CN1022335C - Container for use in hydrothermal synthesis - Google Patents
Container for use in hydrothermal synthesis Download PDFInfo
- Publication number
- CN1022335C CN1022335C CN90101411.7A CN90101411A CN1022335C CN 1022335 C CN1022335 C CN 1022335C CN 90101411 A CN90101411 A CN 90101411A CN 1022335 C CN1022335 C CN 1022335C
- Authority
- CN
- China
- Prior art keywords
- container
- surge chamber
- tubular
- tubular vessel
- space
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Expired - Fee Related
Links
Images
Classifications
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B01—PHYSICAL OR CHEMICAL PROCESSES OR APPARATUS IN GENERAL
- B01J—CHEMICAL OR PHYSICAL PROCESSES, e.g. CATALYSIS OR COLLOID CHEMISTRY; THEIR RELEVANT APPARATUS
- B01J19/00—Chemical, physical or physico-chemical processes in general; Their relevant apparatus
- B01J19/02—Apparatus characterised by being constructed of material selected for its chemically-resistant properties
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B01—PHYSICAL OR CHEMICAL PROCESSES OR APPARATUS IN GENERAL
- B01J—CHEMICAL OR PHYSICAL PROCESSES, e.g. CATALYSIS OR COLLOID CHEMISTRY; THEIR RELEVANT APPARATUS
- B01J3/00—Processes of utilising sub-atmospheric or super-atmospheric pressure to effect chemical or physical change of matter; Apparatus therefor
- B01J3/04—Pressure vessels, e.g. autoclaves
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B01—PHYSICAL OR CHEMICAL PROCESSES OR APPARATUS IN GENERAL
- B01J—CHEMICAL OR PHYSICAL PROCESSES, e.g. CATALYSIS OR COLLOID CHEMISTRY; THEIR RELEVANT APPARATUS
- B01J3/00—Processes of utilising sub-atmospheric or super-atmospheric pressure to effect chemical or physical change of matter; Apparatus therefor
- B01J3/04—Pressure vessels, e.g. autoclaves
- B01J3/046—Pressure-balanced vessels
-
- C—CHEMISTRY; METALLURGY
- C30—CRYSTAL GROWTH
- C30B—SINGLE-CRYSTAL GROWTH; UNIDIRECTIONAL SOLIDIFICATION OF EUTECTIC MATERIAL OR UNIDIRECTIONAL DEMIXING OF EUTECTOID MATERIAL; REFINING BY ZONE-MELTING OF MATERIAL; PRODUCTION OF A HOMOGENEOUS POLYCRYSTALLINE MATERIAL WITH DEFINED STRUCTURE; SINGLE CRYSTALS OR HOMOGENEOUS POLYCRYSTALLINE MATERIAL WITH DEFINED STRUCTURE; AFTER-TREATMENT OF SINGLE CRYSTALS OR A HOMOGENEOUS POLYCRYSTALLINE MATERIAL WITH DEFINED STRUCTURE; APPARATUS THEREFOR
- C30B7/00—Single-crystal growth from solutions using solvents which are liquid at normal temperature, e.g. aqueous solutions
- C30B7/10—Single-crystal growth from solutions using solvents which are liquid at normal temperature, e.g. aqueous solutions by application of pressure, e.g. hydrothermal processes
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B01—PHYSICAL OR CHEMICAL PROCESSES OR APPARATUS IN GENERAL
- B01J—CHEMICAL OR PHYSICAL PROCESSES, e.g. CATALYSIS OR COLLOID CHEMISTRY; THEIR RELEVANT APPARATUS
- B01J2219/00—Chemical, physical or physico-chemical processes in general; Their relevant apparatus
- B01J2219/02—Apparatus characterised by their chemically-resistant properties
- B01J2219/0204—Apparatus characterised by their chemically-resistant properties comprising coatings on the surfaces in direct contact with the reactive components
- B01J2219/0236—Metal based
Abstract
The present invention relates to a container for hydrothermal synthesis, which comprises a container body, an inner tubular container and a buffer chamber, wherein the container body is heated from the outer part of the container body; the inner tubular container is arranged in the container body; a tubular gap is formed between the container body and the inner tubular container; the inner tubular container is provided with an inner surface prepared from noble metals, such as silver, gold or platinum, and a cover; the cover is arranged on the inner surface and provided with a first small hole; the first small hole is covered by the buffer chamber; the buffer chamber is provided with a wall and the other wall with a second small hole, and the wall is limited by the cover.
