NOWKCMXCCJGMRR-UHFFFAOYSA-N 0.000 description 2
- PIICEJLVQHRZGT-UHFFFAOYSA-N Ethylenediamine Chemical compound NCCN PIICEJLVQHRZGT-UHFFFAOYSA-N 0.000 description 2
- CDBYLPFSWZWCQE-UHFFFAOYSA-L Sodium Carbonate Chemical compound [Na+].[Na+].[O-]C([O-])=O CDBYLPFSWZWCQE-UHFFFAOYSA-L 0.000 description 2
- YXFVVABEGXRONW-UHFFFAOYSA-N Toluene Natural products CC1=CC=CC=C1 YXFVVABEGXRONW-UHFFFAOYSA-N 0.000 description 2
- XSQUKJJJFZCRTK-UHFFFAOYSA-N Urea Chemical compound NC(N)=O XSQUKJJJFZCRTK-UHFFFAOYSA-N 0.000 description 2
- 239000002253 acid Substances 0.000 description 2
- 125000003342 alkenyl group Chemical group 0.000 description 2
- 239000003242 anti bacterial agent Substances 0.000 description 2
- 239000007864 aqueous solution Substances 0.000 description 2
- 239000004202 carbamide Substances 0.000 description 2
- 239000007795 chemical reaction product Substances 0.000 description 2
- 239000003795 chemical substances by application Substances 0.000 description 2
- 150000001860 citric acid derivatives Chemical class 0.000 description 2
- 238000004140 cleaning Methods 0.000 description 2
- 239000007859 condensation product Substances 0.000 description 2
- 238000002474 experimental method Methods 0.000 description 2
- 239000003925 fat Substances 0.000 description 2
- 235000019197 fats Nutrition 0.000 description 2
- 125000001183 hydrocarbyl group Chemical group 0.000 description 2
- 230000006872 improvement Effects 0.000 description 2
- 239000002563 ionic surfactant Substances 0.000 description 2
- SUMDYPCJJOFFON-UHFFFAOYSA-N isethionic acid Chemical compound OCCS(O)(=O)=O SUMDYPCJJOFFON-UHFFFAOYSA-N 0.000 description 2
- 239000000463 material Substances 0.000 description 2
- 125000001570 methylene group Chemical group [H]C([H])([*:1])[*:2] 0.000 description 2
- 239000003921 oil Substances 0.000 description 2
- 235000019198 oils Nutrition 0.000 description 2
- BWHMMNNQKKPAPP-UHFFFAOYSA-L potassium carbonate Chemical compound [K+].[K+].[O-]C([O-])=O BWHMMNNQKKPAPP-UHFFFAOYSA-L 0.000 description 2
- 239000002243 precursor Substances 0.000 description 2
- 230000008569 process Effects 0.000 description 2
- 238000004904 shortening Methods 0.000 description 2
- 235000019832 sodium triphosphate Nutrition 0.000 description 2
- 230000003381 solubilizing effect Effects 0.000 description 2
- 125000001273 sulfonato group Chemical group [O-]S(*)(=O)=O 0.000 description 2
- 150000003460 sulfonic acids Chemical class 0.000 description 2
- QAOWNCQODCNURD-UHFFFAOYSA-N sulfuric acid Substances OS(O)(=O)=O QAOWNCQODCNURD-UHFFFAOYSA-N 0.000 description 2
- 150000003892 tartrate salts Chemical class 0.000 description 2
- 235000013311 vegetables Nutrition 0.000 description 2
- NSEXSMYEGCPXLT-UHFFFAOYSA-N (dodecan-3-ylamino) propane-1-sulfonate;sodium Chemical compound [Na].CCCCCCCCCC(CC)NOS(=O)(=O)CCC NSEXSMYEGCPXLT-UHFFFAOYSA-N 0.000 description 1
- HZAXFHJVJLSVMW-UHFFFAOYSA-N 2-Aminoethan-1-ol Chemical compound NCCO HZAXFHJVJLSVMW-UHFFFAOYSA-N 0.000 description 1
- UAZLASMTBCLJKO-UHFFFAOYSA-N 2-decylbenzenesulfonic acid Chemical compound CCCCCCCCCCC1=CC=CC=C1S(O)(=O)=O UAZLASMTBCLJKO-UHFFFAOYSA-N 0.000 description 1
- WBIQQQGBSDOWNP-UHFFFAOYSA-N 2-dodecylbenzenesulfonic acid Chemical compound CCCCCCCCCCCCC1=CC=CC=C1S(O)(=O)=O WBIQQQGBSDOWNP-UHFFFAOYSA-N 0.000 description 1
- JBVOQKNLGSOPNZ-UHFFFAOYSA-N 2-propan-2-ylbenzenesulfonic acid Chemical class CC(C)C1=CC=CC=C1S(O)(=O)=O JBVOQKNLGSOPNZ-UHFFFAOYSA-N 0.000 description 1
- PVXSFEGIHWMAOD-UHFFFAOYSA-N 2-tridecylbenzenesulfonic acid Chemical compound CCCCCCCCCCCCCC1=CC=CC=C1S(O)(=O)=O PVXSFEGIHWMAOD-UHFFFAOYSA-N 0.000 description 1
- UCDCOJNNUVYFKJ-UHFFFAOYSA-N 4-undecylbenzenesulfonic acid Chemical compound CCCCCCCCCCCC1=CC=C(S(O)(=O)=O)C=C1 UCDCOJNNUVYFKJ-UHFFFAOYSA-N 0.000 description 1
- 241000894006 Bacteria Species 0.000 description 1
- LSNNMFCWUKXFEE-UHFFFAOYSA-M Bisulfite Chemical compound OS([O-])=O LSNNMFCWUKXFEE-UHFFFAOYSA-M 0.000 description 1
- OYPRJOBELJOOCE-UHFFFAOYSA-N Calcium Chemical compound [Ca] OYPRJOBELJOOCE-UHFFFAOYSA-N 0.000 description 1
- BHPQYMZQTOCNFJ-UHFFFAOYSA-N Calcium cation Chemical compound [Ca+2] BHPQYMZQTOCNFJ-UHFFFAOYSA-N 0.000 description 1
- UGFAIRIUMAVXCW-UHFFFAOYSA-N Carbon monoxide Chemical compound [O+]#[C-] UGFAIRIUMAVXCW-UHFFFAOYSA-N 0.000 description 1
- SNRUBQQJIBEYMU-UHFFFAOYSA-N Dodecane Natural products CCCCCCCCCCCC SNRUBQQJIBEYMU-UHFFFAOYSA-N 0.000 description 1
- 102000004190 Enzymes Human genes 0.000 description 1
- 108090000790 Enzymes Proteins 0.000 description 1
- WHXSMMKQMYFTQS-UHFFFAOYSA-N Lithium Chemical compound [Li] WHXSMMKQMYFTQS-UHFFFAOYSA-N 0.000 description 1
- WLLGXSLBOPFWQV-UHFFFAOYSA-N MGK 264 Chemical compound C1=CC2CC1C1C2C(=O)N(CC(CC)CCCC)C1=O WLLGXSLBOPFWQV-UHFFFAOYSA-N 0.000 description 1
- 229920003091 Methocel™ Polymers 0.000 description 1
- CTQNGGLPUBDAKN-UHFFFAOYSA-N O-Xylene Chemical compound CC1=CC=CC=C1C CTQNGGLPUBDAKN-UHFFFAOYSA-N 0.000 description 1
- 229910019142 PO4 Inorganic materials 0.000 description 1
- 235000019482 Palm oil Nutrition 0.000 description 1
- 239000002202 Polyethylene glycol Substances 0.000 description 1
- 239000004743 Polypropylene Substances 0.000 description 1
- HVWGGPRWKSHASF-UHFFFAOYSA-N Sulfuric acid, monooctadecyl ester Chemical class CCCCCCCCCCCCCCCCCCOS(O)(=O)=O HVWGGPRWKSHASF-UHFFFAOYSA-N 0.000 description 1
- GSEJCLTVZPLZKY-UHFFFAOYSA-N Triethanolamine Chemical compound OCCN(CCO)CCO GSEJCLTVZPLZKY-UHFFFAOYSA-N 0.000 description 1
- BAECOWNUKCLBPZ-HIUWNOOHSA-N Triolein Natural products O([C@H](OCC(=O)CCCCCCC/C=C\CCCCCCCC)COC(=O)CCCCCCC/C=C\CCCCCCCC)C(=O)CCCCCCC/C=C\CCCCCCCC BAECOWNUKCLBPZ-HIUWNOOHSA-N 0.000 description 1
- PHYFQTYBJUILEZ-UHFFFAOYSA-N Trioleoylglycerol Natural products CCCCCCCCC=CCCCCCCCC(=O)OCC(OC(=O)CCCCCCCC=CCCCCCCCC)COC(=O)CCCCCCCC=CCCCCCCCC PHYFQTYBJUILEZ-UHFFFAOYSA-N 0.000 description 1
- ZZXDRXVIRVJQBT-UHFFFAOYSA-M Xylenesulfonate Chemical compound CC1=CC=CC(S([O-])(=O)=O)=C1C ZZXDRXVIRVJQBT-UHFFFAOYSA-M 0.000 description 1
- 150000007513 acids Chemical class 0.000 description 1
- 230000000996 additive effect Effects 0.000 description 1
- 239000002671 adjuvant Substances 0.000 description 1
- 230000002411 adverse Effects 0.000 description 1
- 230000032683 aging Effects 0.000 description 1
- 238000013019 agitation Methods 0.000 description 1
- 150000001335 aliphatic alkanes Chemical class 0.000 description 1
- 125000001931 aliphatic group Chemical group 0.000 description 1
- 229910000288 alkali metal carbonate Inorganic materials 0.000 description 1
- 150000008041 alkali metal carbonates Chemical class 0.000 description 1
- 150000008044 alkali metal hydroxides Chemical class 0.000 description 1
- 229910052784 alkaline earth metal Inorganic materials 0.000 description 1
- 150000001342 alkaline earth metals Chemical class 0.000 description 1
- 150000001336 alkenes Chemical class 0.000 description 1
- 125000002877 alkyl aryl group Chemical group 0.000 description 1
- 229910021529 ammonia Inorganic materials 0.000 description 1
- 239000004599 antimicrobial Substances 0.000 description 1
- 239000008346 aqueous phase Substances 0.000 description 1
- 125000003710 aryl alkyl group Chemical group 0.000 description 1
- 235000015278 beef Nutrition 0.000 description 1
- LPTWEDZIPSKWDG-UHFFFAOYSA-N benzenesulfonic acid;dodecane Chemical compound OS(=O)(=O)C1=CC=CC=C1.CCCCCCCCCCCC LPTWEDZIPSKWDG-UHFFFAOYSA-N 0.000 description 1
- 229910052791 calcium Inorganic materials 0.000 description 1
- 239000011575 calcium Substances 0.000 description 1
- 229910001424 calcium ion Inorganic materials 0.000 description 1
- 229910052799 carbon Inorganic materials 0.000 description 1
- 229910002091 carbon monoxide Inorganic materials 0.000 description 1
- 230000000052 comparative effect Effects 0.000 description 1
- 239000004064 cosurfactant Substances 0.000 description 1
- CSMFSDCPJHNZRY-UHFFFAOYSA-N decyl hydrogen sulfate Chemical class CCCCCCCCCCOS(O)(=O)=O CSMFSDCPJHNZRY-UHFFFAOYSA-N 0.000 description 1
- 230000008021 deposition Effects 0.000 description 1
- 239000003085 diluting agent Substances 0.000 description 1
- 239000012153 distilled water Substances 0.000 description 1
- MOTZDAYCYVMXPC-UHFFFAOYSA-N dodecyl hydrogen sulfate Chemical class CCCCCCCCCCCCOS(O)(=O)=O MOTZDAYCYVMXPC-UHFFFAOYSA-N 0.000 description 1
- QKHKGSULBQVNMO-UHFFFAOYSA-N dodecyl(dimethyl)azanium;hexanoate Chemical compound CCCCCC([O-])=O.CCCCCCCCCCCC[NH+](C)C QKHKGSULBQVNMO-UHFFFAOYSA-N 0.000 description 1
- 229940060296 dodecylbenzenesulfonic acid Drugs 0.000 description 1
- 238000001035 drying Methods 0.000 description 1
- 239000000975 dye Substances 0.000 description 1
- 239000003792 electrolyte Substances 0.000 description 1
- 238000004945 emulsification Methods 0.000 description 1
- 150000002170 ethers Chemical class 0.000 description 1
- 235000013305 food Nutrition 0.000 description 1
- 125000005456 glyceride group Chemical group 0.000 description 1
- LPTIRUACFKQDHZ-UHFFFAOYSA-N hexadecyl sulfate;hydron Chemical class CCCCCCCCCCCCCCCCOS(O)(=O)=O LPTIRUACFKQDHZ-UHFFFAOYSA-N 0.000 description 1
- FUZZWVXGSFPDMH-UHFFFAOYSA-N hexanoic acid Chemical compound CCCCCC(O)=O FUZZWVXGSFPDMH-UHFFFAOYSA-N 0.000 description 1
- 229930195733 hydrocarbon Natural products 0.000 description 1
- 238000005984 hydrogenation reaction Methods 0.000 description 1
- 230000007062 hydrolysis Effects 0.000 description 1
- 238000006460 hydrolysis reaction Methods 0.000 description 1
- 230000005661 hydrophobic surface Effects 0.000 description 1
- 229940045996 isethionic acid Drugs 0.000 description 1
- 150000002605 large molecules Chemical class 0.000 description 1
- 229910052744 lithium Inorganic materials 0.000 description 1
- 150000002688 maleic acid derivatives Chemical class 0.000 description 1
- 150000004701 malic acid derivatives Chemical class 0.000 description 1
- 150000002690 malonic acid derivatives Chemical class 0.000 description 1
- 239000000693 micelle Substances 0.000 description 1
- DVEKCXOJTLDBFE-UHFFFAOYSA-N n-dodecyl-n,n-dimethylglycinate Chemical compound CCCCCCCCCCCC[N+](C)(C)CC([O-])=O DVEKCXOJTLDBFE-UHFFFAOYSA-N 0.000 description 1
- JRZJOMJEPLMPRA-UHFFFAOYSA-N olefin Natural products CCCCCCCC=C JRZJOMJEPLMPRA-UHFFFAOYSA-N 0.000 description 1
- 230000003647 oxidation Effects 0.000 description 1
- 238000007254 oxidation reaction Methods 0.000 description 1
- 125000000963 oxybis(methylene) group Chemical group [H]C([H])(*)OC([H])([H])* 0.000 description 1
- 239000006179 pH buffering agent Substances 0.000 description 1
- 239000002540 palm oil Substances 0.000 description 1
- 239000012188 paraffin wax Substances 0.000 description 1
- 239000002304 perfume Substances 0.000 description 1
- 239000003208 petroleum Substances 0.000 description 1
- 125000001997 phenyl group Chemical group [H]C1=C([H])C([H])=C(*)C([H])=C1[H] 0.000 description 1
- 235000021317 phosphate Nutrition 0.000 description 1
- XYFCBTPGUUZFHI-UHFFFAOYSA-O phosphonium Chemical compound [PH4+] XYFCBTPGUUZFHI-UHFFFAOYSA-O 0.000 description 1
- 125000001476 phosphono group Chemical group [H]OP(*)(=O)O[H] 0.000 description 1
- 150000003013 phosphoric acid derivatives Chemical class 0.000 description 1
- 229920000233 poly(alkylene oxides) Polymers 0.000 description 1
- 229920005646 polycarboxylate Polymers 0.000 description 1
- 229920001223 polyethylene glycol Polymers 0.000 description 1
- 229920005862 polyol Polymers 0.000 description 1
- 150000003077 polyols Chemical class 0.000 description 1
- 229920001155 polypropylene Polymers 0.000 description 1
- AVTYONGGKAJVTE-OLXYHTOASA-L potassium L-tartrate Chemical compound [K+].[K+].[O-]C(=O)[C@H](O)[C@@H](O)C([O-])=O AVTYONGGKAJVTE-OLXYHTOASA-L 0.000 description 1
- 229910000027 potassium carbonate Inorganic materials 0.000 description 1
- 229940093956 potassium carbonate Drugs 0.000 description 1
- 235000011181 potassium carbonates Nutrition 0.000 description 1
- 239000001508 potassium citrate Substances 0.000 description 1
- 229960002635 potassium citrate Drugs 0.000 description 1
- QEEAPRPFLLJWCF-UHFFFAOYSA-K potassium citrate (anhydrous) Chemical compound [K+].[K+].[K+].[O-]C(=O)CC(O)(CC([O-])=O)C([O-])=O QEEAPRPFLLJWCF-UHFFFAOYSA-K 0.000 description 1
- 235000011082 potassium citrates Nutrition 0.000 description 1
- 229940098424 potassium pyrophosphate Drugs 0.000 description 1
- 239000001472 potassium tartrate Substances 0.000 description 1
- 229940111695 potassium tartrate Drugs 0.000 description 1
- 235000011005 potassium tartrates Nutrition 0.000 description 1
- GHKGUEZUGFJUEJ-UHFFFAOYSA-M potassium;4-methylbenzenesulfonate Chemical compound [K+].CC1=CC=C(S([O-])(=O)=O)C=C1 GHKGUEZUGFJUEJ-UHFFFAOYSA-M 0.000 description 1
- 230000000063 preceeding effect Effects 0.000 description 1
- 239000003352 sequestering agent Substances 0.000 description 1
- 150000004760 silicates Chemical class 0.000 description 1
- 238000002791 soaking Methods 0.000 description 1
- 229940083542 sodium Drugs 0.000 description 1
- 235000015424 sodium Nutrition 0.000 description 1
- 229910000029 sodium carbonate Inorganic materials 0.000 description 1
- 229940001593 sodium carbonate Drugs 0.000 description 1
- 235000017550 sodium carbonate Nutrition 0.000 description 1
- 239000001509 sodium citrate Substances 0.000 description 1
- FQENQNTWSFEDLI-UHFFFAOYSA-J sodium diphosphate Chemical compound [Na+].[Na+].[Na+].[Na+].[O-]P([O-])(=O)OP([O-])([O-])=O FQENQNTWSFEDLI-UHFFFAOYSA-J 0.000 description 1
- URLJMZWTXZTZRR-UHFFFAOYSA-N sodium myristyl sulfate Chemical class CCCCCCCCCCCCCCOS(O)(=O)=O URLJMZWTXZTZRR-UHFFFAOYSA-N 0.000 description 1
- 229940048086 sodium pyrophosphate Drugs 0.000 description 1
- 239000001433 sodium tartrate Substances 0.000 description 1
- 229960002167 sodium tartrate Drugs 0.000 description 1
- 235000011004 sodium tartrates Nutrition 0.000 description 1
- 229940048842 sodium xylenesulfonate Drugs 0.000 description 1
- KVCGISUBCHHTDD-UHFFFAOYSA-M sodium;4-methylbenzenesulfonate Chemical compound [Na+].CC1=CC=C(S([O-])(=O)=O)C=C1 KVCGISUBCHHTDD-UHFFFAOYSA-M 0.000 description 1
- SIXNTGDWLSRMIC-UHFFFAOYSA-N sodium;toluene Chemical compound [Na].CC1=CC=CC=C1 SIXNTGDWLSRMIC-UHFFFAOYSA-N 0.000 description 1
- 239000008234 soft water Substances 0.000 description 1
- 239000007787 solid Substances 0.000 description 1
- 239000002904 solvent Substances 0.000 description 1
- 239000003549 soybean oil Substances 0.000 description 1
- 235000012424 soybean oil Nutrition 0.000 description 1
- 241000894007 species Species 0.000 description 1
- 238000009987 spinning Methods 0.000 description 1
- 239000000126 substance Substances 0.000 description 1
- 150000003890 succinate salts Chemical class 0.000 description 1
- 230000001180 sulfating effect Effects 0.000 description 1
- 239000000271 synthetic detergent Substances 0.000 description 1
- 150000004026 tertiary sulfonium compounds Chemical class 0.000 description 1
- RYCLIXPGLDDLTM-UHFFFAOYSA-J tetrapotassium;phosphonato phosphate Chemical compound [K+].[K+].[K+].[K+].[O-]P([O-])(=O)OP([O-])([O-])=O RYCLIXPGLDDLTM-UHFFFAOYSA-J 0.000 description 1
- FODHIQQNHOPUKH-UHFFFAOYSA-N tetrapropylene-benzenesulfonic acid Chemical compound CC1CC11C2=C3S(=O)(=O)OC(C)CC3=C3C(C)CC3=C2C1C FODHIQQNHOPUKH-UHFFFAOYSA-N 0.000 description 1
- 235000019818 tetrasodium diphosphate Nutrition 0.000 description 1
- 239000001577 tetrasodium phosphonato phosphate Substances 0.000 description 1
- PHYFQTYBJUILEZ-IUPFWZBJSA-N triolein Chemical compound CCCCCCCC\C=C/CCCCCCCC(=O)OCC(OC(=O)CCCCCCC\C=C/CCCCCCCC)COC(=O)CCCCCCC\C=C/CCCCCCCC PHYFQTYBJUILEZ-IUPFWZBJSA-N 0.000 description 1
- 229940117972 triolein Drugs 0.000 description 1
- UNXRWKVEANCORM-UHFFFAOYSA-I triphosphate(5-) Chemical compound [O-]P([O-])(=O)OP([O-])(=O)OP([O-])([O-])=O UNXRWKVEANCORM-UHFFFAOYSA-I 0.000 description 1
- ZXGOACRTCPRVON-UHFFFAOYSA-K trisodium;2-sulfonatobutanedioate Chemical compound [Na+].[Na+].[Na+].[O-]C(=O)CC(C([O-])=O)S([O-])(=O)=O ZXGOACRTCPRVON-UHFFFAOYSA-K 0.000 description 1
- 235000015112 vegetable and seed oil Nutrition 0.000 description 1
- 239000008158 vegetable oil Substances 0.000 description 1
- 238000005406 washing Methods 0.000 description 1
- 230000004584 weight gain Effects 0.000 description 1
- 235000019786 weight gain Nutrition 0.000 description 1
- 239000008096 xylene Substances 0.000 description 1
- 229940071104 xylenesulfonate Drugs 0.000 description 1
- 239000002888 zwitterionic surfactant Substances 0.000 description 1
Classifications
-
- C—CHEMISTRY; METALLURGY
- C11—ANIMAL OR VEGETABLE OILS, FATS, FATTY SUBSTANCES OR WAXES; FATTY ACIDS THEREFROM; DETERGENTS; CANDLES
- C11D—DETERGENT COMPOSITIONS; USE OF SINGLE SUBSTANCES AS DETERGENTS; SOAP OR SOAP-MAKING; RESIN SOAPS; RECOVERY OF GLYCEROL
- C11D3/00—Other compounding ingredients of detergent compositions covered in group C11D1/00
- C11D3/0005—Other compounding ingredients characterised by their effect
- C11D3/0094—High foaming compositions
-
- C—CHEMISTRY; METALLURGY
- C11—ANIMAL OR VEGETABLE OILS, FATS, FATTY SUBSTANCES OR WAXES; FATTY ACIDS THEREFROM; DETERGENTS; CANDLES
- C11D—DETERGENT COMPOSITIONS; USE OF SINGLE SUBSTANCES AS DETERGENTS; SOAP OR SOAP-MAKING; RESIN SOAPS; RECOVERY OF GLYCEROL
- C11D1/00—Detergent compositions based essentially on surface-active compounds; Use of these compounds as a detergent
- C11D1/008—Polymeric surface-active agents
-
- C—CHEMISTRY; METALLURGY
- C11—ANIMAL OR VEGETABLE OILS, FATS, FATTY SUBSTANCES OR WAXES; FATTY ACIDS THEREFROM; DETERGENTS; CANDLES
- C11D—DETERGENT COMPOSITIONS; USE OF SINGLE SUBSTANCES AS DETERGENTS; SOAP OR SOAP-MAKING; RESIN SOAPS; RECOVERY OF GLYCEROL
- C11D1/00—Detergent compositions based essentially on surface-active compounds; Use of these compounds as a detergent
- C11D1/66—Non-ionic compounds
- C11D1/72—Ethers of polyoxyalkylene glycols
- C11D1/721—End blocked ethers
-
- C—CHEMISTRY; METALLURGY
- C11—ANIMAL OR VEGETABLE OILS, FATS, FATTY SUBSTANCES OR WAXES; FATTY ACIDS THEREFROM; DETERGENTS; CANDLES
- C11D—DETERGENT COMPOSITIONS; USE OF SINGLE SUBSTANCES AS DETERGENTS; SOAP OR SOAP-MAKING; RESIN SOAPS; RECOVERY OF GLYCEROL
- C11D3/00—Other compounding ingredients of detergent compositions covered in group C11D1/00
- C11D3/16—Organic compounds
- C11D3/37—Polymers
- C11D3/3703—Macromolecular compounds obtained otherwise than by reactions only involving carbon-to-carbon unsaturated bonds
-
- C—CHEMISTRY; METALLURGY
- C11—ANIMAL OR VEGETABLE OILS, FATS, FATTY SUBSTANCES OR WAXES; FATTY ACIDS THEREFROM; DETERGENTS; CANDLES
- C11D—DETERGENT COMPOSITIONS; USE OF SINGLE SUBSTANCES AS DETERGENTS; SOAP OR SOAP-MAKING; RESIN SOAPS; RECOVERY OF GLYCEROL
- C11D3/00—Other compounding ingredients of detergent compositions covered in group C11D1/00
- C11D3/16—Organic compounds
- C11D3/37—Polymers
- C11D3/3703—Macromolecular compounds obtained otherwise than by reactions only involving carbon-to-carbon unsaturated bonds
- C11D3/3707—Polyethers, e.g. polyalkyleneoxides
Definitions
- the invention relates to aqueous high sudsing liquid detergent compositions containing specified amounts and types of surfactants especially useful in the washing of tableware, kitchenware and other hard surfaces.
- compositions of this invention have superior ability to handle grease.
- the performance of a detergent composition for cleaning tableware and kitchen utensils is evaluated by its ability to handle grease.
- the detergent solution should readily remove grease and minimize its redeposition.
- compositions and methods which can be employed during dishwashing operations to improve the appearance of kitchen utensils and articles.
- Such compositions and methods should provide improved removal of grease in conventional dishwashing soil removal operations while maintaining the sudsing attributes of an acceptable dishwashing detergent composition.
- the present invention comprises a high sudsing liquid detergent composition containing by weight:
- Dishware, glassware, and other tableware and kitchenware are washed in water solutions of the detergent composition, generally at a weight concentration of from about 0.05% to about 0.4% of the composition in water at a temperature of from about 60°F to about 120°F.
- liquid detergent compositions of the present invention contain two essential components:
- Optional ingredients can be added to provide various performance and aesthetic characteristics.