Description
The present invention relates to the water thermal synthesis and produce the container used as synthetic quartz.
Hydro-thermal is synthetic to be shown in Fig. 4 with container usually, and it generally is made up of container body 21, lid 22, clamping element 23, convection current switchboard 24, well heater 25 and thermopair 26.Crystal seed A in the container body 21 and raw material B are dipped in the strong base solution of well heater 25 heating.Quartzy with the hydro-thermal synthesis of artificial for falling, be temperature and 1000~1500Kg/cm at 350-400 ℃
2Pressure use down this container, so this container body 21 must be used the intensity height, good toughness, corrosion resistant metallic substance manufacturing.
The many goods of hydro-thermal synthetic are used for electronics and optical device, require thin day by day and miniaturization.When if little and thin belt product is produced in the water thermal synthesis, the major cause that its quality product reduces is owing to be mingled with outer folder material.The subject matter that should point out in advance is especially, and the internal surface of container body 21 is produced the iron cpd that is called " acmite " by the strong base solution corrosion, and product contains Fe
+Ionic impurity.Adopted several different methods to solve this problem already, one of its way is to isolate the surface of crystal seed A, yet this method is also satisfactory not to the utmost, hinders the growth of crystal through the seed surface of protection, thereby productive rate is reduced.
Another measure of being taked is, on the internal surface of container body 21, be coated with mercury, gold or platinum one class precious metal or in container body 21, put into a tubular vessel by these precious metal systems, this is the generation that stops iron ion in essence, this first method is a coated mercury on the internal surface of container body 21, gold or platinum one class precious metal, must carry out boning between noble coatings and container body 21 internal surfaces guaranteeing of hydraulic buckling or explosive bonding one class measure to this tubular body arranged inside, but when this container of preparation, will run into quite big difficulty.And, according to the structure of container body 21, the situation that it is enough need hold sealed area plate face can take place.In the second approach, be about to as silver, the interior tubular vessel of gold or platinum system is put into container body 21, and the pressure at this moment must making in the tubular vessel equals outside operating pressure, thereby too high external pressure is affacted on this inner jar.Therefore, must keep the liquid volume in the inner jar to equal the outer liquid volume of this container.In view of the foregoing, try hard in order to stop Fe
+What above-mentioned two kinds of methods that ion produces had been suitable for only is small-sized laboratory containers.
In view of above-mentioned, main purpose of the present invention provides a kind of large-scale, is applicable to the container for use in hydrothermal synthesis of industrial operation.According to first purpose of the present invention, container for use in hydrothermal synthesis provided by the invention comprises a tubular vessel that is put in the said container body, one tubular space is arranged therebetween, at least the internal surface of tubular vessel is by preparing as silver, gold or platinum one class precious metal, covering of tubular vessel has an aperture in this, said synthesizing also comprises a surge chamber with container, this surge chamber covers said aperture, a surface of said surge chamber is limited by said lid, also has an aperture on another wall of said surge chamber.
According to another object of the present invention, surge chamber space at said vesse, the useful space of tubular space and the useful space of interior tubular vessel are equipped with the alkaline solution by specified proportion, in accordance with regulations, and the alkaline concentration in the alkaline concentration in surge chamber and the tubular space will be lower than in the tubular vessel.
According to above-mentioned two purposes of the present invention, fill up the poromeric material of good heat conductivity in the said tubular space between container body and the interior tubular vessel.