- compositions of this invention contain from about 5% to about 50% by weight of an anionic surfactant or mixtures thereof preferably comprising at least about 5%, more preferably at least about 8%, and most preferably more than about 10% of an alkyl polyethoxylate (polyethylene oxide) sulfate having from about 10 to about 20, preferably from about 10 to about 16 carbon atoms in the alkyl group and containing from about 7 to about 10, preferably from about 1 to about 8, most preferably from about 1 to about 6 ethoxy groups on the average.
- Preferred compositions contain from about 20% to about 40% of anionic surfactant by weight.
- anionic detergents can be broadly described as the water-soluble salts, particularly the alkali metal, alkaline earth metal, ammonium or amine salts, of organic sulfuric reaction products having in their molecular structure an alkyl radical containing from about 8 to about 22 carbon atoms and a radical selected from the group consisting of sulfonic acid and sulfuric acid ester radicals. Included in the term "alkyl” is the alkyl portion of acyl radicals.
- the anionic synthetic detergents which can form the surfactant component of the compositions of the present invention are the salts of compatible cations, e.g.
- alkyl sulfates especially those obtained by sulfating the higher alcohols (C.-C,. carbon atoms), alkyl benzene, or alkyl toluene, sulfonates, in which the alkyl group contains from about 9 to about 15 carbon atoms, the alkyl radical being either a straight or branched aliphatic chain; paraffin sulfonates or olefin sulfonated in which the alkyl or alkenyl group contains from about 10 to about 20 carbon atoms; sodium C 'O .
- alkyl ether sulfonates especially those ethers of alcohols derived from tallow and coconut oil; coconut oil fatty acid monoglyceride sulfates and sulfonates; alkylphenolpolyethylene oxide ether sulfates with from about 1 to about 10 units of ethylene oxide per molecule on the average in which the alkyl radicals contain from 8 to about 12 carbon atoms; the reaction products of fatty acids esterified with isethionic acid where, for example, the fatty acids are derived from coconut oil; fatty acid amides of a methyl tauride in which the fatty acids, for example, are derived from coconut oil; and beta- acetoxy-or beta-acetamido-alkanesulfonates where the alkane has from 8 to 22 carbon atoms.
- alkyl sulfate salts which can be employed in the instant detergent compositions include sodium, potassium, ammonium, monoethanolammonium, diethanolammonium, triethanolammonium, and magnesium: lauryl sulfates, stearyl sulfates, palmityl sulfates, decyl sulfates, myristyl sulfates, tallow alkyl sulfates, coconut alkyl sulfates, C 12-15 alkyl sulfates and mixtures of these surfactants.
- Preferred alkyl sulfates include the C 12 . 15 alkyl sulfates.
- Suitable alkylbenzene, or alkyltoluene, sulfonates include the alkali metal (lithium, sodium, and/or potassium), alkaline earth (preferably magnesium), ammonium and/or alkanolammonium salts of straight, or branched-chain, alkylbenzene, or alkyltoluene, sulfonic acids.
- Alkylbenzene sulfonic acids useful as precursors for these surfactants include decyl benzene sulfonic acid, undecyl benzene sulfonic acid, dodecyl benzene sulfonic acid, tridecyl benzene sulfonic acid, tetrapropylene benzene sulfonic acid and mixtures thereof.
- Preferred sulfonic acids as precursors of the alkyl-benzene sulfonates useful for compositions herein are those in which the alkyl chain is linear and averages about 11 to 13 carbon atoms in length. Examples of commercially available alkyl benzene sulfonic acids useful in the present invention include Conoco SA 515 and SA 597 marketed by the Continental Oil Company and Calsoft LAS 99 marketed by the Pilot Chemical Company.
- the preferred anionic surfactants herein which are essential if there are no, e.g., magnesium ions or betaine surfactant present, are alkylpolyethoxylate sulfates having the formula RO(C 2 H 4 O) x SO,M wherein R is alkyl, or alkenyl, of from about 10 to about 20 carbon atoms, x is from about to about ten on the average, treating alkyl sulfates as if they had 0 ethoxy groups, preferably from about to about eight, most preferably from about one to about six, and M is a water-soluble compatible cation such as those disclosed hereinbefore.
- the alkylpolyethoxylate sulfates useful in the present invention are sulfates of condensation products of ethylene oxide and monohydric alcohols having from about 10 to about 20 carbon atoms.
- R has 10 to 16 carbon atoms.
- the alcohols can be derived from natural fats, e.g., coconut oil or tallow, or can be synthetic. Such alcohols can be reacted with from about t to about 20, especially from about one to about 14, and more especially from about one to about eight, molar proportions of ethylene oxide and the resulting mixture of molecular species is sulfated and neutralized.
- the computed average degree of ethoxylation should be more than about 0.5, preferably more than about 0.6.
- the other anionic surfactant can be treated as if it were an alkyl sulfate to compute the average degree of ethoxylation.
- alkylpolyethoxylate sulfates of the present invention are sodium coconut alkylpolyethoxylate (3) ether sulfate, magnesium C 12-15 alkylpolyethoxylate (3) ether sulfate, and sodium tallow alkylpolyethoxylate (6) ether sulfate.
- a particularly preferred example is a water soluble, e.g. magnesium, C 12-13 alkylpolyethoxylate (1) ether sulfate.
- Preferred alkyl polyethoxylate sulfates are those comprising a mixture of individual compounds, said mixture having an average alkyl chain length of from about 10 to 16 carbon atoms and an average degree of ethoxylation of from about 1 to about 8 moles of ethylene oxide.
- the compositions should contain magnesium ions, and/or at least about 10%, preferably at least about 15% by weight of the anionic surfactant, of the preferred alkyl polyethoxylate sulfates described hereinbefore. It is preferred that the compositions of this invention, including those that contain the preferred alkylpolyethoxylate sulfates, also contain magnesium and/or calcium ions, most preferably magnesium ions, to act as cations for a portion of the anionic surfactant. If the composition is to be used primarily in water containing more than about 2 grains/gal. of hardness, added magnesium may not be essential. In use, from about 10% to about 100%, preferably from about 20% to about 90%, of the anionic surfactant should be the magnesium salt.
- the surfactant system minus the polymeric surfactant should preferably reduce the interfacial tension to below about 2t dyne/cm, preferably below about 2 dynes/cm, against triolein at a concentration of 0.2% and a temperature of 115°F (46°C) in a spinning drop Tensiometer.
- Interfacial tension is lowered by any detergent surfactant, but the efficiency can be improved by selection of surfactants which have longer alkyl chain lengths, use of cations such as magnesium which minimize charge effects when anionic surfactants are used, and use of anionic surfactants combined with cosurfactants like trialkylamine oxides which form complexes with the anionic surfactant.
- compositions of the present invention contain from about 0.1% to about 10%, more preferably from about 1 ⁇ 2% to about 4%, and most preferably from about 1 ⁇ 2% to about 2%, of the polymeric surfactant described generically hereinbefore and discussed in detail hereinafter.
- B is preferably a polypropylene oxide group, containing more than about 5 propylene oxide groups, which can contain some ethylene oxide groups, n and m are preferably from about 1 to about 2 and the sum of n + m is from about 2 to about 4, the molecule contains from about 20 to about 500 ether linkages, and the molecular weight is from about 1000 to about 40,000.
- the polymeric surfactant is preferably represented by the formula:
- the polymeric surfactant functions by forming complexes with the hydrophilic portions of the anionic surfactants, thereby minimizing the ability of the anionic surfactants to leave a micelle or other interfacial region once formed. Therefore, long terminal hydrocarbon groups are not preferred, and are not acceptable when the formula is of the BA type. Long terminal hydrocarbons pull the polymer into any oil phase, thereby minimizing the number of anionic surfactant molecules that are stabilized. Similarly, if the hydrophilic portion of the molecule is too hydrophilic, the molecule is pulled into the aqueous phase too far.
- the molecule should be balanced between hydrophobicity and hydrophilicity and have enough ether and/or amine linkages spread throughout the structure to complex the anionic surfactant.
- the anionic surfactant also must be one that will form the complex. Magnesium cations, ether linkages, and amine or ammonium groups form stable complexes with the polymeric surfactants.
- the surfactant contains a hydrophilic group comprising polyethylene oxide and/or ethyleneimine groups containing from about 1 to about 500 ethylene oxide and/or ethyleneimine derived moieties. Sulfonate or sulfate groups, can also be present.
- the polymeric surfactant also contains at least one hydrophobic group, preferably comprising polyalkylene oxide groups wherein the alkylene contains from three to about six, most preferably three, carbon atoms and the molecular weight is from about 400 to about 60,000.
- alkylene groups containing from about 7 to about 18, preferably from about 10 to about 18, carbon atoms can also be used, but preferably only short chain relatively nonoleophilic alkyl or acyl groups containing less than about ten carbon atoms are pendant on the polymeric surfactant.
- Preferred surfactants are block copolymers comprising one or more groups that are hydrophilic and which contain mostly ethylene oxide groups and one or more hydrophobic groups which contain mostly propylene oxide groups attached to the residue of a compound that contained one or more hydroxy or amine groups onto which the respective alkylene oxides were polymerized, said polymers having molecular weights of from about 400 to about 60,000, an ethylene oxide content of from about 10% to about 90% by weight and a propylene oxide content of from about 10% to about 90% by weight.
- Preferred surfactants are those in which propylene oxide is condensed with an amine, especially ethylenediamine to provide a hydrophobic base having a molecular weight of from about 350 to about 55,000, preferably from about 500 to about 40,000. This hydrophobic base is then condensed with ethylene oxide to provide from about 10% to about 90%, preferably from about 20% to about 80% ethylene oxide. Reverse structures in which the ethylene oxide is condensed first are also desirable. These structures are especially easy to formulate into desirable single phase liquid compositions.
- the polypropylene glycol portion can be replaced by an alkyl, or alkylene group containing from about 5 to about 18, preferably from about 8 to about 16 carbon atoms and the polyethylene oxide groups can be replaced either totally, or, preferably in part, by other water solubilizing groups, especially sulfate and sulfonate groups.
- compositions of this invention contain from 0% to about 10%, preferably from about 1% to about 8%, of suds stabilizing nonionic surfactant or mixtures thereof.
- Suds stabilizing nonionic surfactants operable in the instant compositions are of two basic types: fatty acid amides and the trialkyl amine oxide semi-polar nonionics.
- the amide type of nonionic surface active agent includes the ammonia, monoethanol and diethanol amides of fatty acids having an acyl moiety of from about 8 to 18 carbon atoms and represented by the general formula: R'-CO-N(H) m (R 2 OH) 2-m wherein R, is a saturated or unsaturated, aliphatic hydrocarbon radical having from 7 to 21, preferably from 11 to 17 carbon atoms; R 2 represents a methylene or ethylene group; and m is 1 or 2.
- Specific examples of said amides are coconut fatty acid monoethanol amide and dodecyl fatty acid diethanol amide.
- acyl moieties may be derived from naturally occurring glycerides, e.g., coconut oil, palm oil, soybean oil and tallow, but can be derived synthetically, e.g., by the oxidation of petroleum, or hydrogenation of carbon monoxide by the Fischer-Tropsch process.
- the monoethanol amides and diethanolamides of C12.14fatty acids are preferred.
- Amine oxide semi-polar nonionic surface active agents comprise compounds and mixtures of compounds having the formula: wherein R' is an alkyl, 2-hydroxyalkyl, 3-hydroxyalkyl, or 3-alkoxy-2-hydroxypropyl radical in which the alkyl and alkoxy, respectively, contain from about 8 to 18 carbon atoms, R 2 and R 3 are each a methyl, ethyl, propyl, isopropyl, 2-hydroxyethyl, 2-hydroxypropyl, or 3-hydroxypropyl radical and n is from 0 to about 10. Particularly preferred are amine oxides of the formula: wherein R' is a C 10 . 14 alkyl and R 2 and R 3 are methyl or ethyl.
- the pref, -red sudsing characteristics of the compositions of the invention are those which will provide the user of the product with an indication of cleaning potential in a dishwashing solution. Soils encountered in dishwashing act as suds depressants and the presence or absence of suds from the surface of a dishwashing solution is a convenient guide to product usage. Mixtures of anionic surfactants and suds stabilizing nonionic surfactants are utilized in the compositions of the invention because of their high sudsing characteristics, their suds stability in the presence of food soils and their ability to indicate accurately an adequate level of product usage in the presence of soil
- the ratio of anionic surfactants to suds stabilizing nonionic surfactants in the composition will be in a molar ratio of from about 11:1 to about 1:1, and more preferably from about 8:1 to about 3:1.
- compositions of the invention can desirably contain optional surfactants, especially ampholytic and/or zwitterionic surfactants.
- optional surfactants especially ampholytic and/or zwitterionic surfactants.
- the level of anionic surfactant is less than about 20%, the composition should not contain any substantial amount of conventional nonionic surfactant, e.g., an alkylpolyethoxylate, in addition to the polymeric surfactant. Large amounts of conventional nonionic surfactants, e.g., more than about three or four percent, tend to harm the sudsing ability of the composition.
- anionic surfactants When larger amounts ( > 20%) of anionic surfactants are present it is sometimes desirable to have a low level, up to about 5%, of conventional nonionic surfactants "conventional" nonionic surfactants are e.g., C 8 . 18 alkyl polyethoxylates (4-15) or C 8 . 15 alkyl phenol polyethoxylates (4-15).
- conventional nonionic surfactants are e.g., C 8 . 18 alkyl polyethoxylates (4-15) or C 8 . 15 alkyl phenol polyethoxylates (4-15).
- Ampholytic surfactants can be broadly described as derivatives of aliphatic amines which contain a long chain of about 8 to 18 carbon atoms and an anionic water-solubilizing group, e.g. carboxylate, sulfonate or sulfate. Examples of compounds falling within this definition are sodium-3-dodecylamino propane sulfonate, and dodecyl dimethylammonium hexanoate.
- Zwitterionic surface agents operable in the instant composition are broadly described as internally- neutralized derivatives of aliphatic quaternary ammonium and phosphonium and tertiary sulfonium compounds in which the aliphatic radical can be straight chain or branched, and wherein one of the aliphatic substituents contains from about 8 to 18 carbon atoms and one contains an anionic water solubilizing group, e.g., carboxy, sulfo, sulfato, phosphato, or phosphono.
- betaine detergent surfactants which synergistically interact with the polymeric surfactant to provide improved grease handling.
- the betaine detergent surfactant has the general formula: wherein R is a hydrophobic group selected from the group consisting of alkyl groups containing from about 10 to about 22 carbon atoms, preferably from about 12 to about 18 carbon atoms, alkyl aryl and aryl alkyl groups containing a similar number of carbon atoms with a benzene ring being treated as equivalent to about 2 carbon atoms, and similar structures interrupted by amido or ether linkages; each R * is an alkyl group containing from one to about 2 carbon atoms; and R' is an alkylene group containing from one to about 6 carbon atoms.
- betaines dodecylamidopropyl dimethylbetaine; dodecyldimethylbetaine; tetradecyldimethylbetaine; cetyldimethylbetaine; cetylamidopropyldimethylbetaine, tetradecyldimethylbetaine, tetradecylamidopropyldimethylbetaine, and docosyldimethylammonium hexanoate and mixtures thereof.
- Betaine surfactants are unique ingredients that provide exceptional benefits. When betaine surfactant and polymeric surfactant are combined with any anionic surfactant, with, or without magnesium ions being present, superior grease holding benefits are provided.
- Betaines containing a C 12 . 14 alkyl provide a much bigger benefit when combined with polymeric surfactant than when used by themselves.
- the betaine is preferably present at a level of from about 7% to about 15% by weight of the formula, preferably from about 1% to about 10%, most preferably from about 1% to about 8%.
- the ratio of anionic detergent surfactants to the betaine is from about 1 to about 80, preferably from about 1 to about 40, more preferably from about 2 to about 40.
- the composition should preferably have a ratio of betaine to polymeric surfactant of more than about 7:1, preferably more than about 9:1.
- Alcohols such as ethyl alcohol, and hydrotropes, such as sodium and potassium toluene sulfonate, sodium and potassium xylene sulfonate, trisodium sulfosuccinate and related compounds (as disclosed in U.S. Patent 3,915,903, incorporated herein by reference) and urea, can be utilized in the interests of achieving a desired product phase stability and viscosity.
- Alkanols containing from one to about six carbon atoms, especially two, and especially ethyl alcohol can be present.
- Ethyl alcohol at a level of from 0% to about 15%, preferably from about 1% to about 6%, and potassium and/or sodium toluene, xylene, and/or cumene sulfonates at a level of from about 1% to about 6% can be used in the compositions of the invention.
- the viscosity should be greater than about 100 centipoise, more preferably more than 150 centipoise, most preferably more than about 200 centipoise for consumer acceptance.
- the polymeric surfactant can be used to reduce the viscosity and provide phase stability, e.g., when either the preferred alkyl polyethoxylate sulfate or magnesium ions are present in the composition.
- the percentage of ethylene oxide in the polymer should be less than about 70%, preferably less than about 50%.
- Preferred compositions contain less than about 2% alcohol and less than about 3% hydrotrope and preferably essentially none while maintaining a viscosity of from about 150 to about 500 centipoise, preferably from about 200 to about 400 centipoise.
- the percentage of ethylene oxide in the polymer should be more than about 50%, preferably more than about 70%.
- the polymeric surfactant reduces viscosity for all water soluble anionic surfactants.
- compositions of this invention contain from about 20% to about 90%, preferably from about 30% to about 80%, water.
- compositions of this invention can contain up to about 10%, by weight of detergency builders either of the organic or inorganic type.
- detergency builders either of the organic or inorganic type.
- water-soluble inorganic builders which can be used, alone or in admixture with themselves and organic alkaline sequestrant builder salts, are alkali metal carbonates, phosphates, polyphosphates, and silicates.
- specific examples of such salts are sodium tripolyphosphate, sodium carbonate, potassium carbonate, sodium pyrophosphate, potassium pyrophosphate, and potassium tripolyphosphate.
- organic builder salts which can be used alone, or in admixture with each other or with the preceding inorganic alkaline builder salts, are alkali metal polycarboxylates, e.g., water-soluble citrates, tartrates, etc. such as sodium and potassium citrate and sodium and potassium tartrate.
- detergency builders have limited value in dishwashing detergent compositions and use at levels above about 10% can restrict formulation flexibility in liquid compositions because of solubility and phase stability considerations. It is preferred than any builder used be relatively specific to control of calcium as opposed to magnesium. Citrates, tartrates, malates, maleates, succinates and malonates are especially preferred.
- the detergent compositions of this invention can contain, if desired, any of the usual adjuvants, diluents and additives, for example, perfumes, electrolytes, enzymes, dyes, antitarnishing agents, antimicrobial agents, and the like, without detracting from the advantageous properties of the compositions.
- adjuvants for example, perfumes, electrolytes, enzymes, dyes, antitarnishing agents, antimicrobial agents, and the like
- Alkalinity sources and pH buffering agents such as monoethanolamine, triethanolamine and alkali metal hydroxides can also be utilized.
- the anionic surfactant is a sulfate surfactant or alkylpolyethoxylate sulfate surfactant
- the pH should ' a above about 6, preferably above about 7 to avoid hydrolysis of the ester linkage.
- the composition be substantially free of antibacterial agents such as N-trichloromethyl-thio-4-cyclohexane-1,2,dicarboximide for safety.
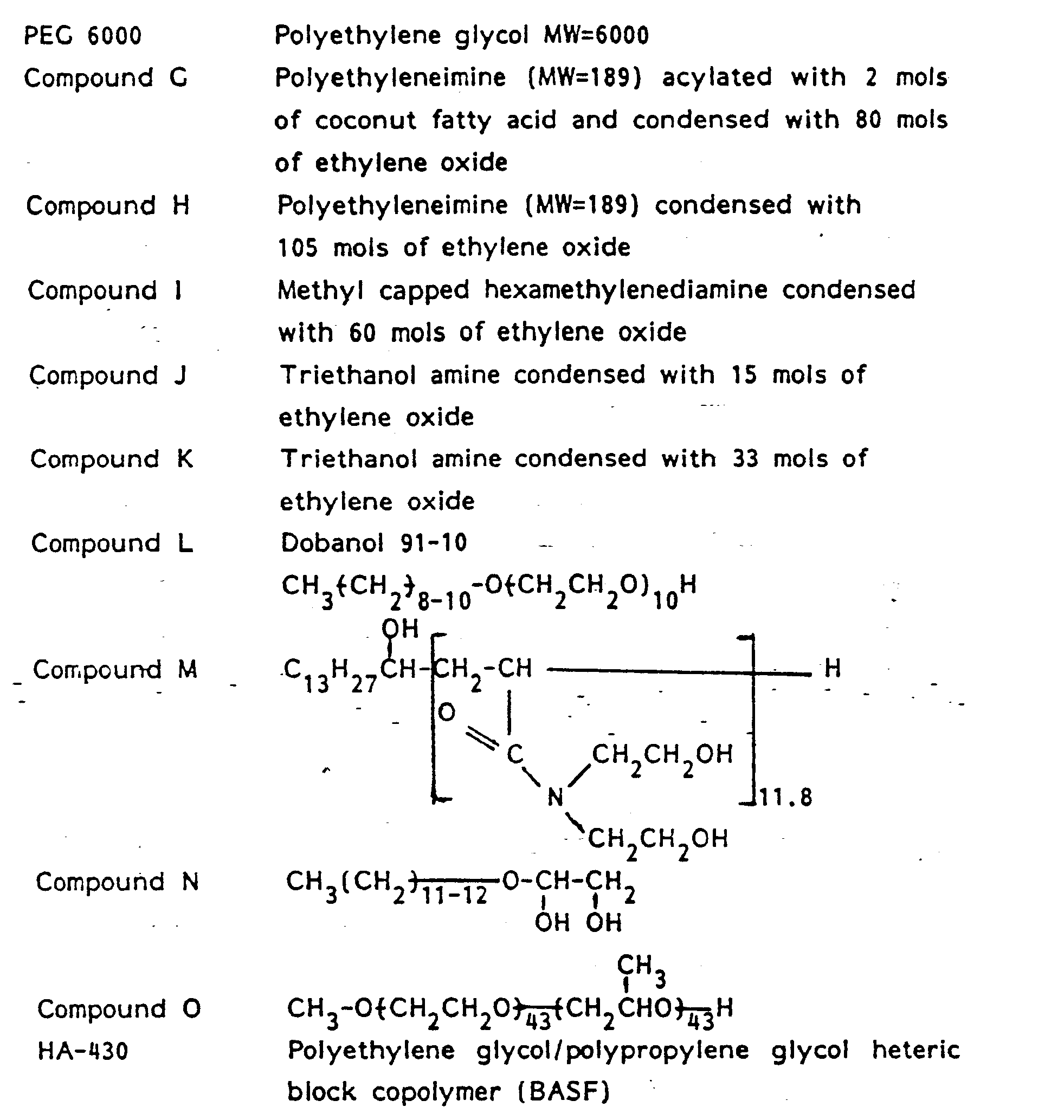
- E stands for an ethoxylate group and P stands for a propoxylate group.
- the base product contains about 5% magnesium C, 2 . 13 alkyl sulfate, about 23% mixed magnesium and ammonium C 12 . 13 alkyl polyethoxylate (1) sulfate, about 2.7% C 12 . 13 alkyl dimethyl amine oxide, about 5% ethyl alcohol, about 3% sodium toluene sulfonate, about 60% water, and the balance being inorganic salts, minor ingredients, etc.
- grey cutting is determined by the following test.
- a preweighed 250 cc. polypropylene cup has 3 cc. of a melted beef grease applied to its inner bottom surface. After the grease has solidified, the cup is reweighed. Then a .4% aqueous solution of the composition to be tested is added to the cup to completely fill it. The aqueous solution has a temperature of 46°C. After 15 minutes, the cup is emptied and rinsed with distilled water. The cup is dried and then weighed to determine the amount of grease removal. The amount removed by the base product is indexed at 100.
- greyness is determined by modifying the above grease cutting test by using 10 ml of an easier to remove fat which is an 80/20 mixture of a solid vegetable shortening and a liquid vegetable shortening, lowering the detergent concentration to about 0.2%, and soaking for 30 minutes to allow equilibrium to occur.
- the viscosity of the composition is greater than about 150 centipoise and less than about 500 centipoise.
- This example demonstrates yet another polymeric surfactant structure that is operable.
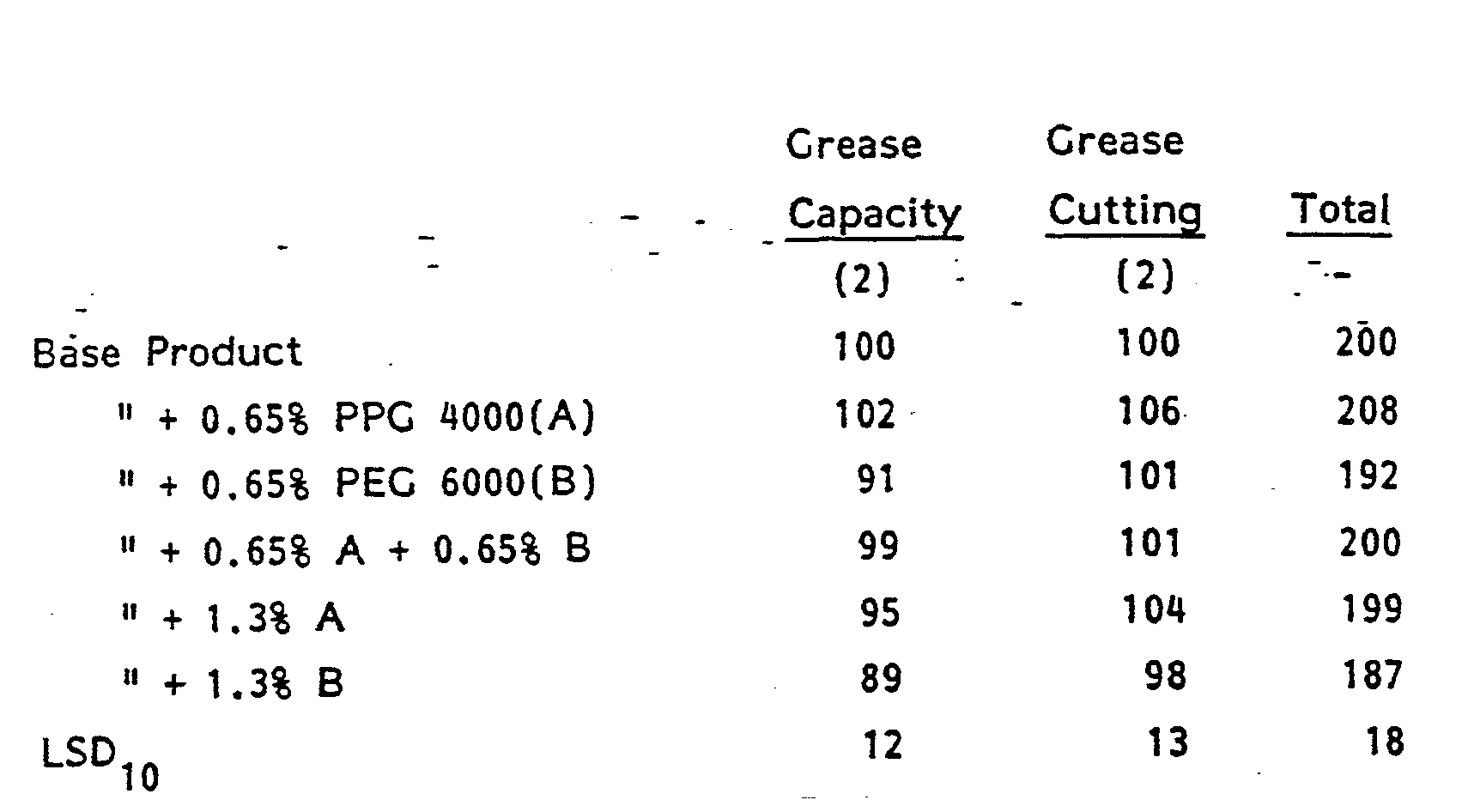
- This example demonstrates that increasing the amount of the polymeric surfactant, a heteric block copolymer of ethylene oxide and propylene oxide on a glycerol base, improves Grease Capacity, but, eventually, lowers the Grease Cutting unacceptably. High levels above about 4%, and especially above about 9%, lose good grease cutting when the basic formula is optimized for grease cutting.