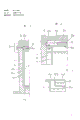
Fig. 1 is the semi-sectional view of the container for use in hydrothermal synthesis of example according to the present invention.
Fig. 2 is the major portion of lid of interior tubular vessel of container shown in Figure 1 and the sectional view of surge chamber.
Fig. 3 improves the major portion of the interior tubular vessel lid that designs and the sectional view of surge chamber.
Fig. 4 is the synthetic sectional view with existing known containers of hydro-thermal.
Tubular vessel dress crystal seed and raw material in of the present invention, synthetic from indirect heating container body to carry out hydro-thermal.The internal surface of interior at least tubular vessel prepares with precious metal, and hydro-thermal just can not produce the deleterious iron ion of crystal seed in the container between synthesis phase like this.
Tubular space between container body and the interior tubular vessel by it lid and the wall of surge chamber on the aperture that is provided with communicate with the inside of interior tubular vessel, thereby the pressure in the tubular vessel will equate to guarantee that moderate external pressure affacts on the interior tubular vessel with the pressure of outside like this.Interior tubular vessel will can not be out of shape or damage.
If the space of surge chamber, the useful space of the useful space of tubular space and interior tubular vessel is equipped with the alkaline solution of specified proportion.If make alkaline concentration in surge chamber and the tubular space be lower than in alkaline concentration in the tubular vessel, the pressure in the tubular space equates with pressure in the interior tubular vessel basically.Any variation of pressure all will be cushioned by the aperture on the gentle locular wall of covering of interior tubular vessel, thereby recovers the balance between tubular space and interior tubular vessel pressure.
If the pressure in the tubular space rises and dashes a spot of Fe that contains
+The ionic alkaline solution will enter surge chamber by the aperture on its wall, just mix the Fe in this alkaline solution with alkaline solution in the surge chamber thereupon
+Ion is also diluted, and then the crystal seed in the only internal tubular vessel of the alkaline solution after this dilution of tubular vessel produces minimum influence in entering.In addition, the alkaline concentration in the tubular space is also enough low does not have corrosive nature down to the internal surface to the container body.Because this effect has reduced Fe
+Ion produces, thereby has further reduced in the interior tubular vessel harmful effect to crystal seed.
As fill the poromeric material of good heat conductivity in tubular space, this not only makes calm body improve to the heat-transfer effect of interior tubular vessel, and the pressure in also making in the tubular vessel equals external pressure.
Come embodiment of the present invention are described with reference to Fig. 1-3, Fig. 1 is one according to embodiment of the present invention, is used for the semi-sectional view of container for use in hydrothermal synthesis.This container comprises following major parts; By high strength, high tenacity, corrosion resistant metallic substance preparation, container body 1 by indirect heating, container body lid 2, it be loaded on movably on the container body 1 by sealing member 2a and some clamping elements 3 and by as silver, gold or the preparation of platinum one class precious metal or by on its internal surface with tubular vessel 4 in the bottom of precious metal covering titanium and so on base metal system.Tubular vessel has removable cover 4a and base plate 4b in this, and they are by with the same material preparation of interior tubular vessel.As shown in Figure 2, aperture 4a ' is located substantially on the centre of covering 4a.The size of aperture 4a ' should make no any pressure effect in the above, and liquid is not because its surface tension can be dirty because of it self weight.As a kind of guide effect, the diameter in this hole is about 0.4mm.Surge chamber 5 be by be placed on aperture 4a ' go up and be fixed in the box-shaped member 5a of tubular vessel lid 4a upper surface formed.Have the aperture 5b that a diameter is about 0.4mm on the top board of box-shaped member 5a.The position of aperture 5b should be than the liquid level height in the surge chamber 5.At least the internal surface of surge chamber 5 should be prepared by precious metal.
The aforesaid interior tubular vessel 4 of its structure is put in the container body 1, but keeps a tubular space 1a between them.