- Example XIV shows the effect of increased (Tetronic) surfactant. Again, above about 4%, there is a loss which becomes substantial before a level of about 9% is reached.
- This example shows the effect of using twice the amount of a commercial detergent.
- the Grease Capacity and Grease Cutting are increased, but at a much greater cost than associated with the invention.
- a high sudsing, light duty liquid detergent composition is as follows
- This example demonstrates the excellent performance of mixtures of betaine surfactants and the polymeric surfactants. At ratios up to about 20:1 grease cutting is improved, but the optimum ratio is lower, e.g. about 9:1 or less where both grease cutting and grease capacity are improved.
- This example demonstrates the large reductions is viscosity obtained by adding the polymeric surfactant.
- the viscosity can be adjusted back up by reducing alcohol and/or hydrotrope levels. As can be seen, the higher the level of ethoxylate moieties in the polymers, the less the reduction in viscosity.
- Viscosities are measured on these compositions at 70°F with a Brookfield LVF viscometer, spindle No. 2, at 60 rpm.
- Results are shown for the three additives and are compared against equal parts of added ethanol also replacing water in the formula.
- Ethanol is typically used to trim viscosity and is already present in the formula at about 4.5 parts/100 prior to the added parts.
- the addition of the polymers all drop the viscosity further than does the added ethanol.
- the Pluronic 61 is even more effective at 1% than is ethanol at 5%.
- the additive compounds provide different levels of viscosity reduction.
- the Compound H in he first experiment is one of the poorer (more hydrophilic) performers of Example IX and, though effective on viscosity reduction, did not show as great a benefit.
- the pluronic compounds of lower HLB (lower second digit) and moderate molecular weight (first digit) are more effective. If the purpose for adding the polymer is to lower viscosity, lower levels provide the biggest benefit per part of polymer added.
- alkyl groups can be used as terminal hydrophobic groups, but do not provide the best results, especially when the hydrophilic portion of the molecule represents less than about 45% of the molecular weight in compounds with saturated groups each of which is longer than about 16 carbon atoms.
- This test determines the effectiveness or strength of the grease emulsification by the detergent by measuring the level of grease deposition on a hydrophobic surface after its exposure to a detergent solution to which a grease has been added. This test models the actual situation of redeposition of greases onto later washed items, especially plastics.
- the reference product used here is the base product.
- the polymeric surfactant is added at the 1% level to the base.
- a " * " indicates a statistically significant (LSD 05 ) reduction in grease redeposition compared to the Base Product.
- Tetronic 704 and Compound F did not excel in this test, but did perform well in the previous examples. Again, the Methocel polymer does not provide sufficient benefit.
- compositions of this invention When some of the compositions of this invention are first made, they are not at equilibrium. They typically require an aging period to reach equilibrium and exhibit the full benefit. A period of about two weeks, which is about equivalent to the normal time between making and use by the consumer is usually sufficient.
Landscapes
- Chemical & Material Sciences (AREA)
- Life Sciences & Earth Sciences (AREA)
- Engineering & Computer Science (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Oil, Petroleum & Natural Gas (AREA)
- Wood Science & Technology (AREA)
- Organic Chemistry (AREA)
- Detergent Compositions (AREA)
- Emulsifying, Dispersing, Foam-Producing Or Wetting Agents (AREA)
Abstract
Description
- This is a continuation-in-part of our copending application, Serial Number 793,530, filed October 31, 1985.
- The invention relates to aqueous high sudsing liquid detergent compositions containing specified amounts and types of surfactants especially useful in the washing of tableware, kitchenware and other hard surfaces.
- The compositions of this invention have superior ability to handle grease.
- The performance of a detergent composition for cleaning tableware and kitchen utensils is evaluated by its ability to handle grease. The detergent solution should readily remove grease and minimize its redeposition.
- There is continuing need for improved compositions and methods which can be employed during dishwashing operations to improve the appearance of kitchen utensils and articles. Such compositions and methods should provide improved removal of grease in conventional dishwashing soil removal operations while maintaining the sudsing attributes of an acceptable dishwashing detergent composition.
- The present invention comprises a high sudsing liquid detergent composition containing by weight:
- (a) from about 5% to about 50% anionic surfactant;
- (b) from about 0.1% to about 12% of polymeric surfactant having the formula selected from the group consisting of AnBAm, BnABm, BA, B and mixtures thereof wherein each B is a hydrophobic group; each A is a hydrophilic group; each n and m are either 0 or an integer from one to about 50; the sum of n + m is from one to about 50; the molecule contains from about 5 to about 1,000 ether linkages; when the formula is BA, B contains from about 5 to about 500 ether linkages; when the formula is B, the ratio of -CH- 2-groups to ether linkages is at least about 2.1:1 and less than about 3:1; the molecular weight is from about 400 to about 60,000; and the percentage of (̵ C2H4O 7- groups in the molecule is less than about 90%;
- (c) from 0% to about 10% of a suds stabilizing nonionic surfactant selected from the group consisting of fatty acid amines, trialkyl amine oxides and mixtures thereof;
- (d) from 0% to about 10% of a detergency builder selected from inorganic phosphates, inorganic polyphosphates, inorganic silicates, and inorganic carbonates, organic carboxylates, organic phosphonates, and mixtures thereof;
- (e) from 0% to about 15% alkanol containing from one to about six carbon atoms; and
- (f) from about 20% to about 90% water, said composition containing sufficient magnesium ions to neutralize at least about 10% of said anionic surfactant when less than about 10% of the anionic surfactant is an alkylpolyethoxylate sulfate surfactant containing from about ? to about ten ethoxy groups per molecule on the average (or there is no betaine surfactant present); said composition having a pH of greater than about six when the composition contains said alkylpolyethoxylate sulfate surfactant; said composition having a viscosity of greater than about 100 cps or being substantially free of alkylpolyethoxylate detergent surfactants when the amount of anionic surfactant is less than about 20% (and there is no betaine surfactant present).
- Dishware, glassware, and other tableware and kitchenware are washed in water solutions of the detergent composition, generally at a weight concentration of from about 0.05% to about 0.4% of the composition in water at a temperature of from about 60°F to about 120°F.
- The liquid detergent compositions of the present invention contain two essential components:
- (a) anionic surfactant which when there is no betaine surfactant present is either a magnesium salt and/ or an alkylpolyethoxylate sulfate containing an average of from about to about ten ethoxy groups per molecule, said average being computed herein by treating any alkyl sulfate surfactant as an alkylpolyethoxylate sulfate containing 0 ethoxy groups, as described hereinbefore, to provide good sudsing, and preferably a low interfacial tension; and
- (b) the polymeric surfactant, which improves grease handling.
- Optional ingredients can be added to provide various performance and aesthetic characteristics.
- The compositions of this invention contain from about 5% to about 50% by weight of an anionic surfactant or mixtures thereof preferably comprising at least about 5%, more preferably at least about 8%, and most preferably more than about 10% of an alkyl polyethoxylate (polyethylene oxide) sulfate having from about 10 to about 20, preferably from about 10 to about 16 carbon atoms in the alkyl group and containing from about 7 to about 10, preferably from about 1 to about 8, most preferably from about 1 to about 6 ethoxy groups on the average. Preferred compositions contain from about 20% to about 40% of anionic surfactant by weight.
- Most anionic detergents can be broadly described as the water-soluble salts, particularly the alkali metal, alkaline earth metal, ammonium or amine salts, of organic sulfuric reaction products having in their molecular structure an alkyl radical containing from about 8 to about 22 carbon atoms and a radical selected from the group consisting of sulfonic acid and sulfuric acid ester radicals. Included in the term "alkyl" is the alkyl portion of acyl radicals. Examples of the anionic synthetic detergents which can form the surfactant component of the compositions of the present invention are the salts of compatible cations, e.g. sodium, ammonium, monoethanolammonium, diethanolammonium, triethanolammonium, potassium and/or, especially, magnesium cations with: alkyl sulfates, especially those obtained by sulfating the higher alcohols (C.-C,. carbon atoms), alkyl benzene, or alkyl toluene, sulfonates, in which the alkyl group contains from about 9 to about 15 carbon atoms, the alkyl radical being either a straight or branched aliphatic chain; paraffin sulfonates or olefin sulfonated in which the alkyl or alkenyl group contains from about 10 to about 20 carbon atoms; sodium C'O.20 alkyl ether sulfonates, especially those ethers of alcohols derived from tallow and coconut oil; coconut oil fatty acid monoglyceride sulfates and sulfonates; alkylphenolpolyethylene oxide ether sulfates with from about 1 to about 10 units of ethylene oxide per molecule on the average in which the alkyl radicals contain from 8 to about 12 carbon atoms; the reaction products of fatty acids esterified with isethionic acid where, for example, the fatty acids are derived from coconut oil; fatty acid amides of a methyl tauride in which the fatty acids, for example, are derived from coconut oil; and beta- acetoxy-or beta-acetamido-alkanesulfonates where the alkane has from 8 to 22 carbon atoms.
- Specific examples of alkyl sulfate salts which can be employed in the instant detergent compositions include sodium, potassium, ammonium, monoethanolammonium, diethanolammonium, triethanolammonium, and magnesium: lauryl sulfates, stearyl sulfates, palmityl sulfates, decyl sulfates, myristyl sulfates, tallow alkyl sulfates, coconut alkyl sulfates, C12-15 alkyl sulfates and mixtures of these surfactants. Preferred alkyl sulfates include the C12.15 alkyl sulfates.
- Suitable alkylbenzene, or alkyltoluene, sulfonates include the alkali metal (lithium, sodium, and/or potassium), alkaline earth (preferably magnesium), ammonium and/or alkanolammonium salts of straight, or branched-chain, alkylbenzene, or alkyltoluene, sulfonic acids. Alkylbenzene sulfonic acids useful as precursors for these surfactants include decyl benzene sulfonic acid, undecyl benzene sulfonic acid, dodecyl benzene sulfonic acid, tridecyl benzene sulfonic acid, tetrapropylene benzene sulfonic acid and mixtures thereof. Preferred sulfonic acids as precursors of the alkyl-benzene sulfonates useful for compositions herein are those in which the alkyl chain is linear and averages about 11 to 13 carbon atoms in length. Examples of commercially available alkyl benzene sulfonic acids useful in the present invention include Conoco SA 515 and SA 597 marketed by the Continental Oil Company and Calsoft LAS 99 marketed by the Pilot Chemical Company.
- The preferred anionic surfactants herein, which are essential if there are no, e.g., magnesium ions or betaine surfactant present, are alkylpolyethoxylate sulfates having the formula RO(C2H4O)x SO,M wherein R is alkyl, or alkenyl, of from about 10 to about 20 carbon atoms, x is from about to about ten on the average, treating alkyl sulfates as if they had 0 ethoxy groups, preferably from about to about eight, most preferably from about one to about six, and M is a water-soluble compatible cation such as those disclosed hereinbefore. The alkylpolyethoxylate sulfates useful in the present invention are sulfates of condensation products of ethylene oxide and monohydric alcohols having from about 10 to about 20 carbon atoms. Preferably, R has 10 to 16 carbon atoms. The alcohols can be derived from natural fats, e.g., coconut oil or tallow, or can be synthetic. Such alcohols can be reacted with from about t to about 20, especially from about one to about 14, and more especially from about one to about eight, molar proportions of ethylene oxide and the resulting mixture of molecular species is sulfated and neutralized.
- There should be more than about 10%, preferably more than about 15% of such molecules containing one to 10 ethoxylate groups calculated as a percentage of the total anionic surfactant in the composition. When these molecules are mixed with alkyl sulfates which are treated as containing 0 ethoxylate groups, the computed average degree of ethoxylation should be more than about 0.5, preferably more than about 0.6. One can use a similar approach in computing the minimum desired amount of the alkyl polyethoxylate sulfate which should be present when admixed with any anionic surfactant. E.g. the other anionic surfactant can be treated as if it were an alkyl sulfate to compute the average degree of ethoxylation.
- Specific examples of alkylpolyethoxylate sulfates of the present invention are sodium coconut alkylpolyethoxylate (3) ether sulfate, magnesium C12-15 alkylpolyethoxylate (3) ether sulfate, and sodium tallow alkylpolyethoxylate (6) ether sulfate. A particularly preferred example is a water soluble, e.g. magnesium, C12-13 alkylpolyethoxylate (1) ether sulfate. Preferred alkyl polyethoxylate sulfates are those comprising a mixture of individual compounds, said mixture having an average alkyl chain length of from about 10 to 16 carbon atoms and an average degree of ethoxylation of from about 1 to about 8 moles of ethylene oxide.
- For use in completely soft water, the compositions should contain magnesium ions, and/or at least about 10%, preferably at least about 15% by weight of the anionic surfactant, of the preferred alkyl polyethoxylate sulfates described hereinbefore. It is preferred that the compositions of this invention, including those that contain the preferred alkylpolyethoxylate sulfates, also contain magnesium and/or calcium ions, most preferably magnesium ions, to act as cations for a portion of the anionic surfactant. If the composition is to be used primarily in water containing more than about 2 grains/gal. of hardness, added magnesium may not be essential. In use, from about 10% to about 100%, preferably from about 20% to about 90%, of the anionic surfactant should be the magnesium salt.
- The formulation of anionic surfactant systems that will reduce the interfacial tension is well within the skill of the typical detergent formulator. For the purposes of this invention, the surfactant system minus the polymeric surfactant should preferably reduce the interfacial tension to below about 2t dyne/cm, preferably below about 2 dynes/cm, against triolein at a concentration of 0.2% and a temperature of 115°F (46°C) in a spinning drop Tensiometer. Interfacial tension is lowered by any detergent surfactant, but the efficiency can be improved by selection of surfactants which have longer alkyl chain lengths, use of cations such as magnesium which minimize charge effects when anionic surfactants are used, and use of anionic surfactants combined with cosurfactants like trialkylamine oxides which form complexes with the anionic surfactant. A more complete discussion of such effects can be found in Milton J. Rosen, Surfactants and Interfacial Phenomena, 149-173 (1978), incorporated herein by reference.
- Preferably, the compositions of the present invention contain from about 0.1% to about 10%, more preferably from about ½% to about 4%, and most preferably from about ½% to about 2%, of the polymeric surfactant described generically hereinbefore and discussed in detail hereinafter.
- In the generic formula for the polymeric surfactant set forth hereinbefore, B is preferably a polypropylene oxide group, containing more than about 5 propylene oxide groups, which can contain some ethylene oxide groups, n and m are preferably from about 1 to about 2 and the sum of n + m is from about 2 to about 4, the molecule contains from about 20 to about 500 ether linkages, and the molecular weight is from about 1000 to about 40,000.
- The polymeric surfactant is preferably represented by the formula:
- [R1 (̵ R2O )̵ n (̵ R3O )̵ m]y[R4] wherein each R' is selected from the group consisting of hydrogen, alkyl groups containing from one to about 18 carbon atoms, acyl groups containing from two to about 18 carbon atoms, -S04M, -SO,M, -COOm, -N(R5)2→ O, -N(R5)+) amide groups, pyrollidone groups, saccharide groups, and hydroxy groups in which each M is a compatible cation and each RS is either an alkyl or hydroxy alkyl group containing from one to about four carbon atoms; wherein each R2 or R is an alkylene group containing from two to about six carbon atoms with no more than about 90% of said molecule comprising R2 and R3 groups containing two carbon atoms; wherein R* is selected from the group consisting of alkylene groups containing from one to about 18 carbon atoms and having from two to about six valences, polyhydroxyalkylene oxide groups wherein each alkylene group has from one to about six hydroxy groups and contains from three to about eight carbon atoms and there are from two to about 50 hydroxyalkylene oxide groups and from two to about 50 hydroxy groups, (=NR2N=), hydrogen, =N (̵ R2NH )̵x, polyester groups containing from one to about 20 ester linkages and each ester group containing from about 4 to about 18 carbon atoms; wherein n is from 0 to about 500, m is from 0 to about 500, n + m is from about 5 to about 1000, x is from about 2 to about 50, and y is from one to about 50 and equal to the valences of R*; wherein the molecular weight is from about 400 to about 60,000; and wherein the (̵ R20 )̵ and the (̵ R3O )̵ groups are interchangeable;
- While not wishing to be bound by theory, it is believed that the polymeric surfactant functions by forming complexes with the hydrophilic portions of the anionic surfactants, thereby minimizing the ability of the anionic surfactants to leave a micelle or other interfacial region once formed. Therefore, long terminal hydrocarbon groups are not preferred, and are not acceptable when the formula is of the BA type. Long terminal hydrocarbons pull the polymer into any oil phase, thereby minimizing the number of anionic surfactant molecules that are stabilized. Similarly, if the hydrophilic portion of the molecule is too hydrophilic, the molecule is pulled into the aqueous phase too far. The molecule should be balanced between hydrophobicity and hydrophilicity and have enough ether and/or amine linkages spread throughout the structure to complex the anionic surfactant. The anionic surfactant also must be one that will form the complex. Magnesium cations, ether linkages, and amine or ammonium groups form stable complexes with the polymeric surfactants.
- Preferably the surfactant contains a hydrophilic group comprising polyethylene oxide and/or ethyleneimine groups containing from about 1 to about 500 ethylene oxide and/or ethyleneimine derived moieties. Sulfonate or sulfate groups, can also be present. The polymeric surfactant also contains at least one hydrophobic group, preferably comprising polyalkylene oxide groups wherein the alkylene contains from three to about six, most preferably three, carbon atoms and the molecular weight is from about 400 to about 60,000. The alkylene groups containing from about 7 to about 18, preferably from about 10 to about 18, carbon atoms can also be used, but preferably only short chain relatively nonoleophilic alkyl or acyl groups containing less than about ten carbon atoms are pendant on the polymeric surfactant.
- Preferred surfactants are block copolymers comprising one or more groups that are hydrophilic and which contain mostly ethylene oxide groups and one or more hydrophobic groups which contain mostly propylene oxide groups attached to the residue of a compound that contained one or more hydroxy or amine groups onto which the respective alkylene oxides were polymerized, said polymers having molecular weights of from about 400 to about 60,000, an ethylene oxide content of from about 10% to about 90% by weight and a propylene oxide content of from about 10% to about 90% by weight.
- Preferred surfactants are those in which propylene oxide is condensed with an amine, especially ethylenediamine to provide a hydrophobic base having a molecular weight of from about 350 to about 55,000, preferably from about 500 to about 40,000. This hydrophobic base is then condensed with ethylene oxide to provide from about 10% to about 90%, preferably from about 20% to about 80% ethylene oxide. Reverse structures in which the ethylene oxide is condensed first are also desirable. These structures are especially easy to formulate into desirable single phase liquid compositions.
- Similar structures in which the ethylenediamine is replaced by a polyol, especially propylene glycol, or glycerine, or condensation products of glycerine, are also desirable.
- In similar compositions, the polypropylene glycol portion can be replaced by an alkyl, or alkylene group containing from about 5 to about 18, preferably from about 8 to about 16 carbon atoms and the polyethylene oxide groups can be replaced either totally, or, preferably in part, by other water solubilizing groups, especially sulfate and sulfonate groups.
- Specific examples of such compounds include:
- A. R' f OCH2CH2 )̵x R2 (̵ OCH2CH2 )̵y OR' where: -R' is H, or CH,, or CH3(CH2)n, or unsaturated analogues
where: n=1-17
-x,y = 2-500 - B. R3R4 (̵ OCH2 CH )̵AR4R3
where: -R' is sulfate or sulfonate
-R' is nothing; -E OCH2CH2 )̵ B; or other groups capable of bonding to propylene oxide, including sulfate or sulfonate groups.
-A is 5-500
-B < A/2 - Specific preferred examples of such compounds include:
- A. H (̵ OCH2CH2)x-O(CH2)z-(OCH2CH2)y-H
- B. CH3(CH2)(OCH2CH2) O(CH2)nCH,
- C. NaO3S (̵ OCH2 CH )̵-A-OSO3Na H3
- D. NaO3S (̵ OCH2CH2 )̵-B-(OCH2 H(OCH2CH2 OSO3Na where: -x, y, z, n, A, B are as previously defined.
- The compositions of this invention contain from 0% to about 10%, preferably from about 1% to about 8%, of suds stabilizing nonionic surfactant or mixtures thereof.
- Suds stabilizing nonionic surfactants operable in the instant compositions are of two basic types: fatty acid amides and the trialkyl amine oxide semi-polar nonionics.
- The amide type of nonionic surface active agent includes the ammonia, monoethanol and diethanol amides of fatty acids having an acyl moiety of from about 8 to 18 carbon atoms and represented by the general formula:
R'-CO-N(H)m (R2OH)2-m
wherein R, is a saturated or unsaturated, aliphatic hydrocarbon radical having from 7 to 21, preferably from 11 to 17 carbon atoms; R2 represents a methylene or ethylene group; and m is 1 or 2. Specific examples of said amides are coconut fatty acid monoethanol amide and dodecyl fatty acid diethanol amide. These acyl moieties may be derived from naturally occurring glycerides, e.g., coconut oil, palm oil, soybean oil and tallow, but can be derived synthetically, e.g., by the oxidation of petroleum, or hydrogenation of carbon monoxide by the Fischer-Tropsch process. The monoethanol amides and diethanolamides of C12.14fatty acids are preferred. - Amine oxide semi-polar nonionic surface active agents comprise compounds and mixtures of compounds having the formula:
- The pref, -red sudsing characteristics of the compositions of the invention are those which will provide the user of the product with an indication of cleaning potential in a dishwashing solution. Soils encountered in dishwashing act as suds depressants and the presence or absence of suds from the surface of a dishwashing solution is a convenient guide to product usage. Mixtures of anionic surfactants and suds stabilizing nonionic surfactants are utilized in the compositions of the invention because of their high sudsing characteristics, their suds stability in the presence of food soils and their ability to indicate accurately an adequate level of product usage in the presence of soil
- In preferred embodiments of the invention, the ratio of anionic surfactants to suds stabilizing nonionic surfactants in the composition will be in a molar ratio of from about 11:1 to about 1:1, and more preferably from about 8:1 to about 3:1.
- The compositions of the invention can desirably contain optional surfactants, especially ampholytic and/or zwitterionic surfactants. However, when the level of anionic surfactant is less than about 20%, the composition should not contain any substantial amount of conventional nonionic surfactant, e.g., an alkylpolyethoxylate, in addition to the polymeric surfactant. Large amounts of conventional nonionic surfactants, e.g., more than about three or four percent, tend to harm the sudsing ability of the composition.
- When larger amounts ( > 20%) of anionic surfactants are present it is sometimes desirable to have a low level, up to about 5%, of conventional nonionic surfactants "conventional" nonionic surfactants are e.g., C8.18 alkyl polyethoxylates (4-15) or C8.15 alkyl phenol polyethoxylates (4-15).
- Ampholytic surfactants can be broadly described as derivatives of aliphatic amines which contain a long chain of about 8 to 18 carbon atoms and an anionic water-solubilizing group, e.g. carboxylate, sulfonate or sulfate. Examples of compounds falling within this definition are sodium-3-dodecylamino propane sulfonate, and dodecyl dimethylammonium hexanoate.
- Zwitterionic surface agents operable in the instant composition are broadly described as internally- neutralized derivatives of aliphatic quaternary ammonium and phosphonium and tertiary sulfonium compounds in which the aliphatic radical can be straight chain or branched, and wherein one of the aliphatic substituents contains from about 8 to 18 carbon atoms and one contains an anionic water solubilizing group, e.g., carboxy, sulfo, sulfato, phosphato, or phosphono.
- Highly preferred are betaine detergent surfactants which synergistically interact with the polymeric surfactant to provide improved grease handling.
- The betaine detergent surfactant has the general formula:
- Examples of preferred betaines are dodecylamidopropyl dimethylbetaine; dodecyldimethylbetaine; tetradecyldimethylbetaine; cetyldimethylbetaine; cetylamidopropyldimethylbetaine, tetradecyldimethylbetaine, tetradecylamidopropyldimethylbetaine, and docosyldimethylammonium hexanoate and mixtures thereof.
- Betaine surfactants are unique ingredients that provide exceptional benefits. When betaine surfactant and polymeric surfactant are combined with any anionic surfactant, with, or without magnesium ions being present, superior grease holding benefits are provided.
- Betaines containing a C12.14 alkyl provide a much bigger benefit when combined with polymeric surfactant than when used by themselves.
- The betaine is preferably present at a level of from about 7% to about 15% by weight of the formula, preferably from about 1% to about 10%, most preferably from about 1% to about 8%. The ratio of anionic detergent surfactants to the betaine is from about 1 to about 80, preferably from about 1 to about 40, more preferably from about 2 to about 40.
- When betaines are present, the composition should preferably have a ratio of betaine to polymeric surfactant of more than about 7:1, preferably more than about 9:1.
- Alcohols, such as ethyl alcohol, and hydrotropes, such as sodium and potassium toluene sulfonate, sodium and potassium xylene sulfonate, trisodium sulfosuccinate and related compounds (as disclosed in U.S. Patent 3,915,903, incorporated herein by reference) and urea, can be utilized in the interests of achieving a desired product phase stability and viscosity. Alkanols containing from one to about six carbon atoms, especially two, and especially ethyl alcohol can be present. Ethyl alcohol at a level of from 0% to about 15%, preferably from about 1% to about 6%, and potassium and/or sodium toluene, xylene, and/or cumene sulfonates at a level of from about 1% to about 6% can be used in the compositions of the invention. The viscosity should be greater than about 100 centipoise, more preferably more than 150 centipoise, most preferably more than about 200 centipoise for consumer acceptance.