Container shown in Figure 1 also has two convection current switchboard 6a and 6b.Plate 6a is within interior tubular vessel 4, and plate 6b is in the tubular space 1a of 1 of interior tubular vessel 4 and container body.Crystal seed is put in the interior tubular vessel 4 of convection current switchboard 6a top, and raw material is put in the below of convection current switchboard.The space of surge chamber 5, the useful space of the tubular space 1a that container body 1 and interior tubular vessel are 4 and in the useful space of tubular vessel 4 alkaline solution of specified proportion is housed, should make alkaline concentration among surge chamber 5 and the tubular space 1a be lower than in alkaline concentration in the tubular vessel 4.
Under these conditions, synthetic from indirect heating container body 1 to cause hydro-thermal.
Container of the present invention is operated as follows, and surge chamber 5 communicates with tubular space 1a via aperture 5b, communicates with the inside of interior tubular vessel 4 via aperture 4a '.Like this, if because the fluidic ratio is slightly different with prescribed value or because heating and cause in the temperature official post pressure in the tubular vessel 4 and external pressure not simultaneously between the fluid of tubular space 1a and interior tubular vessel 4 in tubular space 1a and the interior tubular vessel 4, in the surge chamber 5 alkaline solution will flow in the interior tubular vessel 4 or inflow tubular space 1a in so that pressure in the interior tubular vessel 4 and external pressure restore balance.
And, contain Fe among the tubular space 1a
+Just mix before the tubular vessel 4 in the ionic alkaline solution enters with alkaline solution in the surge chamber 5, so that enter Fe in the alkaline solution in the interior tubular vessel 4
+Ion has obtained sufficient dilution, and will reduce to minimum for the possible harmful effect of end product quality.
Alkaline concentration among the tubular space 1a is transferred to the concentration that is lower than in the interior tubular vessel 4, and this just effectively reduces the corrosion and the Fe of container body 1
+Ionic produces.
The surge chambers 5 different with structure shown in Figure 2 are shown in Fig. 3.This surge chamber is by the box-shaped member 5 lid 4a downside of tubular vessel in being fixed on and that aperture 4a ' is covered ' formed.The about 0.4mm of the diameter of aperture 5 ' b on the side plate of box-shaped member 5 ' a, is positioned on the liquid level of this surge chamber.This surge chamber 5 ' preferred surfaces externally and internally all prepares with precious metal.
Through aperture 4a " flow into surge chamber 5 ' contain Fe
+The ionic alkaline solution will be therewith alkaline solution in the surge chamber mix.Fe in this alkaline solution
+ Tubular vessel 4 in ion enters after fully being diluted again.The same effect of surge chamber 5 ' will play basically and surge chamber 5 like this.
Can understand that from top explanation container of the present invention has following advantages:
(1) use this container, the interior tubular container that wherein is placed with crystal seed will can not produce Fe+Ion.
(2) interior tubulose wall of a container is quite thin, but can make wherein pressure and external pressure keep balance, thereby can guarantee stable operation and do not cause inner tube shape container deformation or damage.
(3) when the container body is corroded by alkaline solution, can produce Fe
+Ion.Yet, if the pressure of interior tubular vessel outside raises the Fe that is produced
+Ion is cushioned alkaline solution in the chamber suitably after the dilution, and alkaline solution just flows into interior tubular vessel, so Fe
+The influence of the crystal seed in the ion pair in the tubular vessel is slight.
(4) alkaline concentration that contacts with the container body is so low, to such an extent as to the corrosive nature of container body is small enough to reduce Fe
+Generation.
In view of above-mentioned advantage, container of the present invention can make hydrothermal synthesis method produce quartzy and other products fine quality.