- However the polymeric surfactant can be used to reduce the viscosity and provide phase stability, e.g., when either the preferred alkyl polyethoxylate sulfate or magnesium ions are present in the composition. For viscosity reduction, the percentage of ethylene oxide in the polymer should be less than about 70%, preferably less than about 50%. Preferred compositions contain less than about 2% alcohol and less than about 3% hydrotrope and preferably essentially none while maintaining a viscosity of from about 150 to about 500 centipoise, preferably from about 200 to about 400 centipoise. If viscosity reduction is not desired the percentage of ethylene oxide in the polymer should be more than about 50%, preferably more than about 70%. The polymeric surfactant reduces viscosity for all water soluble anionic surfactants.
- The compositions of this invention contain from about 20% to about 90%, preferably from about 30% to about 80%, water.
- The compositions of this invention can contain up to about 10%, by weight of detergency builders either of the organic or inorganic type. Examples of water-soluble inorganic builders which can be used, alone or in admixture with themselves and organic alkaline sequestrant builder salts, are alkali metal carbonates, phosphates, polyphosphates, and silicates. Specific examples of such salts are sodium tripolyphosphate, sodium carbonate, potassium carbonate, sodium pyrophosphate, potassium pyrophosphate, and potassium tripolyphosphate. Examples of organic builder salts which can be used alone, or in admixture with each other or with the preceding inorganic alkaline builder salts, are alkali metal polycarboxylates, e.g., water-soluble citrates, tartrates, etc. such as sodium and potassium citrate and sodium and potassium tartrate. In general, however, detergency builders have limited value in dishwashing detergent compositions and use at levels above about 10% can restrict formulation flexibility in liquid compositions because of solubility and phase stability considerations. It is preferred than any builder used be relatively specific to control of calcium as opposed to magnesium. Citrates, tartrates, malates, maleates, succinates and malonates are especially preferred.
- The detergent compositions of this invention can contain, if desired, any of the usual adjuvants, diluents and additives, for example, perfumes, electrolytes, enzymes, dyes, antitarnishing agents, antimicrobial agents, and the like, without detracting from the advantageous properties of the compositions. Alkalinity sources and pH buffering agents such as monoethanolamine, triethanolamine and alkali metal hydroxides can also be utilized.
- When the anionic surfactant is a sulfate surfactant or alkylpolyethoxylate sulfate surfactant, the pH should ' a above about 6, preferably above about 7 to avoid hydrolysis of the ester linkage. Also, it is desirable that the composition be substantially free of antibacterial agents such as N-trichloromethyl-thio-4-cyclohexane-1,2,dicarboximide for safety.
- Low levels of antibacterial agents that will prevent growth of bacteria, molds, etc. in the product, but which have essentially no effect in use can be desirable, especially when low levels of alcohol are present.
- All percentages and ratios herein are by weight unless otherwise indicated.
- The following examples are given to illustrate the compositions of the invention.
-
- The base product contains about 5% magnesium C,2.13 alkyl sulfate, about 23% mixed magnesium and ammonium C12.13 alkyl polyethoxylate (1) sulfate, about 2.7% C 12.13 alkyl dimethyl amine oxide, about 5% ethyl alcohol, about 3% sodium toluene sulfonate, about 60% water, and the balance being inorganic salts, minor ingredients, etc.
- In the following examples, "grease cutting" is determined by the following test. A preweighed 250 cc. polypropylene cup has 3 cc. of a melted beef grease applied to its inner bottom surface. After the grease has solidified, the cup is reweighed. Then a .4% aqueous solution of the composition to be tested is added to the cup to completely fill it. The aqueous solution has a temperature of 46°C. After 15 minutes, the cup is emptied and rinsed with distilled water. The cup is dried and then weighed to determine the amount of grease removal. The amount removed by the base product is indexed at 100.
- In the following examples, "grease capacity" is determined by modifying the above grease cutting test by using 10 ml of an easier to remove fat which is an 80/20 mixture of a solid vegetable shortening and a liquid vegetable shortening, lowering the detergent concentration to about 0.2%, and soaking for 30 minutes to allow equilibrium to occur.
- In the Examples "*" indicates a significant difference and the figures in parentheses under the headings "Grease Capacity" and "Grease Cutting" are the number of replicates run and averaged to give the indicated test scores.
- In all of the Examples, the viscosity of the composition is greater than about 150 centipoise and less than about 500 centipoise.
-
-
-
-
-
-
-
-
-
-
-
-
-
- This example demonstrates that increasing the amount of the polymeric surfactant, a heteric block copolymer of ethylene oxide and propylene oxide on a glycerol base, improves Grease Capacity, but, eventually, lowers the Grease Cutting unacceptably. High levels above about 4%, and especially above about 9%, lose good grease cutting when the basic formula is optimized for grease cutting.
-
-
-
- In a similar composition the urea is replaced by 4% sodium xylene sulfonate and the ethanol is reduced to 3.5%.
-
- This example demonstrates the excellent performance of mixtures of betaine surfactants and the polymeric surfactants. At ratios up to about 20:1 grease cutting is improved, but the optimum ratio is lower, e.g. about 9:1 or less where both grease cutting and grease capacity are improved.
-
- This example demonstrates the large reductions is viscosity obtained by adding the polymeric surfactant. The viscosity can be adjusted back up by reducing alcohol and/or hydrotrope levels. As can be seen, the higher the level of ethoxylate moieties in the polymers, the less the reduction in viscosity.
-
- Polymer compounds are added at 0.5%, 1 %, and 5% to the National Brand composition previously described, replacing water in the 100-part formula. Clear solutions result.
- Viscosities are measured on these compositions at 70°F with a Brookfield LVF viscometer, spindle No. 2, at 60 rpm.
- Results are shown for the three additives and are compared against equal parts of added ethanol also replacing water in the formula. Ethanol is typically used to trim viscosity and is already present in the formula at about 4.5 parts/100 prior to the added parts.
- Surprisingly, the addition of the polymers all drop the viscosity further than does the added ethanol. The Pluronic 61 is even more effective at 1% than is ethanol at 5%.
-
-
- Note that the additive compounds provide different levels of viscosity reduction. The Compound H in he first experiment is one of the poorer (more hydrophilic) performers of Example IX and, though effective on viscosity reduction, did not show as great a benefit. The pluronic compounds of lower HLB (lower second digit) and moderate molecular weight (first digit) are more effective. If the purpose for adding the polymer is to lower viscosity, lower levels provide the biggest benefit per part of polymer added.
-
- This example clearly shows that when a mixture of polymeric surfactant and betaine is used, it is not necessary to have either an alkyl polyethoxylate sulfate surfactant or magnesium ions present.
-
- MAPEG 6000DS (dialkyl polyethoxylate) C18 E136 C18 92% E
- MAPEG 400DS (dialkyl polyethoxylate) C18 E9 C18 44% E
- MAPEG 400DL (dialkyl polyethoxylate) C,2 E9 C12 54% E
- MAPEG 400 DO (dialkylene polyethoxylate) C18 E9 C18 45% E
- This example clearly shows that alkyl groups can be used as terminal hydrophobic groups, but do not provide the best results, especially when the hydrophilic portion of the molecule represents less than about 45% of the molecular weight in compounds with saturated groups each of which is longer than about 16 carbon atoms.
- In this example, a different type of test was used to demonstrate another aspect of grease control by the detergent compositions. In most cases, this test gives a ranking between formulations similar to that of the total index value of the preceeding examples.
- This test determines the effectiveness or strength of the grease emulsification by the detergent by measuring the level of grease deposition on a hydrophobic surface after its exposure to a detergent solution to which a grease has been added. This test models the actual situation of redeposition of greases onto later washed items, especially plastics.
- For this experiment, 2 gallons of median hardness water (6 grains/gallon) were held at 105°F, a common end-of-wash temperature for dishwater. A 0.1% solution of the detergent product was made and mild agitation was begun. Liquid vegetable oil was added in 6cc increments. At totals of 18cc, 36cc, and 54cc, plastic items (3 for each grease level, 9 total) are dipped in succession into the water. After drying, the mean weight gain per plastic item unit area is calculated and indexed to a reference product.
- The reference product used here is the base product. The polymeric surfactant is added at the 1% level to the base.
- A "*" indicates a statistically significant (LSD05) reduction in grease redeposition compared to the Base Product.
- The compounds tested herein that were not previously defined are as follows:
Formula for P-T: - CH3(OCH2CH2)xO (CH2)y O(CH2CH2O)xCH3
- P X=8, Y=4
- Q X=8,Y=14
- R X=43, Y=4
- S X=43, Y=14
- TX=17,Y=10
-
- Note from the above that Tetronic 704 and Compound F did not excel in this test, but did perform well in the previous examples. Again, the Methocel polymer does not provide sufficient benefit.
- Also, certain very high molecular weight compounds (R and S) of the ABA type do not show any advantage.
- Otherwise, all are exemplary of the invention.
- When some of the compositions of this invention are first made, they are not at equilibrium. They typically require an aging period to reach equilibrium and exhibit the full benefit. A period of about two weeks, which is about equivalent to the normal time between making and use by the consumer is usually sufficient.
-R2 = nothing or O(CH2)z or saturated analogue of these where z = 1-18
Claims (22)
wherein R, is a saturated or unsaturated, aliphatic hydrocarbon radical having from 7 to 21, R2 represents a methylene or ethylene group; and m is 1 or 2 and there is from about 2% to about 8% of said fatty acid amide.
[R1 (̵R2O)̵ n (̵ R3O )̵ m]y[R4]
wherein each R' is selected from the group consisting of hydrogen, alkyl groups containing from one to about 18 carbon atoms, acyl groups containing from two to about 18 carbon atoms, -S04M, -SO3M, -COOM, -N(R5)2)̵o,-N(R5)3 (+) , amide groups, pyrollidone groups, saccharide groups, and hydroxy groups in which each M is a compatible cation and each R5 is either an alkyl or hydroxy alkyl group containing from one to about four carbon atoms; wherein each R2 or R3 is an alkylene group containing from two to about six carbon atoms with no more than about 90% of said molecule comprising R2 or R3 groups containing two carbon atoms; wherein R4 is selected from the group consisting of alkylene groups containing from one to about 18 carbon atoms and having from two to about six valences, poly (hydroxyalkylene oxide) groups wherein each alkylene group has from one to about six hydroxy groups and contains from three to about eight carbon atoms and there are from two to about 50 hydroxyalkylene oxide groups and from two to about 50 hydroxy groups, (=NR2N=), hydrogen, = N (̵ R2NH )̵ x, polyester groups containing from one to about 20 ester linkages and each ester group containing from about 4 to about 18 carbon atoms, wherein n is from 0 to about 500, m is from 0 to about 500, n + m is from about 5 to about 1000, x is from about 2 to about 50, and y is from one to about 50 and equal to the valences of R'; wherein the molecular weight is from about 400 to about 60,000; and wherein the (R20) and the (R3O) groups are interchangeable and wherein R' contains no more than about six carbon atoms when R4 is hydrogen.
Priority Applications (1)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| AT86308453T ATE95834T1 (en) | 1985-10-31 | 1986-10-30 | LIQUID DETERGENT COMPOSITION. |
Applications Claiming Priority (4)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US79353085A | 1985-10-31 | 1985-10-31 | |
| US793530 | 1985-10-31 | ||
| US91856786A | 1986-10-20 | 1986-10-20 | |
| US918567 | 1986-10-20 |
Publications (3)
| Publication Number | Publication Date |
|---|---|
| EP0222557A2 true EP0222557A2 (en) | 1987-05-20 |
| EP0222557A3 EP0222557A3 (en) | 1988-09-28 |
| EP0222557B1 EP0222557B1 (en) | 1993-10-13 |
Family
ID=27121400
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| EP86308453A Expired - Lifetime EP0222557B1 (en) | 1985-10-31 | 1986-10-30 | Liquid detergent composition |
Country Status (7)
| Country | Link |
|---|---|
| EP (1) | EP0222557B1 (en) |
| AU (1) | AU589225B2 (en) |
| CA (1) | CA1299962C (en) |
| DE (1) | DE3689165T2 (en) |
| DK (1) | DK522786A (en) |
| FI (1) | FI87086C (en) |
| MX (1) | MX165143B (en) |
Cited By (12)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| GB2197338B (en) * | 1986-09-30 | 1990-09-26 | Kao Corp | Liquid detergent composition |
| WO1991006622A1 (en) * | 1989-10-31 | 1991-05-16 | Unilever N.V. | Detergent compositions |
| US5382376A (en) * | 1992-10-02 | 1995-01-17 | The Procter & Gamble Company | Hard surface detergent compositions |
| WO1995035361A1 (en) * | 1994-06-17 | 1995-12-28 | The Procter & Gamble Company | Hand wash laundry compositions |
| EP0916720A1 (en) * | 1997-11-17 | 1999-05-19 | The Procter & Gamble Company | Anti-bacterial liquid dishwashing detergent compositions |
| EP0906383A4 (en) * | 1996-05-31 | 1999-11-24 | Procter & Gamble | Detergent compositions |
| WO2003031550A1 (en) * | 2001-10-11 | 2003-04-17 | S. C. Johnson & Son, Inc. | Hard surface cleaners containing ethylene oxide/propylene oxide block copolymer surfactants |
| WO2015030768A1 (en) * | 2013-08-29 | 2015-03-05 | Colgate-Palmolive Company | Aqueous liquid compositions |
| USD845638S1 (en) | 2016-07-15 | 2019-04-16 | Colgate-Palmolive Company | Tootbrush |
| US20190161703A1 (en) * | 2017-11-27 | 2019-05-30 | The Procter & Gamble Company | Liquid hand dishwashing detergent composition |
| US11136532B2 (en) | 2017-11-27 | 2021-10-05 | The Procter & Gamble Company | Liquid hand dishwashing detergent composition |
| EP4624558A1 (en) * | 2024-03-26 | 2025-10-01 | The Procter & Gamble Company | Liquid hand dishwashing detergent composition |
Citations (4)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US3915903A (en) | 1972-07-03 | 1975-10-28 | Procter & Gamble | Sulfated alkyl ethoxylate-containing detergent composition |
| EP0024711A1 (en) | 1979-09-01 | 1981-03-11 | Henkel Kommanditgesellschaft auf Aktien | Watery tenside concentrates and process for the improvement of the flowing property of difficultly movable watery tenside concentrates |
| EP0083223A2 (en) | 1981-12-24 | 1983-07-06 | Leisure Products Corporation | Oil and grease emulsification system |
| EP0105556A1 (en) | 1982-09-30 | 1984-04-18 | THE PROCTER & GAMBLE COMPANY | Liquid detergent composition containing nonionic and ionic surfactants |
Family Cites Families (2)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US4483787A (en) * | 1983-12-28 | 1984-11-20 | The Procter & Gamble Company | Concentrated aqueous detergent compositions |
| DE3568455D1 (en) * | 1984-05-23 | 1989-04-06 | Rhone Poulenc Chimie | Detergent compositions containing copolymers based on polyoxyethylene and polyoxyalkylene used as antisoil redeposition agents, and process for their preparation |
-
1986
- 1986-10-30 AU AU64542/86A patent/AU589225B2/en not_active Ceased
- 1986-10-30 DE DE86308453T patent/DE3689165T2/en not_active Expired - Fee Related
- 1986-10-30 EP EP86308453A patent/EP0222557B1/en not_active Expired - Lifetime
- 1986-10-30 FI FI864424A patent/FI87086C/en not_active IP Right Cessation
- 1986-10-30 CA CA000521794A patent/CA1299962C/en not_active Expired - Lifetime
- 1986-10-31 MX MX4241A patent/MX165143B/en unknown
- 1986-10-31 DK DK522786A patent/DK522786A/en not_active Application Discontinuation
Patent Citations (4)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US3915903A (en) | 1972-07-03 | 1975-10-28 | Procter & Gamble | Sulfated alkyl ethoxylate-containing detergent composition |
| EP0024711A1 (en) | 1979-09-01 | 1981-03-11 | Henkel Kommanditgesellschaft auf Aktien | Watery tenside concentrates and process for the improvement of the flowing property of difficultly movable watery tenside concentrates |
| EP0083223A2 (en) | 1981-12-24 | 1983-07-06 | Leisure Products Corporation | Oil and grease emulsification system |
| EP0105556A1 (en) | 1982-09-30 | 1984-04-18 | THE PROCTER & GAMBLE COMPANY | Liquid detergent composition containing nonionic and ionic surfactants |
Cited By (22)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| GB2197338B (en) * | 1986-09-30 | 1990-09-26 | Kao Corp | Liquid detergent composition |
| WO1991006622A1 (en) * | 1989-10-31 | 1991-05-16 | Unilever N.V. | Detergent compositions |
| US5382376A (en) * | 1992-10-02 | 1995-01-17 | The Procter & Gamble Company | Hard surface detergent compositions |
| WO1995035361A1 (en) * | 1994-06-17 | 1995-12-28 | The Procter & Gamble Company | Hand wash laundry compositions |
| CN1082998C (en) * | 1994-06-17 | 2002-04-17 | 普罗格特-甘布尔公司 | Hand wash laundry compositions |
| EP0906383A4 (en) * | 1996-05-31 | 1999-11-24 | Procter & Gamble | Detergent compositions |
| EP0916720A1 (en) * | 1997-11-17 | 1999-05-19 | The Procter & Gamble Company | Anti-bacterial liquid dishwashing detergent compositions |
| WO1999025800A1 (en) * | 1997-11-17 | 1999-05-27 | The Procter & Gamble Company | Antibacterial liquid dishwashing detergent compositions |
| WO2003031550A1 (en) * | 2001-10-11 | 2003-04-17 | S. C. Johnson & Son, Inc. | Hard surface cleaners containing ethylene oxide/propylene oxide block copolymer surfactants |
| US6701940B2 (en) | 2001-10-11 | 2004-03-09 | S. C. Johnson & Son, Inc. | Hard surface cleaners containing ethylene oxide/propylene oxide block copolymer surfactants |
| WO2015030768A1 (en) * | 2013-08-29 | 2015-03-05 | Colgate-Palmolive Company | Aqueous liquid compositions |
| CN105473697A (en) * | 2013-08-29 | 2016-04-06 | 高露洁-棕榄公司 | Aqueous liquid compositions |
| AU2013399106B2 (en) * | 2013-08-29 | 2016-07-07 | Colgate-Palmolive Company | Aqueous liquid compositions |
| US9969960B2 (en) | 2013-08-29 | 2018-05-15 | Colgate-Palmolive Company | Aqueous liquid composition |
| CN105473697B (en) * | 2013-08-29 | 2019-02-15 | 高露洁-棕榄公司 | Aqueous liquid composition |
| USD845638S1 (en) | 2016-07-15 | 2019-04-16 | Colgate-Palmolive Company | Tootbrush |
| US20190161703A1 (en) * | 2017-11-27 | 2019-05-30 | The Procter & Gamble Company | Liquid hand dishwashing detergent composition |
| WO2019104106A1 (en) | 2017-11-27 | 2019-05-31 | The Procter & Gamble Company | Liquid hand dishwashing detergent composition |
| EP3502222A1 (en) | 2017-11-27 | 2019-06-26 | The Procter & Gamble Company | Liquid hand dishwashing detergent composition |
| US11136532B2 (en) | 2017-11-27 | 2021-10-05 | The Procter & Gamble Company | Liquid hand dishwashing detergent composition |
| US11155770B2 (en) | 2017-11-27 | 2021-10-26 | The Procter & Gamble Company | Liquid hand dishwashing detergent composition |
| EP4624558A1 (en) * | 2024-03-26 | 2025-10-01 | The Procter & Gamble Company | Liquid hand dishwashing detergent composition |
Also Published As
| Publication number | Publication date |
|---|---|
| FI864424A0 (en) | 1986-10-30 |
| DK522786A (en) | 1987-05-01 |
| AU6454286A (en) | 1987-05-07 |
| FI87086B (en) | 1992-08-14 |
| AU589225B2 (en) | 1989-10-05 |
| MX165143B (en) | 1992-10-29 |
| CA1299962C (en) | 1992-05-05 |
| DK522786D0 (en) | 1986-10-31 |
| DE3689165T2 (en) | 1994-05-05 |
| FI87086C (en) | 1992-11-25 |
| FI864424L (en) | 1987-05-01 |
| EP0222557B1 (en) | 1993-10-13 |
| EP0222557A3 (en) | 1988-09-28 |
| DE3689165D1 (en) | 1993-11-18 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| US5167872A (en) | Comprising anionic surfactant polymeric nonionic surfactant and betaine surfactant | |
| US4904359A (en) | Liquid detergent composition containing polymeric surfactant | |
| US4316824A (en) | Liquid detergent composition containing alkyl sulfate and alkyl ethoxylated sulfate | |
| US4492646A (en) | Liquid dishwashing detergent containing anionic surfactant, suds stabilizer and highly ethoxylated nonionic drainage promotor | |
| AU728470B2 (en) | Reduced residue hard surface cleaner comprising hydrotrope | |
| US4556509A (en) | Light duty detergents containing an organic diamine diacid salt | |
| CA2002095C (en) | High viscosity detergent gel composition and method of making same | |
| EP3971275B1 (en) | Liquid hand dishwashing cleaning composition | |
| EP0105556A1 (en) | Liquid detergent composition containing nonionic and ionic surfactants | |
| CA2973502A1 (en) | Cleaning composition and method of forming the same | |
| EP0222557B1 (en) | Liquid detergent composition | |
| US20140336094A1 (en) | Cleaning composition and method of forming the same | |
| US6423678B1 (en) | Alcohol ethoxylate-peg ether of glycerin | |
| EP0157443B1 (en) | Detergent composition containing semi-polar nonionic detergent, alkaline earth metal anionic detergent, and amidoalkylbetaine detergent | |
| USH1467H (en) | Detergent formulations containing a surface active composition containing a nonionic surfactant component and a secondary alkyl sulfate anionic surfactant component | |
| EP0034039B1 (en) | Liquid detergent composition | |
| MXPA97003374A (en) | Lig work liquid cleaning compositions | |
| WO1996014378A1 (en) | Light duty liquid cleaning compositions | |
| JPH08503236A (en) | Liquid dishwashing detergent composition | |
| JP2555037B2 (en) | Liquid detergent composition | |
| JPH07116478B2 (en) | Liquid detergent composition | |
| CA1170949A (en) | Liquid detergent composition | |
| EP4481024B1 (en) | Liquid hand dishwashing detergent composition | |
| CA1207210A (en) | Liquid detergent composition | |
| JPH08188794A (en) | Detergent composition |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| PUAI | Public reference made under article 153(3) epc to a published international application that has entered the european phase |
Free format text: ORIGINAL CODE: 0009012 |
|
| AK | Designated contracting states |
Kind code of ref document: A2 Designated state(s): AT BE CH DE FR GB GR IT LI NL SE |
|
| PUAL | Search report despatched |
Free format text: ORIGINAL CODE: 0009013 |
|
| AK | Designated contracting states |
Kind code of ref document: A3 Designated state(s): AT BE CH DE FR GB GR IT LI NL SE |
|
| 17P | Request for examination filed |
Effective date: 19890317 |
|
| 17Q | First examination report despatched |
Effective date: 19900608 |
|
| GRAA | (expected) grant |
Free format text: ORIGINAL CODE: 0009210 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: AT Payment date: 19931007 Year of fee payment: 8 |
|
| AK | Designated contracting states |
Kind code of ref document: B1 Designated state(s): AT BE CH DE FR GB GR IT LI NL SE |
|
| REF | Corresponds to: |
Ref document number: 95834 Country of ref document: AT Date of ref document: 19931015 Kind code of ref document: T |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: CH Payment date: 19931025 Year of fee payment: 8 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: GR Payment date: 19931027 Year of fee payment: 8 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: NL Payment date: 19931031 Year of fee payment: 8 |
|
| REF | Corresponds to: |
Ref document number: 3689165 Country of ref document: DE Date of ref document: 19931118 |
|
| ITF | It: translation for a ep patent filed | ||
| ET | Fr: translation filed | ||
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: BE Payment date: 19931130 Year of fee payment: 8 |
|
| REG | Reference to a national code |
Ref country code: GR Ref legal event code: FG4A Free format text: 3009662 |
|
| PLBE | No opposition filed within time limit |
Free format text: ORIGINAL CODE: 0009261 |
|
| STAA | Information on the status of an ep patent application or granted ep patent |
Free format text: STATUS: NO OPPOSITION FILED WITHIN TIME LIMIT |
|
| 26N | No opposition filed | ||
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: AT Effective date: 19941030 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: LI Effective date: 19941031 Ref country code: CH Effective date: 19941031 Ref country code: BE Effective date: 19941031 |
|
| EAL | Se: european patent in force in sweden |
Ref document number: 86308453.9 |
|
| BERE | Be: lapsed |
Owner name: THE PROCTER & GAMBLE CY Effective date: 19941031 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: GR Free format text: THE PATENT HAS BEEN ANNULLED BY A DECISION OF A NATIONAL AUTHORITY Effective date: 19950430 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: NL Effective date: 19950501 |
|
| NLV4 | Nl: lapsed or anulled due to non-payment of the annual fee | ||
| REG | Reference to a national code |
Ref country code: CH Ref legal event code: PL Ref country code: GR Ref legal event code: MM2A Free format text: 3009662 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: SE Payment date: 19991014 Year of fee payment: 14 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: SE Free format text: THE PATENT HAS BEEN ANNULLED BY A DECISION OF A NATIONAL AUTHORITY Effective date: 20001030 |
|
| EUG | Se: european patent has lapsed |
Ref document number: 86308453.9 |
|
| REG | Reference to a national code |
Ref country code: GB Ref legal event code: IF02 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: GB Payment date: 20040915 Year of fee payment: 19 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: FR Payment date: 20041004 Year of fee payment: 19 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: DE Payment date: 20041029 Year of fee payment: 19 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: IT Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES;WARNING: LAPSES OF ITALIAN PATENTS WITH EFFECTIVE DATE BEFORE 2007 MAY HAVE OCCURRED AT ANY TIME BEFORE 2007. THE CORRECT EFFECTIVE DATE MAY BE DIFFERENT FROM THE ONE RECORDED. Effective date: 20051030 Ref country code: GB Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20051030 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: DE Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20060503 |
|
| GBPC | Gb: european patent ceased through non-payment of renewal fee |
Effective date: 20051030 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: FR Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20060630 |
|
| REG | Reference to a national code |
Ref country code: FR Ref legal event code: ST Effective date: 20060630 |