Claims (6)
1, container for use in hydrothermal synthesis in a kind of alkali solution is characterized in that said container comprises:
Container body from indirect heating;
Place said container body and therebetween form the interior tubular vessel of a tubular space, said in the internal surface of tubular vessel by such as silver, gold or the preparation of platinum one class precious metal, this container has a lid that has first aperture on it;
In order to the surge chamber that said first aperture is covered, said surge chamber has a wall for said lid qualification, and second aperture arranged on another wall of this surge chamber;
The useful space of the space of said surge chamber, the useful space of said tubular space and said interior tubular vessel is equipped with the alkaline solution of specified proportion respectively, and the alkaline concentration in said surge chamber and the said tubular space is lower than the alkaline concentration in the tubular vessel in said;
Said container body and said in the poromeric material of good heat conductivity is housed in the said tubular space between the tubular vessel.
2, container according to claim 1 is characterized in that the outside of wherein said surge chamber tubular vessel in said.
3, container according to claim 1 is characterized in that the inside of wherein said surge chamber tubular vessel in said.
4, container according to claim 1 is characterized in that wherein said second aperture is positioned at the alkaline solution liquid level top that said surge chamber is housed.
5, container according to claim 2 is characterized in that wherein said surge chamber has an internal surface by the precious metal preparation.
6, container according to claim 3 is characterized in that wherein the inside and outside surface of said surge chamber is all prepared by precious metal.
Priority Applications (3)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| JP63194338A JPH0722692B2 (en) | 1988-08-05 | 1988-08-05 | Hydrothermal synthesis container |
| CN90101411.7A CN1022335C (en) | 1988-08-05 | 1990-02-03 | Container for use in hydrothermal synthesis |
| DE4003377A DE4003377C1 (en) | 1988-08-05 | 1990-02-05 |
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| JP63194338A JPH0722692B2 (en) | 1988-08-05 | 1988-08-05 | Hydrothermal synthesis container |
| CN90101411.7A CN1022335C (en) | 1988-08-05 | 1990-02-03 | Container for use in hydrothermal synthesis |
Publications (2)
| Publication Number | Publication Date |
|---|---|
| CN1053819A CN1053819A (en) | 1991-08-14 |
| CN1022335C true CN1022335C (en) | 1993-10-06 |
Family
ID=36754727
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| CN90101411.7A Expired - Fee Related CN1022335C (en) | 1988-08-05 | 1990-02-03 | Container for use in hydrothermal synthesis |
Country Status (3)
| Country | Link |
|---|---|
| JP (1) | JPH0722692B2 (en) |
| CN (1) | CN1022335C (en) |
| DE (1) | DE4003377C1 (en) |
Families Citing this family (13)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| DE4229468C2 (en) * | 1992-09-03 | 1995-06-14 | Streitenberg Hubert Dr Med | Containers for liquid or pasty ingredients threatened by bacterial attack |
| US5552039A (en) * | 1994-07-13 | 1996-09-03 | Rpc Waste Management Services, Inc. | Turbulent flow cold-wall reactor |
| JP3366820B2 (en) * | 1997-02-19 | 2003-01-14 | 株式会社日立製作所 | Oxidation treatment method and apparatus and reaction vessel |
| US6398867B1 (en) * | 1999-10-06 | 2002-06-04 | General Electric Company | Crystalline gallium nitride and method for forming crystalline gallium nitride |
| PL207400B1 (en) | 2001-06-06 | 2010-12-31 | Ammono Społka Z Ograniczoną Odpowiedzialnością | Method of and apparatus for obtaining voluminous, gallium containing, monocrystalline nitride |
| AU2002328130B2 (en) | 2001-06-06 | 2008-05-29 | Ammono Sp. Z O.O. | Process and apparatus for obtaining bulk monocrystalline gallium-containing nitride |
| JP2003063889A (en) * | 2001-08-24 | 2003-03-05 | Tokyo Denpa Co Ltd | Vessel for growing single crystal |
| CA2464083C (en) | 2001-10-26 | 2011-08-02 | Ammono Sp. Z O.O. | Substrate for epitaxy |
| KR100679387B1 (en) | 2001-10-26 | 2007-02-05 | 암모노 에스피. 제트오. 오. | Nitride semiconductor laser devise and manufacturing method thereof |
| EP1514958B1 (en) | 2002-05-17 | 2014-05-14 | Ammono S.A. | Apparatus for obtaining a bulk single crystal using supercritical ammonia |
| AU2002354467A1 (en) | 2002-05-17 | 2003-12-02 | Ammono Sp.Zo.O. | Light emitting element structure having nitride bulk single crystal layer |
| CN1297695C (en) * | 2003-04-25 | 2007-01-31 | 郎丽红 | High pressure still for artificial quartz crystal |
| JP4276627B2 (en) | 2005-01-12 | 2009-06-10 | ソルボサーマル結晶成長技術研究組合 | Pressure vessel for single crystal growth and method for producing the same |
-
1988
- 1988-08-05 JP JP63194338A patent/JPH0722692B2/en not_active Expired - Lifetime
-
1990
- 1990-02-03 CN CN90101411.7A patent/CN1022335C/en not_active Expired - Fee Related
- 1990-02-05 DE DE4003377A patent/DE4003377C1/de not_active Expired - Lifetime
Also Published As
| Publication number | Publication date |
|---|---|
| JPH0722692B2 (en) | 1995-03-15 |
| CN1053819A (en) | 1991-08-14 |
| JPH0243939A (en) | 1990-02-14 |
| DE4003377C1 (en) | 1991-08-08 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| CN1022335C (en) | Container for use in hydrothermal synthesis | |
| ATE66964T1 (en) | PLANT FOR ON-LINE DEGASSING AND FILTRATION OF ALUMINUM AND ALUMINUM ALLOYS. | |
| CA2009656A1 (en) | Molten metal pump with filter | |
| CA1176867A (en) | Liquid level system | |
| US3991929A (en) | Coating and bonding of metals | |
| US4042753A (en) | Composite conductor | |
| US3271114A (en) | Crystal growth container | |
| US5057286A (en) | Vessel for use in hydrothermal synthesis | |
| US4337624A (en) | Cryostatic device | |
| KR920003914B1 (en) | Vessel for use in hydro thermal synthesis | |
| CA2111791A1 (en) | Electrolytic process for dissolving platinum, platinum metal impurities and/or platinum metal alloys | |
| JP2002520645A5 (en) | ||
| DE29724638U1 (en) | Device for raising the temperature of a wine or an alcoholic beverage in a container | |
| Bagster et al. | LXXIII.—Electrolysis of hydrogen bromide in liquid sulphur dioxide | |
| USH580H (en) | Method and apparatus for growing high perfection quartz | |
| CN2504214Y (en) | Sealing valve for vacuum vessel | |
| CN214622438U (en) | Measuring device for chemical reaction thermodynamic function temperature coefficient based on electromotive force method | |
| CN220529717U (en) | Novel container | |
| Fouad et al. | Mass transfer at cylindrical gas evolving electrodes | |
| DE20016484U1 (en) | Housing for electrochemical cells | |
| CN215044488U (en) | Stress-resistant packaging bottle under seasonal temperature difference | |
| CN1017812B (en) | Deposition apparatus | |
| KR100202730B1 (en) | Manufacturing method of infrared sensor | |
| Kurov et al. | Study of the Mechanism of Creep and Fracture of Aluminum in Torsion | |
| EP0335840A3 (en) | A gravity valve device to prevent liquids from draining completely out of vessels |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| C06 | Publication | ||
| PB01 | Publication | ||
| C10 | Entry into substantive examination | ||
| SE01 | Entry into force of request for substantive examination | ||
| C14 | Grant of patent or utility model | ||
| GR01 | Patent grant | ||
| C19 | Lapse of patent right due to non-payment of the annual fee | ||
| CF01 | Termination of patent right due to non-payment of annual fee |